Abstract
Uterine fibroids are the most common tumors of the female reproductive tract, affecting up to 80% of women. Despite their heavy burden and high prevalence, available medical treatment options are limited and are offered to patients assuming equal responsiveness. These benign tumors are complex, originating from potentially diverse pathobiologic processes, yet they are all managed in a rather standardized symptom-oriented approach that does not take into account the underlying processes. With our increasing understanding of the interplay between genes, epigenetics, individual’s lifestyle and the environment in disease development, uterine fibroids management should be geared towards individualized preventive and treatment options. For example, it seems that some subsets of patients with fibroids also suffer from vitamin D deficiency, hypertension, metabolic syndrome or other conditions. It is possible that these subsets may have different underlying processes and different responsiveness to different treatment options. Herein, we call for a futuristic paradigm shift of research to develop a new model to manage uterine fibroids with the treatment approach varying depending on the patient’s perceived underlying processes as assessed by medical, social, family history and relevant investigations. This is only possible through the collaboration of scientists, physicians, and funding agencies and with the help of our patients.
Keywords: uterine fibroids, precision medicine, preventive medicine
Problems can be simple, complicated, or complex. Uterine fibroids are squarely in the complex category. This applies to why they develop and how they present. For example, how many times do we incidentally discover large fibroids barely causing any symptoms while a much smaller tumor in a similar location may cause significant symptoms in another patient? Why do some fibroids grow at a fast clip while others stay stationary over several years? Why do fibroids recur in some women while in others they do not? Are we adopting a “one size fits all” approach that ignores the disease variability and heterogeneity?
With our increasing understanding of the interplay between genes, epigenetics, individual’s lifestyle, and the environment in disease development, precision medicine geared towards individualized prevention and treatment options is expanding, and uterine fibroids should be no exception. Uterine fibroids are the most common tumors of the female reproductive tract, affecting up to 80% of women. Yet, available medical treatment options are not only limited, but they are offered assuming equal responsiveness. To reduce the health, economic, social, and psychological burden of these tumors, a better understanding of different pathogenic pathways is necessary to enable effective prevention and management approaches. It is time to utilize the information we currently know on risk factors and treatment options and use it to manage this benign tumor.
A striking example is the association of low vitamin D with uterine fibroids. Robust evidence suggests that a rigorous clinical trial is not only indicated, but overdue [1, 2]. Another example is the association of cardiometabolic risk factors; including obesity, hypertension, hyperlipidemia, and metabolic syndrome with uterine fibroid development [3]. While more studies are needed to identify the mechanisms of this association, it offers the potential for clinical intervention through lifestyle changes and medications. Our group showed the possible beneficial effects of simvastatin, an anti-hyperlipidemic drug, on uterine fibroids through in vitro, in vivo, and epidemiologic studies [4–7]. Simvastatin was shown to induce calcium dependent apoptosis [5], reduce extracellular matrix deposition [8], and restore the altered mechanotransduction state in uterine fibroids [4]. A nested-case control study showed that statin use was also associated with a lower risk of uterine fibroid development and experiencing fibroid-related symptoms [7]. Similarly, a recent study suggests that angiotensin-converting enzyme inhibitors may reduce uterine fibroid incidence in hypertensive patients [9]. It seems reasonable to think that the underlying pathobiologic processes and the response to treatment options can be different between patients with vitamin D deficiency and those with hypertension/metabolic syndrome.
One of the possible limitations to the development of effective therapeutics for uterine fibroids is the assumption that it is one uniform disease. As mentioned earlier, fibroids behave differently in their growth rate and the symptoms they produce. It is also possible that fibroids are the ultimate presentation of a group of different pathogenic pathways. For example, the observed cellular proliferation and the deposition of excessive extracellular matrix can be the outcome of different disease processes such as cardiometabolic risk factors, hypertension, or low vitamin D levels. One or more of these processes can be predominant in a group of patients. Therefore, it is conceivable that some patients may respond to vitamin D, while others may respond to simvastatin, angiotensin-converting enzyme inhibitors and so on. Thus, it can be helpful to identify the specific disease processes in each patient to deploy its appropriate treatment (Fig. 1).
Figure 1:
A potential stratification paradigm according to the pathobiology patients might have.
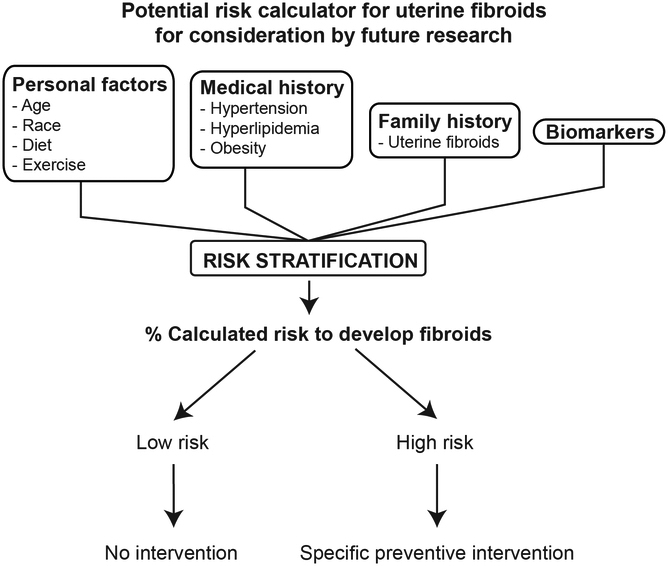
To efficiently prevent the development of uterine fibroids, it would be wise to target the group of women who are at highest risk. More research is needed to develop sensitive and specific biomarkers that can accurately predict future fibroid growth and symptom development beyond the initial work we currently have [10]. These biomarkers should be non-invasive and cost-effective manner for efficient application. The future of uterine fibroid prevention can be developing a reliable risk calculator using these biomarkers and individual risk factors, such as age, race, parity, family history, lifestyle, medication use including hormonal contraception. This would be similar to the extensively used ASCVD (atherosclerotic vascular disease) risk calculator which uses several factors such as age, gender, and cholesterol level to predict cardiovascular events and guide primary prevention management [11]. Similarly, the uterine fibroid risk calculator would serve as a tool to direct management, where a determined cut-off would prompt the use of established preventive medications (Fig. 2). Certainly, more research is needed to develop this risk score calculator and to validate its effectiveness in predicting uterine fibroids, their symptomatology, and responsiveness to management options.
Figure 2:
Potential fibroid risk calculator for consideration by future research. The aim of this calculator would be to guide prevention after stratifying the patients depending on their risk factors.
Despite its high burden, uterine fibroids’ research funding is considerably low, a problem hindering women health research in general. Recently, several women health professional organizations formed a coalition to advocate for increasing funding to propel women health research and discovery [12]. The therapeutic examples discussed in this article are already available drugs with decades of clinical use, established safety and low cost. While drug repurposing is a highly efficient approach to hasten discovery, these very advantages, in fact, discourage pharmaceutical companies as these drugs are already generic. This highlights the critical role of NIH for funding clinical trials and mechanistic studies of these drugs. This is in no way a call to stop developing and examining new molecules, but rather to broaden our search. The currently ongoing clinical trial (NCT03400826) for simvastatin in women with uterine fibroids funded by the Gynecologic Health and Disease Branch (GHDB) of the National Institute of Child Health and Human Development (NICHD) is an example of such highly needed studies.
In summary, this is a call for a new paradigm in uterine fibroid research focused in a more precise approach. Undoubtedly, more clinical, epidemiologic and translational research is needed to further develop these prevention and individualized treatment options. This is only possible through the cooperation of scientists, physicians, funding agencies, and patients. It is critical to prioritize uterine fibroid prevention and management research in an effort to reduce the heavy burden of women suffering from this condition and the billions of dollars spent to manage it.
Funding:
This work was supported, in part, by NIH grant 1R01HD094380 to Mostafa Borahay.
Footnotes
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Ali M, Prince L, Al-Hendy A. Vitamin D and uterine fibroids: preclinical evidence is in; time for an overdue clinical study! Fertil Steril 2020;113(1):89–90. doi: 10.1016/j.fertnstert.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Ali M, Shahin SM, Sabri NA, Al-Hendy A, Yang Q. Hypovitaminosis D exacerbates the DNA damage load in human uterine fibroids, which is ameliorated by vitamin D3 treatment. Acta Pharmacol Sin 2019;40(7):957–70. doi: 10.1038/s41401-018-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AlAshqar A, Patzkowsky K, Afrin S, Wild R, Taylor HS, Borahay MA. Cardiometabolic Risk Factors and Benign Gynecologic Disorders. Obstet Gynecol Surv 2019;74(11):661–73. doi: 10.1097/OGX.0000000000000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afrin S, Islam MS, Patzkowsky K, Malik M, Catherino WH, Segars JH et al. Simvastatin ameliorates altered mechanotransduction in uterine leiomyoma cells. Am J Obstet Gynecol 2020. doi: 10.1016/j.ajog.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borahay MA, Kilic GS, Yallampalli C, Snyder RR, Hankins GD, Al-Hendy A et al. Simvastatin Potently Induces Calcium-Dependent Apoptosis of Human Leiomyoma Cells. J Biol Chem 2014. doi: 10.1074/jbc.M114.583575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borahay MA, Vincent K, Motamedi M, Sbrana E, Kilic GS, Al-Hendy A et al. Novel Effects of Simvastatin on Uterine Fibroids: In vitro and Patient-Derived Xenograft Mouse Model Study. Am J Obstet Gynecol 2015. doi: 10.1016/j.ajog.2015.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borahay MA, Fang X, Baillargeon JG, Kilic GS, Boehning DF, Kuo YF. Statin use and uterine fibroid risk in hyperlipidemia patients: a nested case-control study. Am J Obstet Gynecol 2016. doi: 10.1016/j.ajog.2016.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik M, Britten J, Borahay M, Segars J, Catherino WH. Simvastatin, at clinically relevant concentrations, affects human uterine leiomyoma growth and extracellular matrix production. Fertil Steril 2018;110(7):1398–407 e1. doi: 10.1016/j.fertnstert.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Fischer NM, Nieuwenhuis TO, Singh B, Yenokyan G, Segars JH. Angiotensin-Converting Enzyme Inhibitors Reduce Uterine Fibroid Incidence in Hypertensive Women. J Clin Endocrinol Metab 2020. doi: 10.1210/clinem/dgaa718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy G, Hill MJ, Plowden TC, Catherino WH, Armstrong AY. Biomarkers in uterine leiomyoma. Fertil Steril 2013;99(4):1146–52. doi: 10.1016/j.fertnstert.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563–e95. doi: 10.1161/CIR.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice LW, Cedars MI, Sadovsky Y, Siddiqui NY, Teal SB, Wright JD et al. Increasing NIH funding for academic departments of obstetrics and gynecology: a call to action. Am J Obstet Gynecol 2020;223(1):79 e1–e8. doi: 10.1016/j.ajog.2020.03.022. [DOI] [PubMed] [Google Scholar]