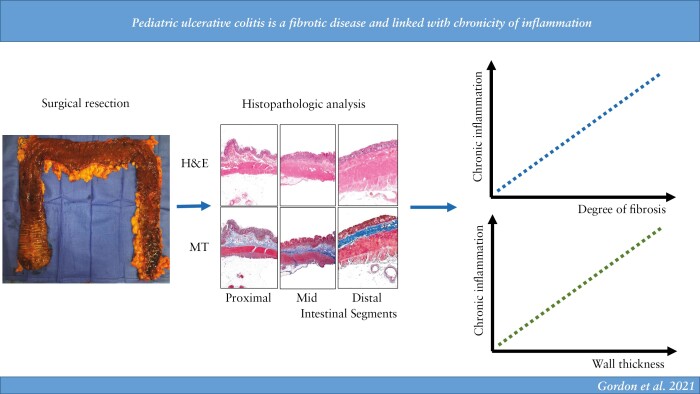
Abstract
Background and Aims
Intestinal fibrosis has recently been characterised in adult ulcerative colitis and may affect motility, diarrhoea, and the symptom of urgency. We aimed to charactersze the presence of fibrosis in paediatric patients with ulcerative colitis, and its link to severity and chronicity of mucosal inflammation, as well as clinical factors of severity.
Methods
We performed a single-centre cross-sectional study in children ages 1–18 years with ulcerative colitis, undergoing colectomy or proctocolectomy. Tissue cross-sections were derived from proximal, mid, and distal colon and rectum, and inflammation and fibrosis were graded based on previously developed scores. Clinical data were collected prospectively.
Results
From 62 patients, 205 intestinal sections were evaluated. Median age at diagnosis was 13 years, 100% had extensive colitis, and all resections were done for refractory disease. The presence, chronicity, and degree of inflammation were linked with the presence of fibrosis. Thickness of the muscularis mucosa was also linked with presence and chronicity of inflammation. The overall submucosal fibrosis burden was associated with prior anti-tumour necrosis factor use.
Conclusions
Paediatric patients with ulcerative colitis exhibit colorectal submucosal fibrosis and muscularis mucosa thickening, which correlate with the presence, chronicity, and degree of mucosal inflammation. Fibrosis should be recognised as a complication of paediatric ulcerative colitis, and ulcerative colitis should be considered a progressive disease.
Keywords: Fibrosis, inflammation, paediatric ulcerative colitis
Graphical Abstract
1. Introduction
In 25% of patients, inflammatory bowel disease [IBD] presents during childhood or adolescence.1 Although overall less prevalent than paediatric Crohn’s disease [CD], paediatric ulcerative colitis [UC] has been significantly increasing in both incidence and prevalence over time.2,3 Childhood onset UC extensively involves the colon in up to 75% of cases.4 Moreover, up to 20% of these cases require a colectomy within 10 years of diagnosis, with refractory disease being the indication in the vast majority of cases.4,5 A key manifestation of IBD in children is growth delay or failure, defined by abnormalities in height velocity.6–8 Especially in children with pre-pubertal disease onset and more severe disease, this may lead to a reduced adult height compared with their healthy peers.6,9,10 Principal factors include poor nutrition, corticosteroid therapy, degree of inflammation, and pro-inflammatory cytokines.6,11
Fibrosis is characterised by the excessive deposition of extracellular matrix [ECM] proteins.12 The expansion of myofibroblasts, the major source of ECM, is the key component in the pathogenesis of fibrosis.13 Repetitive injury leading to chronic inflammation and subsequent abnormal wound healing, characterised by excessive repair without complete restitution, seems to be the driver of fibrosis in all organs, including the intestine.14,15 Strictures in IBD are not limited to CD, and are thought to be a complication in 1% to 11.2% of patients with UC.14 Interestingly, ECM deposition was recently observed throughout the full thickness of the colonic wall in UC.16 This has significant clinical implications in UC, including motility abnormalities, anorectal dysfunction, rectal urgency, and incontinence.14 As more research emerges focusing on the specific molecular pathways underlying fibrosis in IBD, novel potential targets arise for future anti-fibrotic intestinal agents.17
Given the serious implications of fibrosis in UC, and the limited number of studies characterising fibrosis outside of strictures,16,18–20 recent and comprehensive assessment of fibrosis in adult UC21 highlighted that adult UC is a progressive disease. The aim of this study was to characterise the presence of fibrosis in paediatric patients with UC, and its link to severity and chronicity of mucosal inflammation, as well as clinical factors of severity. The current study fills the significant knowledge gap regarding the paediatric population and delineates intestinal fibrosis in paediatric UC, which is linked to chronicity, severity, and extent of inflammation as well as clinical factors.
2. Materials and Methods
2.1. Patient population
In this retrospective study were included 62 paediatric UC patients aged 1–18 years who underwent colectomy or proctocolectomy at the Cleveland Clinic between 2001 and 2016. The diagnosis of UC was based on clinical, endoscopic, radiographic, and histopathological criteria, including absence of transmural inflammation, fistulising disease, and mural granulomatous inflammation, and was confirmed according to the North American Society for Paediatric Gastroenterology, Hepatology, and Nutrition clinical consensus guidelines.22 Infants below the age of 1 year, adults above the age of 18 years, children with another form of IBD [e.g., CD], those with and any history of prior colonic stricture or colonic surgery were excluded.
Clinical data collected included gender, race, age at diagnosis, weight and height at colectomy and at least 6 months prior [for assessment of height and weight velocity], family history of IBD, smoking status, disease duration at colectomy, indication for colectomy, disease extent including presence of backwash ileitis and perianal disease, duration of pre-operative biologics or immunomodulators, administration of immunomodulators or biologics at the time of colectomy, presence of extraintestinal manifestations [EIM], length of colectomy specimen, and post-colectomy follow-up. Height z-score and body mass index [BMI] were obtained for each patient at the time of diagnosis and at the time of colectomy. The 2000 Centers for Disease Control and Prevention [CDC] Growth Charts [ages 0 to <20 years]23 were used as the reference file for height and BMI z-score calculation. Z-scores standardise growth [height and BMI] for age and sex, allowing comparisons for any age group, using a standard deviation from the mean. All data were obtained from the prospectively collected electronic medical record and then transferred and stored in a secure coded semi-anonymised database for analysis [REDCap, Vanderbilt University, Nashville, TN]. The study was approved by the Institutional Review Board of the Cleveland Clinic as a minimal risk study, including a waiver of informed consent.
2.2. Histopathological staining procedure
Three segments per colectomy [proximal, mid, and distal colon], accounting for 113 intestinal sections from 38 UC specimens [one patient did not have an adequate histological section of distal colon], and four segments per proctocolectomy [proximal, mid, and distal colon, and rectum], accounting for 92 intestinal sections from 24 UC specimens [three patients did not have an adequate histological section of rectum], for a total of 205 intestinal sections from 62 patients, were stained for haematoxylin and eosin [H&E] and Masson trichrome [MT] using the Gomori One-Step Trichrome kit [Newcomer Supply, Middleton, WI].
2.3. Grading of inflammation
Inflammation was graded for each segment based on the Geboes score24 and the numerical Geboes score using a continuous scale from 0 to 22 was determined.21,25,26 A categorical interpretation based on Geboes score was also used. For this categorical interpretation, histological features of chronic mucosal injury, defined as crypt architectural distortion and chronic inflammatory infiltrate of the lamina propria, corresponding to the Geboes score categories of ‘structural’ and ‘chronic inflammatory infiltrate’, respectively, were separated from active inflammation and were assessed as described previously.21 Briefly, chronic mucosal injury was considered as present when Geboes score included any structural [Grade 0] or chronic inflammatory infiltrate [Grade 1] greater than 0, i.e., 0.1, 0.2, 0.3, 1.1, 1.2, or 1.3. Active inflammation was considered present when Geboes score included any lamina propria neutrophils [Grade 2A], epithelial neutrophils [Grade 3], crypt destruction [Grade 4], or erosion or ulceration [Grade 5] greater than 0. Chronic active inflammation was considered when both chronic mucosal injury and active inflammation scores each had at least one component >0, and no inflammation was when all grades were 0. Involved segments were defined as having active inflammation and/or chronic mucosal injury, consistent with standard UC pathology terminology27 and European Crohn´s and Colitis Organisation [ECCO] clinical consensus.28 Overall involvement for each patient was defined as involvement in at least one of the three segments.
2.4. Assessment of fibrosis
A qualitative and quantitative assessment of fibrosis was performed in each section, as described previously.21 The quantitative assessment was performed by measuring the thickness in micrometres of each bowel wall layer, using calibrated imaging software [CellSens, Olympus Corporation, Tokyo, Japan] as described previously.21 Briefly, measurements were taken in two areas, the maximum and minimum submucosal thickness as per H&E section, and included mucosa, muscularis mucosa, submucosa, muscularis propria interna, muscularis propria externa, complete muscularis propria, and full thickness [Supplementary Figure 1, available as Supplementary data at ECCO-JCC online]. Muscularis mucosa thickness considered the deepest smooth muscle fibre of this layer as the point of demarcation from muscularis mucosa to submucosa, such that assessment of submucosal fibrosis was based on non-muscular tissue, as confirmed on the MT-stained sections. Qualitative assessment of thickening of the muscularis mucosa was categorised as uniformly thickened, split, duplicated, or splayed. More than one category could be assigned per case due to variation seen within a section. The extent of submucosal fibrosis on both H&E and MT was categorised qualitatively as none, 1–25%, 26–50%, 51–75%, or 76–100%, each corresponding to a specific ‘fibrosis score’ ranging from 0 to 4, respectively.21 A ‘fibrosis burden score’ for each patient was calculated by averaging the fibrosis score from all three segments of the colon.21 We have previously shown that fibrosis can be assessed on H&E stain and that the fibrosis burden score shows agreement with objective measures of fibrosis in UC.21
2.5. Statistical analysis
Shapiro–Wilks testing was conducted for continuous variables to assess normality. Normally distributed measures were summarised as means [standard deviations], non-normally distributed continuous measures were described using medians [quartiles], and categorical factors were presented as frequencies [percentages]. A univariate analysis was performed to assess factors associated with fibrosis burden for MT and H&E. Kruskal–Wallis tests were used to assess association between fibrosis burden score and several categorical factors of interest. Spearman’s correlation coefficients were used to assess association between fibrosis burden and disease duration. The same was done using average muscularis mucosae thickness, average submucosa thickness, average lamina propria thickness, average full muscularis propria thickness, and average full intestinal wall thickness, as a measure of fibrosis burden.
To assess the association between Geboes score and thickness, linear mixed effects models with fixed effect inflammation and random intercepts for intra-patient correlation were used. Similarly, linear mixed effects models were used to assess the association between Geboes score and fibrosis area, thickness and colon location, thickness and involvement, and thickness and degree of inflammation. To assess whether association between thickness and inflammation varied with adjustment of location, linear mixed effects models with fixed effect for inflammation and location, and random intercepts for intra-patient correlation, were used. Mixed models were used to account for within-subject correlation, since multiple colon segments per patient were included. Generalised estimating equations [GEE] were used to assess the association between the fibrosis area and involvement, location, and degree of inflammation. All tests were two-tailed and performed at a significance level of 0.05. R version 3.6.2 [R Foundation for Statistical Computing, Vienna, Austria] and SAS [version 9.4; Cary, NC] were used for all analyses.
3. Results
3.1. Clinical phenotypes of the population
A total of 62 paediatric UC patients who underwent colectomy or proctocolectomy [38.7% female and 91.9% Caucasian] were included in the final analysis [Table 1]. The median age at diagnosis was 13 years, 29.5% had a family history of IBD, 93.8% never smoked, and 51.6% had an EIM, primarily anaemia. Prior to colectomy, 75.9% received at least one immunomodulatory therapy and 64.9% had anti-tumour necrosis factor [TNF] therapy. The median disease duration at time of colectomy was 2.6 years; all patients [100%] underwent surgical resection for refractory disease, which was extensive in all cases. Growth impairment, identified as a z-score <-1 in height, was identified in six patients, and in BMI in seven patients.
Table 1.
Cohort demographics and clinical characteristics.
| Factor | N | Summary |
|---|---|---|
| Gender | 62 | |
| • Female | 24 [38.7] | |
| Race | 62 | |
| • Caucasian | 57 [91.9] | |
| • Hispanic | 3 [4.8] | |
| • African American | 1 [1.6] | |
| • Other | 1 [1.6] | |
| Family history | ||
| • UC | 61 | 12 [19.7] |
| • CD | 61 | 6 [9.8] |
| Smoking | 59 | |
| • Never | 58 [98.3] | |
| • Current | 1 [1.7] | |
| Age at diagnosis | 62 | 12.9 [10.8, 15.5] |
| EIM [non-exclusive] | 62 | |
| • Any EIM | 32 [51.6] | |
| • Joint | 6 [9.7] | |
| • Anaemia | 26 [41.9] | |
| Disease extent of colitis | ||
| • Extensive colitis | 59 | 59 [100] |
| Backwash ileitis present | 61 | 13 [21.3] |
| Medications prior to colectomy | ||
| • Immunomodulators [at any time] | 58 | 44 [75.9] |
| • Duration of immunomodulators [mo] | 8.0 [3.0, 22.0] | |
| • Anti-TNF [at any time] | 57 | 37 [64.9] |
| • Duration of anti-TNF [mo] | 8.5 [1.0, 18.2] | |
| • Vedolizumab [at any time] | 23 | 1 [4.4] |
| • Duration of vedolizumab [mo] | 1 | |
| Disease duration at colectomy [years] | 62 | 2.6 [0.9, 4.8] |
| Reason for colectomy | ||
| • Refractory disease | 62 | 65 [100.0] |
| Medications at time of colectomy | 62 | |
| • 6MP | 6 [9.7] | |
| • AZA | 7 [11.3] | |
| • MTX | 9 [14.5] | |
| • Anti-TNF | 14 [22.6] | |
| Length of colorectum specimen [cm] | 62 | 70.3 ± 18.5 |
| Rectum included | 62 | 24 [38.7] |
| Post-colectomy follow-up [mo] | 62 | 1.0 [1.0, 2.0] |
Values presented as mean ± standard deviation [SD], median [P25, P75], or N [column %].
CD, Crohn’s disease; UC, ulcerative colitis; EIM, extraintestinal manifestations; 6MP, mercaptopurine; AZA, azathioprine; MTX, methotrexate; TNF, tumour necrosis factor; mo, months; P, percentile.
3.2. Presence of inflammation and chronic mucosal injury correlate with presence of fibrosis in paediatric ulcerative colitis
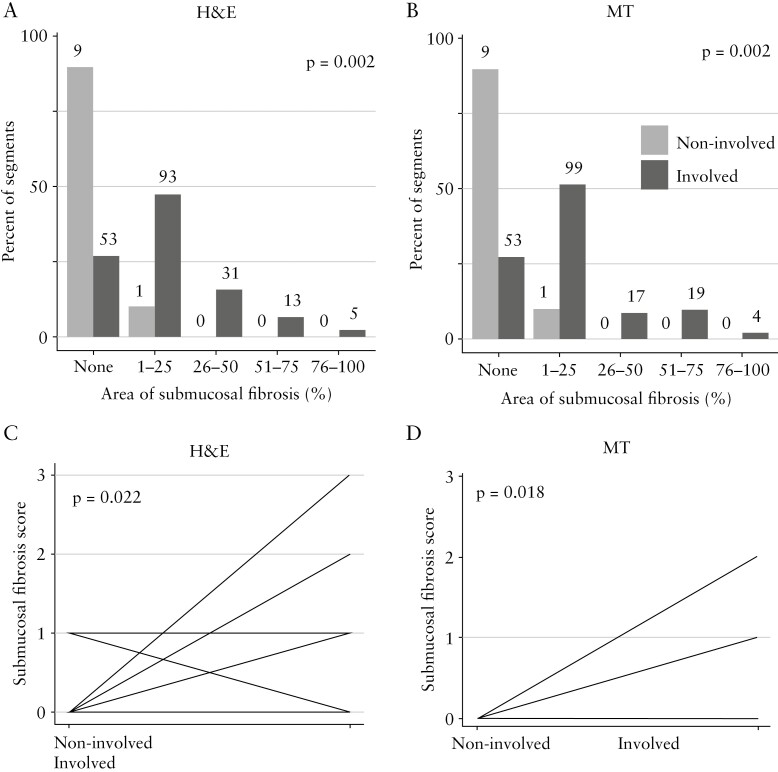
We first analysed segments involved by inflammation versus segments without inflammation, with respect to fibrosis. Overall, segments involved by inflammation showed significantly more submucosal fibrosis than non-involved segments [Figure 1A and B]. When comparing the submucosal fibrosis score in involved with non-involved segments in the same patient, the inflamed segments showed significantly higher submucosal fibrosis [Figure 1C and D]. This suggests a link between inflammation and fibrosis in paediatric UC.
Figure 1.
Fibrosis in paediatric ulcerative colitis is linked with the presence of inflammation. Involved segments showed higher degrees of fibrosis compared with non-involved segments. A and B: All slides together [n = 205]. C and D: Per patient analysis for patients in whom involved and non-involved segments were present [H&E: n = 10; MT: n = 10]; patients with the same findings are depicted together. H&E: haematoxylin and eosin; MT, Masson trichrome.
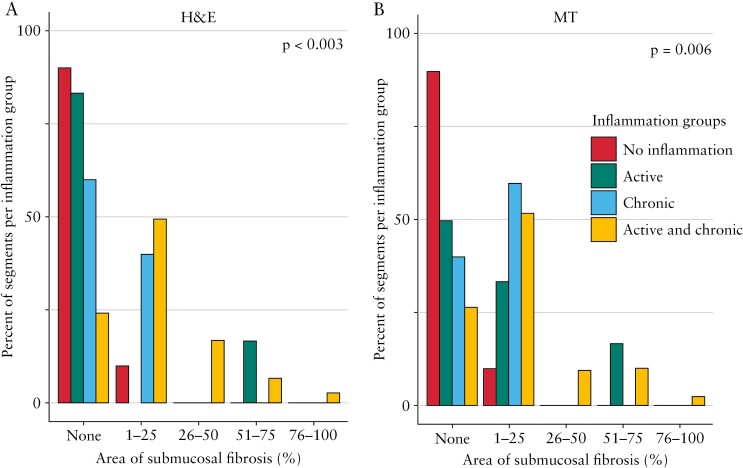
Next, segments involved by inflammation were further sub-classified into active inflammation only, chronic mucosal injury only, and chronic mucosal injury plus active inflammation, as described in Materials and Methods. No patient [of n = 62] exhibited chronic mucosal changes without active disease, and only five segments [of n = 205; three proximal, one mid, and one distal] showed chronic mucosal injury alone, consistent with refractory disease being all patients’ indication for surgery, suggesting heavily pretreated/burned out diseased segments. The degree of submucosal fibrosis was significantly higher in patients with active plus chronic as compared with active only, chronic only, or no inflammation [Figure 2A and B]. This suggests that both chronic inflammatory mucosal changes and active inflammation together are linked with fibrosis in paediatric UC.
Figure 2.
Active inflammation and chronic mucosal injury are associated with the grade of submucosal fibrosis. A. Haematoxylin and eosin [H&E]. B. Masson trichrome [MT].
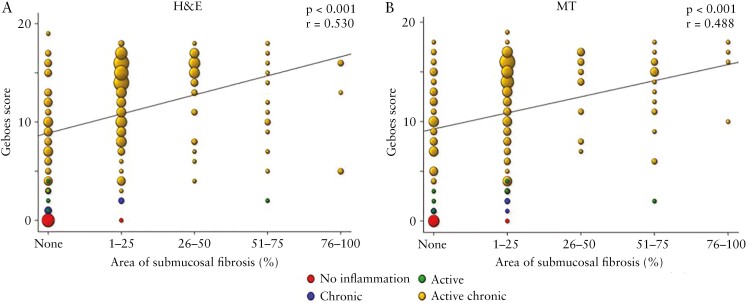
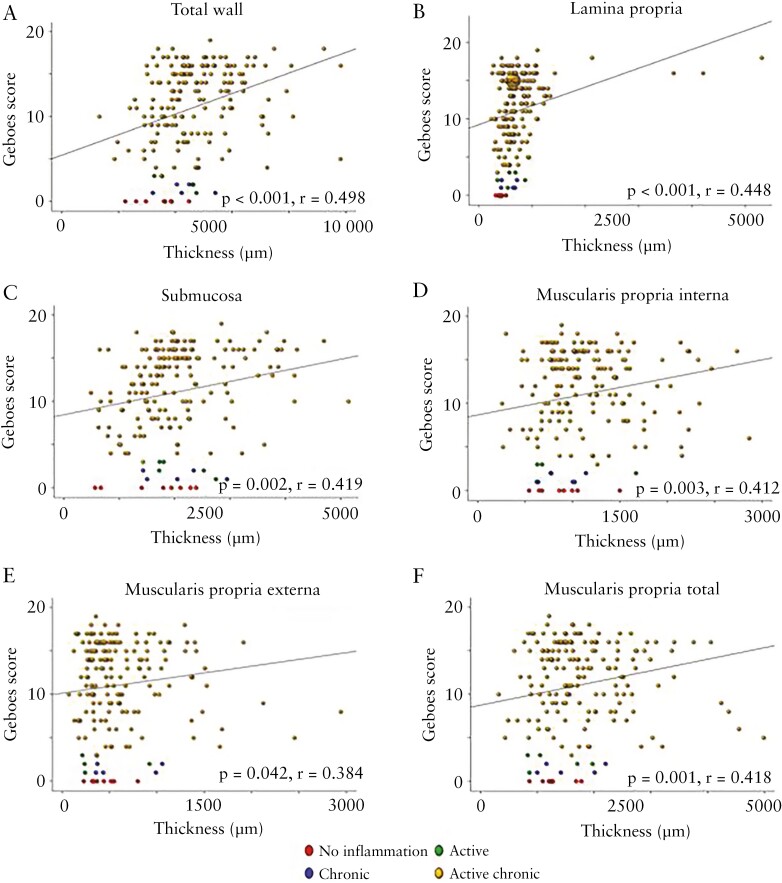
Upon further examination of slides [n = 205] as a single dataset, the majority of sections [142, 69.3%] showed both inflammation and fibrosis, with only one [0.5%] having fibrosis but no inflammation, 53 [25.8%] having inflammation but no fibrosis, and nine [4.4%] having no inflammation and no fibrosis. When both active inflammation and chronic mucosal injury were present [n = 184], 139 [75.5%] of these showed any amount of fibrosis, with only five [2.7%] having the maximal grade of fibrosis. In contrast, only one [16.7%] section with active inflammation only, two [40.0%] sections with chronic mucosal injury only, and one [10.0%] section with no inflammation showed any degree of fibrosis [Table 2]. Furthermore, an increase in Geboes score was linked with increasing grades of fibrosis with a moderate positive association [p <0.001, r = 0.5, Figure 3A and B]. In segments with any inflammation, the area of submucosal fibrosis nominally increased along the length of the colon from proximal to distal, and the frequency of segments without any fibrosis nominally decreased, but this did not reach statistical significance [Figure 4]. This indicates not only that fibrosis is infrequent in the absence of inflammation, but also that the severity of inflammation is associated with the severity of fibrosis.
Table 2.
Fibrosis in the submucosa [on Masson trichrome] in relation to inflammation as assessed by Geboes score.
| Active and chronic | Active | Chronic | No inflammation | |||||
|---|---|---|---|---|---|---|---|---|
| Median [Q1, Q3] | N | Median [Q1, Q3] | N | Median [Q1, Q3] | N | Median [Q1, Q3] | N | |
| No fibrosis | 10 [7, 13] | 45 | 3 [2, 3] | 5 | 1 [1, 1] | 3 | 0 [0, 0] | 9 |
| 1–25% | 14 [10, 15.5] | 91 | NA | 0 | 2 [2, 2] | 2 | 0 [0, 0] | 1 |
| 26–50% | 15 [13, 16] | 31 | NA | 0 | NA | 0 | NA | 0 |
| 51–75% | 11.5 [9.8, 15.3] | 12 | 2 [2, 2] | 1 | NA | 0 | NA | 0 |
| 76–100% | 13 [5, 16] | 5 | NA | 0 | NA | 0 | NA | 0 |
Q, quartile; NA, not available.
Figure 3.
The severity of inflammation is linked with the severity of fibrosis. A. Haematoxylin and eosin [H&E]. B. Masson trichrome [MT].
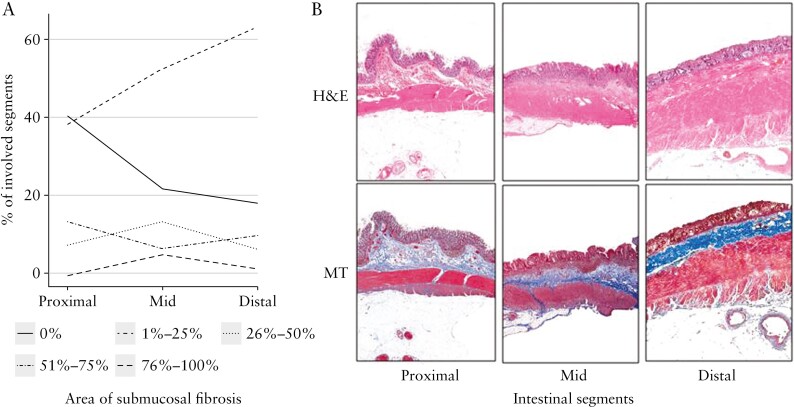
Figure 4.
Submucosal fibrosis in inflamed segments exhibits a gradient from proximal to distal. A. Percent of involved segments in the proximal, mid, and distal colon. B. Representative images of intestinal segments. H&E, haematoxylin and eosin; MT, Masson trichrome.
3.3. Mucosal inflammation correlates with both muscularis mucosa and total wall thickness
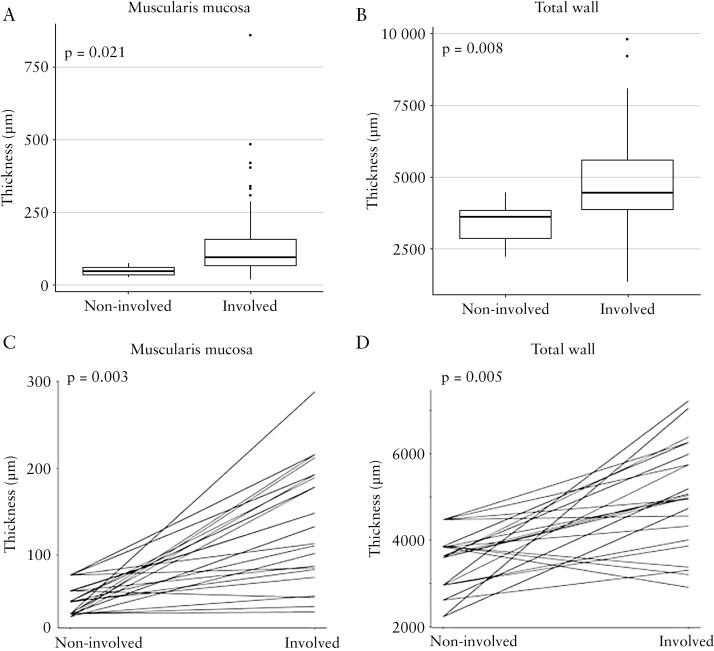
Inflammation and fibrosis have been linked with increased wall thickness in CD29,30 and increased wall thickness is a critical factor of fibrostenosis and in mediating symptoms. We therefore explored if mucosal inflammation is associated with mural thickness in paediatric UC, looking at each bowel wall layer separately. Considering the entire slide set, involved segments had an increased thickness of the muscularis mucosa [p = 0.021] and total wall [p = 0.008, Figure 5A and B], but not the lamina propria, submucosa, or muscularis propria [data not shown], compared with non-involved segments.
Figure 5.
Mucosal inflammation is associated with thickness of muscularis mucosa and of total wall. A and B: All slides together [n = 205]. C and D: Per patient analysis for patients in whom involved and non-involved segments were present [n = 10 patients, 35 slides].
Comparing among patients for whom both involved and non-involved segments were available for analysis, involved segments had significantly increased thickness of lamina propria [p = 0.001], muscularis mucosa [p = 0.003], muscularis propria interna [p = 0.044], total muscularis propria [p = 0.007], and total wall [p = 0.005] [Figure 5C and D and data not shown], but not submucosa or muscularis propria externa [data not shown], compared with non-involved segments.
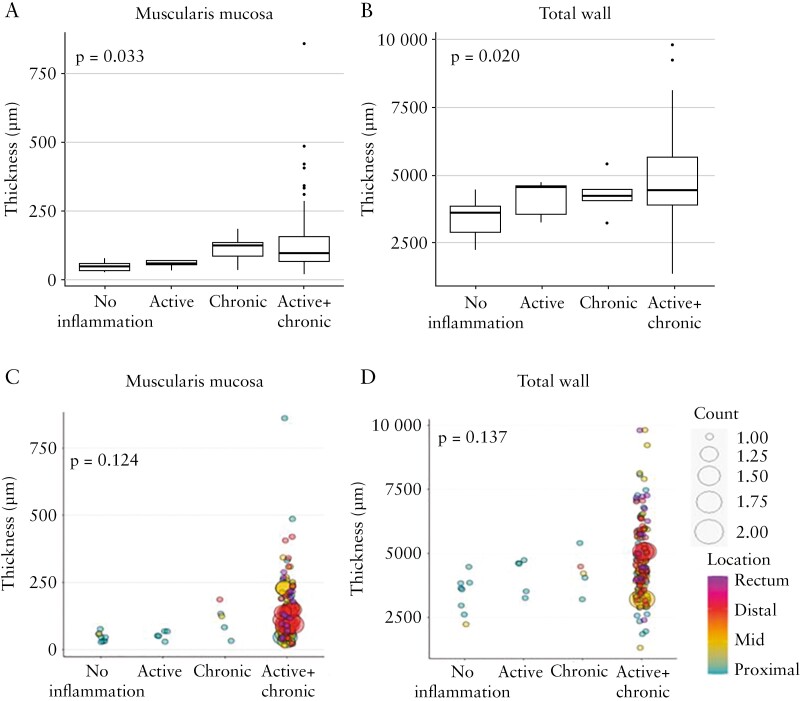
When segments involved by inflammation were further sub-classified into active inflammation only, chronic mucosal injury only and chronic mucosal injury plus active inflammation, as described in Materials and Methods, we found that the thickness of the muscularis mucosa [p = 0.033] and total wall [p = 0.020, Figure 6A and B], but not of the lamina propria, submucosa, muscularis propria interna, muscularis propria externa, or total muscularis propria [data not shown], are increased in the presence of active and chronic changes, with the highest thickness being found when active plus chronic mucosal changes were present. When adjusted for colon location [proximal, mid, distal, rectum], these associations were not significant for any bowel wall layer [Figures 4B, 6C and D and data not shown]. Considering all slides, with an increasing total Geboes score, the thickness of each individual bowel wall layer except the muscularis mucosa increased [all p≤0.042, r range 0.384 to 0.498, Figure 7].
Figure 6.
Active inflammation and chronic mucosal injury are associated with the thickness of particular bowel wall layers, including when adjusted for colon location. A, C. Muscularis mucosa. B, D. Total wall.
Figure 7.
Increasing Geboes score correlates with increased thickness of each of the bowel wall layer. A. Total wall. B. Lamina propria. C. Submucosa. D. Muscularis propria interna. E. Muscularis propria externa. F. Muscularis propria total, except for muscularis mucosa [plot not shown].
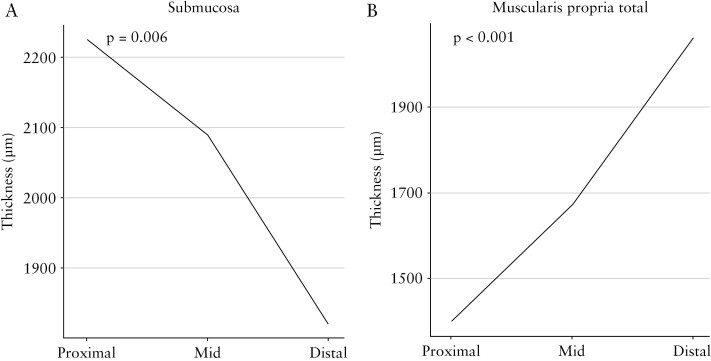
Also considering all colon slides, the thickness of the submucosa [p = 0.006], muscularis propria interna [p ≤0.001], muscularis propria externa [p ≤0.001], and total muscularis propria [p ≤0.001], but not the lamina propria, muscularis mucosa, or total wall, are significantly different, with decreasing thickness of submucosa and increasing thickness of the other indicated layers, between proximal, mid, and distal colon [Figures 4B and 8 and data not shown]. These associations are maintained when adjusted for inflammation and when looking only at inflamed segments [data not shown].
Figure 8.
Thickness of the submucosa decreases [A] and total muscularis propria increases [B] from proximal to distal.
Qualitative assessment of the muscularis mucosa morphology revealed uniform thickening as the most common finding, present in 52% of sections [107 of 205 total slides], and splaying of fibres as being next most common [27%, 56 slides] [data not shown]. A split inner and outer muscularis mucosa, often with loose fibrous tissue between, was present in 11% of cases [23 slides], whereas true duplication was only seen in 5% of cases [11 slides]. Fibrosis and fibrous replacement of smooth muscle in either muscularis mucosa or muscularis propria was not seen.
3.4. Colonic fibrosis in ulcerative colitis is associated with anti-TNF therapy
Looking at fibrosis burden score compared with each clinical factor, fibrosis burden score was associated with both pre-operative anti-TNF therapy and with anti-TNF therapy at the time of colectomy, where patients with anti-TNF therapy had a higher fibrosis burden score compared with patients without [p = 0.005 and p = 0.020, respectively, Table 3]. This study cohort showed an expected difference between pre-operative use of anti-TNF when analysing the colectomy tissues from two separate time periods [2001–2008 compared with 2009–2016; p = 0.026, Supplementary Figure 2A, available as Supplementary data at ECCO-JCC online], with a higher proportion of patients using anti-TNF in the time frame from 2009–2016. However, this difference did not lead to any change in the degree of fibrosis, muscularis mucosa [MM] thickness, or inflammation between the two time periods [Supplementary Figure 2B–D]. Furthermore, the correlation between Geboes score and area of fibrosis, as well as between Geboes score and full wall thickness, maintained significance across both time periods [Supplementary Figure 3A–D, available as Supplementary data at ECCO-JCC online].
Table 3.
Correlation between fibrosis burden score and clinical factors
| Factor | Categories | N | Statistics | p-value |
|---|---|---|---|---|
| Gender | Male | 37 | 0.75 [0.33, 1.00] | 0.136 |
| Female | 24 | 1.00 [0.63, 1.33] | ||
| Family history | ||||
| • CD | Yes | 6 | 1.00 [0.75, 1.50] | 0.654 |
| No | 54 | 1.00 [0.50, 1.33] | ||
| • UC | Yes | 12 | 1.00 [0.33, 1.00] | 0.228 |
| No | 48 | 1.00 [0.67, 1.33] | ||
| Smoker | Current | 1 | 1.33 [1.33, 1.33] | 0.398 |
| Ex-smoker | 0 | - | ||
| Never | 57 | 1.00 [0.50, 1.33] | ||
| Extraintestinal manifestations | ||||
| • Joint | Yes | 6 | 0.67 [0.42, 0.92] | 0.316 |
| No | 55 | 1.00 [0.50, 1.33] | ||
| • Skin | Yes | 1 | 3.00 [3.00, 3.00] | 0.115 |
| No | 60 | 1.00 [0.50, 1.33] | ||
| • Eye | Yes | 0 | - | NA |
| No | 61 | 1.00 [0.50, 1.33] | ||
| • Bone | Yes | 0 | - | NA |
| No | 61 | 1.00 [0.50, 1.33] | ||
| • Anaemia | Yes | 26 | 1.00 [0.69, 1.46] | 0.090 |
| No | 35 | 0.75 [0.50, 1.00] | ||
| • PSC | Yes | 1 | 2.00 [2.00, 2.00] | 0.160 |
| No | 60 | 1.00 [0.50, 1.33] | ||
| • None | Yes | 29 | 0.75 [0.50, 1.00] | 0.068 |
| No | 32 | 1.00 [0.67, 1.33] | ||
| Backwash ileitis | Yes | 12 | 1.00 [0.69, 1.37] | 0.634 |
| No | 48 | 1.00 [0.50, 1.33] | ||
| Extent of colitis | Extensive | 58 | 1.00 [0.50, 1.33] | 0.429 |
| Left | 3 | 0.75 [0.54, 0.88] | ||
| Proctitis | 0 | - | ||
| Medications prior to colectomy | ||||
| • Immunomodulators | Yes | 43 | 1.00 [0.67, 1.33] | 0.822 |
| No | 14 | 1.00 [0.54, 1.33] | ||
| • Anti-TNF | Yes | 37 | 1.00 [0.75, 1.67] | 0.005 |
| No | 19 | 0.67 [0.33, 1.00] | ||
| • Vedolizumab | Yes | 1 | 0.33 [0.33, 0.33] | 0.104 |
| No | 22 | 1.00 [0.69, 1.31] | ||
| Medications at time of colectomy | ||||
| • 5-ASA | Yes | 12 | 1.00 [0.67, 1.42] | 0.602 |
| No | 49 | 1.00 [0.50, 1.33] | ||
| • Systemic steroids | Yes | 52 | 1.00 [0.67, 1.33] | 0.100 |
| No | 9 | 0.50 [0.33, 0.75] | ||
| • 6MP | Yes | 6 | 0.83 [0.35, 1.00] | 0.261 |
| No | 55 | 1.00 [0.50, 1.33] | ||
| • AZA | Yes | 6 | 1.00 [0.81, 2.50] | 0.226 |
| No | 55 | 1.00 [0.50, 1.33] | ||
| • MTX | Yes | 9 | 1.00 [1.00, 1.50] | 0.166 |
| No | 52 | 1.00 [0.50, 1.33] | ||
| • Vedolizumab | Yes | 0 | - | NA |
| No | 61 | 1.00 [0.50, 1.33] | ||
| • Anti-TNF | Yes | 14 | 1.17 [0.75, 2.00] | 0.020 |
| No | 47 | 1.00 [0.50, 1.12] | ||
| • Sulphasalazine | Yes | 1 | 1.00 [1.00, 1.00] | 0.819 |
| No | 60 | 1.00 [0.50, 1.33] | ||
| • Cyclosporine | Yes | 2 | 0.88 [0.81, 0.94] | 0.951 |
| No | 59 | 1.00 [0.50, 1.33] | ||
| • Tacrolimus | No | 60 | 1.00 [0.50, 1.33] | 0.819 |
| Yes | 1 | 1.00 [1.00, 1.00] | ||
| Duration of immunomodulators | 28 | -0.15 [ -0.50, 0.24 ] | 0.45 | |
| Duration of anti-TNF | 28 | -0.28 [-0.59, 0.10] | 0.15 |
Values presented as median [25th, 75th percentile] with Kruskal–Wallis test or Spearman’s rho [95% confidence interval].
BMI, body mass index, CD, Crohn’s disease; UC, ulcerative colitis; PSC, primary sclerosing cholangitis; 5- ASA, 5-aminosalicylic acid, 6MP, mercaptopurine; AZA, azathioprine; MTX, methotrexate; TNF, tumour necrosis factor; NA, not available.
Finally, we assessed whether the thickness [in micrometres] of the different layers of the bowel wall was associated with any clinical characteristics. Patients with backwash ileitis had a significantly higher submucosa thickness compared with patients without [median 2303 vs 1907 µm, p = 0.019]. Patients who had received pre-operative anti-TNF therapy had a significantly higher muscularis mucosa thickness compared with patients who did not receive this therapy [median 116 vs 82 µm, p = 0.021]. Patients reporting no EIM had significantly lower lamina propria thickness [median 590 vs 704 µm, p = 0.025] and significantly higher muscularis mucosa thickness [median 118 vs 94 µm, p = 0.044] than those with any EIM. Patients on azathioprine at the time of colectomy had a significantly higher lamina propria thickness compared with patients who were not [median 889 vs 651 µm, p = 0.037]. Muscularis propria thickness was significantly associated with family history of UC and pre-operative immunomodulators, where patients with a family history of UC had higher muscularis propria thickness than those without [median 2108 vs 1590 µm, p = 0.009], and patients on pre-operative immunomodulators also had a higher muscularis propria thickness than patients without [median 1783 vs 1481 µm, p = 0.015]. There was no association between any clinical factors and total wall thickness [Table 4].
Table 4.
Correlation between bowel wall thickness and clinical factors [see separate file]
| Factor | Levels | N | Lamina propria thickness | Muscularis mucosa thickness | Submucosa thickness | Muscularis propria thickness | Total wall thickness | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statistics | p-value | Statistics | p-value | Statistics | p-value | Statistics | p-value | Statistics | p-value | |||
| Gender | Male | 38 | 680.06 [539.47, 867.12] | 0.298 | 108.42 [78.67, 154.81] | 0.513 | 1950.00 [1644.97, 2439.53] | 0.562 | 1626.71 [1494.19, 2028.34] | 0.964 | 4629.52 [3882.00, 5182.67] | 0.356 |
| Female | 23 | 648.17 [534.19, 702.50] | 108.50 [66.08, 127.50] | 1925.88 [1649.50, 2239.92] | 1592.25 [1504.17, 2099.83] | 4435.00 [3953.92, 4768.58] | ||||||
| Family history | ||||||||||||
| CD | Yes | 6 | 598.67 [502.00, 646.96] | 0.324 | 113.25 [54.67, 119.33] | 0.622 | 1845.67 [1769.17, 1915.54] | 0.522 | 1670.42 [1519.67, 1824.67] | 0.605 | 4432.00 [3956.08, 4857.42] | 0.538 |
| No | 54 | 670.92 [539.47, 811.17] | 100.92 [74.50, 146.75] | 1958.83 [1627.50, 2380.62] | 1626.71 [1490.62, 2095.38] | 4549.50 [3913.75, 5161.58] | ||||||
| UC | Yes | 12 | 656.94 [489.58, 728.38] | 0.318 | 105.67 [77.00, 122.25] | 0.897 | 2009.92 [1516.98, 2565.30] | 0.882 | 2108.17 [1793.78, 2259.04] | 0.009 | 4907.42 [4300.17, 5563.50] | 0.155 |
| No | 48 | 670.42 [548.16, 833.19] | 106.08 [72.53, 156.50] | 1934.44 [1671.91, 2304.69] | 1589.54 [1455.29, 1984.79] | 4495.19 [3896.83, 5062.75] | ||||||
| Smoker | Current | 1 | 497.33 [497.33, 497.33] | 0.244 | 51.33 [51.33, 51.33] | 0.179 | 1212.17 [1212.17, 1212.17] | 0.16 | 2118.17 [2118.17, 2118.17] | 0.269 | 3895.67 [3895.67, 3895.67] | 0.355 |
| Ex smoker | 0 | - | - | - | - | - | ||||||
| Never | 57 | 661.50 [543.50, 812.67] | 108.75 [78.17, 151.25] | 1957.00 [1689.00, 2404.12] | 1621.67 [1486.33, 2034.62] | 4550.00 [3945.00, 5126.83] | ||||||
| EIM | ||||||||||||
| Joint | Yes | 6 | 701.65 [578.41, 852.67] | 0.645 | 117.08 [90.67, 189.38] | 0.265 | 2173.56 [1897.88, 2975.66] | 0.153 | 1708.44 [1573.25, 2128.09] | 0.529 | 5087.83 [4306.62, 5875.29] | 0.208 |
| No | 55 | 660.17 [534.19, 789.33] | 108.50 [69.31, 142.25] | 1925.88 [1618.08, 2290.94] | 1621.67 [1494.92, 2051.65] | 4513.50 [3899.50, 5031.27] | ||||||
| Skin | Yes | 1 | 704.88 [704.88, 704.88] | 0.65 | 108.75 [108.75, 108.75] | 0.955 | 1325.88 [1325.88, 1325.88] | 0.173 | 1681.62 [1681.62, 1681.62] | 0.91 | 3800.38 [3800.38, 3800.38] | 0.256 |
| No | 60 | 659.65 [535.16, 808.17] | 106.08 [72.53, 152.44] | 1950.00 [1671.91, 2333.62] | 1619.40 [1499.21, 2071.88] | 4549.50 [3934.58, 5079.61] | ||||||
| Eye | Yes | 0 | - | NA | - | NA | - | NA | - | NA | - | NA |
| No | 61 | 660.17 [536.12, 806.67] | 108.50 [73.33, 151.25] | 1943.00 [1631.50, 2310.12] | 1621.67 [1503.50, 2068.67] | 4549.00 [3903.33, 5063.88] | ||||||
| Bone | Yes | 0 | - | NA | - | NA | - | NA | - | NA | - | NA |
| No | 61 | 660.17 [536.12, 806.67] | 108.50 [73.33, 151.25] | 1943.00 [1631.50, 2310.12] | 1621.67 [1503.50, 2068.67] | 4549.00 [3903.33, 5063.88] | ||||||
| Anaemia | Yes | 26 | 700.50 [636.12, 867.12] | 0.066 | 93.92 [65.28, 117.44] | 0.027 | 2070.33 [1615.38, 2502.75] | 0.531 | 1727.81 [1506.04, 2107.62] | 0.737 | 4579.19 [3913.75, 5171.08] | 0.705 |
| No | 35 | 607.33 [527.79, 733.67] | 117.25 [83.08, 177.50] | 1925.88 [1716.08, 2225.83] | 1616.50 [1494.92, 1999.58] | 4513.50 [3940.92, 4916.00] | ||||||
| PSC | Yes | 1 | 705.50 [705.50, 705.50] | 0.609 | 53.33 [53.33, 53.33] | 0.211 | 2454.50 [2454.50, 2454.50] | 0.307 | 1395.00 [1395.00, 1395.00] | 0.281 | 4599.83 [4599.83, 4599.83] | 0.91 |
| No | 60 | 659.65 [535.16, 808.17] | 108.62 [76.83, 152.44] | 1934.44 [1630.17, 2304.69] | 1626.71 [1504.50, 2071.88] | 4531.25 [3901.42, 5079.61] | ||||||
| None | Yes | 29 | 590.00 [523.33, 700.12] | 0.025 | 117.50 [85.00, 169.67] | 0.044 | 1918.50 [1626.17, 2160.67] | 0.133 | 1617.12 [1503.50, 1989.67] | 0.74 | 4476.88 [3877.33, 4902.17] | 0.333 |
| No | 32 | 703.62 [628.12, 879.21] | 93.92 [66.51, 118.93] | 2070.33 [1671.91, 2543.94] | 1656.69 [1493.12, 2116.79] | 4604.10 [3934.58, 5252.79] | ||||||
| Backwash ileitis | Yes | 13 | 702.38 [551.75, 949.00] | 0.302 | 117.62 [68.50, 156.50] | 0.823 | 2302.88 [2180.00, 2498.50] | 0.019 | 1557.12 [1486.33, 1983.17] | 0.206 | 4732.50 [4476.88, 5185.83] | 0.129 |
| No | 47 | 654.75 [537.12, 745.00] | 108.50 [79.17, 141.71] | 1906.67 [1570.19, 2238.92] | 1681.62 [1513.71, 2117.25] | 4435.00 [3886.50, 5031.27] | ||||||
| Extent of colitis | Extensive | 58 | 659.65 [536.62, 811.17] | 0.92 | 109.71 [74.50, 154.81] | 0.171 | 1950.00 [1644.97, 2380.62] | 0.333 | 1619.40 [1503.83, 2060.16] | 0.739 | 4549.50 [3913.75, 5111.09] | 0.35 |
| Left- sided | 3 | 723.17 [564.67, 747.58] | 80.17 [58.08, 94.33] | 1784.67 [1443.02, 1950.08] | 2009.50 [1641.58, 2071.56] | 4148.25 [3816.29, 4525.21] | ||||||
| Proctitis | 0 | - | - | - | - | - | ||||||
| Medications prior to colectomy | ||||||||||||
| Immunomodulators | Yes | 44 | 649.29 [532.93, 744.58] | 0.095 | 106.08 [79.67, 139.44] | 0.513 | 1934.44 [1671.91, 2248.17] | 0.292 | 1782.58 [1550.29, 2104.08] | 0.015 | 4574.92 [4016.41, 5062.75] | 0.827 |
| No | 14 | 696.77 [651.17, 945.33] | 97.75 [69.71, 119.59] | 2367.17 [1591.00, 2976.17] | 1481.42 [1272.04, 1665.34] | 4677.17 [3702.97, 5278.96] | ||||||
| Anti-TNF | Yes | 37 | 679.33 [543.50, 812.67] | 0.616 | 115.83 [85.00, 158.83] | 0.021 | 1960.67 [1743.17, 2404.12] | 0.763 | 1770.00 [1549.67, 2068.67] | 0.308 | 4671.67 [4074.67, 5063.88] | 0.263 |
| No | 20 | 658.12 [548.34, 751.00] | 81.50 [66.62, 116.97] | 1941.44 [1574.91, 2353.20] | 1539.88 [1291.35, 2167.80] | 4199.92 [3854.06, 5216.88] | ||||||
| Vedolizumab | Yes | 1 | 463.67 [463.67, 463.67] | 0.175 | 86.17 [86.17, 86.17] | 0.546 | 1846.83 [1846.83, 1846.83] | 0.651 | 2100.00 [2100.00, 2100.00] | 0.366 | 4676.17 [4676.17, 4676.17] | 0.88 |
| No | 22 | 657.42 [537.06, 826.23] | 111.92 [79.88, 166.96] | 1912.58 [1748.38, 2308.31] | 1706.42 [1547.67, 2069.78] | 4640.02 [4104.08, 5182.67] | ||||||
| Medications at time of colectomy | Yes | 13 | 659.12 [532.25, 812.67] | 0.846 | 96.00 [78.17, 158.83] | 0.805 | 1882.83 [1743.17, 2302.88] | 0.846 | 1592.25 [1447.17, 1800.38] | 0.428 | 4549.00 [4074.67, 5002.50] | 0.972 |
| 5-ASA | No | 48 | 661.33 [537.62, 780.67] | 109.71 [72.53, 137.75] | 1958.83 [1602.41, 2345.43] | 1725.56 [1508.46, 2086.12] | 4556.67 [3901.42, 5079.61] | |||||
| Systemic steroids | Yes | 53 | 662.50 [543.50, 812.67] | 0.114 | 103.67 [70.12, 133.25] | 0.347 | 1943.00 [1631.50, 2310.12] | 0.966 | 1621.67 [1458.00, 2034.62] | 0.393 | 4549.00 [3877.33, 5063.88] | 0.358 |
| No | 8 | 535.75 [418.75, 687.34] | 119.50 [84.17, 169.62] | 2052.17 [1660.47, 2310.28] | 1708.44 [1549.38, 2108.41] | 4594.83 [4208.44, 5225.12] | ||||||
| 6MP | Yes | 6 | 605.44 [540.03, 688.75] | 0.716 | 90.15 [47.44, 116.23] | 0.175 | 2210.69 [1959.62, 2378.81] | 0.298 | 1958.35 [1793.47, 2145.52] | 0.069 | 4805.44 [4509.75, 5047.41] | 0.235 |
| No | 55 | 661.50 [535.19, 809.67] | 108.50 [78.08, 156.25] | 1925.88 [1618.08, 2294.56] | 1616.50 [1472.17, 2022.06] | 4513.50 [3886.50, 5095.35] | ||||||
| · AZA | Yes | 7 | 888.83 [692.33, 1174.50] | 0.037 | 86.33 [72.42, 140.67] | 0.635 | 2180.00 [2029.25, 2373.08] | 0.23 | 2009.50 [1627.92, 2051.65] | 0.556 | 5173.17 [4726.08, 5688.92] | 0.067 |
| No | 54 | 651.46 [533.22, 745.42] | 109.71 [74.50, 146.75] | 1912.58 [1615.38, 2308.31] | 1616.81 [1503.83, 2061.28] | 4455.94 [3897.58, 5001.92] | ||||||
| MTX | Yes | 9 | 590.00 [543.50, 812.67] | 0.555 | 110.67 [81.17, 117.50] | 0.887 | 1882.83 [1764.00, 2160.67] | 0.76 | 1891.67 [1558.83, 2000.62] | 0.36 | 4650.67 [3877.33, 4912.67] | 0.871 |
| No | 52 | 662.00 [535.16, 780.67] | 103.33 [69.72, 156.12] | 1958.83 [1622.12, 2415.93] | 1616.81 [1479.25, 2071.88] | 4531.25 [3934.58, 5138.42] | ||||||
| Vedolizumab | Yes | 0 | - | NA | - | NA | - | NA | - | NA | - | NA |
| No | 61 | 660.17 [536.12, 806.67] | 108.50 [73.33, 151.25] | 1943.00 [1631.50, 2310.12] | 1621.67 [1503.50, 2068.67] | 4549.00 [3903.33, 5063.88] | ||||||
| Anti-TNF | Yes | 14 | 702.06 [601.38, 991.31] | 0.237 | 92.42 [65.28, 144.15] | 0.461 | 2021.75 [1705.03, 2400.42] | 0.891 | 1587.67 [1433.00, 1861.25] | 0.493 | 4574.92 [3883.83, 5283.04] | 0.918 |
| No | 47 | 659.12 [527.79, 745.00] | 108.75 [79.08, 144.62] | 1943.00 [1628.83, 2306.50] | 1631.75 [1504.17, 2090.75] | 4513.50 [3974.75, 5063.12] | ||||||
| Sulphasalizine | Yes | 1 | 523.33 [523.33, 523.33] | 0.334 | 503.33 [503.33, 503.33] | 0.088 | 1876.67 [1876.67, 1876.67] | 0.733 | 1447.17 [1447.17, 1447.17] | 0.307 | 4333.83 [4333.83, 4333.83] | 0.776 |
| No | 60 | 660.83 [537.62, 808.17] | 106.08 [72.53, 137.75] | 1950.00 [1630.17, 2333.62] | 1626.71 [1504.50, 2071.88] | 4549.50 [3901.42, 5079.61] | ||||||
| Cyclosporine | Yes | 3 | 700.12 [680.81, 701.25] | 0.641 | 96.00 [90.50, 114.62] | 0.96 | 1631.50 [1628.83, 1778.69] | 0.286 | 1486.33 [1391.79, 1521.73] | 0.133 | 4004.50 [3932.75, 4012.44] | 0.152 |
| No | 58 | 656.94 [533.22, 811.17] | 108.62 [70.93, 154.81] | 1958.83 [1686.28, 2380.62] | 1656.69 [1506.04, 2078.29] | 4574.92 [3913.75, 5111.09] | ||||||
| Tacrolimus | No | 60 | 659.65 [535.16, 808.17] | 0.776 | 108.62 [76.83, 152.44] | 0.281 | 1950.00 [1630.17, 2333.62] | 0.427 | 1619.40 [1499.21, 2043.14] | 0.233 | 4531.25 [3901.42, 5079.61] | 0.865 |
| Yes | 1 | 698.62 [698.62, 698.62] | 67.12 [67.12, 67.12] | 1685.38 [1685.38, 1685.38] | 2155.25 [2155.25, 2155.25] | 4608.38 [4608.38, 4608.38] | ||||||
| Duration of immunomodulators | 29 | -0.10 [-0.45, 0.27] | 0.59 | 0.15 [-0.23, 0.49] | 0.45 | 0.14 [-0.24, 0.48] | 0.46 | 0.19 [-0.19, 0.52] | 0.32 | 0.06 [-0.31, 0.42] | 0.75 | |
| Duration of anti-TNF | 28 | -0.15 [-0.50, 0.23] | 0.44 | 0.15 [-0.23, 0.50] | 0.44 | -0.03 [-0.40, 0.35] | 0.88 | 0.07 [-0.31, 0.43] | 0.73 | -0.12 [-0.47, 0.26] | 0.54 |
CD, Crohn’s disease; UC, ulcerative colitis; PSC, primary sclerosing cholangitis; 5- ASA, 5-aminosalicylic acid, 6MP, mercaptopurine; AZA, azathioprine; MTX, methotrexate; TNF, tumour necrosis factor; NA, not available; EIM, extraintestinal manifestations.
4. Discussion
For the first time, we systematically evaluated full-thickness sections along the colon of paediatric UC patients for fibrosis and its link with inflammation and clinical factors. We found that UC is a progressive disease, submucosal fibrosis is associated with mucosal inflammation, and the degree of mucosal inflammation correlates with the degree of fibrosis and the thickness of the lamina propria and total colonic wall. We discovered a colonic gradient, with increased wall thickness toward the distal colon. The overall fibrosis burden was associated with pre-operative anti-TNF therapy.
Paediatric UC is distinct in presentation from adult UC.31 Paediatric UC can present with overlapping features of Crohn’s disease, such as rectal sparing and gastro-enteric inflammation.32 Fibrosis is a robust feature of adult UC, as previously described and characterised by us and others,20,21 but fibrosis has not previously been described in paediatric UC. This is in stark contrast with paediatric CD, a field in which the prospective paediatric RISK cohort identified clinical and molecular markers associated with the increased risk of developing strictures,33–35 including a specific extracellular matrix gene signature in intestinal biopsies,35 intestinal Ruminococcus species,35 increased plasma COL3A1,36 and plasma ECM1.37 The natural history of paediatric stricturing CD has been examined and the ability to detect fibrosis on imaging in paediatric CD patients explored.38,39 Interestingly in general in the field of intestinal fibrosis, studies outside of clinically apparent stricturing disease are rare, but as shown in adult UC, fibrosis can be marked even in the colon of non-stricturing UC and may be linked with clinical consequences.14,21
Our study hence adds important information to the field of paediatric IBD. In concordance with previous paediatric UC resection studies, the large majority of our patients had extensive disease at the time of colectomy,4 a comparable incidence of family history of IBD,1 and presence of backwash ileitis.40 Given the severity of childhood onset UC, the use of immunosuppressant agents is often recommended for maintenance therapy,31 which is reflected in our cohort with three-quarters of patients having been on an immunosuppressant agent. Over half of our patients were on biologic therapy prior to colectomy; this aligns with a previous study that has established biologic therapy as an independent predictive factor for colectomy, highlighting the aggressive disease phenotype in these patients.41 Moreover, our patients received anti-TNF therapy for a median duration of <1 year prior to colectomy, similar to a previously reported study.42 Only one of our patients had been on vedolizumab prior to colectomy, which is expected as vedolizumab use remains off-label in paediatrics, with only one case series in the USA supporting its use at that time.43 As our patients had ulcerative colitis for a median of 2.3 years prior to colectomy, which is similar to the median of 3.5 years reported by Rinawi, et al.,5 it is not surprising than none of them had dysplasia/colorectal cancer which is typically associated with long-standing [>10 years] colitis.44 Finally, our patients had a low level of growth impairment, similar to a previously reported study.45 This suggests that our cohort is comparable to previous resection studies in terms of their clinical characteristics and is representative of a quaternary centre surgical cohort.
Our first key finding is that fibrosis is linked with inflammation. This is in line with several papers from the adult UC literature in which the presence, chronicity, and degree of fibrosis correlated with the severity and extent of inflammation.16,20,21 In one study, collagen I deposition in the mucosa, muscularis mucosa, and external layer of muscularis propria inversely correlated with the severity of inflammation in the acute UC cases, but not in long-standing disease.20 Colonic wall fibrosis, as measured by types I and III collagen and fibronectin, has been shown to be increased in long-standing [>10 years] UC compared with recently diagnosed UC [<3 years] and controls.16 Our own group has studied fibrosis in adult UC patients and showed association with chronicity and severity of inflammation21 similar to our current findings in paediatric UC. Also aligning with findings from the adult UC fibrosis study, the presence of any degree of fibrosis was almost exclusively seen in involved rather than non-involved segments of the colon of paediatric UC patients.
We also previously reported14 that only a small number of studies delineate the histological characteristics of UC-associated fibrosis in non-stricture specimens. One study, comparing inflammation and fibrosis in resected stenotic specimens compared with non-stenotic sites, found significantly thickened muscularis propria at stenotic sites and the myofibroblast count increased with the degree of inflammation, possibly due to basic fibroblast growth factor-positive neutrophil-induced myofibroblast proliferation.46 Several studies have provided insights into the pathophysiology of fibrosis in IBD, including the role of transforming growth factor-β1,47 nuclear factor erythroid 2–related factor 2 [Nrf2],48 interleukin-13,49 interleukin-36 receptor,50 and multiple other molecular mediators.51 Even in quiescent UC, the effects of fibrosis of the colon from prior bouts of inflammation may persist and lead to clinical symptoms.52
Strikingly, despite a relatively short disease duration of 2.3 years, paediatric UC patients presented with significant amounts of submucosal fibrosis and wall changes. This makes one wonder about the classical paradigm that long-standing chronic disease is a prerequisite to establish fibrosis, and whether fibrosis may in fact be a much earlier phenomenon than previously thought, which also holds true for paediatric UC.
The second important finding is that active and chronic inflammation were linked to additional features consistent with fibrosis, such as thickening of the entire wall as well as the individual layers muscularis mucosa and muscularis propria interna. Thickening of the muscularis mucosa was also found in adult UC patients.21 Interestingly, in CD strictures the muscularis mucosa is also the most thickened bowel wall layer identified, and both the smooth muscle and collagen content were significantly increased in the muscularis mucosa and in the muscularis propria interna.53 Thickening of the muscularis mucosa due to hyperplasia of its smooth muscle is well described in CD.54,55 This suggests that factors associated with inflammation may be affecting smooth muscle similarly in both UC and CD. The exact mechanism of smooth muscle hyperplasia and hypertrophy in IBD is not known, but it is believed to be linked to basic wound healing physiology in the mucosal ulcer bed, where muscularis mucosa smooth muscle cells are activated by inflammatory cytokines and growth factors to secrete ECM proteins and proliferate to increase in numbers. This process is pathologically altered in IBD, perhaps due the chronic relapsing-remitting inflammatory nature in its pathogenesis.15,56
Third, we linked the presence of fibrosis to clinical factors, such as disease duration. Our study found that pre-operative anti-TNF therapy did not prevent the development of fibrosis, in fact anti-TNF treated patients had higher fibrosis burden scores compared with other patients, and this is in contrast with a previous study which showed no association between pre-operative anti-TNF therapy and histological fibrosis in colectomy specimens of adult UC patients.57 This discrepancy may be explained by the higher threshold for a colectomy in a younger patient, resulting in prolonged inflammation and maturation of fibrosis. Interestingly, our finding of higher fibrosis burden scores in anti-TNF treated paediatric UC patients aligns with the previously reported finding of hyalinising submucosal fibrosis in CD patients treated with anti-TNF therapy.58 The prevalence of growth impairment in paediatric UC has previously been reported to be as high as 10% at diagnosis and 17% at follow-up.45 This is much lower than in paediatric Crohn’s disease where 56% have growth impairment at diagnosis and 40% at follow-up.59,60 The proportion of growth impairment in our cohort requiring colectomy was also low.
Our study has multiple strengths. This is the first characterisation of fibrosis in paediatric UC and its link to clinical factors. We use a previously established score that was built against an objective gold standard for ECM expression.21 Given possible variability between histopathological readers, we centrally read all images by a single experienced IBD pathologist following previously developed reading conventions.21 Our manuscript also has limitations. The patient population is representative of a resection study and hence is skewed toward those with more severe disease. In addition, infliximab was not approved for use in paediatric UC until 2011, which likely influenced the timing of colectomy in those with refractory disease in the pre-biologic era. Most patients in our study were on anti-TNF therapy, which could be a limitation in interpreting associations of fibrosis with anti-TNF therapy. However when we separated our patients into two cohorts based on the time of colectomy, the earlier group, which was less likely to have had anti-TNF therapy, and the later group, which was more likely to have had anti-TNF therapy, revealed the exact same results in relation to the association of anti-TNF with histopathological changes. Although we were able to accrue a high number of patients, this is still a single-centre study, and the finding will need to be validated in an external cohort.
In conclusion, we provide a link between chronicity of inflammation and colonic fibrosis in paediatric UC. This is clinically relevant, as the degree of fibrosis was linked to anti-TNF therapy. Paediatric UC is a progressive disease with fibrosis accumulation already early in the disease course, and future studies will need to determine the optimal way to measure colonic fibrosis in vivo. Given the degree and consistency of fibrosis, UC may serve as a model for future anti-fibrotic therapy development.
Supplementary Material
Contributor Information
Ilyssa O Gordon, Department of Pathology, Robert J. Tomsich Pathology and Laboratory Medicine Institute, Cleveland Clinic Foundation, Cleveland, OH, USA.
Suha Abushamma, Department of Gastroenterology, Washington University School of Medicine, Barnes Jewish Hospital, St Louis, MO, USA.
Jacob A Kurowski, Department of Paediatric Gastroenterology, Hepatology and Nutrition, Cleveland Clinic Foundation, Cleveland, OH, USA.
Stefan D Holubar, Department of Colorectal Surgery, Digestive Diseases and Surgery Institute, Cleveland Clinic Foundation, Cleveland, OH, USA.
Lei Kou, Department of Quantitative Health Sciences, Lerner Research Institute, Cleveland Clinic Foundation, Cleveland, OH, USA.
Ruishen Lyu, Department of Quantitative Health Sciences, Lerner Research Institute, Cleveland Clinic Foundation, Cleveland, OH, USA.
Florian Rieder, Department of Gastroenterology, Hepatology and Nutrition, Digestive Diseases and Surgery Institute, and Department of Inflammation and Immunity, Lerner Research Institute, Cleveland Clinic Foundation, Cleveland, OH, USA.
Funding
This work was supported by the Helmsley Charitable Trust through the Stenosis Therapy and Anti-Fibrotic Research [STAR] Consortium [No. 3081 to FR], the Crohn’s and Colitis Foundation [No. 569125 to FR], the National Institute of Health [NIDDK K08DK110415 and R01DK123233 to FR], and Cleveland Clinic Intramural Funding to IOG.
Conflict of Interest
IOG receives no direct funds, but the Cleveland Clinic receives funds on her behalf from UCB, Celgene, and GB004. SDH receives fees as a consultant for Shionogi, Takeda, and Guidepoint. FR discloses Consulting or AdBoard for Adnovate, Agomab, Allergan, AbbVie, Arena, Boehringer-Ingelheim, Celgene/BMS, CDISC, Cowen, Galmed, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Index Pharma, Jannsen, Koutif, Mestag, Metacrine, Morphic, Organovo, Origo, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Surmodics, Surrozen, Takeda, Techlab, Theravance, Thetis, UCB, Ysios, 89Bio, and funding from NIH, Helmsley Charitable Trust, Crohn’s and Colitis Foundation, UCB, Pliant, BMS, AbbVie, Pfizer, Boehringer Ingelheim, Morphic, Kenneth Rainin Foundation.
Author Contributions
IOG: study concept and design, data acquisition, analysis, and interpretation, drafting and revising the article, final approval of the manuscript. SA: data acquisition, analysis, and interpretation, drafting and revising the article, final approval of the manuscript. JAK: data analysis and interpretation, drafting and revising the article, final approval of the manuscript. SDH: data acquisition, revising the article, final approval of the manuscript. LK: data analysis, drafting the article, final approval of the manuscript. RL: data analysis and interpretation, drafting and revising the article, final approval of the manuscript. FR: study concept and design, data acquisition, analysis, and interpretation, drafting and revising the article, final approval of the manuscript.
Partial data from this paper were presented in a poster session at Digestive Disease Week 2019, in San Diego [Gastroenterology 2019;156:S446].
References
- 1. Griffiths AM. Specificities of inflammatory bowel disease in childhood. Best Pract Res Clin Gastroenterol 2004;18:509–23. [DOI] [PubMed] [Google Scholar]
- 2. Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis 2011;17:423–39. [DOI] [PubMed] [Google Scholar]
- 3. Ye Y, Manne S, Treem WR, Bennett D. Prevalence of inflammatory bowel disease in pediatric and adult populations: recent estimates from large national databases in the United States, 2007-2016. Inflamm Bowel Dis 2020;26:619–25. [DOI] [PubMed] [Google Scholar]
- 4. Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008;135:1114–22. [DOI] [PubMed] [Google Scholar]
- 5. Rinawi F, Assa A, Eliakim R, et al. Risk of colectomy in patients with paediatric-onset ulcerative colitis. J Paediatr Gastroenterol Nutr 2017;65:410–5. [DOI] [PubMed] [Google Scholar]
- 6. Wong SC, Macrae VE, McGrogan P, Ahmed SF. The role of pro-inflammatory cytokines in inflammatory bowel disease growth retardation. J Paediatr Gastroenterol Nutr 2006;43:144–55. [DOI] [PubMed] [Google Scholar]
- 7. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314–21. [DOI] [PubMed] [Google Scholar]
- 8. Ishige T. Growth failure in paediatric onset inflammatory bowel disease: mechanisms, epidemiology, and management. Transl Pediatr 2019;8:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mouratidou N, Malmborg P, Sachs MC, et al. Adult height in patients with childhood-onset inflammatory bowel disease: a nationwide population-based cohort study. Aliment Pharmacol Ther 2020;51:789–800. [DOI] [PubMed] [Google Scholar]
- 10. Taddio A, Cont G, Da Dalt E, et al. Final adult height in childhood-onset Inflammatory Bowel Disease. Minerva Pediatr 2019. doi: 10.23736/S0026-4946.19.05183-1. [DOI] [PubMed] [Google Scholar]
- 11. Heuschkel R, Salvestrini C, Beattie RM, Hildebrand H, Walters T, Griffiths A. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis 2008;14:839–49. [DOI] [PubMed] [Google Scholar]
- 12. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Latella G, Rieder F. Intestinal fibrosis: ready to be reversed. Curr Opin Gastroenterol 2017;33:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon IO, Agrawal N, Goldblum JR, Fiocchi C, Rieder F. Fibrosis in ulcerative colitis: mechanisms, features, and consequences of a neglected problem. Inflamm Bowel Dis 2014;20:2198–206. [DOI] [PubMed] [Google Scholar]
- 15. Rieder F, Brenmoehl J, Leeb S, Schölmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut 2007;56:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ippolito C, Colucci R, Segnani C, et al. Fibrotic and vascular remodelling of colonic wall in patients with active ulcerative colitis. J Crohns Colitis 2016;10:1194–204. [DOI] [PubMed] [Google Scholar]
- 17. Bettenworth D, Rieder F. Reversibility of stricturing Crohn’s disease – fact or fiction? Inflamm Bowel Dis 2016;22:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Warren S, Sommers SC. Pathogenesis of ulcerative colitis. Am J Pathol 1949;25:657–79. [PMC free article] [PubMed] [Google Scholar]
- 19. Mitomi H, Okayasu I, Bronner MP, et al. Comparative histologic assessment of proctocolectomy specimens from Japanese and American patients with ulcerative colitis with or without dysplasia. Int J Surg Pathol 2005;13:259–65. [DOI] [PubMed] [Google Scholar]
- 20. de Bruyn JR, Meijer SL, Wildenberg ME, Bemelman WA, van den Brink GR, D’Haens GR. Development of fibrosis in acute and long-standing ulcerative colitis. J Crohns Colitis 2015;9:966–72. [DOI] [PubMed] [Google Scholar]
- 21. Gordon IO, Agrawal N, Willis E, et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment Pharmacol Ther 2018;47:922–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bousvaros A, Antonioli DA, Colletti RB, et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr 2007;44:653–74. [DOI] [PubMed] [Google Scholar]
- 23. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 2002;11:1–190. [PubMed] [Google Scholar]
- 24. Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000;47:404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shi HY, Chan FKL, Chan AWH, et al. Accuracy of faecal immunochemical test to predict endoscopic and histological healing in ulcerative colitis: a prospective study based on validated histological scores. J Crohns Colitis 2017;11:1071–7. [DOI] [PubMed] [Google Scholar]
- 26. Zenlea T, Yee EU, Rosenberg L, et al. Histology grade is independently associated with relapse risk in patients with ulcerative colitis in clinical remission: a prospective study. Am J Gastroenterol 2016;111:685–90. [DOI] [PubMed] [Google Scholar]
- 27. Odze R, Goldblum JR.. Odze and Goldblum Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas. 3rd edn. New York, NY: Elsevier; 2014: 1632. [Google Scholar]
- 28. Magro F, Gionchetti P, Eliakim R, et al. ; European Crohn’s and Colitis Organisation [ECCO]. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017;11:649–70. [DOI] [PubMed] [Google Scholar]
- 29. Zhang X, Ko H-BN, Cai Z, et al. Fibromuscular strictures in ileal Crohn’s disease: a detailed morphometric and histopathologic analysis. Mod Pathol 2017;30:209A–10A. [Google Scholar]
- 30. Bettenworth D, Rieder F. Medical therapy of stricturing Crohn’s disease: what the gut can learn from other organs – a systematic review. Fibrogenesis Tissue Repair 2014;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruemmele FM, Turner D. Differences in the management of paediatric and adult onset ulcerative colitis—lessons from the joint ECCO and ESPGHAN consensus guidelines for the management of paediatric ulcerative colitis. J Crohns Colitis 2014;8:1–4. [DOI] [PubMed] [Google Scholar]
- 32. Hyams JS, Davis S, Mack DR, et al. Factors associated with early outcomes following standardised therapy in children with ulcerative colitis [PROTECT]: a multicentre inception cohort study. Lancet Gastroenterol Hepatol 2017;2:855–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haberman Y, Minar P, Karns R, et al. Mucosal inflammatory and wound healing gene programmes reveal targets for stricturing behaviour in paediatric Crohn’s disease. J Crohns Colitis 2020;15:273–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ta AD, Ollberding NJ, Karns R, et al. Association of baseline luminal narrowing with ileal microbial shifts and gene expression programs and subsequent transmural healing in pediatric Crohn disease. Inflamm Bowel Dis 2021;27:1707–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet 2017;389:1710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ballengee CR, Stidham RW, Liu C, et al. Association between plasma level of collagen type III alpha 1 chain and development of strictures in paediatric patients with Crohn’s disease. Clin Gastroenterol Hepatol 2019;17:1799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu J, Lubman DM, Kugathasan S, et al. Serum protein biomarkers of fibrosis aid in risk stratification of future stricturing complications in pediatric Crohn’s disease. Am J Gastroenterol 2019;114:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quencer KB, Nimkin K, Mino-Kenudson M, Gee MS. Detecting active inflammation and fibrosis in paediatric Crohn’s disease: prospective evaluation of MR-E and CT-E. Abdom Imaging 2013;38:705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kovanlikaya A, Beneck D, Rose M, et al. Quantitative apparent diffusion coefficient [ADC] values as an imaging biomarker for fibrosis in paediatric Crohn’s disease: preliminary experience. Abdom Imaging 2015;40:1068–74. [DOI] [PubMed] [Google Scholar]
- 40. Najarian RM, Ashworth LA, Wang HH, Bousvaros A, Goldsmith JD. Microscopic/“backwash” ileitis and its association with colonic disease in new onset pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr 2019;68:835–40. [DOI] [PubMed] [Google Scholar]
- 41. Martinelli M, Giugliano FP, Russo M, et al. The changing face of pediatric ulcerative colitis: a population-based cohort study. J Pediatr Gastroenterol Nutr 2018;66:903–8. [DOI] [PubMed] [Google Scholar]
- 42. Ihekweazu FD, Fofanova T, Palacios R, et al. Progression to colectomy in the era of biologics: a single center experience with paediatric ulcerative colitis. J Paediatr Surg 2020;55:1815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Conrad MA, Stein RE, Maxwell EC, et al. Vedolizumab therapy in severe pediatric inflammatory bowel disease. Inflamm Bowel Dis 2016;22:2425–31. [DOI] [PubMed] [Google Scholar]
- 44. Olén O, Askling J, Sachs MC, et al. Childhood onset inflammatory bowel disease and risk of cancer: a Swedish nationwide cohort study 1964-2014. BMJ 2017;358:j3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abraham BP, Mehta S, El-Serag HB. Natural history of paediatric-onset inflammatory bowel disease: a systematic review. J Clin Gastroenterol 2012;46:581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamagata M, Mikami T, Tsuruta T, et al. Submucosal fibrosis and basic-fibroblast growth factor-positive neutrophils correlate with colonic stenosis in cases of ulcerative colitis. Digestion 2011;84:12–21. [DOI] [PubMed] [Google Scholar]
- 47. Latella G, Di Gregorio J, Flati V, Rieder F, Lawrance IC. Mechanisms of initiation and progression of intestinal fibrosis in IBD. Scand J Gastroenterol 2015;50:53–65. [DOI] [PubMed] [Google Scholar]
- 48. Latella G. Redox imbalance in intestinal fibrosis: beware of the TGFβ-1, ROS, and Nrf2 connection. Dig Dis Sci 2018;63:312–20. [DOI] [PubMed] [Google Scholar]
- 49. Fichtner-Feigl S, Young CA, Kitani A, Geissler EK, Schlitt HJ, Strober W. IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology 2008;135:2003–13, 2013.e1–7. [DOI] [PubMed] [Google Scholar]
- 50. Scheibe K, Kersten C, Schmied A, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology 2019;156:1082–97.e11. [DOI] [PubMed] [Google Scholar]
- 51. Latella G, Rogler G, Bamias G, et al. Results of the 4th scientific workshop of the ECCO [I]: pathophysiology of intestinal fibrosis in IBD. J Crohns Colitis 2014;8:1147–65. [DOI] [PubMed] [Google Scholar]
- 52. Magro F, António T. Fibrosis in ulcerative colitis. In: Rieder F, editor, Fibrostenotic Inflammatory Bowel Disease. New York, NY: Springer International Publishing;2018: 147–57. [Google Scholar]
- 53. Zhang X, Ko HM, Torres J, et al. Luminally polarized mural and vascular remodeling in ileal strictures of Crohn’s disease. Hum Pathol 2018;79:42–9. [DOI] [PubMed] [Google Scholar]
- 54. Chen W, Lu C, Hirota C, Iacucci M, Ghosh S, Gui X. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn’s fibrostenosing bowel strictures: a semiquantitative analysis by using a novel histological grading scheme. J Crohns Colitis 2017;11:92–104. [DOI] [PubMed] [Google Scholar]
- 55. Koukoulis G, Ke Y, Henley JD, et al. Obliterative muscularization of the small bowel submucosa in Crohn disease: a possible mechanism of small bowel obstruction. Arch Pathol Lab Med 2001;125:1331–4. [DOI] [PubMed] [Google Scholar]
- 56. Stenke E, Bourke B, Knaus U. Crohn’s strictures: moving away from the knife. Front Pediatr 2017;5:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zittan E, Muir J, Milgrom R, et al. Preoperative exposure to anti-tumor necrosis factor therapy in ulcerative colitis patients undergoing ileal pouch-anal anastomosis [IPAA] is not associated with histological fibrosis: a case control study. Int J Surg 2019;65:80–5. [DOI] [PubMed] [Google Scholar]
- 58. Schaeffer DF, Walsh JC, Kirsch R, Waterman M, Silverberg MS, Riddell RH. Distinctive histopathologic phenotype in resection specimens from patients with Crohn’s disease receiving anti-TNF-α therapy. Hum Pathol 2014;45:1928–35. [DOI] [PubMed] [Google Scholar]
- 59. Turunen P, Ashorn M, Auvinen A, Iltanen S, Huhtala H, Kolho KL. Long-term health outcomes in pediatric inflammatory bowel disease: a population-based study. Inflamm Bowel Dis 2009;15:56–62. [DOI] [PubMed] [Google Scholar]
- 60. Gupta N, Liu C, King E, et al. ; ImproveCareNow Network. Continued statural growth in older adolescents and young adults with Crohn’s disease and ulcerative colitis beyond the time of expected growth plate closure. Inflamm Bowel Dis 2020;26:1880–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.