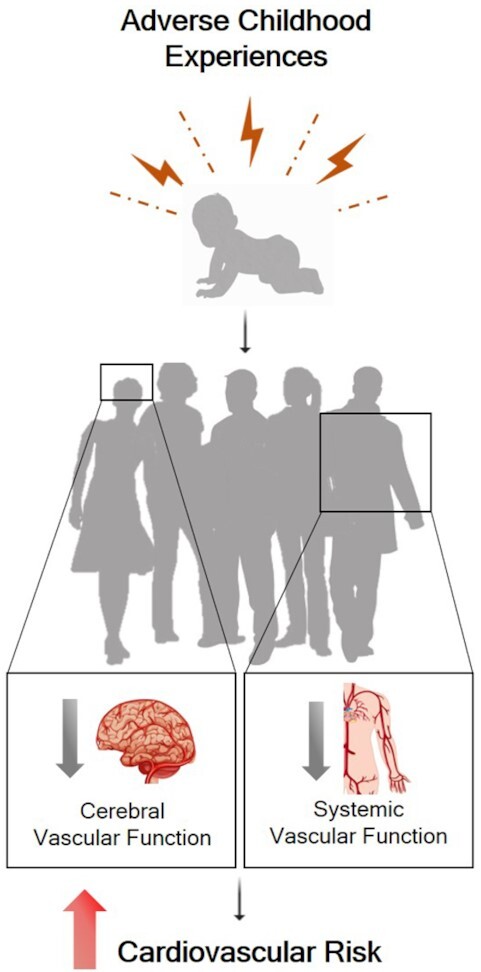
Abstract
Adverse childhood experiences (ACEs) are traumatic events during the first years of life that are associated with a higher risk of developing cardiovascular disease (CVD) during adulthood. The medial prefrontal cortex (mPFC) is a core region in the brain that modulates emotions and is directly involved in the cardiovascular response to stress by increasing vascular resistance. In the present study we examined the relationship between ACEs, mPFC and peripheral vascular function. Forty-five, adults (33±5 yrs.) participated in the present study to evaluate cerebral hemodynamics and peripheral vascular function. The impact of adverse experiences was evaluated through the ACE questionnaire. Among those that experienced ACEs (ACE group, n = 22), there was a significantly (P < 0.001) reduced activation of the mPFC as well as greater peripheral vascular resistance observed in the small (P ≤ 0.035), conduit (P ≤ 0.042) and large (P ≤ 0.001) blood vessels, when compared to those that did not report ACEs (Control group, n = 23). In addition, relationships between the number of ACEs and mPFC activation (rs = −0.428; P = 0.003) and peripheral vascular function (rs≤ −0.373; P ≤ 0.009) were observed. Findings from the present study support that adults who experienced ACEs exhibit a reduced activation of the mPFC along with systemic vascular dysfunction. In addition, individuals exposed to more childhood traumatic events exhibited a progressively greater inactivation of the mPFC and an increased peripheral vasoconstriction in a dose-dependent manner. These findings provide novel insights into the potential role that the brain and the peripheral vasculature may have in connecting adverse childhood events to the increased risk of CVD.
Keywords: adverse childhood event, cerebral blood flow, vascular function, nitric oxide, cardiovacular
Graphical Abstract
Graphical Abstract.
Assessment of fraction new over time reveals insight into proteostatic maintenance in muscle collagen.
Introduction
Childhood adversity, including abuse, neglect, or household dysfunctions, are highly prevalent stressful experiences during the first years of life that have a crucial impact on the individual's psychological and physical health.1,2 Compelling evidence supports that adverse childhood experiences (ACEs) increase the likelihood of developing cardiovascular diseases (CVD) later in life, including ischemic heart disease, hypertension, or atherosclerosis.3–5 Vascular endothelial dysfunction, a well-established precursor of CVD,6 is an important mediator in the ACE-induced increase in CVD risk.7 Prevailing data in the literature supports that young adults exposed to ACE exhibit greater peripheral vascular resistance, augmented arterial stiffness, and reduced endothelial function,8,9 all independent predictors of CVD events.
The brain is directly involved in controlling cardiovascular functions during stressful situations.10 Specifically, the medial prefrontal cortex (mPFC), a core region of the brain that modulates emotions,11 is directly engaged in the cardiovascular response to stress.12 The mPFC closely interacts with subcortical regions, such as the amygdala,13 activating the autonomous nervous system and promoting changes in cardiovascular reactivity that include exacerbated changes in blood pressure, heart rate, or peripheral vascular resistance.10 Data supports that stress-mediated peripheral vasoconstriction is related to reduced activation of the mPFC14 and associated hyperresponsiveness of the amygdala.15 Importantly, this cascade of events is recognized in individuals with depression, anxiety, or post-traumatic stress disorders (PTSD).16,17 However, to the best of our knowledge, no studies have examined these mechanisms as it relates to ACE-related stress.
Thus, the present study sought to test the hypothesis that adults who experienced ACEs had a reduced mPFC activation that was associated with a reduced peripheral vascular function when compared to adults that did not experience ACEs.
Material and Methods
Experimental Design
Volunteers presented to the Laboratory of Integrative Vascular and Exercise Physiology (LIVEP) on two separate occasions: a preliminary day and an experimental day. The preliminary day consisted of assessing body composition, blood pressure, medical history, and overall health status. For the experimental visit, participants reported to the LIVEP at 8:00 AM following an overnight fast and having abstained from tobacco, caffeine, and vigorous physical activity for 24 h and vitamin supplementation for 72 h. Cerebral hemodynamics and peripheral vascular function were evaluated.
Participants
A total of forty-five adult men and women, ages 18–41 yr were enrolled in the present study, following the principals of the Declaration of Helsinki and after approval by the Institutional Review Board at Augusta University. Participants were excluded if they (1) had a body mass index (BMI) greater than 40 kg/m2 (Class III obesity), (2) were pregnant or postmenopausal women, (3) were diagnosed with any cardiovascular, pulmonary, renal, hepatic, cerebral, or metabolic disease, (3) were prescribed any vasoactive medications (i.e., nitrates, β-blockers, angiotensin-converting enzyme inhibitors, PDE-5 inhibitors, etc.), or (4) had symptoms of uncontrolled hypertension. All participants were informed of the objectives, and possible risks of the investigation before written consent for participation was obtained.
Demographic Characteristics and Clinical Laboratory Values
Demographic characteristics were evaluated in all the participants during the preliminary visit. Volunteers completed a standard anthropometric assessment of height, weight, and calculated body mass index (BMI). Fasting concentrations of standard biochemical values for lipids (total cholesterol (TC), high-density lipoproteins (HDL), low-density lipoproteins (LDL), and triglycerides) and glucose levels were obtained using the Cholestech LDX analyzer (Alere, Providence, RI). Hemoglobin and hematocrit values were obtained using the HemoPoint H2 analyzer (Stanbio Laboratory, Boerne, TX). Concentrations of high-sensitivity C-reactive protein (hsCRP) were obtained from standard core laboratory techniques (Laboratory Corporation of America Holdings, Burlington, NC).
Adverse Childhood Experiences
ACEs information was collected in all the participants using the ACE questionnaire from the Behavioral Risk Factor Surveillance System adapted from the original CDC-Kaiser Study, specifically designed to gather information related to specific experiences during childhood. The ACE questionnaire contained detailed questions about childhood abuse, neglect, and household dysfunction divided into eleven items18. The ACE score (the number of categories of ACEs reported) was used to assess the cumulative effect of multiple ACEs, following the ACE scoring guidelines. Each exposure to a specific category of childhood adversity was coded as one ACE. Participants’ ACE score composite indicated how many types of childhood adverse events they were exposed to.
Cerebral Hemodynamics
A Portalite near-infrared spectroscopy (NIRS) device (Artinis, Elst, The Netherlands) was used to non-invasively monitor cerebral hemodynamics in the mPFC. Changes in cerebral hemodynamics are strongly correlated, both spatially and temporally, with changes in neural activity.19,20 NIRS was used as a continuous-wave tissue spectrometer that operates at wavelengths of 760 and 850 nm. The device is designed with three transmitters and one receiver that penetrate between 30 to 40 mm, to avoid signal interference from the scalp, skull, and cerebrospinal fluid. The Portalite device was secured to the subject's forehead and covered with a black cloth to avoid light interference while the participant laid supine in a low-light and temperature-controlled room. Resting assessments were recorded from each participant for 5 min to evaluate cerebral hemodynamics through a modification of the Lambert–Beer Law using individual age-related brain differential pathlength factor.21 The changes in concentrations of oxyhemoglobin (O2Hb) and deoxyhemoglobin (HHb), reflecting brain activity, were obtained. Total hemoglobin (tHb), representing the sum of O2Hb and HHb, was evaluated as an indicator of cerebral blood volume (CBV) and therefore, cerebral hemodynamics.20 Cerebral oxygen saturation (ScO2) was calculated from the ratio of O2Hb to tHb, considering the scattering coefficient of the brain. Estimated measurements of CBF were also computed using a derivation of the Fick equation.22 Oxyhemoglobin concentration, blood concentration of hemoglobin (Hb), arterial saturation (SaO2) measured non-invasively with a pulse oximeter, and a constant (k) related to the mass density of brain tissue were used as follows:
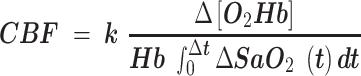 |
Macrovascular Function
Brachial artery endothelial function was evaluated using the flow-mediated dilation (FMD) test. A detailed explanation of the technique has been described previously23. Briefly, using a 12-MHz linear transducer, simultaneous B-mode and blood velocity profiles of the brachial artery were evaluated through ultrasound imaging (Logiq 7, G.E. Medical Systems, Milwaukee, WI). After an initial baseline, a forearm occlusion cuff (D.E. Hokanson, Bellevue, WA) placed immediately distal to the medial epicondyle, was rapidly inflated to 250 mmHg for five minutes (E-20 rapid cuff inflator, D.E. Hokanson, ) to induce arterial occlusion. Then, the pressure of the cuff was release inducing reactive hyperemia of the brachial artery23. R-wave gaiting (Accusync 72, Accusync Medical Research Corporation, Milford, CN) was used to capture end-diastolic arterial diameters and used for automated offline analysis of brachial artery vasodilation (Medical Imaging Applications, Coralville, IA). Peak diameter was determined by the highest five second average following cuff release. FMD is expressed as the % increase in peak diameter from baseline diameter. Cumulative shear rate (area under the curve, AUC) was determined every 4 s for the first 20 s, and every 5 s thereafter for the remainder 2-min data collection period using the trapezoidal rule. FMD was normalized by shear rate and presented as FMD/shear, as previously was reported.23
Microvascular Function
Peripheral vascular function in the cutaneous microvasculature was evaluated using laser Doppler imaging (MoorVMS-LDF, Moor Instruments, DE, USA) of the forearm. Imaging was combined with three reactivity tests to assess microvascular function: (1) local thermal hyperemia (LTH) to determine microvascular maximal dilation primarily mediated by nitric oxide and endothelial-derived hyperpolarization factors (EDHF), (2) post-occlusive reactive hyperemia (PORH) to evaluate microvascular shear-stress response primarily mediated by sensory nerves and EDHF, and (3) iontophoresis of acetylcholine (Ach) to assess microvascular endothelial-dependent vasodilation through nitric oxide, EDHF, and prostaglandins. A detailed explanation of each test has been described elsewhere.24
For this study, baseline (BL) flux was determined prior to every test by calculating a 30 s average. Brownian movement of macromolecules in the cutaneous interstitial space was calculated when a forearm cuff was inflated, reported as biological zero (B0), and subtracted from both baseline and peak responses. Cutaneous blood flow was evaluated as red blood cell flux (RBF) in perfusion units (PU) and as cutaneous vascular conductance (CVC) when mean arterial blood pressure was considered (PU/mmHg). Results are presented as (1) maximal hyperemic response (Peak), (2) area under the curve (Area), and (3) time-to-peak (TTP), which represents the time from the start of the stimuli to the maximal hyperemic response. Skin resistance (SR) was also evaluated for every individual using Ohm's law.
Arterial Stiffness
Peripheral vascular function in the central arteries was determined non-invasively through applanation tonometry using the SphygmoCor Xcel System (AtCor Medical, Sydney, Australia). Augmentation index (AIx) was determined in duplicate and normalized to a heart rate of 75 beats per minute (AIx75).25 Carotid–femoral pulse wave velocity (cfPWV), another index of arterial stiffness, was determined in duplicate by sequentially recording electrocardiographic-gated carotid and femoral artery waveforms using applanation tonometry with the SphygmoCor Xcel system and controlled by blood pressure.26
Statistical Analysis
The data were analyzed using SPSS version 27 (SPSS Inc., Chicago, IL) and expressed as mean ± standard error of mean (SEM), unless otherwise noted. An initial power calculation was performed based on the anticipated effect size estimated for the primary outcomes. The initial proposed sample size yielded a power > 0.89 in the primary outcome for the present study (macrovascular function). A power analysis and sample size calculation were performed before initiating the study, considering that under most circumstances, an α = 0.05 and a statistical power ≥ 0.85 is well accepted.
For all statistical analyses, significance was set at P<0.05. Based on the ACE score obtained in the questionnaire and for analysis purposes, subjects were divided into two groups: those who reported an ACE score equal or greater to 1 (ACE group) and those who reported an ACE score of 0 (Control group), following a similar stratification than for other traumatic-related conditions such as PTSD. The Shapiro-Wilk test was used to analyze the normality of the measurement distribution. When normality was met, independent group t-tests were performed to identify group differences between participants from the ACE and the Control group. If normality was not met, Mann-Whitney U tests were completed. Results are illustrated with box-and-whisker plots with minimum and maximum values. To compare the effects of ACE on cerebral hemodynamics and vascular function, a one-way ANOVA was completed with post hoc comparison using the Tukey's Honest Significant Difference test and group based on ACE scores (0, 1–4, 5–7, and > 8). Relationships between ACEs, cerebral hemodynamics, and peripheral vascular function assessments were evaluated using Pearson's correlation coefficients (r) or Spearman's correlation (rs) when the assumption of normality was not met. Effect size calculations using Cohen's d were reported for primary outcomes to represent small (Cohen's d = 0.2), medium (Cohen's d = 0.5), and large (Cohen's d = 0.8) effect sizes27,28.
Results
Participant Characteristics and Clinical Laboratory Values
Demographic characteristics and laboratory values for participants from the ACE and the Control groups are presented in Table 1. As expected, the only significant (P < 0.001) difference among both groups was the number of ACEs reported. No differences in subject demographics and body composition were observed between participants from both groups. Similarly, no differences were identified in the clinical laboratory values between both groups.
Table 1.
Participant Characteristics and Clinical Laboratory Values
| Variable | ACE | Control | P value |
|---|---|---|---|
| N | 22 | 23 | – |
| ACE score | 4 ± 2 | 0 ± 0 | <0.001 |
| Sex (M/F) | 14/8 | 10/13 | 0.113 |
| Race (C/AA) | 9/13 | 11/12 | 0.291 |
| Age (yrs) | 34 ± 3 | 31 ± 5 | 0.135 |
| Height (cm) | 174 ± 9 | 164 ± 8 | 0.194 |
| Weight (kg) | 88 ± 17 | 79 ± 20 | 0.134 |
| BMI (kg/m2) | 29.5 ± 3.7 | 26.9 ± 4.5 | 0.186 |
| Waist/hip ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.321 |
| Heart rate (bpm) | 63 ± 17 | 59 ± 11 | 0.299 |
| SBP (mmHg) | 121 ± 9 | 112 ± 8 | 0.110 |
| DBP (mmHg) | 75 ± 6 | 72 ± 6 | 0.148 |
| MAP (mmHg) | 90 ± 6 | 85 ± 7 | 0.101 |
| TC (mg/dL) | 175 ± 23 | 162 ± 28 | 0.201 |
| HDL (mg/dL) | 51 ± 12 | 56 ± 18 | 0.201 |
| LDL (mg/dL) | 95 ± 29 | 84 ± 28 | 0.183 |
| TRIG (mg/dL) | 101 ± 29 | 82 ± 32 | 0.106 |
| TC/HDL ratio | 3.5 ± 0.9 | 3.0 ± 1.1 | 0.109 |
| GLU (mg/dL) | 90 ± 8 | 87 ± 8 | 0.106 |
| HbA1C (%) | 5.5 ± 0.3 | 5.4 ± 0.4 | 0.103 |
| Hb (g/dL) | 14.7 ± 1.6 | 14.1 ± 1.4 | 0.112 |
| Hct (%) | 44 ± 4 | 42 ± 4 | 0.791 |
| hs-CRP (mg/L) | 2.3 ± 1.5 | 1.7 ± 1.5 | 0.190 |
Values are mean ± standard deviation (S.D.). Boldfaced value indicates statistical significance. ACE: Adverse childhood event; M: male; F: female; C: Caucasian; AA.: African American; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; MAP: mean arterial blood pressure; TC: total cholesterol; HDL: high density lipoproteins; LDL: low density lipoproteins; TRIG: triglycerides; GLU: glucose; HbA1c: hemoglobin A1c; Hb: hemoglobin; Hct: hematocrit; hs-CRP: high sensitivity C-reactive protein.
Cerebral Hemodynamics
Values related to cerebral hemodynamics in both the ACE and the Control groups are presented in Table 2. Overall, both groups showed similar (P = 0.420) cerebral oxygen saturation, as expected from individuals that are considered apparently healthy. Nonetheless, participants in the ACE group exhibited a significantly lower oxyhemoglobin (P< 0.001; Cohen's d = 1.27) and CBV (P< 0.001; Cohen's d = 0.99, Figure 1A) when compared to the Control group. Similarly, the balance between O2 supply and O2 demand in the cerebral tissue was also significantly lower in the ACE group when compared to the control (ACE: 12±1 µM vs. Control: 17±2 µM, P = 0.033; Cohen's d = 0.69). Cerebral blood flow was also significantly (P< 0.001; Cohen's d = 1.76) reduced in the ACE group than in the Control group (36±2 ml/100g/min vs. 51±2 ml/100g/min, respectively).
Table 2.
Cerebral Hemodynamics
| Variable | ACE | Control | P value |
|---|---|---|---|
| ScO2 (%) | 65 ± 1 | 64 ± 1 | 0.420 |
| O2Hb (µM) | 34 ± 2 | 45 ± 2 | <0.001 |
| HHb (µM) | 22 ± 2 | 28 ± 2 | 0.013 |
| tHb (µM) | 56 ± 3 | 71 ± 3 | <0.001 |
Values are mean ± standard error of mean (SEM). Boldfaced value indicates statistical significance. ACE: Adverse childhood event; ScO2: cerebral oxygen saturation; O2Hb: oxyhemoglobin; HHb: deoxyhemoglobin; tHb: total hemoglobin.
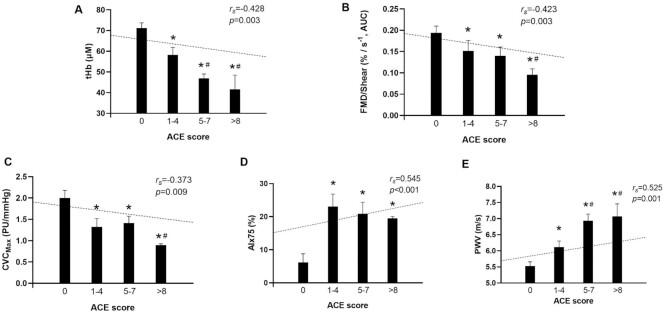
Figure 1.
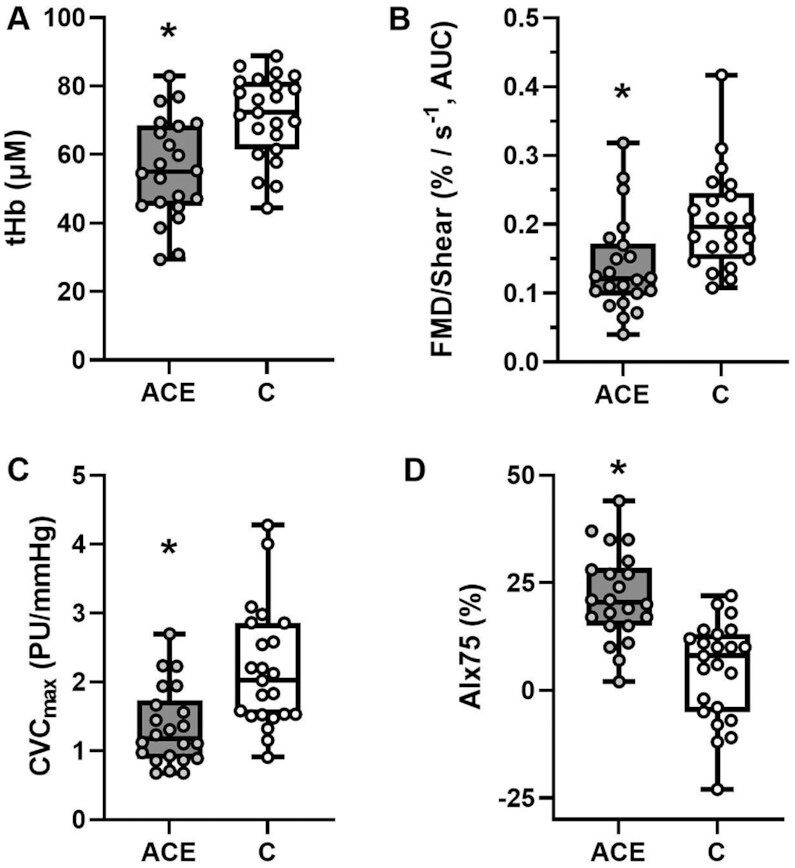
Individual data illustrated as Box-and-Whisker plots with minimum and maximum values for (A) cerebral hemodynamics (total hemoglobin, tHb), (B) macrovascular function (flow mediated dilation normalized for shear rate, FMD/shear), (C) microvascular function (maximal cutaneous vascular conductance, CVCmax), and (D) arterial stiffness (augmentation index normalized for a heart rate of 75 bpm, AIx75). Group differences were determined by independent group t tests or Mann-Whitney U-tests and denoted by * when P < 0.05 vs. Control group. µM: micromolar; AUC: Area under the curve; PU: perfusion units.
Macrovascular Function
Table 3 presents vascular function in the brachial artery in participants from the ACE and the Control groups. Baseline diameter was similar (P = 0.101) between groups. The FMD response was significantly (P = 0.042; Cohen's d = 0.70) lower in the ACE group compared to the Control group. No differences (P = 0.625) in shear rate were identified between both groups; howbeit, FMD normalized for shear rate was significantly (P = 0.030; Cohen's d = 0.96, Figure 1B) lower in the participants from the ACE group compared to the Control. No differences (P = 0.354) were identified between both groups in the time-to-peak vasodilation.
Table 3.
Macrovascular Function
| Variable | ACE | Control | P value |
| Baseline Diameter (mm) | 3.37 ± 0.2 | 3.33 ± 0.2 | 0.101 |
| Peak Diameter (mm) | 3.86 ± 0.2 | 3.73 ± 0.2 | 0.379 |
| FMD (%) | 6.3 ± 0.7 | 8.6 ± 0.8 | 0.042 |
| Shear (s–1, AUC) | 44,606 ± 5261 | 41,517 ± 3632 | 0.625 |
| FMD (%)/Shear (s–1, AUC) | 0.139 ± 0.02 | 0.206 ± 0.02 | 0.030 |
| Time to Peak (s) | 45 ± 3 | 51 ± 5 | 0.354 |
Values are mean ± standard error of men (SEM). Boldfaced value indicates statistical significance. ACE: Adverse childhood event; FMD: flow-mediated dilation; AUC: area under the curve.
Microvascular Function
Data for participants in the ACE and Control groups for red blood flux (RBF) and cutaneous vascular conductance (CVC) is presented in Table 4. Baseline flux and conductance were similar (P ≥ 0.340) between groups for all the reactivity tests completed. Brownian movement of macromolecules in the cutaneous interstitial space was also similar between participants from both groups (B0, ACE: 1.4 ± 0.1 PU vs. Control: 1.8 ± 0.1 PU, P = 0.121). No differences (P = 0.274) in skin resistance were identified between groups (ACE: 176 419 ± 13 094 Ω vs. Control: 159 181 ± 9063 Ω).
Table 4.
Microvascular function
| Variable | ACE | Control | P value |
|---|---|---|---|
| Local Thermal Hyperemia | |||
| BaselineLTH (PU) | 9 ± 1 | 9 ± 1 | 0.923 |
| PeakLTH (PU) | 125 ± 12 | 174 ± 16 | 0.020 |
| AreaLTH (PU s–1) | 151 980 ± 16 706 | 211 190 ± 20 042 | 0.031 |
| BaselineLTH (PU/mm Hg) | 0.10 ± 0.01 | 0.10 ± 0.02 | 0.370 |
| PeakLTH (PU/mm Hg) | 1.34 ± 0.13 | 2.17 ± 0.18 | <0.001 |
| TTPLTH (s) | 1056 ± 55 | 1128 ± 19 | 0.192 |
| Post-Occlusive Reactive Hyperemia | |||
| BaselinePORH (PU) | 8 ± 1 | 8 ± 1 | 0.939 |
| PeakPORH (PU) | 39 ± 5 | 50 ± 4 | 0.096 |
| AreaPORH (PU s–1) | 3048 ± 324 | 4067 ± 310 | 0.029 |
| BaselinePORH (PU/mm Hg) | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.491 |
| PeakPORH (PU/mm Hg) | 0.43 ± 0.05 | 0.64 ± 0.05 | 0.005 |
| TTPPORH (s) | 35 ± 4 | 33 ± 4 | 0.715 |
| Iontophoresis with Acetylcholine | |||
| BaselineACH (PU) | 8 ± 1 | 7 ± 2 | 0.340 |
| PeakACH (PU) | 98 ± 8 | 128 ± 11 | 0.035 |
| AreaACH (PU s–1) | 40 998 ± 4484 | 52 673 ± 4577 | 0.047 |
| BaselineACH (PU/mm Hg) | 0.09 ± 0.01 | 0.08 ± 0.03 | 0.728 |
| PeakACH (PU/mm Hg) | 1.11 ± 0.12 | 1.64 ± 0.50 | 0.002 |
| TTPACH, (s) | 18 ± 3 | 18 ± 2 | 0.933 |
Values are mean ± standard error of men (SEM). Boldfaced value indicates statistical significance. ACE: Adverse childhood event; LTH: Local thermal Hyperemia; PORH: Post occlusive reactive hyperemia; Ach: Acetylcholine; TTP: Time to peak; PU: perfusion units.
For local thermal hyperemia, the overall microvascular response was lower in the ACE group compared to the Control group. Specifically, during the initial peak in response to the thermal provocation, the ACE group exhibited a significantly lower response than the Control group (RBF, ACE: 95±7 PU vs. Control: 126±12 PU, P = 0.056; CVC, ACE: 1.1 ± 0.1 PU/mmHg vs. Control 1.5 ± 0.2 PU/mmHg, P = 0.026). Similarly, maximal dilation achieved during the plateau phase was significantly (P ≤ 0.020) lower in the ACE group than in the Control for both RBF (P = 0.020; Cohen's d = 0.91) and CVC (P < 0.001; Cohen's d = 1.46, Figure 1C).
For the post-occlusive reactive hyperemia test, both RBF and CVC baseline values were similar between groups (P ≥ 0.491). During this reactivity test, the ACE group showed a lower RBF hyperemic response (compared to the Control group (P = 0.096), findings that persisted when controlling for changes in blood pressure (P = 0.005; Cohen's d = 0.90).
During iontophoresis with Ach, both groups showed similar baseline response (P ≥ 0.340) and a progressive increase in perfusion in response to the delivery of Ach. However, the ACE group showed a significantly reduced maximum response compared to the Control group, both for RBF (P = 0.035; Cohen's d = 0.80) and CVC (P = 0.002; Cohen's d = 1.30).
Arterial Stiffness
Parameters of arterial stiffness in both the ACE and Control groups are presented in Table 5. Significant differences (P ≤ 0.001, Cohen's d = 1.51) were identified between both groups in the arterial tonometry assessments for both AIx and adjusted AIx75 (Figure 1D). Similarly, significant (P < 0.001, Cohen's d = 1.40) differences were observed in cfPWV assessment between the ACE and control group.
Table 5.
Arterial Stiffness
| Variable | ACE | Control | P value |
|---|---|---|---|
| AIx (%) | 26 ± 2 | 13 ± 2 | <0.001 |
| AIx75 (%) | 22 ± 2 | 5 ± 3 | <0.001 |
| cfPWV (m/s) | 6.5 ± 0.3 | 5.5 ± 0.1 | <0.001 |
Values are mean ± standard error of men (SEM). Boldfaced value indicates statistical significance. ACE: Adverse childhood event; AIx: Augmentation Index; AIx75: Augmentation index normalized for a heart rate of 75 beats per minute; cfPWV: carotid–femoral pulse wave velocity.
Relationships Between ACEs, Cerebral Hemodynamics, and Peripheral Vascular Function
Associations between ACE scores and markers of cerebral and peripheral vascular function are illustrated in Figure 2. Significant differences between groups were identified in cerebral hemodynamics (tHb, F(3,44) = 4.59, P = 0.007; Figure 2A), with negative associations identified between ACE scores and markers of cerebral hemodynamics including tHb (rs = −0.428; P = 0.003, Figure 2A), O2Hb (rs = −0.442; P = 0.002) and CBF (rs = −0.522; P < 0.001). Similarly, macrovascular function was significantly difference among ACE groups (FMD: F(3,43) = 2.29, P = 0.009; FMD/Shear: F(3, 43) = 2.32, P = 0.009) and ACE scores were negatively associated with FMD (rs = −0.335; P = 0.019) and FMD/Shear (rs = −0.423; P = 0.003, Figure 2B). Likewise, significant differences in microvascular function were also identified among ACE groups (F(3,44) = 3.22, P = 0.032, Figure 2C) and ACE scores negatively associated with maximal dilation (CVCmax: rs = −0.373; P = 0.009, Figure 2C). Significant differences were also identified among ACE groups in arterial stiffness (AIx75: F(3,44) = 6.59, P < 0.001, Figure 2D; PWV: F(3,44) = 13.53, P < 0.001, Figure 2F) and ACE scores were negatively associated with AIx (rs = 0.518; P< 0.001), AIx75 (rs = 0.545; P < 0.001, Figure 2D), and PWV (rs = 0.525; P = 0.001, Figure 2F).
Figure 2.
Relationships between ACE scores and (A) cerebral hemodynamics (tHb); (B) macrovascular function (FMD/Shear); (C) microvascular function (CVCmax); (D) arterial stiffness (AIx75); (E) pulse-wave velocity (PWV). Differences between groups determined by a one-way ANOVA based on ACE score (0, 1 to 4, 5, to 7 and a score greater than 8) and denoted by * P < 0.05 vs. Control group, # P < 0.05 vs. 1–4 group. Relationships between individual ACE scores and each measurement were evaluated using Spearman's correlation coefficients and denoted by a dotted line.
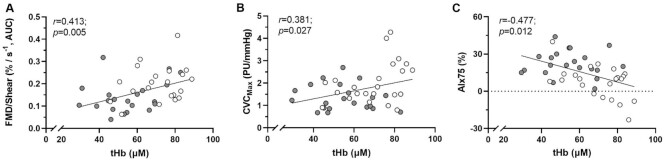
Relationships between cerebral hemodynamics and markers of peripheral vascular function were also evaluated and are illustrated in Figure 3. Specifically, cerebral hemodynamics were significantly associated with markers of peripheral vascular function including FMD (r = 0.335; P = 0.026) and FMD/Shear (r = 0.413; P = 0.005, Figure 3A), microvascular function through maximal dilation (r = 0.381; P = 0.027, Figure 3B) and arterial stiffness (r = −0.477; P = 0.012, Figure 3C).
Figure 3.
Relationships between mPFC hemodynamics and markers of peripheral vascular including (A) macrovascular function (FMD/Shear), (B) microvascular function (CVCmax) and (C) arterial stiffness (AIx75). Relationships were identified using Pearson's correlation coefficients. ACE group, grey circles. Control group, white circles.
Discussion
The experience of traumatic events during the first years of life has tremendous implications for the individual's psychological and physical health. Cumulative evidence supports a link between childhood adversity and the early development of multiple conditions later in life, such as ischemic heart disease, hypertension, or atherosclerosis. The present investigation has expanded prevailing information and has examined whether adversity early in life is associated with dysfunctions on the mPFC region, an area of the brain centrally involved in emotional regulation, as well as reduced peripheral vascular function. Findings of the present investigation also identified that different physiological mechanisms that are involved in central, conduit, and microvascular responses are diminished in individuals exposed to ACEs supporting the notion of multisystemic vascular dysfunction. In addition, present results support that individuals exposed to larger number of childhood traumatic events exhibited a progressively greater inactivation of the mPFC and increased peripheral vasoconstriction in a dose-dependent manner. Thus, the current study provides new and important insights into the link between ACE, mPFC, and peripheral vascular function.
Adversity occurring during the first years of life triggers behavioral and emotional stress that mediate physiological changes and precipitate an adaptive response to environmental stressors.1,3 Acute and intense mental stress leads to structural changes in the mPFC, a critical region of the brain that regulates emotions.29 Changes are detected by the amygdala, which responds to threats and activates physiological mechanisms related to stress.30 Compelling data supports that in conditions such as anxiety or PTSD, there is decreased activity of the mPFC29,31,32 and an increase in amygdala function.33,34 Prevailing data in the literature have also reported that children affected by low socioeconomic status, a known ACE, have a reduced mPFC activity35 and associated greater amygdala volume.36 Findings from the present study are in good agreement with prior data since adults that were exposed to adversity early in life presented with reduced activation of the mPFC. Interestingly, the present results also revealed an inverse relationship between mPFC activity and the number of ACEs suggesting that those individuals who were exposed to a greater number of stressors during childhood exhibited lower activation of the mPFC during adulthood. Considering that both animal and human data support that early exposure to chronic stress produces lasting neurobiological changes in the immature brain37 and the mPFC matures primarily during adolescence,38 it is not surprising that repeated childhood stressors can progressively impact the plasticity and functionality of the mPFC.37
Apart from emotional recognition and processing, the mPFC plays a central role in modulating the cardiovascular response to stress and fear.39 Emotional stress triggers the mPFC-amygdala circuit that prompts a hyperactivation of the sympathetic nervous system.40,41 In response, the body secretes hormones to cope with stress through rapid changes in blood pressure, heart rate, disruption of normal endothelial function, abnormal vasoconstriction, and peripheral vascular resistance.42,43 Inactivation of the mPFC has also been related to reductions in vagal activity and, therefore, exacerbate the cardiovascular response.44 It is important to note that endothelial dysfunction is considered one of the earliest precursors in the development and progression of nearly all types of CVD.45 In this line, data from animal models using maternal separation as an early life stressor, support the presence of vascular endothelial dysfunction in adult mice.46 Similar outcomes have been recently confirmed in young adults exposed to ACEs with comparable associations identified between macrovascular function and the reported number of ACEs.9 Current results extend previous findings and identify that individuals who experienced adversity during childhood also present with a significantly diminished vascular endothelial function both in the macro- and the micro-vasculature. Endothelial cells, and therefore endothelial dysfunction, differ in structure and phenotype, depending on the vessel type, and macro and microvessels respond differently to insults.47 Particularly, endothelial dysfunction in the macrovasculature is frequently associated with a reduced nitric oxide bioavailability,48 while prostaglandins and EDHF play an important role in the microcirculation.49 In the present study, parallel dysfunctions in both the micro and macrovascular response were observed in the ACE cohort, supporting that different physiological mechanisms that govern vascular health are impacted in these individuals. It is also worth noting that 1% reduction in vasodilation during the FMD test has been associated with 8% greater risk of future cardiovascular events.50 Accordingly, based on the FMD data, the ACE group in the present study may present with a ≈ 18% greater risk to develop future cardiovascular outcomes compared to the control group. These results are especially noteworthy since the cohort investigated is still young and all classic clinical cardiovascular markers (i.e., blood pressure, lipid profile, CRP, diabetes) evaluated demonstrate that the participants from both groups are relatively healthy.51
If the trauma is severe and/or repeated, the vasodilatory response of the endothelium is progressively reduced over time, leading to vasoconstriction, increasing blood flow resistance, and stiffening large arteries. Thus, it is not surprising that individuals in the ACE cohort also presented with significantly stiffer arteries compared with those from the control group. Similar results have been obtained from large epidemiological studies supporting that children who face adversity are more likely to exhibit higher blood pressure and augmented arterial stiffness8 during early adulthood,52 thus contributing to a greater risk to develop ischemic heart disease, hypertension or atherosclerosis.3–5 Deficiencies in different mechanisms have been associated with greater aortic stiffness including reduced NO, increased endothelin-1 (ET-1) and/or altered glycosaminoglycans, among others. Prevailing data have already identified elevated concentrations of circulating ET-1 in adolescents exposed to ACEs, along with enhanced arterial stiffness.8 Bearing in mind the role that NO plays in the functionality of blood vessel function, it is possible that a reduced systemic NO bioavailability may contribute, at least in part, to the observed systemic vascular dysfunction identified in the present study. However, further investigations of the role of NO in ACE-mediated vascular dysfunction are needed to confirm that hypothesis. Nonetheless, findings from the present study expand current knowledge, and demonstrate that different physiological mechanisms of endothelial dysfunction, one of the earliest markers of CVD,45 are associated with childhood adversity. These results also emphasize the importance of investigating earlier markers of CVD risk in order to identify premature vascular dysfunction in otherwise healthy individuals.
To our knowledge, few studies have explored the association between stress, brain, and cardiovascular health. Data from a large cohort reported that low socioeconomic status was associated with higher resting amygdala activity and a greater risk of developing cardiovascular events.53,54 Similarly, a relationship between stress-mediated peripheral vasoconstriction and reduced activation of the mPFC was found in patients with coronary artery disease.14 Results from the present study expand previous observations and identify that individuals exposed to childhood adversity exhibited a reduced activation of the mPFC and a progressively greater vascular dysfunction of the small, conduit, and large vessels. Bearing in mind that 23% of children in the U.S. have experienced two or more ACEs,55 these results reinforce the notion that early traumatic experiences represent a threat to cardiovascular health and should be considered an emerging public health concern. Certainly, these findings gain more significance considering that physical and, especially, emotionally stressful events tend to be underrecognized in routine clinical care. Conditions such as stress cardiomyopathy exemplified the limited attention paid to emotional stressors into one's health until severe symptomatology appears. Present findings also highlight that other cardiovascular conditions, where endothelial dysfunction is a primary mechanism,45 may also be impacted by ACEs. These cardiovascular events carry a high risk of a fatal outcome, so increasing clinical and public health awareness of the risks related to repetitive childhood adversity is critical and deserves further attention.
The high prevalence of ACEs and their association with a wide variety of negative outcomes support the need to prioritize ACEs recognition and prevention as early as possible. Primary prevention of childhood adversity through detailed screening and assessment, as well as early identification and intervention, are crucial in order to mitigate the ACE-related negative health consequences.56 The present results also open the door to potential novel interventions targeting psychological wellbeing that could exert benefits on the neuro- and cardio- vascular systems. Interventions aiming at reducing stress and the associated neurobiological changes could also be effective therapies to mitigate childhood stress-related cardiovascular morbidity and mortality prior to the development of severe outcomes.57
Conclusion
In conclusion, for the first time, results from the present study demonstrate that adults who experienced adversity during their childhood exhibit a reduced activation of the mPFC and a concomitant systemic vascular dysfunction that impacts small, conduit, and large vessels. Findings also identified that individuals that were exposed to a greater number of ACEs exhibit a progressively greater inactivation of the mPFC and an associated decreased peripheral vasodilation in a dose-dependent manner. Results from the present study emphasize the importance of early life adversities as a modifiable risk factor for the development of premature CVD. Additionally, these findings identify the brain, and especially the mPFC region, as a potential therapeutic target that can mitigate the CVD risk associated with ACEs. Future studies should expand these results in larger cohorts and evaluate interventions that target stress-induced changes in the brain as well as systemic vascular dysfunction, to further reduce the burden of cardiovascular disease.
ACKNOWLEDGEMENTS
The authors want to thank all the volunteers that participated in this study, including those that were part of the Augusta Heart Study, for their commitment to this research investigation.
Contributor Information
Paula Rodriguez-Miguelez, Department of Kinesiology and Health Sciences, Virginia Commonwealth University, Richmond 23284, VA, USA; Division of Pulmonary and Critical Care, Virginia Commonwealth University, Richmond 23284, VA, USA.
Jacob Looney, Georgia Prevention Institute, Augusta University, Augusta 30912, Georgia, USA.
Marsha Blackburn, Georgia Prevention Institute, Augusta University, Augusta 30912, Georgia, USA.
Jeffrey Thomas, Georgia Prevention Institute, Augusta University, Augusta 30912, Georgia, USA.
Jennifer S Pollock, School of Medicine, University of Alabama at Birmingham, Birmingham 35294, Alabama, USA.
Ryan A Harris, Georgia Prevention Institute, Augusta University, Augusta 30912, Georgia, USA; Sport and Exercise Science Research Institute, University of Ulster, Jordanstown BT37 0QB, Northern Ireland, UK.
Funding
This work was supported in part by the National Institute of Health NIH/NHLBI P01HL06999 (JSP, RAH) and the American Heart Association 16POST31080031 (PRM).
Conflict of Interest Statement
None declared
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Loria AS, Ho DH, Pollock JS. A mechanistic look at the effects of adversity early in life on cardiovascular disease risk during adulthood. Acta Physiologica. 2014;210(2):277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suglia SF, Koenen KC, Boynton-Jarrett R et al. Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American Heart Association. Circulation. 2018;137(5):e15–e28.. doi: 10.1161/CIR.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dong M, Giles WH, Felitti VJ et al. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110(13):1761–1766. [DOI] [PubMed] [Google Scholar]
- 4. Felitti VJ, Anda RF, Nordenberg D et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. [DOI] [PubMed] [Google Scholar]
- 5. Riley EH, Wright RJ, Jun HJ, Hibert EN, Rich-Edwards JW. Hypertension in adult survivors of child abuse: observations from the Nurses' Health Study II. J Epidemiol Community Health. 2010;64(5):413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42(7):1149–1160. [DOI] [PubMed] [Google Scholar]
- 7. Su S, Wang X, Pollock JS et al. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia stress and Heart study. Circulation. 2015;131(19):1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Su S, Wang X, Kapuku GK et al. Adverse childhood experiences are associated with detrimental hemodynamics and elevated circulating endothelin-1 in adolescents and young adults. Hypertension. 2014;64(1):201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jenkins NDM, Rogers EM, Banks NF et al. Childhood psychosocial stress is linked with impaired vascular endothelial function, lower SIRT1, and oxidative stress in young adulthood. Am J Physiol-Heart Circulat Physiol . 2021;321(3):H532–H541.. doi: 10.1152/ajpheart.00123.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ginty AT, Kraynak TE, Fisher JP, Gianaros PJ. Cardiovascular and autonomic reactivity to psychological stress: neurophysiological substrates and links to cardiovascular disease. Auton Neurosci. 2017;207:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9(2):148–158. [DOI] [PubMed] [Google Scholar]
- 12. Gianaros PJ, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41(4):521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lindquist KA, Satpute AB, Wager TD, Weber J, Barrett LF. The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cereb Cortex. 2016;26(5):1910–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah A, Chen C, Campanella C et al. Brain correlates of stress-induced peripheral vasoconstriction in patients with cardiovascular disease. Psychophysiology. 2019;56(2):e13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20(16):6225–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson MA, Liberzon I, Lindsey ML et al. Common pathways and communication between the brain and heart: connecting post-traumatic stress disorder and heart failure. Stress. 2019;22(5):530–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)?. J Affect Disord. 2002;70(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ford DC, Merrick MT, Parks SE et al. Examination of the factorial structure of adverse childhood experiences and recommendations for three subscale scores. Psychol Violence. 2014;4(4):432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He F, Sullender CT, Zhu H et al. Multimodal mapping of neural activity and cerebral blood flow reveals long-lasting neurovascular dissociations after small-scale strokes. Sci Adv. 2020;6(21):eaba1933. doi: 10.1126/sciadv.aba1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ren H, Jiang X, Xu K et al. A review of cerebral hemodynamics during sleep using near-infrared spectroscopy. Front Neurol. 2020;11:524009. doi: 10.3389/fneur.2020.524009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duncan A, Meek JH, Clemence M et al. Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatr Res. 1996;39(5):889–894. [DOI] [PubMed] [Google Scholar]
- 22. Elwell CE, Owen-Reece H, Cope M et al. Measurement of adult cerebral haemodynamics using near infrared spectroscopy. Acta Neurochir Suppl (Wien). 1993;59:74–80. [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez-Miguelez P, Seigler N, Harris RA. Ultrasound assessment of endothelial function: a technical guideline of the flow-mediated dilation test. J Visual Experim. 2016;(110):54011. doi: 10.3791/54011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodriguez-Miguelez P, Looney J, Thomas J, Harshfield G, Pollock JS, Harris RA. Sirt1 during childhood is associated with microvascular function later in life. Am J Physiol-Heart Circulat Physiol. 2020;318(6):H1371–H1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(1):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laurent S, Cockcroft J, Van Bortel L et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. [DOI] [PubMed] [Google Scholar]
- 27. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen J. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educat Psychol Measure. 1973;33(1):107–112. [Google Scholar]
- 29. Clausen AN, Francisco AJ, Thelen J et al. PTSD and cognitive symptoms relate to inhibition-related prefrontal activation and functional connectivity. Depress Anxiety. 2017;34(5):427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32(1):289–313.. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 31. Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156(11):1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45(7):806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. [DOI] [PubMed] [Google Scholar]
- 34. Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164(2):318–327. [DOI] [PubMed] [Google Scholar]
- 35. Sheridan MA, Sarsour K, Jutte D, D'Esposito M, Boyce WT. The impact of social disparity on prefrontal function in childhood. PLoS One. 2012;7(4):e35744. doi: 10.1371/journal.pone.0035744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 2012;15(4):516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. [DOI] [PubMed] [Google Scholar]
- 38. Zimmermann KS, Richardson R, Baker KD. Maturational changes in prefrontal and amygdala circuits in adolescence: implications for understanding fear inhibition during a vulnerable period of development. Brain Sciences. 2019;9(3):65. doi: 10.3390/brainsci9030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Myers B. Corticolimbic regulation of cardiovascular responses to stress. Physiol Behav. 2017;172:49–59.. doi: 10.1016/j.physbeh.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106(1):29–39. [DOI] [PubMed] [Google Scholar]
- 41. Hansel A, von Kanel R. The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity?. BioPsychoSocial Medicine. 2008;2:1, 21. doi: 10.1186/1751-0759-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet North Am Ed. 2007;370(9592):1089–1100. [DOI] [PubMed] [Google Scholar]
- 43. Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865–871. [DOI] [PubMed] [Google Scholar]
- 44. Wong SW, Masse N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage. 2007;35(2):698–708. [DOI] [PubMed] [Google Scholar]
- 45. Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24(6):1468–1474. [DOI] [PubMed] [Google Scholar]
- 46. Ho DH, Burch ML, Musall B, Musall JB, Hyndman KA, Pollock JS. Early life stress in male mice induces superoxide production and endothelial dysfunction in adulthood. Am J Physiol-Heart Circul Physiol. 2016;310(9):H1267–H1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hill CE, Phillips JK, Sandow SL. Heterogeneous control of blood flow amongst different vascular beds. Med Res Rev. 2001;21(1):1–60. [DOI] [PubMed] [Google Scholar]
- 48. Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter?. Hypertension. 2011;57(3):363–369.. HYPERTENSIONAHA.110.167015 [pii]. [DOI] [PubMed] [Google Scholar]
- 49. Cracowski JL, Roustit M. Current methods to assess human cutaneous blood flow: an updated focus on laser-based-techniques. Microcirculation. 2016;23(5):337–344. [DOI] [PubMed] [Google Scholar]
- 50. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26(6):631–640. [DOI] [PubMed] [Google Scholar]
- 51. Ruwanpathirana T, Owen A, Reid CM. Review on cardiovascular risk prediction. Cardiovasc Ther. 2015;33(2):62–70. [DOI] [PubMed] [Google Scholar]
- 52. Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the CARDIA study. Health Psychol. 2009;28(3):338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tawakol A, Osborne MT, Wang Y et al. Stress-associated neurobiological pathway linking socioeconomic disparities to cardiovascular disease. J Am Coll Cardiol. 2019;73(25):3243–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tawakol A, Ishai A, Takx RA et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet North Am Ed. 2017;389(10071):834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Child and Adolescent Health Measurement Initiative. Overview of Adverse Child and Family Experiences among US Children. 2013. [Google Scholar]
- 56. Oral R, Ramirez M, Coohey C et al. Adverse childhood experiences and trauma informed care: the future of health care. Pediatr Res. 2016;79(1-2):227–233. [DOI] [PubMed] [Google Scholar]
- 57. Levine GN, Lange RA, Bairey-Merz CN et al. Meditation and cardiovascular risk reduction: a scientific statement from the American Heart Association. J Am Heart Assoc. 2017;6(10). doi: 10.1161/JAHA.117.002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.