Abstract
Prenatal exposure to drugs of abuse results in neonatal abstinence syndrome (NAS). NAS causes significant morbidity and is associated with costly and lengthy hospitalization. Current pharmacotherapy is suboptimal with no FDA approved treatments. We examined the effect of postnatal oxytocin treatment on survival and neurodevelopmental outcomes in rats prenatally exposed to opioids or benzodiazepines. Sprague-Dawley rat dams were injected with escalating doses of morphine (10–50 mg/kg/day) or diazepam (2–15 mg/kg/day) throughout gestation. In an initial experiment, exposed rat pups received subcutaneous injections of 2 mg/kg oxytocin or saline for the first 10 postnatal days and survival rates were assessed. In a second experiment, exposed rat pups received subcutaneous injections of 0.3, 1, or 2 mg/kg oxytocin or saline for the first 10 postnatal days and survival and body weight were assessed for 30 days. In animals surviving through adolescence, neurodevelopmental outcomes and biological parameters (blood glucose, corticosterone, aldosterone) were also measured. Postnatal oxytocin treatment improved survival in animals prenatally exposed to morphine or diazepam. Preliminary evidence showed that postnatal oxytocin treatment improves long-term learning and memory processes in animals prenatally exposed to morphine or diazepam. These findings highlight the potential of oxytocin as a novel treatment for NAS resulting from prenatal exposure to opioids or benzodiazepines.
Keywords: Oxytocin, Neonatal abstinence syndrome, Withdrawal, Opioids, Benzodiazepines, Survival
Highlights
-
•
Prenatal exposure to drugs of abuse results in neonatal abstinence syndrome (NAS).
-
•
There are no approved pharmacological treatment options for NAS.
-
•
Oxytocin improves survival in animals prenatally exposed to morphine or diazepam.
-
•
Oxytocin improves behavior in animals prenatally exposed to morphine or diazepam.
1. Introduction
Neonatal abstinence syndrome (NAS) is a constellation of signs of withdrawal caused by prenatal exposure to opioids and other psychoactive substances (eg. benzodiazepines, antidepressants). Neonates with NAS experience central and autonomic nervous system dysfunction which if untreated can result in seizures and death [1]. Common symptoms include increased muscle tone, tremors, high-pitched cry, poor feeding, poor weight gain, diarrhea, temperature instability, and irritability. Emerging evidence also suggests that neonates with NAS have a higher incidence of neurodevelopmental problems later in life. Between 2004 and 2014, the incidence of NAS increased over fivefold in the US, affecting 8 neonates per 1000 births and 27 cases per 1000 NICU admissions [2]. Clinically significant signs of NAS occur in up to 80% of opioid-exposed neonates, and some estimates suggest that as many as 50,000 neonates receive pharmacotherapy for the treatment of NAS each year in the US [3]. There are currently no FDA approved treatments for NAS. Standard pharmacotherapy consists of an opioid-based treatment regimen (morphine, methadone, or buprenorphine). Neonates treated pharmacologically for NAS are much more likely to have also been exposed to benzodiazepines prenatally. Due to the high rates of polysubstance use in women using opioids during pregnancy, adjunctive medications (eg. clonidine, phenobarbital) are also used to manage NAS. Efforts to develop therapeutic approaches that eliminate the use of opioids and other toxic substances in neonates are urgently needed.
The neuropeptide oxytocin has been shown to block the development of tolerance and dependence to morphine and heroin and reduces naloxone-induced morphine withdrawal in adult rats [4,5]. Several early-stage clinical trials also highlight the ability of oxytocin to reduce withdrawal symptoms in alcohol and heroin dependent adults [6,7]. The present study aimed to determine the effect of postnatal oxytocin treatment on survival and neurodevelopmental outcomes in rats prenatally exposed to morphine or diazepam.
2. Methods
2.1. Experimental animals
Twenty-two nulliparous, timed-pregnant, gestational day two, Sprague-Dawley rats (∼200g; approximately 70 days of age; Charles River Labs, Hollister, CA) were utilized to establish the NAS models. Another 22 nulliparous timed-pregnant Sprague-Dawley rats were used as drug-naïve surrogates for rat pups prenatally exposed to drugs. All dams were group housed in their respective cohorts until the day before expected parturition (typically 21–23 days). Animal studies were approved by the Institutional Animal Care and Use Committees of AfaSci Research Laboratories, Afasci, Inc., and were performed in conformance with the US Public Health Service Guidelines on Care and Use of Animals in Research.
2.2. Drugs
Morphine sulfate and diazepam were purchased from Sigma Aldrich (St. Louis, MO). Oxytocin peptide was purchased from Phoenix Pharmaceuticals, Inc. (Burlingame, CA). All drugs were dissolved in 0.9% saline for subcutaneous (s.c.) injection.
2.3. Drug administration and assessment of survival
Beginning on gestational day 2, dams received escalating daily doses of morphine (10–50 mg/kg/day, s.c.) or diazepam (2–15 mg/kg/day, s.c.) until parturition. On the day of parturition (postnatal day 0), litters from the morphine or diazepam treated dams were cross-fostered to drug-naïve surrogates. At this time, all drug treated dams and drug naïve pups were humanely euthanized. On postnatal day 1, pups were randomly assigned to oxytocin or saline treatment groups (groups balanced for number of pups). In experiment 1, pups received s.c. injections of either 2 mg/kg/day oxytocin or saline for the first 10 postnatal days and survival was measured daily for 10 days. In experiment 2, pups received s.c. injections of either 0.3, 1, or 2 mg/kg/day oxytocin or saline for the first 10 postnatal days and survival and body weight was measured daily for 30 days, after which animals were weaned and housed according to sex and treatment group.
2.4. Behavioral assay
Animals from experiment 2 that survived through adolescence were subjected to the Passive Avoidance Task on postnatal day 35. In this assay, eight animals from each treatment group were randomly selected and individually placed into the open chamber of a light-dark box for a maximum of 5 min. Upon completely entering the dark chamber, a mild foot shock was delivered through the chamber floor. Twenty-four hours later, memory for the foot-shock was assessed by measuring the latency to enter the dark chamber during a 5-min period. No foot shock was delivered on the test day.
2.5. Blood collection and biological assays
On postnatal day 45, blood (∼2 mL/animal) was collected via terminal cardiac puncture, immediately centrifuged at 5000 RPM for 5 min, and plasma (∼1 mL/animal) was stored at −70 °C. Plasma glucose oxidase levels were measured using an Amplex® Red Glucose/Glucose Oxidase Kit Assay Kit according to manufacturer instructions (Invitrogen, Eugene, OR). Determination of plasma corticosterone and aldosterone levels were performed using competitive Enzyme Immunoassay kits according to manufacturer instructions (Thermo Fischer Scientific, Inc., Carlsbad, CA). Prepared plates were analyzed using a microplate reader (SpectraMax M2 with SoftMax Pro7.1, Molecular Devices, San Jose, CA). Results were expressed as the plasma concentration of glucose oxidase in uM/ml and corticosterone and aldosterone in pg/ml for each sample.
2.6. Statistical analysis
Survival was assessed with Kaplan-Meier analyses and the log-rank (Mantel-Cox) test. One-way ANOVA with Fisher's LSD was used to compare treatment groups for body weight, Passive Avoidance Task entry latencies, and biological parameters in Experiment 2. Data were analyzed using Excel and GraphPad Prism (version 8.0, GraphPad Software, San Diego, CA). The significance level for two-sided analyses was set at P < 0.05.
3. Results
3.1. Experiment 1
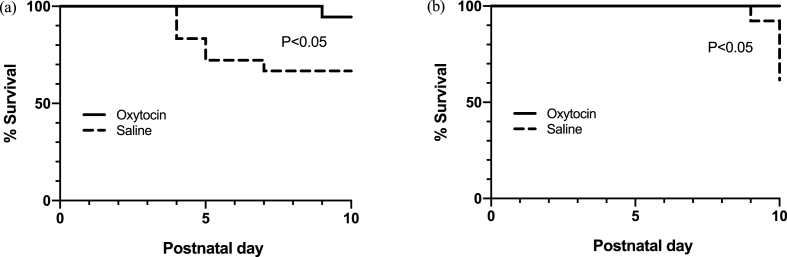
For pups exposed to morphine prenatally, survival was significantly improved with oxytocin treatment (χ2 = 4.597, P < 0.05) (Fig. 1a). At postnatal day 10, 17/18 (94.44%) oxytocin treated animals had survived compared to 12/18 (66.67%) of saline treated animals. For pups exposed to diazepam prenatally, oxytocin treatment significantly improved survival (χ2 = 5.924, P < 0.05) (Fig. 1b). At postnatal day 10, 13/13 (100%) oxytocin treated animals compared to 8/13 (61.5%) saline treated animals had survived.
Fig. 1.
Effects of postnatal oxytocin (2 mg/kg) treatment on survival rates in rat pups exposed to morphine or diazepam prenatally. Pups were injected subcutaneously with oxytocin or saline for the first 10 postnatal days.
3.2. Experiment 2
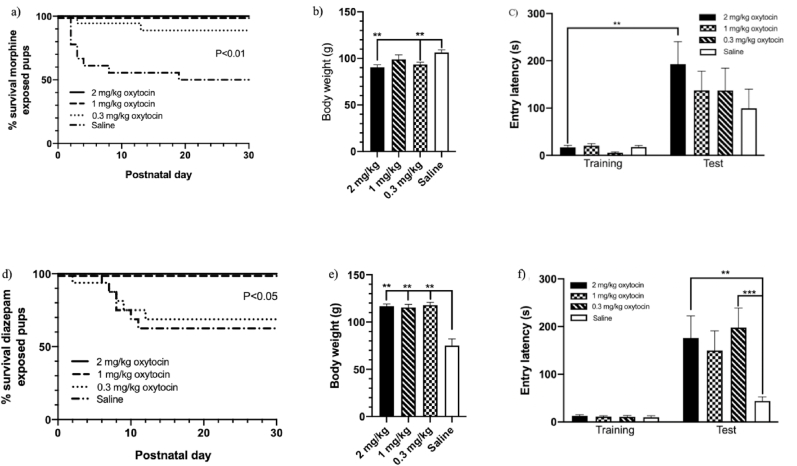
For pups exposed to morphine prenatally, oxytocin treatment significantly improved survival (χ2 = 26.59, P < 0.01; Fig. 2a). Follow-up tests revealed that postnatal treatment with all three oxytocin doses was associated with improvements in survival, compared to saline. At postnatal day 30, 16/18 (88.89%) animals treated with 0.3 mg/kg oxytocin and 18/18 (100%) in each group of animals treated with 1 and 2 mg/kg oxytocin had survived compared to 9/18 (50%) saline treated animals. All oxytocin treatment groups had significantly higher body weight compared to saline treated animals (P < 0.01; Fig. 2b). There was no difference between treatment groups on the test day during the Passive Avoidance Task. Post hoc comparisons revealed that animals treated with 2 mg/kg oxytocin had significantly higher entry latencies at test compared with training, suggesting that this treatment group was the only group that adequately learned the task (P < 0.01; Fig. 2c). Glucose oxidase levels were significantly higher in all oxytocin treatment groups (P < 0.01). No differences in corticosterone or aldosterone were observed.
Fig. 2.
Dose-response assessment of postnatal oxytocin treatment on survival rates, body weight, and learning and memory in rats exposed to morphine (a, b, c) or diazepam (d, e, f) prenatally. Pups were injected subcutaneously with 0.3 mg/kg, 1 mg/mg, or 2 mg/kg oxytocin or saline for the first 10 postnatal days. **P < 0.01, ***P < 0.001; Data shown as mean ± SEM.
For pups exposed to diazepam prenatally, oxytocin treatment significantly improved survival (χ2 = 10.12, P < 0.05; Fig. 2d). Follow-up tests revealed that postnatal treatment with 1 mg/kg and 2 mg/kg improved survival, compared to saline. One dam in the 1 mg/kg treatment group was infanticidal at postnatal day 3 and all pups from this cohort were removed from the analyses. Survival in animals treated with 0.3 mg/kg oxytocin was no different than saline treated animals. At postnatal day 30, 8/8 (100%) animals treated with 1 mg/kg oxytocin and 16/16 (100%) animals treated with 2 mg/kg oxytocin had survived compared to 11/16 (68.75%) animals treated with 0.3 mg/kg oxytocin and 10/16 (62.5%) animals treated with saline. Animals treated postnatally with 0.3 mg/kg (P < 0.01) and 2 mg/kg (P < 0.01), but not 1 mg/kg, oxytocin had significantly lower body weight at postnatal day 30 compared to saline treated animals (Fig. 2e). During the test day on the Passive Avoidance Task, animals treated with 2 mg/kg (P < 0.05) and 0.3 mg/kg (P < 0.01), but not 1 mg/kg, oxytocin had significantly higher entry latencies compared to saline treated animals (Fig. 2f). Animals treated postnatally with 0.3 mg/kg (P < 0.01) and 2 mg/kg (P < 0.01), but not 1 mg/kg, oxytocin had significantly lower glucose oxidase levels compared to saline treated animals. No differences in corticosterone or aldosterone were observed. Biological parameters are not shown graphically.
4. Discussion
Our data highlights the striking ability of oxytocin to significantly improve survival in animals prenatally exposed to drugs of abuse. Although mortality of neonates treated for NAS has declined substantially due to widespread use of pharmacologic treatment protocols, prenatal exposure to opioids still has the potential to result in death and significant perturbations of development, including delayed growth and behavioral problems [1]. While the exact mechanisms remain unclear, our data shows that mortality appears to be restricted to approximately the first 10 postnatal days, suggesting that this phenomenon is driven by factors associated with acute drug withdrawal rather than chronic conditions and that early treatment with oxytocin may therefore provide the greatest benefit for NAS. Our data also highlights that oxytocin may be effective in treating withdrawal symptoms in neonates prenatally exposed to distinct drug classes making it an appealing therapeutic option in the setting of increased polysubstance use amongst opioid using pregnant women. In a clinical setting, neonates concomitantly exposed to benzodiazepines and opioids are at higher risk of developing severe withdrawal symptoms necessitating pharmacological intervention [8]. There are currently no specific treatments for neonatal benzodiazepine withdrawal, so phenobarbital is often used in conjunction with opioids when both prenatal benzodiazepine and opioid exposure is suspected. Polysubstance use during pregnancy poses a major barrier to developing adequate treatment protocols for NAS. The use of potent narcotic agents for the treatment of NAS is undesirable and thus development of safe and effective pharmacotherapeutic treatments that mitigate the negative impact of prenatal and postnatal drug exposure on acute and long-term outcomes is of critical importance.
In the setting of increased metabolic demand, neonates with NAS can experience hypophagia and/or hyperphagia, but overall body weight appears to be comparable to the general population during their first year of life [9]. In the present study, oxytocin treatment significantly increased body weight into early adolescence in animals exposed to morphine prenatally and glucose oxidase levels followed the same trends. Previous research has shown that postnatal oxytocin increases body weight and blood glucose levels and reduces stress hormones in adult rats exposed to food restriction prenatally [10]. However, in animals prenatally exposed to benzodiazepines oxytocin appeared to modestly reduce both body weight and glucose oxidase levels in adolescent animals suggesting a possible anorectic effect in these animals. Further research is needed to elucidate the effect of oxytocin on feeding and growth patterns in animal models of NAS.
A growing body of research shows that early treatment with oxytocin improves complex behavioral processes in both animals and humans [[11], [12], [13]]. Results from our study indicate that postnatal treatment with oxytocin improved learning and memory processes as measured by the Passive Avoidance Task. However, without the use of a drug naïve control group, it is unclear if exposure to morphine or diazepam negatively impacted neurodevelopmental functioning or if oxytocin has beneficial effects irrespective of prenatal exposure to drugs of abuse. Regardless, uncovering the mechanisms by which early life oxytocin treatment positively impacts neurodevelopmental outcomes might provide further insights into the potential therapeutic effect of oxytocin in NAS.
Preclinical research highlights oxytocin's modulatory effects across a broad range of neurochemical processes impacted by drugs of abuse including central monoaminergic activity and control of neural tone through regulation of glutamatergic and GABAergic functioning [12]. A growing body of research also shows that oxytocin reduces markers of stress and inflammation, including inhibition of cortisol production, and mitigates physical and social stressors [14]. Interestingly, the present study provided no evidence for an effect of postnatal oxytocin treatment on plasma corticosterone or aldosterone levels in exposed animals at the time of adolescence. Clinical evidence suggests that salivary cortisol may be a useful biomarker for determining acute withdrawal severity in NAS so it remains possible that measurement of stress hormones during acute stages of withdrawal in the model utilized here may reveal an effect of oxytocin [15]. In a small clinical study investigating the effects of oxytocin as a treatment for acute opioid withdrawal in heroin dependent patients, elevated cortisol levels were reversed following single doses of oxytocin [6].
In conclusion, we provide the first evidence for a potential therapeutic effect of oxytocin in the treatment of NAS. Postnatal oxytocin treatment results in significant improvements to survival in rats prenatally exposed to opioids and benzodiazepines. Effects on body weight, biological processes, and neurodevelopmental outcomes require further investigation before strong conclusions can be made.
Statement of ethics
Animal studies were approved by the Institutional Animal Care and Use Committees of AfaSci Research Laboratories, Afasci, Inc., and were performed in conformance with the US Public Health Service Guidelines on Care and Use of Animals in Research.
Funding sources
All research was funded by Katana Pharmaceuticals Inc.
Author contributions
DSC conceptualized and designed the study and wrote the manuscript. DSC, SJA, ERTC, CP, and XSX provided substantial contribution to overall running of the experiments, data acquisition, analysis and interpretation, and manuscript revisions and final approval.
Conflict of interest statement
DSC is inventor of an issued patent (US 11,266,711 B2) for the use of oxytocin as a treatment for neonatal abstinence syndrome. No other authors have a conflict of interest to declare.
References
- 1.Gomez-Pomar E., Finnegan L.P. The epidemic of neonatal abstinence syndrome, historical references of its' origins, assessment, and management. Front Pediatr. 2018;6:33. doi: 10.3389/fped.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winkelman T.N.A., Villapiano N., Kozhimannil K.B., Davis M.M., Patrick S.W. Incidence and costs of neonatal abstinence syndrome among infants with medicaid: 2004-2014. Pediatrics. 2018;141 doi: 10.1542/peds.2017-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backes C.H., Backes C.R., Gardner D., Nankervis C.A., Giannone P.J., Cordero L. Neonatal abstinence syndrome: transitioning methadone-treated infants from an inpatient to an outpatient setting. J. Perinatol. 2012;32:425–430. doi: 10.1038/jp.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovacs G.L., Horvath Z., Sarnyai Z., Faludi M., Telegdy G. Oxytocin and a C-terminal derivative (Z-prolyl-D-leucine) attenuate tolerance to and dependence on morphine and interact with dopaminergic neurotransmission in the mouse brain. Neuropharmacology. 1985;24:413–419. doi: 10.1016/0028-3908(85)90026-7. [DOI] [PubMed] [Google Scholar]
- 5.Kovács G.L., Izbéki F., Horváth Z., Telegdy G. Effects of oxytocin and a derivative (Z-prolyl-D-leucine) on morphine tolerance/withdrawal are mediated by the limbic system. Behav. Brain Res. 1984;14:1–8. doi: 10.1016/0166-4328(84)90014-7. [DOI] [PubMed] [Google Scholar]
- 6.Moeini M., Omidi A., Sehat M., Banafshe H.R. The effects of oxytocin on withdrawal, craving and stress response in heroin-dependent patients: a randomized, double-blind clinical trial. Eur. Addiction Res. 2019;25:41–47. doi: 10.1159/000496194. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen C.A., Smedley K.L., Leserman J., Jarskog L.F., Rau S.W., Kampov-Polevoi A., Casey R.L., Fender T., Garbutt J.C. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol Clin. Exp. Res. 2013;37:484–489. doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraft W.K., van den Anker J.N. Pharmacologic management of the opioid neonatal abstinence syndrome. Pediatr. Clin. 2012;59:1147–1165. doi: 10.1016/j.pcl.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corr T.E., Schaefer E.W., Paul I.M. Growth during the first year in infants affected by neonatal abstinence syndrome. BMC Pediatr. 2018;18:343. doi: 10.1186/s12887-018-1327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sohlstrom A., Carlsson C., Uvnas-Moberg K. Effects of oxytocin treatment in early life on body weight and corticosterone in adult offspring from ad libitum-fed and food-restricted rats. Biol. Neonate. 2000;78:33–40. doi: 10.1159/000014244. [DOI] [PubMed] [Google Scholar]
- 11.Bowen M.T., Carson D.S., Spiro A., Arnold J.C., McGregor I.S. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carson D.S., Guastella A.J., Taylor E.R., McGregor I.S. A brief history of oxytocin and its role in modulating psychostimulant effects. J. Psychopharmacol. 2013;27:231–247. doi: 10.1177/0269881112473788. [DOI] [PubMed] [Google Scholar]
- 13.Parker K.J., Oztan O., Libove R.A., Sumiyoshi R.D., Jackson L.P., Karhson D.S., Summers J.E., Hinman K.E., Motonaga K.S., Phillips J.M., Carson D.S., Garner J.P., Hardan A.Y. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc. Natl. Acad. Sci. U. S. A. 2017;114:8119–8124. doi: 10.1073/pnas.1705521114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., Zhao S., Liu X., Zheng Y., Li L., Meng S. Oxytocin improves animal behaviors and ameliorates oxidative stress and inflammation in autistic mice. Biomed. Pharmacother. 2018;107:262–269. doi: 10.1016/j.biopha.2018.07.148. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez N., Vining M., Bloch-Salisbury E. Salivary cortisol levels as a biomarker for severity of withdrawal in opioid-exposed newborns. Pediatr. Res. 2020;87:1033–1038. doi: 10.1038/s41390-019-0601-7. [DOI] [PubMed] [Google Scholar]