Abstract
Background:
Both the superficial medial collateral ligament (sMCL) and the deep MCL (dMCL) contribute to the restraint of anteromedial (AM) rotatory instability (AMRI). Previous studies have not investigated how MCL reconstructions control AMRI.
Purpose/Hypothesis:
The purpose was to establish the optimal medial reconstruction for restoring normal knee kinematics in an sMCL- and dMCL-deficient knee. It was hypothesized that AMRI would be better controlled with the addition of an anatomically shaped (flat) sMCL reconstruction and with the addition of an AM reconstruction replicating the function of the dMCL.
Study Design:
Controlled laboratory study.
Methods:
A 6 degrees of freedom robotic system equipped with a force-torque sensor was used to test 8 unpaired knees in the intact, sMCL/dMCL sectioned, and reconstructed states. Four different reconstructions were assessed. The sMCL was reconstructed with either a single-bundle (SB) or a flattened hamstring graft aimed at better replicating the appearance of the native ligament. These reconstructions were tested with and without an additional AM reconstruction. Simulated laxity tests were performed at 0°, 30°, 60°, and 90° of flexion: 10 N·m valgus rotation, 5 N·m internal and external rotation (ER), and an AM drawer test (combined 134-N anterior tibial drawer in 5 N·m ER). The primary outcome measures of this force-controlled setup were anterior tibial translation (ATT; in mm) and axial tibial rotation (in degrees).
Results:
Sectioning the sMCL/dMCL increased valgus rotation, ER, and ATT with the simulated AM draw test at all flexion angles. SB sMCL reconstruction was unable to restore ATT, valgus rotation, and ER at 30°, 60°, and 90° of flexion to the intact state (P < .05). Flat MCL reconstruction restored valgus rotation at all flexion angles to the intact state (P > .05). ER was restored at all angles except at 90°, but ATT laxity in response to the AM drawer persisted. Addition of an AM reconstruction improved control of ATT relative to the intact state at all flexion angles (P > .05). Combined flat MCL and AM reconstruction restored knee kinematics closest to the intact state.
Conclusion:
In a cadaveric model, AMRI resulting from an injured sMCL and dMCL complex could not be restored by an isolated SB sMCL reconstruction. A flat MCL reconstruction or an additional AM procedure, however, better restored medial knee stability.
Clinical Relevance:
In patients evaluated with a combined valgus and AM rotatory instability, a flat sMCL and an additional AM reconstruction may be superior to an isolated SB sMCL reconstruction.
Keywords: MCL, biomechanics, reconstruction, AMRI, ACL
Injuries of the medial collateral ligament (MCL) are common, accounting for 7.9% of all knee injuries as shown in a large observational study over a 10-year period. 22 High-grade MCL injuries frequently occur with anterior cruciate ligament (ACL) rupture.22,40 While most MCL injuries can be treated nonoperatively, medial instability may remain, particularly with higher-grade injuries.16,21 Residual MCL laxity can impair knee function, cause chronic pain, and lead to higher rates of ACL reconstruction failure.1,2,5,31,40 Combined ACL/MCL reconstruction can afford good clinical results,21,32,34 but these are currently inferior to those after isolated ACL reconstruction. 21 In a recent registry study, 1-year postoperative Knee injury and Osteoarthritis Outcome Score and Tegner scores were higher after isolated ACL reconstruction compared with combined ACL/MCL reconstruction. 21
The importance of rotational instability and its influence on successful ACL reconstruction has been compellingly demonstrated by the improvement in stability and reduced rerupture rates seen with combined anterolateral stabilization procedures.10,15,17 However, different rotational instability patterns have been described based on the injury pattern to peripheral structures. 25 Slocum and Larson 30 were the first to define anteromedial (AM) rotatory instability (AMRI) as the AM subluxation of the tibial plateau due to coupled anterior tibial translation (ATT) and external tibial rotation. They attributed AMRI to combined ACL and deep MCL (dMCL) injury. This pattern of instability has been the focus of recent biomechanical studies.4,37 The dMCL functions as a primary restraint to external rotation (ER) in early flexion angles (as well as acting as a secondary restraint to valgus rotation and ATT), 37 but the superficial MCL (sMCL) also plays a key role in restraining AMRI (as well as being the primary restraint to valgus rotation).4,37
The finding of persistent laxity in 60% of knees with combined ACL/sMCL reconstructions and the failure of this procedure to address dMCL laxity has been proposed as a cause of the poor outcomes of combined reconstructions compared with isolated ACL reconstruction. 21 Additionally, recent length-change pattern studies have revealed the reciprocal function of the different regions of the broad, flat appearance of the native sMCL, in which structures attaching posterior to the medial epicondyle are taut in extension, whereas the anterior sMCL proportion is tensioned during flexion.18,41 An isolated, single-bundle (SB) tendon autograft, such as that commonly used for sMCL reconstruction, may not reproduce this complex behavior because of its thinner, rounder appearance and smaller attachment sites.
The purpose of this study was to (1) compare a conventional SB sMCL reconstruction with a flat sMCL reconstruction in restoring normal knee kinematics after injury to the MCL and to (2) evaluate the effect of an additional AM reconstruction oriented to tighten with tibial ER. It was hypothesized that (1) a flat reconstruction would better restore valgus and axial rotational stability and (2) that an additional AM reconstruction would better control external tibial rotation laxity and AMRI.
Methods
Eight unpaired, fresh-frozen human cadaveric knees (mean age, 81.8 years; range, 73-90 years) with no history of previous injury and no fixed flexion deformity or joint disease were used in this study. The specimens were dissected and tested by a single senior orthopaedic surgeon investigator (P.B.) and with the necessary permissions from the “Gesetz über das Leichen-, Bestattungs- und Friedhofswesen (Bestattungsgesetz) des Landes Schleswig–Holstein vom 04.02.2005, Abschnitt II, § 9 (Leichenöffnung, anatomisch).” After testing, specimens were examined to ensure the integrity of the menisci and cruciate ligaments and that no advanced cartilage erosions were present.
Specimen Preparation
Specimens were stored at –20°C and thawed for 24 hours before testing. The tibia and femur were transected 200 mm from the joint line. The skin and subcutaneous tissue were removed, but the fascia and muscles were left intact. The fibula was divided 10 cm distal to the proximal tibiofibular joint and secured to the tibia in its anatomic position with a 3.5-mm positioning screw. 27 The cut ends of the femur and tibia were secured in aluminum tubes using polyurethane resin bone cement. The tibia was centralized in the tube to standardize rotational effects, with the tube axis aligned with the center of the intercondylar eminence visualized via a transpatellar approach.27,37 Specimens were wrapped in phosphate buffered saline–soaked tissue paper to prevent tissue dehydration.
Testing Setup
A 6 degrees of freedom industrial robot (KR 60-3; KUKA Robotics) equipped with a force-torque sensor (Theta; ATI Industrial Automation) was used for biomechanical testing as described previously.7,36,37 The robotic system allowed for repeatability of motion within ±0.06 mm and accuracies of ±0.25 N and 0.05 N·m for forces and torques, respectively, similar to robotic testing systems used in previous biomechanical studies.7,36,37 A custom software for musculoskeletal robotic simulation (SimVitro Cleveland Clinic BioRobotics Lab) was used for testing. A tactile measuring arm (Absolute Arm 8320-7; Hexagon Metrology GmbH) was used to digitize anatomic landmarks and thus define the knee coordinate system based on the description by Grood and Suntay. 13 Differences in translation (in mm) and rotations (in degrees) recorded at the beginning and end of each simulated instability test were calculated.
Biomechanical Testing
All specimens were flexed and extended 10 times to minimize tissue hysteresis. 23 The tibia was fixed into the stationary base unit with the femur fixed to the manipulator arm of the robot. The knee neutral starting position was defined by minimizing forces (<1 N) and torques (<0.5 N·m) at full extension. 17 A recording of a continuous passive flexion-extension path from 0° to 90° of flexion by the robotic system was used to optimize the manually digitized joint coordinate system. Axial force (50 N) was applied to ensure contact between the femur and tibia. This was continuously measured throughout testing by a load cell attached to the tibial baseplate. For biomechanical testing, a force-controlled setup was used. Forces and torques were applied to the knee at 0°, 30°, 60°, and 90° of flexion to simulate clinical laxity examination. The following simulated clinical laxity tests were applied in accordance to previously used forces.4,6,39,42
(1) Anteromedial drawer (AMD) test: 134-N anterior tibia force with tibial ER (5 N·m) simulating the Slocum test. 30
(2) Valgus stress test: 10 N·m torque.
(3) ER: 5 N·m torque.
(4) Internal tibial rotation (IR): 5 N·m torque.
Each test was performed 3 times at each flexion angle with the knee in its intact state, after cutting the sMCL, and dMCL, and after different MCL reconstruction techniques, which are described in detail below. The first cycle was used for preconditioning and the recorded force/displacement data for the other 2 cycles for each laxity test were plotted and the maximum displacement was averaged. For the AMD test, ATT was quantified as the change in distance between the midpoint of the tibial plateau and the midpoint between the femoral condyles. The primary outcome measures of this force-controlled setup were ATT (in mm) and axial tibial rotations (in degrees).
Cutting Protocol
After testing the knee in its intact state, the sMCL and dMCL were cut (Figure 1). The satorius fascia was incised horizontally, just proximal to the gracilis tendon, and both the gracilis and the semitendinosus tendons were harvested, exposing the tibial attachment of the sMCL. More proximally, the fascia overlying the sMCL was carefully reflected by sharp dissection. Eyelet pins were then used to mark the anterior and posterior borders and the center of both the femoral and tibial sMCL attachments. Care was taken not to damage the posterior oblique ligament (POL), posteromedial capsule, AM capsule, and longitudinal AM retinaculum. The sMCL was divided distally, by sharply dissecting the descending anterior-to-posterior tibial sMCL attachment from the tibia3,19 approximately 6 cm distal to the joint line. The dissection of the sMCL was continued proximally, removing the proximal tibial attachment.3,38 Further proximally, the femoral attachment was located, enveloping the medial epicondyle and extending just proximal and posterior to it. 28 The sMCL was divided from its femoral attachment by sharp dissection; thus, the entire sMCL was excised. The underlying dMCL was then identified and in a similar manner excised from its tibial to its femoral attachment. The meniscofemoral and meniscotibial attachments of the dMCL were then divided just proximal and distal to the meniscus.
Figure 1.
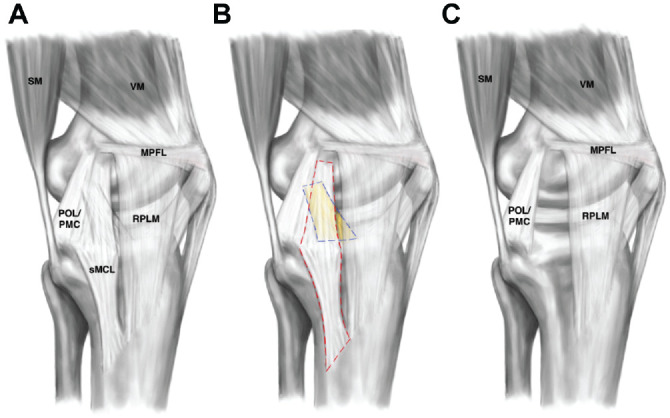
Illustration of the cutting protocol. (A) Intact knee state. (B) Dissection of the superficial (sMCL; red dashed line) and deep (dMCL; colored in yellow; blue dashed line) medial collateral ligaments. (C) Cut state. After removal of the dMCL and sMCL, the posterior oblique ligament (POL), posteromedial capsule (PMC), vastus medialis (VM), semimembranosus muscle (SM), medial patellofemoral ligament (MPFL), and medial longitudinal patellar retinaculum (RPLM) remained intact.
Medial Reconstructions
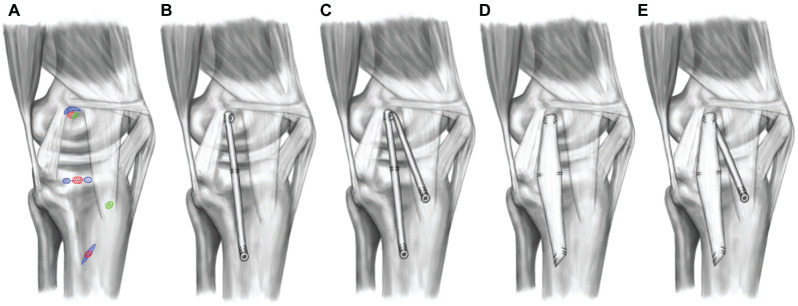
Each knee was tested in the intact and sectioned state and then tested after 4 different medial reconstructions (Figure 2), performed in a random order: (1) SB sMCL reconstruction; (2) SB sMCL + AM reconstruction; (3) MCL reconstruction with flattened tendon (flat MCL); and (4) flat MCL + AM.
Figure 2.
Illustration of reconstructions. (A) Reconstruction insertion sites: (A) single-bundle (SB) superficial medial collateral ligament (sMCL; red), flat MCL (blue), and anteromedial (AM) reconstruction (green). (B) SB sMCL reconstruction, which was inserted in close proximity to the anatomic insertion sites of the native sMCL. (C) SB with additional AM reconstruction simulating the deep MCL. (D) Flat sMCL reconstruction (using flattened tendon autograft) simulating the appearance of the native sMCL. (E) Flat MCL reconstruction with AM reconstruction as described in panel C. MPFL, medial patellofemoral ligament; PMC, posteromedial capsule; POL, posterior oblique ligament; RPLM, medial longitudinal patellar retinaculum; SM, semimembranosus muscle; VM, vastus medialis.
To reproduce the broad anatomic sMCL attachments, the femoral and tibial eyelet pins were overdrilled with a 4.5-mm drill. These transosseous drill holes were connected at the medial cortex using a straight osteotome at the tibial attachment site and a curved osteotome at the femoral attachment site. For the flat MCL reconstruction, the harvested semitendinosus was prepared according to Domnick et al. 8 The tendon sheath was incised along its length and a raspatory was then used to flatten the graft. The length of the graft was determined by measuring the length of the native sMCL and adding 30 mm. No. 2 sutures (Arthrex Inc.) were placed at all 4 corners of the flat MCL graft, and an additional central suture was placed proximally. Passing loop sutures were placed through the transosseous tunnels and used to shuttle the graft 15 mm into the curved femoral socket. The sutures were tied over 3 suspensory buttons at the anterolateral femoral cortex. On the tibial side, the graft was similarly shuttled into the oblique rectangular socket. The anterior tibial sutures were independently tensioned using a custom-made tensioning device (Figure 3). The anterior sutures were tensioned at 60 N with the knee at 50° of knee flexion and the posterior sutures tensioned at 60 N at 20° of flexion (both with the tibia in neutral IR/ER). This tensioning method was based on recent ligament length-change studies showing a reciprocal tensioning profile of the sMCL with the posterior fibers becoming tighter near extension and the anterior fibers in flexion.18,41
Figure 3.
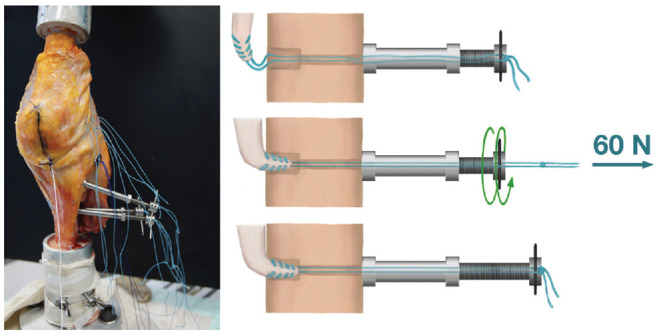
With the knee in upright position (femur on top, tibia at the bottom), custom-made tensioning devices were installed at the opposite cortex to allow for separate tensioning of each medial reconstruction.
For the SB sMCL reconstructions, a transosseous K-wire was placed in the center of the tibial and femoral attachment sites and was overreamed with a 7-mm reamer to a depth of 20 mm. The shared femoral insertion of the SB sMCL and AM reconstructions was placed slightly more distally compared with that of the flat MCL reconstruction. This technical limitation was optimized by orienting the tendon proximally with the interference screw distal. A 20-cm semitendinosus graft was then whipstitched proximally and distally using a suture. The graft was then doubled and baseball stitched on the femoral side over a length of 1 cm to create an inverse V-shaped graft for the combined SB sMCL and AM reconstruction. The looped graft end was shuttled into the femoral socket and fixed using a bioabsorbable interference screw (7 × 23 mm). The suture whipstitches were additionally tied over a cortical fixation button at the anterolateral femoral cortex. For the sMCL reconstruction, one of the free graft ends was shuttled into the tibial tunnel and fixed to another tensioning device at the anterolateral tibial cortex. Tension (60 N) was applied with the knee at 20° of flexion and in neutral tibial rotation. To reproduce the proximal tibial attachment of the sMCL, a No. 2 suture was placed in both SB sMCL and flat MCL reconstructions 1.2 cm below the joint line 19 and passed through the transosseous tunnel to the anterolateral tibial cortex, where it was tied over a cortical button.
For the AM reconstructions, the tibial attachment was positioned halfway between the anterior border of the sMCL and the tibial tubercle, 2 cm below the joint line (Figure 2C). A K-wire was drilled from this position to the anterolateral tibial surface and overreamed with a 4.5-mm drill. The distal end of the graft was passed into the tibial tunnel and fixed to a tensioning device. With the knee in 20° of flexion and neutral tibial rotation, 40 N of tension was applied. Pilot testing with 2 knee joints was conducted to test tensioning forces for AM reconstruction with 60 N, resulting in overconstrained kinematics (ATT at 0° up to 4 mm less that the intact knee) compared with the intact joint.
Before each simulated clinical laxity test and each change in knee flexion, the knee was returned to 20° of flexion and the reconstructions retensioned using the custom tensioning devices (the anterior part of the flat MCL graft was also retensioned at 50° of flexion).
Statistical Analysis
Differences from the intact state of the knee were determined using a 2-factor repeated-measures analysis of variance and post hoc Bonferroni correction. Statistical analysis was performed with SPSS Version 24 (IBM Corp) with a significance level set to .05. Based on previous work, 37 a power analysis (G*Power 3.1; α = .05; power 0.8) was conducted, which indicated 7 specimens were needed to identify a 2° and 2-mm difference with an effect size of 1.2.
Results
AMD (Simulated Slocum Test)
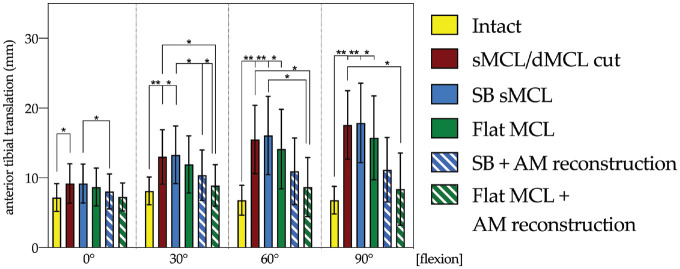
In the intact state, ATT in response to the AMD test (Figure 4) did not vary significantly (P < .05 at each flexion angle) between different flexion angles. Cutting the sMCL + dMCL increased ATT compared with the intact state at all flexion angles (0°, P < .05; 30°, 60°, and 90°, P < .01), but more markedly in deeper flexion (Figure 4). After SB sMCL and flat MCL reconstruction, ATT at all flexion angles was not significantly different from the sMCL/dMCL sectioned state (P > .05) and intact knee kinematics were not restored. However, ATT was reduced with the addition of an AM reconstruction for the flat MCL reconstruction and for the SB sMCL reconstruction. Flat MCL reconstruction with additional AM reduced the ATT significantly compared with the sMCL/dMCL cut state (P < .05) above 30° of flexion, while SB sMCL with additional AM reconstruction did not reach a significant level.
Figure 4.
Changes (in mm) in anterior tibial translation in response to a 134-N anterior drawer in 5 N·m external rotation in the intact and medial collateral ligament (MCL) sectioned and reconstructed states. The sequence of reconstructions was randomized among the experiments (n = 8): (1) single-bundle (SB) superficial MCL (sMCL) reconstruction; (2) SB + anteromedial reconstruction (SB sMCL + AM); (3) flat MCL reconstruction; and (4) flat MCL + AM. Statistically significant differences are indicated (*P < .05, **P < .01). Error bars indicate mean ± SD. dMCL, deep MCL.
Valgus Rotation
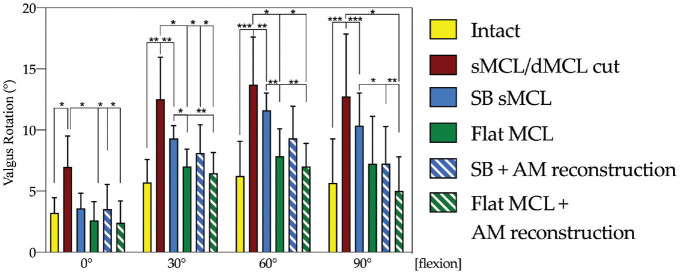
Cutting the sMCL and dMCL resulted in a statistically significant increased valgus rotation compared with the intact state at all tested flexion angles (0°, P < .05; 30°, P < .01; 60° and 90°, P < .001) (Figure 5). SB sMCL reconstruction restored valgus laxity similar to the intact state (Figure 5) only at 0° (0°, not significant; 30° and 60°, P < .01; 90°, P < .001), whereas the flat MCL reconstruction restored valgus rotation laxity similar to the intact state at all flexion angles (not significant at all flexion angles). Combined SB sMCL and AM reconstruction resulted in restoration of valgus laxity to the intact state at all flexion angles (intact vs SB MCL at 0°, P > .05; 30°, P > .05; 60°, P > .05; 90°, P > .05). Flat MCL reconstruction improved control of valgus laxity at 30° and 60° compared with SB sMCL reconstruction. Flat MCL + AM reconstruction restored valgus rotation laxity close to normal at all flexion angles.
Figure 5.
Changes (in degrees) in valgus rotation with an applied 10 N·m valgus torque in the intact and superficial (sMCL) and deep (dMCL) medial collateral ligament sectioned and reconstructed states. Statistically significant differences compared with the previous states are indicated (*P < .05, **P < .01, ***P < .001). Error bars indicate mean ± SD. AM, anteromedial; SB, single bundle.
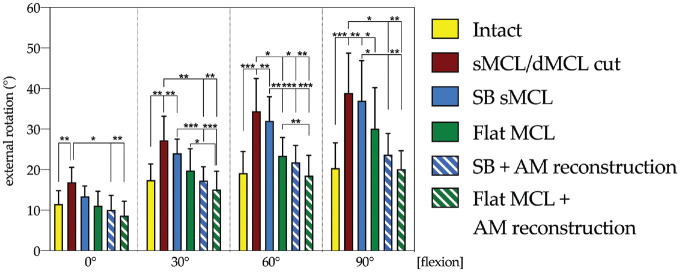
Tibial ER
SB sMCL reconstruction failed to restore tibial ER laxity to the intact state at 30°, 60°, and 90° of flexion (P < .01) (Figure 6). Flat MCL reconstruction better restrained ER laxity compared with SB sMCL reconstruction at 60° of flexion (P < .01) and restored tibial ER to the intact state at 0°, 30°, and 60° of knee flexion. AM reconstruction and SB sMCL reconstruction had a more potent effect on the control of ER laxity than isolated SB sMCL reconstruction at 30°, 60°, and 90° of flexion. Flat MCL with AM reconstruction reduced tibial ER laxity to the intact state at 0°, 30°, and 60° (P > .05).
Figure 6.
Changes (in degrees) in external rotation with an applied 5 N·m external rotation torque in the intact and medial collateral ligament (MCL) sectioned and reconstructed states. Statistically significant differences compared with the previous states are indicated (*P < .05, **P < .01, ***P < .001). Error bars indicate mean ± SD. AM, anteromedial; dMCL, deep MCL; SB, single bundle; sMCL, superficial MCL.
Tibial IR
IR increased up to 22.3° ± 6.9° (sMCL/dMCL cut) at 30° of flexion, which was significantly different from the intact state at 30° (intact, 18.6° ± 5.2°; P < .05). All reconstruction techniques restored IR at 0° to 60° similar to the intact state. There was no significant difference between the different reconstruction techniques. AM reconstruction was not found to overconstrain IR compared with the intact state.
Discussion
The main finding of this study was that a conventional SB sMCL reconstruction could restore neither translational nor rotational knee kinematics in an MCL-deficient knee. A more anatomic flat MCL reconstruction was more effective in controlling valgus and ER laxity, particularly in deeper flexion, but was unable to fully restore AMRI. AM reconstruction, when combined with SB sMCL or a flat MCL reconstruction, was seen to restrain AMRI, restoring knee kinematics similar to the intact state.
The inability to restore intact knee kinematics with a single point-to-point sMCL reconstruction has been suggested by previous MCL length-change pattern studies.3,13 These revealed the complex behavior of the broad sMCL, with the anterior and posterior parts exhibiting different tension patterns throughout the range of flexion.18,41 Additionally, the importance of the dMCL as a primary restraint to tibial ER in extension and as a secondary restraint to valgus rotation has been recently demonstrated.4,27,37 Older studies using different cutting protocols may have underestimated the role of the dMCL in restraining secondary coupled movements of the tibia, 12 and in many studies examining MCL reconstruction techniques, the dMCL was left intact.6,33,35,39,42
We found that cutting both the dMCL and the sMCL led to an increase in tibial ER from 20.4° to 38.9° with the knee at 90° of flexion. This was comparable with previous studies, which also applied 5 N·m ER torque, that found similar increases in tibial ER after cutting both the sMCL and dMCL27,37 and was more pronounced than in studies that left the dMCL intact.6,39 Our finding that both the dMCL and the sMCL are important in restraining AMRI is also consistent with previous reports.4,27,37
There is clinical evidence that a more differentiated approach to MCL reconstruction techniques may be necessary. Lind et al 21 showed, in a recent registry study, that clinical outcomes after combined ACL/MCL reconstruction were worse than after isolated ACL reconstruction and hypothesized that a possible cause for this observation was that sMCL reconstructions fail to address dMCL-related laxity. Insufficiency of the dMCL is an important component of AMRI and has not been investigated before in the context of MCL reconstruction techniques. Like Zhu et al, 42 we found that SB sMCL reconstruction was unable to restore intact knee kinematics. While valgus laxity in extension was controlled, SB sMCL reconstruction was unable to restore ER laxity and AMRI. Flat MCL reconstruction better controlled ER and valgus laxity throughout the range of flexion, although it did not control AMRI. The finding that valgus laxity was better controlled by a flat MCL reconstruction than SB sMCL reconstruction at 30° and 60° of flexion is likely because the flatter, wider graft better represents the native ligament. As in previous studies, the SB sMCL reconstruction was tensioned at 20° of knee flexion,6,20,39 whereas for the flat MCL graft, the anterior part was tensioned at 50° while the posterior part was tensioned at 20°. This is likely to better represent the reciprocal tensioning pattern of the different fiber regions of the native sMCL.18,41
Only with the addition of an AM reconstruction to both SB sMCL and flat MCL reconstruction was the ATT seen to match that of the intact knee when applying coupled ER with anterior drawer. The course of the AM reconstruction, running distally and anterior from the medial femoral epicondyle, is well-oriented to control AMRI, and it appears to effectively reproduce the role of the dMCL. It is similar in concept to the pes anserinus transplantation described by Slocum and Larson. 29
We did not investigate the effect of the POL. While the POL contributes significantly to the restraint of valgus in the extended knee, it plays no significant role in AMRI.27,37 Previous studies described MCL reconstruction techniques with SB sMCL and POL grafts and reported restoration of normal knee ER kinematics, but these studies did not section the dMCL or simulate an AM drawer test.6,42 Although we did not section the POL in our study, we found that an SB sMCL reconstruction in combination with an intact native POL failed to control tibial ER laxity or AMRI. The POL is not well-oriented to control external tibial rotation, and indeed, the POL and posteromedial capsule are slack at knee flexion angles greater than 15°.11,37 The POL acts to control tibial IR and valgus rotation in extension. 37 Restraint of IR tibial laxity may be more important in knees with posteromedial rotatory instability (PMRI) where the medial structures contribute to the control of posteromedial rotational laxity, particularly in the PCL-deficient knee. 26 In patients with ACL rupture, the dMCL is found to be injured twice as often as the POL. 40 It is also noteworthy that significant injuries to the dMCL and sMCL in the context of ACL rupture that might lead to AMRI may be missed, as there may not be valgus laxity in extension if the POL remains intact. 40 Given the different contributions of each of the individual components of the MCL complex, individual reconstruction techniques may be necessary. While a flat sMCL with additional AM reconstruction was able to restore intact knee kinematics in this study, in knees with significant valgus laxity in extension and posteromedial instability a POL reconstruction, as previously described,6,20 may be necessary. In this regard, AMRI and PMRI must be identified as different entities that may necessitate different reconstruction strategies. Based on the data presented in this study, the presence of AMRI may suggest an AM rather than a posteromedial reconstruction. It is possible that for ACL injuries with a combined extensive medial injury also involving the POL, that AMRI, PMRI, and valgus laxity may need to be addressed. Further research is also needed to define an ideal AM reconstruction technique, its insertion points, and tensioning profile.
To the authors’ knowledge, this is the first biomechanical study that investigated an AM reconstruction and its effects on control of AMRI. While nonanatomic, the orientation of the AM reconstruction graft is similar to that of the dMCL. Although anatomic studies have shown that the femoral attachment of the dMCL is posterior and inferior to the medial epicondyle, 3 the AM reconstruction described in this study was chosen because of its practicability with a concomitant sMCL reconstruction. The tibial insertion was chosen to be on an ideal path of the Burmester curve 14 and was positioned more distally to the native dMCL tibial attachment. As hypothesized by Menschik, 24 repaired or reconstructed ligaments should be placed on the ideal path of the Burmester curve in order not to fail or elongate. The AM reconstruction was potent in restraining AMRI, and pilot testing suggested tensioning the AM reconstruction with 40 N instead of 60 N to avoid overconstraint. Different graft positions and tensions could be the subject of further work. Translation of the described AM reconstruction into clinical practice should be considered with caution, as all reconstructions were performed in cadaveric conditions with precise tensioning and positioning of the grafts.
There are some limitations of our study. Specimen age and tissue quality may have negatively affected the rigid fixation of multiple reconstruction techniques in close proximity to the MCL footprint, which was in part compensated by double-fixation techniques. Differences in graft femoral attachment sites can affect kinematics, 9 and we acknowledge that the femoral attachment of the SB sMCL and flat MCL reconstructions differed slightly, potentially influencing results. However, to overcome this small attachment site discrepancy, the interference screw used for SB sMCL femoral fixation was positioned to push the graft proximally in the tunnel such that the graft lay proximal to the screw head in close approximation to the native sMCL attachment and flat MCL socket. This methodology also allowed comparison of different reconstruction techniques in the same specimens. In addition, this study represents a time-zero analysis, and the effects of loss of ligament tension through fixation slippage during the process of graft healing were not considered. Also, the ACL was left intact and considered equivalent to an ideal ACL reconstruction. With respect to anatomic structures relevant for AMRI, the AM capsule and longitudinal retinaculum were not considered in this study. Both structures, as well as the hamstring muscles as dynamic medial stabilizers, are relevant for controlling AMRI and should be embraced in future studies.
Conclusion
In a cadaveric model, AMRI resulting from an injured sMCL and dMCL complex could not be restored by an isolated SB sMCL reconstruction. A flat MCL reconstruction or an additional AM procedure, however, better restored medial knee stability. These findings may have important implications for improving medial reconstructions and reducing the risk of ACL graft rupture in combined ACL/MCL injuries.
Acknowledgments
The authors thank Andre Frank, Julia Sußiek, and Luise Hägerich for superb technical assistance.
Footnotes
Submitted September 27, 2021; accepted March 16, 2022.
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
ORCID iD: Jens Wermers  https://orcid.org/0000-0002-3448-5259
https://orcid.org/0000-0002-3448-5259
References
- 1. Alm L, Drenck TC, Frings J, et al. Lower failure rates and improved patient outcome due to reconstruction of the MCL and revision ACL reconstruction in chronic medial knee instability. Orthop J Sports Med. 2021;9(3):2325967121989312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alm L, Krause M, Frosch KH, Akoto R. Preoperative medial knee instability is an underestimated risk factor for failure of revision ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2020;28(8):2458-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Athwal KK, Willinger L, Shinohara S, Ball S, Williams A, Amis AA. The bone attachments of the medial collateral and posterior oblique ligaments are defined anatomically and radiographically. Knee Surg Sports Traumatol Arthrosc. 2020;28(12):3709-3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ball S, Stephen JM, El-Daou H, Williams A, Amis AA. The medial ligaments and the ACL restrain anteromedial laxity of the knee. Knee Surg Sports Traumatol Arthrosc. 2020;28(12):3700-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Battaglia MJ, II, Lenhoff MW, Ehteshami JR, et al. Medial collateral ligament injuries and subsequent load on the anterior cruciate ligament: a biomechanical evaluation in a cadaveric model. Am J Sports Med. 2009;37(2):305-311. [DOI] [PubMed] [Google Scholar]
- 6. Coobs BR, Wijdicks CA, Armitage BM, et al. An in vitro analysis of an anatomical medial knee reconstruction. Am J Sports Med. 2010;38(2):339-347. [DOI] [PubMed] [Google Scholar]
- 7. Domnick C, Frosch KH, Raschke MJ, et al. Kinematics of different components of the posterolateral corner of the knee in the lateral collateral ligament-intact state: a human cadaveric study. Arthroscopy. 2017;33(10):1821-1830.e1821. [DOI] [PubMed] [Google Scholar]
- 8. Domnick C, Herbort M, Raschke MJ, et al. Converting round tendons to flat tendon constructs: does the preparation process have an influence on the structural properties? Knee Surg Sports Traumatol Arthrosc. 2017;25(5):1561-1567. [DOI] [PubMed] [Google Scholar]
- 9. Feeley BT, Muller MS, Allen AA, Granchi CC, Pearle AD. Isometry of medial collateral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1078-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Getgood AMJ, Bryant DM, Litchfield R, et al. Lateral extra-articular tenodesis reduces failure of hamstring tendon autograft anterior cruciate ligament reconstruction: 2-year outcomes from the STABILITY Study Randomized Clinical Trial. Am J Sports Med. 2020;48(2):285-297. [DOI] [PubMed] [Google Scholar]
- 11. Griffith CJ, Wijdicks CA, LaPrade RF, Armitage BM, Johansen S, Engebretsen L. Force measurements on the posterior oblique ligament and superficial medial collateral ligament proximal and distal divisions to applied loads. Am J Sports Med. 2009;37(1):140-148. [DOI] [PubMed] [Google Scholar]
- 12. Grood ES, Noyes FR, Butler DL, Suntay WJ. Ligamentous and capsular restraints preventing straight medial and lateral laxity in intact human cadaver knees. J Bone Joint Surg Am. 1981;63(8):1257-1269. [PubMed] [Google Scholar]
- 13. Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105(2):136-144. [DOI] [PubMed] [Google Scholar]
- 14. Hirschmann MT, Müller W. Complex function of the knee joint: the current understanding of the knee. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2780-2788. [DOI] [PubMed] [Google Scholar]
- 15. Inderhaug E, Stephen JM, Williams A, Amis AA. Biomechanical comparison of anterolateral procedures combined with anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(2):347-354. [DOI] [PubMed] [Google Scholar]
- 16. Kannus P. Long-term results of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103-112. [PubMed] [Google Scholar]
- 17. Kittl C, El-Daou H, Athwal KK, et al. The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee. Am J Sports Med. 2016;44(2):345-354. [DOI] [PubMed] [Google Scholar]
- 18. Kittl C, Robinson J, Raschke MJ, et al. Medial collateral ligament reconstruction graft isometry is effected by femoral position more than tibial position. Knee Surg Sports Traumatol Arthrosc. 2021;29(11):3800-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010. [DOI] [PubMed] [Google Scholar]
- 20. LaPrade RF, Wijdicks CA. Surgical technique: development of an anatomic medial knee reconstruction. Clin Orthop Relat Res. 2012;470(3):806-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lind M, Jacobsen K, Nielsen T. Medial collateral ligament (MCL) reconstruction results in improved medial stability: results from the Danish Knee Ligament Reconstruction Registry (DKRR). Knee Surg Sports Traumatol Arthrosc. 2020;28(3):881-887. [DOI] [PubMed] [Google Scholar]
- 22. Majewski M, Susanne H, Klaus S. Epidemiology of athletic knee injuries: a 10-year study. Knee. 2006;13(3):184-188. [DOI] [PubMed] [Google Scholar]
- 23. Martin RB, Burr DB, Sharkey NA, Fyhrie DP. Skeletal Tissue Mechanics. Vol 2. Springer; 2015. [Google Scholar]
- 24. Menschik A. Mechanics of the knee-joint. Menschik A, trans. Article in German. Z Orthop Ihre Grenzgeb. 1974;112(3):481-495. [PubMed] [Google Scholar]
- 25. Müller W, Hughston JC, Muspach R, Telger TC. The Knee: Form, Function, and Ligament Reconstruction: Springer; 2012. [Google Scholar]
- 26. Petersen W, Loerch S, Schanz S, Raschke M, Zantop T. The role of the posterior oblique ligament in controlling posterior tibial translation in the posterior cruciate ligament-deficient knee. Am J Sports Med. 2008;36(3):495-501. [DOI] [PubMed] [Google Scholar]
- 27. Robinson JR, Bull AM, Thomas RR, Amis AA. The role of the medial collateral ligament and posteromedial capsule in controlling knee laxity. Am J Sports Med. 2006;34(11):1815-1823. [DOI] [PubMed] [Google Scholar]
- 28. Robinson JR, Sanchez-Ballester J, Bull AM, Thomas RW, Amis AA. The posteromedial corner revisited. An anatomical description of the passive restraining structures of the medial aspect of the human knee. J Bone Joint Surg Br. 2004;86(5):674-681. [DOI] [PubMed] [Google Scholar]
- 29. Slocum DB, Larson RL. Pes anserinus transplantation. A surgical procedure for control of rotatory instability of the knee. J Bone Joint Surg Am. 1968;50(2):226-242. [PubMed] [Google Scholar]
- 30. Slocum DB, Larson RL. Rotatory instability of the knee. Its pathogenesis and a clinical test to demonstrate its presence. J Bone Joint Surg Am. 1968;50(2):211-225. [PubMed] [Google Scholar]
- 31. Svantesson E, Hamrin Senorski E, Alentorn-Geli E, et al. Increased risk of ACL revision with non-surgical treatment of a concomitant medial collateral ligament injury: a study on 19,457 patients from the Swedish National Knee Ligament Registry. Knee Surg Sports Traumatol Arthrosc. 2019;27(8):2450-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tapasvi S, Shekhar A, Patil S, Getgood A. Anatomic medial knee reconstruction restores stability and function at minimum 2 years follow-up. Knee Surg Sports Traumatol Arthrosc. 2022;30(1):280-287. [DOI] [PubMed] [Google Scholar]
- 33. Van den Bogaerde JM, Shin E, Neu CP, Marder RA. The superficial medial collateral ligament reconstruction of the knee: effect of altering graft length on knee kinematics and stability. Knee Surg Sports Traumatol Arthrosc. 2011;19(suppl 1):S60-S68. [DOI] [PubMed] [Google Scholar]
- 34. Varelas AN, Erickson BJ, Cvetanovich GL, Bach BR., Jr. Medial collateral ligament reconstruction in patients with medial knee instability: a systematic review. Orthop J Sports Med. 2017;5(5):2325967117703920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang X, Liu H, Duan G, Niu Y, Liu C, Wang F. A biomechanical analysis of triangular medial knee reconstruction. BMC Musculoskelet Disord. 2018;19(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wermers J, Schliemann B, Raschke MJ, et al. The glenolabral articular disruption lesion is a biomechanical risk factor for recurrent shoulder instability. Arthrosc Sports Med Rehabil. 2021;3(6):e1803-e1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wierer G, Milinkovic D, Robinson JR, et al. The superficial medial collateral ligament is the major restraint to anteromedial instability of the knee. Knee Surg Sports Traumatol Arthrosc. 2021;29(2):405-416. [DOI] [PubMed] [Google Scholar]
- 38. Wijdicks CA, Griffith CJ, LaPrade RF, et al. Radiographic identification of the primary medial knee structures. J Bone Joint Surg Am. 2009;91(3):521-529. [DOI] [PubMed] [Google Scholar]
- 39. Wijdicks CA, Michalski MP, Rasmussen MT, et al. Superficial medial collateral ligament anatomic augmented repair versus anatomic reconstruction: an in vitro biomechanical analysis. Am J Sports Med. 2013;41(12):2858-2866. [DOI] [PubMed] [Google Scholar]
- 40. Willinger L, Balendra G, Pai V, et al. High incidence of superficial and deep medial collateral ligament injuries in ‘isolated’ anterior cruciate ligament ruptures: a long overlooked injury. Knee Surg Sports Traumatol Arthrosc. 2022;30(1):167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Willinger L, Shinohara S, Athwal KK, Ball S, Williams A, Amis AA. Length-change patterns of the medial collateral ligament and posterior oblique ligament in relation to their function and surgery. Knee Surg Sports Traumatol Arthrosc. 2020;28(12):3720-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu W, Zhu J, Marshall B, Linde MA, Smolinski P, Fu FH. Single-bundle MCL reconstruction with anatomic single-bundle ACL reconstruction does not restore knee kinematics. Knee Surg Sports Traumatol Arthrosc. 2020;28(8):2687-2696. [DOI] [PubMed] [Google Scholar]