Abstract
Pregnancy and childbirth are among the most dramatic physiological and emotional transformations of a lifetime. Despite their central importance to human survival, many gaps remain in our understanding of the temporal progression of and mechanisms underlying the transition to new parenthood. The goal of this paper is to outline the physiological and emotional development of the maternal-infant dyad from late pregnancy to the postpartum period, and to provide a framework to investigate this development using non-invasive timeseries. We focus on the interaction among neuroendocrine, emotional, and autonomic outputs in the context of late pregnancy, parturition, and post-partum. We then propose that coupled dynamics in these outputs can be leveraged to map both physiologic and pathologic pregnancy, parturition, and parenthood. This approach could address gaps in our knowledge and enable early detection or prediction of problems, with both personalized depth and broad population scale.
Keywords: Dyad, Mood, Biological rhythm, Mother, Child, Reproduction
Highlights
-
•
Giving birth and caring for offspring are dynamic processes that can instill both love and fear.
-
•
Maternal physiology continuously integrates fetal, social, and environmental cues.
-
•
The result is coupled change in hormonal, autonomic nervous, and emotional output.
-
•
Coupling may allow internal state to be assessed from peripheral autonomic markers.
-
•
Such markers may identify healthy or pathologic pregnancy, parturition, and parenting, and enable creation of real-world tools.
1. Introduction & hypothesis
Few naturally occurring phenomena instill equal measures of fear and love as the experience of human childbirth. Throughout history, the documented dangers associated with pregnancy and birth stand alongside the narrative of the beauty, mystique, and reverence for giving birth as an act of love [1,2]. The idea that birth is a loving act likely stems from the notion that to do so risks one's own health and possibly life, and therefore could be seen as the ultimate sacrifice. Yet physical (e.g., endocrine and autonomic) processes enable parturition, as well as adaptation to caring for a newborn. How physiological and emotional states are linked in the context of reproduction and if such links can be measured through peripheral “surrogate markers” is a largely unanswered question. In this paper, we review how precisely coordinated fluctuations of steroid hormones, reproductive peptides, and autonomic nervous system (ANS) outputs within the maternal body integrate signals from the placenta, fetus (newborn), and environment. We propose that these coupled, that is, co-regulated, dynamics contribute to emotional experience and, potentially, peripheral biomarkers of these formative transitions [3,4].
Pregnancy, parturition, and parenting are enabled by hormonal and neural adaptations that influence behavior, permitting otherwise selfish humans to make themselves vulnerable. Human pregnancy has long been viewed from the perspective of life history evolution, with the mother and fetus in a tug-of-war for resources, driven by the extensive or invasive nature of hemochorial placentation and the demands of human brain development [[5], [6], [7]]. This conflict does not always culminate with the survival of parent and child, thereby infusing fear and uncertainty to the process of reproduction [8]. However, the process of pregnancy and birth may be viewed as cooperative rather than competitive or sacrificial; it is a well-timed physiologic dance between partners that usually results in a healthy newborn and a tired but accomplished mother ready to face the primal challenges of lactating and child care [9]. Parenting requires selflessness, creates vulnerabilities for the family, and requires a large investment of resources. Together, for humans to reproduce, fears of pregnancy, birth, and the work of parenting must be overcome by stronger forces: those of bonding, social motivation, and love [1,10,11].
In this essay, we will highlight how the mother adapts to each of these phases of reproduction, and how these adaptations manifest across neuroendocrine, emotional, and autonomic networks. In section 1, we review the basis of interaction among these three systems. We will then discuss three parts of the transition to parenthood: section 2 on pregnancy, section 3 on childbirth, and section 4 on early postpartum. Within each part of this transition, we will briefly review a) physiological adaptations, b) emotional changes (with an emphasis on the balance of states of love and fear), and c) examples of how autonomic timeseries can reflect these processes and, potentially, their pathology. These arguments rely on the existence of consistent temporal relationships among metrics that are difficult to measure (e.g., emotional and neuroendocrine timeseries) and autonomic metrics that are easier to measure (e.g., autonomic influence on skin temperature, heart rate (HR), and heart rate variability (HRV)) [12,13]. We propose that such timeseries, measurable by wearable devices, may reflect the network reorganization that occurs during the transition to parenthood, and therefore offer non-invasive insights into the associated emotional and hormonal states. Due to the complexity of these processes and the number of systems discussed, this essay is intended to introduce a general framework for the study of becoming a parent, as opposed to providing a comprehensive review. Throughout, the reader will be referred to relevant reviews of each system and reproductive stage.
Additionally, we hypothesize that poor outcomes may be predicted or detected in HR, HRV, or temperature timeseries. Network disruption caused by abnormalities in pregnancy and postpartum physiology might allow us to better understand antecedents of preterm birth, preeclampsia, postpartum depression, or understand critical temporal and social influences (e.g., stress). If future studies support this hypothesis, then monitoring peripheral signals would create a unique opportunity to examine real-time neurobiological manifestations in humans. This knowledge could be integrated into personalized therapies for psychological or physiological conditions and inform the basic science of reproduction. Furthermore, the same methods could apply to studying the postnatal development of a newborn/mother dyad with important implications for early life.
2. Emotional, neuroendocrine, and autonomic co-regulation in women
2.1. Synchrony in emotional, neuroendocrine, and autonomic regulation
Reproduction and the ANS are closely coordinated and have measurable associations with emotional state. Outputs of these systems, including reproductive steroid and peptide hormones, corticosteroids, body temperature, and cardiovascular output exhibit coupled oscillations within and across days (Table 1) [12,13]. Although the mechanisms underlying these interactions are incompletely understood, analysis of their synchronized, rhythmic change over time (i.e., biological rhythms) has enabled the development of tools for monitoring and making predictions about emotional and female reproductive state. At present, these include anticipating episodes of depression and mania [14,15], detecting stress [16], improving fertility awareness [[17], [18], [19]], optimizing substance use disorder treatment [20], and potentially providing early indications of pregnancy [21,22].
Table 1.
Known Acute Hormonal Associations Among Body Temperature, HR, HRV, and Emotional State. Representative citations (reviews, where available, or primary literature) are included for each relationship in the context of female reproduction.
| Hormone | Temperature | HR | HRV | Emotional State |
|---|---|---|---|---|
| Estrogens | lower [27] | lower [17] | elevate [28] | mixed effects [29] |
| Oxytocin | lower [30] | lower [31] | elevate [31] | love, bonding, anxiolytic [32,33] |
| Progesterone | elevate [34] | elevate [35] | lower [35] | fatigue [36] |
| Prolactin/hCS | elevate [37] | elevate [38] | lower [38,39] | love, bonding [40,41] |
| Cortisol/pCRH | lower [42] | elevate [16,43] | lower [44] | contextual arousal, fear [45] |
| hCS, human chorionic somatomammotropin; pCRH, placental corticotrophin releasing hormone | ||||
To date, physiological synchrony (e.g., temporal coupling among hormonal, emotional and autonomic systems) has been studied largely in non-pregnant contexts. However, synchrony across systems appears to be at least partially maintained during pregnancy and postpartum. This is remarkable, as gestation and parturition are associated with marked changes in emotional regulation, hypothalamic-pituitary-gonadal feedback, the highest lifetime concentrations of sex and corticosteroids, and a rapid decline to a hypogonadal state after parturition [23,24]. If coupling across systems is sufficiently maintained during and after pregnancy, then continuous monitoring of peripheral outputs using a growing number of available wearable devices/monitors may reflect, and even be used to anticipate, the continuous dynamics of more difficult-to-assess hormonal or emotional states [25,26] with implications for understanding potential events (e.g., birth) or problems (e.g., depression, preterm birth).
2.2. Substrates for interactions between the reproductive and autonomic systems
Research on interactions between the ANS and the reproductive system has focused largely on the impact of sex steroids and peptides on skin and core temperature, HR, and HRV. Briefly, estradiol reduces core and skin temperature [27,46,47], promotes vasodilation, reduces HR [17,[48], [49], [50]] and increases parasympathetic tone (cholinergic activity)/HRV [17], (reviewed in: [51,52]). In cycling women, progesterone combined with estradiol raises core and skin body temperature and increases sympathetic tone and HR (adrenergic activity) [[51], [52], [53]], and lowers HRV [35,54,55]. These dynamics are more complicated in cases of exogenous hormone delivery, via hormonal contraceptive or hormone replacement therapy, or ovarian hyper stimulation (see: [28,[56], [57], [58]]). During most of pregnancy, progesterone dominance triggers nitric oxide production that, combined with elevated estriol (the dominant form of estrogen from placenta), leads to relaxation of maternal smooth muscle of the vasculature, venous distensibility and decreased systemic vascular resistance [59,60]. These adaptations likely reinforce the impact of high estrogen and progesterone to trigger higher cardiac output, HR, and reduced HRV [61].
Peptide hormones including prolactin and oxytocin also influence thermoregulation and autonomic tone. Oxytocin dose-dependently reduces body temperature and HR [30], and increases HRV [62]. Although less studied, prolactin appears to be relatively elevated during higher temperature states, including metabolic elevation to support milk production [63], exercise [37], and the luteal phase [64]. Moreover, prolactin opposes dopamine's temperature-lowering effects [65,66]. Accordingly, in rodents, the spike in prolactin (along with estradiol and progesterone) on estrous days is associated with a plateau of high core body temperature [[67], [68], [69]]. This phenomenon has also been observed in women following ovulation, along with increased HR and decreased HRV across the luteal phase [17,28,70]. Furthermore, elevation of basal body temperature during pregnancy, and its relationship to progesterone, has been recognized for about a century [[71], [72], [73]]. These changes are thought to be centrally mediated, likely via direct feedback of reproductive hormones and peptides on hypothalamic arcuate tuberoinfundibular dopaminergic, kisspeptin, neurokinin B, and dynorphin (KNDy) neurons that regulate pulsatile release of gonadotropin releasing hormone; via direct feedback medial preoptic populations regulating body temperature, HR, and vascular tone; and through direct synaptic coupling of the arcuate and medial preoptic area [27,46]. How these general principles of coordination among hormones and autonomic outputs are modified during pregnancy, parturition, and the early postpartum period are discussed in individual sections, below.
2.3. Reproductive and autonomic impacts on emotional regulation in women
Reproductive hormones and the ANS exhibit complex but predictable interactions with emotional state. Although absolute hormone levels do not exhibit consistent relationships with emotional regulation, direction of hormonal change reveals more consistent associations [74]. Broadly, rapid declines in estrogen and progesterone that occur in the late luteal phase of the ovulatory cycle [75], in cyclic hormone replacement therapy [76,77], and that follow birth [78], are associated with more variable emotional state and elevated amygdalar activity, including depressive/anxious symptoms, less suppression of stress, and potentially greater fear responses [[79], [80], [81]]).
Conversely, states of relatively elevated or rising estradiol (e.g., early-to mid-follicular phase) [82] or estradiol and progesterone (e.g., early to mid-luteal phase) may be associated with relatively elevated mood, and greater suppression of negative emotions [83]. These complex effects may be mediated through changes (largely increases) in serotonin (5-HT) receptor expression [82,84]. Perhaps the context in which the relationship among hormones, peripheral physiology, and mood has been most been studied is that of oxytocin and vasopressin in dyadic synchrony and bond formation (reviewed in: [85]), discussed later in Section 3. Briefly, oxytocin interacts with estrogen to facilitate bonding and onset of maternal behavior [86], increase parasympathetic activity [87,88] and coordinate “adaptive fear and stress responses” [[89], [90], [91]] (e.g., aggression toward intruders [92]). In contrast, maternal anxiety/hypervigilance (fear) may be linked to either variation in oxytocin function and/or in response to patterns of steroid hormone secretion [91,93,94]. Much remains to be learned about the relationship among reproductive hormones and emotional states, including the impacts of untimed, a static dose versus endogenous, naturally contextualized hormone fluctuations [82]. Further, more work has been devoted to negative affect and stress reactivity as opposed to resilience and love, providing broad opportunities for future research.
3. Physiological and emotional coordination in pregnancy: the power of the placenta
Interactions among neuroendocrine, emotional, and autonomic fluctuations have been largely studied in cycling humans and in experimental animal models. Pregnancy presents at least two important challenges in comparison to ovulatory cycles. First, the ovulatory cycles of the non-pregnant female are recurrent, relatively short, and internally controlled by an individual's hypothalamic axes via negative and positive feedback. By contrast, pregnancy is long, recurs relatively rarely, and requires significant remodeling of the HPG axis (reviewed in: [23]). Second, the placenta functions as both the conductor of and conduit between the developing fetus' metabolic, neuroendocrine, and autonomic state; and the maternal state [95]. We briefly review placentation to provide context regarding how pregnancy and parturition are influenced directly by the neuroendocrine processes of the placenta, in addition to the responses of the maternal body.
3.1. Placentation: physiological adaptation and cooperation between mother and fetus
Establishing the placenta is the primary task of early pregnancy, assuming fertilization and implantation occurred normally. Placentation is a complex and carefully regulated process which is influenced by paternally contributed genes and genetic imprints [96,97]. However, in principle, both maternal immune-tolerant factors within the decidua (transformed endometrium during the luteal phase) and the antigenic factors of the trophoblastic tissue (early placental tissue) cooperate to maintain ovarian progesterone production [98]. Luteal phase ovarian progesterone promotes uterine ‘quiescence’ or a lack of contractions. This allows the developing trophoblast tissue to produce β-hCG (human chorionic gonadotropin), which will further sustain production of maternal ovarian progesterone until the placenta assumes this task [[99], [100], [101]]. An overabundance of localized immune factors or thrombotic activity on the maternal side, or abnormal antigen expression by the trophoblast would lead to a failure of successful placentation and thus, pregnancy loss [102,103]. Mature placentation requires significant remodeling of maternal spiral arteries (removal of vasoconstrictive endothelial smooth muscle) located deep within the uterine lining and myometrium to support fetal development to term. The mother's uterus therefore must be tolerant of this process immunologically [104,105]. This conceptualization of placentation as an example of synchrony between maternal and embryonic features counters the dominant view that the placenta is dangerously invasive with tumor-like properties.
As the pregnancy progresses, the placenta primarily serves to maintain the pregnant state, producing high quantities of human chorionic somatomammotropin (HCS) (also known as human placental lactogen) and progesterone derived from maternal cholesterol [95,106]. This dominance of progesterone leads to significant reductions in reduced vascular resistance and proximal renal tubular reabsorption, permitting increased blood volume and cardiac output [61,107,108]. These critical adaptations necessarily occur very early in gestation and, if inadequate or absent, are thought to contribute to the early pathogenesis of inadequate fetal growth and some forms of preeclampsia [61] if the embryo survives. Later in pregnancy, the metabolic needs of the fetus are met as HCS prompts adaptation of the typical maternal response to insulin, causing relative insulin insensitivity leading to less maternal cellular uptake of nutrients and more nutrient availability for rapid fetal growth in the third trimester [106]. Progesterone dominance also leads to a shift toward Th2 (T Helper) cytokine profiles and responses, leading to a relatively anti-inflammatory state [109,110] and supporting pregnancy to full-term by suppressing contractile gene expression in the uterus. To initiate labor however, this progesterone dominance needs to be tempered, which is through the action of fetal maturation and rising placental estriol production (reviewed in section 3.1 below).
3.2. Emotional regulation in pregnancy
Emotions associated with pregnancy are influenced by many interconnected factors (reviewed in: [111]). Individual life circumstances (e.g., unwanted pregnancy, unwanted single parenthood, social support, stress, presence of any pregnancy complications, and mental health history) as well as placenta-driven physiologic changes will influence pregnant individuals' emotional states and their vulnerability to mood disorders. Overall, depressive symptomatology across pregnancy has been shown to be stable or to generally improve [112]. This improvement may be attributed to a woman's anticipation of the newborn's arrival (particularly when pregnancy is a positive life circumstance) and/or the increased estriol signaling as the pregnancy advances [113,114]. Despite this possible improvement in depression across pregnancy, several studies still demonstrate that around 20% or pregnant women do report depression and/or higher anxiety [112,115]. Importantly, poor mental health or emotional regulation during pregnancy is associated with altered immune pathways [116,117], promoting higher inflammation which can trigger spontaneous preterm birth [118]. The association of emotional regulation and mood disorder development with the early onset of labor exemplifies the connection between emotional state, endocrine changes, and possibly adverse birth outcomes.
3.3. Autonomic correlates of neuroendocrine and emotional adaptation in pregnancy
Wearable timeseries may aid the investigation of the full range of emotional and hormonal states in pregnancy. The value of timeseries is not only for observing the relationship between internal state and levels of autonomic output. Timeseries are particularly valuable in revealing synchronized rhythmic patterning [119], direction of change, and rate of change of hormones that uniquely characterize each stage of reproductive life and associated emotional experiences [120,121]. By the third trimester, for example, sex steroids [101], cortisol [122], prolactin, and HCS reach historically high levels [95]. The hormone changes result in altered autonomic output [123] and are also associated with unique dynamics of emotional state [124]. Varied mechanisms enable these global hormonal elevations, including placental production of hypothalamic releasing hormones (e.g., placental corticotrophin releasing hormone (CRH)). Placental CRH overrides typical negative feedback [125] and increases burst frequency of arcuate and PVN populations regulating these hormones in the mother/fetus. We propose that the profound immunologic shifts [126] and changes in sex steroid production occurring in pregnancy (e.g., progesterone dominance) [127] should result in changes in peripheral outputs that reflect these adaptations (see: Table 2).
Table 2.
Hypothesized impacts of pregnancy adaptations and complications on autonomic metrics.
| Physiologic or Pathologic Change in Pregnancy | Temperature | HR | HRV | Emotional State |
|---|---|---|---|---|
| 1st Trimester rising progesterone | elevate [34] | elevate [35] | lower [35] | fatigue [36] |
| Suboptimal progesterone production prior to miscarriage [21] | relatively lower [137] | relatively lower, hypothesized [138] | relatively elevated, hypothesized [139] | ? |
| Reduced plasma volume increase preceding preeclampsia [107] | relatively elevated, hypothesized | relatively elevated [16,43] | relatively lower [44] | anxiety [140] |
| Peripartum depression | circadian destabilization, reduced amplitude, hypothesized | relatively elevated, hypothesized | relatively lower, hypothesized | depressive symptom onset |
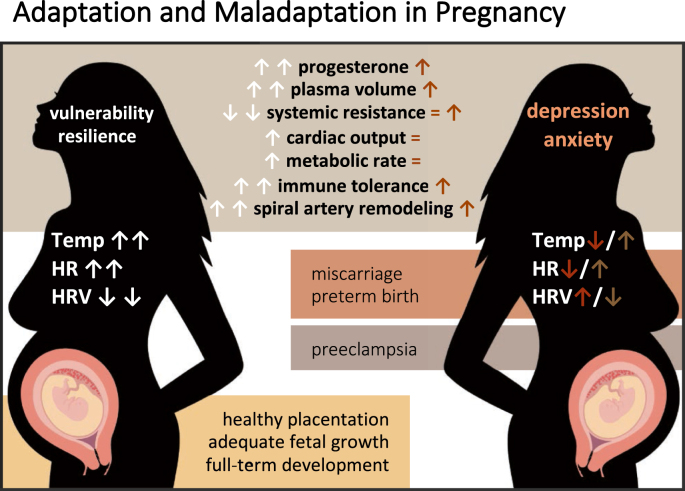
Extrapolating from the non-pregnant literature, we would expect higher body temperatures and lower HRV due to the progesterone effects with healthy placental development during pregnancy. If true, what patterns of network outputs would correlate with abnormal adaptation or placentation? Fig. 1 demonstrates some of these possible adaptations and hypothesized timeseries with maladaptation. For example, could we predict a future miscarriage or preterm birth if suboptimal progesterone production were visible through body temperature monitoring? Could a lack of plasma volume increase in the second trimester [107] (with expected concomitant cardiac output increases and decreased HRV) be a reliable predictor of a future pregnancy complications like preeclampsia or fetal growth restriction? At present, these complications are only diagnosed once they manifest later in pregnancy, yet it is purported that these obstetric problems originate early in the process of placentation [128].
Fig. 1.
Role of neuroendocrine hormones and associated mechanisms in the adaptation to pregnancy. Opportunities to monitor through timeseries and potential for observing maladaptation. Direction of hormonal, autonomic, and underlying physiological change during adaptation to pregnancy (left). Modifications to these changes in pregnancy complications (right). Arrows direction (up, down) indicates direction of change of the metric (increase, decrease).
A growing number of wearable sensors offer non-invasive, continuous temperature, HR, and HRV data that may be used across the reproductive phases to investigate the relationship between emotional and physiological state [[17], [18], [19],129]. Peripheral metrics generated from wearable devices, and in particular, metrics of biological rhythmic stability, are used to assess risk of depression and bipolar disorder [15,130]. For example, reductions in stability and amplitude of daily (circadian) temperature and cortisol rhythms are associated with mood imbalances [14] and elevated risk of major depression in peripartum women [131]. Additionally, reduced circadian amplitude of temperature, reduced activity and HRV, and elevated HR are predictors of the onset depressive episodes [14,[132], [133], [134]]. Such timeseries, if appropriately measured and interpreted contextually [135,136], may provide a window onto potential problems with great temporal granularity, and create an opportunity for precise mapping of patterns of change in pregnancy and birth.
4. Parturition: maternal, fetal, and placental factors inform the timing and experience of birth
4.1. Neuroendocrine physiology of parturition: preparing the mother and fetus for birth
Progesterone inhibits the ability of the uterus to contract effectively and helps maintain the rigid collagen structure of the cervix [101]. This inhibition of labor persists for an average of 268 days in healthy gestations (estimated ovulation to birth) [141]. The body transitions away from the maintenance of pregnancy to actively giving birth via the phases of parturition [142]. This transition is necessarily tied to the processes of fetal maturation, representing the synchrony between the maternal and fetal networks [143]. Inhibition needs to be removed or counteracted to initiate the labor and birth process. The events characterized by the “activation phase” of labor likely occur prior to true labor symptoms being detected by the mother. In contrast to other mammals, human progesterone does not fall in absolute quantities [144]. Instead, the effects of progesterone are counteracted against by the measurable increases in estriol (also manufactured by the placenta). The production of estriol is directly influenced by fetal maturation of the hypothalamic-pituitary-adrenal (HPA) axis as the placenta requires a precursor (dehydroepiandrosterone sulfate; DHEA-S) from the maturing fetal adrenal gland/liver as a substrate [142].
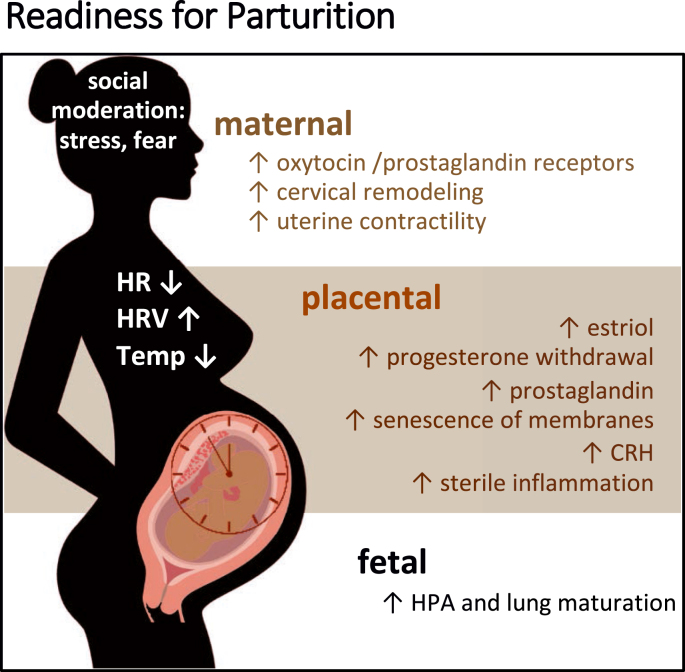
In concert with rising estriol, and the functional progesterone withdrawal, the placenta, the maternal HPA system and fetal systems (again driven by adrenal and CNS maturation) all drive a surge in CRH production. This feed-forward system from maternal and fetal glucocorticoid production to placental CRH increase is associated with fetal lung maturation and upregulation of prostaglandin synthesis (which raises body temperature) and receptors important for uterine receptivity (oxytocin receptors, gap junctions) at the time of labor onset [[145], [146], [147]]. As such, activation of the processes of labor, when occurring in a healthy pregnancy, tend to occur when the fetus has achieved a level of maturation compatible with extrauterine viability (Fig. 2), both neurologically and for respiratory transition (glucocorticoids increase production of surfactant, which allows for alveolar expansion and gas exchange) [[148], [149], [150], [151]].
Fig. 2.
Timing of parturition depends upon hormonal and autonomic mechanisms signaling readiness for labor and extrauterine life from maternal, placental and fetal compartments.
A diminished role of progesterone as a protective ‘anti-inflammatory’ mediator and the rising role of estriol augments higher CRH, which further contributes to the rise in prostaglandin receptors, oxytocin receptors and connexin-43 (gap junctions) [101]. This cluster of contraction-associated proteins allow for the coordinated uterine contractions to finally occur with an expected and clinically measurable rhythm and pattern [146,152]. Other pro-inflammatory processes occur simultaneously with the rebalancing of steroid hormones and CRH surges. These include chorionic/amniotic and decidua cellular senescence as well as the presence of ascending inflammation as cervical softening and cervical smooth muscle apoptosis begins to occur [101,153]. Lastly, in the active stimulation of the labor itself (stimulation phase), we witness/experience the symptoms and observe the behaviors that accompany the complete expulsion of a fetus and placenta [154].
Although inflammation and increased glucocorticoid production help transition the body away from quiescence, to prepare for the processes of parturition, mammalian parturition is best accomplished when the female is not under duress [[155], [156], [157]]. Parasympathetic activity dominates the process of parturition [[158], [159], [160]]. For example, transecting vagal nerves leading to the cervix results in delayed birth, compared to sham procedures in rats [160]. Studies from many species indicate that causing disruptions in the birthing environment, inducing stress or blocking parasympathetic nervous signaling leads to delayed or problematic parturition [159,161]. Further to this point, while many mammals give birth alone, humans tend to seek the companionship of other trusted humans.
4.2. Love and fear: emotional state and support during parturition
The powers of the psychological state of the mother on labor progression are not well understood. The role of subjective experiences of stress on onset of labor has been a focus of research, with researchers noting higher CRH production either from the placenta or mother contributes to shorter gestational lengths or preterm birth [116,162,163]. It has also been noted that acute stress may initiate shorter gestation (preterm labor) and that individuals living in chronic stress conditions (physical and/or psychological) trend toward earlier gestation [116,164,165]. The case of Black or African American individuals experiencing earlier onset of parturition than European-American/White counterparts in the United States speaks to the potential role for chronically higher glucocorticoids in triggering earlier maturation of the fetus and processes of labor as a consequence of racism, discrimination, or socio-economic or factors of the built environment [[166], [167], [168]].
Labor is both physically and emotionally intense, even under ideal social and environmental circumstances. Human birth is characterized by moving a relatively snug fitting fetal body through the pelvis [[169], [170], [171]], as well as by a highly vascularized placental attachment site (exposing one to possible hemorrhage) [5]. The challenges and fears that have historically accompanied childbirth are tempered by social support in the birthing process (family, midwives, physicians, doulas, partners) [172,173]. Fear results in higher levels of pain, may inhibit progression of labor, and can exacerbate postnatal depressive symptoms [[174], [175], [176], [177]]. The absence of social support, even among healthy women, is associated with more problematic labor and birth [[178], [179], [180]]. Thus, supportive care could be an adaptive human strategy to help the labor to progress normally by countering sympathetic/adrenergic stimulation. This may be particularly important given that, birth, often occurs under distressing circumstances, for example, in the presence of obstetric violence (mistreatment or racism) [[180], [181], [182], [183], [184]]. Giving birth also happens in the context of an individual's life, which may not be overwhelmingly characterized by love [185,186] or a wanted child. Social support, therefore, confers protection through physiologic means (oxytocin expression and modulation of stress hormones) [187,188]. From this view, bonds between the support persons and the birthing parent could be viewed as being intertwined in the ‘network physiology’ of parturition itself and could be observed with autonomic output.
4.3. Observing parturition through autonomic correlates of neuroendocrine and emotional regulation
Physiological preparations for birth are associated with changes in autonomic output, including autonomic contributions to thermoregulation (See Table 3). In many mammalian species, females’ core body temperature drops prior to the onset of parturition (orca, dog, cow, sheep, horse, rabbit, wolverine) [127,[189], [190], [191], [192], [193], [194]], and rises during the active birthing process [195]. This decrease is likely due to diminished influence of progesterone, but may also be lowered by oxytocin secretion [30,144]. The span of time over which the temperature change occurs varies from species to species but may provide a forecast of impending birth in days [192,193]. If a similar phenomenon occurred in humans, it may be possible to utilize measurement of these signals via wearable devices to improve delivery planning for women in a multitude of circumstances (workforce leave, high-risk delivery timing, travel required for delivery, induction of labor planning). In support of this hypothesis, lower than normal body temperatures were noted in a sample of laboring women upon hospital admission [195]. Not only was the mean temperature lower than normal in this study, but the distribution had a significant skew to the left, with nearly 80% falling under 98 °F.
Table 3.
Hypothesized impacts of labor, delivery, and complications on autonomic metrics.
| Physiologic or Pathologic Change around Birth | Temperature | HR | HRV | Emotional State |
|---|---|---|---|---|
| Functional progesterone withdrawal | relatively lower across third trimester [[51], [72], [73]] | relatively lower across third trimester [52] | relatively elevated across third trimester [[52], [197]] | potential depressive symptoms [78] |
| Activation phase of parturition at term or preterm | relatively lower [127,[189], [190], [191], [192], [193], [194]] | relatively lower [197] | relative elevated [197] | variable and influenced by social support structures [78] |
| Labor | elevate [195] | elevate [[197], [200], [201]] | decrease [[197], [200], [201]] | fear/stress, joy, influenced by support structures and obstetric condition [78] |
Although low-temporal-resolution studies, or those done at the population level, have not consistently identified pregnancy-related patterns in HRV [196], within individual and continuous HRV data suggest that HRV is progressively more suppressed (effects of progesterone) and rises following birth [197] (likely oxytocin-mediated) [90]. Additionally, as body temperature, HR, and HRV levels and patterning broadly reflect reproductive hormone status, such a biomarker may help elucidate the underlying mechanisms of labor onset timing, or potentially preterm birth [198,199].
Autonomic physiology during active phases of labor has not been described in detail in humans, although vagally-mediated activity mirroring that observed in other animals has been reported [200,201]. The metabolic work of labor, coping with pain, and increased ventilation will be reflected in increased body temperature as the duration of labor lengthens, alongside inflammation as assessed by white blood cell count [195]. Heart rate increases are seen as a physiologic adaptation but may also be visible relative to levels of coping, anxiety, fear, or other physical effects of labor complications (i.e., infection). How a person's subjective birth experience could be monitored within the timeseries is a compelling area for research as it may reflect not only the physiologic manifestations of fear (or love/support) but also a possible key connection to understanding the connection between labor dysfunction, intrauterine infection, and autonomic physiology.
For pregnant individuals and their families, the uncertainty of when labor could begin can be complex and anxiety-provoking [[202], [203], [204]]. Future labor prediction could be informative and valuable to individuals and families. Advance knowledge of the onset of parturition could lead to a fundamental shift in the way in which we approach birth care. This is partly because it would allow families to gather the necessary social support for the birth process or move closer to medical facilities, if necessary. Not only would this ensure that appropriate medical care is available but having additional preparation time may help alleviate anxiety and promote parasympathetic activation.
5. Dyadic synchrony: neuroendocrine and autonomic markers of bonding and maternal-infant coordination
5.1. Neuroendocrine adaptations in early parenthood
The immediate postpartum state (particularly following vaginal birth or physiologic labor) is dominated by steroid hormone withdrawal and a significant surge of pulsatile prolactin and oxytocin secretion. Oxytocin facilitates involution of the uterus [205], prevents hemorrhage [146], promotes prolactin secretion, and coordinates lactation with milk-ejection [40]. Furthermore, oxytocin plays a significant role in modulating stress reactivity and contributes to mood regulation, given sex-steroid suppression and the potential for provoking depressive or anxious symptoms [32,206,207].
Temporal coordination between the mother and a new infant is influenced by numerous factors. The most salient of these factors is that the infant inhabits a world physically distinct from the mother. The nervous and hormonal connection of the placenta is removed and is replaced by endocrine, autonomic, and emotional cues from intermittent breast feeding, touch, and other sensory inputs. Despite physical separation, mothers and infants can maintain a high degree of behavioral, emotional, and physiological coordination. This coordination is influenced by numerous factors, including physical contact, stress, medical interventions, light exposure, and feeding practices (reviewed in: [26,208,209]). Absence of (or overly tight) synchrony between parent-infant dyads leads to pronounced impacts on bonding and development.
5.2. The emotional process of synchrony and bonding
Dyadic or biobehavioral synchrony here refers to the temporal coordination of physiology and behavior during interactions between mothers and children (e.g., Ref. [210]). Dyadic synchrony was historically assessed behaviorally via observation and coding of dyads' touch, gaze, attention, and vocalization [[211], [212], [213]]. These investigations consistently report that “detectable” synchrony leads to better emotional regulation in toddlers, including greater empathy and longer attention spans in childhood [214], and greater social bonding within dyads [215,216]. These findings, as well as studies of dyadic synchrony in couples, led to the theory that human physiology benefits from the loving presence of another human regulator, and that synchrony promotes bonding and survival [217,218]. Although these studies might lead one to assume that greater synchrony is linearly associated with better outcomes, an inverse-U-curve of the benefits of synchrony has since been proposed for mothers and infants [26]. In this hypothesized framework, both lack of synchrony and perfect synchrony are associated with inferior emotional and cognitive outcomes in comparison to an intermediate amount described as “appropriate maternal responsiveness to the infant's needs” [26,219], or a “cooperative” vs. “controlling” dyadic interaction [220]. This “some is more” concept may enable children and parents to develop awareness of, and practice refining the anticipation of one another's needs.
5.3. Autonomic correlates of dyadic synchrony
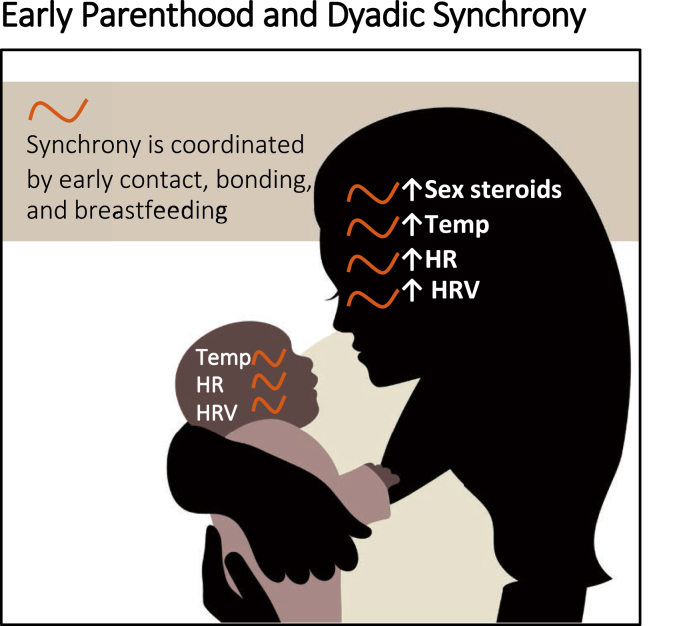
Neuroendocrine and autonomic synchrony may be initiated by skin-to-skin contact and breastfeeding and maintained through maternal and infant interactions and familial support (Fig. 3). Behavioral synchrony has been observed at approximately two to three months of age [[221], [222], [223]], and may both arise from and reinforce underlying physiological synchrony. Hormonal and autonomic synchrony are facilitated via skin-to-skin contact, and breastfeeding [224]. Dyadic synchrony has been recorded across myriad physiological outputs including melatonin (immediately post-partum) [[225], [226], [227]], oxytocin (4–6 months) [228], cortisol (4–11 months) [208,210,229], various measures and correlates of neural activity [230,231], and sleep and circadian rhythms (reviewed in: [232,233]). Additionally, coupled patterns of HR and HRV emerge by about six months of age [210,[234], [235], [236]], apparently coordinated via gaze and vocalizations [218]. Finally, the presence or absence, and degree of this coupling appears to be influenced by a) the presence of breastfeeding [227,229], b) whether breast milk is fresh or previously collected [237] and c) environmental light [238,239] and d) touch [26].
Fig. 3.
Synchrony between mother/infant, role of neuroendocrine systems and autonomic physiology observable via timeseries.
There are several common cases of intentional or unintentional disruption of maternal-infant synchrony that may be observable through peripheral autonomic metrics. These include routine separation of mothers and infants during the critical window after delivery (e.g., for cleaning, weighing of infant), which is associated with increased maternal-infant stress, reduced metrics of maternal-infant bonding (reviewed in: [240,241]) and, we hypothesize, reduced physiological synchrony. Additional disruptors of synchrony include cases in which formula is used, either due to the choice, feeding difficulties (i.e., hypotonia or ankyloglossia) or an inability to lactate [242]; these are especially likely to occur in a neonatal intensive care unit (NICU) [243] as well as cases in which mothers must work outside the home early in the child's life [244]. In each of these cases, a degree of additional separation is placed between the intrinsic physiological, emotional, and behavioral rhythms of the mother and those of the child. Use of real-time physiological monitoring to understand relationships among these types of separation on dyadic synchrony, short term bonding, and long-term outcomes has the potential to inform our understanding of both mechanisms underlying bonding, and for early detection and intervention to promote healthier dyadic relationships.
These factors also reinforce the role of the environment on dyads; especially in cases of early maternal-infant separation, a triadic framework of parent-infant-environmental monitoring may prove useful for deciphering outcomes [245,246]. Parent-infant-environment interactions are a powerful example of how external inputs can “train” and “reinforce” an individual's physiological networks, and how physiological networks appear to be key for forming bonds and facilitating emotional communication. Much remains to be learned about the association among physiological, behavioral, and emotional correlates of synchrony under different parenting conditions, as well as their long-term outcomes for maternal mood and child development/interactions, which have implications for shaping the emotional perceptions (love and fear).
Leveraging knowledge of the physiological networks described above may allow for development of new tools to study variability, structure, and temporal progression of dyadic synchrony and its connection to health outcomes (See Table 4). Numerous wearable devices [17,18] and smart phone application-based platforms have recently been developed for pregnancy, birth, parent, and infant monitoring. They include smart bassinets [249], sock-based infant monitors [250,251], and sleep tracking apps. Challenges of data access, device safety, and data-interpretation standards for infants [26] may limit the speed of investigations into the dynamics of maternal-infant health. Moreover, the complex temporal disruptions of the modern world (e.g., light-at-night) likely hinder the coordination across systems and may make autonomic signal interpretation more difficult. However, the array of powerful tools may allow us to address these questions at scale, in a wide variety of individuals, and in participants’ home environments. Combining these powerful sensor platforms with signal-processing and questions targeted at mapping maternal and maternal-infant dynamics may reveal peripheral markers of healthy or pathological pregnancy, birth and bonding, and eventually enable objective, continuous assessment of interventions [252].
Table 4.
Hypothesized impacts of dyadic synchrony formation on autonomic outputs in mothers and infants.
| Physiologic or Pathologic Change in Early Parenthood | Temperature | HR | HRV | Emotional State |
|---|---|---|---|---|
| Skin-to-skin contact and family nurturing | decrease; oscillation with breast-feeding | Lower [247]; synchronized oscillation with breast-feeding | elevate [248]; synchronized oscillation with breast feeding | fatigue, relaxation, bonding, love |
| Early postpartum maternal-infant separation, formula use, short maternity leave | attenuated decrease, reduced synchrony of oscillation | attenuated decrease, reduced synchrony of oscillation | attenuated elevation, reduced synchrony of oscillation | fatigue, stress, attenuated bonding |
| Development of healthy dyadic synchrony | appropriate oscillatory synchrony | appropriate oscillatory synchrony | appropriate oscillatory synchrony | fatigue, stress, relaxation, bonding, love |
| Postpartum depression | circadian destabilization, reduced amplitude, decreased oscillatory synchrony | elevate | decrease | depressive symptom onset |
6. Conclusions
Maternal physiology and mental state adapt dynamically across pregnancy, parturition, and postpartum. These adaptations integrate across systems and environmental conditions and, remarkably, may do so in a coordinated manner. Such coordination may enable information about internal systems to be gleaned from peripheral autonomic timeseries. These measures may lend insight into the etiology of physiologic and pathologic reproduction, from miscarriage to pre-term labor, healthy pregnancy adaptation to maternal-infant bonding. Observing the development of new life in real-time will help us address the uncertainties and fears surrounding birth and promote loving interactions around the time of birth and between parents and children.
Funding source
This research was supported in part by the Oregon Health and Science School of Nursing Innovations Grant, The Oregon Clinical Translational Research Institute: Biomedical Innovations Program Device and Diagnostic Award, and Dr. Erickson is also supported on a non-related grant by National Institutes for Health National Institute for Nursing Research under Award Number 1K99NR019596-01.
Author contributions
Elise N. Erickson: Conceptualization, Investigation, Writing, Visualization, Funding acquisition, Project Administration. Azure D. Grant: Investigation, Methodology, Writing, Visualization.
Declaration of interests
EE is conducting a research study in pregnancy with devices from Oura Inc., EE does not receive any research funding or salary support from the company. AG previously received payment from Oura Inc. as part of a research internship.
References
- 1.Golmakani N., Gholami M., Shaghaghi F., Safinejad H., Kamali Z., Mohebbi-Dehnavi Z. Relationship between fear of childbirth and the sense of cohesion with the attachment of pregnant mothers to the fetus. J. Educ. Health Promot. 2020;9:261. doi: 10.4103/jehp.jehp_46_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noriuchi M., Kikuchi Y., Senoo A. The functional neuroanatomy of maternal love: mother's response to infant's attachment behaviors. Biol. Psychiatr. Feb. 2008;63(4):415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Sharma S.R., Gonda X., Dome P., Tarazi F.I. What's Love Got to do with it: role of oxytocin in trauma, attachment and resilience. Pharmacol. Ther. Oct. 2020;214 doi: 10.1016/j.pharmthera.2020.107602. [DOI] [PubMed] [Google Scholar]
- 4.Quintana D.S., Guastella A.J. An allostatic theory of oxytocin. Trends Cognit. Sci. 2020 doi: 10.1016/j.tics.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Abrams E.T., Rutherford J.N. Framing postpartum hemorrhage as a consequence of human placental biology: an evolutionary and comparative perspective. Am. Anthropol. Aug. 2011;113(3):417–430. doi: 10.1111/j.1548-1433.2011.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells J.C.K. Life history trade-offs and the partitioning of maternal investment: implications for health of mothers and offspring. Evol. Med. Public Health. 2018;2018(1):153–166. doi: 10.1093/emph/eoy014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisbecker V., Goswami A. Reassessing the relationship between brain size, life history, and metabolism at the marsupial/placental dichotomy. Zool. Sci. (Tokyo) Sep. 2014;31(9):608–612. doi: 10.2108/zs140022. [DOI] [PubMed] [Google Scholar]
- 8.Small M.J., Allen T.K., Brown H.L. Global disparities in maternal morbidity and mortality. Semin. Perinatol. Aug. 2017;41(5):318–322. doi: 10.1053/j.semperi.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakala C., Romano A.M., Buckley S.J. Hormonal physiology of childbearing, an essential framework for maternal–newborn nursing. JOGNN - J. Obstet. Gynecol. Neonatal Nurs. 2016 doi: 10.1016/j.jogn.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Bell A.F., Erickson E.N., Carter C.S. Beyond labor: the role of natural and synthetic oxytocin in the transition to motherhood. J. Midwifery Wom. Health. 2014;59(1):35–42. doi: 10.1111/jmwh.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman R., Monakhov M., Pratt M., Ebstein R.P. Oxytocin pathway genes: evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol. Psychiatr. 2016;79(3):174–184. doi: 10.1016/j.biopsych.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Shannahoff-Khalsa D.S., Kennedy B., Yates F.E., Ziegler M.G. Ultradian rhythms of autonomic, cardiovascular, and neuroendocrine systems are related in humans. Am. J. Physiol. Apr. 1996;270(4 Pt 2):R873–R887. doi: 10.1152/ajpregu.1996.270.4.R873. [DOI] [PubMed] [Google Scholar]
- 13.Grant A.D., Wilsterman K., Smarr B.L., Kriegsfeld L.J. Evidence for a coupled oscillator model of endocrine ultradian rhythms. J. Biol. Rhythm. Aug. 2018;33(5):475–496. doi: 10.1177/0748730418791423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdullah S., Matthews M., Frank E., Doherty G., Gay G., Choudhury T. Automatic detection of social rhythms in bipolar disorder. J. Am. Med. Inf. Assoc. May 2016;23(3):538–543. doi: 10.1093/jamia/ocv200. [DOI] [PubMed] [Google Scholar]
- 15.Cho C.-H., Lee T., Kim M.-G., In H.P., Kim L., Lee H.-J. Mood prediction of patients with mood disorders by machine learning using passive digital phenotypes based on the circadian rhythm: prospective observational cohort study. J. Med. Internet Res. Apr. 2019;21(4):e11029. doi: 10.2196/11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong S., et al. Circadian rhythm of heart rate assessed by wearable devices tends to correlate with the circadian rhythm of salivary cortisol concentration in healthy young adults. Chronobiol. Med. Sep. 2020;2(3):109–114. doi: 10.33069/cim.2020.0022. [DOI] [Google Scholar]
- 17.Grant A.D., Newman M., Kriegsfeld L.J. Ultradian rhythms in heart rate variability and distal body temperature anticipate onset of the luteinizing hormone surge. Sci. Rep. Nov. 2020;10(1) doi: 10.1038/s41598-020-76236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maijala A., Kinnunen H., Koskimäki H., Jämsä T., Kangas M. Nocturnal finger skin temperature in menstrual cycle tracking: ambulatory pilot study using a wearable Oura ring. BMC Wom. Health. 2019;19(1):150. doi: 10.1186/s12905-019-0844-9. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster W.W., Smarr B. Using circadian rhythm patterns of continuous core body temperature to improve fertility and pregnancy planning. J. Circadian Rhythms. Sep. 2020;18:5. doi: 10.5334/jcr.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen A.M., al'Absi M., Lando H., Hatsukami D., Allen S.S. Menstrual phase, depressive symptoms, and allopregnanolone during short-term smoking cessation. Exp. Clin. Psychopharmacol. Dec. 2013;21(6):427–433. doi: 10.1037/a0034075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smarr B.L., Zucker I., Kriegsfeld L.J. Detection of successful and unsuccessful pregnancies in mice within hours of pairing through frequency analysis of high temporal resolution core body temperature data. PLoS One. 2016;11(7):e0160127. doi: 10.1371/journal.pone.0160127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant A., Smarr B. Feasibility of continuous distal body temperature for passive. Early Pregnancy Detection. 2021;24 doi: 10.1371/journal.pdig.0000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grattan D.R., Ladyman S.R. Handbook of Clinical Neurology. vol. 171. 2020. Neurophysiological and cognitive changes in pregnancy; pp. 25–55. Elsevier. [DOI] [PubMed] [Google Scholar]
- 24.Altemus M. Neuroendocrine networks and functionality. Med. Clin. Jul. 2019;103(4):601–612. doi: 10.1016/j.mcna.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 25.McFarland D.H., Fortin A.J., Polka L. Physiological measures of mother-infant interactional synchrony. Dev. Psychobiol. Jan. 2020;62(1):50–61. doi: 10.1002/dev.21913. [DOI] [PubMed] [Google Scholar]
- 26.Bell M.A. Mother-child behavioral and physiological synchrony. Adv. Child Dev. Behav. 2020;58:163–188. doi: 10.1016/bs.acdb.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Mittelman-Smith M.A., Williams H., Krajewski-Hall S.J., McMullen N.T., Rance N.E. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc. Natl. Acad. Sci. U.S.A. Nov. 2012;109(48):19846–19851. doi: 10.1073/pnas.1211517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenan M.S., Brothers R.M., Tweedell A.J., Hackney A.C., Griffin L. Changes in resting heart rate variability across the menstrual cycle. Psychophysiology. Oct. 2014;51(10):996–1004. doi: 10.1111/psyp.12250. [DOI] [PubMed] [Google Scholar]
- 29.Le J., Thomas N., Gurvich C. Cognition, the menstrual cycle, and premenstrual disorders: a review. Brain Sci. Mar. 2020;10(4):E198. doi: 10.3390/brainsci10040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hicks C., et al. Body temperature and cardiac changes induced by peripherally administered oxytocin, vasopressin and the non-peptide oxytocin receptor agonist WAY 267,464: a biotelemetry study in rats. Br. J. Pharmacol. Jun. 2014;171(11):2868–2887. doi: 10.1111/bph.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissman A., Tobia R.S., Burke Y.Z., Maxymovski O., Drugan A. The effects of oxytocin and atosiban on the modulation of heart rate in pregnant women. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. Feb. 2017;30(3):329–333. doi: 10.3109/14767058.2016.1172564. [DOI] [PubMed] [Google Scholar]
- 32.Ebner N.C., Horta M., Lin T., Feifel D., Fischer H., Cohen R.A. Oxytocin modulates meta-mood as a function of age and sex. Front. Aging Neurosci. Sep. 2015;7:175. doi: 10.3389/fnagi.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter C.S. The oxytocin-vasopressin pathway in the context of love and fear. Front. Endocrinol. 2017;8:356. doi: 10.3389/fendo.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stachenfeld N.S., Silva C., Keefe D.L. Estrogen modifies the temperature effects of progesterone. J. Appl. Physiol. Bethesda Md 1985. May 2000;88(5):1643–1649. doi: 10.1152/jappl.2000.88.5.1643. [DOI] [PubMed] [Google Scholar]
- 35.Schmalenberger K.M., et al. Menstrual cycle changes in vagally-mediated heart rate variability are associated with progesterone: evidence from two within-person studies. J. Clin. Med. Feb. 2020;9(3) doi: 10.3390/jcm9030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bei B., Coo S., Trinder J. Sleep and mood during pregnancy and the postpartum period. Sleep Med. Clin. Mar. 2015;10(1):25–33. doi: 10.1016/j.jsmc.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Melin B., Curé M., Pequignot J.M., Bittel J. Body temperature and plasma prolactin and norepinephrine relationships during exercise in a warm environment: effect of dehydration. Eur. J. Appl. Physiol. 1988;58(1–2):146–151. doi: 10.1007/BF00636618. [DOI] [PubMed] [Google Scholar]
- 38.Triebel J., et al. Principles of the prolactin/vasoinhibin axis. Am. J. Physiol. Regul. Integr. Comp. Physiol. Nov. 2015;309(10):R1193–R1203. doi: 10.1152/ajpregu.00256.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenke L., Martínez de la Escalera G., Clapp C., Bertsch T., Triebel J. A dysregulation of the prolactin/vasoinhibin Axis Appears to contribute to preeclampsia. Front. Endocrinol. 2020;10:893. doi: 10.3389/fendo.2019.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uvnäs Moberg K., et al. Maternal plasma levels of oxytocin during breastfeeding-A systematic review. PLoS One. 2020;15(8):e0235806. doi: 10.1371/journal.pone.0235806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snowdon C.T., Ziegler T.E. Variation in prolactin is related to variation in sexual behavior and contact affiliation. PLoS One. Mar. 2015;10(3):e0120650. doi: 10.1371/journal.pone.0120650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chowers I., Conforti N., Feldman S. Local effect of cortisol in the preoptic area on temperature regulation. Am. J. Physiol.-Leg. Content. Mar. 1968;214(3):538–542. doi: 10.1152/ajplegacy.1968.214.3.538. [DOI] [PubMed] [Google Scholar]
- 43.Nagel C., et al. Cortisol and progestin release, heart rate and heart rate variability in the pregnant and postpartum mare, fetus and newborn foal. Theriogenology. Sep. 2012;78(4):759–767. doi: 10.1016/j.theriogenology.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 44.Pulopulos M.M., Vanderhasselt M.-A., De Raedt R. Association between changes in heart rate variability during the anticipation of a stressful situation and the stress-induced cortisol response. Psychoneuroendocrinology. Aug. 2018;94:63–71. doi: 10.1016/j.psyneuen.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costa-Martins J.M., et al. Adult attachment style and cortisol responses in women in late pregnancy. BMC Psychol. Jan. 2016;4(1) doi: 10.1186/s40359-016-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rance N.E., Dacks P.A., Mittelman-Smith M.A., Romanovsky A.A., Krajewski-Hall S.J. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front. Neuroendocrinol. Aug. 2013;34(3):211–227. doi: 10.1016/j.yfrne.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams H., Dacks P.A., Rance N.E. An improved method for recording tail skin temperature in the rat reveals changes during the estrous cycle and effects of ovarian steroids. Endocrinology. Nov. 2010;151(11):5389–5394. doi: 10.1210/en.2010-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fregly M.J., Thrasher T.N. Response of heart rate to acute administration of isoproterenol in rats treated chronically with norethynodrel, ethinyl estradiol, and both combined. Endocrinology. Jan. 1977;100(1):148–154. doi: 10.1210/endo-100-1-148. [DOI] [PubMed] [Google Scholar]
- 49.Eckstein N., Nadler E., Barnea O., Shavit G., Ayalon D. Acute effects of 17 beta-estradiol on the rat heart. Am. J. Obstet. Gynecol. Sep. 1994;171(3):844–848. doi: 10.1016/0002-9378(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 50.Raddino R., Manca C., Poli E., Bolognesi R., Visioli O. Effects of 17 beta-estradiol on the isolated rabbit heart. Arch. Int. Pharmacodyn. Ther. May 1986;281(1):57–65. [PubMed] [Google Scholar]
- 51.Charkoudian N., Stachenfeld N.S. Comprehensive Physiology. John Wiley & Sons, Inc.; 2011. Reproductive hormone influences on thermoregulation in women. [DOI] [PubMed] [Google Scholar]
- 52.Charkoudian N., Hart E.C.J., Barnes J.N., Joyner M.J. Autonomic control of body temperature and blood pressure: influences of female sex hormones. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. Jun. 2017;27(3):149–155. doi: 10.1007/s10286-017-0420-z. [DOI] [PubMed] [Google Scholar]
- 53.Barnes J.N., Charkoudian N. Integrative cardiovascular control in women: regulation of blood pressure, body temperature, and cerebrovascular responsiveness. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. Feb. 2021;35(2):e21143. doi: 10.1096/fj.202001387R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tada Y., et al. The impact of menstrual cycle phases on cardiac autonomic nervous system Activity: an observational study considering lifestyle (diet, physical activity, and sleep) among female college students. J. Nutr. Sci. Vitaminol. 2017;63(4):249–255. doi: 10.3177/jnsv.63.249. [DOI] [PubMed] [Google Scholar]
- 55.Visrutha K., Harini N., Ganaraja B., Pavanchand A., Veliath S. A study of cardiac autonomic control and pulmonary functions in different phases of menstrual cycle. Int. J. Appl. Biol. Pharmaceut. Technol. Jul. 2012;3(3):306–311. [Google Scholar]
- 56.Lantto H., et al. Vasomotor hot flashes and heart rate variability: a placebo-controlled trial of postmenopausal hormone therapy. Menopause N. Y. N. Jan. 2012;19(1):82–88. doi: 10.1097/gme.0b013e318221bae8. [DOI] [PubMed] [Google Scholar]
- 57.Tomczy R., Paluch K., Gałuszka-Bednarczyk A., Milewicz T., Janeczko J., Klocek M. [Changes in blood pressure and heart rate by an increase in serum estradiol in women undergoing controlled ovarian hyperstimulation] Przegl. Lek. 2015;72(4):174–177. [PubMed] [Google Scholar]
- 58.Harvey P.J., O'Donnell E., Picton P., Morris B.L., Notarius C.F., Floras J.S. After-exercise heart rate variability is attenuated in postmenopausal women and unaffected by estrogen therapy. Menopause N. Y. N. Apr. 2016;23(4):390–395. doi: 10.1097/GME.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 59.Zullino S., Buzzella F., Simoncini T. Nitric oxide and the biology of pregnancy. Vasc. Pharmacol. Nov. 2018;110:71–74. doi: 10.1016/j.vph.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Bai J., et al. Estrogen receptors and estrogen-induced uterine vasodilation in pregnancy. Int. J. Mol. Sci. Jun. 2020;21(12):4349. doi: 10.3390/ijms21124349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanghavi M., Rutherford J.D. Cardiovascular physiology of pregnancy. Circulation. Sep. 2014;130(12):1003–1008. doi: 10.1161/CIRCULATIONAHA.114.009029. [DOI] [PubMed] [Google Scholar]
- 62.Kemp A.H., Quintana D.S., Kuhnert R.-L.L., Griffiths K., Hickie I.B., Guastella A.J. Oxytocin increases heart rate variability in humans at rest: implications for social approach-related motivation and capacity for social engagement. PLoS One. Aug. 2012;7(8) doi: 10.1371/journal.pone.0044014. e44014–e44014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopez-Vicchi F., De Winne C., Brie B., Sorianello E., Ladyman S.R., Becu-Villalobos D. Metabolic functions of prolactin: physiological and pathological aspects. J. Neuroendocrinol. 2020;32(11):e12888. doi: 10.1111/jne.12888. [DOI] [PubMed] [Google Scholar]
- 64.Franchimont P., et al. Prolactin levels during the menstrual cycle. Clin. Endocrinol. Nov. 1976;5(6):643–650. doi: 10.1111/j.1365-2265.1976.tb03867.x. [DOI] [PubMed] [Google Scholar]
- 65.Lee T.F., Mora F., Myers R.D. Dopamine and thermoregulation: an evaluation with special reference to dopaminergic pathways. Neurosci. Biobehav. Rev. 1985;9(4):589–598. doi: 10.1016/0149-7634(85)90005-3. [DOI] [PubMed] [Google Scholar]
- 66.Folgueira C., et al. Hypothalamic dopamine signaling regulates brown fat thermogenesis. Nat. Metab. Aug. 2019;1(8):811–829. doi: 10.1038/s42255-019-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prendergast B.J., Beery A.K., Paul M.J., Zucker I. Enhancement and suppression of ultradian and circadian rhythms across the female hamster reproductive cycle. J. Biol. Rhythm. Jun. 2012;27(3):246–256. doi: 10.1177/0748730412441315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smarr B.L., Grant A.D., Zucker I., Prendergast B.J., Kriegsfeld L.J. Sex differences in variability across timescales in BALB/c mice. Biol. Sex Differ. 2017;8:7. doi: 10.1186/s13293-016-0125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanchez-Alavez M., Alboni S., Conti B. Sex- and age-specific differences in core body temperature of C57Bl/6 mice. Age Dordr. Neth. Mar. 2011;33(1):89–99. doi: 10.1007/s11357-010-9164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sims S.T., Ware L., Capodilupo E.R. Patterns of endogenous and exogenous ovarian hormone modulation on recovery metrics across the menstrual cycle. BMJ Open Sport Exerc. Med. 2021;7(3):e001047. doi: 10.1136/bmjsem-2021-001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van de Velde T. H. Van de Velde.,” Wellcome Collection; 1904. Ueber den Zusammenhang zwischen Ovarialfunction, Wellenbewegung und Menstrualblutung, und ueber die Entstehung des sogenannten Mittelschmerzes/von Th.https://wellcomecollection.org/works/tcxermmu [PubMed] [Google Scholar]
- 72.Buxton C.L., Atkinson W.B. Hormonal factors involved in the regulation of basal body temperature during the menstrual cycle and pregnancy. J. Clin. Endocrinol. Metab. Jul. 1948;8(7):544–549. doi: 10.1210/jcem-8-7-544. [DOI] [PubMed] [Google Scholar]
- 73.Burt CatherineC. Peripheral SKIN temperature IN normal pregnancy. Lancet. Oct. 1949;254(6583):787–790. doi: 10.1016/S0140-6736(49)91371-9. [DOI] [PubMed] [Google Scholar]
- 74.Schiller C.E., Johnson S.L., Abate A.C., Schmidt P.J., Rubinow D.R. Reproductive steroid regulation of mood and behavior. Compr. Physiol. Jun. 2016;6(3):1135–1160. doi: 10.1002/cphy.c150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ossewaarde L., et al. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. Jan. 2010;35(1):47–55. doi: 10.1016/j.psyneuen.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 76.Hammarbäck S., Bäckström T., Holst J., von Schoultz B., Lyrenäs S. Cyclical mood changes as in the premenstrual tension syndrome during sequential estrogen-progestagen postmenopausal replacement therapy. Acta Obstet. Gynecol. Scand. 1985;64(5):393–397. doi: 10.3109/00016348509155154. [DOI] [PubMed] [Google Scholar]
- 77.Andréen L., Sundström-Poromaa I., Bixo M., Andersson A., Nyberg S., Bäckström T. Relationship between allopregnanolone and negative mood in postmenopausal women taking sequential hormone replacement therapy with vaginal progesterone. Psychoneuroendocrinology. Feb. 2005;30(2):212–224. doi: 10.1016/j.psyneuen.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Buckwalter J.G., Buckwalter D.K., Bluestein B.W., Stanczyk F.Z. Progress in Brain Research. vol. 133. 2001. Chapter 22 Pregnancy and postpartum: changes in cognition and mood; pp. 303–319. Elsevier. [DOI] [PubMed] [Google Scholar]
- 79.Epperson C.N., Pittman B., Czarkowski K.A., Stiklus S., Krystal J.H., Grillon C. Luteal-phase Accentuation of acoustic startle response in women with premenstrual dysphoric disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. Oct. 2007;32(10):2190–2198. doi: 10.1038/sj.npp.1301351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sundström Poromaa I., Gingnell M. Menstrual cycle influence on cognitive function and emotion processing—from a reproductive perspective. Front. Neurosci. Nov. 2014;8:380. doi: 10.3389/fnins.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sundström Poromaa I., Gingnell M. Menstrual cycle influence on cognitive function and emotion processing—from a reproductive perspective. Front. Neurosci. Nov. 2014;8:380. doi: 10.3389/fnins.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lasiuk G.C., Hegadoren K.M. The effects of estradiol on central serotonergic systems and its relationship to mood in women. Biol. Res. Nurs. Oct. 2007;9(2):147–160. doi: 10.1177/1099800407305600. [DOI] [PubMed] [Google Scholar]
- 83.Chung Y.S., et al. A preliminary study of association between adolescent estradiol level and dorsolateral prefrontal cortex activity during emotion regulation. Psychoneuroendocrinology. Nov. 2019;109 doi: 10.1016/j.psyneuen.2019.104398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fink G., Sumner B.E., McQueen J.K., Wilson H., Rosie R. Sex steroid control of mood, mental state and memory. Clin. Exp. Pharmacol. Physiol. Oct. 1998;25(10):764–775. doi: 10.1111/j.1440-1681.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 85.Campbell A. Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biol. Psychol. Jan. 2008;77(1):1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 86.Pedersen C.A., Prange A.J. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Natl. Acad. Sci. Unit. States Am. Dec. 1979;76(12):6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Washio H., Takeshita D., Sakata S. Parasympathetic nervous activity is associated with oxytocin in multiparous, but not primiparous, women during the perinatal period. Clin. Exp. Pharmacol. Physiol. Jun. 2020;47(6):955–965. doi: 10.1111/1440-1681.13267. [DOI] [PubMed] [Google Scholar]
- 88.Gamer M., Büchel C. Oxytocin specifically enhances valence-dependent parasympathetic responses. Psychoneuroendocrinology. Jan. 2012;37(1):87–93. doi: 10.1016/j.psyneuen.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 89.McQuaid R.J., McInnis O.A., Paric A., Al-Yawer F., Matheson K., Anisman H. Relations between plasma oxytocin and cortisol: the stress buffering role of social support. Neurobiol. Stress. Jun. 2016;3:52–60. doi: 10.1016/j.ynstr.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cox E.Q., Stuebe A., Pearson B., Grewen K., Rubinow D., Meltzer-Brody S. Oxytocin and HPA stress axis reactivity in postpartum women. Psychoneuroendocrinology. May 2015;55:164–172. doi: 10.1016/j.psyneuen.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janeček M., Dabrowska J. Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies - potential interaction with the corticotropin releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST) Cell Tissue Res. Jan. 2019;375(1):143–172. doi: 10.1007/s00441-018-2889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Debiec J. Peptides of love and fear: vasopressin and oxytocin modulate the integration of information in the amygdala. BioEssays News Rev. Mol. Cell. Dev. Biol. Sep. 2005;27(9):869–873. doi: 10.1002/bies.20301. [DOI] [PubMed] [Google Scholar]
- 93.Olivera-Pasilio V., Dabrowska J. Oxytocin promotes accurate fear discrimination and adaptive defensive behaviors. Front. Neurosci. 2020;14 doi: 10.3389/fnins.2020.583878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tang S., Graham B.M. In: Anxiety Disorders: Rethinking and Understanding Recent Discoveries. Kim Y.-K., editor. Springer; Singapore: 2020. The role of hormonal and reproductive status in the treatment of anxiety disorders in women; pp. 523–541. [DOI] [PubMed] [Google Scholar]
- 95.Pasca A.M., Penn A.A. The placenta: the lost neuroendocrine organ. NeoReviews. Feb. 2010;11(2):e64–e77. doi: 10.1542/neo.11-2-e64. [DOI] [Google Scholar]
- 96.Burton G.J., Fowden A.L. The placenta: a multifaceted, transient organ. Philos. Trans. R. Soc. B Biol. Sci. 2015;370(1663) doi: 10.1098/rstb.2014.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kroener L., Wang E.T., Pisarska M.D. Predisposing factors to abnormal first trimester placentation and the impact on fetal outcomes. Semin. Reprod. Med. 2015;34(1):27–35. doi: 10.1055/s-0035-1570029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun J.-Y., Wu R., Xu J., Xue H.-Y., Lu X.-J., Ji J. Placental immune tolerance and organ transplantation: underlying interconnections and clinical implications. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.705950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tan H., Yi L., Rote N.S., Hurd W.W., Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J. Clin. Endocrinol. Metab. May 2012;97(5):E719–E730. doi: 10.1210/jc.2011-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li X., Chen C., Luo H., Van Velkinburgh J.C., Ni B., Chang Q. Decreased DNA methylations at the progesterone receptor promoter a induce functional progesterone withdrawal in human parturition. Reprod. Sci. 2014;21(7):898–905. doi: 10.1177/1933719113518982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vannuccini S., Bocchi C., Severi F.M., Challis J.R., Petraglia F. Endocrinology of human parturition. Ann. Endocrinol. Jun. 2016;77(2):105–113. doi: 10.1016/j.ando.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 102.Larsen E.C., Christiansen O.B., Kolte A.M., Macklon N. New insights into mechanisms behind miscarriage. BMC Med. 2013;11(1) doi: 10.1186/1741-7015-11-154. 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lucas E.S., Dyer N.P., Fishwick K., Ott S., Brosens J.J. Success after failure: the role of endometrial stem cells in recurrent miscarriage. Reproduction. Nov. 2016;152(5):R159–R166. doi: 10.1530/REP-16-0306. [DOI] [PubMed] [Google Scholar]
- 104.Liu S., Diao L., Huang C., Li Y., Zeng Y., Kwak-Kim J.Y.H. The role of decidual immune cells on human pregnancy. J. Reprod. Immunol. Nov. 2017;124:44–53. doi: 10.1016/j.jri.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 105.Mor G., Aldo P., Alvero A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. Aug. 2017;17(8):469–482. doi: 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- 106.Newbern D., Freemark M. Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. Dec. 2011;18(6):409–416. doi: 10.1097/MED.0b013e32834c800d. [DOI] [PubMed] [Google Scholar]
- 107.Reijnders I.F., et al. First-trimester maternal haemodynamic adaptation to pregnancy and placental, embryonic and fetal development: the prospective observational Rotterdam Periconception cohort. BJOG An Int. J. Obstet. Gynaecol. Oct. 2021 doi: 10.1111/1471-0528.16979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wiegel R.E., et al. Corpus luteum number and the maternal renin-angiotensin-aldosterone system as determinants of utero-placental (vascular) development: the Rotterdam Periconceptional Cohort. Reprod. Biol. Endocrinol. RBE. Nov. 2021;19(1):164. doi: 10.1186/s12958-021-00843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Piccinni M.-P., Raghupathy R., Saito S., Szekeres-Bartho J. Cytokines, hormones and cellular regulatory mechanisms favoring successful reproduction. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.717808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spence T., Allsopp P.J., Yeates A.J., Mulhern M.S., Strain J.J., McSorley E.M. Maternal serum cytokine concentrations in healthy pregnancy and preeclampsia. J. Pregnancy. 2021;2021 doi: 10.1155/2021/6649608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Penner F., Rutherford H.J.V. Emotion regulation during pregnancy: a call to action for increased research, screening, and intervention. Arch. Womens Ment. Health. Jan. 2022 doi: 10.1007/s00737-022-01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Choi C., Mersky J.P., Janczewski C.E., Goyal D. Advancing research on perinatal depression trajectories: evidence from a longitudinal study of low-income women. J. Affect. Disord. Mar. 2022;301:44–51. doi: 10.1016/j.jad.2022.01.026. [DOI] [PubMed] [Google Scholar]
- 113.Suri R., Hellemann G., Cohen L., Aquino A., Altshuler L. Saliva estriol levels in women with and without prenatal antidepressant treatment. Biol. Psychiatr. Sep. 2008;64(6):533–537. doi: 10.1016/j.biopsych.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ruiz R.J., Marti C.N., Pickler R., Murphey C., Wommack J., Brown C.E.L. Acculturation, depressive symptoms, estriol, progesterone, and preterm birth in Hispanic women. Arch. Womens Ment. Health. Feb. 2012;15(1):57–67. doi: 10.1007/s00737-012-0258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang L., Wang L., Cui S., Yuan Q., Huang C., Zhou X. Prenatal depression in women in the third trimester: prevalence, predictive factors, and relationship with maternal-fetal attachment. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.602005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Christian L.M. Psychoneuroimmunology in pregnancy: immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neurosci. Biobehav. Rev. Jan. 2012;36(1):350–361. doi: 10.1016/j.neubiorev.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mehta D., et al. Genome-wide gene expression changes in postpartum depression point towards an altered immune landscape. Transl. Psychiatry. Mar. 2021;11(1):155. doi: 10.1038/s41398-021-01270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Venkatesh K.K., Ferguson K.K., Smith N.A., Cantonwine D.E., McElrath T.F. Association of antenatal depression with clinical subtypes of preterm birth. Am. J. Perinatol. May 2019;36(6):567–573. doi: 10.1055/s-0038-1675646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Backstrom C., McNeilly A.S., Leask R., Baird D. Pulsatile secretion of LH, FSH, Prolactin, oestradiol, and progesterone during the human menstrual cycle. Clin. Endocrinol. 1982;17:29–42. doi: 10.1111/j.1365-2265.1982.tb02631.x. [DOI] [PubMed] [Google Scholar]
- 120.McEwen B.S., Woolley C.S. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp. Gerontol. Aug. 1994;29(3–4):431–436. doi: 10.1016/0531-5565(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 121.Toffoletto S., Lanzenberger R., Gingnell M., Sundström-Poromaa I., Comasco E. Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: a systematic review. Psychoneuroendocrinology. Dec. 2014;50:28–52. doi: 10.1016/j.psyneuen.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 122.Thomson M. The physiological roles of placental corticotropin releasing hormone in pregnancy and childbirth. J. Physiol. Biochem. Sep. 2013;69(3):559–573. doi: 10.1007/s13105-012-0227-2. [DOI] [PubMed] [Google Scholar]
- 123.Fu Q., Levine B.D. Autonomic circulatory control during pregnancy in humans. Semin. Reprod. Med. Jun. 2009;27(4):330–337. doi: 10.1055/s-0029-1225261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Markon K.E., Brunette C.A., Whitney B.M., O'Hara M.W. Mood during pregnancy: trends, structure, and invariance by gestational day. J. Psychiatr. Res. Aug. 2021;140:260–266. doi: 10.1016/j.jpsychires.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 125.Power M.L., Schulkin J. Functions of corticotropin-releasing hormone in anthropoid primates: from brain to placenta. Am. J. Hum. Biol. Off. J. Hum. Biol. Counc. Aug. 2006;18(4):431–447. doi: 10.1002/ajhb.20521. [DOI] [PubMed] [Google Scholar]
- 126.Shah N.M., Lai P.F., Imami N., Johnson M.R. Progesterone-related immune modulation of pregnancy and labor. Front. Endocrinol. Mar. 2019;10:198. doi: 10.3389/fendo.2019.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Katsumata E., et al. Body temperature and circulating progesterone levels before and after parturition in killer whales (Orcinus orca) J. Reprod. Dev. Feb. 2006;52(1):65–71. doi: 10.1262/jrd.17063. [DOI] [PubMed] [Google Scholar]
- 128.Farina A. Biophysical markers for abnormal placentation: first and/or second trimester. Prenat. Diagn. Jul. 2014;34(7):628–634. doi: 10.1002/pd.4377. [DOI] [PubMed] [Google Scholar]
- 129.Hasselberg M.J., McMahon J., Parker K. The validity, reliability, and utility of the iButton® for measurement of body temperature circadian rhythms in sleep/wake research. Sleep Med. Jan. 2013;14(1):5–11. doi: 10.1016/j.sleep.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 130.Doherty A. Circadian rhythms and mental health: wearable sensing at scale. Lancet Psychiatr. Jun. 2018;5(6):457–458. doi: 10.1016/S2215-0366(18)30172-X. [DOI] [PubMed] [Google Scholar]
- 131.Szpunar M.J., Parry B.L. A systematic review of cortisol, thyroid-stimulating hormone, and prolactin in peripartum women with major depression. Arch. Womens Ment. Health. Apr. 2018;21(2):149–161. doi: 10.1007/s00737-017-0787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nováková M., Praško J., Látalová K., Sládek M., Sumová A. The circadian system of patients with bipolar disorder differs in episodes of mania and depression. Bipolar Disord. May 2015;17(3):303–314. doi: 10.1111/bdi.12270. [DOI] [PubMed] [Google Scholar]
- 133.Kripke D.F., Elliott J.A., Welsh D.K., Youngstedt S.D. Photoperiodic and circadian bifurcation theories of depression and mania. F1000Research. May 2015;4 doi: 10.12688/f1000research.6444.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schiweck C., et al. Twenty-four-hour heart rate is a trait but not state marker for depression in a pilot randomized controlled trial with a single infusion of ketamine. Front. Psychiatr. 2021;12:1170. doi: 10.3389/fpsyt.2021.696170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Krauchi K., et al. Diurnal and menstrual cycles in body temperature are regulated differently: a 28-day ambulatory study in healthy women with thermal discomfort of cold extremities and controls. Chronobiol. Int. Feb. 2014;31(1):102–113. doi: 10.3109/07420528.2013.829482. [DOI] [PubMed] [Google Scholar]
- 136.Batinga H., et al. Ontogeny and aging of the distal skin temperature rhythm in humans. Age Dordr. Neth. 2015;37(2):29. doi: 10.1007/s11357-015-9768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cohen J., Iffy L., Keyser H.H. Basal body temperature recordings in spontaneous abortion. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 1976;14(2):117–122. doi: 10.1002/j.1879-3479.1976.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 138.Odendaal H., et al. Effects of low maternal heart rate on fetal growth and birthweight. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. Aug. 2019;146(2):250–256. doi: 10.1002/ijgo.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Asri H., Mousannif H., Al Moatassime H. Comprehensive miscarriage dataset for an early miscarriage prediction. Data Brief. Aug. 2018;19:240–243. doi: 10.1016/j.dib.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qiu C., Williams M.A., Calderon-Margalit R., Cripe S.M., Sorensen T.K. Preeclampsia risk in relation to maternal mood and anxiety disorders diagnosed before or during early pregnancy. Am. J. Hypertens. Apr. 2009;22(4):397–402. doi: 10.1038/ajh.2008.366. [DOI] [PubMed] [Google Scholar]
- 141.Jukic A.M., Baird D.D., Weinberg C.R., McConnaughey D.R., Wilcox A.J. Length of human pregnancy and contributors to its natural variation. Hum. Reprod. Oxf. Engl. Oct. 2013;28(10):2848–2855. doi: 10.1093/humrep/det297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Snegovskikh V., Park J.S., Norwitz E.R. Endocrinology of parturition. Endocrinol Metab. Clin. N. Am. Mar. 2006;35(1):173–191. doi: 10.1016/J.ECL.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 143.Reinl E.L., England S.K. Fetal-to-maternal signaling to initiate parturition. J. Clin. Investig. 2015;125(7):2571. doi: 10.1172/JCI82576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nadeem L., Shynlova O., Matysiak-Zablocki E., Mesiano S., Dong X., Lye S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat. Commun. Dec. 2016;7(1) doi: 10.1038/ncomms11565. 11565–11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mitsuya K., Singh N., Sooranna S.R., Johnson M.R., Myatt L. Epigenetics of human myometrium: DNA methylation of genes encoding contraction-associated proteins in term and preterm Labor1. Biol. Reprod. May 2014;90(5):590–598. doi: 10.1095/biolreprod.113.113209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Arrowsmith S., Wray S. Oxytocin: its mechanism of action and receptor signalling in the myometrium. J. Neuroendocrinol. 2014;26(6):356–369. doi: 10.1111/jne.12154. [DOI] [PubMed] [Google Scholar]
- 147.Lamming G., Mann G. Control of endometrial oxytocin receptors and prostaglandin F2 alpha production in cows by progesterone and oestradiol. J. Reprod. Fertil. Jan. 1995;103(1):69–73. doi: 10.1530/jrf.0.1030069. [DOI] [PubMed] [Google Scholar]
- 148.Graves B.W., Haley M.M. Newborn transition. J. Midwifery Wom. Health. Dec. 2013;58(6):662–670. doi: 10.1111/jmwh.12097. [DOI] [PubMed] [Google Scholar]
- 149.Jain L., Dudell G.G. Respiratory transition in infants delivered by cesarean section. Semin. Perinatol. Oct. 2006;30(5):296–304. doi: 10.1053/j.semperi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 150.Han Y.M., et al. Surfactant protein-A mRNA expression by human fetal membranes is increased in histological chorioamnionitis but not in spontaneous labour at term. J. Pathol. Mar. 2007;211(4):489–496. doi: 10.1002/path.2131. [DOI] [PubMed] [Google Scholar]
- 151.Pinto S., Malheiro M.F., Vaz A., Rodrigues T., Montenegro N., Guimarães H. Neonatal outcome in preterm deliveries before 34-week gestation - the influence of the mechanism of labor onset. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. Nov. 2019;32(21):3655–3661. doi: 10.1080/14767058.2018.1481038. [DOI] [PubMed] [Google Scholar]
- 152.Smith R., Imtiaz M., Banney D., Paul J.W., Young R.C. Why the heart is like an orchestra and the uterus is like a soccer crowd. Am. J. Obstet. Gynecol. 2015 doi: 10.1016/j.ajog.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 153.Menon R., Bonney E.A., Condon J., Mesiano S., Taylor R.N. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum. Reprod. Update. Sep. 2016;22(5):535–560. doi: 10.1093/humupd/dmw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.American College of Nurse-Midwives Supporting healthy and normal physiologic childbirth: a consensus statement by the american college of nurse-midwives, midwives alliance of north America, and the national association of certified professional midwives. J. Midwifery Wom. Health. 2012;57(5):529–532. doi: 10.1111/j.1542-2011.2012.00218.x. [DOI] [PubMed] [Google Scholar]
- 155.Petraglia F., Imperatore A., Challis J.R.G. Neuroendocrine mechanisms in pregnancy and parturition. Endocr. Rev. Dec. 2010;31(6):783–816. doi: 10.1210/er.2009-0019. [DOI] [PubMed] [Google Scholar]
- 156.Olcese J. Circadian aspects of mammalian parturition: a review. Mol. Cell. Endocrinol. Feb. 2012;349(1):62–67. doi: 10.1016/j.mce.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 157.Martínez-Burnes J., Muns R., Barrios-García H., Villanueva-García D., Domínguez-Oliva A., Mota-Rojas D. Parturition in mammals: animal models, pain and distress. Anim. Open Access J. MDPI. Oct. 2021;11(10):2960. doi: 10.3390/ani11102960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nagel C., et al. Parturition in horses is dominated by parasympathetic activity of the autonomous nervous system. Theriogenology. Jul. 2014;82(1):160–168. doi: 10.1016/j.theriogenology.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 159.Nagel C., Aurich C., Aurich J. Stress effects on the regulation of parturition in different domestic animal species. Anim. Reprod. Sci. Aug. 2019;207:153–161. doi: 10.1016/j.anireprosci.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 160.Clyde L.A., Lechuga T.J., Ebner C.A., Burns A.E., Kirby M.A., Yellon S.M. Transection of the pelvic or vagus nerve forestalls ripening of the cervix and delays birth in Rats1. Biol. Reprod. 2011;84(3):587–594. doi: 10.1095/biolreprod.110.086207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stark M.A., Remynse M., Zwelling E. Importance of the birth environment to support physiologic birth. JOGNN - J. Obstet. Gynecol. Neonatal Nurs. 2016 doi: 10.1016/j.jogn.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 162.Wetzka B., et al. Expression patterns of CRH, CRH receptors, and CRH binding protein in human gestational tissue at term. Exp. Clin. Endocrinol. Diabetes. May 2003;111(3):154–161. doi: 10.1055/s-2003-39778. [DOI] [PubMed] [Google Scholar]
- 163.Cathey A.L., et al. Gestational hormone concentrations are associated with timing of delivery in a fetal sex-dependent manner. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.742145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.KRAMER M.R., HOGUE C.J., DUNLOP A.L., MENON R. Preconceptional stress and racial disparities in preterm birth: an overview. Acta Obstet. Gynecol. Scand. Dec. 2011;90(12):1307–1316. doi: 10.1111/j.1600-0412.2011.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Almeida J., Bécares L., Erbetta K., Bettegowda V.R., Ahluwalia I.B., Almeida Joannaalmeida J. Racial/ethnic inequities in low birth weight and preterm birth: the role of multiple forms of stress. Matern. Child Health J. 2018;22:1154–1163. doi: 10.1007/s10995-018-2500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Latendresse G. The interaction between chronic stress and pregnancy: preterm birth from A biobehavioral perspective. J. Midwifery Wom. Health. 2009 doi: 10.1016/j.jmwh.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Slaughter-Acey J.C., Brown T.N., Keith V.M., Dailey R., Misra D.P. A tale of two generations: maternal skin color and adverse birth outcomes in Black/African American women. Soc. Sci. Med. Nov. 2020;265 doi: 10.1016/j.socscimed.2020.113552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dorner R.A., Rankin K.M., Collins J.W. Early preterm birth across generations among whites and african-Americans: a population-based study. Matern. Child Health J. 2017;21(11):2061–2067. doi: 10.1007/s10995-017-2311-2. [DOI] [PubMed] [Google Scholar]
- 169.Stansfield E., Fischer B., Grunstra N.D.S., Pouca M.V., Mitteroecker P. The evolution of pelvic canal shape and rotational birth in humans. BMC Biol. Oct. 2021;19(1):224. doi: 10.1186/s12915-021-01150-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fischer B., Mitteroecker P. Covariation between human pelvis shape, stature, and head size alleviates the obstetric dilemma. Proc. Natl. Acad. Sci. U.S.A. 2015;112(18):5655–5660. doi: 10.1073/pnas.1420325112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pavličev M., Romero R., Mitteroecker P. Evolution of the human pelvis and obstructed labor: new explanations of an old obstetrical dilemma. Am. J. Obstet. Gynecol. 2019:1–14. doi: 10.1016/j.ajog.2019.06.043. 29397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jones P.M., Olsan L.T. Performative rituals for conception and childbirth in england, 900-1500. Bull. Hist. Med. 2015;89(3):406–433. doi: 10.1353/bhm.2015.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bohren M.A., Berger B.O., Munthe-Kaas H., Tunçalp Ö. Perceptions and experiences of labour companionship: a qualitative evidence synthesis. Cochrane Database Syst. Rev. Mar. 2019;3:CD012449. doi: 10.1002/14651858.CD012449.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Deng Y., et al. A comparison of maternal fear of childbirth, labor pain intensity and intrapartum analgesic consumption between primiparas and multiparas: a cross-sectional study. Int. J. Nurs. Sci. Oct. 2021;8(4):380–387. doi: 10.1016/j.ijnss.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hildingsson I. The trajectory of fear of birth during and after pregnancy in women living in a rural area far from the hospital and its labour ward. Rural Rem. Health. Nov. 2021;21(4):6974. doi: 10.22605/RRH6974. [DOI] [PubMed] [Google Scholar]
- 176.Mandar O., Idrees M.B., Ahmed A., ALhabardi N., Hassan B., Adam I. Prevalence and associated factors of fear for childbirth among pregnant women in eastern Sudan. J. Reprod. Infant Psychol. Oct. 2021:1–11. doi: 10.1080/02646838.2021.1995598. [DOI] [PubMed] [Google Scholar]
- 177.Zhou X.-L., Liu H., Li X.-H., Li F., Zhang S.-M., Zhang S.-R. Mediating effects of social support between antenatal depression and fear of childbirth among nulliparous woman. Ann. Palliat. Med. Jun. 2021;10(6):6399–6409. doi: 10.21037/apm-21-854. [DOI] [PubMed] [Google Scholar]
- 178.Kennell J., Klaus M., McGrath S., Robertson S., Hinkley C. Continuous emotional support during labor in a US hospital. A randomized controlled trial. JAMA. May 1991;265(17):2197–2201. [PubMed] [Google Scholar]
- 179.Stjernholm Y.V., Charvalho P. da S., Bergdahl O., Vladic T., Petersson M. Continuous support promotes obstetric labor progress and vaginal delivery in primiparous women - a randomized controlled study. Front. Psychol. 2021;12 doi: 10.3389/fpsyg.2021.582823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Balde M.D., et al. Labour companionship and women's experiences of mistreatment during childbirth: results from a multi-country community-based survey. BMJ Glob. Health. Nov. 2020;5(Suppl 2) doi: 10.1136/bmjgh-2020-003564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bohren M.A., et al. The mistreatment of women during childbirth in health facilities globally: a mixed-methods systematic review. PLoS Med. Jun. 2015;12(6) doi: 10.1371/journal.pmed.1001847. discussion e1001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vedam S., et al. The Giving Voice to Mothers study: inequity and mistreatment during pregnancy and childbirth in the United States. Reprod. Health. Jun. 2019;16(1):77. doi: 10.1186/s12978-019-0729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Attanasio L.B., Hardeman R.R. Declined care and discrimination during the childbirth hospitalization. Soc. Sci. Med. 1982. Jul. 2019;232:270–277. doi: 10.1016/j.socscimed.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 184.Davis D.-A. Obstetric racism: the racial politics of pregnancy, labor, and birthing. Med. Anthropol. Oct. 2019;38(7):560–573. doi: 10.1080/01459740.2018.1549389. [DOI] [PubMed] [Google Scholar]
- 185.Moghaddam Hossieni V., Toohill J., Akaberi A., HashemiAsl B. Influence of intimate partner violence during pregnancy on fear of childbirth. Sex. Reprod. Healthc. Off. J. Swed. Assoc. Midwives. Dec. 2017;14:17–23. doi: 10.1016/j.srhc.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 186.Shamblaw A.L., Sommer J.L., Reynolds K., Mota N., Afifi T.O., El-Gabalawy R. Pregnancy and obstetric complications in women with a history of childhood maltreatment: results from a nationally representative sample. Gen. Hosp. Psychiatr. Jun. 2021;70:109–115. doi: 10.1016/j.genhosppsych.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 187.Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatr. Dec. 2003;54(12):1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 188.Anacker A.M.J., Beery A.K. Life in groups: the roles of oxytocin in mammalian sociality. Front. Behav. Neurosci. 2013;7 doi: 10.3389/fnbeh.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Thiel A., Evans A.L., Fuchs B., Arnemo J.M., Aronsson M., Persson J. Effects of reproduction and environmental factors on body temperature and activity patterns of wolverines. Front. Zool. 2019;16 doi: 10.1186/s12983-019-0319-8. 21–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Koyama K., et al. Prediction of calving time in Holstein dairy cows by monitoring the ventral tail base surface temperature. Vet. J. Oct. 2018;240:1–5. doi: 10.1016/j.tvjl.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 191.Jilge B., Kuhnt B., Landerer W., Rest S. Circadian temperature rhythms in rabbit pups and in their does. Lab. Anim. 2001;35(4):364–373. doi: 10.1258/0023677011911831. [DOI] [PubMed] [Google Scholar]
- 192.Korosue K., Murase H., Sato F., Ishimaru M., Endo Y., Nambo Y. Assessment for predicting parturition in mares based on prepartum temperature changes using a digital rectal thermometer and microchip transponder thermometry device. J. Vet. Med. Sci. Jul. 2012;74(7):845–850. doi: 10.1292/jvms.11-0497. [DOI] [PubMed] [Google Scholar]
- 193.Geiser B., Burfeind O., Heuwieser W., Arlt S. Prediction of parturition in bitches utilizing continuous vaginal temperature measurement. Reprod. Domest. Anim. Feb. 2014;49(1):109–114. doi: 10.1111/rda.12236. [DOI] [PubMed] [Google Scholar]
- 194.Nabenishi H., Yamazaki A. Decrease in body surface temperature before parturition in ewes. J. Reprod. Dev. Apr. 2017;63(2):185–190. doi: 10.1262/jrd.2016-097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Acker D.B., Schulman E.B., Ransil B.J., Sachs B.P., Friedman E.A. The normal parturient's admission temperature. Am. J. Obstet. Gynecol. 1987;157(2):308–311. doi: 10.1016/S0002-9378(87)80158-8. [DOI] [PubMed] [Google Scholar]
- 196.Al-Shafei A.I., Musa S.M., Rayis D.A., Lutfi M.F., El-Gendy O.A., Adam I. Heart rate variability and hematological parameters in pregnant women. J. Clin. Lab. Anal. Jun. 2020;34(6):e23250. doi: 10.1002/jcla.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Carpenter R.E., D'Silva L.A., Emery S.J., Uzun O., Rassi D., Lewis M.J. Changes in heart rate variability and QT variability during the first trimester of pregnancy. Physiol. Meas. Mar. 2015;36(3):531–545. doi: 10.1088/0967-3334/36/3/531. [DOI] [PubMed] [Google Scholar]
- 198.Goldenberg R., Culhane J., Iams J., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wallace M.E., Mendola P., Chen Z., Hwang B.S., Grantz K.L. Preterm birth in the context of increasing income inequality. Matern. Child Health J. 2016 doi: 10.1007/s10995-015-1816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Reyes-Lagos J.J., et al. A comparison of heart rate variability in women at the third trimester of pregnancy and during low-risk labour. Physiol. Behav. Oct. 2015;149:255–261. doi: 10.1016/j.physbeh.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 201.Musa S.M., Adam I., Hassan N.G., Rayis D.A., Lutfi M.F. Maternal heart rate variability during the first stage of labor. Front. Physiol. 2017;8:774. doi: 10.3389/fphys.2017.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Eri T.S., Blystad A., Gjengedal E., Blaaka G. ‘The waiting mode’: first-time mothers' experiences of waiting for labour onset. Sex. Reprod. Healthc. Nov. 2010;1(4):169–173. doi: 10.1016/j.srhc.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 203.Henderson J., Redshaw M. Worries about labor and birth: a population-based study of outcomes for young primiparous women. Birth. Jun. 2016;43(2):151–158. doi: 10.1111/birt.12219. [DOI] [PubMed] [Google Scholar]
- 204.Borrelli S.E., Walsh D., Spiby H. First-time mothers' expectations of the unknown territory of childbirth: uncertainties, coping strategies and ‘going with the flow. Midwifery. Aug. 2018;63:39–45. doi: 10.1016/j.midw.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 205.Sulistiana M.P., Marfuah D., Mutiar A., Nurhayati N. KnE Life Sci.; Mar. 2021. The Effect of Oxytocin and Endorphin Massage to Uterine Involution in Post-Partum Mothers: A Literature Review; pp. 680–688. [DOI] [Google Scholar]
- 206.Becker M., Weinberger T., Chandy A., Schmukler S. Depression during pregnancy and postpartum. Curr. Psychiatr. Rep. Mar. 2016;18(3):32. doi: 10.1007/s11920-016-0664-7. [DOI] [PubMed] [Google Scholar]
- 207.Cardaillac C., Rua C., Simon E.G., El-Hage W. [Oxytocin and postpartum depression] J. Gynecol. Obstet. Biol. Reprod. Oct. 2016;45(8):786–795. doi: 10.1016/j.jgyn.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 208.Pratt M., Apter-Levi Y., Vakart A., Kanat-Maymon Y., Zagoory-Sharon O., Feldman R. Mother-child adrenocortical synchrony; Moderation by dyadic relational behavior. Horm. Behav. Mar. 2017;89:167–175. doi: 10.1016/j.yhbeh.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 209.Thomas K.A., Burr R.L., Spieker S., Lee J., Chen J. Mother-infant circadian rhythm: development of individual patterns and dyadic synchrony. Early Hum. Dev. Dec. 2014;90(12):885–890. doi: 10.1016/j.earlhumdev.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Davis M., West K., Bilms J., Morelen D., Suveg C. A systematic review of parent–child synchrony: it is more than skin deep. Dev. Psychobiol. 2018;60(6):674–691. doi: 10.1002/dev.21743. [DOI] [PubMed] [Google Scholar]
- 211.Northrup J.B., Iverson J.M. The development of mother–infant coordination across the first year of life. Dev. Psychol. 2020;56(2):221–236. doi: 10.1037/dev0000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Provenzi L., Scotto di Minico G., Giusti L., Guida E., Müller M. Disentangling the dyadic dance: theoretical, methodological and outcomes systematic review of mother-infant dyadic processes. Front. Psychol. Mar. 2018;9:348. doi: 10.3389/fpsyg.2018.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Leclère C., et al. Why synchrony matters during mother-child interactions: a systematic review. PLoS One. Dec. 2014;9(12) doi: 10.1371/journal.pone.0113571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gaertner B.M., Spinrad T.L., Eisenberg N. Focused attention in toddlers: measurement, stability, and relations to negative emotion and parenting. Infant Child Dev. Aug. 2008;17(4):339–363. doi: 10.1002/ICD.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yaniv A.U., Salomon R., Waidergoren S., Shimon-Raz O., Djalovski A., Feldman R. Synchronous caregiving from birth to adulthood tunes humans’ social brain. Proc. Natl. Acad. Sci. Unit. States Am. Apr. 2021;118(14) doi: 10.1073/pnas.2012900118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Feldman R. Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. JCPP (J. Child Psychol. Psychiatry) Apr. 2007;48(3–4):329–354. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- 217.Atzil S., Hendler T., Zagoory-Sharon O., Winetraub Y., Feldman R. Synchrony and specificity in the maternal and the paternal brain: relations to oxytocin and vasopressin. J. Am. Acad. Child Adolesc. Psychiatry. Aug. 2012;51(8):798–811. doi: 10.1016/j.jaac.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 218.McFarland D.H., Fortin A.J., Polka L. Physiological measures of mother-infant interactional synchrony. Dev. Psychobiol. Jan. 2020;62(1):50–61. doi: 10.1002/dev.21913. [DOI] [PubMed] [Google Scholar]
- 219.Bornstein M.H., Manian N. Maternal responsiveness and sensitivity Re-considered: some is more. Dev. Psychopathol. Nov. 2013;25(4 0 1) doi: 10.1017/S0954579413000308. 10.1017/S0954579413000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Forcada-Guex M., Pierrehumbert B., Borghini A., Moessinger A., Muller-Nix C. Early dyadic patterns of mother-infant interactions and outcomes of prematurity at 18 months. Pediatrics. Jul. 2006;118(1):e107–114. doi: 10.1542/peds.2005-1145. [DOI] [PubMed] [Google Scholar]
- 221.Jaffe J., Beebe B., Feldstein S., Crown C.L., Jasnow M.D. Rhythms of dialogue in infancy: coordinated timing in development. Monogr. Soc. Res. Child Dev. 2001;66(2):1–132. i–viii. [PubMed] [Google Scholar]
- 222.Tronick E.Z. Emotions and emotional communication in infants. Am. Psychol. Feb. 1989;44(2):112–119. doi: 10.1037//0003-066x.44.2.112. [DOI] [PubMed] [Google Scholar]
- 223.Bell L., Goulet C., St-Cyr Tribble D., Paul D., Boisclair A., Tronick E.Z. Mothers' and fathers' views of the interdependence of their relationships with their infant: a systems perspective on early family relationships. J. Fam. Nurs. May 2007;13(2):179–200. doi: 10.1177/1074840707300774. [DOI] [PubMed] [Google Scholar]
- 224.Perlman J., Kjaer K. Neonatal and maternal temperature regulation during and after delivery. Anesth. Analg. Jul. 2016;123(1):168–172. doi: 10.1213/ANE.0000000000001256. [DOI] [PubMed] [Google Scholar]
- 225.Qin Y., et al. Variations in melatonin levels in preterm and term human breast milk during the first month after delivery. Sci. Rep. Nov. 2019;9(1):17984. doi: 10.1038/s41598-019-54530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Italianer M.F., et al. Circadian variation in human milk composition, a systematic review. Nutrients. Aug. 2020;12(8) doi: 10.3390/nu12082328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cohen Engler A., Hadash A., Shehadeh N., Pillar G. Breastfeeding may improve nocturnal sleep and reduce infantile colic: potential role of breast milk melatonin. Eur. J. Pediatr. Apr. 2012;171(4):729–732. doi: 10.1007/s00431-011-1659-3. [DOI] [PubMed] [Google Scholar]
- 228.Feldman R., Gordon I., Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Dev. Sci. Jul. 2011;14(4):752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- 229.Benjamin Neelon S.E., Stroo M., Mayhew M., Maselko J., Hoyo C. Correlation between maternal and infant cortisol varies by breastfeeding status. Infant Behav. Dev. Aug. 2015;40:252–258. doi: 10.1016/j.infbeh.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Levy J., Lankinen K., Hakonen M., Feldman R. The integration of social and neural synchrony: a case for ecologically valid research using MEG neuroimaging. Soc. Cognit. Affect Neurosci. Jan. 2021;16(1–2):143–152. doi: 10.1093/scan/nsaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Levy J., Goldstein A., Feldman R. Perception of social synchrony induces mother-child gamma coupling in the social brain. Soc. Cognit. Affect Neurosci. Jul. 2017;12(7):1036–1046. doi: 10.1093/scan/nsx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.de Graag J.A., Cox R.F.A., Hasselman F., Jansen J., de Weerth C. Functioning within a relationship: mother-infant synchrony and infant sleep. Infant Behav. Dev. Apr. 2012;35(2):252–263. doi: 10.1016/j.infbeh.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 233.Blyton D.M., Sullivan C.E., Edwards N. Lactation is associated with an increase in slow-wave sleep in women. J. Sleep Res. 2002;11(4):297–303. doi: 10.1046/j.1365-2869.2002.00315.x. [DOI] [PubMed] [Google Scholar]
- 234.Busuito A., Quigley K.M., Moore G.A., Voegtline K.M., DiPietro J.A. “In sync: physiological correlates of behavioral synchrony in infants and mothers. Dev. Psychol. May 2019;55(5):1034–1045. doi: 10.1037/dev0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ostlund B.D., Measelle J.R., Laurent H.K., Conradt E., Ablow J.C. Shaping emotion regulation: attunement, symptomatology, and stress recovery within mother-infant dyads. Dev. Psychobiol. Jan. 2017;59(1):15–25. doi: 10.1002/dev.21448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Feldman R., Magori-Cohen R., Galili G., Singer M., Louzoun Y. Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behav. Dev. Dec. 2011;34(4):569–577. doi: 10.1016/j.infbeh.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 237.Hahn-Holbrook J., Saxbe D., Bixby C., Steele C., Glynn L. Human milk as ‘chrononutrition’: implications for child health and development. Pediatr. Res. Jun. 2019;85(7):936–942. doi: 10.1038/s41390-019-0368-x. [DOI] [PubMed] [Google Scholar]
- 238.Rivkees S.A., Mayes L., Jacobs H., Gross I. Rest-activity patterns of premature infants are regulated by cycled lighting. Pediatrics. Apr. 2004;113(4):833–839. doi: 10.1542/peds.113.4.833. [DOI] [PubMed] [Google Scholar]
- 239.Tsai S.-Y., Thomas K.A., Lentz M.J., Barnard K.E. Light is beneficial for infant circadian entrainment: an actigraphic study. J. Adv. Nurs. Aug. 2012;68(8):1738–1747. doi: 10.1111/j.1365-2648.2011.05857.x. [DOI] [PubMed] [Google Scholar]
- 240.Bergman N.J. Birth practices: maternal-neonate separation as a source of toxic stress. Birth Defects Res. Sep. 2019;111(15):1087–1109. doi: 10.1002/bdr2.1530. [DOI] [PubMed] [Google Scholar]
- 241.Moore E.R., Bergman N., Anderson G.C., Medley N. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst. Rev. Nov. 2016;11:CD003519. doi: 10.1002/14651858.CD003519.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Walburg V., Goehlich M., Conquet M., Callahan S., Schölmerich A., Chabrol H. Breast feeding initiation and duration: comparison of French and German mothers. Midwifery. Feb. 2010;26(1):109–115. doi: 10.1016/j.midw.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 243.Rivkees S.A. The development of circadian rhythms: from animals to humans. Sleep Med. Clin. Sep. 2007;2(3):331–341. doi: 10.1016/j.jsmc.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jou J., Kozhimannil K.B., Abraham J.M., Blewett L.A., McGovern P.M. Paid maternity leave in the United States: associations with maternal and infant health. Matern. Child Health J. Feb. 2018;22(2):216–225. doi: 10.1007/s10995-017-2393-x. [DOI] [PubMed] [Google Scholar]
- 245.Bystrova K., et al. Early contact versus separation: effects on mother-infant interaction one year later. Birth Berkeley Calif. Jun. 2009;36(2):97–109. doi: 10.1111/j.1523-536X.2009.00307.x. [DOI] [PubMed] [Google Scholar]
- 246.Molmen Lichter M., Peled Y., Levy S., Wiznitzer A., Krissi H., Handelzalts J.E. The associations between insecure attachment, rooming-in, and postpartum depression: a 2 months' longitudinal study. Infant Ment. Health J. Jan. 2021;42(1):74–86. doi: 10.1002/imhj.21895. [DOI] [PubMed] [Google Scholar]
- 247.Ludwig R.J., et al. Preterm infant heart rate is lowered after Family Nurture Intervention in the NICU: evidence in support of autonomic conditioning. Early Hum. Dev. Oct. 2021;161 doi: 10.1016/j.earlhumdev.2021.105455. [DOI] [PubMed] [Google Scholar]
- 248.Porges S.W., et al. Autonomic regulation of preterm infants is enhanced by Family Nurture Intervention. Dev. Psychobiol. Sep. 2019;61(6):942–952. doi: 10.1002/dev.21841. [DOI] [PubMed] [Google Scholar]
- 249.Parga J.J., et al. A prospective observational cohort study of exposure to womb-like sounds to stabilize breathing and cardiovascular patterns in preterm neonates. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. Sep. 2018;31(17):2245–2251. doi: 10.1080/14767058.2017.1339269. [DOI] [PubMed] [Google Scholar]
- 250.Dangerfield M.I., Ward K., Davidson L., Adamian M. Initial experience and usage patterns with the owlet smart sock monitor in 47,495 newborns. Glob. Pediatr. Health. 2017;4 doi: 10.1177/2333794X17742751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Anjewierden S., Humpherys J., LaPage M.J., Asaki S.Y., Aziz P.F. Detection of tachyarrhythmias in a large cohort of infants using direct-to-consumer heart rate monitoring. J. Pediatr. May 2021;232:147–153. doi: 10.1016/j.jpeds.2020.12.080. e1. [DOI] [PubMed] [Google Scholar]
- 252.Gomez-Pomar E., Blubaugh R. The Baby Friendly Hospital Initiative and the ten steps for successful breastfeeding. a critical review of the literature. J. Perinatol. Off. J. Calif. Perinat. Assoc. Jun. 2018;38(6):623–632. doi: 10.1038/s41372-018-0068-0. [DOI] [PubMed] [Google Scholar]