Abstract
Objective
Formononetin is a bioactive isoflavone that has numerous medicinal benefits. We explored the feasibility and its mechanism of formononetin on treating acute deep vein thrombosis (DVT) in rats.
Materials and methods
Inferior vena cava (IVC) stenosis was performed to establish the DVT rat model. First, different doses of formononetin were used to observe the feasibility of formononetin on treating DVT. In sham and DVT groups, rats were orally treated with vehicle. In the remaining groups, formononetin (10 mg/kg, 20 mg/kg, and 40 mg/kg) was orally treated once a day for 7 days at 24 h after IVC. After 7 days, the levels of thrombosis and inflammation related factors in plasma were measured. The expression of endothelial nitric oxide synthase (eNOS) was analyzed by western blot and immunofluorescence. Molecular docking was used to evaluate the interaction between the formononetin and eNOS. Further, the NOS inhibitor (L-NAME) was used to explore the mechanism of formononetin for DVT.
Result
After treatment with formononetin, the average weights of thrombosis were decreased, and the levels of thrombosis and inflammation related factors were also significantly decreased. Additionally, phosphorylation of eNOS was increased with the formononetin administration. There is a good activity of formononetin to eNOS (total score = −6.8). However, the effects of 40 mg/kg formononetin were concealed by the NOS inhibitor (L-NAME).
Conclusion
Formononetin reduces vascular endothelium injury induced by DVT through increasing eNOS in rats, which provides a potential drug for treatment of venous thrombosis.
Keywords: inferior vena cava, molecular docking, nuclear factor-kappa B, venous thrombosis
Introduction
Deep vein thrombosis (DVT), also known as venous thromboembolism, globally constitutes a major fraction of diseases and frequently complicates the postoperative recovery of surgical patients with recognized or unrecognized risk factors.1,2 Estimation of clinical probability, measurement of D-dimer levels, and ultrasonography are common approaches for diagnosing symptomatic DVT of the lower extremities. 3 Clinically, unfractionated heparin, low-molecular weight heparin, fondaparinux, and direct oral anticoagulants are used to treat acute vein thrombosis. 4 Moreover, inflammatory reactions are also closely associated with DVT.
It is well known that activation of immune system strongly influences blood coagulation and pathological thrombus formation. 5 Adhesion of neutrophils has been identified as the initial step of thrombosis. D-dimer, prothrombin fragment 1 + 2 (F1 + 2), thrombomodulin (TM), interleukin (IL)-1β, IL-18, and tissue factor (TF) are some of the factors that are reported to participate in the process of thrombosis and inflammation.6–8 As a prototypical proinflammatory signaling pathway, nuclear factor-kappa B (NF-κB) pathway plays a major role in the expression of proinflammatory genes, including cytokines, chemokines, and adhesion molecules. 9 These immune cascades lead to expression of adhesion receptors on vascular endothelial cells. The vascular endothelium maintains the balance between vasodilatation and vasoconstriction, procoagulant and anticoagulant, and prothrombotic and antithrombotic mechanisms. 10 Phosphorylation status and expression of endothelial nitric oxide synthase (eNOS) are markers of vascular endothelium function.10,11
Formononetin (7-hydroxy-40-methoxyisoflavone) is a bioactive isoflavone that has numerous medicinal benefits, including antioxidant, anti-inflammatory, and antitumor activities.12,13 Interestingly, formononetin could improve arterial endothelium function by upregulating eNOS through estrogen receptors and mitogen-activated protein kinase (MAPK) pathway. 14 In addition, formononetin can suppress NF-κB and phosphatidylinositol 3-kinase (PI3K)/AKT in myeloma cells. 15 These research studies suggested that formononetin might play an active role in reducing thrombus formation through regulating arterial endothelium function.
In addition, we found that eNOS is one of the targets of formononetin through Traditional Chinese Medicine Database and Analysis Platform (TCMSP) (https://www.tcmsp-e.com/). In this study, we evaluated the effects of formononetin on thrombus formation and eNOS expression in DVT rats in an attempt to elucidate its potential mechanism.
Materials and methods
Experimental subjects
This study was approved by the ethics committee of the Weihai Municipal Hospital. Eighty Sprague-Dawley male and female rats (age, 8 weeks; weight, 200–220 g) were purchased from Jinan Pengyue Experimental Animal Breeding Co, Ltd (SCXK (Lu) 20,190,003). All rats had free access to food and water with a 12 h cyclic lighting schedule at 20–26°C and 40–70% relative humidity. The experiments followed the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no.85–23, revised 1996).
DVT model
Referring to the existing protocols, 7 we established an inferior vena cava (IVC) stenosis-induced DVT model in rats. In Figure S1, it showed the situation of IVC stenosis-induced DVT model. Briefly, rats were anesthetized by intraperitoneal injection of 1% pentobarbital sodium (40 mg/kg) after 1 week of adaptive feeding. The limbs were fixed at a supine position on the operating table. A 2-cm incision was made along the midline of the abdomen. The IVC was disserted and exposed, and stenosed by putting a 20 G needle (d = 0.91 mm) outside the vein, tying with a 7-0 silk at about 1 mm below the left renal vein, and removing the needle. The abdominal muscles and skin were sutured after surgery. Ceftriaxone sodium was evenly sprayed on the incision line to prevent bacterial infection.
Animal groups
Experiment 1
Forty rats were randomly divided into five groups, eight in each group. In sham group, the IVC was exposed and then sutured without any invasive treatment. In DVT group, the IVC stenosis was properly established. In formononetin groups, 10, 20, 40 mg/kg formononetin (F828304, Macklin, Shanghai, China) was orally administrated at 24 h after IVC stenosis surgery, 13 once per day for 7 days. In the sham and DVT groups, the rats were treated with the same volume of 0.5% carboxymethyl cellulose (CMC). The formononetin was dissolved in 0.5% CMC.
Experiment 2
In order to analyze whether formononetin improved the DVT via regulating eNOS, the NOS selected inhibitor L-NAME (HY-18729A, MedChemExpress, China) was used to the following study. Forty rats were divided into sham, DVT, L-NAME, formononetin, and formononetin plus L-NAME groups (n = 8). In the L-NAME group, the rats were orally treated with 40 mg/kg L-NAME at 24 h after IVC stenosis surgery, once per day for 7 days. 16 In the formononetin group, the rats were orally treated with 40 mg/kg formononetin at 24 h after IVC stenosis surgery, once a day for 7 days. In the formononetin plus L-NAME groups, the rats were orally treated with 40 mg/kg formononetin and 40 mg/kg L-NAME at 24 h after IVC stenosis surgery, once per day for 7 days. Similarly, the rats were treated with the same volume of vehicle in the sham and DVT groups. The L-NAME was dissolved in the water.
Sample collection
On day 8 after operation, the rats were anesthetized with 1% pentobarbital sodium, then collected venous plasma from the femoral vein in vacuum blood tubes containing sodium citrate additives as anticoagulants and shook it repeatedly immediately. Then, rats were killed by an intraperitoneal injection of 3% pentobarbital sodium (150 mg/kg). The thrombus-containing IVC was carefully removed from the test subjects for measuring weight and length. After washing out unclotted blood with 0.9% saline carefully, one part of the thrombosed IVC were fixed with 4% paraformaldehyde (C111000222, Macklin, Shanghai, China) for 24 h, while the rest were stored at −80°C for downstream analysis.
Enzyme linked immunosorbent assay (ELISA)
For the D-dimer (0623R2, Meimian, Jiangsun, China), F1 + 2 (70174R2, Meimian, Jiangsun, China), TF (0082R2, MeiMian, Jiangsun, China), and TM (0075r2, Meimian, Jiangsun, China), their levels were analyzed after collection and marking within 60 min. For the IL-1β (0047r2, Meimian, Jiangsun, China) and IL-18 (0194R2, MeiMian, Jiangsun, China), the plasma was centrifuged at 1000×g for 30 min at 4°C to collect supernatants. The supernatants were identified by ELISA kit within 4 h. The multiscan spectrum of the Labsystems Multiskan MS 352 Microplate Reader (Thermo Fisher Scientific) was used for the analysis.
Hematoxylin-eosin (H&E) staining
The thrombus-containing IVC, fixed with 4% paraformaldehyde for 24 h, were embedded in paraffin and sectioned into slices (3 μm). After dewaxing with xylene, the slices were dehydrated using ethanol (anhydrous ethanol, 5 min; 95% ethanol, 2 min; 80% ethanol, 2 min; 70% ethanol, 2 min). The slices were processed for hematoxylin staining (G1120, Solarbio, Beijing, China) for 15 min, differentiated for 30 s, and soaked at 50°C for 5 min. The slices were stained with eosin for 40 s. After washing with phosphate-buffered saline (PBS), the slices were again dehydrated with ethanol and cleared with xylene (X820585, Macklin, Shanghai, China). The sections were then observed under a light microscope (DM1000 LED, Leica, Germany).
Immunofluorescence
The thrombus-containing IVC, fixed with 4% paraformaldehyde for 24 h, were embedded in paraffin and sectioned into slices (5 μm). After dewaxing with xylene, the slices were dehydrated using ethanol. The sections were then treated with citrate buffer (pH 6.0, m053201, mreda, Beijing, China) at 95°C for 10 min and sealed with 5% bovine serum albumin (BSA) (A8010, Solarbio, Beijing, China) for 60 min at 25°C. Thereafter, sections were incubated with rabbit anti-phospho-eNOS (Thr113) (1:200, bs-3589R, Bioss, Beijing, China) at 4°C overnight. After washing with PBS, slices were incubated with a second antibody, lgG, labeled with Cy3 (rabbit anti-rat lgG/Cy3, 1:1000, bs-0293R, Bioss, Beijing, China) for 60 min at room temperature (25°C). After washing with PBS, sections were stained with 4′, 6-diamidino-2-phenylindole (DAPI) for 5 min. The fluorescent images were obtained using a laser confocal microscope (LSM800, Zeiss, Germany) and analyzed using Image J software (version 6; National Institutes of Health).
Western blot
The thrombus-containing IVC, previously stored at −80°C, were dissolved in cold RIPA (Radioimmunoprecipitation assay) buffer (R0010, Solarbio) containing protease and phosphatase inhibitors for 15 min and centrifuged at 12,000 g for 25 min at 4°C. Total protein was extracted using a total protein extraction kit (BC3640-50T, Solarbio, Beijing, China). The protein (40 μg) was separated by 10% SDS-PAGE (Bio-Rad Laboratories, Inc.), transferred to a polyvinylidene fluoride (PVDF) membrane (EMD Millipore), and sealed with 5% skimmed milk at 5°C for 1 h. The primary antibodies were diluted with 5% BSA at 4°C, including rabbit anti-Iκκβ (1:800,bs-4880R,Bioss, Beijing, China), rabbit anti-p-eNOS (1:800, bs-3589R, Bioss, Beijing, China), rabbit anti-eNOS (1:800, bs-20608R, Bioss, Beijing, China), rabbit anti-intercellular cell adhesion molecule-1 (ICAM-1, 1:800, bs-4617R,Bioss, Beijing, China), rabbit anti-NF-κB p65 (1:800, bs-23217R, Bioss, Beijing, China), rabbit anti-p-NF-κB p65 (1:800, bs-230303R, Bioss, Beijing, China), and rabbit anti-GAPDH (1:1000, bs-0755R, Bioss, Beijing, China). The membranes were incubated overnight at 4°C. Thereafter, they were washed thrice (10 min/wash) in TRIS-buffered saline with 0.1% Tween 20 (TBST). The membranes were subsequently incubated with goat anti-rabbit IgG/HRP (1:1000, bs-0295G-HRP, Bioss, Beijing, China) at 25°C for 1 h. Following three washes with TBST, enhanced chemiluminescence (ECL) reagent (C05-07004, Bioss, Beijing, China) was used to observe the protein bands. Protein expression was quantified using the ImageJ software (version 6; National Institutes of Health).
Molecular docking analysis
The formononetin ligand was retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov/) with 3D structure, and the three-dimensional structure of eNOS was downloaded from PDB database (https://www.rcsb.org/). Mechanical optimization, hydrogenation, and charging of ligand were carried out by UCSF chimera software. At the same time, the acceptor was extracted from the ligand small molecule, repaired the side chain and hydrogenated using UCSF chimera software. 17 Then the molecular docking was analyzed using AutoDock Vina tool. The docking results were evaluated using total score. The total score means inter molecular energy (kcal/mol), which represents the stability between the ligand and receptor. The value is more negative, and the binding is more stable.
Statistical analysis
Data were processed using SPSS software (version 19.0; National Institutes of Health), and the results are expressed as mean ± standard deviation. One-way analysis of variance followed by Tukey’s post hoc test was used for data analysis among groups. p value of <0.05 was considered statistically significant.
Results
Effects of formononetin on IVC thrombosis in DVT rats
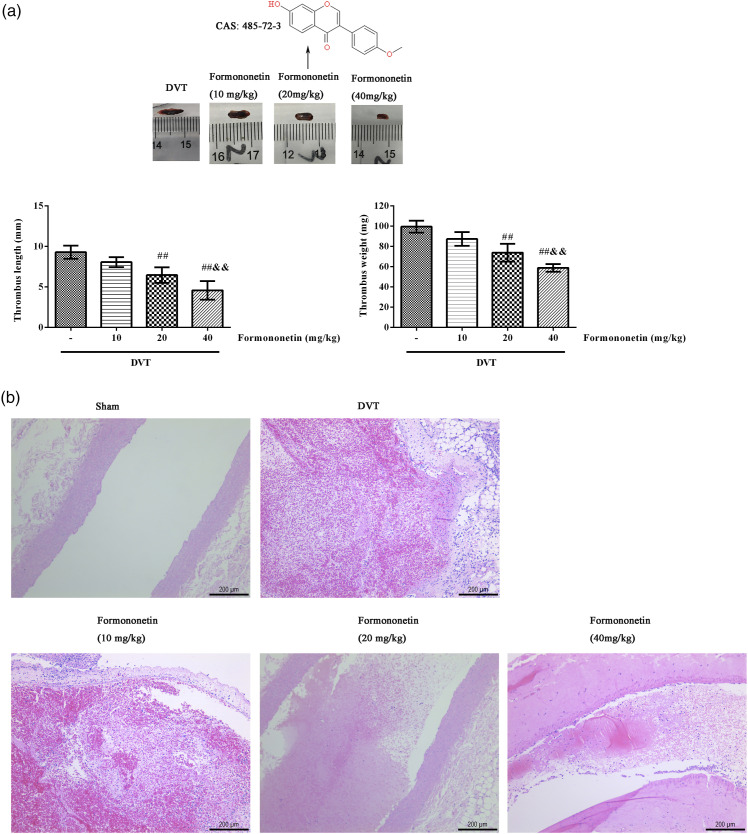
The lengths and weights of the IVC thrombus in each group were observed and measured (Figure 1(a)). After treatment of formononetin, the length and weight of thrombosis were significantly decreased in a dose-dependent manner. The pathological changes of venous thrombosis in each group were observed by H&E staining (Figure 1(b)), which showed longitudinal sections. In the sham group, the IVC wall structure was intact, and no thrombosis was observed in the vascular lumen. In the DVT group, there was a full thrombus, while in the formononetin groups, the amounts of thrombus in the vascular lumen were declined with the doses increasing. These findings suggest that formononetin could reduce DVT.
Figure 1.
Effects of formononetin on IVC thrombosis in DVT rats. (a) The images, lengths and weights of IVC thrombosis in each group. (b) The pathological changes of venous thrombosis in each group were analyzed by H&E (scale = 200 μm), which showed longitudinal sections. The arrow represents the thrombosis. Compared with the DVT group, ##p < 0.01. Compared with the 10 mg/kg formononetin group, &&p < 0.01.
Effects of formononetin on inflammatory and coagulation factors in DVT rats
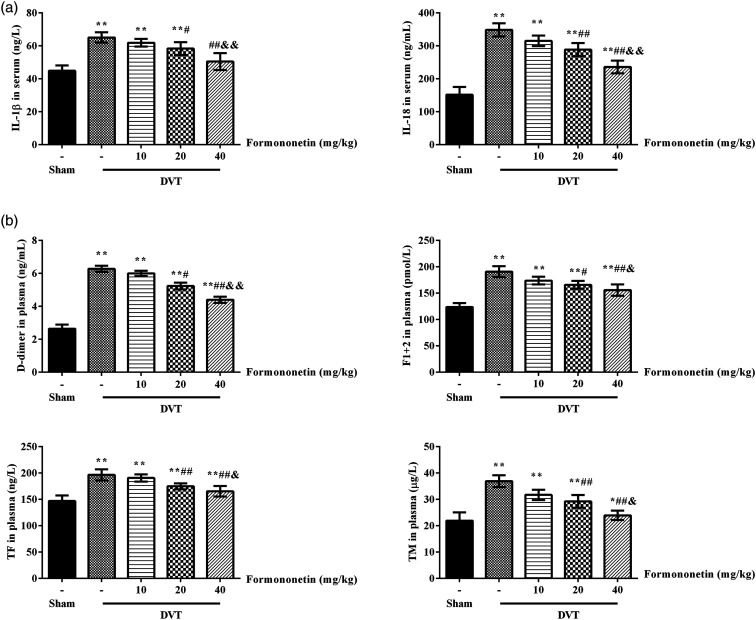
The expression of inflammatory factors, IL-1β and IL-18 (Figure 2(a)), and coagulation factors, D-dimer, F1 + 2, TF, and TM (Figure 2(b)) were analyzed by ELISA. Compared with the sham group, the above indexes were significantly increased. Compared to the DVT group, the contents of inflammatory factors and coagulation factors in the formononetin groups were significantly low. In addition, the levels of inflammatory factors and coagulation factors were decreased with the doses of formononetin increasing. These results indicate that formononetin reduced inflammatory and coagulation factors in DVT rats.
Figure 2.
Effects of formononetin on inflammatory factors and coagulation factors in DVT rats. (a) The contents of IL-1β and IL-18 in serum analyzed by ELISA. (b) The contents of D-dimer, F1 + 2, TM, and TF in plasma analyzed by ELISA. Compared to the sham group, *p < 0.05, **p < 0.01. Compared to the DVT group, #p < 0.05, ##p < 0.01. Compared to the 10 mg/kg formononetin group, &p < 0.05, &&p < 0.01.
Effects of formononetin on p-eNOS expression in IVC thrombus
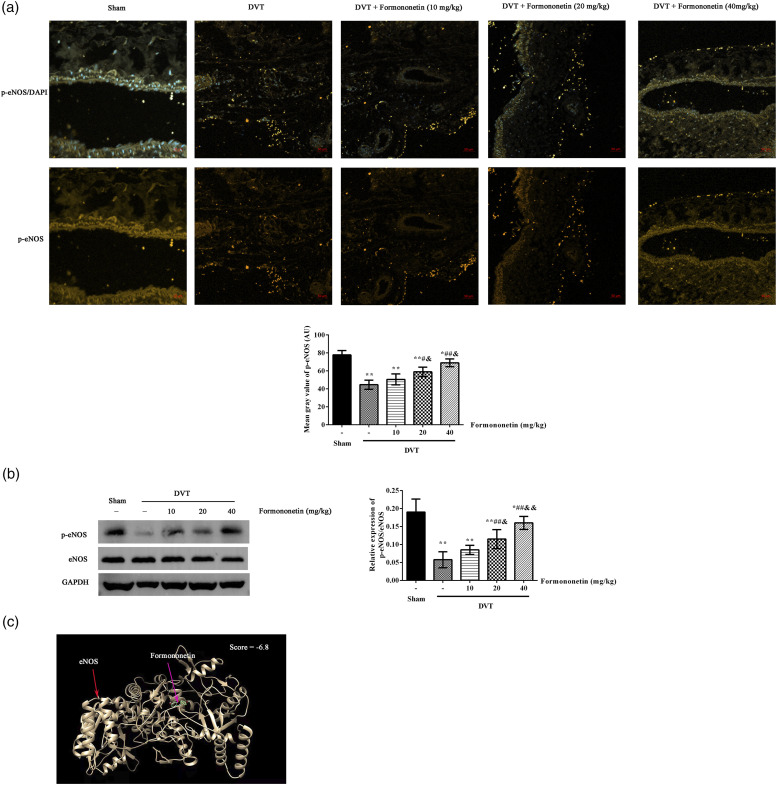
The expression of p-eNOS in IVC thrombus was analyzed using immunofluorescence (Figure 3(a)) and western blot (Figure 3(b)). The expression level of p-eNOS was significantly lower in the DVT group than in the sham group. However, after formononetin treatment, the expression level of p-eNOS was significantly increased compared with the DVT group. The levels of p-eNOS were increased with the doses of formononetin increasing. In order to confirm the relationship between the formononetin and eNOS, the molecular docking analysis showed that there is a good activity of formononetin to eNOS (score = −6.8, Figure 3(c)). The absolute value of score greater than six is used as the screening condition.
Figure 3.
Effects of formononetin on p-eNOS expression in the venous endothelial cells of DVT rats. The expression of eNOS was analyzed by immunofluorescence ((a), yellow) and western blot (b). Scale = 50 μm. The results were analyzed by Image J software. Compared to the sham group, *p < 0.05, **p < 0.01. Compared to the DVT group, #p < 0.05, ##p < 0.01. Compared to the 10 mg/kg formononetin group, &p < 0.05, &&p < 0.01. The activity of formononetin to eNOS was predicted by the molecular docking analysis (c).
Formononetin improved DVT via regulating the p-eNOS
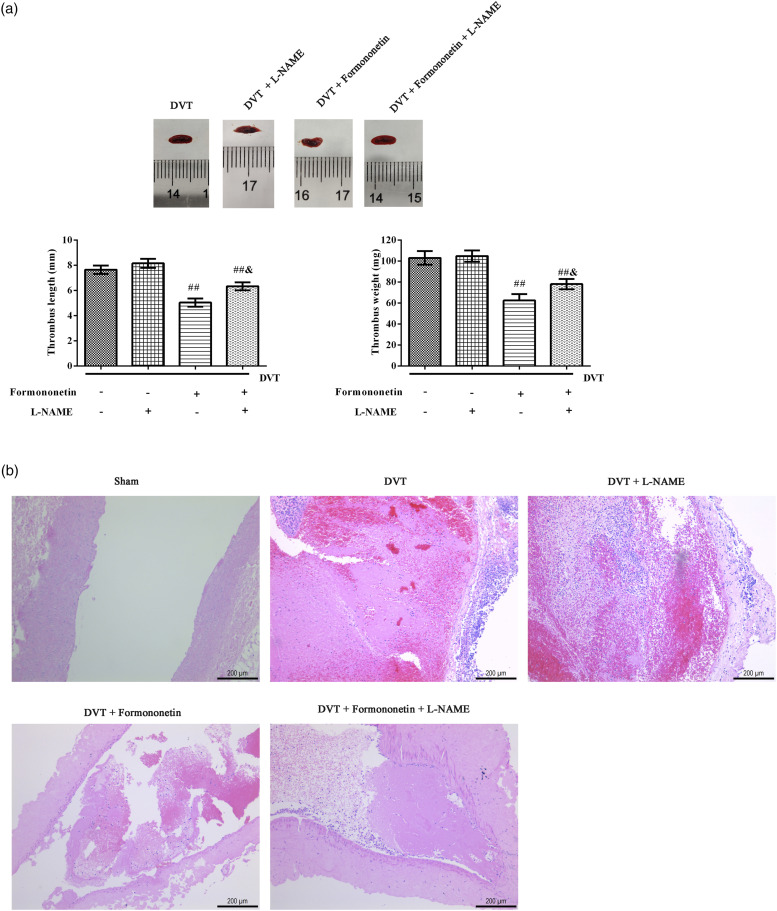
In order to confirm that formononetin improves DVT through regulating eNOS, the eNOS inhibitor L-NAME was used to carry out the experiment 2. At the same time, there was no significant difference between the DVT group and the L-NAME alone treatment group. Compared with the DVT group, the length and weight of thrombus were remarkably declined (Figure 4(a)), and the thrombosis in the vascular lumen (Figure 4(b)) was reduced after 40 mg/kg formononetin treatment. However, the effects of 40 mg/kg formononetin were clearly counteracted by L-NAME.
Figure 4.
Formononetin improved DVT via regulating the p-eNOS in rats. (a) The images, lengths and weights of IVC thrombosis in each group. (b) The pathological changes of venous thrombosis in each group were analyzed by H&E (scale = 200 μm), which showed longitudinal sections. 40 mg/kg formononetin and 40 mg/kg NOS inhibitor L-NAME were orally treated after surgery. Compared to the sham group, **p < 0.01. Compared to the DVT group, ##p < 0.01. Compared to the formononetin group, &p < 0.05.
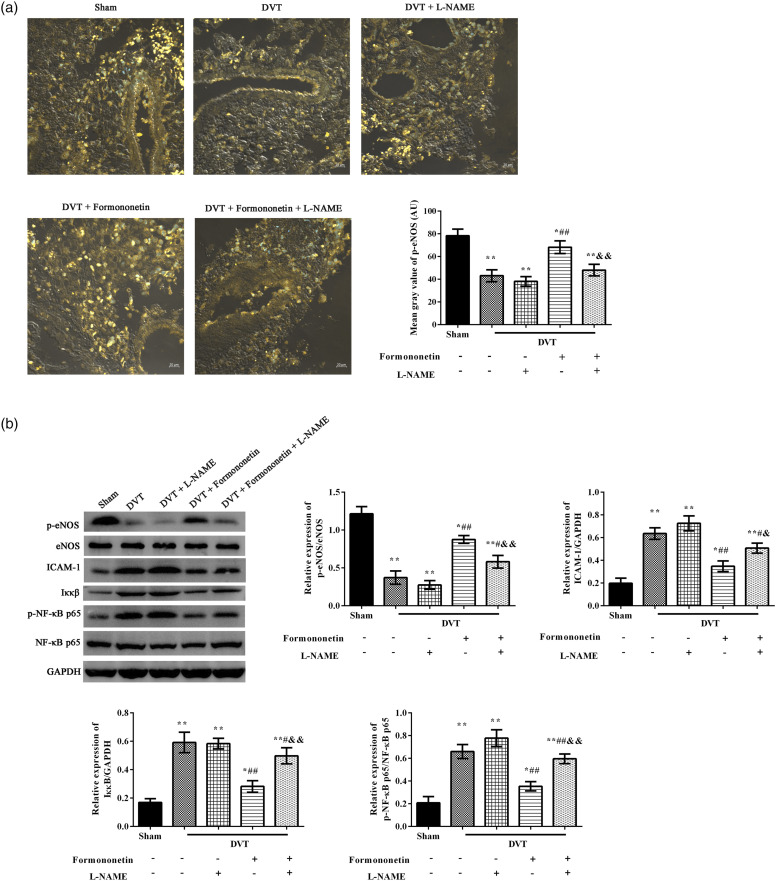
Formononetin decreased NF-κB pathway via regulating the p-eNOS
The expression of eNOS was measured by immunofluorescence (Figure 5(a)) and western blot (Figure 5(b)), and the results showed that eNOS inhibitor L-NAME obviously inhibited the effects of formononetin on eNOS expression when contrasted to the formononetin group. Meanwhile, the expression of ICAM-1 was significantly increased because of the IVC thrombosis, and its expression was clearly inhibited by 40 mg/kg formononetin treatment (Figure 5(b)). In addition, the NF-κB pathway-related proteins Iκκβ, p-NF-κB p65/NF-κB p65 were observed in each group by western blot (Figure 5(b)). The activated NF-κB pathway by DVT was notably suppressed after 40 mg/kg formononetin treatment. However, the suppression of 40 mg/kg formononetin was weakened when treated with the eNOS inhibitor L-NAME at the same time.
Figure 5.
Formononetin decreased NF-κB pathway via regulating the p-eNOS in the DVT rats. (a) The expression of p-eNOS was measured by Immunofluorescence ((a), yellow) and western blot (b). Scale = 20 μm. (b) Western blot was used to analyze the expression of ICAM-1, Iκκβ and p-NF-κB p65/NF-κB p65. Compared to the sham group, *p < 0.05, **p < 0.01. Compared to the DVT group, #p < 0.05, ##p < 0.01. Compared to the formononetin group, &&p < 0.01.
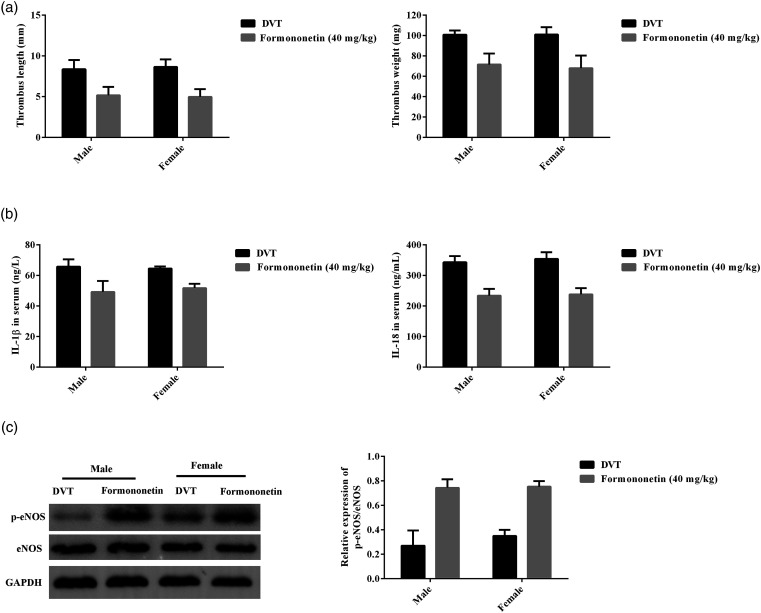
Gender-related differences in DVT rats after formononetin treatment
In order to find the potential influence of formononetin treatment in DVT rats on the basis of gender, the thrombus length and weights (Figure 6(a)), IL-1β and IL-18 in serum (Figure 6(b)), and p-eNOS/eNOS in thrombus-containing IVC (Figure 6(c)) were compared both DVT rats and 40 mg/kg formononetin treated rats. The results showed that there was no difference between male and female rats with IVC stenosis-induced DVT.
Figure 6.
Gender-related differences in DVT rats after formononetin (40 mg/kg) treatment. (a) The differences of thrombus length and weights between male and female rats. (b) The difference of levels of IL-1β and IL-18 in serum between male and female rats. (c) The difference of p-eNOS/eNOS protein expression between male and female rats was analyzed by western blot. No difference between male and female was found. Male = 8 and female = 8.
Discussion
In this study, we used different doses of formononetin to explore its roles in IVC thrombus of DVT rats and found that formononetin could decrease the length and weight of IVC thrombus and alleviate venous thrombosis in IVC stenosis-induced DVT rats with the dose of increasing. These findings suggest that formononetin could alleviate DVT in rats. As is well known, formononetin could prevent inflammation in many diseases, such as kidney injury, 13 type 2 diabetes, 18 and cancers. 12 Many studies also have shown that inflammation is activated and contributes to the progression of venous thrombosis.6,7,19 Once a thrombus forms, inflammatory cells are important for thrombus resolution, along with fibrinolytic agents and proinflammatory mediators. In our study, formononetin treatment reduced the secretion of proinflammatory mediators, such as IL-1β and IL-18, suggesting that formononetin improves venous thrombosis by inhibiting inflammation in rats.
In the context of venous thrombosis, the levels of D-dimer, F1 + 2, TM, and TF are important indicators for the diagnosis and evaluation of thrombotic diseases. D-dimer is a marker of endogenous fibrinolysis and has a high negative predictive value for patients with DVT.2,20 As a sensitive direct marker of thrombin formation, F1 + 2 is used to monitor anticoagulant therapies. 21 TM plays the role of an anticoagulant by mediating the interaction between thrombin and protein C. 22 TF is activated to increase the levels of blood borne in the vascular compartment when risk factors for thrombosis, such as inflammatory mediators, are present. 23 Through our study, the levels of D-dimer, F1 + 2, TM, and TF were clearly increased, and formononetin administration significantly decreased the above factors in the DVT rats. These data revealed that formononetin has a positive role in anti-thrombosis.
Endothelial dysfunction is a crucial mechanism of DVT, which could damage the antithrombotic function of venous wall through downregulation of eNOS expression or stimulation of adhesion receptor expression. 24 Upregulation of eNOS could increase NO release and improve arterial endothelial cell function, which could contribute to the prevention of cardiovascular diseases. 14 In our study, the levels of p-eNOS were continuously upregulated in the venous thrombosis induced by DVT with the doses of formononetin increasing. Meanwhile, there is a good activity of formononetin to eNOS from the results of the molecular docking. These results were consistent with findings of Tseng and his colleagues 25 that formononetin upregulates eNOS expression in endothelial cells to improve endothelial functions. As a component of endothelial cell-to-cell adherens junctions, ICAM-1 plays an important role in maintaining vascular integrity. 26 The expression of ICAM-1 is upregulated in response to inflammatory stimulation, which regulates leukocyte rolling and adhesive interactions with the vessel wall and guides leukocyte crossing of the endothelial layer. Studies on formononetin and ICAM-1 have reported in the human umbilical vein endothelial cells (HUVEC)27–29 that formononetin exhibits a protective effect on HUVEC through reducing ICAM-1. Consistent with these studies, we also found that formononetin reduced the expression of ICAM-1 in the IVC thrombus in rats.
Classical activation of NF-κB is dependent on the degradation of inhibitor of κB (IκB), which sequesters NF-κB in the cytoplasm during homeostasis. 30 After stimulation, the inhibitor of κB kinase (Iκκ) is activated, which contains three subunits: Iκκα, Iκκβ, and Iκκγ. Iκκγ activates Iκκβ to phosphorylate IκB, which, in turn, causes a conformational change, thereby releasing NF-κB subunits.12,13 NF-κB not only plays an important role in the expression of proinflammatory cytokines but also promotes the metabolism of glycolytic energy and the production of angiogenic factors.29,31 Zhou et al. 28 reported that formononetin could reverse the abnormal levels of phosphorylated ICAM-1, IL-1β, and NO by inhibiting Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling. In addition, Kwon et al. 32 found that formononetin blocked cytokine-induced endothelial cell adhesion molecule expression through NF-κB pathway disruption. Consistent with these studies, we found that formononetin suppressed the levels of Iκκβ and p-NF-κB p65 in venous thrombosis of DVT rats.
Previous reports have found that formononetin has a strong estrogenic effect and that estrogens could activate estrogen receptors.14,27 In endothelial cells, eNOS is influenced by various stimuli, such as estrogen receptors. 14 In our study, no significant difference on eNOS protein expression was found in thrombus-containing IVC after 40 mg/kg formononetin treatment between male and female rats. However, the estrogen-related respects with respect to the formononetin in venous thrombosis are needed to study in the future. The IVC stenosis model can reproduce the clinical scenario where a thrombus has reopened after DVT in patients, but its significant disadvantage is the degree of stenosis is inconsistent leading to a constant variable in thrombus sizes. 33 An electrolytic model may be used to analyze the effects of formononetin in the acute and chronic DVT because of the constant presence of flow, which more closely resembles the clinical scenario. 33 Besides, sample size calculation is an important consideration and a necessary component for animal research studies, which provides critical information for assessing feasibility and implications of each study. However, the sample size calculation and justification were not done in this study, which is another limitation of this research.
Conclusion
The results of our study indicate that formononetin prevents venous thrombosis in IVC stenosis-induced DVT rats, and its mechanism is related to upregulation of eNOS expression to improve endothelial functions. This potential of formononetin might provide a potential novel drug for treatment of venous thrombosis.
Supplemental Material
Supplemental Material for Formononetin regulates endothelial nitric oxide synthase to protect vascular endothelium in deep vein thrombosis rats by Zhongxiao Zhou, Haimeng Zhou, Xin Zou, Xiaowei Wang, and Mengjun Yan in International Journal of Immunopathology and Pharmacology
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Availability of data and material: All data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Supplementary Material: Supplementary material for this article is available online.
ORCID iD
Haimeng Zhou https://orcid.org/0000-0002-7063-0337
References
- 1.Di Nisio M, Van Es N, Büller HR. (2016). Deep vein thrombosis and pulmonary embolism. Lancet 388: 3060–3073. [DOI] [PubMed] [Google Scholar]
- 2.Niranjan A, Agarwal N, Agarwal T, et al. (2010) Deep vein thrombosis: review and update. J Indian Med Assoc 108: 248–251. [PubMed] [Google Scholar]
- 3.Bernardi E, Camporese G. (2018). Diagnosis of deep-vein thrombosis. Thromb Res 163: 201–206. [DOI] [PubMed] [Google Scholar]
- 4.Kyrle PA, Eichinger S. (2005). Deep vein thrombosis. Lancet 365: 1163–1174. [DOI] [PubMed] [Google Scholar]
- 5.Swystun LL, Liaw PC. (2016). The role of leukocytes in thrombosis. Blood 128: 753–762. [DOI] [PubMed] [Google Scholar]
- 6.Yao X, Chen W, Liu J, et al. (2019) Deep vein thrombosis is modulated by inflammation regulated via sirtuin 1/NF-κB signalling pathway in a rat model. Thromb Haemost 119: 421–430. [DOI] [PubMed] [Google Scholar]
- 7.Li G, Zhou R, Zhao X, et al. (2018) Correlation between the expression of IL-18 and deep venous thrombosis. Int J Mol Med 42: 2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peternel P, Terbizan M, Tratar G, et al. (2002) Markers of hemostatic system activation during treatment of deep vein thrombosis with subcutaneous unfractionated or low-molecular weight heparin. Thromb Res 105: 241–246. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence T. (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1: a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamwal S, Sharma S. (2018) Vascular endothelium dysfunction: a conservative target in metabolic disorders. Inflamm Res 67:391–405. [DOI] [PubMed] [Google Scholar]
- 11.Siragusa M, Fleming I. (2016) The eNOS signalosome and its link to endothelial dysfunction. Pflugers Arch 468: 1125–1137. [DOI] [PubMed] [Google Scholar]
- 12.Tay KC, Tan LT, Chan CK, et al. (2019) Formononetin: a review of its anticancer potentials and mechanisms. Front Pharmacol 10: 820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aladaileh SH, Hussein OE, Abukhalil MH, et al. (2019) Formononetin upregulates Nrf2/HO-1 signaling and prevents oxidative stress, inflammation, and kidney injury in methotrexate-induced rats. Antioxidants 8: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun T, Cao L, Ping NN, et al. (2016) Formononetin upregulates nitric oxide synthase in arterial endothelium through estrogen receptors and MAPK pathways. J Pharm Pharmacol 68: 342–351. [DOI] [PubMed] [Google Scholar]
- 15.Kim C, Lee JH, Ko JH, et al. (2019) Formononetin regulates multiple oncogenic signaling cascades and enhances sensitivity to bortezomib in a multiple myeloma mouse model. Biomolecules 9: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maneesai P, Prasarttong P, Bunbupha S, et al. (2016) Synergistic antihypertensive effect of carthamus tinctorius L. Extract and captopril in L-NAME-induced hypertensive rats via restoration of eNOS and AT-R expression. Nutrients 8: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettersen EF, Goddard TD, Huang CC, et al. (2004) UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612. [DOI] [PubMed] [Google Scholar]
- 18.Oza MJ, Kulkarni YA. (2018) Formononetin treatment in type 2 diabetic rats reduces insulin resistance and hyperglycemia. Front Pharmacol 9: 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukhopadhyay S, Johnson TA, Duru N, et al. (2019) Fibrinolysis and inflammation in venous thrombus resolution. Front Immunol 10: 1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumagai G, Wada K, Kudo H, et al. (2020) D-dimer monitoring combined with ultrasonography improves screening for asymptomatic venous thromboembolism in acute spinal cord injury. J Spinal Cord Med 43: 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semeraro F, Ammollo CT, Caironi P, et al. (2020) D-dimer corrected for thrombin and plasmin generation is a strong predictor of mortality in patients with sepsis. Blood Transfus 18: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiler H, Isermann BH. (2003) Thrombomodulin. J Thromb Haemost 1: 1515–1524. [DOI] [PubMed] [Google Scholar]
- 23.Müller-Calleja N, Hollerbach A, Ritter S, et al. (2019) Tissue factor pathway inhibitor primes monocytes for antiphospholipid antibody-induced thrombosis. Blood 134: 1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atochin DN, Huang PL. (2010) Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflügers Archiv 460: 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng HH, Vong CT, Leung GP, et al. (2016) Calycosin and formononetin induce endothelium-dependent vasodilation by the activation of large-conductance Ca2+-activated K+channels (BKCa). Evid Based Complement Alternat Med 2016: 5272531–5272613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bui TM, Wiesolek HL, Sumagin R. (2020) ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol 108: 787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrade CM, Sá MF, Toloi MR. (2012) Effects of phytoestrogens derived from soy bean on expression of adhesion molecules on HUVEC. Climacteric 15: 186–194. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Z, Zhou X, Dong Y, et al. (2019) Formononetin ameliorates high glucose-induced endothelial dysfunction by inhibiting the JAK/STAT signaling pathway. Mol Med Rep 20: 2893–2901. [DOI] [PubMed] [Google Scholar]
- 29.Liang C, Zhou A, Sui C, et al. (2019) The effect of formononetin on the proliferation and migration of human umbilical vein endothelial cells and its mechanism. Biomed Pharmacother 111: 86–90. [DOI] [PubMed] [Google Scholar]
- 30.Mackos AR, Allen JM, Kim E, et al. (2019) Mice deficient in epithelial or myeloid cell Iκκβ have distinct colonic microbiomes and increased resistance to citrobacter rodentium infection. Front Immunol 10: 2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rius J, Guma M, Schachtrup C, et al. (2008) NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453: 807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon HM, Choi YJ, Choi JS, et al. (2007) Blockade of cytokine-induced endothelial cell adhesion molecule expression by licorice isoliquiritigenin through NF-kappaB signal disruption. Exp Biol Med 232: 235–245. [PubMed] [Google Scholar]
- 33.Diaz JA, Obi AT, Myers DD, Jr, et al. (2012) Critical review of mouse models of venous thrombosis. Arterioscler Thromb Vasc Biol 32: 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Formononetin regulates endothelial nitric oxide synthase to protect vascular endothelium in deep vein thrombosis rats by Zhongxiao Zhou, Haimeng Zhou, Xin Zou, Xiaowei Wang, and Mengjun Yan in International Journal of Immunopathology and Pharmacology