Abstract
Few studies, if any, have addressed the adhesion of enterococci to the intestinal mucosa and their interference with the adhesion of pathogens, although more than 60% of probiotic preparations in the market contain strains of enterococci. The objective of this study was to investigate if Enterococcus faecium 18C23 has the ability to inhibit the adhesion of Escherichia coli K88ac and K88MB to the small intestine mucus of piglets. Approximately 9% of E. faecium 18C23 organisms adhered to the small intestine mucus, and the adhesion was found to be specific. Living E. faecium 18C23 culture efficiently inhibited the adhesion of E. coli K88ac and K88MB to the piglet intestine mucus. Inhibition of the adhesion of E. coli K88ac to the small intestine mucus was found to be dose dependent. Inhibition of >90% was observed when 109 CFU or more of living E. faecium 18C23 culture per ml was added simultaneously with E. coli to immobilized mucus. The substances from both the 18C23 cells and the spent culture supernatant contributed to the inhibition of adhesion of E. coli K88 to the small intestine mucus receptors. The inhibiting effect was not solely a pH effect since considerable inhibitory action was demonstrated after neutralizing the mixture or spent culture supernatant to pH 7.0. Part of the inhibition of adhesion of E. coli K88ac by E. faecium 18C23 or its supernatant might occur through steric hindrance.
Enterotoxigenic Escherichia coli (ETEC) strains are frequent causes of piglet diarrhea during the preweaning and immediate postweaning periods. Among the different ETEC strains (K88-, K99-, or 987P-expressing strains), those expressing K88 fimbrial antigen are the most prevalent (1, 13). These fimbriae mediate the adhesion of E. coli K88-expressing strains to the intestinal epithelial mucosa and also to the mucus layer lining the small intestine (7), and thereafter the organism elaborates one or two enterotoxins, heat-stable toxin and heat-labile toxin, which induce massive fluid and electrolyte secretion into the gut lumen (1, 13). Among ETEC variants expressing the ab, ac, or ad K88 fimbriae, those possessing the K88ac fimbrial antigen are the most common variant found in pathogenic E. coli isolates in the United States (26). Antibiotics are routinely used in an attempt to control pathogens, but the organisms are becoming resistant to the more commonly used treatments, making antibiotic therapy unreliable (10). Furthermore, the use of antimicrobial growth promoters may cause the development of resistance in a number of important pathogenic bacterial species (28). Recently, the European Union decided to ban the use of four widely used antibiotics, i.e., tylosin, virginiamycin, spiramycin, and zinc bacitracin, as growth promoters from July 1999. As a consequence, there is an urgent need to seek an alternative to antibiotics for the purpose of enhancing the health status and production performance of domestic animals.
Probiotics have been used to reduce the colonization of the intestines of animals by pathogens. This, in turn, reduces the prophylactic use of antibiotics as feed additives in animal production (12, 15, 23). Most probiotic bacteria are of intestinal origin and belong to the lactic acid-producing bacteria (LAB) such as bifidobacteria, lactobacilli, and enterococci. Many studies have focused on lactobacilli and bifidobacteria and have been carried out to elucidate the mechanism of bacterial adhesion and the ability of these bacteria to inhibit the adhesion of pathogens to the intestinal mucosa. However, there are very few studies, if any, on the adhesion of enterococci and their inhibition of the adhesion of pathogens to the intestine, although more than 60% of probiotic preparations in the market contain strains of enterococci (9, 11, 25). Recently, Netherwood et al. (21) reported that probiotics containing both genetically modified (GM) and non-GM Enterococcus faecium changed the bacterial flora of the chicken gastrointestinal tract, but with conflicting results: the relative amount of E. faecalis in the total eubacterial population increased in the presence of the non-GM strain and decreased in the presence of the GM probiotic was used compared with the results obtained with an untreated control group (21).
It has been established that receptors for the K88 fimbriae are detectable in the mucus overlying the epithelial cells of the piglet small intestine (17). The presence of receptors in mucus may play a role in the pathogenesis of K88-bearing strains (7). The objective of the present study was to investigate if E. faecium 18C23 has the ability to inhibit the adhesion of E. coli K88ac and K88MB to the small intestinal mucus of piglets.
MATERIALS AND METHODS
Bacteria and culture conditions.
Three strains of LAB, namely, E. faecium 18C23, Lactobacillus acidophilus 80H10, and Lactobacillus casei subsp. rhamnosus 47G19, were used. These bacteria were originally isolated from cottage cheese and obtained from StarLab Inc. (St. Joseph, Mo.). E. faecium 18C23 was grown in KF Streptococcus broth (Difco, Detroit, Mich.), and the Lactobacillus strains were cultured in MRS broth (Difco). ETEC K88ac was obtained from the Pennsylvania State University E. coli Reference Center (University Park, Pa.). A strain of the hemolytic ETEC K88+ bacterium (K88+MB) was obtained from the Animal Health Center, Veterinary Services Branch, Manitoba Agriculture, Winnipeg, Canada. The isolate, which was obtained from the feces of a piglet suffering from diarrhea, was shown to give a positive agglutination test with anti-K88 ETEC antibody but not with anti-K99 ETEC antibody. E. coli K88+MB was tested as the K88ac phenotype (16). Primary cultures of ETEC strains were grown overnight in tryptic soy broth (Difco) at 37°C using a 1% innoculum from stocks stored at −20°C. All strains of bacteria were stored at −20°C in the appropriate medium containing 30% glycerol.
Preparation of labeled bacteria.
Both LAB and E. coli K88 were labeled as described by Laux et al. (18). E. coli K88 was grown overnight and LAB were incubated for 10, 15, 25, and 40 h at 37°C in their respective media containing 10 μCi of [methyl-1,2-3H]thymidine (118 Ci mmol−1; Amersham International, Little Chalfont, United Kingdom). Cells were harvested by centrifugation at 2,000 × g for 15 min, washed twice in phosphate-buffered saline (PBS; pH 7.2), and resuspended in PBS. The suspension of ETEC K88ac and K88MB was adjusted to an optical density of 1.0 at 600 nm (approximately 109 CFU ml−1) and was used in the following tests.
Preparation of small intestine mucus from piglets.
Four 14 ± 2-day-old healthy Cotswold piglets were obtained from the Glenlea Swine Research Unit, University of Manitoba, Winnipeg, Canada. The piglets used in this study were of the K88-susceptible phenotype, since K88-bearing E. coli MB was able to bind to the intestinal epithelial cells when tested microscopically using the procedure described by Wilson and Hohmann (27). The small intestine mucus used in the present study was isolated from piglets by the procedure of Jin et al. (16). Briefly, a microscope slide was used to gently scrape the mucosal surface into HEPES-Hanks' buffer (HH buffer; pH 7.4). The mucosal scrapings were then centrifuged twice at 27,000 × g at 4°C for 15 min to remove solids. The supernatant, containing crude intestinal mucus, was then analyzed for protein content by the method of Lowry et al. (20). Bovine serum albumin (BSA) was used as a standard.
Adhesion assay.
The adhesion of radioactively labeled bacteria to the intestinal mucus receptor was studied by using a modification of the methods of Blomberg et al. (3). All adhesion assays were performed in triplicate in multiwell polythene tissue culture plates (Linbro, flat bottom, 1.6 cm in diameter; Nalge Nunc International, Roskilde, Denmark). Briefly, the intestinal mucus was immobilized overnight at 4°C in wells at 0.5 mg of protein per ml. Control wells were immobilized with bovine serum albumen (BSA, Sigma, St. Louis, Mo.) at 1 mg of protein per ml or with 0.2 ml of PBS as a polythene control. After immobilization, the wells were washed twice with 0.5 ml of HH buffer (pH 7.4) containing 0.5% mannose (Sigma), 0.2 ml of [methyl-1,2-3H]thymidine-labeled LAB or E. coli was added to each well, and the plates were incubated for 1 h at 37°C. The wells were washed three times with 1.0 ml of HH buffer. Adherent bacteria were recovered by adding 0.5 ml of 0.5% sodium dodecyl sulfate (SDS). The samples, after incubation for 3 h at 37°C, were collected and mixed thoroughly with 10 ml of scintillation cocktail (ICN Biomedicals Inc., Aurora, Ohio). The radioactivity of the SDS-extracted sample was enumerated using a liquid scintillation spectrophotometer (LKB-Wallac RackBeta).
Characterization of the intestinal mucus receptor for E. faecium adhesion.
To examine the nature of the intestinal mucus receptor for E. faecium adhesion, three proteolytic enzymes (Sigma), i.e., pronase, proteinase K, and trypsin (0.2 ml of solutions containing 800 μg of enzyme per ml), were added individually to wells containing immobilized mucus and the plates were incubated for 2 h at 37°C and then overnight at 4°C. The plates were then washed, and the adhesion assay was performed with E. faecium 18C23 culture incubated for 25 h. Controls were the immobilized mucus that was treated with BSA (800 μg ml−1) rather than enzymes. Immobilized mucus on tissue culture wells was also treated with 0.2 ml of 0.01 M sodium metaperiodate or 0.2 ml of 0.01 M sodium iodate in 0.2 M sodium acetate buffer (pH 4.5), incubated in the dark for 3 h at 4°C, washed twice with HH buffer, and assayed for bacterial adhesion.
Assay of inhibition of adhesion.
The assay of inhibition of adhesion was similar to that carried out previously (16). The E. faecium culture was prepared by incubation in KF Streptococcus broth for 25 h at 37°C. The final concentration of E. faecium was approximately 5 × 108 CFU ml−1. The intestinal mucus was immobilized overnight at 4°C in wells at a concentration of 0.5 mg of protein per ml. After immobilization, the wells were washed twice with 0.5 ml of HH buffer (pH 7.4) containing 0.5% mannose (Sigma). For the competition test, suspensions of the broth cultures of E. faecium (0.1 ml) and E. coli K88ac (0.1 ml) or K88MB (0.1 ml) were added simultaneously to the immobilized mucus and the mixture was incubated for 60 min at 37°C. For the displacement test, E. coli was added to immobilized mucus and incubated for 60 min. After the mucus was washed three times with 0.5 ml of HH buffer, 0.2 ml of the suspension of E. faecium was added and the mixture was incubated for another 60 min at 37°C. After the reaction, the plates were washed three times with HH buffer (1 ml). Adherent bacteria were recovered by adding 0.5 ml of 0.5% SDS. Attachment of labeled E. coli to mucus in wells treated for the same period with sterile KF broth (control for culture and supernatant inhibition) and PBS buffer (control for inhibition by bacterial cells) was taken as 100%. The inhibition of adhesion was assessed as the percentage of radioactivity in the test culture relative to the respective control. All assays were performed three times each in duplicate.
A dose dependence test was conduced on the inhibition of adhesion of E. coli K88 to the intestinal mucus by the original E. faecium 18C23 culture. The concentrations of E. faecium 18C23 used were 106, 107, 108, 109, and 1010 CFU ml−1.
To further identify the component of the E. faecium 18C23 culture that inhibited the attachment of E. coli to the mucus receptor, the following preparations (0.1-ml aliquots) were tested: pH 4.0 KF broth culture, neutralized (with 2 M NaOH) KF broth culture of EF (pH 7.0), cell-free supernatant (pH 4.0) from the E. faecium 18C23 culture, neutralized cell-free supernatant (pH 7.0) from the E. faecium 18C23 culture, washed bacterial cells from the E. faecium 18C23 culture in PBS buffer (pH 7.0), recombination of washed bacterial cells and cell-free supernatant (pH 4.0), citric acid-sodium citrate buffer (pH 4.0), and PBS (pH 7.0). Bacterial cells were obtained by centrifuging the E. faecium 18C23 culture at 650 × g for 20 min at 4°C and washing it twice with PBS. The supernatant was sterilized by filtration (0.22-μm-pore-size cellulose acetate filter; Millipore) and used immediately or stored at −70°C until used.
Statistical analysis.
The data were analyzed using the SAS program (24). Tukey's test was used to identify differences among groups after analysis of variance. In the dose-dependent inhibition test, data were analyzed using regression with dummy coding.
RESULTS
Bacterial adhesion to small intestine mucus receptors.
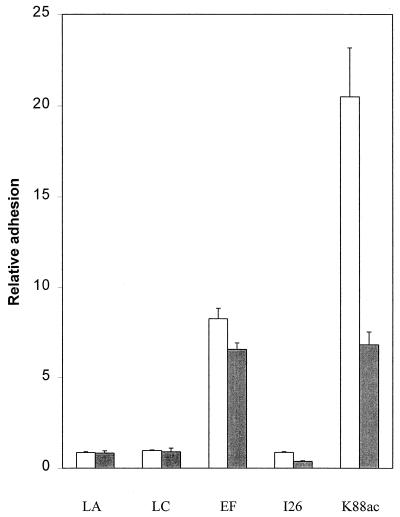
The three strains of LAB, i.e., E. faecium 18C23, L. acidophilus 80H10, and L. casei 47G19, were tested for their ability to adhere to the immobilized intestinal mucus of piglets, and the results obtained using the LAB culture incubated for 25 h are presented in Fig. 1. E. coli K88ac, which showed a strong ability to attach to the intestinal mucus in our previous study (16), was used as a positive control, while L. acidophilus I26, which was isolated from the chicken intestine (14), was used as a negative control. The result showed that the adhesion of 3H-labeled E. faecium 18C23 to the small intestine mucus was much higher than the adhesion of L. acidophilus 80H10 and L. casei 47G19 (Fig. 1). Similar patterns were obtained using LAB cultures incubated for 10, 15, and 40 h (data not shown). Approximately 9% of E. faecium 18C23 adhered to the small intestine mucus, while less than 1.5% of the other two strains did so. The adhesion of E. faecium 18C23 to the small intestine mucus was 8.5-fold higher than that to BSA, while the adhesion of L. acidophilus 80H10 and L. casei 47G19 was similar to (0.5-fold to 1.5-fold higher) their adhesion to BSA. A similar attaching pattern of these strains to polythene was also observed. The ratio of E. faecium 18C23 attachment to mucus and to polythene was much higher than the ratios for L. acidophilus 80H10 and L. casei 47G19 (Fig. 1). These results indicate that the binding of E. faecium 18C23 to the piglet intestinal mucus receptor was specific. Since incubation for 25 h yielded the highest attachment of E. faecium 18C23 to the mucus receptor (1.5 to 1.8 times higher than the values for cultures incubated for shorter or longer periods), this incubation time was used in the following experiments unless stated otherwise.
FIG. 1.
Relative adhesion of bacteria to the intestinal epithelial mucus. The result is expressed as the ratio between the number of bacteria attached to mucus and the number attached to BSA albumin (open bars) or polythene (solid bars). LA, L. acidophilus 80H10; LC, L. casei 47G19; EF, E. faecium 18C23; I26, L. acidophilus I26 (a chicken isolate, as a negative control); K88ac, E. coli K88ac (as a positive control).
Pretreatment of the porcine small intestine receptor with trypsin or sodium metaperiodate produced a similar pattern of inhibition (Table 1). Trypsin treatment reduced the level of adhesion of E. faecium 18C23 by 42.4%, and more than 60% of E. faecium 18C23 adhesion was inhibited by treatment with sodium metaperiodate. However, surprisingly, treatment with the other two proteolytic enzymes (pronase and proteinase) increased the adhesion of E. faecium 18C23 to the intestinal mucus receptor.
TABLE 1.
Effect of proteolytic enzymes and iodination on the adhesion of E. faecium to the porcine intestinal mucus receptor
| Treatment | Relative adhesion (%)a |
|---|---|
| Proteolytic enzymesb | |
| Control | 100 |
| Trypsin | 57.6 ± 5.5* |
| Protease | 162.3 ± 5.8* |
| Proteinase K | 130.0 ± 6.3* |
| Iodate | |
| Acetate buffer (pH 4.5) | 100.7 ± 4.3 |
| Sodium iodate | 78.3 ± 3.8* |
| Metaperiodate | 39.0 ± 4.8* |
Adhesion is expressed as the percentage of radioactivity in the treatment relative to the control (treated with PBS buffer [pH 7.0]). Values are mean ± standard error of three separate experiments. *, Significantly different from the control (P < 0.01).
In vitro competitive exclusion study.
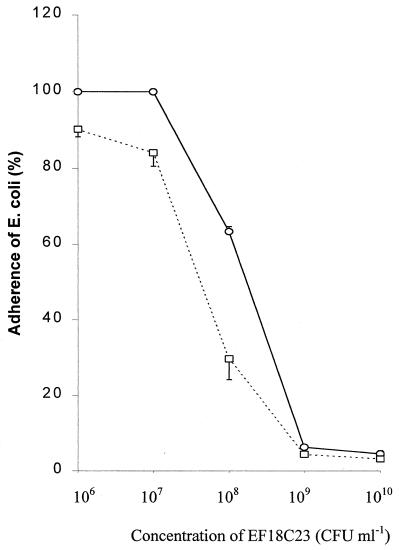
Living E. faecium 18C23 culture efficiently inhibited the adhesion of E. coli K88ac and K88MB to the piglet intestine mucus receptor. Inhibition of adhesion of E. coli K88ac to the small intestine mucus receptor was found to be dose dependent (Fig. 2). Inhibition of >90% was observed with 109 CFU or more of living E. faecium 18C23 culture when E. faecium 18C23 and E. coli were simultaneously added to immobilized mucus. The adhesion-inhibiting effects of the E. faecium 18C23 culture declined dramatically when the E. faecium 18C23 culture was used at 107 CFU ml−1 or less. In addition, the E. faecium 18C23 culture was more effective in inhibition of adhesion of K88MB than of K88ac (P = 0.02 [Fig. 2]). On the other hand, in the displacement test, there was no significant reduction in the adhesion of E. coli K88ac and K88MB to mucus with culture, washed cells, or supernatant (data not shown).
FIG. 2.
Inhibition of adhesion of E. coli K88ac (open circles) and K88MB (open squares) to piglet intestinal mucus by E. faecium 18C23. The results are expressed as a percentage of E. coli K88 binding to mucus relative to the control (treated with PBS buffer [pH 7.0]).
Characterization of inhibitory substances.
The bacterial cells, supernatant, or original culture of E. faecium 18C23 were tested separately to identify which part was involved in the inhibition. The original EF18C23 culture, washed bacterial cells, or culture supernatant remarkably reduced the attachment of E. coli K88ac and K88MB to the intestinal mucus receptor (Table 2). Compared to the original EF18C23 culture, washed cells or culture supernatant inhibited the adhesion of E. coli to a lesser degree. However, this inhibiting activity was recovered after the washed cells and the supernatant were recombined, with the recombined mixture having a similar inhibitory ability to that of the original E. faecium 18C23 culture.
TABLE 2.
Inhibition of adhesion of E. coli K88ac and K88MB to the intestinal mucus receptor of piglets by E. faecium 18C23
| Treatment | Adhesion (%)a of:
|
|||
|---|---|---|---|---|
|
E. coli K88ac
|
E. coli K88MB
|
|||
| Incubation for 24 h | Incubation for 72 h | Incubation for 24 h | Incubation for 72 h | |
| Control (PBS; pH 7.0) | 100 | 100 | 100 | 100 |
| Citrate buffer (pH 4.0) | 107 ± 2.5 | 107 ± 2.5 | 102 ± 1.4 | 102 ± 1.6 |
| Fresh KF broth (pH 4.0) | 101 ± 3.6 | 102 ± 2.9 | 99.1 ± 0.9 | 103 ± 3.4 |
| E. faecium 18C23 culture (pH 4.0) | 6.5 ± 0.75 | 4.0 ± 1.0 | 3.0 ± 0.2 | 3.1 ± 0.3 |
| Neutralized mixture (pH 7.0) | 26.0 ± 2.0* | 25.0 ± 2.6* | 12.5 ± 2.1* | 20.2 ± 2.2* |
| Supernatant only (pH 4.0)b | 21.0 ± 3.0 | 19.0 ± 4.0 | 18.0 ± 2.8 | 14.0 ± 2.1 |
| Neutralized supernatant (pH 7.0) | 39.5 ± 4.7* | 34.5 ± 3.9* | 27.0 ± 3.1* | 36.0 ± 2.9* |
| Pellet only (pH 7.0)c | 26.0 ± 8.0 | 27.3 ± 6.2 | 3.0 ± 0.2 | 3.0 ± 0.19 |
| Recombinant of supernatant and pellet (pH 4.0)d | 7.5 ± 0.25 | 8.5 ± 0.51 | 3.1 ± 0.25 | 2.9 ± 0.11 |
Adhesion is expressed as the percentage of E. coli K88 binding to mucus relative to the control. Values are expressed as the mean ± standard error of three experiments, each performed in triplicate. *, significantly different from their nonneutralized counterparts (P < 0.05).
Supernatant was prepared by filtration through a 0.22-μm-pore-size filter after the E. faecium 18C23 culture was centrifuged at 650 × g.
The bacterial cells were washed three times and suspended in PBS buffer (pH 7.0).
The washed bacterial cells were resuspended in the spent culture.
Neutralized E. faecium 18C23 culture or its supernatant still inhibited the adhesion of E. coli K88ac and K88MB to the mucus receptor but to a lesser degree (P < 0.05) compared with their nonneutralized counterparts (Table 2). On the other hand, a low pH value of 4.0, as is found in neutralized KF broth or citric acid-sodium citrate buffer, had no effect on the inhibition of adhesion of E. coli K88ac and K88MB. Consistent with the result in Fig. 2, E. coli K88ac was more resistant to the inhibition of adhesion by E. faecium 18C23 culture than was K88MB. The degree of inhibition of adhesion of K88ac and K88MB by E. faecium 18C23 culture or its supernatant was similar for incubation periods of either 24 or 72 h.
DISCUSSION
The genus Enterococcus is a member of the normal microflora of the alimentary tracts of pigs. E. faecium, E. faecalis, E. hirae, and E. cecorum are the most frequently found species in the swine intestine (8). Together with the other LAB, i.e., lactobacilli and bifidobacteria, enterococci are widely used in probiotic products (9, 11). The results of the present study showed that the culture of E. faecium 18C23, washed bacterial cells or its supernatant, inhibited the adhesion of E. coli K88ac or K88MB to the small intestine mucus of piglets. Somewhat surprisingly, this is, to our knowledge, the first report of its kind. With other LAB such as lactobacilli or bifidobacteria, similar results have been reported, that LAB inhibits the attachment of pathogenic bacteria in both in vitro and in vivo (2–4, 12). Conway (6) found that the adhesion of pathogenic E. coli to piglet ileal epithelial cells can be inhibited by pretreating the ileal cells with whole Lactobacillus cells. Blomberg et al. (3) also reported that three Lactobacillus strains of porcine origin reduced the adhesion of E. coli K88ab and K88ac by approximately 50% in an in vitro study.
The inhibitory effects of LAB on pathogens have been attributed to steric hindrance of binding sites (22), pH values (19), or certain components of the lysed cell wall (19, 22). The results of the present study support the idea that E. faecium 18C23 cells or the substances released in its culture might occupy the binding sites, although these binding sites are not necessarily the same epitope as that for E. coli K88. The mucus receptor for E. coli K88 is involved with glycoprotein and had been characterized as an 80-kDa protein in our previous study (17). Apparently the nature of the mucus receptor for E. faecium 18C23 seems to be a glycoprotein, at least in part based on the results that (i) treatment with trypsin reduced the adhesion of this organism, indicating the protein nature of the receptor, and (ii) treatment with metaperiodate decreased the adhesion of E. faecium 18C23 to the mucus receptor, also indicating the carbohydrate is involved with the attachment to the receptor. However, the mucus receptor for E. faecium 18C23 may not be same as that for E. coli K88ac, because treatment of intestinal mucus with pronase and proteinase reduced the adhesion of E. coli K88ac (17) but increased the adhesion of E. faecium 18C23 (see above). This result may imply that E. coli and E. faecium 18C23 might not attach to the same domain of receptor. Therefore, the inhibition of adhesion of E. coli K88ac by E. faecium 18C23 or its supernatant might be through steric hindrance. This type of inhibition has been previously proposed by Ouwehand and Conway (22) for the inhibition of E. coli K88 by Lactobacillus spp. or the compounds released into their cultures, by Chauviere et al. (5) for the inhibition of human ETEC adhesion by heat-killed L. acidophilus cells, by Bernet et al. (2) for the inhibition of cell attachment and invasion of enterovirulent bacteria by L. acidophilus and its spent culture supernatant fluid, and by Chan et al. (4) for the inhibition of adhesion of uropathogenic E. coli by Lactobacillus whole cells or their cell wall fragments.
The culture pH has been proposed to be an important factor in the inhibition of adhesion of pathogens to the mucosa (19). In the present study, the inhibiting effect was not solely a pH effect since considerable inhibitory action was demonstrated after neutralizing the mixture or spent culture supernatant to pH 7.0. In addition, citrate buffer at pH 4.0 or acidified fresh KF broth at pH 4.0 did not reduce the adhesion of E. coli K88ac or K88MB to the intestinal mucus receptor. The results disagree with the results of Lehto and Salminen (19), who reported that inhibition of Salmonella enterica serovar Typhimurium adhesion to Caco-2 cell cultures by Lactobacillus strain GG spent culture supernatant was only a pH effect. It should be noted that the two studies used different intestinal receptors (human Caco-2 cell line versus porcine mucus) and different LAB (Lactobacillus versus E. faecium). A synergic inhibition effect by the E. faecium 18C23 cells and the spent culture supernatant was obtained in the present study. The inhibition effect became weaker when using the E. faecium 18C23 cells or the spent culture supernatant individually compared with the original E. faecium 18C23 culture. However, a similar inhibitory ability with the original E. faecium 18C23 culture was observed when the washed cells and the spent culture supernatant were recombined. This result may imply that the substances from both E. faecium 18C23 cells and the spent culture supernatant contribute to the inhibition of adhesion of E. coli K88 to the small intestine mucus receptors.
In conclusion, the present study found that (i) living E. faecium 18C23 efficiently inhibited the adhesion of E. coli K88ac and K88MB to the piglet intestinal mucus and that the inhibition of adhesion of E. coli K88ac to the mucus was dose dependent; (ii) the substances from both the intact E. faecium 18C23 cells and the spent culture supernatant contribute to the inhibition of adhesion of E. coli K88 to the small intestine mucus; (iii) the inhibition of adhesion of E. coli K88ac by E. faecium 18C23 or its supernatant might occur by steric hindrance and altered pH; and (iv) the results of this in vitro study warrant further testing to determine if E. faecium 18C23 can prevent diarrhea caused by E. coli K88, possibly by inhibiting the colonization of the host intestine by these pathogens.
ACKNOWLEDGMENTS
This research was financially supported by StarLab Inc. (St. Joseph, Mo.), the Manitoba Rural Adaptation Council (MRAC), and the National Science and Engineering Council of Canada (NSERC).
REFERENCES
- 1.Alexander T J L. Neonatal diarrhea in pigs. In: Gyles C L, editor. Escherichia coli in domestic animals and humans. Wallingford, United Kingdom: CAB International; 1994. pp. 151–170. [Google Scholar]
- 2.Bernet M F, Brassart D, Neeser J R, Servin A L. Lactobacillus acidophilus LA1 binds to cultured human intestinal cells and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483–489. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blomberg L, Henriksson A, Conway P L. Inhibition of adhesion of Escherichia coli K88 to piglet ileal mucus by Lactobacillus spp. Appl Environ Microbiol. 1993;59:34–39. doi: 10.1128/aem.59.1.34-39.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan R C Y, Reid G, Irvin R T, Bruce A W, Costerton J W. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect Immun. 1985;47:84–89. doi: 10.1128/iai.47.1.84-89.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauviere G, Coconnier M H, Kerneis S, Darfeuille-Michaud A, Joly B, Servin A L. Competitive exclusion of diarrheagenic Escherichia coli (ETEC) from human enterocyte-like Caco-2 cells by heat-killed Lactobacillus. FEMS Microbiol Lett. 1992;91:213–218. doi: 10.1016/0378-1097(92)90700-x. [DOI] [PubMed] [Google Scholar]
- 6.Conway P L. Lactobacilli: fact and fiction. In: Grubbe R, Midtvedt T, Norin E, editors. The regulatory and protective role of the normal microflora. New York, N.Y: Macmillan; 1989. pp. 263–281. [Google Scholar]
- 7.Conway P L, Blomberg L, Welin A, Cohen P S. The role of piglet intestinal mucus in pathogenicity of Escherichia coli K88. FEMS Symp. 1991;59:335–337. [Google Scholar]
- 8.Devriese L A, Hommez J, Pot B, Haesebrouck F. Identification and composition of the streptococcal and enterococcal flora of tonsils, intestines and faeces of pigs. J Appl Bacteriol. 1994;77:31–36. doi: 10.1111/j.1365-2672.1994.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 9.Ewing W, Haresign W. Probiotics UK. Bucks, United Kingdom: Chalcombe Publications; 1989. [Google Scholar]
- 10.Fairbrother J. Worldwide pig progress. Amsterdam, The Netherlands: Elsevier International; 1999. Severe E. coli outbreak on the increase; pp. 16–17. [Google Scholar]
- 11.Franz C M A P, Holzapfel W H, Stiles M E. Enterococci at the crossroads of food safety. Int J Food Microbiol. 1999;47:1–24. doi: 10.1016/s0168-1605(99)00007-0. [DOI] [PubMed] [Google Scholar]
- 12.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 13.Hampson D J. Postweaning Escherichia coli diarrhoea in pigs. In: Gyles C L, editor. Escherichia coli in domestic animals and humans. Wallingford, United Kingdom: CAB International; 1994. pp. 171–191. [Google Scholar]
- 14.Jin L Z, Ho Y W, Abdullah N, Jalaludin S. Effect of adherent Lactobacillus spp. on in vitro adherence of salmonellae to the intestinal epithelial cells of chicken. J Appl Bacteriol. 1996;81:201–206. doi: 10.1111/j.1365-2672.1996.tb04501.x. [DOI] [PubMed] [Google Scholar]
- 15.Jin L Z, Ho Y W, Abdullah N, Jalaludin S. Probiotics in poultry: mode of action. World's Poult Sci J. 1997;53:351–363. [Google Scholar]
- 16.Jin L Z, Baidoo S K, Marquardt R R, Frohlich A A. In vitro inhibition of adhesion of enterotoxigenic Escherichia coli K88 to piglet intestinal mucus by egg-yolk antibodies. FEMS Immunol Med Microbiol. 1998;21:313–322. doi: 10.1111/j.1574-695X.1998.tb01179.x. [DOI] [PubMed] [Google Scholar]
- 17.Jin L Z, Marquardt R R, Baidoo S K, Frohlich A A. Characterization and purification of porcine small intestinal mucus receptor for Escherichia coli K88ac fimbrial adhesin. FEMS Immunol Med Microbiol. 2000;27:17–22. doi: 10.1111/j.1574-695X.2000.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 18.Laux D C, McSweegan E F, Cohen P S. Adhesion of enterotoxigenic Escherichia coli to immobilised intestinal mucosal preparations: a model for adhesion to mucosal surface components. J Microbiol Methods. 1984;2:27–39. [Google Scholar]
- 19.Lehto E M, Salminen S J. Inhibition of Salmonella typhimurium adhesion to Caco-2 cell cultures by Lactobacillus strain GG spent culture supernate: only a pH effect? FEMS Immunol Med Microbiol. 1997;18:125–132. doi: 10.1111/j.1574-695X.1997.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 20.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Netherwood T, Gilbert H J, Parker D S, O'Donnell A G. Probiotics shown to change bacterial community structure in the avian gastrointestinal tract. Appl Environ Microbiol. 1999;65:5134–5138. doi: 10.1128/aem.65.11.5134-5138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouwehand A C, Conway P L. Purification and characterization of a component produced by Lactobacillus fermentum that inhibits the adhesion of K88-expressing Escherichia coli to porcine ileal mucus. J Appl Bacteriol. 1996;80:311–318. doi: 10.1111/j.1365-2672.1996.tb03225.x. [DOI] [PubMed] [Google Scholar]
- 23.Reid C. The scientific basis for probiotic strains of Lactobacillus. Appl Environ Microbiol. 1999;65:3763–3766. doi: 10.1128/aem.65.9.3763-3766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SAS Institute. SAS user's guide. Statistics, version 5.0 edition. Cary, N.C: Statistical Analysis System Institute; 1985. [Google Scholar]
- 25.Underdahl N R. The effect of feeding Streptococcus faecium upon Escherichia coli induced diarrhea in gnotobiotic pigs. Prog Fed Nutr Sci. 1983;7:5–12. [PubMed] [Google Scholar]
- 26.Westerman R B, Mills K W, Phillips R M, Forter G W, Greenwood J M. Predominance of the ac variant in K88-positive Escherichia coli isolates from swine. J Clin Microbiol. 1988;26:149–150. doi: 10.1128/jcm.26.1.149-150.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson M R, Holmann A W. Immunity to Escherichia coli in pigs: adhesion of enteropathogenic Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1974;10:776–782. doi: 10.1128/iai.10.4.776-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witte W. Medical consequences of antibiotic use in agriculture. Science. 1998;279:996–997. doi: 10.1126/science.279.5353.996. [DOI] [PubMed] [Google Scholar]