Abstract
Urea hydrogen peroxide (UHP) at a concentration of 30 to 32 mmol/liter reduced the numbers of five Lactobacillus spp. (Lactobacillus plantarum, L. paracasei, Lactobacillus sp. strain 3, L. rhamnosus, and L. fermentum) from ∼107 to ∼102 CFU/ml in a 2-h preincubation at 30°C of normal-gravity wheat mash at ∼21 g of dissolved solids per ml containing normal levels of suspended grain particles. Fermentation was completed 36 h after inoculation of Saccharomyces cerevisiae in the presence of UHP, even when wheat mash was deliberately contaminated (infected) with L. paracasei at ∼107 CFU/ml. There were no significant differences in the maximum ethanol produced between treatments when urea hydrogen peroxide was used to kill the bacteria and controls (in which no bacteria were added). However, the presence of L. paracasei at ∼107 CFU/ml without added agent resulted in a 5.84% reduction in the maximum ethanol produced compared to the control. The bactericidal activity of UHP is greatly affected by the presence of particulate matter. In fact, only 2 mmol of urea hydrogen peroxide per liter was required for disinfection when mashes had little or no particulate matter present. No significant differences were observed in the decomposition of hydrogen peroxide in normal-gravity wheat mash at 30°C whether the bactericidal agent was added as H2O2 or as urea hydrogen peroxide. NADH peroxidase activity (involved in degrading H2O2) increased significantly (P = 0.05) in the presence of 0.75 mM hydrogen peroxide (sublethal level) in all five strains of lactobacilli tested but did not persist in cells regrown in the absence of H2O2. H2O2-resistant mutants were not expected or found when lethal levels of H2O2 or UHP were used. Contaminating lactobacilli can be effectively managed by UHP, a compound which when used at ca. 30 mmol/liter happens to provide near-optimum levels of assimilable nitrogen and oxygen that aid in vigorous fermentation performance by yeast.
Bacterial contamination is a major cause for the reduction in yeast growth, yeast viability, and ethanol yield during the fermentation of starch- or sugar-based feedstocks by Saccharomyces cerevisiae. Lactic acid bacteria are the most persistent contaminants because of their tolerance of ethanol, low pH, and high temperature and their ability to therefore survive alcoholic fermentation. Predominant contaminants isolated from distilleries and fuel alcohol plants belong to the genus Lactobacillus. These microbes are able to ferment carbohydrates for growth and energy production, the latter leading to the production of lactic acid and small amounts of acetic acid which cause reductions in both yeast growth and ethanol yield (19). A number of commercially isolated lactobacilli are extremely fast growing and are able to grow in ethanol concentrations exceeding 10% (vol/vol) (10, 19).
The management of bacterial contaminants is often achieved in industry by using antibiotics such as penicillin G, streptomycin, tetracycline (4, 9), virginiamycin, and monensin or mixtures of these compounds. Virginiamycin may be a better choice for treatment since this antibiotic, unlike penicillin, retains its activity at lower pH values (10). However, antibiotics are expensive, and the concept of antibiotic use in an industrial process is in question in spite of the absence of antibiotic residues in spent grains subsequent to distillation. General misuse of antibiotics in society has contributed to a buildup of reservoirs of antibiotic-resistant bacteria (17), providing an incentive to examine other antimicrobials which are not antibiotics.
The physiological differences between yeast and lactobacilli suggest the use of hydrogen peroxide to manage these bacteria in mashes used for alcoholic fermentations. Literature concerning the antibacterial effects of hydrogen peroxide covers a period of more than 100 years. Lactobacilli lack the enzyme catalase, which decomposes hydrogen peroxide, and therefore are unable to eliminate its toxic effect. In an attempt to evaluate sulfite and hydrogen peroxide as bacterial-contamination control agents, Chang et al. (7) have reported that the viability of Lactobacillus fermentum could be selectively controlled by hydrogen peroxide at concentrations of 1 to 10 mM in an ethanol fermentation process with cell recycling.
For maximal bactericidal activity, hydrogen peroxide should be electrolytically pure and allowed to come into contact with only stainless steel or other corrosion-resistant materials (18). At higher temperatures, bactericidal efficiency increases (1). The stability of the compound also decreases as the pH increases (18). Moreover, in contact with organic matter, hydrogen peroxide breaks up into nascent oxygen and water. To avoid the problem of instability, Banerjee (5) prepared a solid compound containing hydrogen peroxide and urea in his laboratory and claimed that this compound, urea hydrogen peroxide (UHP), was perfectly stable in a dry state at ordinary temperatures. UHP has since been used as an antiseptic for topical application on wounds and against gingivitis and dental plaque (25).
Apart from bacterial contamination, “stuck” or sluggish fermentations, common in the alcohol industry, lead to reductions in ethanol yield. Stuck fermentations are most often caused by inadequate levels of yeast nutrients that lead to a cessation of yeast growth with a concomitant reduction in ethanol yield (12). Two such nutrients usually deficient in fermentation mashes are usable (assimilable) nitrogen and oxygen. Yeasts used in alcohol production are not proteolytic and can use only low-molecular-weight nitrogenous compounds such as ammonium ion, urea, amino acids, or dipeptides (11, 20). Urea and liquid ammonia are being used in the fuel alcohol industry as inexpensive sources of nitrogen for yeast (12); diammonium phosphate is often added to must in wine making. In addition to the requirement for usable nitrogen, oxygen is required in small quantities for the synthesis of unsaturated fatty acids and sterols, which are both essential components of the yeast cell membrane (3). Unfortunately, oxygen is not available at optimal levels due to industrial practices and its lower solubility in mashes (12). Deficiencies of usable nitrogen and oxygen also affect the ethanol tolerance of yeast (12, 22). The judicious use of nutrients can lead to the production of more than 23% (vol/vol) ethanol by commercial yeast strains in batch fermentation (22).
The use of UHP in fuel and industrial alcohol production offers the advantages of providing yeast with a nitrogen source (in the form of urea) and a supply of oxygen, in addition to its bactericidal activity against lactic bacteria and other microbial contaminants in the mash. This study therefore investigates the use of UHP to control lactobacilli in the fermentation of starch-based feedstock (wheat mash) by yeast.
(This work is covered by U.S. patent application 09/271,877 [W. M. Ingledew, K. C. Thomas, and N. V. Narendranath, 18 March 1999, U.S. Patent Office] and Canadian patent application 2,300,807 [W. M. Ingledew, K. C. Thomas, and N. V. Narendranath, 17 March 2000, Canadian Patent Office].)
MATERIALS AND METHODS
Organisms.
An industrial strain of Saccharomyces cerevisiae (Allyeast Superstart; Alltech, Inc., Nicholasville, Ky.) was used. Five species of industrially important lactobacilli capable of extensive growth in mash within 36 h at 30°C and tolerance to >10% (vol/vol) ethanol were used. Two species were obtained from the Centro de Technologia Copersucar, Piracicaba, São Paulo, Brazil, and were tentatively identified to the species level and numbered biotype by API 50 CHL test kits (bioMérieux, Montreal, Quebec, Canada) as L. plantarum 1 and L. paracasei subsp. paracasei 2 (referred to here as L. paracasei). Two other strains, L. rhamnosus (ATCC 15280) and L. fermentum (ATCC 14931), were obtained from the American Type Culture Collection. The fifth strain was an industrial isolate labeled Cargill 3 from Cargill Corn Milling (Eddyville, Iowa). An API 50 CHL test kit for Lactobacillus identified this latter strain as L. paracasei subsp. paracasei 2, but it differed in microscopic and colony morphology compared to the L. paracasei strain obtained from Copersucar. Therefore, for the purposes of this study, we used the designation Lactobacillus sp. strain 3.
Preparation of inocula. (i) Yeast.
Eleven grams of Saccharomyces cerevisiae active dry yeast was dispersed into 99 ml of prewarmed (38°C), sterile, 0.1% (wt/vol) peptone water and incubated at 38°C for 20 min with periodic shaking. Aliquots (0.25 ml) of this suspension were added to each fermentor to obtain ∼106 viable yeast cells/ml.
(ii) Bacteria.
Lactobacilli were grown in 50 ml of MRS broth (Unipath, Nepean, Ontario, Canada) contained in 250-ml screw-capped side-arm Erlenmeyer flasks. Then, 4 ml of late-log-phase cultures was transferred to 1-liter screw-capped flasks containing 200 ml of MRS broth. The headspace of each flask was flushed with filter-sterilized (0.22-μm-pore-size membrane filter) CO2 gas, and the flasks were incubated in a G25 Controlled Environmental shaker (New Brunswick Scientific Co., Inc., Edison, N.J.) at 150 rpm and at 30°C. Growth of these organisms was monitored by measuring absorbance using a Klett-Summerson colorimeter (Klett Manufacturing Co., New York, N.Y.) equipped with a no. 66 red filter (420 to 660 nm), and the time for growth to early stationary phase was determined. A relationship between Klett units and the number of CFU per milliliter in mid-log-phase cultures was established for each strain. Bacterial cells from 1,000-ml cultures were aseptically harvested by centrifugation at 10,200 × g for 15 min at 4°C. The pellets were washed twice with sterile 0.1% (wt/vol) peptone water (Difco Laboratories, Detroit, Mich.), and the cells were then resuspended in 50 ml of sterile 0.1% (wt/vol) peptone water and chilled in ice until they were dispensed.
Viable cell count.
Viable cell counts were monitored by the membrane filtration technique (14). For enumeration of yeast cells, the membranes were incubated aerobically at 30°C for 48 h on the surface of YPD plates (10 g of yeast extract per liter, 10 g of peptone per liter, 20 g of dextrose per liter, 15 g of agar per liter) supplemented with 0.005% (wt/vol) gentamicin and 0.01% (wt/vol) oxytetracycline (Sigma Chemical Co.) to suppress the growth of bacteria. The plating was done in triplicate for each dilution used.
For enumeration of bacteria, membrane-filtered samples were placed on plates of MRS agar containing 0.001% cycloheximide (Sigma Chemical Co.) to inhibit the growth of yeast and incubated in a CO2 incubator (National Appliance Co., Portland, Oreg.) at 30°C for 48 h after two cycles of evacuating and refilling the chamber with commercial-grade (>99.5%) CO2. The results were expressed as CFU per milliliter.
Mashing of wheat.
Normal-gravity (∼21 g of dissolved solids/100 ml) wheat mash (with particulates left in the mash) was prepared as described by Narendranath et al. (19).
Determination of dissolved solids.
Portions of samples were centrifuged at 10,300 × g for 30 min, and the supernatants were collected and stored at −20°C until analyzed. Total dissolved solids in these supernatants were determined by measuring the specific gravity at 20°C with a DMA45 density meter (Anton Parr KG, Graz, Austria). The readings were converted to grams of dissolved solids per 100 ml.
Determination of the most suitable concentration of UHP.
Wheat mash was distributed into sterile, 250-ml screw-capped Erlenmeyer flasks at 50 ml/flask. For this particular set of experiments, L. paracasei was used since it is well adapted to fermentation conditions and tolerant of higher concentrations of ethanol. Appropriate quantities of the bacterial suspension were added to the mashes so that the bacterial numbers were approximately 107 CFU/ml. Six different concentrations of UHP (Sigma Chemical Co.) were tested in triplicate (Table 1). A 40% (wt/vol) solution of UHP was made in deionized water, filter sterilized through a 0.22-μm-pore-size membrane filter, and dispensed. The inoculated flasks were then placed at 30°C in an incubator shaker at 150 rpm. After 48 h, samples were withdrawn from the flasks and centrifuged at 10,200 × g for 30 min, and the supernatants were analyzed for lactic acid. It was previously established that there is a linear relationship between final lactic acid concentration and initial viable bacterial cell numbers (19).
TABLE 1.
Treatment combinations for evaluating the use of UHP in batch fermentation of unclarified wheat mash
| Treatment | Presence (+) or absence (−) of:
|
||||
|---|---|---|---|---|---|
| Yeasta | Bacteriab | Ureac | Hydrogen peroxidec | UHPc | |
| 1 | + | − | + | − | − |
| 2 | + | + | + | − | − |
| 3 | + | − | − | − | + |
| 4 | + | + | − | − | + |
| 5 | + | − | + | + | − |
| 6 | + | + | + | + | − |
Inoculated to give an initial number of 1.0 × 106 CFU/ml.
Inoculated to give an initial number of 1.0 × 107 CFU/ml.
Applied at a concentration of 30 mM.
Evaluation of the effects of UHP on lactobacilli.
Unclarified wheat mash was distributed in 500-ml quantities into 10 jacketed, sterile glass fermentors. Immediately after the 0-h sample was withdrawn, the mashes were inoculated with L. plantarum, L. paracasei, L. rhamnosus, L. fermentum, and Lactobacillus sp. strain 3 at approximately 107 CFU/ml, followed by the addition of a solution of 40% (wt/vol) UHP (a volume to give a final concentration of 32 mM). All tests were done in duplicate. Samples were withdrawn at 0, 2, and 4 h and analyzed in triplicate for viable bacterial numbers (CFU/milliliter) by the membrane filtration technique.
Use of UHP in batch fermentation of unclarified wheat mash.
Jacketed glass fermentors containing 500-ml quantities of wheat mash were connected to a circulating water bath maintained at 30°C throughout the fermentation and stirred magnetically (IKA-Labortechnik, Staufen, Germany). The experimental setup is shown in Table 1. Mashes were infected with L. paracasei at ∼107 CFU/ml. Samples were immediately withdrawn from infected fermentors for the determination of initial viable numbers of bacteria. After 90 min, 0.4 ml of glucoamylase (Allcoholase II; Alltech, Inc.) was added to all of the fermentors for saccharification. Exactly 30 min after the addition of glucoamylase, yeast was inoculated into all fermentors at approximately 106 CFU/ml (so that there was a preincubation period of 2 h for the UHP and hydrogen peroxide before any yeast inoculation). Then, samples were withdrawn at 0 h (immediately after yeast inoculation) and at 12, 24, 36, 48, and 72 h (after yeast inoculation) for analysis.
Bactericidal effectiveness of UHP in the presence of mash particles.
Liquefied wheat mash (α-amylase treated) was filtered through Whatman no. 1 filter paper, and the insoluble mash solids were collected, washed three times with sterile distilled water, and refiltered. Collected solids were spread on stainless steel trays and frozen at −40°C. Trays were placed in a tray dryer (Labconco Corp., Kansas City, Mo.) and lyophilized for 48 h. Once the particles were dry, the lumps were broken and powdered with a mortar and pestle and stored at room temperature.
The experiment was done in 250-ml screw-capped, side-arm flasks with 50 ml of MRS broth in each flask. The treatments included the use of UHP at two different doses (2 and 42.6 mM) in the presence or absence of wheat mash particles (10% [wt/vol]). Doses were chosen based on the observations made by Anders et al. (2) that (i) >1.5 mmol/liter of H2O2 would induce the cell death of lactic acid bacteria and (ii) 42.6 mmol/liter was the maximum concentration of UHP tested to manage lactic acid bacteria in grain mashes (although 30 mmol/liter is quite effective). All treatments contained L. paracasei inoculated at ∼107 CFU/ml, and all were done in duplicate. In treatments in which clear media were used, the growth of the organism was measured by determining the optical density using a Klett-Summerson colorimeter. In the presence of particles, samples were withdrawn at 0, 2, 4, 6, 12, and 24 h, and viable CFU counts (per milliliter) were assessed (in triplicate) using the membrane filtration technique.
Decomposition of hydrogen peroxide and UHP in wheat mash.
Normal-gravity wheat mash was prepared and distributed into two, jacketed glass fermentors in 400-ml quantities. The fermentors were connected through a circulating water bath maintained at 30°C and magnetically stirred. Hydrogen peroxide at 40 mM was added to one fermentor, while UHP at 40 mM was added to the other. This experiment was repeated three times. Samples were withdrawn at 0.5, 1, 1.5, 2, 3, and 5 h after addition of the agents and analyzed for the presence of hydrogen peroxide by fluorometry. The method involved (i) the hydrolysis of the stable reagent l-dichlorofluorescein diacetate by sodium hydroxide to the less-stable nonfluorescent compound l-dichlorofluorescein and (ii) the subsequent oxidation of l-dichlorofluorescein and measurement of the formed fluorescent compound dichlorofluorescein by the horseradish peroxidase-catalyzed reaction with hydrogen peroxide (18). Fluorescence was measured by using the primary filter 405 and the secondary filter 2A-12 (corresponding to a 468-nm excitation wavelength and a 519-nm emission wavelength) using a fluorometer (Model 111; GK Turner Associates, Palo Alto, Calif.). Concentrations of hydrogen peroxide were calculated from the standard curve prepared by using different known concentrations of hydrogen peroxide.
HPLC analysis.
Ethanol and lactic acid were determined by high-performance liquid chromatographic (HPLC) analysis. A 5-μl aliquot from a suitably diluted fermentation sample was analyzed using an HPX-87H column maintained at 40°C (Bio-Rad Laboratories, Ltd., Mississauga, Ontario, Canada) which analyzes sugars, alcohols, and organic acids. Sulfuric acid (5 mM) was used as the mobile phase at a flow rate of 0.7 ml/min. The components were detected with a differential refractometer (Model 410; Waters Chromatographic Division, Milford, Mass.). Boric acid (2% [wt/vol]) was used as the internal standard. The data were processed using the Maxima 810 computer program (Waters Chromatographic Division).
Preparation of cell extracts.
Cells of lactobacilli were grown, harvested, washed, and resuspended at (∼1.5 × 1010 cells/ml) in 40 mM potassium phosphate buffer (pH 7.2). The cell suspensions were passed three times through a French pressure cell (American Instrument Co., Inc., Silver Spring, Md.) at 20,000 lb/in2. Cell debris was removed from each extract by centrifugation at 10,300 × g for 30 min. The supernatants (cell extracts) were used as the enzyme source. The entire procedure was carried out at 4°C. The enzyme assays were done immediately, and for the estimation of total protein cell extracts were stored at 4°C for no longer than 24 h.
Specific activity of NADH peroxidase.
Oxidation of NADH (Sigma Chemical Co.) by hydrogen peroxide at 30°C was monitored spectrophotometrically at 340 nm. The NADH oxidizing activity (in the absence of H2O2) was subtracted. The reaction mixture (1 ml) contained 40 mM potassium phosphate buffer (pH 7.2), 0.2 mM EDTA, 0.17 mM NADH, 0.02 mM FAD, cell extract (0.05 ml), and 1.3 mM H2O2. The reaction was initiated by adding H2O2. All solutions used in the assay were flushed with oxygen-free nitrogen gas for about 10 min. Total protein in the cell extract was measured using the Bio-Rad Protein Assay Kit II. The specific activities presented are the means of three separate assays using a different cell extract for each assay.
RESULTS
Most suitable concentration of UHP for controlling lactobacilli.
UHP, when used at a concentration of 32 mmol/liter in unclarified wheat mash contaminated with L. paracasei, reduced the final lactic acid produced (after 48 h at 30°C) from 1.14% (wt/vol) (in the control) to 0% (wt/vol) (Table 2). When added to mash, UHP breaks down into urea and hydrogen peroxide. If yeast is added at the beginning or immediately after the addition of UHP, the hydrogen peroxide would be decomposed into water and molecular oxygen, resulting in the loss of the bactericidal effect of hydrogen peroxide on contaminating lactobacilli. Therefore, it is necessary to preincubate the mash with UHP for a specified duration prior to yeast inoculation. This may be done during saccharification of the mash or postsaccharification (in the fermentor) prior to yeast addition. For maximal bactericidal action on high levels of contaminating bacteria, a preincubation period for 2 h with UHP was required before mashes were inoculated with yeast (data not shown).
TABLE 2.
The 48-h concentration of lactic acid produced by L. paracasei inoculated at ∼107 CFU/ml into unclarified wheat mash at 30°C in the presence of UHP at various levels
| UHP (mmol/liter) | Mean lactic acid concn (% [wt/vol]) ± SDa |
|---|---|
| 0 | 1.14 ± 0.03 |
| 2.1 | 1.10 ± 0.01 |
| 5.4 | 1.05 ± 0.01 |
| 10.7 | 1.01 ± 0.03 |
| 21.3 | 0.57 ± 0.03 |
| 32.1 | 0.00 ± 0.00 |
| 42.6 | 0.00 ± 0.00 |
Mean of triplicate samples ± the standard deviation.
Reduction of lactobacilli in the presence of UHP.
All five industrially important isolates of lactobacilli exhibited 4- to 5-log reductions in viable cell numbers in 2 h in the presence of UHP (Table 3). The results indicate that UHP is effective in preventing the growth of yield-reducing bacterial contaminants in the fuel alcohol industry. The bactericidal effectiveness of UHP did not differ significantly when added as a powder or in the form of a filter-sterilized 40% (wt/vol) solution in deionized water (data not shown).
TABLE 3.
Survival of various Lactobacillus strains in unclarified wheat mash at 30°C in the presence of UHP at 32.1 mmol/liter
| Time (h) | Survival (CFU/ml)a of:
|
||||
|---|---|---|---|---|---|
| L. plantarum | L. paracasei | Lactobacillus sp. strain 3 | L. rhamnosus | L. fermentum | |
| 0 | 1.09 × 107 | 1.15 × 107 | 0.88 × 107 | 0.85 × 107 | 0.83 × 107 |
| 2 | 5.67 × 102 | 8.67 × 102 | 7.67 × 102 | 3.00 × 102 | 7.50 × 102 |
| 4 | 5.83 × 102 | 8.33 × 102 | 7.67 × 102 | 3.33 × 102 | 7.00 × 102 |
All values are the mean of duplicate samples. Plating was done in triplicate.
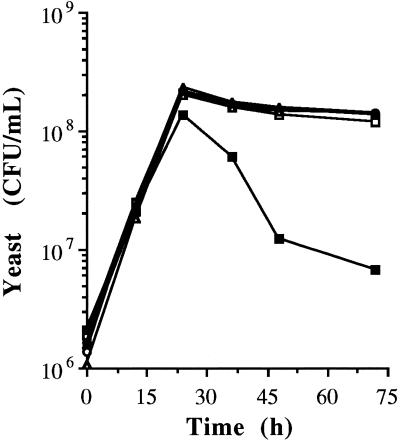
Batch fermentations of unclarified wheat mash contaminated (infected) with L. paracasei at ∼107 CFU/ml were carried out in the presence or absence of UHP. The details of the treatments are given in Materials and Methods. The fermentations were complete (<0.05 g of dissolved solids remaining/100 ml) within 36 h in all of the treatments. Viable yeast counts reached a maximum during the first 24 h. Compared to the controls, the yeast viable numbers were higher in treatments with UHP and with hydrogen peroxide. The numbers of viable yeast cells were lowest in samples treated with bacteria but in which no agents were added (Fig. 1). In samples with bacteria but without agents this is likely due to the competition for nutrients by the bacteria as well as the production of lactic acid, which at levels of 0.8% (wt/vol) begin to stress the yeast (13).
FIG. 1.
Growth of yeast during the fermentation of wheat mash at 30°C. Symbols: □, control (yeast plus 30 mM urea, no bacteria); ■, yeast plus L. paracasei plus 30 mM urea (no agents); ○, yeast plus UHP at 30 mM; ●, yeast plus L. paracasei plus UHP at 30 mM; ▵, yeast plus 30 mM H2O2 plus 30 mM urea (added separately); ▴, yeast plus L. paracasei plus 30 mM H2O2 plus 30 mM urea (added separately). In all cases, yeast was inoculated at ∼106 CFU/ml. Where added, L. paracasei was inoculated at ∼107 CFU/ml.
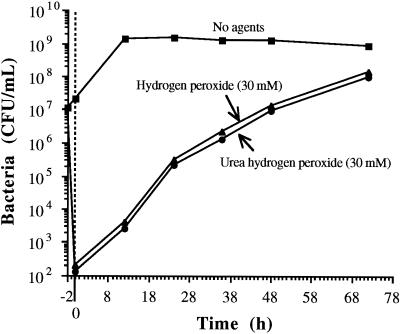
The number of viable bacteria in mash dropped from ∼107 to ∼2 × 102 CFU/ml in the first 2 h when treated with UHP or hydrogen peroxide. Once yeast was inoculated, residual hydrogen peroxide in the medium was decomposed by the action of catalase known to be present in microbodies of yeast cells (24). We have verified the presence of catalase in this yeast strain by using the traditional catalase test. Once hydrogen peroxide is degraded, the remaining viable bacteria are able to reinitiate growth (Fig. 2). Even though the fermentation was effectively over at 36 h, however, the bacteria continue to grow (Fig. 2), presumably on substrates not used by yeast and on lytic products from yeast. At 36 h, the bacterial numbers were too low to have caused significant reductions of ethanol yield; control of the fermentation at the critical time was effected by this practice.
FIG. 2.
Growth of L. paracasei in fermenting wheat mash at 30°C in the presence or absence of hydrogen peroxide or UHP. All treatments had yeast inoculated at ∼106 CFU/ml (at 0 h) following a 2-h preincubation with the antimicrobials. UHP (30 mM) yields 30 mmol/liter of H2O2 and 30 mmol/liter of urea.
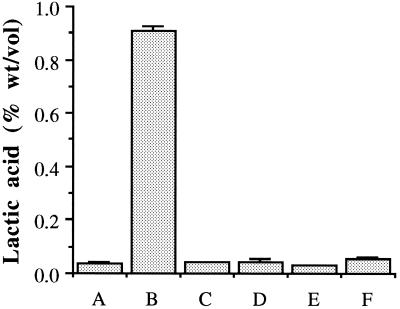
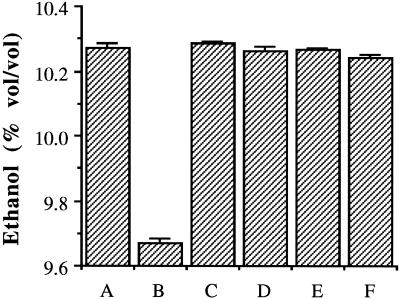
The lactic acid concentration in the medium when UHP was used was as low as that in the treatment with yeast alone with no bacteria (∼0.03% [wt/vol]) (Fig. 3). In the treatment in which hydrogen peroxide alone was used, 0.05% (wt/vol) lactic acid was detected. In the medium in which neither of the bactericidal agents was used, however, 0.9% (wt/vol) lactic acid was found at the time when the ethanol production was maximal in all treatments. This level (0.9% [wt/vol]) severely affected yeast viability (Fig. 1). The maximum concentrations of ethanol produced in all of the treated fermentors were not significantly different from each other, but in the fermentor that had neither UHP nor hydrogen peroxide to kill the L. paracasei there was a 5.84% reduction in ethanol yield compared to the control with yeast alone and no agents added (Fig. 4).
FIG. 3.
Concentration of lactic acid produced (after 36 h) by L. paracasei in the presence or absence of UHP or hydrogen peroxide in fermenting wheat mash at 30°C. Columns: A, control (yeast plus 30 mM urea, no bacteria); B, yeast plus L. paracasei plus 30 mM urea (no agents); C, yeast plus UHP at 30 mM; D, yeast plus L. paracasei plus UHP at 30 mM; E, yeast plus 30 mM H2O2 plus 30 mM urea (added separately); F, yeast plus L. paracasei plus 30 mM H2O2 plus 30 mM urea (added separately). In all cases, yeast was inoculated at ∼106 CFU/ml. Where added, L. paracasei was inoculated at ∼107 CFU/ml. Error bars indicate the mean of duplicate values ± the standard deviation.
FIG. 4.
Concentration of ethanol after 36 h of fermentation of wheat mash by S. cerevisiae at 30°C. Columns are as defined in Fig. 3. Error bars indicate mean of duplicate values ± the standard deviation.
Decomposition and bactericidal effectiveness of UHP in the presence of particulate matter.
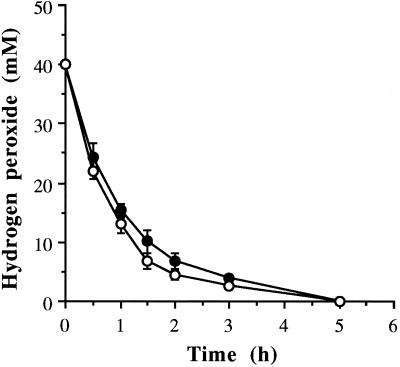
Hydrogen peroxide (released from the breakdown of UHP in the medium) is presumed to be quickly decomposed in the presence of particulate materials. Results indicated that a dose of 2 mmol of UHP per liter is enough to kill L. paracasei in a clear medium (MRS broth), whereas a much higher dose is needed in the presence of grain particles (Table 4). A similar kind of experiment was conducted with clarified wheat mash (in which the mash particles were filtered out using Whatman no. 4 filter paper, followed by a filtration using diatomaceous earth). The clarified mash was then passed through a 0.45-μm (pore-size) Versaflow filter (Gelman Sciences, Ltd.) and used. It was then found that UHP at a dose as low as 2 mmol/liter killed L. paracasei when inoculated at ∼107 CFU/ml. There was absolutely no bacterial growth observed for up to 48 h after the addition of UHP (data not shown). It was previously found that UHP in wheat mash at a concentration of 40 mM was totally decomposed in 5 h. No significant differences were observed in the decomposition of hydrogen peroxide, whether added as H2O2 or as UHP (Fig. 5).
TABLE 4.
Survival of L. paracasei in the presence or absence of particulate matter at two different doses of UHP
| Time (h) |
L. paracasei survival (CFU/ml)a in:
|
|||
|---|---|---|---|---|
| MRS broth at:
|
MRS broth plus 10% (wt/vol) wheat mash particles at:
|
|||
| 2 mmol/liter | 42.6 mmol/liter | 2 mmol/liter | 42.6 mmol/liter | |
| 0 | 1.07 × 107 | 1.02 × 107 | 1.06 × 107 | 1.08 × 107 |
| 2 | 2.92 × 106 | <102 | 1.83 × 106 | 3.21 × 104 |
| 4 | <102 | – | 2.47 × 106 | 4.17 × 102 |
| 6 | – | – | 2.82 × 106 | 5.67 × 102 |
| 12 | – | – | 9.15 × 107 | 7.83 × 102 |
| 24 | – | – | 8.57 × 108 | 1.27 × 103 |
Values are the means of duplicate samples. A value of <102 indicates that no colonies were seen on the plates. –, the Klett value remained at 0, indicating no regrowth of the bacteria (since media was clear, growth was measured by determining the optical density when MRS broth was used).
FIG. 5.
Decomposition of hydrogen peroxide when applied as H2O2 (○) or in the form of UHP (●) in normal-gravity wheat mash at 30°C. The error bars indicate ± the standard deviation obtained from triplicate analyses.
NADH peroxidase activity.
The NADH peroxidase activities of all five selected industrially important lactobacilli were assayed. Assays were performed on cultures grown in MRS broth at 30°C in screw-capped Erlenmeyer flasks flushed with sterile CO2 gas, on cultures grown in MRS broth with H2O2 at a sublethal level (0.75 mM), and on cultures transferred to fresh MRS broth without H2O2 from those grown in the presence of 0.75 mM H2O2. The final set of experiments was done to see if the activity was reduced in the absence of hydrogen peroxide. The data were subjected to Duncan's multiple range test (SAS Institute, Cary, N.C.). A significant increase (P = 0.05) in NADH peroxidase activity was observed with all five Lactobacillus strains studied, when grown in the presence of a sublethal concentration (0.75 mM) of H2O2 (Table 5). Upon transferring them back to fresh MRS broth without H2O2 (for ∼18 h), there was a significant loss (P = 0.05) in the specific activity. These data suggest that if a high-enough concentration of H2O2 was administered, the organisms would be unable to adapt quickly enough to enzymatically degrade the antimicrobial at a meaningful rate and would therefore be killed.
TABLE 5.
NADH peroxidase activity in various Lactobacillus strains grown in MRS broth at 30°C in the presence or absence of hydrogen peroxide (0.75 mmol/liter)
| Organism | Mean sp act (μmol of NADH oxidized/min/mg of total protein)a ± SD
|
||
|---|---|---|---|
| No hydrogen peroxide (control) | Hydrogen peroxide (0.75 mM) added | No hydrogen peroxideb | |
| L. plantarum | 0.143 ± 0.012C | 0.233 ± 0.031A | 0.159 ± 0.018B |
| L. paracasei | 0.093 ± 0.020C | 0.241 ± 0.023A | 0.119 ± 0.031B |
| Lactobacillus sp. strain 3 | 0.217 ± 0.012C | 0.324 ± 0.021A | 0.215 ± 0.030B |
| L. rhamnosus | 0.051 ± 0.029C | 0.347 ± 0.018A | 0.104 ± 0.007B |
| L. fermentum | 0.062 ± 0.003C | 0.149 ± 0.021A | 0.052 ± 0.009B |
The values given are the mean of three separate analyses ± the standard deviation. The superscript capital letters indicate the levels of significance (P = 0.05) between treatments and not between organisms (based on Duncan's multiple range test).
In this case, cells were grown in MRS broth containing 0.75 mM H2O2 until late log phase and then transferred to fresh media for ∼18 h to stationary phase without hydrogen peroxide.
DISCUSSION
The occurrence of contaminants in an industrial-scale ethanol fermentation process using starch- or sugar-based feedstocks is unavoidable. UHP (which eliminates the risk of the emergence of antibiotic-resistant microorganisms) appears to be an ecologically friendly agent for effectively managing lactic acid bacteria and other bacterial contaminants in the production of industrial or fuel ethanol.
Batch fermentations of mashes were complete by 36 h in all cases in which the effect of UHP on L. paracasei was studied. This was due to the increased availability of assimilable nitrogen under all conditions. Since grain mashes are, in general, deficient in usable nitrogen, both yeast growth and fermentation rate benefit from urea whether it is added as free urea or as UHP. It was reported by Thomas and Ingledew (23) that the fermentation of wheat mash without supplementation with an assimilable nitrogen source is slow (ca. 120 h), but with added urea the fermentation completed in less than 72 h. The fermentation rate in the present study, in which urea from UHP served as the assimilable nitrogen source, was similar to that reported by these workers. Jones and Ingledew (15) have shown complete utilization of urea by yeast during the early stages of fermentation. In experiments done to compare diammonium phosphate with urea (in equimolar quantities, so that both would provide yeast with equal amounts of nitrogen), along with hydrogen peroxide, urea appeared to be a better source of nitrogen in combination with hydrogen peroxide at 30 mmol/liter (unpublished results). Interestingly, the availability of UHP (in a solid, stable form) makes this compound a good choice for use in the production of industrial or fuel ethanol.
Hydrogen peroxide is a natural product of the action of some flavoprotein oxidases of lactic acid bacteria with oxygen, and it may accumulate in the “aerobic” cultures of many strains. In lactococci sensitive to hydrogen peroxide, preexposure to a sublethal concentration of the compound allowed the organism to grow in the presence of a lethal concentration of hydrogen peroxide (8). Codon (8) also observed a simultaneous induction of NADH peroxidase and, to a lesser extent, of NADH oxidase. Since lactic acid bacteria lack catalase (due to their inability to synthesize hemoporphyrins), they use NADH peroxidase to rid themselves of hydrogen peroxide when it is present at sublethal levels (2, 21). NADH peroxidase catalyzes the following reaction: NADH + H+ + H2O2→2H2O + NAD. As the activity of NADH peroxidase is rapidly lost when the organism grows in the absence of H2O2 (Table 5), the chances of resistant mutants (organisms that would constitutively express high levels of NADH peroxidase) is reduced.
UHP not only exhibits excellent bactericidal activity against lactobacilli but also has the important advantage of providing the fermentation yeast with usable nitrogen in the form of urea which, with oxygen, are essential nutrients for stimulating yeast growth and fermentation rate (12). This serves to prevent “sluggish” or “stuck” fermentations that would lead to a reduction in alcohol yields. UHP leaves no residues when added to the fermentation medium. The pH of the mash is not affected as it would be if ammonium salts were employed, nor are there residues in the whole or thin stillage. Moreover, hydrogen peroxide (at the dose recommended) can also eliminate a wide variety of contaminating bacteria that are present in low levels in the mash. We found that UHP (2 mM) was enough to kill Pediococcus damnosus (ATCC 29358), Pediococcus sp. (BSO 77), and Zymomonas anaerobia in MRS broth when inoculated at ∼107 CFU/ml. Block (6) has shown hydrogen peroxide to be lethal to other bacterial species, such as Staphylococcus aureus, Escherichia coli, Streptococcus spp., and spore-forming Bacillus spp.
UHP proves to be an ideal additive for use in the production of industrial or fuel ethanol. We advocate the use of 2 mmol of urea hydrogen peroxide or hydrogen peroxide per liter as a disinfectant only in clear mashes. Yeast nutrients would still be required. For mashes with particulates, ∼32 mmol of UHP (or hydrogen peroxide) per liter is required. At this concentration, UHP provides all of the usable nitrogen and oxygen needed to ensure predictable, trouble-free fermentations; hydrogen peroxide at 32 mmol/liter would only serve as a disinfectant.
ACKNOWLEDGMENTS
We thank J. Finguerut, Centro de Technologia Copersucar, Piracicaba, SP, Brazil, and Cargill Corn Milling, Eddyville, Iowa (D. Schisler) for the strains provided and D. A. Bautista for aid with the statistical analyses.
N.V.N. thanks the University of Saskatchewan for providing scholarship support. The Western Grains Research Foundation, the College of Agriculture, and the Natural Sciences and Engineering Research Council are acknowledged for grant support (to W.M.I.).
REFERENCES
- 1.Amin V M, Olson N F. Effect of temperature on stability of hydrogen peroxide in milk. J Dairy Sci. 1967;50:1336–1338. doi: 10.3168/jds.S0022-0302(67)87452-6. [DOI] [PubMed] [Google Scholar]
- 2.Anders R F, Hogg D M, Jago G R. Formation of hydrogen peroxide by group N streptococci and its effects on their growth and metabolism. Appl Microbiol. 1970;19:608–612. doi: 10.1128/am.19.4.608-612.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreasen A A, Stier T. Anaerobic nutrition of Saccharomyces cerevisiae. J Cell Comp Physiol. 1954;43:271–281. doi: 10.1002/jcp.1030430303. [DOI] [PubMed] [Google Scholar]
- 4.Aquarone E. Penicillin and tetracycline as contamination control agents in alcoholic fermentation of sugarcane molasses. Appl Microbiol. 1960;8:263–268. doi: 10.1128/am.8.5.263-268.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee N L. Use of hydrogen peroxide as a milk preservative. Ind Med Gazette. 1947;1947:156–159. [PMC free article] [PubMed] [Google Scholar]
- 6.Block S S. Peroxygen compounds. In: Block S S, editor. Disinfection, sterilization, and preservation. 4th ed. Philadelphia, Pa: Lea & Febiger; 1991. pp. 167–181. [Google Scholar]
- 7.Chang I S, Kim B H, Shin P K. Use of sulfite and hydrogen peroxide to control bacterial contamination in ethanol fermentation. Appl Environ Microbiol. 1997;63:1–6. doi: 10.1128/aem.63.1.1-6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condon S. Responses of lactic acid bacteria to oxygen. FEMS Microbiol Rev. 1987;46:269–280. [Google Scholar]
- 9.Day W H, Serjak W C, Stratton J R, Stone L. Antibiotics as contamination control agents in grain alcohol fermentations. J Agric Food Chem. 1954;2:252–258. [Google Scholar]
- 10.Hynes S H, Kjarsgaard D M, Thomas K C, Ingledew W M. Use of virginiamycin to control the growth of lactic acid bacteria during alcoholic fermentation. J Ind Microbiol Biotechnol. 1997;18:284–291. doi: 10.1038/sj.jim.2900381. [DOI] [PubMed] [Google Scholar]
- 11.Ingledew W M. Yeast for the production of fuel ethanol. In: Rose A H, Harrison J S, editors. The yeasts. 5. Yeast technology. London, United Kingdom: Academic Press; 1993. pp. 245–291. [Google Scholar]
- 12.Ingledew W M. The Biochemistry of alcohol production. In: Lyons T P, Kelsall D R, Murtagh J E, editors. The alcohol textbook. 2nd ed. Nottingham, United Kingdom: Nottingham University Press; 1995. pp. 55–79. [Google Scholar]
- 13.Ingledew W M. Alcohol production by Saccharomyces cerevisiae: a yeast primer. In: Jacques K, Lyons T P, Kelsall D R, editors. The alcohol textbook. 3rd ed. Nottingham, United Kingdom: Nottingham University Press; 1999. pp. 49–87. [Google Scholar]
- 14.Ingledew W M, Burton J D, Hysert D W, Van Gheluwe G. Membrane filtration: survival of brewing microbes on membranes during storage at reduced humidities. J Am Soc Brew Chem. 1980;38:125–129. [Google Scholar]
- 15.Jones A M, Ingledew W M. Fuel alcohol production: appraisal of nitrogenous yeast foods for very high gravity wheat mash fermentation. Proc Biochem. 1994;29:483–488. [Google Scholar]
- 16.Keston A S, Brandt R. The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal Biochem. 1965;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- 17.Khachatourians G G. Agricultural use of antibiotics and the evolution and transfer of antibiotic-resistant bacteria. Can Med Assoc J. 1998;159:1129–1136. [PMC free article] [PubMed] [Google Scholar]
- 18.Luck H. The use of hydrogen peroxide as a dairy preservative. Dairy Sci Abstr. 1956;18:363–386. [Google Scholar]
- 19.Narendranath N V, Hynes S H, Thomas K C, Ingledew W M. Effects of lactobacilli on yeast-catalyzed ethanol fermentations. Appl Environ Microbiol. 1997;63:4158–4163. doi: 10.1128/aem.63.11.4158-4163.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson C A, Ingledew W M. Utilization of peptides by a lager brewing yeast. J Am Soc Brew Chem. 1999;57:1–8. [Google Scholar]
- 21.Piard J C, Desmazeaud M. Inhibiting factors produced by lactic acid bacteria. 1. Oxygen metabolites and catabolism end products. Lait. 1991;71:525–541. [Google Scholar]
- 22.Thomas K C, Hynes S H, Jones A M, Ingledew W M. Production of fuel ethanol from wheat by VHG technology: effect of sugar concentration and fermentation temperature. Appl Biochem Biotechnol. 1993;43:211–226. [Google Scholar]
- 23.Thomas K C, Ingledew W M. Relationship of low lysine and high arginine concentrations to efficient ethanolic fermentation of wheat mash. Can J Microbiol. 1992;38:626–634. doi: 10.1139/m92-103. [DOI] [PubMed] [Google Scholar]
- 24.Walker G M. Yeast physiology and biotechnology. Chichester, England: John Wiley & Sons, Ltd.; 1998. [Google Scholar]
- 25.Zinner D D, Duany L F, Liorente M. Effects of urea peroxide in anhydrous glycerol on gingivitis and dental plaque. J Preventive Dent. 1978;5:38–40. [PubMed] [Google Scholar]