Abstract
The growing interest concerning the role of metabolic sensors in various eating disorders requires the implementation of a strict methodology to collect, store and process blood samples in clinical studies. In particular, measurement of isoforms of the appetite-stimulating hormone, ghrelin, has been challenging in clinical settings. Indeed the acyl ghrelin (AG) isoform is rapidly degraded into desacyl ghrelin (DAG) by blood esterases, thus optimal conditions for the conservation of AG and accurate determination of AG/DAG ratio should be used. Here, we compared different protease inhibitors (Aprotinin, PHMB, AEBSF) during blood collection, increasing delays (0–180 min) before centrifugation, plasma supplementation with various HCl concentrations, storage durations of frozen plasma (8 and 447 days) and immunoenzyme-assay procedures (one-step versus sequential) in healthy subjects. Optimal conditions were obtained by collecting blood with aprotinin and supplementation of plasma with 0.1 N HCl with subsequent freezing for at least 8 days and using one-step assay. Under such conditions, different patterns of secretion of ghrelin isoforms were characterized in patients with restrictive-type anorexia nervosa (AN-R) before and after nutritional recovery. We illustrate the pulsatile variations of ghrelin isoforms according to the time around a meal and hunger rates in 3 patients with AN-R. This study offers a comprehensive comparison of various conditions using selective and specific immunoassays for both ghrelin isoforms in order to optimize assay sensitivity and consistency among procedures. These assay conditions could therefore be widely used to elucidate precisely the role of ghrelin isoforms on eating behavior in physiological and pathological situations.
Keywords: Ghrelin, Immunoassay, Pulsatile secretion, Anorexia nervosa, Appetite
Highlights
-
•
Treatment conditions and assay procedures are critical for ghrelin isoforms level determination in plasma.
-
•
Specific treatment conditions allow long term stability of ghrelin isoforms.
-
•
HCl supplementation of plasma before freezing improves AG/DAG ratio on the short and long term.
-
•
Variations of ghrelin isoforms occur with meal timing and chronic nutritional status in patients with anorexia nervosa.
1. Introduction
Preproghrelin is a complex gastrointestinal prohormone which produces two isoforms, acylated (AG) and desacylated (DAG) ghrelin. AG is the endogenous agonist of the Growth Hormone Secretagogue Receptor (GHS-R). It displays pleiotropic effects, being a powerful growth hormone secretagogue and orexigenic peptide with an ultradian pattern of secretion that is increased in anticipation of meals in healthy humans and rodents [1,2]. AG also plays a key role in reinforcing and motivational aspects of food [3]. Conversion of AG to DAG in blood is dependent on esterase activities while the enzyme Ghrelin-O-Acyl-Transferase (GOAT) catalyzes ghrelin acylation [1,4,5]. High fasting plasma AG and DAG concentrations were reported in patients with restrictive-type anorexia nervosa (AN-R) [6], a psychiatric condition characterized by a compulsive self-restriction of food intake. Recent genome wide association studies also demonstrated genetic associations of metabolic traits with AN suggesting an uncovered role of metabolic sensors of undernutrition in the pathophysiology of AN [7]. Although most studies focused on deciphering AG physiological role, both AG and DAG regulate feeding, physical activity and energy metabolism but they have distinct and sometimes opposite actions [[8], [9], [10]]. Higher plasma DAG in AN-R has been recently correlated with reward dysfunctions but in sampling conditions that did not allow proper assessment of AG [11]. Therefore, deciphering the specific interaction of ghrelin isoforms is essential to help elucidating which metabo-psychiatric mechanisms contribute to AN-R. However, the rapid action of blood esterases impairs a correct estimation of the AG/DAG ratio [1,12]. The validation of reproducible methods to collect, process and store human blood samples to assess AG and DAG plasma variations in clinical studies is still pending. Furthermore, the limited utilization of sensitive and selective immunoassays for ghrelin isoforms has been until recently a limitation to the accurate determination of these ghrelin isoforms in clinical studies. Finally, most studies assess ghrelin after an overnight fast without taking into account its physiological pattern of secretion in the course of a meal. Thus, validated sampling and assay methods to assess ultradian variations of ghrelin isoforms in relationship with meal and appetite are critical for a better understanding of their relevance in pathological eating.
The aim of this study was (1) to compare different methods of collection, processing and storage of blood samples in healthy women and (2) apply these conditions to study the ultradian variations of ghrelin isoforms in relationship with the nutritional state, meal patterns and meal-associated insulin surge in AN-R patients, in conditions that are compatible within a clinical practice.
2. Material and methods
2.1. Subjects
Study 1. Evaluation of optimal conditions of sampling, processing and storage of human blood for validation of ghrelin immunoassays. Healthy female subjects (HS) were recruited in the Centre de Recherche Clinique (GHU Paris). Blood samples were withdrawn either after an overnight fast (9:00 h) or before lunchtime (12:00 h).
Study 2. Impact of nutritional status on longitudinal variations of AG and DAG in female patients with restrictive-type AN (AN-R). Patients attended a structured in-patient program in the Eating Disorders Unit of Clinique des Maladies Mentales et de l’Encéphale (CMME, GHU Paris). Blood samples were performed in undernourished conditions (acute phase of the disorder) and after complete weight recovery (100% reached target BMI, i.e. range between 18.5 and 25), either after an overnight fast thereby providing baseline morning concentrations (n = 13 patients) or around lunchtime (n = 3 patients). Motivation to eat and hunger were evaluated with a validated visual analogue scale. The study protocols were approved by Comité de Protection des Personnes Ile de France III (EUDRACT N°: 2008-A008 17–48; CPP N°Am5355-2-2592; CPP 19.07.26.54412) (See Supplementary file for details). Written informed consent was obtained from all participants before recruitment.
2.2. Conditions of sampling, processing and storage of blood samples
Blood samples were collected in tubes containing 15% EDTA and supplemented with different protease inhibitors: p-hydroxymercuribenzoic acid 1 mM (PHMB, a cystine protease inhibitor), Aprotinin 250 KIU (a selective serine protease inhibitor) or 4-(2-aminoethyl)benzene sulfonyl fluoride hydrochloride 0.2–2 mg/ml (AEBSF, an irreversible serine protease inhibitor). Blood samples were placed on ice after withdrawal then immediately centrifuged at 4 °C (1000 g during 15 min) unless otherwise stated. The impact of delayed centrifugation times (immediate, 15, 30, 60, 120 and 180 min after) on acylation preservation following blood withdrawal was also assessed. In addition, plasma samples were aliquoted and supplemented or not with HCl 0.1 N or 0.2 N immediately after collection, then stored at either −20 °C or −80 °C. Plasma were assayed within 3 months following collection, unless otherwise stated. Finally, short-term (8 days) versus long-term (447 days) freezing conditions were compared (See Supplementary file for details).
2.3. Hormone immunoassays
AG and DAG concentrations were assayed with selective two-sites sandwich enzyme-immunoassays (human AG and DAG Easy Sampling Elisa kits, Ref A05306 and Ref A05319, respectively, Bertin Bioreagent, Montigny-le-Bretonneaux, France), either in the condition of one-step (sample or standard incubated with anti-ghrelin AChE tracer) [9] or sequential (sample or standard incubated on the plate with a washing step before adding the anti-ghrelin AChE tracer) protocol. Insulin was assayed using a competitive enzyme-immunoassay (Ref A05322, Bertin Bioreagents, Montigny-le-Bretonneaux, France) (See Supplementary file for details).
2.4. Statistical analysis
Values are given as Mean ± SEM. Statistical analyses were performed using Student t-test or 1-way or 2-way ANOVAs followed by Tukey's post-hoc analysis using GraphPad Prism (GraphPad software). Data were tested for normality and p values < 0.05 were considered significant.
3. Results
3.1. Effect of blood treatment and processing, and plasma acidification and storage conditions on ghrelin stability
3.1.1. Protease inhibitor and HCl supplementation
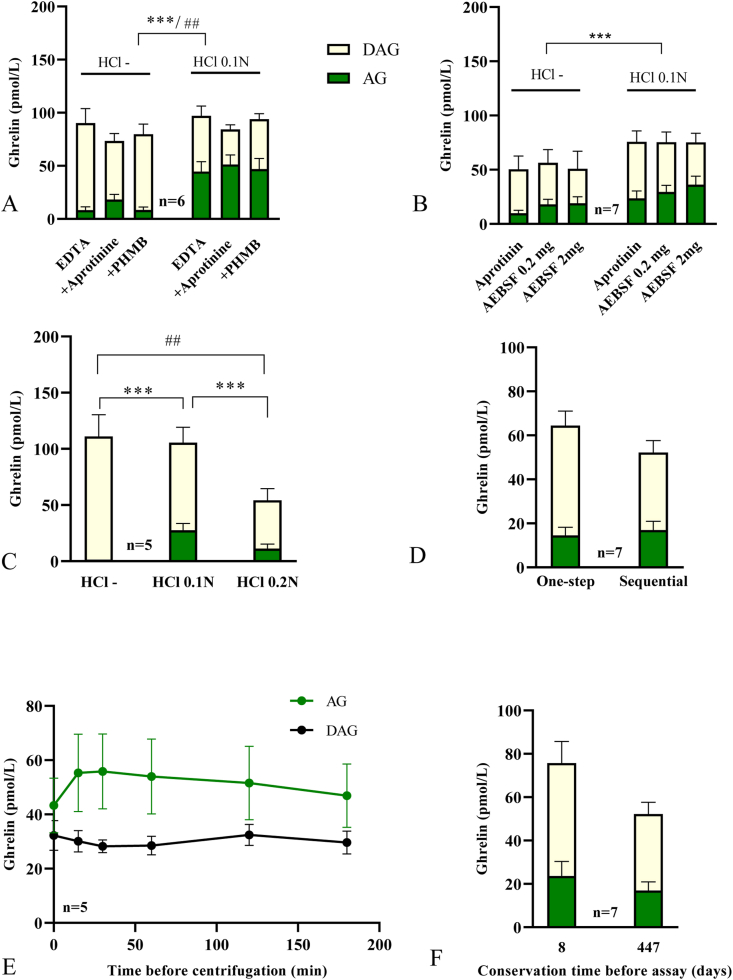
We first evaluated the stability of ghrelin under various conditions comparing the concentration of AG, DAG and the AG/DAG ratio (Fig. 1) (See also Supplementary file, section 1.4, for the description of the protocol used for each experimental data). Treatment of plasma samples with HCl 0.1 N increased AG over DAG concentrations (ANOVA effect: AG: p < 0.001; DAG: p = 0.0013) and AG/DAG ratio (p < 0.0001) regardless of the inhibitors (no HCl x Protease inhibitor interaction) using either one-step assay (Fig. 1A) or sequential assay (Fig. 1B and Supplementary Table 1). Under HCl 0.1N-treated conditions, regardless of the type of assay (one-step vs sequential), results showed no significant effect of protease inhibitors on AG concentrations (Fig. 1A, p = 0.46; Fig. 1B, p = 0.12) but increased AG/DAG ratio (p = 0.0002) due to lower DAG concentrations (p = 0.0013). Post-hoc analysis revealed a significant increase of AG/DAG ratio in aprotinin conditions compared to no inhibitor or PHMB (p < 0.05) (Supplementary Table 1).
Fig. 1.
Treatment of blood samples with various protease inibitors and plasma acidification before storage at -80°C increasesghrelin stability. (A) Adjunction of HCl and use of protease inhibitors (Aprotinin or PHMB). (B) Sequential assay comparing different protease inhibitors adjunction to acidified or non acidified plasma. (C) Comparison of 0.1 and 0.2 N HCl concentrations of plasma samples in blood collected with aprotinin. (D) Comparison of one step versus sequential assay on samples kept at −80 °C for 18 months. (E) Effect of time delays before centrifugation after blood collection with samples kept on ice. (F) Impact of storage at −20°C°C for 8 days or at −80°C for 18 months of samples collected with HCl and aprotinin. Blood was collected with aprotinin (C–F) and plasma was treated with 0.1 N HCl (D-F). Data were obtained during one-step assay (A, C and E) or sequential assay (B and F). Asterisks indicate significant differences for AG levels (***p < 0.0001), hashtag indicate significant differences for DAG (##: p < 0.001). AG and DAG plasma concentrations are represented in pmol/L. AG: acyl-ghrelin; DAG: desacyl-ghrelin.
3.1.2. HCl concentrations
Furthermore, higher HCl concentrations (0.2 N) did not increase AG or DAG concentrations compared to HCl 0.1 N. Indeed, HCl 0.2 N significantly reduced AG compared to HCl 0.1 N (p < 0.05) and DAG concentrations compared to non-HCl treated conditions (p < 0.05), as well as the AG/DAG ratio (p < 0.05) (Fig. 1C and Supplementary Table 1).
3.1.3. One-step versus sequential assay
Using aprotinin-treated conditions, AG and DAG were similar and AG/DAG ratio was slightly higher in the sequential compared to the one-step assay (p = 0.04) (Fig. 1D and Supplementary Table 1).
3.1.4. Delay of centrifugation
Delayed centrifugation of samples had no significant effect on DAG concentrations nor AG/DAG ratio over time (Supplementary Table 1). For AG, although repeated analyses showed an overall significant effect of time on AG concentrations only (p = 0.028), comparison between each time point was not significant (Fig. 1E).
3.1.5. Duration of freezing
Samples treated with aprotinin and HCl 0.1 N were assayed immediately, after 8 days at −20 °C or after 18 months at −80 °C. Immediate assay did not increase AG, DAG nor AG/DAG levels compared to frozen samples (not shown), Thus, −80 °C seemed to be an appropriate sample conservation procedure. Moreover, long-term conservation at −80 °C did not significantly reduce AG nor DAG level (8 days versus 18 months conservation AG: p = 0.11, DAG: p = 0.14, AG/DAG: p = 0.76) (Fig. 1F). With AEBSF, however, a reduction in AG/DAG ratio was observed over time (not shown).
3.2. Plasma AG and DAG variations with nutritional state in anorexia nervosa and ultradian rhythmic variations around a meal in three patients
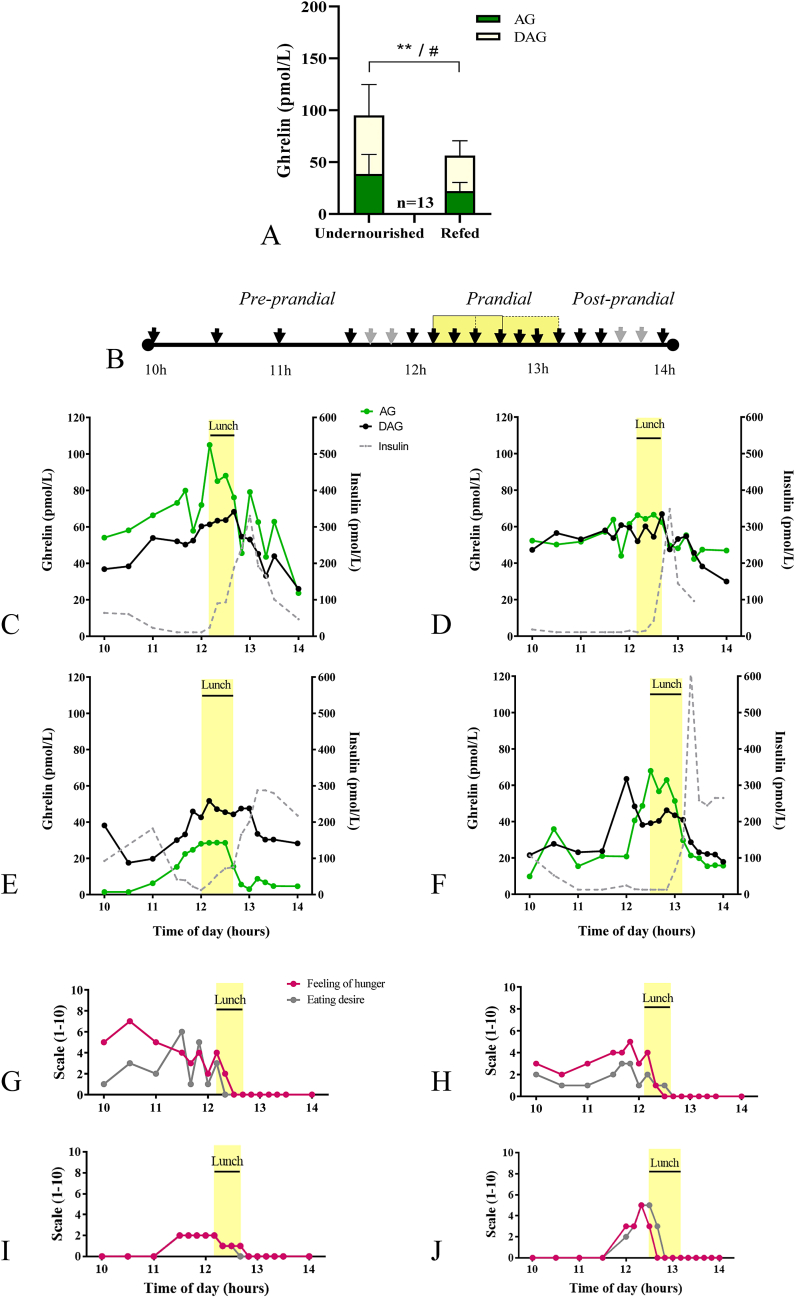
We next measured AG and DAG plasma concentrations and AG/DAG ratio in 13 patients with AN-R under different nutritional status (undernourished or refed) and during the course of a meal in 3 different patients (Fig. 2). Blood was collected on EDTA coated tubes supplemented with aprotinin, centrifuged within 15 min, plasma were acidified with HCl 0.1 N and stored at −80 °C prior to performing immunoassays in conditions of one-step. In the undernourished state (IMC = 15.0 ± 0.3), patients showed significantly greater levels of AG (p = 0.0039) and DAG (p = 0.0219) compared to refed state (IMC = 20.1 ± 0.1) (Fig. 2A). Furthermore, decreased AG and DAG concentrations coincided with the occurrence of a post-prandial insulin peak and was associated with a reduction in hunger and motivation to eat in the course of a meal in three patients with AN-R (Fig. 2B–F).
Fig. 2.
AG and DAG plasma concentrations in AN-R in the undernourished and refed states after an overnight fast or during the course of a meal. (A) Baseline morning plasma concentration of both ghrelin isoforms in a sample of 13 AN-R patients in the undernourished state and after refeeding. (B) Experimental paradigm of multiple blood sampling during the course of a meal to evaluate ultradian variations of ghrelin isoforms; each arrow indicates a blood sample. (C–J) Ultradian variations of AG, DAG and insulin plasma concentrations in relation with appetite and food motivation in 3 patients with AN-R. (C–F) Plasma concentrations of AG, DAG and insulin around lunchtime in undernourished conditions (IMC = 16,1 kg/m2, C or IMC = 14.5 kg/m2, E), immediately after refeeding (100% target BMI, IMC = 20,5 kg/m2, D, patient matched to panel C) or 6-months following refeeding (IMC = 19.0 kg/m2, F). (G-J) Feeling of hunger and eating desire evaluated on a 1–10 scale in undernourished conditions (G, I), immediately after refeeding (H) or 6-months following refeeding (J). Lunchtime lasted 30-40 min (yellow shade). Asterisks indicate significant differences for AG (**p < 0.001). Hashtag indicates significant differences for DAG (#p < 0.05). AG, DAG and insulin plasma concentrations are represented in pmol/L. AG: acyl-ghrelin;DAG: desacyl-ghrelin; undernourished condition (admission)vs refed(100% target BMI).
4. Discussion
Ghrelin is a hormone highly sensitive to nutritional status but the exploration of its ultradian variation is limited by non-standardized assay techniques. To our knowledge, only a few studies compared ghrelin assay procedures in humans providing partial results, mainly focusing on AG in non-clinical cohorts. For example, Trivedi et al. [13] tested various protease inhibitors but not acidification of samples; Blatnik et al. [12] used assay methods insensitive to low DAG levels and Delhanty et al. [14] compared various conservation techniques using only AEBSF as a protease inhibitor. The present study provides a methodology for blood sampling and processing, storage conditions and immunoassay procedures compatible with routine clinical practices combining high feasibility and high relevance to assay both ghrelin isoforms. We advise collecting blood on a tube containing EDTA and keep samples on ice to limit AG degradation [1]. We recommend centrifugation of blood samples as soon as possible but ghrelin is stable up to 180 min when blood is conserved on ice, facilitating routing to a technical platform. Acidification of centrifuged plasma before storage increases AG recovery and lowers ghrelin degradation without affecting total ghrelin concentrations, regardless of the protease inhibitor used. Acidification and protease inhibition (amongst other sample processing) was previously described in the RAPID (“Reduced temperatures, Acidification, Protease inhibition, Isotopic exogenous controls, and Dilution”) method. It increased AG's recovery over total ghrelin compared to standard procedures in rodents (i.e. EDTA-blood on ice) [15]. In the present study, the AG/DAG ratio was increased by 5-fold when blood was treated with EDTA + aprotinin and by 17-fold when plasma was supplemented with HCl compared to EDTA conditions only.
Treatment with HCl inhibits the activity of butyrylcholinesterase, a major esterase known as a key factor of ghrelin degradation [1]. Adjunction of a protease inhibitor (PI) is also preferable and not all PI are equivalent. AEBSF integrates a cholinesterase inhibitor and interacts with the tracer AChE, thus requires sequential assay (i.e. incubation with sample or standard alone followed by incubation with anti-ghrelin AChE tracer). This heavier procedure did not show significantly different results between AEBSF and aprotinin neither with a one-step assay using aprotinin. Moreover, aprotinin slightly increased AG/DAG ratio compared to other inhibitors, both after short-term or long-term storage, enabling us to get rather high AG/DAG ratio around 1.5 in the one-step assay. These results are consistent with previous studies, suggesting that aprotinin is the optimal protease inhibitor to our point of view [14].
In such conditions, samples can be kept for various months at −80 °C with a limited impact, which is convenient in case of longitudinal follow up studies as, for example, explorations in AN-R patients at multiple time-points over the course of renutrition. The sandwich assay used in the present study presents two advantages for clinical studies. Firstly, it has been validated using HPLC and mass spectrometry and demonstrates high specificity for AG and DAG [16] and secondly, its sensitivity allows to use minimal volumes of plasma within serial sampling protocols, particularly necessary in undernourished patients. Possible limitations of the current study are that it does not include systematically the same individuals in all different experimental comparisons, likely increasing the variability in AG/DAG ratio. Indeed, the AG/DAG ratio varies considerably (from 0.02 to 0.5 in non HCl-treated conditions and from 0.5 to almost 3 in HCl-treated conditions). Within a set of samples that are collected, processed and assayed exactly in the same conditions (See Supplementary Fig. 1), the most probable explanation is that this reflects biological variability in individual subjects.
An usual limitation to the study of ghrelin role in pathophysiological conditions is its ultradian mode of secretion [1,17]. We here report for the first time that AG and DAG follow a rhythmic pattern of secretion in anticipation of meals in a case study of three patients with AN-R, similar to previous results on healthy subjects [1]. Furthermore, the variations of AG and DAG can be precisely assessed within a longitudinal perspective and around a meal. Such validated sampling and assay conditions enable the detection of ghrelin variations around mealtime in AN-R patient under undernourished and refed conditions, crucial for clinical research devoted to eating disorders. Although we only provide here a proof of concept that ghrelin rhythmic variations can be detected in 3 different AN patients, using our methodology, evaluation in a sufficient number of patients will be necessary to confirm this pattern and for comparative analysis between undernourished and refed conditions. To conclude, technical improvements in ghrelin assays may open new windows for routine applications in clinical research. The significance of ghrelin variations in correlation with meal patterns and behavioral responses need to be further assessed to elucidate the complex interaction between metabolic sensing and clinical phenotypes of patients with AN.
Author contributions
Tezenas du Montcel C: Investigation, Visualization, Formal analysis, Writing -original draft & editing. Duriez P: Conceptualization, Investigation. Lebrun N: Data curation, Validation, Formal analysis. Grouselle D: Investigation, Methodology, Data curation. Degrimaudet B: Validation, Formal analysis. Dardennes R: Resources, Investigation.. Epelbaum J: Conceptualization, Writing – Review & Editing. Cuenca M: Resources, Data curation. Viltart O: Writing – Review & Editing. Gorwood P: Resources, Funding Acquisition, Supervision. Tolle V: Conceptualization, Project administration, Methodology, Supervision, Writing – Review & Editing.
Funding
This work was supported by Université Paris Cité and Institut National de la Santé et de la Recherche Médicale (INSERM) and by Fondation de France – Maladies Psychiatriques (to P.G.). C.T. is the recipient of a PhD fellowship (N°FDM202006011161) funded by « Fondation pour la Recherche Médicale » (FRM).
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Benoît de Grimaudet is working at Bertin Biotechnologies.
Acknowledgments
We warmly acknowledge Dr Alexandra Pham-Scottez, Cécile Bergot, Emilie Grasset and Etienne Kimmel (Clinique des Maladies Mentales et de l’Encéphale, GHU Paris Psychiatrie et Neurosciences, Hôpital Sainte-Anne, Paris, France) for the care of the patients and their contribution to the clinical study. We would like to thank the “Centre de Recherche Clinique” and “Centre de Ressources biologiques” for their help in conducting this study. We are also grateful to Amel Aloulou (Centre de Recherche Clinique et Centre de Ressources biologiques, GHU Paris Psychiatrie et Neurosciences, Hôpital Sainte-Anne, Paris, France) for technical support. We warmly acknowledge Patrick Brune, Patrick Vitaux and Carole Travers (Bertin Technologies, Montigny-le-Bretonneux) for methodological inputs on ghrelin assays.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpnec.2022.100140.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Liu J., Prudom C.E., Nass R., Pezzoli S.S., Oliveri M.C., Johnson M.L., Veldhuis P., Gordon D.A., Howard A.D., Witcher D.R., Geysen H.M., Gaylinn B.D., Thorner M.O. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J. Clin. Endocrinol. Metab. 2008;93:1980–1987. doi: 10.1210/jc.2007-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zizzari P., Hassouna R., Grouselle D., Epelbaum J., Tolle V. Physiological roles of preproghrelin-derived peptides in GH secretion and feeding. Peptides. 2011;32:2274–2282. doi: 10.1016/j.peptides.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Perelló M., Zigman J.M. The role of ghrelin in reward-based eating. Biol. Psychiatr. 2012;72:347–353. doi: 10.1016/j.biopsych.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutierrez J.A., Solenberg P.J., Perkins D.R., Willency J.A., Knierman M.D., Jin Z., Witcher D.R., Luo S., Onyia J.E., Hale J.E. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl. Acad. Sci. Unit. States Am. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satou M., Nishi Y., Yoh J., Hattori Y., Sugimoto H. Identification and characterization of acyl-protein thioesterase 1/lysophospholipase I as a ghrelin deacylation/lysophospholipid hydrolyzing enzyme in fetal bovine serum and conditioned medium. Endocrinology. 2010;151:4765–4775. doi: 10.1210/en.2010-0412. [DOI] [PubMed] [Google Scholar]
- 6.Koyama K.-I., Yasuhara D., Nakahara T., Harada T., Uehara M., Ushikai M., Asakawa A., Inui A. Changes in acyl ghrelin, des-acyl ghrelin, and ratio of acyl ghrelin to total ghrelin with short-term refeeding in female inpatients with restricting-type anorexia nervosa. Horm. Metab. Res. Horm. Stoffwechselforschung Horm. Metab. 2010;42:595–598. doi: 10.1055/s-0030-1252017. [DOI] [PubMed] [Google Scholar]
- 7.Duncan L., Yilmaz Z., Gaspar H., Walters R., Goldstein J., Anttila V., Bulik-Sullivan B., Ripke S., Eating Disorders Working Group of the Psychiatric Genomics Consortium. Thornton L., Hinney A., Daly M., Sullivan P.F., Zeggini E., Breen G., Bulik C.M. Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am. J. Psychiatr. 2017 doi: 10.1176/appi.ajp.2017.16121402. appiajp.201716121402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delhanty P.J.D., Neggers S.J., van der Lely A.J. Should we consider des-acyl ghrelin as a separate hormone and if so, what does it do? Front. Horm. Res. 2014;42:163–174. doi: 10.1159/000358345. [DOI] [PubMed] [Google Scholar]
- 9.Duriez P., Robichon L., Dardennes R., Lavoisy G., Grouselle D., Epelbaum J., Ramoz N., Gorwood P., Tolle V., Viltart O. Unexpected association of desacyl-ghrelin with physical activity and chronic food restriction: a translational study on anorexia nervosa. J. Clin. Med. 2020;9 doi: 10.3390/jcm9092782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez G., Cabral A., Cornejo M.P., De Francesco P.N., Garcia-Romero G., Reynaldo M., Perello M. Des-acyl ghrelin directly targets the arcuate nucleus in a ghrelin-receptor independent manner and impairs the orexigenic effect of ghrelin. J. Neuroendocrinol. 2016;28:12349. doi: 10.1111/jne.12349. [DOI] [PubMed] [Google Scholar]
- 11.Bernardoni F., Bernhardt N., Pooseh S., King J.A., Geisler D., Ritschel F., Boehm I., Seidel M., Roessner V., Smolka M.N., Ehrlich S. Metabolic state and value-based decision-making in acute and recovered female patients with anorexia nervosa. J. Psychiatr. Neurosci. 2020;45(4):253–261. doi: 10.1503/jpn.190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blatnik M., Soderstrom C.I., Dysinger M., Fraser S.A. Prandial ghrelin attenuation provides evidence that des-acyl ghrelin may be an artifact of sample handling in human plasma. Bioanalysis. 2012;4:2447–2455. doi: 10.4155/bio.12.248. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi A., Babic S., Chanoine J.-P. Pitfalls in the determination of human acylated ghrelin plasma concentrations using a double antibody enzyme immunometric assay. Clin. Biochem. 2012;45:178–180. doi: 10.1016/j.clinbiochem.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Delhanty P.J.D., Huisman M., Julien M., Mouchain K., Brune P., Themmen A.P.N., Abribat T., van der Lely A.J. The acylated (AG) to unacylated (UAG) ghrelin ratio in esterase inhibitor-treated blood is higher than previously described. Clin. Endocrinol. 2015;82:142–146. doi: 10.1111/cen.12489. [DOI] [PubMed] [Google Scholar]
- 15.Stengel A., Keire D., Goebel M., Evilevitch L., Wiggins B., Taché Y., Reeve J.R. The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology. 2009;150:5113–5118. doi: 10.1210/en.2009-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassouna R., Grouselle D., Chiappetta G., Lipecka J., Fiquet O., Tomasetto C., Vinh J., Epelbaum J., Tolle V. Combination of selective immunoassays and mass spectrometry to characterize preproghrelin-derived peptides in mouse tissues. Front. Neurosci. 2017;11:211. doi: 10.3389/fnins.2017.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams D.L., Cummings D.E. Regulation of ghrelin in physiologic and pathophysiologic states. J. Nutr. 2005;135:1320–1325. doi: 10.1093/jn/135.5.1320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.