Abstract
Nanocomposite materials have acquired great importance, as have similar composite materials on a macroscopic scale, because the reinforcement complements the defects in the properties of the matrix, thus obtaining materials with better mechanical, thermal, and electrical properties, among others. At the same time, the importance and research of polymeric nanocomposites reinforced with nanoparticles of various types have grown. Among those that have stood out the most in the electronics industry are polymeric matrices reinforced with nanoparticles that present dual behavior, that is, both magnetic and semiconductor. This property has been very well used in developing electronic devices such as televisions, computers, and smartphones, which are part of everyday life. In this sense, this review presents a compilation of the synthetic methods to produce polymer nanocomposites with dual magnetic and semiconductor behavior and their potential applications within electronic fields and new relevant trends.
Keywords: polymer nanocomposite, reinforcement, magnetic, semiconductor
1. Introduction
There is no doubt that magnetism is historically linked to the development of humanity. Since ancient times, magnetic materials and the phenomena related to the impulses to materialize novel ideas in various fields of application have played a role of great importance in the development of science and technology [1]. Nowadays, magnetic materials are involved in all aspects of daily human activities because they are used in a large list of optoelectronic-magnetic applications, for instance, data storing, data reading, electromagnets, transformers, motors, generators, actuators, microphones, relays, inductors, microwave isolators, antenna rods, and phase shifters [2]. Additionally, magnetic materials are used for magnetic resonance imaging, sensors, radiation sources, bioremediation, that is, for mineral separation and metal separation [3], biomedicine [4], and even in energies field [5].
In the last years, scientists have shown a strong interest in nanostructured materials because they show unusual and improved chemical and physical properties compared with their bulk counterparts [6]. This unconventional behavior shown by nanomaterial appears when they reach the quantum size regime; their size is comparable with the de Broglie wavelengths of exciton [7]. However, the factor responsible for the unique properties of nanomaterials such as nanoparticles, nanowires, and quantum dots, is the increasing predominance of the proportion of atoms present on the material surface with respect to the number of atoms belonging to the interior of the bulk as the size of the systems decreases [8,9,10]. Particularly, in the case of magnetic nanoparticles (MNPs), there is a significant change in the properties such as Curie (TC) or Neel (TN) temperatures and hysteresis behavior because, on the nanoscale, each atom will suffer higher magnetization than in bulk and anisotropy of magnetization caused by the smallness of domain which, in ferromagnetic nanoparticles, will produce superparamagnetic properties [7,11].
The tunable properties of magnetic nanoparticles allow their use in several applications, for instance, biomedicine [12], environmental remediation [13], catalysis and imaging [14,15], and others. However, MNPs tend to lose their colloidal stability and generate agglomerates to reduce their surface energy. To assure the colloidal stability of MNPs, some approaches have been proved, including mechanical dispersion, an increase of viscosity of the medium, and modifying the surface with macromolecules such as polymers [16]. In that context, imbibing MNPs as reinforcement phase into a polymeric matrix to generate a stable nanocomposite with fashionable properties and with a promising practical application have been highly explored [17].
Conducting polymer and hydrogel-based matrices have special importance because these types of organic macromolecules can be easily synthesized, are highly tunable and versatile materials [18], and provide nanocomposites with a semiconductor behavior that allows them to be used for optoelectronics [19]. In that way, joining MNPs into a polymer matrix allows obtaining a nanocomposite that is sensitive to exposure to magnetic fields with improved conducting and optical properties that widely expand that potential application in fields of science and industry. Therefore, this document pretends to be a comprehensive review of polymer–magnetic semiconductor nanocomposites focused mainly on the new synthetic methods and current trends in reported industrial applications.
2. Fundamentals
2.1. Composites
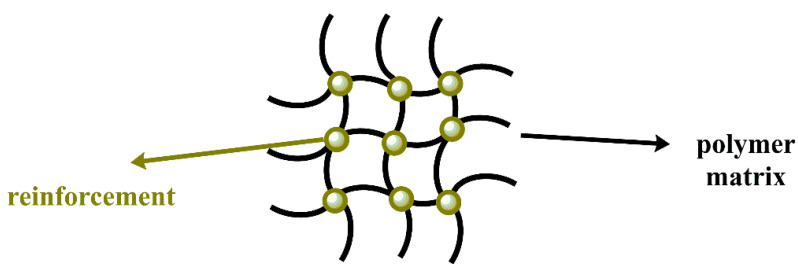
Composite materials emerged in the middle of the 20th century as a promising class of engineering materials providing new prospects for modern technology [20]. Composite materials combine at least two materials, often with different properties, to get unique characteristics in which the strength-to-weight ratio is high and there is significant flexibility in the design [21]. These compounds can be selected to achieve unusual stiffness, strength, weight, high-temperature performance, corrosion resistance, toughness, or conductivity. In all composite materials, two components are distinguished: the matrix, which is the component that appears as a continuous phase and acts as a binder, and the reinforcement or filler, which is the discontinuous phase. Generally, it is said that material is composite when it meets the requirements of being made up of two or more physically different and mechanically separable components [22]. It has several chemically different phases, insoluble among themselves and separated by an interface. By carefully choosing the matrix, the reinforcement, and the manufacturing process that brings them together, the engineers can tailor the properties to meet specific requirements [23]. The modifications inside the new nanocomposite are explained by an increase in the surface/volume ratio, which increases the contact area between the reinforcement and the matrix.
2.1.1. Polymer Matrix
Any material can serve as a matrix material for composite; however, the most common materials employed as a matrix are ceramics, metals, and polymers. Most matrix materials on the composites market are polymers [24]. Polymer matrices (PMs) have been widely used as a matrix for composites production because they have great properties, including excellent toughness and adhesion [25]. The polymer matrix is the continuous phase in the composites used to hold the reinforcing agent in its place. Additionally, its properties determine most of the degradative processes (delamination, impact damage, chemical resistance, water absorption, and high-temperature creep) [26].
Hydrogels
One of the most common hydrophilic PMs is those known as hydrogels. A hydrogel is a tridimensional polymeric structure with swelling and collapse properties, flexibility, biodegradability, biocompatibility, and softness [27,28,29]. Some hydrogels can respond to external stimuli such as pH, temperature, electricity, light, magnetism, and biological molecules such as enzymes during the swelling and shrinking [30]. In electronics, conductive hydrogels are good prospects for use in this field due to their remarkable properties, such as good electronic properties, mechanical flexibility, ease of processing, and the previously mentioned biological characteristics, which can be used to satisfy specific requirements and demands [31,32]. The main disadvantage of these polymeric materials is their relatively low (compared to metals) temperature resistance limited by the matrix, which can be enhanced with reinforcement [20].
Conducting and Semiconducting Polymers
Scientific and technological development has given rise to two types of electronic conductive materials with a polymeric matrix: extrinsic and intrinsic conductors. Extrinsic conductors have a polymer matrix with composite materials made up of a polymer, generally thermoplastic, and a filler. The extrinsically conducting polymers are insulators that enable their electrical conduction by introducing a conducting mediator inside their polymeric chains [33].
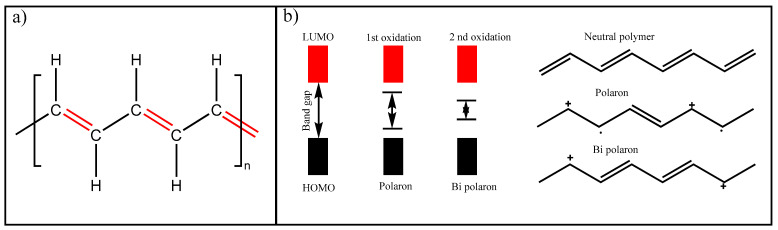
Intrinsic conducting polymers are also called conjugated polymers since a generalized characteristic is the successive alternation of a single bond with double bonds (Figure 1a) along the main chains. In that context, the inherent electrical conductivity can be explained by the facility of π electron to be transferred to vicinal carbon allowing the electron flow [33,34]. However, this mechanism produces a low conductivity (between 10−10 to 101 S/cm): thus, it is required to generate a charge carrier by oxidation (p-doping) or reducing (n-doping) the polymer using chemical or electrochemical methods, which in most of the cases are reversible processes [35,36]. Doping improves the conducting character of polymers considerably in that way is possible to generate a semiconductor with relatively high conductivity (1 to 104 S/cm) because this modification produces a substantial change in the band-gap value [37]. The band-gap theory approach will be used to understand the doping mechanism. As Figure 1b shows, an electron is extracted from the HOMO (p-type doping) of the valence band in the oxidation. Additionally, a polaron (cation-radical specie) is created when two electronic levels inside the band gap are generated, reducing its value. A bipolaron (bi-cationic specie) is formed in second oxidation, and the band gap is reduced even more [38]. These mentioned charge carriers allow a strong delocalization of electrons that can now flow freely along with polymer conjugation without much resistance to the band gap. In some cases, the conductivity observed in intrinsically CP is close to metallic materials [39]. Regarding reduction (p-type doping), it is not ccommon because the intrinsically conducting polymers are electron-rich components, which means that they will not allow accepting a negative charge.
Figure 1.
(a) Graphical representation of intrinsically conducting polymers and (b) p-doping process.
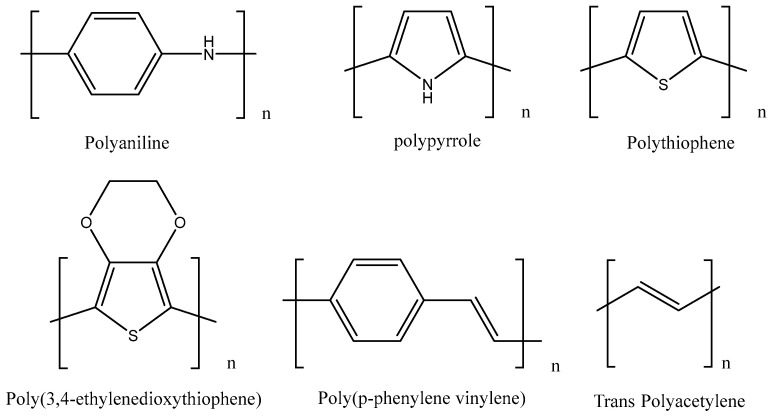
In this sense, semiconducting polymers, that is, polymers with a broadband gap and lesser degree of conjugation, have acquired great relevance in electronics due to their rich optical and electrical properties, they are used as organic photovoltaics [40], organic light-emitting diodes, stretchable electronic devices [41], supercapacitors [42], fuel cells [43], and electrochemical sensors [18]. Figure 2 shows some examples of intrinsically conducting materials that will be observed as a part of nanocomposites in the future in this document.
Figure 2.
Structure of the most used intrinsically conductive polymers.
2.1.2. Reinforcement
The reinforcement material is added to the matrix material to enhance the physical properties of the final composite material [44]. In other words, the reinforcing phase provides strength and stiffness because the reinforcement materials are more rigid, stronger, and stiffer than the matrix [45]. These fillers or reinforcement materials might be classified according to their chemical nature and physical structure, but usually, they are classified based on the shape of the particles [46]. Reinforcement is typically added to polymeric matrices to improve their chemical and physical properties. Of these properties, the optimization of the mechanical characteristics has been the most desired objective, leading to the development of reinforcements that range from inorganic fibers (glass and carbon fibers) to organic (aramid) and inorganic particles (SiO2, Al2O3, CaCO3), carbon black, and silicate sheets [47]. Despite having materials with exceptional properties, the practical applications are reduced by factors that greatly increase their costs, such as the difficulty of manufacture or the incompatibility between materials.
Nanoparticles as Reinforcement
Nanoparticles (NPs) are ultrafine particles that have sizes between 1 and 100 nm [48]. These NPs can serve as a filler in the polymeric matrix. The NPs exhibit higher specific surface area, surface energy, and density than microparticles; hence lower nanofiller concentrations are needed to attain properties comparable to or even better than those obtained by conventional micro filler loadings, which facilitate processing and minimize the increase in composite weight [49]. After adding NPs to the PM, a nanocomposite is obtained, which will present a specific structure with physical properties that depend on the content and type of nanometric charge incorporated [50]. NPs display many atoms that make them up and belong to their surface. Therefore, these atoms can easily interact with the matrix where they are embedded [51]. A significant reason for using NPs is to reduce the cost of the composite. They are also readily available in the required quantities, sizes, and shapes [52].
2.2. Magnetic Nanoparticles
Magnetic nanoparticles (MNPs) show excellent features such as small size, high surface area, an active surface that can successfully be modified, low toxicity, and superparamagnetism (magnetic behavior with characteristics of ferromagnetism and paramagnetism) [53]. Currently, magnetic nanostructures exhibit a great variety of applications ranging from electronics, mechanics, and optics to reaching more complex areas such as biomedicine, where superparamagnetic nanoparticles are used for the diagnosis [54] and treatment of diseases by the transport and release of drugs [55] and immobilization and magnetic separation of biological entities or treatment of tumors using hyperthermia therapies [56]. However, the functionalization of the MNPs with organic and inorganic materials is indispensable for their applications [57].
The size reduction brings about a change in the magnetic behavior of the material since it goes from being ferro- or ferrimagnetic to superparamagnetic, where, due to the effect of thermal energy, the magnetic moment of each particle fluctuates in the direction being the net magnetic moment equal to zero [58]. The unique physicochemical properties of MNPs, especially their large surface areas, ease of synthesis and modification, and inherent superparamagnetic properties, could lead to improved technologies. Moreover, these MNPs present an excellent capability to achieve a synergic union with other compounds, such as polymers [57].
2.2.1. Types of Magnetic Semiconductor Nanoparticles
Metal and Metal Oxide Nanoparticles
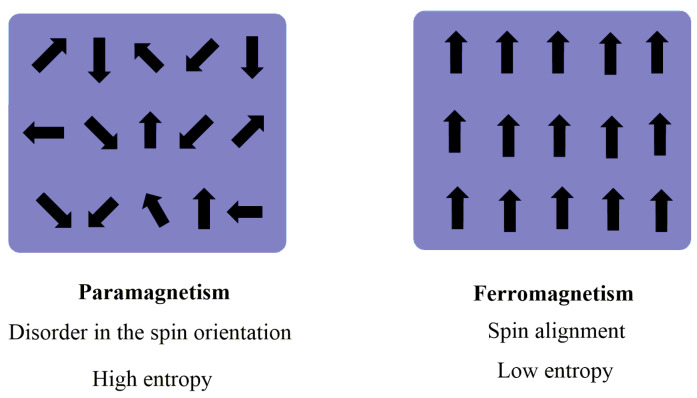
Metal oxides have always aroused technological and scientific interest due to their various properties (optical, magnetic, electrical, and some others) and their general characteristics of hardness, thermal stability, and chemical resistance [17]. Metallic elements can form many oxides capable of adopting many structural geometries and with electronic properties that can exhibit conductive, semiconductor, and even insulating characteristics. Ferromagnetic oxides are the preferred materials for information storage and transmission. Transition metal oxides such as Fe, Ni, and Co have a peculiar magnetic behavior since they are ferromagnetic at low and ambient temperatures and show paramagnetism at high temperatures, as is shown in Figure 3 [59,60]. Some oxides with variable electron mobility are used as semiconductors (V2O5) or superconductors (YBa2Cu3O7). Ferroelectric and dielectric oxides with perovskite structure (BaTiO3, PbZrTiO3) are extensively used in electronic devices [61,62,63,64].
Figure 3.
Graphic illustration of paramagnetic and ferromagnetic behaviors.
At the macroscopic level, metal oxides constitute robust and stable systems with well-defined crystallographic structures. In nanomaterials, however, changes in thermodynamic stability can be observed, including modifications in cell parameters and in the crystal structure of these materials. As a result, phases with very low macroscopic stability can be formed into highly stable nanostructures (TiO2, VOx, Al2O3, MoOx) [65]. Non-stoichiometric structure phenomena (widespread in manganese oxides) and the formation of non-existent phases at the macroscopic level can also be observed.
Metal oxides at the nanoscale exhibit unique chemical and physical properties due to their limited size and the high density of active sites on their surface. The surface atoms, with a coordination number lower than those located inside the mass, together with the existence of oxygen vacancies on the surface of the oxide, give rise to structural arrangements that generally improve the chemical activity of the systems involved. The decrease in size strongly influences the conductivity and chemical reactivity properties [66]. The strongly modified surface properties give rise to solids with unprecedented acid/base and adsorption characteristics. Furthermore, the oxides of the metals, as mentioned earlier, are used in biomedical and industrial applications due to their magnetic and electronic versatility [66,67,68].
Ferrite
Ferromagnetic oxides, commonly called ferrites, are ceramic materials composed mainly of iron oxide combined with other metallic elements. They are ferromagnetic materials; they can be magnetized or attracted by magnets [69].
The magnetic properties of ferrites derive from their crystalline structure, where the metallic atoms occupy well-defined positions about the oxygen atoms. Soft ferrites generally have a cubic structure of the generic spinel-type MFe2O4, where M represents one or more of the transition metals Mn, Fe, Co, Ni, Cu, or Zn. Several scientists and researchers have reported some properties and applications of these particles with ferromagnetic properties [70,71].
Manganite
Manganites are compounds of the type A1-xA’xMnO3, where A is rare-earth (La, Pr, Y) and A’ is alkaline earth (Sr, Ca, Ba). Type A cations have a valence of +3, and A’s have a valence of +2 [72]. Therefore, when substituting a fraction x of cations, A for A’ in the AMnO3 material, the valence of an equivalent fraction of Mn ions changes from +3 to +4. The crystalline structure is perovskite-type with distortions concerning the cube according to the A and A’ ions present, giving rise to orthorhombic or rhombohedral structures [73]. A great variety of magnetic behaviors have been found in different manganites and, associated with them, different properties of electrical transport and presenting various fields of industrial and magnetic applications [74,75].
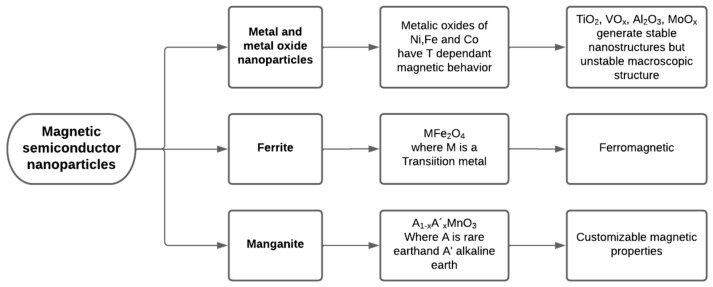
Magnetic semiconductor nanoparticles discussed in this section with characteristics and applications are summarized in Figure 4.
Figure 4.
Summary of magnetic semiconductor nanoparticles.
2.3. Polymer Nanocomposites
Currently, researchers have led studies approaching the use of conventional polymers as one of the components of NCs, forming in a particular type of hybrid materials called polymer nanocomposites (PNCs) (Figure 5) [76]. Moreover, the incorporation of NPs with remarkable properties in polymeric matrices has gained great interest thanks to the significant changes in thermal, optical, electrical, and magnetic properties that are conferred on the final nanocomposites compared to the pure matrix [61]. The interesting properties of the PNCs will depend either on the morphology, surface properties, and arrangement, in a wide range of the NPs, as well as on the structure of the NCs, which depends on the compatibility between the organic and inorganic phase and the mixing, and the dispersion method used during its preparation. One of the significant challenges when preparing PNCs is the homogeneous dispersion of nanofillers in a polymer [77].
Figure 5.
Polymer nanocomposite.
Magnetic Polymer Nanocomposites
Polymers can serve as an excellent matrix for the processing of NCs; however, they have no magnetic properties. Therefore, incorporating nanofillers with magnetic properties is an excellent solution to improve the characteristics of the NCs. Nanosized particles of ferromagnetic and ferrimagnetic materials have been incorporated into extended matrix materials to create integrated functional systems with additional magnetic properties [78].
Recently, polymeric nanocomposites with magnetic properties have aroused great interest in the scientific value of understanding their properties and their numerous applications [79]. Among them, the most common are in the fields of sensors and transducers, electronic devices, magnetic storage, electromagnetic and microwave absorption, and magnetic actuators [80,81]. In the first decade of the 2000s, studies were carried out on obtaining these materials by incorporating iron carbide particles in micrometric sizes into elastomeric matrices, where the increase in the elastic modulus of the magnetic elastomer was evidenced under the application of an external magnetic field [82]. Furthermore, Muñoz-Bonilla et al. reported using magnetite NPs to obtain NCs using polyaniline as a conductive polymer matrix with superparamagnetic behavior for electromagnetic shielding applications [83].
As mentioned earlier, ferrite combines magnetic material properties with those of an electrical insulator. It is a good option due to its flexibility to modulate their magnetic and electromagnetic properties, low cost, and ease of obtaining [84]. Ferrite-reinforced composite materials vary in their properties depending on the amount of ferrite added to the polymer matrix. Bellucci et al., for example, produced natural rubber nanocomposites reinforced with zinc ferrite nanoparticles using a rubber mill [85]. The study shows that the magnetic hysteresis loops are tight, which shows little material loss during the magnetization/demagnetization process. The remanence magnetization values are small, as are those of the coercive field, a typical behavior of soft ferrites.
Hard ferrites such as barium ferrite (BaFe12O19), unlike soft ferrites, have broad hysteresis loops. These materials have been used to make compounds with matrices of natural rubber or reused rubber from used tires [86,87]. In these works, the saturation magnetization and remanence magnetization vary linearly with the content of the reinforcement, as do materials reinforced with soft ferrites. The main difference lies in the wide hysteresis loops typical of hard ferrites. Makled et al. found that the coercive field tends to decrease for charges equal to or greater than 100 phr due to the increasing magnetic interaction between the ferrite particles as the average distances between them decrease [86].
Conductive reinforcements such as carbon-based composites are required to have adequate reflection values of the incident electromagnetic radiation in a polymeric matrix composite material. The composite material must have a reinforcing material with abundant electric or magnetic dipoles to obtain significant absorption values of the same radiation. Sunny et al. synthesized nanocomposites from natural rubber and nickel ferrite (NiFe2O4) to evaluate their magnetic and electromagnetic properties, finding that there is a constant increase in saturation magnetization and dielectric permittivity as the content of the reinforcement increases, as with magnetic permeability, although the latter decreases with increasing frequency [88]. For its part, the highest reflective losses that quantify the electromagnetic energy absorbed or dissipated by the composite material in the event of incident waves, for this case, were −5.9 dB for low frequencies (2–3.5 GHz) and −16 dB for high frequencies (9.66 GHz), both for a 120 phr nickel ferrite load and 12 mm thick sheets.
Magnetite is a soft ferrite commonly found in nature and easily obtained in the laboratory; it has ferrimagnetic properties and acceptable electrical conductivity values generated by its inverse spinel structure, where the divalent cation Fe2+ is found in the octahedral sites, while the trivalent cation Fe3+ is found in the tetrahedral and octahedral sites. The constant jump of electrons between the Fe2+ and Fe3+ ions found at the octahedral sites impart some metallic properties to the magnetite even at room temperature. Kong et al. manufactured and evaluated the microwave absorption properties of compounds made from natural thermoplastic rubber reinforced with magnetite nanoparticles (Fe3O4) in different concentrations of 4–12 wt% using an internal mixer [89]. The thermoplastic rubber matrix was synthesized from polypropylene (PP) and natural rubber. The real and complex permittivity values, the imaginary permeability, and the saturation magnetization values increase while the real permeability decreases with increasing reinforcement content. The microwave absorption properties and the absorption bandwidth of the compounds can be easily modulated by changing the magnetite concentration in the matrix and the thickness of the sample.
Ramapo et al. also found that high dielectric losses and high permittivity values are obtained at low frequencies when high concentrations of magnetite are added to thermoset matrices (40 vol%) [90]. The magnetite particles were processed through a ball mill to obtain micrometric sizes. Later these particles are added to epoxy resin matrices. The method of obtaining the particles generates a wide distribution of sizes and appearance of the particles that directly affect the shielding properties of the material.
Other properties such as the volumetric resistivity of this type of material depend on the magnetite content added to the matrix. Weidenfeller et al. added different proportions by volume of magnetite in polypropylene and polyamide matrices using an extruder as a manufacturing tool [91]. A decrease of up to seven orders of magnitude was evidenced for magnetite contents of 47 vol%. For volume contents above 30%, contact points begin to exist inside the matrices, which translates into a noticeable drop in the resistivity of the compounds [91]. The different grain sizes and the additives used during mixing do not influence the conductive behavior of the compound. Similar results of volumetric resistivity were obtained in another study in which compounds with high-density polyethylene matrices were reinforced with magnetite. They found a significant drop in the material resistivity of up to six orders of magnitude for samples of pure HDPE, up to magnitudes of 106 Ω*cm for samples with a content of 40% by volume of magnetite. In this same work, it was also observed how the saturation and remanence magnetization of the compound increased linearly with the amount of reinforcement added to the matrix [92].
3. Synthesis of MNCs
The synthesis of hybrid nanocomposites (magnetic and polymers) has significant advances in the last years. New strategies have been developed for magnetic nanocomposites with desirable properties. However, the homogeneity of MNPs into the polymeric matrix is still a challenge. Several processes have been designed to accomplish this objective, such as molding, coprecipitation, in situ precipitation, chemical vapor deposition, blending, and grafting onto the method. Practically, magnetic composites can be synthesized by embedding magnetic particles into a non-magnetic matrix [93].
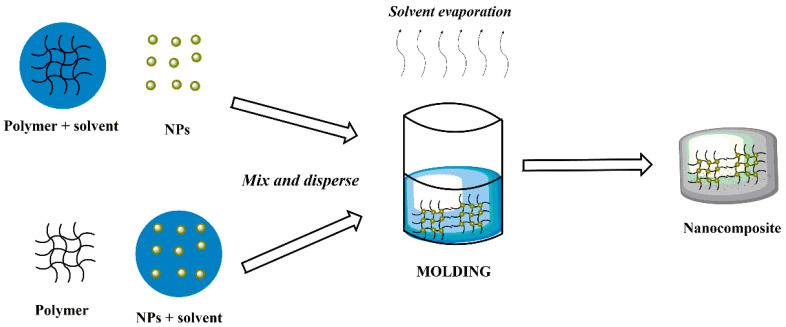
3.1. Molding
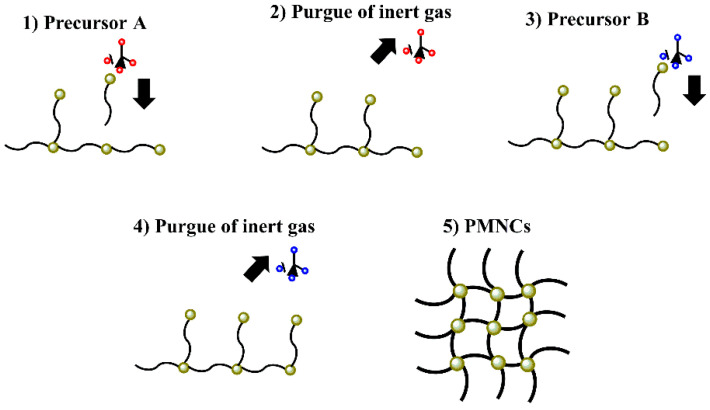
Molding is a widely used fabrication process of magnetic composites that is accomplished by mixing magnetic fillers and polymeric precursors thoroughly and curing them to form specific shapes or structures in molds [94] (Figure 6). There are several types of molding processes, such as injection [95], resin transfer [96], and compression molding techniques [97], which practically use a mold that is filled under pressure and temperatures. Chen et al. reported the synthesis of highly crosslinked poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) (PZS) used to coat ferric oxide (Fe3O4) nanoparticles directly and then were loaded with carbon nanotubes (CNTs) to get CNTs/Fe3O4@PZS as the photothermal magnetic filler. The PDMS/CNTs/Fe3O4@PZS surfaces with micron-scale truncated cones were prepared using compression molding and magnetic attraction [98].
Figure 6.
Schematic representation of molding technique.
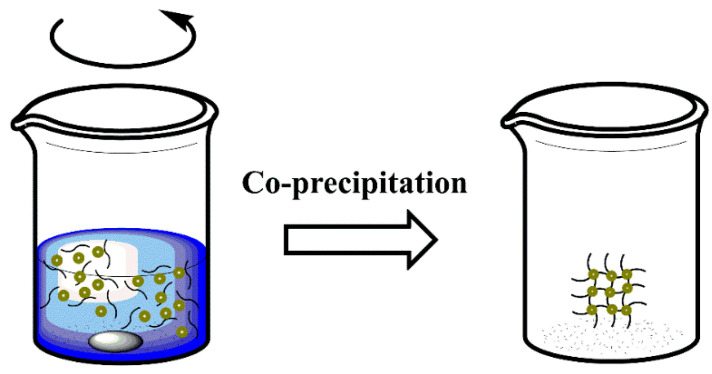
3.2. Coprecipitation
Coprecipitation is a facile and convenient approach to preparing MPNs [99] (either Fe3O4 or γ-Fe2O3) from aqueous Fe2+/Fe3+ salt solutions [100]. This process generally involves forming simultaneous nucleation, growth, coarsening, and agglomeration processes [79] (Figure 7). Generally, MNPs synthesized by coprecipitation will result in polydispersed particles that are spherical and have a size distribution between 5 and 40 nm [57,59]. Moreover, magnetic nanocomposites have been studied by the coprecipitation method, which is generally synthesized from salt species like Fe2+ and Fe3+ in alkali solution and under non-oxidizing conditions [101]. For example, Mehra et al. used an in situ coprecipitation method to achieve a homogeneous melamine-cyanurate (MC) distribution in a polymer matrix. As a result of this incorporation, a 65% enhancement of thermal conductivity was achieved (0.66 W/m·K), offering a new strategy for developing new thermally conductive materials [102].
Figure 7.
Schematic representation of coprecipitation method.
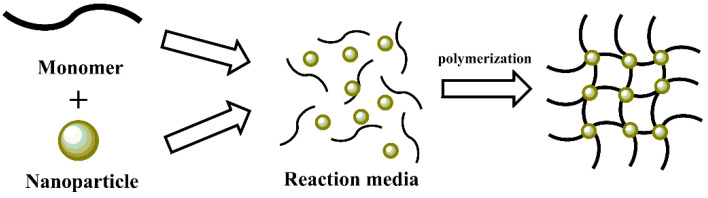
3.3. In Situ Polymerization
The in situ polymerization method is widely used and reported in the literature to synthesize MNPs based on their application in many fields [103,104,105]. This simple and straightforward method loads MNPs into a polymeric matrix because this method involves the inclusion of nanoparticles into a polymer matrix in the presence of a precipitation medium [106] (Figure 8). The selective precipitation of small amounts of inorganic nanoparticles within the porous matrix reduces the accessible pore volume [107]. Pu et al. synthesized a superparamagnetic graphene oxide (GO)/Fe3O4 nanocomposite by a facile in situ coprecipitation strategy for bioremediation [108]. Wang et al. reported the synthesis of novel composite hydrogels using in situ method. First, they proposed the synthesis of polypyrrole (PPy) and Fe3O4 nanoparticles and then the inclusion of this composite within the polyvinyl alcohol (PVA) matrix. The result was a hybrid hydrogel, named Fe3O4/PPy/PVA hydrogel, which shows interesting mechanical, conductive, and magnetic properties, making it a potential candidate for biomedical electronic devices [109]. Additionally, Hermán et al. prepared a core double-shell cobalt/graphene//polystyrene nanocomposite (Co/C//PS) by in situ polymerization, obtaining highly protected Co particles in the nanocomposite with a high mass-magnetization, that is, ~49 emu/g for 94% wt Co/C [110]. In another interesting research study, Shabzendedar et al. synthesized a superparamagnetic core-shell nanocomposite of poly(m-aminobenzenesulfonic acid) (PABS) embedded with Fe3O4 nanoparticles by an in situ polymerization method. This novel material exhibit excellent magnetic properties showing a saturation magnetization value of 40 emu/g and improved power-conversion efficiency (η = 4.24% and 660% enhanced efficiency), which can be exploited in polymer solar cells [111].
Figure 8.
Schematic representation of in situ polymerization.
3.4. Chemical Vapor Deposition (CVD)
CVD is utilized to produce composite material films and infiltrate fabric to produce different nanomaterials [112]. This technique involves the deposition of a solid in a matrix through a chemical reaction considering one or several gases (Figure 9). There are few reports on magnetic oxide films fabricated using CVD compared to other methods; however, some interesting magnetic materials can only be deposited via CVD [113]. In this technique, the precursors, gas or vapor, can react or decompose on the preselected substrate at high temperatures and vacuum in a chamber [114]. Several gases are admitted into the vacuum chamber through the inlet, and after dissociation between the species, the newly formed chemical molecules are deposited on the heated substrate [115]. CVD has proved to be a method that can produce iron oxide nanocomposites with high performance as a solid material and produce one-dimensional nanomaterials with a high purity [101].
Figure 9.
Schematic representation of the CVD method.
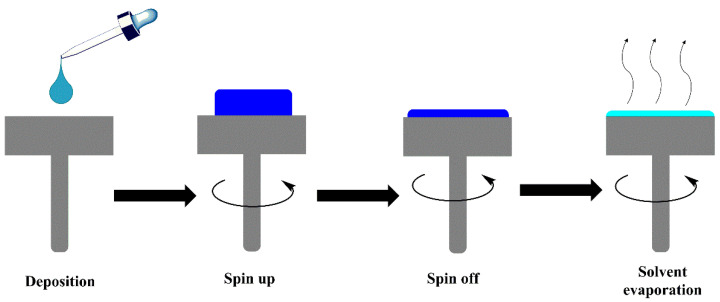
3.5. Spin Coating
Spin coating is a technique used in the microelectronics industry to disperse thin films or nanoparticles on solid substrates through centrifugal force. The rotation continues until the applied solvent evaporates and the thickness of the layer on the nanocomposite is reached [116,117,118] (Figure 10). Using this method, Sasikumar et al. prepared a highly sensitive resistive type of humidity sensor of TiO2/PANI nanocomposite. The exciting properties obtained can be reflected in the obtained values of room temperature resistivity (2.56 × 103 Ω.cm), fast response (20 s), and recovery time (15 s) [119]. In another research, Chou et al. dispersed Fe3O4 magnetic nanoparticles in poly(3-hexylthiophene-2,5-diyl) (P3HT), obtaining a novel polymer ferromagnetic semiconductor with a coercivity of 300 Oe. This value indicates a strong interaction between matrix and reinforcement, which was achieved using this method [120]. Additionally, Sharif et al. incorporated Mg0.5Zn0.5Fe2O4 nanoparticles by spin coating method into a PVA network matrix, obtaining good dielectric properties with potential applications in memristors and random access memories (RAM) [121].
Figure 10.
Schematic representation of Spin coating method.
4. Electronic Applications
In recent years, nanotechnology has been widely linked to the design and production of advanced devices focused on electronic applications. In this sense, magnetic polymer nanocomposites have been very useful due to their versatility, low weight, low cost, and easy preparation [122,123,124]. This section details certain applications that have found these promising materials.
4.1. Supercapacitors
Supercapacitors are electrochemical capacitors with energy densities an order of magnitude greater than the densities of conventional capacitors. Compared to batteries, these devices have low internal resistance, which means they can achieve high power densities [122]. Currently, supercapacitors are used to stabilize varying charges and to provide fast charging for mobile electronics. Additionally, these capacitive devices serve as a power buffer to mitigate voltage swings. Supercapacitors will not replace batteries in most applications, but they are likely to co-exist with them [125,126]. According to Altai et al., supercapacitor devices will be used where high levels of instantaneous power are required and fast recharging. On the other hand, batteries will be needed when renewable energy sources are intermittent [127].
To meet these needs, metal oxide coupled conducting polymer nanocomposites are a pseudocapacitive material that exhibits high-performance electrochemical properties for charge storage applications [128]. Conductive polymers such as polyaniline, polypyrrole, polythiophene, and some others, have been shown to have great flexibility, controllable thickness, and a high conductivity value (>103 S/cm) but lower stability [129]. In this sense, Prasankumar et al. prepared a polyaniline (PANI)/Fe3O4 nanocomposite by an in situ method. This nanocomposite showed improved electrochemical properties and high specific capacitance value, that is, 572 F/g at 0.5 A/g, and pronounced cycling stability (>5000 cycles at 1 A/g) with excellent capacitance retention of 82%, proving that this material can be exploited as a highly efficient electrode for supercapacitor [130]. Additionally, Sadeghinia et al. deposited BaFe12O19 nanoparticles into the PANI matrix to obtain a nanocomposite with a specific capacitance in the range of 225 and 330F/g, depending on the content of the reinforcement [131]. On the other hand, Asen et al. incorporated Cr2O3-graphene oxide into conducting polymer matrices such as polyaniline (PANI) and polypyrrole (PPy). The materials obtained showed high specific capacitance values of 525 F/g for the PANI-based nanocomposite and 495 F/g for the PPy-based nanocomposite, both at 5 A/g. Interestingly, these nanocomposites retain up to 84 and 80% of the initial capacitance after 4000 charge–discharge periods, suggesting improved electrochemical stability [132].
Among metal oxides, titanium oxide (SnO2) is an n-type semiconductor with a band gap of 3.6 eV, and its inclusion in PANI arrays has demonstrated applications in electronics [133]. Utilizing cyclic voltammetry, Prasanna et al. found that this inclusion to form a nanocomposite shows a specific capacitance of 337 F/g at a current density of 0.2 A/g and capacitance retention of 73% after 2000 cycles [134]. Even the inclusion of this reinforcement within a ternary nanocomposite shows improved electrochemical properties, as demonstrated by Ghebache et al. in the preparation of a nanohybrid electrode consisting of HY zeolite/SnO2/PANI, which shows a maximum capacitance of 1085 F/g at 5 mV/s, a good result derived from the synergistic effect with the complementary properties of both components of the filler [135]. Additionally, Kandasamy et al. synthesized a ternary nanocomposite consisting of carbon nanotubes/SiO2/PANI. This nanocomposite showed a specific capacitance value of 221 F/g at a current density of 2 A/g, with 95% capacitance retention after 1000 charge–discharge cycles due to an increase in quantum capacitance induced by interactions between PANI and the dual reinforcement [136].
Another conductive polymer matrix widely used for the development of supercapacitors is poly(3,4-ethylene dioxythiophene) (PEDOT), a polymer that has some advantages over other conductive polymers due to its long-term electrical stability, low band gap, improved mechanical properties, lower cost, and good electrochemical activity [137,138]. In this way, Ates et al. synthesized a new nanocomposite electrode by incorporating reduced graphene oxide (rGO) and tin oxide (TiO2) into the PEDOT matrix. This inclusion increases the specific capacitance to 652 F/g at 1 mV/s in a ratio of [rGO]/[TiO2] = 1/5. Another result for SC varied depending on the content of filler, that is, [rGO]/[TiO2] = 1/2 (SC = 475.33 F/g), [rGO]/[TiO2] = 1/1 (SC = 48.02 F/g), rGO/PEDOT (SC = 114.09 F/g), which demonstrates the distinct possibilities of this new nanocomposite to be incorporated as future supercapacitor application [139]. Gupta et al. replaced TiO2 with Mn ferrite (MnFe2O4) by an in situ polymerization, obtaining a material with EDLC and pseudocapacitive behavior with a good specific capacitance of 298.97 F/g at 1 A/g, and retention of these values at 81% over 5000 cycles. This ternary nanocomposite is a promising possibility as an electrode for supercapacitor fabrication [140].
4.2. Sensors
Industrial sensors are a crucial part of factory automation and Industry 4.0. A sensor is an electronic device that detects and responds to input from the physical environment and converts these output signals into a human-readable display. In industrial automation, sensors play a vital role in making products exceptionally automatic. They can also confer improved performance for energy sources such as fuel cells and batteries, and solar power, thus conferring better lifestyles for human beings [141,142,143].
With the growing industrial population, several environmental challenges have arisen due to the emission of toxic and dangerous gases [144]. For this reason, its detection by employing sensors has become essential. With this approach, Saaedi et al. dispersed zinc oxide (ZnO) into the PANI matrix, obtaining a nanocomposite to detect methanol, a highly volatile, flammable, and toxic alcohol. This study showed that the nanocomposite synthesized at a magnetic flux of 0.5 T obtained a resistance of 865 k, response time of 18.2 s, and recovery time of 5.1 s [145]. Wang et al. developed a material with potential application in detecting ammonia, a harmful gas to human health. For this purpose, Wang et al. synthesized a CuFe2O4/PANI nanocomposite, which showed an improved response time (67%) compared to pristine PANI and CuFe2O4 films [146]. Additionally, Husain et al. prepared a polythiophene/zirconium oxide (PTH/ZrO2) nanocomposite with high thermal stability and high electrical conductivity (9.42 × 10−4 S/cm) that can be applied to the detection of ethane [147].
The detection of contaminants such as insecticides is important for their subsequent removal. In this sense, this review shows two striking studies in which sensors based on magnetic polymer nanocomposites are used. The first was developed by Miao et al., who synthesized a nanocomposite based on Fe3O4 and polydopamine to detect dichlorodiphenyltrichloroethane (DDT) insecticide. This obtained sensor exhibited a limit of detection of DDT of 6 × 10−12 M, which was proved to be more efficient than other sensors [148]. Sohrabi et al. prepared an imprinted polymer nanocomposite for adsorption of diazinon insecticides. It was achieved by including graphene oxide, clay, and Fe3O4 into polydiacetylene (PDA), showing a magnetic saturation of 8.28 emu/g, detection of diazinon at 1.45 ppm, and adsorption capacity of more than 98% up to nine cycles of adsorption/desorption process [149].
4.3. Light-Emitting Diodes (LED)
A light-emitting diode (LED) is a light source that emits photons when an electrical current of very low intensity is received. An LED is usually encased in a colored plastic material that accentuates the length of light generated by the diode and helps focus the light into a beam [150]. Observing these properties, LEDs became popular, and advantages were found over other technologies. These advantages include long life, low power consumption, high level of efficiency, small dimensions, immediate light when turned on, and resistance to shocks or vibrations, which can be used in smart TV screens, smartphones, PC monitors, billboards, and some others [150,151].
This technology has been studied over the years. It has been tried to couple the use of magnetic polymer nanocomposites-based LED, due to its versatility and properties, including magnetic and optoelectronic properties [152]. In this sense, Skoda et al. synthesized a nanocomposite with deposition of CoxZn1-xO (x = 0.01, 0.05, and 0.1) nanoparticles into poly[2 -methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] (MEH-PPV). The inclusion of Co2+ into the ZnO lattice provides different optoelectronic properties such as band gap narrowing and luminescence quenching. Then, the addition of obtained nanoparticles into the polymer matrix gives a homogeneous solution for spin coating of thin nanocomposite layers, with application in optoelectronic devices, including hybrid polymer light-emitting diodes [153]. In the same research team, Jamatia et al. included FexZn1-xO in the stoichiometric relationships in the previous research. By a spin coating method, nanoparticles were dispersed into MEH-PPV, showing a significant enhancement of electroluminescence of the PLED devices due to the inclusion of nanoparticles into the active layer [154].
Including some relevant reinforcements into polymer matrices has been widely reported to obtain improved optoelectronic properties. Kumar et al. synthesized polymeric nanocomposites based on poly(p-phenylenediamine) filled with ZnO, Fe3O4, and TiO2 nanoparticles, describing an electrical conductivity of 10−7 S/cm, which makes it a semiconducting material. With complementary studies, the Fe3O4 nanocomposite exhibits improved soft magnetic properties reflected from the coercivity, retentivity, magnetization, and moment [155]. In another research team, Hadavand et al. deposited CdS/ZnS nanoparticles into poly(3,4-ethylene dioxythiophene) polystyrene sulfonate (PEDOT: PSS) polymer as a hole transport layer in samples of LEDs. The measurements of voltage-current density show that the turn-on voltage of obtained LED (0.4% of nanocomposite included) was decreased from more than 3 V for pristine LED to 1 V [156]. Additionally, Chen et al. fabricated a nanocomposite encapsulating CsPbX3 (X = Cl/Br, Br, Br/I) perovskite nanocrystals into superhydrophobic matrix-like poly(styrene-ethylene-butylene-styrene) (SEBS). The obtained CsPbX3@SEBS flexible films were used as color conversion materials to fabricate LED devices, exhibiting a wide color gamut (113% according to the National Television System Committee), a promising value for highly efficient color conversion materials for white LED [157].
On the other hand, the inclusion of other particles, like quantum dots (QDs), into polymer matrices can be beneficial to obtaining better light performance due to their great potential in optoelectronic devices, derived from their excellent optical performance [158]. Zhu et al. synthesized a nanocomposite from perovskite (FAPbBr3) QDs, as filler, and poly(methylmethacrylate) (PMMA), as a matrix. The final product showed a Lumen Efficiency of 80.4 m/W and a Color Rendering Index of 90, which satisfy commercial expectations and suggest application as light-emitting diode material [159].
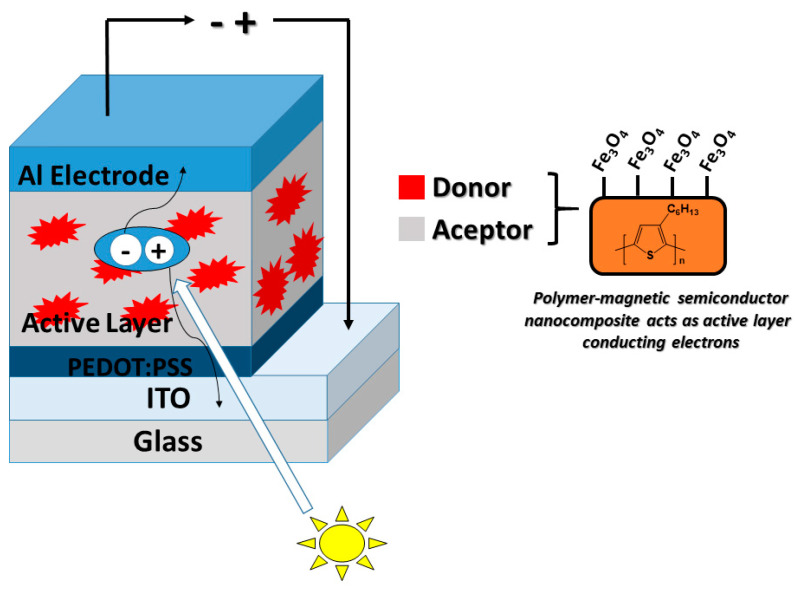
4.4. Solar Cells
Solar cells are energy converters in the form of electromagnetic radiation into electrical energy. Once this radiation makes contact with the semiconductor material (donor in the active layer), it transforms it into electrical energy in the form of direct current to be used immediately and, in turn, can be stored in a battery bank through load control [160] (Figure 11). Within these materials for harnessing solar energy, those solar cells based on organic semiconductors represent a promising technology due to their low cost, low weight, and versatility. Thus, polymer solar cell (PSC) devices have gained ground by using a π-conjugated polymer donor with a nanoscale coating; it forms a nanocomposite [161]. These materials have advantages compared to conventional solar cells, such as improved stability and efficiency [160,162]. Meng et al. describe the fabrication of composite nanofibers based on Fe3O4 as filler and blends of regio-regular poly(3-hexylthiophene) (P3HT) as the matrix. The resulting hybrid nanocomposite shows electronic properties related to the matrix and improved magnetic responsiveness. Despite having controlled and stable morphologies, there is no significant improvement in organic solar cells’ performance with this obtained material [163].
Figure 11.
The basic scheme of an organic solar cell.
Polymer matrices, magnetic fillers, properties of nanocomposites, applications, and corresponding references explained in this section are summarized in Table 1.
Table 1.
Summary of polymer nanocomposites with their corresponding properties.
| Application | Polymer Matrix | Magnetic Filler | Properties | References |
|---|---|---|---|---|
| Supercapacitors | PANI | Fe3O4 | High specific capacitance Cycling stability Excellent capacitance retention |
[130] |
| BaFe12O19 | Specific capacitance (225–330 F/g) | [131] | ||
| Cr2O3-graphene oxide | A high specific capacitance value of 525 F/g | [132] | ||
| SnO2 | A specific capacitance of 337 F/g | [134] | ||
| HY zeolite/SnO2 | A maximum capacitance of 1085 F/g | [135] | ||
| CN/SiO2 | A specific capacitance value of 221 F/g | [136] | ||
| PPy | Cr2O3-graphene oxide | A high specific capacitance value of 495 F/g | [132] | |
| PEDOT | rGO/TiO2 | Improved specific capacitance depending on the ratio content | [139] | |
| rGO/MnFe2O4 | A good specific capacitance of 298.97 F/g | [140] | ||
| Sensors | PANI | ZnO | A magnetic flux of 0.5 T Resistance of 865 k Response time of 18.2 s Recovery time of 5.1 s |
[145] |
| CuFe2O4 | Fast response time | [146] | ||
| PTh | ZrO2 | High thermal stability Highest electrical conductivity |
[147] | |
| Polydopamine | Fe3O4 | Enhanced limit of detection of DDT | [148] | |
| PDA | Clay/Fe3O4 | Improved magnetic saturation Highest detection of diazinon Enhanced adsorption capacity |
[149] | |
| LED | MEH-PPV | CoxZn1−xO | Band gap narrowing Luminescence quenching |
[153] |
| FexZn1−xO | Enhanced electroluminescence | [154] | ||
| Poly(p-phenylenediamine | ZnO, Fe3O4, or TiO2 | Improved soft magnetic properties Semiconducting material |
[155] | |
| PEDOT: PSS | CDs/ZnS | Decreased turn-on voltage | [156] | |
| PSEBS | CsPbX3 (X = Cl/Br, Br, Br/I) | Flexible films Wide color gamut |
[157] | |
| PMMA | FAPbBr3 QDs | Enhanced Lumen Efficiency Improved Color Rendering Index |
[159] | |
| Solar cells | π-conjugated polymer donor | Nanoscale coating | Improved stability and efficiency | [160,162] |
| P3HT | Fe3O4 | Improved magnetic responsiveness Controlled and stable morphologies |
[163] |
5. Perspectives and Conclusions
The incorporation of nanoparticles with remarkable properties in polymeric matrices has gained great interest thanks to the significant changes in thermal, optical, electrical, and magnetic properties that are conferred on the final nanocomposites compared to the pure matrix. These unique properties depend not only on the morphology (size and shape), surface properties, and organization in a wide range of the nanoparticles but also on the structure of the nanocomposites. Nanocomposite materials with inorganic–organic phases can no longer be solely considered reinforced material. It will depend on the compatibility between the organic and inorganic phases and the mixing and dispersion method used for its preparation.
The brittleness, great weight, and processing difficulty of conventional solid materials with magnetic and conductive properties limit their use in many potential applications. On the other hand, polymeric materials are lightweight, easily processable, and mechanically robust, but generally not magnetic. Therefore, the combination of polymers and inorganic nanofillers with magnetic properties is presented as a solution for integrating magnetic properties into a soft matrix.
It is essential to provide nanocomposites with good processability properties to exploit the great potential of their technological applications. This factor, in recent years, has led researchers to use conventional polymers as one of the components of nanocomposites, resulting in a particular type of hybrid material. Polymeric nanocomposites with magnetic properties have attracted great interest, not only for the scientific value of understanding their properties but also for their many applications. The most common are in the fields of sensors and transducers, electronic devices, magnetic storage, electromagnetic and microwave absorption, and magnetic actuators.
Acknowledgments
The authors would like to thank M. Cruz-Villafañe, J. Gutiérrez-Romero, and Y. Gamboa-Calderón from ICN-UNAM for technical assistance.
Author Contributions
D.R.-F., M.B.-T., F.B.-P., H.M. and E.B. participated in the review, writing, and revision, E.B., funding acquisition. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Dirección General de Asuntos del Personal Académico (DGAPA), Universidad Nacional Autónoma de México under Grant IN202320. Call for basic scientific research CONACYT 2017–2018 del “Fondo Sectorial de Investigación para la educación CB2017-2018” (grant A1-S-29789).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ghouse H., Slewa L., Mahmood M., Rehmat S., Musharrat S., Dahman Y. Nanotechnology for Electronic Applications. Springer Nature Singapore; Singapore: 2022. Importance of Nanotechnology, Various Applications in Electronic Field; pp. 1–28. [Google Scholar]
- 2.Coey J.M.D. Magnetism and Magnetic Materials. Cambridge University Press; Cambridge, UK: 2001. Applications of soft magnets; pp. 439–463. [Google Scholar]
- 3.Coey J.M.D. Magnetism and Magnetic Materials. Cambridge University Press; Cambridge, UK: 2001. Applications of hard magnets; pp. 464–493. [Google Scholar]
- 4.Zverev V.I., Pyatakov A.P., Shtil A.A., Tishin A.M. Novel applications of magnetic materials and technologies for medicine. J. Magn. Magn. Mater. 2018;459:182–186. doi: 10.1016/j.jmmm.2017.11.032. [DOI] [Google Scholar]
- 5.Lewis L.H., Jiménez-Villacorta F. Perspectives on Permanent Magnetic Materials for Energy Conversion and Power Generation. Metall. Mater. Trans. A. 2013;44:2–20. doi: 10.1007/s11661-012-1278-2. [DOI] [Google Scholar]
- 6.Sadiku M.N.O., Ashaolu T.J., Ajayi-Majebi A., Musa S.M. Future of Nanotechnology. Int. J. Sci. Adv. 2021;2 doi: 10.51542/ijscia.v2i2.9. [DOI] [Google Scholar]
- 7.Gubin S.P., Koksharov Y.A., Khomutov G.B., Yurkov G.Y. Magnetic nanoparticles: Preparation, structure and properties. Russ. Chem. Rev. 2005;74:489–520. doi: 10.1070/RC2005v074n06ABEH000897. [DOI] [Google Scholar]
- 8.Payal, Pandey P. Role of Nanotechnology in Electronics: A Review of Recent Developments and Patents. Recent Pat. Nanotechnol. 2022;16:45–66. doi: 10.2174/1872210515666210120114504. [DOI] [PubMed] [Google Scholar]
- 9.Pandey G., Jain P. Assessing the nanotechnology on the grounds of costs, benefits, and risks. Beni-Suef Univ. J. Basic Appl. Sci. 2020;9:63. doi: 10.1186/s43088-020-00085-5. [DOI] [Google Scholar]
- 10.Nasrollahzadeh M., Sajadi S.M., Sajjadi M., Issaabadi Z. An Introduction to Green Nanotechnology. Academic Press; Delhi, India: 2019. An Introduction to Nanotechnology; pp. 1–27. [Google Scholar]
- 11.Vatta L.L., Sanderson R.D., Koch K.R. Magnetic nanoparticles: Properties and potential applications. Pure Appl. Chem. 2006;78:1793–1801. doi: 10.1351/pac200678091793. [DOI] [Google Scholar]
- 12.Pankhurst Q.A., Thanh N.T.K., Jones S.K., Dobson J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009;42:224001. doi: 10.1088/0022-3727/42/22/224001. [DOI] [Google Scholar]
- 13.Tang S.C.N., Lo I.M.C. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013;47:2613–2632. doi: 10.1016/j.watres.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 14.Faraji M., Yamini Y., Rezaee M. Magnetic nanoparticles: Synthesis, stabilization, functionalization, characterization, and applications. J. Iran. Chem. Soc. 2010;7:1–37. doi: 10.1007/BF03245856. [DOI] [Google Scholar]
- 15.Kudr J., Haddad Y., Richtera L., Heger Z., Cernak M., Adam V., Zitka O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials. 2017;7:243. doi: 10.3390/nano7090243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeap S.P., Lim J., Ooi B.S., Ahmad A.L. Agglomeration, colloidal stability, and magnetic separation of magnetic nanoparticles: Collective influences on environmental engineering applications. J. Nanopart. Res. 2017;19:368. doi: 10.1007/s11051-017-4065-6. [DOI] [Google Scholar]
- 17.Kalia S., Kango S., Kumar A., Haldorai Y., Kumari B., Kumar R. Magnetic polymer nanocomposites for environmental and biomedical applications. Colloid Polym. Sci. 2014;292:2025–2052. doi: 10.1007/s00396-014-3357-y. [DOI] [Google Scholar]
- 18.Terán-Alcocer Á., Bravo-Plascencia F., Cevallos-Morillo C., Palma-Cando A. Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules. Nanomaterials. 2021;11:252. doi: 10.3390/nano11010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naveen M.H., Gurudatt N.G., Shim Y.-B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today. 2017;9:419–433. doi: 10.1016/j.apmt.2017.09.001. [DOI] [Google Scholar]
- 20.Vasiliev V.V., Morozov E.V. Advanced Mechanics of Composite Materials and Structures. Elsevier; Canberra, Australia: 2018. Introduction; pp. xvii–xxv. [Google Scholar]
- 21.Amir S.M.M., Sultan M.T.H., Jawaid M., Ariffin A.H., Mohd S., Salleh K.A.M., Ishak M.R., Shah A.U.M. Durability and Life Prediction in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites. Elsevier; Amsterdam, The Netherlands: 2019. Nondestructive testing method for Kevlar and natural fiber and their hybrid composites; pp. 367–388. [Google Scholar]
- 22.Sapuan S.M. Composite Materials. In: Sapuan S.M., editor. Composite Materials. Elsevier; Boston, MA, USA: 2017. pp. 57–93. [Google Scholar]
- 23.Abbas Q. Reference Module in Materials Science and Materials Engineering. Elsevier; Durhan, NC, USA: 2020. Advances of Electrode Materials. [Google Scholar]
- 24.Ngo T.-D. Composite and Nanocomposite Materials—From Knowledge to Industrial Applications. IntechOpen; Edmonton, AB, Canada: 2020. Introduction to Composite Materials. [Google Scholar]
- 25.Rahman M.R., Hamdan S. Silica and Clay Dispersed Polymer Nanocomposites. Elsevier; New Delhi, India: 2018. bin Study on physical, mechanical, morphological and thermal properties of styrene- co -glycidyl methacrylate/fumed silica/clay nanocomposites; pp. 71–85. [Google Scholar]
- 26.Saba N., Jawaid M., Sultan M.T.H. Lignocellulosic Fibre and Biomass-Based Composite Materials. Elsevier; New Delhi, India: 2017. Thermal properties of oil palm biomass based composites; pp. 95–122. [Google Scholar]
- 27.Chen Z., Chen Y., Hedenqvist M.S., Chen C., Cai C., Li H., Liu H., Fu J. Multifunctional conductive hydrogels and their applications as smart wearable devices. J. Mater. Chem. B. 2021;9:2561–2583. doi: 10.1039/D0TB02929G. [DOI] [PubMed] [Google Scholar]
- 28.Fu F., Wang J., Zeng H., Yu J. Functional Conductive Hydrogels for Bioelectronics. ACS Mater. Lett. 2020;2:1287–1301. doi: 10.1021/acsmaterialslett.0c00309. [DOI] [Google Scholar]
- 29.Zhou Y., Wan C., Yang Y., Yang H., Wang S., Dai Z., Ji K., Jiang H., Chen X., Long Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2019;29:1806220. doi: 10.1002/adfm.201806220. [DOI] [Google Scholar]
- 30.Bustamante-Torres M., Romero-Fierro D., Arcentales-Vera B., Palomino K., Magaña H., Bucio E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels. 2021;7:182. doi: 10.3390/gels7040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rong Q., Lei W., Liu M. Frontispiece: Conductive Hydrogels as Smart Materials for Flexible Electronic Devices. Chem. A Eur. J. 2018;24:chem.201886461. doi: 10.1002/chem.201801302. [DOI] [PubMed] [Google Scholar]
- 32.Peng Q., Chen J., Wang T., Peng X., Liu J., Wang X., Wang J., Zeng H. Recent advances in designing conductive hydrogels for flexible electronics. InfoMat. 2020;2:843–865. doi: 10.1002/inf2.12113. [DOI] [Google Scholar]
- 33.Rawat N.K., Ahmad S. Nanomaterials-Based Coatings. Elsevier; Amsterdam, The Netherlands: 2019. Unveiling nanoconducting polymers and composites for corrosion protection; pp. 373–395. [Google Scholar]
- 34.Le T.-H., Kim Y., Yoon H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers. 2017;9:150. doi: 10.3390/polym9040150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibanez J.G., Rincón M.E., Gutierrez-Granados S., Chahma M., Jaramillo-Quintero O.A., Frontana-Uribe B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical–Chiral Sensors. Chem. Rev. 2018;118:4731–4816. doi: 10.1021/acs.chemrev.7b00482. [DOI] [PubMed] [Google Scholar]
- 36.Heinze J., Frontana-Uribe B.A., Ludwigs S. Electrochemistry of Conducting Polymers—Persistent Models and New Concepts. Chem. Rev. 2010;110:4724–4771. doi: 10.1021/cr900226k. [DOI] [PubMed] [Google Scholar]
- 37.Mishra A.K. Conducting Polymers: Concepts and Applications. J. At. Mol. Condens. Nano Phys. 2018;5:159–193. doi: 10.26713/jamcnp.v5i2.842. [DOI] [Google Scholar]
- 38.Roth S. Conductive polymers. Phys. B+C. 1984;127:151–157. doi: 10.1016/S0378-4363(84)80023-6. [DOI] [Google Scholar]
- 39.Pham Truong T.N., Banet P., Aubert P. Conjugated Polymer Nanostructures for Energy Conversion and Storage Applications. Wiley; Weinheim, Germany: 2021. Conducting Polymers Nanowires with Carbon Nanotubes or Graphene-Based Nanocomposites for Supercapacitors Applications; pp. 445–497. [Google Scholar]
- 40.Murad A.R., Iraqi A., Aziz S.B., N. Abdullah S., Brza M.A. Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review. Polymers. 2020;12:2627. doi: 10.3390/polym12112627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phan S., Luscombe C.K. Recent Advances in the Green, Sustainable Synthesis of Semiconducting Polymers. Trends Chem. 2019;1:670–681. doi: 10.1016/j.trechm.2019.08.002. [DOI] [Google Scholar]
- 42.Han Y., Dai L. Conducting Polymers for Flexible Supercapacitors. Macromol. Chem. Phys. 2019;220:1800355. doi: 10.1002/macp.201800355. [DOI] [Google Scholar]
- 43.Tajik S., Beitollahi H., Nejad F.G., Shoaie I.S., Khalilzadeh M.A., Asl M.S., Van Le Q., Zhang K., Jang H.W., Shokouhimehr M. Recent developments in conducting polymers: Applications for electrochemistry. RSC Adv. 2020;10:37834–37856. doi: 10.1039/D0RA06160C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arumugaprabu V., Ko T.J., Uthayakumar M., Joel Johnson R.D. Failure Analysis in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites. Elsevier; Amsterdam, The Netherlands: 2019. Failure analysis in hybrid composites prepared using industrial wastes; pp. 229–244. [Google Scholar]
- 45.Hasan Z. Tooling for Composite Aerospace Structures. Elsevier; Oxford, UK: 2020. Composite materials; pp. 21–48. [Google Scholar]
- 46.Abhilash M., Thomas D. Biopolymer Composites in Electronics. Elsevier; Amsterdam, The Netherlands: 2017. Biopolymers for Biocomposites and Chemical Sensor Applications; pp. 405–435. [Google Scholar]
- 47.Fu S.-Y., Feng X.-Q., Lauke B., Mai Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008;39:933–961. doi: 10.1016/j.compositesb.2008.01.002. [DOI] [Google Scholar]
- 48.Khan I., Saeed K., Khan I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019;12:908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- 49.Díez-Pascual A. Nanoparticle Reinforced Polymers. Polymers. 2019;11:625. doi: 10.3390/polym11040625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bustamante-Torres M., Romero-Fierro D., Arcentales-Vera B., Pardo S., Bucio E. Interaction between Filler and Polymeric Matrix in Nanocomposites: Magnetic Approach and Applications. Polymers. 2021;13:2998. doi: 10.3390/polym13172998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yurkov G.Y., Baranov D.A., Dotsenko I.P., Gubin S.P. New magnetic materials based on cobalt and iron-containing nanoparicles. Compos. Part B Eng. 2006;37:413–417. doi: 10.1016/j.compositesb.2006.02.007. [DOI] [Google Scholar]
- 52.Kapranos P., Brabazon D., Midson S.P., Naher S., Haga T. Comprehensive Materials Processing. Elsevier; Amsterdam, The Netherlands: 2014. Advanced Casting Methodologies; pp. 3–37. [Google Scholar]
- 53.Keçili R., Büyüktiryaki S., Dolak İ., Hussain C.M. Handbook of Nanomaterials in Analytical Chemistry. Elsevier; Amsterdam, The Netherlands: 2020. The use of magnetic nanoparticles in sample preparation devices and tools; pp. 75–95. [Google Scholar]
- 54.Jeong J.-R., Shin S.-C., Lee S.-J., Kim J.-D. Magnetic properties of superparamagnetic γ-Fe2O3 nanoparticles prepared by coprecipitation technique. J. Magn. Magn. Mater. 2005;286:5–9. doi: 10.1016/j.jmmm.2004.09.129. [DOI] [Google Scholar]
- 55.Acar H.Y.C., Garaas R.S., Syud F., Bonitatebus P., Kulkarni A.M. Superparamagnetic nanoparticles stabilized by polymerized PEGylated coatings. J. Magn. Magn. Mater. 2005;293:1–7. doi: 10.1016/j.jmmm.2005.01.035. [DOI] [Google Scholar]
- 56.Mikhaylova M., Kim D.K., Bobrysheva N.P., Osmolowsky M., Semenov V., Tsakalakos T., Muhammed M. Superparamagnetism of Magnetite Nanoparticles: Dependence on Surface Modification. Langmuir. 2004;20:2472–2477. doi: 10.1021/la035648e. [DOI] [PubMed] [Google Scholar]
- 57.Bustamante-Torres M., Romero-Fierro D., Estrella-Nuñez J., Arcentales-Vera B., Chichande-Proaño E., Bucio E. Polymeric Composite of Magnetite Iron Oxide Nanoparticles and Their Application in Biomedicine: A Review. Polymers. 2022;14:752. doi: 10.3390/polym14040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sommertune J., Sugunan A., Ahniyaz A., Bejhed R.S., Sarwe A., Johansson C., Balceris C., Ludwig F., Posth O., Fornara A. Polymer/iron oxide nanoparticle composites—A straight forward and scalable synthesis approach. Int. J. Mol. Sci. 2015;16:19752–19768. doi: 10.3390/ijms160819752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ansari S.A.M.K., Ficiarà E., Ruffinatti F.A., Stura I., Argenziano M., Abollino O., Cavalli R., Guiot C., D’Agata F. Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System. Materials. 2019;12:465. doi: 10.3390/ma12030465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maicas M., Sanz M., Cui H., Aroca C., Sánchez P. Magnetic properties and morphology of Ni nanoparticles synthesized in gas phase. J. Magn. Magn. Mater. 2010;322:3485–3489. doi: 10.1016/j.jmmm.2010.06.050. [DOI] [Google Scholar]
- 61.Hanemann T., Szabó D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials. 2010;3:3468–3517. doi: 10.3390/ma3063468. [DOI] [Google Scholar]
- 62.Gao W., Zhu Y., Wang Y., Yuan G., Liu J.-M. A review of flexible perovskite oxide ferroelectric films and their application. J. Mater. 2020;6:1–16. doi: 10.1016/j.jmat.2019.11.001. [DOI] [Google Scholar]
- 63.Fan Z., Sun K., Wang J. Perovskites for photovoltaics: A combined review of organic–inorganic halide perovskites and ferroelectric oxide perovskites. J. Mater. Chem. A. 2015;3:18809–18828. doi: 10.1039/C5TA04235F. [DOI] [Google Scholar]
- 64.Tian M., Xu L., Yang Y. Perovskite Oxide Ferroelectric Thin Films. Adv. Electron. Mater. 2022;2101409 doi: 10.1002/aelm.202101409. [DOI] [Google Scholar]
- 65.Bui V.K.H., Tran V.V., Moon J.-Y., Park D., Lee Y.-C. Titanium Dioxide Microscale and Macroscale Structures: A Mini-Review. Nanomaterials. 2020;10:1190. doi: 10.3390/nano10061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kryszewski M., Jeszka J.K. Nanostructured conducting polymer composites—Superparamagnetic particles in conducting polymers. Synth. Met. 1998;94:99–104. doi: 10.1016/s0379-6779(97)04152-0. [DOI] [Google Scholar]
- 67.Koo J.H. Polymer Nanocomposites: Processing, Characterization, and Applications. McGraw-Hill Education; New York, NY, USA: 2006. [Google Scholar]
- 68.Petkov P., Tsiulyanu D., Popov C., Kulisch W. Advanced Nanotechnologies for Detection and Defence against CBRN Agents. Springer; New York, NY, USA: 2018. [Google Scholar]
- 69.Mazrouaa A.M., Mohamed M.G., Fekry M. Physical and magnetic properties of iron oxide nanoparticles with a different molar ratio of ferrous and ferric. Egypt. J. Pet. 2019;28:165–171. doi: 10.1016/j.ejpe.2019.02.002. [DOI] [Google Scholar]
- 70.Xuan Y., Li Q., Yang G. Synthesis and magnetic properties of Mn-Zn ferrite nanoparticles. J. Magn. Magn. Mater. 2007;312:464–469. doi: 10.1016/j.jmmm.2006.11.200. [DOI] [Google Scholar]
- 71.Ichiyanagi Y., Kubota M., Moritake S., Kanazawa Y., Yamada T., Uehashi T. Magnetic properties of Mg-ferrite nanoparticles. J. Magn. Magn. Mater. 2007;310:2378–2380. doi: 10.1016/j.jmmm.2006.10.737. [DOI] [Google Scholar]
- 72.Brommer P.E., Duc N.H. Magnetoelasticity in Nanoscale Heterogeneous Materials. Elsevier; Oxford, UK: 2004. pp. 1–6. [Google Scholar]
- 73.Fiebig M., Lottermoser T., Kneip M.K., Bayer M. Correlations between magnetic and electrical orderings in multiferroic manganites. J. Appl. Phys. 2006;99:08E302. doi: 10.1063/1.2172198. [DOI] [Google Scholar]
- 74.Roy S.B. Materials Under Extreme Conditions. Elsevier; Amsterdam, The Netherlands: 2017. Materials in a High Magnetic Field; pp. 755–789. [Google Scholar]
- 75.Joshi R.S., Kumar P.S.A. Comprehensive Inorganic Chemistry II. Elsevier; Amsterdam, The Netherlands: 2013. Magnetic Solid-State Materials; pp. 271–316. [Google Scholar]
- 76.Pirzada B.M., Sabir S. Polymer-Based Nanocomposites for Significantly Enhanced Dielectric Properties and Energy Storage Capability. Elsevier Ltd.; Duxford, UK: 2018. [Google Scholar]
- 77.Mishra R., Militky J. Nanotechnology in Textiles. Elsevier; Duxford, UK: 2019. Nanocomposites; pp. 263–310. [Google Scholar]
- 78.Parhi R. Applications of Nanocomposite Materials in Drug Delivery. Elsevier; Duxford, UK: 2018. Nanocomposite for transdermal drug delivery; pp. 353–389. [Google Scholar]
- 79.Nathani H., Gubbala S., Misra R.D.K. Magnetic behavior of nickel ferrite-polyethylene nanocomposites synthesized by mechanical milling process. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2004;111:95–100. doi: 10.1016/j.mseb.2004.03.002. [DOI] [Google Scholar]
- 80.Passaglia E., Bertoldo M., Coiai S., Augier S., Savi S., Ciardelli F. Nanostructured polyolefins/clay composites: Role of the molecular interaction at the interface. Polym. Adv. Technol. 2008;19:560–568. doi: 10.1002/pat.1107. [DOI] [Google Scholar]
- 81.Jiles D.C., Lo C.C.H. The role of new materials in the development of magnetic sensors and actuators. Sens. Actuators A Phys. 2003;106:3–7. doi: 10.1016/S0924-4247(03)00255-3. [DOI] [Google Scholar]
- 82.Varga Z., Filipcsei G., Zrínyi M. Magnetic field sensitive functional elastomers with tuneable elastic modulus. Polymer. 2006;47:227–233. doi: 10.1016/j.polymer.2005.10.139. [DOI] [Google Scholar]
- 83.Muñoz-Bonilla A., Sánchez-Marcos J., Herrasti P. Magnetic Nanoparticles-Based Conducting Polymer Nanocomposites. Springer; Dehradun, India: 2017. [Google Scholar]
- 84.Fulco A.P.P., Melo J.D.D., Paskocimas C.A., de Medeiros S.N., de Araujo Machado F.L., Rodrigues A.R. Magnetic properties of polymer matrix composites with embedded ferrite particles. NDT E Int. 2016;77:42–48. doi: 10.1016/j.ndteint.2015.10.002. [DOI] [Google Scholar]
- 85.Bellucci F.S., Lobato de Almeida F.C., Lima Nobre M.A., Rodríguez-Pérez M.A., Paschoalini A.T., Job A.E. Magnetic properties of vulcanized natural rubber nanocomposites as a function of the concentration, size and shape of the magnetic fillers. Compos. Part B Eng. 2016;85:196–206. doi: 10.1016/j.compositesb.2015.09.013. [DOI] [Google Scholar]
- 86.Makled M.H., Matsui T., Tsuda H., Mabuchi H., El-Mansy M.K., Morii K. Magnetic and dynamic mechanical properties of barium ferrite–natural rubber composites. J. Mater. Process. Technol. 2005;160:229–233. doi: 10.1016/j.jmatprotec.2004.06.013. [DOI] [Google Scholar]
- 87.Junliang L., Liu P., Zhang X., Lu P., Jivio Z., Zhang M. Fabrication of Magnetic Rubber Composites by Recycling Waste Rubber Powders via a Microwave-assisted In Situ Surface Modification and Semi-devulcanization Process. Chem. Eng. J. 2016;295:79. doi: 10.1016/j.cej.2016.03.025. [DOI] [Google Scholar]
- 88.Sunny V., Kurian P., Mohanan P., Joy P.A., Anantharaman M.R. A flexible microwave absorber based on nickel ferrite nanocomposite. J. Alloys Compd. 2010;489:297–303. doi: 10.1016/j.jallcom.2009.09.077. [DOI] [Google Scholar]
- 89.Kong I., Ahmad S., Abdullah M., Hui D., Yusoff A., Puryanti D. Magnetic and microwave absorbing properties of magnetite–thermoplastic natural rubber nanocomposites. J. Magn. Magn. Mater. 2010;322:3401–3409. doi: 10.1016/j.jmmm.2010.06.036. [DOI] [Google Scholar]
- 90.Ramajo L.A., Cristóbal A.A., Botta P.M., Porto López J.M., Reboredo M.M., Castro M.S. Dielectric and magnetic response of Fe3O4/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2009;40:388–393. doi: 10.1016/j.compositesa.2008.12.017. [DOI] [Google Scholar]
- 91.Weidenfeller B., Höfer M., Schilling F. Thermal and electrical properties of magnetite filled polymers. Compos. Part A Appl. Sci. Manuf. 2002;33:1041–1053. doi: 10.1016/S1359-835X(02)00085-4. [DOI] [Google Scholar]
- 92.Garzón A.O., Landínez D.A., Roa-Rojas J., Fajardo-Tolosa F.E., Peña-Rodríguez G., Parra-Vargas C.A. Production and structural, electrical and magnetic characterization of a composite material based on powdered magnetite and high density polyethylene. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 2017;41:154. doi: 10.18257/raccefyn.422. [DOI] [Google Scholar]
- 93.Palmero E.M., Bollero A. Encyclopedia of Materials: Composites. Elsevier; Amsterdam, The Netherlands: 2021. 3D and 4D Printing of Functional and Smart Composite Materials; pp. 402–419. [Google Scholar]
- 94.Wu S., Hu W., Ze Q., Sitti M., Zhao R. Multifunctional magnetic soft composites: A review. Multifunct. Mater. 2020;3:042003. doi: 10.1088/2399-7532/abcb0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trochu F., Gauvin R., Gao D.-M. Numerical analysis of the resin transfer molding process by the finite element method. Adv. Polym. Technol. 1993;12:329–342. doi: 10.1002/adv.1993.060120401. [DOI] [Google Scholar]
- 96.Mallick P.K. Failure Mechanisms in Polymer Matrix Composites. Elsevier; Cambridge, UK: 2012. Failure of polymer matrix composites (PMCs) in automotive and transportation applications; pp. 368–392. [Google Scholar]
- 97.Marques A.T. Fibrous materials reinforced composites production techniques. In: Fangueiro R., editor. Fibrous and Composite Materials for Civil Engineering Applications. Elsevier; Cambridge, UK: 2011. pp. 191–215. [Google Scholar]
- 98.Chen A., Wang Q., Li M., Peng Z., Lai J., Zhang J., Xu J., Huang H., Lei C. Combined Approach of Compression Molding and Magnetic Attraction to Micropatterning of Magnetic Polydimethylsiloxane Composite Surfaces with Excellent Anti-Icing/Deicing Performance. ACS Appl. Mater. Interfaces. 2021;13:48153–48162. doi: 10.1021/acsami.1c15428. [DOI] [PubMed] [Google Scholar]
- 99.Hui B.H., Salimi M.N. Production of Iron Oxide Nanoparticles by Co-Precipitation method with Optimization Studies of Processing Temperature, pH and Stirring Rate. IOP Conf. Ser. Mater. Sci. Eng. 2020;743:012036. doi: 10.1088/1757-899X/743/1/012036. [DOI] [Google Scholar]
- 100.Gautam R.K., Chattopadhyaya M.C. Nanomaterials for Wastewater Remediation. Elsevier; Oxford, UK: 2016. Functionalized Magnetic Nanoparticles: Adsorbents and Applications; pp. 139–159. [Google Scholar]
- 101.Fatimah I., Fadillah G., Yudha S.P. Synthesis of iron-based magnetic nanocomposites: A review. Arab. J. Chem. 2021;14:103301. doi: 10.1016/j.arabjc.2021.103301. [DOI] [Google Scholar]
- 102.Mehra N., Jeske M., Yang X., Gu J., Kashfipour M.A., Li Y., Baughman J.A., Zhu J. Hydrogen-Bond Driven Self-Assembly of Two-Dimensional Supramolecular Melamine-Cyanuric Acid Crystals and Its Self-Alignment in Polymer Composites for Enhanced Thermal Conduction. ACS Appl. Polym. Mater. 2019;1:1291–1300. doi: 10.1021/acsapm.9b00111. [DOI] [Google Scholar]
- 103.Adnan M., Dalod A., Balci M., Glaum J., Einarsrud M.-A. In Situ Synthesis of Hybrid Inorganic–Polymer Nanocomposites. Polymers. 2018;10:1129. doi: 10.3390/polym10101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Omanović-Mikličanin E., Badnjević A., Kazlagić A., Hajlovac M. Nanocomposites: A brief review. Health Technol. 2020;10:51–59. doi: 10.1007/s12553-019-00380-x. [DOI] [Google Scholar]
- 105.Rane A.V., Kanny K., Abitha V.K., Thomas S. Synthesis of Inorganic Nanomaterials. Elsevier; Duxford, UK: 2018. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites; pp. 121–139. [Google Scholar]
- 106.Harito C., Bavykin D.V., Yuliarto B., Dipojono H.K., Walsh F.C. Polymer nanocomposites having a high filler content: Synthesis, structures, properties, and applications. Nanoscale. 2019;11:4653–4682. doi: 10.1039/C9NR00117D. [DOI] [PubMed] [Google Scholar]
- 107.Yadav R., Tirumali M., Wang X., Naebe M., Kandasubramanian B. Polymer composite for antistatic application in aerospace. Def. Technol. 2020;16:107–118. doi: 10.1016/j.dt.2019.04.008. [DOI] [Google Scholar]
- 108.Pu S., Xue S., Yang Z., Hou Y., Zhu R., Chu W. In situ co-precipitation preparation of a superparamagnetic graphene oxide/Fe3O4 nanocomposite as an adsorbent for wastewater purification: Synthesis, characterization, kinetics, and isotherm studies. Environ. Sci. Pollut. Res. 2018;25:17310–17320. doi: 10.1007/s11356-018-1872-y. [DOI] [PubMed] [Google Scholar]
- 109.Wang Y., Zhu Y., Xue Y., Wang J., Li X., Wu X., Qin Y., Chen W. Sequential in-situ route to synthesize novel composite hydrogels with excellent mechanical, conductive, and magnetic responsive properties. Mater. Des. 2020;193:108759. doi: 10.1016/j.matdes.2020.108759. [DOI] [Google Scholar]
- 110.Hermán V., Takacs H., Duclairoir F., Renault O., Tortai J.H., Viala B. Core double–shell cobalt/graphene/polystyrene magnetic nanocomposites synthesized by in situ sonochemical polymerization. RSC Adv. 2015;5:51371–51381. doi: 10.1039/C5RA06847A. [DOI] [Google Scholar]
- 111.Shabzendedar S., Modarresi-Alam A.R., Noroozifar M., Kerman K. Core-shell nanocomposite of superparamagnetic Fe3O4 nanoparticles with poly(m-aminobenzenesulfonic acid) for polymer solar cells. Org. Electron. 2020;77:105462. doi: 10.1016/j.orgel.2019.105462. [DOI] [Google Scholar]
- 112.Tahir M.B., Rafique M., Rafique M.S., Nawaz T., Rizwan M., Tanveer M. Nanotechnology and Photocatalysis for Environmental Applications. Elsevier; Amsterdam, The Netherlands: 2020. Photocatalytic nanomaterials for degradation of organic pollutants and heavy metals; pp. 119–138. [Google Scholar]
- 113.Yamaguchi A., Hirohata A., Stadler B.J.H. Nanomagnetic Materials: Fabrication, Characterization and Application. Elsevier; Amsterdam, The Netherlands: 2021. Synthesis and processing; pp. 57–118. [Google Scholar]
- 114.Pottathara Y.B., Grohens Y., Kokol V., Kalarikkal N., Thomas S. Nanomaterials Synthesis. Elsevier; Amsterdam, The Netherlands: 2019. Synthesis and Processing of Emerging Two-Dimensional Nanomaterials; pp. 1–25. [Google Scholar]
- 115.Madhuri K.V. Metal Oxide Powder Technologies. Elsevier; Amsterdam, The Netherlands: 2020. Thermal protection coatings of metal oxide powders; pp. 209–231. [Google Scholar]
- 116.Augello C., Liu H. Surface Modification of Magnesium and its Alloys for Biomedical Applications. Elsevier; Cambridge, UK: 2015. Surface modification of magnesium by functional polymer coatings for neural applications; pp. 335–353. [Google Scholar]
- 117.Mammeri F. Frontiers of Nanoscience. Elsevier; Amsterdam, The Netherlands: 2019. Nanostructured flexible PVDF and fluoropolymer-based hybrid films; pp. 67–101. [Google Scholar]
- 118.Yilbas B.S., Al-Sharafi A., Ali H. Self-Cleaning of Surfaces and Water Droplet Mobility. Elsevier; Amsterdam, The Netherlands: 2019. Surfaces for Self-Cleaning; pp. 45–98. [Google Scholar]
- 119.Sasikumar M., Subiramaniyam N.P. Microstructure, electrical and humidity sensing properties of TiO2/polyaniline nanocomposite films prepared by sol–gel spin coating technique. J. Mater. Sci. Mater. Electron. 2018;29:7099–7106. doi: 10.1007/s10854-018-8697-9. [DOI] [Google Scholar]
- 120.Chou W.-Y., Fang P.-H., Chiang W.-C., Cheng H.-L. Room temperature ferromagnetism in Fe3O4 nanoparticle-embedded polymer semiconductors. J. Phys. Chem. Solids. 2022;167:110750. doi: 10.1016/j.jpcs.2022.110750. [DOI] [Google Scholar]
- 121.Sharif A., Mustaqeem M., Saleh T.A., ur Rehman A., Ahmad M., Warsi M.F. Synthesis, structural and dielectric properties of Mg/Zn ferrites -PVA nanocomposites. Mater. Sci. Eng. B. 2022;280:115689. doi: 10.1016/j.mseb.2022.115689. [DOI] [Google Scholar]
- 122.Bogue R. Nanocomposites: A review of technology and applications. Assem. Autom. 2011;31:106–112. doi: 10.1108/01445151111117683. [DOI] [Google Scholar]
- 123.Talaat A., Suraj M.V., Byerly K., Wang A., Wang Y., Lee J.K., Ohodnicki P.R. Review on soft magnetic metal and inorganic oxide nanocomposites for power applications. J. Alloys Compd. 2021;870:159500. doi: 10.1016/j.jallcom.2021.159500. [DOI] [Google Scholar]
- 124.Peng S., Yu Y., Wu S., Wang C.-H. Conductive Polymer Nanocomposites for Stretchable Electronics: Material Selection, Design, and Applications. ACS Appl. Mater. Interfaces. 2021;13:43831–43854. doi: 10.1021/acsami.1c15014. [DOI] [PubMed] [Google Scholar]
- 125.Hossain S.K.S., Hoque M.E. Polymer-Based Nanocomposites for Energy and Environmental Applications. Elsevier; Duxford, UK: 2018. Polymer nanocomposite materials in energy storage: Properties and applications; pp. 239–282. [Google Scholar]
- 126.Arunachalam P. Polymer-Based Nanocomposites for Energy and Environmental Applications. Elsevier; Duxford, UK: 2018. Polymer-based nanocomposites for energy and environmental applications; pp. 185–203. [Google Scholar]
- 127.Alatai S., Salem M., Ishak D., Das H.S., Alhuyi Nazari M., Bughneda A., Kamarol M. A Review on State-of-the-Art Power Converters: Bidirectional, Resonant, Multilevel Converters and Their Derivatives. Appl. Sci. 2021;11:10172. doi: 10.3390/app112110172. [DOI] [Google Scholar]
- 128.Magu T., Agobi A., Hitler L., Dass P. A Review on Conducting Polymers-Based Composites for Energy Storage Application. J. Chem. Rev. 2019;1:19–34. doi: 10.33945/SAMI/JCR.2019.1.1934. [DOI] [Google Scholar]
- 129.Chen W., Rakhi R.B., Alshareef H.N. Capacitance enhancement of polyaniline coated curved-graphene supercapacitors in a redox-active electrolyte. Nanoscale. 2013;5:4134. doi: 10.1039/c3nr00773a. [DOI] [PubMed] [Google Scholar]
- 130.Prasankumar T., Wiston B.R., Gautam C.R., Ilangovan R., Jose S.P. Synthesis and enhanced electrochemical performance of PANI/Fe3O4 nanocomposite as supercapacitor electrode. J. Alloys Compd. 2018;757:466–475. doi: 10.1016/j.jallcom.2018.05.108. [DOI] [Google Scholar]
- 131.Sadeghinia M., Shayeh J.S., Fatemi F., Rahmandoust M., Ehsani A., Rezaei M. Electrochemical study of perlite-barium ferrite/conductive polymer nano composite for super capacitor applications. Int. J. Hydrogen Energy. 2019;44:28088–28095. doi: 10.1016/j.ijhydene.2019.09.085. [DOI] [Google Scholar]
- 132.Asen P., Shahrokhian S., Zad A.I. Ternary nanostructures of Cr2O3/graphene oxide/conducting polymers for supercapacitor application. J. Electroanal. Chem. 2018;823:505–516. doi: 10.1016/j.jelechem.2018.06.048. [DOI] [Google Scholar]
- 133.Sun L., Shi Y., He Z., Li B., Liu J. Synthesis and characterization of SnO2/polyaniline nanocomposites by sol–gel technique and microemulsion polymerization. Synth. Met. 2012;162:2183–2187. doi: 10.1016/j.synthmet.2012.10.004. [DOI] [Google Scholar]
- 134.Prasanna B.P., Avadhani D.N., Raj V., Kumar K.Y., Raghu M.S. Fabrication of PANI/SnO2 Hybrid Nanocomposites via Interfacial Polymerization for High Performance Supercapacitors Applications. Surf. Eng. Appl. Electrochem. 2019;55:463–471. doi: 10.3103/S1068375519040100. [DOI] [Google Scholar]
- 135.Ghebache Z., Hamidouche F., Safidine Z., Trari M., Bellal B. Synthesis and Electrical Conducting Properties of Poly(aniline) Doped With Zeolite HY Nanocomposites Containing SnO2 for High-Performance Supercapacitor Electrode. J. Inorg. Organomet. Polym. Mater. 2019;29:1548–1558. doi: 10.1007/s10904-019-01118-y. [DOI] [Google Scholar]
- 136.Kandasamy M., Seetharaman A., Babu I.M., William J.J., Muralidharan G., Sivasubramanian D., Jothivenkatachalam K., Imran M., Chakraborty B. Experimental and theoretical investigations of a multiwalled carbon nanotubes/SnO2/polyaniline ternary nanohybrid electrode for energy storage. Surf. Interfaces. 2022;30:101978. doi: 10.1016/j.surfin.2022.101978. [DOI] [Google Scholar]
- 137.Fan X., Nie W., Tsai H., Wang N., Huang H., Cheng Y., Wen R., Ma L., Yan F., Xia Y. PEDOT:PSS for Flexible and Stretchable Electronics: Modifications, Strategies, and Applications. Adv. Sci. 2019;6:1900813. doi: 10.1002/advs.201900813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jin L., Wang T., Feng Z.-Q., Leach M.K., Wu J., Mo S., Jiang Q. A facile approach for the fabrication of core–shell PEDOT nanofiber mats with superior mechanical properties and biocompatibility. J. Mater. Chem. B. 2013;1:1818. doi: 10.1039/c3tb00448a. [DOI] [PubMed] [Google Scholar]
- 139.Ates M., Bayrak Y., Ozkan H., Yoruk O., Yildirim M., Kuzgun O. Synthesis of rGO/TiO2/PEDOT nanocomposites, supercapacitor device performances and equivalent electrical circuit models. J. Polym. Res. 2019;26:49. doi: 10.1007/s10965-018-1692-2. [DOI] [Google Scholar]
- 140.Gupta M., Tyagi S., Kumari N. Electrochemical evaluation of highly stable Mn ferrite/PEDOT/rGO ternary nanocomposite for supercapacitor electrodes. J. Mater. Sci. Mater. Electron. 2022;33:7838–7852. doi: 10.1007/s10854-022-07935-1. [DOI] [Google Scholar]
- 141.Megha R., Ali F.A., Ravikiran Y.T., Ramana C.H.V.V., Kiran Kumar A.B.V., Mishra D.K., Vijayakumari S.C., Kim D. Conducting polymer nanocomposite based temperature sensors: A review. Inorg. Chem. Commun. 2018;98:11–28. doi: 10.1016/j.inoche.2018.09.040. [DOI] [Google Scholar]
- 142.Chow W.S., Mohd Ishak Z.A. Smart polymer nanocomposites: A review. Express Polym. Lett. 2020;14:416–435. doi: 10.3144/expresspolymlett.2020.35. [DOI] [Google Scholar]
- 143.Muhammed Shameem M., Sasikanth S.M., Annamalai R., Ganapathi Raman R. A brief review on polymer nanocomposites and its applications. Mater. Today Proc. 2021;45:2536–2539. doi: 10.1016/j.matpr.2020.11.254. [DOI] [Google Scholar]
- 144.Péres L.O., Li R.W.C., Yamauchi E.Y., Lippi R., Gruber J. Conductive polymer gas sensor for quantitative detection of methanol in Brazilian sugar-cane spirit. Food Chem. 2012;130:1105–1107. doi: 10.1016/j.foodchem.2011.08.014. [DOI] [Google Scholar]
- 145.Sáaedi A., Shabani P., Yousefi R. High performance of methanol gas sensing of ZnO/PAni nanocomposites synthesized under different magnetic field. J. Alloys Compd. 2019;802:335–344. doi: 10.1016/j.jallcom.2019.06.088. [DOI] [Google Scholar]
- 146.Wang X., Gong L., Zhang D., Fan X., Jin Y., Guo L. Room temperature ammonia gas sensor based on polyaniline/copper ferrite binary nanocomposites. Sens. Actuators B Chem. 2020;322:128615. doi: 10.1016/j.snb.2020.128615. [DOI] [Google Scholar]
- 147.Husain A., Ahmad S., Mohammad F. Thermally stable and highly sensitive ethene gas sensor based on polythiophene/zirconium oxide nanocomposites. Mater. Today Commun. 2019;20:100574. doi: 10.1016/j.mtcomm.2019.100574. [DOI] [Google Scholar]
- 148.Miao J., Liu A., Wu L., Yu M., Wei W., Liu S. Magnetic ferroferric oxide and polydopamine molecularly imprinted polymer nanocomposites based electrochemical impedance sensor for the selective separation and sensitive determination of dichlorodiphenyltrichloroethane (DDT) Anal. Chim. Acta. 2020;1095:82–92. doi: 10.1016/j.aca.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 149.Sohrabi N., Mohammadi R., Ghassemzadeh H.R., Heris S.S.S. Design and synthesis of a new magnetic molecularly imprinted polymer nanocomposite for specific adsorption and separation of diazinon insecticides from aqueous media. Microchem. J. 2022;175:107087. doi: 10.1016/j.microc.2021.107087. [DOI] [Google Scholar]
- 150.Song J., Lee H., Jeong E.G., Choi K.C., Yoo S. Organic Light-Emitting Diodes: Pushing Toward the Limits and Beyond. Adv. Mater. 2020;32:1907539. doi: 10.1002/adma.201907539. [DOI] [PubMed] [Google Scholar]
- 151.Kandulna R., Das U., Rimpi M., Kachhap B., Prasad N. Hybrid Polymeric Nanocomposites Based High Performance Oleds: A Review. Shodh Sankalp J. 2021;1:16–34. doi: 10.54051/shodh.2021.1.3.1. [DOI] [Google Scholar]
- 152.Alarifi I., Prasad B., Uddin M.K. Self-Standing Substrates. Springer; Cham, Switzerland: 2020. Conducting Polymer Membranes and Their Applications; pp. 147–176. [Google Scholar]
- 153.Skoda D., Urbanek P., Sevcik J., Munster L., Nadazdy V., Cullen D.A., Bazant P., Antos J., Kuritka I. Colloidal cobalt-doped ZnO nanoparticles by microwave-assisted synthesis and their utilization in thin composite layers with MEH-PPV as an electroluminescent material for polymer light emitting diodes. Org. Electron. 2018;59:337–348. doi: 10.1016/j.orgel.2018.05.037. [DOI] [Google Scholar]
- 154.Jamatia T., Skoda D., Urbanek P., Sevcik J., Maslik J., Munster L., Kalina L., Kuritka I. Microwave-assisted synthesis of FexZn1−xO nanoparticles for use in MEH-PPV nanocomposites and their application in polymer light-emitting diodes. J. Mater. Sci. Mater. Electron. 2019;30:11269–11281. doi: 10.1007/s10854-019-01473-z. [DOI] [Google Scholar]
- 155.Kumar S., Baruah S., Puzari A. Poly(p-phenylenediamine)-based nanocomposites with metal oxide nanoparticle for optoelectronic and magneto-optic application. Polym. Bull. 2020;77:441–457. doi: 10.1007/s00289-019-02760-9. [DOI] [Google Scholar]
- 156.Hadavand M., Jafari M.R., Pakpour F., Ghanbari D. CdS/ZnS nanocomposite: Synthesis and utilization in organic light-emitting diodes for a lower turn-on voltage. J. Nanopart. Res. 2021;23:61. doi: 10.1007/s11051-021-05174-9. [DOI] [Google Scholar]
- 157.Chen Y., Liang X., Liu J., Chen S., Xiang W. Environmentally Friendly and Ultrastable Narrow-Band Emitter: CsPbX 3 @SEBS Flexible Film for Light-Emitting Diode Displays. ACS Sustain. Chem. Eng. 2021;9:10291–10298. doi: 10.1021/acssuschemeng.1c02981. [DOI] [Google Scholar]
- 158.Xue L., Liu Y., Li F., Sun K., Chen W., Yang K., Hu H., Lin J., Chen H., Yang Z., et al. Highly flexible light emitting diodes based on a quantum dots-polymer composite emitting layer. Vacuum. 2019;163:282–286. doi: 10.1016/j.vacuum.2019.02.033. [DOI] [Google Scholar]
- 159.Zhu H., Cheng M., Li J., Yang S., Tao X., Yu Y., Jiang Y. Independent dispersed and highly water-oxygen environment stable FAPbBr3 QDs-polymer composite for down-conversion display films. Chem. Eng. J. 2022;428:130974. doi: 10.1016/j.cej.2021.130974. [DOI] [Google Scholar]
- 160.Wang G., Melkonyan F.S., Facchetti A., Marks T.J. All-Polymer Solar Cells: Recent Progress, Challenges, and Prospects. Angew. Chem. Int. Ed. 2019;58:4129–4142. doi: 10.1002/anie.201808976. [DOI] [PubMed] [Google Scholar]
- 161.Tessema Mola G., Mbuyise X.G., Oseni S.O., Dlamini W.M., Tonui P., Arbab E.A.A., Kaviyarasu K., Maaza M. Nanocomposite for Solar Energy Application. Nano Hybrids Compos. 2018;20:90–107. doi: 10.4028/www.scientific.net/NHC.20.90. [DOI] [Google Scholar]
- 162.Hou W., Xiao Y., Han G., Lin J.-Y. The Applications of Polymers in Solar Cells: A Review. Polymers. 2019;11:143. doi: 10.3390/polym11010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Meng L., Watson B.W., Qin Y. Hybrid conjugated polymer/magnetic nanoparticle composite nanofibers through cooperative non-covalent interactions. Nanoscale Adv. 2020;2:2462–2470. doi: 10.1039/D0NA00191K. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.