Abstract
A microbial consortium which rapidly mineralized the environmentally persistent pollutant benzo[a]pyrene was recovered from soil. The consortium cometabolically converted [7-14C]benzo[a]pyrene to 14CO2 when it was grown on diesel fuel, and the extent of benzo[a]pyrene mineralization was dependent on both diesel fuel and benzo[a]pyrene concentrations. Addition of diesel fuel at concentrations ranging from 0.007 to 0.2% (wt/vol) stimulated the mineralization of 10 mg of benzo[a]pyrene per liter 33 to 65% during a 2-week incubation period. When the benzo[a]pyrene concentration was 10 to 100 mg liter−1 and the diesel fuel concentration was 0.1% (wt/vol), an inoculum containing 1 mg of cell protein per liter (small inoculum) resulted in mineralization of up to 17.2 mg of benzo[a]pyrene per liter in 16 days. This corresponded to 35% of the added radiolabel when the concentration of benzo[a]pyrene was 50 mg liter−1. A radiocarbon mass balance analysis recovered 25% of the added benzo[a]pyrene solubilized in the culture suspension prior to mineralization. Populations growing on diesel fuel most likely promoted emulsification of benzo[a]pyrene through the production of surface-active compounds. The consortium was also analyzed by PCR-denaturing gradient gel electrophoresis of 16S rRNA gene fragments, and 12 dominant bands, representing different sequence types, were detected during a 19-day incubation period. The onset of benzo[a]pyrene mineralization was compared to changes in the consortium community structure and was found to correlate with the emergence of at least four sequence types. DNA from 10 sequence types were successfully purified and sequenced, and that data revealed that eight of the consortium members were related to the class Proteobacteria but that the consortium also included members which were related to the genera Mycobacterium and Sphingobacterium.
Sites which are contaminated with polycyclic aromatic hydrocarbons (PAHs) pose challenges to industries and regulators alike. PAHs with more than four fused benzene rings are more resistant to biodegradation than their three- and four-ring counterparts are (12). Generally, an increase in the number of fused rings increases the chemical stability and hydrophobicity of PAH molecules, thus making them less amenable to biodegradation (11). In the last 10 years, significant advances in describing the bacterial catabolism of high-molecular-weight (HMW) PAHs with four fused rings have been made; however, information regarding the biodegradation of PAHs with five or more rings is limited (26).
Benzo[a]pyrene is a globally distributed five-ring PAH that is both environmentally recalcitrant (11) and a potent carcinogen following bioactivation (48). Benzo[a]pyrene is included in both the U.S. Environmental Protection Agency's Priority Pollutant List (29) and the agency's new strategy for controlling persistent, bioaccumulative, and toxic pollutants (43). High concentrations of benzo[a]pyrene at contaminated sites usually occur as a result of activities which involve processing, combustion, and disposal of fossil fuels and fossil fuel-derived products. Benzo[a]pyrene is hydrophobic, exists in the aqueous phase only sparingly at such sites, and commonly occurs as a constituent in heterogeneous non-aqueous-phase liquids (NAPLs) comprised of complex mixtures of hydrocarbons. Examples of these NAPLs include creosote and products of crude oil refinery processes, such as diesel fuel (14, 15, 46). When benzo[a]pyrene is present in such a mixture, the rate and extent of benzo[a]pyrene biodegradation are guided by a variety of factors, including mass transfer processes, the production of surface-active compounds by bacteria during growth on other hydrocarbons in the mixture, and/or cometabolic processes. Inhibition and stimulation of biodegradation of various hydrocarbons have been shown to occur when the hydrocarbon being studied was dissolved in a NAPL (16, 17, 23, 25, 27, 31).
There have been few reports in the literature which document benzo[a]pyrene biodegradation by either pure or mixed cultures of bacteria (4, 20, 21, 24, 44, 49), and there have been still fewer reports which describe extensive benzo[a]pyrene mineralization by bacteria. Ye et al. (53) showed that a resting cell suspension of Sphingomonas paucimobilis EPA505 (36) cometabolically converted 10 mg of [7-14C]benzo[a]pyrene per liter to 28% 14CO2 in 48 h. Recently, Boonchan et al. (7) described a bacterium-fungus coculture which cometabolically mineralized 50 mg of [7-14C]benzo[a]pyrene per liter to 58.1% 14CO2 in 56 days in the presence of 250 mg of pyrene per liter. Even more remarkable, this coculture mineralized 25.5% [7-14C]benzo[a]pyrene as a sole source of carbon and energy under the same conditions.
From an environmental perspective, bacteria which grow on hydrocarbon NAPLs and simultaneously mineralize benzo[a]pyrene may be especially useful for bioremediation because benzo[a]pyrene usually occurs in such a matrix at contaminated sites. However, studying such a complex system may pose considerable challenges. Due to the rapid nature of benzo[a]pyrene biotransformation in NAPLs by a consortium, we were interested in investigating the population structure of the consortium and the mechanisms of the biodegradation process. Advances in molecular biology have led to the development of methods such as PCR-denaturing gradient gel electrophoresis (DGGE) which allow for cultivation-independent analysis of microbial populations in situ and which may be applied to the study of microbial ecology (10, 40). In the bioremediation field, DGGE analyses have already been employed to monitor population changes during oil spill bioremediation (33). In our study, DGGE was used to analyze a bacterial community which mineralized benzo[a]pyrene during biodegradation of a multicomponent hydrocarbon mixture. Here, the results of radiorespirometry, radiocarbon mass balance analyses, and molecular analyses are presented in order to describe a microbial consortium which grows on diesel fuel and rapidly mineralizes [7-14C]benzo[a]pyrene.
MATERIALS AND METHODS
Chemicals.
[7-14C]benzo[a]pyrene (58.78 mCi/mmol) was purchased from Chemsyn Science Laboratories (Lenexa, Kans.). The reported radiochemical purity was ≥98%; this was verified by our own thin-layer chromatographic (TLC) determination, which showed that 99% of the total radioactivity traveled with the unlabeled benzo[a]pyrene standard. Unlabeled benzo[a]pyrene (>99% pure, gold label) was purchased from Aldrich Chemical Co. (Milwaukee, Wis.). [14C]sodium bicarbonate (9.2 mCi/mmol), with a reported radiochemical purity of ≥98%, was purchased from New England Nuclear (Boston, Mass.). Diethyl ether was purchased from Fisher Scientific (Pittsburgh, Pa.). Jet fuel and diesel fuel were obtained from Exxon Co. (Houston, Tex.). Inipol EAP-22 was obtained from Elf Atochem (Philadelphia, Pa.). Methylene chloride and chloroform were purchased from Wako Chemical (Osaka, Japan).
Enrichment of the benzo[a]pyrene-degrading consortium.
Biodegradation of benzo[a]pyrene in soil was demonstrated previously when the indigenous microbiota was supplied with a suitable primary substrate, such as crude oil (25). The origin of the soil (1.2% organic matter; sand-silt-clay, 33:51:16) was an active cattle pasture in the Gulf region of Texas that had been used for this purpose for more than 18 years and had no recorded history of chemical contamination (25). The same soil was used in benzo[a]pyrene biodegradation experiments in which soil was exposed to benzo[a]pyrene (80 μg/g of soil) and jet fuel (1.0%, wt/wt) (27).
The consortium originated from seven precultures from soil which had been exposed to benzo[a]pyrene and jet fuel for 3 months (27). Approximately 3 g of this soil was transferred into 25 ml of Stanier's basal medium (SBM) (2) which contained 250 μl of jet fuel (1.0%, wt/wt) plus 80 mg of [7-14C]benzo[a]pyrene per liter. Similar enrichments were started, and some of them were supplemented with small amounts of additional carbon sources, such as 0.01% (wt/vol) BBL nutrient broth (Becton Dickinson, Cockeysville, Md.). The enrichments were incubated at 28°C with rotary shaking (300 rpm) in modified micro-Fernbach flasks (Bellco Glass Co., Vineland, N.J.) that were monitored daily for 14CO2 evolution (35). The microbial consortium used in this study originated from a jet fuel–nutrient broth-supplemented enrichment that evolved 14CO2 from radiolabeled benzo[a]pyrene even after repeated serial transfers had eliminated the added soil. Analysis of this consortium began after it was observed that the soil-free consortium consistently mineralized benzo[a]pyrene in repeated experiments and that the mineralization patterns did not change after multiple transfers. The consortium was maintained in 300 ml of SBM which contained 10 mg of benzo[a]pyrene per liter and 0.2% (wt/vol) diesel fuel with continuous shaking at 300 rpm on a rotary shaker at 28°C in the dark. Every 14 to 21 days, 10 ml of the culture suspension was transferred to fresh medium.
Monitoring benzo[a]pyrene mineralization in liquid culture.
All operations were carried out in dim yellow light in order to avoid photodegradation of benzo[a]pyrene. Customized micro-Fernbach flasks were charged with benzo[a]pyrene, [7-14C]benzo[a]pyrene, and diesel fuel and monitored for 14CO2 evolution as follows. A stock solution containing a known mass of benzo[a]pyrene and [7-14C]benzo[a]pyrene was prepared by dissolving the components in a known volume of diethyl ether in a 10-ml glass vial sealed with a Teflon septum and aluminum crimp top. A known volume of this mixture was added to the flasks with a gas-tight microsyringe (Hamilton, Reno, Nev.). The diethyl ether was evaporated under a gentle N2 stream, and the diesel fuel was applied directly to the bottom of the flask. Negative controls were prepared in the same manner but without diesel fuel. SBM and inoculum were added to the flask following diesel fuel application. Benzo[a]pyrene and diesel fuel were in physical contact for a total of approximately 5 to 10 min prior to the SBM and inoculum additions. All incubations were started with 24.5 ml of SBM plus 0.5 ml of stock culture inoculum which contained approximately 8 × 108 cells per ml. The Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.) was utilized for the determination of bacterial cell biomass. The starting cell protein concentration in each flask was approximately 1 mg liter−1. The flasks were sealed and incubated with rotary shaking at 300 rpm and 28°C. The flasks were flushed with air through a series of traps at appropriate time intervals, and 14CO2 was trapped in the CO2-trapping scintillation cocktail Oxosol C14 (National Diagnostics, Atlanta, Ga.). Radioactivity was measured with a Beta Trac model 6895 liquid scintillation counter (TM Analytic, Elk Grove Village, Ill.). All counts were corrected for background values after pure Oxosol C14 was measured. The trapping efficiency of the CO2-trapping apparatus was checked routinely by transferring 250-μl aliquots of an aqueous solution of NaH14CO3 (pH 10) to Erlenmeyer flasks in triplicate. The flasks were connected to the CO2-trapping apparatus, and air was flushed through each flask. After airflow was begun, 5 ml of 5.0 N HCl (Fisher Scientific, Pittsburgh, Pa.) was added to each flask through the inlet valve; this HCl reacted with the NaH14CO3 and 14CO2 was produced. The flushing was continued for approximately 15 min, and the 14CO2 was trapped and measured. The trapping efficiency ranged from 96 to 99%.
Benzo[a]pyrene radiocarbon mass balance.
Three-hundred-milliliter Erlenmeyer flasks designed to trap headspace gas as described above were charged with benzo[a]pyrene, [7-14C]benzo[a]pyrene, and diesel fuel in 30 ml of SBM and monitored for 14CO2 evolution. The final diesel fuel and benzo[a]pyrene concentrations were 0.1% (wt/vol) and 10 mg liter−1, respectively. Negative controls contained benzo[a]pyrene, [7-14C]benzo[a]pyrene, and SBM plus inoculum. Duplicate samples were sacrificed and used for mass balance analyses at four time points over a 2-week incubation period. At the sampling times, the flask contents were mixed with a stainless steel spatula and six 3-ml aliquots of the culture suspension were passed through six 0.22-μm-pore-size nitrocellulose filters (Millipore) under negative pressure. The aqueous filtrate was collected, aliquots were added to Scintiverse BD scintillation cocktail, and radioactivity was measured. The filters were gently rinsed with SBM and air dried, and the sample was split; the radioactivity on three filters was measured directly by using Scintiverse BD, and three filters were extracted in the dark for approximately 24 h in 30 ml of methylene chloride with rotary shaking at 150 rpm. The methylene chloride extracts were concentrated to 100 μl under N2 gas at 30°C to amplify the 14C signal, and then radioactivity was measured by using Scintiverse BD. Depending on the 14C activity present in the sample extract, a known volume was applied to a 0.25-mm-thick silica gel TLC plate (Macherey-Nagel, Düren, Germany), which was developed for approximately 30 min in the dark in 1:1 hexane-benzene. Bands which represented benzo[a]pyrene were identified in the sample extracts by comparison to a benzo[a]pyrene standard under UV light (254 nm). The silica gel bands were scraped from the TLC plate with a stainless steel spatula and added to Scintiverse BD, and radioactivity was measured, thus quantifying the mass of [7-14C]benzo[a]pyrene present in the filter extract. Biomass-associated 14C consisted of biotransformed 14C which was incorporated into biomass or which was associated with biomass. To complete the mass balance analysis for each pair of flasks, the residue in each flask was extracted with chloroform and concentrated under N2 gas at 40°C and the radioactivity in the extract was measured. In some cases, extracts of flask residue were also subjected to TLC analysis as described above to confirm that the flask residue radioactivity was indeed intact benzo[a]pyrene radioactivity. In all cases, the flask residues were confirmed to be mostly whole benzo[a]pyrene (average, >95%) after flask extraction and TLC analyses.
DGGE analysis.
One-liter flasks which contained 100 ml of SBM and 35 mg of benzo[a]pyrene per liter with and without 0.007% (wt/vol) diesel fuel were inoculated with 0.1 ml of stock culture (0.1% [vol/vol] inoculum) and incubated on a rotary shaker at 150 rpm and 28°C for 19 days. The stock culture was maintained as described above; this “stable” consortium was maintained in this fashion for more than 2 years and mineralized benzo[a]pyrene consistently after transfers. DNA was amplified directly from the cultures on eight sampling days. PCR primers P2 and P3 (containing 40 bp of GC clamp) (40) were used to amplify the variable V3 region of bacterial 16S ribosomal DNA (corresponding to positions 341 to 534 in the Escherichia coli sequence) as described previously (50). Taq DNA polymerase (Amplitaq Gold; Perkin-Elmer) was used; our technique involved 10 min of activation of the polymerase at 94°C before PCR and contributed to cell lysis. Amplification of PCR products of the proper size to confirm the absence of by-products was performed by electrophoresis through a 2% (wt/vol) agarose gel (LO3 agarose; Takara Shuzo) in TBE buffer, followed by staining with ethidium bromide. DGGE was performed as recommended by the manufacturer with a Protean II system (Bio-Rad) at 200 V and 58°C. Eight microliters of PCR-amplified mixture was loaded on a 10% (wt/vol) polyacrylamide gel in 1× TAE (20 mM Tris-acetate [pH 7.4], 10 mM acetate, 0.5 mM disodium EDTA). The denaturing gradient contained 40 to 55% denaturant (100% denaturant corresponded to 6 M urea and 40% [vol/vol] formamide). After electrophoresis, the gel was stained with SYBR Gold I (FMC Bioproducts, Princeton, N.J.) for 30 min as recommended by the manufacturer and was visualized by using a Gel Doc 2000 UV table and digital camera (Bio-Rad). Sequencing of DGGE bands was performed as described previously (50), and a search of the GenBank database was conducted by using BLAST (1).
Cloning of PCR-amplified products.
Amplification products from three bands (bands 1, 8, and 10 [see Fig. 4]) which failed to generate complete sequences by direct sequencing of DNA from excised DGGE bands were cloned by using a pGEM-T cloning kit (Promega Corp., Madison, Wis.). Ligation with T4 DNA ligase and transformation of JM109 high-efficiency competent cells were performed according to the manufacturer's instructions. Recombinants were picked from indicator plates and grown overnight in liquid medium (Luria-Bertani broth), and a small amount of cells was picked from the plate and used directly as a DNA template for PCR performed with primers 341GC-clamp and 534R. The PCR conditions were the same as those described above for DGGE analysis, and the PCR products were analyzed by DGGE as described above. The clones which resulted in a DGGE band at the same position as a band in the sample profiles were sequenced as described above.
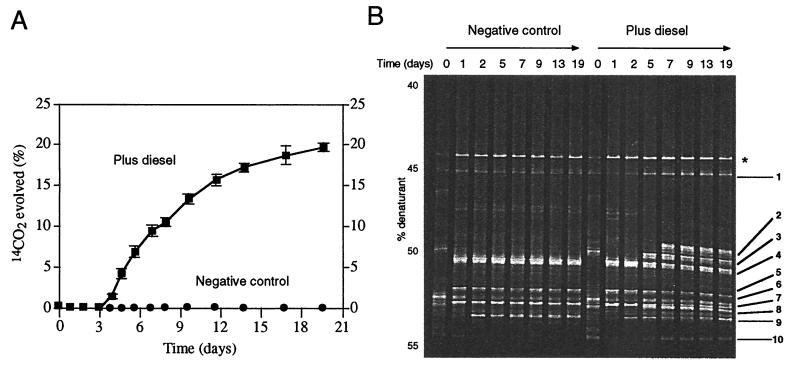
FIG. 4.
Nineteen-day time course analysis of the consortium community structure compared to [7-14C]benzo[a]pyrene mineralization. Treated samples contained 0.007% (wt/vol) diesel fuel and 35 mg of benzo[a]pyrene per liter. Negative controls contained 35 mg of benzo[a]pyrene per liter and no diesel fuel. (A) Cumulative amount of 14CO2 evolved from [7-14C]benzo[a]pyrene after 19 days. Each point represents the average value obtained with triplicate flasks, and the error bars indicate the standard deviations (omitted when they were smaller than the symbol). (B) DGGE analysis of bacterial communities in the negative control and a treated sample. Ten of the dominant sequence types detected by day 19 were successfully sequenced following band excision, reamplification, and purification or by cloning and screening, and their positions are indicated on the right (see text for details).
Nucleotide sequence accession numbers.
The sequences obtained in this study have been deposited in the GenBank database under accession no. AF247773 to AF247779.
RESULTS AND DISCUSSION
Mineralization of benzo[a]pyrene radiocarbon.
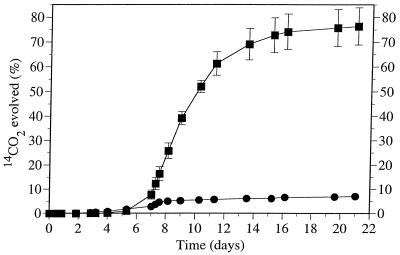
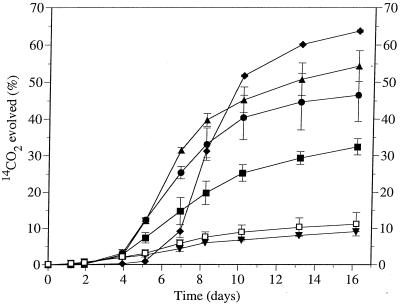
Figure 1 shows that approximately 75% mineralization of 10 mg of benzo[a]pyrene per liter by the consortium occurred in 3 weeks when it was grown on 0.2% (wt/vol) diesel fuel. Without diesel fuel, the level of benzo[a]pyrene mineralization by the consortium was less than 8%. To gain a better understanding of the metabolic capabilities of the consortium, the rates of mineralization of 10 mg of benzo[a]pyrene per liter in the presence of a range of diesel fuel concentrations were measured, as shown in Fig. 2. As the diesel fuel concentration increased, the extent of benzo[a]pyrene mineralization also increased. However, at the highest diesel fuel concentration (0.2%), the lag period prior to the onset of benzo[a]pyrene mineralization was also the longest. Increasing lag periods prior to the onset of benzo[a]pyrene mineralization as the petroleum hydrocarbon concentration increased were also noted in soil (27). A negative control which contained benzo[a]pyrene without diesel fuel addition produced only approximately 10% 14CO2, as did a second control which contained 0.1% (wt/vol) Inipol EAP-22 as the carbon and nutrient source in lieu of diesel fuel. Inipol EAP-22 is an oleophilic fertilizer designed to stimulate the biodegradation of polluting oil. It consists of a microemulsion of urea in an oleic acid-lauryl phosphate matrix. Although microbial growth was observed in the culture containing Inipol EAP-22, this compound did not stimulate benzo[a]pyrene mineralization any differently than the negative control. Previous experiments had shown that the consortium was unable to mineralize benzo[a]pyrene in the presence of diesel fuel when the consortium was pregrown in 0.8% (wt/vol) nutrient broth instead of SBM containing benzo[a]pyrene and diesel fuel.
FIG. 1.
Cumulative amount of 14CO2 evolved from [7-14C]benzo[a]pyrene (10 mg/liter) by a bacterial consortium recovered from soil. Benzo[a]pyrene and SBM were incubated with (■) and without (●) diesel fuel (0.2%, wt/vol). Each point represents the average value obtained with triplicate flasks, and the error bars indicate the standard deviations (omitted when they were smaller than the symbol).
FIG. 2.
Cumulative amount of 14CO2 evolved from [7-14C]benzo[a]pyrene (10 mg/liter) by a bacterial consortium grown on a range of diesel fuel concentrations or on Inipol EAP-22. The concentrations of diesel fuel used were 0.007% (wt/vol) (■), 0.05% (wt/vol) (●), 0.1% (wt/vol) (▴), and 0.2% (wt/vol) (⧫). ▾, no diesel fuel; □, Inipol EAP-22. Each point represents the average value obtained with triplicate flasks, and the error bars indicate the standard deviations (omitted when they were smaller than the symbol).
Table 1 shows the benzo[a]pyrene mineralization rates when the concentration of benzo[a]pyrene in the culture medium was varied from 10 to 100 mg liter−1 in the presence of 0.1% (wt/vol) diesel fuel. Cultures treated with 10, 30, 50, and 100 mg of benzo[a]pyrene per liter cumulatively mineralized 5.4, 13.1, 17.2, and 17.0 mg of benzo[a]pyrene per liter, respectively. Cultures treated with 50 mg liter−1 and cultures treated with 100 mg liter−1 mineralized similar masses of benzo[a]pyrene, which suggests that a minimal ratio of benzo[a]pyrene to primary substrate (diesel fuel) was necessary for benzo[a]pyrene mineralization.
TABLE 1.
Amounts of benzo[a]pyrene mineralized by the consortium at the 7 position when it was incubated with different benzo[a]pyrene concentrations in the presence of 0.1% (wt/vol) diesel fuel
| Amt of Benzo[a]pyrene added
|
Total amt of benzo[a]pyrene mineralized (μg) aftera:
|
Concn of benzo[a]pyrene mineralized after 16 days (mg/liter) | ||||
|---|---|---|---|---|---|---|
| μg | mg/liter | 4 Days | 8 Days | 13 Days | 16 Days | |
| 250 | 10 | NDb | 99 ± 4 | 127 ± 11 | 136 ± 10 | 5.4 |
| 750 | 30 | ND | 172 ± 18 | 299 ± 10 | 329 ± 20 | 13.2 |
| 1,250 | 50 | ND | 191 ± 15 | 355 ± 29 | 432 ± 37 | 17.2 |
| 2,500 | 100 | ND | 229 ± <0.5 | 390 ± <0.5 | 426 ± <0.5 | 17.0 |
The values are the average masses ± standard deviations of benzo[a]pyrene mineralized in triplicate samples, as calculated from 14CO2 evolution data. The concentrations of benzo[a]pyrene ranged from 10 to 100 mg/liter and were much greater than the aqueous solubility of this compound (approximately 4 μg/liter).
ND, not detected.
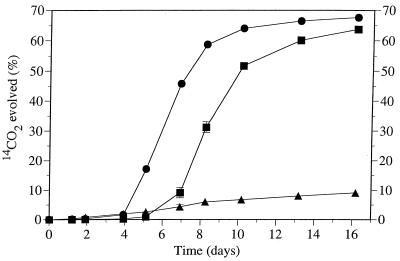
Figure 3 shows the rates of mineralization of 10 mg of benzo[a]pyrene per liter in the presence of 0.2% (wt/vol) diesel fuel when the starting inoculum size was increased from 1 to 10 mg of cell protein per liter. An increase in inoculum size shortened the lag period prior to the onset of mineralization, but, as expected, there was little difference in the maximal benzo[a]pyrene mineralization rates or in the total amounts of benzo[a]pyrene mineralized at the end of the 16-day incubation period. Similar lag periods are also evident in Fig. 1 and 2 and in Table 1 and were observed in previous work conducted with soil (25, 27). Some parts of the lag periods may reflect the periods required for microbial proliferation on the primary substrate (diesel fuel), but more importantly, the complex hydrocarbon mixtures used contained competitive inhibitors of benzo[a]pyrene mineralization in addition to hydrocarbon substrates essential for the cometabolism of benzo[a]pyrene. The volatile portion of diesel fuel has been demonstrated to contain such competitive inhibitors (27), and the primary effect of the 10× inoculum may have been faster depletion of these inhibitors, thus shortening the lag period.
FIG. 3.
Cumulative amount of 14CO2 evolved from [7-14C]benzo[a]pyrene by a bacterial consortium when the inoculum contained 1 mg (■) or 10 mg (●) of cell protein per liter. Benzo[a]pyrene was mineralized in the presence of 0.2% (wt/vol) diesel fuel, which was omitted in a control (▴) that received a 1-mg/liter inoculum. Each point represents the average value obtained with triplicate flasks, and the error bars indicate the standard deviations (omitted when they were smaller than the symbol).
A 2-week radiocarbon mass balance study (Table 2) revealed that benzo[a]pyrene was first mobilized from the glass surface of the flask into the culture suspension, where it was presumably made more bioavailable to the microbial community. Detection of benzo[a]pyrene in the culture suspension was unexpected because the aqueous solubility of benzo[a]pyrene is very low (approximately 0.004 mg liter−1). This event most likely occurred as a result of the action of surface-active compounds produced by consortium members growing on diesel fuel. The benzo[a]pyrene level detected in the culture suspension increased to 26.4% by day 4, and this correlated with an independently measured proportional decrease in the amount of benzo[a]pyrene attached to the flask surface. Following the transfer of benzo[a]pyrene into the culture suspension, biodegradation and subsequent detection of biotransformation products occurred.
TABLE 2.
Mass balance of benzo[a]pyrene radiocarbon during a 14-day incubation period
| Day | Mass balance (%)
|
Total recovery (%) | ||||
|---|---|---|---|---|---|---|
| Biomass-associated 14Ca | 14C-aqueous metabolites | Suspended [7-14C]benzo[a]pyreneb | Attached [7-14C]benzo[a]pyrenec | 14CO2 | ||
| Diesel fuel added | ||||||
| 0 | NDd | ND | 0.4 ± 0.1 | 98.5 ± 4.1 | ND | 98.9 ± 4.0 |
| 4 | 1.6 ± 1.5 | 0.2 ± <0.1 | 26.4 ± 1.9 | 70.0 ± 1.5 | 0.3 ± 0.1 | 98.5 ± 1.9 |
| 7 | 16.0 ± 3.8 | 3.0 ± 0.6 | 25.4 ± 13.3 | 29.5 ± 1.0 | 25.4 ± 2.5 | 99.3 ± 7.3 |
| 14 | 8.8 ± 2.0 | 2.6 ± 0.3 | 7.3 ± 1.0 | 17.8 ± 2.8 | 51.0 ± 3.3 | 87.4 ± 1.9 |
| No diesel fuel added | ||||||
| 14e | 2.1 ± <0.1 | 0.5 ± <0.1 | 14.6 ± 8.8 | 82.2 ± 12.3 | 2.8 ± 0.5 | 102.7 ± 3.9 |
14C in biomass or in transformation products which were associated with biomass.
14C trapped on filters, extracted, and determined to be 14C in [7-14C]benzo[a]pyrene by TLC.
More than 95% of the attached 14C was determined to be 14C in [7-14C]benzo[a]pyrene by TLC.
ND, not detected (below the detection limit).
The negative control consisted of benzo[a]pyrene and inoculum with no diesel fuel added.
When Fig. 1 and 3 are compared to Table 1, it is evident that 65 to 75% mineralization of benzo[a]pyrene occurred only when 10 mg liter−1 was added. Such levels of mineralization may be considered to signify complete biodegradation of the compound in many cases, with the remaining carbon either incorporated into biomass or present in the form of biodegradation intermediates. This degree of mineralization had to be supported by at least 0.1 to 0.2% (wt/vol) diesel fuel. If the benzo[a]pyrene concentration was increased (Table 1) or the amount of diesel fuel added was reduced (Fig. 2), the percentage of total added benzo[a]pyrene which was mineralized became limited by the primary substrate concentration. As shown in Table 2, approximately 18% of the added radiocarbon was in intact benzo[a]pyrene after 51% mineralization to 14CO2 had occurred by day 14. As diesel fuel was depleted, the rates of benzo[a]pyrene radiocarbon biotransformation and mineralization decreased. Depending on the amount of diesel fuel present, it is likely that the maximum concentration of benzo[a]pyrene in the culture suspension was reached between days 4 and 7 because approximately 40% of the benzo[a]pyrene radiocarbon was brought into suspension over this 3-day period and the amount of biomass-associated 14C peaked on day 7. These observations also coincide with the period when benzo[a]pyrene mineralization was most rapid (Fig. 2, 0.1% diesel fuel application). The amount of aqueous 14C, presumed to be in polar metabolites of benzo[a]pyrene biodegradation, never exceeded 3% of the total amount of radiocarbon applied. The only carbon added to the negative control was the small amount transferred during inoculation; therefore, only approximately 15% of the benzo[a]pyrene was detected in suspension and was accompanied by less than 3% biomass-associated 14C and 3% 14CO2. Transformation of benzo[a]pyrene did not occur in abiotic controls.
Prior to this communication, the most rapid rate of benzo[a]pyrene mineralization reported at a comparable concentration (10 mg liter−1) was 28% in 48 h (53) and was accomplished by a resting cell suspension of Sphingomonas paucimobilis EPA505 having a cell density of 1 mg (wet weight) per ml. Using commonly accepted conversion factors (cell protein accounts for 50% of the cell dry weight and cell dry weight is 20% of the cell wet weight), the biomass of S. paucimobilis EPA505 was 100-fold greater than that of the inoculum (1 mg of cell protein liter−1) that was routinely used in our mineralization experiments whose results are shown in Fig. 1 through 3 and Table 1.
Community analysis.
The population dynamics of the diesel fuel-utilizing consortium were investigated by DGGE (Fig. 4). A 2:1 ratio of diesel fuel to benzo[a]pyrene was used in the treated samples so that the concentration of benzo[a]pyrene would exceed the concentrations of all the individual compounds in diesel fuel. The intent was to expose the bacterial populations to a high concentration of benzo[a]pyrene relative to the concentrations of diesel fuel compounds and yet still obtain detectable levels of 14CO2 production over the 19-day incubation period. Following an initial lag period of 3 days, benzo[a]pyrene mineralization was detected, as shown in Fig. 4A. Only 20% of the benzo[a]pyrene radiocarbon was detected as 14CO2 in the treated sample after 19 days due to the low concentration of diesel fuel and high concentration of benzo[a]pyrene used. This observation was in accordance with what we expected from our previous results regarding the effects of diesel fuel and benzo[a]pyrene concentrations on the extent of benzo[a]pyrene mineralization (Fig. 2 and Table 1). The negative control did not produce 14CO2 at detectable levels during the 19-day experiment.
The DGGE patterns for the negative control and the treated sample were similar for the first 2 days (Fig. 4B). After 2 days, additional sequence types were detected in the DGGE pattern of the treated sample, while the pattern for the negative control remained virtually unchanged during the 19-day experiment. By day 19 approximately 13 dominant bands were observed in the treated sample. Ten of the 13 bands, each representing a different sequence type, were successfully sequenced. One band (indicated by an asterisk in Fig. 4B) was most likely a result of PCR by-products or heteroduplex formation (18, 39) because repeated excision, amplification, and electrophoresis attempts resulted in multiple band patterns. We were unable to confirm the sequence identities of the remaining two dominant bands. As shown in Table 3, sequence data indicated that 9 of the 10 consortium members were related to gram-negative organisms which belonged to class Proteobacteria. Sequence types 7, 9, and 10 were 100% identical to bacteria belonging to the genera Sphingomonas, Mycobacterium, and Alcaligenes, respectively, and these three genera are known to include strains which biodegrade HMW PAHs. Specifically, the versatile organisms S. paucimobilis EPA505 and Sphingomonas yanoikuyae B1 and B8/36 (formerly Beijerinckia sp. [19, 30]) have been shown to oxidize and/or mineralize a variety of HMW PAHs (9, 20, 22, 34, 36–38, 53). Mycobacterium strains, including strains PYR-1 (11), RJGII-135 (44), BB1 (6), KR2 (42), CH1 (13), and VF1 (28), are also known HMW PAH degraders, and at least one member of the genus Alcaligenes, Alcaligenes denitrificans WW1, was shown to biodegrade a four-ring PAH (51, 52). Of the remaining sequence types, six were most closely related to Proteobacteria species and included a member of the genus Burkholderia, another genus whose members are known to degrade PAHs (38), including HMW PAHs (24).
TABLE 3.
Sequence similarities of the excised DGGE bands that appear in Fig. 4
| Band | % Similarity | Phylogenetically related organism | Accession no. | Taxonomic position |
|---|---|---|---|---|
| 1 | 94 | Sphingobacterium sp. strain OM-E81 | AB020206 | Cytophagales |
| 2 | 91 | Pseudomonas halophila | AB021383 | γ-Proteobacteriaa |
| 3 | 96 | Aquabacterium sp. | AF089857 | β-Proteobacteria |
| 4 | 98 | Burkholderia phenazinium | U96936 | β-Proteobacteria |
| 5 | 98 | Type II methanotroph strain AML-A6 | AF177299 | α-Proteobacteria |
| 6 | 99 | Ralstonia eutropha | M32021 | β-Proteobacteria |
| 7 | 100 | Sphingomonas paucimobilis | X94100 | α-Proteobacteria |
| 8 | 97 | Ralstonia eutropha | M32021 | β-Proteobacteria |
| 9 | 100 | Mycobacterium ratisbonense | AF055331 | Actinomycetes |
| 10 | 100 | Alcaligenes sp. isolate 151 | AJ002802 | β-Proteobacteria |
γ-Proteobacteria, γ subclass of the class Proteobacteria.
DGGE and mass balance interpretation.
The mass balance results indicated that the first step in the biodegradation of benzo[a]pyrene involved a rapid transfer of the molecule into the culture suspension. This transfer was postulated to occur predominantly due to the action of surface-active compounds produced by consortium members. After benzo[a]pyrene was transferred into suspension, cometabolic biodegradation of benzo[a]pyrene occurred, ultimately resulting in mineralization. Benzo[a]pyrene mineralization which began on day 3 correlated with the emergence or reemergence of four sequence types in the DGGE analysis on day 5 (Fig. 4B). Notably, sequence types 3 and 10 detected at time zero became undetectable 24 h after the addition of diesel fuel and reemerged after 5 days. This observation indicated that preferential growth of populations other than sequence types 3 and 10 occurred after the addition of diesel fuel. Although the order of biodegradation of compounds in hydrocarbon mixtures depends on a variety of factors, including the microbial populations present (3, 5, 41), it has been shown that low-molecular-weight compounds may be removed first and that low-molecular-weight PAHs may inhibit the degradation of HMW PAHs (3, 8, 32, 45, 47). As stated previously, benzo[a]pyrene mineralization was inhibited by the volatile components of diesel fuel in the same soil from which the consortium was derived. Sequence types which reemerged on day 5 possibly followed biodegradation of this fraction of diesel fuel. Previous reports showed that cometabolic benzo[a]pyrene mineralization occurred when the cosubstrate was another HMW PAH. It was therefore postulated in our case that low-molecular-weight hydrocarbons were metabolized during the lag period, that HMW PAHs were biodegraded by emergent populations on day 5, that this biodegradation was linked to the biodegradation of benzo[a]pyrene by a cometabolic mechanism, and therefore that the whole process of benzo[a]pyrene mobilization and biodegradation required the combined efforts of different populations.
Conclusions.
Extensive and rapid mineralization of substantial concentrations of [7-14C]benzo[a]pyrene by a microbial consortium growing on an NAPL was demonstrated. The nature of the mineralization was cometabolic, and mineralization required proportional amounts of a primary substrate (diesel fuel) that served as the source of carbon and energy. A microbial consortium which biodegrades benzo[a]pyrene while growing on an NAPL hydrocarbon mixture may have applications in the development of strategies for bioremediation of PAH-contaminated sites.
Due to the complex nature of the system which we studied, DGGE proved to be a valuable first step for obtaining information regarding the dynamics of the consortium and for tentatively identifying the relevant consortium members. The results confirmed the presence of sequence types which were closely related to known HMW-PAH-degrading genera. Interestingly, the consortium was recovered from a site that had no history of chemical contamination. In a comprehensive study of the bacterial diversity of PAH-contaminated sites, Mueller et al. (38) showed that PAH degradation capabilities of bacteria were associated with a few phylogenetically distinct genera and were independent of geographic location. They also discussed the fact that although uncontaminated aquatic sites may naturally harbor bacteria which are capable of PAH degradation (S. Komukai, personal communication), it was unclear if PAH-degrading organisms could be recovered from uncontaminated soil sites. In our case, a bacterial community with the capability to mineralize benzo[a]pyrene was recovered from such a soil site. Although this may be one of the only examples of such an occurrence, it indicates that organisms which can degrade HMW PAHs may be recovered from such environments.
By utilizing the information obtained from DGGE, efforts are being made to determine which individual populations are most important in the benzo[a]pyrene biodegradation process and which components of diesel fuel specifically induce the cometabolic biotransformation of benzo[a]pyrene.
ACKNOWLEDGMENTS
We gratefully acknowledge Fusako Numazaki and Maki Teramoto for technical assistance.
This work was supported in part by Hazardous Substance Management Research Center project BICM-48, by National Science Foundation project 9726687, and as a part of The Industrial Science and Technology Project, Technological Development of Biological Resources in Bioconsortia, supported by the New Energy and Industrial Technology Development Organization (NEDO).
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Atlas R M. Handbook of microbiological media. Boca Raton, Fla: CRC Press, Inc.; 1993. [Google Scholar]
- 3.Atlas R M, Bartha R. Hydrocarbon biodegradation and oil spill bioremediation. In: Marshall K C, editor. Advances in microbial ecology. Vol. 12. New York, N.Y: Plenum Press; 1992. pp. 287–338. [Google Scholar]
- 4.Barnsley E A. The bacterial degradation of fluoranthene and benzo[a]pyrene. Can J Microbiol. 1975;21:1004–1008. doi: 10.1139/m75-148. [DOI] [PubMed] [Google Scholar]
- 5.Bauer J E, Capone D G. Effects of co-occurring aromatic hydrocarbons on degradation of individual polycyclic aromatic hydrocarbons in marine sediment slurries. Appl Environ Microbiol. 1988;54:1649–1655. doi: 10.1128/aem.54.7.1649-1655.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boldrin B, Tiehm A, Fritzsche C. Degradation of phenanthrene, fluorene, fluoranthene, and pyrene by a Mycobacterium sp. Appl Environ Microbiol. 1993;59:1927–1930. doi: 10.1128/aem.59.6.1927-1930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boonchan S, Britz M L, Stanley G A. Degradation and mineralization of high-molecular-weight polycyclic aromatic hydrocarbons by defined fungal-bacterial cocultures. Appl Environ Microbiol. 2000;66:1007–1019. doi: 10.1128/aem.66.3.1007-1019.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouchez M, Blanchet D, Vandecasteele J-P. Degradation of polycyclic aromatic hydrocarbons by pure strains and by defined strain associations: inhibition phenomena and cometabolism. Appl Microbiol Biotechnol. 1995;43:156–164. doi: 10.1007/BF00170638. [DOI] [PubMed] [Google Scholar]
- 9.Boyd D R, Sharma N D, Hempenstall F, Kennedy M A, Malone J F, Allen C C R, Resnick S M, Gibson D T. bis-cis-Dihydrodiols: a new class of metabolites from biphenyl dioxygenase-catalyzed sequential asymmetric cis-dihydroxylation of polycyclic arenes and heteroarenes. J Org Chem. 1999;64:4005–4011. [Google Scholar]
- 10.Cariello N F, Swenberg J A, De Bellis A, Skopek T R. Analysis of mutations using PCR and denaturing gradient gel electrophoresis. Environ Mol Mutagen. 1991;18:249–254. doi: 10.1002/em.2850180408. [DOI] [PubMed] [Google Scholar]
- 11.Cerniglia C E. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation. 1992;3:351–368. [Google Scholar]
- 12.Cerniglia C E. Biodegradation of polycyclic aromatic hydrocarbons. Curr Opin Biotechnol. 1993;4:331–338. [Google Scholar]
- 13.Churchill S A, Harper J P, Churchill P F. Isolation and characterization of a Mycobacterium species capable of degrading three- and four-ring aromatic and aliphatic hydrocarbons. Appl Environ Microbiol. 1999;65:549–552. doi: 10.1128/aem.65.2.549-552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deschênes L, Lafrance P, Villeneuve J-P, Samson R. Adding sodium dodecyl sulfate and Pseudomonas aeruginosa UG2 biosurfactants inhibits polycyclic aromatic hydrocarbon biodegradation in a weathered creosote-contaminated soil. Appl Microbiol Biotechnol. 1996;46:638–646. doi: 10.1007/s002530050874. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly K C, Anderson C S, Thomas J C, Brown K W, Manek D J, Safe S H. Bacterial mutagenicity of soil extracts from a bioremediation facility treating wood-preserving waste. J Hazard Mater. 1992;30:71–81. [Google Scholar]
- 16.Efroymson R A, Alexander M. Biodegradation by an Arthrobacter species of hydrocarbons partitioned into an organic solvent. Appl Environ Microbiol. 1991;57:1441–1447. doi: 10.1128/aem.57.5.1441-1447.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Efroymson R A, Alexander M. Biodegradation in soil of hydrophobic pollutants in nonaqueous-phase liquids (NAPLs) Environ Toxicol Chem. 1994;13:405–411. [Google Scholar]
- 18.Ferris M J, Ward D M. Seasonal distributions of dominant 16S rRNA-defined populations in a hot spring microbial mat examined by denaturing gradient gel electrophoresis. Appl Environ Microbiol. 1997;63:1375–1381. doi: 10.1128/aem.63.4.1375-1381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson D T. Beijerinckia sp. strain B1: a strain by any other name … . J Ind Microbiol Biotechnol. 1999;23:284–293. doi: 10.1038/sj.jim.2900715. [DOI] [PubMed] [Google Scholar]
- 20.Gibson D T, Venkatanayarana M, Jerina D M, Yagi H, Yeh H. Oxidation of the carcinogens benzo[a]pyrene and benzo[a]anthracene to dihydrodiols by a bacterium. Science. 1975;189:295–297. doi: 10.1126/science.1145203. [DOI] [PubMed] [Google Scholar]
- 21.Heitkamp M A, Cerniglia C E. Mineralization of polycyclic aromatic hydrocarbons by a bacterium isolated from sediment below an oil field. Appl Environ Microbiol. 1988;54:1612–1614. doi: 10.1128/aem.54.6.1612-1614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jerina D M, van Bladeren P J, Yagi H, Gibson D T, Mahadevan V, Neese A S, Koreeda M, Sharma N D, Boyd D R. Synthesis and absolute configuration of the bacterial cis-1,2-, cis-8,9-, and cis-10,11-dihydrodiol metabolites of benz[a]anthracene formed by a strain of Beijerinckia. J Org Chem. 1984;49:3621–3628. [Google Scholar]
- 23.Jimenez I Y, Bartha R. Solvent-augmented mineralization of pyrene by a Mycobacterium sp. Appl Environ Microbiol. 1996;62:2311–2316. doi: 10.1128/aem.62.7.2311-2316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juhasz A L, Britz M L, Stanley G A. Degradation of fluoranthene, pyrene, benz[a]anthracene and dibenz[a,h]anthracene by Burkholderia cepacia. J Appl Microbiol. 1997;83:189–198. [Google Scholar]
- 25.Kanaly R A, Bartha R, Fogel S, Findlay M. Biodegradation of [14C]benzo[a]pyrene added in crude oil to uncontaminated soil. Appl Environ Microbiol. 1997;63:4511–4515. doi: 10.1128/aem.63.11.4511-4515.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanaly R A, Harayama S. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol. 2000;182:2059–2067. doi: 10.1128/jb.182.8.2059-2067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanaly R A, Bartha R. Cometabolic mineralization of benzo[a]pyrene caused by hydrocarbon additions to soil. Environ Toxicol Chem. 1999;18:2186–2190. doi: 10.1002/etc.5620181010. [DOI] [PubMed] [Google Scholar]
- 28.Kästner M, Breuer-Jammali M, Mahro B. Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons (PAH) Appl Microbiol Biotechnol. 1994;41:267–273. [Google Scholar]
- 29.Keith L H, Telliard W A. Priority pollutants I—a perspective view. Environ Sci Technol. 1979;13:416–423. [Google Scholar]
- 30.Khan A A, Wang R-F, Cao W-W, Franklin W, Cerniglia C E. Reclassification of a polycyclic aromatic hydrocarbon-metabolizing bacterium, Beijerinckia sp. strain B1, as Sphingomonas yanoikuyae by fatty acid analysis, protein pattern analysis, DNA-DNA hybridization, and 16S ribosomal DNA sequencing. Int J Syst Bacteriol. 1996;46:466–469. doi: 10.1099/00207713-46-2-466. [DOI] [PubMed] [Google Scholar]
- 31.Labare M P, Alexander M. Enhanced mineralization of organic compounds in nonaqueous-phase liquids. Environ Toxicol Chem. 1995;14:257–265. [Google Scholar]
- 32.Leahy J G, Colwell R R. Microbial degradation of hydrocarbons in the environment. Microbiol Rev. 1990;54:305–315. doi: 10.1128/mr.54.3.305-315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macnaughton S J, Stephen J R, Venosa A D, Davis G A, Chang Y-J, White D C. Microbial population changes during bioremediation of an experimental oil spill. Appl Environ Microbiol. 1999;65:3566–3574. doi: 10.1128/aem.65.8.3566-3574.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahaffey W R, Gibson D T, Cerniglia C E. Bacterial oxidation of chemical carcinogens: formation of polycyclic aromatic acids from benz[a]anthracene. Appl Environ Microbiol. 1988;54:2415–2423. doi: 10.1128/aem.54.10.2415-2423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marinucci A C, Bartha R. Apparatus for monitoring the mineralization of volatile 14C-labeled compounds. Appl Environ Microbiol. 1979;38:1020–1022. doi: 10.1128/aem.38.5.1020-1022.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller J G, Chapman P J, Blattmann B O, Pritchard P H. Isolation and characterization of a fluoranthene-utilizing strain of Pseudomonas paucimobilis. Appl Environ Microbiol. 1990;56:1079–1086. doi: 10.1128/aem.56.4.1079-1086.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller J G, Chapman P J, Pritchard P H. Action of a fluoranthene-utilizing bacterial community on polycyclic aromatic hydrocarbon components of creosote. Appl Environ Microbiol. 1989;55:3085–3090. doi: 10.1128/aem.55.12.3085-3090.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller J G, Devereux R, Santavy D L, Lantz S E, Willis S G, Pritchard P H. Phylogenetic and physiological comparisons of PAH-degrading bacteria from geographically diverse soils. Antonie Leeuwenhoek. 1997;71:329–343. doi: 10.1023/a:1000277008064. [DOI] [PubMed] [Google Scholar]
- 39.Murray A E, Hollibaugh J T, Orrego C. Phylogenetic compositions of bacterioplankton from two California estuaries compared by denaturing gradient gel electrophoresis of 16S rDNA fragments. Appl Environ Microbiol. 1996;62:2676–2680. doi: 10.1128/aem.62.7.2676-2680.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muyzer G, de Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson J J, Mills G L, Herbert B E, Morris P J. Biodegradation rates of separated diesel components. Environ Toxicol Chem. 1999;18:2448–2453. [Google Scholar]
- 42.Rehmann K, Noll H P, Steinberg C E, Kettrup A A. Pyrene degradation by Mycobacterium sp. strain KR2. Chemosphere. 1998;36:2977–2992. doi: 10.1016/s0045-6535(97)10240-5. [DOI] [PubMed] [Google Scholar]
- 43.Renner R. EPA to strengthen persistent, bioaccumulative, and toxic pollutant controls—mercury first to be targeted. Environ Sci Technol. 1999;33:62. doi: 10.1021/es992653p. [DOI] [PubMed] [Google Scholar]
- 44.Schneider J, Grosser R, Jayasimhulu K, Xue W, Warshawsky D. Degradation of pyrene, benz[a]anthracene, and benzo[a]pyrene by Mycobacterium sp. strain RJGII-135, isolated from a former coal gasification site. Appl Environ Microbiol. 1996;62:13–19. doi: 10.1128/aem.62.1.13-19.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shuttleworth K L, Cerniglia C E. Bacterial degradation of low concentrations of phenanthrene and inhibition by naphthalene. Microb Ecol. 1996;31:305–317. [PubMed] [Google Scholar]
- 46.Smith J R, Nakles D V, Sherman D F, Neuhauser E F, Loehr R C. The Third International Conference on New Frontiers for Hazardous Waste Management. U.S. Washington, D.C.: Environmental Protection Agency; 1989. Environmental fate mechanisms influencing biological degradation of coal-tar derived polynuclear aromatic hydrocarbons in soil systems; pp. 397–405. [Google Scholar]
- 47.Stringfellow W T, Aitken M D. Competitive metabolism of naphthalene, methylnaphthalenes, and fluorene by phenanthrene-degrading pseudomonads. Appl Environ Microbiol. 1995;61:357–362. doi: 10.1128/aem.61.1.357-362.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutherland J B, Rafii F, Khan A A, Cerniglia C E. Mechanisms of polycylic aromatic hydrocarbon degradation. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss; 1995. pp. 269–306. [Google Scholar]
- 49.Trzesicka-Mlynarz D, Ward O P. Degradation of polycyclic aromatic hydrocarbons (PAHs) by a mixed culture and its component pure cultures, obtained from PAH-contaminated soil. Can J Microbiol. 1995;41:470–476. doi: 10.1139/m95-063. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe K, Teramoto M, Futamata H, Harayama S. Molecular detection, isolation, and physiological characterization of functionally dominant phenol-degrading bacteria in activated sludge. Appl Environ Microbiol. 1998;64:4396–4402. doi: 10.1128/aem.64.11.4396-4402.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weissenfels W D, Beyer M, Klein J. Degradation of phenanthrene, fluorene and fluoranthene by pure bacterial cultures. Appl Microbiol Biotechnol. 1990;32:479–484. doi: 10.1007/BF00903787. [DOI] [PubMed] [Google Scholar]
- 52.Weissenfels W D, Beyer M, Klein J, Rehm H J. Microbial metabolism of fluoranthene: isolation and identification of ring fission products. Appl Microbiol Biotechnol. 1991;34:528–535. [Google Scholar]
- 53.Ye D, Siddiqi M A, Maccubbin A E, Kumar S, Sikka H C. Degradation of polynuclear aromatic hydrocarbons by Sphingomonas paucimobilis. Environ Sci Technol. 1996;30:137–142. [Google Scholar]