Abstract
We have studied inactivation of four strains each of Escherichia coli and Listeria innocua in milk by the combined use of high hydrostatic pressure and the lactoperoxidase-thiocyanate-hydrogen peroxide system as a potential mild food preservation method. The lactoperoxidase system alone exerted a bacteriostatic effect on both species for at least 24 h at room temperature, but none of the strains was inactivated. Upon high-pressure treatment in the presence of the lactoperoxidase system, different results were obtained for E. coli and L. innocua. For none of the E. coli strains did the lactoperoxidase system increase the inactivation compared to a treatment with high pressure alone. However, a strong synergistic interaction of both treatments was observed for L. innocua. Inactivation exceeding 7 decades was achieved for all strains with a mild treatment (400 MPa, 15 min, 20°C), which in the absence of the lactoperoxidase system caused only 2 to 5 decades of inactivation depending on the strain. Milk as a substrate was found to have a considerable effect protecting E. coli and L. innocua against pressure inactivation and reducing the effectiveness of the lactoperoxidase system under pressure on L. innocua. Time course experiments showed that L. innocua counts continued to decrease in the first hours after pressure treatment in the presence of the lactoperoxidase system. E. coli counts remained constant for at least 24 h, except after treatment at the highest pressure level (600 MPa, 15 min, 20°C), in which case, in the presence of the lactoperoxidase system, a transient decrease was observed, indicating sublethal injury rather than true inactivation.
The use of antimicrobial compounds from a wide variety of natural sources is being explored as a means to improve the safety and stability of several foods while maintaining an image of natural, high quality, and healthy products (11). Many different compounds have been isolated from and tested with a variety of products, including fresh and cooked meat, vegetable products, and dairy products, such as milk and cheese (5, 8, 33). However, the effectiveness is limited for several reasons. First, the antimicrobial spectrum of most natural preservative systems is restricted to a narrow group of microorganisms. For instance, nisin and other bacteriocins are effective against only some gram-positive bacteria, but not against gram-negative bacteria. Further, sensitive bacterial strains may develop resistance when exposed to sublethal doses of an antimicrobial. A well-studied example of this is nisin resistance (22, 23). Finally, protein, fat, or other components in complex food substrates may protect target microorganisms, for instance, by adsorbing antimicrobial components. One way to overcome these limitations is to use combinations of two or more biopreservatives with different targets (36) or to combine antimicrobials with other preservation techniques. In this respect, emerging nonthermal preservation techniques, such as high hydrostatic pressure and pulsed electrical fields in combination with natural biomolecules, have received particular attention (13, 16, 32). A major advantage of nonthermal methods of food preservation is that they inactivate microorganisms without the need of severe heating and therefore cause minimal damage to the flavor, color, texture, and nutritional value of the food (18). Mild pressure treatment (300 to 600 MPa) at ambient temperature was widely believed to sufficiently inactivate vegetative bacteria for the purpose of food pasteurization; however, this view has been challenged recently by a number of findings, including the development by mutation of high levels of baroresistance in certain vegetative bacteria (14, 34), the large strain variation in pressure sensitivity (1, 2), and the protection against pressure inactivation provided by some food matrices, such as milk (9). Thus, pressure treatment at ambient temperature may in itself not be a safe pasteurization process under all conditions, and the combination of pressure with other hurdles seems to be necessary to increase safety. High pressure has been reported by several authors to increase the bactericidal spectrum of lysozyme and some bacteriocins against vegetative bacteria, and vice versa, these compounds increased the sensitivity of bacteria to pressure inactivation (9, 13, 16, 17, 21, 25). This type of synergy offers an interesting perspective for the development of mild food preservation techniques for producing safe and high quality products.
Besides the above mentioned bacteriocins and lysozyme, another interesting biopreservative is lactoperoxidase (LP). LP is a native milk enzyme that catalyzes the oxidation of thiocyanate (SCN−) by peroxide into short-lived reactive oxidation products, such as the hypothiocyanite anion (OSCN−), that in turn rapidly oxidize many biomolecules. Most relevant for microbial inactivation is probably the oxidation of enzymes and other proteins in the bacterial cell membrane that have exposed sulfhydryl groups (⩵SH). The first direct effect of LP action on the cell is membrane damage resulting in loss of pH gradient, K+ leakage, and inhibition of transport of solutes, such as amino acids and glucose (6, 20, 26). Native LP is the basis for extension of the keeping-quality of milk by addition of low concentrations of hydrogen peroxide in countries having limited cold storage facilities (19), but more recently, novel applications in preservation of milk and other products have also been investigated (6). Because the mode of action and cellular targets of LP are totally different from those of bacteriocins and lysozyme, and because it has a broad working spectrum, LP may be an interesting additional hurdle to improve the safety of high pressure food preservation. In the present study, we investigated the combined action of the LP system and high pressure treatment on four strains of Escherichia coli and Listeria innocua inoculated in milk.
MATERIALS AND METHODS
Growth of bacteria and preparation of inocula.
Four strains of E. coli and L. innocua were used in this study (Table 1). Cultures were grown at 37°C using Luria-Bertani (27) broth or agar and tryptic soy broth or agar (Oxoid, Basingstoke, United Kingdom) for E. coli and L. innocua, respectively. Permanent stocks were maintained at −80°C as broth cultures containing 25% glycerol. Agar plates were streaked every 7 to 10 days from these stocks and stored at 4°C. Cultures for inactivation experiments were inoculated from single colonies on these agar plates and grown to stationary phase for 21 h with shaking (200 rpm). Cells were harvested by centrifugation (3,000 × g, 5 min) and resuspended in ultrahigh-temperature-treated (UHT) skim milk or in 100 mM potassium phosphate buffer (pH 6.7) at a cell density of approximately 106 CFU/ml for survival experiments not preceded by a pressure treatment and 109 CFU/ml for pressure treatment experiments. In some experiments, survival was monitored during storage at 20°C after pressure treatment. In that case, a sufficient number of replicate bags was prepared and simultaneously treated to allow destructive sampling.
TABLE 1.
Bacterial strains used in this work
| Species | Strain | Source or reference |
|---|---|---|
| E. coli | MG1655 | 12 |
| ATCC 11775 | American Type Culture Collection, Manassas, Va. | |
| ATCC 11303 | American Type Culture Collection, Manassas, Va. | |
| ATCC 43888a | American Type Culture Collection, Manassas, Va. | |
| L. innocua | LMG 11387 | Belgian Coordinated Collection of Microorganisms, Ghent, Belgium |
| LMG 13568 | Belgian Coordinated Collection of Microorganisms, Ghent, Belgium | |
| CIP 78.44 | Institut Pasteur Collection, Paris, France | |
| CIP 79.45 | Institut Pasteur Collection, Paris, France |
Serotype O157:H7. This particular strain does not produce either Shiga-like toxin I or II and does not possess the genes for these toxins.
Application of the LP system.
A stock solution of 10 mg of LP (EC 1.11.1.7; Sigma, Bornem, Belgium) per ml was prepared in a suspension of 50% glycerol and 50% phosphate-buffered saline (0.1 M potassium phosphate buffer [pH 6.0], 150 mM NaCl). Aqueous 25 mM stock solutions of the substrates of the LP system, KSCN (Acros, Geel, Belgium) and H2O2 (Vel, Leuven, Belgium), were sterilized by passage through 0.22-μm-pore-size filters and stored at 4°C. In experiments with the LP system, the enzyme was used at 5 μg/ml together with both substrates at 0.25 mM. Two control experiments were always performed, one without addition of enzyme or substrates and one with addition of 0.25 mM H2O2 only. The latter allowed us to see whether inactivation was caused by the toxic effect of H2O2 alone. The ABTS [2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid] method of Shindler et al. (28) was used to measure LP activity. Assays were conducted in 1 ml of sodium acetate buffer (0.1 M, pH 4.4) with 1 mM ABTS reagent (Sigma) and 0.05 mM H2O2. The increase in absorbance at 412 nm was recorded at 20°C as a measure of enzymatic activity.
High-pressure treatment.
Samples in heat-sealed polyethylene bags were pressurized in a small 8-ml pressure autoclave driven by a manual spindle pump and thermostatically controlled with a water jacket connected to a cryostat (Resato, Roden, The Netherlands). The pressure liquid was a mixture of water and glycol. All pressure treatments were conducted at 20°C. Although temperatures of 45 to 50°C ensure rapid and efficient inactivation of vegetative bacteria under pressure (1, 13), we have focused in our work on a truly nonthermal high pressure process, in which such temperature elevations are to be avoided in order to benefit from a maximal retention of sensory and nutritional food properties. It should be noted, however, that sample temperatures in our experiments may temporarily reach up to 30°C during adiabatic compression (21). Refrigeration, on the other hand, was also avoided because it might have resulted in ice formation during adiabatic decompression.
Determination of bacterial survival and reproducibility of results.
Viability was determined by plating the appropriate decimal dilutions on tryptic soy agar with a spiral plater (Spiral Systems, Cincinnati, Ohio) and incubating at 37°C for 24 to 48 h. For pressure inactivation experiments, viability of pressure-treated samples and untreated controls was determined 2 h after pressure release except when otherwise mentioned. Reduction of viable cells was expressed as the difference between the logarithms of the colony counts of the untreated and treated samples (log N0 − log N). All experiments were repeated three times independently. For the time course experiments, representative results are shown. For the other experiments, in addition, three replicate samples of one E. coli strain and one L. innocua strain were included within one of these three experiments, to allow an estimation of experimental reproducibility. For these strains, error bars representing standard deviations are given in the figures.
RESULTS
Effect of LP system on L. innocua and E. coli in milk.
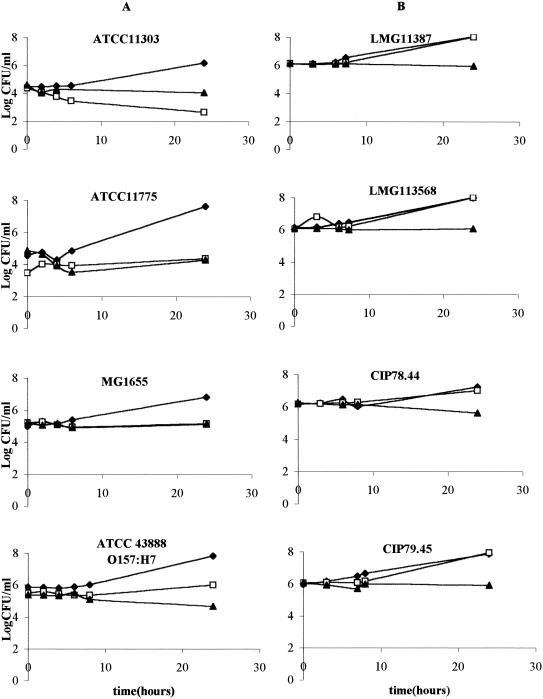
In an introductory experiment, the effect of the LP system was studied on the panel of four E. coli and four L. innocua strains inoculated in ultrahigh-temperature-treated skim milk during storage at 20°C (Fig. 1). During the first 6 h of storage, cell numbers remained constant in the samples with the active LP system and also in the control samples with H2O2 alone or without additives. Upon further incubation, growth resumed in untreated milk for all the strains of both species, resulting in a population increase of 1 to 2 log units. The LP system did not cause inactivation even after 24 h of exposure but effectively inhibited growth of all the strains during this period. Under the conditions of this experiment, no difference with respect to LP system sensitivity was seen between E. coli and L. innocua. However, a remarkable difference occurred in sensitivity to H2O2 alone, without LP enzyme, with a bacteriostatic effect on three E. coli strains and even a bactericidal effect on one E. coli strain (ATCC 11303), but no inhibitory effect on any of the L. innocua strains.
FIG. 1.
Evolution of viable counts of E. coli (strains ATCC 11303, ATCC 11775, MG1655, and ATCC 43888) (A) and of L. innocua (strains LMG 11387, LMG 13568, CIP 78.44, and CIP 79.45) (B) inoculated in skim milk held at 20°C for 24 h without any additives (⧫), supplemented with H2O2 (□), or supplemented with the full LP system (▴).
Effect of combined treatment with LP system and high pressure on L. innocua and E. coli in milk.
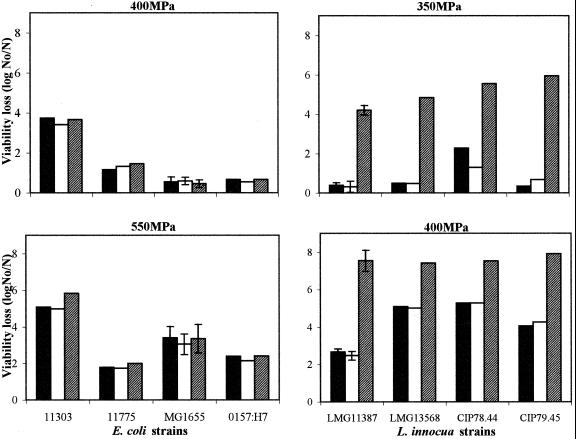
The actual purpose of this work was to investigate whether the combined use of high pressure and the LP system would result in a synergetic bactericidal effect that could lead to useful applications. Therefore, we subjected all the strains suspended in milk to pressures of 200 to 600 MPa for 15 min at 20°C. In a control experiment without bacteria, the LP enzyme was found to retain 100% of its activity when treated in milk for 15 min at 600 MPa and 20°C (data not shown). Therefore, in the experiments involving the combined treatment, the LP system was added before pressure treatment. A general observation from the results is that in the absence of the LP system or H2O2, the E. coli strains generally showed a higher barotolerance than the L. innocua strains. At 400 MPa, a 10-fold or lower viability loss was achieved for the E. coli strains except for the most sensitive one (ATCC 11303), whereas all four L. innocua strains were reduced from 100-fold to more than 105-fold (Fig. 2). The most pressure-sensitive E. coli strain was slightly more sensitive than the most pressure-resistant strain of L. innocua (LMG 11387).
FIG. 2.
Inactivation of E. coli and L. innocua strains inoculated in skim milk by high pressure (black), by high pressure and H2O2 (white), or by high pressure and the full LP system (grey). Data with error bars are means ± standard deviations.
For E. coli, neither the presence of peroxide nor the LP system increased inactivation by pressure (Fig. 2). For only strain ATCC 11303, a slightly increased inactivation of 1 log unit seemed to occur in the presence of the LP system at 550 MPa, but this is probably below the significance level. Increasing the pressure up to 600 MPa or increasing the concentrations of the components of the LP system up to 20 μg of LP/ml, 0.5 mM KSCN, and 0.5 mM H2O2 did not render E. coli sensitive to the system (data not shown).
The combined effect of the LP system and pressure on the destruction of L. innocua is also shown in Fig. 2. On these bacteria, as opposed to E. coli, a strong synergistic effect was observed when both treatments were combined. Complete inactivation (≥7 log units) was achieved at mild pressure (400 MPa) in the presence of the activated LP system, whereas in unamended and peroxide-treated milk at the same pressure, the reduction was only 2 to 5 log units, depending on the strain. The results clearly show that all tested L. innocua strains are highly sensitized to the LP system by pressure treatment and that E. coli and L. innocua differ in sensitivity to the combined effect of high pressure and the LP system.
Effect of LP system under pressure on L. innocua and E. coli in phosphate buffer versus milk.
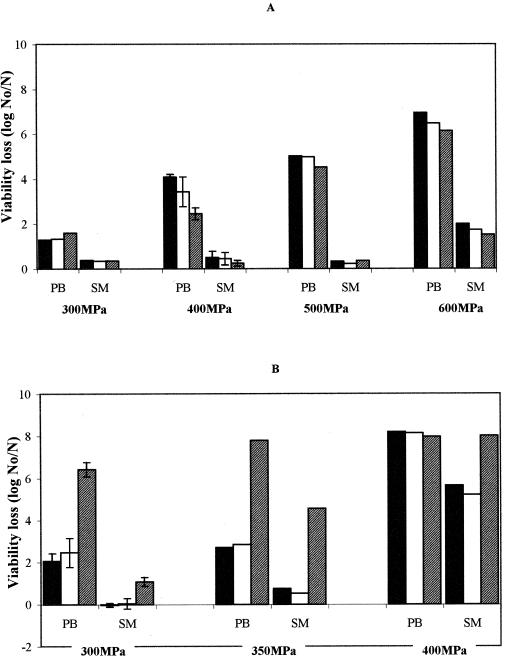
As mentioned in the introduction, the efficacy of antimicrobials is often reduced in complex media, such as milk, compared to water or buffer systems. This is also the case for the efficacy of certain antimicrobials in synergistic combinations (9, 35). Therefore, we further investigated whether the complexity of milk as a matrix would interfere with the bactericidal effect of the LP system under pressure, in particular for E. coli, in our experiments. We subjected cell suspensions of E. coli MG1655 and L. innocua LMG 11387 to different pressures at 20°C for 15 min in milk and in 100 mM phosphate buffer adjusted to the same pH as milk (Fig. 3). The results show that also in buffer, E. coli could not be sensitized to the LP system at pressures in the range of 300 to 600 MPa. For L. innocua, the sensitization for the LP system under high pressure was even stronger in phosphate buffer than in milk. Finally, milk also clearly reduced the effectiveness of high pressure treatment alone, and this was the case for both bacteria. For instance, at 600 MPa, E. coli was reduced 7 log units in buffer, but only 2 log units in milk.
FIG. 3.
Comparison of inactivation of E. coli MG1655 (A) and L. innocua LMG 11387 (B) in skim milk (SM) versus 100 mM phosphate buffer (pH 6.7) (PB) by high pressure (black), by high pressure and H2O2 (white), or by high pressure and the full LP system (grey). Data with error bars are means ± standard deviations.
Time course of L. innocua and E. coli viable counts after combined treatment with pressure and the LP system in milk.
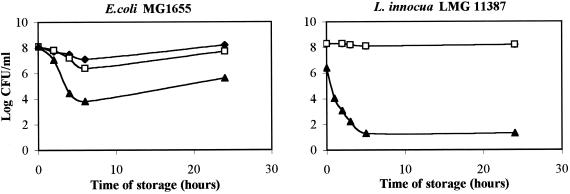
In a final experiment, we examined for E. coli MG1655 and L. innocua LMG 11387 the time course of inactivation by the LP system after pressure treatment. In the previous experiments, we always determined the effect of high pressure combined with the LP system at one point in time, namely 2 h after pressure release. Here, we followed in detail the inactivation from immediately after pressure release up to 24 h of storage at room temperature. Pressure treatments (20°C, 15 min) were in milk at 600 and 350 MPa for E. coli and L. innocua, respectively, either without additives or supplemented with H2O2 or with the complete LP system. Interestingly, the results revealed sensitization of E. coli resulting in a viable count reduction of 4 log units 6 h after pressure treatment (Fig. 4). The decline did not occur immediately but only 4 to 6 h after pressure treatment, and it was partly reversed upon further incubation up to 24 h. When pressures lower than 600 MPa were used, no inactivation was observed in the subsequent hours (data not shown). The finding that bacterial counts increased again after 24 h of storage is surprising in view of the efficient growth inhibition by the LP system in unpressurized inoculated milk seen in the first experiment (Fig. 1). To make the conditions of the current experiment more comparable to those of the first experiment, we subjected milk samples inoculated with E. coli MG1655 without any additive to pressure of up to 600 MPa and added the LP system immediately after pressure treatment. Again, a similar pattern of decline in viable counts was observed, and again the effect was transient, showing increased counts upon longer storage (data not shown). L. innocua, on the other hand, was sensitized to the LP system much more rapidly (Fig. 4). In this organism, sensitization was already apparent immediately after pressure treatment. Sensitization was also much more extensive than in E. coli, in spite of the much lower pressure applied, yielding complete inactivation 4 to 5 h after pressure treatment. In addition, no recovery of viable counts was observed at up to 24 h of incubation.
FIG. 4.
Evolution of viable counts during storage at 20°C after pressure treatment (15 min, 20°C) of E. coli MG1655 (600 MPa) and L. innocua LMG 11387 (350 MPa) in milk without any additives (⧫), supplemented with H2O2 (□), or supplemented with the full LP system (▴).
DISCUSSION
In this work, we have explored the potential of a combined treatment with high hydrostatic pressure and the LP system as a mild preservation technique for producing microbiologically safe foods. There has been recently a growing awareness of large variations in pressure sensitivity between strains of the same bacterial species and hence a growing consensus that evaluations of bacterial high pressure sensitivity should be based on multiple-strain studies. For instance, upon pressurization for 5 min at 25°C at 345 MPa, Alpas et al. (1) observed inactivation levels of between 2.8 and 5.6 log units among six E. coli O157:H7 strains, between 0.7 and 7.8 log units among seven Staphylococcus aureus strains, and between 0.9 and 3.5 log units among nine Listeria monocytogenes strains. Benito et al. (2) found up to 5 log units of difference in inactivation between six E. coli strains treated at 500 MPa and 25°C. Large strain variations in pressure resistance for L. monocytogenes have also been reported by other authors (29, 31). In our own work, we observed up to 3 log units of difference in inactivation between the most sensitive and the most resistant strains of both E. coli and L. innocua (Fig. 2). In addition, we have also observed that the sensitization of E. coli to lysozyme and nisin under high pressure, which we reported earlier (13), is subject to strain differences (21). Therefore, to allow interstrain comparisons, we limited our study to two bacterial species, but included four strains of each. L. innocua was chosen as a nonpathogenic model organism for L. monocytogenes, which is an important foodborne pathogen in refrigerated pasteurized foods. The suitability of L. innocua as a substitute for L. monocytogenes in our work is supported by the fact that both species appear to be equally susceptible to the LP system (3), as well as to high pressure when we compare our results with those of Patterson et al. (24) and Simpson and Gilmour (29). E. coli was chosen because it includes foodborne pathogenic strains, such as those of serotype O157:H7, which have a low infective dose and therefore must be efficiently inactivated.
Exposure to the LP system without pressure treatment clearly revealed a bacteriostatic effect for at least 24 h on E. coli and L. innocua in skim milk at room temperature. The effect was not strain dependent since similar results were obtained for all four strains tested of each species. None of the strains was inactivated to significant degree. On the other hand, E. coli and L. innocua showed a clearly different response towards hydrogen peroxide alone (0.25 mM). L. innocua growth was not at all affected after 24 h, while growth of all E. coli strains was inhibited, and for one strain there was even some inactivation. These results suggest that there is a fundamental difference in hydrogen peroxide sensitivity between the species. The basis of this difference is not obvious, since both bacterial species can produce a hydrogen peroxide-degrading catalase enzyme. Numerous studies have been performed on the action of the LP system on a wide range of gram-negative and gram-positive bacteria, including E. coli and L. monocytogenes or L. innocua, sometimes with opposing conclusions with regard to its bacteriostatic or bactericidal action. However, the experimental conditions in these studies were usually different, and it is known that the effect of the enzyme may depend on several factors, such as temperature, substrate, bacterial strain, and inoculum size (3, 4, 7, 15, 30). The general conclusion from most studies, however, is that the LP system can be used to delay spoilage of milk owing to its antibacterial effect, and our results support this conclusion.
On the other hand, since we failed to show a bactericidal effect on any of the tested strains, including one strain of the low-infective-dose pathogen E. coli O157:H7, the LP system alone cannot be used to process raw milk for safety. In an attempt to sensitize bacteria to the LP system and thus to potentiate the LP system as a natural preservative system contributing to product safety, we studied the combined application of moderately high pressure with the LP system. Experiments with the four E. coli and four L. innocua strains inoculated in milk revealed a consistently different response of both species towards this combined treatment when survivors were analyzed 2 h after the treatment. All L. innocua strains were strongly sensitized to the LP system, even at a pressure that in the absence of LP caused only very low levels of inactivation, ranging from less than 1 to 2 decades depending on the strain (Fig. 2). In contrast, none of the E. coli strains was sensitized to the LP system, not even at a pressure causing 2 to 5 decades of inactivation in the absence of the LP system (Fig. 2). When the same experiments were conducted in phosphate buffer instead of milk to exclude the protective effect of complex food components, higher levels of inactivation were achieved for all treatments, as expected. For L. innocua, the contribution of the LP system to the total inactivation was higher in phosphate buffer than in milk, and this can be explained either by a reduced efficiency of the LP system in milk or by a reduced sensitization of the bacteria by pressure in milk. In contrast, even in phosphate buffer, no sensitization for the LP system was observed for E. coli (Fig. 3). If L. innocua is a good model for L. monocytogenes, this result is remarkable, because a comparison of literature data suggests E. coli to be intrinsically more sensitive to the LP system in milk than L. monocytogenes (3, 7, 10, 15, 30). It can therefore be speculated that high pressure inactivates one or more components required for protection against the LP system in L. innocua, but does not inactivate such component(s) in E. coli, because high pressure has different targets in both bacteria, or the LP system.
More detailed time course experiments revealed a transient decline in viable E. coli counts during storage at 20°C of samples that had been treated at 600 MPa in the presence of the LP system. In the previous experiments, this decline went unnoticed because it occurs only more than 2 h after pressure treatment, and not with pressures lower than 600 MPa. In addition, the effect was transient and bacterial numbers started to increase again after a few hours, in spite of the presence of growth-inhibiting concentrations of the LP system. The transient decline is therefore probably due to a form of transient sublethal injury that is subsequently repaired, but that prevents the cells to develop colonies even on a beneficial growth medium like tryptic soy agar. With L. innocua, the presence of the LP system caused the viable count to further decrease immediately after pressure treatment, and this effect occurred even when using moderately high pressures, and the decrease was irreversible within 24 h. In conclusion, high pressure and the LP system exhibit a strongly synergetic interaction enhancing the inactivation of L. innocua, but not E. coli. The addition of an LP system therefore will not allow the use of lower pressures for the pasteurization of milk by high hydrostatic pressure, in spite of the efficient inactivation of L. innocua that can be achieved.
ACKNOWLEDGMENTS
This work was supported by a fellowship from the European Union to C.G.-G. (FAIR-CT96-5065) and by grants from the Research Fund of the KULeuven (OT/97/31 and VIS/98/009) and the Fund for Scientific Research Flanders (F.W.O. G.0395.98).
REFERENCES
- 1.Alpas H, Kalchayanand N, Bozoglu F, Sikes A, Dunne C P, Ray B. Variation in resistance to hydrostatic pressure among strains of food-borne pathogens. Appl Environ Microbiol. 1999;65:4248–4251. doi: 10.1128/aem.65.9.4248-4251.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benito A, Ventoura G, Casadei M, Robinson T, Mackey B. Variation in resistance of natural isolates of Escherichia coli O157 to high hydrostatic pressure, mild heat, and other stresses. Appl Environ Microbiol. 1999;65:1564–1569. doi: 10.1128/aem.65.4.1564-1569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibi W, Bachmann M R. Antibacterial effect of the lactoperoxidase-thiocyanate-hydrogen peroxide system on the growth of Listeria spp. in skim milk. Milchwissenschaft. 1990;45:26–28. [Google Scholar]
- 4.Björck L, Rosén C-G, Marshall V, Reiter B. Antibacterial activity of the lactoperoxidase system in milk against Pseudomonas and other gram-negative bacteria. Appl Microbiol. 1975;30:199–204. doi: 10.1128/am.30.2.199-204.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delves-Broughton J. Nisin and its uses as a food preservative. Food Technol. 1990;44:100–112. [Google Scholar]
- 6.Ekstrand B. Lactoperoxidase and lactoferrin. In: Dillon V M, Board R G, editors. Natural antimicrobial systems and food preservation. Oxon, United Kingdom: CAB International; 1994. pp. 15–23. [Google Scholar]
- 7.Farrag S A, El-Gazzar F E, Marth E H. Use of the lactoperoxidase system to inactivate Escherichia coli O157:H7 in a semi-synthetic medium and in raw milk. Milchwissenschaft. 1992;47:15–17. [Google Scholar]
- 8.Fuglsang C C, Johansen C, Christgau S, Adlernissen J. Antimicrobial enzymes: applications and future potential in the food industry. Trends Food Sci Technol. 1995;6:390–396. [Google Scholar]
- 9.García-Graells C, Masschalck B, Michiels C W. Inactivation of Escherichia coli in milk by high-hydrostatic-pressure treatment in combination with antimicrobial peptides. J Food Prot. 1999;62:1248–1254. doi: 10.4315/0362-028x-62.11.1248. [DOI] [PubMed] [Google Scholar]
- 10.Gaya P, Medina M, Nuñez M. Effect of the Lactoperoxidase system on Listeria monocytogenes behavior in raw milk at refrigeration temperature. Appl Environ Microbiol. 1991;57:3355–3360. doi: 10.1128/aem.57.11.3355-3360.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gould G W. Industry perspectives on the use of natural antimicrobials and inhibitors for food applications. J Food Prot. 1996;1996(Suppl.):82–86. doi: 10.4315/0362-028X-59.13.82. [DOI] [PubMed] [Google Scholar]
- 12.Guyer M S, Reed R R, Steitz J A, Low K B. Identification of a sex-factor-affinity site in Escherichia coli as γς. Cold Spring Harbor Symp Quant Biol. 1981;45:135–140. doi: 10.1101/sqb.1981.045.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Hauben K J A, Wuytack E Y, Soontjens C C F, Michiels C W. High-pressure transient sensitisation of Escherichia coli to lysozyme and nisin by disruption of outer-membrane permeability. J Food Prot. 1996;59:350–355. doi: 10.4315/0362-028X-59.4.350. [DOI] [PubMed] [Google Scholar]
- 14.Hauben K J A, Barlett D H, Soontjes C C F, Cornelis K, Wuytack E Y, Michiels C W. Escherichia coli mutants resistant to inactivation by high hydrostatic pressure. Appl Environ Microbiol. 1997;63:945–950. doi: 10.1128/aem.63.3.945-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heuvelink A E, Bleumink B, van den Biggelaar F L A M, Te Giffel M C, Beumer R R, De Boer E. Occurrence and survival of verocytotoxin-producing Escherichia coli O157 in raw cow's milk in the Netherlands. J Food Prot. 1998;61:1597–1601. doi: 10.4315/0362-028x-61.12.1597. [DOI] [PubMed] [Google Scholar]
- 16.Kalchayanand N, Sikes T, Dunne C P, Ray B. Hydrostatic pressure and electroporation have increased bactericidal efficiency in combination with bacteriocins. Appl Environ Microbiol. 1994;60:4174–4177. doi: 10.1128/aem.60.11.4174-4177.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalchayanand N, Sikes A, Dunne C P, Ray B. Interaction of hydrostatic pressure, time and temperature of pressurization and pediocin AcH on inactivation of foodborne bacteria. J Food Prot. 1998;61:425–431. doi: 10.4315/0362-028x-61.4.425. [DOI] [PubMed] [Google Scholar]
- 18.Knorr D, Mertens B. Development of nonthermal processes for food preservation. Food Technol. 1992;46:123–134. [Google Scholar]
- 19.Korhonen H. A new method for preserving raw milk, the lactoperoxidase antibacterial system. World Anim Rev. 1980;35:23–29. [Google Scholar]
- 20.Marshall V M E, Reiter B. Comparison of the antibacterial activity of the hypothiocyanite anion towards Streptococcus lactis and Escherichia coli. J Gen Microbiol. 1980;120:513–516. doi: 10.1099/00221287-120-2-513. [DOI] [PubMed] [Google Scholar]
- 21.Masschalck B, García-Graells C, Van Haver E, Michiels C W. Inactivation of high pressure resistant Escherichia coli by lysozyme and nisin under high pressure. Innovat Food Sci Emerg Technol. 2000;1:39–48. [Google Scholar]
- 22.Mazzotta A S, Montville T J. Characterization of fatty acid composition, spore germination, and thermal resistance in a nisin-resistant mutant of Clostridium botulinum 169B and in the wild-type strain. Appl Environ Microbiol. 1999;65:659–664. doi: 10.1128/aem.65.2.659-664.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendoza F, Maqueda M, Galvez A, Martinez-Bueno M, Valdivia E. Antilisterial activity of peptide AS-48 and study of changes induced in the cell envelope properties of an AS-48-adapted strain of Listeria monocytogenes. Appl Environ Microbiol. 1999;65:618–625. doi: 10.1128/aem.65.2.618-625.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patterson M F, Quinn M, Simpson R, Gilmour A. Sensitivity of vegetative pathogens to high hydrostatic pressure treatment in phosphate-buffered saline and foods. J Food Prot. 1995;58:524–529. doi: 10.4315/0362-028X-58.5.524. [DOI] [PubMed] [Google Scholar]
- 25.Ponce E, Pla R, Sendra E, Guamis B, Mor-Mur M. Combined effect of nisin and high hydrostatic pressure on destruction of Listeria innocua and Escherichia coli in liquid whole egg. Int J Microbiol. 1998;43:15–19. doi: 10.1016/s0168-1605(98)00088-9. [DOI] [PubMed] [Google Scholar]
- 26.Reiter B. The lactoperoxidase-thiocyanate-hydrogen peroxide antibacterium system. Ciba Found Symp. 1978;65:285–294. doi: 10.1002/9780470715413.ch16. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 28.Shindler J S, Childs R E, Barley W G. Peroxidase from human cervical mucus. Isolation and characterisation. Eur J Biochem. 1976;65:325–331. doi: 10.1111/j.1432-1033.1976.tb10345.x. [DOI] [PubMed] [Google Scholar]
- 29.Simpson R K, Gilmour G. The effect of high hydrostatic pressure on Listeria monocytogenes in phosphate-buffered saline and model food systems. J Appl Microbiol. 1997;83:181–188. doi: 10.1046/j.1365-2672.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- 30.Siragusa G R, Johnson M G. Inhibition of Listeria monocytogenes growth by the Lactoperoxidase-thiocyanate-H2O2 antimicrobial system. Appl Environ Microbiol. 1989;55:2802–2805. doi: 10.1128/aem.55.11.2802-2805.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Styles M F, Hoover D G, Farkas D F. Response of Listeria monocytogenes and Vibrio parahaemolyticus to high hydrostatic pressure. J Food Sci. 1991;56:1404–1407. [Google Scholar]
- 32.ter Steeg P F, Hellemons J C, Kok A E. Synergistic action of nisin, sublethal ultrahigh pressure, and reduced temperature on bacteria and yeast. Appl Environ Microbiol. 1999;65:4148–4154. doi: 10.1128/aem.65.9.4148-4154.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tranter H S. Lysozyme, ovotransferrin and avidin. In: Dillon V M, Board R G, editors. Natural antimicrobial systems and food preservation. Oxon, United Kingdom: CAB International; 1994. pp. 65–97. [Google Scholar]
- 34.Verroens P, Hauben K, Michiels C. Acquired resistance of microorganisms to inactivation by high hydrostatic pressure. In: Isaacs N, editor. High pressure food science, bioscience and chemistry. Cambridge, United Kingdom: Royal Society of Chemistry; 1998. pp. 370–375. [Google Scholar]
- 35.Yuste J, Mor-Mur M, Capellas M, Guamis B, Pla R. Microbial quality of mechanically recovered poultry meat treated with high hydrostatic pressure and nisin. Food Microbiol. 1998;15:407–414. [Google Scholar]
- 36.Zapico P, Medina M, Gaya P, Nuñez M. Synergistic effect of nisin and the lactoperoxidase system on Listeria monocytogenes in skim milk. Int J Food Microbiol. 1998;40:35–42. doi: 10.1016/s0168-1605(98)00008-7. [DOI] [PubMed] [Google Scholar]