Abstract
Consumption of coffee, tea, wine, curry, and soybeans has been linked to a lower risk of cancer in epidemiological studies. Several cell-based and animal studies have shown that dietary polyphenols like chlorogenic acid, curcumin, epigallocatechin-3-O-gallate, genistein, quercetin and resveratrol play a major role in these anticancer effects. Several mechanisms have been proposed to explain the anticancer effects of polyphenols. Depending on the cellular microenvironment, these polyphenols can exert double-faced actions as either an antioxidant or a prooxidant, and one of the representative anticancer mechanisms is a reactive oxygen species (ROS)-mediated mechanism. These polyphenols can also influence microRNA (miR) expression. In general, they can modulate the expression/activity of the constituent molecules in ROS-mediated anticancer pathways by increasing the expression of tumor-suppressive miRs and decreasing the expression of oncogenic miRs. Thus, miR modulation may enhance the anticancer effects of polyphenols through the ROS-mediated pathways in an additive or synergistic manner. More precise human clinical studies on the effects of dietary polyphenols on miR expression will provide convincing evidence of the preventive roles of dietary polyphenols in cancer and other diseases.
Keywords: dietary polyphenols, microRNA, cancer, reactive oxygen species, anticancer pathway
1. Introduction
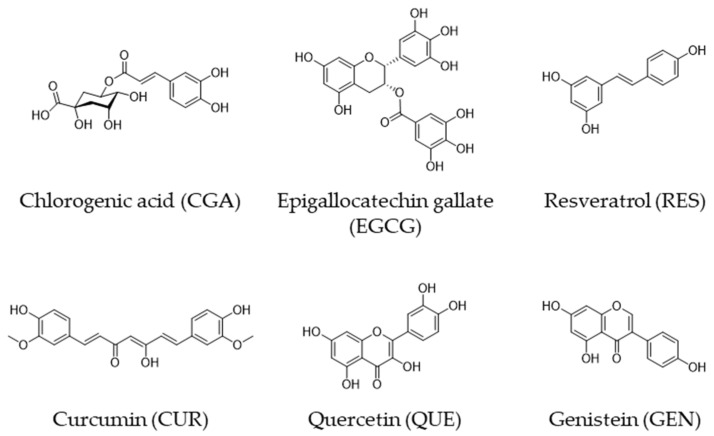
Human epidemiological studies have shown that diets high in plant polyphenols have beneficial effects on various diseases including cancer [1,2]. We have discussed the anticancer effects of coffee, tea, wine, and curry based on recent evidence from human studies, in which chlorogenic acid (CGA), (-)-epigallocatechin gallate (EGCG), resveratrol (RES), and curcumin (CUR), respectively, are believed to be major contributors to the activity [3] (Figure 1 and Table 1).
Figure 1.
Chemical structures of CGA, EGCG, RES, CUR, QUE, and GEN.
Table 1.
Major food sources of polyphenols.
| Polyphenol | Major Food Source |
|---|---|
| Chlorogenic acid (CGA) | Coffee bean |
| (−)-Epigallocatechin gallate (EGCG) | Green tea |
| Resveratrol (RES) | Red wine |
| Curcumin (CUR) | Curry |
| Quercetin (QUE) | Onion |
| Genistein (GEN) | Soy |
Quercetin (QUE) is a flavonol found in a variety of fruits and vegetables including apples, grapes, broccoli, green tea, and onions [4,5] (Figure 1), and several human studies have shown that QUE-rich diets have anticancer effects [5,6,7,8]. For example, Ekström et al. [7] discovered that QUE intake had a strong inverse association with the risk of noncardia gastric adenocarcinoma, with an adjusted odds ratio (OR) of 0.57 (95% confidence interval [CI] = 0.40–0.83) when the highest quintile (≥11.9 mg/day) was compared to the lowest quintile (<4 mg).
Epidemiologic studies have also shown that a soy-rich diet reduces the risk of various diseases, including cancer, and one of the main contributors is thought to be genistein (GEN), a phenolic compound [9,10,11] (Figure 1). Wang et al. [12] discovered a lower risk of papillary macrocarcinomas in women who consumed 1860–3110 μg/day of GEN (OR = 0.26, CI = 0.08–0.85) compared to women who consumed <760 μg/day in a population-based case-control study in Connecticut from 2010 to 2011. A meta-analysis conducted by Applegate et al. [13] revealed that the pooled relative risk for GEN in the risk of prostate cancer was 0.90 (CI: 0.84–0.97).
Many epidemiological studies, on the other hand, have found that these foods have no anticancer effects [1,14]. The inconsistent results could be due to a number of confounding factors, including the quantity and quality of plant foods consumed, as well as residual pesticides and acrylamide formed during preparation, cigarette smoking, alcohol consumption, differences in ingredients, hormonal activities, microbiota, and genetic background [1,14,15]. Human intervention studies that are well-designed could provide significant evidence for the anticancer effects of dietary foods containing these polyphenols.
The anticancer properties of these polyphenols have been demonstrated in a large number of cell-based and animal studies, and their possible anticancer mechanisms have been proposed. Of them, one involving reactive oxygen species (ROS) appears to be the most likely, in which these polyphenols can act as both an ROS-generator and an ROS-scavenger [16].
In our previous review, we presented putative anticancer pathways that CGA, CUR, EGCG, or RES can trigger [3], as well as the roles of microRNAs (miRs) modulated by these polyphenols in the pathways. As GEN and QUE share some properties similar to CGA, CUR, EGCG, and RES, this review focuses on their ROS-mediated anticancer properties, which may include their miR-modulating activity.
2. Anticancer Pathways
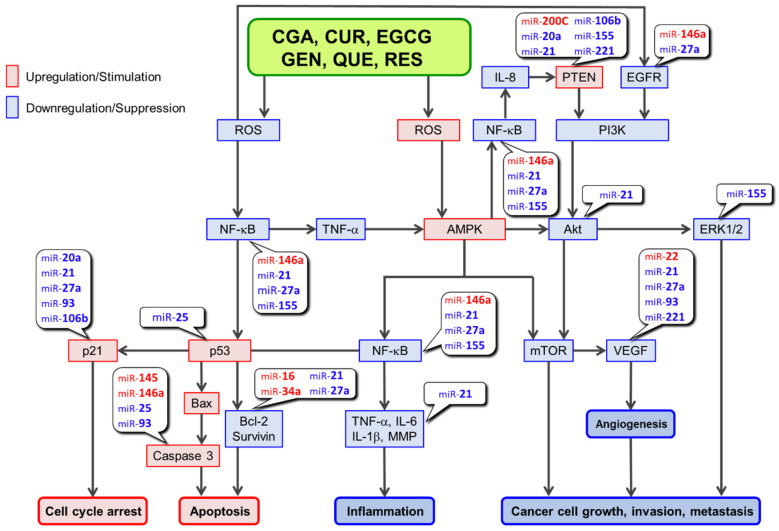
Based on our previous discussions [3,17,18], Figure 2 depicts a putative ROS-mediated anticancer mechanism in which polyphenols may be involved [19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Based on the findings of Zhang et al. [33], a pathway involving AMP-activated protein kinase (AMPK), SIRT1, p53, and p21 is depicted in this figure. They discovered that S-nitrosoglutathione, an endogenous nitric oxide carrier, induces apoptosis in lung cancer A549 cells by inhibiting SIRT1 deacetylase activity toward p53 and thus increasing p53 acetylation, which leads to an increased expression of p21 and apoptosis in A549 cells. According to our previous discussion [3], links of miRs to the constituting molecules in the pathways are also presented in Figure 2.
Figure 2.
ROS-mediated anti-cancer activities associated with miRs regulated by polyphenols.
As shown in Table 2 [14,21,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96], these six polyphenols are similar in that they can act as an ROS-generator and an ROS-scavenger, respectively, leading to AMPK upregulation and NF-κB downregulation. GEN and QUE also influence other molecular components of the anticancer pathways depicted in Figure 2 and Table 3 [50,97,98,99,100,101,102,103,104,105,106,107,108]. At present, it is not clear what can direct a polyphenol to act as an ROS-generator or ROS-scavenger. Differences in cell types, concentrations of polyphenols and metal ions such as Fe(II) and Cu(II); antioxidant enzymes such as glutathione S-transferase, glutathione peroxidase, and hemeoxygenase-1; and small molecules such as glutathione [18,109] are all possible candidates.
Table 2.
Modulatory effects of CGA, CUR, GEN, EGCG, QUE, and RES on ROS, AMPK, and NF-κB.
| ROS Up | AMPK Up | ROS Down | NF-κB Down | |
|---|---|---|---|---|
| Polyphenols | Stimulation/ upregulation |
Stimulation/ upregulation |
Suppression/ downregulation |
Suppression/ downregulation |
| CGA | Rakshit et al. [44] Hou et al. [55] Yang et al. [66] |
Sudeep et al. [77] Lukitasari et al. [88] Santana-Galvez et al. [94] |
Cha et al. [95] Wang et al. [96] Santana-Galvez et al. [94] |
Zeng et al. [21] Chen et al. [34] Zatorski et al. [35] |
| CUR | Nakamae et al. [36] Gupta et al. [37] Gersey et al. [38] |
Yu et al. [39] Hamidie et al. [40] Pan et al. [41] |
Abadi et al. [42] Park et al. [43] Wang et al. [45] |
Pimentel-Gutierrez et al. [46] Zhou et al. [47] Shao et al. [48] |
| GEN | Lee et al. [49] Zhang et al. [50] Park et al. [51] |
Gasparrini et al. [52] Ikeda et al. [53] Lee et al. [54] |
Cai et al. [56] Lee et al. [57] Lagunes et al. [58] |
Mukund et al. [59] Mukund et al. [60] Javed et al. [61] |
| EGCG | Wei et al. [62] Ouyang et al. [63] Yang et al. [14] |
Yang et al. [64] Ouyang et al. [63] Kim et al. [65] |
Na et a. [67] Yang et al. [14] Wada et al. [68] |
Shen et al. [69] Reddy et al. [70] Ohishi et al. [71] |
| QUE | Kim et al. [72] Lagunes et al. [58] Wang et al. [73] |
Kim et al. [72] Zhang et al. [74] Fukaya et al. [75] |
Bahar et al. [76] Priyadarsini et al. [78] Rezaei-Sadabady et al. [79] |
Bahar, et al. [76] Cheng et al. [80] Chen et al. [81] |
| RES | Costa et al. [82] Fu et al. [83] Li et al. [84] |
Wang et al. [45] Wang et al. [85] Baur et al. [86] |
Giordo et al. [87] Perez-Torres et al. [89] Mathieu et al. [90] |
Subedi et al. [91] Hsu et al. [92] Ginés et al. [93] |
Table 3.
Modulation by GEN and QUE of the molecules constituting the ROS-mediated anticancer pathway.
| GEN | QUE | ||
|---|---|---|---|
| p53 | Upregulation | Ye et al. [97] | Priyadarsini et al. [101] |
| p21 | Ye et al. [102] | Clemente-Soto et al. [103] | |
| PTEN | Bilir et al. [104] | Boadi et al. [105] | |
| EGFR | Downregulation | Gao et al. [106] | Pani et al. [107] |
| ERK | Li et al. [108] | Pan et al. [98] | |
| VEGF | Yazdani et al. [99] | Lai et al. [100] | |
| Bcl-2 | Zhang et al. [50] | Pan et al. [98] |
miRs in red and in blue are upregulated and downregulated by polyphenols, respectively.
For example, Kanadzu et al. [110] demonstrated a concentration-dependent dual function of EGCG by showing that EGCG at 1–100 µM enhanced DNA strand breakage induced by bleomycin and hydrogen peroxide, whereas a lower concentration at 0.1 to 0.01 µM suppressed DNA breakage in human lymphocytes. CUR was shown to increase superoxide production in MCF-7, HepG2, and MDAMB cancer cells, but not in normal rat hepatocytes [111]. Low concentrations of GEN promoted primary muscle cell proliferation, whereas high concentrations inhibited their proliferation by causing intracellular ROS production [112].
3. Modulation of miRs by Dietary Polyphenols
Polyphenols can influence the expression of miRs, which are 20–22 nucleotide long single-stranded non-coding RNAs [3]. As miRs regulate a wide range of biological processes, including cell proliferation, apoptosis, and cell differentiation, changes in their expression levels are linked to disease progression, including cancer [113]. When compared to normal cells or tissues, the expression of miRs is upregulated (oncogenic miRs) or downregulated (tumor suppressor miRs) in cancers, indicating their important roles in cancer.
We previously discussed the modulatory activity of CGA, CUR, EGCG, and RES [3], and dietary polyphenols can affect miR expression. At least three of these polyphenols can modulate the same nine miRs, five of which are downregulated (miR-20a, 21, 25, 93, and 106b) and four of which are upregulated (miR-16, 34a, 145, and 200c). Based on our previous discussion [3] and information on caspase 3 [114], we depict Figure 2 for the ROS-mediated anticancer pathways. As mentioned earlier, GEN and QUE share many similar properties with the other four polyphenols, implying that they have similar miR-modulatory effects. Table 4 and Table 5 compare the effects of these dietary polyphenols on miRs reported in the literature [21,23,24,26,27,29,30,31,32,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182]. The addition of GEN and QUE data increased the number of miRs modulated similarly by at least three polyphenols from 9 to 15, as expected. The effects of miRs modulated by these polyphenols on the molecular constituents in the ROS-mediated pathways are also provided in these tables and incorporated in Figure 2.
Table 4.
Tumor-suppressor miRs upregulated by polyphenols, cell types examined, and effects of miR upregulation.
| miR | CUR | EGCG | GEN | QUE | RES | Effects of miRs Upregulated by Polyphenols on Molecules in the ROS-Mediated Pathway: ↑, Upregulation; ↓ Downregulation |
|---|---|---|---|---|---|---|
| miR-16 |
MCF-7 (breast cancer) (Yang, et al.) [182] |
HepG2 (liver cancer) (Tsang, et al.) [115] |
A549 (lung cancer) (Sonoki, et al.) [116] HSC-6 SCC-9 (oral cancer) (Zhao, et al.) [117] |
MCF7-ADR MCF10A MDA-MB-231-luc-D3H2LN (breast cancer) (Hagiwara, et al.) [118] CCRF-CEM (acute lymphoblastic leukemia) (Azimi, et al.) [119] |
↓Bcl-2 [115,182] | |
| miR-22 | BxPC-3 (pancreatic carcinoma) (Sun, et al.) [120] Y79 (retinoblastoma) (Sreenivasan, et al.) [121] Downregulated * MyLa2059, SeAx (malignant cutaneous lymphoma) (Sibbesen, et al.) [122] |
CNE2 (nasopharyngeal carcinoma) (Li, et al.) [123] |
Tca8113 SAS (oral squamous cell carcinoma) (Zhang, et al.) [124] |
↓VEGF via↓Sp1 [120] | ||
| miR-34a | MDA-MB-231 MDA-MB-435 (breast cancer) (Guo, et al.) [125] SGC-7901 (gastric cancer) (Sun, et al.) [126] HCT116 (colorectal cancer) (Toden, et al.) [127] BxPC-3 (pancreatic cancer) (Sun, et al.) [120] Downregulated * TE-7 (esophageal adenocarcinoma) (Subramaniam, et al.) [128] |
SK-N-BE2 IMR-32 (malignant neuroblastoma) (Chakrabarti, et al.) [129] SH-SY5Y SK-N-DZ (malignant neuroblastoma) (Chakrabarti, et al.) [130] HCT116 HCT116-5FUR (colorectal cancer, 5FU resistant) (Toden, et al.) [131] CNE2 (nasopharyngeal carcinoma) (Li, et al.) [123] HepG2 (hepatocellular carcinoma) (Mostafa, et al.) [132] |
HNC-TICs (tumor-initiating cells of head and neck cancer) (Hsieh, et al.) [133] DU145 (prostate cancer) (Chiyomaru, et al.) [134] AsPC-1 MiaPaCa-2 (pancreatic cancer) (Xia, et al.) [135] |
MDA-MB-231-luc-D3H2LN (breast cancer) (Hagiwara, et al.) [118] DLD-1 (colon cancer) (Kumazaki, et al.) [136] MCF-7 (breast cancer) (Otsuka, et al.) [137] SKOV-3 OV-90 (ovarian cancer) (Yao, et al.) [138] |
↓Bcl-2 [125,126,127,138] ↓NF-κB via Notch-1 [135] |
|
| miR-141 | HCT116-5FUR (colorectal cancer, 5FU resistant) (Toden, et al.) [139] |
Downregulated * MM1.s (multiple myeloma) (Gordon, et al.) [140] |
786-O ACHN (renal carcinoma) (Chiyomaru, et al.) [141] |
MCF7-ADR MCF-7 MCF10A MDA-MB-231-luc-D3H2LN (breast cancer) (Hagiwara, et al.) [118] |
||
| miR-145 | U-87 MG (glioblastoma) Mirgani, et al.) [142] DU145 22RV1 (prostate cancer) (Liu, et al.) [143] |
HCT116 HCT116-5FUR (colorectal cancer, 5FU resistant) (Toden, et al.) [131] |
Y79 (retinoblastoma) (Wei, et al.) [144] |
SKOV-3 A2780 (ovarian cancer) (Zhou, et al.) [145] |
BT-549 MDA-MB-231 MCF-7 (breast cancer) (Sachdeva, et al.) [146] |
↑Caspase-3 [145] |
| miR-146a | U-87 MG (glioblastoma) (Wu, et al.) [31] AsPC-1 (pancreatic cancer) CDF (analog) (Bao, et al.) [147] |
Colo357 Panc-1 (pancreatic cancer) G2535 (mixture of genistein and other isoflavones) (Li, et al.) [148] |
MCF-7 MDA-MB-231 (breast cancer) (Tao, et al.) [26] |
↓NF-κB [31] ↑Caspase-3 [26] ↓EGFR [26] |
||
| miR-200c | HCT116-5FUR SW480-5FUR (colorectal cancer, 5FU resistant) (Toden, et al.) [139] MiaPaCa-2 MiaPaCa-2-GR BxPC-3 (pancreatic cancer) CDF (analog) (Soubani, et al.) [149] |
HCT116-5FUR (colorectal cancer, 5FU resistant) (Toden, et al.) [131] |
Cancer stem cells of nasopharyngeal carcinoma (Shen, et al.) [150] MCF7-ADR MCF-7 MCF10A MDA-MB-231-luc-D3H2LN (breast cancer) (Hagiwara, et al.) [118] HCT116 (colorectal cancer) (Dermani, et al.) [151] |
↑PTEN [149] |
* The items shown in italics are different findings from other reported results (see Text).
Table 5.
miRs downregulated by polyphenols, cell types examined, and effects of miR downregulation.
| miR | CGA | CUR | EGCG | GEN | QUE | RES | Effects of miRs Downregulated by Polyphenols on Molecules in the ROS-Mediated Pathway: ↑, Upregulation; ↓, Downregulation |
|---|---|---|---|---|---|---|---|
| miR-20a | Huh7 (Hepatoma) H446 (lung carcinoma) (Huang, et al.) [152] |
RKO (colon cancer) (Gandhy, et al.) [27] |
HUVEC (umbilical vascular endothelial cell cocultured with A549) (Mirzaaghaei, et al.) [153] |
DU145 22RV1 (prostate cancer) (Dhar, et al.) [154] (CCL4-induced liver fibrotic cells) (Zhu, et al.) [155] DU145 (prostate cancer) (Dhar, et al.) [156] |
↑ p21 [152] ↑ PTEN [154] ↑ PTEN/PI3K/AKT [155] |
||
| miR-21 | LX2 (hepatic stellate) (Wang, et al.) [157] |
HCT116 RKO (colorectal cancer) (Mudduluru, et al.) [158] AsPC-1 MiaPaCa-2 (pancreatic cancer) CDF (analog) (Bao, et al.) [147] TE-7 (esophageal cancer) (Subramaniam, et al.) [128] PC-3 LNCaP (prostate cancer) Hypoxia CDF (analog) (Bao, et al.) [23] A549 (lung cancer) (Zhang, et al.) [159] K562 LAMA84 (chronic myelogenous leukemia) (Taverna, et al.) [160] DU145 C4-2 (prostate cancer) (Yallapu, et al.) [161] |
MCF-7 (breast cancer) Polyphenon-60 (Fix, et al.) [162] 22Rv1 xenograft (prostate tumor) (Siddiqui, et al.) [163] |
A-498 xenograft (renal cancer) (Zaman, et al.) [164] |
SW480 (colon cancer) (Tili, et al.) [165] PC-3M-MM2 (prostate cancer) (Sheth, et al.) [166] PANC-1 CFPAC-1 MiaPaCa-2 (pancreatic cancer) (Liu, et al.) [167] U251 (glioblastoma) (Li, et al.) [32] T24 5637 (bladder cancer) (Zhou, et al.) [29] |
↓VEGF [23] ↓IL-6 [23] ↑PTEN [159,160] ↑p21 [164] ↓Bcl-2 [29,167] ↓NF-κB [32] ↓Akt [29] |
|
| miR-25 | BxPC-3 (pancreatic cancer) (Sun, et al.) [120] |
MCF-7 (breast cancer) Polyphenon-60 (Fix, et al.) [162] MM1.s (multiple myeloma) (Gordon, et al.) [140] MCF-7 (breast cancer) (Zan, et al.) [168] |
SW480 (colon cancer) (Tili, et al.) [165] |
↑ p53 [140] ↑Caspase-3 [168] |
|||
| miR-27a | HCT116p53± SW480 (Toden, et al.) [127] SW480 (colon cancer) (Noratto, et al.) [169] RKO (colon cancer) (Gandhy, et al.) [27] |
MCF-7 (breast cancer) Polyphenon-60 (Fix, et al.) [162] |
PANC-1 BxPC-3 (pancreatic cancer) (Cheng, et al.) [170] SKOV3 (ovarian cancer) (Xu, et al.) [171] C918 (uveal melanoma) (Sun, et al.) [172] Upregulated * A549 (lung cancer) (Yang, et al.) [173] |
↓VEGF via Sp1 [169] ↓VEGF via Sp1 [27] ↓EGFR [27] ↓Survivin [27] ↓Bcl-2 [27] ↓NF-κB [27] ↑FOXO1 [170] |
|||
| miR-93 | Huh7 (Hepatoma) H446 (Lung carcinoma) (Huang, et al.) [152] |
SK-N-BE2 IMR-32 (malignant neuroblastoma) (Chakrabarti, et al.) [129] SH-SY5Y SK-N-DZ (malignant neuroblastoma) (Chakrabarti, et al.) [130] |
MCF-10A (breast cancer) (Singh, et al.) [174] |
↑ p21 [152] ↑Caspase-3 [129,130] |
|||
| miR-106b | Huh7 (Hepatoma) H446 (Lung carcinoma) (Huang, et al.) [152] |
SK-N-BE2 IMR-32 (malignant neuroblastoma) (Chakrabarti, et al.) [129] SH-SY5Y SK-N-DZ (malignant neuroblastoma) (Chakrabarti, et al.) [130] |
LNCaP DU145 (prostate cancer) (Dhar, et al.) [156] DU145 22RV1 (prostate cancer) (Dhar, et al.) [154] |
↑ p21 [152] ↑ PTEN [154,156] |
|||
| miR-155 | RAW264.7 (mouse macrophage) (Zeng, et al.) [21] |
RAW264.7 (mouse macrophage) THP1 (acute monocyte leukemia) (Ma, et al.) [30] |
MDA-MB-435 Hs578t (breast cancer) (Parra, et al.) [175] (Basu, et al.) [196] |
RAW264.7 (mouse macrophage) (Boesch-Saadatmandi, et al.) [176] |
THP-1 (monocyte) (Tili, et al.) [177] |
↓ NF-κB [21] ↑ PTEN [175] |
|
| miR-221 | MiaPaCa-2 (pancreatic cancer) CDF (analog) (Sarkar, et al.) [178] HepG2 tumor (HCC orthotopic mouse model) (Zhang, et al.) [24] SW1736 (Anaplastic thyroid carcinoma) (Allegri, et al.) [179] |
SW1736 (Anaplastic thyroid carcinoma) (Allegri, et al.) [179] Upregulated * HepG2 (liver cancer) (Tsang, et al.) [115] |
PC-3 (prostate cancer) (Chen, et al.) [180] SW1736 (Anaplastic thyroid carcinoma) (Allegri, et al.) [179] |
WI-38 (lung fibroblast) (Wang, et al.) [181] |
↑ PTEN [178] ↓VEGF [24] |
* The items shown in italics are different findings from other reported results (see Text).
4. Anticancer Mechanism of Tumor Suppressor miRs Upregulated by Polyphenols
Table 4 summarizes the available data for tumor-suppressor miRs that are commonly upregulated by at least three different polyphenols in cancer cells. Figure 2 shows that several molecules involved in the anticancer mechanism are found in ROS-mediated pathways. Table 4 also provides information on the modulatory effects of miRs upregulated by these polyphenols on these molecules.
4.1. miR-16
CUR, EGCG, QUE, and RES have been shown to have anticancer properties [3,17,183]. These polyphenols have been shown to increase the expression of the tumor suppressor miR-16. miR-16 has the ability to reduce the expression of the target Bcl-2 [115]. Claudin-2 expression is decreased by QUE-induced miR-16, which may downregulate Bcl-2 [116]. Bcl-2 is an anti-apoptotic protein, and its inhibition would result in an anticancer effect. QUE may increase miR-16 expression to decrease Homeobox A10 expression, which is involved in cancer proliferation, migration, and invasion [117]. RES increased the expression and activity of Argonaute2, a central RNA interference component, which resulted in anticancer effects by increasing the expression of several tumor-suppressor miRs including miR-16 [118].
4.2. miR-22
CUR, EGCG, and QUE have been shown to upregulate miR-22, which may downregulate specificity protein 1 (Sp1), estrogen receptor 1 (ESR1) [120], erythoblastic leukemia viral oncogene homolog 3 (Erbb3) [121], and nuclear receptor coactivator 1 (NCoA1) [122]. Sun et al. [120] discovered that CUR increased miR-22 expression in PxBC-3 pancreatic cancer cells using oligonucleotide microarray analysis. Transfection with miR-22 mimetics reduced expression of the target genes Sp1 and ESR1, whereas antisense inhibition of miR-22 increased Sp1 and ESR1 expression. Sp1 is overexpressed in various cancers and has the potential to be a chemotherapeutic drug target [184]. Sp1 can upregulate VEGF to promote cancer cell growth, angiogenesis, and metastasis [185,186], downregulation of miR-22 upregulated by these polyphenols may contribute to the anticancer effects of these polyphenols.
In malignant T cells, transfection of recombinant miR-22 resulted in the inhibition of its targets including NCoA1, HDAC6, MAX, MYCBP, and PTEN [122]. As PTEN is known to be tumor suppressing [187], its downregulation by CUR does not appear to be consistent with CUR’s anticancer properties. Downregulation of other cancer-promoting molecules such as HDAC6, required for efficient oncogenic tumorigenesis [188], and NCoA1, whose overexpression increases the number of circulating cancer cells and the metastasis [189], may overwhelm PTEN’s efficacy in this case.
Zhang et al. [124] showed that overexpression of miR-22 increased cancer cell apoptosis by targeting WNT1, and that the miR-22/WNT1/β-catenin axis is the downstream pathway for QUE to exert an antitumor effect in oral squamous cell carcinoma.
4.3. miR-34a
CUR upregulation of miR-34 resulted in Bcl-2 downregulation, cell cycle arrest, and/or c-Myc downregulation [125,126,127]. RES increased apoptosis and miR-34a expression in ovarian cancer cells [138]. miR-34a inhibition experiments revealed that miR-34a downregulates Bcl-2, upregulates Bax, and activates caspase-3.
EGCG has been shown to exert anticancer effects by upregulating tumor-suppressing miRs including miR-34a and downregulating oncogenic miRs such as miR-92, miR-93, and miR-106b [130].
In an experiment with HNC-TICs cells from head and neck cancer, GEN inhibited their proliferation, downregulated epithelial–mesenchymal transition (EMT), and induced upregulation of miR-34a, which resulted in ROS production [133]. Caspase-3 activation induced by overexpression of miR-34a was inhibited by N-acetylcysteine, indicating that ROS are involved in the anticancer effects of GEN.
In, GEN induced apoptosis in prostate cancer PC3 and DU145 cells, increased miR-34a expression levels, and reduced those of oncogenic HOX transcript antisense RNA (HOTAIR), a target of miR-34a [134]. HOTAIR is a non-coding RNA that has been shown to induce cell cycle arrest in the G2/M phase [190]. The GEN-mediated upregulation of miR-34a in pancreatic cancer cells also inhibited the Notch-1 signaling pathway [135], whose activation promotes cancer cell growth and metastasis [191,192]. Inhibition of Notch-1 would result in down regulation of NF-κB, leading to cancer suppression [193].
RES increased the expression of tumor suppressor miR-34a, 424, and 503 in breast cancer cells [137]. HNRNPA1, a heterogeneous nuclear ribonucleoprotein associated with tumorigenesis and progression, was directly downregulated by miR-424 and miR-503, but indirectly by miR-34a [137]. According to Kumazaki et al. [136], RES upregulates miR-34a, which causes downregulation of the target gene E2F3 and its downstream SIRT1, leading to inhibition of colon cancer cell growth.
Thus, polyphenols appear to upregulate miR-34 in general, but Subrama-niam et al. [128] found that CUR decreased expression of miR-34a in esophageal cancer TE-7 cells. One possible explanation for the difference is that the p53 status of different cell lines differs, as TE-7 cells are p53-deficient and p53 is an upstream regulator of miR-34a.
4.4. miR-141
CUR upregulated the expression of EMT-suppressing miRs such as miR-34a, 101, 141, 200c, and 429 in 5-fluorouracil (5FU)-resistant HCT116 cells, but not in 5FU-resistant SW480 cells [139]. EMT is a crucial step in the generation of cancer stem cells and the progression of cancer. The extent to which miR-141 contributes to EMT suppression is not known.
Chiyomaru et al. [141] discovered that treatment of renal carcinoma cells with GEN increased miR-141 expression and decreased HOTAIR, which is known to promote malignancy. HOTAIR expression was reduced in cells transfected with pre-miR-141. By increasing the expression of a number of tumor-suppressive miRs, including miR-16, 141, 143, and 200c, RES reduced the viability of breast cancer cells and inhibited cancer stem-like cell characteristics [118]. The miR-141 inhibitor reduced the efficacy of RES’s inhibitory effect against cancer invasion, implying that miR-141 plays a role in RES’ anticancer effect.
Gordon et al. [140] reported that treatment of multiple myeloma, MM1.s cells, with the carcinogen benzo[a]pyrene upregulated the expression of miR-15a, 16, 25, 92, 125b, 141, and 200a, all of which are p53 targets. EGCG inhibited the expression of tumor-suppressive miR-141 which upregulates p53. The finding appears inconsistent with EGCG’s anticancer activity. It is possible that EGCG’s downregulation of oncogenic miR-25 may be more effective in the anticancer effect than downregulation of miR-141 in these cells.
4.5. miR-145
Curcumin encapsulated in a non-toxic nanocarrier inhibited the proliferation of glioblastoma U-87 MG cells, increased miR-145 expression, and decreased the expression of transcription factors Oct4, SOX-2, and Nanog, all of which are upregulated and result in increased metastasis, invasion, and recurrence [142,194].
CUR inhibited the proliferation, invasion, and tumorigenicity of prostate cancer stem cells HuPCaSCs (CD44+/CD133+ subpopulation isolated from prostate cancer cell lines Du145 and 22RV1) by increasing the expression of miR-145, which prevents cell proliferation by decreasing Oct4 expression [143]. In colorectal cancer cells, EGCG increased apoptosis and cell cycle arrest, and upregulated miR-145 [131].
In GEN-treated retinoblastoma Y79 cells, miR-145 was found to be significantly upregulated [144]. The siRNA downregulated miR-145 and the target of miR-145 has been identified as ABCE1 which has oncogene-like properties. By increasing the expression of miR-145, QUE was found to induce apoptosis in human ovarian carcinoma cells. The increased expression levels of cleaved caspase-3 induced by QUE were further increased by overexpression of miR-145 [145].
4.6. miR-146a
CUR upregulated miR-146a in human U-87 MG glioblastoma cells, and overexpression of miR-146a increased apoptosis and decreased NF-κB activation in cells treated with the anticancer drug temozolomide [31]. miR-146a expression is lower in pancreatic cancer cells compared to normal human pancreatic duct epithelial cells. GEN treatment increased miR-146a expression with decreasing EGFR and NF-κB expression in these cancer cells. Transfection of miR-146a inhibited these cells’ invasive ability by downregulating EGFR and NF-κB, implying that upregulation of miR-146a is involved in the anticancer effect of GEN [148]. The results of experiments with or without transfection of miR-146a mimic or anti-miR-146a revealed that QUE increased miR-146a, leading to apoptosis induction through downregulation of EGFR and activation of caspase-3 in a study of QUE’s anticancer effect [26].
4.7. miR-200c
Experiments on overexpression or silencing of miR-200c in pancreatic cancer MiaPaCa-2 cells showed that a CUR analog upregulated PTEN expression, increased levels of MT1-MMP, and reduced tumor cell aggressiveness through upregulation of miR-200c [149]. Toden et al. [139] discovered that CUR improved the efficacy of 5-FU in suppressing tumor growth and EMT in 5FU-resistant colorectal cancer cells. miR-200c, a key EMT-suppressing miR, was upregulated by CUR, and miR-200c was found to downregulate BMI1, SUZ12, and EZH2 in a transfection experiment.
Upregulation of miR-200c was also observed in RES-treated nasopharyngeal carcinoma cancer stem cells [150], EGCG-treated 5FU-resistant colorectal cancer cells [131], and RES-treated breast cancer cells [118]. Dermani et al. [151] discovered that RES increased the expression of miR-200c and decreased the viability of colorectal cancer cells. Transfection with anti-miR-200c increased vimentin and ZEB1 expression, while decreasing E-cadherin expression and apoptosis. These changes were reversed by RES, indicating that RES induces apoptosis and inhibits EMT in colorectal cancer by regulating miR-200c.
5. Anticancer Mechanism of Oncogenic miRs Downregulated by Polyphenols
Table 5 summarizes the available data for oncogenic miRs that are commonly modulated by at least three different polyphenols in cancer cells. Among these molecules, Figure 2 shows that several molecules involved in the anticancer mechanism are found in the ROS-mediated pathways. Table 5 also shows the effects of miRs downregulated by polyphenols on the molecules involved in ROS-mediated anticancer pathways (Figure 2).
5.1. miR-20a
CGA inhibited hepatoma and lung cancer cells by causing cell cycle arrest in the G0/G1 phase [152]. CGA increased KHSRP, p53, and p21 expression while decreasing c-Myc and CD44 expression. The microarray analysis revealed that the expression of the miR-17 family members miR-20a, 93, and 106b was downregulated in cells treated with CGA. An inhibitor of miR-20a increased p21 mRNA expression, and transfection of CGA-treated cells with a mimic of miR-20a which cancelled CGA’s p21 upregulation effect while increasing c-Myc, indicating that p21 is the miR’s target.
Dhar et al. [156] discovered that RES reduced the expression of miRs-17, 20a, 106a, and 106b in prostate cancer cells. In an extended study, they discovered that RES downregulation of these miRs increased the expression of their target PTEN. These miRs, when expressed ectopically, directly targeted PTEN 3’UTR, leading to the reduction of its expression [154].
Liver fibrosis is often linked to the development of cancer [157]. RES was shown to attenuate liver fibrosis in an animal model in a study to investigate its role in this pathology. Cell-based experiments with an miR-20a mimic revealed that RES induces autophagy and activates the miR-20a-mediated PTEN/PI3K/AKT signaling pathway, resulting in fibrosis prevention [155].
5.2. miR-21
Increases in the mRNA levels of miR-21 and connective tissue growth factor (CTGF) and a decrease in the level of Smad7 were caused by IL-13 stimulation of LX-2 cells, which were reversed by CGA [195]. miR-21 knockdown resulted in lower mRNA levels of miR-21 and CTGF expression, while Smad7 levels increased in line with the findings on the protein expression levels of CTGF, p-Smad1, p-Smad2, p-Smad2/3, and TGF-β receptor 1. The affected tissues had increased mRNA levels of miR-21 and CTGF with a decrease in the level of Smad7 and CGA, which prevented these changes and liver fibrosis in an animal model of liver fibrosis induced by Schistosoma japonicum cercaria infection. Since liver fibrosis is intimately related to liver cancer, these findings suggest anticancer effects of CGA as well [157].
CUR inhibited colorectal cancer cell proliferation by inducing G2/M arrest [158]. CUR inhibited AP-1 binding to the promoter of miR-21 and induced the expression of the tumor suppressor programmed cell death protein 4, which is a target of miR-21.
In pancreatic cancer AsPC-1 and MiaPaCa-2 cells, Bao et al. [147] discovered that a CUR analog CDF suppressed the expression of histone methyltransferase EZH2, EpCAM, ABCG2, Shh, MMP-9, cleaved Notch-1, and Hes-1, while increasing the miR expressions of let-7 family miRs, miR-26a, 101, 146a, and 200. The expression of miR-21 was extremely high in these cells, and CDF suppressed its expression. The same group of researchers also discovered that hypoxia increases the expression of VEGF, IL-6, and CSC marker genes such as Nanog, Oct4, and EZH2, as well as the expression of miR-21 in prostate cancer cells [23].
CUR inhibited esophageal cancer cell proliferation and colony formation by inducing apoptosis through caspase 3 activation [128]. CUR also inhibited Notch-1 activation, Jagged-1 expression and its downstream target Hes-1, as well as downregulation of miR-21 and miR-34a expression and upregulation of tumor suppressor let-7a miR.
CUR inhibited cell proliferation, induced apoptosis and suppressed miR-21 expression in A549 cells. PTEN, a putative miR-21 target, was upregulated by CUR. miR-21 transfection suppressed CUR’s effects on cell proliferation and apoptosis in these cells, suggesting that miR-21 suppression may have anticancer therapeutic benefits [159]. Similarly, CUR reduced cell viability and miR-21 expression in chronic myelogenous leukemia cells [160]. PTEN was upregulated by CUR, while VEGF was downregulated. miR-21 mimic transfection increased VEGF expression, while miR-21 inhibitor decreased VEGF expression. CUR reversed the effect of a miR-21 mimic, while increasing the effect of an miR-21 inhibitor, indicating that VEGF is a target of miR-21 in CUR’s anticancer effects.
CUR inhibited cell growth and miR-21 expression in prostate cancer cells [161]. Western blot analysis showed that CUR caused increased levels of the cleaved PARP, and decreased levels of Bcl-xL, Mcl-1, and p-Akt, respectively.
Polyphenon -60, which contains EGCG as a major component, caused downregulation of miR-21 expression, which can downregulate the tumor suppressor gene tropomyosin-1 in MCF-7 breast cancer cells [162]. EGCG inhibits prostate cancer cell growth. The tumor xenograft tissues from EGCG-treated mice had decreased levels of miR-21 and increased levels of miR-330 [163].
Zaman et al. [164] discovered that GEN inhibited tumor formation by inhibiting miR-21 expression in kidney cancer A-498 cells and xenografts. Inhibition of cell growth, induction of G0/G1 arrest, and upregulation of p21 and p38 MAP kinase were all observed when miR-21 was knocked down in these cells, indicating that p21 could be a target of miR-21.
A microarray analysis showed that RES downregulated several oncogenic miRs including miR-21 and upregulated tumor-suppressing miRs, including miR-663 in colon cancer cells, suggesting that RES’ anticancer effects may be influenced by changes in the composition of miR populations in cancer cells [165]. A similar study in prostate cancer cells revealed that RES reduced the expression of miR-21, which was confirmed by qRT-PCR [166]. Transfection with pre-miR-21 resulted in the downregulation of tumor-suppressing PDCD4 and the upregulation of cancer cell invasion, which were both reversed by RES.
RES reduced the viability of pancreatic cancer cells and suppressed miR-21 expression [167]. Bcl-2 expression was reduced when miR-21 expression decreased. Transfection of a miR-21 mimic reversed RES-induced downregulation of Bcl-2 and apoptosis, indicating that miR-21 is a target of the RES’s anticancer action. Similar results were reported for RES’s anticancer effects in bladder cancer cells [29]. miR-21 overexpression attenuated the inhibition of p-Akt activity and downregulated Bcl-2 expression and apoptosis induced by RES. Furthermore, RES’s anticancer mechanism against glioma cells was reported to involve miR-21 [32]. RES decreased IκB phosphorylation, nuclear p65 protein levels, and NF-κB activity. miR-21 expression was inhibited by RES, and miR-21 downregulation reduced NF-κB activity. The effect of RES on NF-κB activity and apoptosis was reversed when miR-21 was overexpressed.
5.3. miR-25
According to a microarray analysis, CUR-treated pancreatic cancer cells had lower expression of miR-25 and other miRs than untreated pancreatic cancer cells [120]. In colon cancer cells, RES was found to downregulate several oncogenic miRs, including miR-25 [165]. Fix et al. [162] showed that breast cancer cells treated with Polyphenon-60 exhibited upregulated expression of let-7a, 107, 548m, 720, 1826, 1978, and 1979 and downregulated expression of let-7c, let-7e, let-7g, miR-21, 25, 26b, 27a, 27b, 92a, 125a-5p, 200b, 203, 342-3p, 454, 1469, and 1977.
Gordon et al. [140] discovered that the carcinogen benzo[a]pyrene upregulated the expression of p53-targeting miRs including miR-25 in MM1.s cells. EGCG inhibited the expression of miR-25 in these cells as well as the induction of miR-25 by the carcinogen, suggesting that miR-25 is involved in EGCG’s anticarcinogenic activity. In breast cancer cells, Zan et al. [168] discovered that EGCG inhibited miR-25 expression, as well as induction of apoptosis and disruption of cell cycle progression at G2/M phase. The apoptotic effects of EGCG, such as caspase-3 and caspase-9 activation and an increase in PARP expression, were reduced when cells were transfected with miR-25 mimic.
5.4. miR-27a
CUR inhibited the expression of miR-27a and had cytotoxic effects on colorectal cancer cells [127]. In colorectal cancer cells, knockdown of miR-27a increased apoptosis and G2/M phase arrest. Curcuminoids inhibited the growth of colon cancer cells and suppressed miR-27a while downregulating Sp1, Sp3, and Sp4 and Sp-regulated genes [169]. Treatment of breast cancer cells with Polyphenon-60 inhibited growth and decreased miR-27a expression [162]. As miR-27a has been shown to promote cancer cell proliferation in osteosarcoma cells [197], suppressing miR-27a may help these polyphenols to have anticancer effects. Downregulation of Sp1 may be linked to VEGF downregulation [22342309, 29048687], which can also explain the anticancer effects of these polyphenols.
Antitumor GEN has been shown to suppress miR-27a expression in pancreatic cancer cells [170]. Inhibiting miR-27a induced cell growth inhibition and apoptosis, implying that miR-27a is involved in GEN’s anticancer effect. Similarly, Xu et al. [171] discovered that GEN inhibited ovarian cancer cell growth and migration with downregulating miR-27a expression and increasing the expression of Sprouty2, a putative miR-27a target gene. GEN was also shown to inhibit uveal melanoma cell growth, which was accompanied by a decrease in miR-27a and an increase in its target gene ZBTB10 [172].
Apoptosis induction enhanced by miR-27a downregulation may be explained by its effect on caspase-9 activation through Apaf-1 upregulation, as demonstrated by experiments in which miR-27a antioligonucleotides promoted the formation of Apaf1-caspase-9 complex in TRAIL-treated colorectal cancer stem cells [198]. Yang et al. [173] reported that GEN have anticancer effects in lung cancer A549 cells by upregulating miR-27a and downregulating the proto-oncogene MET. The reason for the disparity in the results on GEN’s modulation of miR-27a is currently not known, but it could be due to the use of different cancer cells.
5.5. miR-93
CGA inhibited hepatoma and lung cancer cells by causing cell cycle arrest at the G0/G1 phase. Transfection of CGA-treated cells with mimics of miR-93 cancelled the p21 upregulation effect of CGA while increasing c-Myc, indicating that p21 is the target of miR-93 as reported in experiments for miR-20a [152].
EGCG inhibited cell growth and induced apoptosis in malignant neuroblastoma SK-N-BE2 and IMR-32 cells by decreasing Bcl-2 expression, increasing Bax expression, and activating caspase-8 and caspase-3 [129]. miR-92, 93, and 106b were downregulated by EGCG, while miR-7-1, miR-34a, and miR-99a were upregulated. miR-93 overexpression prevented EGCG-induced apoptosis, which was accompanied by an increase in Bcl-2 expression and a decrease in caspase-8 and caspase-3 activation. The findings suggest that miR-93 plays a role in EGCG-mediated apoptosis. Similarly, in neuroblastoma SH-SY5Y and SK-N-DZ cells, EGCG caused the downregulation of oncogenic miR-92, 93, and 106b and upregulation of tumor-suppressing miR-7-1, 34a, and 99a [130]. Prolonged exposure to estrogen is known to increase the risk of breast cancer [199]. Singh et al. [174] demonstrated that RES inhibited mammary carcinogenesis in a rat model of 17-estradiol-induced mammary tumors. Hormone-treatment induced increased tumor formation and expression of miR-93 in mammary tissues compared to control levels. The RES treatment had no effect on miR-93 expression levels.
5.6. miR-106b
CGA inhibited hepatoma and lung cancer cells by causing cell cycle arrest at the G0/G1 phase and transfection of CGA-treated cells with mimics of miR-106b reduced the CGA’s upregulation effect of p21 while increasing c-Myc [152].
As previously stated, EGCG inhibited the growth of malignant neuroblastoma cells, induced apoptosis, and reduced the expression of oncogenic miR-92, 93, and 106b [129,130]. In prostate cancer, RES exhibited anticancer activity and miR microarrays revealed that RES downregulated 23 miRs and upregulated 28 miRs [156]. Downregulation of miR-106b was confirmed by qRT-PCR. PTEN is one of the targets of downregulated miRs, including miR-106b and RES upregulated PTEN, suggesting that downregulation of miR-106b can lead to PTEN upregulation in the anticancer effect of RES. This notion is clearly demonstrated by Dhar et al. [154], who showed that RES decreased the levels of miR-17, miR-20a. and miR-106b, leading to upregulation of their target PTEN in prostate cancer cells. PTEN protein expression was downregulated when miR-106b was overexpressed, but it was upregulated in the presence of RES, indicating that PTEN is a direct target of miR-106b.
5.7. miR-155
CGA downregulated NK-κB and the nucleotide-binding domain like receptor protein 3 inflammasome-related proteins in a model of inflammation using LPS/ATP-stimulated RAW264.7 cells, which was dependent on the downregulation of miR-155 expression [21]. Ma et al. [30] showed that CUR suppressed LPS-induced cytokines (TNF-α, IL-6) and miR-155 expression in Raw264.7 and THP-1 cells in a similar experiment. Transfection of miR-155 mimics suppressed these effects, indicating that CUR suppresses LPS-induced inflammatory response by inhibiting miR-155. In experiments using a similar inflammation model, QUE was shown to downregulate cytokines such as TNF-α, IL-1β, and IL-6, as well as miR-155 [176]. Tili et al. [177] discovered that pretreatment with RES reduced the upregulation of miR-155 in LPS-treated THP-1 cells. As the results of several studies indicate a correlation between elevated levels of miR-155 and the development of tumors such as breast, lung, or gastric cancers, as well as leukemias, RES may be useful as an anti-inflammatory and anticancer agent. In metastatic breast cancer cells, GEN reduced cell viability and induced apoptosis by downregulating miR-155, FOXO3, PTEN, casein kinase, and p27. Overexpression of miR-155 in cells infected with miR-155 lentiviral vectors reduced the effects of GEN [175].
5.8. miR-221
Sarkar et al. [178] discovered that pancreatic cancer patients with high miR-221 expression have a lower rate of survival. Transfection of an miR-221 inhibitor suppressed pancreatic cancer cell growth while also upregulating PTEN, p27, and p57. A curcumin analogue CDF and isoflavone mixture containing 70.54% GEN mimicked the miR-221 inhibitor.
CUR reduced tumor weight and tumor microvessel count in a xenograft model inoculated with HepG2 cells compared to a vehicle control [24]. CUR decreased miR-221 expression while increasing miR-222 expression. miR-221 may be a target of anticancer strategies because it is involved in the angiogenesis mechanism. Expression of the tumor suppressor gene aplysia ras homolog I (ARHI) was found to be inversely associated with the expression of miR-221 and 222 in prostate cancer cell lines [180]. ARHI expression was significantly induced by transfection of miR-221 and 222 inhibitors. GEN upregulated ARHI expression in these cells by downregulating miR-221 and 222.
Wang et al. [181] discovered that QUE reduced LPS-induced inflammatory damage in WI-38 lung fibroblasts by increasing cell viability, suppressing cell apoptosis, and decreasing the production of inflammatory cytokines IL-6 and TNF-α. QUE inhibited LPS-induced upregulation of miR-221 in these cells, and miR-221 overexpression reversed QUE’s anti-inflammatory effects. Through downregulation of miR-221, QUE inhibited NF-B activity and the JNK pathway in LPS-treated cells. In human hepatocellular carcinoma HepG2 cells, EGCG inhibited cancer cell growth and induced apoptosis [115]. miR-let-7a, 16, and 221 were upregulated while miR-18a, 34b, 193b, 222, and 342 were downregulated, according to a microarray analysis and qRT-PCR results. Tumor-promoting effects of the minor upregulation of oncogenic miR-221 may be overcome by increased expression of tumor suppressive miR-16 and/or the anticancer effects of other miRs’ modulation, leading to EGCG’s eventual anticancer effects [115].
6. Conclusions
Consumption of coffee, tea, wine, curry, and soybeans has been linked to cancer prevention in epidemiological studies. A number of cell-based and animal studies have shown that polyphenols such as CGA, CUR, EGCG, GEN, QUE, and RES are major contributors to anticancer effects. Depending on their cellular microenvironments, these dietary polyphenols can act as both an antioxidant and a prooxidant, and several mechanisms have been proposed to explain their anticancer effects, one of which is an ROS-mediated mechanism (Figure 2). Furthermore, these polyphenols have been shown to modulate miRs expression. In general, they can increase the expression of tumor-suppressive miRs while decreasing the expression of oncogenic miRs, resulting in modulation of the expression/activity of constituents in ROS-mediated anticancer pathways (Figure 2) [3]. As a result, modulations by these miRs may enhance the anticancer effects of polyphenols in an additive or synergistic manner. In addition, other mechanisms such as EMT modulation by miRs may be involved in the anticancer effects of these polyphenols.
Several xenograft experiments such as those described above have shown that polyphenols modulate miRs in vivo [32,164,165]. However, only a few human studies have been conducted on this subject. miR-21 in the plasma of postmenopausal women with low bone density after CUR supplementation [200], miR-17, 27, and 146a in regulatory T cells from inflammatory rheumatic disease patients treated with CUR [201], and inflammation-responsive miRs such as miR-21, 34a, and 155 in peripheral blood mononuclear cells from type 2 diabetes and hypertensive patients who consumed RES-enriched grape extract [202] are just a few examples. Similar future studies in humans will provide convincing information on the effects of dietary polyphenols on cancer and other diseases.
A limitation of this review is that something other than what we have shown here may be found, as the results were obtained from a search of two databases: PubMed and Web of Science.
Author Contributions
Y.Y., literature search, text preparation of Abstract, Introduction, Section 4 and Section 5, preparation of Table 4 and Table 5; T.O., corresponding author, text preparation of Abstract, Introduction, Section 2, Section 3, Section 4 and Section 5, drawing of Figure 2, preparation of Table 2 and Table 3; Y.N., literature search, preparation of references, text preparation of Section 2; R.F., literature search, drawing of Figure 1, text preparation of Section 2 and Section 3; N.M., corresponding author, project design, outline of the whole manuscript, literature search, text preparation of Abstract, Introduction, Section 2, Section 3, Section 4, Section 5 and Section 6, and preparation of Table 1. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
Funding Statement
This research was funded in part by a Grant-in-Aid for Scientific Research (KAKENHI, No. 20H04109 to N.M.) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT, Tokyo).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wahle K.W.J., Brown I., Rotondo D., Heys S.D. Plant Phenolics in the Prevention and Treatment of Cancer. Adv. Exp. Med. Biol. 2010;698:36–51. doi: 10.1007/978-1-4419-7347-4_4. [DOI] [PubMed] [Google Scholar]
- 2.Kumar N., Goel N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019;24:e00370. doi: 10.1016/j.btre.2019.e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohishi T., Hayakawa S., Miyoshi N. Involvement of MicroRNA Modifications in Anticancer Effects of Major Polyphenols from Green Tea, Coffee, Wine, and Curry. Crit. Rev. Food Sci. Nutr. 2022:1–32. doi: 10.1080/10408398.2022.2038540. [DOI] [PubMed] [Google Scholar]
- 4.Tanabe H., Pervin M., Goto S., Isemura M., Nakamura Y. Beneficial Effects of Plant Polyphenols on Obesity. Obes. Control Ther. 2017;4:1–16. doi: 10.15226/2374-8354/4/3/00142. [DOI] [Google Scholar]
- 5.Lam T.K., Rotunno M., Lubin J.H., Wacholder S., Consonni D., Pesatori A.C., Bertazzi P.A., Chanock S.J., Burdette L., Goldstein A.M., et al. Dietary Quercetin, Quercetin-Gene Interaction, Metabolic Gene Expression in Lung Tissue and Lung Cancer Risk. Carcinogenesis. 2010;31:634–642. doi: 10.1093/carcin/bgp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandera E.V., Williams M.G., Sima C., Bayuga S., Pulick K., Wilcox H., Soslow R., Zauber A.G., Olson S.H. Phytoestrogen Consumption and Endometrial Cancer Risk: A Population-Based Case-Control Study in New Jersey. Cancer Causes Control. 2009;20:1117–1127. doi: 10.1007/s10552-009-9336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekström A.M., Serafini M., Nyrén O., Wolk A., Bosetti C., Bellocco R. Dietary Quercetin Intake and Risk of Gastric Cancer: Results from a Population-Based Study in Sweden. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011;22:438–443. doi: 10.1093/annonc/mdq390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo H.D., Kim J. Dietary Flavonoid Intake and Smoking-Related Cancer Risk: A Meta-Analysis. PLoS ONE. 2013;8:e75604. doi: 10.1371/journal.pone.0075604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang Y.W., Kim S.Y., Jee S.H., Kim Y.N., Nam C.M. Soy Food Consumption and Risk of Prostate Cancer: A Meta-Analysis of Observational Studies. Nutr. Cancer. 2009;61:598–606. doi: 10.1080/01635580902825639. [DOI] [PubMed] [Google Scholar]
- 10.Spagnuolo C., Russo G.L., Orhan I.E., Habtemariam S., Daglia M., Sureda A., Nabavi S.F., Devi K.P., Loizzo M.R., Tundis R., et al. Genistein and Cancer: Current Status, Challenges, and Future Directions. Adv. Nutr. 2015;6:408–419. doi: 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamagata K., Yamori Y. Potential Effects of Soy Isoflavones on the Prevention of Metabolic Syndrome. Molecules. 2021;26:5863. doi: 10.3390/molecules26195863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q., Huang H., Zhao N., Ni X., Udelsman R., Zhang Y. Phytoestrogens and Thyroid Cancer Risk: A Population-Based Case-Control Study in Connecticut. Cancer Epidemiol. Biomarkers Prev. 2020;29:500–508. doi: 10.1158/1055-9965.EPI-19-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Applegate C.C., Rowles J.L., Ranard K.M., Jeon S., Erdman J.W. Soy Consumption and the Risk of Prostate Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients. 2018;10:40. doi: 10.3390/nu10010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang C.S., Wang X., Lu G., Picinich S.C. Cancer Prevention by Tea: Animal Studies, Molecular Mechanisms and Human Relevance. Nat. Rev. Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahinfar H., Jayedi A., Khan T.A., Shab-Bidar S. Coffee Consumption and Cardiovascular Diseases and Mortality in Patients with Type 2 Diabetes: A Systematic Review and Dose-Response Meta-Analysis of Cohort Studies. Nutr. Metab. Cardiovasc. Dis. 2021;31:2526–2538. doi: 10.1016/j.numecd.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Slika H., Mansour H., Wehbe N., Nasser S.A., Iratni R., Nasrallah G., Shaito A., Ghaddar T., Kobeissy F., Eid A.H. Therapeutic Potential of Flavonoids in Cancer: ROS-Mediated Mechanisms. Biomed. Pharmacother. 2022;146:112442. doi: 10.1016/j.biopha.2021.112442. [DOI] [PubMed] [Google Scholar]
- 17.Hayakawa S., Ohishi T., Miyoshi N., Oishi Y., Nakamura Y., Isemura M. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules. 2020;25:4553. doi: 10.3390/molecules25194553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayakawa S., Saito K., Miyoshi N., Ohishi T., Oishi Y., Miyoshi M., Nakamura Y. Anti-Cancer Effects of Green Tea by Either Anti- or Pro- Oxidative Mechanisms. Asian Pac. J. Cancer Prev. 2016;17:1649–1654. doi: 10.7314/APJCP.2016.17.4.1649. [DOI] [PubMed] [Google Scholar]
- 19.Hu H., Li H., He Y. MicroRNA-17 Downregulates Expression of the PTEN Gene to Promote the Occurrence and Development of Adenomyosis. Exp. Ther. Med. 2017;14:3805–3811. doi: 10.3892/etm.2017.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan J., Su Z., Gu W., Shen X., Zhao Q., Shi L., Jin C., Wang X., Cong H., Ju S. MiR-19b and MiR-20a Suppress Apoptosis, Promote Proliferation and Induce Tumorigenicity of Multiple Myeloma Cells by Targeting PTEN. Cancer Biomark. 2019;24:279–289. doi: 10.3233/CBM-182182. [DOI] [PubMed] [Google Scholar]
- 21.Zeng J., Zhang D., Wan X., Bai Y., Yuan C., Wang T., Yuan D., Zhang C., Liu C. Chlorogenic Acid Suppresses MiR-155 and Ameliorates Ulcerative Colitis through the NF-ΚB/NLRP3 Inflammasome Pathway. Mol. Nutr. Food Res. 2020;64:e2000452. doi: 10.1002/mnfr.202000452. [DOI] [PubMed] [Google Scholar]
- 22.Kumar M., Lu Z., Takwi A.A.L., Chen W., Callander N.S., Ramos K.S., Young K.H., Li Y. Negative Regulation of the Tumor Suppressor P53 Gene by MicroRNAs. Oncogene. 2011;30:843–853. doi: 10.1038/onc.2010.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao B., Ahmad A., Kong D., Ali S., Azmi A.S., Li Y., Banerjee S., Padhye S., Sarkar F.H. Hypoxia Induced Aggressiveness of Prostate Cancer Cells Is Linked with Deregulated Expression of VEGF, IL-6 and MiRNAs That Are Attenuated by CDF. PLoS ONE. 2012;7:e43726. doi: 10.1371/journal.pone.0043726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S., Tang D., Zang W., Yin G., Dai J., Sun Y.U., Yang Z., Hoffman R.M., Guo X. Synergistic Inhibitory Effect of Traditional Chinese Medicine Astragaloside IV and Curcumin on Tumor Growth and Angiogenesis in an Orthotopic Nude-Mouse Model of Human Hepatocellular Carcinoma. Anticancer Res. 2017;37:465–473. doi: 10.21873/anticanres.11338. [DOI] [PubMed] [Google Scholar]
- 25.Dey N., Ghosh-Choudhury N., Kasinath B.S., Choudhury G.G. TGFβ-Stimulated MicroRNA-21 Utilizes PTEN to Orchestrate AKT/MTORC1 Signaling for Mesangial Cell Hypertrophy and Matrix Expansion. PLoS ONE. 2012;7:e42316. doi: 10.1371/journal.pone.0042316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao S.-F., He H.-F., Chen Q. Quercetin Inhibits Proliferation and Invasion Acts by Up-Regulating MiR-146a in Human Breast Cancer Cells. Mol. Cell. Biochem. 2015;402:93–100. doi: 10.1007/s11010-014-2317-7. [DOI] [PubMed] [Google Scholar]
- 27.Gandhy S.U., Kim K., Larsen L., Rosengren R.J., Safe S. Curcumin and Synthetic Analogs Induce Reactive Oxygen Species and Decreases Specificity Protein (Sp) Transcription Factors by Targeting MicroRNAs. BMC Cancer. 2012;12:564. doi: 10.1186/1471-2407-12-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trompeter H.-I., Abbad H., Iwaniuk K.M., Hafner M., Renwick N., Tuschl T., Schira J., Müller H.W., Wernet P. MicroRNAs MiR-17, MiR-20a, and MiR-106b Act in Concert to Modulate E2F Activity on Cell Cycle Arrest during Neuronal Lineage Differentiation of USSC. PLoS ONE. 2011;6:e16138. doi: 10.1371/journal.pone.0016138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou C., Ding J., Wu Y. Resveratrol Induces Apoptosis of Bladder Cancer Cells via MiR-21 Regulation of the Akt/Bcl-2 Signaling Pathway. Mol. Med. Rep. 2014;9:1467–1473. doi: 10.3892/mmr.2014.1950. [DOI] [PubMed] [Google Scholar]
- 30.Ma F., Liu F., Ding L., You M., Yue H., Zhou Y., Hou Y. Anti-Inflammatory Effects of Curcumin Are Associated with down Regulating MicroRNA-155 in LPS-Treated Macrophages and Mice. Pharm. Biol. 2017;55:1263–1273. doi: 10.1080/13880209.2017.1297838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu H., Liu Q., Cai T., Chen Y.-D., Wang Z.-F. Induction of MicroRNA-146a Is Involved in Curcumin-Mediated Enhancement of Temozolomide Cytotoxicity against Human Glioblastoma. Mol. Med. Rep. 2015;12:5461–5466. doi: 10.3892/mmr.2015.4087. [DOI] [PubMed] [Google Scholar]
- 32.Li H., Jia Z., Li A., Jenkins G., Yang X., Hu J., Guo W. Resveratrol Repressed Viability of U251 Cells by MiR-21 Inhibiting of NF-ΚB Pathway. Mol. Cell. Biochem. 2013;382:137–143. doi: 10.1007/s11010-013-1728-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Sun C., Xiao G., Shan H., Tang L., Yi Y., Yu W., Gu Y. S-Nitrosylation of the Peroxiredoxin-2 Promotes S-Nitrosoglutathione-Mediated Lung Cancer Cells Apoptosis via AMPK-SIRT1 Pathway. Cell Death Dis. 2019;10:329. doi: 10.1038/s41419-019-1561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J., Luo Y., Li Y., Chen D., Yu B., He J. Chlorogenic Acid Attenuates Oxidative Stress-Induced Intestinal Epithelium Injury by Co-Regulating the PI3K/Akt and IκBα/NF-ΚB Signaling. Antioxidants. 2021;10:1915. doi: 10.3390/antiox10121915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zatorski H., Sałaga M., Zielińska M., Piechota-Polańczyk A., Owczarek K., Kordek R., Lewandowska U., Chen C., Fichna J. Experimental Colitis in Mice Is Attenuated by Topical Administration of Chlorogenic Acid. Naunyn. Schmiedebergs. Arch. Pharmacol. 2015;388:643–651. doi: 10.1007/s00210-015-1110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamae I., Morimoto T., Shima H., Shionyu M., Fujiki H., Yoneda-Kato N., Yokoyama T., Kanaya S., Kakiuchi K., Shirai T., et al. Curcumin Derivatives Verify the Essentiality of ROS Upregulation in Tumor Suppression. Molecules. 2019;24:4067. doi: 10.3390/molecules24224067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta N., Verma K., Nalla S., Kulshreshtha A., Lall R., Prasad S. Free Radicals as a Double-Edged Sword: The Cancer Preventive and Therapeutic Roles of Curcumin. Molecules. 2020;25:5390. doi: 10.3390/molecules25225390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gersey Z.C., Rodriguez G.A., Barbarite E., Sanchez A., Walters W.M., Ohaeto K.C., Komotar R.J., Graham R.M. Curcumin Decreases Malignant Characteristics of Glioblastoma Stem Cells via Induction of Reactive Oxygen Species. BMC Cancer. 2017;17:99. doi: 10.1186/s12885-017-3058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu H., Xie Y., Zhou Z., Wu Z., Dai X., Xu B. Curcumin Regulates the Progression of Colorectal Cancer via LncRNA NBR2/AMPK Pathway. Technol. Cancer Res. Treat. 2019;18:1533033819870781. doi: 10.1177/1533033819870781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamidie R.D.R., Shibaguchi T., Yamada T., Koma R., Ishizawa R., Saito Y., Hosoi T., Masuda K. Curcumin Induces Mitochondrial Biogenesis by Increasing Cyclic AMP Levels via Phosphodiesterase 4A Inhibition in Skeletal Muscle. Br. J. Nutr. 2021;126:1642–1650. doi: 10.1017/S0007114521000490. [DOI] [PubMed] [Google Scholar]
- 41.Pan W., Yang H., Cao C., Song X., Wallin B., Kivlin R., Lu S., Hu G., Di W., Wan Y. AMPK Mediates Curcumin-Induced Cell Death in CaOV3 Ovarian Cancer Cells. Oncol. Rep. 2008;20:1553–1559. [PubMed] [Google Scholar]
- 42.Abadi A.J., Mirzaei S., Mahabady M.K., Hashemi F., Zabolian A., Hashemi F., Raee P., Aghamiri S., Ashrafizadeh M., Aref A.R., et al. Curcumin and Its Derivatives in Cancer Therapy: Potentiating Antitumor Activity of Cisplatin and Reducing Side Effects. Phytother. Res. 2022;36:189–213. doi: 10.1002/ptr.7305. [DOI] [PubMed] [Google Scholar]
- 43.Park J.H., Lee B.M., Kim H.S. Potential Protective Roles of Curcumin against Cadmium-Induced Toxicity and Oxidative Stress. J. Toxicol. Environ. Health. B Crit. Rev. 2021;24:95–118. doi: 10.1080/10937404.2020.1860842. [DOI] [PubMed] [Google Scholar]
- 44.Rakshit S., Mandal L., Pal B.C., Bagchi J., Biswas N., Chaudhuri J., Chowdhury A.A., Manna A., Chaudhuri U., Konar A., et al. Involvement of ROS in Chlorogenic Acid-Induced Apoptosis of Bcr-Abl+ CML Cells. Biochem. Pharmacol. 2010;80:1662–1675. doi: 10.1016/j.bcp.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 45.Wang S., Moustaid-Moussa N., Chen L., Mo H., Shastri A., Su R., Bapat P., Kwun I., Shen C.-L. Novel Insights of Dietary Polyphenols and Obesity. J. Nutr. Biochem. 2014;25:1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pimentel-Gutiérrez H.J., Bobadilla-Morales L., Barba-Barba C.C., Ortega-De-La-Torre C., Sánchez-Zubieta F.A., Corona-Rivera J.R., González-Quezada B.A., Armendáriz-Borunda J.S., Silva-Cruz R., Corona-Rivera A. Curcumin Potentiates the Effect of Chemotherapy against Acute Lymphoblastic Leukemia Cells via Downregulation of NF-ΚB. Oncol. Lett. 2016;12:4117–4124. doi: 10.3892/ol.2016.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou T., Wang Y., Liu M., Huang Y., Shi J., Dong N., Xu K. Curcumin Inhibits Calcification of Human Aortic Valve Interstitial Cells by Interfering NF-ΚB, AKT, and ERK Pathways. Phytother. Res. 2020;34:2074–2081. doi: 10.1002/ptr.6674. [DOI] [PubMed] [Google Scholar]
- 48.Shao W., Yu Z., Chiang Y., Yang Y., Chai T., Foltz W., Lu H., Fantus I.G., Jin T. Curcumin Prevents High Fat Diet Induced Insulin Resistance and Obesity via Attenuating Lipogenesis in Liver and Inflammatory Pathway in Adipocytes. PLoS ONE. 2012;7:e28784. doi: 10.1371/journal.pone.0028784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee Y.-K., Park O.J. Soybean Isoflavone Genistein Regulates Apoptosis through NF-ΚB Dependent and Independent Pathways. Exp. Toxicol. Pathol. 2013;65:1–6. doi: 10.1016/j.etp.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q., Bao J., Yang J. Genistein-Triggered Anticancer Activity against Liver Cancer Cell Line HepG2 Involves ROS Generation, Mitochondrial Apoptosis, G2/M Cell Cycle Arrest and Inhibition of Cell Migration. Arch. Med. Sci. 2019;15:1001–1009. doi: 10.5114/aoms.2018.78742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park C., Cha H.-J., Lee H., Hwang-Bo H., Ji S.Y., Kim M.Y., Hong S.H., Jeong J.-W., Han M.H., Choi S.H., et al. Induction of G2/M Cell Cycle Arrest and Apoptosis by Genistein in Human Bladder Cancer T24 Cells through Inhibition of the ROS-Dependent PI3k/Akt Signal Transduction Pathway. Antioxidants. 2019;8:327. doi: 10.3390/antiox8090327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gasparrini M., Giampieri F., Alvarez Suarez J., Mazzoni L., Y Forbes Hernandez T., Quiles J.L., Bullon P., Battino M. AMPK as a New Attractive Therapeutic Target for Disease Prevention: The Role of Dietary Compounds AMPK and Disease Prevention. Curr. Drug Targets. 2016;17:865–889. doi: 10.2174/1573399811666150615150235. [DOI] [PubMed] [Google Scholar]
- 53.Ikeda T., Watanabe S., Mitani T. Genistein Regulates Adipogenesis by Blocking the Function of Adenine Nucleotide Translocase-2 in the Mitochondria. Biosci. Biotechnol. Biochem. 2022;86:260–272. doi: 10.1093/bbb/zbab203. [DOI] [PubMed] [Google Scholar]
- 54.Lee S.R., Kwon S.W., Lee Y.H., Kaya P., Kim J.M., Ahn C., Jung E.-M., Lee G.-S., An B.-S., Jeung E.-B., et al. Dietary Intake of Genistein Suppresses Hepatocellular Carcinoma through AMPK-Mediated Apoptosis and Anti-Inflammation. BMC Cancer. 2019;19:6. doi: 10.1186/s12885-018-5222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hou N., Liu N., Han J., Yan Y., Li J. Chlorogenic Acid Induces Reactive Oxygen Species Generation and Inhibits the Viability of Human Colon Cancer Cells. Anticancer Drugs. 2017;28:59–65. doi: 10.1097/CAD.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 56.Cai Q., Rahn R.O., Zhang R. Dietary Flavonoids, Quercetin, Luteolin and Genistein, Reduce Oxidative DNA Damage and Lipid Peroxidation and Quench Free Radicals. Cancer Lett. 1997;119:99–107. doi: 10.1016/S0304-3835(97)00261-9. [DOI] [PubMed] [Google Scholar]
- 57.Lee S.-H., Kim J.-K., Jang H.-D. Genistein Inhibits Osteoclastic Differentiation of RAW 264.7 Cells via Regulation of ROS Production and Scavenging. Int. J. Mol. Sci. 2014;15:10605–10621. doi: 10.3390/ijms150610605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lagunes I., Trigos Á. Photo-Oxidation of Ergosterol: Indirect Detection of Antioxidants Photosensitizers or Quenchers of Singlet Oxygen. J. Photochem. Photobiol. B. 2015;145:30–34. doi: 10.1016/j.jphotobiol.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 59.Mukund V., Behera S.K., Alam A., Nagaraju G.P. Molecular Docking Analysis of Nuclear Factor-ΚB and Genistein Interaction in the Context of Breast Cancer. Bioinformation. 2019;15:11–17. doi: 10.6026/97320630015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukund V. Genistein: Its Role in Breast Cancer Growth and Metastasis. Curr. Drug Metab. 2020;21:6–10. doi: 10.2174/1389200221666200120121919. [DOI] [PubMed] [Google Scholar]
- 61.Javed Z., Khan K., Herrera-Bravo J., Naeem S., Iqbal M.J., Sadia H., Qadri Q.R., Raza S., Irshad A., Akbar A., et al. Genistein as a Regulator of Signaling Pathways and MicroRNAs in Different Types of Cancers. Cancer Cell Int. 2021;21:388. doi: 10.1186/s12935-021-02091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei R., Hackman R.M., Wang Y., Mackenzie G.G. Targeting Glycolysis with Epigallocatechin-3-Gallate Enhances the Efficacy of Chemotherapeutics in Pancreatic Cancer Cells and Xenografts. Cancers. 2019;11:1496. doi: 10.3390/cancers11101496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ouyang J., Zhu K., Liu Z., Huang J. Prooxidant Effects of Epigallocatechin-3-Gallate in Health Benefits and Potential Adverse Effect. Oxid. Med. Cell. Longev. 2020;2020:9723686. doi: 10.1155/2020/9723686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang C.S., Zhang J., Zhang L., Huang J., Wang Y. Mechanisms of Body Weight Reduction and Metabolic Syndrome Alleviation by Tea. Mol. Nutr. Food Res. 2016;60:160–174. doi: 10.1002/mnfr.201500428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim S.-N., Kwon H.-J., Akindehin S., Jeong H.W., Lee Y.-H. Effects of Epigallocatechin-3-Gallate on Autophagic Lipolysis in Adipocytes. Nutrients. 2017;9:680. doi: 10.3390/nu9070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang J.-S., Liu C.-W., Ma Y.-S., Weng S.-W., Tang N.-Y., Wu S.-H., Ji B.-C., Ma C.-Y., Ko Y.-C., Funayama S., et al. Chlorogenic Acid Induces Apoptotic Cell Death in U937 Leukemia Cells through Caspase- and Mitochondria-Dependent Pathways. In Vivo. 2012;26:971–978. [PubMed] [Google Scholar]
- 67.Na H.-K., Surh Y.-J. Modulation of Nrf2-Mediated Antioxidant and Detoxifying Enzyme Induction by the Green Tea Polyphenol EGCG. Food Chem. Toxicol. 2008;46:1271–1278. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 68.Wada Y., Takata A., Ikemoto T., Morine Y., Imura S., Iwahashi S., Saito Y., Shimada M. The Protective Effect of Epigallocatechin 3-Gallate on Mouse Pancreatic Islets via the Nrf2 Pathway. Surg. Today. 2019;49:536–545. doi: 10.1007/s00595-019-1761-0. [DOI] [PubMed] [Google Scholar]
- 69.Shen X., Zhang Y., Feng Y., Zhang L., Li J., Xie Y.-A., Luo X. Epigallocatechin-3-Gallate Inhibits Cell Growth, Induces Apoptosis and Causes S Phase Arrest in Hepatocellular Carcinoma by Suppressing the AKT Pathway. Int. J. Oncol. 2014;44:791–796. doi: 10.3892/ijo.2014.2251. [DOI] [PubMed] [Google Scholar]
- 70.Reddy A.T., Lakshmi S.P., Maruthi Prasad E., Varadacharyulu N.C., Kodidhela L.D. Epigallocatechin Gallate Suppresses Inflammation in Human Coronary Artery Endothelial Cells by Inhibiting NF-ΚB. Life Sci. 2020;258:118136. doi: 10.1016/j.lfs.2020.118136. [DOI] [PubMed] [Google Scholar]
- 71.Ohishi T., Goto S., Monira P., Isemura M., Nakamura Y. Anti-Inflammatory Action of Green Tea. Antiinflamm. Antiallergy. Agents Med. Chem. 2016;15:74–90. doi: 10.2174/1871523015666160915154443. [DOI] [PubMed] [Google Scholar]
- 72.Kim G.T., Lee S.H., Kim J.I., Kim Y.M. Quercetin Regulates the Sestrin 2-AMPK-P38 MAPK Signaling Pathway and Induces Apoptosis by Increasing the Generation of Intracellular ROS in a P53-Independent Manner. Int. J. Mol. Med. 2014;33:863–869. doi: 10.3892/ijmm.2014.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Z.-X., Ma J., Li X.-Y., Wu Y., Shi H., Chen Y., Lu G., Shen H.-M., Lu G.-D., Zhou J. Quercetin Induces P53-Independent Cancer Cell Death through Lysosome Activation by the Transcription Factor EB and Reactive Oxygen Species-Dependent Ferroptosis. Br. J. Pharmacol. 2021;178:1133–1148. doi: 10.1111/bph.15350. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Q., Song W., Zhao B., Xie J., Sun Q., Shi X., Yan B., Tian G., Liang X. Quercetin Attenuates Diabetic Peripheral Neuropathy by Correcting Mitochondrial Abnormality via Activation of AMPK/PGC-1α Pathway in Vivo and in Vitro. Front. Neurosci. 2021;15:636172. doi: 10.3389/fnins.2021.636172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fukaya M., Sato Y., Kondo S., Adachi S.-I., Yoshizawa F., Sato Y. Quercetin Enhances Fatty Acid β-Oxidation by Inducing Lipophagy in AML12 Hepatocytes. Heliyon. 2021;7:e07324. doi: 10.1016/j.heliyon.2021.e07324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bahar E., Kim J.-Y., Yoon H. Quercetin Attenuates Manganese-Induced Neuroinflammation by Alleviating Oxidative Stress through Regulation of Apoptosis, INOS/NF-ΚB and HO-1/Nrf2 Pathways. Int. J. Mol. Sci. 2017;18:1989. doi: 10.3390/ijms18091989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sudeep H.V., Venkatakrishna K., Patel D., Shyamprasad K. Biomechanism of Chlorogenic Acid Complex Mediated Plasma Free Fatty Acid Metabolism in Rat Liver. BMC Complement. Altern. Med. 2016;16:274. doi: 10.1186/s12906-016-1258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Priyadarsini R.V., Nagini S. Quercetin Suppresses Cytochrome P450 Mediated ROS Generation and NFκB Activation to Inhibit the Development of 7,12-Dimethylbenz[a]Anthracene (DMBA) Induced Hamster Buccal Pouch Carcinomas. Free Radic. Res. 2012;46:41–49. doi: 10.3109/10715762.2011.637204. [DOI] [PubMed] [Google Scholar]
- 79.Rezaei-Sadabady R., Eidi A., Zarghami N., Barzegar A. Intracellular ROS Protection Efficiency and Free Radical-Scavenging Activity of Quercetin and Quercetin-Encapsulated Liposomes. Artif. Cells Nanomed. Biotechnol. 2016;44:128–134. doi: 10.3109/21691401.2014.926456. [DOI] [PubMed] [Google Scholar]
- 80.Cheng S.-C., Huang W.-C., S Pang J.-H., Wu Y.-H., Cheng C.-Y. Quercetin Inhibits the Production of IL-1β-Induced Inflammatory Cytokines and Chemokines in ARPE-19 Cells via the MAPK and NF-ΚB Signaling Pathways. Int. J. Mol. Sci. 2019;20:2957. doi: 10.3390/ijms20122957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen T., Zhang X., Zhu G., Liu H., Chen J., Wang Y., He X. Quercetin Inhibits TNF-α Induced HUVECs Apoptosis and Inflammation via Downregulating NF-KB and AP-1 Signaling Pathway in Vitro. Medicine. 2020;99:e22241. doi: 10.1097/MD.0000000000022241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Da Costa P.S., Ramos P.S., Ferreira C., Silva J.L., El-Bacha T., Fialho E. Pro-Oxidant Effect of Resveratrol on Human Breast Cancer MCF-7 Cells Is Associated with CK2 Inhibition. Nutr. Cancer. 2021:1–10. doi: 10.1080/01635581.2021.1977834. [DOI] [PubMed] [Google Scholar]
- 83.Fu Y., Ye Y., Zhu G., Xu Y., Sun J., Wu H., Feng F., Wen Z., Jiang S., Li Y., et al. Resveratrol Induces Human Colorectal Cancer Cell Apoptosis by Activating the Mitochondrial Pathway via Increasing Reactive Oxygen Species. Mol. Med. Rep. 2021;23:170. doi: 10.3892/mmr.2020.11809. [DOI] [PubMed] [Google Scholar]
- 84.Li B., Hou D., Guo H., Zhou H., Zhang S., Xu X., Liu Q., Zhang X., Zou Y., Gong Y., et al. Resveratrol Sequentially Induces Replication and Oxidative Stresses to Drive P53-CXCR2 Mediated Cellular Senescence in Cancer Cells. Sci. Rep. 2017;7:208. doi: 10.1038/s41598-017-00315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L., Li Q., Yan H., Jiao G., Wang H., Chi H., Zhou H., Chen L., Shan Y., Chen Y. Resveratrol Protects Osteoblasts Against Dexamethasone-Induced Cytotoxicity Through Activation of AMP-Activated Protein Kinase. Drug Des. Devel. Ther. 2020;14:4451–4463. doi: 10.2147/DDDT.S266502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A., Prabhu V.V., Allard J.S., Lopez-Lluch G., Lewis K., et al. Resveratrol Improves Health and Survival of Mice on a High-Calorie Diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giordo R., Nasrallah G.K., Al-Jamal O., Paliogiannis P., Pintus G. Resveratrol Inhibits Oxidative Stress and Prevents Mitochondrial Damage Induced by Zinc Oxide Nanoparticles in Zebrafish (Danio Rerio) Int. J. Mol. Sci. 2020;21:3838. doi: 10.3390/ijms21113838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lukitasari M., Nugroho D.A., Widodo N. Chlorogenic Acid: The Conceivable Chemosensitizer Leading to Cancer Growth Suppression. J. Evid.-Based Integr. Med. 2018;23:2515690X18789628. doi: 10.1177/2515690X18789628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pérez-Torres I., Castrejón-Téllez V., Soto M.E., Rubio-Ruiz M.E., Manzano-Pech L., Guarner-Lans V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021;22:1786. doi: 10.3390/ijms22041786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mathieu L., Lopes Costa A., Le Bachelier C., Slama A., Lebre A.-S., Taylor R.W., Bastin J., Djouadi F. Resveratrol Attenuates Oxidative Stress in Mitochondrial Complex I Deficiency: Involvement of SIRT3. Free Radic. Biol. Med. 2016;96:190–198. doi: 10.1016/j.freeradbiomed.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 91.Subedi L., Baek S.-H., Kim S.Y. Genetically Engineered Resveratrol-Enriched Rice Inhibits Neuroinflammation in Lipopolysaccharide-Activated BV2 Microglia Via Downregulating Mitogen-Activated Protein Kinase-Nuclear Factor Kappa B Signaling Pathway. Oxid. Med. Cell. Longev. 2018;2018:8092713. doi: 10.1155/2018/8092713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hsu Y.-A., Chen C.-S., Wang Y.-C., Lin E.-S., Chang C.-Y., Chen J.J.-Y., Wu M.-Y., Lin H.-J., Wan L. Anti-Inflammatory Effects of Resveratrol on Human Retinal Pigment Cells and a Myopia Animal Model. Curr. Issues Mol. Biol. 2021;43:52. doi: 10.3390/cimb43020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ginés C., Cuesta S., Kireev R., García C., Rancan L., Paredes S.D., Vara E., Tresguerres J.A.F. Protective Effect of Resveratrol against Inflammation, Oxidative Stress and Apoptosis in Pancreas of Aged SAMP8 Mice. Exp. Gerontol. 2017;90:61–70. doi: 10.1016/j.exger.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 94.Santana-Gálvez J., Cisneros-Zevallos L., Jacobo-Velázquez D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules. 2017;22:358. doi: 10.3390/molecules22030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cha J.W., Piao M.J., Kim K.C., Yao C.W., Zheng J., Kim S.M., Hyun C.L., Ahn Y.S., Hyun J.W. The Polyphenol Chlorogenic Acid Attenuates UVB-Mediated Oxidative Stress in Human HaCaT Keratinocytes. Biomol. Ther. 2014;22:136–142. doi: 10.4062/biomolther.2014.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang J., Li J., Liu J., Xu M., Tong X., Wang J. Chlorogenic Acid Prevents Isoproterenol-Induced DNA Damage in Vascular Smooth Muscle Cells. Mol. Med. Rep. 2016;14:4063–4068. doi: 10.3892/mmr.2016.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ye R., Bodero A., Zhou B.B., Khanna K.K., Lavin M.F., Lees-Miller S.P. The Plant Isoflavenoid Genistein Activates P53 and Chk2 in an ATM-Dependent Manner. J. Biol. Chem. 2001;276:4828–4833. doi: 10.1074/jbc.M004894200. [DOI] [PubMed] [Google Scholar]
- 98.Pan H.-C., Jiang Q., Yu Y., Mei J.-P., Cui Y.-K., Zhao W.-J. Quercetin Promotes Cell Apoptosis and Inhibits the Expression of MMP-9 and Fibronectin via the AKT and ERK Signalling Pathways in Human Glioma Cells. Neurochem. Int. 2015;80:60–71. doi: 10.1016/j.neuint.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 99.Yazdani Y., Sharifi Rad M.R., Taghipour M., Chenari N., Ghaderi A., Razmkhah M. Genistein Suppression of Matrix Metalloproteinase 2 (MMP-2) and Vascular Endothelial Growth Factor (VEGF) Expression in Mesenchymal Stem Cell Like Cells Isolated from High and Low Grade Gliomas. Asian Pac. J. Cancer Prev. 2016;17:5303–5307. doi: 10.22034/APJCP.2016.17.12.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lai W.-W., Hsu S.-C., Chueh F.-S., Chen Y.-Y., Yang J.-S., Lin J.-P., Lien J.-C., Tsai C.-H., Chung J.-G. Quercetin Inhibits Migration and Invasion of SAS Human Oral Cancer Cells through Inhibition of NF-ΚB and Matrix Metalloproteinase-2/-9 Signaling Pathways. Anticancer Res. 2013;33:1941–1950. [PubMed] [Google Scholar]
- 101.Vidya Priyadarsini R., Senthil Murugan R., Maitreyi S., Ramalingam K., Karunagaran D., Nagini S. The Flavonoid Quercetin Induces Cell Cycle Arrest and Mitochondria-Mediated Apoptosis in Human Cervical Cancer (HeLa) Cells through P53 Induction and NF-ΚB Inhibition. Eur. J. Pharmacol. 2010;649:84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 102.Ye D., Li Z., Wei C. Genistein Inhibits the S-Phase Kinase-Associated Protein 2 Expression in Breast Cancer Cells. Exp. Ther. Med. 2018;15:1069–1075. doi: 10.3892/etm.2017.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clemente-Soto A.F., Salas-Vidal E., Milan-Pacheco C., Sánchez-Carranza J.N., Peralta-Zaragoza O., González-Maya L. Quercetin Induces G2 Phase Arrest and Apoptosis with the Activation of P53 in an E6 Expression-independent Manner in HPV-positive Human Cervical Cancer-derived Cells. Mol. Med. Rep. 2019;19:2097–2106. doi: 10.3892/mmr.2019.9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bilir B., Sharma N.V., Lee J., Hammarstrom B., Svindland A., Kucuk O., Moreno C.S. Effects of Genistein Supplementation on Genome-wide DNA Methylation and Gene Expression in Patients with Localized Prostate Cancer. Int. J. Oncol. 2017;51:223–234. doi: 10.3892/ijo.2017.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boadi W.Y., Myles E.L., Garcia A.S. Phospho Tensin Homolog in Human and Lipid Peroxides in Peripheral Blood Mononuclear Cells Following Exposure to Flavonoids. J. Am. Coll. Nutr. 2020;39:135–146. doi: 10.1080/07315724.2019.1616234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao J., Xia R., Chen J., Gao J., Luo X., Ke C., Ren C., Li J., Mi Y. Inhibition of Esophageal-Carcinoma Cell Proliferation by Genistein via Suppression of JAK1/2-STAT3 and AKT/MDM2/P53 Signaling Pathways. Aging (Albany NY) 2020;12:6240–6259. doi: 10.18632/aging.103019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pani S., Mohapatra S., Sahoo A., Baral B., Debata P.R. Shifting of Cell Cycle Arrest from the S-Phase to G2/M Phase and Downregulation of EGFR Expression by Phytochemical Combinations in HeLa Cervical Cancer Cells. J. Biochem. Mol. Toxicol. 2022;36:e22947. doi: 10.1002/jbt.22947. [DOI] [PubMed] [Google Scholar]
- 108.Li K., Hong S., Lin S., Chen K. Genistein Inhibits the Proliferation, Migration and Invasion of the Squamous Cell Carcinoma Cells via Inhibition of MEK/ERK and JNK Signalling Pathways. J. BU ON. 2020;25:1172–1177. [PubMed] [Google Scholar]
- 109.Suzuki T., Pervin M., Goto S., Isemura M., Nakamura Y. Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-Gallate on Obesity. Molecules. 2016;21:1305. doi: 10.3390/molecules21101305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kanadzu M., Lu Y., Morimoto K. Dual Function of (--)-Epigallocatechin Gallate (EGCG) in Healthy Human Lymphocytes. Cancer Lett. 2006;241:250–255. doi: 10.1016/j.canlet.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 111.Syng-Ai C., Kumari A.L., Khar A. Effect of Curcumin on Normal and Tumor Cells: Role of Glutathione and Bcl-2. Mol. Cancer Ther. 2004;3:1101–1108. doi: 10.1158/1535-7163.1101.3.9. [DOI] [PubMed] [Google Scholar]
- 112.Chen W., Lin Y.C., Ma X.Y., Jiang Z.Y., Lan S.P. High Concentrations of Genistein Exhibit Pro-Oxidant Effects in Primary Muscle Cells through Mechanisms Involving 5-Lipoxygenase-Mediated Production of Reactive Oxygen Species. Food Chem. Toxicol. 2014;67:72–79. doi: 10.1016/j.fct.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 113.Arora I., Sharma M., Tollefsbol T.O. Combinatorial Epigenetics Impact of Polyphenols and Phytochemicals in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2019;20:4567. doi: 10.3390/ijms20184567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chota A., George B.P., Abrahamse H. Interactions of Multidomain Pro-Apoptotic and Anti-Apoptotic Proteins in Cancer Cell Death. Oncotarget. 2021;12:1615–1626. doi: 10.18632/oncotarget.28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsang W.P., Kwok T.T. Epigallocatechin Gallate Up-Regulation of MiR-16 and Induction of Apoptosis in Human Cancer Cells. J. Nutr. Biochem. 2010;21:140–146. doi: 10.1016/j.jnutbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 116.Sonoki H., Sato T., Endo S., Matsunaga T., Yamaguchi M., Yamazaki Y., Sugatani J., Ikari A. Quercetin Decreases Claudin-2 Expression Mediated by Up-Regulation of MicroRNA MiR-16 in Lung Adenocarcinoma A549 Cells. Nutrients. 2015;7:4578–4592. doi: 10.3390/nu7064578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao J., Fang Z., Zha Z., Sun Q., Wang H., Sun M., Qiao B. Quercetin Inhibits Cell Viability, Migration and Invasion by Regulating MiR-16/HOXA10 Axis in Oral Cancer. Eur. J. Pharmacol. 2019;847:11–18. doi: 10.1016/j.ejphar.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 118.Hagiwara K., Kosaka N., Yoshioka Y., Takahashi R.-U., Takeshita F., Ochiya T. Stilbene Derivatives Promote Ago2-Dependent Tumour-Suppressive MicroRNA Activity. Sci. Rep. 2012;2:314. doi: 10.1038/srep00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Azimi A., Hagh M.F., Talebi M., Yousefi B., Hossein pour feizi A.A., Baradaran B., Movassaghpour A.A., Shamsasenjan K., Khanzedeh T., Ghaderi A.H., et al. Time-and Concentration-Dependent Effects of Resveratrol on MiR 15a and MiR16-1 Expression and Apoptosis in the CCRF-CEM Acute Lymphoblastic Leukemia Cell Line. Asian Pac. J. Cancer Prev. 2015;16:6463–6468. doi: 10.7314/APJCP.2015.16.15.6463. [DOI] [PubMed] [Google Scholar]
- 120.Sun M., Estrov Z., Ji Y., Coombes K.R., Harris D.H., Kurzrock R. Curcumin (Diferuloylmethane) Alters the Expression Profiles of MicroRNAs in Human Pancreatic Cancer Cells. Mol. Cancer Ther. 2008;7:464–473. doi: 10.1158/1535-7163.MCT-07-2272. [DOI] [PubMed] [Google Scholar]
- 121.Sreenivasan S., Thirumalai K., Danda R., Krishnakumar S. Effect of Curcumin on MiRNA Expression in Human Y79 Retinoblastoma Cells. Curr. Eye Res. 2012;37:421–428. doi: 10.3109/02713683.2011.647224. [DOI] [PubMed] [Google Scholar]
- 122.Sibbesen N.A., Kopp K.L., Litvinov I.V., Jønson L., Willerslev-Olsen A., Fredholm S., Petersen D.L., Nastasi C., Krejsgaard T., Lindahl L.M., et al. Jak3, STAT3, and STAT5 Inhibit Expression of MiR-22, a Novel Tumor Suppressor MicroRNA, in Cutaneous T-Cell Lymphoma. Oncotarget. 2015;6:20555–20569. doi: 10.18632/oncotarget.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li B.-B., Huang G.-L., Li H.-H., Kong X., He Z.-W. Epigallocatechin-3-Gallate Modulates MicroRNA Expression Profiles in Human Nasopharyngeal Carcinoma CNE2 Cells. Chin. Med. J. 2017;130:93–99. doi: 10.4103/0366-6999.196586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang C., Hao Y., Sun Y., Liu P. Quercetin Suppresses the Tumorigenesis of Oral Squamous Cell Carcinoma by Regulating MicroRNA-22/WNT1/β-Catenin Axis. J. Pharmacol. Sci. 2019;140:128–136. doi: 10.1016/j.jphs.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 125.Guo J., Li W., Shi H., Xie X., Li L., Tang H., Wu M., Kong Y., Yang L., Gao J., et al. Synergistic Effects of Curcumin with Emodin against the Proliferation and Invasion of Breast Cancer Cells through Upregulation of MiR-34a. Mol. Cell. Biochem. 2013;382:103–111. doi: 10.1007/s11010-013-1723-6. [DOI] [PubMed] [Google Scholar]
- 126.Sun C., Zhang S., Liu C., Liu X. Curcumin Promoted MiR-34a Expression and Suppressed Proliferation of Gastric Cancer Cells. Cancer Biother. Radiopharm. 2019;34:634–641. doi: 10.1089/cbr.2019.2874. [DOI] [PubMed] [Google Scholar]
- 127.Toden S., Okugawa Y., Buhrmann C., Nattamai D., Anguiano E., Baldwin N., Shakibaei M., Boland C.R., Goel A. Novel Evidence for Curcumin and Boswellic Acid-Induced Chemoprevention through Regulation of MiR-34a and MiR-27a in Colorectal Cancer. Cancer Prev. Res. 2015;8:431–443. doi: 10.1158/1940-6207.CAPR-14-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Subramaniam D., Ponnurangam S., Ramamoorthy P., Standing D., Battafarano R.J., Anant S., Sharma P. Curcumin Induces Cell Death in Esophageal Cancer Cells through Modulating Notch Signaling. PLoS ONE. 2012;7:e30590. doi: 10.1371/journal.pone.0030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chakrabarti M., Khandkar M., Banik N.L., Ray S.K. Alterations in Expression of Specific MicroRNAs by Combination of 4-HPR and EGCG Inhibited Growth of Human Malignant Neuroblastoma Cells. Brain Res. 2012;1454:1–13. doi: 10.1016/j.brainres.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chakrabarti M., Ai W., Banik N.L., Ray S.K. Overexpression of MiR-7-1 Increases Efficacy of Green Tea Polyphenols for Induction of Apoptosis in Human Malignant Neuroblastoma SH-SY5Y and SK-N-DZ Cells. Neurochem. Res. 2013;38:420–432. doi: 10.1007/s11064-012-0936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Toden S., Tran H.-M., Tovar-Camargo O.A., Okugawa Y., Goel A. Epigallocatechin-3-Gallate Targets Cancer Stem-like Cells and Enhances 5-Fluorouracil Chemosensitivity in Colorectal Cancer. Oncotarget. 2016;7:16158–16171. doi: 10.18632/oncotarget.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mostafa S.M., Gamal-Eldeen A.M., Maksoud N.A.E., Fahmi A.A. Epigallocatechin Gallate-Capped Gold Nanoparticles Enhanced the Tumor Suppressors Let-7a and MiR-34a in Hepatocellular Carcinoma Cells. An. Acad. Bras. Cienc. 2020;92:e20200574. doi: 10.1590/0001-3765202020200574. [DOI] [PubMed] [Google Scholar]
- 133.Hsieh P.-L., Liao Y.-W., Hsieh C.-W., Chen P.-N., Yu C.-C. Soy Isoflavone Genistein Impedes Cancer Stemness and Mesenchymal Transition in Head and Neck Cancer through Activating MiR-34a/RTCB Axis. Nutrients. 2020;12:1924. doi: 10.3390/nu12071924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chiyomaru T., Yamamura S., Fukuhara S., Yoshino H., Kinoshita T., Majid S., Saini S., Chang I., Tanaka Y., Enokida H., et al. Genistein Inhibits Prostate Cancer Cell Growth by Targeting MiR-34a and Oncogenic HOTAIR. PLoS ONE. 2013;8:e70372. doi: 10.1371/journal.pone.0070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xia J., Duan Q., Ahmad A., Bao B., Banerjee S., Shi Y., Ma J., Geng J., Chen Z., Rahman K.M.W., et al. Genistein Inhibits Cell Growth and Induces Apoptosis through Up-Regulation of MiR-34a in Pancreatic Cancer Cells. Curr. Drug Targets. 2012;13:1750–1756. doi: 10.2174/138945012804545597. [DOI] [PubMed] [Google Scholar]
- 136.Kumazaki M., Noguchi S., Yasui Y., Iwasaki J., Shinohara H., Yamada N., Akao Y. Anti-Cancer Effects of Naturally Occurring Compounds through Modulation of Signal Transduction and MiRNA Expression in Human Colon Cancer Cells. J. Nutr. Biochem. 2013;24:1849–1858. doi: 10.1016/j.jnutbio.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 137.Otsuka K., Yamamoto Y., Ochiya T. Regulatory Role of Resveratrol, a MicroRNA-Controlling Compound, in HNRNPA1 Expression, Which Is Associated with Poor Prognosis in Breast Cancer. Oncotarget. 2018;9:24718–24730. doi: 10.18632/oncotarget.25339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yao S., Gao M., Wang Z., Wang W., Zhan L., Wei B. Upregulation of MicroRNA-34a Sensitizes Ovarian Cancer Cells to Resveratrol by Targeting Bcl-2. Yonsei Med. J. 2021;62:691–701. doi: 10.3349/ymj.2021.62.8.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Toden S., Okugawa Y., Jascur T., Wodarz D., Komarova N.L., Buhrmann C., Shakibaei M., Boland C.R., Goel A. Curcumin Mediates Chemosensitization to 5-Fluorouracil through MiRNA-Induced Suppression of Epithelial-to-Mesenchymal Transition in Chemoresistant Colorectal Cancer. Carcinogenesis. 2015;36:355–367. doi: 10.1093/carcin/bgv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gordon M.W., Yan F., Zhong X., Mazumder P.B., Xu-Monette Z.Y., Zou D., Young K.H., Ramos K.S., Li Y. Regulation of P53-Targeting MicroRNAs by Polycyclic Aromatic Hydrocarbons: Implications in the Etiology of Multiple Myeloma. Mol. Carcinog. 2015;54:1060–1069. doi: 10.1002/mc.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chiyomaru T., Fukuhara S., Saini S., Majid S., Deng G., Shahryari V., Chang I., Tanaka Y., Enokida H., Nakagawa M., et al. Long Non-Coding RNA HOTAIR Is Targeted and Regulated by MiR-141 in Human Cancer Cells. J. Biol. Chem. 2014;289:12550–12565. doi: 10.1074/jbc.M113.488593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tahmasebi Mirgani M., Isacchi B., Sadeghizadeh M., Marra F., Bilia A.R., Mowla S.J., Najafi F., Babaei E. Dendrosomal Curcumin Nanoformulation Downregulates Pluripotency Genes via MiR-145 Activation in U87MG Glioblastoma Cells. Int. J. Nanomedicine. 2014;9:403–417. doi: 10.2147/IJN.S48136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu T., Chi H., Chen J., Chen C., Huang Y., Xi H., Xue J., Si Y. Curcumin Suppresses Proliferation and in Vitro Invasion of Human Prostate Cancer Stem Cells by CeRNA Effect of MiR-145 and LncRNA-ROR. Gene. 2017;631:29–38. doi: 10.1016/j.gene.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 144.Wei D., Yang L., Lv B., Chen L. Genistein Suppresses Retinoblastoma Cell Viability and Growth and Induces Apoptosis by Upregulating MiR-145 and Inhibiting Its Target ABCE1. Mol. Vis. 2017;23:385–394. [PMC free article] [PubMed] [Google Scholar]
- 145.Zhou J., Gong J., Ding C., Chen G. Quercetin Induces the Apoptosis of Human Ovarian Carcinoma Cells by Upregulating the Expression of MicroRNA-145. Mol. Med. Rep. 2015;12:3127–3131. doi: 10.3892/mmr.2015.3679. [DOI] [PubMed] [Google Scholar]
- 146.Sachdeva M., Liu Q., Cao J., Lu Z., Mo Y.-Y. Negative Regulation of MiR-145 by C/EBP-β through the Akt Pathway in Cancer Cells. Nucleic Acids Res. 2012;40:6683–6692. doi: 10.1093/nar/gks324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bao B., Ali S., Banerjee S., Wang Z., Logna F., Azmi A.S., Kong D., Ahmad A., Li Y., Padhye S., et al. Curcumin Analogue CDF Inhibits Pancreatic Tumor Growth by Switching on Suppressor MicroRNAs and Attenuating EZH2 Expression. Cancer Res. 2012;72:335–345. doi: 10.1158/0008-5472.CAN-11-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li Y., Vandenboom T.G., Wang Z., Kong D., Ali S., Philip P.A., Sarkar F.H. MiR-146a Suppresses Invasion of Pancreatic Cancer Cells. Cancer Res. 2010;70:1486–1495. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Soubani O., Ali A.S., Logna F., Ali S., Philip P.A., Sarkar F.H. Re-Expression of MiR-200 by Novel Approaches Regulates the Expression of PTEN and MT1-MMP in Pancreatic Cancer. Carcinogenesis. 2012;33:1563–1571. doi: 10.1093/carcin/bgs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shen Y.-A., Lin C.-H., Chi W.-H., Wang C.-Y., Hsieh Y.-T., Wei Y.-H., Chen Y.-J. Resveratrol Impedes the Stemness, Epithelial-Mesenchymal Transition, and Metabolic Reprogramming of Cancer Stem Cells in Nasopharyngeal Carcinoma through P53 Activation. Evid. Based Complement. Alternat. Med. 2013;2013:590393. doi: 10.1155/2013/590393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Karimi Dermani F., Saidijam M., Amini R., Mahdavinezhad A., Heydari K., Najafi R. Resveratrol Inhibits Proliferation, Invasion, and Epithelial-Mesenchymal Transition by Increasing MiR-200c Expression in HCT-116 Colorectal Cancer Cells. J. Cell Biochem. 2017;118:1547–1555. doi: 10.1002/jcb.25816. [DOI] [PubMed] [Google Scholar]
- 152.Huang S., Wang L.-L., Xue N.-N., Li C., Guo H.-H., Ren T.-K., Zhan Y., Li W.-B., Zhang J., Chen X.-G., et al. Chlorogenic Acid Effectively Treats Cancers through Induction of Cancer Cell Differentiation. Theranostics. 2019;9:6745–6763. doi: 10.7150/thno.34674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mirzaaghaei S., Foroughmand A.M., Saki G., Shafiei M. Combination of Epigallocatechin-3-Gallate and Silibinin: A Novel Approach for Targeting Both Tumor and Endothelial Cells. ACS Omega. 2019;4:8421–8430. doi: 10.1021/acsomega.9b00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dhar S., Kumar A., Rimando A.M., Zhang X., Levenson A.S. Resveratrol and Pterostilbene Epigenetically Restore PTEN Expression by Targeting OncomiRs of the MiR-17 Family in Prostate Cancer. Oncotarget. 2015;6:27214–27226. doi: 10.18632/oncotarget.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu L., Mou Q., Wang Y., Zhu Z., Cheng M. Resveratrol Contributes to the Inhibition of Liver Fibrosis by Inducing Autophagy via the MicroRNA-20a-mediated Activation of the PTEN/PI3K/AKT Signaling Pathway. Int. J. Mol. Med. 2020;46:2035–2046. doi: 10.3892/ijmm.2020.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dhar S., Hicks C., Levenson A.S. Resveratrol and Prostate Cancer: Promising Role for MicroRNAs. Mol. Nutr. Food Res. 2011;55:1219–1229. doi: 10.1002/mnfr.201100141. [DOI] [PubMed] [Google Scholar]
- 157.Wang Y., Yang F., Xue J., Zhou X., Luo L., Ma Q., Chen Y.-F., Zhang J., Zhang S.-L., Zhao L. Antischistosomiasis Liver Fibrosis Effects of Chlorogenic Acid through IL-13/MiR-21/Smad7 Signaling Interactions In Vivo and In Vitro. Antimicrob. Agents Chemother. 2017;61:e01347-16. doi: 10.1128/AAC.01347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mudduluru G., George-William J.N., Muppala S., Asangani I.A., Kumarswamy R., Nelson L.D., Allgayer H. Curcumin Regulates MiR-21 Expression and Inhibits Invasion and Metastasis in Colorectal Cancer. Biosci. Rep. 2011;31:185–197. doi: 10.1042/BSR20100065. [DOI] [PubMed] [Google Scholar]
- 159.Zhang W., Bai W., Zhang W. MiR-21 Suppresses the Anticancer Activities of Curcumin by Targeting PTEN Gene in Human Non-Small Cell Lung Cancer A549 Cells. Clin. Transl. Oncol. 2014;16:708–713. doi: 10.1007/s12094-013-1135-9. [DOI] [PubMed] [Google Scholar]
- 160.Taverna S., Giallombardo M., Pucci M., Flugy A., Manno M., Raccosta S., Rolfo C., De Leo G., Alessandro R. Curcumin Inhibits in Vitro and in Vivo Chronic Myelogenous Leukemia Cells Growth: A Possible Role for Exosomal Disposal of MiR-21. Oncotarget. 2015;6:21918–21933. doi: 10.18632/oncotarget.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yallapu M.M., Khan S., Maher D.M., Ebeling M.C., Sundram V., Chauhan N., Ganju A., Balakrishna S., Gupta B.K., Zafar N., et al. Anti-Cancer Activity of Curcumin Loaded Nanoparticles in Prostate Cancer. Biomaterials. 2014;35:8635–8648. doi: 10.1016/j.biomaterials.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fix L.N., Shah M., Efferth T., Farwell M.A., Zhang B. MicroRNA Expression Profile of MCF-7 Human Breast Cancer Cells and the Effect of Green Tea Polyphenon-60. Cancer Genom. Proteom. 2010;7:261–277. [PubMed] [Google Scholar]
- 163.Siddiqui I.A., Asim M., Hafeez B.B., Adhami V.M., Tarapore R.S., Mukhtar H. Green Tea Polyphenol EGCG Blunts Androgen Receptor Function in Prostate Cancer. FASEB J. 2011;25:1198–1207. doi: 10.1096/fj.10-167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zaman M.S., Shahryari V., Deng G., Thamminana S., Saini S., Majid S., Chang I., Hirata H., Ueno K., Yamamura S., et al. Up-Regulation of MicroRNA-21 Correlates with Lower Kidney Cancer Survival. PLoS ONE. 2012;7:e31060. doi: 10.1371/annotation/6662579f-3a41-4bce-9298-9d15f6582ef5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tili E., Michaille J.-J., Alder H., Volinia S., Delmas D., Latruffe N., Croce C.M. Resveratrol Modulates the Levels of MicroRNAs Targeting Genes Encoding Tumor-Suppressors and Effectors of TGFβ Signaling Pathway in SW480 Cells. Biochem. Pharmacol. 2010;80:2057–2065. doi: 10.1016/j.bcp.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sheth S., Jajoo S., Kaur T., Mukherjea D., Sheehan K., Rybak L.P., Ramkumar V. Resveratrol Reduces Prostate Cancer Growth and Metastasis by Inhibiting the Akt/MicroRNA-21 Pathway. PLoS ONE. 2012;7:e51655. doi: 10.1371/journal.pone.0051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu P., Liang H., Xia Q., Li P., Kong H., Lei P., Wang S., Tu Z. Resveratrol Induces Apoptosis of Pancreatic Cancers Cells by Inhibiting MiR-21 Regulation of BCL-2 Expression. Clin. Transl. Oncol. 2013;15:741–746. doi: 10.1007/s12094-012-0999-4. [DOI] [PubMed] [Google Scholar]
- 168.Zan L., Chen Q., Zhang L., Li X. Epigallocatechin Gallate (EGCG) Suppresses Growth and Tumorigenicity in Breast Cancer Cells by Downregulation of MiR-25. Bioengineered. 2019;10:374–382. doi: 10.1080/21655979.2019.1657327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Noratto G.D., Jutooru I., Safe S., Angel-Morales G., Mertens-Talcott S.U. The Drug Resistance Suppression Induced by Curcuminoids in Colon Cancer SW-480 Cells Is Mediated by Reactive Oxygen Species-Induced Disruption of the MicroRNA-27a-ZBTB10-Sp Axis. Mol. Nutr. Food Res. 2013;57:1638–1648. doi: 10.1002/mnfr.201200609. [DOI] [PubMed] [Google Scholar]
- 170.Xia J., Cheng L., Mei C., Ma J., Shi Y., Zeng F., Wang Z., Wang Z. Genistein Inhibits Cell Growth and Invasion through Regulation of MiR-27a in Pancreatic Cancer Cells. Curr. Pharm. Des. 2014;20:5348–5353. doi: 10.2174/1381612820666140128215756. [DOI] [PubMed] [Google Scholar]
- 171.Xu L., Xiang J., Shen J., Zou X., Zhai S., Yin Y., Li P., Wang X., Sun Q. Oncogenic MicroRNA-27a Is a Target for Genistein in Ovarian Cancer Cells. Anticancer. Agents Med. Chem. 2013;13:1126–1132. doi: 10.2174/18715206113139990006. [DOI] [PubMed] [Google Scholar]
- 172.Sun Q., Cong R., Yan H., Gu H., Zeng Y., Liu N., Chen J., Wang B. Genistein Inhibits Growth of Human Uveal Melanoma Cells and Affects MicroRNA-27a and Target Gene Expression. Oncol. Rep. 2009;22:563–567. doi: 10.3892/or_00000472. [DOI] [PubMed] [Google Scholar]
- 173.Yang Y., Zang A., Jia Y., Shang Y., Zhang Z., Ge K., Zhang J., Fan W., Wang B. Genistein Inhibits A549 Human Lung Cancer Cell Proliferation via MiR-27a and MET Signaling. Oncol. Lett. 2016;12:2189–2193. doi: 10.3892/ol.2016.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Singh B., Shoulson R., Chatterjee A., Ronghe A., Bhat N.K., Dim D.C., Bhat H.K. Resveratrol Inhibits Estrogen-Induced Breast Carcinogenesis through Induction of NRF2-Mediated Protective Pathways. Carcinogenesis. 2014;35:1872–1880. doi: 10.1093/carcin/bgu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.De la Parra C., Castillo-Pichardo L., Cruz-Collazo A., Cubano L., Redis R., Calin G.A., Dharmawardhane S. Soy Isoflavone Genistein-Mediated Downregulation of MiR-155 Contributes to the Anticancer Effects of Genistein. Nutr. Cancer. 2016;68:154–164. doi: 10.1080/01635581.2016.1115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Boesch-Saadatmandi C., Loboda A., Wagner A.E., Stachurska A., Jozkowicz A., Dulak J., Döring F., Wolffram S., Rimbach G. Effect of Quercetin and Its Metabolites Isorhamnetin and Quercetin-3-Glucuronide on Inflammatory Gene Expression: Role of MiR-155. J. Nutr. Biochem. 2011;22:293–299. doi: 10.1016/j.jnutbio.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 177.Tili E., Michaille J.-J., Adair B., Alder H., Limagne E., Taccioli C., Ferracin M., Delmas D., Latruffe N., Croce C.M. Resveratrol Decreases the Levels of MiR-155 by Upregulating MiR-663, a MicroRNA Targeting JunB and JunD. Carcinogenesis. 2010;31:1561–1566. doi: 10.1093/carcin/bgq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sarkar S., Dubaybo H., Ali S., Goncalves P., Kollepara S.L., Sethi S., Philip P.A., Li Y. Down-Regulation of MiR-221 Inhibits Proliferation of Pancreatic Cancer Cells through up-Regulation of PTEN, P27(Kip1), P57(Kip2), and PUMA. Am. J. Cancer Res. 2013;3:465–477. [PMC free article] [PubMed] [Google Scholar]
- 179.Allegri L., Rosignolo F., Mio C., Filetti S., Baldan F., Damante G. Effects of Nutraceuticals on Anaplastic Thyroid Cancer Cells. J. Cancer Res. Clin. Oncol. 2018;144:285–294. doi: 10.1007/s00432-017-2555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chen Y., Zaman M.S., Deng G., Majid S., Saini S., Liu J., Tanaka Y., Dahiya R. MicroRNAs 221/222 and Genistein-Mediated Regulation of ARHI Tumor Suppressor Gene in Prostate Cancer. Cancer Prev. Res. 2011;4:76–86. doi: 10.1158/1940-6207.CAPR-10-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang C., Qu Z., Kong L., Xu L., Zhang M., Liu J., Yang Z. Quercetin Ameliorates Lipopolysaccharide-Caused Inflammatory Damage via down-Regulation of MiR-221 in WI-38 Cells. Exp. Mol. Pathol. 2019;108:1–8. doi: 10.1016/j.yexmp.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 182.Yang J., Cao Y., Sun J., Zhang Y. Curcumin Reduces the Expression of Bcl-2 by Upregulating MiR-15a and MiR-16 in MCF-7 Cells. Med. Oncol. 2010;27:1114–1118. doi: 10.1007/s12032-009-9344-3. [DOI] [PubMed] [Google Scholar]
- 183.Khan F., Niaz K., Maqbool F., Ismail Hassan F., Abdollahi M., Nagulapalli Venkata K.C., Nabavi S.M., Bishayee A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients. 2016;8:529. doi: 10.3390/nu8090529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vellingiri B., Iyer M., Devi Subramaniam M., Jayaramayya K., Siama Z., Giridharan B., Narayanasamy A., Abdal Dayem A., Cho S.-G. Understanding the Role of the Transcription Factor Sp1 in Ovarian Cancer: From Theory to Practice. Int. J. Mol. Sci. 2020;21:1153. doi: 10.3390/ijms21031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Huang C., Xie K. Crosstalk of Sp1 and Stat3 Signaling in Pancreatic Cancer Pathogenesis. Cytokine Growth Factor Rev. 2012;23:25–35. doi: 10.1016/j.cytogfr.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Su F., Geng J., Li X., Qiao C., Luo L., Feng J., Dong X., Lv M. SP1 Promotes Tumor Angiogenesis and Invasion by Activating VEGF Expression in an Acquired Trastuzumab-resistant Ovarian Cancer Model. Oncol. Rep. 2017;38:2677–2684. doi: 10.3892/or.2017.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang Q., Wang J., Xiang H., Ding P., Wu T., Ji G. The Biochemical and Clinical Implications of Phosphatase and Tensin Homolog Deleted on Chromosome Ten in Different Cancers. Am. J. Cancer Res. 2021;11:5833–5855. [PMC free article] [PubMed] [Google Scholar]
- 188.Lee Y.-S., Lim K.-H., Guo X., Kawaguchi Y., Gao Y., Barrientos T., Ordentlich P., Wang X.-F., Counter C.M., Yao T.-P. The Cytoplasmic Deacetylase HDAC6 Is Required for Efficient Oncogenic Tumorigenesis. Cancer Res. 2008;68:7561–7569. doi: 10.1158/0008-5472.CAN-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Qin L., Wu Y.-L., Toneff M.J., Li D., Liao L., Gao X., Bane F.T., Tien J.C.-Y., Xu Y., Feng Z., et al. NCOA1 Directly Targets M-CSF1 Expression to Promote Breast Cancer Metastasis. Cancer Res. 2014;74:3477–3488. doi: 10.1158/0008-5472.CAN-13-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jiang J., Wang S., Wang Z., Cai J., Han L., Xie L., Han Q., Wang W., Zhang Y., He X., et al. HOTAIR Promotes Paclitaxel Resistance by Regulating CHEK1 in Ovarian Cancer. Cancer Chemother. Pharmacol. 2020;86:295–305. doi: 10.1007/s00280-020-04120-1. [DOI] [PubMed] [Google Scholar]
- 191.Yu L., Li W. Abnormal Activation of Notch 1 Signaling Causes Apoptosis Resistance in Cervical Cancer. Int. J. Clin. Exp. Pathol. 2022;15:11–19. [PMC free article] [PubMed] [Google Scholar]
- 192.Cui L., Dong Y., Wang X., Zhao X., Kong C., Liu Y., Jiang X., Zhang X. Downregulation of Long Noncoding RNA SNHG1 Inhibits Cell Proliferation, Metastasis, and Invasion by Suppressing the Notch-1 Signaling Pathway in Pancreatic Cancer. J. Cell Biochem. 2019;120:6106–6112. doi: 10.1002/jcb.27897. [DOI] [PubMed] [Google Scholar]
- 193.Kiesel V.A., Stan S.D. Modulation of Notch Signaling Pathway by Bioactive Dietary Agents. Int. J. Mol. Sci. 2022;23:3532. doi: 10.3390/ijms23073532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Alemohammad H., Asadzadeh Z., Motafakker Azad R., Hemmat N., Najafzadeh B., Vasefifar P., Najafi S., Baradaran B. Signaling Pathways and MicroRNAs, the Orchestrators of NANOG Activity during Cancer Induction. Life Sci. 2020;260:118337. doi: 10.1016/j.lfs.2020.118337. [DOI] [PubMed] [Google Scholar]
- 195.Yang F., Luo L., Zhu Z.-D., Zhou X., Wang Y., Xue J., Zhang J., Cai X., Chen Z.-L., Ma Q., et al. Chlorogenic Acid Inhibits Liver Fibrosis by Blocking the MiR-21-Regulated TGF-Β1/Smad7 Signaling Pathway in Vitro and in Vivo. Front. Pharmacol. 2017;8:929. doi: 10.3389/fphar.2017.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Basu P., Maier C. Phytoestrogens and Breast Cancer: In Vitro Anticancer Activities of Isoflavones, Lignans, Coumestans, Stilbenes and Their Analogs and Derivatives. Biomed. Pharmacother. 2018;107:1648–1666. doi: 10.1016/j.biopha.2018.08.100. [DOI] [PubMed] [Google Scholar]
- 197.Pan W., Wang H., Jianwei R., Ye Z. MicroRNA-27a Promotes Proliferation, Migration and Invasion by Targeting MAP2K4 in Human Osteosarcoma Cells. Cell Physiol. Biochem. 2014;33:402–412. doi: 10.1159/000356679. [DOI] [PubMed] [Google Scholar]
- 198.Zhang R., Xu J., Zhao J., Bai J. Knockdown of MiR-27a Sensitizes Colorectal Cancer Stem Cells to TRAIL by Promoting the Formation of Apaf-1-Caspase-9 Complex. Oncotarget. 2017;8:45213–45223. doi: 10.18632/oncotarget.16779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mense S.M., Remotti F., Bhan A., Singh B., El-Tamer M., Hei T.K., Bhat H.K. Estrogen-Induced Breast Cancer: Alterations in Breast Morphology and Oxidative Stress as a Function of Estrogen Exposure. Toxicol. Appl. Pharmacol. 2008;232:78–85. doi: 10.1016/j.taap.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Farshbaf-Khalili A., Farajnia S., Pourzeinali S., Shakouri S.K., Salehi-Pourmehr H. The Effect of Nanomicelle Curcumin Supplementation and Nigella Sativa Oil on the Expression Level of MiRNA-21, MiRNA-422a, and MiRNA-503 Gene in Postmenopausal Women with Low Bone Mass Density: A Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. Phytother. Res. 2021;35:6216–6227. doi: 10.1002/ptr.7259. [DOI] [PubMed] [Google Scholar]
- 201.Ahmadi M., Hajialilo M., Dolati S., Eghbal-Fard S., Heydarlou H., Ghaebi M., Ghassembaglou A., Aghebati-Maleki L., Samadi Kafil H., Kamrani A., et al. The Effects of Nanocurcumin on Treg Cell Responses and Treatment of Ankylosing Spondylitis Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Cell. Biochem. 2020;121:103–110. doi: 10.1002/jcb.28901. [DOI] [PubMed] [Google Scholar]
- 202.Tomé-Carneiro J., Larrosa M., Yáñez-Gascón M.J., Dávalos A., Gil-Zamorano J., Gonzálvez M., García-Almagro F.J., Ruiz Ros J.A., Tomás-Barberán F.A., Espín J.C., et al. One-Year Supplementation with a Grape Extract Containing Resveratrol Modulates Inflammatory-Related MicroRNAs and Cytokines Expression in Peripheral Blood Mononuclear Cells of Type 2 Diabetes and Hypertensive Patients with Coronary Artery Disease. Pharmacol. Res. 2013;72:69–82. doi: 10.1016/j.phrs.2013.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.