Abstract
This article provides a comprehensive assessment of dioxins contaminating the soil and evaluates the bioremediation technology currently being widely used, and also offers recommendations for future prospects. Soil pollution containing dioxins is extremely toxic and hazardous to human health and the environment. Dioxin concentrations in soils around the world are caused by a variety of sources and outcomes, but the main sources are from the consequences of war and human activities. Bioremediation technology (bioaugmentation, biostimulation, and phytoremediation) is considered an optimal and environmentally friendly technology, with the goal of applying native microbial communities and using plant species with a high biomass to treat contaminated dioxins in soil. The powerful bioremediation system is the growth of microorganisms that contribute to the increased mutualistic and competitive relationships between different strains of microorganisms. Although biological treatment technology can thoroughly treat contaminated dioxins in soil with high efficiency, the amount of gas generated and Cl radicals dispersed after the treatment process remains high. Further research on the subject is required to provide stricter control over the outputs noted in this study.
Keywords: dioxins, soil, contamination, bioremediation, toxic
1. Introduction
Dioxins are environmentally stable solid substances with high melting and boiling points and very low vapor pressure [1]. Dioxins are almost insoluble in water, have high thermal stability that can only be completely decomposed at temperatures above 1200 °C, and are resistant to strong acids and alkalis and adhere to the surface of organic resources, especially in soil [2,3]. In addition, dioxins are also substances which are man-made through activities such as the production of 2,4,5-T herbicides, chlorine-containing plant protection agents, combustion processes (the burning municipal waste, medical waste, industrial waste, especially waste containing PVC and metallurgical processes), and pulp bleaching with chlorine substances [4,5,6]. Dioxins are dangerous threat agents, even at low concentrations (one part per billion), which are enough to wreak havoc on human health and the environment [3]. In humans, dioxin exposure in humans effects the endocrine glands and reproductive functions, causes diseases in the central and peripheral areas of the nervous system, and impedes fetal development, especially in the nervous system and joints [7,8]. Dioxins can be remain in the environment for a long time, seeping deeply into soil and sediments [2]. Therefore, dioxins remaining in the soil and sediments will seep into water sources and ecosystems, including those that produce fish, shrimp, vegetables, and crops, posing a risk of poisoning for future generations [9].
Several studies have been conducted on physical, chemical, and biological dioxin remediation technologies. In physical treatment, physical processes, such as radioactive material degradation using solvent extraction and liquefied petroleum gas extraction methods [10,11], steam distillation [12], thermal desorption [13], and in situ vitrification [14] are used to degrade persistent organic pollutants,. Chemical reactions, such as basic catalytic decomposition, above and below extreme water treatment [15], photolysis at the spot level [16,17], electronic solvation technology [18], and K-polyethylene glycol technology [19] are used in chemical treatments to degrade persistent organic pollutants in the soil. Recently, nanotechnology has also been applied, using nanoscale zero-valent iron [20] to decompose persistent organic pollutants. Bioremediation aims to remove persistent organic compounds from contaminated soil using the anaerobic and aerobic decomposition of microorganisms [21,22]. Indigenous microorganisms enriched from dioxin-contaminated sites are believed to be able to remove dioxins [23,24]. The dechlorination of dioxins by microbial metabolism under anaerobic and aerobic conditions are the two main mechanisms of dioxin degradation [25]. Microbial strains can use dioxins as a carbon and energy source [26] to effectively dechlorinate dioxins against highly chlorinated congeners [27]. Phytoremediation is more effective and widespread, but only with the use of plants that have a large biomass, and is capable of dioxin adsorption in many laboratory and field studies [28,29].
There are many studies on airborne and food dioxins as the main sources in different countries, but there are not many general reports on dioxin-contaminated soil, especially for the bioremediation of dioxins in soil. Furthermore, this paper focuses on analyzing, comparing, and discussing a global overview of the situation of dioxin-contaminated soil. This review provides a comprehensive summary and discussion of relevant studies on dioxin-contaminated soil bioremediation. An assessment will contribute to the optimization parameters of bioaugmentation, biostimulation, and phytoremediation in the remediation of dioxin-contaminated soils. Current knowledge, research challenges/gaps, and prospects for future research are presented in this study.
2. Overview of Dioxins
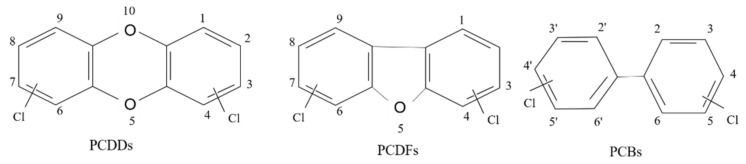
Dioxins are persistent organic pollutants produced by both natural and human activities [30]. Dioxins are also the common name for a group of hundreds of chemical compounds that persist in the environment, as well as in the human body and other organisms [31]. Dioxins are very stable compounds with low polar, lipophilic, and hydrophobic qualities. They are classified into three groups: polychlorinated dibenzo-p-dioxins (PCDDs, referred to as dioxins), polychlorinated dibenzofurans (PCDFs, referred to as furans), and coplanar polychlorinated biphenyls (dioxin-like PCBs, referred to as dl-PCBs). The chemical structures of PCDDs, PCDFs, and PCBs are shown in Figure 1 [1]. Depending on the number of chlorine atoms and the spatial position, dioxins have 75 congeners PCDD (poly-chloro-dibenzo-dioxins) and 135 congeners PCDFs (poly-chloro-dibenzo-furans) with different toxicity. In 210 congeners, 17 congeners are known to be highly toxic because they can have chlorine atoms at positions 2, 3, 7, and 8, (at least) on the benzene ring [3]. Based on the international toxicity equivalence factor (I-TEF), 2,3,7,8-TCDD/TCDFs are known to be the most toxic compounds [32].
Figure 1.
The basic structures of Polychlorinated dibenzo-p-dioxin (PCDD), dibenzofuran (PCDF), and biphenyls (PCBs).
2.1. Sources, Fate, and Transportation of Dioxins in Soil
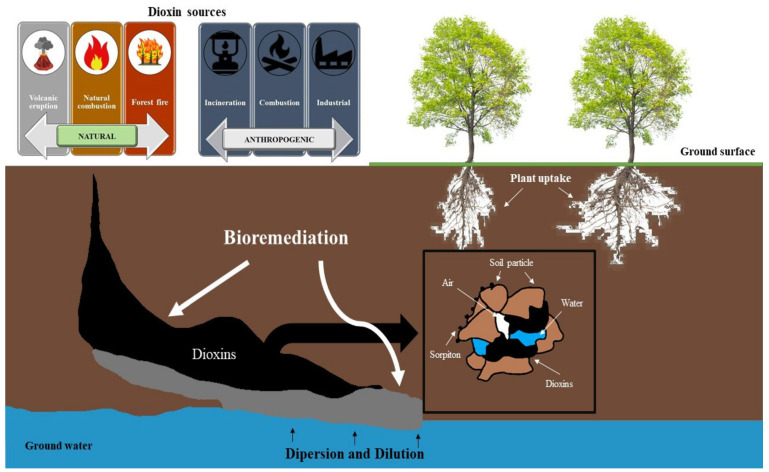
Dioxin sources are typically found in both natural and anthropogenic environments (Figure 2). Dioxins are a typical examples of persistent organic compounds with extremely complex structures and many different toxicities. Dioxins are naturally emitted from volcanic eruptions [33], forest fires [34], and natural combustion [35]. However, previous studies have shown that the emission of dioxins in the environment is mainly caused by humans. The main anthropogenic origins of dioxins are classified into three sources: incineration, combustion, and industrial processes [30,36,37].
Figure 2.
Sources, fate, and transportation of dioxins in soil.
Dioxins in soil are typically solid and cling to soil particles [38]. The fate and transport of dioxins in soil are depicted in Figure 2. Dioxins undergo diffusion and dispersion, as well as biodegradation processes (bioaugmentation, biostimulation, and phytoremediation) [39]. In addition, the fate and transport of dioxins in the soil media is affected by soil characteristics (moisture content, soil texture, pH, and organic matter) and environmental conditions (ground surface, groundwater, plants, weather conditions, and biological activity) [40]. Since soil particles often adsorb dioxins based on their low mobility and biodegradability [38], understanding the fate and transportation of dioxins in contaminated soil is essential in developing bioremediation technologies.
2.2. Toxicity and Health Risk Assessment
The World Health Organization (WHO-2005) has recommended the standard exposure levels of dioxins as 1–4 picograms WHO-TEQ/kg of body weight per one day, or 0.07 ppt in blood; the general environmental limit in most countries is 1200 ppt TEQ in soil and 150 ppt in sediment [3]. The United States Environmental Protection Agency (US-EPA) considers reducing the dioxins limit to 72 ppt TEQ to increase the volume of contaminated soil to be treated [3]. Dioxins, as a dangerous threat agent to the environment and humans, are associated with severe damage to human health, shortening the lives of those exposed and potentially shortening the lives of their children and future generations [3]. When dioxin levels in humans exceed the allowable threshold of 0.0064 picograms/kg of the human body (US-EPA), dark patches of skin appear quickly as a result of cell death, mutated pigment cells, and impaired liver and kidney function [8,41]. If long-term exposure to levels exceeds the threshold, dioxins will affect the immune system, endocrine glands, and reproductive functions, leading to some cancers and diseases of the central and peripheral nervous systems, thyroid disorders, immune system damage, endometriosis, diabetes, etc. [8,42]. As a result, children of exposed parents were born with many tragic deformities, and some children lived in a vegetative state from birth; the association between dioxins exposure and five diseases, such as soft tissue cancer, benign lymphoma, chronic lymphocytic leukemia, hairy leukemia cancer, and chlorosis was also noted [2,43]. Some diseases associated with dioxin exposure, such as acute, chronic, and subacute peripheral neuropathy; chlorosis; type 2 diabetes; liver cancer; lipid metabolism disorders; reproductive abnormalities and birth defects, such as cleft lip and palate; congenital malformations of the legs, hydrocephalus, neural tube defects, adhesions (sticky fingers or toes), muscle malformations, and paralysis [43] have also been observed. The half-life of dioxins in the soil is from 60 to 80 years, and at the same time, it persists for a long time in the environment, seeps into the soil and sediments, and migrates into vegetation and aquatic life, leading to bioaccumulation in the soil and food chain [2,9,44].
3. Situation of Dioxin-Contaminated Soil and Standard Limits
Environmental pollution in general, and dioxins pollution in the soil in particular, are markedly on the rise. The main sources of dioxin emissions are industrial activities (such as combustion) which are an important part of human production. Dioxin emissions from G20 industrial activities account for more than 80% of the estimated annual emissions [41]. According to Miguel Dopico and Alberto Gómez et al., 2015, annual global dioxin emissions were 17,226 kg, equivalent to about 287 kg-TEQ. The main sources of dioxins in the soil environment are fuel combustion, metal production, pesticide production and use, waste incineration, accidental fires, landfill disposal, combustion, and herbicide runoff in agricultural uses [42].
Many countries around the world are currently hotspots for dioxin contamination, including Germany [43], Korea [44], the Mediterranean [45], South Africa [46], Poland [47], China [48], Vietnam [3,49], etc. In China, soil dioxin concentrations are primarily found in soils in the vicinity of production areas, such as urban, agricultural, and mountain soils [48]. Dioxin concentrations in soil in China have varied ranges at the provincial sampling points, listed in the Table 1 below, used to assess the concentration and health risks of people living in the area. According to research by Gene J. Zheng et al. in the mainland of China, Hong Kong, and Taiwan, the main source of PCDD/Fs pollution is from industrial waste activities, with total PCDD/Fs up to 967,500 ppt of dry weight in a soil sample located in the eastern part of Guangdong Province [50]. Soil samples in urban and rural areas in Liaoning province were also compared, and the results revealed that the concentration of dioxin-like PCBs in urban areas is higher than in rural areas [51]. In general, the dioxin concentrations in the east of China are lower than in the rest of China, and the urban and manufacturing areas are higher than the rural areas. While dioxins in the soil are primarily found in China due to industrial activities, high concentrations of dioxins have been found in Vietnam in areas affected by previous wars due to Agent Orange [49]. The majority of research documents on dioxin content in Japanese soil come from agricultural sources and incinerators; there have not been many studies published on this topic in recent years. A study of soil samples taken in China and Korea’s coastal areas revealed that the concentrations of PCDD/Fs at the sampling sites in Korea were higher than those in China, but both countries are lower than Japan [52].
Table 1.
The average concentration of dioxins (homogeneous unit calculated in ppt TEQ in dry weight) in some different nations in Asia.
| Country | Year | Type of Soil | Source Area | Concentration | References |
|---|---|---|---|---|---|
| China (Sichuan) | 2013 | Soil | High mountain area | 2.48–4.30 ppt | [53] |
| China (mainland Hong Kong and Tai wan) | 2008 | Soil | Schistosomiasis disease area | 244.8–33,660 ppt | [50] |
| Soil | E-waste recycling | 799,000–967,500 ppt | [50,54] | ||
| Paddy soil | E-waste recycling | 2552–2726 ppt | [50] | ||
| Soil | Pentachlorophenol manufacturing factory | 606,000 ppt | [50,55,56] | ||
| South China | 2022 | Surface Soil | Municipal solid waste incinerator | 114–2440 ppt | [57] |
| North China | 2020 | Soil | Urban green space in a metropolis | 11.5–91.4 ppt | [58] |
| Eastern China | 2009 | Surface Soil | Electronic solid-waste with incinerators | 0.017–5.04 ppt | [59] |
| North China | 2011 | Topsoil | Coastal areas | 6.78–12.3 ppt | [52] |
| Central Vietnam | 2019 | Surface soil | The storage of Agent Orange in A-So Airbase during the Vietnam War | 2.7 to 746 ppt | [60] |
| Southern Vietnam | 2007 | Topsoil | Bien Hoa Airbase was a former storage depot for Agent Orange | 4.6–184 ppt | [61] |
| Japan (Osaka) | 2013 | Surface soil | Incineration plant | >1000 ppt | [62] |
| Paddy field soil | Former herbicide use | 38–110 ppt | |||
| Japan (Akita) | 2007 | Paddy soil | Agricultural area | 18,000–540,000 ppt | [63] |
| Non-agricultural soil samples | Parks | 950–1400 ppt | |||
| South Korea | 2021 | Soil | Industrial sites | 77.73 ppt | [64] |
| West Korea | 2011 | Topsoil | Coastal areas | 14.2–27 ppt | [52] |
Dioxin concentrations have not decreased in Europe in recent years, but instead, have increased significantly due to new dioxins emission sources, such as the illegal disposal of electric transformers in Italy [65] and Sweden [66]. Besides the main sources of pollution from manufacturing industries, motor vehicle emissions are one of the sources of pollution in Russia, because motor fuel combustion is dependent on the dopes used [67]. The results of dioxin concentrations in various regions of European countries are compared in Table 2; it is revealed that the concentration of dioxins in the soil in Spain, Slovakia, and Austria was lower than in other countries. Similarly, another study in Spain on dioxin levels affected by various sources, including municipal solid waste incineration, clinical waste incineration, and industrial areas, found results ranging from 0.45 to 14.41 ppt-TEQ (dry weight), with the effects of uncontrolled combustion processes being the most severe [68]. Research results on dioxin content in the soil in the UK by C.S. Creaser et al. showed that uncontrolled combustion leads to higher results in urban areas than in rural areas [69,70].
Table 2.
The average concentration of dioxins (homogeneous unit calculated in ppt TEQ in dry weight) in some different nations in Europe.
| Country | Year | Type of Soil | Source Area | Concentration | References |
|---|---|---|---|---|---|
| Sweden | 2013 | Soil | Contaminated sawmill site | 0.62–690,000 ppt | [66] |
| Russia | 2011 | Soil | Urban site | 8.2 ppt | [67] |
| Poland | 2015 | Soil | Urban site | 475.48–3039.27 ppt | [47] |
| Germany | 2007 | Soil | Alluvial flood plain of the river |
7680 ppt | [57] |
| Spain | 2006 | Topsoil | High industrial activity zones | 0.33–9.99 ppt | [71] |
| Slovakia | 2012 | Topsoil | Industrial site | 0.34 to 18.05 ppt | [72] |
| Austria | 2004 | Soil | Agricultural site | 0.05–23 ppt | [73] |
Similar to the results of searching for research documents on the situation of dioxin pollution in the soils of European countries, there are few data on the situation of soil research in America in recent years. In general, across the United States, dioxin concentrations in urban soil are generally higher than in rural soil, with maximum concentrations reaching 186 ng/kg according to TEQ in urban areas [74]. Another study conducted in Washington state discovered relatively low dioxin concentrations in soils, ranging from 0.14 to 4.1 ng/kg by TEQ, with agricultural land having the lowest dioxin value and urban land having the highest dioxin value, in accordance with other studies [75].
Research data on dioxins in countries in Africa are limited because the cost of dioxin analysis is expensive, and the analytical capacity is limited in this country [76]. According to research by C. Nieuwoudt et al., the dioxin concentrations in the soil of the sampled areas in Africa ranged from 0.34 to 20 ng/kg by TEQ, lower than those in Europe and the US [46,77]. The study also found that dioxin concentrations at industrial sites were higher than in agricultural soils and higher than in non-industrial sites, with combustion sources being the primary polluters [77].
Depending on soil pollution and effective land-use planning, several organizations around the world, such as the US EPA and WHO, have issued regulations on the concentration of pollutants in the soil (e.g., dioxins). However, some countries have developed industries in which the emission of pollutants into the soil is high (or have been affected by war, such as Vietnam), so each country will have its own regulations on PCDD/Fs concentrations in soil. The details are presented in Table 3.
Table 3.
Some standards limitations for PCDD/Fs (ppt TEQ) in different nations.
| National | Standard Limitation | Comments | Regulation/Guideline Values | References |
|---|---|---|---|---|
| US EPA Region 5 | 11 ppt 38.6 ppt |
PCDD in soil PCDFs in soil |
US EPA Region 5 ecological screening levels | [78] |
| US EPA Region 9 | 39 ppt | Residential soil | US EPA Region 9 preliminary remediation goal for 2,3,7,8-TCDD | [79] |
| China (Taiwan) | 1000 ppt | General soil | The standard limit—Taiwan EPA | [80] |
| Vietnam | 100 ppt 300 ppt 1200 ppt |
Forest soil Agricultural soil Commercial soil |
National technical regulation on the permissible limit of dioxins in soil | [81] |
| Finland | 500 ppt | Agricultural and residential soil | Finland Ministry of the Environment, Department for Environmental Protection |
[82] |
| Sweden | 10 ppt 250 ppt |
Land with sensitive use, Land with less sensitive use and groundwater extraction |
Sweden Generic Guidance Value | [82] |
| Netherlands | 10 ppt 1000 ppt |
Dairy farming Agricultural and residential soil |
The Netherlands Guidelines | [82] |
| Germany | 5–40 ppt 100 ppt 1000 ppt 10,000 ppt |
Agriculture Landscape Residential soil Industrial soil |
Germany regulatory limit and recommendation | [82] |
| New Zealand | 100 ppt 1500 ppt 18,000 ppt 90,000 ppt 21,000 ppt |
Agricultural soil Residential soil Industrial soil Industrial-paved soil Maintenance |
New Zealand Interim Acceptance Criteria | [83] |
| Canada | 4 ppt | Alert soil | Canadian Environmental Quality Guidelines | [84] |
4. Biodegradation Technologies of Dioxins
Biological treatment methods to reduce pollutants in different environments are considered effective and environmentally friendly [39]. Bioaugmentation is the addition of a degradation capacity into the soil to increase contaminant degradation, with effective potential for the bioremediation of organic-contaminated soils [85,86]. The addition of nutrients can encourage microbial activity by adjusting soil nutrients, which is known as biostimulation [87]. Composting is used to convert organic waste into simple organic substances. Bio-composting has traditionally been considered an eco-friendly remedy for organic soil contaminants, including petroleum, dioxins, and furans. Composting incubation is divided into mesophilic, thermophilic, cooling, and maturation stages, depending on microbial metabolism and heat production [88]. The mesophilic phase (<45 °C) occurs when the microbial community adapts to the initial conditions, and their numbers increase rapidly due to the readily degradable organic substrates [89]. Phytoremediation is frequently studied for its potential for immediate soil use with persistent organic compounds [28]. The main mechanisms involved in phytoremediation are based on the combined effects of plant uptake and the accumulation of toxic substances [90]. In this paper, bioremediation technology, including bioaugmentation, biostimulation, and phytoremediation, is analyzed and discussed below for the treatment of dioxin contamination in soil.
4.1. Bioaugmentation
Using microorganisms and fungus is currently an active application trend in dioxin-contaminated soil because of its low cost and environmental friendliness. The dechlorination of dioxins by microbial metabolism under anaerobic and aerobic conditions are the two main mechanisms of dioxin degradation in biological treatment [25]. Microbial strains can use dioxins as a carbon and energy source [26] to effectively dechlorinate dioxins from highly chlorinated isomers [27]. Table 4 lists microorganisms strains capable of degrading dioxins. Certain microorganisms such as Pseudomonas, Mendocino, and Dehalococcoides have been shown to effectively dechlorinate dioxins under anaerobic conditions [25,91,92]. Furthermore, aerobic microorganisms were discovered to degrade dioxins more efficiently and quickly than typical dioxin-contaminated soil anaerobes. Catechol 2,3-dioxygenase (C23O) is an important enzyme that catalyzes the reaction using molecular oxygen to destroy benzene rings [22], and Bacillus (Firmicutes) is the most dominant strain in aerobic degradation [93]. In addition to the strains of microorganisms that have been found to degrade dioxins with high efficiency, fungi also play a similar role, with their high mass and rapid environmental metabolism. Fungi are a diverse group of organisms, present in most environments and playing an integral role in ecosystems. In addition, fungi can regulate the flow of nutrients and energy through their network [89]. Furthermore, fungi are also unique organisms that can be used in the remediation of persistent organic wastes (POPs) in different environments, such as soil, water, and air [94]. Soils heavily contaminated with dioxins can also use fungi to decompose (with high efficiency) some typical strains such as Cordyceps sinensis strain A [15], Phlebia lindtneri [95,96], Phanerochaete sordida YK-264 [97], etc. Table 5 presents some fungal strains capable of decomposing dioxins in soil, with high efficiency.
Table 4.
Bacterial strains capable of biodegrading dioxins in a soil matrix.
| Bacterial Strains | PCDD/Fs Congeners | Concentration | Removal Average (%) | Time | References |
|---|---|---|---|---|---|
| Terrabacter sp. strain DBF63 | 2-CDD | 10 μg/mL | 75 | 18 h | [98] |
| 2,3-CDD | 80 | ||||
| 2-CDF | 82.5 | ||||
| 2,8-DCDF | 85 | ||||
| Pseudomonas sp. strain CA10 | 2-CDF | 60 | |||
| Pseudomonas sp. strain CA10 | 2-CDD | 1 μg/mL | 97 | 5 d | [98] |
| 2,3-CDD | 89 | ||||
| Sphingomonas sp. strain RW1 | DD | 10 ppm | 90 | 24 h | [99] |
| 2-CDD | 90 | ||||
| Sphingomonas sp. strain KA1 | 2-CDD | 1 μg/g | 96 | 7 d | [100,101] |
| 2,3-DCDD | 70 | ||||
| Rhodococcus opacus SAO 101 | 1-CDD | 1 ppm | 92 | 7 d | [102] |
| Dioxin (DD) | 97 | ||||
| Pseudomonas aeruginosa | 3,6-DCDF | 10 mg/L | 60 | 5 d | [103] |
| 1,2,3,4-TCDD | 84 | ||||
| DBF | 90 | ||||
| Pseudomonas veronii PH-03 | 1-MCDD | 1 μM | 88.3 | 60 h | [104] |
| 2-MCDD | 78.6 | ||||
| DD | 90.7 | ||||
| DF | 79.7 | ||||
| Sphingomonas sp. wittichi RW1 | DD | 1 mM | 81 | 72 h | [105] |
| PCDD | 29 ppt | 75.5 | 15 d | ||
|
Pseudomonas resinovorans strain CA10 |
2,3-DCDD | 1 μg/kg | 90.95 | 7–14 d | [106] |
|
Pseudomonas resinovorans strain CA10 |
2,3-DCDD | 1000 μg/L | 100 | 14 d | [106] |
| Pseudomonas sp. CA10 | 2-CDD | 10,000 μg/L | 98.5 | 7 d | [99] |
| Pseudallescheria boydii | 2,3,7,8-TCDD | 125 ng/g | 92 | 15 d | [107] |
| Stropharia rugosoannulata | 1,2,3,4,6,7,8-HpCDF | 200 μg/L | 64 | 3 m | [91] |
| Bacillus-Firmicutes | 2,3,7,8-TCDD | 136.33 ng/g | 75 | 42 d | [108] |
| Bosea BHBi7 | 2,3,7,8-TCDD | 170 ng/g | 59.1 | 21 d | [109] |
| Hydrocarboniphaga BHBi4 | |||||
| Pseudomonas mendocina NSYSU | OCDD | 20.1 mg/kg | 74 | 60 d | [92] |
Table 5.
Degradation of dioxins by fungi strains in soil matrix.
| Fungi sp. Name | Pollutants Compounds | Nutrients/Conditions | Removal (%) | Time | References |
|---|---|---|---|---|---|
|
Cordyceps sinensis strain A |
DD | Glucose or 1,4-dioxane | 50 | 4 d | [15] |
| 2,3,7-CDD | 50 | ||||
| octaCDD | 50 | ||||
| Phanerochaete sordida YK-624 | 2,3,7,8-TetraCDD | Glucose | 70 | 7 d | [97] |
| 1,2,3,7,7-PentaCDD | 70 | ||||
| 1,2,3,4,7,8-HexaCDD | 75 | ||||
| 1,2,3,4,6,7,8-HeptaCDD | 70 | ||||
| 1,2,3,4,6,7,8,9-OctaCDD | 70 | ||||
| 2,3,7,8-TetraCDF | 45 | ||||
| 1,2,3,7,8-PentaCDF | 45 | ||||
| 1,2,3,4,7,8-HexaCDF | 75 | ||||
| 1,2,3,4,6,7,8-HeptaCDF | 70 | ||||
| 1,2,3,4,6,7,8,9-OctaCDF | 70 | ||||
|
Acremonium sp. strain 622 |
T4CDD | Activated sludge and effluent | 73 | 24 h | [110] |
| P5CDD | 85 | ||||
| H6CDD | 79 | ||||
| H7CDD | 76 | ||||
| O8CDD | 88 | ||||
| T4CDF | 81 | ||||
| P5CDF | 88 | ||||
| H6CDF | 84 | ||||
| H7CDF | 84 | ||||
| O8CDF | 71 | ||||
|
Phanerochaete chrysosporium strain PcCYP11a3 |
1-MCDD | Glucose | 100 | 2 h | [111] |
| 2-MCDD | 38.2 | ||||
| 2,3-DCDD | 6.1 | ||||
| Pleurotus pulmonarius strain BCRC36906 | HexaCDD/Fs | Solid state fermentation (SSF) | 80 | 72 d | [112] |
| HeptaCDD/Fs | 97 | ||||
| OctaCDD/Fs | 90 | ||||
|
Phlebia radiata strain 267 |
1,2,3,4,7,8-H6CDD | Laccase, Tween-80 50 mL | 28 | 30 d | [113,114] |
| 1,2,3,7,8-P5CDF | 29 | ||||
| 2,3,7,8-T4CDF | 60 | ||||
|
Phlebia radiata strain PL1 |
1,2,3,7,8-P5CDD | Laccase, 50 mL Tween-80 | 76.3 | 30 d | [113,114] |
| 1,2,3,4,7,8-H6CDD | 75.6 | ||||
| 1,2,3,6,7,8-H6CDD | 79.4 | ||||
| 1,2,3,7,8,9-H6CDD | 79.3 | ||||
| 1,2,3,4,6,7,8-H7CDD | 79 | ||||
| octaCDD | 80 | ||||
| 1,2,3,4,7,8-H6CDF | 100 | ||||
| 1,2,3,6,7,8-H6CDF | 100 | ||||
| 2,3,4,6,7,8-H6CDF | 82.3 | ||||
| 1,2,3,4,6,7,8-H7CDF | 70.2 | ||||
| 1,2,3,4,7,8,9-H7CDF | 100 | ||||
| octaCDF | 67.4 | ||||
|
P. brevispora strain BMC3014 |
2,7-DiCDD | Glucose and ammonium tartrate | 33.8 | 14 d | [95,114] |
| 2,3,7-TriCDD | 20 | ||||
| 1,2,8,9-TetraCDD | 15 | ||||
| 1,2,6,7-TetraCDD | 18 | ||||
|
P. brevispora strain BMC9152 |
2,7-DiCDD | 54 | |||
| 2,3,7-TriCDD | 30 | ||||
| 1,2,8,9-TetraCDD | 16.5 | ||||
| 1,2,6,7-TetraCDD | 26 | ||||
|
P. brevispora strain BMC9160 |
2,7-DiCDD | 40 | |||
| 2,3,7-TriCDD | 27 | ||||
| 1,2,8,9-TetraCDD | 23 | ||||
| 1,2,6,7-TetraCDD | 16.5 |
4.2. Biostimulation
Microbial adsorption highly contributes to the mineralization and co-transformation of organic contaminants during composting [115]. Therefore, microbial activity is the most important factor for the biodegradation of organic contaminants. In addition, the effects of operational parameters, such as humidity, growing conditions, and C/N ratio on the bio-incubation process are very important. Humidity is known as the main factor affecting the biodegradation of organic contaminants because it affects the microbial activity and the physicochemical properties of the contaminants [22]. Oxygen molecules participate in the catabolism and mineralization of hydrocarbon compounds by microbial and fungal activities. In addition, the C/N ratio plays an important role in the biodegradation of contaminants because it controls the composition of the microbial community. Previous studies have shown that the optimal C/N ratio for the biodegradation of organic contaminants by bio-incubation is between 10 and 40 [116]. Bio-composting has been successfully used in the biodegradation of dioxin-contaminated soils on a laboratory scale [25]. Chen et al., 2016, reported that the biodegradation efficiency of PCDD/PCDFs was approximately 95.8–99.7%, from an initial toxic concentration of 1580–3660 μg I-TEQ/kg dw after 42 days of incubation. Table 6 summarizes the organic compositions used to degrade dioxins in the soil by biological composting.
Table 6.
Summary of biodegradation statistics for dioxins in contaminated soil.
| Initial Concentration | Mechanical Components | Materials | Removal (%) | Time (days) | Conditions | References |
|---|---|---|---|---|---|---|
| 16,004 ng-TEQ/kg | Sandy loam | Food waste, sawdust, and compost |
75 | 42 | Aerobic | [108] |
| 840–5300 ng-TEQ/kg | Sandy | Wood chips and compost | 85 | 360 | Semi-aerobic | [25] |
| 30,000–60,000 ng-TEQ/kg | Sandy loam | Lime granules, Nutrients, and bark |
21 | 175 | Anaerobic | [117] |
| 88.8–912.7 μmol/kg | Sandy loam | Sewage sludge | 61.2 | 42 | Aerobic | [32] |
| Leaves | 36.8 | |||||
| Animal manure | 32.5 | |||||
| Sewage sludge and compost | 53 | 280 | ||||
| Sewage sludge and animal manure |
79 | |||||
| 6048 ng-TEQ/kg | Sandy loam | Food waste, sawdust, and compost |
70 | 49 | Aerobic | [118] |
| 300–660 ngTEQ/kg | Sandy loam | Straw manure, bark chips, and wood chips | 75 | 175 | Semi-aerobic | [91] |
4.3. Phytoremediation
The application of phytoremediation is widely used by plants that grow in the wild, with features such as round and fat stems, many gaseous roots, or large roots, which can crawl on the ground or crawl on the trunk of another tree. These trees can grow, completely covering the ground, and remain green all year, are less deciduous, and yield a large biomass, which can withstand harsh environmental conditions, making it an ideal habitat for microorganisms and fungi in the rhizosphere, etc. They can form a favorable combination that optimizes the absorption and decomposition of toxic chemicals in the soil [119]. In addition, biological products, such as DECOM1 (a mixture of nutrient salts and organic humus), can be applied to the soil to increase the decay time of toxic substances to reduce the concentration of difficult pollutants, degrading, or in other words, helping plants absorb organic toxins more quickly in the soil [120,121]. Especially for the phytoremediation of persistent organic compounds in the soil, it takes a long time considering practical experimental conditions, such as weather, climate, other anthropogenic factors, etc. [122]. Table 7 below reviews some plants with the highest removal performance used for the treatment of dioxins in the soil.
Table 7.
Degradation of dioxins by phytoremediation.
| Names | Pollutant Compounds | Concentration | Removal (%) | Time | References |
|---|---|---|---|---|---|
| Arabidopsis thaliana | TCDD | 10 ppt | 72 | 30 d | [123] |
| 50 ppt | 58 | ||||
| 100 ppt | 55 | ||||
| Black Beauty | Total PCDDs | 43 ppt-TEQ | 46 | 32 d | [124] |
| Total PCDFs | 50 | ||||
| Gold Rush | Total PCDDs | 45 ppt-TEQ | 60 | 32 d | |
| Total PCDFs | 62 | ||||
| Spinach | Total PCDDs | 3.42 ppt | 48.6 | ND | [125] |
| Total PCDFs | 0.519 ppt | 37.9 | |||
| Garland Chrysanthemum | Total PCDDs | 0.543 ppt | 36.1 | ||
| Total PCDFs | 0.622 ppt | 48.8 | |||
| Mitsuba | Total PCDDs | 0.765 ppt | 38 | ||
| Total PCDFs | 0.161 ppt | 43.8 | |||
| Chingentsuai | Total PCDDs | 0.268 ppt | 39.2 | ||
| Total PCDFs | 0.166 ppt | 41.6 | |||
| Rice leaf and stem | Total dioxins | 317 ppt | 90 | 5 m | [126] |
| Rice paddy chaff | Total dioxins | 44 ppt | 98 | ||
| Atena Polka | PCDD/Fs | 7 ppt-TEQ dw | 66 | 5 w | [127] |
| Zucchini | PCDD/Fs | 155 ppt-TEQ dw | 37 | 5 w | [127,128] |
| Cucumber | PCDD/Fs | 122 ppt-TEQ dw | 24 | 5 w | |
| Zucchini | 2,4,8-TrCDF | 0.0089 TSCF | 64 | 4 d | [129,130] |
| 2,3,7,8-TeCDD | 70 | ||||
| Pumpkin | 2,4,8-TrCDF | 0.0064 TSCF | 77 | ||
| 2,3,7,8-TeCDD | 79 |
5. Prospects for Future Research
Bio-composting can degrade dioxins in contaminated soil. However, to achieve high efficiency of the decomposition process, many different strains of microorganisms are required. Therefore, the biodegradation mechanisms of microbial strains are still poorly understood, and further research efforts are needed. To better understand the microbial diversity and structural changes associated with bio-composting, next-generation sequencing is proposed to identify the respective microbial strains. Lastly, the biodegradation process is designed to enhance the biodegradation process and shorten the processing time.
The growth and activity of microorganisms during the composting process determine the efficiency of the biodegradation of dioxins, which can be optimized through operational parameters, such as aeration rate, humidity, incubation time, pH, and C/N ratio. Optimal values of operational parameters vary with laboratory scale, pilot scale, composting material, and soil properties relative to the properties of dioxins. Currently, the knowledge from the literature is not sufficient to achieve commonly used optimal values. In addition, one of the major challenges of composting is that microbial activity is very time-consuming, which demonstrates why field-scale studies are very rare.
Additional studies are required to accurately and completely evaluate dioxin contamination sites of various origins and locations in order to provide effective treatment options. Furthermore, the bioremediation approaches for various contamination sites should be investigated. Other methods currently remain limited, such as hybrid bioremediation strategies in developing some transgenic plants to express dioxin-degrading enzymes, or nano-phytoremediation by combining the nanoparticles and vegetal species, which should be emphasized to improve the biodegradation efficiency of dioxins. In addition, the combination of chemical and biological measures or the combination of physicochemical and biological technologies should be utilized to improve efficiency in the degradation of pollutants.
Overall, future studies should provide more insight into the microbial relationships in the biodegradation of dioxins, in addition to the biodegradation mechanisms outlined above. The performance parameters also need to be studied more deeply for study scaling purposes.
6. Conclusions
With the current rate of industrial development and urbanization, the land area is shrinking, land quality is deteriorating, and land area per capita is decreasing. Currently, there are many hotspots of dioxin pollution in the soils of some countries around the world; the main source of dioxin-contaminated soil is industrial production activities, followed by the consequences of war, producing high dioxin concentrations and widespread infection. Dioxin contamination in the soil not only has a negative impact on industrial production, agriculture, and service activities, but it also has an indirect impact on human and animal health through food, vegetables, etc. Composting is full of economic benefits, it can treat dioxins in contaminated soil with high efficiency, and it is environmentally friendly. Parameters such as temperature, humidity, pH, oxygen content, aeration rate, and C/N ratio need to be continuously monitored and controlled during the composting process. The microbial community is primarily responsible for the biodegradation of dioxins in the soil. Some microbials can use dioxins as a source of carbon and energy to break down these compounds. The correlation between the microbial communities and the breakdown of dioxins during the composting process needs to be further studied so that the metabolic and congeners mechanisms can be elucidated. Ultimately, current knowledge is insufficient to achieve an optimal set of values for the treatment of soils contaminated with dioxins on a laboratory scale, pilot scale, and field scale.
Author Contributions
N.T.H.N.: writing, investigation, formal analysis; X.-T.T.N.: writing and investigation; V.D.L.: writing and investigation; Y.W.: investigation, validation, writing—review and editing; T.F.: supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This research was funded by the Natural Science Foundation, number NSFC 21976039.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kulkarni P.S., Du T. Encyclopedia of Environmental Health. Volume 2. Elsevier; Amsterdam, The Netherlands: 2019. Dioxins; pp. 83–92. [DOI] [Google Scholar]
- 2.Consonni D., Sindaco R., Alberto P. Blood levels of dioxins, furans, dioxin-like PCBs, and TEQs in general populations: A review, 1989–2010. Environ. Int. 2012;44:151–162. doi: 10.1016/j.envint.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 3.USAID . Dioxin Remediation Bien Hoa Airbase Area Project. Volume 1 USAID; Washington, DC, USA: 2020. Final Masterplan—Volume 1. [Google Scholar]
- 4.Mckay G. Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration: Review. Chem. Eng. J. 2002;86:343–368. doi: 10.1016/S1385-8947(01)00228-5. [DOI] [Google Scholar]
- 5.Stanmore B.R. An Empirical Model for the De Novo Formation of PCDD/F in Medical Waste Incinerators. Environ. Sci. Technol. 2000;34:4538–4544. doi: 10.1021/es001160d. [DOI] [Google Scholar]
- 6.Anderson D.R., Fisher R. Sources of dioxins in the United Kingdom: The steel industry and other sources. Chemosphere. 2002;46:371–381. doi: 10.1016/S0045-6535(01)00178-3. [DOI] [PubMed] [Google Scholar]
- 7.Ahn Y., Liu F., Fennell D.E. Biostimulation and bioaugmentation to enhance dechlorination of polychlorinated dibenzo-p-dioxins in contaminated sediments. FEMS Microbiol Ecol. 2008;66:271–281. doi: 10.1111/j.1574-6941.2008.00557.x. [DOI] [PubMed] [Google Scholar]
- 8.Thi T., Hanh T., Anh L.V., Bich N.N., Hung N.V. Environmental health risk assessment by exposure to dioxin in food at dioxin hot site in Da Nang. J. Prev. Med. 2011;4:4–6. [Google Scholar]
- 9.Manh H.D., Kido T., Okamoto R., XianLiang S., Anh L.T., Supratman S., Maruzeni S., Nishijo M., Nakagawa H., Honma S., et al. Serum Dioxin Levels in Vietnamese Men more than 40 Years after Herbicide Spraying. Am. Chem. Soc. 2014;48:3496–3503. doi: 10.1021/es404853h. [DOI] [PubMed] [Google Scholar]
- 10.Haglund P. Methods for Treating Soils Contaminated with Polychlorinated Dibenzo-p-Dioxins, Dibenzofurans, and Other Polychlorinated Aromatic Compounds. Ambio. 2007;36:467–474. doi: 10.1579/0044-7447(2007)36[467:MFTSCW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Marklund S., Andersson R., Tysidind M., Bjrkman K.E.E., Grigoriadis V. Emissions of PCDDs and PCDFs in gasoline and diesel fueled cars. Chemosphere. 1990;20:553–561. doi: 10.1016/0045-6535(90)90111-6. [DOI] [Google Scholar]
- 12.Mino Y., Moriyama Y. Possible Remediation of Dioxin-Polluted Soil by Steam Distillation. Chem. Pharm. Bull. 2001;49:1050–1051. doi: 10.1248/cpb.49.1050. [DOI] [PubMed] [Google Scholar]
- 13.Harjanto S., Kasai E., Terui T., Nakamura T. Formation and transport of PCDD/Fs in the packed bed of soil containing organic chloride during a thermal remediation process. Chemosphere. 2002;49:217–224. doi: 10.1016/S0045-6535(02)00198-4. [DOI] [PubMed] [Google Scholar]
- 14.Gray K.A., Hilarides R.J. Radiolytic treatment of dioxin contaminated soils. Pt 2Radiat. Phys. Chem. 1995;46:1081–1084. doi: 10.1016/0969-806X(95)00326-S. [DOI] [Google Scholar]
- 15.Nakamiya K., Hashimoto S., Ito H., Edmonds J.S., Yasuhara A., Morita M. Degradation of dioxins by cyclic ether degrading fungus, Cordyceps sinensis. Fed. Eur. Microbiol. Soc. 2005;248:17–22. doi: 10.1016/j.femsle.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Isosaaril P., Tuhkanen T., Vartiainen T. Photodegradation of Polychlorinated Dibenzo-p-dioxins and Dibenzofurans in Soil with Vegetable Oil. Environ. Sci. Pollut. Res. 2004;11:181–185. doi: 10.1007/BF02979673. [DOI] [PubMed] [Google Scholar]
- 17.Isosaari P., Laine O., Tuhkanen T., Vartiainen T. Photolysis of polychlorinated dibenzo-p-dioxins and dibenzofurans dissolved in vegetable oils: Influence of oil quality. Sci. Total Environ. 2005;340:1–11. doi: 10.1016/j.scitotenv.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Pittman C.U., Jr., He J. Dechlorination of PCBs, CAHs, herbicides and pesticides neat and in soils at 25 °C using Na/NH3. J. Hazard. Mater. 2002;92:51–62. doi: 10.1016/S0304-3894(01)00372-7. [DOI] [PubMed] [Google Scholar]
- 19.Alassali A., Moon H., Picuno C., Meyer R.S.A., Kuchta K. Assessment of polyethylene degradation after aging through anaerobic digestion and composting. Polym. Degrad. Stab. 2018;158:14–25. doi: 10.1016/j.polymdegradstab.2018.10.014. [DOI] [Google Scholar]
- 20.Nam I., Kim Y., Murugesan K., Jeon J., Chang Y., Chang Y. Bioremediation of PCDD/Fs-contaminated municipal solid waste incinerator fly ash by a potent microbial biocatalyst. J. Hazard. Mater. 2008;157:114–121. doi: 10.1016/j.jhazmat.2007.12.086. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki S., Hiraishi A. Novosphingobium naphthalenivorans sp. nov., a naphthalene-degrading bacterium isolated from polychlorinated-dioxin-contaminated. J. Gen. Appl. Microbiol. 2007;228:221–228. doi: 10.2323/jgam.53.221. [DOI] [PubMed] [Google Scholar]
- 22.Field J.A., Sierra-Alvarez R. Microbial degradation of chlorinated dioxins. Chemosphere. 2008;71:1005–1018. doi: 10.1016/j.chemosphere.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 23.Bunge M., Adrian L., Kraus A., Opel M. Reductive dehalogenation of chlorinated dioxins by an anaerobic bacterium. Nature. 2003;421:357–360. doi: 10.1038/nature01237. [DOI] [PubMed] [Google Scholar]
- 24.Barkovskii A.L. Microbial Dechlorination of Historically Present and Freshly Spiked Chlorinated Dioxins and Diversity of Dioxin-Dechlorinating Populations. Appl. Environ. Microbiol. 1996;62:4556–4562. doi: 10.1128/aem.62.12.4556-4562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narihiro T., Kaiya S., Futamata H., Hiraishi A. Removal of polychlorinated dioxins by semi-aerobic fed-batch composting with biostimulation of “Dehalococcoides”. J. Biosci. Bioeng. 2010;109:249–256. doi: 10.1016/j.jbiosc.2009.08.498. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida N., Takahashi N., Hiraishi A. Phylogenetic Characterization of a Polychlorinated-Dioxin-Dechlorinating Microbial Community by Use of Microcosm Studies. Appl. Environ. Microbiol. 2005;71:4325–4334. doi: 10.1128/AEM.71.8.4325-4334.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X., Pan S., Zhang Z., Lin X., Zhang Y., Chen S. Effects of the feeding ratio of food waste on fed-batch aerobic composting and its microbial community. Bioresour. Technol. 2016;224:397–404. doi: 10.1016/j.biortech.2016.11.076. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Echartea E., Macek T., Demnerova K., Uhlik O. Bacterial Biotransformation of Pentachlorophenol and Micropollutants Formed during Its Production Process. Int. J. Environ. Res. Public Health. 2016;13:1146. doi: 10.3390/ijerph13111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnoor J.L. Phytoremediation of Polychlorinated Biphenyls: New Trends and Promises. Environ. Sci. Technol. 2010;44:2767–2776. doi: 10.1021/es902514d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mudhoo A., Thayalan G., Muthoora N.J., Muthoora M.N., Oozeer B.Z. Dioxins and Furans: Sources, Impacts and Remediation. Springer International Publishing; Cham, Switzerland: 2013. [Google Scholar]
- 31.Kulkarni P.S., Crespo J.G., Afonso C.A.M. Dioxins sources and current remediation technologies—A review. Environ. Int. 2008;34:139–153. doi: 10.1016/j.envint.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Chen W., Wu J., Lin S., Chang J. Bioremediation of polychlorinated-p-dioxins/dibenzofurans contaminated soil using simulated compost-amended landfill reactors under hypoxic conditions. J. Hazard. Mater. 2016;312:159–168. doi: 10.1016/j.jhazmat.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 33.Feshin D., Poberezhnaya T., Shelepchikov A., Brodsky E., Levin B. PCDD/Fs in emissions of dirt volcano. Organohalogen Compd. 2006;68:2240–2243. [Google Scholar]
- 34.Salamanca M., Chandía C., Hernández A. Impact of forest fi res on the concentrations of polychlorinated dibenzo-p-dioxin and dibenzofurans in coastal waters of central Chile. Sci. Total Environ. 2016;573:1397–1405. doi: 10.1016/j.scitotenv.2016.07.113. [DOI] [PubMed] [Google Scholar]
- 35.Reiner E.J., Clement R.E., Okey A.B., Marvin C.H. Advances in analytical techniques for polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans and dioxin-like PCBs. Anal. Bioanal. Chem. 2006;386:791–806. doi: 10.1007/s00216-006-0479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rathna R., Varjani S., Nakkeeran E. Recent developments and prospects of dioxins and furans remediation. J. Environ. Manag. 2018;223:797–806. doi: 10.1016/j.jenvman.2018.06.095. [DOI] [PubMed] [Google Scholar]
- 37.Holtzer M., Dańko J., Dańko R. Possibilities of formation of dioxin and furans in metallurgical processes as well as methods of their reduction. Metalurgija. 2007;46:285–290. [Google Scholar]
- 38.Committee on the Implications of Dioxin in the Food Supply-National Research Council . Dioxins and Dioxin-Like Compounds. The National Academies Press; Washington, DC, USA: 2003. [Google Scholar]
- 39.Halden R.U., Dwyer D.F., Halden R.U., Dwyer D.F. Biodegradation of Dioxin-Related Compounds: A Review Biodegradation of Dioxin-Related Compounds: A Review. Bioremediat. J. 2008;1:11–25. doi: 10.1080/10889869709351314. [DOI] [Google Scholar]
- 40.Baveye P.C., Otten W., Kravchenko A., Balseiro-Romero M., Beckers É., Chalhoub M., Darnault C., Eickhorst T., Garnier P., Hapca S., et al. Emergent Properties of Microbial Activity in Heterogeneous Soil Microenvironments: Different Research Approaches Are Slowly Converging, Yet Major Challenges Remain. Front. Microbiol. 2018;9:1929. doi: 10.3389/fmicb.2018.01929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Booth S., Hui J., Alojado Z., Lam V., Cheung W., Zeller D., Steyn D., Pauly D. Global deposition of airborne dioxin. Mar. Pollut. Bull. 2013;75:182–186. doi: 10.1016/j.marpolbul.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 42.Dopico M., Gómez A. Review of the current state and main sources of dioxins around the world. J. Air Waste Manag. Assoc. 2015;65:1033–1049. doi: 10.1080/10962247.2015.1058869. [DOI] [PubMed] [Google Scholar]
- 43.Nijenhuis I., Stollberg R., Lechner U. Anaerobic microbial dehalogenation and its key players in the contaminated Bitterfeld-Wolfen megasite. FEMS Microbiol. Ecol. 2018;94:fiy012. doi: 10.1093/femsec/fiy012. [DOI] [PubMed] [Google Scholar]
- 44.Moon H., Choi M., Choi H., Kannan K. Severe pollution of PCDD/Fs and dioxin-like PCBs in sediments from Lake Shihwa, Korea: Tracking the source. Mar. Pollut. Bull. 2012;64:2357–2363. doi: 10.1016/j.marpolbul.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 45.Capanni F., Muñoz-Arnanz J., Marsili L., Fossi M.C. Assessment of PCDD/Fs, dioxin-like PCBs and PBDEs in Mediterranean striped dolphins. Mar. Pollut. Bull. 2020;156:111207. doi: 10.1016/j.marpolbul.2020.111207. [DOI] [PubMed] [Google Scholar]
- 46.Matovu H., Mubiru E., Ssebugere P., Sillanp M. Human and environmental exposure to PCDD/Fs and dioxin-like PCBs in Africa: A review. Chemosphere. 2019;223:483–493. doi: 10.1016/j.chemosphere.2019.02.065. [DOI] [PubMed] [Google Scholar]
- 47.Zielinski M., Czerska M., Urbaniak M., Gromadzinska J. Soil contamination of PCDDs/PCDFs and dl-PCBs in the urban catchment in central Poland. Toxicol. Lett. 2015;238:S379–S380. doi: 10.1016/j.toxlet.2015.08.1083. [DOI] [Google Scholar]
- 48.Liu G., Cai Z., Wu Y., Jiang G. Dioxin analysis in China. Trends Anal. Chem. 2013;46:178–188. doi: 10.1016/j.trac.2012.05.012. [DOI] [Google Scholar]
- 49.Stellman J.M., Stellman S.D., Christian R., Weber T., Tomasallo C. The extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature. 2003;422:681–687. doi: 10.1038/nature01537. [DOI] [PubMed] [Google Scholar]
- 50.Zheng G.J., Leung A.O.W., Jiao L.P., Wong M.H. Polychlorinated dibenzo-p-dioxins and dibenzofurans pollution in China: Sources, environmental levels and potential human health impacts. Environ. Int. 2008;34:1050–1061. doi: 10.1016/j.envint.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Wang D.G., Yang M., Jia H.L., Zhou L., Li Y.F. Levels, distributions and profiles of polychlorinated biphenyls in surface soils of Dalian, China. Chemosphere. 2008;73:38–42. doi: 10.1016/j.chemosphere.2008.05.055. [DOI] [PubMed] [Google Scholar]
- 52.Naile J.E., Khim J.S., Wang T., Wan Y., Luo W., Hu W., Jiao W., Park J., Ryu J., Hong S., et al. Sources and distribution of polychlorinated-dibenzo-p-dioxins and -dibenzofurans in soil and sediment from the Yellow Sea region of China and Korea. Environ. Pollut. 2011;159:907–917. doi: 10.1016/j.envpol.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 53.Pan J., Yang Y., Zhu X., Yeung LW Y., Taniyasu S., Miyake Y., Falandysz J., Yamashita N. Science of the Total Environment Altitudinal distributions of PCDD/Fs, dioxin-like PCBs and PCNs in soil and yak samples from Wolong high mountain area, eastern Tibet-Qinghai Plateau, China. Sci. Total Environ. 2013;444:102–109. doi: 10.1016/j.scitotenv.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Leung A.O.W., Wong A.S. Spatial Distribution of Polybrominated Diphenyl Ethers and Polychlorinated Dibenzo-p-dioxins and Dibenzofurans in Soil and Combusted Residue at Guiyu, an Electronic Waste Recycling Site in Southeast China. Environ. Sci. Technol. 2007;41:2730–2737. doi: 10.1021/es0625935. [DOI] [PubMed] [Google Scholar]
- 55.Lee C.C., Lin W.T., Liao P.C., Su H.J., Chen H.L. High average daily intake of PCDD/Fs and serum levels in residents living near a deserted factory producing pentachlorophenol (PCP) in Taiwan: Influence of contaminated fish consumption. Environ. Pollut. 2006;141:381–386. doi: 10.1016/j.envpol.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 56.Lee C.C., Guo Y.L., Kuei C.H., Chang H.Y., Hsu J.F., Wang S.T., Liao P.C. Human PCDD/PCDF levels near a pentachlorophenol contamination site in Tainan, Taiwan. Chemosphere. 2006;65:436–448. doi: 10.1016/j.chemosphere.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 57.Song A., Li H., Liu M., Hu J., Sheng G., Ying G. Polybrominated dibenzo-p-dioxins/furans (PBDD/Fs) in soil around municipal solid waste incinerator: A comparison with polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs) Environ. Pollut. 2022;293:118563. doi: 10.1016/j.envpol.2021.118563. [DOI] [PubMed] [Google Scholar]
- 58.Die Q., Lu A., Li C., Li H., Kong H., Li B. Occurrence of dioxin-like POPs in soils from urban green space in a metropolis, North China: Implication to human exposure. Environ. Sci. Pollut. Res. 2021;28:5587–5597. doi: 10.1007/s11356-020-10953-3. [DOI] [PubMed] [Google Scholar]
- 59.Liu J., Liu W. Distribution of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDDs/Fs) and dioxin-like polychlorinated biphenyls (dioxin-like PCBs) in the soil in a typical area of eastern China. J. Hazard. Mater. 2009;163:959–966. doi: 10.1016/j.jhazmat.2008.07.080. [DOI] [PubMed] [Google Scholar]
- 60.Le L.T.H., Dat N.D., Minh N.H., Nguyen K.A. Characteristics of PCDD/Fs in soil and sediment samples collected from A-So former airbase in Central Vietnam. Sci. Total Environ. 2019;661:27–34. doi: 10.1016/j.scitotenv.2019.01.163. [DOI] [PubMed] [Google Scholar]
- 61.Anh T., Vu T., Tarradellas J. Dioxin contamination in soils of Southern Vietnam. Chemosphere. 2007;67:1802–1807. doi: 10.1016/j.chemosphere.2006.05.086. [DOI] [PubMed] [Google Scholar]
- 62.Takeda N., Takaoka M. An assessment of dioxin contamination from the intermittent operation of a municipal waste incinerator in Japan and associated remediation. Environ. Sci. Pollut. Res. 2013;20:2070–2080. doi: 10.1007/s11356-012-1412-0. [DOI] [PubMed] [Google Scholar]
- 63.Kiguchi O., Kobayashi T., Wada Y., Saitoh K., Ogawa N. Polychlorinated dibenzo-p-dioxins and dibenzofurans in paddy soils and river sediments in Akita, Japan. Chemosphere. 2007;67:557–573. doi: 10.1016/j.chemosphere.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 64.Cho H.-K. Master’s Thesis. Ulsan National Institute of Science and Technology; Ulsan, Korea: 2021. Distribution of PCDD/Fs and PCBs in Soils and Pine Needles in Ulsan, South Korea. [Google Scholar]
- 65.Liberatori G., Cotugno P., Sturba L., Vannuccini M.L., Capasso G., Velardo R., Besselink H., Massari F., Tursi A., Corbelli V., et al. Chemosphere Occurrence and spatial distribution of dioxin and dioxin-like compounds in topsoil of Taranto (Apulia, Italy) by GC-MS analysis and DR-CALUX® bioassay. Chemosphere. 2021;279:130576. doi: 10.1016/j.chemosphere.2021.130576. [DOI] [PubMed] [Google Scholar]
- 66.Henriksson S., Hagberg J., Bäckström M., Persson I., Lindström G. Assessment of PCDD/Fs levels in soil at a contaminated sawmill site in Sweden—A GIS and PCA approach to interpret the contamination pattern and distribution. Environ. Pollut. 2013;180:19–26. doi: 10.1016/j.envpol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Shelepchikov A.A., Brodskii E.S., Feshin D.B., Zhil’nikov V.G., Mir-Kadyrova E.Y., Balashova S.P. Polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in soils of Moscow. Eurasian Soil Sci. 2011;44:286–296. doi: 10.1134/S1064229311030124. [DOI] [Google Scholar]
- 68.Eljarrat E., Caixach J., Rivera J. Levels of polychlorinated dibenzo-p-dioxins and dibenzofurans in soil samples from Spain. Chemosphere. 2001;44:1383–1387. doi: 10.1016/S0045-6535(00)00373-8. [DOI] [PubMed] [Google Scholar]
- 69.Creaser C.S., Fernandes A.R., Harrad S.J., Cox E.A. Levels and Sources of PCDDs and PCDFs in urban British soils. Chemosphere. 1990;21:931–938. doi: 10.1016/0045-6535(90)90116-B. [DOI] [Google Scholar]
- 70.Creaser C.S., Fernandes A.R., Al-Haddad A., Harrad S.J., Homer R.B., Skett P.W., Cox E.A. Survey of background levels of PCDDs & PCDFs in UK soils. Chemosphere. 1989;18:767–776. doi: 10.1016/0045-6535(89)90194-X. [DOI] [Google Scholar]
- 71.Martínez K., Abad E., Rivera J. Surveillance programme on dioxin levels in soils in the Campo de Gibraltar (Southwest Spain) Chemosphere. 2006;65:382–389. doi: 10.1016/j.chemosphere.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 72.Dömötörová M., Sejáková Z.S., Kočan A., Čonka K., Chovancová J., FabiŠiková A. PCDDs, PCDFs, dioxin-like PCBs and indicator PCBs in soil from five selected areas in Slovakia. Chemosphere. 2012;89:480–485. doi: 10.1016/j.chemosphere.2012.05.106. [DOI] [PubMed] [Google Scholar]
- 73.Ivory A., Mobbs C. Dioxins levels in Australia: Key findings of studies. Organohalogen Compd. 2004;66:3398–3403. [Google Scholar]
- 74.Urban J.D., Wikoff D.S., Bunch A.T.G., Harris M.A., Haws L.C. A review of background dioxin concentrations in urban/suburban and rural soils across the United States: Implications for site assessments and the establishment of soil cleanup levels. Sci. Total Environ. 2014;466–467:586–597. doi: 10.1016/j.scitotenv.2013.07.065. [DOI] [PubMed] [Google Scholar]
- 75.Rogowski D.L., Yake W. Typical dioxin concentrations in agriculture soils of Washington State and potential sources. Environ. Sci. Technol. 2005;39:5170–5176. doi: 10.1021/es047945r. [DOI] [PubMed] [Google Scholar]
- 76.Pius C., Sichilongo K., Mswela P.K., Dikinya O. Monitoring polychlorinated dibenzo-p-dioxins/dibenzofurans and dioxin-like polychlorinated biphenyls in Africa since the implementation of the Stockholm Convention—An overview. Environ. Sci. Pollut. Res. 2019;26:101–113. doi: 10.1007/s11356-018-3629-z. [DOI] [PubMed] [Google Scholar]
- 77.Nieuwoudt C., Quinn L.P., Pieters R., Jordaan I., Visser M., Kylin H., Borge A.R., Gieasy J.P., Bouwman H. Dioxin-like chemicals in soil and sediment from residential and industrial areas in central South Africa. Chemosphere. 2009;76:774–783. doi: 10.1016/j.chemosphere.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 78.USEPA . US EPA Region 5 Soil and Sediment Ecological Screening Levels. USEPA; San Francisco, CA, USA: 2003. [Google Scholar]
- 79.USEPA . USEPA Region 9 Preliminary Remediation Goals. US Environmental Protection Agency; San Francisco, CA, USA: 2000. [Google Scholar]
- 80.Environmental Protection Agency (EPA) of Taiwan Control Standards of Soil Pollution. [(accessed on 2 February 2022)];2011 Available online: http://ivy5.epa.gov.tw/%0Aepalaw/search/LordiDispFull.aspx?ltype¼14&lname¼0120.
- 81.Ministry of Natural Resources and Environment . National Technique Regulation on Allowed Limits of Dioxin in Soils. Ministry of Natural Resources and Environment; Hanoi, Vietnam: 2012. [Google Scholar]
- 82.UK Department of the Environment Transport and the Regions (DETR) Compilation of EU Dioxin Exposure and Health Data. Task 1—Member State Legislation and Programmes. Report Produced for the European Commission DG. 1999. [(accessed on 6 February 2022)]. Available online: http://ec.europa.eu/environment/dioxin/pdf/task1.pdf.
- 83.New Zealand Ministry of Environment Health and Environmental Guidelines for Selected Timber Treatment Chemicals—Chapter 5. New Zealand. [(accessed on 23 February 2022)];1997 Available online: http://www.mfe.govt.nz/publications/hazardous/timber-guide-jun97/timber-guide-jun97.pdf.
- 84.Canadian Council of Ministers of the Environment . Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health—Polychlorinated Dibenzo-p-Dioxins and Polychlorinated Dibenzofurans (PCDD/Fs) Canadian Council of Ministers of the Environment; Winnipeg, MB, Canada: 2002. [Google Scholar]
- 85.Garbisu C., Garaiyurrebaso O., Epelde L., Grohmann E. Plasmid-Mediated Bioaugmentation for the Bioremediation of Contaminated Soils. Front. Microbiol. 2017;8:1966. doi: 10.3389/fmicb.2017.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coppotelli B.M., Ibarrolaza A., del Panno M.T. Effects of the Inoculant Strain Sphingomonas paucimobilis 20006FA on Soil Bacterial Community and Biodegradation in Phenanthrene-contaminated Soil. Microb. Ecol. 2008;55:173–183. doi: 10.1007/s00248-007-9265-7. [DOI] [PubMed] [Google Scholar]
- 87.Lyon D.Y., Vogel T.M., de Lyon U. 6.08 Bioaugmentation as a Strategy for the Treatment of Persistent Pollutants. 2nd ed. Volume 1 Elsevier B.V.; Amsterdam, The Netherlands: 2011. [Google Scholar]
- 88.Lin C., Kao C., Grasso D., Sheu D., Lin T. Thermophilic Biodegradation of Diesel Oil in Food Waste Composting Processes Without Bioaugmentation. Environ. Eng. Sci. 2012;29:117–123. doi: 10.1089/ees.2010.0212. [DOI] [Google Scholar]
- 89.Chang Y. Recent Developments in Microbial Biotransformation and Biodegradation. J. Mol. Microbiol. Biotechnol. 2008;15:152–171. doi: 10.1159/000121327. [DOI] [PubMed] [Google Scholar]
- 90.Xie H., Zhu L., Wang J. Combined treatment of contaminated soil with a bacterial Stenotrophomonas strain DXZ9 and ryegrass (Lolium perenne) enhances DDT and DDE remediation. Environ. Sci. Pollut. Res. 2018;25:31895–31905. doi: 10.1007/s11356-018-1236-7. [DOI] [PubMed] [Google Scholar]
- 91.Tu Y.T., Liu J.K., Lin W.C., Lin J.L., Kao C.M. Enhanced anaerobic biodegradation of OCDD-contaminated soils by Pseudomonas mendocina NSYSU: Microcosm, pilot-scale, and gene studies. J. Hazard. Mater. 2014;278:433–443. doi: 10.1016/j.jhazmat.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 92.Lin J.L., Lin W.C., Liu J.K., Surampalli R.Y., Zhang T.C., Kao C.M. Aerobic Biodegradation of OCDD by P. Mendocina NSYSU: Effectiveness and Gene Inducement Studies. Water Environ. Res. 2017;89:2113–2121. doi: 10.2175/106143017X15054988926415. [DOI] [PubMed] [Google Scholar]
- 93.Liu N., Hou T., Yin H., Han L., Huang G. Effects of amoxicillin on nitrogen transformation and bacterial community succession during aerobic composting. J. Hazard. Mater. 2018;362:258–265. doi: 10.1016/j.jhazmat.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 94.Deshmukh R., Khardenavis A.A., Purohit H.J. Diverse Metabolic Capacities of Fungi for Bioremediation. Indian J. Microbiol. 2016;56:247–264. doi: 10.1007/s12088-016-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kamei I., Suhara H., Kondo R. Phylogenetical approach to isolation of white-rot fungi capable of degrading polychlorinated dibenzo-p-dioxin. Appl. Microbiol. Biotechnol. 2005;69:358–366. doi: 10.1007/s00253-005-0052-4. [DOI] [PubMed] [Google Scholar]
- 96.Kamei I., Kondo R. Biotransformation of dichloro-, trichloro-, and tetrachlorodibenzo-p-dioxin by the white-rot fungus Phlebia lindtneri. Appl. Microbiol. Biotechnol. 2005;68:560–566. doi: 10.1007/s00253-005-1947-9. [DOI] [PubMed] [Google Scholar]
- 97.Takada S., Nakamura M., Matsueda T. Degradation of Polychlorinated Dibenzo-p-Dioxins and Polychlorinated Dibenzofurans by the White Rot Fungus Phanerochaete sordida YK-624. Appl. Environ. Microbiol. 1996;62:4323–4328. doi: 10.1128/aem.62.12.4323-4328.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Habe H., Chung J.S., Lee J.H., Kasuga K., Yoshida T., Nojiri H., Omori T. Degradation of Chlorinated Dibenzofurans and Dibenzo-p-Dioxins by Two Types of Bacteria Having Angular Dioxygenases with Different Features. Appl. Environ. Microbiol. 2001;67:3610–3617. doi: 10.1128/AEM.67.8.3610-3617.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Halden R.U., Halden B.G., Dwyer D.F. Removal of Dibenzofuran, Dibenzo-p-Dioxin, and 2-Chlorodibenzo-p-Dioxin from Soils Inoculated with Sphingomonas sp. Strain RW1. Appl. Environ. Microbiol. 1999;65:2246–2249. doi: 10.1128/AEM.65.5.2246-2249.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Habe H., Ide K., Yotsumoto M., Tsuji H., Yoshida T., Nojiri H., Omori T. Degradation characteristics of a dibenzofuran-degrader Terrabacter sp. strain DBF63 toward chlorinated dioxins in soil. Chemosphere. 2002;48:201–207. doi: 10.1016/S0045-6535(02)00064-4. [DOI] [PubMed] [Google Scholar]
- 101.Habe H., Ashikawa Y., Saiki Y., Yoshida T., Nojiri H. Sphingomonas sp. strain KA1, carrying a carbazole dioxygenase gene homologue, degrades chlorinated dibenzo-p-dioxins in soil. FEMS Microbiol. Lett. 2002;211:43–49. doi: 10.1111/j.1574-6968.2002.tb11201.x. [DOI] [PubMed] [Google Scholar]
- 102.Kimura N., Kitagawa W., Mori T., Nakashima N., Tamura T., Kamagata Y. Genetic and biochemical characterization of the dioxygenase involved in lateral dioxygenation of dibenzofuran from Rhodococcus opacus strain SAO101. Appl. Microbiol. Biotechnol. 2006;73:474–484. doi: 10.1007/s00253-006-0481-8. [DOI] [PubMed] [Google Scholar]
- 103.Ishiguro T., Ohtake Y., Nakayama S., Inamori Y., Amagai T., Soma M., Matsusita H. Biodegradation of Dibenzofuran and Dioxins by Pseudomonas aeruginosa and Xanthomonas maltophilia. Environ. Technol. 2000;21:1309–1316. doi: 10.1080/09593330.2000.9619020. [DOI] [Google Scholar]
- 104.Hong H., Nam I., Murugesan K., Kim Y. Biodegradation of dibenzo-p-dioxin, dibenzofuran, and chlorodibenzo-p-dioxins by Pseudomonas veronii PH-03. Biodegradation. 2004;15:303–313. doi: 10.1023/B:BIOD.0000042185.04905.0d. [DOI] [PubMed] [Google Scholar]
- 105.Nam I., Hong H., Kim Y., Kim B., Murugesan K., Chang Y.S. Biological removal of polychlorinated dibenzo-p-dioxins from incinerator fly ash by Sphingomonas wittichii RW1. Water Res. 2005;39:4651–4660. doi: 10.1016/j.watres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 106.Widada J., Omori T. Enhanced degradation of carbazole and 2, 3-dichlorodibenzo-p-dioxin in soils by Pseudomonas resinovorans strain CA10. Chemosphere. 2002;49:485–491. doi: 10.1016/S0045-6535(02)00334-X. [DOI] [PubMed] [Google Scholar]
- 107.Ishii K., Furuichi T. Development of bioreactor system for treatment of dioxin-contaminated soil using Pseudallescheria boydii. J. Hazard. Mater. 2007;148:693–700. doi: 10.1016/j.jhazmat.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 108.Huang W.Y., Ngo H.H., Lin C., Vu C.T., Kaewlaoyoong A., Boonsong T., Tran H.-T., Bui X.-T., Vo T.-D.-H., Chen J.-R. Aerobic co-composting degradation of highly PCDD/F-contaminated field soil. A study of bacterial community. Sci. Total Environ. 2019;660:595–602. doi: 10.1016/j.scitotenv.2018.12.312. [DOI] [PubMed] [Google Scholar]
- 109.Huy P.Q., Thoa N.K., Thi D., Ha C. Degradation of 2,3,7,8-TCDD by a consortium of bacterial strains isolated from heavil herbicide/Dioxin contaminated soil in Bienhoa airbase. J. Biotechnol. 2018;16:777–784. [Google Scholar]
- 110.Nakamiya K., Furuichi T., Ishii K. Isolation of a fungus from denitrifying activated sludge that degrades highly chlorinated dioxins. J. Mater. Cycles Waste Manag. 2002;4:127–134. [Google Scholar]
- 111.Kasai N., Ikushiro S., Shinkyo R., Yasuda K., Hirosue S. Metabolism of mono- and dichloro-dibenzo-p-dioxins by Phanerochaete chrysosporium cytochromes P450. Appl Microbiol Biotechnol. 2010;86:773–780. doi: 10.1007/s00253-009-2413-x. [DOI] [PubMed] [Google Scholar]
- 112.Kaewlaoyoong A., Cheng C., Lin C., Chen J., Huang W., Sriprom P. Science of the Total Environment White rot fungus Pleurotus pulmonarius enhanced bioremediation of highly PCDD/F-contaminated fi eld soil via solid state fermentation. Sci. Total Environ. 2020;738:139670. doi: 10.1016/j.scitotenv.2020.139670. [DOI] [PubMed] [Google Scholar]
- 113.Tachibana M.K.S., Kiyota Y. Bioremediation of Dioxin-Contaminated Soil by Fungi Screened from Nature. Pak. J. Biol. Sci. 2007;10:486–491. doi: 10.3923/pjbs.2007.486.491. [DOI] [PubMed] [Google Scholar]
- 114.Pinedo-Rivilla C., Aleu J., Collado I.G. Pollutants Biodegradation by Fungi. Curr. Org. Chem. 2009;13:1194–1214. doi: 10.2174/138527209788921774. [DOI] [Google Scholar]
- 115.Zafar U., Nzeram P., Langarica-Fuentes A., Houlden A., Heyworth A., Saiani A., Robson G.D. Bioresource Technology Biodegradation of polyester polyurethane during commercial composting and analysis of associated fungal communities. Bioresour. Technol. 2014;158:374–377. doi: 10.1016/j.biortech.2014.02.077. [DOI] [PubMed] [Google Scholar]
- 116.Obuekwe C.O., Al-jadi Z.K., Al-saleh E.S. International Biodeterioration & Biodegradation Hydrocarbon degradation in relation to cell-surface hydrophobicity among bacterial hydrocarbon degraders from petroleum-contaminated Kuwait desert environment. Int. Biodeterior. Biodegrad. 2009;63:273–279. doi: 10.1016/j.ibiod.2008.10.004. [DOI] [Google Scholar]
- 117.Laine M.M., Ahtiainen J., Wågman N., Öberg L.G., Jørgensen K.S. Fate and toxicity of chlorophenols, polychlorinated dibenzo-p-dioxins, and dibenzofurans during composting of contaminated sawmill soil. Environ. Sci. Technol. 1997;31:3244–3250. doi: 10.1021/es970233z. [DOI] [Google Scholar]
- 118.Lin C., Kaewlaoyoong A., Vu C.T., Huang W.Y. Treatment of dioxin-contaminated soil by organic waste co-composting system. Springer Proc. Phys. 2018;207:619–623. doi: 10.1007/978-3-319-78919-4_48. [DOI] [Google Scholar]
- 119.Alkorta I., Garbisu C. Phytoremediation of organic contaminants in soils. Bioresour. Technol. 2001;79:273–276. doi: 10.1016/S0960-8524(01)00016-5. [DOI] [PubMed] [Google Scholar]
- 120.Macek J.K.T., Macková M. Exploitation of plants for the removal of organics in environmental remediation. Biotechnol. Adv. 2000;18:23–34. doi: 10.1016/S0734-9750(99)00034-8. [DOI] [PubMed] [Google Scholar]
- 121.Reichenauer T.G., Germida J.J. Phytoremediation of Organic Contaminants in Soil and Groundwater. ChemSusChem. 2008;8:708–717. doi: 10.1002/cssc.200800125. [DOI] [PubMed] [Google Scholar]
- 122.Kurzawová M.M.V., Uhlík O., Macek T. Interactions of microorganisms and plants in soil contaminated with PCBs and their importance in phyto/rhizoremediation. Remediat. Phytoremediation Technol. 2010;126:396–398. [Google Scholar]
- 123.Hanano A., Almousally I., Shaban M. Chemosphere Phytotoxicity effects and biological responses of Arabidopsis thaliana to 2, 3, 7, 8-tetrachlorinated dibenzo-p-dioxin exposure. Chemosphere. 2014;104:76–84. doi: 10.1016/j.chemosphere.2013.10.060. [DOI] [PubMed] [Google Scholar]
- 124.Inui H., Wakai T., Gion K., Kim Y., Eun H. Chemosphere Differential uptake for dioxin-like compounds by zucchini subspecies. Chemosphere. 2008;73:1602–1607. doi: 10.1016/j.chemosphere.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 125.Nakagawa R., Hori T., Tobiishi K., Iida T., Tsutsumi T., Sasaki K., Toyoda M. Levels and tissue-dependent distribution of dioxin in Japanese domestic leafy vegetables—From the 1999 national investigation. Chemosphere. 2002;48:247–256. doi: 10.1016/S0045-6535(02)00059-0. [DOI] [PubMed] [Google Scholar]
- 126.Uegaki R., Seike N., Otani T. Dioxin-like polychlorinated biphenyls in rice plants: Possible contaminated pathways. Chemosphere. 2006;65:1537–1543. doi: 10.1016/j.chemosphere.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 127.Urbaniak M., Zieliński M., Wyrwicka A. The influence of the Cucurbitaceae on mitigating the phytotoxicity and PCDD/PCDF content of soil amended with sewage sludge. Int. J. Phytoremediation. 2017;19:207–213. doi: 10.1080/15226514.2016.1207606. [DOI] [PubMed] [Google Scholar]
- 128.Campanellaand B., Paul R. Presence, in the Rhizosphere and Leaf Extracts of Zucchini (Cucurbita pepo L.) and Melon (Cucumis melo L.), of Molecules Capable of Increasing the Apparent Aqueous Solubility of Hydrophobic Pollutants. Int. J. Phytoremediation. 2006;2:145–158. doi: 10.1080/15226510008500036. [DOI] [Google Scholar]
- 129.Hulster A., Muller J.F., Marschner H. Soil-Plant Transfer of Polychlorinated Dibenzo-p-dioxins and Dibenzofurans to Vegetables of the Cucumber Family (Cucurbitaceae) Environ. Sci. Technol. 1994;28:1110–1115. doi: 10.1021/es00055a021. [DOI] [PubMed] [Google Scholar]
- 130.Zhang H., Chen J., Ni Y., Zhang Q., Zhao L. Chemosphere Uptake by roots and translocation to shoots of polychlorinated dibenzo-p-dioxins and dibenzofurans in typical crop plants. Chemosphere. 2009;76:740–746. doi: 10.1016/j.chemosphere.2009.05.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.