Abstract
In this review, we focus on some interesting and recent examples of various applications of organic azides such as their intermolecular or intramolecular, under thermal, catalyzed, or noncatalyzed reaction conditions. The aforementioned reactions in the aim to prepare basic five-, six-, organometallic heterocyclic-membered systems and/or their fused analogs. This review article also provides a report on the developed methods describing the synthesis of various heterocycles from organic azides, especially those reported in recent papers (till 2020). At the outset, this review groups the synthetic methods of organic azides into different categories. Secondly, the review deals with the functionality of the azido group in chemical reactions. This is followed by a major section on the following: (1) the synthetic tools of various heterocycles from the corresponding organic azides by one-pot domino reaction; (2) the utility of the chosen catalysts in the chemoselectivity favoring C−H and C-N bonds; (3) one-pot procedures (i.e., Ugi four-component reaction); (4) nucleophilic addition, such as Aza-Michael addition; (5) cycloaddition reactions, such as [3+2] cycloaddition; (6) mixed addition/cyclization/oxygen; and (7) insertion reaction of C-H amination. The review also includes the synthetic procedures of fused heterocycles, such as quinazoline derivatives and organometal heterocycles (i.e., phosphorus-, boron- and aluminum-containing heterocycles). Due to many references that have dealt with the reactions of azides in heterocyclic synthesis (currently more than 32,000), we selected according to generality and timeliness. This is considered a recent review that focuses on selected interesting examples of various heterocycles from the mechanistic aspects of organic azides.
Keywords: organic azides, click reaction, catalysis, five membered rings, six membered rings, organo-metal heterocycles
1. Introduction
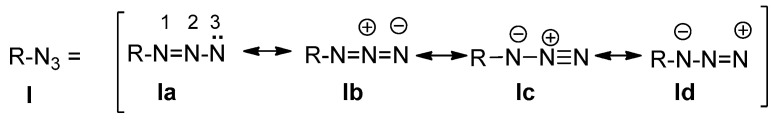
Organic azides are organic compounds containing the azide (N3) functional group. Due to the hazards associated with their use, few azides are commercially used, although they display interesting applications in organic chemistry. Organic azides have four mesomeric structures (1a–1d, Figure 1), and their structure is also described as isoelectronic with carbon dioxide.
Figure 1.
Mesomeric structures of organic azides.
The polar character of the azido group has a remarkable effect on their bond lengths and angles. In methyl azide, as an example, the angles of CH3-N1–N2N3 and CH3N1–N2–N3 are approximately 115.28 and 172.58 Å, respectively [1]. Aromatic azides show slightly shorter bond lengths between N2 and N3 [1]. Accordingly, an almost linear azide structure is present, with sp2 hybridization at N1. The polar resonance structures Ib–Id illustrated that strong IR absorption by a band at nearly 2114 cm−1 (phenyl azide [2]). Alkyl azides show absorption in the UV region at 287 nm and 216 nm [2]. They also exhibit weak dipole moment (1.44 D for phenyl azide) [2]. Azido group in aromatic substitution reactions directs to ortho- and para-positions.
Organic azides engage in useful organic reactions, as the terminal nitrogen is mildly nucleophilic. Generally, nucleophiles attack the azide at the terminal nitrogen Nγ, while electrophiles react at the internal atom Nα [3]. Azides easily extrude diatomic nitrogen, a tendency that is engaged in many reactions, such as the Staudinger ligation or the Curtius rearrangement [4]. Azides can be reduced to amines by hydrogenolysis [5] or with a phosphine (e.g., triphenylphosphine) in the Staudinger reaction [5]. Organic azides can react with phosphines to give iminophosphoranes, which can be hydrolyzed into primary amines (the Staudinger reaction) [6]. They react with carbonyl compounds to give imines (the aza-Wittig reaction) [7,8] or undergo other transformations. Thermal decomposition of azides gives nitrenes, which participate in various reactions; vinyl azides decompose into 2H-azirines [9].
Since organic azides are highly reactive and have been long established as versatile building blocks in assembling structurally diverse N-containing heterocycles, converting organic azides into high-value compounds, such as heterocycles, would be greatly valued and a subject of enormous current interest. Currently, well over 32,000 total and nearly 1000 in 2021 showed interest in this type of chemistry.
2. Some Synthetic Procedures of Organic Azides
2.1. From Diazonium Salts
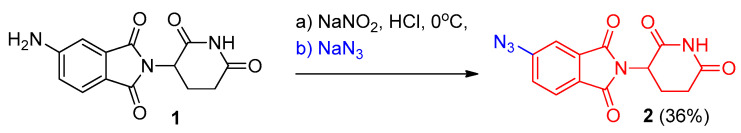
The aryl diazonium salts were decomposed readily on reacting with azide ions (NaN3 or Me3SiN3) to the corresponding aryl azide without a catalyst. As an example, the facile conversion of 5-amino-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (1) into 5-azido-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (2) via a two-step reaction involving diazotization followed by azidation using sodium azide as a precursor of azide ion [10] (Scheme 1).
Scheme 1.
Synthesis of aromatic azide 2 from diazonium salt of 1.
2.2. Via SNAr Reactions (Nucleophilic Aromatic Substitution Reactions)
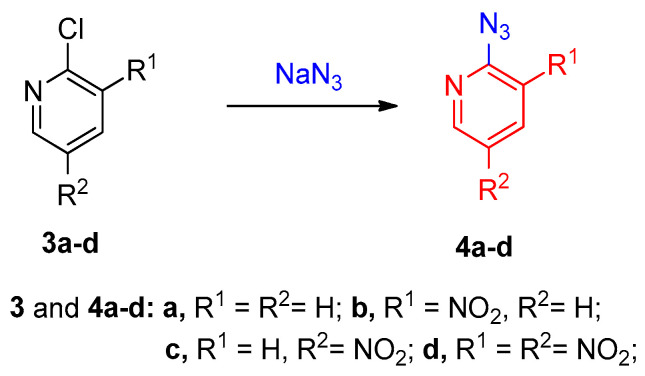
As an example, synthesis of 2-azido-3-nitropyridines (4) from 2-chloro-3-nitropyridines (3) using NaN3 as the source of nucleophile (Scheme 2) was established as shown in Scheme 2 [11,12].
Scheme 2.
Synthesis of aryl azides 4a–d via SNAr reaction.
2.3. From Lithium-Reagent
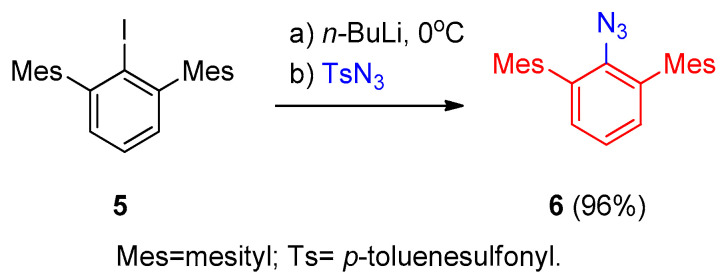
The reaction of aromatic halides 5 with lithium reagent (t-BuLi) followed by the reaction with tosyl azide gave the corresponding aryl azide 6 in 96% yield (Scheme 3) [13].
Scheme 3.
Synthesis of aryl azide 6 using lithium reagent.
2.4. From Aryl Hydrazines
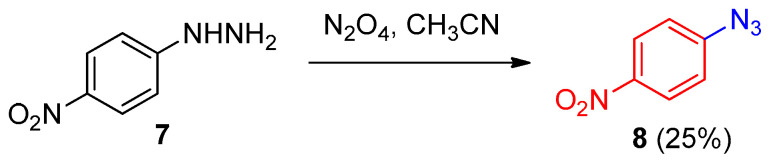
Kim et al. [14] reported the synthesis of aromatic azide 8 from the reaction of arylhydrazine 7 with nitrosyl ion (Scheme 4).
Scheme 4.
Formation aryl azide 8 from the corresponding hydrazide 7.
3. Chemistry of Azides
3.1. Azide as Aminating Group
3.1.1. Synthesis of 8-Aminoquinoline
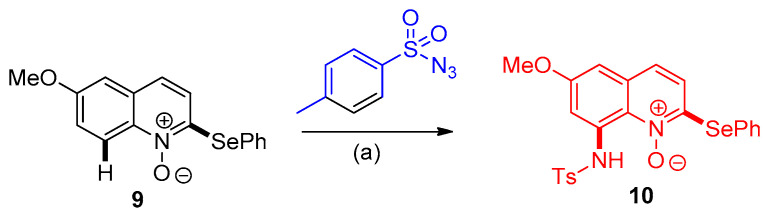
The biologically active 8-aminoquinoline 10 was obtained through Ir(III)-catalyzed C8-amination of C2-selenylated quinoline N-oxide 9 with tosyl azide [15] (Scheme 5).
Scheme 5.
Formation of biologically active 8-aminoquinoline 10. Reagents and conditions: (a) [IrCp*Cl2]2 (2.0 mol%), AcOH, AgNTf2, DCE, room temperature.
3.1.2. Synthesis of Amino Furo/Pyrroloindole Derivatives
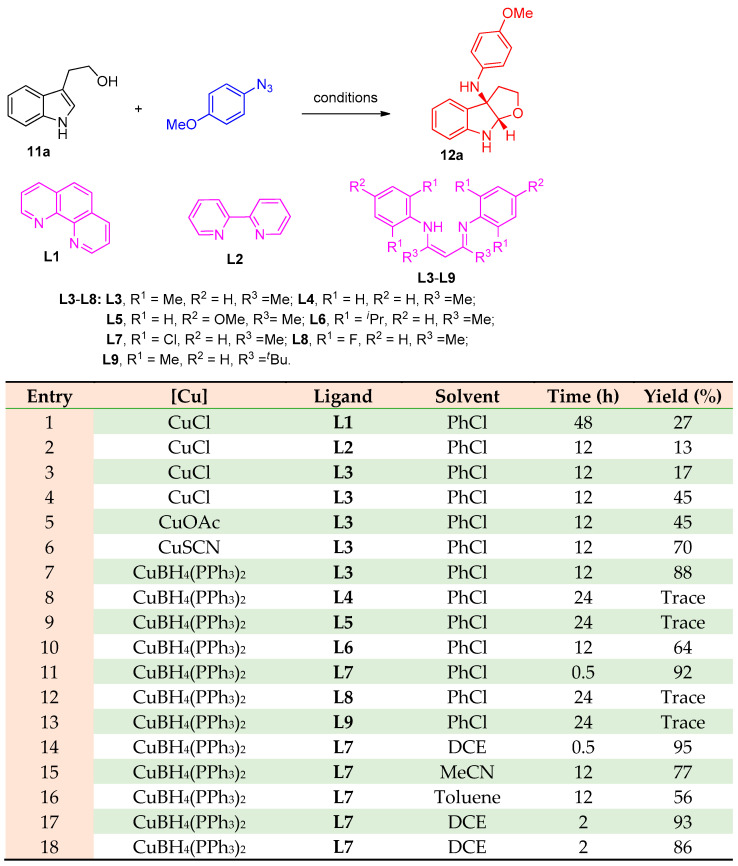
Zhang and others [16] reported that tryptophols or tryptamines reacted with aryl azides to produce 3a-nitrogenous furoindolines and pyrroloindolines 3a-nitrogenous indole alkaloids. Using a nitrogen source, the reaction proceeded via nitrene transfer/cyclization under copper-catalyzed conditions. Firstly, the reaction was investigated to stand at the optimal reaction conditions indicated in Scheme 6. Starting with tryptophol (2-(1H-indol-3-yl)ethanol) (11a) and 1-azido-4-methoxybenzene, the corresponding furoindole 12a was obtained (Scheme 6). The investigation revealed that the conditions mentioned in entry 14 were chosen to be the optimal reaction conditions for all substrates (CuBH4(PPh3)2 + L7 (12 mol%), DCE 0.5 h).
Scheme 6.
Synthesis furoindoline 12a. Reagents and conditions: (a) [Cu] (20 mol%), ligand (24 mol%), solvent, 80 °C, N2.
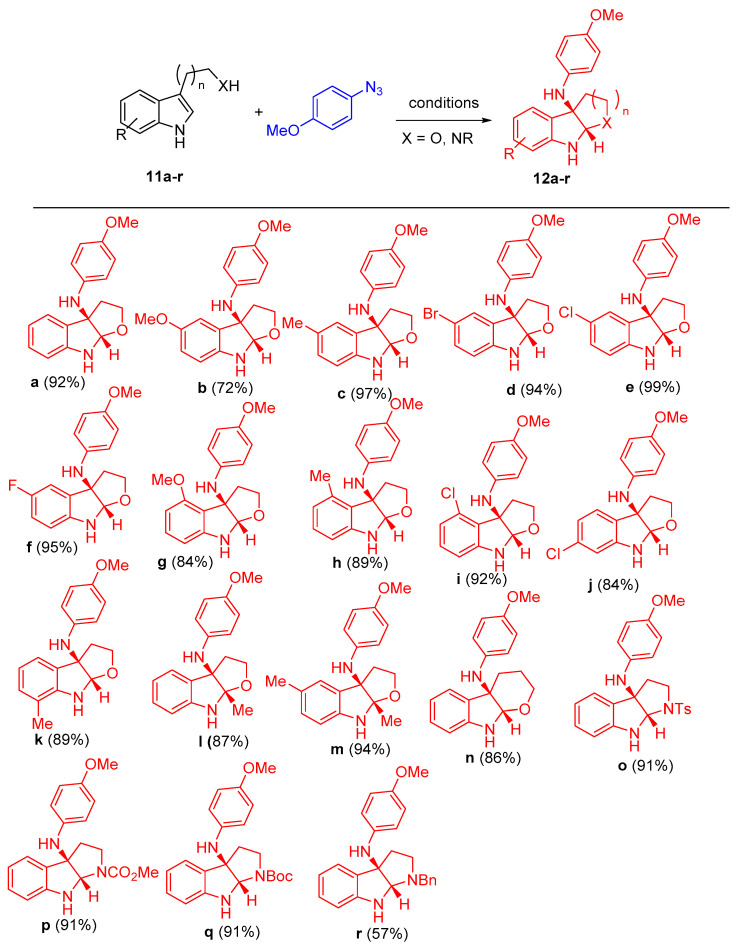
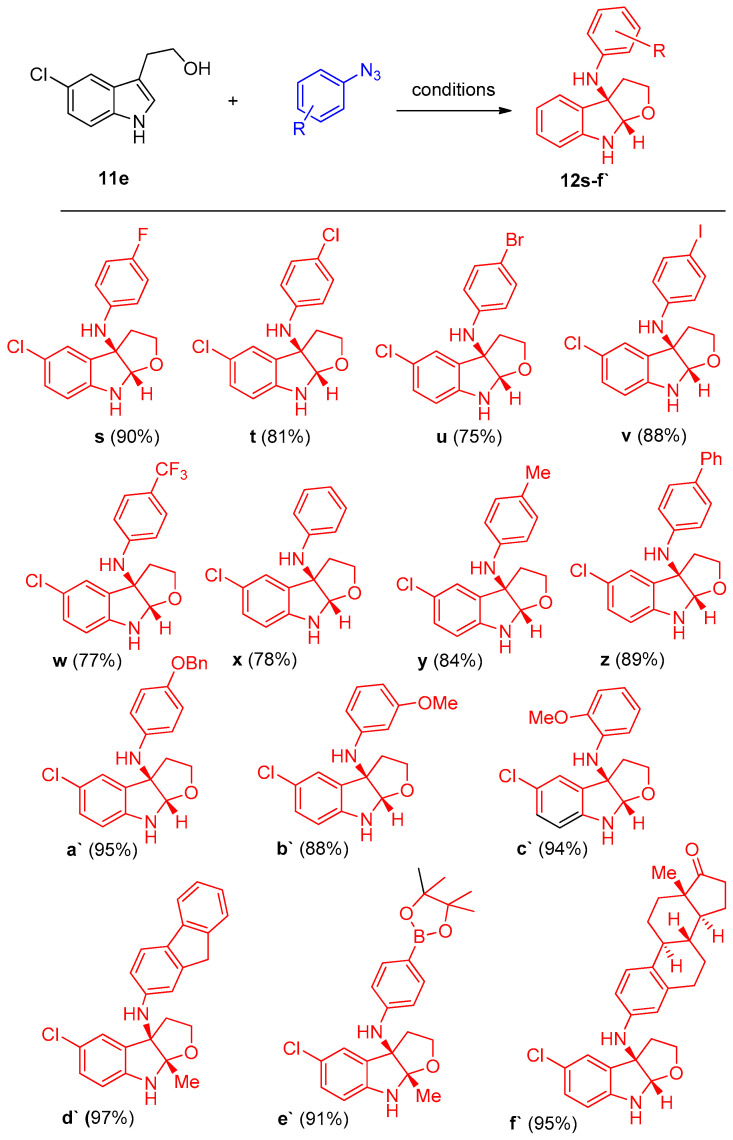
Utilizing by the aforementioned optimized condition, a variety of tryptophols (11a–n) and tryptamine substrates (11o–r) were taken to react with 1-azido-4-methoxybenzene, as shown in Scheme 7. According to the electronic nature or positions of the substituents, the reactions proceeded smoothly to give the target products 12a–r in high yields ranging from 72 to 99%. Moreover, different aryl azides were selected to react with tryptophol (2-(5-chlorobenzofuran-3-yl)ethanol) (11e) under the optimized reaction conditions to investigate the effect of substituents on the products’ yields.
Scheme 7.
Synthesis of furoindolines 12a–n and pyrroloindolines (120-r). Reagents and conditions: (a) CuBH4(PPh3)2 (12 mol%), L7 (14 mol%), DCE, 80 °C, N2.
Another series of compound 12 was prepared, with azides having electron-donating and -withdrawing groups. The reaction proceeded smoothly to give the corresponding products 12s–f` in moderate to excellent yields [16] (Scheme 8).
Scheme 8.
Synthesis of furoindolines 12s–f` Reagents and conditions: (a) CuBH4(PPh3)2 (12 mol%), L7 (14 mol%), DCE, 80 °C, N2.
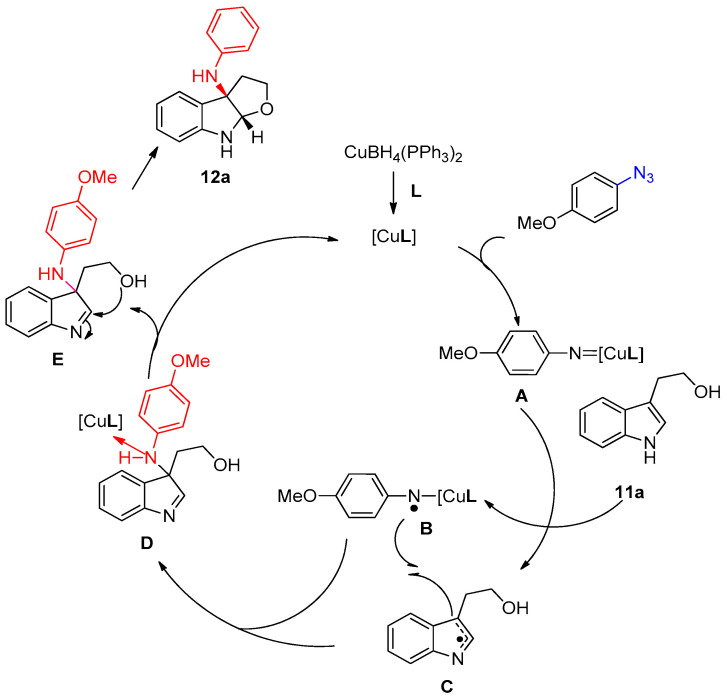
The suggested mechanism describes the formation of furroindole 12a, as shown in Scheme 9 [16]. Firstly, the azide moiety reacted with copper complex to produce copper nitrene complex A, which abstracts a hydrogen atom from compound 11a to form the copper aminyl species B and imine radical C, which combines to produce the intermediate D. The catalyst moiety was then excluded from the intermediate D to form the imine species E. Finally, E was converted to the desired product 12a via an intramolecular nucleophilic addition [16].
Scheme 9.
A plausible mechanism described the formation of compound 12a.
3.2. Azidation
Synthesis of 3-Azido-Tetralins, Chromanes, and -Tetrahydroquinolines
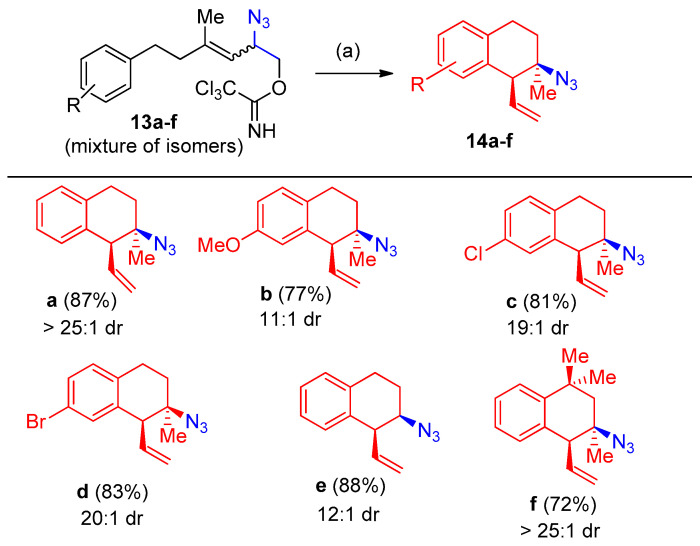
Porter et al. [17] reported the stereoselective synthesis of 3-azido-tetralins, chromanes, and -tetrahydroquinolines via a tandem allylic azide rearrangement/Friedel-Crafts alkylation. The allylic azides 13a–f were cyclized to the corresponding tetralines 14a–f in 58–88% (Scheme 10).
Scheme 10.
Azidation by azido group and synthesis of 3-azido-tetralins 14a–f. Reagents and conditions: (a) 10 mol% AgSbF6, CHCl3, 40–60 °C, 24–48 h.
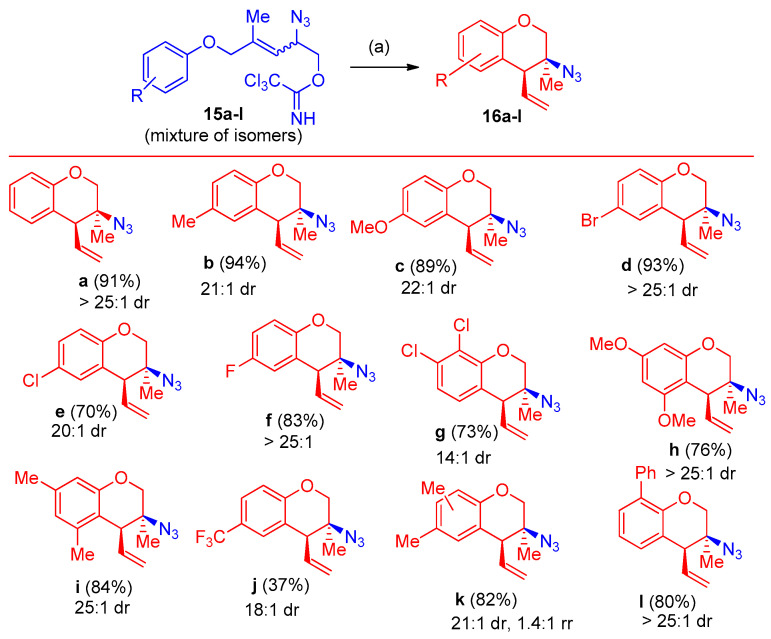
In continuation of the optimized procedure [17], the ethereal allylic azides 15a–i were converted into chromanes 16a–i in 34–97% yields (Scheme 11).
Scheme 11.
Azidation by azido group and synthesis of ethereal allylic azides 15a–i. Reagents and conditions: (a) 10 mol% AgSbF6, CHCl3, 40–60 °C, 24–48 h.
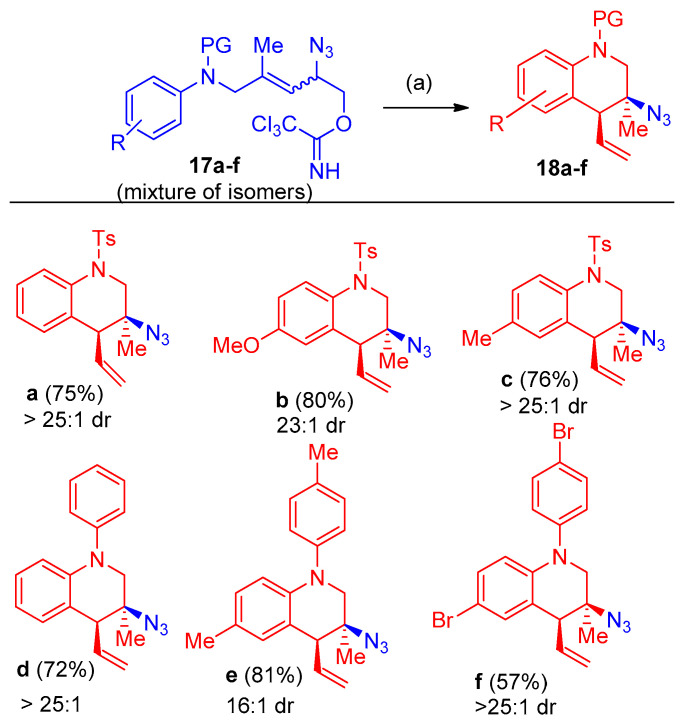
On the other hand, aniline-derived allylic azides 17a–f carrying the N-protecting group were also cyclized using the tandem process to tetrahydroquinolines 18a–f in good yields of 57–81% [17] (Scheme 12).
Scheme 12.
Azidation and cyclization; Synthesis of compounds 18a–f. Reagents and conditions: (a) 10 mol% AgSbF6, CHCl3, 40–60 °C, 24–48 h.
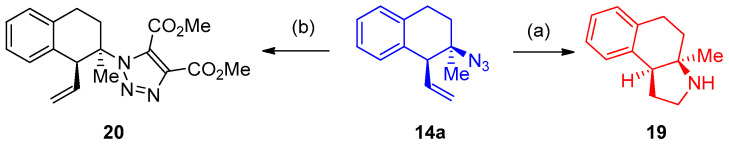
Finally, the tetraline 14a was converted into pyrrolidine 19 [17]. At the same time, the cycloaddition reaction of the tetraline 14a with dimethyl acetylene dicarboxylate gave the triazole 20 (Scheme 13).
Scheme 13.
Synthesis of pyrrole 19. Reagents and conditions: (a) HBCy2 (dicyclohexyl borane) (b) DMAD (dimethylacetylene.dicarboxylate).
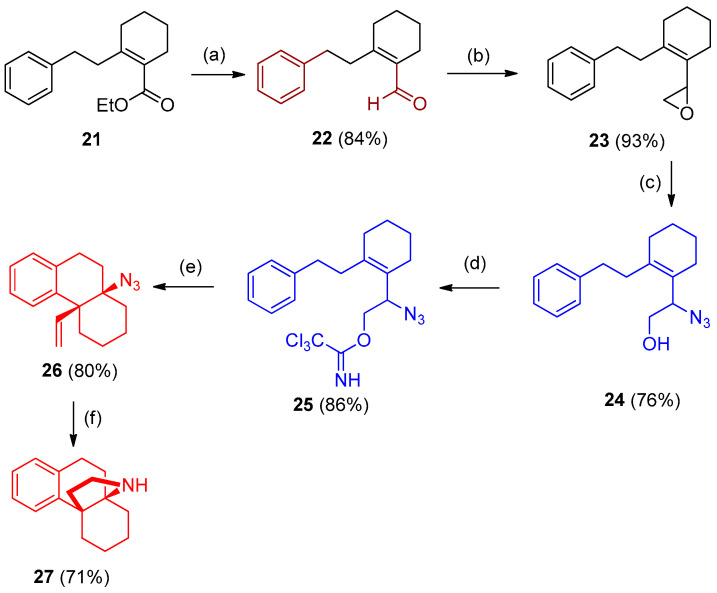
An interest application of previously reported work, was directed towards synthesizing a large family of botanical natural products group named husbanan [17]. Husbanan was synthesized from ethyl 2-phenethylcyclohex-1-enecarboxylate (21), which initially underwent reduction followed by partial re-oxidation to the aldehyde 22 (i.e., from ester to alcohol then aldehyde using tetrapropylammonium perruthenate (TPAP), and N-methylmorpholine N-oxide (NMO)). Aldehyde 22 was elaborated by Corey-Chaykovsky epoxidation. Epoxide 23 was opened with NaN3 in acetone/water yielding the allylic azide 24. Imidate 25 was isolated after activation with trichloroacetonitrile. Finally, reduction of imidate 25 gave 26 on the presence of dicyclohexyl borane ring closer to the hasubanan product 27 [17] (Scheme 14).
Scheme 14.
Synthesis of husbanan 27. Reagents and conditions: (a) (i) LiAlH4; (ii) tetrapropylammonium perruthenate (TPAP, NMO); (b) Me3S+ MeOSO3-, NaOH; (c) NaN3, acetone/H2O; (d) CCl3CN, DBU; (e) 10 mol% AgSbF6;(f) HBCy2 (dicyclohexyl borane).
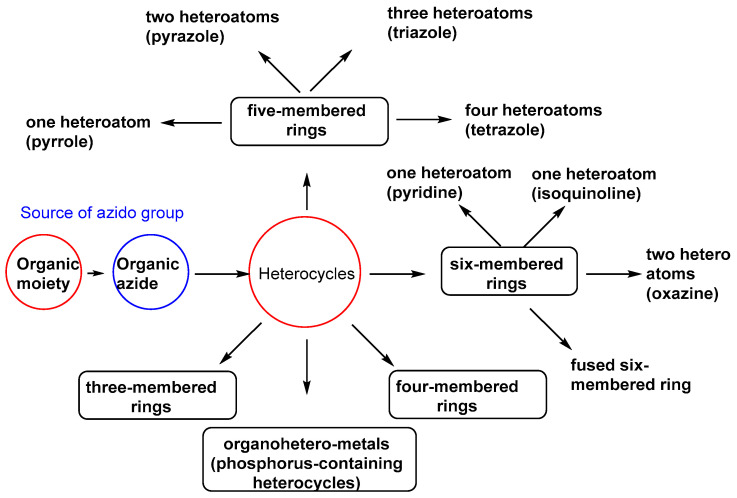
4. Organic Azides in the Synthesis of Heterocycles
Organic azides have synthesized various heterocycles of the five-member ring with one heteroatom, such as pyrroles. They are also involved in synthesizing heterocycles with two heteroatoms, such as pyrazole and isoxazole, oxazole, thiazole, oxazine, and pyrimidine. In addition, heterocycles containing three heteroatoms, such as 1,2,3-triazoles and thiadiazole, are also included. Furthermore, organic azides are used in synthesizing heterocycles of six-membered rings and with one heteroatom, such as pyridine, isoquinoline, and phenanthridine. Heterocycles with four heteroatoms, such as tetrazole, are also discussed. Synthetic interest applications of organometal heterocycles (i.e., phosphorus-, boron-, and aluminum-containing heterocycles) were also investigated. Figure 2 indicates the contribution of organic azides in heterocyclic synthesis.
Figure 2.
Contribution of organic azides in heterocyclic synthesis, exemplary cases of the heterocycles.
4.1. Synthesis of the Pyrrole Ring
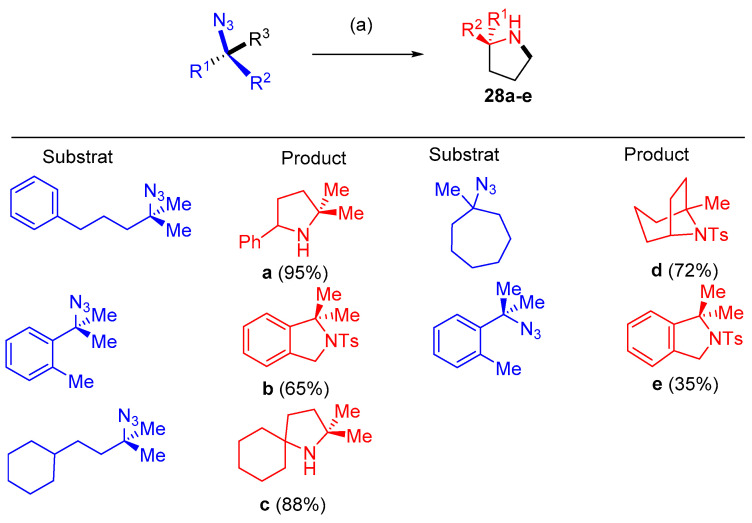
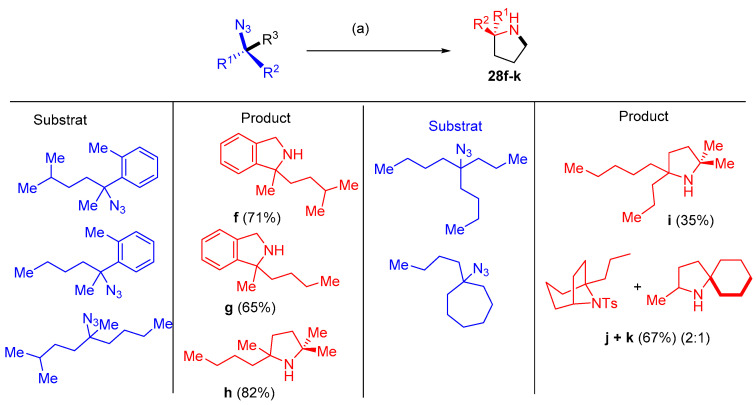
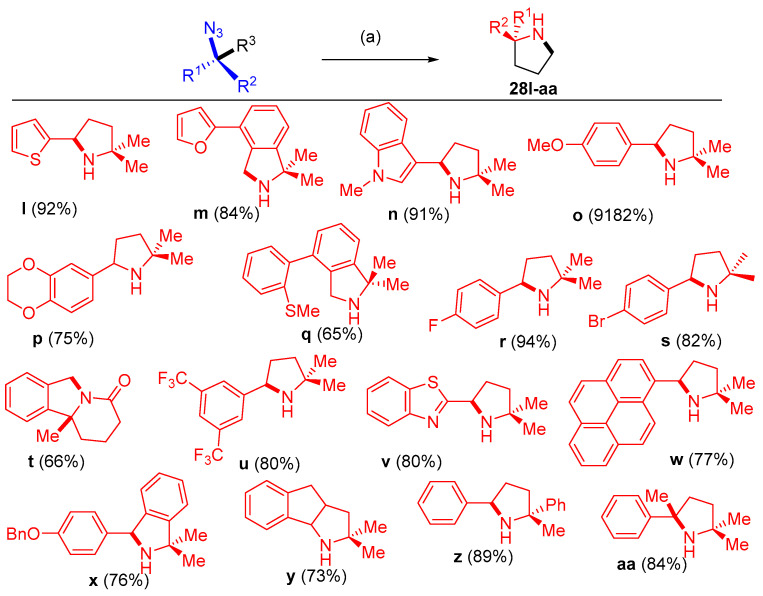
Dong and others [18] reported that dipyrrin-supported nickel catalyst (AdFL)Ni(py) (AdFL: 1,9-di(1-adamantyl)-5-perfluorophenyldipyrrin; py: pyridine) catalyzed the productive intramolecular C−H bond amination to give N-heterocyclic products 28a–k using aliphatic azide substrates. The catalytic amination conditions were mild, requiring 0.1−2 mol% catalyst, and occurred at room temperature. The amination process occurred using different substrates having multiple activatable C−H bonds (Scheme 15 and Scheme 16). The selective catalyst showed high chemoselectivity favoring C−H bonds in ethers, halides, thioethers, esters, etc. (Scheme 17). Sequential cyclization of substrates with ester groups was achieved to provide facile preparation of indolizidine derivatives found in various alkaloids [18].
Scheme 15.
Synthesis of pyrroles 28a–e. Reagents and conditions: (a) 1 mol% (AdFL)Ni(py) (AdFL), C6D6, 25–80 °C.
Scheme 16.
Synthesis of pyrroles 28f–k. Reagents and conditions: (a) 1 mol% (AdFL)Ni(py) (AdFL), C6D6, 60 °C.
Scheme 17.
Synthesis of pyrroles and its fused systems 28l–aa. Reagents and conditions: (a) 1 mol% (AdFL)Ni(py), C6D6, 60 °C.
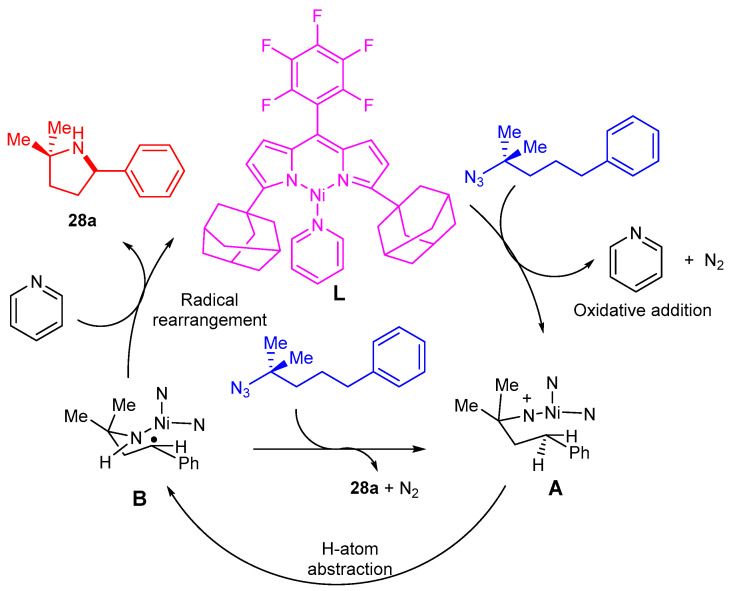
The amination cyclization reaction mechanism is illustrated in Scheme 18. Benzene (4-azido-4-methyl pentyl), as an example, releases pyridine and N2 from L to give the corresponding nickel iminyl species A. The next step would be a hydrogen atom abstraction to give radical B, followed by radical recombination to give the cyclized product 28a [18].
Scheme 18.
The postulated reaction mechanism for the formation of pyrroles and C-H amination.
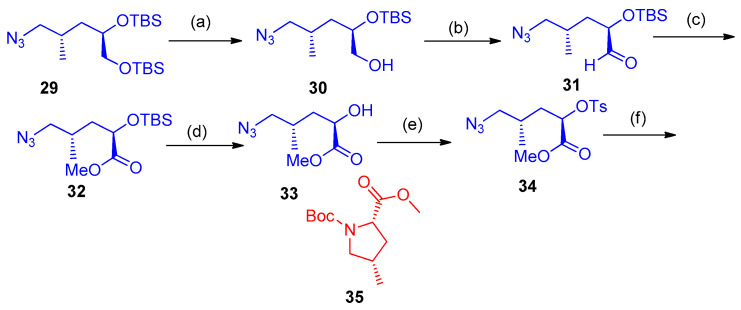
Sun et al. [19] have reported the diastereoselective synthesis of Boc-protected 4-methylproline carboxylates 35, starting with the reduction of the azido compound 29. Selective cleavage of the primary TBS-alcohol 30 was performed using NH4F in MeOH at room temperature for 6 h. Oxidation of the alcohol 30 was achieved using 2-iodoxybenzoic acid (IBX) in DMSO at 30 °C, and the resulting aldehyde 31 was subsequently transformed into the corresponding methyl ester 32 by adding KOH/I2/MeOH. Deprotection of ester 32 with camphorsulfonic acid (CSA) afforded the corresponding alcohol 33. Tosylation of alcohol 33 with p-toluenesulfonyl chloride in the presence of 1,4-diazabicyclo[2.2.2]octane (DABCO) as a base, gave compound 34 in high yield (90%). Finally, the two-step azide reduction/intramolecular SN2 cyclization procedure was performed to obtain the desired Boc-protected (2S,4S)-4-methylproline carboxylate (35) in 90% yield (Scheme 19) [19].
Scheme 19.
Synthesis of pyrrole 35. Reagents and conditions: (a) NH4F (50 eq.), MeOH, room temperature., 6 h (75%); (b) IBX, DMSO, room temperature, 2 h; (c) I2, KOH, MeOH, 0 °C, 1 h (95%); (d) CSA, MeOH, room temperature, 2 h; (e) TsCl, DABCO, CH2Cl2, room temperature, 18 h, (90%); (f) 10% NaHCO3, H2, MeOH, room temperature, 2 h, then NaHCO3, Boc2O, 12 h (90%).
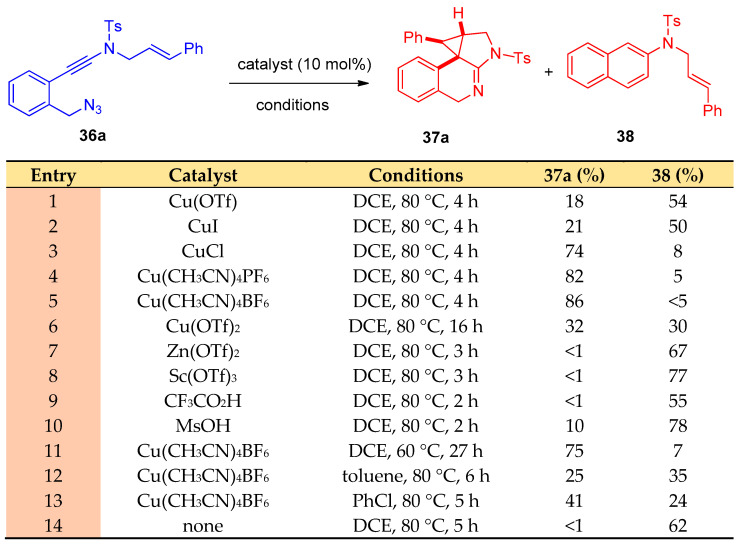
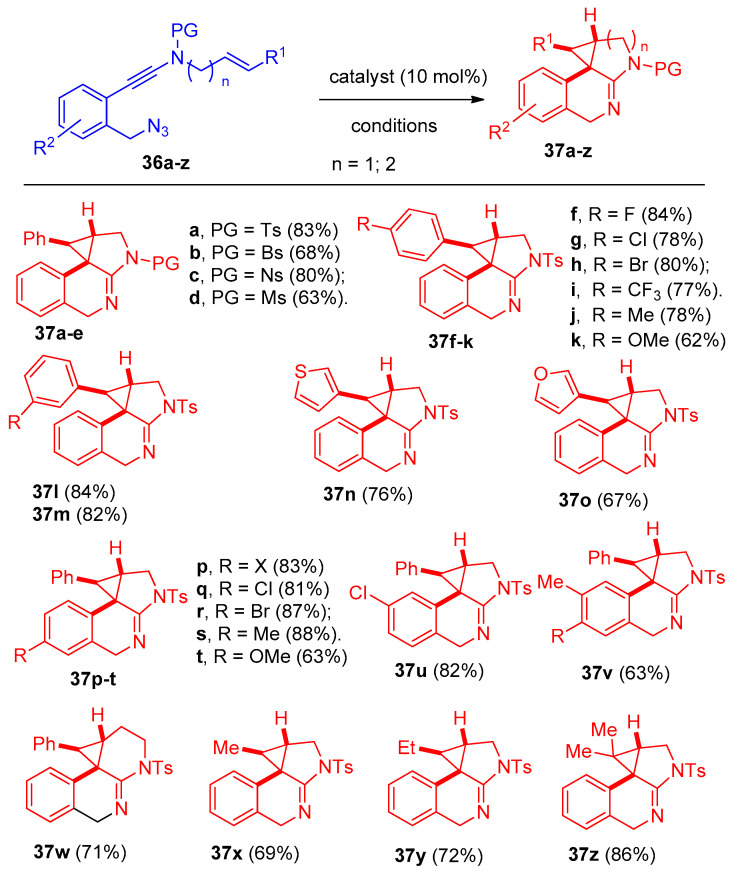
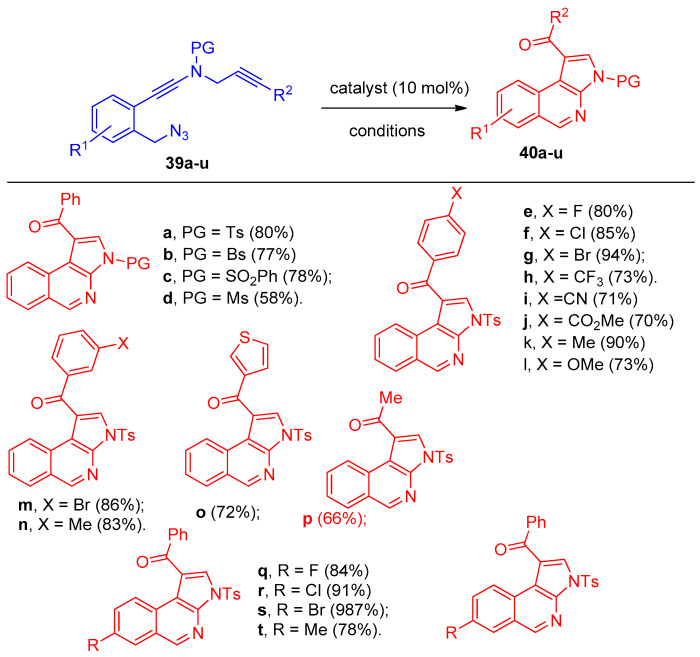
Using chiral bisoxazoline-copper (BOX-Cu) complexes as catalysts, the cyclization process of asymmetric azide-ynamides via α-imino copper carbene intermediates polycyclic N-heterocycles with high enantioselectivities (up to 98:2 e.r) was performed [20]. N-Styryl benzyl-tethered(azido)ynamide(N-((2-(azidomethyl)phenyl)ethynyl)-N-cinnamyl-4-methylbenzene sulfonamide) (36a) was selected as the model substrate, and Cu(CH3CN)4BF4 was used as a catalyst to give ((8bR,9R,9aS)-9-phenyl-2-tosyl-2,4,9,9a-tetrahydro-1H-benzo[e]cyclopropa[c]indole) (37a) in 86% yield and N-cinnamyl-4-methyl-N-(naphthalen-2-yl)benzenesulfonamide (38) as a side product (Scheme 20). The reaction was applied to various N-(azido)ynamide 36a–z. Firstly, different selected N-protecting groups of the ynamides 36a–e were examined, and the reaction proceeded smoothly when Ts-,Bs-, Ns-, SO2Ph-, and Ms- were used as protecting groups (PG = protecting group, Bs = 4-bromobenzene-sulfonyl, Ns = 4-nitrobenzene-sulfonyl)(azido)ynamides) producing the desired tetracyclic heterocycles 37a–e in 63–83% yields. In addition, various aryl-substituted benzyl-tethered (azido)ynamides 36f–m, having either electron-withdrawing and/or electron-donating groups, were also examined, and the corresponding cyclopropanation products 37f–m were obtained in good yields. The reaction was also applied to the thienyl- and furanyl-substituted (azido)ynamides 36n,o to give 37n and 37o in 76% and 67% yields, respectively. Different substituents on the phenyl ring 36p–v (F, Cl, Br, Me, and OMe) were also examined, and products 37p–v were obtained in 63–88% yields. Piperidine fused tetracyclic heterocycle 37w was also obtained in 71% yield. Moreover, methyl-, ethyl-, and even dimethyl-substituted N-allyl (azido)ynamides 36x–z were also suitable substrates for this type of cyclization to give products 37x–z in 69–86% yields (Scheme 21). In addition, the reaction was extended to investigate the copper-catalyzed cyclization for N-propargyl benzyl-tethered (azido)-ynamides 39a–u (Scheme 22) to synthesize 3H-pyrrolo[2,3-c]isoquinolines 40a–u using 10 mol% of Cu(CH3CN)4PF6 as the catalyst and 2 equiv. of DDQ (4,5-dichloro-3,6-dioxocyclohexa-1,4-diene-1,2-dicarbonitrile) as oxidant [20].
Scheme 20.
Synthesis of pyrrole 37a. Reagents and conditions: 36a (0.1 mmol), catalyst (0.01 mmol), solvent (2 mL), 60 °C to 80 °C, 2–27 h, in vials.
Scheme 21.
Synthesis of pyrroles 37a–z. Reagents and conditions: Cu(CH3CN)4BF4 (0.02 mmol), DCE (4 mL), 80 °C, 4 h.
Scheme 22.
Synthesis of pyrroles 40a–u. Reagents and conditions: DDQ, Cu-(CH3CN)4PF6, DCE, room temperature, 5 h.
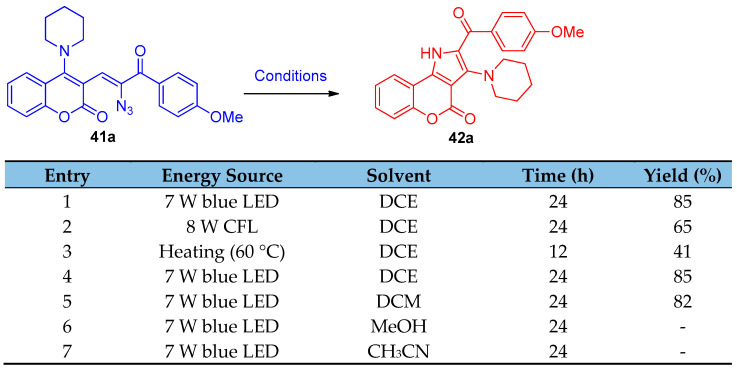
Under thermal and UV light exposure, vinyl azides have been known to decompose into nitrenes and/or 2H-azirines, and they have been widely utilized to synthesize various N-heterocycles [21]. A photocatalyst-free visible light synthesized substituted pyrroles 42a–i from α-keto vinyl azides 41a–i. The optimized reaction condition was determined by applying the reaction on compound 41a, and it was observed that the optimal reaction condition was irradiation of 0.1 M solution of 41a in DCE with a blue LED (7 W) light (Scheme 23 and Scheme 24).
Scheme 23.
Optimized reaction condition of the decomposition of vinyl azide 41a.
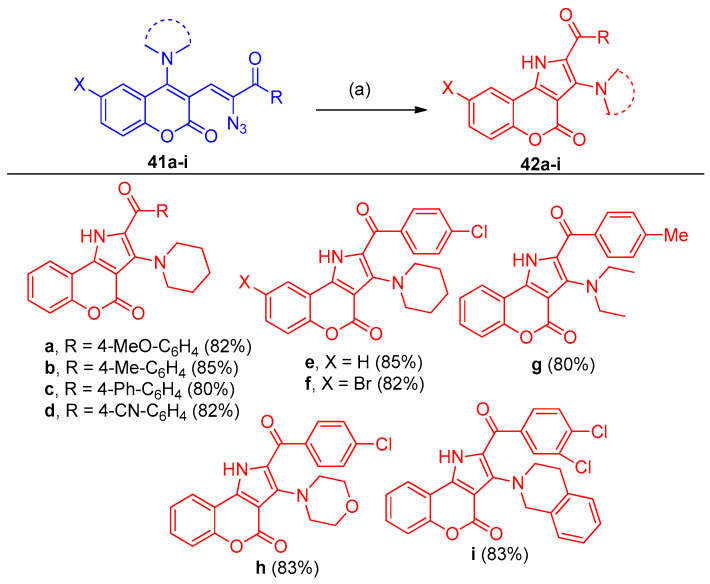
Scheme 24.
Synthesis of chromenopyrroles 42a–i. Scope of the photodecomposition of vinyl azides 41 to access substituted pyrroles 42. Reagents and conditions: (a) 7 W blue LED light, DCE, 24 h.
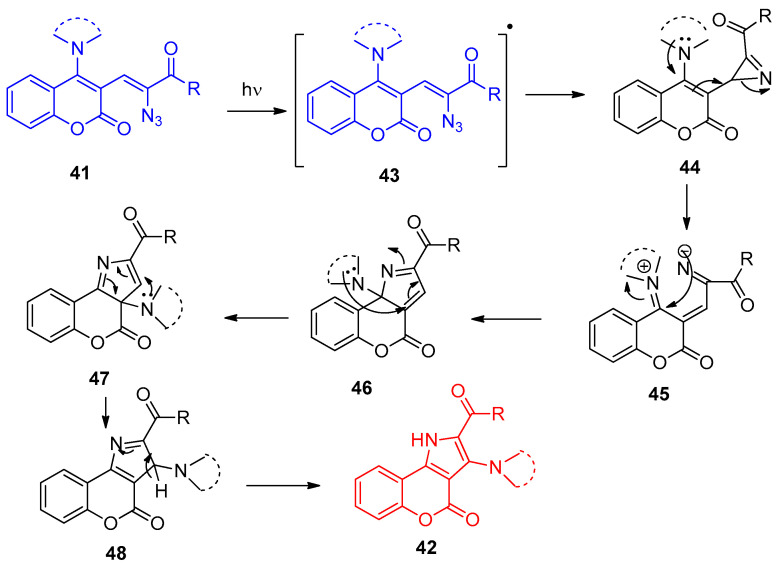
The reaction mechanism was described as a result of the denitrogenative photodecomposition process of α-keto vinyl azides, 1,3-amino group migration, and coupling of intermediates 43–48 with secondary amines, as shown in Scheme 25 [22].
Scheme 25.
Postulated mechanistic steps for the conversion of vinyl azides 41 into chromenopyrroles 42.
4.2. Synthesis of the Pyrazole Ring
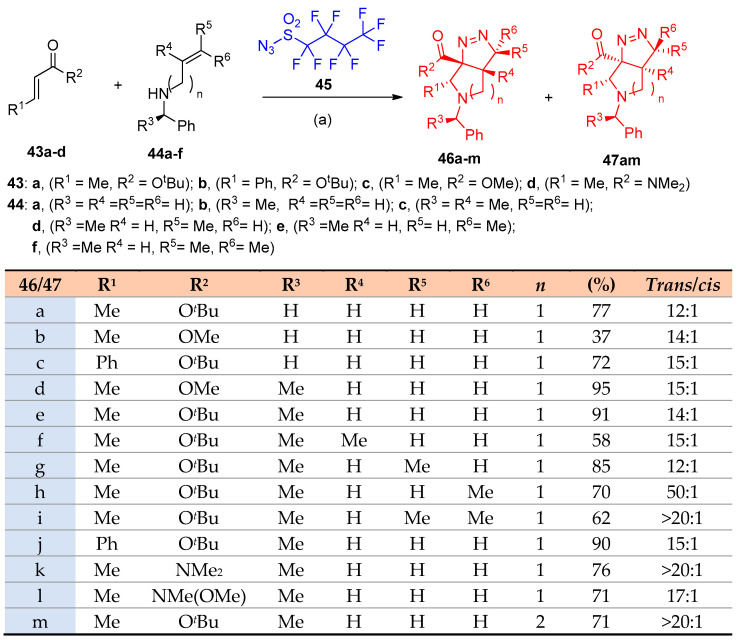
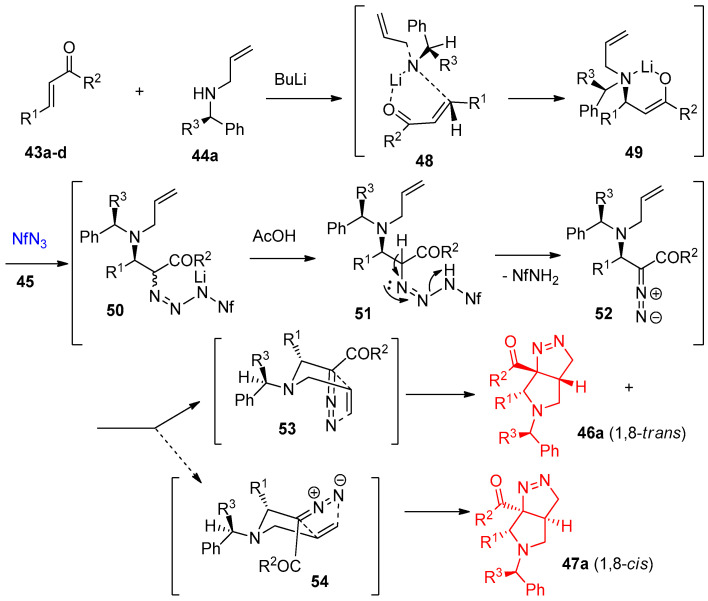
Quiclet-Sire et al. [23] reported syntheses of tetrahydropyrrolo-pyrazole from hydrazones of pendant alkene using iodine in a basic medium as a catalyst. In contrast, Jahn et al. [24] demonstrated the construction of tetrahedropyrrolo-pyrazole 46, 47a–m via Aza-Michael addition/[3+2]cycloaddition between α,β-unsaturated esters 43a–c or amide 43d and allylic amines 44a–f using nonaflyl azides 45 ((F3C(CF2)3SO2N3, NfN3), acting as aza- transfer reagent) in the presence of n-BuLi. The reaction proceeded regioselectively with more than 12:1 1,8-trans/1,8-cis selectivity (Scheme 26) [24].
Scheme 26.
Synthesis of pyrrolopyrazoles 46a–m and 47am. Reagents and conditions: (a) n-BuLi, THF. −78 °C, then AcOH, −78–25 °C.
The mechanism for this reaction is illustrated in Scheme 27. Initially, lithiation of the amine 44 gave the lithium amide 48, which coordinates to the carbonyl group of 43, followed by transfer of the amide group to the β-position via the formation of intermediate 49. (Z)-Enolate then 49 couples with nonaflyl azide (NfN3) to form the unstable triazenide 50. Protonation 50 would give the intermediate 51, followed by the formation of diazo intermediate 52. Finally, diastereoselective cycloaddition step takes place via transition state 53 and 54 to give the diastereoisomers 46 (1,8-trans) and 47 (1,8-cis) (Scheme 27) [24].
Scheme 27.
The proposed mechanism for the formation of pyrrolotriazoles 46 and 47.
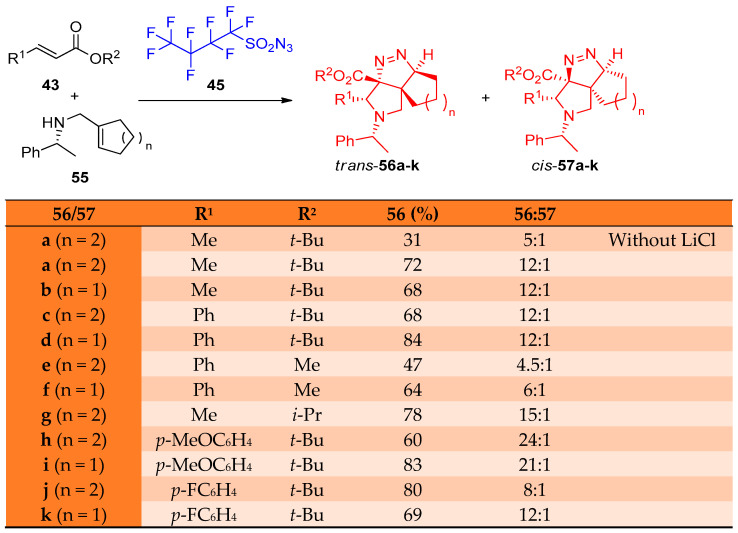
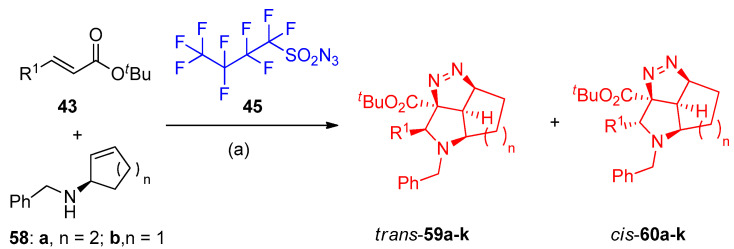
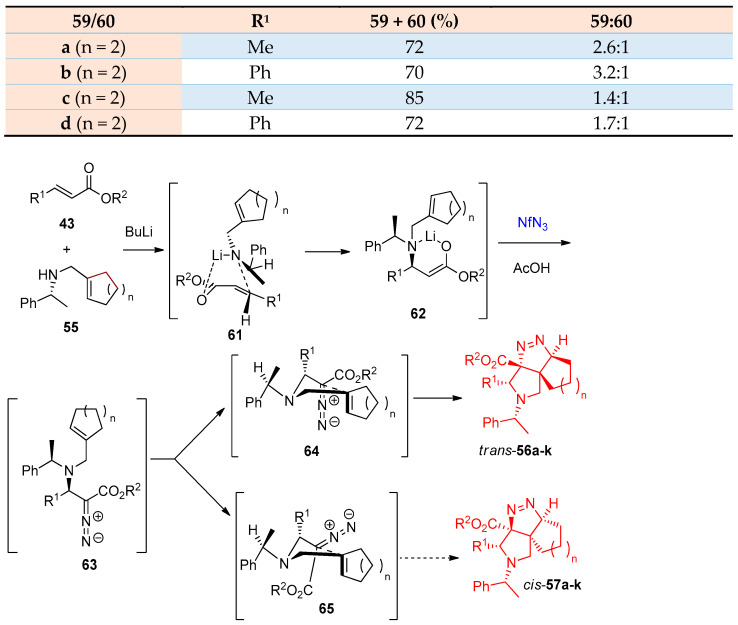
Moreover, Just and others [25] reported the catalytic syntheses of fused tricyclic pyrrolidinopyrazolines via aza-Michael cycloaddition of cyclic amines 55 as well as (R)-N-benzyl-cycloalkenyl amines 58 with NfN3 45; the reaction was catalyzed by lithium chloride (LiCl). Diastereoselective products have been obtained in good yields (68–84%) for the tricyclic products (trans)-56a–f and (cis)-57a–f, while in the case of 5,5,5-tricyclic (trans)-59a–d and (cis)-60a–d, the yield ranged from 72 to 85%. Regarding the optimized reaction conditions (Scheme 28 and Scheme 29), it was observed that the diastereomers’ yields depend on the nature of cycloalkenylmethyl amines having five- or six-membered rings and α,β-unsaturated esters bearing alkyl or aryl substituents in β-position. In Scheme 30, the proposed mechanism for forming the tetrahedral-pyrrolo-pyrazole from the reaction of cycloalkenyl amines and α,β-unsaturated esters via intermediates 61–65 was postulated [25].
Scheme 28.
Synthesis of cis/trans-pyrrolotriazoles 56a–k and 57a–k. Reagents and conditions: (a) BuLi, THF, 1 mol% LiCl, −78 °C, then AcOH, −78 to 25 °C.
Scheme 29.
Synthesis of cis/trans-pyrrolotriazoles 59a–k and 60a–k. Reagents and conditions: (a) BuLi, THF, 1 mol% LiCl, −78 °C, then AcOH, −78 to 25 °C.
Scheme 30.
The suggested stereochemical rationale for the formation of tricyclic compounds 56 and 57a–k.
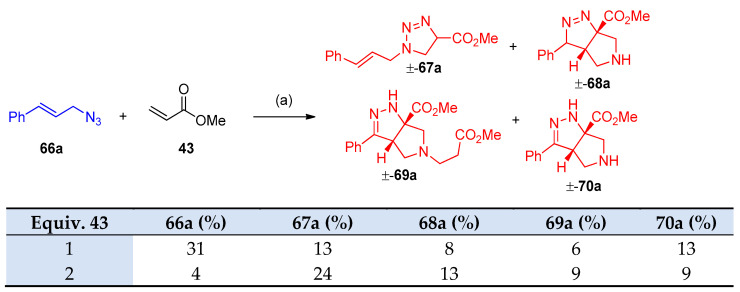
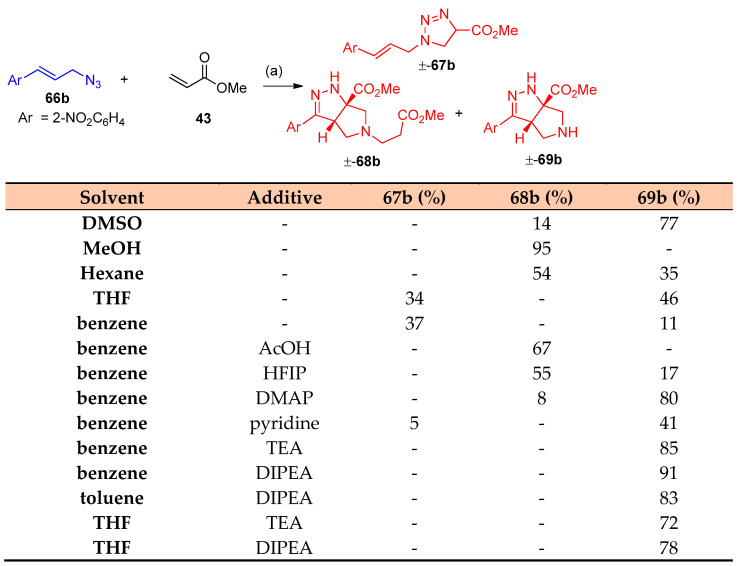
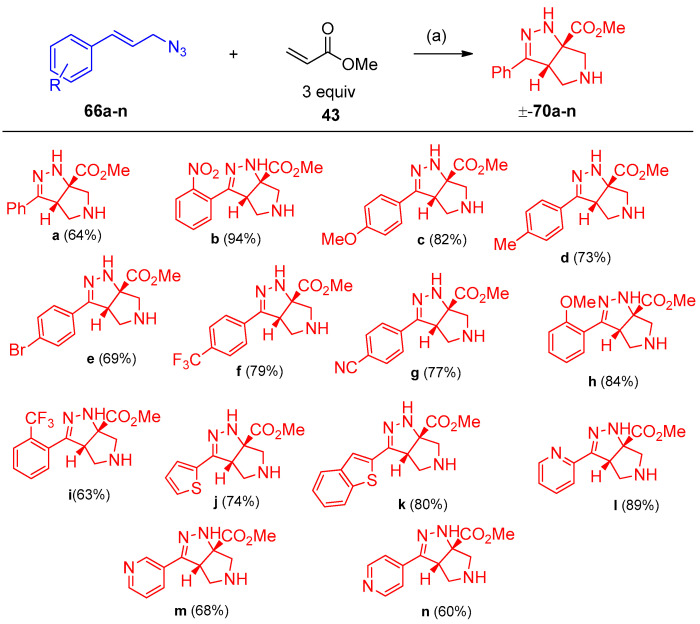
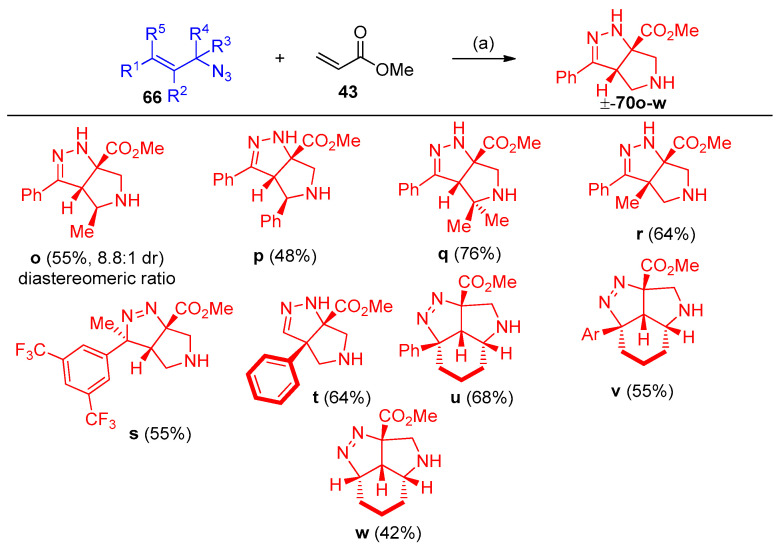
Previously, a cycloaddition reaction was reported between cinnamyl azide and methyl acrylates to obtain the tetrahydro-pyrrole-pyrazole [26,27]. Recently, Carlson et al. [28] developed the previously mentioned procedure via stereoselective interaction between allylic azides and acrylates in high yields. The development includes (i) secondary and tertiary azides, (ii) the use of an enantioenriched azide, (iii) cinnamyl azides substituted at the α or β-carbon, (iv) derivatization of the products, and (v) additional Michael acceptors. Interestingly, it was found the following sequences: (A) the reaction was not completed during the reaction with cinnamoyl azide 66a, (B) 1 equivalent of acrylate 43 did not consume the azide at room temperature for three d, and (C) quantitative intermediates 67a–69a were obtained (Scheme 31). Optimization of acrylates 43 with cinnamoyl azides with aryl group of electrons withdrawing character has been investigated in Scheme 32. Re-optimization of reaction conditions such as (i) solvent, (ii) concentration, (iii) temperature, (iv) equivalents of acrylate, (v) time, and (vi) addition of DIPEA. It was found that the reaction proceeds well with a variety of cinnamoyl azides and the yields were improved. When DIPEA was used as a solvent compound, 70b was obtained with a 94% yield (Scheme 33). Optimization of the reaction in the scope of the substrate, incorporating methyl or phenyl group adjacent to the azide, for compound 70o a diastereomer was observed. Furthermore, cyclic azide resulted in the formation of tricyclic compounds 70u–w, as demonstrated in Scheme 34 [28].
Scheme 31.
Synthesis of tetrahydro-pyrrole-pyrazoles 67a–70a. Reagents and conditions: (a) THF 5 mL, r.t 3 d.
Scheme 32.
Synthesis of tetrahydro-pyrrole-pyrazoles 67b–69b. Reagent and conditions: (a) Solvent (0.2 mL), additive (0.5 equiv.), 70 °C, 24 h.
Scheme 33.
Synthesis of pyrrole-pyrazoles 70a–n. Reagent and conditions: (a) 0.5 equiv. DIPEA, benzene, 70 °C, 24 h.
Scheme 34.
Synthesis of pyrrole-pyrazoles 70o–w. Reagents and conditions: (a) (i) 3.5 equiv. acrylate, neat; (ii) 0.5 DIPEA, 70 °C.
4.3. Synthesis of Heterocycles Containing Two Heteroatoms
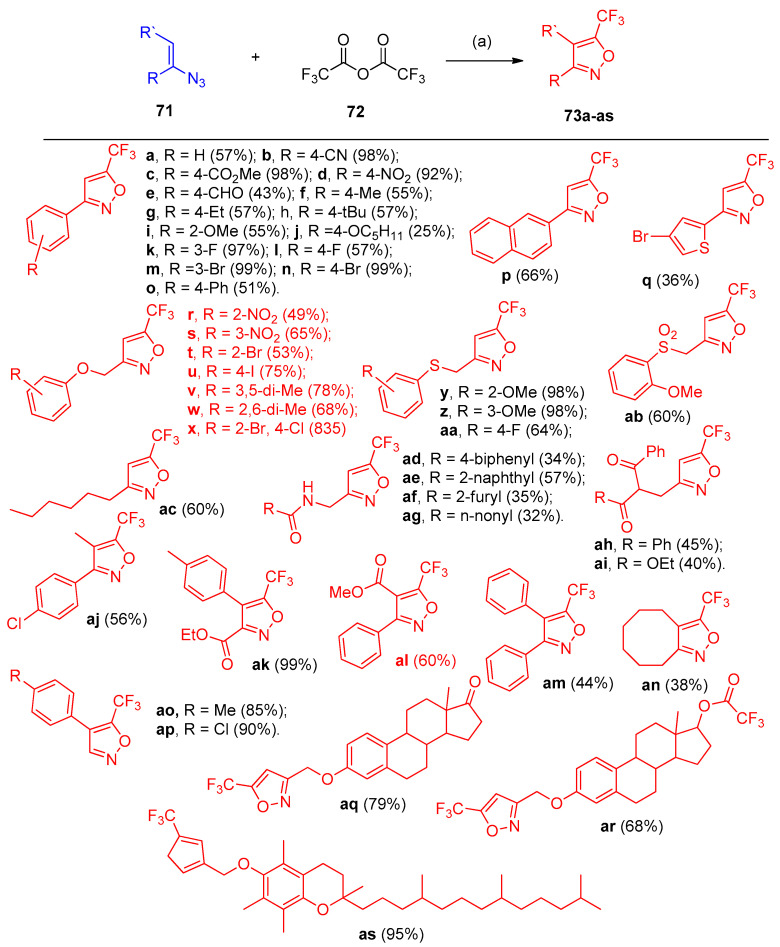
Vinyl azides 71 reacted with trifluoroacetic anhydride 72 in the presence of NEt3 to give 5-(trifluoromethyl)isoxazoles 73a–as via denitrogenative cyclization processes (Scheme 35) [29].
Scheme 35.
Syntheses of 5-trifluoromethyl isoxazoles 73a–73as. Reagent and conditions: (a) Et3N, 1,4-dioxane, under N2 atmosphere.
4.4. Synthesis of Oxazole, Thiazole, and Oxazine Derivatives
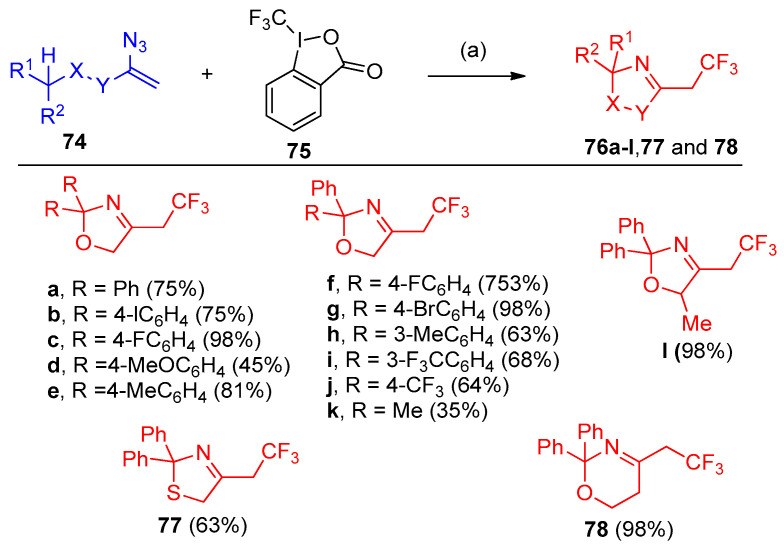
The reaction of substituted vinyl azides 74 with a combination of substoichiometric amounts of iron(II) chloride and Togni’s trifluomethylating reagent 75 resulted in the formation of 2,2,2-trifluoroethyl-substituted 3-oxazolines 76, 3-thiazolines 77, and 5,6-dihydro-2H-1,3-oxazines 78. It was found that the optimal reaction conditions clarified that DCM, DCE, DMF, and 1,4-dioxane were solvents of choice, and the temperature varied from 80°C to ambient temperature (Scheme 36) [30].
Scheme 36.
Azides in the synthesis of oxazole, thiazole, and oxazine derivatives. Reagents and conditions: (a) FeCl2 (20 mol%), DCM, room temperature, 30 min.
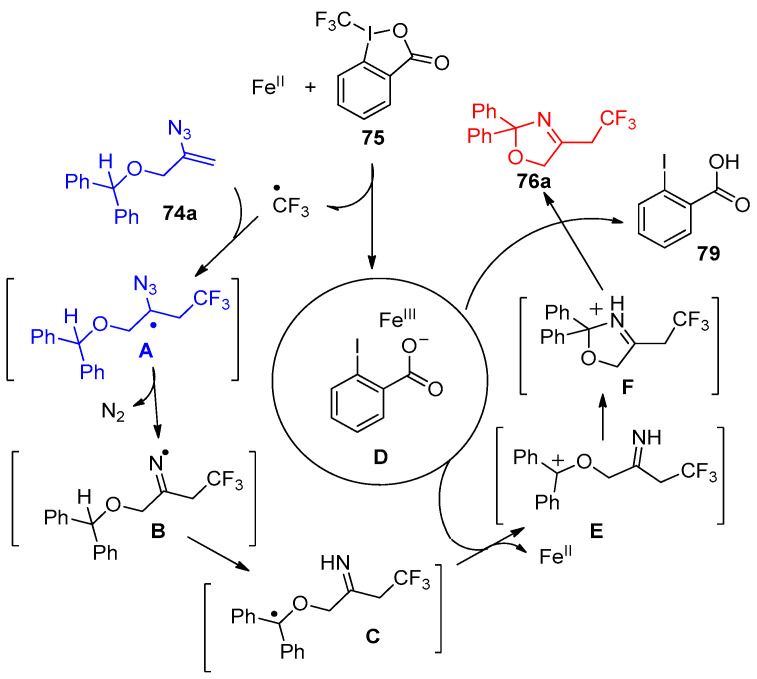
The proposed mechanism described the formation of compound 76a. It showed the role of FeII in the reaction steps and its activation of Togni’s reagent via the formation of intermediates A–C; deprotonation was then achieved by the FeIII iodobenzoate complex (D) to give compound 76a and iodobenzoic acid 79 (Scheme 37) [30].
Scheme 37.
Plausible Mechanism for the formation of compound 76a.
4.5. Synthesis of the Triazole Ring
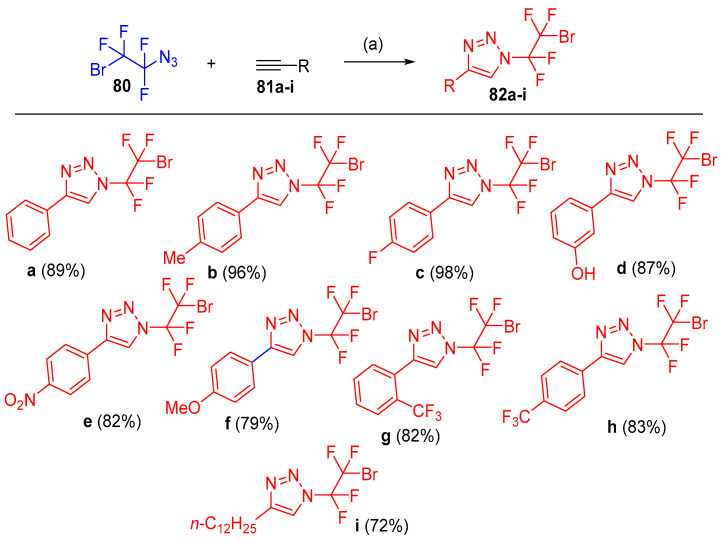
Reactivity of azides 80 in [3 + 2] cycloaddition with aromatic or aliphatic terminal alkynes 81a–i clarified that 5 mol% of CuMeSal (copper(I) 3-methylsalicylate), with azidotrifluoromethane and other azidoperfluoroalkanes, afforded a range of N-bromo tetrafluoroethyl-substituted 1,2,3-triazoles 82a–i in good to high yields (Scheme 38). Since the reaction gave only one regioisomer, it was described as highly efficient and regiospecific (the reaction exclusively afforded only the 1,4-disubstetuted triazole derivatives) at room temperature [31].
Scheme 38.
Synthesis of N-bromotetrafluoroethyl-substituted 4-aryl-1,2,3-triazoles 82a–i. Reagents and conditions: (a) THF, CuMeSal (0.05 mmol), r.t. overnight.
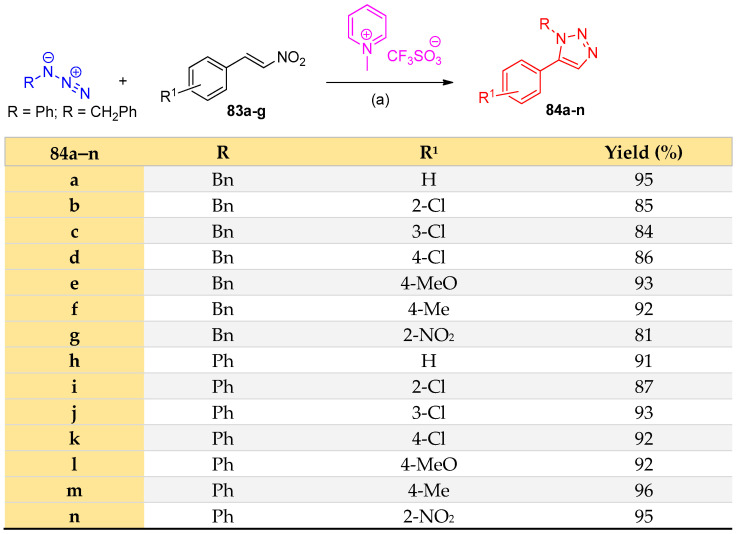
Ionic liquid/iron(III) chloride as a homogeneous catalyst was applied in the synthesis of 1 1,2,3-triazoles 84a–n from the reaction of substituted azides and substituted styrenes 83a–g [32] (Scheme 39).
Scheme 39.
Synthesis of 1,2,3-triazoles 84a–n. Reagents and conditions: (a) FeCl3 (20%), 100 °C.
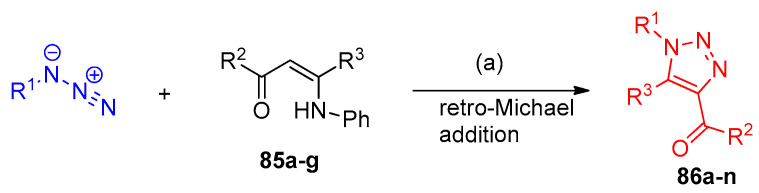
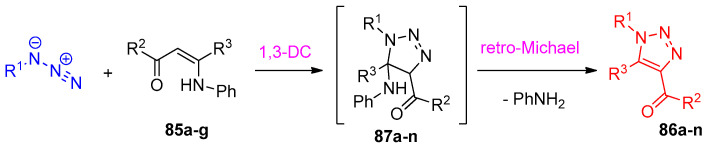
Regioselective synthesis of 1,4,5-trisubstituted-1,2,3-triazoles 86a–p from the catalyzed reaction between enaminones 85 and aryl azides using 1-methyl pyridinium trifluoromethanesulfonate [mPy]OTf as the ionic liquid in basic medium via 1,3-dipolar cycloaddition (Scheme 40). Herein, the reaction selectively generates only the 1,5-disubstituted triazoles as the only possible product over 1,4-disubstituted triazoles. As illustrated in Scheme 41, the proposed mechanism demonstrated that the formation of triazoles 86a–n from the reaction of the aryl azides and enaminones 85a–g was taken via the formation of intermediate 87a–n [33]. The reaction was described as retro-Michael addition to give two regioisomers. Elimination of aniline from the two regiosomers afforded the corresponding triazoles 86a–n. Moreover, the reaction took place with complete regioselectivity yielding the regioisomer with the electron-deficient group of the enaminone in position 4 and the alkyl substituent in position 5 as the only product of the reaction.
Scheme 40.
Synthesis of 1,2,3-triazoles 86a–n. Reagents and conditions: (a) [mPy]OTf/H2O, Et3N, 100 °C, 4–10 h.
Scheme 41.
Reaction mechanism for the reaction between enaminones and azides to form triazoles 86a–n.
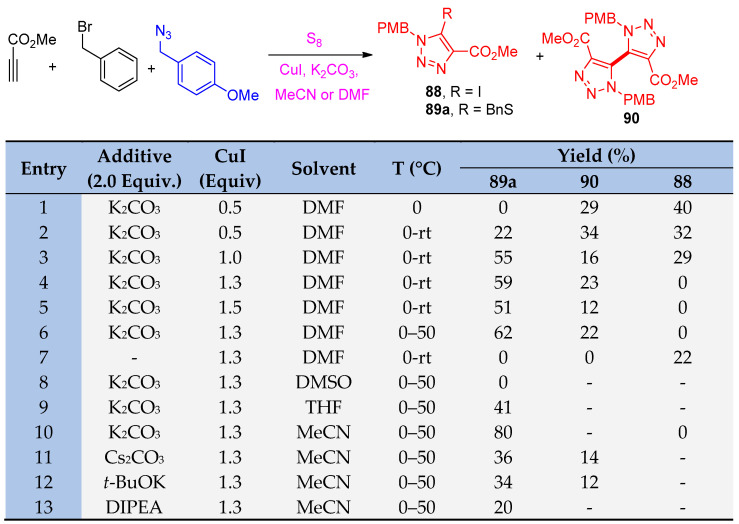
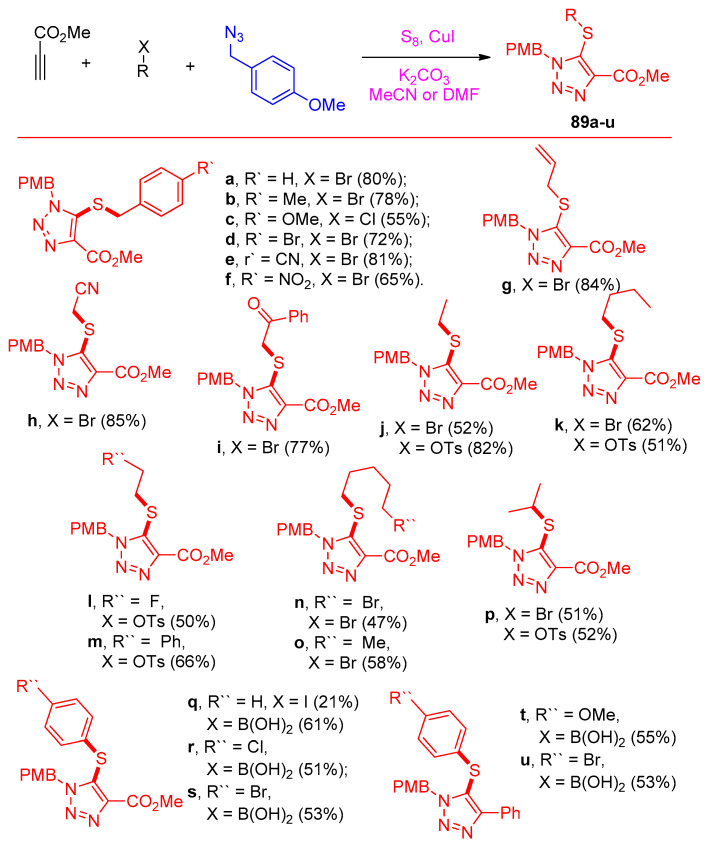
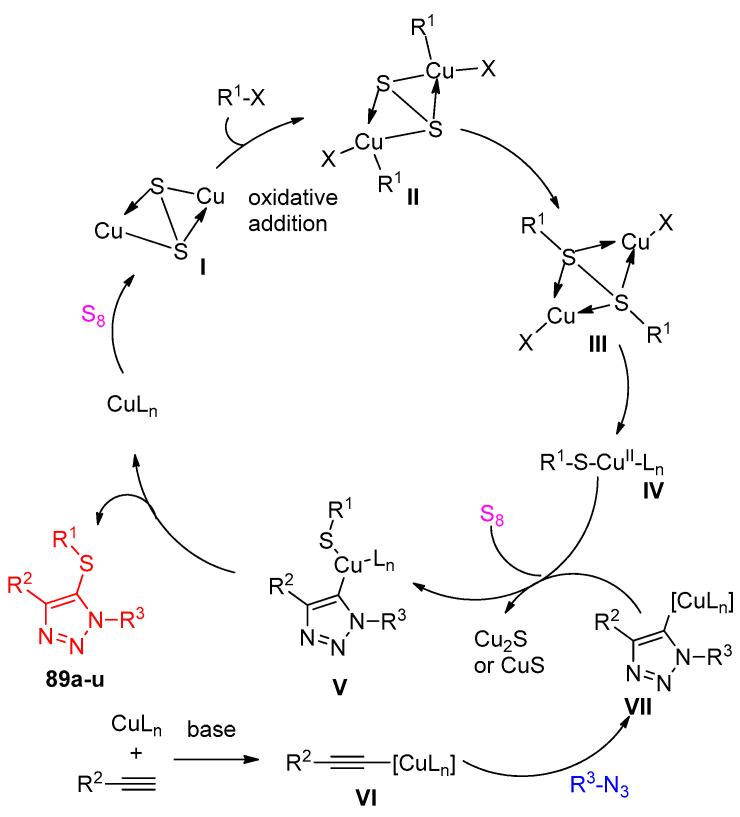
Zhang et al. [34] reporoom temperatureed the one-pot multicomponent reaction for the syntheses of 5-thiotriazoles 89a–u, 91a–m, and 5-selenotriazole 92a–l scaffolds using sulfur and selenium elements. Firstly, the reaction was displayed between methyl propiolate, benzyl bromide, S8, and 4-methoxybenzyl azide (PMBN3) and was selected to optimize the reaction conditions. It was clear that the optimized condition was achieved for 89a in the following conditions: CuI (1.3 equiv), K2CO3 (2.0 equiv), and S8 (3.0 equiv) in MeCN (or DMF), first at 0 °C for 1 h and then at 50 °C for 10 h (Scheme 42). The yield was increased with an increasing amount of CuI to 1.3 equivalent and at 50 °C using DMF or MeCN as a solvent. Accordingly, the yields of compounds 89a–u became good compared with the previous method, as shown in Scheme 43.
Scheme 42.
The optimization of the multicomponent reaction for the formation of thiotriazoles 88, 89a, and 90.
Scheme 43.
Substrate effect on the formation of 5-thiotriazoles 89a–u under the optimal conditions.
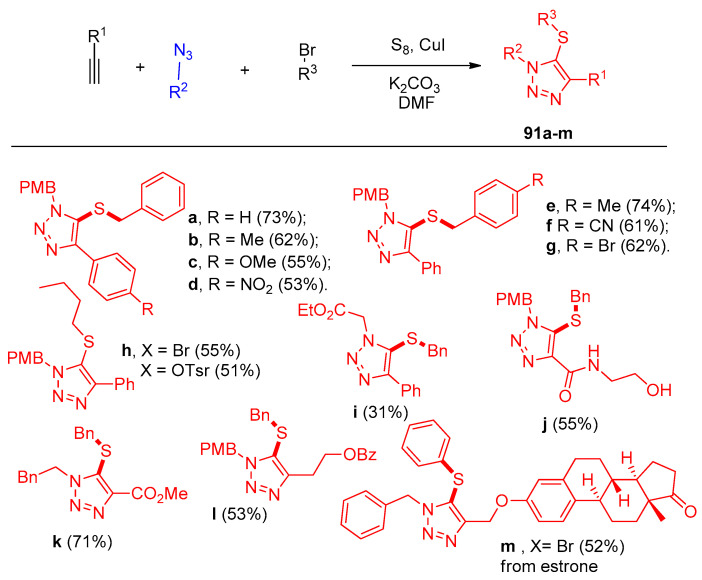
Next, the influence of the alkynes and azides was examined using DMF as the solvent at temperatures ranging from room temperature to 70 °C for the generation of the sulfenylating agent (Scheme 44). Aromatic alkynes with methyl, methoxy, and nitro groups on the benzene ring worked well to produce the corresponding 5-thiotriazoles 91a–i. The efficiency of acylacetylenes was demonstrated by the generation of 91j bearing a reactive hydroxyl group. Aliphatic alkynes also proved to be effective for this process 91l. The excellent availability of this multistep reaction has been well demonstrated by the generation of products 91i and 91k from α-azidoacetate and 2-phenylethyl azide, respectively. Under the previous mild sequential copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) and thiolization reaction conditions, the estrone derivative of an alkyne was easily transformed into the corresponding 5-thiotriazole 91m in 52% yield.
Scheme 44.
Substrate effect of alkynes and azides in the synthesis triazolothiols 91a–m.
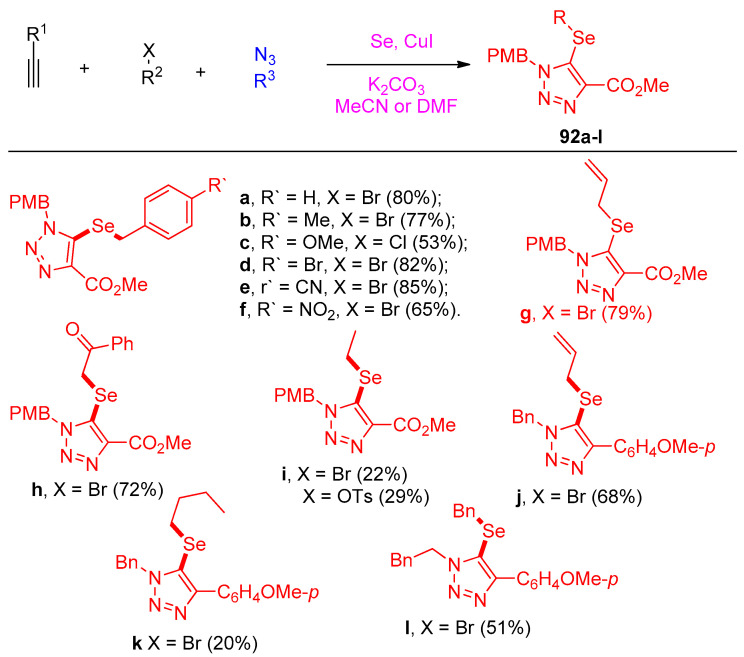
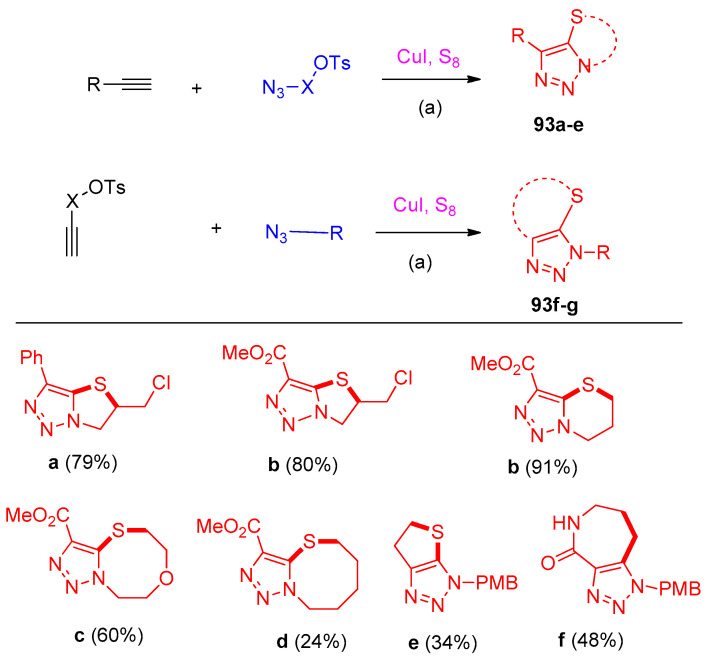
5-Selenotriazoles 92a–l were prepared using selenium as a reagent under the standard conditions in DMF or MeCN, as shown in Scheme 45. The examination was extended to synthesize fused bicyclic 5-thiotriazoles 93a–g [34] (Scheme 46).
Scheme 45.
Synthesis of selenotriazoles 92a–l.
Scheme 46.
Synthesis of fused bicyclic 5-thiotriazoles. Reagents and conditions: (a) CuI (0.65 mmol), S8 (1.5 mmol), K2CO3 (1.0 mmol), DMF, 0 °C, 30 min, room temperature or 70 °C, 10 h or overnight.
Copper(I) acetylide VI was formed from a terminal alkyne and CuI under basic conditions. The proposed mechanism for this work is shown in Scheme 47. In the beginning, CuI reacted with sulfur to give copper sulfide I. Subsequently, the oxidative addition with a halide forms II, transformed into copper(II) thiolate IV via reductive organic group transfer and oxidation. The cycloaddition of VI with the azide produced the copper(I) triazolide intermediate VI1. Reaction of copper(II) thiolate IV with the copper(I) triazolide would give the intermediate V. Finally, reductive elimination of V would then lead to the expected 5-thiotriazole 89a−u (Scheme 47) [34].
Scheme 47.
A plausible mechanism for the formation of thiotriazoles.
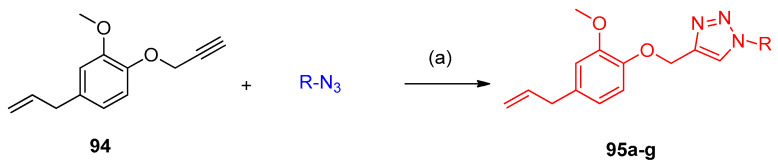
Copper catalyzed 1,3-dipolar cycloaddition between azides and 4-allyl-2-methoxy-1-(prop-2-yn-1yloxy)benzene (94) in refluxing acetonitrile afforded the corresponding monocycloadduct 95a–g with regioselectivity in high yields between 78 and 90% (Scheme 48) [35].
Scheme 48.
Synthesis of triazoles 95a–g. Reagents and conditions: (a) CuI, MeCN, room temperature, 2 h.
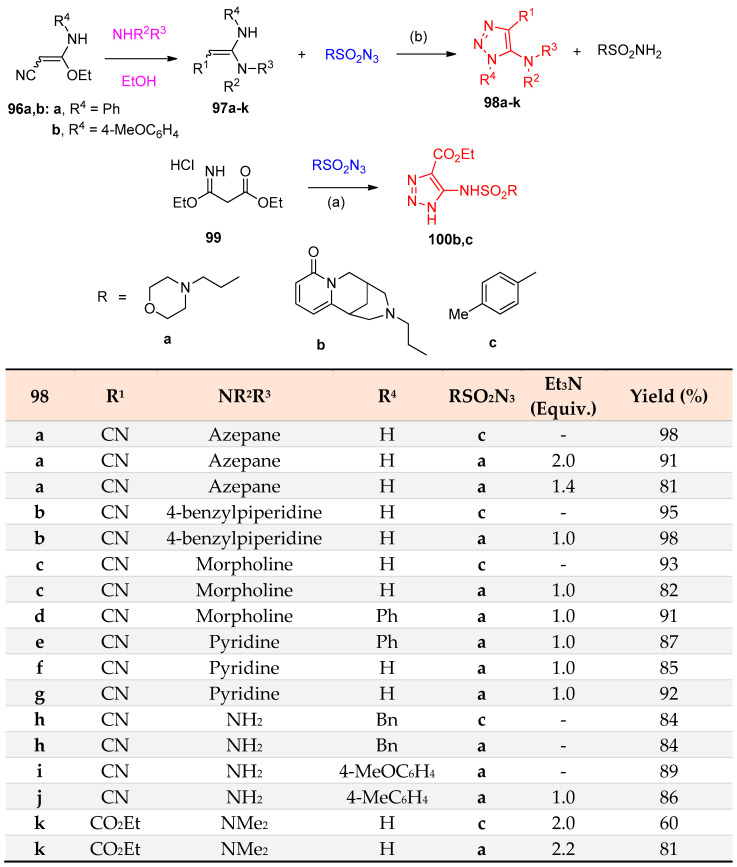
5-Amino-1,2,3-triazoles 98a–k were prepared from the corresponding amidines 97a−k reacting with tosyl azides in a methanolic basic medium (Scheme 49) [36].
Scheme 49.
Synthesis of 5-amino-1,2,3-triazoles 98a–k. Reagents and conditions: (a) EtOH, reflux; (b) Et3N, MeOH, room temperature.
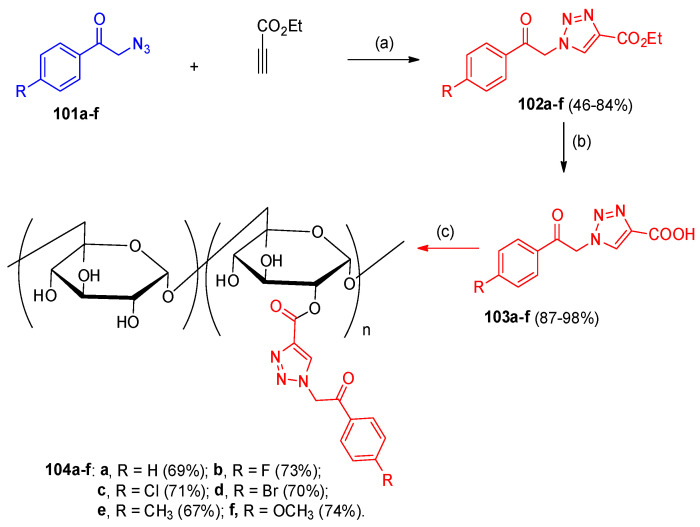
Stanciu et al. [37] reported that N, N′-carbonyldiimidazole (CDI) synthesized amphiphilic esters based on dextran via a one-pot procedure based on the reaction between the polysaccharide and different substituted 1,2,3-triazoles-4-carboxylates 102a–f. Firstly, the triazole derivatives 102a–f were obtained through copper alkyne azide cycloaddition (CuAAC) between azide 101a–f and ethyl propiolate. Basic hydrolysis of the triazole ester 102a–f using KOH(aq), MeOH/H2O gave 1,2,3-triazol-4-carboxylic acid derivatives 103a–f [37]. Esterification of the dextran (polysaccharide) with the triazole ester activated in situ with 1,1′-carbonyldiimidazole (CDI) to give the dextran esters 104a–f (Scheme 50).
Scheme 50.
Synthesis of esters of dextran-triazole esters 104a–f. Reagents and conditions: (a) CuSO4·5H2O, sodium ascorbate, t-BuOH/MeOH/H2O; (b) KOH (aq), MeOH/H2O; (c) (i) CDI (DMSO, room temperature, 24h); (ii) Dex40 (DMSO, 80 °C, 24 h).
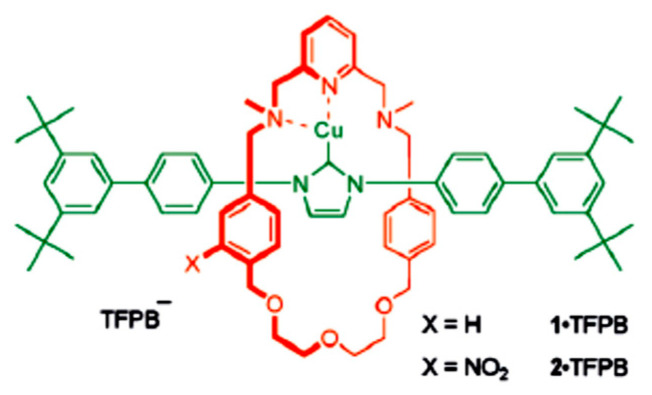
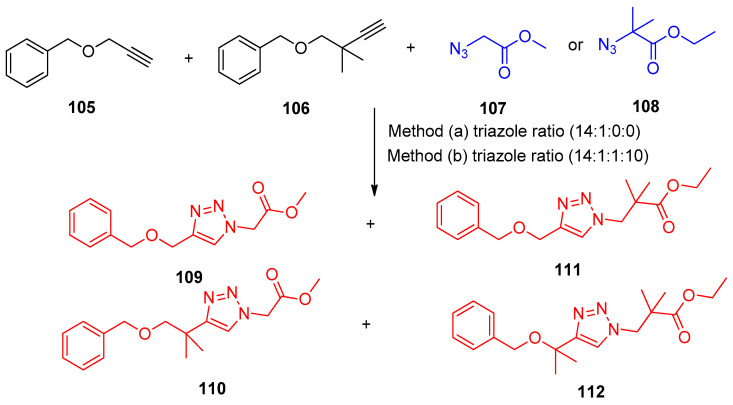
N-Heterocyclic carbene-copper (NHC-Cu) complexes were known as organometallic catalysts that could differentiate the reactivities of simple terminal alkynes and azides through amplified steric discrimination, allowing efficient sequential ligations of a diyne with two different azides under conditions of premixing all of the reaction partners in solution [38,39]. The interlocked NHC-CuI complexes were found as 1-TFPB and 2-TFPB (TFPB: tetrakis[3,5-bis(trifluoromethyl)phenyl] borate) (Figure 3). The rotaxane 1-TFPB catalyzed a competition reaction involving two pairs of individual alkynes and azides. Therefore, a heating mixture of the non-bulky alkyne 105, the bulky alkyne 106, the non-bulky azide 107, and the bulky azide 108 in THF at 323 K for 48 h in the presence of rotaxane 1-TFPB (15 mol%), four possible triazole products were formed 109–112 with good selectivity. The triazole 109 was predominant, with the ratio of the triazoles 109–112 being 14:1:0:0 (Scheme 51) [40].
Figure 3.
Structure of the interlocked NHC-CuI complexes 1-TFPB and 2-TFPB [40].
Scheme 51.
Synthesis of triazoles 109–112. Reagents and conditions: Method (a) 1-TFPB (15 mol%), THF-d6, 50 °C, 48 h; Method (b) (i)1-TFPB (15 mol%), THF, 50 °C, 48 h; (ii) Cu(MeCN)4PF6/2,6-lutidine.
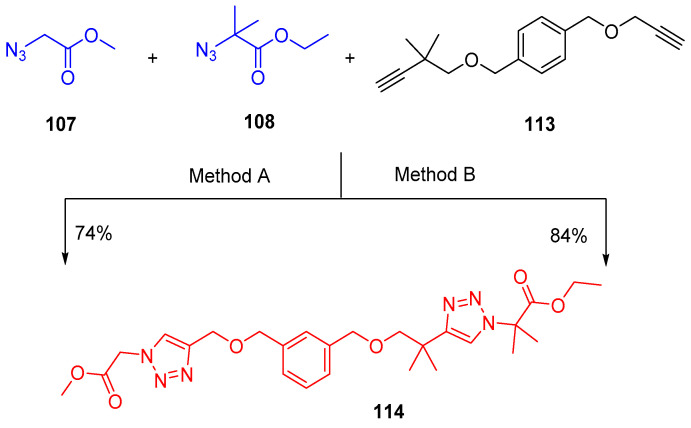
Moreover, when azides 107 and 108 were reacted with diyne 113, under the same conditions in the presence of rotaxane 1-TFPB (15 mol%) and then adding [Cu(MeCN)4]PF6 and 2,6-lutidine to the intermediate mixture bis-triazole, 114 was isolated in 74% yield (Method A) (Scheme 52). When the last reaction was performed in the presence of 2-TFPB (15 mol%) under the same conditions in the dark for 48 h, the less bulky alkyne and azide had disappeared, while those of the bulky alkyne and azide remained, exhibiting good chemoselectivity toward the coupling of the less bulky parts. Irradiation of this intermediate mixture with light (350 nm, 5 min) cleaved approximately half of the macrocyclic components formed. Heating the resulting mixture (323 K, 12 h) led to the coupling of the bulky pair of alkyne/azide partners and the formation of triazole product 114 in 84% yield (Scheme 52) [40].
Scheme 52.
Synthesis of ethyl 2-(4-(1-((3-(((1-(2-methoxy-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)benzyl)oxy)-2-methylpropan-2-yl)-1H-1,2,3-triazol-1-yl)-2-methylpropanoate (114). Reagents and conditions: Method A (i)1-TFPB (15 mol%), THF-d6, 50 °C, 48 h; (ii) Cu(MeCN)4PF6/2,6-lutidine. Method B (i) 2-TFPB (15 mol%), THF-d6, 50 °C, 48 h, dark; (ii) hν, (350 nm), 5 min, room temperature; (iii) hν, 15 min, room temperature, then 50 °C, 12 h.
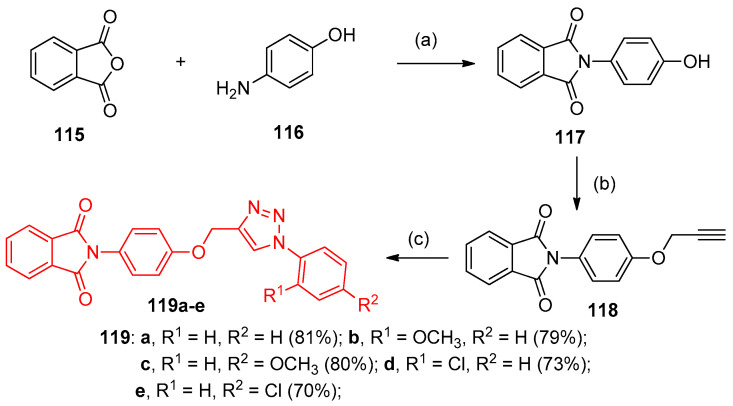
Syntheses of 2-(4-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)isoindoline-1,3-dione derivatives 119a–f were involved in three steps. 4-Aminophenol 116 reacted with phthalic anhydride (115) in acetic acid at 100 °C to give compound 117 (Scheme 53). Propargylation of 117 afforded the corresponding 2-(4-(prop-2-yn-1-yloxy)phenyl)isoindoline-1,3-dione (118), which in the presence of potassium carbonate and subsequently treated with various azides via 1,3-dipolar cycloaddition (click reaction) during treatment with 10 mol% of sodium ascorbate and 10 mol% of copper sulfate. Consequently, the reaction afforded 1,2,3-triazolyisoindoline-1,3-dione derivatives 119a–e in excellent 70% to 81% yield, as shown in Scheme 53 [41].
Scheme 53.
Synthesis of 1,2,3-triazolyisoindoline-1,3-dione derivatives 119a–f. Reagents and conditions: (a) Acetic acid, 100 °C, 3 h; (b) K2CO3, Propargyl bromide, DMF, room temperature, 3 h; (c) Azides, 10 mol% of sodium ascorbate, 10 mol% of CuSO4·H2O, DMF: Water (1:1), room temperature, 10 to 12 h.
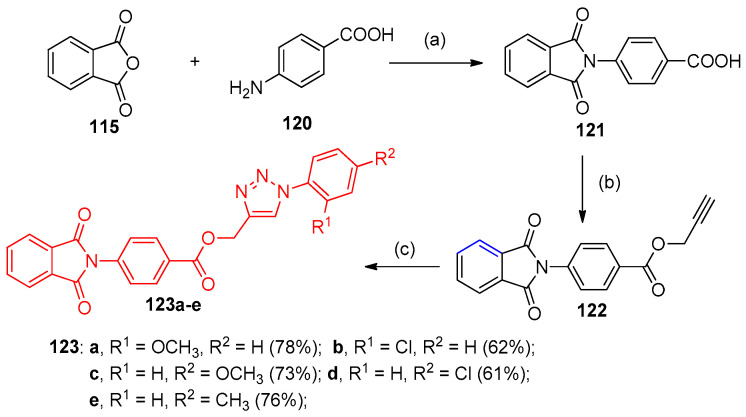
Applying the same procedure mentioned, the synthesis of triazoles 123a–e was also established in the same three steps. Compound 122 was obtained, which in the presence of potassium carbonate and subsequently treated with various azides and 10 mol% of sodium ascorbate and 10 mol% of copper sulfate, afforded the final compounds 123a–e via 1,3-dipolar cycloaddition (Click reaction) (Scheme 54) [41].
Scheme 54.
Synthesis of triazoles 123a–e. Reagents and Conditions: (a) acetic acid, 100 °C, 3 h; (b) K2CO3, propargyl bromide, DMF, room temperature, 3 h; (c) Azides, 10 mol% of sodium ascorbate, 10 mol% of CuSO4·5H2O, DMF:H2O (1:1), room temperature, 10 to 12 h.
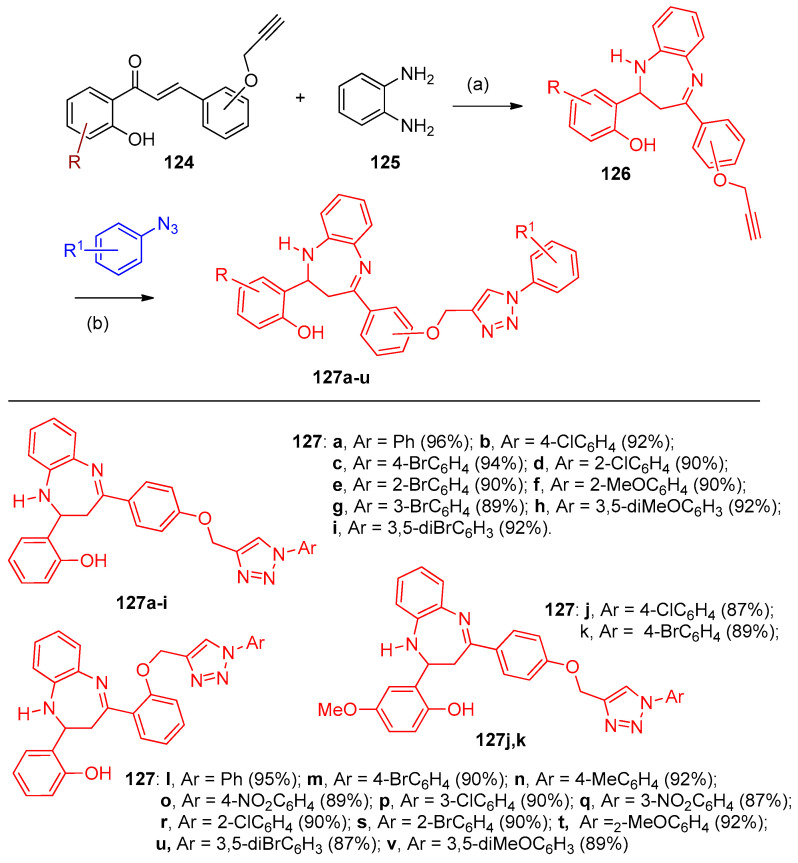
CuFe2O4@MIL-101(Cr) was used as a catalyst in synthesizing benzodiazepine triazole derivatives during the reaction of chalcones containing the acetylene group in the o or p positions 124a–c with substituted azides containing both electron-withdrawing groups and electron-donating groups (Scheme 55). Firstly, the chalcones 124 reacted with o-phenylene diamine 125 to furnish the corresponding diazepine acetylene derivatives 126, which on click reaction underwent cyclization to give the triazole derivatives 127a–u [42].
Scheme 55.
Synthesis of triazoles 127a–v. Reagents and conditions: (a) CuFe2O4@MIL-101(Cr), water reflux; (b) H2O, reflux.
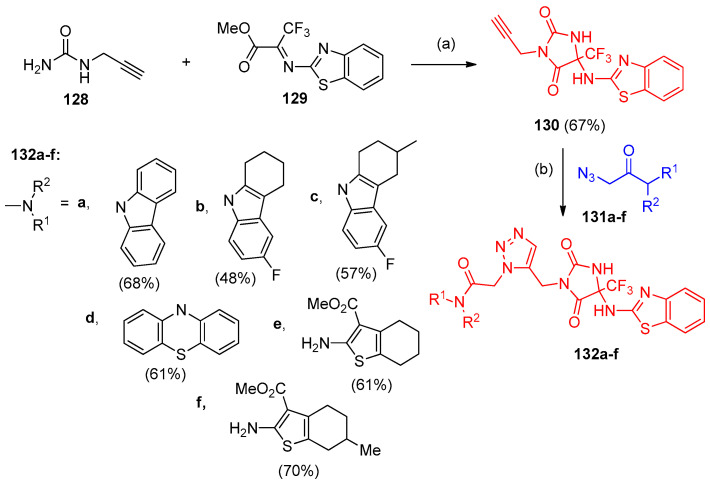
Propargylurea (128) underwent cyclocondensation with methyl trifluoropyruvate benzothiazolylimine (129) in the presence of Et3N to give 5-alkynyl-substituted trifluoromethyl hydantoin 130 in 67% yield. The alkyne 130 was subjected to CuAAC reaction with 2-azidoacetamides 131 to give the corresponding 1,4-substituted 1,2,3-triazoles 132a–f (Scheme 56) [43].
Scheme 56.
Synthesis of 1,2,3-triazoles 132a–f. Reagent and conditions: (a) (i) DMF, 20 °C, stirring then at 90 °C for 1 h; (ii) E3N, 90 °C, 2 h; (b) CH2Cl2, CuSO4 (0.05 mmol), sodium ascorbate (0.05 mmol), stirring 3 h, 40 °C.
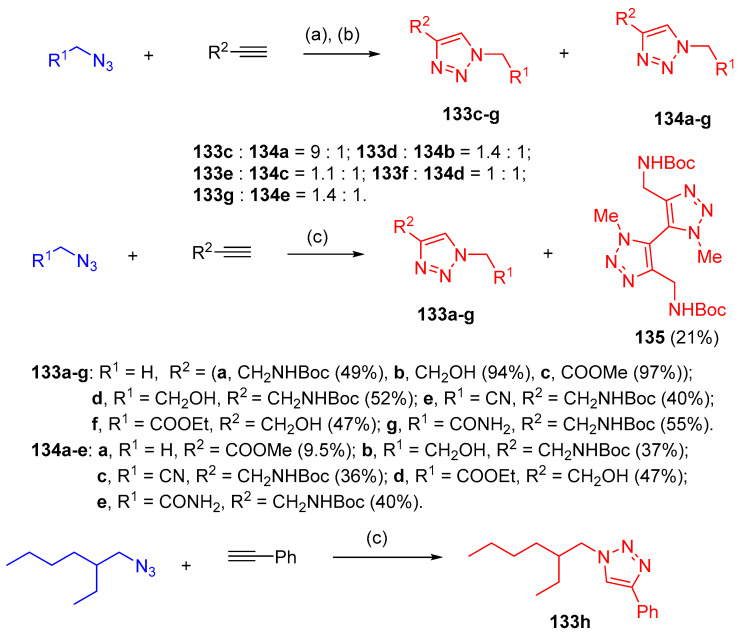
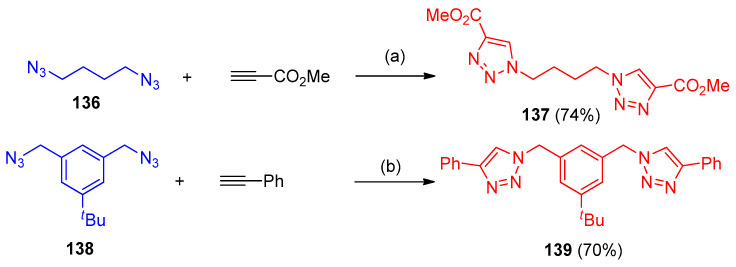
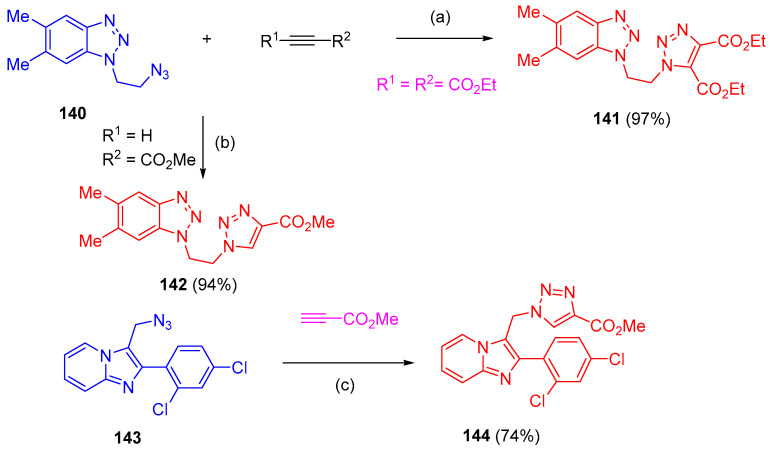
Azides reacted regioselectivity with alkynes via CuAAC 1,3-dipolar cycloaddition using CuI to form triazoles 133a–g as a major product and triazoles 134a–g as minor products. An exception was the reaction of methyl azide with tert-butyl prop-2-yn-1-ylcarbamate, which resulted in a mixture of triazole 133a and 5,5′-bitriazole 135 at a ratio of 2.4:1. Under similar conditions, the longer aliphatic chain azide (3-(azidomethyl)heptane) reacted with phenylacetylene to give triazole 133h in low yield by using copper(I) iodide as a catalyst in combination with a catalytic amount of benzoic acid furnished 133h in a high yield (90%) (Scheme 57). Bis-triazoles 137 and 139 were obtained in high yields with a faster rate of reaction via click reaction of 1,3-diazidopropane (136) and diazide 138 with ethyl propiolate and phenylacetylene, respectively (Scheme 58). Furthermore, azide 140 (1-(2-azidoethyl)-5,6-dimethyl-1H-benzo[d][1,2,3]triazole) was reacted with diethyl acetylenedicarboxylate and methyl propiolate afforded triazole 141 (97%) and triazole 142 (94%) yields, respectively (Scheme 59). Azide 143 was reacted even with a reactive dipolarophile, such as acetylenecarboxylate in t-BuOH in the presence of a basic cocatalyst gave triazole 144 (74%) (Scheme 59) [44].
Scheme 57.
Synthesis of various regioisomers of triazoles 133–135. Reagent and conditions: (a): R1 = H, R2 = CO2Me, PhH, 20 °C to room temperature, 12 h; (b): R1 ≠ H, PhMe, 80–90 °C, 24 h; (c) CuI, PhCOOH, i-PrOH–H2O.
Scheme 58.
Synthesis of bis-triazoles 137 and 139. Reagent and conditions: (a) CuI (1 mol%), Et3N, THF; (b) CuSO4·5 H2O, NaAsc, DMSO–H2O.
Scheme 59.
Synthesis of bis-triazole carboxylate derivatives 141, 142 and 144. Reagents and conditions: (a) PhMe; (b) CuI (1 mol%), THF; (c) CuI (10 mol%), Et3N, THF.
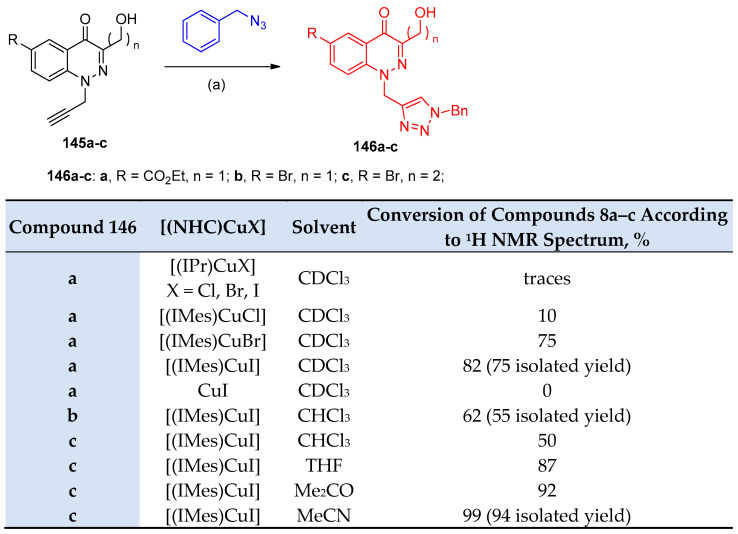
N-Propargylated cinnolinones 145a–c reacted with benzyl azide to afford the corresponding triazole derivatives 146a–c via CuAAC in CHCl3. It was noted that the complexes of the [(IPr)CuX] series (X = Cl, Br, I) did not exhibit catalytic activity. However, [(IMes)CuX] complexes (X = Cl, Br, I) containing a less sterically hindered ligand showed higher activity under the same reaction conditions; this was attributed to the electronic nature of the halides, and the catalytic reactivity increased in the order of Cl− < Br− ≈ I−, (Scheme 60) [45].
Scheme 60.
Synthesis of 1,2,3-triazoles 146a–c. Reagents and conditions: (a) [(NHC)CuX] (2 mol%), solvent, room temperature, 18 h.
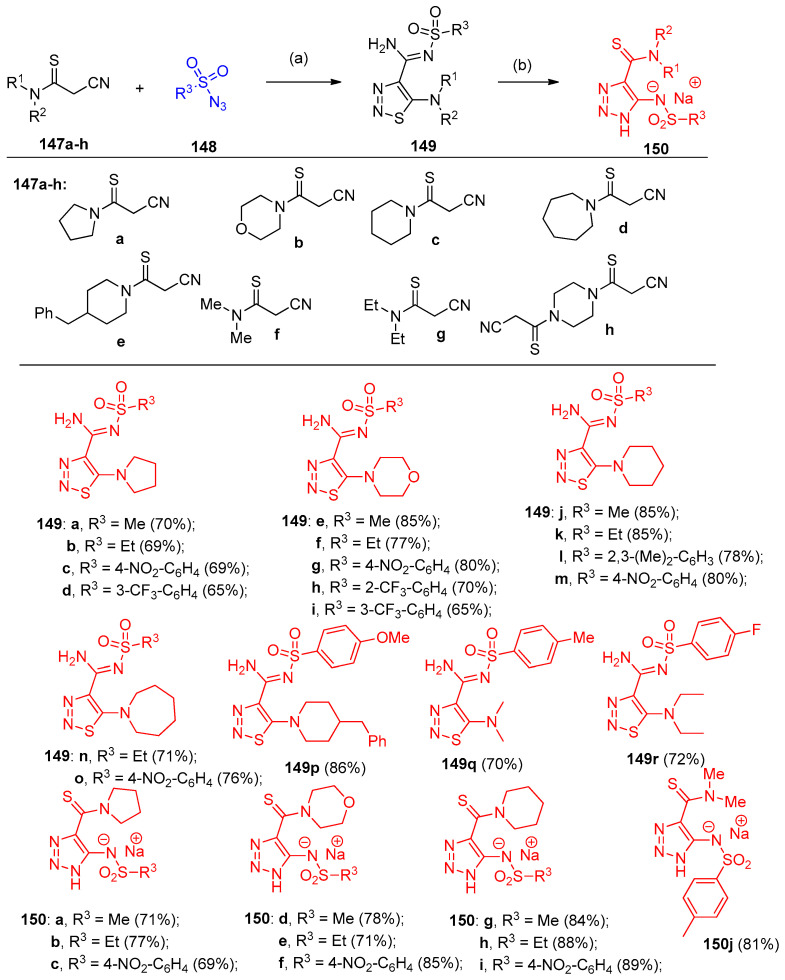
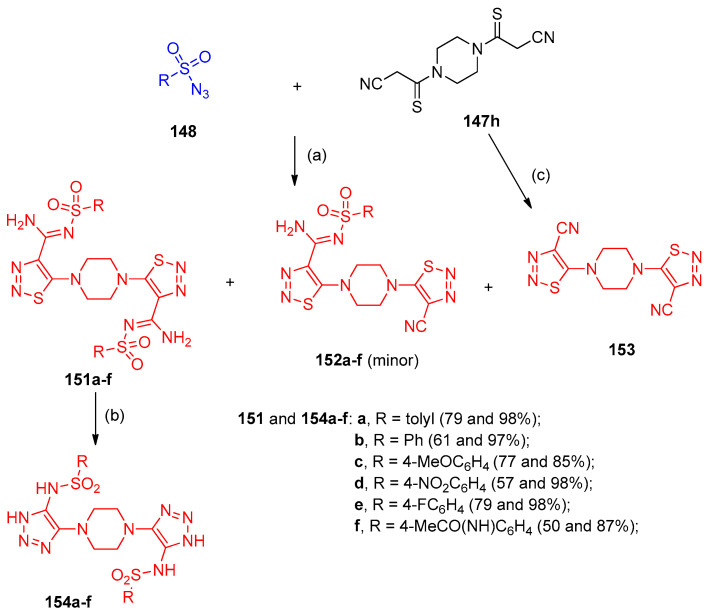
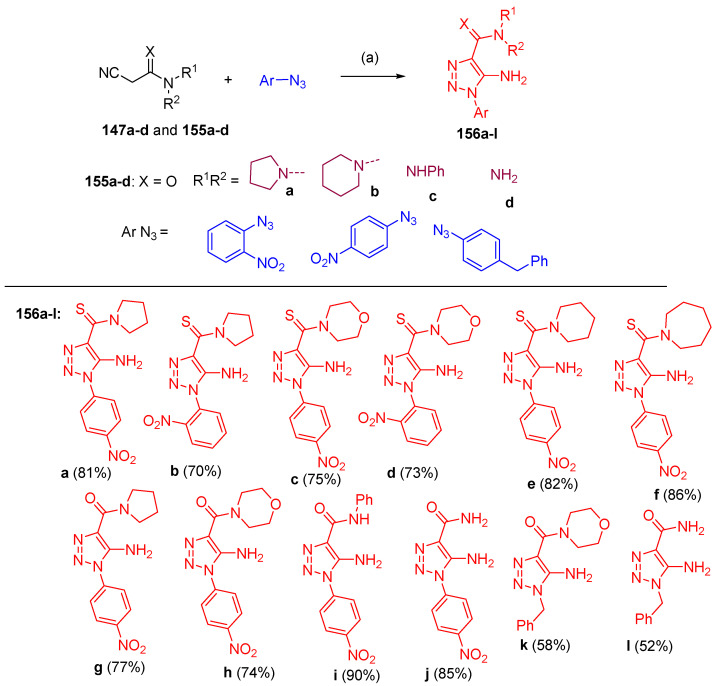
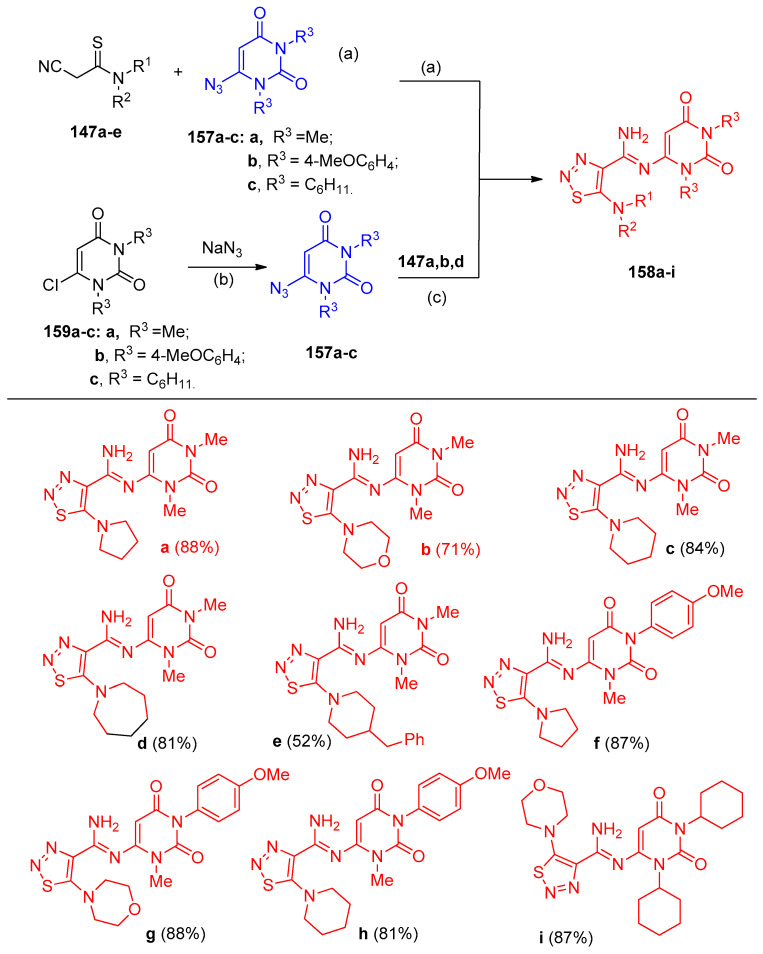
Filimonov et al. [46] reported a one-step, eco-friendly method for synthesizing 1,2,3-thiadiazol-4-carbimidamides 149a–r and 1,2,3-triazole-4-carbothioamides 150a–j, during the reactions of 2-cyanothioacetamides 147a–g with various types of azides 148 in water in the presence of alkali (Scheme 61). Furthermore, N,N`-bis-(2-cyanothiocarbonyl)-pyrazine 147h was reacted with sulfonyl azides 148 to give the bicyclic 1,2,3-thiadiazoles 151–153, and 1,2,3-triazoles 154a–f connected via a 1,1`-piperazinyl linker (Scheme 62). On the other hand, 2-cyanothioacetamides 155 reacted with aromatic azides in water in the presence of alkali to afford 1-aryl-5-amino-1,2,3-triazole-4-carbothioamides 156a–l (Scheme 63). In contrast to aromatic azides and sulfonyl azides, 6-azidopyrimidine-2,4-diones 157a–c reacted with cyanothioacetamides 147a–e to give N-pyrimidine-6-yl-5-dialkylamino-1,2,3-thiadiazole-4-N-l-carbimidamides 158a–i. Additionally, compounds 158a–i were obtained in two step-reaction starting with 6-chloro-1,3-disubstituted-pyrimidine-2,4-dione 159 (Scheme 64).
Scheme 61.
Synthesis of triazoles 147–150. Reagents and conditions: (a) NaOH, H2O, 0 °C; (b) EtONa, EtOH, 23 °C, 1 h.
Scheme 62.
Synthesis of bis-triazoles 154a–f. Reagents and conditions: (a) H2O, NaOH; (b) NaOMe, MeOH; (c) TsN3, pyridine.
Scheme 63.
Synthesis of triazoles 156a–l. Reagents and conditions: (a) NaOH, H2O, 50–60 °C.
Scheme 64.
Synthesis of N-pyrimidin-6-yl-5-dialkylamino-1,2,3-thiadiazole-4-N-l-carbimidamides 158a–i. Reagents and conditions: (a) H2O, NaOH, 0 °C; (b) NaN3, H2O, r.t., 24 h; (c) H2O, 0 °C, 24 h.
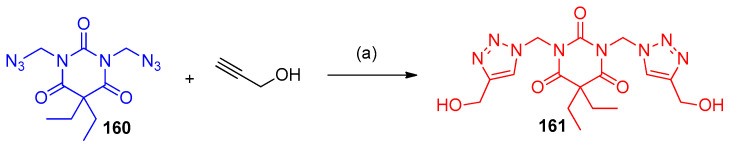
Bis(azidomethyl)-5,5-diethylpyrimidinetrione (160) underwent CuAAC 1,3-dipolar cycloaddition with alkyne (prop-2-yn-1-ol) to afford bis((4-(hydroxymethyl)-1H-1,2,3-triazol-1-yl)methyl)pyrimidinetrione (161) (Scheme 65) [46].
Scheme 65.
Synthesis of bistriazolylpyrimidinetrione 161. Reagents and conditions: (a) CuSO4·5H2O, NaAsc, DMF, 50 °C, stirring 15–50 h.
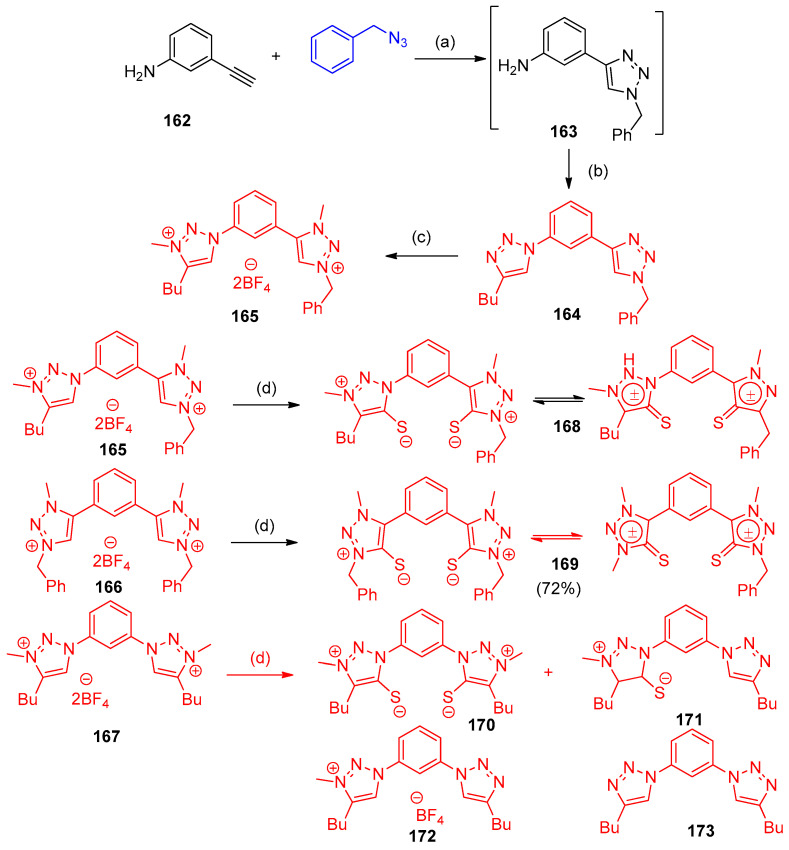
The copper-mediated click reaction using 3-aminophenyl-acetylene (162) and benzyl azide as the starting materials gave the monotriazole 163 using a well-defined copper carbene complex [CuCl (IPr)] (IPr = 1,3-bis-(2,6-diisopropylphenyl)imidazolin-2-ylidene) as a catalyst. Compound 163 underwent diazotization and azidation followed by [3 + 2] click reaction to afford the non-symmetrical bis(triazoles) 164. Alkylation of 164 using Meerwein’s salt (CH3)3OBF4 gave the dicationic pro-ligand salt 165. The non-symmetrical triazolium salt 165 and symmetrical 1,2,3-triazolium salts [47,48,49,50] 166 and 167 were utilized to synthesize mesoionic carbene-sulfur adducts 168a, 169b, and 170c. Firstly, the triazolium salts 165/166 were reacted with elemental sulfur in a base (KOtBu and K2CO3) non-symmetrical mesoionic bis (NHT) compound 168 the symmetrical analog 169 in good yields, 76% and 72%, respectively. Complexes 170–173 were formed with the treatment of 167 with elemental sulfur in the presence of K2CO3 as a base (Scheme 66) [51].
Scheme 66.
Synthesis of cationic-anionic triazoles. Reagents and conditions: (a) [CuCl(IPr)], EtOH/H2O; (b): NaNO2, HCl, and then NaN3, and then 1-hexyne, [CuCl (IPr)], EtOH/H2O, 82% (two-step combined yield); (c): (CH3)3OBF4, dichloroethane (DCE), 56%; (d) K2CO3, S8, MeCN, 90 °C, 24 h.
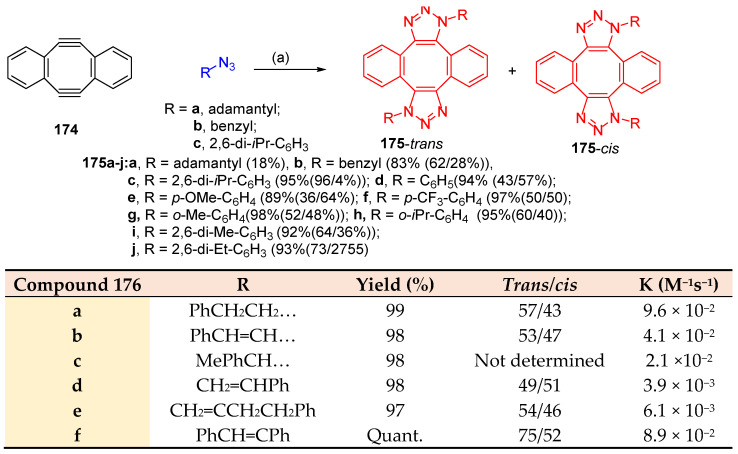
Yoshida et al. [52] reported that the double-click reaction between aryl azides and the diyne (183) afforded a regioisomeric mixture of bicyclo-adduct 184 (trans/cis) in an excellent yield. It was observed that 1-adamantyl azide was bulky, caused retention to the cycloaddition and gave the bis-cyclo-adduct with 18% for 4 h. The 2,6-diisopropylphenyl azide gave the bis-cyclo-adduct in quantitative yields, while the unhindered benzyl azide gave the bis-cyclo-adduct in 83% yields for 1 h. The studies were prolonged to cover the effect of the substituent in the aryl azide to clarify that the bulkiness groups enhanced the reaction rate, and the electronic nature of substituted groups in both o- and p-positions showed a limited effect on the reaction rate. In contrast, 2,6-disubstituted phenyl azides had accelerated reaction rates as the size of substituents became bulkier (the lower click ability of adamantyl azide than that of diisopropylphenyl azide was attributed to the stabilization of the azido group by hyper-conjugation with C-H bonds, which decreases the distort ability of the azido group) (Scheme 67) [52]. The click ability of the alkyl and alkenyl azides with Sondheimer diyne (174) afforded the regioisomeric bicyclo adducts 176 (trans/cis). The studies showed that the reaction rate was faster in the case of alkyl azides than in the alkenyl azides, indicating that resonance retarded the reaction rate and that both the inductive effect and hyper-conjugation increased the reaction rate (Scheme 67) [52,53].
Scheme 67.
Cycloaddition of Sondheimer diyne 174 with aryl azides. Reagents and conditions: (a) 2.4 equiv. of azide, MeOH room temperature.
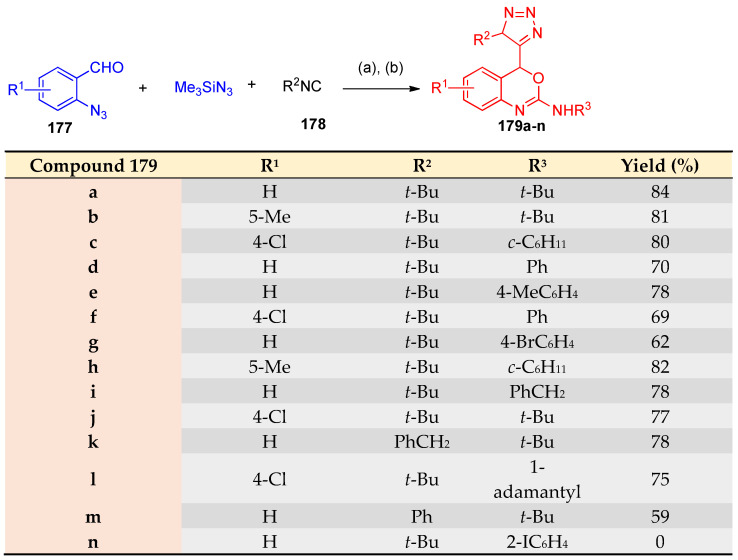
The synthesis of triazolyl benzoxazine derivatives 179a-n via one-pot reaction (e.g., Ugi reaction [54]) using the so-called Passerini-azide reactions (a method to prepare tetrazoles by substituting hydrazoic acid generated in situ from NaN3 or TMS-N3 (177), has been reported [54,55,56]. The reaction of 2-azidobenzaldehydes 176, 177, and isocyanides 178 gave 4H-3,1-benzoxazine derivatives by the in-situ formation of azide intermediate (Scheme 68) [56].
Scheme 68.
Synthesis of 4-triazolylbenzoxazoline derivatives. Reagents and conditions: (a) CH2Cl2; (b) R3NC 178, Pd(PPh3)4, THF, 60 °C.
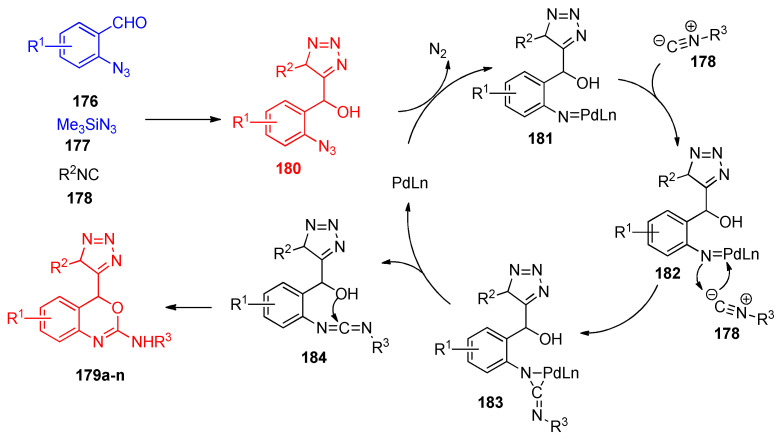
The proposed mechanism for the formation of compounds 179a–n was started from the Passerini-azide adduct 180, which reacted with the palladium reagent to form the palladium–nitrogen intermediate 181 with the elimination of the nitrogen molecule. Insertion of isocyanide 178 to the formed intermediate 181 gave the three-membered ring intermediate 182. The carbodiimide intermediate 183 was formed via reductive elimination of intermediate 182. Finally, intermolecular cyclization of intermediate 184 resulted in the formation of the benzoxazine derivatives 179a–n, as illustrated in Scheme 69 [56].
Scheme 69.
A plausible mechanism for the formation of oxazine derivatives 179a–n.
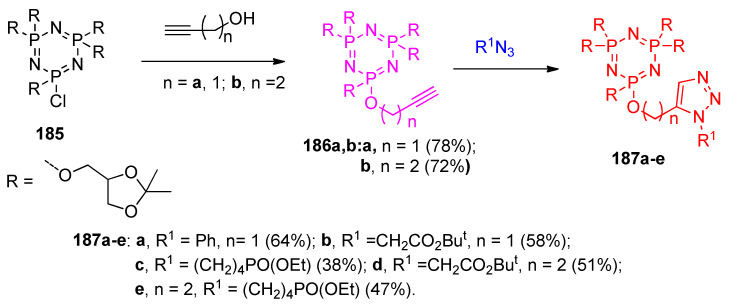
Regioselective syntheses of functionalized cyclotriphosphazenes linked via 1,2,3-triazole 187a–e. Firstly, 1,3,3,5,5-penta[1-(2,2-dimethyl-1,3-dioxolan-4-yl)methoxy]-1-chlorocyclotri phosphazene (185) reacted with 2-propyn-1-ol and 3-butyn-1-ol in the presence of NaH to give the alkynyl derivatives 186a,b. That was followed by the cycloaddition click reaction with phenyl azide, azido acetic acid tert-butyl ester or diethyl (4-azidobutyl) phosphonate at 20 °C in the presence of Cu(I) (Scheme 70) [57].
Scheme 70.
Synthesis of 1,2,3-triazoles 187a–e. Reagents and conditions: (i) THF, NaH, 0 °C stirring, then 20 °C, 1 h; (ii) CuSO4·5H2O (0.06 mmol, 20 mol%) of sodium ascorbate (0.12 mmol, 20 mol%).
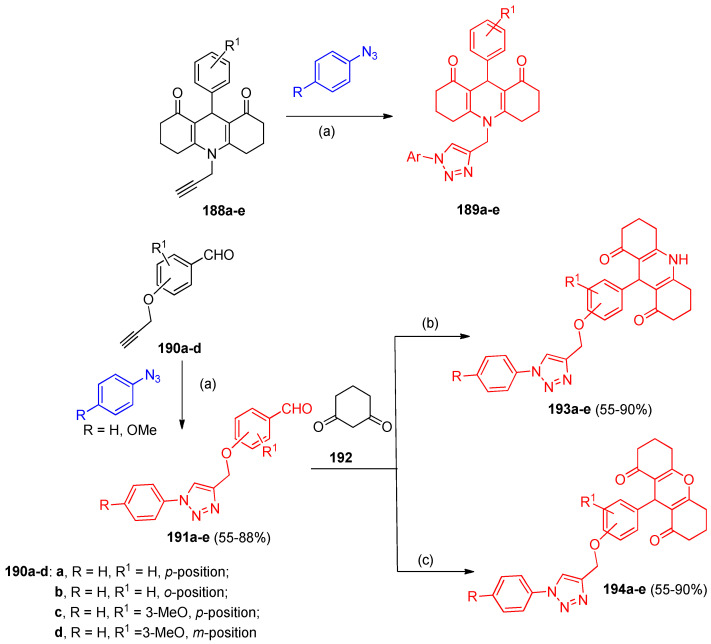
Another regioselective 1,3-dipolar cycloaddition reaction was established between N-propargyl-substituted-1,8-dioxodecahydroacridines 188a–d with aromatic azides in the presence of CuSO4.5H2O/ascorbic acid as catalyst (Click reaction) in a 2:1 mixture of CH2Cl2:H2O at room temperature gave 1,2,3-triazole-dioxodecahydroacridine hybrids 189a–e in high yields (70–86%). Furthermore, the click reaction was subjected to propargyloxy-benzaldehydes 190a–d to give 1,4-disubstituted 1,2,3-triazolealdehydes 191a–e in 55–88% yields (Scheme 71). The application of the Hantzsch route on the corresponding 1,2,3-triazolealdehydes 191a–e with 1,3-cyclohexanedione 192 produced 1,4-disubstituted 1,2,3-triazole-O-acridinedione 193a–e (55–90% yields). Treatment of 1,2,3-triazolealdehydes 191a–e with two molecules of 1,3-cyclohexanedione in triethylamine and acetic acid gave the corresponding 1,2,3-triazole-O-xanthenediones 194a–e in 67–91% yields (Scheme 71) [58].
Scheme 71.
Regioselective synthesis of 1,2,3-triazoles 193a–e and 194a–e. Reagents and conditions: (a) CuSO4·5H2O, sodium ascorbate, DCM:H2O (1:1), 12 h; (b) AcONH4, Et3N, EtOH, 12 h; (c) Et3N, AcOH, 12 h.
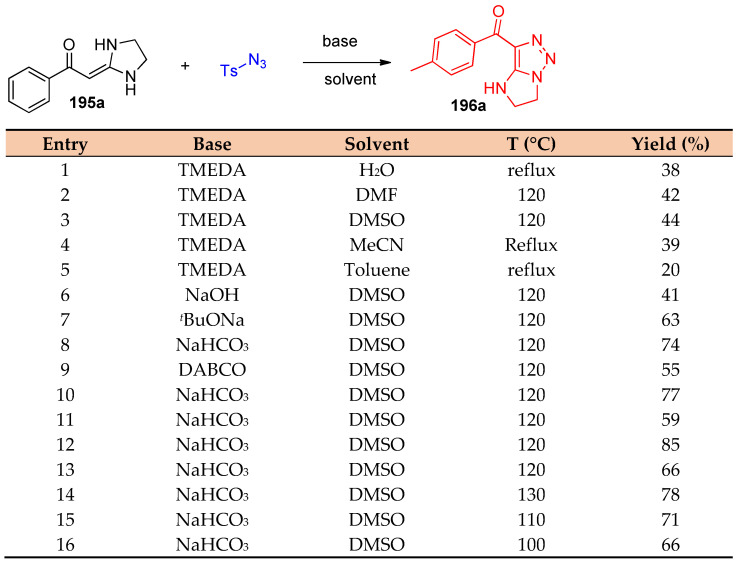
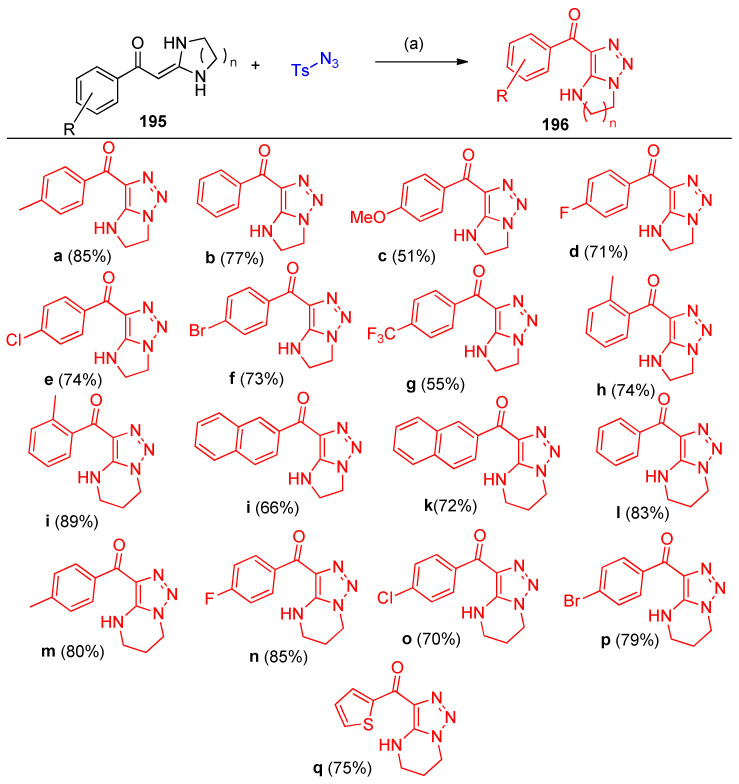
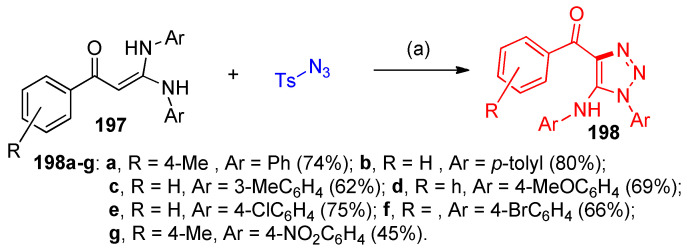
Annulation reactions between gem-diamino enaminones 195 and 197 (ketene aminals) and tosyl azide furnished N-heterocycle fused 196a–q and 5-amino side-chain 198a–g functionalized 1,2,3-triazoles under transition metal-free conditions, using NaHCO3 as a catalyst to promote the reaction (Scheme 72, Scheme 73 and Scheme 74). The reaction was screened to optimize the reaction conditions. Firstly, the reaction was performed using different solvents, such as water, DMSO, DMF, toluene, and MeCN; it was observed that DMSO is the solvent of choice. Additionally, the reaction was performed under different basic conditions using NaOH, tBuONa, NaHCO3, and DBACO; it was found that using NaHCO3 is favorable for obtaining high yields (Scheme 72) [58].
Scheme 72.
Screening of the optimized reaction conditions for the reaction of enaminones and tosyl azide.
Scheme 73.
Syntheses of fused imidazotriazoles 197a–q. Reagent and conditions: (a) NaHCO3, DMSO, 120 °C, 12 h.
Scheme 74.
Syntheses of 5-amino-1,2,3-triazoles 198a–g. Reagent and conditions: (a) NaHCO3, DMSO, 120 °C.
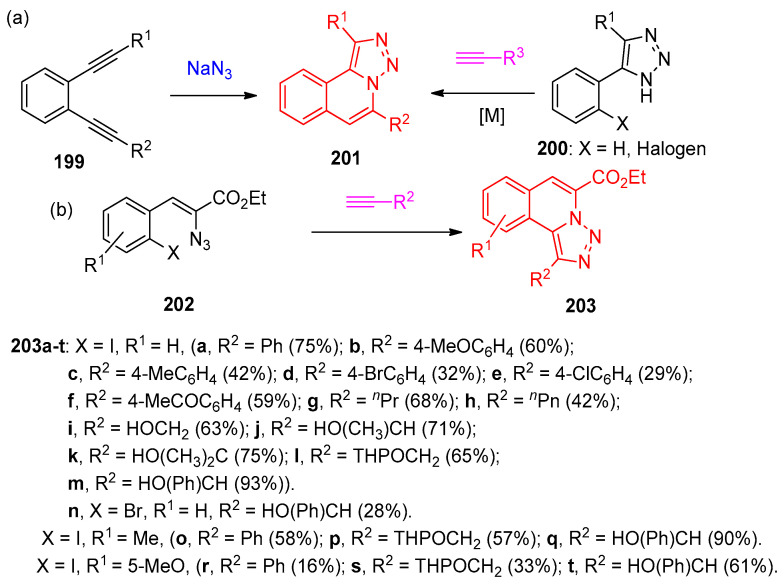
1,2-Diacetylenic benzenes 199 were cyclized with sodium azide (NaN3) furnished the corresponding [1,2,3]triazolo[5,1-a]isoquinolines 201, but with low regioselectivity for substrates bearing two different alkyne substituents (R1 ≠ R2) [59,60,61]. Additionally, the same triazoloisoquinolines 201 were obtained via annulation of acetylenes with (2-halo)phenyl-1,2,3-triazoles 200 under transition-metal catalyzed conditions [62,63,64,65]. On the other hand, the annulation of 2-azido-3-(2-iodophenyl)acrylates 202 to the corresponding triazolo-isoquinolines 203 was achieved using copper chloride as a transition metal catalyst in these heterocyclization [66,67] (Scheme 75).
Scheme 75.
Synthesis of triazoloisquinolines 203.
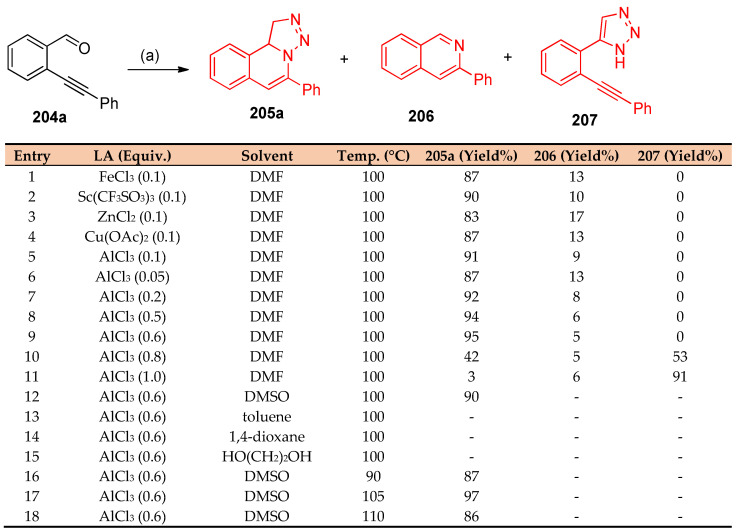
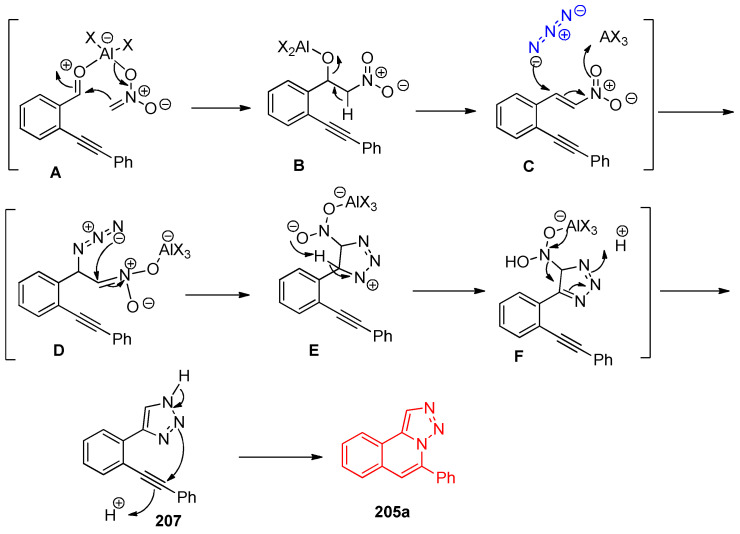
Recently, Wu et al. [68] reported that AlCl3 syntheses of triazoloisoquinolines via three-component Henry reaction–triazole formation–intramolecular 6-endo-dig cyclization were successfully achieved. Upon reacting 2-(phenylethynyl)-benzaldehyde 204a, nitromethane, and sodium azide in the presence of Lewis acid in DMF at 100 °C, a mixture of [1,2,3]triazolo[5,1-a]isoquinoline 205a and isoquinoline 206 was obtained. However, triazole 207 was obtained when an excess of AlCl3 was used. Among these studies, it was found that Sc(CF3SO3)3 and AlCl3 were the preferable Lewis acids to promote the formation of triazoloisoquinolones, as shown in Scheme 76.
Scheme 76.
Synthesis of triazoloisoquinoline 205a. Reagent and conditions: (a) CH3NO2 (3.0 equiv.), NaN3 (2.5 equiv.), Lewis acid.
The type of solvent and Lewis acid affected the yield and the regioselectivity of the product, as in 205a. Additionally, the substituted group affected the yield of the target product in which the presence of electron-donating groups gave higher yields than electron-withdrawing groups. On replacing the phenyl ring with pyridine ring (an electron-deficient) and naphthalene (π-electron delocalized group), the triazoloisoquinolones 205h and 205i were synthesized in moderate yields of 32 and 53%, respectively, as shown in Scheme 77 [68].
Scheme 77.
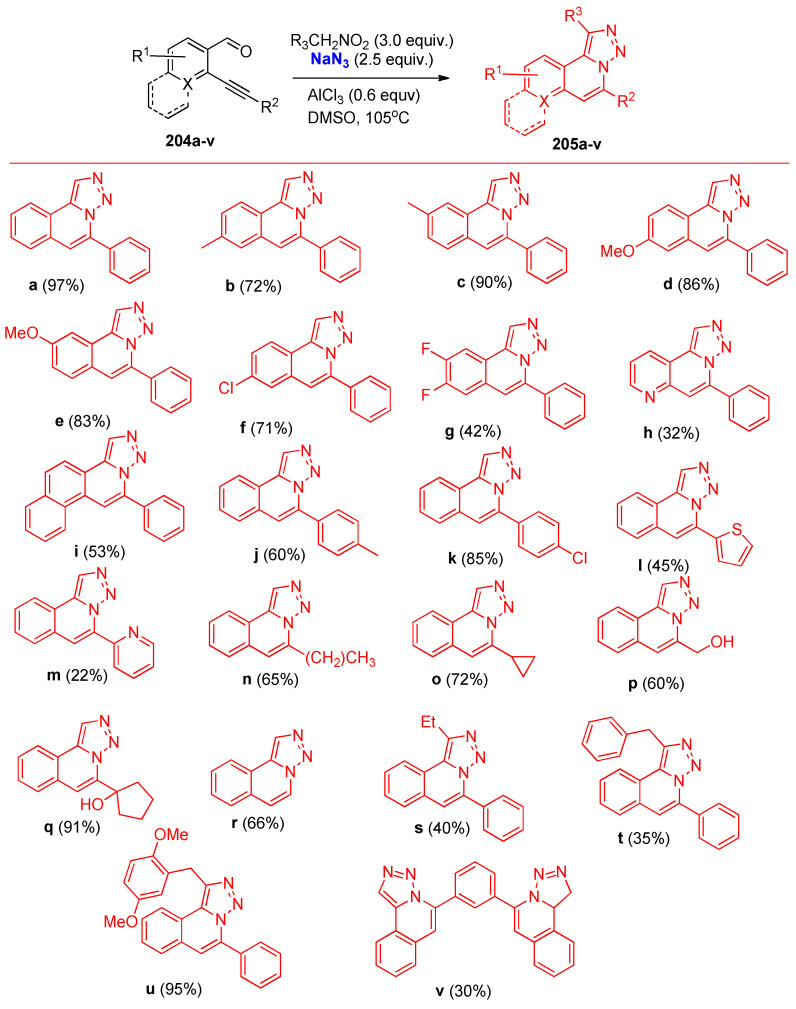
Substituent affected the yields of the synthesized triazoloquinolines 205a–v. The plausible mechanism for the formation of compound 205a is illustrated in Scheme 78 [68].
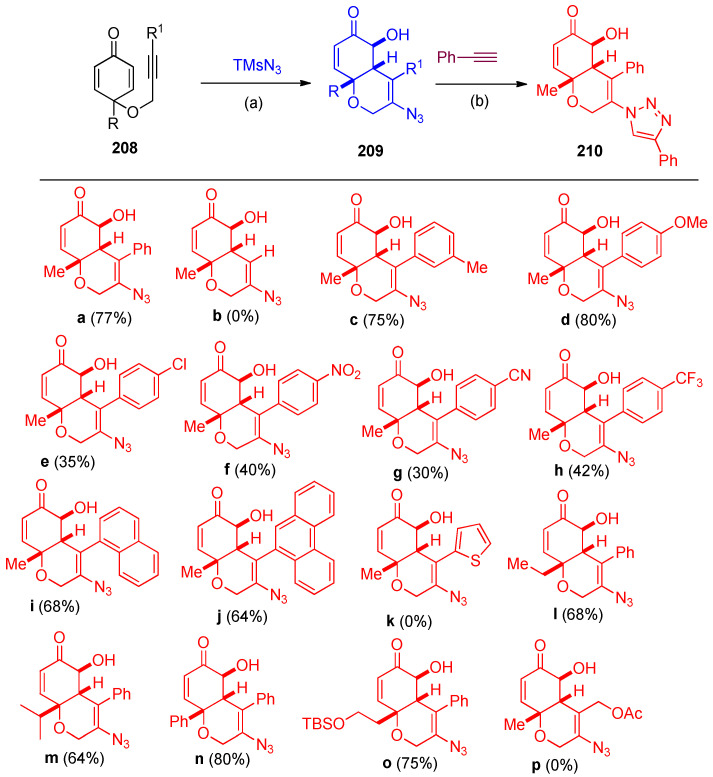
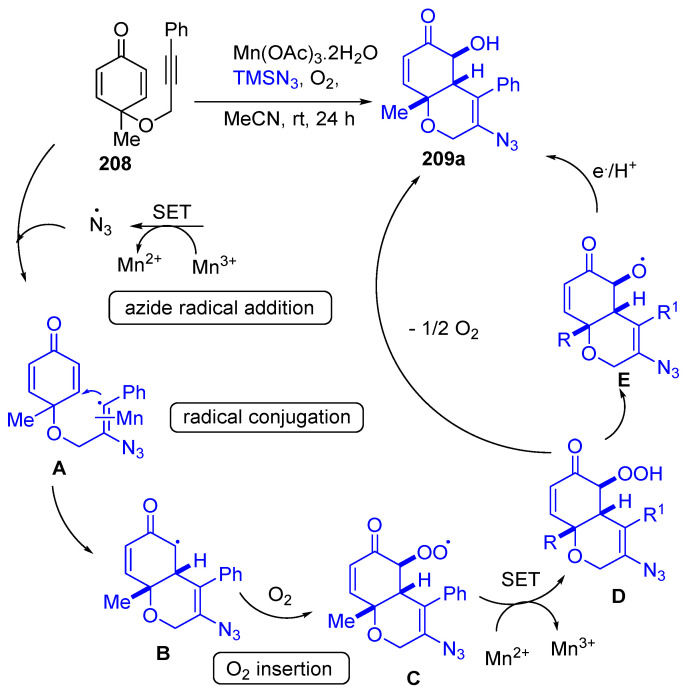
Mn(OAc)3·2H2O were used as catalysts in the syntheses of bicyclic azido alcohol 208 via azide radical addition/cyclization/oxygen insertion reaction of alkyne-tethered cyclohexadienones 208 with TMSN3 under mild conditions. The azido alcohol 209a was led to react with phenylacetylene via Cu-catalyzed click reaction 1,2,3-triazole 210 was obtained in 84% yield (Scheme 79) [69]. The plausible mechanism for forming the azido alcohol 209a is shown in Scheme 80. It was described due to azide radical addition, then radical conjugation, and lastly, oxygen insertion process through the formation of the intermediates A–E [69].
Scheme 79.
Synthesis of 1,2,3-triazoles 210a–p. Reagents and conditions: (a) Mn(OAc)3·2H2O (50 mol%), TMSN3 (2.1 mmol, 5 eq.), CH3CN (2 mL), O2 balloon, room temperature, 6–24 h. (b) CuSO4·H2O, Sodium ascorbate, tBuOH:H2O (1:1), room temperature, 24 h.
Scheme 80.
Proposed mechanism for the formation of the azido alcohol 188a.
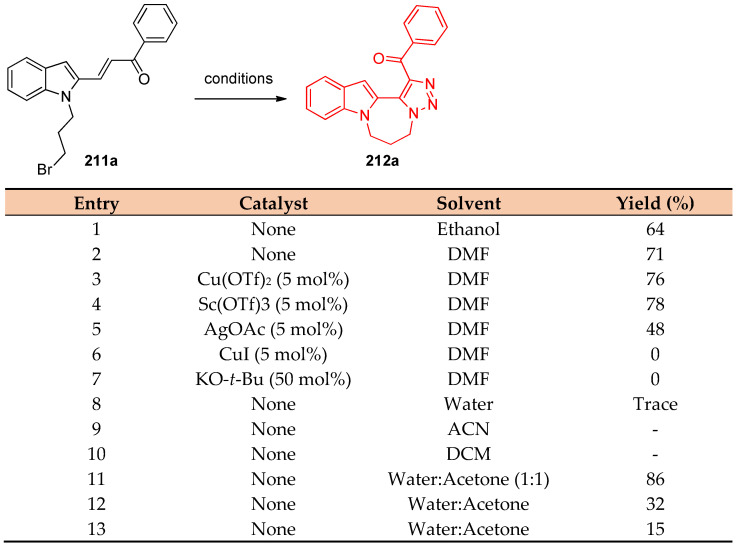
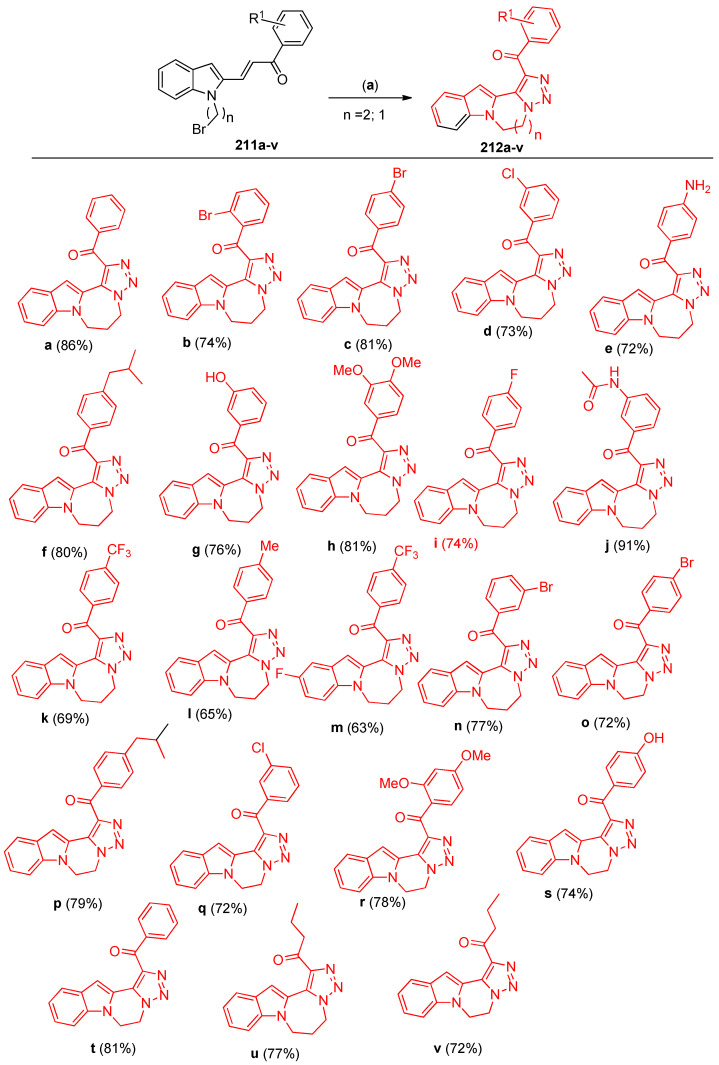
Intramolecular azide–alkene cycloaddition of N-bromoalkyl indole and pyrrole derivatives 211a–v resulted in the formation of polycyclic fused 1,2,3-triazoles 212a–v [70]. As a model example, the reaction progress was investigated to determine the optimized conditions via the reaction of 211a (0.5 mmol) with sodium azide (0.6 mmol) in ethanol at room temperature for 20 h and under catalyst-free conditions. The reaction proceeded smoothly to give (6,7-dihydro-5H-[1,2,3]triazolo[5`,1`:3,4][1,4]diazepino[1,2-a]indol-1-yl)(phenyl)-methanone 212a in 64% yield (Scheme 81). When the reaction was first applied to the seven-membered ring annulated indole by varying substituents (R1) on the benzoyl group. It was observed that electron-donating groups, such as amine, methoxy, hydroxyl, and isobutyl, under the optimal reaction conditions, gave the desired products in 65–91% yields (Scheme 82). Similarly, halogen substituents were also produced the appropriate products (212b–d, 212i, and 212m) in good to high yields (73–81%). However, highly electron-poor substituents, such as CF3, showed lower efficiency (212k, 69% yield), and the reaction of 3,5-ditrifluoromethyl acetophenone failed to give the corresponding product. Additionally, the investigation was extended to prepare six-membered ring annulated indoles by using N-bromoethyl substituent on the indole under the optimized conditions. It was shown that electron-donating and electron-withdrawing groups produced the corresponding fused polycyclic N-heterocycles (212o–t) in slightly lower yields (72–81%). Alkyl groups on the ketone derivatives led to the desired products 212u and 212v in good yields (77% and 72%, respectively) [70].
Scheme 81.
Optimization of reaction conditions in the synthesis of 1,2,3-triazolo-diazapine 134a.
Scheme 82.
Synthesis 1,2,3-triazolo-diazapines 212a–j and 1,2,3-triazolopyrazines 212k–v. Reagents and conditions: (a) NaN3 (0.6 mmol), solvent (5 mL), room temperature 18–20 h.
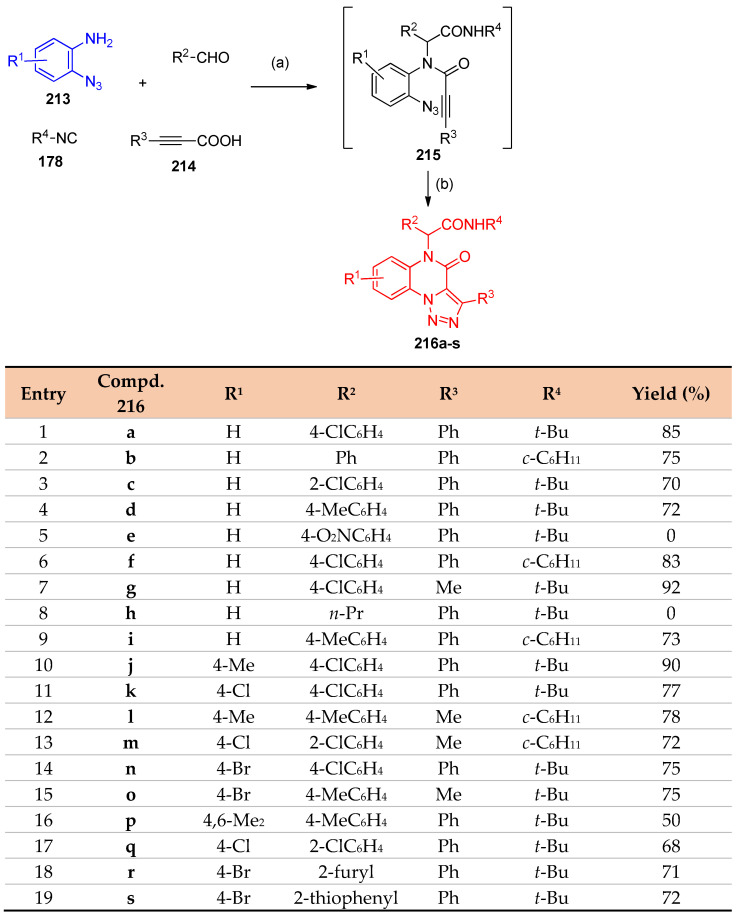
Ugi four-component reaction/alkyne–azide cycloaddition reaction was applied to synthesize triazoloquinoxalines. Reacting 2-azidobenzenamines 213, isocyanide 178, aldehydes, and propiolic acids 214 afforded [1,2,3]triazolo[1,5-a]quinoxalin-4(5H)-ones 216a–s via the formation of Ugi adducts 215. The cyclization occurs via an alkyne–azide cycloaddition reaction (Scheme 83) [71].
Scheme 83.
Synthesis of triazoloisoquinoxalines 216a–s. Reagents and conditions: (a) MeOH, r.t., 12–24 h; (b) DMF, 90 °C.
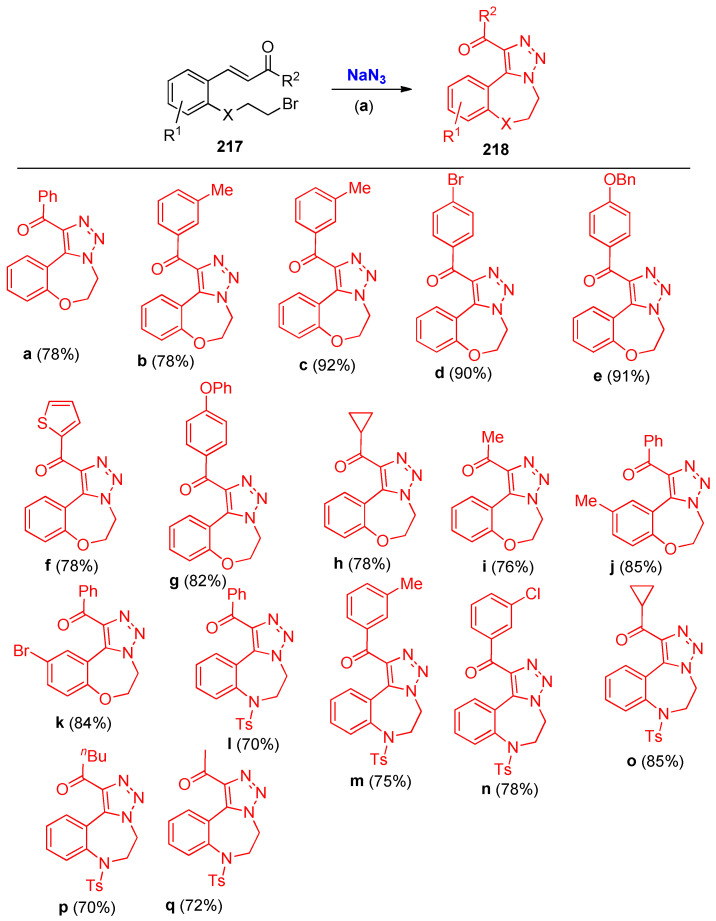
Gangaprasad et al. [72] reported the syntheses of 1,2,3-triazole fused benzooxazepine and benzodiazepine analogs 218a–q via one-pot azide substitution and intramolecular azide-olefin 217 oxidative cycloaddition sequence under metal-free conditions (Scheme 84) [72].
Scheme 84.
Synthesis of 1,2,3-triazole fused benzooxazepine and benzodiazepines analogs 218a–q. Reagents and conditions: (a) DMF, 90 °C, 6 h.
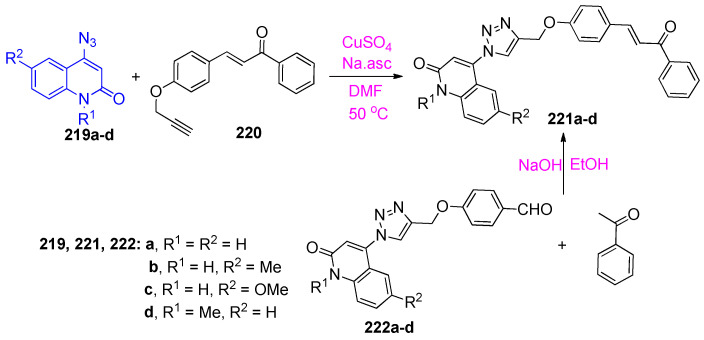
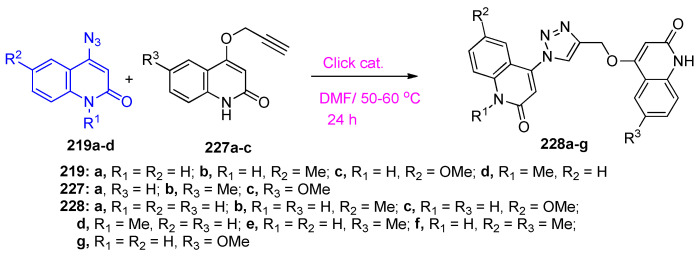
Aly et al. [73] reported that copper(I)-catalyzed azide-alkyne [3+2] dipolar cycloaddition reaction (CuAAC) between 219a–d and 220 to afford the target hybrids 221a–d, in good to excellent yields depending on the concentration of catalyst (Scheme 85). Additionally, the target compounds 221a–d were synthesized, in very good yields, via the reaction of 4-{[1-(2-oxo-1,2-dihydroquinolin-4-yl)-1H-1,2,3-triazol-4-yl]methoxy}benzaldehydes 222a–d [73] with acetophenone (Scheme 85).
Scheme 85.
Reaction of 4-azido-2-quinolinones 219a–d with chalcone 220.
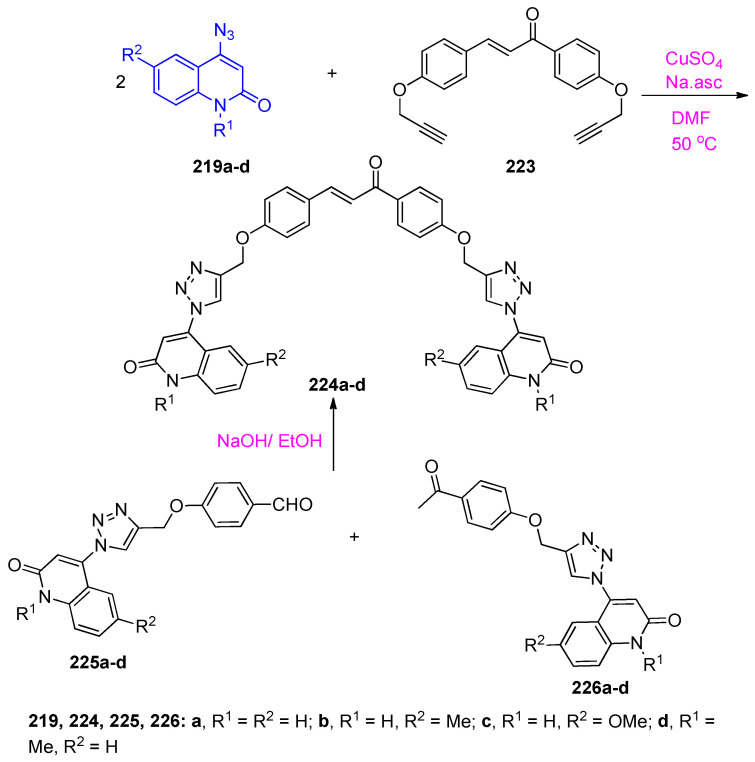
Similarly, doubly derivatized chalcones were prepared by the interaction between (E)-1,3-bis[4-(prop-2-yn-1-yloxy)phenyl]prop-2-en-1-one (223) and 4-azidoquinolin-2(1H)-ones 219a–d in the presence of CuAAC to obtain 1,2,3-triazoles 224a–d [73]. The 1,2,3-triazoles 224a–d were also synthesized by the reactions of aldehydes 225a–d with 4-{4-[(4-acetylphenoxy)-methyl]-1H-1,2,3-triazol-1-yl}-quinolin-2(1H)-ones 226a–d in basic medium, as shown in Scheme 86.
Scheme 86.
Synthesis of compounds 224a–d.
Aly et al. [74] also reported that the synthesis of hybrids 228a–g through click chemistry which is a powerful tool for a quick, highly selective, and reliable access to a reaction product with high yields. The [3+2] cycloadditions of 4-azidoquinolin-2(1H)-ones 219a–d with 4-(prop-2-yn-1-yloxy)quinolin-2(1H)-ones 227a–c, gave the corresponding 4-((1-(2-oxo-1,2-dihydroquinolin-4-yl)-1H-1,2,3-triazol-4-yl)methoxy)quinolin-2(1H)-ones 228a–g (Scheme 87). Compounds 228a–c were found to be the most active antiapoptotic hybrids with significant measurements for the antioxidant parameters (malondialdehyde (MDA), total antioxidant capacity (TAC), and the apoptotic biomarkers (testicular testosterone, tumor necrosis factor (TNFα) and caspase-3) in comparison to the reference. A preliminary mechanistic study was performed in order to improve the antiapoptotic activity through caspase-3 inhibition. A compound assigned as 6-methoxy-4-(4-(((2-oxo-1,2-dihydroquinolin-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)quinolin-2(1H)-one (228c) was selected as a representative of the most active hybrids in comparison to N-acetyl cysteine (NAC). Assay of cytochrome C for 228c revealed a down expression of cytochrome C level by about 3.54 fold, comparable to NAC (4.13 fold). In caspases-3,8,9 assays, 228c was found to exhibit more potency and selectivity toward caspase-3 than other caspases. Testicular histopathological investigation was carried out on all targeted compounds 228a–g, indicating a significant improvement in spermatogenesis process for compounds 228a–c if compare with the reference relative to the control [74].
Scheme 87.
Click reactions between 4-azido-quinolin-2(1H)-ones 219a–d and alkynes 227a–c.
4.6. Synthesis of Tetrazole Ring
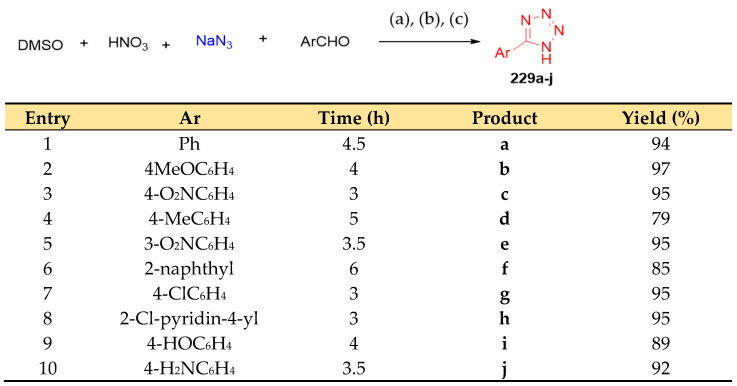
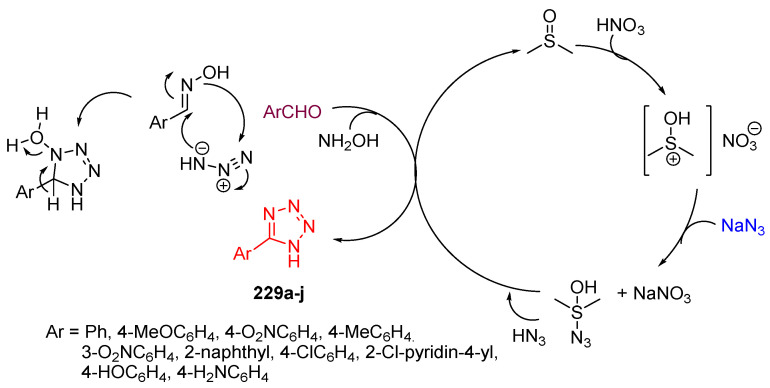
One-pot syntheses of 5-substituted 1H-tetrazole derivatives 229a–j [75] were achieved using a dimethyl sulfoxide–nitric acid combination in an aldehyde, hydroxylamine combination hydrochloride, and sodium azide under mild conditions (Scheme 88). The proposed mechanism is illustrated in Scheme 89 [75].
Scheme 88.
5-substituted 1H-tetrazole derivatives 229a–j. Reagents and conditions: (a) 20 min, 40 °C; (b) NaN3(10 mmol), 20 min, H2O, 40 °C; (c) ArCHO (0.05 mmol), NH2OH·HCl (0.05 mmol), DMSO, 40 °C, 3–6 h.
Scheme 89.
The proposed mechanism for the formation of tetrazoles 229a–j.
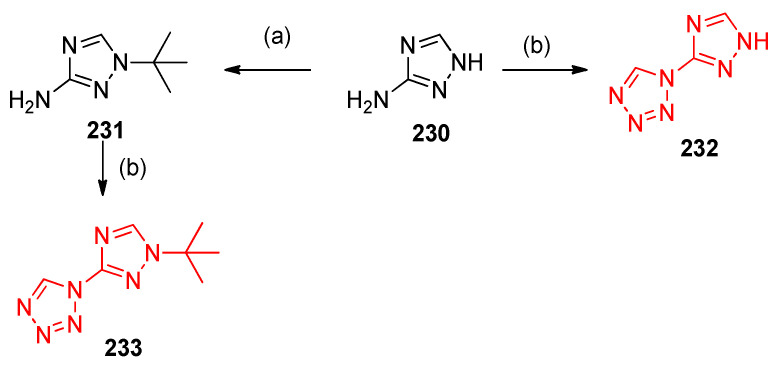
Heterocyclization of 1,2,4-triazol-3-amine 230 and 3-amino-1-tert-butyl-1,2,4-triazole 231 was established via alkylation of 3-amino-1,2,4-triazole 230 using t-BuOH-HClO4 with triethyl orthoformate and sodium azide in absolute ethanol. The reaction gave 1-(1,2,4-triazol-3-yl)-1H-tetrazole 232 and 1-(1-tert-butyl-1,2,4-triazol-3-yl)-1H-tetrazole 233, respectively, as depicted in Scheme 90 [76].
Scheme 90.
Formation of 1-(1,2,4-triazol-3-yl)-1H-tetrazole 232 and 1-(1-tert-butyl-1,2,4-triazol-3-yl)-1H-tetrazole 233. Reagents and conditions: (a) t-BuOH, HClO4; (b) HC(OEt)3, NaN3, AcOH.
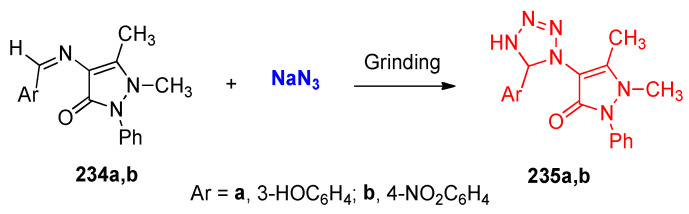
Grinding a mixture of Schiff bases: 4-(3-hydroxybenzylideneamino)antipyrine and 4-(4-nitrobenzylideneamino)antipyrine 234a,b with sodium azide (NaN3) gave the corresponding tetrazoles 235a,b (Scheme 91) [77].
Scheme 91.
Synthesis of tetrazoles 235a,b.
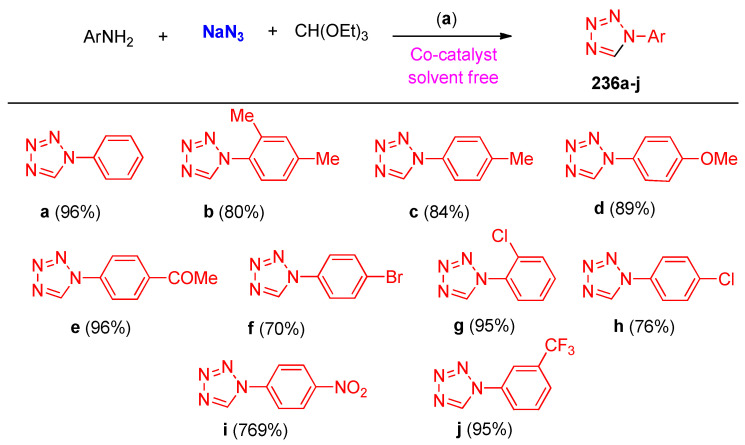
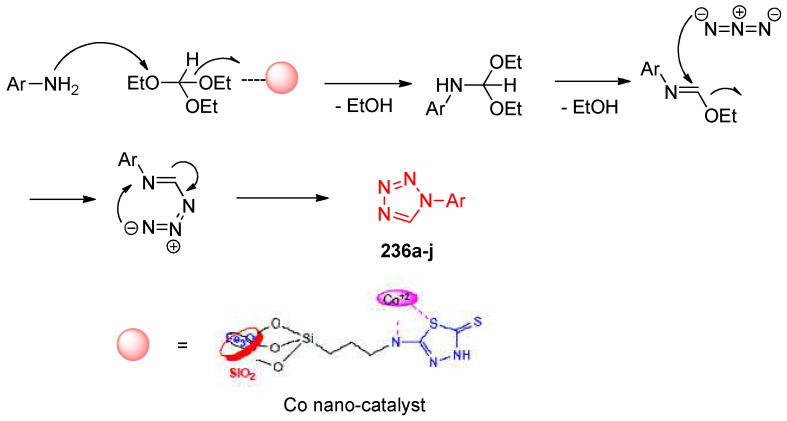
Cobalt nano-particles, a heterogeneous catalyst, catalyzed the synthesis of tetrazoles 236a–j from a multicomponent reaction of amines, sodium azide, and triethyl orthoformate under solvent-free conditions at 100 °C (Scheme 92). The reaction was screened for the effects of the amount of both catalyst and solvent; it was found that carrying the reaction using 50 mg of the catalyst under solvent-free conditions gave the tetrazole 236a a 96% yield. The proposed mechanism is illustrated in Scheme 93, which involved condensation between the amine and ethyl orthoformate followed by cycloaddition ([1,3]-dipolar cycloaddition) of azide and imine to give the tetrazole product [78].
Scheme 92.
Cobalt nano-particles, a heterogeneous catalyst, catalyzed the synthesis of tetrazoles 236a–j. Reagents and conditions: (a) Co-nano-catalyst, Solvent-free.
Scheme 93.
Proposed mechanism for the formation of tetrazoles 236a–j.
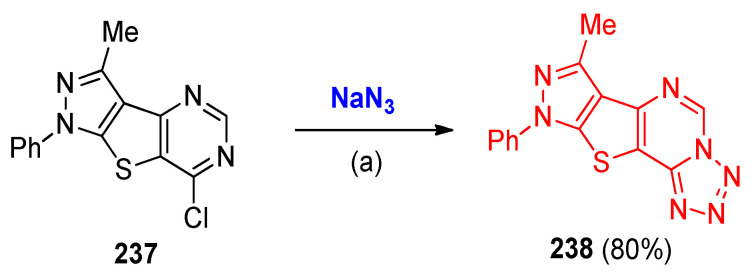
Reaction of the chloropyrimidine (7-chloro-3-methyl-1-phenyl-1H-pyrazolo [4’,3’:4,5] thieno[3,2-d]pyrimidine) (237) with sodium azide in DMF in presence of NH4Cl gave the tetrazole derivative 238, which was identified as (7-methyl-9-phenyl-9H-pyrazolo-[4’,3’:4,5]thieno[2,3-e]tetrazolo[1,5-c]pyrimidine) (Scheme 94) [79].
Scheme 94.
Synthesis of tetrazole derivative 238. Reagents and conditions: (a) NH4Cl, DMF.
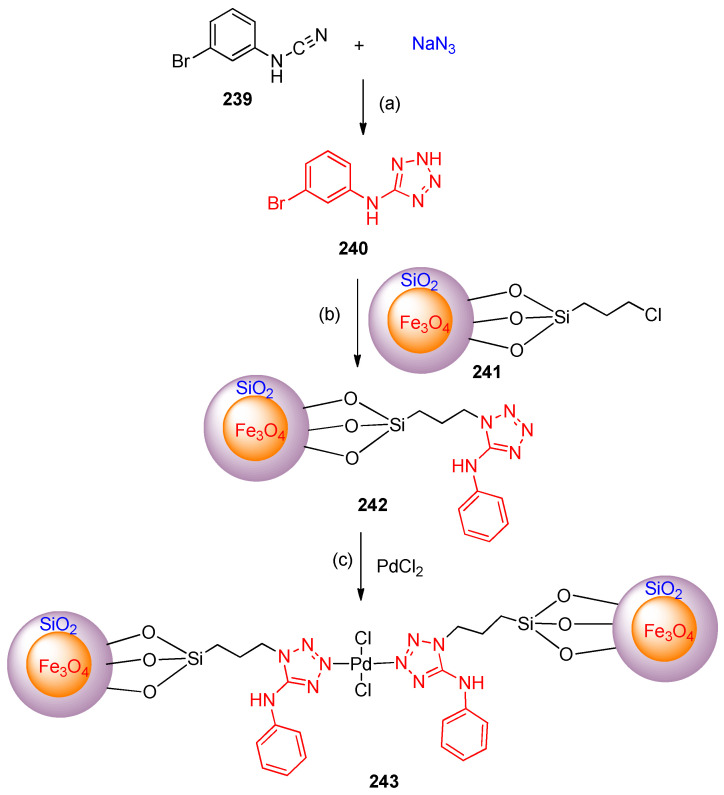
Ag/Fe3O4 nanocomposite catalyzed the synthesis of 5-(3-bromophenyl)amino-1H-tetrazole240 from 3-bromophenyl cyanamide 239 and sodium azide in DMF at 110 °C [78]. Aminotetrazole–palladium (II) complex 243 was prepared via the nucleophilic substitution between 5-(3-bromophenyl)amino-1H-tetrazole 240 and Fe3O4@SiO2@(CH2)3-Cl (241), followed by incorporation of the Pd-ions using PdCl2.2H2O in EtOH under reflux for 24 h [80] (Scheme 95).
Scheme 95.
Synthesis of tetrazole 243. Reagents and conditions: (a) Ag/Fe3O4 nanocomposite, DMF, 110 °C; (b) DMF, K2CO3; (c) EtOH, reflux, 24 h.
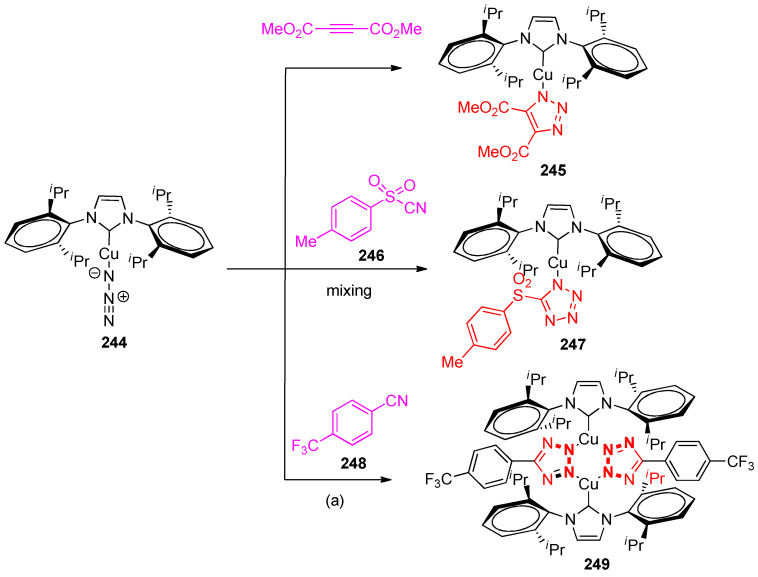
Trose et al. [79] have reported that the reaction of N-Heterocyclic carbene (NHC)-based copper azide complex [Cu(N3)(IPr)] (244) (IPr = N,N`-bis[(2,6-(di-isopropyl)phenyl)]-imidazole-2-ylidene) with dimethyl acetylenedicarboxylate (as an activated alkyne), produced triazolate copper complex 245 (Scheme 96). However, complex 244 was found that it was reacted with the activated p-toluenesulfonyl cyanide (246) to give the tetrazole complex 247 in quantitative yield upon mixing (98%). In contrast, the reaction of complex 244 with the less activated 4-(trifluoromethyl)benzonitrile (248) needed heating and longer reaction times (50 °C, 16 h) to form the bis tetrazole complex 249 in high yield (93%) (Scheme 96) [81].
Scheme 96.
Syntheses of triazolate and tetrazolate copper complexes 245, 247, and 249, Reagents and conditions: (a) heating 50 °C, 16 h.
4.7. Synthesis of Thiadiazole
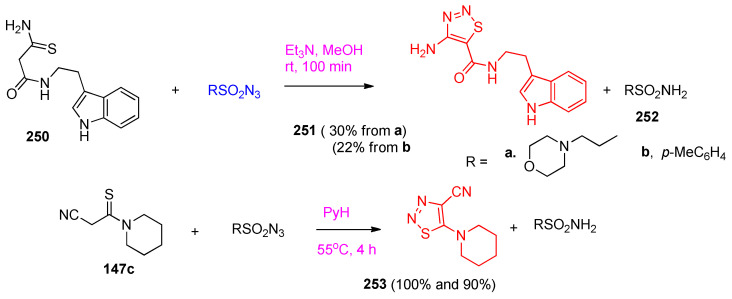
The more reactive thioamide 250 was reacted with tosyl azide in the presence of Et3N at room temperature to afford 1,2,3-thiadiazole 251 (30 and 22% yield) together with compounds 252. At the same time, the thioamide 147c reacted with tosyl azide (R = p-Me-C6H4) to produce thiadiazole 253 (100 and 90% yield) (Scheme 97) [36].
Scheme 97.
Synthesis of thiadiazole 253. Reagents and conditions: (a) Et3N, MeOH, room temperature, 100 min; (b) PyH, 55 °C, 4 h.
4.8. Synthesis of Pyridine and Isoquinoline Derivatives
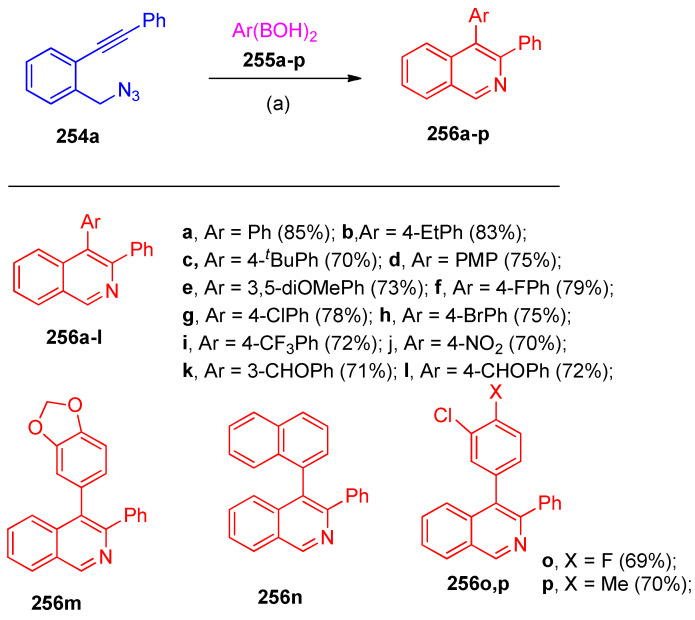
Singam et al. [82] reported the regioselective arylnicalation of ortho functional diaryl acetylene 254a with Ar(BOH)2 255a–p to synthesize substituted di-aryl isoquinolines 256a–p (Scheme 98).
Scheme 98.
Synthesis of isoquinoline derivatives 256a–p. Reagents and conditions: (a) Ni(acac)2 (10 mol%), PPh3 (10 mol%), Cs2CO3 (20 mol%), dioxane, 90 °C.
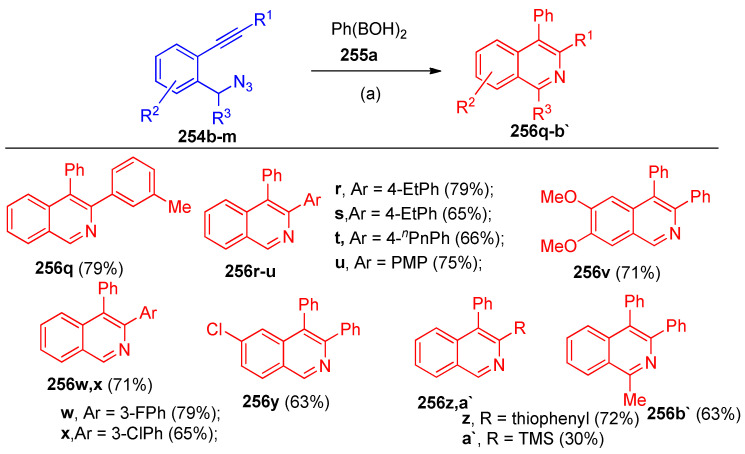
Additionally, ortho diarylacetylene derivatives 254b–m were investigated under the same reaction conditions, which on reacting with Ph(BOH)2 255a gave the desired product 256q–b` in high yields, as depicted in Scheme 99.
Scheme 99.
Synthesis of isoquinoline derivatives 256q–b`. Reagents and conditions: (a) Ni(acac)2 (10 mol%), PPh3 (10 mol%), Cs2CO3 (20 mol%), dioxane, 90 °C.
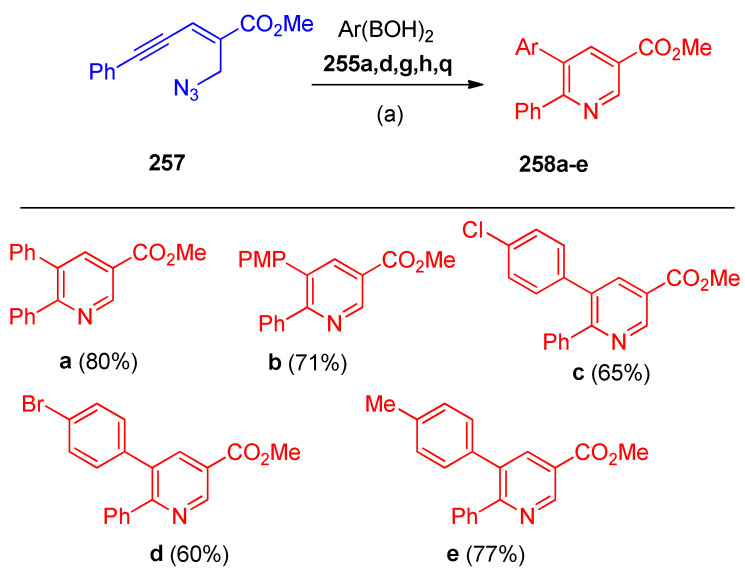
The 5,6-diarylnicotinates 258a–e were performed from enynyl azides 257 with 255a,d,g,h,q under the same standard conditions (Scheme 100) [82].
Scheme 100.
Synthesis of 5,6-diarylnicotinates 258. Reagents and conditions: (a) Ni(acac)2 (10 mol%), PPh3 (10 mol%), Cs2CO3 (20 mol%), dioxane, 90 °C.
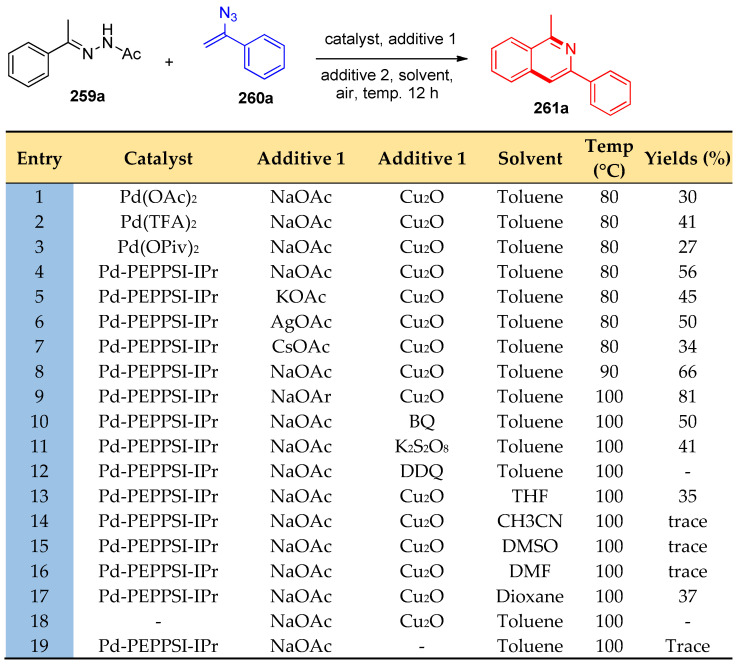
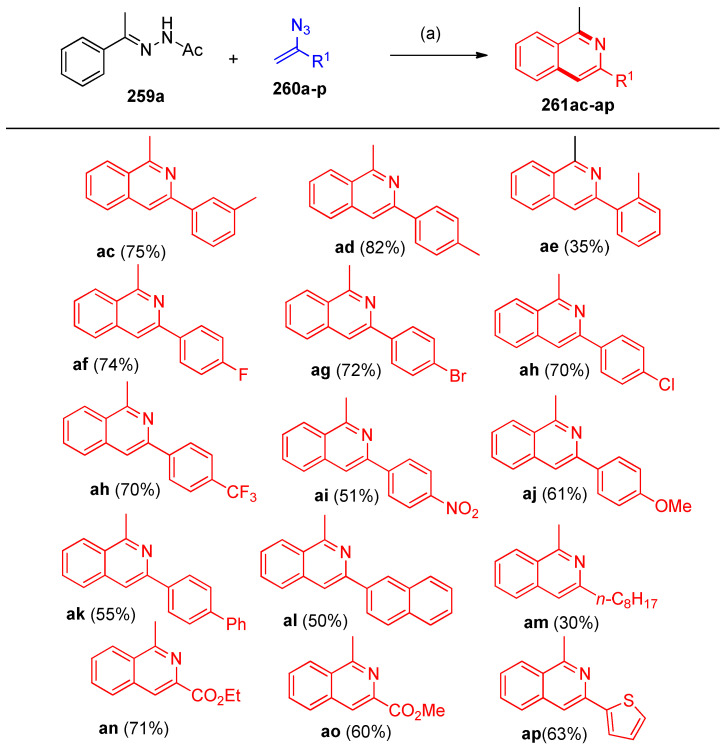
Pd-PEPPSI-IPr was used as a catalyst to reach the optimal reaction conditions during the reaction of acetophenone-N-acetylhydrazone (259a) and (1-azidovinyl) benzene (260a) (Scheme 101) [83]. It was concluded that toluene was the best solvent of choice, and heating to 100 °C gave 81% yield of 261a (Scheme 101)
Scheme 101.
Optimization of reaction conditions described the formation of 261a.
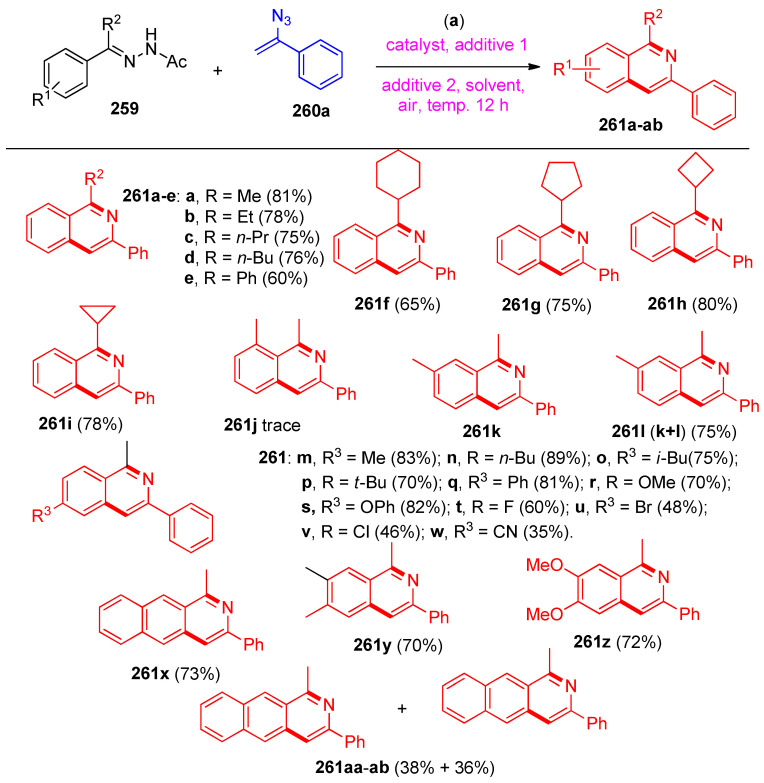
The procedure showed that N-acetyl hydrazones 259 were screened to react with (1-azidovinyl)benzene (260a), as shown in Scheme 102. Variation from alkyl aryl ketones to benzophenone and cycloalkyl aryl ketones hydrazones reacted smoothly with 260a to afford isoquinolines 261a–ab in 60–81% yields (Scheme 102) [83]. The C-H functionalization occurred regioselectively at the less hindered site for meta-substituted substrate (Me, 259k), yielding a mixture of two isomers, 261k (major) and 261l [83]. Either electron-donating (Me, Bu, Ph, OMe, OPh) or electron-withdrawing (F, Br, Cl, CN) group on the para-position of the phenyl ring of acetophenone N-acetylhydrazones were transformed to the desired products in moderate yields.
Scheme 102.
Synthesis of isoquinolines 261a–ab. Reagents and conditions: (a) Pd-PFPPSI-IPr (10 mol%), Cu2O (2.0 equiv.), NaOAc (1.0 equiv), toluene, 100 °C, 12 h.
The reaction of various vinyl azides 260a–p with N-acetyl hydrazone 259a under the standard reaction conditions was examined (Scheme 103). Fused isoquinolines 261ac–ap were obtained via the same previous procedure [83].
Scheme 103.
Syntheses of isoquinolines 261ac–ap. Reagents and conditions: (a) Pd-PFPPSI-IPr (10 mol%), Cu2O (2.0 equiv.), NaOAc (1.0 equiv), toluene, 100 °C, 12 h.
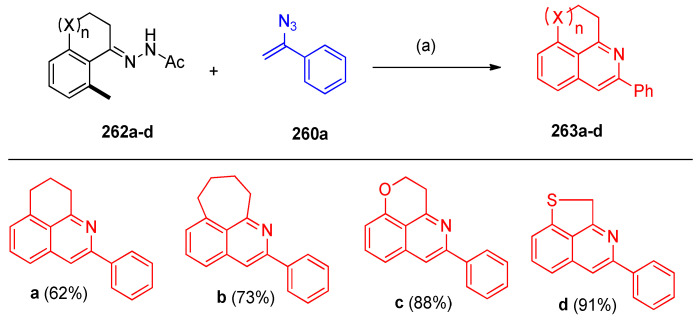
The transformations of 1-tetralone, 1-benzosuberone hydrazones 262a–d proceeded smoothly to give the desired polycyclic product 263a,b in moderate yields. Moreover, chroman-4-one and thiochroman-4-one-hydrazone substrates were converted to polyheterocyclic products 263c,d in 88% and 91% yields (Scheme 104).
Scheme 104.
Synthesis of fused isoquinolines 263a–d. Reagents and conditions: (a) Pd-PFPPSI-IPr (10 mol%), Cu2O (2.0 equiv.), NaOAc (1.0 equiv), toluene, 100 °C, 12 h.
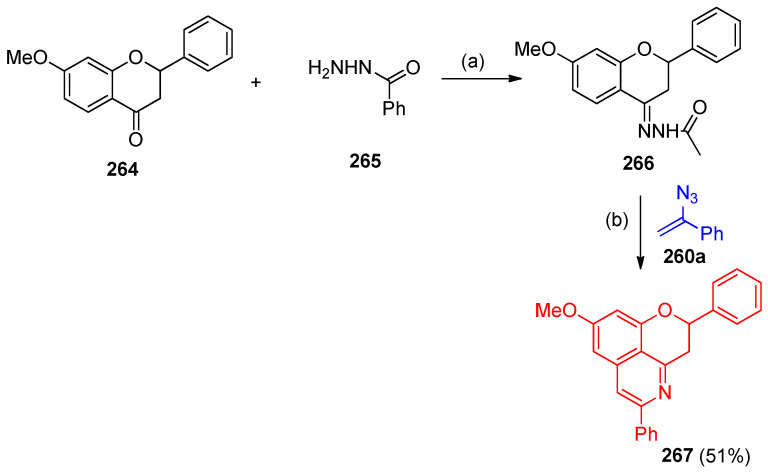
7-Methoxyflavanone 264 was reacted with acetohydrazide 265 to hydrazone 266, which, when treated with vinyl azide 260a, provided the isoquinoline product 267 in 51% yield (Scheme 105) [83].
Scheme 105.
Formation of isoquinoline product 267. Reagents and conditions: (a) EtOH, reflux; (b) Pd-PFPPSI-IPr (10 mol%), Cu2O (2.0 equiv.), NaOAc (1.0 equiv), toluene, 100 °C, 12 h.
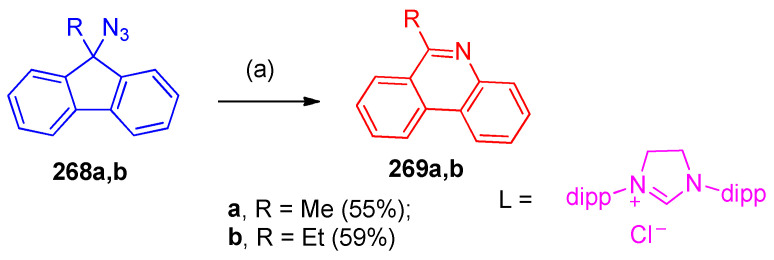
4.9. Synthesis of Phenanthridine
Phenanthridines 269a,b were synthesized using the catalytic system of FeCl2/N-heterocyclic carbene (NHC) SIPr-HCl (1,3-bis-(2,6-diisopropylphenyl)imidazolinium chloride) from 9-azidofluorenes 268,b via 1,2-aryl migration (Scheme 106) [84].
Scheme 106.
Formation of phenanthridines 269a,b. Reagents and conditions: (a) FeCl2 (10 mol%), ligand (10 mol%), PhCl, Ar, 80 °C.
4.10. Synthesis of Imidazoindoles
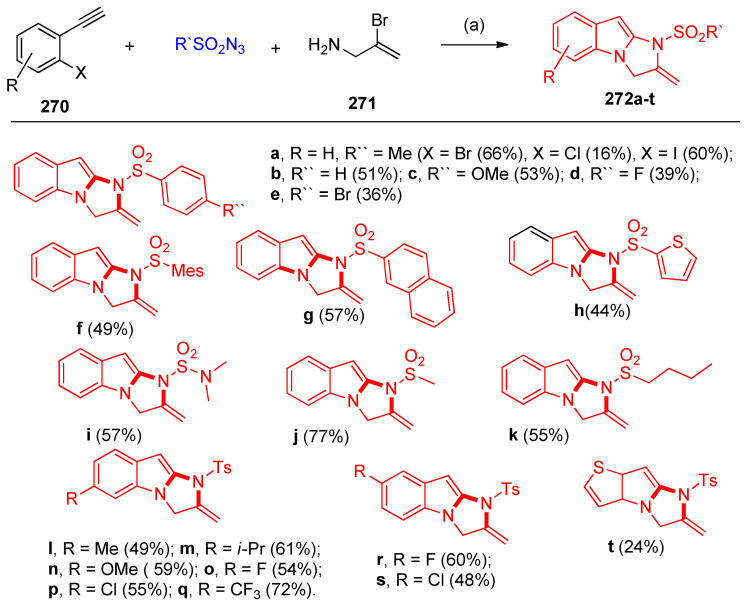
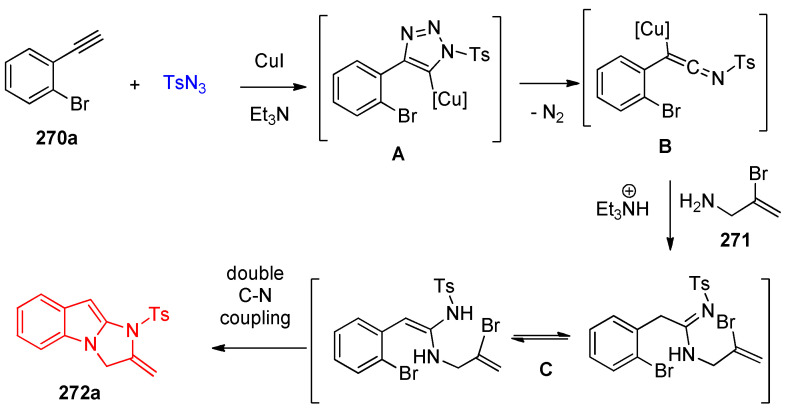
Jin and others [85] reported that the multicomponent reaction of sulfonyl azides, alkynes 270, and allylamines 271 was catalyzed by copper iodide in the presence of triethylamine in DMSO/K2CO3 and dimethyl ethylenediamine as a ligand (L), affording 2,3-dihydro-1H-imidazo[1,2-a]indoles 272a–t (Scheme 107). Four C–N bonds were formed by way of azide-alkyne cycloaddition (CuAAC) and double Ullmann-type coupling reactions in a one-pot process, as illustrated in the reaction mechanism (Scheme 107) [85].
Scheme 107.
Synthesis of 2,3-dihydro-1H-imidazo[1,2-a]indoles 272a–t. Substrate scope of azides and alkynes. Reagents and conditions: (a) CuI (10 mol%), Et3N (1.0 equiv), 270 (0.5 mmol), azide (0.6 mmol), 271a (0.5 mmol), DMSO (3 mL) at r.t. for 1 h; 2) CuI (20 mol%),L (0.3 mmol), K2CO3 (2 equiv) at 80 °C for 6 h. Mes = 2,4,6-Trimethylphenyl.
The proposed mechanistic steps were proposed, as shown in Scheme 108. First, a copper-catalyzed azide-alkyne cycloaddition reaction (CuAAC) takes place to generate intermediate A, which then transforms to ketenimine B by the extrusion of N2. Nucleophilic addition of 2-bromoprop-2-en-1-amine (270a) to intermediate B affords carboxamidine C and/or its tautomer. Finally, a consecutive coppercatalyzed C–N coupling reactions proceeds to provide 2,3-dihydro-1H-imidazo[1,2-a]indole 272a (Scheme 108) [85].
Scheme 108.
Proposed mechanism for the formation of imidazoindoles 272a.
4.11. Synthesis of Quinazoline Derivatives
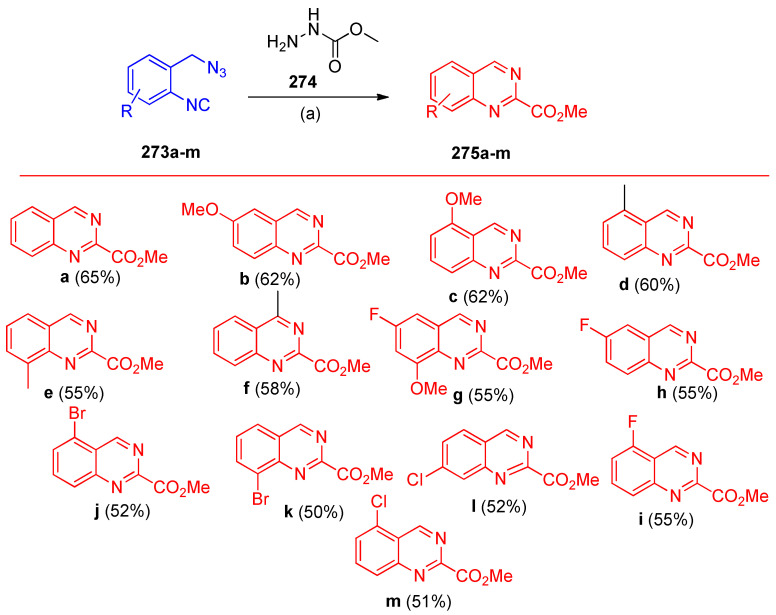
Kumar et al. [86] reported the tandem synthesis of 2-quinazoline carboxylates 275a–m using 2-(azidomethyl)phenyl isocyanides 273a–m along with carbazates 274 in the presence of Mn(OAc)3·2H2O (Scheme 109).
Scheme 109.
Synthesis of quinazoline derivatives 275a–m. Reagents and conditions: (a) Mn(OAc)3·2H2O (0.63 mmol), TBHP (1.89 mmol, 5 M in decan), and EtOAc, 12 h at 80 °C.
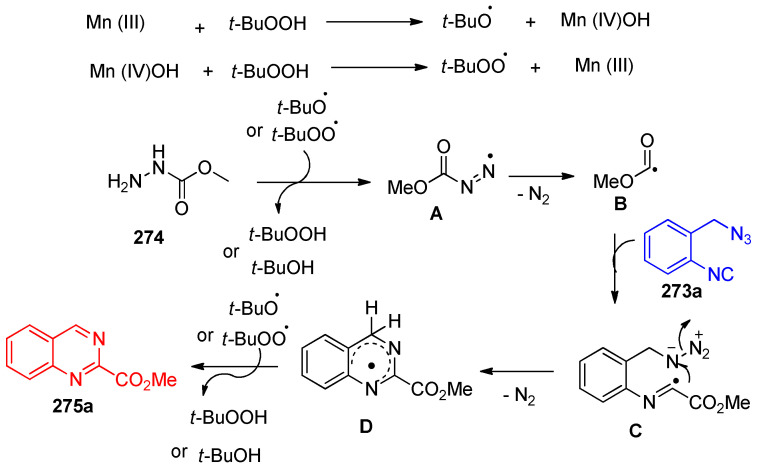
The postulated mechanism is illustrated in Scheme 110. Initially, the Mn(OAc)3·2H2O-assisted homolysis of tert-butyl hydroperoxide (TBHP) generates the tert-butoxy and the tert-butyl peroxy radicals. Bond cleavage of the C-N in methyl carbazate 274 forms the alkoxycarbonyl radical (B), which loses molecular N2. Radical (B) then attacks the R–NC bond of 2-(azidomethyl)phenyl isocyanide (273a) to form an imidoyl radical intermediate (C). The intermediate C then undergoes intermolecular cyclization with the azido group to give a cyclized aminyl radical (D) by nitrogen loss. Finally, a hydrogen abstraction of the radical intermediate (D) leads to the desired product (275a) (Scheme 110) [86].
Scheme 110.
Proposed mechanism for the formation of quinazoline 275a via the radical pathway.
4.12. Synthesis of Borane Containing Heterocycles
4.12.1. Synthesis of Diborylaniline and Diboryl-Fused Pyrimidine
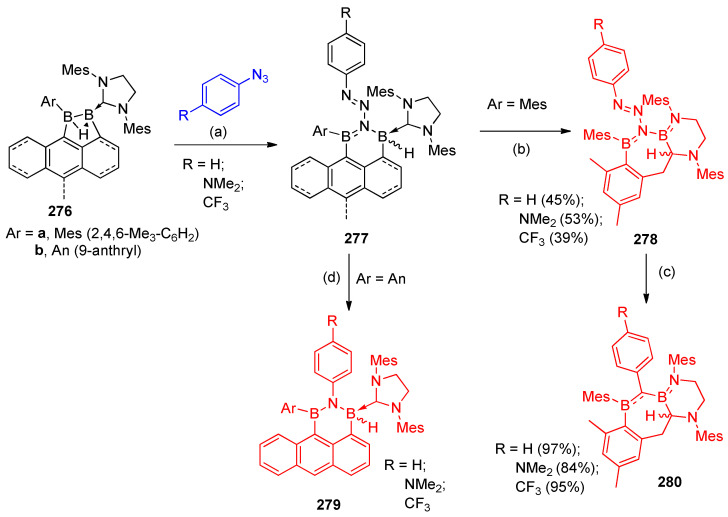
Prieschl et al. [87] reported that the c-nitrogen insertion of aryl azides into the B–B bond of electron-rich cyclic l-hydridodiboranes 276 was stabilized by one N-heterocyclic carbene (NHC) ligand leads to the expansion of the central C3B2 ring, yielding unsymmetrical polyheterocyclic 1,1-diboryltriazenes 278 and 279 via the intermediate 277. The 2-benzyl-bridged analogs undergo further NHC ring expansion and thermally-induced loss of N2 to give polyheterocyclic diborylanilines 280 (Scheme 111) [87].
Scheme 111.
Syntheses of diborylfused pyrimidines 279 and diborykanilines 280. Reagents and conditions: (a) benzene, 60 °C; (b) benzene 80 °C; (c) benzene, 80–100 °C, 12–15 d; (d) benzene, room temperature.
4.12.2. Synthesis of Triazaphosphaborolidine
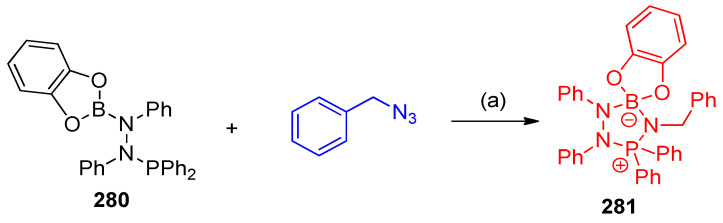
1-(Benzo[d][1,3,2]dioxaborol-2-yl)-2-(diphenylphosphino)-1,2-diphenylhydrazine (280) showed frustrated Lewis pairs (FLPs) reacted with benzyl azide, forming 4’-benzyl-1’,2’,3’,3’-tetraphenylspiro[benzo[d][1,3,2]dioxaborole-2,5’-[1,2,4,3,5]triazaphosphaborolidin]-3’-ium-12-uide (281) in 73% yield (Scheme 112) [88].
Scheme 112.
Formation of 4’-benzyl-1’,2’,3’,3’-tetraphenylspiro[benzo[d][1,3,2]dioxaborole-2,5’-[1,2,4,3,5]triazaphosphaborolidin]-3’-ium-12-uide (281). Reagents and conditions: (a) CH2Cl2, room temperature, 24 h stirring.
4.13. Synthesis of Aluminum-Containing Heterocyclic
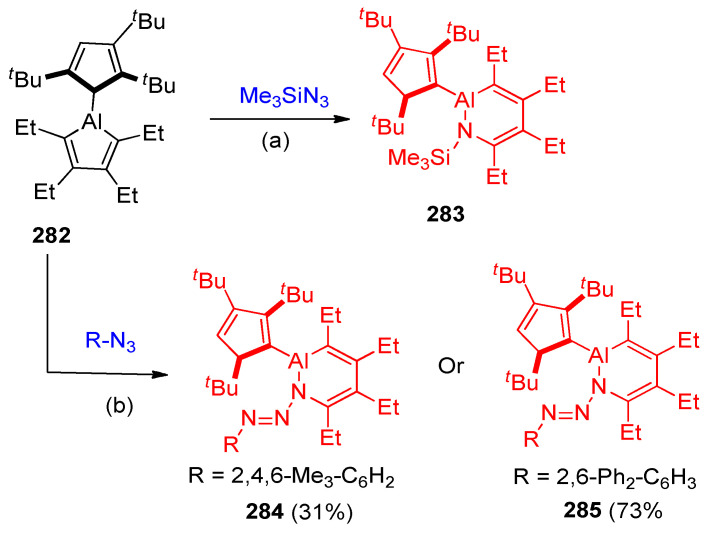
Drescher and others [89] reported the ring expansion of alumina cyclopentadienes (alumoles) on treatment with organic azides. Treatment of alumole 282 and trimethylsilyl azide in benzene at 60 °C gave cycloadduct 283 in 61% yield, while mesityl (Mes = 2,4,6-Me3C6H2) or 2,6-diphenylphenyl azide forming the aza-cycloadducts 284 (31%) or 285 (73%), respectively (Scheme 113) [89].
Scheme 113.
Synthesis of aluminum heterocycles 283–285. Reagents and conditions: (a) benzene, 60 °C. (b) room temperature.
4.14. Synthesis of Phosphorus-Containing Heterocycles
4.14.1. Synthesis of Triazphosphocine
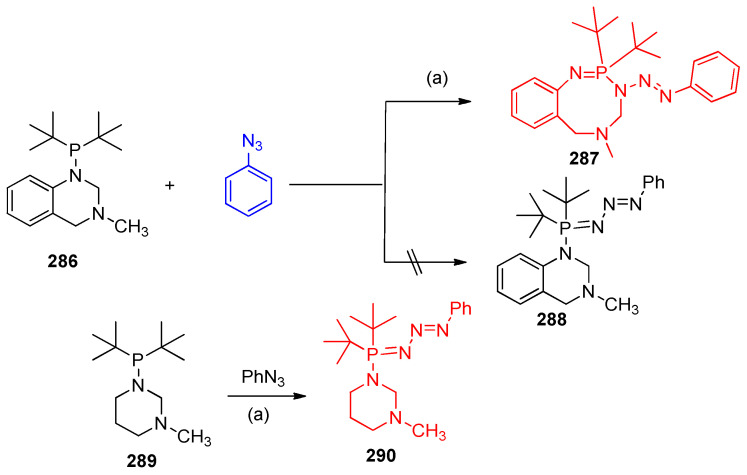
Treatment of 1-(di-tert-butylphosphino)-3-methyl-1,2,3,4-tetrahydroquin azoline (286) with phenyl azide gave benzo[g][1,3,5,2]triazaphosphocine (287) instead of phosphazide derivative ((E)-1-(di-tert-butyl(phenyltriaz-2-en-1-ylidene)phosphoranyl)-3-methyl-1,2,3,4-tetrahydroquinazoline) (288). The reaction of compound 286 with phenyl azide takes place on P(III) of compound 286 was believed to give P(V) product phosphazide 288, which underwent ring enlargement to give benzotriazaphosphocine 287. Derivative 289 reacted readily with phenyl azide to give compound 290 in a high yield (Scheme 114) [90].
Scheme 114.
Synthesis of triazphosphocine 287. Reagents and conditions: (a) Benzene, r.t., 3 h.
4.14.2. Synthesis of Benzothiazaphosphole
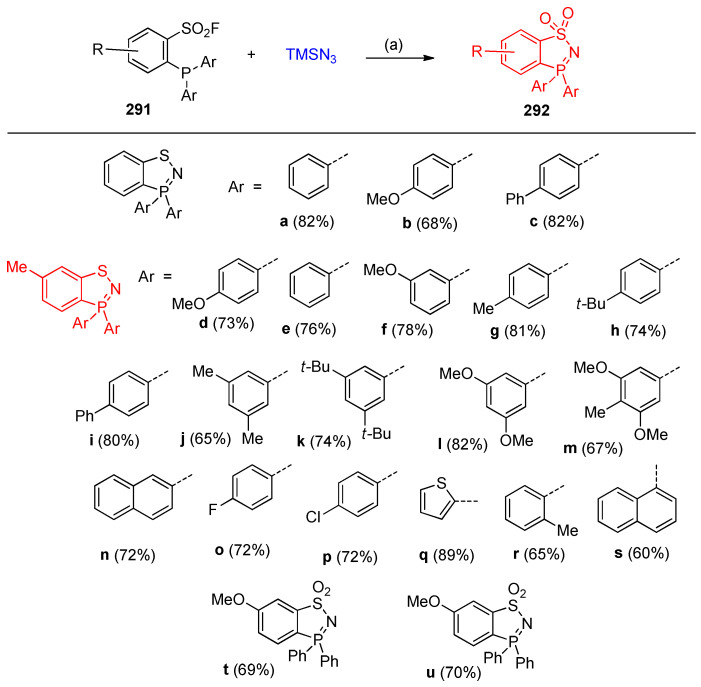
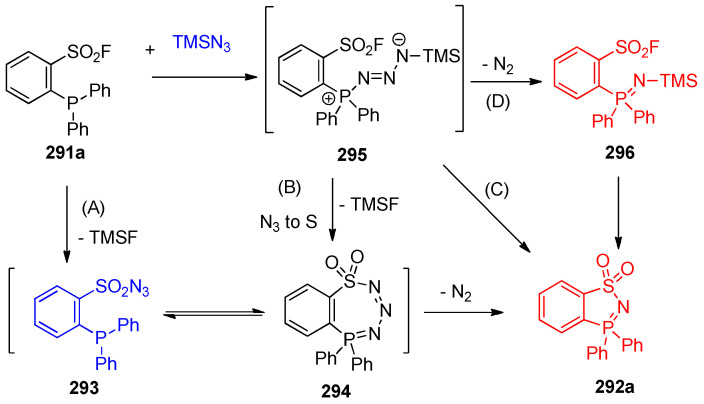
The reaction of ortho-phosphinoarenesulfonyl fluorides 291 with trimethylsilyl azide resulted in benzo-1,2,3-thiazaphosphole 292 [91]. To optimize the reaction condition, it was found that a mixture of acetonitrile and 10 equivalents of trimethylsilyl azide at 60 °C was the optimal reaction chosen condition (Scheme 115) [92]. The three possible mechanistic pathways (A), (B) and (C) for forming the benzo-thiazaphosphole 292a are illustrated in Scheme 116 [91].
Scheme 115.
Syntheses of benzo-1,2,3-thiazaphospholes 292a–u. Reagents and conditions: (a) MeCN, 10 equiv. Azide, 60 °C, 12 h.
Scheme 116.
Mechanistic pathways for the formation of compound 292a.
5. Conclusions
In summary, the azido group in organic substrates are effectively served in the synthesis of various heterocycles through different mechanistic steps, such as one-pot reactions, nucleophilic additions (such as Aza-Michael addition), cycloaddition reactions (such as [3+2] cycloaddition), mixed addition/cyclization/oxygen, and insertion reactions of C-H amination. The selectivity of the chosen catalyst plays an important role in the chemoselectivity favoring C−H and C-N bonds, as it can be seen that organic azides have been used in the synthesis of various types of natural products producing good to excellent yields. Most indicative is the utility of organic azides in the synthetic procedures of fused heterocycles, such as quinazoline derivatives along with organo-metal heterocycles (i.e., phosphorus-, boron-, and aluminum-containing heterocycles). This review focused on synthesizing various heterocycles using azide chemistry and mechanistic aspects.
Scheme 78.
Mechanistic equations for the formation of 5-phenyl-[1,2,3]triazolo[5,1-a]isoquinoline 205a.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Jouf University under grand No. (DSR-2021–03-0369). We acknowledge support from the KIT-Publication Fund of the Karlsruhe Institute of Technology. Stefan Bräse is grateful for support from the DFG-funded cluster program “3D Matter Made To Order” under Germany’s Excellence Strategy-2082/1-390761711.
Abbreviations
TMS: trimethylsilyl: pmp: pentamethylpiperidines; DCE dichloroethane; Tf: trifluorosulfonyl; Nf: nonafluorbutanesulfonyl; Py: pyridinyl; Ms: mesyl; Bs: brosyl; Ts: tosyl; Ns: nosyl; CuMeSal: copper 3-methylsalicylate; NHC: N-heterocyclic carbene; Boc: ter-butyloxycarbonyl; IBX: 2-Iodoxybenzoic acid; DABCO: diazobicyclooctane; BOX: bisoxazoline; CSA: camphorsulfonic acid; TBS: tribuylsilyl; IMes. 1,3-Bis(2,4,6-trimethylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene; TFPB: tetrakis[3,5-bis(trifluoromethyl)phenyl] borate.
Author Contributions
A.A.A. Conceptualization, writing, editing, and submitting), A.A.N. (Editing, fund acquisition, and project administration, W.A.A.A., I.M.A., A.I.A.-E., E.M.E.-F. and M.A.A. (Project administration), S.B. (Editing, project administration). H.N.T. (Writing and editing). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Hazardous Information
A qualified scientist with appropriate safety precautions should be mandatory for using azides. The website azide.org should be consulted [92].
Funding Statement
This work was funded by the Deanship of Scientific Research at Jouf University under grand No. (DSR-2021–03-0369).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nguyen M.T., Sengupta D., Ha T.-K. Another Look at the Decomposition of Methyl Azide and Methanimine: How Is HCN Formed? J. Phys. Chem. 1996;100:6499–6503. doi: 10.1021/jp953022u. [DOI] [Google Scholar]
- 2.Breuning A., Vicik R., Schirmeister T. An improved synthesis of aziridine-2,3-dicarboxylates via azido alcohols—epimerization studies. Tetrahedron Asymmetry. 2003;14:3301–3312. doi: 10.1016/j.tetasy.2003.09.015. [DOI] [Google Scholar]
- 3.Bräse S., Banert K. Chichester. John Wiley; West Sussex, UK: 2010. Organic azides: Syntheses and applications; p. 507. [Google Scholar]
- 4.Kölmel D.K., Jung N., Bräse S. Azides-Diazonium ions-Triazenes: Versatile nitrogen-rich functional groups. Austr. J. Chem. 2014;67:328–336. doi: 10.1071/CH13533. [DOI] [Google Scholar]
- 5.Monguchi Y., Sajiki H. Catalytic Reduction in Organic. Synthesis. 2018;217:353. doi: 10.1055/sos-SD-227-00156. [DOI] [Google Scholar]
- 6.Gololobov Y.G., Kasukhin L.F. Recent advances in the Staudinger reaction. Tetrahedron. 1992;48:1353–1406. doi: 10.1016/S0040-4020(01)92229-X. [DOI] [Google Scholar]
- 7.Fresneda P.M., Molina P. Application of Iminophosphorane-Based Methodologies for the Synthesis of Natural Products. Synlett. 2004;35:1–17. doi: 10.1055/s-2003-43338. [DOI] [Google Scholar]
- 8.Palacios F., Alonso C., Aparicio D., Rubiales G., de Los S., Jesús M. The aza-Wittig reaction: An efficient tool for the construction of carbon–nitrogen double bonds. Tetrahedron. 2007;63:523–575. doi: 10.1016/j.tet.2006.09.048. [DOI] [Google Scholar]
- 9.Álvares Y.S.P., Alves M.J., Azoia N.G., Bickley J.F., Gilchrist T.L. Diastereoselective synthesis of aziridines from (1R)-10-(N,N-dialkyl-sulfamoyl)isobornyl 2H-azirine-3-carboxylates. J. Chem. Soc. Perkin Trans. 2022;1:1911–1919. doi: 10.1039/B202321K. [DOI] [Google Scholar]
- 10.Capitosti S.M., Hansen T.P., Brown M.L. Facile Synthesis of an Azido-Labeled Thalidomide Analogue. Org. Lett. 2003;5:2865–2867. doi: 10.1021/ol034906w. [DOI] [PubMed] [Google Scholar]
- 11.Lowe-Ma C.K., Nissan R.A., Wilson W.S. Tetrazolo [1,5-a]pyridines and Furazano [4,5-b]pyridine 1-Oxides. J. Org. Chem. 1990;55:3755–3761. doi: 10.1021/jo00299a014. [DOI] [Google Scholar]
- 12.Stadlbauer W., Fiala W., Fischer M., Hojas G. Thermal cyclization of 4-azido-3-nitropyridines to furoxanes. J. Heterocycl. Chem. 2000;37:1253–1256. doi: 10.1002/jhet.5570370537. [DOI] [Google Scholar]
- 13.Gavenonis J., Tilley T.D. Tantalum Alkyl and Silyl Complexes of the Bulky (Terphenyl)imido Ligand [2,6-(2,4,6-Me3C6H2)2C6H3N]2- ([Ar*N]2-). Generation and Reactivity of [(Ar*N)(Ar*NH)Ta(H)(OSO2CF3)], Which Reversibly Transfers Hydride to an Aromatic Ring of the Arylamide Ligand. Organometallics. 2002;21:5549–5563. doi: 10.1021/om020509y. [DOI] [Google Scholar]
- 14.Kim Y.H., Kim K., Shim S.B. Reaction of arylhydrazines with nitric oxide in the presence of oxygen. Tetrahedron Lett. 1986;27:4749–4752. doi: 10.1016/S0040-4039(00)85055-8. [DOI] [Google Scholar]
- 15.Lee S.H., Kwon N.Y., Lee J.Y., An W., Jung Y.H., Mishra N.K., Ghosh P., Park J.S., Kim I.S. Transition-Metal-Free and Site-Selective Selenylation of Heterocyclic N-Oxides in Anisole as a Green Solvent. Eur. J. Org. Chem. 2020;2020:4886–4892. doi: 10.1002/ejoc.202000610. [DOI] [Google Scholar]
- 16.Zhang G.-Y., Peng Y., Xue J., Fan Y.-H., Deng Q.-H. Copper-catalyzed nitrene transfer/cyclization cascade to synthesize 3a-nitrogenous furoindolines and pyrroloindolines. Org. Chem. Front. 2019;6:3934–3938. doi: 10.1039/C9QO01124B. [DOI] [Google Scholar]
- 17.Porter M.R., Shaker R.M., Calcanas C., Topczewski J.J. Stereoselective Dynamic Cyclization of Allylic Azides: Synthesis of Tetralins, Chromanes, and Tetrahydroquinolines. J. Am. Chem. Soc. 2018;140:1211–1214. doi: 10.1021/jacs.7b11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Y., Clarke R.M., Porter G.J., Betley T.A. Efficient C−H Amination Catalysis Using Nickel-Dipyrrin Complexes. J. Am. Chem. Soc. 2020;142:10996–11005. doi: 10.1021/jacs.0c02126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun K., Tao C., Long B., Zeng X., Wu Z., Zhang R. Highly stereoselective gram scale synthesis of all the four diastereoisomers of Boc-protected 4-methylproline carboxylates. RSC Adv. 2019;9:32017–32020. doi: 10.1039/C9RA06827A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X., Wang Z.-S., Zhai T.-Y., Luo C., Zhang Y.-P., Chen Y.-B., Deng C., Liu R.S., Ye L.-W. Copper-Catalyzed Azide–Ynamide Cyclization to Generate α-Imino Copper Carbenes: Divergent and Enantioselective Access to Polycyclic N-Heterocycles. Angew. Chem. Int. Ed. 2020;59:2–9. doi: 10.1002/anie.202007206. [DOI] [PubMed] [Google Scholar]
- 21.Fu J.G., Zanoni G., Anderson E.A., Bi X. α-Substituted vinyl azides: An emerging functionalized alkene. Chem. Soc. Rev. 2017;46:7208–7228. doi: 10.1039/C7CS00017K. [DOI] [PubMed] [Google Scholar]
- 22.Borra S., Borkotoky L., Newar U.D., Das B., Maurya R.A. Photocatalyst-Free Visible-Light Enabled Synthesis of Substituted Pyrroles from α-Keto Vinyl Azides. Adv. Synth. Catal. 2020;362:3364–3368. doi: 10.1002/adsc.202000562. [DOI] [Google Scholar]
- 23.Quiclet-Sire B., Zard S.Z. Observations on the Reaction of Hydrazones with Iodine: Interception of the Diazo Intermediates. Chem. Commun. 2006:1831–1832. doi: 10.1039/b602580c. [DOI] [PubMed] [Google Scholar]
- 24.Kapras V., Pohl R., Císařová I., Jahn U. Asymmetric Domino Aza-Michael Addition/[3+2] Cycloaddition Reactions as a Versatile Approach to α,β,γ-Triamino Acid Derivatives. Org. Lett. 2014;16:1088–1091. doi: 10.1021/ol403660w. [DOI] [PubMed] [Google Scholar]
- 25.Just D., Hernandez-Guerra D., Kritsch S., Pohl R., Císařová I., Jones P.G., Mackman R., Bahador G., Jahn U. Lithium Chloride Catalyzed Asymmetric Domino Aza-Michael Addition/[3+2] Cycloaddition Reactions for the Synthesis of Spiro- and Bicyclic α,β,γ-Triamino Acid Derivatives. Eur. J. Org. Chem. 2018;2018:5213–5221. doi: 10.1002/ejoc.201800585. [DOI] [Google Scholar]
- 26.Yang C., Shen H. One Pot Multiple-Steps Reactions of Allyl Azide and Alkenes Carrying Electron-Withdrawing Groups. Tetrahedron Lett. 1993;34:4051–4054. doi: 10.1016/S0040-4039(00)60613-5. [DOI] [Google Scholar]
- 27.Yang C.-H., Sherf H.-J., Wang R.-H., Wang J.-C. 2,3,7-Triazabicyclo [3.3.0]Octenes Prepared by Tandem Cascade Reaction of Allyl Azides and Olefinic Dipolarophiles. J. Chin. Chem. Soc. 2002;49:95–102. doi: 10.1002/jccs.200200016. [DOI] [Google Scholar]
- 28.Carlson A.S., Liu E.-C., Topczewski J.J. A Cascade Reaction of Cinnamyl Azides with Acrylates Directly Generates Tetrahydro-Pyrrolo-Pyrazole Heterocycles. J. Org. Chem. 2020;85:6044–6059. doi: 10.1021/acs.joc.0c00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu W., Chen Q., Tian Y., Xu Y., Huang Y., You Y., Weng Z. Synthesis of polysubstituted 5-trifluoromethyl isoxazoles via denitrogenative cyclization of vinyl azides with trifluoroacetic anhydride. Org. Chem. Front. 2020;7:1878–1883. doi: 10.1039/D0QO00243G. [DOI] [Google Scholar]
- 30.Terhorst S., Tiwari D.P., Meister D., BPetran B., Rissanen K., Bolm C. Syntheses of Trifluoroethylated N-Heterocycles from Vinyl Azides and Togni’s Reagent Involving 1,n-Hydrogen-Atom Transfer Reactions. Org. Lett. 2020;22:4766–4770. doi: 10.1021/acs.orglett.0c01566. [DOI] [PubMed] [Google Scholar]
- 31.Tichý D., Košťál V., Motornov V., Klimánková I., Beier P. Preparation of 1-Azido-2-Bromo-1,1,2,2-Tetrafluoroethane and Its Use in the Synthesis of N-Fluoroalkylated Nitrogen Heterocycles. J. Org. Chem. 2020;85:11482–11489. doi: 10.1021/acs.joc.0c01610. [DOI] [PubMed] [Google Scholar]
- 32.De Nino A., Merino P., Algieri V., Nardi M., Di Gioia M.L., Russo B., Tallarida M.A., Maiuolo L. Synthesis of 1,5-Functionalized 1,2,3-Triazoles Using Ionic Liquid/Iron(III) Chloride as an Efficient and Reusable Homogeneous Catalyst. Catalysts. 2018;8:364. doi: 10.3390/catal8090364. [DOI] [Google Scholar]
- 33.De Nino A., Algieri V., Tallarida M.A., Costanzo P., Pedrón M., Tejero T., Merino P., Maiuolo L. Regioselective Synthesis of 1,4,5-Trisubstituted-1,2,3-Triazoles from Aryl Azides and Enaminones. Eur. J. Org. Chem. 2019;2019:5725–5731. doi: 10.1002/ejoc.201900889. [DOI] [Google Scholar]
- 34.Zhang L.-L., Li Y.-T., Gao T., Guo S.S., Yang B., Meng Z.-H., Dai Q.P., Xu Z.-B., Wu Q.P. Efficient Synthesis of Diverse 5-Thio- or 5-Selenotriazoles: One-Pot Multicomponent Reaction from Elemental Sulfur or Selenium. Synthesis. 2019;51:4170–4182. doi: 10.1055/s-0039-1690618. [DOI] [Google Scholar]
- 35.Taia A., Essaber M., Oubella A., Aatif A., Bodiguel J., Grégoire B.J., Itto M.Y.A., Morjani H. Synthesis, characterization, and biological evaluation of new heterocyclic systems 1,2,3-triazole-isoxazoline from eugenol by the mixed condensation reactions. Synth. Commu. 2020;50:2052–2065. doi: 10.1080/00397911.2020.1762224. [DOI] [Google Scholar]
- 36.Shafran Y.M., Silaichev P.S., Bakulev V.A. β-(Cycloalkylamino)ethanesulfonyl azides as novel water-soluble reagents for the synthesis of diazo compounds and heterocycles. Chem. Heterocycl. Compd. 2019;55:1251–1261. doi: 10.1007/s10593-019-02609-z. [DOI] [Google Scholar]
- 37.Stanciu M.C., Belei D., Bicu E., Tuchilus C.G., Nichifor M. Novel amphiphilic dextran esters with antimicrobial activity. Internat. J. Biol. Macromol. 2020;150:746–755. doi: 10.1016/j.ijbiomac.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 38.Denis M., Goldup S.M. The active template approach to interlocked molecules. Nat. Rev. Chem. 2017;1:61. doi: 10.1038/s41570-017-0061. [DOI] [Google Scholar]
- 39.Liu Y., Zhao W., Chen C.-H., Flood A.H. Chloride capture using a C-H hydrogen-bonding cage. Science. 2019;365:159–161. doi: 10.1126/science.aaw5145. [DOI] [PubMed] [Google Scholar]
- 40.Hsueh F.-C., Tsai C.-Y., Lai C.-C., Liu Y.-H., Peng S.-M., Chiu S.-H. N Heterocyclic Carbene Copper(I) Rotaxanes Mediate Sequential Click Ligations with All Reagents Premixed. Angew. Chem. 2020;132:11374–11378. doi: 10.1002/ange.202001398. [DOI] [PubMed] [Google Scholar]
- 41.Boddu L., Potlapati V., Subhashini N.J.P. Synthesis of novel isoindolone-based medium-sized macromolecules and triazole containing heterocyclic compounds. J. Heterocycl. Chem. 2019;56:3197–3205. doi: 10.1002/jhet.3704. [DOI] [Google Scholar]
- 42.Gupta A., Sarkar F.K., Sarkar R., Jamatia R., Lee C.Y., Gupta G., Pal A.K. Development of a new catalytic and sustainable methodology for the synthesis of benzodiazepine triazole scaffold using magnetically separable CuFe2O4@MIL-101(Cr) nano-catalyst in aqueous medium. Appl. Organomet. Chem. 2020;34:e5782. doi: 10.1002/aoc.5782. [DOI] [Google Scholar]
- 43.Sokolov V.B., Aksinenko A.Y., Goreva T.V., Epishina T.A., Bachurin S.O. Modification of the drug “riluzole” with an alkyne-azide “click”-reaction with pharmacologically active fragments. Russ. Chem. Bull. Inter. 2019;68:2241–2244. doi: 10.1007/s11172-019-2693-y. [DOI] [Google Scholar]
- 44.Pokhodylo N.T., Tupychak M.A., Shyyka O.Y., Obushak M.D. Some Aspects of the Azide–Alkyne 1,3-Dipolar Cycloaddition Reaction. Russ. J. Org. Chem. 2019;55:1310–1321. doi: 10.1134/S1070428019090082. [DOI] [Google Scholar]
- 45.Mikhaylov V.N., Pavlov A.O., Ogorodnov Y.V., Spiridonova D.V., Sorokoumov V.N., Balova I.A. N-Propargylation and copper(I)-catalyzed azide-alkyne cycloaddition as a convenient strategy for directed post-synthetic modification of 4-oxo-1,4-dihydrocinnoline derivatives. Chem. Heterocycl. Comp. 2020;56:915–922. doi: 10.1007/s10593-020-02750-0. [DOI] [Google Scholar]
- 46.Filimonov V.O., Dianova L.N., Beryozkina T.V., Mazur D., Beliaev N.A., Volkova N.N., Ilkin V.G., Dehaen W., Lebedev A.T., Bakulev V.A. Water/Alkali-Catalyzed Reactions of Azides with 2-Cyanothioacetamides. Eco-Friendly Synthesis of Monocyclic and Bicyclic 1,2,3-Thiadiazole-4-carbimidamides and 5-Amino-1,2,3-triazole-4-carbothioamides. J. Org. Chem. 2019;84:13430–13446. doi: 10.1021/acs.joc.9b01599. [DOI] [PubMed] [Google Scholar]
- 47.Hussain B.J., Salim A.T. Synthesis of mannich and triazole derivatives for barbiturate derivatives with some analytical applications. J. Phys. Conf. Series. 2019;1294:052058–052073. doi: 10.1088/1742-6596/1294/5/052058. [DOI] [Google Scholar]
- 48.Kilpin K.J., Paul U.S.D., Lee A.-L., Crowley J.D. Gold(I) ‘‘click’’ 1,2,3-triazolylidenes: Synthesis, self-assembly and catalysis. Chem. Commun. 2011;47:328–330. doi: 10.1039/C0CC02185G. [DOI] [PubMed] [Google Scholar]
- 49.Crowley J.D., Bandeen P.H., Hanton L.R. A one pot multi-component CuAAC “click” approach to bidentate and tridentate pyridyl-1,2,3-triazole ligands: Synthesis, X-ray structures and copper(II) and silver(I) complexes. Polyhedron. 2010;29:70. doi: 10.1016/j.poly.2009.06.010. [DOI] [Google Scholar]
- 50.Yan X., Wang H., Guo S. Employing Aryl-Linked Bis-mesoionic Carbenes as a Pincer-Type Platform to A cess Ambient-Stable Palladium(IV) Complexes. Angew. Chem. Int. Ed. 2019;58:16907. doi: 10.1002/anie.201911180. [DOI] [PubMed] [Google Scholar]
- 51.Yan X., Zhang B., Zhang X., Wang H., Duan Y.-A., Guo S. Symmetrical and Non-symmetrical Pd (II) Pincer Complexes Bearing Mesoionic N-heterocyclic Thiones: Synthesis, Characterizations and Catalytic Properties. Appl. Organomet. Chem. 2020;34:e5885. doi: 10.1002/aoc.5885. [DOI] [Google Scholar]
- 52.Yoshida S., Shiraishi A., Kanno K., Matsushita T., Johmoto K., Uekusa H., Hosoya T. Enhanced clickability of doubly sterically-hindered aryl azides. Sci. Rep. 2011;1:82. doi: 10.1038/srep00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshida S., Goto S., Nishiyama Y., Hazama Y., Kondo M., Matsushita T., Hosoya T. Effect of Resonance on the Clickability of Alkenyl Azides in the Strain-promoted Cycloaddition with Dibenzo-fused Cyclooctynes. Chem. Lett. 2019;48:1038–1041. doi: 10.1246/cl.190400. [DOI] [Google Scholar]
- 54.Ugi I., Meyr R., Isonitrile V. Erweiterter Anwendungsbereich der Passerini-Reaktion. Chem. Ber. 1961;94:2229. doi: 10.1002/cber.19610940844. [DOI] [Google Scholar]
- 55.Neochoritis C.G., Zhao T., Dömling A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019;119:1970–2042. doi: 10.1021/acs.chemrev.8b00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng Q.-X., Mu Z.-Y., Yao G., Zhang J.-A., He H.-T., Pang Y.-L., Xiong J. Efficient Synthesis of 4H-3, 1-Benzoxazine Derivatives via One-Pot Sequential Passerini-Azide/Palladium-Catalyzed Azide–Isocyanide Coupling/Cyclization Reaction. Synlett. 2020;31:1003–1006. doi: 10.1055/s-0039-1690831. [DOI] [Google Scholar]
- 57.Morgalyuk V.P., Strelkova T.S., Kononevich Y.N., Brel V.K. Synthesis of 1,3,3,5,5-Penta [1-(2,2-dimethyl-1,3-dioxolan-4-yl)methoxy]-1-[(1H-1,2,3-triazol-4-yl)alkoxy]cyclotriphosphazenes. Rus. J. Gen. Chem. 2019;89:1620–1624. doi: 10.1134/S1070363219080115. [DOI] [Google Scholar]
- 58.Naouri A., Djemoui A., Ouahran M.R., Lahrech M.B., Lemouari N., Rocha D.H.A., Albuquerque H., Mendes R.F., Paz F.A.A., Helguero L.A., et al. Multicomponent and 1,3-dipolar cycloaddition synthesis of triazoleand isoxazole-acridinedione/xanthenedione heterocyclic hybrids: Cytotoxic effects on human cancer cells. J. Mol. Struct. 2020;1217:128325–128333. doi: 10.1016/j.molstruc.2020.128325. [DOI] [Google Scholar]
- 59.Deng L., Liu Y., Zhu Y., Wan I.P. Transition-Metal-Free Annulation of Enamines and Tosyl Azide toward N-Heterocycle Fused and 5-Amino-1,2,3-Triazoles. Eur. J. Org. Chem. 2020;2020:5606–5609. doi: 10.1002/ejoc.202000938. [DOI] [Google Scholar]
- 60.Gulevskaya A.V., Tyaglivy A.S., Pozharskii A.F., Nelina-Nemtseva J.I., Steglenko D.V. Heterocyclization of Enediynes Promoted by Sodium Azide: A Case of Ambiguity of X-ray Data and Structure Revision. Org. Lett. 2014;16:1582–1585. doi: 10.1021/ol500125j. [DOI] [PubMed] [Google Scholar]
- 61.Ning Y., Wu N., Yu H., Liao P., Li X., Bi X. Silver-Catalyzed Tandem Hydroazidation/Alkyne–Azide Cycloaddition of Diynes with TMS-N3: An Easy Access to 1,5-Fused 1,2,3-Triazole Frameworks. Org. Lett. 2015;17:2198–2201. doi: 10.1021/acs.orglett.5b00784. [DOI] [PubMed] [Google Scholar]
- 62.Tao S., Hu Q., Li H., Ma S., Chen Y. Synthesis of [1,2,3]Triazolo [5,1-a]isoquinoline Derivatives via a Selective Cascade Cyclization Sequence of 1,2-bis(Phenylethynyl)benzene Derivatives. Synth. Commun. 2015;45:1354–1361. doi: 10.1080/00397911.2015.1020952. [DOI] [Google Scholar]
- 63.Zhao S., Yu R., Chen W., Liu M., Wu H. Efficient Approach to Mesoionic Triazolo [5,1-a]isoquinolium through Rhodium-Catalyzed Annulation of Triazoles and Internal Alkynes. Org. Lett. 2015;17:2828–2831. doi: 10.1021/acs.orglett.5b01247. [DOI] [PubMed] [Google Scholar]
- 64.Fan M., Liu Y., Hu Q., Jia L., Chen Y. Facile Synthesis of [1,2,3]Triazolo [5,1-a]isoquinolines through a Copper-Catalyzed Tandem Sonogashira Coupling/Cyclization Reaction. Eur. J. Org. Chem. 2016;2016:5470–5473. doi: 10.1002/ejoc.201601188. [DOI] [Google Scholar]
- 65.Chen Y., Zhou S., Ma S., Liu W., Pan Z., Shi X. A facile synthesis of 5- amino-[1,2,3]triazolo[5,1-a]isoquinoline derivatives through copper-catalyzed cascade reactions. Org. Biomol. Chem. 2013;11:8171–8174. doi: 10.1039/C3OB41774C. [DOI] [PubMed] [Google Scholar]
- 66.Hu Y.Y., Hu J., Wang X.C., Guo L.N., Shu X.Z., Niu Y.N., Liang Y.M. Copper-catalyzed tandem synthesis of [1,2,3]triazolo [5,1-a]isoquinolines and their transformation to 1,3-disubstituted isoquinolines. Tetrahedron. 2010;66:80–86. doi: 10.1016/j.tet.2009.11.043. [DOI] [Google Scholar]
- 67.Maurya R.A., Adiyala P.R., Chandrasekhar D., Reddy C.N., Kapure J.S., Kamal A. Rapid Access to Novel 1,2,3-Triazolo-Heterocyclic Scaffolds via Tandem Knoevenagel Condensation/Azide–Alkyne 1,3-Dipolar Cycloaddition Reaction in One Pot. ACS Comb. Sci. 2014;16:466–477. doi: 10.1021/co500070e. [DOI] [PubMed] [Google Scholar]
- 68.Wu Y., Meng Y., Liu Z., Zhang M., Song C. AlCl3-promoted three-component cascade reaction for rapid access to [1,2,3]triazolo [5,1-a]isoquinolines. Tetrahedron Lett. 2019;60:151287. doi: 10.1016/j.tetlet.2019.151287. [DOI] [Google Scholar]
- 69.Pal P., Mainkar P.S., Nayani K., Chandrasekhar S. Mn-catalyzed radical initiated domino transformation of alkynylated cyclohexadienones with TMSN3 and O2 to bicyclic azido alcohols. Chem. Commun. 2020;56:3453–3456. doi: 10.1039/D0CC00102C. [DOI] [PubMed] [Google Scholar]
- 70.Gour J., Gatadi S., Akunuri R., Yaddanapudi M.V., Nengroo M.A., Datta D., Chopra S., Nanduri S. Catalyst-free facile synthesis of polycyclic indole/pyrrole substituted-1,2,3-triazoles. Org. Biomol. Chem. 2019;17:8153–8165. doi: 10.1039/C9OB01560D. [DOI] [PubMed] [Google Scholar]
- 71.Yan Y.-M., Li H.-Y., Zhang M., Wang R.-X., Zhou C.-G., Ren Z.-X., Ding M.-W. One-Pot Synthesis of [1,2,3]Triazolo [1,5-a]quinoxalin-4(5H)-ones by a Metal-Free Sequential Ugi-4CR/Alkyne–Azide Cycloaddition Reaction. Synlett. 2020;31:73–76. doi: 10.1055/s-0037-1610737. [DOI] [Google Scholar]
- 72.Gangaprasad D., Raj J.P., Karthikeyan K., Rengasamy R., Kesavan M., Vajjiravel M., Elangovan J. A new route to 1,2,3-triazole fused benzooxazepine and benzodiazepine analogues through metal-free intramolecular azide-olefin oxidative cycloaddition. Tetrahedron Lett. 2019;60:151164–151166. doi: 10.1016/j.tetlet.2019.151164. [DOI] [Google Scholar]
- 73.Alshammari M.B., Aly A.A., Brown A.B., Bakht M.A., Shawky A.M., Abdelhakem A.M., El-Sheref E.M. An Efficient Click Synthesis of Chalcones Derivatized with Two 1-(2-quinolon-4-yl)-1,2,3-triazoles. Z. Für Nat. 2021;76:395–403. doi: 10.1515/znb-2021-0028. [DOI] [Google Scholar]
- 74.El-Sheref E.M., Aly A.A., Alshammari M.B., Brown A.B., Abdel-Hafez S.M.N. Design, Synthesis, Molecular Docking, Antiapoptotic and Caspase-3 Inhibition of New 1,2,3-Triazole/Bis-2(1H)-Quinolinone Hybrids. Molecules. 2020;25:5057. doi: 10.3390/molecules25215057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Voitekhovich S.V., Grigoriev Y.V., Grigoriev Y.V., Lyakhov A.S., Matulis V.E., Ivashkevich L.S., Bogomyakov A.S., Lavrenova L.G., Ivashkevich O.A. 1-(1,2,4-Triazol-3-yl)-1H-tetrazoles and their complexation with copper(II) chloride. Polyhedron. 2020;176:114299. doi: 10.1016/j.poly.2019.114299. [DOI] [Google Scholar]
- 76.Abdullahi J.A., Rabeaa M.A. Grinding-Assisted Synthesis of Some Heterocyclic Compounds. Asian J. Chem. 2020;32:1713–1718. doi: 10.14233/ajchem.2020.22268. [DOI] [Google Scholar]
- 77.Sarrafiouna F., Jamehbozorgib S., Ramezani M. Synthesis of Tetrazoles Catalyzed by Novel Cobalt Magnetic Nanoparticles. Russ. J. Org. Chem. 2019;55:1777–1784. doi: 10.1134/S1070428019110216. [DOI] [Google Scholar]
- 78.Saber A.F., Kamal El-Dean A.M., Redwan S.M., Zaki R.M. Synthesis, spectroscopic characterization, and in vitro antimicrobial activity of fused pyrazolo [40,30:4,5]thieno [3,2-d]pyrimidine. J. Chin. Chem. Soc. 2020;67:1239–1246. doi: 10.1002/jccs.201900374. [DOI] [Google Scholar]
- 79.Sajjadi M., Nasrollahzadeh M., Sajadi S.M. Green synthesis of Ag/Fe3O4 nanocomposite using Euphorbia peplus Linn leaf extract and evaluation of its catalytic activity. J. Colloid Interface Sci. 2017;497:1–13. doi: 10.1016/j.jcis.2017.02.037. [DOI] [PubMed] [Google Scholar]
- 80.Nasrollahzadeh M., Sajjadi M., Varma R.S. Magnetically recoverable nanocatalyst based on N-heterocyclic ligands: Efficient treatment of environmental pollutants in aqueous media. Clean Technol. Environ. Policy. 2020;22:423–440. doi: 10.1007/s10098-019-01794-x. [DOI] [Google Scholar]
- 81.Trose M., Nahra F., Cordes D.B., Slawin A.M.Z., Cazin C.S.I. Cu–NHC azide complex: Synthesis and reactivity. Chem. Commun. 2019;55:12068–12071. doi: 10.1039/C9CC04844H. [DOI] [PubMed] [Google Scholar]
- 82.Singam M.K.R., Nagireddy A., Rajesh M., Ganesha V., Reddy M.S. Ni-Catalyzed electrophile driven regioselective arylative cyclization of ortho-functional diaryl acetylenes for the synthesis of pyridine and indene derivatives. Org. Chem. Front. 2020;7:30–34. doi: 10.1039/C9QO01266D. [DOI] [Google Scholar]
- 83.Nie B., Wu W., Zeng W., Ren Q., Zhang J., Zhang Y., Jiang H. Synthesis of Isoquinoline Derivatives via Palladium-Catalyzed C-H/C-N Bond Activation of N-Acyl Hydrazones with α-Substituted Vinyl Azides. Adv. Synth. Catal. 2020;362:1362–1369. doi: 10.1002/adsc.201901394. [DOI] [Google Scholar]
- 84.Wei K., Yang T., Chen Q., Liang S., Yu W. Iron-catalysed 1,2-aryl migration of tertiary azides. Chem. Commun. 2020;56:11685–11688. doi: 10.1039/D0CC04579A. [DOI] [PubMed] [Google Scholar]
- 85.Jin H., Liu D., Zhou B., Liu Y. One-Pot Copper-Catalyzed Three-Component Reaction of Sulfonyl Azides, Alkynes, and Allylamines To Access 2,3-Dihydro-1H-imidazo [1,2-a]indoles. Synthesis. 2020;52:1417–1424. doi: 10.1055/s-0037-1610739. [DOI] [Google Scholar]
- 86.Kumar G.R.Y., Begum N.S., Imran K.M. Mn-mediated oxidative radical cyclization of 2-(azidomethyl)phenyl isocyanides with carbazate: Access to quinazoline-2-carboxylates. New J. Chem. 2020;44:7001–7006. doi: 10.1039/D0NJ00479K. [DOI] [Google Scholar]
- 87.Prieschl D., Arrowsmith M., Dietz M., Rempel A., Müller M., Braunschweig H. Synthesis of polyheterocyclic 1,1-diboryltriazenes by γ-nitrogen insertion of azides into activated B–B single bonds. Chem. Commun. 2020;56:5681–5684. doi: 10.1039/D0CC02305A. [DOI] [PubMed] [Google Scholar]
- 88.LaFortune J.H.W., Trofimova A., Cummings H., Westcott S.A., Stephan D.W. Phosphinoboration of Diazobenzene: Intramolecular FLP Synthon for PN2B-Derived Heterocycles. Chem. Eur. J. 2019;25:12521–12525. doi: 10.1002/chem.201903670. [DOI] [PubMed] [Google Scholar]
- 89.Drescher R., Lin S., Hofmann A., Lenczyk C., Kachel S., Krummenacher L.Z., Braunschweig H. Ring expansion of alumoles with organic azides: Selective formation of six-membered aluminum–nitrogen heterocycles. Chem. Sci. 2020;11:5559–5564. doi: 10.1039/D0SC02032J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marchenko A., Koidan G., Hurieva A.N., Shishkina S., Rusanov E., Kostyuk A. “Ring-enlargement of N-phosphanyl-1,2,3,4-tetrahydroquinazolines. J. Org. Chem. 2020;85:14467–14472. doi: 10.1021/acs.joc.0c00750. [DOI] [PubMed] [Google Scholar]
- 91.Luo W., Wang Z., Cao X., Liang D., Wei M., Yin K., Li L. Construction of Benzo-1,2,3-thiazaphosphole Heterocycles by Annulations of ortho-Phosphinoarenesulfonyl Fluorides with Trimethylsilyl Azide. J. Org. Chem. 2020;85:14785–14794. doi: 10.1021/acs.joc.0c01309. [DOI] [PubMed] [Google Scholar]
- 92.Schock M., Bräse S. Reactive & Efficient: Organic Azides as Cross-Linkers in Material Sciences. Molecules. 2020;25:1009. doi: 10.3390/molecules25041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.