Abstract
Introduction
No large‐scale characterizations of neurofilament light chain (NfL) have been conducted in diverse populations.
Methods
Baseline data were analyzed among n = 890 Mexican Americans and n = 813 non‐Hispanic Whites from the multi‐ethnic Health & Aging Brain among Latino Elders (HABLE) study. Plasma NfL was measured on the Simoa platform.
Results
In unadjusted models, NfL was significantly associated with age (P < .001), hypertension (P < .001), dyslipidemia (P = .02), and diabetes (P < .001). Covarying for age and sex, NfL was associated with neurodegeneration (P < .001) and global amyloid burden levels (P = .02) in a subset with available data. NfL levels were significantly associated with diagnostic groups (Normal Cognition [NC], mild cognitive impairment [MCI], Dementia; P < .001); however, there was no cut‐score that yielded acceptable diagnostic accuracy. NfL levels produced a sensitivity of 0.60 and specificity of 0.78 with negative predictive value of 89% for detecting amyloid positivity.
Discussion
Plasma NfL levels are significantly impacted by age and medical co‐morbidities that are common among older adults, which complicate its utility as a diagnostic biomarker
Keywords: Alzheimer's disease, diversity, Hispanic, Mexican American, plasma NfL
1. INTRODUCTION
Although cerebrospinal fluid (CSF) and positron emission tomography (PET) biomarkers will likely serve as the ultimate diagnostic tools for detecting Alzheimer's disease (AD), there remains an urgent need for a cost‐effective, non‐invasive, multi‐tiered approach for determining who should and should not undergo these expensive and more invasive procedures. Our team has proposed that a multi‐tiered neurodiagnostic process can meet these needs, 1 and have shown that blood‐based biomarker profiles can be an accurate first step in detecting AD, 2 , 3 mild cognitive impairment (MCI), 4 Parkinson's disease, 5 dementia with Lewy Bodies, 6 as well as AD and MCI among adults with Down syndrome. 7 , 8 , 9
With the shift in diagnostic framework for AD to AT(N), increased work has been conducted looking at the utility of neurodegenerative biomarkers (N) in addition to amyloid beta (A) and tau (T). 10 One of the more interesting putative neurodegenerative biomarkers in recent years has been neurofilament light chain (NfL), a cytoskeleton protein expressed in large‐caliber myelinated axons released into the extra‐cellular fluid as a consequence of axonal damage. 11 Although NfL can be detected across the lifespan, it has received a great deal of attention as a potential biomarker for AD due to numerous studies showing an increase in levels associated with this diagnostic state. 12 , 13 , 14 , 15 , 16 A recent meta‐analysis found that blood and CSF NfL levels correlated well (r = 0.59). 17 Among patients with amnestic mild cognitive impairment (aMCI), plasma NfL was found to be elevated when compared to cognitively normal older adults and higher among those who had lower hippocampal volume and total gray matter volume in the middle temporal and left inferior gyrus as measured on magnetic resonance imaging (MRI). 18 Findings have not always been consistent, though, as Mielke et al. 19 recently found no cross‐sectional associations between CSF or plasma NfL with any neuroimaging or cognitive measures among non‐demented older adults. However, higher baseline plasma NfL was associated with greater cortical thinning and decreased diffusion on MRI fractional anisotropy, indicating loss of microstructural integrity over time. Change in plasma NfL was also associated with change in global cognition, attention, and amyloid PET levels. 20 , 21 , 22 , 23
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional methods and sources (eg, PubMed). No large‐scale studies characterizing plasma neurofilament light chain (NfL) among diverse cohorts was identified.
Interpretation: Our findings indicate the plasma NfL is significantly impacted by a number of demographic and medical factors that must be taken into account if this marker is to become clinically useful. Plasma NfL was significantly associated with a broad range of cognitive and imaging outcomes across ethnic groups; however, the links frequently varied by ethnic group.
Future directions: Plasma NfL may have multiple clinical uses; however, additional work on the clinical parameter is required for the creation of clinical guidelines. Additional work is needed investigating the cross‐sectional and longitudinal links of plasma NfL with cognitive‐related outcomes across diverse populations if this marker is to be clinically implemented.
Few research studies have examined plasma NfL in diverse populations despite the emerging literature demonstrating the impact of race/ethnicity on AD biomarkers. 20 , 24 , 25 , 26 Here we examined data from the Health & Aging Brain among Latino Elders (HABLE) study to: (1) characterize the impact of demographic factors and medical co‐morbidities on plasma NfL levels, (2) examine the link between plasma NfL and cognitive and neuroimaging outcomes, (3) determine if any of these findings vary by ethnicity, and (4) explicitly test two potential context of uses (COUs) for plasma NfL. COU1 was plasma NfL as a diagnostic biomarker for MCI and AD and COU2 was plasma NfL as a first step in a multi‐tiered process for detecting cerebral amyloid.
2. METHODS
2.1. Participants
The HABLE study is an ongoing, longitudinal, community‐based project seeking to understand health disparities in MCI and AD among Hispanic, Mexican Americans. Participant recruitment for HABLE includes a community‐based participatory research (CBPR) approach. 27 The CBPR approach has been used successfully as a recruitment modality for reaching underserved and minority populations. It involves collaborating with local communities through outreach (holding community events, seminars), word of mouth, marketing modalities (newspaper, television, radio), and providing back information (clinical lab work, MRI clinical reads, neuropsychological test results) to the participants and their health care providers. Baseline visits of the HABLE study will continue until n = 1000 per group are enrolled. Recruitment has been slowed due to coronavirus disease 2019 (COVID‐19). The response rate was high with the CBPR approach, with 82% scheduled for a visit who were reached by recruitment methods and qualified for the study. 28 The HABLE protocol includes an interview, functional exam, blood draw for clinical labs and biobanking, neuropsychological testing, and 3T MRI of the brain. A subset of participants underwent amyloid PET scans using florbetaben (18F) as part of a pilot study. All aspects of the study protocol can be conducted in Spanish or English, depending on the participant's preference. The HABLE study is conducted under institutional review board (IRB)–approved protocols and each participant (or his/her legal representative) signs written informed consent. The HABLE database is publicly available through the University of North Texas Health Science Center (UNTHSC) Institute for Translational Research (ITR) webpage.
2.2. Neuropsychological assessment
The neuropsychological test battery includes the following: Mini Mental Status Exam (MMSE), 29 Wechsler Memory Scale‐Third Edition (WMS‐III) Digit Span and Logical Memory (LM1, LM2), 30 Digit Symbol Substitution, Trail Making Test Parts A and B (TMTA, TMTB), 31 Spanish‐English Verbal Learning Test (SEVLT), 32 Animal Naming (semantic fluency), 32 FAS (phonemic fluency), 33 as well as the American National Adult Reading Test (English‐speakers), 34 and Word Accentuation Test (Spanish‐speakers). 35 An informant interview is also conducted for completion of the Clinical Dementia Rating (CDR) Scale. 36
2.3. Diagnostic classification
Cognitive diagnoses are assigned algorithmically (decision tree) and verified at consensus review per as follows: Normal Control (NC) = no cognitive complaints, CDR sum of boxes score of 0 and cognitive tests scores broadly within normal limits; Mild Cognitive Impairment (MCI): cognitive complaint (self or other), CDR sum of boxes score between 0.5 and 2.0, and at least one cognitive test score falling 1.5 standard deviation (SD) below normative ranges; Dementia: CDR sum of boxes score > = 2.5 and at least two cognitive test scores 2 SD below normative ranges. Medical diagnoses are assigned by licensed professionals (NP, DO, and/or MD) during consensus review based on fasting clinical labs, objective measures, self‐report, and current medications.
2.4. Neuroimaging
MRI Data. The HABLE MRI results were acquired on a 3T Siemens Magnetom Skyra whole‐body scanner according to the Alzheimer's Disease Neuroimaing Initiative (ADNI) 3 protocols. The following scan sequences are acquired: T1‐weighted whole brain volumetric (SPoiled Gradient–Recalled [SPGR]; 1.1 × 1.1 × 1.2 mM; rotational time [TR] = 2300 ms; echo time [TE] = 2.93 ms, T1 = 900 ms), susceptibility weighted imaging (SWI), diffusion tensor imaging (DTI), 3D Arterial Spin Labeling (3D ASL), and high resolution (0.4 × 0.4 mM × 2 mM) T2‐weighted hippocampal high‐resolution (HHR) scans. For this study, the “meta‐ROI” for examination of the neurodegeneration (ie, N) component of the AT(N) framework 37 was examined. PET Scans. In a pilot study, n = 55 participants (n = 33 Mexican American, n = 22 non‐Hispanic white) underwent amyloid PET scans using Siemens Biograph Vision 450 whole‐body PET/CT (computed tomography) scanner following the ADNI3 protocol for florbetaben scans. Scans were processed to derive standardized uptake value ratio (SUVR) levels at the USC Stevens Neuroimaging and Informatics Institute per ADNI protocols, using FreeSurfer‐derived regions of interest (ROIs) and whole cerebellum as the reference region. Consistent with the ADNI3 protocols, a global region of interest was composed of frontal, anterior/posterior cingulate, lateral parietal, and lateral temporal cortex. A global SUVR >1.08 was considered to be amyloid positive.
2.5. Blood collection and processing procedures
Fasting blood collection and processing were completed based on the international guidelines for AD biomarker studies and processed within 2 hours (stick‐to‐freezer). 38 Plasma NfL was assayed on a single‐plex plate using the ultra‐sensitive Simoa (single molecule array) technology platform HD‐1 (Quanterix.com). The ITR Biomarker Core has conducted >5000 NfL assays using this platform and all coefficients of variability (CVs) were <4%.
2.6. Statistical analyses
Statistical analyses were conducted using SPSS 25 (IBM) statistical software. To avoid significant effect of outliers, a cut‐score of 4 SD above the mean was set for a maximum NfL value of 74.75 pg/mL. Pearson correlations were conducted to examine correlations between NfL levels and specific demographic characteristics (age and education). Analyses of variance (ANOVAs) were also conducted to evaluate additional demographic factors (ethnicity, sex) as well as the medical co‐morbidity index. Linear regression models were utilized to further determine the relationship between NfL and demographic as well as clinical variables (medical co‐morbidities), neuropsychological test performance, MRI biomarkers, and amyloid PET global SUVR. Logistic regression models were conducted to determine the predictive diagnostic performance of plasma NfL in detecting diagnosis and amyloid positivity. Age and sex were included as covariates in all models. Note, analyses were also run using BoxCox transformed NfL values and all significant findings remained as presented.
3. RESULTS
A total sample of 1705 participants had available plasma NfL levels in the database and were included in this study. The cohort had an average age of 66.49 (SD = 8.76) and education of 12.35 (SD = 4.82). Sixty percent were female and 52% self‐identified as Mexican American. With regard to medical diagnosis, 59% were diagnosed with hypertension, 63% with dyslipidemia, and 25% with diabetes. The demographic characteristics of the cohort can be found in Table 1.
TABLE 1.
Descriptive statistics of cohort (n = 1705)
| Total Cohort | Mexican American N = 890 | Non‐Hispanic White N = 813 | P‐value | |
|---|---|---|---|---|
| Age, Mean (SD) | 66.49 (8.76) | 63.87 (8.02) | 69.34 (8.65) | <.001* |
| Years of Education, Mean (SD) | 12.35 (4.82) | 9.48 (4.61) | 15.49 (2.55) | <.001* |
| Sex (% female) | 60% | 66% | 54% | <.001* |
| Hypertension (% yes) | 59% | 63% | 55% | =.001* |
| Diabetes (% yes) | 25% | 36% | 13% | <.001* |
| Dyslipidemia (% yes) | 63% | 64% | 61% | =.120 |
| Plasma NfL pg/mL, Mean (SD) | 19.06 (11.77) | 17.43 (11.97) | 20.87 (11.29) | <.001* |
| Cognitive Diagnosis % | =.005* | |||
| NC | 79% (n = 1,351) | 76% (n = 676) | 83% (n = 673) | |
| MCI | 14% (n = 243) | 17% (n = 152 ) | 11% (n = 91) | |
| Dementia | 7% (n = 111) | 7% (n = 62) | 6% (n = 49) |
Abbreviations: NfL, neurofilament light chain; NC, normal cognition; MCI, mild cognitive impairment.
* P < .05.
3.1. Demographic factors and NfL levels
In unadjusted models, age (r2 = 0.417, P < .001) was significantly correlated with higher NfL levels, whereas education was not (r2 = 0.05, P = .05). Females also had (in unadjusted models), on average, lower NfL levels (18.50 pg/mL, SD = 11.25 pg/mL) as compared to males (19.91 pg/mL, SD = 12.48 pg/mL), P = .02. Mexican Americans had, on average, lower levels of NfL (17.42 pg/mL, SD = 11.96 pg/mL) as compared to non‐Hispanic whites (20.88 pg/mL, SD = 11.29 pg/mL), P < .001 again in an unadjusted model. When entered into a combined linear regression model, the overall model (shown by the omnibus test) was significant (F[4,1618] = 86.01, P < .001; adjusted R2 = 0.173), with only age remaining significantly related to plasma NfL levels (unstandardized regression coefficient [B] = 0.550, standardized error [SE] = 0.032, t = 17.09, P < .001); sex (B = −0.519, SE = 0.549, t = −0.946, P = .344), education (B = −0.035, SE = 0.071, t = −0.490, P = .624) and ethnicity (B = −0.587, SE = 0.716, t = −0.820, P = .412) were no longer significant.
3.2. Medical co‐morbidities and NfL levels
Next, when examining the relationship between medical co‐morbidities and NfL levels, participants were stratified based on medical diagnosis (presence/absence). In the total sample, participants with hypertension had higher mean NfL levels (20.35 pg/mL, SD = 13.04 pg/mL) as compared to those without the presence of hypertension (17.21 pg/mL, SD = 9.32 pg/mL), P < .001 in an unadjusted model. Similarly, in an unadjusted model, those with dyslipidemia had, on average, higher levels of plasma NfL (19.58 pg/mL, SD = 12.08 pg/mL) as compared to those without dyslipidemia (18.21 pg/mL, SD = 11.19 pg/mL), P = .02. Participants with a diagnosis of diabetes (22.25 pg/mL, SD = 14.08 pg/mL) had significantly higher NfL levels when compared to those without diabetes (18.01 pg/mL, SD = 10.69 pg/mL), P < .001 again in an unadjusted model. Because age was shown to be significantly related to plasma NfL level, it was included as a covariate along with sex in the next subsequent models. In a linear regression model including each of the three medical co‐morbidities, the overall model was significant (F[4, 1618] = 102.50; adjusted R2 = 0.20) whereas only diabetes remained significant (B = 4.36, SE = 0.62, t = 6.89, P < .001) after covarying for age and sex When separated by ethnic group (covarying for age and sex), NfL levels were found to be significantly related to the diagnosis of hypertension (P = .019) and diabetes (P < .001) among those with Mexican American ethnicity and only diabetes (P = .027) among those with non‐Hispanic white ethnicity (see Table 2).
TABLE 2.
Link between plasma NfL and medical co‐morbidities by ethnic group adjusting for age and sex
| B | Std. Error | Standardized B | t‐score | P ‐value | |
|---|---|---|---|---|---|
| Hypertension | |||||
| NHW (n = 451) | 1.116 | 0.743 | 0.049 | 1.502 | .134 |
| MA (n = 564) | 1.962 | 0.832 | 0.079 | 2.360 | .019* |
| Diabetes | |||||
| NHW (n = 106) | 2.379 | 1.072 | 0.071 | 2.220 | .027* |
| MA (n = 319) | 6.248 | 0.781 | 0.251 | 8.000 | <.001* |
| Dyslipidemia | |||||
| NHW(n = 490) | ‐0.024 | 0.750 | ‐0.001 | ‐0.032 | .974 |
| MA(n = 569) | 1.283 | 0.808 | 0.052 | 1.588 | .113 |
| Medical Co‐morbidity Index | |||||
| Total Sample | |||||
| 0 (n = 302) | |||||
| 1 (n = 569) | |||||
| 2 (n = 566) | |||||
| 3 (n = 266) | 1.450 | 0.279 | 0.118 | 5.204 | <.001* |
| NHW | |||||
| 0 (n = 172) | |||||
| 1 (n = 302) | |||||
| 2 (n = 272) | |||||
| 3 (n = 67) | 0.694 | 0.414 | 0.055 | 1.676 | .094 |
| MA | |||||
| 0 (n = 130) | |||||
| 1 (n = 267) | |||||
| 2 (n = 294) | |||||
| 3 (n = 199) | 2.353 | 0.397 | 0.193 | 5.920 | <.001* |
Note: A co‐morbidity index was created by combining presence/absence of all three conditions with a range of scores from 0 (ie, none of the three diagnoses) to 3 (all three diagnoses present). Non‐Hispanic white (NHW). Mexican American (MA). * P < 0.05.
Next, a co‐morbidity index was created by combining presence/absence of all three conditions with a range of scores from 0 (ie, none of the three diagnoses) to 3 (all three diagnoses present). There was a significant difference between groups: group 0 (n = 286, 17.6%) mean NfL = 16.40 pg/mL (SD = 8.37 pg/mL), group 1 (n = 542, 33.4%) mean NfL = 18.14 pg/mL (SD = 10.73 pg/mL), group 2 (n = 542, 33.4%) mean NfL = 19.55 pg/mL (SD = 12.17 pg/mL), and group 3 (n = 253, 15.6%) mean NfL = 23.03 pg/mL (SD = 14.68 pg/mL). All medical co‐morbidity index groups were found to be individually statistically significant (P < .05), with age and sex entered as covariates into the model. When ethnicity was included in the model, there was shown to be a significant interaction with the medical co‐morbidity index (P = .004); age remained a significant covariate in this model (see Figure 1). In the total sample, the medical co‐morbidity index was found to be significantly associated with plasma NfL (after covarying for age and sex) (P < .001). Among non‐Hispanic whites, the medical co‐morbidity index was non‐significant (P = .094), whereas it was highly significant among Mexican Americans (P < .001) (see Table 2).
FIGURE 1.
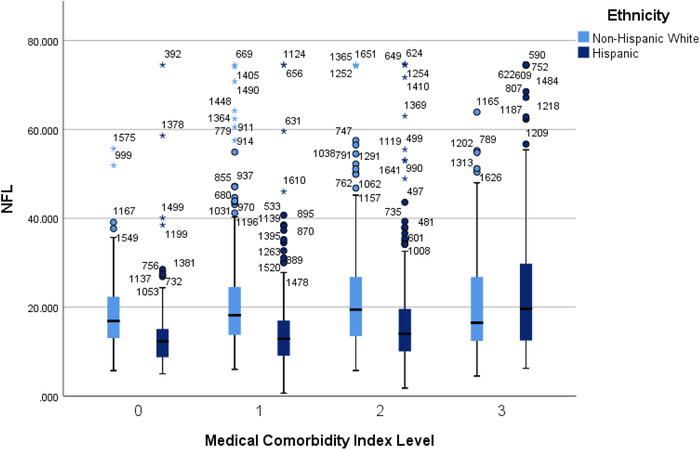
F 1 NfLl levels by medical co‐morbidity index level split by ethnicity
3.3. NfL levels by diagnostic group
Plasma NfL levels varied by diagnostic group: NC NfL = 18.07 pg/mL (SD = 10.57 pg/mL), MCI NfL = 20.93 pg/mL (SD = 13.75 pg/mL), and dementia NfL = 27.28 pg/mL (SD = 16.53 pg/mL). The overall ANCOVA (covarying for age and sex) was statistically significant (P < .001) (Figure 2). The NC group had significantly lower NfL values than the MCI group (mean difference I‐J = −2.427, P = .001) and dementia group (mean difference I‐J = −7.14, P < .001). In addition, the MCI group had significantly lower NfL values than the dementia group (mean difference I‐J = −4.72, P < .001).
FIGURE 2.
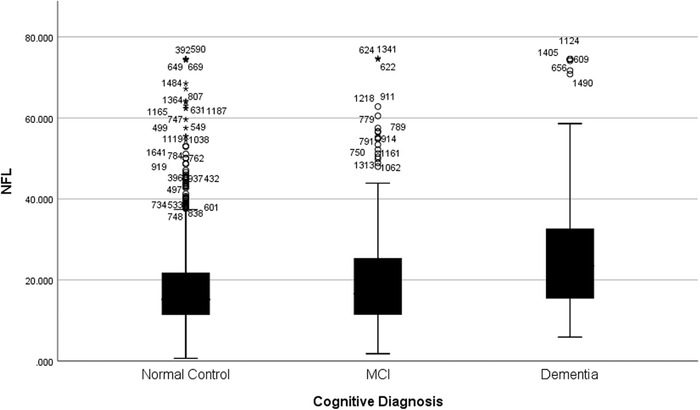
NfL levels by cognitive diagnosis
3.4. NfL levels in relation to MRI markers of neurodegeneration
Plasma NfL was significantly negatively associated with the “meta ROI” (B = −0.002, SE = 0.000, t = −5.178, P < .001) as well as the ROIs that made up the meta ROI: entorhinal thickness (B = −0.003, SE = 0.001, t = −3.567, P < .001), mean inferior temporal thickness (B = −0.002, SE = 0.000, t = −4.503, P < .001), mean middle temporal thickness (B = −0.002, SE = 0.000, t = −4.819, P < .001) and mean fusiform thickness (B = ‐0.002, SE = 0.000, t = −5.133, P < .001). A combined model was conducted with ethnicity entered along with covariates of age and sex, which found that ethnicity was significant in models examining “Meta ROI” (B = −0.023, SE = 0.009; t = −2.451, P = .014), mean inferior temporal thickness (B = −0.022, SE = 0.008, t = −2.630, P = .009), mean middle temporal thickness (B = −0.033, SE = 0.008, t = ‐4.166, P < .001), and mean fusiform thickness (B = −0.017, SE = 0.008, t = −2.058, P = .040); however, ethnicity was not shown to be a significant predictor for entorhinal thickness (B = −0.004, SE = 0.019, t = −0.193, P = .847). As most of the MRI markers were shown to be impacted by ethnicity, further analyses were conducted split by ethnic group. Associations between NfL and MRI markers of neurodegeneration covarying for age and sex are reported in Table 3.
TABLE 3.
Association between plasma NfL and MRI markers of neurodegeneration by ethnicity covarying for age and sex
| B | Std. Error | Standardized B | t‐score | P ‐value | |
|---|---|---|---|---|---|
| MetaROI | |||||
| NHW (n = 621) | ‐0.001 | 0.001 | ‐0.090 | ‐2.174 | .030* |
| MA (n = 695) | ‐0.002 | 0.001 | ‐0.166 | ‐4.441 | <.001* |
| Entorhinal (mean L and R) | |||||
| NHW | ‐0.004 | 0.001 | ‐0.109 | ‐2.739 | .006* |
| MA | ‐0.002 | 0.001 | ‐0.082 | ‐2.177 | .030* |
| Inferior Temporal (mean L and R) | |||||
| NHW | ‐0.001 | 0.001 | ‐0.091 | ‐2.237 | .026* |
| MA | ‐0.002 | 0.001 | ‐0.163 | ‐4.408 | <.001* |
| Middle Temporal (mean L and R) | |||||
| NHW | ‐0.002 | 0.001 | ‐0.115 | ‐2.986 | .003* |
| MA | ‐0.002 | 0.000 | ‐0.162 | ‐4.532 | <.001* |
| Fusiform (mean L and R) | |||||
| NHW | ‐0.002 | 0.001 | ‐0.119 | ‐3.040 | .002* |
| MA | ‐0.002 | 0.000 | ‐0.157 | ‐4.348 | <.001* |
Abbreviations: NfL, neurofilament light chain; MRI, magnetic resonance imaging; NHW, non‐Hispanic White; MA, Mexican American; L, Left; R, Right.
* P < .05.
3.5. NfL levels in relation to amyloid PET
A subsample of the study (n = 55 [non‐Hispanic white {NHW} n = 22; Mexican American {MA} n = 33]) had available amyloid PET scans. Of those, n = 11 were amyloid positive while n = 44 were amyloid negative. The mean age of those with an available amyloid PET scans was 65.72 (SD = 9.77), education level was 11.49 (5.41), and NfL level was 21.22 pg/mL (SD = 13.04 pg/mL). There was no significant difference between demographic variables or NfL levels between those who received an amyloid PET scan versus those who did not (P > .05). After covarying for age and sex, plasma NfL levels were significantly positively associated with amyloid global SUVR levels (B = 0.004, SE = 0.002, t = 2.38, P = .021) as well as the cortical regions that compose the global cortical region per the ADNI3 protocol: frontal region (B = 0.004, SE = 0.002, t = 2.12, P = .031), lateral parietal region (B = 0.004, SE = 0.002, t = 2.28, P = .027), lateral temporal region (B = 0.003, SE = 0.001, t = 2.44, P = .018), and anterior/posterior cingulate (B = 0.004, SE = 0.002, t = 2.45, P = .018) (see Table 4). A combined model conducted with ethnicity entered along with covariates of age and sex found that it was not a significant predictor in any of the amyloid PET regions examined (P < .05); however, the limited sample size likely impacted the power of the analyses.
TABLE 4.
Link between plasma NfL and amyloid PET values adjusting for age and sex (n = 55)
| B | Std. Error | Standardized B | t‐score | P ‐value | |
|---|---|---|---|---|---|
| Frontal | 0.004 | 0.002 | 0.296 | 2.217 | .031* |
| Lateral parietal | 0.004 | 0.002 | 0.285 | 2.278 | .027* |
| Lateral temporal | 0.003 | 0.001 | 0.311 | 2.446 | .018* |
| Anterior/posterior cingulate | 0.004 | 0.002 | 0.316 | 2.450 | .018* |
| Global | 0.004 | 0.002 | 0.305 | 2.387 | .021* |
Abbreviation: NfL, Neurofilament light chain. * P < .05.
3.6. NfL levels in relation to neuropsychological test performance
After covarying for age, sex, years of education, and primary language, plasma NfL was significantly associated with all neuropsychological measures (see Table S1). When separated by diagnostic group, most significant findings went away. Among non‐Hispanic whites who were diagnosed as cognitively normal, there was a significant association between NfL and poorer performance on tests of global cognition (P = .023), working memory (.004), attention and processing speed (P = .015), verbal fluency (P = .042), and immediate verbal (rote) memory (P = .049). Among this same ethnic group but for those diagnosed with MCI, a similar association was found on across measures of working memory (P = .005), attention and processing speed (P = .016), as well as immediate (P = .021) and delayed episodic memory (P = .016). For those with dementia, no significant associations were found. When examined among Mexican Americans, a significant association between NfL levels and poorer performance was similarly found on a measure of working memory; however, this finding was shown across diagnostic classifications (NC P = .029; MCI P = .026; dementia P = .009). In this same ethnic group but for those diagnosed with MCI, a significant association was only additionally found for delayed verbal (rote) memory (P = .019). In contrast, a number of significant associations were found among those with a diagnosis of dementia, spanning measures of global cognition (P = .013), attention and working memory (P = .021), verbal fluency (P = .001), and verbal immediate (rote) memory (P = .041) (see Table 5).
TABLE 5.
Association of plasma NfL with neuropsychological outcomes covarying for age, education, sex, and primary language separated by diagnostic status
| B | Std. Error | Standardized B | P ‐value | |
|---|---|---|---|---|
| MMSE | ||||
| NHW | ||||
| NC (n = 673) | ‐0.010 | 0.004 | ‐0.096 | .023* |
| MCI (n = 91) | ‐0.004 | 0.013 | ‐0.037 | .771 |
| Dementia (n = 49) | ‐0.046 | 0.042 | ‐0.166 | .279 |
| MA | ||||
| NC (n = 676) | 0.003 | 0.008 | 0.013 | .702 |
| MCI (n = 152) | 0.003 | 0.018 | 0.012 | .851 |
| Dementia (n = 62) | ‐0.100 | 0.039 | ‐0.254 | .013* |
| Digit Symbol | ||||
| NHW | ||||
| NC | ‐0.113 | 0.040 | ‐0.110 | .004* |
| MCI | ‐0.220 | 0.076 | ‐0.338 | .005* |
| Dementia | 0.060 | 0.098 | 0.087 | .543 |
| MA | ||||
| NC | ‐0.062 | 0.028 | ‐0.054 | .029* |
| MCI | ‐0.134 | 0.060 | ‐0.134 | .026* |
| Dementia | ‐0.206 | 0.075 | ‐0.261 | .009* |
| WMS Digit Span | ||||
| NHW | ||||
| NC | ‐0.023 | 0.015 | ‐0.064 | .140 |
| MCI | ‐0.009 | 0.027 | ‐0.042 | .738 |
| Dementia | ‐0.043 | 0.027 | ‐0.263 | .120 |
| MA | ||||
| NC | 0.002 | 0.011 | 0.007 | .838 |
| MCI | ‐0.027 | 0.019 | ‐0.106 | .159 |
| Dementia | ‐0.051 | 0.021 | ‐0.292 | .021* |
| TMTA | ||||
| NHW | ||||
| NC | 0.105 | 0.043 | 0.098 | .015* |
| MCI | 0.357 | 0.145 | 0.270 | .016* |
| Dementia | ‐0.028 | 0.506 | ‐0.009 | .957 |
| MA | ||||
| NC | 0.126 | 0.067 | 0.064 | .061 |
| MCI | 0.128 | 0.211 | 0.041 | .546 |
| Dementia | 0.687 | 0.354 | 0.216 | .058 |
| TMTB | ||||
| NHW | ||||
| NC | 0.154 | 0.147 | 0.041 | .294 |
| MCI | 0.620 | 0.594 | 0.116 | .300 |
| Dementia | 1.262 | 0.982 | 0.197 | .209 |
| MA | ||||
| NC | 0.399 | 0.231 | 0.055 | .086 |
| MCI | 0.423 | 0.498 | 0.057 | .396 |
| Dementia | 0.698 | 0.597 | 0.152 | .250 |
| FAS | ||||
| NHW | ||||
| NC | ‐0.091 | 0.045 | ‐0.084 | .042* |
| MCI | ‐0.103 | 0.097 | ‐0.140 | .289 |
| Dementia | ‐0.156 | 0.100 | ‐0.262 | .127 |
| MA | ||||
| NC | ‐0.056 | 0.034 | ‐0.059 | .095 |
| MCI | ‐0.039 | 0.058 | ‐0.052 | .499 |
| Dementia | ‐0.217 | 0.064 | ‐0.374 | .001* |
| Animals | ||||
| NHW | ||||
| NC | ‐0.017 | 0.019 | ‐0.037 | .382 |
| MCI | ‐0.065 | 0.036 | ‐0.205 | .079 |
| Dementia | ‐0.086 | 0.053 | ‐0.245 | .113 |
| MA | ||||
| NC | ‐0.006 | 0.015 | ‐0.016 | .659 |
| MCI | ‐0.013 | 0.028 | ‐0.039 | .630 |
| Dementia | ‐0.075 | 0.037 | ‐0.249 | .050 |
| LM1 | ||||
| NHW | ||||
| NC | ‐0.025 | 0.039 | ‐0.026 | .528 |
| MCI | 0.187 | 0.080 | 0.276 | .021* |
| Dementia | ‐0.031 | 0.102 | ‐0.049 | .762 |
| MA | ||||
| NC | ‐0.026 | 0.032 | ‐0.031 | .409 |
| MCI | 0.067 | 0.063 | 0.084 | .289 |
| Dementia | ‐0.076 | 0.091 | ‐0.113 | .403 |
| LM2 | ||||
| NHW | ||||
| NC | ‐0.037 | 0.030 | ‐0.051 | .220 |
| MCI | 0.153 | 0.062 | 0.293 | .016* |
| Dementia | ‐0.038 | 0.063 | ‐0.092 | .552 |
| MA | ||||
| NC | ‐0.006 | 0.025 | ‐0.009 | .803 |
| MCI | 0.067 | 0.051 | 0.108 | .193 |
| Dementia | ‐0.052 | 0.055 | ‐0.123 | .34 |
| SEVLT 1‐5 total | ||||
| NHW | ||||
| NC | ‐0.055 | 0.030 | ‐0.069 | .070 |
| MCI | 0.021 | 0.048 | 0.051 | .660 |
| Dementia | ‐0.079 | 0.065 | ‐0.182 | .231 |
| MA | ||||
| NC | ‐0.021 | 0.024 | ‐0.031 | .382 |
| MCI | ‐0.011 | 0.041 | ‐0.022 | .786 |
| Dementia | ‐0.150 | 0.072 | ‐0.284 | .041* |
| SEVLT delayed recall | ||||
| NHW | ||||
| NC | ‐0.022 | 0.011 | ‐0.079 | .049* |
| MCI | 0.004 | 0.023 | 0.022 | .855 |
| Dementia | 0.004 | 0.020 | 0.026 | .857 |
| MA | ||||
| NC | ‐0.011 | 0.010 | ‐0.039 | .277 |
| MCI | 0.047 | 0.020 | 0.194 | .019* |
| Dementia | ‐0.036 | 0.022 | ‐0.229 | .098 |
3.7. CONTEXT OF USE (COU) ANALYSES
3.7.1. COU1 – NfL as a diagnostic biomarker for MCI and dementia
Plasma NfL was significantly associated with MCI diagnosis (B = 0.020, SE = 0.006, Wald = 12.459, Exp(B) = 1.020 [95% CI = 1.009‐1.031], P = .003) and dementia diagnosis (B = 0.047, SE = 0.006, Wald = 52.034, Exp(B) = 1.048 [95% CI = 1.035‐1.061, P < .001). In ROC analysis, the area under the curve (AUC) for predicting MCI was 0.551 (95% CI = 0.509‐0.593; P = .013) and for predicting dementia the AUC was 0.701 (95% CI = 0.648‐0.754; P < .001). Although statistically significant, there was no cut‐score that provided an acceptable diagnostic accuracy for detecting MCI or dementia.
3.7.2. COU2 – NfL as a biomarker for screening out plasma amyloid
In a logistic regression covarying for age and sex, plasma NfL was associated with amyloid positivity (global SUVR >1.08) (B = 0.071, SE = 0.031, Exp(B) = 1.074 [95% CI = 1.010‐1.142], P = .023). With a logistic regression cut‐score of 0.20 to match the base rate of amyloid positivity, the sensitivity was 0.60 and specificity was 0.78 and the positive predictive value (PPV) was 0.40 and negative predictive value (NPV) was 0.89.
4. DISCUSSION
This is, to our knowledge, the largest and most comprehensive characterization of plasma NfL in relation to cognitive and imaging factors associated with AD in a community‐based multi‐ethnic cohort. The current findings highlight the significant impact of a range of factors on plasma NfL values. Age was found to be significantly related to NfL values, which is consistent with recent work by Kaeser and colleagues. 39 When we covaried for age and sex, diabetes was also found to be significantly related to plasma NfL levels. Of note, significant differences in NfL levels were seen across medical co‐morbidities of hypertension, dyslipidemia, and diabetes between those with and without the medical condition. A medical co‐morbidity index was significantly related to plasma NfL, suggesting that there is an additive effect of multiple co‐morbidities.
Ours is not the first study to document the significant impact of medical co‐morbidities on NfL values. Korley and colleagues 40 found higher hemoglobin A1C and systolic blood pressure to be independent predictors of NfL levels. Serum NfL levels were also found in this study to be higher among those with a history of stroke, with cardiovascular disease risk factors accounting for 19% of variability in baseline NfL levels. 19 Mielke and colleagues also found in their work higher baseline plasma NfL levels in those with a history of hypertension. 41 Relative to these findings, additional work is underway to more explicitly examine the link between vascular pathology including cardiovascular factors (ie, stroke, white matter hyperintensities) and NfL levels in this cohort.
Our findings were also consistent with prior work demonstrating a link between plasma NfL levels and neuropsychological functioning. Osborn and colleagues, 1 identified an inverse association between plasma NfL and cognitive test performance across measures of language, executive functioning, visual spatial, and memory among those with MCI; however, of note, no relation between cognitive test performance and NfL was found for those with normal cognition. Our findings are also consistent with prior work demonstrating a significant link between plasma NfL and MRI‐based markers of neurodegeneration, as prior work has shown elevations in NfL to be associated with poorer white matter integrity (higher MD and lower FA), 40 although this has not been consistently found in cross‐sectional work. 19 Additionally, our findings are consistent with prior work demonstrating a significant link between plasma NfL levels and cerebral amyloid levels. Barker et al. 41 recently found that plasma NfL was higher in those who had greater cerebral amyloid, but was not associated with Hispanic ethnicity.
Because plasma NfL values are (1) significantly related to MRI outcomes of neurodegeneration and (2) significantly related to neuropsychological functioning, an examination as a possible diagnostic biomarker makes logical sense. Therefore, we conducted analyses specific to this COU. COU1 was the potential for plasma NfL to serve as a diagnostic biomarker for MCI and/or dementia. As we have articulated previously, COU1 would potentially serve as the first step in a multi‐tiered neurodiagnostic process for detecting dementia. 1 Although statistically significant, plasma NfL did not meet COU1. There was no optimal cut‐score to provide an adequate diagnostic accuracy. For example, if a cut‐score of plasma NfL < = 6.83 (to rule OUT dementia) was selected it would yield a 97% sensitivity and a specificity of 4%. When considering a base rate of ∼12% for those 65 and above with dementia, the negative predictive value (NPV) would be 91%. However, if this cut‐score were applied to this cohort, it would only rule out 65 total participants. If a higher cut‐score of > = 23.75 was selected, specificity would be 80% and sensitivity was 30%. Again, NPV was high (89%) and this time and n = 1474 participants would have been screened out. However, 88% of the dementia cases were screened out as false negatives. Therefore, plasma NfL does not meet COU1. It is likely that the lack of utility of plasma NfL as a diagnostic biomarker is due to the significant impact of demographic and medical co‐morbidities on biomarker levels. It is possible that a more sophisticated approach that provides specific cut‐values by co‐morbidity and age may have additional utility. Such work is currently being examined using the HABLE data.
The availability of a blood‐test to serve as the first step in a multi‐tiered process for screening into novel clinical trials would be of tremendous benefit. As with a primary care screen, the emphasis on COU2 is also a high NPV with the capacity to rule out as many true negative cases as possible. Therefore, we sought to examine COU2—the utility of plasma NfL as a means of ruling out cerebral amyloid. Taking the sensitivity of 0.60 and specificity of 0.78 outlined above and applying those diagnostic statistics to n = 10,000 patients screened for a clinical trial, the NPV (assuming a 20% amyloid positivity base rate) was 89%. A total n = 7040 participants would have been screened out and 89% would be true negatives. Of the n = 2960 screened forward for a second step in the screening process, 41% would be amyloid positive. Therefore, plasma NfL may have potential as a blood‐based screening tool for ruling out those participants who should not undergo additional expensive and/or invasive procedures for screening into novel amyloid targeting trials.
There are several other potential COUs where plasma NfL may be more suitable. A COU3 for plasma NfL may be as a progression biomarker. That is, baseline NfL values, or change in NfL values over time, may serve as a marker to indicate likelihood of cognitive decline and/or progression within a specific timeframe (eg, the timeframe of a clinical trial). This is in line with longitudinal work by Mattsson and colleagues, 16 who found longitudinal changes in NfL to be correlated with AD changes across measures of cognition as well as neuroimaging, and suggested its potential use as a marker for neurodegenerative progression. A COU4 would be a possible surrogate outcome biomarker for clinical trials. That is, it is possible that changes in plasma NfL over a briefer time (eg, 24‐months) will be predictive of long‐term (eg, 48‐month) cognitive outcomes. If successful in either of these COUs, NfL would offer tremendous cost‐ and time‐savings to novel clinical trials as well as reduced patient burden. Each of these COUs will be examined with future waves of HABLE data.
Overall, the current findings support prior work suggesting a link between plasma NfL and cognitive and imaging outcomes. The impact of ethnicity on the relationship between NfL levels and amyloid PET was likely limited by sample size in the present study, which affects COU recommendations. Future work is planned as part of the HABLE study to increase sample size and further examine this potential relationship. The current findings highlight the need to understand the impact of demographic factors and medical co‐morbidities if plasma NfL is to become clinically useful. Based on this work, and that of others, it is unlikely that plasma NfL will serve as a diagnostic COU; however, there are several possible clinical uses for plasma NfL that require further investigation.
Supporting information
Supporting material
ACKNOWLEDGMENTS
Research reported here was supported by the National Institute on Aging of the National Institutes of Health under Award Numbers R01AG054073 and R01AG058533. This work was also supported in part by NIH/NIBIB award P41‐EB015992. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The research team also thanks the local Fort Worth community and participants of the HABLE study.
O'Bryant S, Petersen M, Hall J, et al. Characterizing plasma NfL in a community‐dwelling multi‐ethnic cohort: Results from the HABLE study. Alzheimer's Dement. 2022;18:240–250. 10.1002/alz.12404
REFERENCES
- 1. O'Bryant SE, Edwards M, Johnson L, et al. A blood screening test for Alzheimer's disease. Alzheimers Dement (Amst). 2016;3(1):83‐90. 10.1016/j.dadm.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Bryant SE, Xiao G, Barber R, et al. A Blood‐Based Screening Tool for Alzheimer's Disease That Spans Serum and Plasma: Findings from TARC and ADNI. PLoS One 2011;6(12):e28092. 10.1371/journal.pone.0028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Bryant SE, Lista S, Rissman RA. Comparing biological markers of Alzheimer's disease across blood fraction and platforms: comparing apples to oranges. Alzheimers Dement (Amst). 2016;3:27‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Villarreal AE, O'Bryant SE, Edwards M, et al. Serum‐based protein profiles of Alzheimer's disease and mild cognitive impairment in elderly Hispanics. Neurodegener Dis Manag. 2016;6(3):203‐213. 10.2217/nmt-2015-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Bryant SE, Edwards M, Zhang F, et al. Potential two‐step proteomic signature for Parkinson's disease: Pilot analysis in the Harvard Biomarkers Study. Alzheimers Dement (Amst). 2019;11(1):374‐382. 10.1016/j.dadm.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Bryant SE, Ferman TJ, Zhang F, et al. A proteomic signature for dementia with Lewy bodies. Alzheimers Dement (Amst). 2019;11(1):270‐276. 10.1016/j.dadm.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petersen ME, Zhang F, Schupf N, et al. Proteomic profiles for Alzheimer's disease and mild cognitive impairment among adults with Down syndrome spanning serum and plasma: An Alzheimer's Biomarker Consortium–Down Syndrome (ABC–DS) study. Alzheimers Dement (Amst). 2020;12(1). 10.1002/dad2.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petersen ME, Rafii MS, Zhang F., et al. Plasma Total‐Tau and Neurofilament Light Chain as Diagnostic Biomarkers of Alzheimer's Disease Dementia and Mild Cognitive Impairment in Adults with Down Syndrome. J Alzheimer's Dis. 2021;79(2):671‐681. 10.3233/jad-201167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Bryant SE, Zhang F, Silverman W, et al. Proteomic profiles of incident mild cognitive impairment and Alzheimer's disease among adults with Down syndrome. Alzheimers Dement (Amst). 2020;12(1). 10.1002/dad2.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jack CR Jr, Bennett DA, Blennow K. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Massa F, Meli R, Morbelli S, Nobili F, Pardini M. Serum neurofilament light chain rate of change in Alzheimer's disease: potentials applications and notes of caution. Ann Transl Med. 2019;7(S3):S133‐S133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lewczuk P, Ermann N, Andreasson U. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer's disease. Alzheimer's Res Ther. 2018;10(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blennow K. A Review of Fluid Biomarkers for Alzheimer's Disease: Moving from CSF to Blood. Neurology and Therapy 2017;6(S1):15‐24. 10.1007/s40120-017-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blennow K, Zetterberg H. Biomarkers for Alzheimer's disease: current status and prospects for the future. J Int Med. 2018;284(6):643‐663. [DOI] [PubMed] [Google Scholar]
- 15. Mattsson N, Andreasson U, Zetterberg H, et al. Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol. 2017;74(5):557. 10.1001/jamaneurol.2016.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mattsson N, Cullen NC, Andreasson U, et al. Association Between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol. 2019;76(7):791. 10.1001/jamaneurol.2019.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin M, Cao L, Dai YP. Role of Neurofilament Light Chain as a Potential Biomarker for Alzheimer's Disease: A Correlative Meta‐Analysis. Front Aging Neurosci. 2019;11. 10.3389/fnagi.2019.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi Y, Lu X, Zhang L, et al. Potential Value of Plasma Amyloid‐β, Total Tau, and Neurofilament Light for Identification of Early Alzheimer's Disease. ACS Chem Neurosci. 2019;10(8):3479‐3485. 10.1021/acschemneuro.9b00095. [DOI] [PubMed] [Google Scholar]
- 19. Mielke MM, Syrjanen JA, Blennow K, et al. Plasma and CSF neurofilament light. Neurology. 2019;93(3):e252–e260. 10.1212/wnl.0000000000007767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Bryant SE, Xiao G, Edwards M, et al. Biomarkers of Alzheimer's Disease Among Mexican Americans. J Alzheimer's Dis. 2013;34(4):841‐849. 10.3233/jad-122074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer's disease and related dementias: Update and areas of immediate need. Alzheimer's & Dementia. 2019;15(2):292‐312. 10.1016/j.jalz.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris JC, Schindler SE, McCue LM, et al. Assessment of Racial Disparities in Biomarkers for Alzheimer Disease. JAMA Neurol. 2019;76(3):264. 10.1001/jamaneurol.2018.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Howell JC, Watts KD, Parker MW, et al. Race modifies the relationship between cognition and Alzheimer's disease cerebrospinal fluid biomarkers. Alzheimer's Res Ther. 2017;9(1). 10.1186/s13195-017-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson LA, Gamboa A, Vintimilla R, et al. A Depressive Endophenotype for Predicting Cognitive Decline among Mexican American Adults and Elders. J Alzheimer's Dis. 2016;54(1):201‐206. 10.3233/jad-150743. [DOI] [PubMed] [Google Scholar]
- 25. Vintimilla R, Hall J, Johnson L, et al. The relationship of CRP and cognition in cognitively normal older Mexican Americans. Medicine (Baltimore) 2019;98(19):e15605. 10.1097/md.0000000000015605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Fallon LR, Dearry A. Community‐based participatory research as a tool to advance environmental health sciences. Environ Health Perspect. 2002;110(suppl 2):155‐159. 10.1289/ehp.02110s2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Munoz HI, Large SE, Wong L, et al. P1‐543: ethnic differences in recruitment of older adults into aging research. Alzheimer Dementia. 2019;15:P479‐P480. [Google Scholar]
- 28. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 29. Weschler D. WMS‐IV: Weschler Memory Scale. Psychological Corporation. 2009. [Google Scholar]
- 30. Reitan RM, Wolfson D. The Halstead‐Reitan Neuropsychological Test Battery: Theory and Interpretation. Neuropsychol Press. 1985. [Google Scholar]
- 31. González HM, Mungas D, Haan MN. A verbal learning and memory test for English‐ and Spanish‐speaking older Mexican‐American adults. Clin Neuropsychol. 2002;16(4):439‐451. [DOI] [PubMed] [Google Scholar]
- 32. Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. Oxford University Press; 2004. [Google Scholar]
- 33. O'Bryant SE, Edwards M, Johnson L, et al. Texas Mexican American adult normative studies: Normative data for commonly used clinical neuropsychological measures for Englishand Spanish‐speakers. Dev Neuropsychol. 2018;43(1):1‐26. 10.1080/87565641.2017.1401628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sierra SN, Montañes P, Sierra MFA, et al. Estimating Intelligence in Spanish: Regression Equations With the Word Accentuation Test and Demographic Variables in Latin America. Applied Neuropsychology: Adult. 2015;22(4):252‐261. 10.1080/23279095.2014.918543. [DOI] [PubMed] [Google Scholar]
- 35. Berg L. Clinical dementia rating (CDR). Psychopharmacol Bull. 1988. Published online. [PubMed] [Google Scholar]
- 36. O'Bryant SE, Gupta V, Henriksen K, et al. Guidelines for the standardization of preanalytic variables for blood‐based biomarker studies in Alzheimer's disease research. Alzheimer's & Dementia. 2015;11(5):549‐560. 10.1016/j.jalz.2014.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Korley FK, Goldstick J, Mastali M, et al. Serum NfL (Neurofilament Light Chain) Levels and Incident Stroke in Adults With Diabetes Mellitus. Stroke. 2019;50(7):1669‐1675. 10.1161/strokeaha.119.024941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Osborn KE, Khan OA, Kresge HA. Cerebrospinal fluid and plasma neurofilament light relate to abnormal cognition. Alzheimers Dement (Amst). 2019;11:700‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaeser SA, Lehallier B, Thinggaard M. A neuronal blood marker is associated with mortality in old age. Nature Aging. 2021;1:218‐225. [DOI] [PubMed] [Google Scholar]
- 40. Moore EE, Hohman TJ, Badami FS. Neurofilament relates to white matter microstructure in older adults. Neurobiol Aging. 2018;70:233‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barker W, Quinonez C, Greig MT, et al. Utility of Plasma Neurofilament Light in the 1Florida Alzheimer's Disease Research Center (ADRC). J Alzheimer's Dis. 2021;79(1):59‐70. 10.3233/jad-200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting material


