Abstract
Cytochrome c3 from Desulfovibrio vulgaris Miyazaki F was successfully expressed in the facultative aerobe Shewanella oneidensis MR-1 under anaerobic, microaerophilic, and aerobic conditions, with yields of 0.3 to 0.5 mg of cytochrome/g of cells. A derivative of the broad-host-range plasmid pRK415 containing the cytochrome c3 gene from D. vulgaris Miyazaki F was used for transformation of S. oneidensis MR-1, resulting in the production of protein product that was indistinguishable from that produced by D. vulgaris Miyazaki F, except for the presence of one extra alanine residue at the N terminus.
Cytochromes c3 are low-potential tetraheme proteins found almost exclusively in anaerobic bacteria, including sulfate-reducing bacteria in the genus Desulfovibrio. These cytochromes (either cell free or in vivo) have many potential uses, including pollutant degradation (6) and bioelectronics (4, 9), so an efficient production system would be very useful. One approach has been to take the cytochrome c3 (cyc) genes from Desulfovibrio vulgaris from which it was cloned (5, 17) and express it in other organisms (1, 11, 16). Such an approach has met with considerable difficulty, although some success has been obtained in several systems, including Rhodobacter sphaeroides (1) and another species of Desulfovibrio (16). However, although expression was obtained, a user-friendly system for protein production has not yet been achieved. Because Shewanella oneidensis MR-1 is known to produce cytochrome c3 (14), and because it is a facultative aerobe that can be easily and rapidly grown to high cell densities, we reasoned that it might be useful as an expression vehicle, and we report here that the gene for cytochrome c3 from D. vulgaris Miyazaki F can be expressed under either aerobic or anaerobic conditions in MR-1.
Cell growth and reagents.
S. oneidensis MR-1 (formerly called Shewanella putrefaciens MR-1 [15]) and its rifampin-resistant strain, TSP-C, were cultured aerobically overnight at 30°C using Luria-Bertani (LB) liquid medium, and rifampin was added at 10 μg/ml for the TSP-C strain. For anaerobic cultures of MR-1, glass bottles with butyl rubber caps containing degassed LB media with 30 mM sodium fumarate as the terminal electron acceptor were used. All enzymes, as well as low- and high-gelling-temperature agaroses, were obtained from TaKaRa Shuzo Co., Ltd. (Kyoto, Japan), while radioactive compounds were purchased from ICN Biomedicals Inc. (Irvine, Calif.) and were used for dideoxynucleotide sequencing. Molecular mass markers for electrophoresis were obtained from Bio-Rad Laboratories (Richmond, Calif.) and Amersham Pharmacia Biotech (Uppsala, Sweden). Both polyvinylidene difluoride membranes (0.2-μm pore size) and horseradish peroxidase color detection reagents (goat anti-rabbit immunoglobulin G secondary antibody conjugated with horseradish peroxidase, 4-chloro-1-naphthol, and hydrogen peroxide) used for Western blotting analysis were obtained from Bio-Rad Laboratories. Albumen was purchased from Seikagaku Corporation (Tokyo, Japan). Columns (SP-Sepharose [2.6 by 10 cm] and Hiload Superdex 75 [2.6 by 60 cm]) were purchased from Amersham Pharmacia Biotech. All other reagent-grade chemicals and antibiotics were obtained from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan).
Preparation of plasmids and transfer of the cyc genes to MR-1.
Two plasmids (pRKM3F and pRKMα) were used in these studies, giving essentially identical results. The former contained the wild-type cytochrome c3 gene from D. vulgaris Miyazaki F, while the latter contained the same gene modified with regard to codon usage by R. sphaeroides (K. Ozawa et al., unpublished results). Both plasmids were derivatives of plasmid pRK415 (3) and were constructed as follows.
Plasmid pKM300 containing the cyc gene from D. vulgaris Miyazaki F (900-bp AatII-SphI fragment in the AatII-SphI site of pUC18) (5) was digested with AatII and SphI. The resultant AatII-SphI fragment (900 bp) was treated with T4 DNA polymerase to produce blunt ends and was ligated into the SmaI site of plasmid pUC118. The plasmid containing the cytochrome c3 gene in the opposite direction to the lac promoter was selected. The constructed vector was called pUKM300. Plasmid pUKM300 was then digested with XbaI and EcoRI, and the 900-bp fragment was isolated from the gel and ligated into the vector pRK415. The ligation product was transformed into Escherichia coli JM109 and plated onto LB plates with 15 μg of tetracycline per ml, 40 μg of X-Gal (5-bromo-4-chloro-3-indolyl-α-d-galactoside) per ml, and 0.1 mM IPTG (isopropyl-α-d-(−)-thiogalactopyranoside. Recombinant plasmids were isolated from white colonies and analyzed by restriction digestion with XbaI and EcoRI, resulting in excision of the cyc gene as a 900-bp fragment. The recombinant plasmid, pRKM3F, was then used for transformation of MR-1.
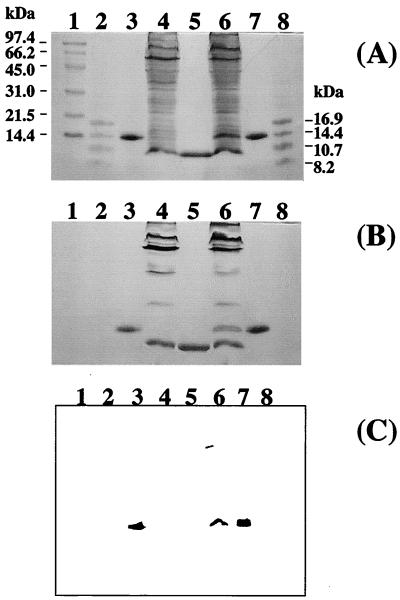
Both pRKM3F and pRKMα were transformed into E. coli S17-1 (13) and subsequently transferred to S. oneidensis TSP-C by conjugation. In order to confirm the presence of the cyc gene from D. vulgaris Miyazaki F, the soluble protein fractions of S. oneidensis MR-1 and the exconjugant S. oneidensis TSP-C (pRKMα) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie brilliant blue R-250 (CBB) (Fig. 1A) for proteins or o-tolidine dihydrochloride for hemes (Fig. 1B). Comparison of lanes 4 and 6 in Fig. 1A and B shows that S. oneidensis TSP-C(pRKMα) expressed the product of the D. vulgaris Miyazaki F cytochrome c3 gene as a c-type cytochrome of 14 kDa. The position of the band of interest in lane 6 was identical to the D. vulgaris Miyazaki F cytochrome c3 marker (lanes 3 and 7) and clearly was different from that of cytochrome c3 from S. oneidensis (lane 5). The 14-kDa band seen in lane 6 was increased approximately twofold when the concentration of tetracycline in the culture of S. oneidensis TSP-C(pRKMα) was raised from 15 to 30 μg/ml (data not shown). Heme staining of the gel (Fig. 1B) revealed a variety of c-type cytochromes present in S. oneidensis MR-1, consistent with previous reports (7). Western blot analysis of these bands was also performed using anti-D. vulgaris Miyazaki F cytochrome c3 serum (Fig. 1C). Cross-reactivity was revealed for the bands in lanes 3, 6, and 7. The 14-kDa band in lane 6 indicates the presence of D. vulgaris Miyazaki F cytochrome c3 in S. oneidensis TSP-C, while the absence of cross-reactivity in the negative controls (Fig. 1C, lanes 4 and 5) indicates that the serum has no cross-reactivity with cytochromes produced by MR-1.
FIG. 1.
SDS-PAGE analyses of cytochrome c3 in S. oneidensis. Cell preparations were loaded onto SDS–15% PAGE gels and electrophoresed. (A) Protein staining with CBB; (B) heme staining with o-tolidine dihydrochloride heme stain prepared as previously described (2); and (C) antigenically active material via Western blotting with antibody to cytochrome c3 from D. vulgaris Miyazaki F. Lanes 3, 5, and 7 were loaded with ca. 0.005 mg of protein, and lanes 4 and 6 were loaded with ca. 1 mg of protein. Lane 1, high-molecular-mass markers (phosphorylase b [97.4 kDa], bovine serum albumin [66.2 kDa], ovalbumin [45 kDa], carbonic anhydrase [31 kDa], soybean trypsin inhibitor [21.5 kDa], and lysozyme [14 kDa]); lanes 2 and 8, low-molecular-mass markers (globin [16.95 kDa], globins I and II [14.4 kDa], globins I and III [10.7 kDa], and globin I [8.16 kDa]); lanes 3 and 7, wild-type cytochrome c3 from D. vulgaris Miyazaki F; lane 4, cell lysate from S. oneidensis MR-1; lane 5, wild-type cytochrome c3 from S. oneidensis MR-1; lane 6, cell lysate from S. oneidensis TSP-C(pRKMα).
Isolation and characterization of recombinant D. vulgaris Miyazaki F cytochrome c3 from S. oneidensis.
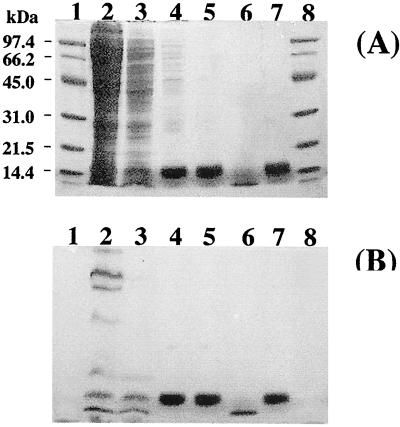
Recombinant D. vulgaris Miyazaki F cytochrome c3 was purified (Fig. 2, lane 5) from a supernatant obtained after centrifugation of the broken-cell supernatant treated with streptomycin sulfate (0.16 g per g of cells). Purification was carried out at 4°C and pH 7.0. The recombinant cytochrome c3 was purified in two steps. First, after dialysis against 10 mM sodium phosphate buffer, the supernatant was loaded onto an SP-Sepharose column (2.6 by 10 cm) previously equilibrated with the same buffer. Under these conditions, D. vulgaris Miyazaki F cytochrome c3 (pI = 10.6) binds to the ion-exchange resin, while endogenous S. oneidensis cytochrome c3 (pI = 5.8) is eluted together with other proteins. A gradient of 0 to 500 mM NaCl in 10 mM sodium phosphate buffer was then used to remove the D. vulgaris Miyazaki F cytochrome c3, which was eluted at 150 mM NaCl. Second, the eluted cytochrome c3 fraction was further purified by gel filtration on fast protein liquid chromatography system (Amersham Pharmacia Biotech) using a Hiload Superdex 75 column (2.6 by 60 cm) equilibrated with 50 mM NaCl–10 mM sodium phosphate buffer. Relative purity was confirmed by the absence of other bands after SDS–15% PAGE using CBB staining and a purity index (A552red/A280ox of ≅3.0.
FIG. 2.
SDS-PAGE analyses of recombinant cytochrome c3 in the purification process. Enzyme preparations were loaded onto SDS–15% PAGE gels and electrophoresed. Two different treatments are shown, CBB staining (A) and heme staining (B). Lane 2 was loaded with ca. 1 mg of protein, lane 3 was loaded with 0.3 mg of protein, lanes 4, 5, and 7 were loaded with ca. 0.01 mg of protein, and lane 6 was loaded with 0.005 mg of protein. Lanes 1 and 8, high-molecular-mass markers that are the same as those in lane 1 of Fig. 1; lane 2, supernatant of S. oneidensis TSP-C(pRKMα) extract; lane 3, supernatant after dialysis; lane 4, cytochrome c3 fraction after SP-Sepharose column chromatography; lane 5, cytochrome c3 fraction after Superdex 75 column chromatography; lane 6, S. oneidensis cytochrome c3 marker; lane 7, D. vulgaris Miyazaki F cytochrome c3 marker.
The UV and visible spectra were recorded with a Shimadzu UV-2200 spectrophotometer at room temperature. The recombinant D. vulgaris Miyazaki F cytochrome c3 showed a peak at 409 nm and a broad band centered at 530 nm in the oxidized state and peaks at 552, 523, and 419 nm in the dithionite-reduced state in 10 mM sodium phosphate buffer (Table 1), identical with those of the wild-type cytochrome c3 isolated from D. vulgaris Miyazaki F (18).
TABLE 1.
Properties of cytochromes c3 from different sources
| Host organism | UV spectraa | D. vulgaris Miyazaki F Abb | N terminusc | Mw | pI |
|---|---|---|---|---|---|
| D. vulgaris Miyazaki F | 409, 419, 523, 552 | + | APKAPADGLKM | 14,000 | 10.6 |
| MR-1(pRKM3F) | 409, 419, 523, 552 | + | AAPKAPADGLKM | 14,000 | Alkaline |
| S. oneidensis MR-1 | 408, 419, 522, 551 | − | ADQKLSDFHAES | 12,000 | 5.8 |
The first value is the peak in the oxidized state, and the other values are the peaks in the dithionite-reduced state.
Interaction with antibody (Ab) against D. vulgaris Miyazaki F.
The N-terminal sequences were determined with a Shimadzu PPSQ-10 protein sequencer coupled to a Shimadzu C-R7A analyzer.
The sequence of the N-terminal 15 amino acid residues of the recombinant D. vulgaris Miyazaki F cytochrome c3 purified from S. oneidensis(pRKMα) was determined by sequential Edman degradation. The sequence was identical to that of wild-type cytochrome c3 (12) isolated from D. vulgaris Miyazaki F, except for the addition of an extra alanine at the N terminus, and distinctly different from that reported for cytochrome c3 from S. oneidensis MR-1 (Table 1).
For nuclear magnetic resonance (NMR) studies, the sample was lyophilized three times with 99.9% 2H2O and resuspended in deuterated 10 mM sodium phosphate buffer, pH 7.0. One-dimensional 1H NMR spectra at 600 MHz were recorded at 303 K on a Bruker DRX-600 NMR spectrometer. The NMR spectra of the oxidized form of wild-type and recombinant cytochromes c3 in the low-field region (10 to 40 ppm, i.e., the region most commonly used to detect chemical or physical changes in cytochromes) were virtually identical to, but easily distinguishable from, that of the cytochrome c3 from S. oneidensis (data not shown). The macroscopic redox potentials of the recombinant cytochrome c3 were identical to those of the wild-type D. vulgaris Miyazaki F cytochrome c3 (10). Furthermore, the recombinant cytochrome c3 easily could be reduced by hydrogen in the presence of D. vulgaris Miyazaki F hydrogenase, just like the wild-type cytochrome c3. On the basis of these data, we conclude that the expressed protein is fully functional and identical to the wild-type D. vulgaris Miyazaki F cytochrome c3, except for the additional alanine residue at the N terminus.
Yield of recombinant D. vulgaris Miyazaki F cytochrome c3.
Cells were grown under three different conditions: (i) aerobic, with strong aeration in a 5-liter fermentor; (ii) microaerobic, with intermediate aeration (2 liters of culture in a 3-liter Erlenmeyer flask); and (iii) anaerobic, with fumarate as the terminal electron acceptor. Yields of cytochrome c3 were compared with those obtained during anaerobic growth of D. vulgaris (Table 2). On a per-weight basis (milligrams of cytochrome per gram [wet weight] of cells), D. vulgaris yielded 0.15 mg, while S. oneidensis(pRKMα) yielded 0.29 mg under aerobic conditions and 0.5 mg under microaerobic or anaerobic conditions.
TABLE 2.
Cytochrome c3 production under different conditions
| Host strain (condition) | Cytochrome c3 concn
|
|
|---|---|---|
| mg/g of cells | mg/liter of culture | |
| D. vulgaris Miyazaki F (anaerobic) | 0.15 | 0.30 |
| D. desulfuricans G200 (anaerobic) | 0.2a | |
| S. oneidensis TSP-C (aerobic) | 0.29 | 1.9 |
| S. oneidensis TSP-C (microaerobic) | 0.5 | 1.0 |
| S. oneidensis TSP-C (anaerobic) | 0.5b | 1.0b |
Expression of the D. vulgaris Hildenborough cyc gene (16).
Similar to the amounts obtained under microaerobic conditions, judged from the intensity of the bands on SDS-PAGE.
Per liter of culture, D. vulgaris yielded 0.3 mg of cytochrome c3. In comparison, S. oneidensis(pRKMα) yielded 1.9 mg aerobically and 1 mg for microaerobic and anaerobic culture. These results reflect the major differences obtained in growth yield under these different conditions. Because of the ease of aerobic growth, high cell yield, and good production of cytochrome c3, this system offers an easy and efficient vehicle for cytochrome c3 production.
The system may also have utility for the expression of other multiheme protein genes, because Shewanella produces a variety of c-type multiheme cytochromes of its own (7, 14), suggesting that it has a very good heme ligase system. MR-1 is capable of reduction of both elemental sulfur and thiosulate (8), an unusual ability even for many anaerobes, and mutants lacking cytochromes c are unable to reduce these compounds. Furthermore, even though D. vulgaris Miyazaki F and S. oneidensis belong to different groups of the Proteobacteria (delta and gamma, respectively) based on 16S rRNA analysis, MR-1 was able to transcribe the genes efficiently, with or without codon modification (data not shown).
Acknowledgments
We thank Kin-ichiro Miura at Gakushuin University and Izumi Kumagai at Tohoku University for helpful discussions. K.H.N. thanks the exobiology and astrobiology programs at NASA for support of this work.
REFERENCES
- 1.Cannack V, Caffrey M S, Voordouw G, Cusanovich M A. Expression of the gene encoding cytochrome c3 from the sulfate-reducing bacterium Desulfovibrio vulgaris in the purple photosynthetic bacterium Rhodobacter sphaeroides. Arch Biochem Biophys. 1991;286:629–632. doi: 10.1016/0003-9861(91)90091-v. [DOI] [PubMed] [Google Scholar]
- 2.Connelly J L, Morrison M, Stolz E. Hemins of beef heart muscle. J Biol Chem. 1958;233:743–747. [PubMed] [Google Scholar]
- 3.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 4.Kimura K, Nakahara Y, Yagi T, Inokuchi H. Electrical conduction of hemoprotein in the solid phase: anhydrous cytochrome c3 film. J Chem Physiol. 1979;70:3317–3323. [Google Scholar]
- 5.Kitamura M, Ozawa K, Kojima S, Kumagai I, Akutsu H, Miura K. The primary structure of pre-cytochrome c3 from Desulfovibrio vulgaris (Miyazaki F) as determined by nucleotide sequencing of its gene and partial amino acid sequencing. Protein Seq Data Anal. 1993;5:193–196. [Google Scholar]
- 6.Lovley D R, Widman P K, Woodward J C, Phillips E J P. Reduction of uranium by cytochrome c3 of Desulfovibrio vulgaris. Appl Environ Microbiol. 1993;59:3572–3576. doi: 10.1128/aem.59.11.3572-3576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris C J, Black A C, Pealing S L, Manson F D C, Chapman S K, Reid G A, Gibson D M, Ward F B. Purification and properties of a novel cytochrome: flavocytochrome c from Shewanella putrefaciens. Biochem J. 1994;302:587–593. doi: 10.1042/bj3020587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moser D P, Nealson K H. Growth of the facultative anaerobe Shewanella putrefaciens by elemental sulfur reduction. Appl Environ Microbiol. 1996;62:2100–2105. doi: 10.1128/aem.62.6.2100-2105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakahara Y, Kimura K, Inokuchi H, Yagi T. Electrical conductivity of an anhydrous cytochrome c3 film as a function of temperature and ambient pressure. Chem Phys Lett. 1980;73:31–34. [Google Scholar]
- 10.Niki K, Kawasaki K, Nishimura N, Higuchi Y, Yasuoka N, Kakudo M. Electrochemical and structural studies of tetraheme proteins from Desulfovibrio—standard potentials of redox sites and heme-heme interactions. J Electroanal Chem. 1984;168:275–286. [Google Scholar]
- 11.Pollock W B R, Chemerika P J, Forrest M E, Beatty J T, Voordouw G. Expression of the gene encoding cytochrome c3 from Desulfovibrio vulgaris (Hildenborough) in Escherichia coli: export and processing of the apoprotein. J Gen Microbiol. 1989;135:2319–2328. doi: 10.1099/00221287-135-8-2319. [DOI] [PubMed] [Google Scholar]
- 12.Shinkai W, Hase T, Yagi T, Matsubara H. Amino acid sequence of cytochrome c3 from Desulfovibrio vulgaris. J Biochem (Tokyo) 1980;87:1747–1756. doi: 10.1093/oxfordjournals.jbchem.a132919. [DOI] [PubMed] [Google Scholar]
- 13.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 14.Tsapin A I, Nealson K H, Meyers T, Cusanovich M A, Van Beeumen J, Crosby L D, Feinberg B A, Zhang C. Purification and properties of a low-redox-potential tetraheme cytochrome c3 from Shewanella putrefaciens. J Bacteriol. 1996;178:6386–6388. doi: 10.1128/jb.178.21.6386-6388.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkateswaren K, Moser D P, Dollhopf M E, Lies D P I, Saffarini D A, MacGregor B J, Ringelberg D B, White D C, Nishijima M, Sano H, Burghardt J, Stackebrandt E, Nealson K H. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int J Syst Bacteriol. 1999;49:705–724. doi: 10.1099/00207713-49-2-705. [DOI] [PubMed] [Google Scholar]
- 16.Voordouw G, Pollock W B R, Bruschi M, Guerlesquin F, Rapp-Giles B J, Wall J D. Functional expression of Desulfovibrio vulgaris Hildenborough cytochrome c3 in Desulfovibrio desulfuricans G200 after conjugational gene transfer from Escherichia coli. J Bacteriol. 1990;172:6122–6126. doi: 10.1128/jb.172.10.6122-6126.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voordouw G, Sydney B. Cloning and sequencing of the gene encoding cytochrome c3 from Desulfovibrio vulgaris (Hildenborough) Eur J Biochem. 1986;159:347–351. doi: 10.1111/j.1432-1033.1986.tb09874.x. [DOI] [PubMed] [Google Scholar]
- 18.Yagi T, Maruyama K. Purification and properties of cytochrome c3 of Desulfovibrio vulgaris, Miyazaki F. Biochim Biophys Acta. 1971;243:214–224. doi: 10.1016/0005-2795(71)90078-x. [DOI] [PubMed] [Google Scholar]