Abstract
A biofloc technology-based 75-day indoor growth trial in an 80 L glass aquaria was conducted to evaluate the effects of two different carbon sources (sugarcane bagasse, SB, and wheat flour, WF) on the biofloc composition, bacterial abundance, and growth of whiteleg shrimp (Litopenaeus vannamei) juveniles (0.23 ± 0.04 g). Three different levels of dietary protein content (250, 300, and 350 g protein kg−1 diet) and two carbon sources (SB and WF) were applied (SB250, WF250, SB300, WF300, SB350, and WF350, respectively), comparing to a controlled diet without biofloc and fed on a 450 g protein kg−1 diet (C450). With the addition of SB and WF, water quality was in the ideal recommended ranges for L. vannamei culture. At the end of the experiment, the biofloc volume increased with increasing dietary protein levels. The nutritional value of biofloc in different treatments was influenced by dietary protein and added SB and WF. Increasing dietary protein significantly increased the protein and lipid contents of the produced biofloc. The use of WF as a carbon source significantly increased lipids and nitrogen-free extract in the biofloc. The total heterotrophic bacterial (THB) count was significantly higher (p < 0.05) in WF300 and WF350 than in the other treatments. The mean effect of the protein levels and carbon source was significantly reported, whereas the highest significant THB count was recorded with 300 dietary protein and using WF as a carbon source. The growth performances of L. vannamei fed with biofloc treatments were significantly (p < 0.05) higher than the C450 group. The highest final weight and weight gain were recorded in SB350 treatment. The feed conversion ratio was not affected by reducing dietary protein levels; meanwhile, the protein efficiency ratio increased significantly in biofloc treatments than in the control. Overall, the results demonstrate that, compared to the control treatment of 450 dietary protein, the biofloc treatments using WF as a carbon source could compensate for the reduction in the dietary protein levels in the diet of L. vannamei and maintain higher zootechnical performance.
Keywords: protein requirement, biofloc quality, crustacean farming, total heterotrophic bacteria, zero-water exchange
1. Introduction
The shrimp aquaculture industry has recently gained considerable attention, due to aquaculture development, expansion, the high economic values of shrimp products, and the increase in human demand [1,2]. However, shrimp farming still has critical limiting factors regarding expansion, such as the cost of a shrimp diet, among other aquaculture activities [3,4]. The most important obstacle is that the diet consists of protein content (%), which has the highest feed component price [5,6]. Biofloc technology (BFT) is a realistic solution for efficiently managing water quality with low or no water exchange, enhancing shrimp growth performance and establishing an efficient and healthy shrimp culture with a better food conversion ratio in the shrimp aquaculture business [7,8,9,10,11,12]. In general, in shrimp culture, the BFT is successfully consumed, digested, and may replace a significant portion of protein in the diet [13,14,15]. BFT can be grown and produced in the culture water column and effectively reduce ammonium and nitrite accumulation in culture systems, as well as being employed as a supplemental food source for cultured shrimp [16], such as protein [17], lipid [18], amino acids [19], and fatty acids [20].
The BFT can be defined as an aggregation of microorganisms, microalgae, zooplankton, uneaten feeds, and trapped organic particles [21,22]. The BFT system needs external inputs of exogenous carbon sources to sustain the optimum carbon-to-nitrogen (C/N) ratio required for microbial production. In the BFT system, the effectiveness of BFT is determined not only by the carbon sources, but also by the ratio of carbon to nitrogen (C/N ratio) [11,23]. The goal of BFT research is to determine and describe the ideal circumstances for a diverse and stable BFT microbial community, as well as to create strategies for establishing diverse and stable BFT microbial growth [20]. The appendance of aquatic bacterial communities in ponds has a substantial impact on the internal bacterial microbiota of farmed species, which can aid in animal growth, feed consumption, water quality control, and disease resistance [24,25,26,27,28,29,30].
Several studies have evaluated the impact of using carbon sources, concentrations, and ingestions on the water quality growth performance and feed utilization of shrimp in BFT-based farms [30]. Many carbon sources were extensively investigated as sources for BFT production, such as starch, glucose, sugarcane bagasse, sugarcane molasses, rice bran, rice flour, wheat bran, wheat flour, cassava flour, gram flour, corn flour [15,22,30,31,32,33,34], as well as agriculture waste (by-products) [35,36]. Although not all carbon sources support the production of BFT with the same efficiency [15,20,30], some carbon sources are considered promising substrates for BFT aggregation due to their ability to support rapid ammonia elimination and a higher production of BFT volume [15,37]. In BFT, some carbon sources resulted in improved growth, feed utilization, water quality control, and enhanced total heterotrophic bacteria (THB) [30]. On the other hand, aquaculture wastewater contains high amounts of pollutants from unconsumed feeds and fish feces [38], resulting in high levels of total ammonia nitrogen (TAN), nitrite–nitrogen (NO2-N), and nitrate–nitrogen (NO3-N), which are considered as good applicable sources for BFT [30].
The production of whiteleg shrimp, Litopenaeus vannamei, in BFT-based intensive systems with zero-water exchange has become popular and has achieved sustainable growth over the last decade [12,39,40]. Several researchers have calculated the various ideal dietary protein requirements for L. vannamei under various farming conditions, which could be exploited to optimize growth performance, which ranged between 32–40% [14,41,42,43,44,45,46,47]. In addition, BFT consumption can improve feed utilization efficiency by recovering a portion of voided nutrients and boosting nitrogen retention from additional feed by 7 to 13% [48,49,50]. In the present study, low-dietary protein diets were formulated to examine the compensation effect of BFT, in comparison to a commercial diet, whereas the recommended dietary protein level for L. vannamei is high, accounting for 35% [51]. Moreover, the development of BFT using a carbon source should be cost effective and not competitive to human consumption. Accordingly, in the present study, we use low-cost carbon sources, such as sugarcane bagasse (SB) and wheat flour (WF). According to the best available knowledge, little is known about how to improve shrimp performance by managing functional BFT by the addition of diverse carbon sources and the correct dietary protein amount. Therefore, the present study aims to investigate the effect of dietary protein levels (250, 300, and 350 g protein kg−1) and two different carbon sources (SB and WF) to improve L. vannamei production, feed utilization, and water quality.
2. Materials and Methods
2.1. Whiteleg Shrimp Juveniles
A 75-day feeding experiment was conducted at the National Institute of Oceanography and Fisheries (NIOF), Suez Branch. Juveniles of L. vannamei (average initial weight, 0.23 ± 0.04 g) were obtained from a commercial shrimp hatchery in the Al-Deba triangle, Damietta, Egypt. Shrimp were acclimatized in an indoor glass aquarium for 14 days before the feeding trial began at a temperature of 29.1 ± 0.2 °C, pH of 8 ± 0.01, and salinity of 20 ± 0.1 g L−1; during this period, they were fed twice daily with a control diet containing 450 protein (C450).
2.2. Experimental Design and Set-Up
The current experiment was conducted in glass aquaria (30 × 40 × 60 cm) filled with 80 L of filtered (50 µm) seawater. To adjust the salinity at 20 g L−1, the seawater was diluted with de-chloride tap water. At the beginning of the trial, juveniles of whiteleg shrimp were carefully selected from the stock tanks and directly distributed into 21 aquaria (3 replicates per treatment) with an initial stocking density of 13 shrimp/aquarium. Three levels of dietary protein (250, 300, and 350 g protein kg−1 diet) were used under two different carbon sources (SB and WF) to produce six treatments (SB250, WF250, SB300, WF300, SB350, and WF350, respectively), compared to the control diet without carbon addition and fed a practical diet (450 protein; C450). The formulated experimental diets were isolipidic (≈87.7 g kg−1) and isocaloric (gross energy ≈ 19.27 MJ kg−1 diet). The formula and proximate chemical composition of the experimental diets are presented in Table 1.
Table 1.
Ingredients and proximate composition of the experimental diets (g kg−1).
| Ingredients (g kg−1) |
Experimental Diets (g Protein kg−1 Diet) | Control Diet (450 g Protein kg−1 Diet) |
||
|---|---|---|---|---|
| 250 | 300 | 350 | ||
| Fish meal | 90 | 270 | 270 | 450 |
| Soybean meal | 330 | 230 | 330 | 330 |
| Yellow corn | 280 | 250 | 205 | 90 |
| Wheat bran | 195 | 155 | 100 | 45 |
| Fish oil | 60 | 50 | 50 | 40 |
| Cholesterol | 5 | 5 | 5 | 5 |
| Di-calcium phosphate | 20 | 20 | 20 | 20 |
| Vitamin and minerals 1 | 20 | 20 | 20 | 20 |
| Proximate analysis (g kg−1) | ||||
| Dry matter | 893.5 | 899.6 | 901.8 | 906 |
| Crude protein | 256.2 | 319.6 | 352.1 | 451.7 |
| Crude lipid | 71.8 | 92.6 | 89.3 | 81.2 |
| Ash content | 78.1 | 81.2 | 90.1 | 112.3 |
| Crude fiber | 49.8 | 39.3 | 39.8 | 33.8 |
| Nitrogen-free extract 2 | 544.1 | 467.3 | 428.7 | 321 |
| Gross energy (MJ kg−1 diet) 3 | 18.70 | 19.22 | 19.20 | 19.38 |
1 Vitamin premix (mg or IU kg−1 diet): vitamin A, 6000 IU; vitamin D3, 2000 IU; ascorbic acid, 200 mg; vitamin E, 50 mg; menadione, 5 mg; thiamine, 15 mg; riboflavin, 15 mg; nicotinic acid, 30 mg; pantothenic acid, 35 mg; pyridoxine HCl, 6 mg; cyanocobalamin, 0.03 mg; biotin, 0.2 mg; inositol, 200 mg; folic acid, 3 mg; iodine, 0.4 mg; cobalt, 0.1 mg; copper, 4 mg; iron, 150 mg; zinc, 80 mg; manganese, 20 mg; selenium, 0.1 mg; magnesium, 100 mg. 2 Nitrogen-free extract (%) = 100 − (crude protein + ether extract + crude fiber + ash). 3 Gross energy (MJ kg−1) = (crude protein × 23.6 + ether extract × 39.5 + Nitrogen-free extract × 17.2)/100.
The experimental diets were prepared in the laboratory. Briefly, raw materials were crushed in an electric grain grinder (HR-40B Wuyi, Zhejiang, China) and sieved and weighed on a digital scale according to the formula (Table 1). All the ingredients of each diet were mixed with an electric blender until they formed a homogenous mixture. During the mixing process, 300 mL of warm water per kilogram of raw material was added and mixed for 15 min. This mixture was formed into pellets by extrusion through a pelleting machine (MG-2000 Tornado, Cairo, Egypt) with a 1 mm die. Finally, the pellets were dried in an oven at 55–60 °C (FD 260 Binder, Tuttlingen, Germany), then stored in plastic bags at 4 °C. The proximate compositions of experimented diets were analyzed, as described by AOAC [52].
Shrimp were fed with experimental diets at 10% of live body weight, which decreased gradually according to the increase in body weight by 3% at the end of the experiment. The daily-feed ratios were divided into three equal amounts given three times at 8:00, 14:00, and 20:00 h to all aquaria. During the experiment, all aquaria were supported with 5 cm air stones, which were fitted to the air compressor (220 W). Aeration was provided for 24 h throughout the experiment for ensuring better flocculation. Shrimp were held under a lighting regime (12:12 h, light:dark). All aquaria were covered with a plastic hab net to prevent jumping shrimp.
To initiate the development of BFT in the aquaria, 100 mL of BFT from an old shrimp tank was used to inculcate the experimental aquaria. The C/N ratio was 16:1 [48,53] after adding external carbon sources in the BFT treatments as SB (SB250, SB300, and SB350) and WF (WF250, WF300, and WF350). To convert 1 g of TAN into bacterial biomass, an average of 10 g of carbon is required [9]. In the control treatment (without carbon sources), water was exchanged two times per week. Meanwhile, BFT treatments were maintained without any water exchange, except for the addition of dechlorinated fresh water to compensate for evaporation loss and keep the salinity at 20 g L−1.
2.3. Water Quality Analysis
Water temperature, salinity, pH, TAN, and total suspended solids (TSSs) were all measured during the experiment to keep water quality within the shrimp optimum range. Water temperature was recorded daily at 13:00 h using a mercury thermometer suspended at a 30 cm depth. The salinity and pH were measured daily between 9:00 and 10:00 h by using a refractometer and pH meter (Orion pH meter, Abilene, TX, USA), respectively. From each tank, a 500 mL water sample was collected weekly and divided into two portions; the first was used to spectrophotometrically analyze TAN, NO2-N, and NO3-N, as described by Parsons [54]. Under vacuum pressure, the remaining portion of water sample was filtered using pre-dried and pre-weighed Whatman GF/C filter paper, then dried in an oven at 105 °C, and weighed to 0.01 mg. The weight difference was determined and a TSS estimate was obtained [55].
2.4. Carbon Sources and Biofloc Analysis
The locally available sources of SB and WF were selected as carbon sources for BFT treatments. SB was purchased as a by-product from a local vegetable market, then dried in an oven at 60 °C until totally dry, powdered, sieved at 35 μm, and then kept until further use [15]. WF powder was purchased from the local store: Suez Government, Egypt. The biochemical compositions (% dry-weight basis) of both SB and WF were determined following the standard methods of AOAC [52], while the organic carbon content (%) in SB and WF was determined as in Jackson [56]. However, the biochemical compositions and organic carbon content of both carbon sources (SB and WF) are presented in Table 2.
Table 2.
Biochemical compositions and organic carbon content (g kg−1) of used carbon sources.
| Carbon Sources | Total Organic Carbon |
Total Protein |
Total Lipid |
Nitrogen-Free Extract | Ash | Fiber |
|---|---|---|---|---|---|---|
| Sugarcane bagasse | 395 ± 2.1 | 15.2 ± 0.1 | 15.3 ± 0.1 | 244 ± 0.3 | 76 ± 0.2 | 650 ± 2.1 |
| Wheat flour | 411 ± 1.1 | 122 ± 0.3 | 12.5 ± 0.2 | 812 ± 11 | 41.5 ± 0.2 | 13 ± 0.2 |
The volume of the BFT was measured biweekly using the Imhoff cone by registering the volume of the BFT in 1000 mL of tank water after 15–20 min of sedimentation, according to Avnimelech and Kochba [55]. The proximate composition of the BFT was determined following the standard methods of AOAC [52]. Briefly, the moisture content was determined by oven drying to a constant weight at 105 °C. Crude protein was estimated using Kjeldahl techniques and the factor of N × 6.25 [57] after acid digestion using an auto Kjeldahl system (Kelplus, DXVA, Pelican Equipments, Chennai, India). Using a Soxtec system (Socs plus, SCS-6, Pelican Equipment, Chennai, India), crude lipid was determined by the ether extraction method [58]. In a muffle furnace, ash content was determined by incineration at 600 °C for 6 h. Fibertec (Foss Tecator 2022, Sweden) was used to determine crude fiber utilizing sequential digestion with H2SO4 and NaOH.
2.5. Total Heterotrophic Bacteria
The total heterotrophic bacteria (THB) count was determined at the end of the experiment. From the center of the tanks, all samples were collected in a sterilized polypropylene bottle. Following that, a 10-fold serial dilution using sterilized distilled water was prepared. A total of 0.1 mL of appropriate dilutions was applied in duplicate over the tryptone soya agar containing 1.0% w/v NaCl. All plates were incubated at 37 °C for 24–48 h and colonies in the range of 30–300 counted and expressed as a bacterial colony-forming unit (cfu) according to [59,60].
2.6. Growth Performance and Feed Utilization Parameters
At the end of the experiment, shrimps were collected after draining the tanks’ water. Shrimp weight gain (WG), specific growth rate (SGR), feed conversion ratio (FCR), the protein efficiency ratio (PER), and survival rate (SR %) were calculated using the following equations:
| Weight gain (WG) = Final body weight (g) − Initial body weight (g) | (1) |
| Specific growth rate (SGR %) = [(Log final weight − log initial weight) × 100]/rearing period in days | (2) |
| Feed conversion ratio (FCR) = Dry weight of feed consumed (g)/weight gain (g) | (3) |
| Protein efficiency ratio (PER) = Weight gain (g)/Protein fed (g) | (4) |
| Survival rate (SR % day−1) = (Final number of shrimp juveniles/Initial number of shrimp juveniles) × 100 | (5) |
2.7. Statistical Analysis
The presented data are means ± SD. All data were tested for homogeneity by Levene’s test, and the normal distribution was also checked using the Shapiro–Wilk’s test and all data met the parametric test requirements. All measured variables were performed using the SPSS ver. 16 to analyze the statistical difference one-way ANOVA, followed by the Duncan test [61]. In addition, two-way ANOVA was used to determine the effects of the protein level, carbon source, and their interaction [62]. Significance was set as p < 0.05.
3. Results
3.1. Water Quality
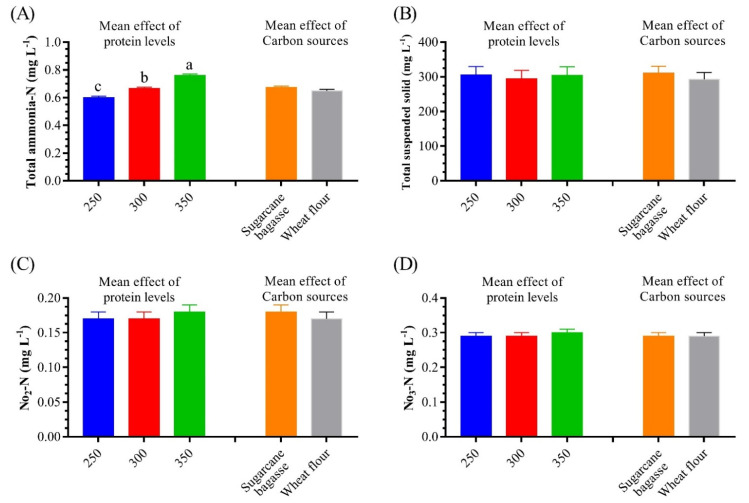
All water quality conditions were at the permissible range for cultured L. vannamei, according to Mohanty et al. [63]. The different water parameters’ mean of temperature, pH, salinity, TTS, TAN, NO2-N, and NO3-N were 27.4 ± 0.17 °C, 7.55 ± 0.04, 20.6 ± 0.10 g L−1, 314.2 mg L−1, 0.674 ± 0.03 mg L−1, 0.181 ± 0.01 mg L−1, and 0.299 ± 0.003 mg L−1, respectively. Figure 1 does not show any significant effects of protein levels and carbon sources on the water quality of the BFT systems. Meanwhile, the main effect of increasing dietary protein levels shows a significant increase in TAN.
Figure 1.
Mean effect of dietary protein levels and carbon sources on some water quality parameters of whiteleg shrimp L. vannamei juveniles reared in water. (A) Total ammonia nitrogen, (B) total suspended solids, (C) nitrate, and (D) nitrite. Different letters indicate significantly different values (p < 0.05).
3.2. Biofloc Volume and Nutritional Values
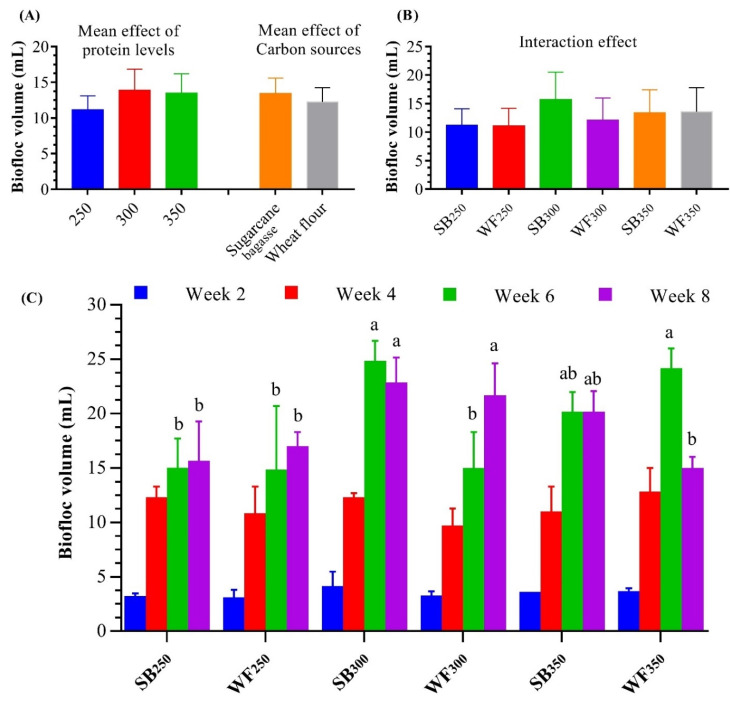
Figure 2 shows the BFT volume (BFV) produced biweekly for 8 weeks (W2, W4, W6, and W8) in water tanks with BFT treatments. In W2 and W4, no significant differences (p < 0.05) in BFV were obtained for any BFT treatments. In W6 and W8, the highest significant (p < 0.05) BFV was obtained for SB300, followed by SB350, WF350, and WF300, while the lowest significant (p < 0.05) BFV was obtained for WF250 and SB250 (Figure 2), respectively. In general, among all BFT treatments, the highest BFV average of all weeks was reported at SB300 (9.63 mg L−1), followed by WF350 (8.42 mg L−1), SB350 (8.02 mg L−1), WF300 (7.81 mg L−1), WF250 (6.12 mg L−1), and SB250 (5.73 mg L−1). The two-way ANOVA study showed an increase in BFT volume with increasing dietary protein levels and SB as a carbon source.
Figure 2.
Effect of dietary protein levels and carbon sources on biofloc volume of whiteleg shrimp, L. vannamei, juveniles reared in water. (A) Mean effects of protein levels and carbon source, (B) interaction effect of protein levels and carbon source, and (C) biweekly in biofloc development. Presented data are means ± SD (n = 3). Different letters in the same week indicate significantly different values (p < 0.05).
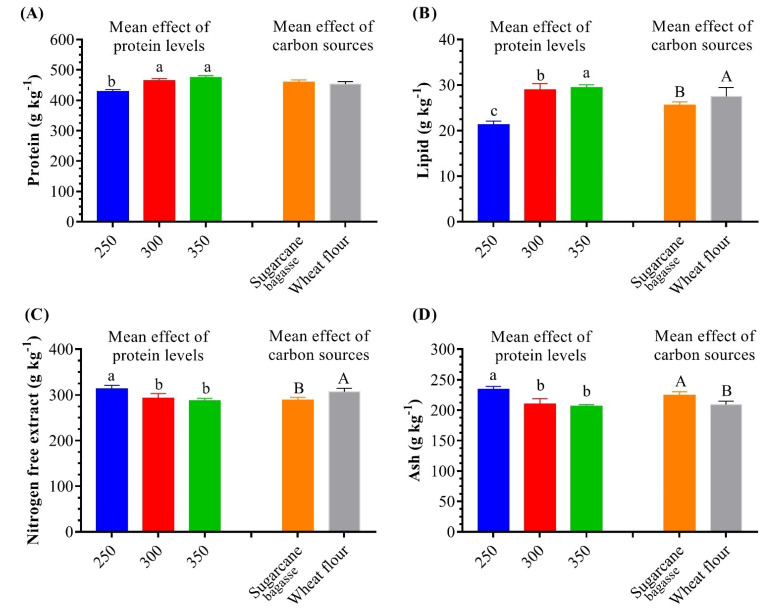
Figure 3 and Table 3 show the biochemical compositions of different BFT treatments, depending on both protein levels and different carbon sources. In all BFT treatments (% dry-weight basis), crude protein content ranged from 42.63% to 47.98%, lipid content ranged from 1.98% to 3.2%, and nitrogen-free extract content ranged from 27.48 to 32.49%, while ash content ranged from 19.18 to 24.13%. The highest (p < 0.05) protein values were recorded for groups fed diets with protein contents of 300 g protein kg−1 (SB300 and WF300) and 350 (SB350 and WF350), compared to 250 (SB250 and WF250). The crude lipid content was significantly (p < 0.05) higher in WF300, SB350, and WF350 than SB300 and SB250. The nitrogen-free extract was higher (p < 0.05) in groups WF250, WF250, and WF300 than the other BFT treatments. The ash content significantly decreased with increased dietary protein levels, and using WF than SB.
Figure 3.
Mean effect of dietary protein levels and carbon sources on biofloc proximate chemical composition of whiteleg shrimp, L. vannamei, juveniles reared in water. (A) Protein content, (B) lipid content, (C) nitrogen-free-extract content, and (D) ash content. Presented data are means ± SD (n = 3). Different letters indicate significantly different values (p < 0.05).
Table 3.
Effect of dietary protein levels and carbon sources on nutritional value (g kg−1) of the biofloc produced in biofloc treatments.
| Protein Levels (g kg−1) | Control (450) * | 250 | 300 | 350 | p-Value of Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carbon Sources | SB | WF | SB | WF | SB | WF | Protein Levels | Carbon Source | Interaction | |
| Protein | – | 432.20 ± 13.00 b | 426.30 ± 18.50 c | 467.80 ± 17.70 a | 463.80 ± 15.20 a | 479.80 ± 15.60 a | 471.6 ± 16.30 a | 0.001 | 0.436 | 0.972 |
| Lipid | – | 23.00 ± 0.10 b | 19.80 ± 0.30 c | 26.00 ± 0.20 b | 32.00 ± 0.10 a | 28.00 ± 0.30 a | 31.00 ± 0.40 a | <0.001 | <0.001 | <0.001 |
| Nitrogen-free extract | – | 303.50 ± 13.30 a | 324.90 ± 19.00 a | 274.80 ± 15.10 c | 312.40 ± 13.6 b | 290.30 ± 15.50 b | 285.50 ± 12.80 b | 0.028 | 0.026 | 0.090 |
| Ash | – | 241.3 ± 7.80 a | 229.00 ± 9.90 a | 231.40 ± 8.40 a | 191.80 ± 6.30 b | 201.90 ± 6.90 b | 211.90 ± 8.80 c | <0.001 | 0.002 | 0.003 |
Means (mean ± SD) in the same row having superscript differ significantly (p < 0.05); two-way ANOVA significant at p < 0.05. * The data of residues collected from control tanks are excluded, however they do not represent any importance to the study. Means with different letters is significantly different (p < 0.05).
The results show that increasing dietary protein levels significantly increases BFT protein and lipid contents, and decreases nitrogen-free extract and ash contents in the produced BFT. In addition, using WF as a carbon source significantly increased lipid and nitrogen-free extract contents. The interaction of dietary protein levels and different carbon sources was significant for lipids and ash and nonsignificant for protein content and nitrogen-free extract.
3.3. Microbial Community/Total Bacteria Count
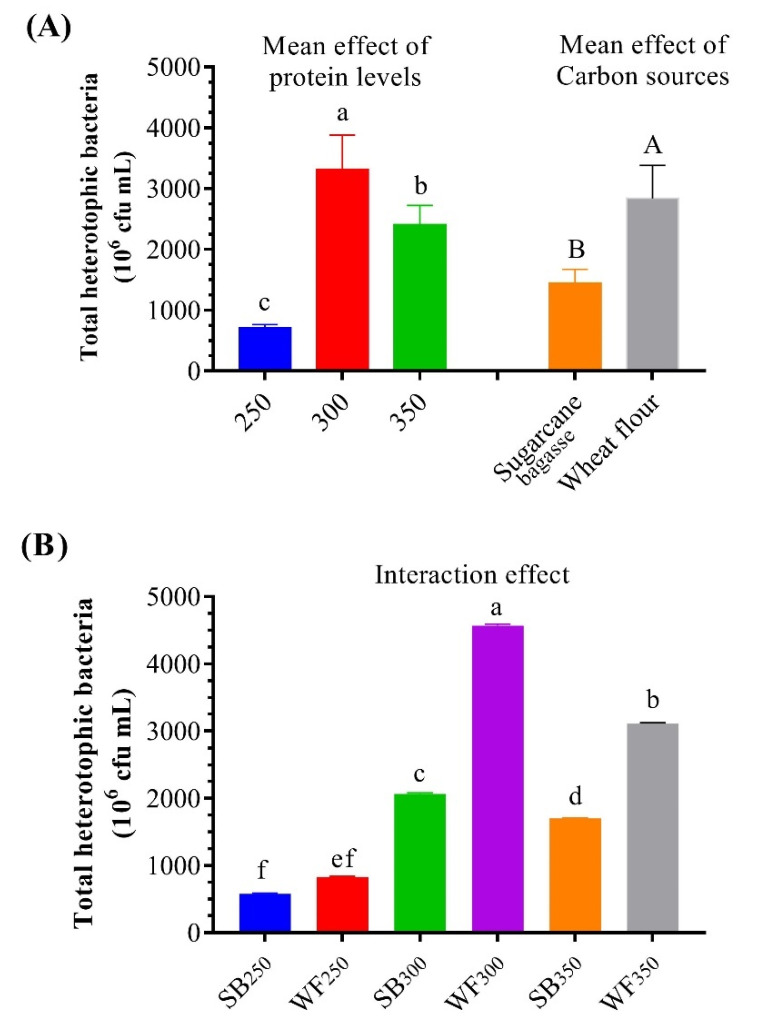
The THB count was developed for overall treatments, especially BFT treatments. The addition of different carbon sources significantly (p < 0.05) affected the growth of the THB count, whereas WF significantly increased the THB count compared to SB. The highest significant THB count was recorded for 300 g protein kg−1. The mean effect of the protein levels showed a significant increase in the THB count with 300 g protein kg−1. In addition, the interaction effect of dietary protein levels and carbon sources revealed that the THB count was significantly higher (p < 0.05) in WF300 and WF350 than in the other treatments (Figure 4).
Figure 4.
Effect of dietary protein levels and carbon sources on the total heterotrophic bacterial count in experimental tanks’ water. Presented data are means ± SD (n = 3). (A) Mean effect of protein levels and carbon source, and (B) interaction effect. Column bearing different letters is significantly different (p < 0.05).
3.4. Growth Performance
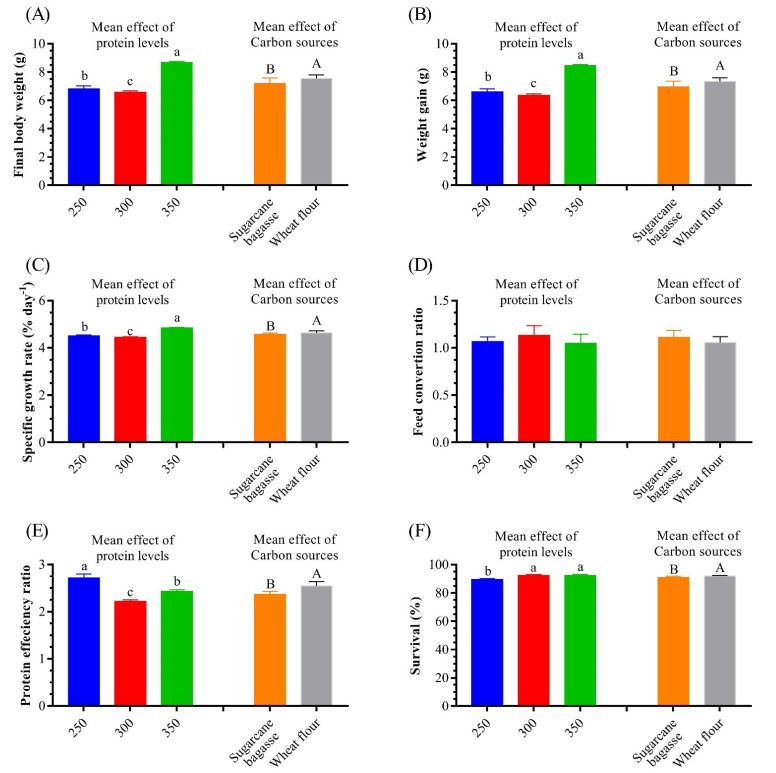
The results of the growth performance are presented in Figure 5 and Table 4. The main effects of increasing dietary protein levels showed a significant increase in FW, WG, SGR, and survival with the highest dietary protein levels. In addition, the shrimp reared in WF-based BFT systems had a higher growth performance than the shrimp reared in BS-based BFT. The interaction of both variables showed that there was a significant difference in the FBW, WG, PER, and SR% among the BFT treatments and the control. FBW and WG were significantly higher in SB350. However, there was no significant difference in FCR in all treatments. PER was significantly higher (p < 0.05) in WF250 than in the other treatments. The highest significant (p < 0.05) SR% was recorded at C450 (control diet), SB300, and WF350 (93.94%, 93.97%, and 93.97%, respectively), as presented in Table 4. The two-way ANOVA revealed high significant effects of both dietary protein levels and carbon sources in the BFT systems on growth performance, whereas the shrimp that were fed the highest protein levels had the highest FBW, WG, and SR.
Figure 5.
Mean effect of dietary protein levels and carbon sources on growth performance and feed utilization of whiteleg shrimp, L. vannamei, juveniles. (A) Final weight, (B) weight gain, (C) specific growth rate, (D) feed conversion ratio, (E) protein efficiency ratio, and (F) survival. Presented data are means ± SD (n = 3). Different letters indicate significantly different values (p < 0.05).
Table 4.
Growth indicators of whiteleg shrimp, L. vannamei, juveniles fed with different protein levels for the experimental period (75 days).
| Protein Levels (g kg−1) | Control (450) |
250 | 300 | 350 | p-Value of Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carbon Sources | SB | WF | SB | WF | SB | WF | Protein Levels | Carbon Source | Interaction | |
| FBW | 6.83 ± 0.04 d | 6.35 ± 0.02 e | 7.31 ± 0.01 c | 6.37 ± 0.07 e | 6.80 ± 0.02 d | 8.83 ± 0.03 a | 8.55 ± 0.07 b | 0.001 | 0.001 | 0.001 |
| WG | 6.60 ± 0.04 d | 6.12 ± 0.02 e | 7.08 ± 0.01 c | 6.14 ± 0.07 e | 6.57 ± 0.02 d | 8.60 ± 0.03 a | 8.32 ± 0.07 b | 0.001 | 0.001 | 0.001 |
| SGR | 4.52 ± 0.01 | 4.43 ± 0.01 | 4.61 ± 0.01 | 4.43 ± 0.02 | 4.52 ± 0.01 | 4.86 ± 0.01 | 4.82 ± 0.01 | 0.001 | 0.001 | 0.10 |
| PER | 1.52 ± 0.01 e | 2.54 ± 0.01 b | 2.92 ± 0.01 a | 2.12 ± 0.01 d | 2.26 ± 0.01 c | 2.52 ± 0.01 b,c | 2.44 ± 0.01 b | 0.001 | 0.001 | 0.001 |
| FCR | 1.30 ± 0.36 | 1.09 ± 0.11 | 0.97 ± 0.09 | 1.25 ± 0.34 | 1.03 ± 0.09 | 0.93 ± 0.08 | 1.17 ± 0.32 | 0.74 | 0.55 | 0.11 |
| SR | 93.94 ± 3.32 a | 87.88 ± 5.92 c | 90.91 ± 4.57 b | 93.97 ± 5.74 a | 90.91 ± 6.55 b | 90.91 ± 4.57 b | 93.97 ± 3.45 a | 0.001 | 0.001 | 0.001 |
Means (mean ± SD) in the same row have superscripts that differ significantly (p < 0.05); two-way ANOVA significant at p < 0.05. FBW: final body weight (g), WG: weight gain (g), SGR: specific growth rate (% day−1), FCR: feed conversion ratio, PER: protein efficiency ratio, and SR: survival rate (%). Means with different letters is significantly different (p < 0.05).
4. Discussion
The importance of BFT technology for shrimp culture has been reported, particularly in intense culture systems with negligible water exchange [10,12,30,36,53,64]. In the current study, the BFT was promoted through the appropriate addition of different carbon sources. The BFT volume and TSS, in addition to the other water quality conditions, were gradually increased and kept within optimum ranges. The formation and development of BFT in shrimp water depended on the assimilation of dissolved nitrogenous substances from uneaten feed and shrimp excretions by THB [55,65]. The optimum C/N ratio (16:1) promoted the growth of THB in the water column and improved the BFT composition, in comparison to the control diet [48,53]. The increased bacterial load resulted in a faster degradation of organic wastes, releasing inorganic nutrients, which stimulated the development of protein-rich new bacterial cells [53]. As a result of the development of the heterotrophic community, single-cell bacterial protein was formed, which served as a protein source for shrimp, leading to a higher growth rate. In the present study, the higher THB load/count developed in BFT treatments increased the growth of L. vannamei compared to the control treatment. This could be due to the assimilation of the shrimp to the formed BFT as additional supplemental food [30,53]. In addition, Heo et al. [66] suggested that the Lactobacillus strain that is abundant in BFT is a good candidate feed additive to improve fish growth and enhance immune responses. Bacteriocins produced by lactic acid bacteria have gained a lot of interest because they are used as natural preservatives in food [67].
The current results show that the THB count increases gradually at the end of the experiment, which agrees with the study conducted by de Paiva Maia et al. [68], who found that the THB count increased in BFT treatments in the 10th and 12th weeks, indicating that THB might effectively assimilate the TAN to generate bacterial protein and new cells if an appropriate carbon source was used [9,59,60]. In the current study, the additions of different carbon sources of SB and WF stimulated THB growth in the aquarium water. The increase in THB could improve water quality via immobilizing inorganic nitrogen [60,69], which is the most common waste material in aquaculture, and by converting this waste into microbial protein [9], it could be used as a supplemental meal for shrimp and improve digestive enzyme activity [11].
The nutritional composition of BFT has been observed to be influenced by the carbon source [22]. However, there is a scarcity of information on the effect of dietary protein levels and the addition of carbon sources in penaeid shrimp farming on growth performance and immunological response. Although the involvement of BFT in stimulating shrimp growth is mainly unknown, it could produce a variety of effects. The whiteleg shrimp were found to be able to utilize and digest BFT in situ, which was available 24 h a day in the rearing water [53]. As a result, BFT could improve shrimp growth rates in culture systems as an essential and extra natural food supply [16,18]. It not only provided vital macronutrients (protein, lipids, and carbohydrates), but it also met the shrimp’s mineral and vitamin needs [13,70,71].
From the results of the present study, it can be observed that the BFT crude protein content is significantly high in the treatments of SB300, WF300, SB350, and WF350, which means that the BFT protein content increases with increasing the dietary protein level. However, it could be noted that reducing the dietary protein level from 350 to 250 g protein kg−1 could not affect the BFT protein composition [14]. In a proximate analysis, the used carbon source, TSS level, salinity, stocking density, light intensity, phytoplankton, zooplankton, and bacterial populations influenced the nutritional characteristics of BFT [72]. In addition, Xu and Pan [13] found that the proximate chemical analysis of the BFT produced in L. vannamei water showed that BFT had a crude protein content comparable with their practical diet, with a range of 25–320 g protein kg−1. Our results agree with Rajkumar et al. [70] who found that the protein, lipid, and fiber contents of BFT produced in BFT with WF in L. vannamei tanks were significantly higher than those of the control. The high protein content was observed in the BFT produced in the BFT with WF followed by BFT with sugarcane molasses. BFT with more than 25% crude protein, 4% fiber, and 7% ash can be regarded as acceptable for aquatic animal nutrition, particularly for herbivorous/omnivorous fish and shrimp species [73].
Generally, the protein level in the shrimp diet is the most influential factor in shrimp culture. In this study, the shrimp fed on SB350 and WF350 g protein kg−1 had a better growth performance, compared to the shrimp fed on C450. This means that shrimp fed lower levels of protein with BFT could compensate for the higher protein diet (control diet). In agreement with the present study, the growth performance of L. vannamei reared in BFT systems was not affected by using different dietary protein levels, such as 30 and 450 g protein kg−1 for post-larvae in ponds [74], 250–350 and 350–400 g protein kg−1 for juveniles in tanks [13,23], and 350–400 g protein kg−1 for juveniles in raceways [75]. On the other hand, several studies found that increasing the protein content in food improves shrimp performance over a lower range of 210–310 g protein kg−1 for juveniles in tanks and 250–350 g protein kg−1 for adults [14,45].
The addition of the appropriate amount of carbon aided L. vannamei growth and survival. This could be due to the combined impact of better water quality and higher bacterial and zooplankton concentrations [76]. Several studies have indicated that carbon-source addition has been linked to the formation and accumulation of BFT [77,78], which could provide an essential food source or a safe shelter for zooplankton, boosting fish and shrimp growth, in addition to their environmental role in aquatic ecosystems [79,80]. Furthermore, zooplankton has been shown to be a supplemental food supply for L. vannamei, possibly increasing the efficiency of microbial protein conversion to animal protein [81,82].
In the current study, improved values of PE were observed in the BFT treatments. PER values were highly significant in SB250 and WF250 than in the other treatments. When there is a high abundance of BFT in the culture system, feeding shrimp a high-protein diet may be unnecessary and uneconomical. In other words, a low level of dietary protein might be compensated for by consuming BFT, allowing for the dietary protein content to be reduced. The BFT influence on protein feeding could have contributed to the shrimps’ good development in the BFT treatments. In accordance, L. vannamei and P. monodon juveniles had higher growth rates in the BFT-based system than others reared in clear water [16,18].
In the current study, the final weight, WG, and SGR of L. vannamei in the BFT treatments fed diets containing 250, 300, and 400 g protein kg−1 were markedly higher than those obtained in the control groups that were fed diets containing 450 g protein kg−1. Accordingly, rearing shrimps in the BFT system could compensate for the reduction in dietary protein. More significantly, by decreasing diet protein, the fishmeal level decreased; a low-protein diet is more cost-effective and environmentally beneficial [5]. The reduction in dietary protein levels without affecting shrimp growth has been reported in several studies, whereas microbial proteins could provided an alternative source of protein for shrimp in the BFT systems [48,83]. In a similar study, Decamp et al. [84] reported no significant changes in the growth performance of L. vannamei grown in unfiltered pond water and fed either a 25 or 35 percent CP diet. In addition, the SGR of P. monodon fed on a 250 and 400 g protein kg−1 diet did not significantly differ when reared in a BFT system, even under intensive culture [48]. The better growth and survival rates of L. vannamei may be due to the improved water quality as a result of flocculation in a zero-water exchange system, which is caused by adding organic carbon sources in a correct C/N ratio to the system [85]. In addition, Yun et al. [14] and Browdy et al. [74] found that the growth of juvenile whiteleg shrimp L. vannamei was not affected by decreasing dietary protein from 350 to 300 or lower. Moreover, FCR could be maintained in shrimp reared in a BFT-based system, even with reducing dietary protein by around 10% [23].
5. Conclusions
The results of the present study reveal that different carbon sources and protein levels could effectively influence the nutritional value of biofloc. The current study suggested that, by rearing whiteleg shrimp, Litopenaeus vannamei, on a biofloc system, dietary protein levels may be reduced from 450 to 350 g protein kg−1 diet with improving the growth performance and feed utilization of shrimp using biofloc technology. In addition, the applied biofloc improved the growth performance and feed utilization of the shrimp, probably by providing a supplemental food source with appropriate protein and lipid contents. The total heterotrophic bacterial count in WF300 and WF350 g protein kg−1 (using wheat flour as a carbon source) was significantly higher than in the other treatments. The 350 g protein kg−1 provided the best performance indices, demonstrating the greater economic viability of shrimp.
Acknowledgments
The authors acknowledge Al Bilad Bank Scholarly Chair for Food Security in Saudi Arabia, the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant No. CHAIR42). Additionally, the authors would like to acknowledge the Science, Technology, and Innovation Funding Authority (STDF), Egypt, through a project titled: Development and Research Application of bioFloc Technology for increasing shrimp production in Egypt (EGY-DRAFT), Agreement No. 25305 (STDF-RG)/STDF-Youth.
Author Contributions
Conceptualization: O.A.A. and Z.Z.S.; methodology: O.A.A. and Z.Z.S.; software: O.A.A., Z.Z.S., A.T.M. and M.A.; validation: O.A.A., Z.Z.S., A.T.M. and M.A.; formal analysis: O.A.A., Z.Z.S. and M.A.; investigation: O.A.A., Z.Z.S., A.T.M. and M.A.; resources: Z.Z.S., A.T.M., A.S.A. and K.M.A.R.; data curation: M.A., Z.Z.S. and A.T.M.; writing—original draft preparation: O.A.A., Z.Z.S. and M.A.; writing—review and editing: M.A., Z.Z.S. and A.T.M.; visualization: O.A.A., Z.Z.S. and M.A.; supervision: M.A. and Z.Z.S.; project administration: Z.Z.S., A.T.M., A.S.A. and K.M.A.R.; funding acquisition: Z.Z.S., A.T.M., A.S.A. and K.M.A.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Compliance with ethical standards in the experimental setup and shrimp handling was approved by the Research Committee of the NIOF, Egypt, approval no.: NIOF/AQ3/1/22/R/012 at 22 January 2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Al Bilad Bank Scholarly Chair for Food Security in Saudi Arabia, the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant No. CHAIR42).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abbas E.M., Ali F.S., Desouky M.G., Ashour M., El-Shafei A., Maaty M.M., Sharawy Z.Z. Novel Comprehensive Molecular and Ecological Study Introducing Coastal Mud Shrimp (Solenocera Crassicornis) Recorded at the Gulf of Suez, Egypt. J. Mar. Sci. Eng. 2020;9:9. doi: 10.3390/jmse9010009. [DOI] [Google Scholar]
- 2.Mabrouk M.M., Ashour M., Labena A., Zaki M.A.A., Abdelhamid A.F., Gewaily M.S., Dawood M.A.O., Abualnaja K.M., Ayoub H.F. Nanoparticles of Arthrospira platensis improves growth, antioxidative and immunological responses of Nile tilapia (Oreochromis niloticus) and its resistance to Aeromonas hydrophila. Aquac. Res. 2022;53:125–135. doi: 10.1111/are.15558. [DOI] [Google Scholar]
- 3.Sharawy Z.Z., Ashour M., Abbas E., Ashry O., Helal M., Nazmi H., Kelany M., Kamel A., Hassaan M., Rossi W., et al. Effects of dietary marine microalgae, Tetraselmis suecica, on production, gene expression, protein markers and bacterial count of Pacific white shrimp Litopenaeus vannamei. Aquac. Res. 2020;51:2216–2228. doi: 10.1111/are.14566. [DOI] [Google Scholar]
- 4.Sharawy Z.Z., Ashour M., Labena A., Alsaqufi A.S., Mansour A.T., Abbas E.M. Effects of dietary Arthrospira platensis nanoparticles on growth performance, feed utilization, and growth-related gene expression of Pacific white shrimp, Litopenaeus vannamei. Aquaculture. 2022;551:737905. doi: 10.1016/j.aquaculture.2022.737905. [DOI] [Google Scholar]
- 5.Ashour M., Mabrouk M.M., Abo-Taleb H.A., Sharawy Z.Z., Ayoub H.F., Van Doan H., Davies S.J., El-Haroun E., Goda A.M.S.A. A liquid seaweed extract (TAM®) improves aqueous rearing environment, diversity of zooplankton community, whilst enhancing growth and immune response of Nile tilapia, Oreochromis niloticus, challenged by Aeromonas hydrophila. Aquaculture. 2021;543:736915. doi: 10.1016/j.aquaculture.2021.736915. [DOI] [Google Scholar]
- 6.Mansour A.T., Ashour M., Alprol A.E., Alsaqufi A.S. Aquatic plants and aquatic animals in the context of sustainability: Cultivation techniques, integration, and blue revolution. Sustainability. 2022;14:3257. doi: 10.3390/su14063257. [DOI] [Google Scholar]
- 7.De Schryver P., Crab R., Defoirdt T., Boon N., Verstraete W. The basics of bio-flocs technology: The added value for aquaculture. Aquaculture. 2008;277:125–137. doi: 10.1016/j.aquaculture.2008.02.019. [DOI] [Google Scholar]
- 8.Stokstad E. Down on the Shrimp Farm. American Association for the Advancement of Science; Washington, DC, USA: 2010. [Google Scholar]
- 9.Avnimelech Y. Biofloc Technology: A Practical Guide Book. World Aquaculture Society; Baton Rouge, LA, USA: 2009. [Google Scholar]
- 10.Crab R., Defoirdt T., Bossier P., Verstraete W. Biofloc technology in aquaculture: Beneficial effects and future challenges. Aquaculture. 2012;356:351–356. doi: 10.1016/j.aquaculture.2012.04.046. [DOI] [Google Scholar]
- 11.Xu W.-J., Pan L.-Q. Enhancement of immune response and antioxidant status of Litopenaeus vannamei juvenile in biofloc-based culture tanks manipulating high C/N ratio of feed input. Aquaculture. 2013;412:117–124. doi: 10.1016/j.aquaculture.2013.07.017. [DOI] [Google Scholar]
- 12.Burford M.A., Thompson P.J., McIntosh R.P., Bauman R.H., Pearson D.C. The contribution of flocculated material to shrimp (Litopenaeus vannamei) nutrition in a high-intensity, zero-exchange system. Aquaculture. 2004;232:525–537. doi: 10.1016/S0044-8486(03)00541-6. [DOI] [Google Scholar]
- 13.Xu W.-J., Pan L.-Q. Effects of bioflocs on growth performance, digestive enzyme activity and body composition of juvenile Litopenaeus vannamei in zero-water exchange tanks manipulating C/N ratio in feed. Aquaculture. 2012;356:147–152. doi: 10.1016/j.aquaculture.2012.05.022. [DOI] [Google Scholar]
- 14.Yun H., Shahkar E., Katya K., Jang I.K., Kim S.K., Bai S.C. Effects of bioflocs on dietary protein requirement in juvenile whiteleg shrimp, Litopenaeus vannamei. Aquac. Res. 2016;47:3203–3214. doi: 10.1111/are.12772. [DOI] [Google Scholar]
- 15.Sharawy Z.Z., Abbas E.M., Abdelkhalek N.K., Ashry O.A., Abd El-Fattah L.S., El-Sawy M.A., Helal M.F., El-Haroun E. Effect of organic carbon source and stocking densities on growth indices, water microflora, and immune-related genes expression of Litopenaeus vannamei Larvae in intensive culture. Aquaculture. 2022;546:737397. doi: 10.1016/j.aquaculture.2021.737397. [DOI] [Google Scholar]
- 16.Arnold S.J., Coman F.E., Jackson C.J., Groves S.A. High-intensity, zero water-exchange production of juvenile tiger shrimp, Penaeus monodon: An evaluation of artificial substrates and stocking density. Aquaculture. 2009;293:42–48. doi: 10.1016/j.aquaculture.2009.03.049. [DOI] [Google Scholar]
- 17.Emerenciano M., Ballester E.L., Cavalli R.O., Wasielesky W. Biofloc technology application as a food source in a limited water exchange nursery system for pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817) Aquac. Res. 2012;43:447–457. doi: 10.1111/j.1365-2109.2011.02848.x. [DOI] [Google Scholar]
- 18.Wasielesky Jr W., Atwood H., Stokes A., Browdy C.L. Effect of natural production in a zero exchange suspended microbial floc based super-intensive culture system for white shrimp Litopenaeus vannamei. Aquaculture. 2006;258:396–403. doi: 10.1016/j.aquaculture.2006.04.030. [DOI] [Google Scholar]
- 19.Ju Z.Y., Forster I., Conquest L., Dominy W., Kuo W.C., David Horgen F. Determination of microbial community structures of shrimp floc cultures by biomarkers and analysis of floc amino acid profiles. Aquac. Res. 2008;39:118–133. doi: 10.1111/j.1365-2109.2007.01856.x. [DOI] [Google Scholar]
- 20.Ekasari J., Crab R., Verstraete W. Primary nutritional content of bio-flocs cultured with different organic carbon sources and salinity. Hayati J. Biosci. 2010;17:125–130. doi: 10.4308/hjb.17.3.125. [DOI] [Google Scholar]
- 21.Lara G., Krummenauer D., Abreu P.C., Poersch L.H., Wasielesky W. The use of different aerators on Litopenaeus vannamei biofloc culture system: Effects on water quality, shrimp growth and biofloc composition. Aquac. Int. 2017;25:147–162. doi: 10.1007/s10499-016-0019-8. [DOI] [Google Scholar]
- 22.Crab R., Chielens B., Wille M., Bossier P., Verstraete W. The effect of different carbon sources on the nutritional value of bioflocs, a feed for Macrobrachium rosenbergii postlarvae. Aquac. Res. 2010;41:559–567. doi: 10.1111/j.1365-2109.2009.02353.x. [DOI] [Google Scholar]
- 23.Xu W.J., Pan L.Q. Dietary protein level and C/N ratio manipulation in zero-exchange culture of Litopenaeus vannamei: Evaluation of inorganic nitrogen control, biofloc composition and shrimp performance. Aquac. Res. 2014;45:1842–1851. [Google Scholar]
- 24.Kim S.K., Pang Z., Seo H.C., Cho Y.R., Samocha T., Jang I.K. Effect of bioflocs on growth and immune activity of Pacific white shrimp, Litopenaeus vannamei postlarvae. Aquac. Res. 2014;45:362–371. doi: 10.1111/are.12319. [DOI] [Google Scholar]
- 25.Haslun J.A., Correia E., Strychar K., Morris T., Samocha T. Characterization of bioflocs in a no water exchange super-intensive system for the production of food size pacific white shrimp Litopenaeus vannamei. Int. J. Aquac. 2012;2:29–38. [Google Scholar]
- 26.Zhao P., Huang J., Wang X.-H., Song X.-L., Yang C.-H., Zhang X.-G., Wang G.-C. The application of bioflocs technology in high-intensive, zero exchange farming systems of Marsupenaeus japonicus. Aquaculture. 2012;354:97–106. doi: 10.1016/j.aquaculture.2012.03.034. [DOI] [Google Scholar]
- 27.Luo G., Gao Q., Wang C., Liu W., Sun D., Li L., Tan H. Growth, digestive activity, welfare, and partial cost-effectiveness of genetically improved farmed tilapia (Oreochromis niloticus) cultured in a recirculating aquaculture system and an indoor biofloc system. Aquaculture. 2014;422:1–7. doi: 10.1016/j.aquaculture.2013.11.023. [DOI] [Google Scholar]
- 28.Liu L., Hu Z., Dai X., Avnimelech Y. Effects of addition of maize starch on the yield, water quality and formation of bioflocs in an integrated shrimp culture system. Aquaculture. 2014;418:79–86. doi: 10.1016/j.aquaculture.2013.10.005. [DOI] [Google Scholar]
- 29.Browdy C.L., Ray A.J., Leffler J.W., Avnimelech Y. Biofloc-Based Aquaculture Systems. Wiley-Blackwell; New Delhi, India: 2012. [Google Scholar]
- 30.El-Sayed A.F.M. Use of biofloc technology in shrimp aquaculture: A comprehensive review, with emphasis on the last decade. Rev. Aquac. 2021;13:676–705. doi: 10.1111/raq.12494. [DOI] [Google Scholar]
- 31.Panigrahi A., Sundaram M., Saranya C., Swain S., Dash R., Dayal J.S. Carbohydrate sources deferentially influence growth performances, microbial dynamics and immunomodulation in Pacific white shrimp (Litopenaeus vannamei) under biofloc system. Fish Shellfish Immunol. 2019;86:1207–1216. doi: 10.1016/j.fsi.2018.12.040. [DOI] [PubMed] [Google Scholar]
- 32.Abakari G., Luo G., Kombat E.O., Alhassan E.H. Supplemental carbon sources applied in biofloc technology aquaculture systems: Types, effects and future research. Rev. Aquac. 2021;13:1193–1222. doi: 10.1111/raq.12520. [DOI] [Google Scholar]
- 33.Elabd H., Wang H.P., Shaheen A., Yao H., Abbass A. Astragalus membranaceus (AM) enhances growth performance and antioxidant stress profiles in bluegill sunfish (Lepomis macrochirus) Fish Physiol. Biochem. 2016;42:955–966. doi: 10.1007/s10695-015-0188-9. [DOI] [PubMed] [Google Scholar]
- 34.Zhao D., Pan L., Huang F., Wang C., Xu W. Effects of different carbon sources on bioactive compound production of biofloc, immune response, antioxidant level, and growth performance of Litopenaeus vannamei in zero-water exchange culture tanks. J. World Aquac. Soc. 2016;47:566–576. doi: 10.1111/jwas.12292. [DOI] [Google Scholar]
- 35.Mansour A.T., Esteban M.Á. Effects of carbon sources and plant protein levels in a biofloc system on growth performance, and the immune and antioxidant status of Nile tilapia (Oreochromis niloticus) Fish Shellfish Immunol. 2017;64:202–209. doi: 10.1016/j.fsi.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 36.Ekasari J., Azhar M.H., Surawidjaja E.H., Nuryati S., De Schryver P., Bossier P. Immune response and disease resistance of shrimp fed biofloc grown on different carbon sources. Fish Shellfish Immunol. 2014;41:332–339. doi: 10.1016/j.fsi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Khanjani M.H., Sharifinia M. Biofloc technology as a promising tool to improve aquaculture production. Rev. Aquac. 2020;12:1836–1850. doi: 10.1111/raq.12412. [DOI] [Google Scholar]
- 38.Abualnaja K.M., Alprol A.E., Abu-Saied M., Ashour M., Mansour A.T. Removing of anionic dye from aqueous solutions by adsorption using of multiwalled carbon nanotubes and poly (acrylonitrile-styrene) impregnated with activated carbon. Sustainability. 2021;13:7077. doi: 10.3390/su13137077. [DOI] [Google Scholar]
- 39.Tacon A., Cody J., Conquest L., Divakaran S., Forster I., Decamp O. Effect of culture system on the nutrition and growth performance of Pacific white shrimp Litopenaeus vannamei (Boone) fed different diets. Aquac. Nutr. 2002;8:121–137. doi: 10.1046/j.1365-2095.2002.00199.x. [DOI] [Google Scholar]
- 40.Ashour M., Alprol A.E., Heneash A.M., Saleh H., Abualnaja K.M., Alhashmialameer D., Mansour A.T. Ammonia bioremediation from aquaculture wastewater effluents using Arthrospira platensis NIOF17/003: Impact of biodiesel residue and potential of ammonia-loaded biomass as rotifer feed. Materials. 2021;14:5460. doi: 10.3390/ma14185460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kureshy N., Davis D.A. Protein requirement for maintenance and maximum weight gain for the Pacific white shrimp, Litopenaeus vannamei. Aquaculture. 2002;204:125–143. doi: 10.1016/S0044-8486(01)00649-4. [DOI] [Google Scholar]
- 42.Shahkar E., Yun H., Park G., Jang I.-K., kyoung Kim S., Katya K., Bai S.C. Evaluation of optimum dietary protein level for juvenile whiteleg shrimp (Litopenaeus vannamei) J. Crust. Biol. 2014;34:552–558. doi: 10.1163/1937240X-00002267. [DOI] [Google Scholar]
- 43.Hu Y., Tan B., Mai K., Ai Q., Zheng S., Cheng K. Growth and body composition of juvenile white shrimp, Litopenaeus vannamei, fed different ratios of dietary protein to energy. Aquac. Nutr. 2008;14:499–506. doi: 10.1111/j.1365-2095.2007.00555.x. [DOI] [Google Scholar]
- 44.Martinez-Cordova L.R., Porchas-Cornejo M.A., Villarreal-Colemnares H., Calderon-Perez J.A., Naranjo-Paramo J. Evaluation of three feeding strategies on the culture of white shrimp Penaeus vannamei Boone 1931 in low water exchange ponds. Aquac. Eng. 1998;17:21–28. doi: 10.1016/S0144-8609(97)00010-1. [DOI] [Google Scholar]
- 45.Hamidoghli A., Yun H., Shahkar E., Won S., Hong J., Bai S.C. Optimum dietary protein-to-energy ratio for juvenile whiteleg shrimp, Litopenaeus vannamei, reared in a biofloc system. Aquac. Res. 2018;49:1875–1886. doi: 10.1111/are.13643. [DOI] [Google Scholar]
- 46.Smith L.L., Lee P.G., Lawrence A.L., Strawn K. Growth and digestibility by three sizes of Penaeus vannamei Boone: Effects of dietary protein level and protein source. Aquaculture. 1985;46:85–96. doi: 10.1016/0044-8486(85)90193-0. [DOI] [Google Scholar]
- 47.Liu D., He J., Liu Y., Zheng S., Tian L. Effects of dietary protein levels on growth performance and immune condition of Pacific white shrimp Litopenaeus vannamei juveniles at very low salinity. Acta Sci. Nat. Univ. Sunyatseni. 2005;44:217–223. [Google Scholar]
- 48.Hari B., Kurup B.M., Varghese J.T., Schrama J., Verdegem M. Effects of carbohydrate addition on production in extensive shrimp culture systems. Aquaculture. 2004;241:179–194. doi: 10.1016/j.aquaculture.2004.07.002. [DOI] [Google Scholar]
- 49.Schneider O., Sereti V., Eding E., Verreth J. Analysis of nutrient flows in integrated intensive aquaculture systems. Aquac. Eng. 2005;32:379–401. doi: 10.1016/j.aquaeng.2004.09.001. [DOI] [Google Scholar]
- 50.Hargreaves J.A. Photosynthetic suspended-growth systems in aquaculture. Aquac. Eng. 2006;34:344–363. doi: 10.1016/j.aquaeng.2005.08.009. [DOI] [Google Scholar]
- 51.Lee C., Lee K.-J. Dietary protein requirement of Pacific white shrimp Litopenaeus vannamei in three different growth stages. Fish. Aquat. Sci. 2018;21:30–36. doi: 10.1186/s41240-018-0105-0. [DOI] [Google Scholar]
- 52.AOAC . Official Methods of Analysis of the Association of Official Analytical Chemists. 17th ed. Volume I The Association of Official Analytical Chemists; Washington, DC, USA: 2003. [Google Scholar]
- 53.Avnimelech Y. Biofloc-Based Aquaculture Systems. Aquaculture Production Systems. Wiley-Blackwell; New Delhi, India: 2012. [Google Scholar]
- 54.Parsons T.R. A Manual of Chemical & Biological Methods for Seawater Analysis. Elsevier; Amsterdam, The Netherlands: 2013. [Google Scholar]
- 55.Avnimelech Y., Kochba M. Evaluation of nitrogen uptake and excretion by tilapia in bio floc tanks, using 15N tracing. Aquaculture. 2009;287:163–168. doi: 10.1016/j.aquaculture.2008.10.009. [DOI] [Google Scholar]
- 56.Jackson M.L. Soil Chemical Analysis: Advanced Course. UW-Madison Libraries Parallel Press; Madison, WI, USA: 2005. [Google Scholar]
- 57.Bremner J. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960;55:11–33. doi: 10.1017/S0021859600021572. [DOI] [Google Scholar]
- 58.Matsler A., Siebenmorgen T. Evaluation of operating conditions for surface lipid extraction from rice using a soxtec system. Cereal Chem. 2005;82:282–286. doi: 10.1094/CC-82-0282. [DOI] [Google Scholar]
- 59.Kumar S., Anand P.S.S., De D., Deo A.D., Ghoshal T.K., Sundaray J.K., Ponniah A.G., Jithendran K.P., Raja R.A., Biswas G. Effects of biofloc under different carbon sources and protein levels on water quality, growth performance and immune responses in black tiger shrimp Penaeus monodon (Fabricius, 1978) Aquac. Res. 2017;48:1168–1182. doi: 10.1111/are.12958. [DOI] [Google Scholar]
- 60.Kumar S., Shyne Anand P., De D., Sundaray J., Ananda Raja R., Biswas G., Ponniah A., Ghoshal T., Deo A., Panigrahi A. Effects of carbohydrate supplementation on water quality, microbial dynamics and growth performance of giant tiger prawn (Penaeus monodon) Aquac. Int. 2014;22:901–912. doi: 10.1007/s10499-013-9715-9. [DOI] [Google Scholar]
- 61.Duncan D.B. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- 62.Wang L., Yin N., Sagada G., Hua Y., Li H., Zhang J., Shao Q. Partial replacement of fishmeal with corn gluten meal, pea protein isolate and their mixture in diet of black sea bream (Acanthopagrus schlegelii) juveniles: Effects on growth performance, feed utilization and haematological parameters. Aquac. Res. 2020;51:2071–2083. doi: 10.1111/are.14558. [DOI] [Google Scholar]
- 63.Mohanty R.K., Ambast S., Panigrahi P., Mandal K. Water quality suitability and water use indices: Useful management tools in coastal aquaculture of Litopenaeus vannamei. Aquaculture. 2018;485:210–219. doi: 10.1016/j.aquaculture.2017.11.048. [DOI] [Google Scholar]
- 64.Emerenciano M., Cuzon G., Paredes A., Gaxiola G. Biofloc technology applied to intensive broodstock farming of pink shrimp Farfantepenaeus duorarum (Part I): Growout, water quality, microorganisms profile and proximate analysis of biofloc. Aquac. Res. 2012;45:1713–1726. doi: 10.1111/are.12117. [DOI] [Google Scholar]
- 65.Chen Y., Avnimelech Y. The Role of Organic Matter in Modern Agriculture. Volume 25 Springer Science & Business Media; Berlin/Heidelberg, Germany: 2012. [Google Scholar]
- 66.Heo W.-S., Kim Y.-R., Kim E.-Y., Bai S.C., Kong I.-S. Effects of dietary probiotic, Lactococcus lactis subsp. lactis I2, supplementation on the growth and immune response of olive flounder (Paralichthys olivaceus) Aquaculture. 2013;376:20–24. doi: 10.1016/j.aquaculture.2012.11.009. [DOI] [Google Scholar]
- 67.Cleveland J., Montville T.J., Nes I.F., Chikindas M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001;71:1–20. doi: 10.1016/S0168-1605(01)00560-8. [DOI] [PubMed] [Google Scholar]
- 68.de Paiva Maia E., Modesto G.A., Brito L.O., Galvez A.O., Gesteira T.C.V. Intensive culture system of Litopenaeus vannamei in commercial ponds with zero water exchange and addition of molasses and probiotics. Rev. Biol. Mar. Oceanogr. 2016;51:61–67. doi: 10.4067/S0718-19572016000100006. [DOI] [Google Scholar]
- 69.Anand P., Kumar S., Panigrahi A., Ghoshal T., Syama Dayal J., Biswas G., Sundaray J., De D., Ananda Raja R., Deo A. Effects of C: N ratio and substrate integration on periphyton biomass, microbial dynamics and growth of Penaeus monodon juveniles. Aquac. Int. 2013;21:511–524. doi: 10.1007/s10499-012-9585-6. [DOI] [Google Scholar]
- 70.Rajkumar M., Pandey P.K., Aravind R., Vennila A., Bharti V., Purushothaman C.S. Effect of different biofloc system on water quality, biofloc composition and growth performance in Litopenaeus vannamei (Boone, 1931) Aquac. Res. 2016;47:3432–3444. doi: 10.1111/are.12792. [DOI] [Google Scholar]
- 71.Becerril-Cortés D., Monroy-Dosta M.D.C., Emerenciano M.G.C., Castro-Mejía G., Sofia B., Bermúdez S., Correa G.V. Effect on nutritional composition of produced bioflocs with different carbon sources (molasses, coffee waste and rice bran) in Biofloc system. Int. J. Fish. Aquat. Stud. 2018;6:541–547. [Google Scholar]
- 72.Emerenciano M., Cuzon G., Paredes A., Gaxiola G. Evaluation of biofloc technology in pink shrimp Farfantepenaeus duorarum culture: Growth performance, water quality, microorganisms profile and proximate analysis of biofloc. Aquac. Int. 2013;21:1381–1394. doi: 10.1007/s10499-013-9640-y. [DOI] [Google Scholar]
- 73.Webster C.D., Lim C. Nutrient Requirements and Feeding of Finfish for Aquaculture. CABI; Wallingford, UK: 2002. [Google Scholar]
- 74.Browdy C.L., Bratvold D., Stokesland A.D., McIntosh P. Perspective on the Application of Closed Shrimp Culture Systems. The World Aquaculture Society; Baton Rouge, LA, USA: 2001. [Google Scholar]
- 75.Prangnell D.I., Castro L.F., Ali A.S., Browdy C.L., Samocha T.M. The performance of juvenile Litopenaeus vannamei fed commercial diets of differing protein content, in a super-intensive biofloc-dominated system. J. Appl. Aquac. 2022;34:1–22. doi: 10.1080/10454438.2020.1766632. [DOI] [Google Scholar]
- 76.Ahmad I., Babitha Rani A., Verma A., Maqsood M. Biofloc technology: An emerging avenue in aquatic animal healthcare and nutrition. Aquac. Int. 2017;25:1215–1226. doi: 10.1007/s10499-016-0108-8. [DOI] [Google Scholar]
- 77.Avnimelech Y. Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds. Aquaculture. 2007;264:140–147. doi: 10.1016/j.aquaculture.2006.11.025. [DOI] [Google Scholar]
- 78.Emerenciano M., Ballester E.L., Cavalli R.O., Wasielesky W. Effect of biofloc technology (BFT) on the early postlarval stage of pink shrimp Farfantepenaeus paulensis: Growth performance, floc composition and salinity stress tolerance. Aquac. Int. 2011;19:891–901. doi: 10.1007/s10499-010-9408-6. [DOI] [Google Scholar]
- 79.Alprol A.E., Ashour M., Mansour A.T., Alzahrani O.M., Mahmoud S.F., Gharib S.M. Assessment of water quality and phytoplankton structure of eight alexandria beaches, southeastern mediterranean sea, Egypt. J. Mar. Sci. Eng. 2021;9:1328. doi: 10.3390/jmse9121328. [DOI] [Google Scholar]
- 80.Alprol A.E., Heneash A.M.M., Soliman A.M., Ashour M., Alsanie W.F., Gaber A., Mansour A.T. Assessment of water quality, eutrophication, and zooplankton community in lake burullus, Egypt. Diversity. 2021;13:268. doi: 10.3390/d13060268. [DOI] [Google Scholar]
- 81.Anderson R.K., Parker P.L., Lawrence A. A 13C/12C tracer study of the utilization of presented feed by a commercially important shrimp Penaeus vannamei in a pond growout system. J. World Aquac. Soc. 1987;18:148–155. doi: 10.1111/j.1749-7345.1987.tb00433.x. [DOI] [Google Scholar]
- 82.Lee Chen Y., Chen H. Juvenile Penaeus monodon as effective zooplankton predators. Aquaculture. 1992;103:35–44. doi: 10.1016/0044-8486(92)90276-Q. [DOI] [Google Scholar]
- 83.Ballester E., Abreu P., Cavalli R., Emerenciano M., De Abreu L., Wasielesky W., Jr. Effect of practical diets with different protein levels on the performance of Farfantepenaeus paulensis juveniles nursed in a zero exchange suspended microbial flocs intensive system. Aquac. Nutr. 2010;16:163–172. doi: 10.1111/j.1365-2095.2009.00648.x. [DOI] [Google Scholar]
- 84.Decamp O., Conquest L., Forster I., Tacon A. The Nutrition and Feeding of Marine Shrimp within Zero-Water Exchange Aquaculture Production Systems: Role of Eukaryotic Microorganisms. The World Aquaculture Socety; Baton Rouge, LA, USA: 2002. [Google Scholar]
- 85.Santacruz-Reyes R.A., Chien Y.-H. The potential of Yucca schidigera extract to reduce the ammonia pollution from shrimp farming. Bioresour. Technol. 2012;113:311–314. doi: 10.1016/j.biortech.2012.02.132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.