Abstract
Tick-borne pathogens (TBPs) include a wide range of bacteria, parasites and viruses that cause a large spectrum of animal, human and zoonotic tick-borne diseases (TBDs). The object of this review was to establish an inventory and an analysis of TBPs found in domestic animals in the countries of the Mediterranean Basin. This geographic area occupies a central position between several continents and is an area of movement for animals, humans and pathogens of interest and their vectors, which is important in terms of animal and human health. In this systematic review, we included a total of 271 publications produced between 2000–2021 concerning TBPs in domestic animals. Among this literature, we found a total of 90 pathogen species (known as TBPs) reported in the 20 countries of the area; these were detected in tick species from domestic animals and were also directly detected in domestic animals. In all, 31 tick species were recorded and 12 domestic animal species, the latter comprising nine livestock and three pet species. More than 50% of the publications were from Western Europe. Island data were extracted and assessed, as islands of the Mediterranean Basin were represented in 16% of the publications and 77.8% of the TBPs reported. Our results show the importance of islands in the monitoring of TBPs, despite the low percentage of publications.
Keywords: TBPs, pathogens, ticks, domestic animals, Mediterranean Basin, islands
1. Introduction
Pathogens are one of the largest threats to health worldwide. They can be divided into four main groups: bacteria, parasites, viruses and fungi. These groups are present on all the continents and in the oceans, and target all types of plants and animals as well as humans, depending on their tropism and life cycle [1]. These pathogens have different transmission strategies: (i) direct contact between individuals from the same species or between individuals from different species; (ii) indirect contact through the environment or dissemination in the air; and (iii) through vectors such as hematophagous arthropods, such as mosquitoes, ticks or sandflies that can transmits pathogens via blood-sucking from one host to another [2,3].
Among the different pathogens, many are zoonotic and are transmitted between animals and humans, while others are non-zoonotic and specifically target either humans or animals. Zoonotic pathogens account for about 60% of pathogens worldwide and are particularly present in emerging diseases [4]. Transmission of these pathogens by hematophagous insects has become one of the main threats to global health in recent decades [4].
Hematophagous arthropod vectors can transmit pathogens called vector-borne pathogens (VBPs), which may be zoonotic or non-zoonotic, from one individual to another. They can transmit bacteria, parasites and viruses [5]. For example, the role of ticks in the circulation of Lyme disease between animal and human populations in North America has been established [6]. Annually, vector-borne diseases (VBDs) cause more than 17% of infections and over 700,000 deaths across the globe [7]. The two predominant vectors, in order of importance, are mosquitoes and ticks. However, ticks also contain bacterial endosymbionts, such as Rickettsia-like, Francisella-like or Coxiella-like organisms. These are endosymbiotic intracellular bacteria that are harmless to mammals and may be required for tick survival [8]. In previous research, they may have been misidentified as pathogens harmful to human and animals.
Ticks (Ixodida) rank first for veterinary vector-borne pathogens and second for human pathogens, just after mosquitoes [1,9]. Ticks are hematophagous arthropods of the Ixodida order. They are composed of three families distributed across the world. The Ixodidae family is divided into the Prostriasta (subfamily Ixodinae, genus Ixodes) and Metastriata (all other subfamilies, genera: Anamalohimalaya, Cosmiomma, Dermacentor, Hyalomma, Margaropus, Nosomma, Rhipicentor and Rhipicephalus). These are also named “hard ticks”, and the family includes most species. The Argasidae family is divided into two subfamilies, the Argasinae (genus Argas) and the Ornithodorinae (genera: Ornithodoros, Otobius and Carios), also named “soft ticks”. The Nuttallielliedae family is composed of just one species [10]. The tick’s life cycle is divided into four stages: egg, six-legged larva, eight-legged nymph without sexual organs, and eight-legged adult male or female. Tick tropism depends on the species and stage. They can be monotropic, ditropic or teleotropic, with one, two or several hosts, respectively. They can transmit pathogens by feeding on a broad spectrum of terrestrial species, including wild animals (mammalian, avifauna, reptilian and amphibian), livestock and pets. These pathogens are named tick-borne pathogens (TBPs) and are known to be both pathogens of veterinary importance and zoonotic pathogens harmful for humans [1].
Ticks can transmit a wide range of bacteria, parasites and viruses [1]. An example is Lyme disease in Europe. This zoonotic disease is caused by spirochetes bacteria from the group Borrelia burgdorferi sensu lato and the genus Borrelia, and could be transmitted by a wide range of ticks [11]. In Europe, this includes Borrelia burgdorferi sensu stricto, Borrelia afzelii and Borrelia garinii, with approximately 65,500 cases every year [6]. One reported tick-borne virus is the Crimean-Congo Hemorrhagic Fever virus from the genus Orthonairovirus, which is responsible for many outbreaks internationally, with a high fatality rate of 40% [12]. It is mainly transmitted by ticks of the genus Hyalomma, which act as both vector and reservoir [13]. Regarding parasites, causative pathogens such as the Babesia genus are transmitted by ticks and can threaten human and veterinary health [14].
Given its geographic position between Europe and Africa, the Mediterranean Sea is bordered by a significant number of countries with a high variation of biotopes, ranging from Mediterranean to arid climates. The Mediterranean Basin is an area highly affected by climatic change, animal migration and human activity [15,16]. While increasing temperatures in Northern Europe favor the prevalence of ticks such as Ixodes ricinus, in the Mediterranean Basin the development of dry areas with arid types of vegetation favors the proliferation of ticks such as Hyalomma marginatum [17]. This development is also supported by the feeding of immature-stage ticks on birds and the long duration of their attachment to hosts during these stages [18]. This supports the theory of the key role of migratory birds in tick dissemination [19]. Other migration, such as the movement of dogs without restriction in the Mediterranean Basin, also favors the circulation of Rhipicephalus sanguineus s.l. [20].
We focused our review on the countries of the Mediterranean Basin where the different TBPs detected in domestic animals and their ticks were screened. Moreover, in order to compare the geographical distribution of these TBPs, and to highlight possible changes in their spread in the future, four areas were considered: Western Europe, composed of France, Italy, Malta, Monaco and Spain; the Balkans, composed of Albania, Bosnia-Herzegovina, Croatia, Greece, Montenegro and Slovenia; the Middle East, composed of Cyprus, Israel, Lebanon, Palestine, Syria, and Turkey; and finally North Africa, composed of Algeria, Egypt, Libya, Morocco and Tunisia. The different areas were determined by common biotope and geographic proximity. The last part of our review was to investigate the potential role of the western and eastern islands in the monitoring of TBPs in domestic animals and their ticks, according to their geographic position, surface areas and potential role in TBP circulation through animal migration. In this part, we focused on the distribution of TBPs in the Mediterranean islands in order to determine a potential role of the islands in the distribution of TBPs.
The aim of this study was to review, according to PRISMA guidelines, the literature published between 2000 and early 2021 addressing the presence of TBPs on domestic animals and their ticks in the countries of the Mediterranean Basin, with the following objectives:
Perform a bibliometric analysis of TBP studies.
Review the diversity of TBPs, positive engorged tick species, domestic animal hosts of TBPs and positive tick species.
Compare the distribution of TBPs from domestic animals and their ticks in the four main areas defined.
Focus on the distribution of TBPs in the Mediterranean islands.
2. Materials and Methods
We undertook a literature review concerning tick-borne pathogens in all countries of the Mediterranean Basin (n = 20). We followed PRISMA guidelines and used explicit and systematic methods to identify, select and evaluate the studies relevant to the topic [20]. We compiled and evaluated the data from the studies included in this review.
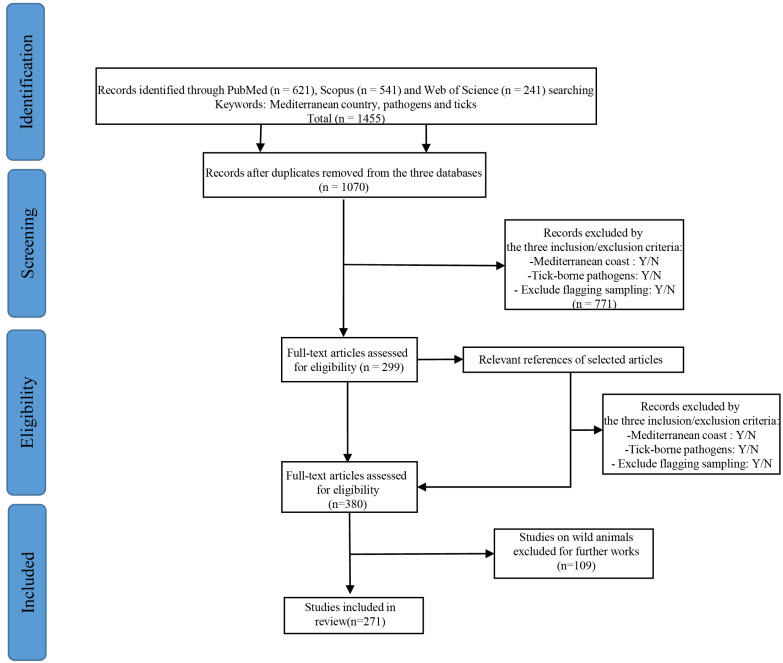
All articles published in English in international journals indexed by PubMed, Scopus and Web of Science were considered (Figure 1). The date range was from 1 January 2000 through 31 February 2021. We used keywords for each country of the Mediterranean Basin, classified in alphabetical order: Albania OR Algeria OR Bosnia-Herzegovina OR Cyprus OR Croatia OR Egypt OR France OR Greece OR Israel OR Italy OR Libya OR Malta OR Monaco OR Montenegro OR Morocco OR Palestine OR Slovenia OR Spain OR Tunisia OR Turkey AND Pathogens AND Ticks, with the option “all fields” to recover articles in which search items appeared in the title, abstract and keywords. First, all papers considered to be “grey” literature, such as literature reviews, case studies, manuscripts and abstracts of posters from conferences and guides from relevant organizations, were excluded. We also discarded clinical descriptions of disease and diagnosis in humans and animals. We selected all papers focusing on the distribution and circulation of tick-borne pathogens and their vectors. We focused on studies dealing with the distribution of these pathogens and ticks in the countries of the Mediterranean Basin and their islands. Second, we excluded duplicate and inaccessible articles (due to language or unavailable full text). We reviewed the titles and abstracts and applied inclusion/exclusion criteria on 1070 articles. Filtering was carried out by responding to the following questions:
Did the study include a country with a Mediterranean coast: Yes/No
Did the study include tick-borne pathogens: Yes/No
Did the study exclude ticks collected in vegetation: Yes/No
Figure 1.
Methodological diagram of the bibliographic research following the PRISMA 2009 Flow according to Moher et al., 2015 [21].
Only publications considering TBPs in domestic animals and/or TBPs in engorged ticks collected on domestic animals in Mediterranean countries were included. This made it possible to conduct an overview of research about TBPs potentially infesting and circulating in the domestic animal population only. The articles were saved if the answers to the three questions were “yes”; otherwise they were eliminated. For the next step, we reviewed the full-text and entered the information of interest into a database for 299 articles. We also excluded articles that we found did not fit the criteria after reading the full text.
We reviewed the bibliography of each selected article in order to check for new articles to include in the review and relevant articles in the field of research. We followed the same steps as previously described for new articles (n = 81).
For the last step, we excluded articles dealing with wild animals (n = 109) and retained only articles dealing with domestic animals (n = 271). The selection steps are summarized in Figure 1, with an explanation of the inclusion/exclusion of articles for this review.
The data of interest were captured in an Excel table that was tested in advance with 15 articles, and included the following information:
Main characteristics of the studies: article ID, years, authors, analytical and statistical methodology
Pathogen-related information: pathogens screened and detected, species, number of species, zoonotic status, host
Tick-related information: species, type, number, stage
Host-related information: groups, sedentary or migratory
Area of interest: country, type of area, number of sampling sites
The different outputs of the data worksheet were selected following mutual agreement from all the authors.
3. Results
3.1. Bibliographic Analysis
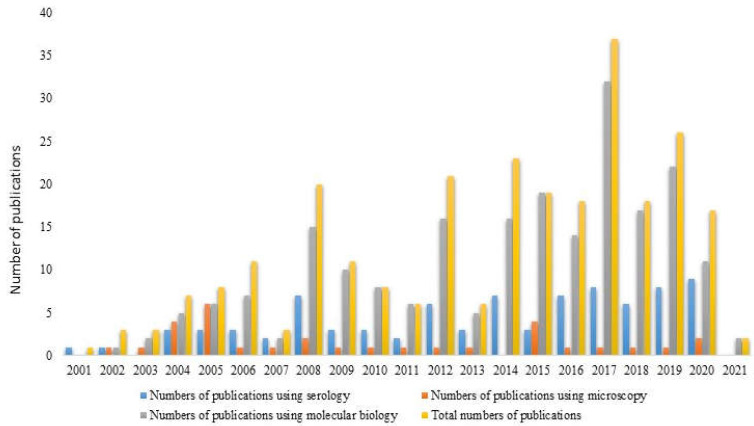
The distribution of the publications through the years is shown in Figure 2. During the first decade, the number of papers increased slightly until 2008, with a maximum of 20 publications, and decreased in the years 2009 and 2010 (11 and nine papers, respectively). During the second decade, there was first a low rate of publication, except in 2012, with a peak of 21 publications. Between 2014 and 2021, the mean number of published papers per year was approximatively 20, accounting for 60% of the published papers about tick-borne pathogens on domestic animals, with a peak of 37 papers in 2017. In 2021, the number of papers was low because we stopped the research in February 2021. The rate of publication of research on tick-borne pathogens increased notably during this period.
Figure 2.
Number of publications through the years (2000–February 2021). The total number is indicated in yellow, and the other colors indicate the number of publications according to the detection method used (serology; microscopy; molecular biology).
This trend can be explained by different factors:
First, there is increased interest from the scientific community due to the importance of this subject in terms of human and animal health.
Second, there is improved accessibility of scientific journals and publications.
Third, there have been changes in pathogen detection methodology through the years, and particularly developments in the field of molecular biology.
Concerning this last point, tick-borne pathogens were typically detected using three kinds of methods (Figure 2). The first category of method was serological analysis, involving detection of pathogens in blood and tissue samples by way of antibodies; such methods include ELISA and immunofluorescence. The second category was the microscopy approach, which is more common for parasites. The third category was molecular biology, which covered techniques involving the detection of pathogen nucleic acids in both tick and animal hosts, such as polymerase chain reaction (PCR), next-generation sequencing (NGS), and high-throughput sequencing techniques. Even though researchers may use more than one approach to detect and monitor TBPs, we observed that over the years, molecular biology rapidly became the main analytical approach. The increase in publications over the last ten years seems to be linked to the development and accessibility of molecular biology techniques, and to their ability to simultaneously detect a large number of pathogens.
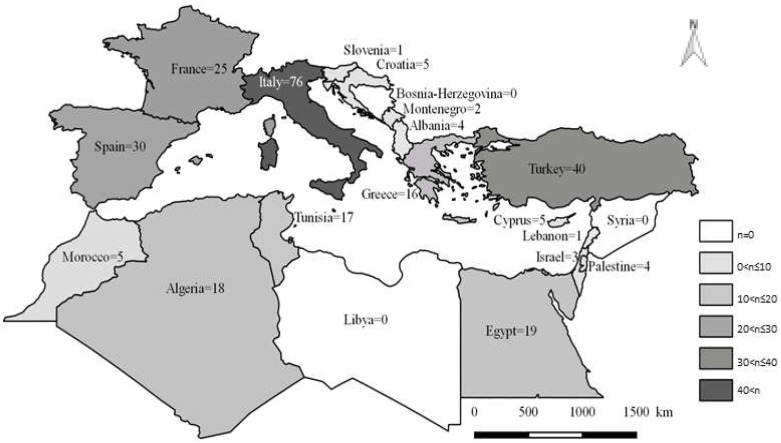
In addition to changes in the amount of data available depending on the year, we can also note variability of these data according to their geographic origin. In total, 271 publications and 20 countries of the Mediterranean Basin were considered in this review. The mean number of publications per country was about 13, and the number of publications varied from 0 to 76, depending on the country (Figure 3).
Figure 3.
Number of publications dealing with TBPs in domestic animals and their ticks in the Mediterranean Basin according to country of origin.
3.2. Tick-Borne Pathogens in Countries of the Mediterranean Basin
A total of 271 publications were analyzed: 56.5% were about bacteria, 37.7% were about parasites and 5.80% were about viruses. A total of 90 pathogens from 18 genera were detected in domestic animals and their ticks from the Mediterranean Basin: 11 genera of bacteria, four genera of parasites, and three genera of viruses (Table 1). Among these 90 TBPs, 60% were zoonotic and 40% were non-zoonotic or of unknown status (Table 1). Among the 90 TBPs detected, 73.3% were found in ticks and 76.7% were found in animal hosts. Of the TBPs reported, 50% were detected in both (51.1%).
Table 1.
Tick-borne pathogens detected in domestic animals or engorged ticks found on domestic animals in the Mediterranean Basin.
| Pathogen | Zoonotic (Yes/No) | Engorged Positive Ticks Collected from Hosts | Positive Tick Hosts | Positive Pathogen Hosts | Countries | References |
|---|---|---|---|---|---|---|
| Parasites: Nematoda | ||||||
| Cercopithifilaria | ||||||
| Cercopithifilaria bainae | No | Rhipicephalus sanguineus s.l. | Dog | Dog | Greece, Italy | [22,23] |
| Parasites: Apicomplexa | ||||||
| Babesia | ||||||
| Babesia spp. | Hyalomma excavatum, Hyalomma marginatum, Rhipicephalus sanguineus s.l. | Cattle | Cattle, Dog, Donkey, Goats, Pigs, Sheep | Egypt, Italy, Morocco, Turkey | [24,25,26,27,28,29,30,31,32,33] | |
| Babesia bigemina | No |
Hyalomma marginatum, Rhipicephalus (B) annulatus, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. |
Cattle, Dog, Sheep | Cattle, Pig, Sheep | Algeria, Egypt, Italy, Turkey | [29,32,33,34,35,36,37,38,39,40,41,42] |
| Babesia bovis | Yes | Dermacentor marginatus, Hyalomma marginatum, Ixodes ricinus, Rhipicephalus sanguineus s.l. | Cattle, Goat, Sheep | Buffalo, Cattle | Algeria, Egypt, Italy, Turkey | [29,30,32,34,35,39,40,41,43,44] |
| Babesia caballi | No | Rhipicephalus (B) annulatus | Cattle | Dog, Donkey, Horse | Croatia, France, Italy, | [32,42,45,46,47] |
| Babesia canis | No | Dermacentor reticulatus, Ixodes hexagonus, Rhipicephalus sanguineus s.l. | Dog | Dog | Croatia, France, Italy, Spain, Turkey | [27,32,37,41,45,48,49,50,51,52,53,54,55,56,57,58,59,60] |
| Babesia capreoli | No | Ixodes hexagonus, Ixodes ricinus, Rhipicephalus sanguineus s.l. | Dog | data not found | Italy | [60] |
| Babesia divergens | Yes | Ixodes ricinus, Rhipicephalus sanguineus s.l. | Dog | Cattle | France, Italy | [60,61,62] |
| Babesia equi | No | data not found | data not found | Horse | Italy | [63] |
| Babesia gibsoni | No | Dermacentor reticulatus, Ixodes hexagonus, Ixodes ricinus, Rhipicephalus sanguineus s.l. | Dog | Dog | Croatia, Italy, Spain | [45,48,51,59] |
| Babesia major | No | data not found | data not found | Cattle | France, Turkey | [36,50] |
| Babesia microti | Yes | Dermacentor marginatus, Ixodes hexagonus, Ixodes ricinus, Rhipicephalus sanguineus s.l. | Dog, Goat, Sheep | Cat, Dog | Italy, Turkey | [32,37,41,60,64] |
| Babesia motasi | No | data not found | data not found | Sheep | Spain | [65] |
| Babesia occultans | No | data not found | data not found | Cattle | Egypt | [35] |
| Babesia ovis | No | Dermacentor marginatus, Haemaphysalis concinna, Haemaphysalis parva, Ixodes ricinus, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. | Dog, Goat, Sheep | Cattle, Goat, Sheep | Algeria, Italy, Palestine, Spain, Turkey | [25,33,36,37,41,65,66,67,68] |
| Babesia venatorum | Yes | Ixodes hexagonus, Ixodes ricinus, Rhipicephalus sanguineus s.l. | Dog | data not found | Italy | [60] |
| Babesia vogeli | No | Dermacentor marginatus, Ixodes ricinus, Rhipicephalus sanguineus s.l. | Cat, Dog, Goat, Sheep | Dog | Croatia, Cyprus, France, Italy, Palestine, Spain, Turkey | [27,41,45,49,51,56,57,59,67,69,70,71,72,73] |
| Hepatozoon | ||||||
| Hepatozoon spp. | Rhipicephalus sanguineus s.l. | Dog | Cat, Dog | Croatia, Cyprus, Italy, Spain, Turkey | [22,23,27,51,52,67,69,72,74,75,76,77] | |
| Hepatozoon canis | No | Haemaphysalis parva, Ixodes hexagonus, Ixodes ricinus, Rhipicephalus sanguineus s.l. | Dog | Cat, Dog | Croatia, Cyprus, France, Greece, Italy, Palestine, Spain, Turkey | [22,23,27,50,51,52,67,69,72,75,77,78,79] |
| Hepatozoon felis | No | Haemaphysalis concinna, Rhipicephalus sanguineus s.l. | Dog | Cat | Greece, Italy, Turkey | [22,75,80] |
| Theileria | ||||||
| Theileria spp. | Hyalomma anatolicum, Hyalomma dromedarii, Rhipicephalus sanguineus s.l. | Cattle, Dromedary | Cat, Cattle, Dogs, Donkey, Goat, Pigs, Sheep | Algeria, Egypt, Italy, Spain, Turkey | [26,30,32,51,65,68,81,82,83,84,85] | |
| Theileria annae | No | Dermacentor reticulatus, Ixodes hexagonus, Ixodes ricinus, Rhipicephalus sanguineus s.l. | Dog | Cattle, Dog, Donkey, Horse | Croatia, France, Italy, Spain | [29,45,47,48,56,86] |
| Theileria annulata | No | Hyalomma anatolicum, Hyalomma detritum, Hyalomma dromedarii, Hyalomma excavatum, Rhipicephalus (B) annulatus | Cattle, Dromedary, Sheep | Buffalo, Cattle, Dromedary, Donkey, Goat, Pig, Sheep | Algeria, Egypt, Italy, Spain, Tunisia, Turkey | [28,32,33,34,35,36,37,40,44,83,84,86,87,88,89,90,91,92,93,94,95] |
| Theileria buffeli | No |
Dermacentor marginatus, Haemaphysalis punctata, Hyalomma marginatum, Ixodes hexagonus, Ixodes ricinus, Rhipicephalus (B) annulatus, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. |
Cat, Cattle, Dog, Goat | Cattle, Horse | Algeria, France, Italy, Spain, Turkey | [36,38,42,44,50,60,86] |
| Theileria cervi | No | Ixodes ricinus, Rhipicephalus sanguineus s.l. | Dog | Data not found | Italy | [60] |
| Theileria equi | No | Hyalomma marginatum, Ixodes ricinus, Rhipicephalus (B) annulatus, Rhipicephalus sanguineus s.l. | Cattle, Dog, Horse | Cattle, Dog, Donkey, Horse | Algeria, Croatia, Italy, Spain | [32,38,41,42,44,45,47,51,63,78,96] |
| Theileria lestoquardi | No | data not found | data not found | Buffalo, Sheep | Egypt, Turkey | [82,89] |
| Theileria luwenshuni | No | data not found | data not found | Goat, Sheep | Turkey | [68] |
| Theileria orientalis | No | Ixodes hexagonus, Ixodes ricinus, Rhipicephalus (B) annulatus, Rhipicephalus sanguineus s.l. | Cattle, Dog | Buffalo, Cattle | Algeria, Egypt, Italy | [40,42,60,95] |
| Theileria ovis | No | Ixodes ricinus, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. | Goat, Sheep | Buffalo, Goat, Sheep | Algeria, France, Greece, Palestine, Spain, Turkey | [22,37,44,50,65,66,67,68,81,97] |
| Theileria uilenbergi | No | data not found | data not found | Buffalo, Goat, Sheep | Egypt, Turkey | [68,89] |
| Theileria sergenti | No | Ixodes hexagonus, Ixodes ricinus, Rhipicephalus (B) annulatus, Rhipicephalus sanguineus s.l. | Cattle, Dog | Cattle, Horse | Italy, Spain | [42,47,60,86] |
| Bacteria | ||||||
| Anaplasma | ||||||
| Anaplasma spp. | Argas persicus, Hyalomma excavatum, Hyalomma marginatum, Rhipicephalus spp., Rhipicephalus (B) annulatus, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. | Cattle, Chicken, Dogs, Dromedary, Goats, Horse, Sheep | Cat, Cattle, Dog, Donkey, Dromedary, Goat, Horse, Pig, Sheep | Algeria, France, Greece, Italy, Morocco, Spain, Tunisia, Turkey, Palestine | [24,26,68,98,99,100,101,102,103,104,105,106,107,108,109,110,111] | |
| Anaplasma bovis | No | data not found | data not found | Cattle, Goat, Sheep | Italy, Tunisia | [30,100] |
| Anaplasma centrale | No | Rhipicephalus bursa, Rhipicephalus sanguineus s.l. | Cattle, Dog | Cattle, Goat | Italy, Morocco, Turkey | [24,30,98,100,112,113] |
| Anaplasma marginale | No |
Haemaphysalis punctata, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. |
Cattle, Dog | Buffalo, Cat, Cattle, Dog, Donkey, Goat, Horse, Pig, Sheep | Algeria, Egypt, France, Italy, Morocco, Tunisia, Turkey | [30,32,33,34,35,39,43,44,98,100,103,105,112,113,114,115] |
| Anaplasma ovis | No | Haemaphysalis punctata, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. | Cattle, Dog, Horse, Goat, Sheep | Cattle, Dog, Goat, Horse, Sheep | Algeria, France, Italy, Morocco, Tunisia, Turkey | [44,60,66,68,100,101,105,113,115,116,117,118,119,120,121] |
| Anaplasma phagocytophilum | Yes |
Haemaphysalis sulcata, Hyalomma marginatum, Ixodes hexagonus, Ixodes ricinus, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. |
Cat, Dog, Goat, Horse, Sheep | Cat, Cattle, Dog, Donkey, Goat, Horse, Sheep | Algeria, Croatia, Egypt, France, Greece, Italy, Morocco, Spain, Tunisia, Turkey | [24,32,46,51,53,55,63,68,78,96,100,104,105,112,115,117,120,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141] |
| Anaplasma platys | Yes | Hyalomma spp., Ixodes hexagonus, Ixodes ricinus, Rhipicephalus (B) annulatus, Rhipicephalus sanguineus s.l. | Cattle, Dog, Dromedary, Goat, Horse, Sheep | Buffalo, Cattle, Dog, Dromedary | Algeria, Croatia, Cyprus, Egypt, Greece, Italy, Morocco, Palestine, Spain, Tunisia, Turkey | [22,23,35,44,51,60,69,71,72,101,104,108,111,113,116,123,133,140,142,143,144,145,146,147,148] |
| Bartonella | ||||||
| Bartonella spp. |
Rhipicephalus (B) annulatus, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. |
Cattle, Dog, Goat | Cat, Dog | Italy, Spain | [85,119,139,149,150] | |
| Bartonella clarridgeiae | Yes | data not found | data not found | Cat | Italy | [151] |
| Bartonella henselae | Yes | Ixodes ricinus | Cat, Dog | Cat, Dog | Algeria, Cyprus, Greece, Italy, Spain | [69,99,126,138,140,144,150,151,152,153,154,155,156] |
| Bartonella vinsonii | Yes | data not found | data not found | Cattle, Dog, Goat | Greece, Morocco | [157,158] |
| Bartonella vinsonii berkhoffi | Yes | Rhipicephalus sanguineus s.l. | Dog | Dog | Italy, Spain | [72,78,140] |
| Borrelia | ||||||
| Borrelia spp. | Argas persicus, Hyalomma aegyptium, Rhipicephalus (B) annulatus | Cattle, chicken, Dog, Goat, Horses, Sheep | Cattle | Algeria, Turkey | [44,102,159] | |
| Borrelia afzelii | Yes | Ixodes hexagonus, Ixodes ricinus | Dog | data not found | Italy, Spain | [51,60] |
| Borrelia burgdoferi sensus lato | Yes | Hyalomma marginatum, Ixodes hexagonus, Ixodes ricinus, Rhipicephalus (B) annulatus, Rhipicephalus bursa | Cattle, Dog | Dog, Horse | Croatia, Italy | [53,60,110,128,149] |
| Borrelia garinii | Yes | Ixodes ricinus | Dog | data not found | Spain | [51] |
| Borrelia theileri | No | data not found | data not found | Goat, Sheep | Algeria | [66] |
| Borrelia valaisiana | Yes | Ixodes ricinus | Dog | data not found | Spain | [51] |
| Chlamydia | ||||||
| Chlamydia abortus | Yes | Ixodes ricinus, Rhipicephalus (B) annulatus, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. | Cat, Cattle, Dog, Goat, Sheep | data not found | Italy | [154,160] |
| Chlamydophila | ||||||
| Chlamydophila psittaci | Yes | Ixodes ricinus, Rhipicephalus sanguineus s.l. | Cat, Cattle, Dog, Goat, Sheep | data not found | Italy | [154,160] |
| Coxiella | ||||||
| Coxiella burnetii | Yes | Argas persicus, Dermacentor marginatus, Haemaphysalis punctata, Haemaphysalis sulcata, Hyalomma spp., Hyalomma dromedarii, Hyalomma marginatum, Ixodes ricinus, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. | Cattle, Chicken, Dog, Dromedary, Goat, Rabbit, Sheep | Buffalo, Cat, Cattle, Dromedary, Goat, Horse, Sheep | Algeria, Cyprus, Egypt, Greece, Italy, Montenegro, Slovenia, Spain, Tunisia | [32,66,74,78,119,139,153,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177] |
| Ehrlichia | ||||||
| Ehrlichia spp. | Haemaphysalis parva, Hyalomma spp., Hyalomma excavatum, Hyalomma marginatum, Ixodes hexagonus, Ixodes ricinus, Rhipicephalus (B) annulatus, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. | Buffalo, Cattle, Dog, Sheep | Cattle, Dog, Goat, Sheep | Egypt, France, Italy, Palestine, Spain, Turkey | [26,60,68,85,99,110,111,112,149,170] | |
| Ehrlichia canis | Yes |
Dermacentor marginatus, Haemaphysalis punctata, Haemaphysalis sulcata Hyalomma spp., Hyalomma excavatum, Ixodes hexagonus, Ixodes ricinus, Ixodes ventalloi, Rhipicephalus (B) annulatus, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. |
Buffalo, Cat, Cattle, Dog, Goat, Sheep | Cat, Dog | Algeria, Croatia, Cyprus, Egypt, France, Greece, Italy, Palestine, Spain, Turkey |
[32,53,55,60,72,73,74,78,103,106,107,111,119,123,126,127,129,138,139,143,144,146,153,170,178,179,180,181,182] |
| Ehrlichia equi | Yes | data not found | data not found | Horse | Italy | [32] |
| Ehrlichia ewingii | Yes | data not found | data not found | Dog | Cyprus | [143] |
| Ehrlichia minancensis | No | Hyalomma marginatum, Rhipicephalus bursa | Cattle, Goat | data not found | France | [183] |
| Candidatus | ||||||
| Candidatus Ehrlichia urmitei | No | Rhipicephalus (B) annulatus | Cattle, Goat, Horse, Sheep | data not found | Algeria | [44] |
| Noehrlichia | ||||||
| Noehrlichia mikurensis | Yes | Ixodes ricinus | Cattle | data not found | Spain | [137] |
| Francisella | ||||||
| Francisella spp. | Yes | Hyalomma marginatum, Rhipicephalus (B) annulatus, Rhipicephalus bursa | Cattle | data not found | Italy | [149] |
| Leptopsira | ||||||
| Leptopsira spp. | Yes | data not found | data not found | Buffalo, Cattle, Dromedary, Sheep | Egypt | [184] |
| Mycoplasma | ||||||
| Mycoplasma spp. | data not found | data not found | Goat, Sheep | Morocco | [109] | |
| Mycoplasma haemocanis | No | data not found | data not found | Dog | Greece, Turkey | [26,52,185,186] |
| Mycoplasma haemofelis | No | data not found | data not found | Cat | Cyprus | [178] |
| Candidatus | ||||||
| Candidatus Mycoplasma haemonutum | No | data not found | data not found | Cat | Cyprus | [178] |
| Candidatus Mycoplasma haematoparvum | No | data not found | data not found | Dog | Greece, Turkey | [26,52,185,186] |
| Candidatus Mycoplasma turicensis | No | data not found | data not found | Cat | Cyprus | [178] |
| Rickettsia | ||||||
| Rickettsia spp. | Dermacentor marginatus, Haemaphysalis sulcata, Hyalomma spp., Hyalomma aegyptium, Hyalomma detritum, Hyalomma dromaderii, Hyalomma impeltatum, Hyalomma marginatum, Ixodes ricinus, Rhipicephalus spp. Rhipicephalus (B) annulatus, Rhipicephalus bursa, Rhipicephalus pusillus, Rhipicephalus sanguineus s.l. | Cat, Cattle, Chicken, Dog, Donkey, Dromedary, Goat, Horse, Sheep | Cat, Dog, Horse | Algeria, Egypt, Israel, Italy, Palestine, Spain, Tunisia, Turkey | [85,99,102,138,139,149,159,170,187,188,189,190,191,192,193] | |
| Rickettsia aeschlimannii | Yes |
Hyalomma dromedarii, Hyalomma impeltatum, Hyalomma marginatum, Hyalomma rufipes, Ixodes ricinus |
Cattle, Dromedary, Goat | Dromedary, Horse | Egypt, France, Israel, Spain, Tunisia | [170,175,193,194,195,196,197,198] |
| Rickettsia africae | Yes | Amblyomma variegatum, Hyalomma spp., Hyalomma anatolicum, Hyalomma dromedarii, Hyalomma excavatum, Hyalomma impeltatum, Hyalomma marginatum, Hyalomma turanicum | Cattle, Donkey, Dromedary, Goat, Sheep | Dromedary | Egypt, France, Israel, Italy, Lebanon, Palestine, Tunisia, Turkey | [189,190,194,197,199,200,201] |
| Rickettsia conorii | Yes | Hyalomma marginatum, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. | Cattle, Dog, Donkey, Goat, Sheep | Cat, Dog | Greece, Italy, Spain, Tunisia | [106,119,138,140,141,173,179,182,189,191,202,203,204,205,206] |
| Rickettsia conorii israelensis | Yes | Rhipicephalus sanguineus s.l. | Dog, Goat | data not found | Italy | [119,207] |
| Rickettsia felis | Yes | Rhipicephalus sanguineus s.l. | Cat, Dog, Sheep | data not found | Italy, Spain | [208,209] |
| Rickettsia helvetica | Yes | Hyalomma impeltatum, Ixodes ricinus, Ixodes ventalloi | Cat, Cattle, Dromedary | Dromedary | Algeria, Italy, Tunisia | [73,154,201,210] |
| Rickettsia hoogstraalii | Unknown | Haemaphysalis parva, Haemaphysalis sulcata | Dog, Sheep | data not found | Greece, Italy | [22,119] |
| Rickettsia massiliae | Yes |
Haemaphysalis adleri, Haemaphysalis parva, Rhipicephalus (B) annulatus, Rhipicephalus bursa Rhipicephalus pusillus, Rhipicephalus sanguineus s.l. |
Cattle, Dog, Goat, Horse, Sheep | Cat, Dog, Dromedary | Algeria, Cyprus, France, Greece, Israel, Italy, Lebanon, Palestine, Spain, Tunisia | [22,44,51,58,137,153,173,190,191,192,193,195,201,205,207,209,210,211,212,213,214,215,216,217] |
| Rickettsia monacensis | Yes | Hyalomma impeltatum, Ixodes ricinus, Rhipicephalus sanguineus s.l. | Cat, Cattle, Dog, Dromedary, Goat | Dromedary | Algeria, France, Greece, Italy, Spain, Tunisia | [51,137,195,201,210,216,218,219] |
| Rickettsia raoultii | Yes | Dermacentor reticulatus, Ixodes ricinus, Rhipicephalus sanguineus s.l. | Cattle, Dog | data not found | Algeria, Spain, Turkey | [200,210,220] |
| Rickettsia rickettsii | Yes | data not found | data not found | Dog | Italy | [141] |
| Rickettsia rhipicephali | Unkown | Rhipicephalus bursa, Rhipicephalus sanguineus s.l. | Cattle, Dog, Goat | data not found | Greece | [173,205] |
| Rickettsia sibirica mongolotimonae | Yes | Hyalomma excavatum | Goat, Sheep | data not found | Cyprus | [212] |
| Rickettsia slovaca | Yes | Dermacento marginatus, Haemaphysalis punctata | Cattle, Dog, Donkey, Sheep, Pig | Cattle, Goat, Sheep | France, Italy, Spain, Turkey | [189,192,193,195,200,209,221,222] |
| Rickettsia typhi | Yes | data not found | data not found | Cat | Greece | [155] |
| Candidatus | ||||||
| Candidatus Rickettsia goldwasserii | Unknown | Haemaphysalis adleri, Haemaphysalis parva, Rhipicephalus sanguineus s.l. | Dog, Goat, Horse, Sheep | data not found | Palestine | [190] |
| Candidatus Rickettsia barbariae | Unknown | Hyalomma dromedarii, Rhipicephalus (B) annulatus, Rhipicephalus bursa, Rhipicephalus sanguineus s.l. | Cattle, Dog, Goat, Horse, Sheep | data not found | Cyprus, France, Italy, Lebanon, Palestine | [119,190,195,212] |
| Viruses | ||||||
| Capripoxvirus | ||||||
| Lumpy skin disease virus | Yes | Amblyomma spp., Amblyomma hebreaum, Hyalomma truncatum, Rhipicephalus (B) annulatus, Rhipicephalus appendiculatus, Rhipicephalus (B) microplus | Cattle | data not found | Egypt | [223] |
| Flavivirus | ||||||
| Tick-borne encephalitis | Yes | Ixodes ricinus | Goat | Goat | Greece, Italy | [224,225] |
| Orthonairovirus | ||||||
| Crimean-Congo Hemorrhagic Fever | Yes | Dermacentor marginatus, Haemaphysalis parva, Hyalomma anatolicum, Hyalomma dromedarii, Hyalomma excavatum, Hyalomma lusitanicum, Hyalomma marginatum, Ixodes ricinus, Rhipicephalus spp., Rhipicephalus bursa s.l., Rhipicephalus sanguineus s.l. | Buffalo, Cattle, Dromedary, Dog, Goat, Sheep | Buffalo, Cattle, Goat, Sheep | Albania, Greece, Egypt, Spain, Turkey | [184,226,227,228,229,230,231,232,233,234,235] |
3.2.1. Parasites
Nematoda
Cercopithifilaria
Pathogens in the genus Cercopithifilaria are microfilariae parasites that mainly infect wild and domestic animals. Among the three species Cercopithifilaria grassii, Cercopithifilaria spp. sensu and Cercopithifilaria bainae, only the latter was reported in our research [236]. It was detected in two countries: Greece, with a prevalence of 7% from Rh. sanguineus s.l. collected from dogs; and Italy, at 25.86% from dogs [22,23]. This parasite was found in both ticks and animals, but from two distinct publications. The genus was found in only 0.7% of the publications and 1.9% of the publications concerning parasites.
Apicomplexa
Babesia
Babesia is a genus of erythrocytic protozoal parasites transmitted by ticks that cause babesiosis in both animals and humans. The main Babesia species transmitted by ticks are B. divergens, B. duncani, B. microti, B. venatorum. B. vogeli and B. canis (responsible for canine babesiosis). Babesia vogeli was the species found in the largest range of countries among the 15 Babesia species found in domestic animals. Babesia vogeli was found in seven countries (Croatia, Cyprus, France, Italy, Palestine, Spain and Turkey) and was not detected in countries of North Africa. Among these seven countries, the highest prevalence of B. vogeli in animals was 14% from dogs in Italy and 10.5% in Rh. sanguineus s.l. ticks collected from dogs in France [56,59]. The next most commonly found species were B. canis and B. ovis. Babesia canis was found in five countries (Croatia, France, Italy, Spain and Turkey). The highest prevalence was 71.4% from dogs in Italy along with 5.65% from Rh. sanguineus s.l. and Dermacentor reticulatus from dogs in France. Babesia ovis was found in five countries (Algeria, Italy, Palestine, Spain and Turkey). The genus Babesia is one of the most frequently screened or found pathogens, along with Rickettsia and Anaplasma, featuring in 17.7% of the publications. It was the most commonly screened or found of the parasites, featuring in 46.2% of the publications concerning parasites. It showed the highest species diversity, just after the genus Rickettsia.
Hepatozoon
Parasites in the genus Hepatozoon are intracellular protozoa belonging to the phylum Apicomplexa that infect amphibians, birds, mammals and reptiles [237]. Two species were found in domestic animals from countries on the Mediterranean Rim. Hepatozoon canis was found in cats or dogs in eight countries (Croatia, Cyprus, France, Greece, Italy, Palestine, Spain and Turkey). The highest prevalence was 22.3% in dogs along with 20.58% in Rh. sanguineus s.l. from dogs, both occurring in Turkey [80,238]. The second species was Hepatozoon felis, with the highest prevalence found to be 5.1% from cats in Italy and 1.7% from Rh. sanguineus s.l. from dogs in Turkey [75,80]. The genus Hepatozoon was the third most commonly found or screened parasite, featuring in a total of 7% of the overall publications and 18.2% of the publications concerning parasites. Pathogens in the genus Hepatozoon were found only in two pet hosts (cats and dogs).
Theileria
Along with Babesia and Hepatozoon, the genus Theileria belongs to the phylum Apicomplexa, and with Babesia, also to the piroplasmids group. Species in this genus infect mammals and have an obligatory cycle in ticks. They cause benign to fatal theileriosis in breeding animals [239]. A total of 11 species were found in domestic animals from the Mediterranean Basin: T. annae, T. annulata, T. buffeli, T. cervi, T. equi, T. lestoquardi, T. luwenshuni, T. orientalis, T. ovis, T. uilenberg and T. sergenti. The species found in the most countries were T. ovis, T. annulata and T. buffeli. T. ovis was found in six countries (Algeria, France, Greece, Palestine, Spain, and Turkey), as was T. annulata (Algeria, Egypt, Italy, Spain, Tunisia and Turkey), while T. buffeli was found in five countries (Algeria, France, Italy, Spain, and Turkey). The highest prevalence of T. ovis was 53.3% in goats and sheep from Algeria, along with 37.35% in ticks from the genus Rhipicephalus taken from goats and sheep in Algeria [44,66]. T. annulata was found in 64% of cattle from Turkey and in 5% of Rh. (Bo.) annulatus collected from cattle, goats and sheep from Algeria [44,92]. The highest prevalence of T. buffeli was 11.56% from cattle in Turkey along with 2.8% in three tick species, I. hexagonus, I. ricinus and Rh. sanguineus s.l., from cattle in Italy [60,240]. Similarly to Babesia, the genus Theileria has widespread distribution in a large number of countries. It was found in 17.3% of the overall publications and 45.2% of the publications concerning parasites.
3.2.2. Bacteria
Anaplasma
The genus Anaplasma includes intracellular Gram-negative bacteria belonging to the family Anaplasmataceae from the Rickettsiales order. Most of these bacteria are zoonotic and have a high impact on veterinary health; for instance, Anaplasma marginale, Anaplasma bovis and Anaplasma ovis; and also on human health; for instance, Anaplasma phagocytophilum and Anaplasma platys [241,242]. The most common species is A. platys, responsible for canine cyclic thrombocytopenia, which was found in 11 countries out of 20 in the Mediterranean Basin (Algeria, Croatia, Cyprus, Egypt, Greece, Italy, Morocco, Palestine, Spain, Tunisia and Turkey). Six different tick species were found to be positive and to transmit this pathogen, with Rhipicephalus sanguineus s.l. as the main tick species, and with the highest prevalence in Morocco at 6.25%. The most common host was the dog, with a prevalence of 40.8% and 33% positive blood samples in Italy [72,113,148]. The second most common species is A. phagocytophilum, the causative agent of human granulocytic anaplasmosis, found in 10 countries (Algeria, Croatia, Egypt, France, Greece, Italy, Morocco, Spain, Tunisia and Turkey). Out of six tick species found to be positive for this bacterium, Rh. sanguineus s.l. was the main tick species, especially on dogs. The highest prevalence of A. phagocytophilum in Rh. sanguineus s.l. was 13.7% in Egypt. The main pathogen host was cattle, with a prevalence of 40.6% in blood samples in Algeria [104,130].
As seen above, the Anaplasma genus is both highly represented in the Mediterranean Basin and also highly screened, with detection in more than 25.8% of the publications analyzed and in 44.9% of the publications concerning bacteria. This can be explained by the number of countries (12) and hosts affected (11), and shows the public health and veterinary importance of this genus in the countries of the Mediterranean Basin, especially for the two zoonotic species A. phagocytophilum and A. platys.
Bartonella
Bartonella are Gram-negative bacteria belonging to the family Bartonellaceae from the Rhizobiales order, and half of them are known to be zoonotic. They are responsible for diseases such as trench fever and cat-scratch disease, and frequently cause endocarditis [243]. The most commonly detected species in domestic animals is Bartonella henselae, responsible for cat-scratch disease, detected in five countries (Algeria, Cyprus, Greece, Italy, and Spain). Bartonella henselae were mainly found in Ixodes ricinus, with the highest prevalence in Italy at 5.4% [154]. The ticks reported positive were mainly collected on cats. The host reported with the highest prevalence was the cat, with 83.5% in Italy [151]. The other four Bartonella species detected were observed in only one or two countries, mainly Italy and Spain.
The Bartonella genus is less often screened for and less often found than the previous genus, featuring in only 7.7% of the publications and 13.5% of the publications concerning bacteria. It is mainly screened for in pets (cats and dogs). This genus is mainly transmitted by biting flies and by fleas; however, it is evident that ticks can also be involved [244]. This shows both unequal presence and unequal screening for the Bartonella genus in the countries of the Mediterranean Basin. Veterinary interest in the genus focuses on pets.
Borrelia
The Borrelia genus includes spirochetes bacteria belonging to the family Spirochaetaceae. It is divided into two groups: the Lyme borreliosis group responsible for Lyme disease, which is mainly caused by bacteria of the Borrelia burgdorferi sensu lato group, and the relapsing fever group, which includes Borrelia miyamotoi [245]. The main species found in our study were from the B. burgdorferi sensu lato group, with the highest prevalence in animal hosts at 1.47% in dogs from Italy; and in ticks at 53% and 26% in Rh. sanguineus s.l. and Rh. annulatus, respectively, from cattle in Italy. Concerning B. afzeeli, B. garinii and B. valaisiana, the highest occurrence rates in ticks were 4.3%, 4.3% and 6.4%, respectively, from Ixodes ricinus collected from dogs in Spain [51,53,60,127]. The last species detected in the Mediterranean Basin from domestic animals was Borrelia theileri, which is a relapsing fever bacterium responsible for bovine borreliosis [246]. It was detected in goats and sheep with 10.8% and 5.8% prevalence, respectively in Algeria [66].
Borrelia found in domestic animals from the Mediterranean Basin were mainly from the Lyme Borreliosis group, except for Bo. theileri. They were detected in only 4.1% of the publications analyzed and in 7.1% of the publications concerning bacteria. Among these publications, they were more commonly found in ticks (72.7%) than in animal hosts (27.8%). On the basis of these results, research seems to be more focused on the possible circulation of infested ticks than on the potential role of domestic animals as reservoirs of the pathogen. Borrelia is either poorly screened or not common in domestic animals from countries of the Mediterranean Basin.
Chlamydia/Chlamydophila
The genera Chlamydia and Chlamydophila belong to the Chlamydiales, which are Gram-negative bacteria responsible for a wide range of diseases throughout almost the entire animal realm [160]. Recently, a few studies carried out in Italy showed that ticks could be vectors of Chlamydia/Chlamydophila [154,160]. Two bacterial species have been found. The first is Chlamydia abortus, from the genus Chlamydia, with the highest prevalence at 40.5% in Ixodes ricinus from cats in Italy. The second, belonging to the genus Chlamydophila, is Chlamydia psittaci, with the highest prevalence at 4.4% in Rh. sanguineus s.l. from dogs and breeding animals in Italy. No bacteria were found in animal hosts. The Chlamydia genus was found in 0.7% of the overall publications and in 1.3% of the publications concerning bacteria. This shows a possible role of ticks in the circulation of Chlamydia species; however, transmission and circulation in the domestic animal population in the countries of the Mediterranean Rim cannot be confirmed.
Coxiella
Only one pathogen is representative of this genus: Coxiella burnetii, responsible for Q fever, which is transmitted by ticks and affects both humans and animals. This disease has a worldwide distribution and can cause febrile illness, endocarditis, meningoencephalitis or pneumonia in humans, while it is mainly asymptomatic in animals apart from sporadic cases of abortion in pregnant animals [247]. It has been found in nine countries of the Mediterranean Basin (Algeria, Cyprus, Egypt, Greece, Italy, Montenegro, Slovenia, Spain and Tunisia), with the highest prevalence in animals at 71.2% from camels in Algeria and 10.2% in Rh. sanguineus s.l. from goats and sheep in Cyprus [163,177]. This genus was found in 8.9% of the publications and in 15.4% of the publications concerning bacteria. For C. burnetii, most of the publications investigated the ticks as well as their hosts, indicating a level of public health and veterinary interest.
Ehrlichia
Ehrlichia spp. is a genus closely related to Anaplasma spp., and is responsible for human monocytotropic ehrlichiosis (Ehrlichia chaffeensis and Ehrlichia canis) and for canine ehrlichiosis (Ehrlichia canis) [248]. The main species in domestic animals from the Mediterranean Basin is Ehrlichia canis, which is responsible for disease in both humans and dogs. It has been detected in 10 countries (Algeria, Croatia, Cyprus, Egypt, France, Greece, Italy, Palestine, Spain, and Turkey) and its highest prevalence was found at 48.5% in dogs from Italy and 6.6% in Rh. sanguineus s.l. collected from dogs in Turkey [123,206]. The other four species found in our review, E. equi, E. ewingii, E. minacensis and Candidatus E. urmitei, have a minor health impact and were each found in only one country. The Ehrlichia genus was the third most common bacterial genus found or screened for after Anaplasma and Rickettsia, featuring in 11.8% of the overall publications and 20.5% of the publications concerning bacteria. Among the publications dealing with the Ehrlichia genus, 93.5% concerned E. canis.
Neoehrlichia
Of the four bacteria in this genus, only one is a human pathogen: Neoehrlichia mikurensis, which causes chronic lymphocytic leukemia, for example. Its vectors are from the Ixodes genus, and rodents are the most well-known hosts [249]. In domestic animals, it has been detected only in Spain with 1% prevalence in I. ricinus from cattle [136]. This species was rarely found or poorly screened for, featuring in only 0.4% of the publications and 0.6% of the publications concerning bacteria.
Francisella
Bacteria from the genus Francisella are Gram-negative bacteria with one important species, Francisella tularensis, responsible for tularemia in humans and animals [250]. In the domestic animals of the Mediterranean Basin, only the genus Francisella spp. level was identified. It was found only in Italy, with a prevalence of 66%, 21% and 8% in Hyalomma marginatum, Rh. bursa and Rh. (Boophilus) annulatus from cattle, respectively. As with the Neoehrlichia genus, no Francisella was detected directly in animal hosts [149].
Leptospira
The genus Leptospira includes spirochetes and zoonotic bacteria that are responsible for leptospirosis worldwide. The first case was documented over 100 years ago. The bacteria are usually transmitted by direct or indirect contact with a contaminated element, but can also be found in ticks [251,252]. Again, only the genus Leptospira spp. was detected. It was found in Egypt with a prevalence of 50%, 41%, 40% and 29% from camel, sheep, cattle and buffalo, respectively [184]. Unlike the two previous genera, Leptospira spp. was only found in domestic animals and not in ticks. However, similarly to the two previous genera, it was found in 0.4% of the overall publications and in 0.6% of publications concerning bacteria. Nevertheless, the Leptospira genus has already been found in ticks in Europe [252], but this was not found to be reported in the present review.
Mycoplasma
The genus Mycoplasma is composed of commensal and pathogenic bacteria that can cause anemia in a wide range of mammals [253,254,255]. Mycoplasma haemocanis, My. haemofelis, Candidatus My. haemonutum, Candidatus My. haematoparvum and Candidatus My. turicensis were found, but only My. heamocanis and Candidatus My. haematoparvum were found in two countries (Greece and Turkey). The prevalence of My. haemocanis was 5.6% in dogs from Greece and 26.2% in dogs from Turkey. For Candidatus My. haematoparvum, the prevalence was 4.2% from dogs in Greece and 6.7% from dogs in Turkey [185,186]. The other three species were each found in only one country. As with the Leptospira genus, the Mycoplasma genus was found only in animals in this review. It was found in 2.2% of the publications and in 1.3% of the publications concerning bacteria.
Rickettsia
The genus Rickettsia is one of the most important tick-borne pathogen genera. It is divided into two groups: the spotted fever group (SFG), including Rickettsia conorii, causative agent of Mediterranean spotted fever, and the typhus group (TG), which is less well-known and includes, for example, Rickettsia typhi [256,257]. Seventeen species were detected in domestic animals in this study. Sixteen of these were from the SFG (R. aeschlimannii, R. africae, R. conorii, R. conorii israelensis, R. felis, R. helvetica, R. hoogstraalii, R. massiliae, R. monacensis, R. raoultii, R. rickettsia, R. rhipicephali, R. sibirica mongolotimonae, R. slovaca, Candidatus R. barbariae and Candidatus R. goldwasserii), and one was from the TG: R. typhi. Of these Rickettsia spp., Rickettsia massiliae (SFG group) was the most widespread bacterium and was detected in 10 countries (Algeria, Cyprus, France, Greece, Israel, Italy, Lebanon, Palestine, Spain and Tunisia). Its highest prevalence was 40.4% in Rh. sanguineus s.l. collected from cattle, dogs and sheep from Algeria, and 2.7% from camel blood in Tunisia [175,213]. The second most common bacterium was R. africae (SFG group), found in eight countries (Egypt, France, Israel, Italy, Lebanon, Palestine, Tunisia and Turkey), with the highest prevalence at 26.7% in Hyalomma impeltatum collected from cattle and camels in Egypt, and 0.3% in camel blood from Tunisia [194,201]. Rickettsia typhi was the only bacterium from the TG group, detected with a prevalence of 29.7% from cats in Greece [155]. On the Mediterranean Rim, the genus Rickettsia is widespread and diversified in domestic animals and their ticks, and is widely screened; it was found in 25.3% of the publications overall, and in 44.2% of the publications concerning bacteria. This genus is the second most commonly studied after Anaplasma spp. However, the SFG group (98.6% of the publications) was of the greatest scientific interest compared to the TG group (1.4% of the publications).
3.2.3. Viruses
Capripoxvirus
This genus includes species that affect a broad range of domestic ruminants and that have a considerable economic impact, especially in Africa and the Middle East. It covers three species: lumpy skin disease virus (LSDV), goatpox virus (GTPV) and sheeppox virus (SPV) [258]. Among these three species, only LSDV was found in ticks from domestic animals in this review. It was found in ticks from the genera Amblyomma, Hyalomma and Rhipicephalus from cattle in Egypt, with a prevalence of 65.5% [224]. It was the least commonly found virus (0.4% of the overall publications and 7.2% of the publications concerning viruses). This genus does not represent a major threat in veterinary health in the countries of the Mediterranean Rim. Nonetheless, it should still be considered a potential threat, considering the range of ticks in which it has been found.
Flavivirus
The flaviviruses are an important part of the arboviruses. They are transmitted by both mosquitoes and ticks. They mainly infect mammals and account for a large proportion of the recent outbreaks of public health and veterinary concern in terms of morbidity and mortality [259]. The most well-known tick-borne flavivirus, tick-borne encephalitis virus (TBEV), was found in two countries in domestic animals: in Greece, with a prevalence of 1.4% from I. ricinus ticks from goats, and in Italy, with a prevalence of 16.43% in goats [224,225]. TBEV was the second most common virus species, found in a total of 0.7% of publications and 14.3% of the publications concerning viruses.
Orthonairovirus
Globally, this genus is mainly represented by one species: Crimean–Congo hemorrhagic fever virus (CCHFV). This virus is transmitted by ticks, mainly from the genus Hyalomma, causes severe or even fatal human disease across almost all of the Old World, and its range has expanded with climate change [260]. In domestic animals from countries of the Mediterranean Basin, the virus has been found in five different countries (Albania, Greece, Egypt, Spain, and Turkey). The highest rates of occurrence were 90% from sheep in Albania and 6.88% from three tick genera (Hyalomma spp., Rhipicephalus spp. and Ixodes spp.) collected from buffalo, cattle, goats and sheep in Turkey [226,230]. CCHFV was the most commonly screened or found virus, featuring in about 78.5% of the publications concerning viruses and in 4.1% of the overall publications. In order to determine the expansion of the virus, numerous research studies have been performed on the tick species that are potential vectors of the virus.
3.3. Ticks Positive for Tick-Borne Pathogens from Domestic Animals in the Mediterranean Basin
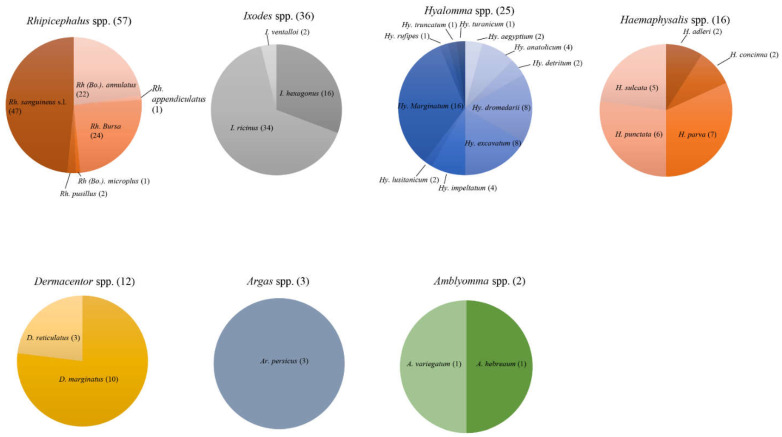
In all, 35 tick species from seven genera found on domestic animals in the Mediterranean Basin were positive for TBPs. Out of the seven genera, six belonged to the hard ticks (Ixodidae): Amblyomma, Dermacentor, Haemaphysalis, Hyalomma, Ixodes and Rhipicephalus. These genera are widely distributed in this region. The last genus, which was collected in Algeria and Egypt, represents the soft ticks (Argasidae): Argas (Table S1). The diversity of tick species positive for TBPs varies between these genera (Figure 4).
Figure 4.
Engorged ticks collected from domestic animals and positive for TBPs (the number of pathogens identified per taxon is indicated in brackets).
3.3.1. Ixodidae
Genus Rhipicephalus
The top-ranking genus in terms of TBP diversity detected in the Mediterranean Basin was the genus Rhipicephalus (Table S1). It has a worldwide distribution and is very broadly present in the Mediterranean Basin, where it can target a large range of wild and domestic animals [261,262]. This genus transmits pathogens that cause diseases with global health impacts, such as anaplasmosis, babesiosis, ehrlichiosis and rickettsiosis [261,263]. However, only six species were identified for the detection of 57 pathogenic species in a total of 13 countries: Rh (Bo.). annulatus, Rh. appendiculatus, Rh. bursa, Rh (Bo.). microplus, Rh. pusillus and Rh. sanguineus s.l. Out of these 57 TBP species, 47 were detected in Rh. sanguineus s.l., which was the tick species with the highest number of pathogenic species found in our review (Figure 4). It is a tick species known for the transmission of a number of TBPs, such as E. canis, R. conorii and B. canis, f-ound in our review. This tick species is highly present in domestic animals and has a tropism for pets, such as dogs, as well as livestock, such as cattle [261]. This tropism and the TBPs transmitted by this species could be the reasons underlying both the scientific interest in this species and the number of TBPs found in domestic animals.
Genus Ixodes
The second-ranking genus in terms of pathogen diversity was the genus Ixodes (Table S1). This genus is mainly known for the transmission of Lyme disease. The ticks are present mainly in Europe, where due to their pleiotropic feeding, they are responsible for the spread of numerous TBPs [262]. They target a broad range of hosts (ubiquity) and have a high impact on human and animal health [261,263]. Only three species were detected in countries of the Mediterranean Basin: I. hexagonus, I. ricinus and I. ventalloi. A total of 36 pathogens were found on six animal hosts. This genus has the highest number of pathogens detected for the lowest number of tick species, with a total of 36 pathogen species for just three tick species: I. hexagonus, I. ricinus and I. ventalloi. The main species is I. ricinus, in which 34 TBPs were found out of the 36 found in the genus (Figure 4). This species is ubiquitous and can be found on mammals, reptiles and avifauna. Ixodes ricinus is mainly known for the transmission of B. burgdorferi s.l., the causative agent of Lyme disease. However, as shown in our review, it can be positive for a wide range of pathogens. This could be due to the large array of hosts on which this species can feed.
Genus Hyalomma
The genus Hyalomma ranks third in terms of TBPs detected. It is known for the transmission of CCHFV and of bacteria such as Rickettsiae. The ticks are large in size (5–6 mm) with tropism for large mammals upon reaching their adult forms, and for small mammals and birds while in their immature stages. Hyalomma species are mainly distributed in the southern part of the Mediterranean Basin, with a slow increase in range in Western Europe and the Balkans [261,263]. In the Mediterranean Basin, a total of 11 species were found, which is the highest number of species among all the tick genera. They were found on nine hosts in 12 countries and were found to be infected by a total of 25 TBPs (Table S1). The number of hosts, countries and pathogens associated with this genus indicates its public health importance and the considerable threat it poses. Among the 11 species of the genus Hyalomma, the species found positive for the largest number of TBPs was Hy. marginatum (Figure 4). Out of 25 species found in the genus, a total of 16 TBPs were found. Concerning domestic animals, Hy. marginatum affects a large range of livestock and can be responsible for TBDs of veterinary importance, such as infections with Rickettsia spp., CCHFV, Babesia spp. and Theileria spp. These characteristics, along with the tropism of the immature-stage ticks for birds, could underpin the public health importance of this species.
Genus Haemaphysalis
The genus Haemaphysalis is the fourth most common genus in terms of pathogens detected (Table S1). The ticks are small in size and target mammals upon reaching their adult stage, and a wide array of hosts when still in their immature stages. They are known to have veterinary importance in livestock and are present in Asia [263]. On domestic animals from the Mediterranean Basin, the genus Haemaphysalis was detected in seven different countries, and ticks were positive for 16 TBPs. Five different species were found: H. adleris, H. concinna, H. parva, H. punctata and H. sulcata. Among these, the species found to be positive for the highest number of TBPs was H. parva (Figure 4). This species is mainly distributed in the Mediterranean Basin, with key tropism for domestic ungulates when the ticks are in the adult stage. It can transmit a wide range of TBPs, such as Rickettsia spp., C. burnetii and F. tularensis [264]. Unlike the genus Hyalomma, the pathogen distribution in the different species of Haemaphysalis is more uniform, especially between H. parva, H. punctata and H. sulcata. This could explain the overall interest in the genus Haemaphysalis on domestic animals across the countries of the Mediterranean Basin.
Genus Dermacentor
The genus Dermacentor ranked fifth in terms of TBPs detected (Table S1). Similarly to the genus Ixodes, it is mainly present in Europe, where it transmits a broad range of TBPs [261,262]. The genus is represented by two common species in the Mediterranean Basin: D. marginatus and D. reticulatus. They transmit a wide array of pathogens, including Rickettsia spp. and Babesia spp., which are of veterinary and human health importance. All the development stages were found in six different domestic animals from eight different countries. Twelve pathogen species have been identified in the two tick species. The main species was D. marginatus, which was found to be positive for 9 TBPs (Figure 4). This species is known for its tropism on wild and domestic ungulates, but also for transmission of B. canis and R. slovaca [261].
Genus Amblyomma
The genus Amblyomma was the last-ranking genus of hard ticks in terms of TBPs detected (Table S1). It can be found in nearly all terrestrial animals and occurs mainly in the tropical and sub-tropical areas of Asia, Africa and Oceania [265]. Recently, it appeared in the Mediterranean Basin, for instance in Corsica [199,266]. Due to the geographical distribution of this genus, it was rarely found in the Mediterranean Basin and only two species (A. hebreaum and A. variegatum) have been identified. Positive ticks were only found on cattle. The virus and bacteria detected were lumpy skin disease virus and Rickettsia africae (Table 2, Figure 4).
Table 2.
TBP diversity in domestic animals or engorged ticks collected on these animals from the Mediterranean Basin.
| Categories of Domestic Animals | Livestock | Pets | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal names (number of countries with data available) | Cattle (13) (Bos taurus) 
|
Goat (13) (Capra hircus) 
|
Sheep (15) (Ovis aries) 
|
Dromedary (6) (Camelus dromedarius) 
|
Horse (7) (Equs caballus) 
|
Buffalo (1) (Bubalus spp.) 
|
Chicken (2) (Gallus gallus) 
|
Donkey (1) (Equs asinus) 
|
Pig (1) (Sus domesticus) 
|
Dog (14) (Canis lupus familiaris) 
|
Cat (5) (Felis silvestris) 
|
Rabbit (1) (Oryctolagus sp.) 
|
| Pathogens found in ticks | ||||||||||||
| Number of studies | 51 | 42 | 42 | 20 | 14 | 5 | 4 | 1 | 0 | 63 | 15 | 1 |
| Number of TBPs found in ticks | Bacteria: 29 Parasite: 10 |
Bacteria: 25 Parasite: 5 Virus: 2 |
Bacteria: 21 Parasite: 7 Virus: 1 |
Bacteria: 8 Parasite: 2 Virus: 1 |
Bacteria: 12 Parasite: 1 |
Bacteria: 2 Virus: 1 |
Bacteria: 3 | Bacteria: 4 Parasite: 4 |
0 | Bacteria: 26 Parasite: 17 Virus: 1 |
Bacteria: 9 Parasite: 2 |
Bacteria: 1 |
| Percentage of TBPs in ticks | 59.1% | 48.5% | 43.9% | 16.7% | 19.7% | 4.5% | 4.5% | 12.1% | 0% | 66.7% | 16.7% | 1.5% |
| Pathogens found in animals | ||||||||||||
| Number of studies | 51 | 33 | 39 | 15 | 20 | 8 | 0 | 4 | 5 | 77 | 24 | 0 |
| Number of TBPs found in animals | Bacteria: 13 Parasites: 13 Viruses: 2 |
Bacteria: 12 Parasites: 5 Viruses: 2 |
Bacteria: 11 Parasites: 10 Viruses: 1 |
Bacteria: 9 Parasites: 1 |
Bacteria: 9 Parasites: 6 |
Bacteria: 4 Parasites: 6 Viruses: 1 |
0 | Bacteria: 3 Parasites: 2 |
Parasites: 2 | Bacteria: 19 Parasites: 13 |
Bacteria: 15 Parasites: 5 |
0 |
| Percentage of TBPs in animals | 40.6% | 27.5% | 21.7% | 14.5% | 21.7% | 15.9% | 0% | 7.2% | 2.9% | 46.4% | 29% | 0% |
3.3.2. Argasidae
Among the soft ticks, only one species was found: Argas persicus. This species is mainly found on domestic birds. It is mainly found in the Mediterranean Basin but is primarily involved in the transmission of bird-related pathogens [261,263,267]. In our review, it was found on two hosts, and three pathogen species were found (Table S1, Figure 4). This genus is one of the less commonly collected ticks in the countries of the Mediterranean Basin, along with the genus Amblyomma of the hard ticks group.
3.4. Domestic Animal Hosts of Both Positive Ticks and TBPs in the Countries of the Mediterranean Basin
All the information in this part brings together data about domestic animals infested by TBPs and about domestic animal hosts of positive engorged ticks. However, the data about positive engorged ticks do not confirm the vector character of the ticks for the different TBPs. The domestic animals from countries of the Mediterranean Basin identified in this review can be divided into two groups: livestock animals and pets.
Livestock was composed of nine species (Table 2), which were reasonably well studied in the literature under review. It appears that cattle, sheep and goats were studied in more than 13 countries and were the subject of the highest number of studies. This can be explained by the wide range of tick-borne pathogen genera targeting these species (Table 2), and the economic importance of livestock in the countries of this region [268]. Among the 90 TBPs identified in this review, about 59% were found in positive ticks from cattle, with more than 40% found directly on the animal. On the other hand, some species (buffalo, chickens, donkeys and pigs) were well studied, and available data were found in only one or two countries. The number of TBPs detected in these animals or in their ticks is much lower (Table 2).
The pet group was composed of only three species: cats, dogs and rabbits. Among these three species, the dog was clearly the more commonly studied pet. Available data about the detection of TBPs in dogs (77 studies) and their ticks (63 studies) were found for 14 countries, while cat and rabbit data were found in only five countries and one country, respectively (Table 2).
Among the 90 TBPs listed in this review (in livestock or pets), 66 were detected in ticks from animals and 69 directly in the animals. The highest number of pathogens was found in ticks on dogs (66.7%) (Table 2). The list of TBPs reported in each domestic animal host and their ticks is summarized in the Table S2.
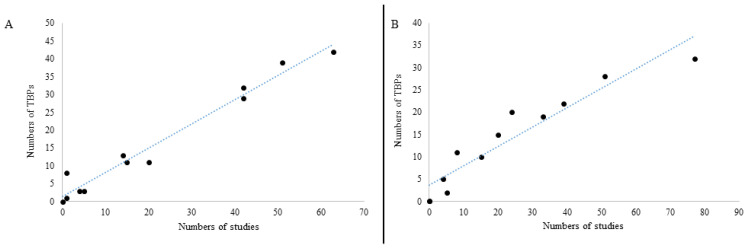
The research effort, expressed by the number of publications, varies greatly from one animal to another. This is why it is difficult to conclude that an animal, or its ticks, are more or less susceptible to being infected by TBPs. In this sense, Figure 5 shows a positive correlation between the number of studies and the number of TBPs found in animals (Figure 5B) or in their ticks (Figure 5A). The very high correlation coefficient (0.95 and 0.98, respectively) expresses a strong correlation between these variables. If we assume that correlation does not imply causation, we can nevertheless observe a correlation between the number of TBPs found and the number of publications focusing on the different domestic animal species, and conclude what may be obvious: the more we seek, the more we find.
Figure 5.
ScatterPlot diagrams showing a strong linear correlation between (A) the number of studies and the number of TBPs from positive ticks by domestic animal (correlation coefficient R = 0.98), and (B) between the number of studies and the number of TBPs by positive domestic animal (correlation coefficient R = 0.95).
3.5. Biogeography, Diversity and Distribution in the Mediterranean Basin
3.5.1. Overall Analysis of the Four Main Regions in the Mediterranean Basin
In this section, we divide the countries of the Mediterranean Basin into four different areas to allow for better comparison of the data found. Western Europe is made up of France, Italy, Malta, Monaco and Spain, which represented 45.7% of the publications. North Africa, which includes Algeria, Egypt, Libya, Morocco and Tunisia, had a percentage of 26.1% of the total publications included in this review. The Middle East, which is composed of Cyprus, Israel, Lebanon, Palestine, Syria and Turkey, represented 17.7%. The Balkans area is made up of Albania, Bosnia-Herzegovina, Croatia, Greece, Montenegro and Slovenia, and had 10.33% of the publications. The five countries in which no pathogens were found on domestic animals or their ticks were Bosnia-Herzegovina, Libya, Malta, Monaco and Syria.
Western Europe
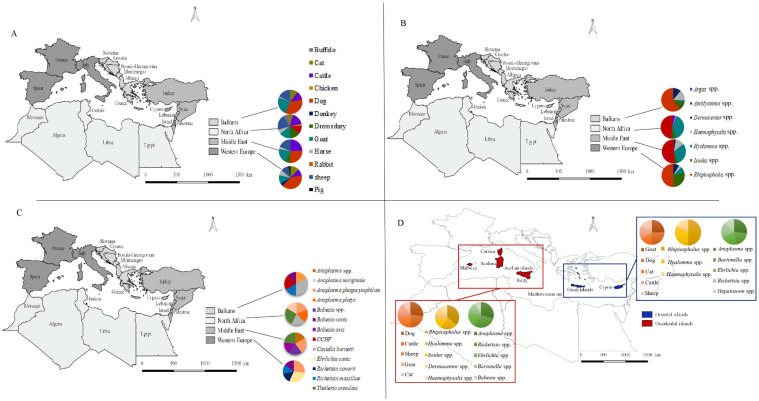
In the five countries of this area, tick-borne pathogens on domestic animals were detected in Italy, France and Spain. These are also the three countries with the highest number of publications in the Mediterranean Basin. A total of eight domestic animal species were found to be carriers of positive ticks and pathogens: cats, cattle, dogs, donkeys, goats, horses, sheep and pigs. From our data, six tick genera from domestic animals were found to be positive for TBPs: Amblyomma spp., Dermacentor spp., Haemaphysalis spp., Hyalomma spp., Ixodes spp. and Rhipicephalus spp. The most commonly found host was the dog (Figure 6A), which was the main target of the genus Rhipicephalus. In addition, the most predominant tick genus found out of the seven genera collected was Rhipicephalus spp. (Figure 6B), a vector of pathogens, such as B. canis and E. canis. The other main tick genera were Ixodes, a ubiquitous genus, and Hyalomma, mainly found on livestock. Of 68 pathogens detected in this region, five were included in nearly 60% of the publications in Western Europe. They belonged to the bacteria and parasite groups E. canis, A. phagocytophilum, R. conorii, B. canis and R. massiliae (Figure 6C). It appears that the papers found in Western Europe mainly focused on dogs, which could explain the high percentage of Rhipicephalus ticks found and TBPs related to dogs. This focus can be explained by the central place of the dog in human activities, as pets, hunting dogs or stray dogs.
Figure 6.
Map of the main domestic animal hosts (A), main positive tick genera (B), main TBPs in the four areas of the Mediterranean Basin (C), and main domestic animal hosts, positive tick genera and TBP genera in the western and eastern islands (D).
North Africa
The North Africa area has the largest diversity of domestic animal hosts of pathogens and positive ticks reported in the countries of the Mediterranean Basin, with a total of 10 domestic animal hosts: cats, cattle, chickens, buffalo, dogs, dromedaries, goats, horses, rabbits and sheep. The main animals reported were from the livestock group: cattle, sheep, goats and dromedaries (Figure 6A). From these hosts, five tick genera were found to be positive for TBPs: Argas spp., Haemaphysalis spp., Hyalomma spp., Ixodes spp. and Rhipicephalus spp. Even though the main tick genus reported was the same as in Western Europe (genus Rhipicephalus), it was found both in livestock and pets, with equal importance in terms of health. The other main tick genus was Hyalomma spp. which was mainly found on livestock (Figure 6B). The main TBPs reported belong to the bacteria and parasite groups: A. marginale, A. phagocytophilum, A. platys, C. burnetii and T. annulata (Figure 6C). They are mainly related to livestock rather than pets. In North Africa, research seems to be more focused on livestock veterinary health monitoring compared to Western Europe.
The Middle East
In this area, a total of six domestic animals were reported to be carriers of positive ticks and TBPs: cattle, dogs, dromedaries, goats, horses and sheep. Similarly to Western Europe, the main domestic animal host found was the dog, closely followed by cattle, featuring in 66.7% and 62.5% of the total publications in the area, respectively (Figure 6A). From the domestic animal hosts, five tick genera were reported to be positive for TBPs: Dermacentor spp., Haemaphysalis spp., Hyalomma spp., Ixodes spp. and Rhipicephalus spp. As with the two previous areas, Rhipicephalus, which can be found both on livestock and pets, was the main tick genus found. The other main tick genera were Hyalomma and Haemaphysalis, which also infested livestock (Figure 6B). Of the 45 pathogens found in the Middle East, the most predominant were Anaplasma spp., A. phagocytophilum, Babesia spp., B. ovis and T. annulata. They were identified in 62.5% of the publications in the area (Figure 6C). In the Middle East, the data available were related to the veterinary health of both pets and livestock to equal extents. However, they were mainly from Turkey (75.5%).
The Balkans
A total of six domestic animal hosts were reported to be carriers of positive ticks and TBPs: cats, cattle, dogs, goats, horses and sheep. Of the six domestic animal hosts reported, the main host was the dog, followed by the goat, which both seemed to be the focus of veterinary research in the four areas, probably due to their economic and social importance (Figure 6A). Five tick genera were reported to be positive for TBPs: Dermacentor spp., Haemaphysalis spp., Hyalomma spp., Ixodes spp. and Rhipicephalus spp. Of the five genera found in the Balkans, the main positive tick genus collected on domestic animals was Rhipicephalus spp., followed by the genera Haemaphysalis, Ixodes and Dermacentor (Figure 6B). A total of 32 pathogens were found in the seven countries of the Balkans area. The most frequently detected were the bacteria C. burnetii, A. platys and R. massiliae, the parasite B. canis and the CCHF virus (Figure 6C). These were reported in 90% of the publications. As for the Middle East, these TBPs can be found in livestock and pets.
3.5.2. Focus on Insular Tick-Borne Pathogens in Domestic Animals and Their Ticks
The Mediterranean islands are an important place for animal migrations, human activities and pathogen circulation due to their geographical situation. A total of 44 publications were reported on islands, representing 16% of the publications taken into account in this review. These 44 publications were considered separately from the previous dataset. Out of these 44 publications, most came from the largest islands of the Mediterranean Basin: Sardinia, Sicily, Corsica and Cyprus, while some data were reported from smaller islands, such as the Greeks islands. We decided to split the islands of the western Basin, including the Aeolian islands, Corsica, Sardinia, Sicily and Mallorca (36 publications), from the eastern Basin, including Crete, Cyprus, Ios, Mykonos, Santorini, Skiathos, Skopelos and Tinos (8 publications) (Table 3).
Table 3.
Tick-borne pathogens from insular domestic animals or engorged ticks collected from these animals in the Mediterranean Basin.
| Country | Island | Surface Area | Western Basin/Eastern Basin | Pathogen (Found in A for Positive Animal and/or T for Positive Tick) |
Positive Ticks | Positive Tick Hosts | Positive Pathogen Hosts | References |
|---|---|---|---|---|---|---|---|---|
| Cyprus | Cyprus | 9251 km2 | Eastern | A. platysA, B. vogeliA, Ba. henselaeA, C. burnetiiT, E. canisA, E. ewingiiA, H. canis, H. felisAM. haemofelisA, Candidatus M. haemotumum A, Candidatus M. turicensis A, R. aeschlimanii T, R. hoostraalii T, R. sibirica mongolotimonae T and Candidatus R. barbariae T | H. punctata, H sulcata, Hyalomma spp., Hy. excavatum, Hy. marginatum, Hy. rufipes, I. gibbosus, Rh. bursa and Rh. sanguineus s.l. | Cattle, Goat and Sheep | Cat and Dog | [69,143,177,178,212] |
| France | Corsica | 8722 km2 | Western | Anaplasma spp. A,T, A. ovis A, A. marginale A,T, A. phagocytophilum T, B. bigemina T, B. canis A,T, B. vogeli A,T, Ba. henselae T, Bo. afzelii T, Bo. burgdoferi s.l. T, Bo. miyamotoi T, E. canis T, E. minancensis, R. aeschlimanii T, R. africae T, R. helvetica T, R. massiliae T, R. monacensis T, R. slovaca T, Candidatus R. barbariae T, T. annae A and T. equi T | A. variegatum, D. marginatus, D. reticulatus, I. ricinus, Hy. aegyptium, Hy. marginatum, Hy. rufipes, Rh. bursa and Rh. sanguineus s.l. | Cat, Cattle, Dog and Sheep | Cattle, dog, Horse, goat and sheep | [56,103,183,195,198,199,272] |
| Greece | Crete | 8450 km2 | Eastern | Ba. henselaeA, Ba. vinsoniiT, R. felisAand R. TyphiA | Rh. bursa and Rh. sanguineus s.l. | Goat, Cattle | Cat | [155,157] |
| Ios | 109 km2 | Eastern | Anaplasma spp. A, B. canis A, Bo. burgdoferi A, E. canis A and R. conorii A | data not found | data not found | Dog | [106] | |
| Mykonos | 85.5 km2 | Eastern | Ba. henselae A and Rickettsia spp. A | data not found | data not found | Cat | [155] | |
| Santorini | 76.19 km2 | Eastern | Anaplasma spp. A, B. canis A, Bo. burgdoferi A, E. canis A and R. conorii A | data not found | data not found | Dog | [106] | |
| Skiathos | 49.9 km2 | Eastern | Anaplasma spp.A, B. canis A, Bo. burgdoferi A, E. canis A and R. conorii A | data not found | data not found | Dog | [106] | |
| Skopelos | 96.3 km2 | Eastern | Ba. henselaeAand Rickettsia spp. A | data not found | data not found | Cat | [155] | |
| Tinos | 197 km2 | Eastern | Anaplasma spp. A, B. canis A, Bo. burgdoferi A, E. canis A and R. conorii A | data not found | data not found | Dog | [106] | |
| Italy | Aeolian Island | 114.7 km2 | Western | Anaplasma spp. A, B. vogeli A, Bartonella spp. A, Ba. clarridgeiae A, Ba. henselae A, Ehrlichia canis A, H. canis A, H. felis A, R. helvetica A and R. monacensis A | I. ricinus, I. ventalloi. Rh. pusillus and Rh. sanguineus s.l. | Cat | Cat and Dog | [73,150] |
| Sardinia | 24090 km2 | Western | A. phagocytophilumA, A. ovisA, B. bigeminaTBartonella spp. T, Ba. henselae A,T, Chlamydia abortus T, Chlamydophila psittaci T, C. burnetii A,T, E. canis T, Rickettsia spp. T, R. aeschlimannii T, R. conorii israelensis T, R. helvetica T, R. hoogstralii T, R. massiliae T, R. slovaca T, Candidatus R. barbariae T, T. buffeli T, T. equi T, T. orientalis Tand T. sergenti T | D. marginatus, H. punctata, H. sulcata, Hy. marginatum, I. festai. Rhipicephalus spp., Rh (B). annulatus, Rh. bursa, Rh. sanguineus s.l. | Cat, Cattle, Dog, Goat, Horse, Pig and Sheep | Cat, Dog, Goat, Horse and Sheep | [38,119,122,139,156,160,171,207,215,221] | |
| Sicily | 25711 km2 | Western | Anaplasma spp. A, A. marginale A, A. phagocytophilum A, A. platys A, A. ovis A, Babesia spp. A, B. bigemina A, B. bovis A, B. caballi A, B. canis A, B. microti A, B. vogeli T, Ba. claridgeiae A,T, Ba. henselae A, Bo. burgdoferi s.l A, Cercopithifilaria spp. A,T, C. burnetii A, Ehrlichia spp. A, E. canis A, E. equi A, Hepatozoon spp. A,T, Rickettsia spp. A,T, R. aeschlimannii T, R. africae T, R. conorii A,T, R. felis A, R. helvetica T, R. monacensis T, R. rickettsii A, R. slovaca T, Theileria spp. A, T. annulata A and T. equi A | D. reticulatus, D. marginatus, H. punctata, Hy. lusitacum, Hy. marginatum, Ixodes spp., I. ricinus, I. ventalloi, Rh. bursa, Rh. pusillus, Rh. sanguineus s.l. | Cat, Cattle, Dog, Donkey and Sheep | Cat, Cattle, Dog, Donkey, Goat, Horse, Pig and Sheep | [32,91,105,115,131,138,145,146,151,179,189,273,274] | |
| Spain | Mallorca | 3640 km2 | Western | A. phagocytophilumA, Ba. henselaeA, Ba. vinsonii berkhoffiA, E. canisAand R. conoriiA | data not found | data not found | Cat and Dog | [140,182] |
On the islands, a total of nine domestic animal hosts were studied: cats, cattle, dogs, donkeys, goats, horses, pigs, rabbits and sheep. Despite this host diversity, the majority of the islands followed the same schema as in the rest of the Mediterranean Basin, with the dog predominating as host of positive ticks and TBPs (43.2% of the island publications), followed by cattle, sheep and goats. We also observed a higher diversity of domestic animal hosts (eight species) in the data reported in the western islands (cats, cattle, dogs, donkeys, goats, horses, pigs and sheep) than in the eastern islands (five species: cats, cattle, dogs, goats and sheep) (Table 3). In the western islands, most of the publications found were about dogs (44.4%), followed by cattle and sheep (each featuring in 36.1% of the publications). In the eastern islands, the main domestic animal hosts reported were goats and dogs, each featuring in 37.5% of the publications (Figure 6D).
A total of six hard tick genera, and no soft ticks, were found to be positive in the data from the islands: Amblyomma spp., Dermacentor spp., Haemaphysalis spp., Hyalomma spp., Ixodes spp. and Rhipicephalus spp. (Table 3). Of these genera, the main positive tick genus reported was Rhipicephalus spp., featuring in 43.2% of the island publications. This follows the same schema as the overall data. Nevertheless, the second most common genus reported was Hyalomma. This corresponds to the observations made in North Africa and the Middle East, and differs from Western Europe and the Balkans. This finding could be due to the wide distribution of Hyalomma in the Mediterranean Basin, especially on the south and east borders, with some species also on the north border [261]. This could also testify to the extension of the distribution area of this genus through animal migration and with the facilitation of climate change. This may result in the spread of TBPs, such as the CCHF virus transmitted by these ticks [19,269,270]. In this dissemination, islands may play an important role as places of transition between the different borders of the Mediterranean Basin [271]. This suggests a potential sentinel role of islands when monitoring dissemination of this tick genus and their TBPs on a continental scale (Figure 6D).
In the western islands, TBPs were screened and found in ticks from domestic animals in 63.9% of the papers. Meanwhile, the corresponding value was only 37.5% of the publications in the eastern islands. For both groups of islands, the main positive tick genus found was Rhipicephalus spp., in 69.6% and 100% of the publications, respectively. This shows the importance of this genus on islands across the whole Mediterranean Basin. For both western and eastern islands, this genus was succeeded in prevalence by the genus Hyalomma (Figure 6D). From the data analyzed, it seems that there was a stronger focus on TBPs in ticks from domestic animals in the western islands than in the eastern islands.
Of the 18 genera of pathogens reported in the data, 14 genera were reported in the islands, representing 77.8% of the total genera (Table 3). The main TBP genera detected were Anaplasma spp. and Rickettsia spp., reported in 43.2% and 40.9% of the publications concerning islands, respectively. This follows the same schema as the overall data. In the western islands, the 14 TBP genera were reported (66.7% bacteria and 33.3% parasites), while only nine were reported (77.8% bacteria and 22.2% parasites) in the eastern islands. In both groups, the main reported genus was Anaplasma, followed by Rickettsia (Figure 6D). They were both reported in 41.7% of the western island publications, and in 50% and 37.5% of the eastern island publications, respectively. No viruses were reported in either of the island groups.
Even though few data were reported for the islands (16%), a high diversity of TBPs was reported. This may indicate the importance of islands in the monitoring of TBPs in domestic animals in the Mediterranean Basin.
4. Conclusions
In this study, 90 TBPs from 18 genera of bacteria, parasites and viruses were reported in domestic animals and their ticks in the countries of the Mediterranean Basin. Most pathogens were bacteria, followed by parasites and viruses. The main genera detected were bacteria: Anaplasma spp. and Rickettsia spp. The data collected reflected a high diversity of TBPs in domestic animals and their ticks in the countries of the Mediterranean Basin, which shows their importance in veterinary and human health. A wide range of pathogens was reported in seven positive tick genera (six hard ticks and one soft tick) and 31 tick species. The most-reported genus was Rhipicephalus spp., a genus found in a large range of domestic animals, from livestock to pets. This genus is also known for the transmission of TBPs from the genera Anaplasma and Rickettsia. These TBPs and positive tick genera were reported in 12 different domestic animal hosts divided into two groups: livestock and pets. The main domestic animal hosts were both dogs and cattle, from which the highest diversity of TBPs was reported. This seems linked to the quantity of data reported for these hosts. In the four areas of the Mediterranean Basin (Western Europe, North Africa, the Middle East and the Balkans), the main studied host was the dog (except in North Africa) and the main positive tick genus was Rhipicephalus. Depending on the area, the second most important genera were Ixodes or Hyalomma. This could be due to the distribution area of Ixodes spp. in Europe and the distribution area of Hyalomma spp. on the south border of the Mediterranean Basin and its expansion through the islands [260,267]. The diversity of the TBPs identified in this review was linked to the domestic animals targeted in the studies and to the animals’ veterinary and social importance. In all, 16% of the publications concerned TBPs from domestic animals and their ticks on islands, but high diversity of domestic animal hosts (nine of 12), positive tick genera (six of seven) and TBP genera (14 of 18) was reported for the islands. This shows the importance of the Mediterranean islands in the monitoring of TBPs in this region as sentinel territories. The development of research on the islands could provide a better understanding of their role as a hotspot for the circulation of ticks and tick-borne pathogens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10061236/s1, Table S1: Positive engorged ticks for TBPs collected on domestic animals and their distribution in the Mediterranean Basin; Table S2: TBP species reported in domestic animals or engorged ticks collected on these animals from the Mediterranean Basin.
Author Contributions
B.D., S.M., V.P. and Y.Q. conceived the scientific ideas. B.D., S.M., V.P. and Y.Q. performed and discussed the work and edited the manuscript. All the authors reviewed the manuscript and provided critical feedback. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The research work of B.D.: Y.Q. and V.P. was supported by UMR 6134, University of Corsica and the CNRS. B.D. was awarded a grant from the Corsican Regional Council. This study was supported by funding from the French Government and from the Corsican Regional Council (CPER project), and also by the GERHYCO interdisciplinary project dedicated to water management, ecology and hydro-ecosystem services in insular context. S.M.’s research was supported by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). UMR BIPAR is supported by the French Government’s Investissement d’Avenir program, Laboratoire d’Excellence “Integrative Biology of Emerging Infectious Diseases” (grant No. ANR-10LABEX-62-IBEID).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nicholson W.L., Sonenshine D.E., Noden B.H., Brown R.N. Chapter 27—Ticks (Ixodida) In: Mullen G.R., Durden L.A., editors. Medical and Veterinary Entomology. 3rd ed. Academic Press; Cambridge, MA, USA: 2019. pp. 603–672. [DOI] [Google Scholar]
- 2.Anderson J.F., Magnarelli L.A. Biology of Ticks. Infect. Dis. Clin. N. Am. 2008;22:195–215. doi: 10.1016/j.idc.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Bellekom B., Hackett T.D., Lewis O.T. A Network Perspective on the Vectoring of Human Disease. Trends Parasitol. 2021;37:391–400. doi: 10.1016/j.pt.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 4.CDC Zoonotic Diseases|One Health|CDC. Published 19 February 2020. [(accessed on 11 February 2021)]; Available online: https://www.cdc.gov/onehealth/basics/zoonotic-diseases.html.
- 5.Rosenberg R., Ben Beard C. Vector-borne Infections. Emerg. Infect. Dis. 2011;17:769–770. doi: 10.3201/eid1705.110310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chomel B. Lyme disease. Rev. Sci. Tech. OIE. 2015;34:569–576. doi: 10.20506/rst.34.2.2380. [DOI] [PubMed] [Google Scholar]
- 7.WHO Vector-Borne Diseases. [(accessed on 10 February 2020)]. Published 2 March 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases.
- 8.Ahantarig A., Trinachartvanit W., Baimai V., Grubhoffer L. Hard ticks and their bacterial endosymbionts (or would be pathogens) Folia Microbiol. 2013;58:419–428. doi: 10.1007/s12223-013-0222-1. [DOI] [PubMed] [Google Scholar]
- 9.Parola P., Raoult D. Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Clin. Infect. Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 10.Beati L., Klompen H. Phylogeography of Ticks (Acari: Ixodida) Annu. Rev. Entomol. 2019;64:379–397. doi: 10.1146/annurev-ento-020117-043027. [DOI] [PubMed] [Google Scholar]
- 11.Eisen L. Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: A review. Ticks Tick-Borne Dis. 2020;11:101359. doi: 10.1016/j.ttbdis.2019.101359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Crimean-Congo Haemorrhagic Fever. [(accessed on 11 February 2020)]. Published 31 January 2013. Available online: https://www.who.int/news-room/fact-sheets/detail/crimean-congo-haemorrhagic-fever.
- 13.Perveen N., Muzaffar S., Al-Deeb M. Ticks and Tick-Borne Diseases of Livestock in the Middle East and North Africa: A Review. Insects. 2021;12:83. doi: 10.3390/insects12010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hildebrandt A., Zintl A., Montero E., Hunfeld K.P., Gray J. Human Babesiosis in Europe. Pathogens. 2021;10:1165. doi: 10.3390/pathogens10091165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannakopoulos C., Bindi M., Moriondo M., LeSager P., Tin T. Climate Change Impacts in the Mediterranean Resulting from a 2 °C Global Temperature Rise. Volume 1 WWF; Gland, Switzerland: 2005. [Google Scholar]
- 16.Ochoa-Hueso R., Munzi S., Alonso R., Arróniz-Crespo M., Avila A., Beremjo V., Bobbink R., Branquinho C., Concostrina-Zubiri L., Cruz C., et al. Ecological impacts of atmospheric pollution and interactions with climate change in terrestrial ecosystems of the Mediterranean Basin: Current research and future directions. Environ. Pollut. 2017;227:194–206. doi: 10.1016/j.envpol.2017.04.062. [DOI] [PubMed] [Google Scholar]
- 17.Al-Abri S.S., Abaidani I.A., Fazlalipour M., Mostafavi E., Leblebicioglu H., Pshenichnayah N., Memish Z.A., Hewson R., Petersen E., Mala P., et al. Current status of Crimean-Congo haemorrhagic fever in the World Health Organization Eastern Mediterranean Region: Issues, challenges, and future directions. Int. J. Infect. Dis. 2017;58:82–89. doi: 10.1016/j.ijid.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasle G., Bjune G., Edvardsen E., Jakobsen C., Linnehol B., Røer J., Mehl R., Røed K., Perdersen J. Transport of Ticks by Migratory Passerine Birds to Norway. J. Parasitol. 2009;95:1342–1351. doi: 10.1645/GE-2146.1. [DOI] [PubMed] [Google Scholar]
- 19.Estrada-Peña A., De La Fuente J., Latapia T., Ortega C. The Impact of Climate Trends on a Tick Affecting Public Health: A Retrospective Modeling Approach for Hyalomma marginatum (Ixodidae) PLoS ONE. 2015;10:e0125760. doi: 10.1371/journal.pone.0125760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray J., Dantas-Torres F., Estrada-Peña A., Levin M. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick-Borne Dis. 2013;4:171–180. doi: 10.1016/j.ttbdis.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A., Prisma-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latrofa M.S., Angelou A., Giannelli A., Annoscia G., Ravagnan S., Dantas-Torres F., Capelli G., Halos L., Beugnet F., Papadopoulos E., et al. Ticks and associated pathogens in dogs from Greece. Parasites Vectors. 2017;10:301. doi: 10.1186/s13071-017-2225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos R.A.N., Giannelli A., Lia R.P., Branti E., Tarallo V.D., Breitshwerdt E.B., Dantas-Torres F., Stanneck D., Otranto D. Incidence of Cercopithifilaria bainae in dogs and probability of co-infection with other tick-borne pathogens. PLoS ONE. 2014;9:e88198. doi: 10.1371/journal.pone.0088198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aktas M., Altay K., Ozubek S., Dumanli N. A survey of ixodid ticks feeding on cattle and prevalence of tick-borne pathogens in the Black Sea region of Turkey. Vet. Parasitol. 2012;187:567–571. doi: 10.1016/j.vetpar.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 25.Aktas M., Altay K., Dumanli N. Determination of prevalence and risk factors for infection with Babesia ovis in small ruminants from Turkey by polymerase chain reaction. Parasitol. Res. 2007;100:797–802. doi: 10.1007/s00436-006-0345-2. [DOI] [PubMed] [Google Scholar]
- 26.Aktas M., Ozubek S. A molecular survey of hemoplasmas in domestic dogs from Turkey. Vet. Microbiol. 2018;221:94–97. doi: 10.1016/j.vetmic.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Aktas M., Ozubek S. A survey of canine haemoprotozoan parasites from Turkey, including molecular evidence of an unnamed Babesia. Comp. Immunol. Microbiol. Infect. Dis. 2017;52:36–42. doi: 10.1016/j.cimid.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Al-Hosary A., Ahmed L., Ahmed J., Nijhof A., Clausen P.H. Epidemiological study on tropical theileriosis (Theileria annulata infection) in the Egyptian Oases with special reference to the molecular characterization of Theileria spp. Ticks Tick Borne Dis. 2018;9:1489–1493. doi: 10.1016/j.ttbdis.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Cassini R., Marcer F., di Regalbono A.F., Cancrini G., Gabrielli S., Moretti A., Galuppi R., Tampieri M.P., Pietrobelli M. New insights into the epidemiology of bovine piroplasmoses in Italy. Vet. Parasitol. 2012;184:77–82. doi: 10.1016/j.vetpar.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Ceci L., Iarussi F., Greco B., Lacinio R., Fornelli S., Carelli G. Retrospective study of hemoparasites in cattle in southern Italy by reverse line blot hybridization. J. Vet. Med. Sci. 2014;76:869–875. doi: 10.1292/jvms.13-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guven E., Avcioglu H., Cengiz S., Hayirli A. Vector-Borne Pathogens in Stray Dogs in Northeastern Turkey. Vector-Borne Zoonotic Dis. 2017;17:610–617. doi: 10.1089/vbz.2017.2128. [DOI] [PubMed] [Google Scholar]
- 32.Torina A., Vicente J., Alongi A., Scimence S., Turlá R., Nicosia S., Marco V.D., Caracappa S., De la Fuente J. Observed Prevalence of Tick-borne Pathogens in Domestic Animals in Sicily, Italy during 2003–2005. Zoonoses Public Health. 2007;54:8–15. doi: 10.1111/j.1863-2378.2007.00989.x. [DOI] [PubMed] [Google Scholar]
- 33.Ziam H., Kernif T., Saidani K., Kelanemer R., Hammaz Z., Geysen D. Bovine piroplasmosis-anaplasmosis and clinical signs of tropical theileriosis in the plains of Djurdjura (north Algeria) Vet. Med. Sci. 2020;6:720–729. doi: 10.1002/vms3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Açici M., Bölükbaş C.S., Pekmezcï G.Z., Gürler A.T., Umur Ş., Karaer K.Z., Çakmam A., Nalbantoğlu A.S., Nisbet C. Seroepidemiological survey of bovine tick-borne infections in the Black Sea Region of Turkey. Turk. J. Vet. Anim. Sci. 2016;40:170–174. doi: 10.3906/vet-1506-3. [DOI] [Google Scholar]
- 35.Al-Hosary A., Răileanu C., Tauchmann O., Fischer S., Nijhof A.M., Silaghi C. Epidemiology and genotyping of Anaplasma marginale and co-infection with piroplasms and other Anaplasmataceae in cattle and buffaloes from Egypt. Parasites Vectors. 2020;13:495. doi: 10.1186/s13071-020-04372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altay K., Aktas M., Dumanli N. Detection of Babesia ovis by PCR in Rhipicephalus bursa collected from naturally infested sheep and goats. Res. Vet. Sci. 2008;85:116–119. doi: 10.1016/j.rvsc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Aydin M.F., Aktas M., Dumanli N. Molecular identification of Theileria and Babesia in ticks collected from sheep and goats in the Black Sea region of Turkey. Parasitol. Res. 2015;114:65–69. doi: 10.1007/s00436-014-4160-x. [DOI] [PubMed] [Google Scholar]
- 38.Chisu V., Alberti A., Zobba R., Foxi C., Masala G. Molecular characterization and phylogenetic analysis of Babesia and Theileria spp. in ticks from domestic and wild hosts in Sardinia. Acta Trop. 2019;196:60–65. doi: 10.1016/j.actatropica.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 39.El-Ashker M., Hotzel H., Gwida M., El-Beskawy M., Silaghi C., Tomaso H. Molecular biological identification of Babesia, Theileria, and Anaplasma species in cattle in Egypt using PCR assays, gene sequence analysis and a novel DNA microarray. Vet. Parasitol. 2015;207:329–334. doi: 10.1016/j.vetpar.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 40.Elsify A., Sivakumar T., Nayel M., Salama A., Elkhtam A., Rizk M., Mosaab O., Sultan K., Elsayed S., Igarashi I., et al. An epidemiological survey of bovine Babesia and Theileria parasites in cattle, buffaloes, and sheep in Egypt. Parasitol. Int. 2015;64:79–85. doi: 10.1016/j.parint.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Iori A., Gabrielli S., Calderini P., Moretti A., Pietrobelli M., Tampieri M.P., Galuppi R., Cancrini G. Tick reservoirs for piroplasms in central and northern Italy. Vet. Parasitol. 2010;170:291–296. doi: 10.1016/j.vetpar.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 42.Toma L., Di Luca M., Mancini F., Severini F., Mariano C., Nicolai G., Laghezza Masci V., Ciervo A., Fausto A.M., Cacciò S.M. Molecular characterization of Babesia and Theileria species in ticks collected in the outskirt of Monte Romano, Lazio Region, Central Italy. Ann Ist. Super Sanita. 2017;53:30–34. doi: 10.4415/ANN_17_01_07. [DOI] [PubMed] [Google Scholar]
- 43.Cringoli G., Otranto D., Testini G., Buono V., Di Giulio G., Traversa D., Lia R., Rinaldi L., Veneziano V., Puccini V. Epidemiology of bovine tick-borne diseases in southern Italy. Vet. Res. 2002;33:421–428. doi: 10.1051/vetres:2002028. [DOI] [PubMed] [Google Scholar]
- 44.Sadeddine R., Diarra A.Z., Laroche M., Mediannikov O., Righi S., Benakhla A., Dahmana H., Raoult D., Parola P. Molecular identification of protozoal and bacterial organisms in domestic animals and their infesting ticks from north-eastern Algeria. Ticks Tick-Borne Dis. 2020;11:101330. doi: 10.1016/j.ttbdis.2019.101330. [DOI] [PubMed] [Google Scholar]
- 45.Beck R., Vojta L., Mrljak V., Marinculić A., Beck A., Živičnjak T., Cacciò S.M. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int. J. Parasitol. 2009;39:843–848. doi: 10.1016/j.ijpara.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Leblond A., Pradier S., Pitel P.H., Fortier G., Chadoeuf J., Savatier P. An epidemiological survey of equine anaplasmosis (Anaplasma phagocytophilum) in Southern France. Rev. Sci. Tech. OIE. 2006;24:899–908. doi: 10.20506/rst.24.3.1612. [DOI] [PubMed] [Google Scholar]
- 47.Moretti A., Mangili V., Salvatori R., Maresca C., Scoccia E., Torina A., Moretta I., Gabrielli S., Tampieri M.P., Pietrobelli M. Prevalence and diagnosis of Babesia and Theileria infections in horses in Italy: A preliminary study. Vet. J. 2010;184:346–350. doi: 10.1016/j.tvjl.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 48.Camacho A.T., Pallas E., Gestal J.J., Guitiàn F.J., Olmeda A.S., Telford S.R., Speilman A. Ixodes hexagonus is the main candidate as vector of Theileria annae in northwest Spain. Vet. Parasitol. 2003;112:157–163. doi: 10.1016/S0304-4017(02)00417-X. [DOI] [PubMed] [Google Scholar]
- 49.Cassini R., Zanutto S., di Regalbono A.F., Gabrielli S., Calderini P., Moretti A., Tampieri M.P., Pietrobelli M. Canine piroplasmosis in Italy: Epidemiological aspects in vertebrate and invertebrate hosts. Vet. Parasitol. 2009;165:30–35. doi: 10.1016/j.vetpar.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 50.Criado-Fornelio A., Buling A., Pingret J.L., Boucraut-Baralon C., Alongi A., Agnone A., Torina A. Hemoprotozoa of domestic animals in France: Prevalence and molecular characterization. Vet. Parasitol. 2009;159:73–76. doi: 10.1016/j.vetpar.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Estrada-Peña A., Roura X., Sainz A., Miró G., Solano-Gallego L. Species of ticks and carried pathogens in owned dogs in Spain: Results of a one-year national survey. Ticks Tick-Borne Dis. 2017;8:443–452. doi: 10.1016/j.ttbdis.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Guo H., Sevinc F., Ceylan O., Sevinc M., Ince E., Gao Y., Moumouni P.F., Liu M., Efstratiou A., Wang G., et al. A PCR survey of vector-borne pathogens in different dog populations from Turkey. Acta Parasitol. 2017;62:533–540. doi: 10.1515/ap-2017-0064. [DOI] [PubMed] [Google Scholar]
- 53.Mrljak V., Kuleš J., Mihaljević Ž., Torti M., Gotić J., Crnogaj M., Živičnjak T., Mayer I., Šmit I., Bhide M., et al. Prevalence and Geographic Distribution of Vector-Borne Pathogens in Apparently Healthy Dogs in Croatia. Vector-Borne Zoonotic Dis. 2017;17:398–408. doi: 10.1089/vbz.2016.1990. [DOI] [PubMed] [Google Scholar]
- 54.Olivieri E., Zanzani S.A., Latrofa M.S., Lia R.P., Dantas-Torres F., Otranto D., Manfredi M.T. The southernmost foci of Dermacentor reticulatus in Italy and associated Babesia canis infection in dogs. Parasites Vectors. 2016;9:213. doi: 10.1186/s13071-016-1502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pennisi M.G., Caprì A., Solano-Gallego L., Lombardo G., Torina A., Masucci M. Prevalence of antibodies against Rickettsia conorii, Babesia canis, Ehrlichia canis, and Anaplasma phagocytophilum antigens in dogs from the Stretto di Messina area (Italy) Ticks Tick-Borne Dis. 2012;3:315–318. doi: 10.1016/j.ttbdis.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 56.René-Martellet M., Moro C.V., Chêne J., Bourdoiseau G., Chabanne L., Mavingui P. Update on epidemiology of canine babesiosis in Southern France. BMC Vet. Res. 2015;11:223. doi: 10.1186/s12917-015-0525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solano-Gallego L., Trotta M., Carli E., Carcy B., Caldin M., Furlanello T. Babesia canis canis and Babesia canis vogeli clinicopathological findings and DNA detection by means of PCR-RFLP in blood from Italian dogs suspected of tick-borne disease. Vet. Parasitol. 2008;157:211–221. doi: 10.1016/j.vetpar.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 58.Trotta M., Nicetto M., Fogliazza A., Montarsi F., Caldin M., Furlanello T., Solano-Gallego L. Detection of Leishmania infantum, Babesia canis, and rickettsiae in ticks removed from dogs living in Italy. Ticks Tick-Borne Dis. 2012;3:294–297. doi: 10.1016/j.ttbdis.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 59.Veneziano V., Piantedosi D., Ferrari N., Neola B., Santoro M., Pacifico L., Sgroi G., D’Alessio N., Panico T., Leutenegger C.M., et al. Distribution and risk factors associated with Babesia spp. infection in hunting dogs from Southern Italy. Ticks Tick-Borne Dis. 2018;9:1459–1463. doi: 10.1016/j.ttbdis.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Zanet S., Battisti E., Pepe P., Cuica L., Colombo L., Trisciuoglio A., Ferroglio E., Cringoli G., Rinaldi L., Maurelli M.P. Tick-borne pathogens in Ixodidae ticks collected from privately-owned dogs in Italy: A country-wide molecular survey. BMC Vet. Res. 2020;16:46. doi: 10.1186/s12917-020-2263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agoulon A., Malandrin L., Lepigeon F., Vénisse M., Bonnet S., Becker C.A.M., Hoch T., Bastian S., Plantard O., Beaudeau F. A Vegetation Index qualifying pasture edges is related to Ixodes ricinus density and to Babesia divergens seroprevalence in dairy cattle herds. Vet. Parasitol. 2012;185:101–109. doi: 10.1016/j.vetpar.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 62.Devos J., Geysen D. Epidemiological study of the prevalence of Babesia divergens in a veterinary practice in the mid-east of France. Vet. Parasitol. 2004;125:237–249. doi: 10.1016/j.vetpar.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 63.Passamonti F., Fabrizia V., Katia C., Giacomo C., Luisa M.M., Daniela P.F., Andrea V.S., Mauro C. Anaplasma phagocytophilum in horses and ticks: A preliminary survey of Central Italy. Comp. Immunol. Microbiol. Infect. Dis. 2010;33:73–83. doi: 10.1016/j.cimid.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Spada E., Proverbio D., Galluzzo P., Perego R., De Giorgi G.B., Roggero N., Caracappa S. Frequency of Piroplasms Babesia microti and Cytauxzoon felis in Stray Cats from Northern Italy. BioMed Res. Int. 2014;2014:943754. doi: 10.1155/2014/943754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagore D., García-Sanmartín J., García-Pérez A.L., Juste R.A., Hurtado A. Identification, genetic diversity and prevalence of Theileria and Babesia species in a sheep population from Northern Spain. Int. J. Parasitol. 2004;34:1059–1067. doi: 10.1016/j.ijpara.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 66.Aouadi A., Leulmi H., Boucheikhchoukh M., Benakhla A., Raoult D., Parola P. Molecular evidence of tick-borne hemoprotozoan-parasites (Theileria ovis and Babesia ovis) and bacteria in ticks and blood from small ruminants in Northern Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2017;50:34–39. doi: 10.1016/j.cimid.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 67.Azmi K., Ereqat S., Nasereddin A., Al-Jawabreh A., Baneth G., Abdeen Z. Molecular detection of Theileria, Babesia, and Hepatozoon spp. in ixodid ticks from Palestine. Ticks Tick-Borne Dis. 2016;7:734–741. doi: 10.1016/j.ttbdis.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 68.Bilgic H.B., Bakırcı S., Kose O., Unlu A.H., Hacilarlioglu S., Eren H., Weir W., Karagenc T. Prevalence of tick-borne haemoparasites in small ruminants in Turkey and diagnostic sensitivity of single-PCR and RLB. Parasites Vectors. 2017;10:211. doi: 10.1186/s13071-017-2151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Attipa C., Papasouliotis K., Solano-Gallego L., Baneth G., Nachum-Biala Y., Sarvani E., Knowles T.G., Mengi S., Morris D., Helps C., et al. Prevalence study and risk factor analysis of selected bacterial, protozoal and viral, including vector-borne, pathogens in cats from Cyprus. Parasites Vectors. 2017;10:130. doi: 10.1186/s13071-017-2063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bilgic H.B., Pekel G.K., Hosgor M., Karagenc T. A Retrospective Epidemiological Study: The Prevalence of Ehrlichia canis and Babesia vogeli in Dogs in the Aegean Region of Turkey. Acta Vet. 2019;69:164–176. doi: 10.2478/acve-2019-0013. [DOI] [Google Scholar]
- 71.Dyachenko V., Pantchev N., Balzer H.J., Meyersen A., Straubinger R.K. First case of Anaplasma platys infection in a dog from Croatia. Parasites Vectors. 2012;5:49. doi: 10.1186/1756-3305-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Otranto D., Testini G., Dantas-Torres F., Latrofa M.S., Dniz P.P.V., De Caprariis D., Lia R.P., Mencke N., Stanneck D., Capelli G., et al. Diagnosis of canine vector-borne diseases in young dogs: A longitudinal study. J. Clin. Microbiol. 2010;48:3316–3324. doi: 10.1128/JCM.00379-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pennisi M.G., Persichetti M.F., Serrano L., Altet L., Reale S., Gulotta L., Solano-Gallego L. Ticks and associated pathogens collected from cats in Sicily and Calabria (Italy) Parasites Vectors. 2015;8:512. doi: 10.1186/s13071-015-1128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ebani V.V., Bertelloni F., Turchi B., Filogari D., Cerri D. Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy. Asian Pac. J. Trop. Med. 2015;8:714–717. doi: 10.1016/j.apjtm.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 75.Giannelli A., Latrofa M.S., Nachum-Biala Y., Hodžić A., Greco G., Attanasi A., Annoscia G., Otranto D., Baneth G. Three different Hepatozoon species in domestic cats from southern Italy. Ticks Tick-borne Dis. 2017;8:721–724. doi: 10.1016/j.ttbdis.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 76.Ortuño A., Castellà J., Criado-Fornelio A., Buling A., Barba-Carretero J. Molecular detection of a Hepatozoon species in stray cats from a feline colony in North-eastern Spain. Vet. J. 2008;177:134–135. doi: 10.1016/j.tvjl.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 77.Pacifico L., Braff J., Buono F., Beall M., Neaola B., Buch J., Sgroi G., Piantedosi D., Santoror M., Tyrell P., et al. Hepatozoon canis in hunting dogs from Southern Italy: Distribution and risk factors. Parasitol. Res. 2020;119:3023–3031. doi: 10.1007/s00436-020-06820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ebani V.V., Nardoni S., Fognani G., Bertelloni F., Vasta V., Papini R.A., Verin R., Poli A., Mancianti F. Molecular detection of vector-borne bacteria and protozoa in healthy hunting dogs from Central Italy. Asian Pac. J. Trop. Biomed. 2015;5:108–112. doi: 10.1016/S2221-1691(15)30153-2. [DOI] [Google Scholar]
- 79.Vojta L., Mrljak V., Ćurković S., Živičnjak T., Marinculić A., Beck R. Molecular epizootiology of canine hepatozoonosis in Croatia. Int. J. Parasitol. 2009;39:1129–1136. doi: 10.1016/j.ijpara.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 80.Aktas M., Özübek S., Ipek D.N.S. Molecular investigations of Hepatozoon species in dogs and developmental stages of Rhipicephalus sanguineus. Parasitol. Res. 2013;112:2381–2385. doi: 10.1007/s00436-013-3403-6. [DOI] [PubMed] [Google Scholar]
- 81.Aktas M., Altay K., Dumanli N. PCR-based detection of Theileria ovis in Rhipicephalus bursa adult ticks. Vet. Parasitol. 2006;140:259–263. doi: 10.1016/j.vetpar.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 82.Aktas M., Dumanli N., Angin M. Cattle infestation by Hyalomma ticks and prevalence of Theileria in Hyalomma species in the east of Turkey. Vet. Parasitol. 2004;119:1–8. doi: 10.1016/j.vetpar.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 83.Hassan M., Gabr H., Abdel-Shafy S., Hammad K., Mokhtar M. Prevalence of tick-vectors of Theileria annulata infesting the one humped camels in Giza, Egypt. J. Egypt. Soc. Parasitol. 2017;47:425–432. doi: 10.21608/jesp.2017.77797. [DOI] [Google Scholar]
- 84.Ziam H., Kelanamer R., Aissi M., Ababou A., Berkvens D., Geysen D. Prevalence of bovine theileriosis in North Central region of Algeria by real-time polymerase chain reaction with a note on its distribution. Trop. Anim. Heal. Prod. 2015;47:787–796. doi: 10.1007/s11250-015-0772-0. [DOI] [PubMed] [Google Scholar]
- 85.Tabar M.D., Altet L., Francino O., Sánchez A., Ferrer L., Roura X. Vector-borne infections in cats: Molecular study in Barcelona area (Spain) Vet. Parasitol. 2008;151:332–336. doi: 10.1016/j.vetpar.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 86.Giménez Pardo C., Casado N., Criado-Fornelio A., de Miguel F.Á., Dominguez-Peñafiel G. A molecular survey of Piroplasmida and Hepatozoon isolated from domestic and wild animals in Burgos (Northern Spain) Vet. Parasitol. 2009;162:147–150. doi: 10.1016/j.vetpar.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 87.Aktas M., Dumanli N., Çetinkaya B., Çakmak A. Field evaluation of PCR in detecting Theileria annulata infection in cattle in eastern Turkey. Vet. Rec. 2002;150:548–549. doi: 10.1136/vr.150.17.548. [DOI] [PubMed] [Google Scholar]
- 88.Al-Hosary A.A.T., Ahmed L., Seitzer U. First Report of Molecular Identification and Characterization of Theileria spp. from Water Buffaloes (Bubalus bubalis) in Egypt. Adv. Anim. Vet. Sci. 2015;3:629–633. doi: 10.14737/journal.aavs/2015/3.12.629.633. [DOI] [Google Scholar]
- 89.AL-Hosary A., Abdel-Rady A., Ahmed L., Al-Hosary A. Using Polymerase chain reaction (PCR) for Diagnosis of Bovine Theileriosis in Upper Egypt. IJAVMS. 2010;4:67–74. doi: 10.13140/RG.2.1.4479.7600. [DOI] [Google Scholar]
- 90.Dumanli N., Aktas M., Cetinkaya B., Cakmak A., Koroglu E., Saki C.E., Erdogmus Z., Nalbantoglu S., Ongor H., Simşek S., et al. Prevalence and distribution of tropical theileriosis in eastern Turkey. Vet. Parasitol. 2005;127:9–15. doi: 10.1016/j.vetpar.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 91.Gargano V., Blanda V., Gambino D., La Russa F., Di Cataldo S., Gentile A., Schirò G., Torina A., Millàn J., Vicari D. Serological Survey and Molecular Characterization of Theileria annulata in Sicilian Cattle. Pathogens. 2021;10:101. doi: 10.3390/pathogens10020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Inci A., Ica A., Yildirim A., Vatansever Z., Cakmak A., Albasan H., Çam Y., Atasever A., Duzlu O. Epidemiology of tropical theileriosis in the Cappadocia region. Turk J. Vet. Anim. Sci. 2008;32:57–64. doi: 10.1007/s00436-007-0693-6. [DOI] [PubMed] [Google Scholar]
- 93.Sallemi S., Rjeibi M.R., Rouatbi M., Amairia S., Ben Said M., Khamassi Khbou M., Gharbi M. Molecular prevalence and phylogenetic analysis of Theileria annulata and Trypanosoma evansi in cattle in Northern Tunisia. Vet. Med. Sci. 2017;4:17–25. doi: 10.1002/vms3.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sayin F., Dinçer S., Karaer Z., Cakmak A., INci A., Yukari B.A., Eren H., Vatansever Z., Nalbantoğlu S. Studies on the Epidemiology of Tropical Theileriosis (Theileria annulata Infection) in Cattle in Central Anatolia, Turkey. Trop. Anim. Health Prod. 2003;35:521–539. doi: 10.1023/A:1027348708038. [DOI] [PubMed] [Google Scholar]
- 95.Ziam H., Benaouf H. Prevalence of blood parasites in cattle from Wilayates of Annaba and El Tarf East Algeria. Archs Inst. Pasteur. Tunis. 2004;81:1–4. [PubMed] [Google Scholar]
- 96.Laus F., Veronesi F., Passamonti F., Paggi E., Cerquetella M., Hyatt D., Tesei B., Fioretti D.P. Prevalence of Tick Borne Pathogens in Horses from Italy. J. Vet. Med. Sci. 2013;75:715–720. doi: 10.1292/jvms.12-0449. [DOI] [PubMed] [Google Scholar]
- 97.Altay K., Dumanli N., Aktas M. A study on ovine tick-borne hemoprotozoan parasites (Theileria and Babesia) in the East Black Sea Region of Turkey. Parasitol. Res. 2012;111:149–153. doi: 10.1007/s00436-011-2811-8. [DOI] [PubMed] [Google Scholar]
- 98.Aydın M.F., Özübek S., Aktaş M. Molecular survey of Anaplasma and Ehrlichia species in cattle from Karaman of Turkey, including a novel tandem report of Anaplasma marginale msp1a gene. Ankara. Üniv. Vet. Fak. Derg. 2019;66:255–260. doi: 10.33988/auvfd.456594. [DOI] [Google Scholar]
- 99.Ayllón T., Diniz P.P.V.P., Breitschwerdt E.B., Villaescusa A., Rodríguez-Franco F., Sainz A. Vector-Borne Diseases in Client-Owned and Stray Cats from Madrid, Spain. Vector-Borne Zoonotic Dis. 2011;12:143–150. doi: 10.1089/vbz.2011.0729. [DOI] [PubMed] [Google Scholar]
- 100.Belkahia H., Ben Said M., Ghribi R., Selmi R., Ben Asker A., Yahiaoui M., Bousrih M., Daaloul-Jedidi M., Messadi L. Molecular detection, genotyping and phylogeny of Anaplasma spp. in Rhipicephalus ticks from Tunisia. Acta Trop. 2018;191:38–49. doi: 10.1016/j.actatropica.2018.12.034. [DOI] [PubMed] [Google Scholar]
- 101.Belkahia H., Said M.B., Sayahi L., Alberti A., Messadi L. Detection of novel strains genetically related to Anaplasma platys in Tunisian one-humped camels (Camelus dromedarius) J. Infect. Dev. Ctries. 2015;9:1117–1125. doi: 10.3855/jidc.6950. [DOI] [PubMed] [Google Scholar]
- 102.Boucheikhchoukh M., Laroche M., Aouadi A., Dib L., Benakhla A., Raoult D., Parola P. MALDI-TOF MS identification of ticks of domestic and wild animals in Algeria and molecular detection of associated microorganisms. Comp. Immunol. Microbiol. Infect. Dis. 2018;57:39–49. doi: 10.1016/j.cimid.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 103.Dahmani M., Davoust B., Tahir D., Raoult D., Fenollar F., Mediannikov O. Molecular investigation and phylogeny of Anaplasmataceae species infecting domestic animals and ticks in Corsica, France. Parasites Vectors. 2017;10:302. doi: 10.1186/s13071-017-2233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dahmani M., Davoust B., Benterki M., Fenollar F., Raoult D., Mediannikov O. Development of a new PCR-based assay to detect Anaplasmataceae and the first report of Anaplasma phagocytophilum and Anaplasma platys in cattle from Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2015;39:39–45. doi: 10.1016/j.cimid.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 105.de la Fuente J., Torina A., Caracappa S., Tumino G., Furlà R., Almazàn C., Kocan K.M. Serologic and molecular characterization of Anaplasma species infection in farm animals and ticks from Sicily. Vet. Parasitol. 2005;133:357–362. doi: 10.1016/j.vetpar.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 106.Diakou A., Di Cesare A., Morelli S., Colombo M., Halos L., Simonato G., Tamvakis A., Beugnet F., Paoletti B., Traversa D. Endoparasites and vector-borne pathogens in dogs from Greek islands: Pathogen distribution and zoonotic implications. PLOS Neglected Trop. Dis. 2019;13:e0007003. doi: 10.1371/journal.pntd.0007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Díaz-Regañón D., Roura X., Suárez M.L., León M., Sainz Á. Serological evaluation of selected vector-borne pathogens in owned dogs from northern Spain based on a multicenter study using a commercial test. Parasites Vectors. 2020;13:301. doi: 10.1186/s13071-020-04172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Elhamiani Khatat S., Daminet S., Kachani M., Leutenegger C.M., Duchateau L., El Amri H., Hing M., Azrib R., Sahibi H. Anaplasma spp. in dogs and owners in north-western Morocco. Parasites Vectors. 2017;10:202. doi: 10.1186/s13071-017-2148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lbacha H.A., Alali S., Zouagui Z., Mamoun L.E.I., Rhalem A., Petit E., Haddad N., Gandoin C., Gandoin C., Boulouis H.-J., et al. High Prevalence of Anaplasma spp. in Small Ruminants in Morocco. Transbound. Emerg. Dis. 2017;64:250–263. doi: 10.1111/tbed.12366. [DOI] [PubMed] [Google Scholar]
- 110.Petruccelli A., Ferrara G., Iovane G., Schettini R., Ciarcia R., Caputo V., Pompamea M., Pagnini U., Montagnaro S. Seroprevalence of Ehrlichia spp., Anaplasma spp., Borrelia burgdorferi sensu lato, and Dirofilaria immitis in Stray Dogs, from 2016 to 2019, in Southern Italy. Animals. 2020;11:9. doi: 10.3390/ani11010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zaid T., Ereqat S., Nasereddin A., Al-Jawabreh A., Abdelkader A., Abdeen Z. Molecular characterization of Anaplasma and Ehrlichia in ixodid ticks and reservoir hosts from Palestine: A pilot survey. Vet. Med. Sci. 2019;5:230–242. doi: 10.1002/vms3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aktas M., Altay K., Dumanli N. Molecular detection and identification of Anaplasma and Ehrlichia species in cattle from Turkey. Ticks Tick-Borne Dis. 2011;2:62–65. doi: 10.1016/j.ttbdis.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 113.Seng P., Sarih M., Socolovschi C., Boudebouch N., Hassar M., Paraola P., Raoult D., Brouqui P. Detection of Anaplasmataceae in ticks collected in Morocco. Clin. Microbiol. Infect. 2009;15:86–87. doi: 10.1111/j.1469-0691.2008.02251.x. [DOI] [PubMed] [Google Scholar]
- 114.Elhariri M.D., Elhelw R.A., Hamza D.A., Soliman D.E. Molecular detection of Anaplasma marginale in the egyptian water buffaloes (Bubalus bubalis) based on major surface protein 1. J. Egypt Soc. Parasitol. 2017;47:247–252. doi: 10.21608/jesp.2017.77758. [DOI] [Google Scholar]
- 115.Torina A., Alongi A., Naranjo V., Estrada-Peña A., Vincente J., Scimeca S., Marino A.M.F., Salina F., Caracappa S., de la Fuente J. Prevalence and Genotypes of Anaplasma Species and Habitat Suitability for Ticks in a Mediterranean Ecosystem. Appl. Environ. Microbiol. 2008;74:7578–7584. doi: 10.1128/AEM.01625-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aktas M., Altay K., Dumanli N., Kalkan A. Molecular detection and identification of Ehrlichia and Anaplasma species in ixodid ticks. Parasitol Res. 2009;104:1243–1248. doi: 10.1007/s00436-009-1377-1. [DOI] [PubMed] [Google Scholar]
- 117.Altay K., Dumanlı N., Aktas M., Ozubek S. Survey of anaplasma infections in small ruminants from east part of Turkey. Kafkas Univ. Vet. Fak. Derg. 2014;20:1–4. doi: 10.9775/kvfd.2013.9189. [DOI] [Google Scholar]
- 118.Cabezas-Cruz A., Gallois M., Fontugne M., Allain E., Denoual M., Moutailler S., Devillers E., Zientara S., Memmi M., Chauvin A., et al. Epidemiology and genetic diversity of Anaplasma ovis in goats in Corsica, France. Parasites Vectors. 2019;12:3. doi: 10.1186/s13071-018-3269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chisu V., Foxi C., Mannu R., Satta G., Masala G. A five-year survey of tick species and identification of tick-borne bacteria in Sardinia, Italy. Ticks Tick-Borne Dis. 2018;9:678–681. doi: 10.1016/j.ttbdis.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 120.Oter K., Cetinkaya H., Vurusaner C., Toparlak M., Ergunay K. Molecular Detection and Typing Of Anaplasma Species In Small Ruminants In Thrace Region Of Turkey. Kafkas Univ. Vet. Fak. Derg. 2016;22:133–138. doi: 10.9775/kvfd.2015.14075. [DOI] [Google Scholar]
- 121.Selmi R., Ben Said M., Dhibi M., Ben Yahia H., Abdelaali H., Messadi L. Genetic diversity of groEL and msp4 sequences of Anaplasma ovis infecting camels from Tunisia. Parasitol. Int. 2020;74:101980. doi: 10.1016/j.parint.2019.101980. [DOI] [PubMed] [Google Scholar]
- 122.Alberti A., Addis M.F., Sparagano O., Zobba R., Chessa B., Cubeddu T., Parpaglia M.L.P., Ardu M., Pittau M. Anaplasma phagocytophilum, Sardinia, Italy. Emerg. Infect. Dis. 2005;11:1322–1324. doi: 10.3201/eid1108.050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Çetinkaya H., Matur E., Akyazi İ., Ekiz E.E., Aydin L., Toparlak M. Serological and molecular investigation of Ehrlichia spp. and Anaplasma spp. in ticks and blood of dogs, in the Thrace Region of Turkey. Ticks Tick-Borne Dis. 2016;7:706–714. doi: 10.1016/j.ttbdis.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 124.Chastagner A., Dugat T., Vourc’h G., Verheyden H., Legrand L., Bachy V., Chabanne L., Joncour G., Maillard R., Boulouis H.-J., et al. Multilocus sequence analysis of Anaplasma phagocytophilum reveals three distinct lineages with different host ranges in clinically ill French cattle. Vet. Res. 2014;45:114. doi: 10.1186/s13567-014-0114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ebani V., Cerri D., Fratini F., Ampola M., Andreani E. Seroprevalence of Anaplasma phagocytophilum in domestic and wild animals from central Italy. New Microbiol. 2008;31:371–375. [PubMed] [Google Scholar]
- 126.Ebani V.V., Guardone L., Marra F., Altomonte I., Nardoni S., Mancianti F. Arthropod-Borne Pathogens in Stray Cats from Northern Italy: A Serological and Molecular Survey. Animals. 2020;10:2334. doi: 10.3390/ani10122334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ebani V.V., Bertelloni F., Torracca B., Cerri D. Serological survey of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, and Ehrlichia canis infections in rural and urban dogs in Central Italy. Ann. Agric. Environ. Med. 2014;21:671–675. doi: 10.5604/12321966.1129912. [DOI] [PubMed] [Google Scholar]
- 128.Ebani V.V., Bertelloni F., Turchi B., Cerri D. Serological and molecular survey of Anaplasma phagocytophilum in Italian hunting dogs. Ann. Agric. Environ. Med. 2013;20:289–292. [PubMed] [Google Scholar]
- 129.Ebani V.V. Serological Survey of Ehrlichia canis and Anaplasma phagocytophilum in Dogs from Central Italy: An Update (2013–2017) Pathogens. 2019;8:3. doi: 10.3390/pathogens8010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ghafar M.W., Amer S.A. Prevalence and first molecular characterization of Anaplasma phagocytophilum, the agent of human granulocytic anaplasmosis, in Rhipicephalus sanguineus ticks attached to dogs from Egypt. J. Adv. Res. 2012;3:189–194. doi: 10.1016/j.jare.2011.08.002. [DOI] [Google Scholar]
- 131.Giudice E., Giannetto C., Furco V., Alongi A., Torina A. Anaplasma phagocytophilum seroprevalence in equids: A survey in Sicily (Italy) Parasitol. Res. 2012;111:951–955. doi: 10.1007/s00436-012-2854-5. [DOI] [PubMed] [Google Scholar]
- 132.Gokce I., Genç O., Akca A., Vatansever Z., Unver A., Erdogan H. Molecular and serological evidence of Anaplasma phagocytophilum infection of farm animals in the Black Sea Region of Turkey. Acta. Vet. Hung. 2008;56:281–292. doi: 10.1556/avet.56.2008.3.2. [DOI] [PubMed] [Google Scholar]
- 133.Huber D., Reil I., Duvnjak S., Jurković D., Lukačević D., Pilat M., Beck A., Mihaljević Ž., Vojta L., Polkinghorne A. Molecular detection of Anaplasma platys, Anaplasma phagocytophilum and Wolbachia sp. but not Ehrlichia canis in Croatian dogs. Parasitol. Res. 2017;116:3019–3026. doi: 10.1007/s00436-017-5611-y. [DOI] [PubMed] [Google Scholar]
- 134.Laamari A., Azzag N., Tennah S., Derdour S.Y., China B., Bouadballah R., Ghalmi F. Seroprevalence of Antibodies Against Anaplasma Phagocytophilum and Borrelia Burgdorferi in Horses (Equus Caballus) from Northern Algeria. J. Vet. Res. 2020;64:413–419. doi: 10.2478/jvetres-2020-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.M’ghirbi Y., Bèji M., Oporto B., Khrouf F., Hurtado A., Bouattour A. Anaplasma marginale and A. phagocytophilum in cattle in Tunisia. Parasites Vectors. 2016;9:556. doi: 10.1186/s13071-016-1840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Palomar A., García-Álvarez L., Santibáñez S., Portillo A., Oteo J.A. Detection of tick-borne ‘Candidatus Neoehrlichia mikurensis’ and Anaplasma phagocytophilum in Spain in 2013. Parasites Vectors. 2014;7:57. doi: 10.1186/1756-3305-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Papa A., Tsioka K., Kontana A., Papadopoulos C., Giadinis N. Bacterial pathogens and endosymbionts in ticks. Ticks Tick Borne Dis. 2017;8:31–35. doi: 10.1016/j.ttbdis.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 138.Persichetti M.F., Solano-Gallego L., Serrano L., Altet L., Reale S., Masucci M., Pennisi M.-G. Detection of vector-borne pathogens in cats and their ectoparasites in southern Italy. Parasites Vectors. 2016;9:247. doi: 10.1186/s13071-016-1534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Satta G., Chisu V., Cabras P., Fois F., Masala G. Pathogens and symbionts in ticks: A survey on tick species distribution and presence of tick-transmitted micro-organisms in Sardinia, Italy. Pt 1J. Med. Microbiol. 2011;60:63–68. doi: 10.1099/jmm.0.021543-0. [DOI] [PubMed] [Google Scholar]
- 140.Solano-Gallego L., Hegarty B., Espada Y., Llull J., Breitschwerdt E. Serological and molecular evidence of exposure to arthropod-borne organisms in cats from northeastern Spain. Vet. Microbiol. 2006;118:274–277. doi: 10.1016/j.vetmic.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 141.Vascellari M., Ravagnan S., Carminato A., Cazzin S., Carli E., Da Rold G., Lucchese L., Natale A., Otranto D., Capelli G. Exposure to vector-borne pathogens in candidate blood donor and free-roaming dogs of northeast Italy. Parasites Vectors. 2016;9:369. doi: 10.1186/s13071-016-1639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aktas M., Özübek S., Altay K., Ipek N.D.S., Balkaya I., Utuk A.E., Kirbas A., Şimsek S., Dumanli N. Molecular detection of tick-borne rickettsial and protozoan pathogens in domestic dogs from Turkey. Parasites Vectors. 2015;8:157. doi: 10.1186/s13071-015-0763-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Attipa C., Solano-Gallego L., Leutenegger C.M., Papasouliotis K., Soutter F., Balzer J., Carver S., Buch J.S., Tasker S. Associations between clinical canine leishmaniosis and multiple vector-borne co-infections: A case-control serological study. BMC Vet. Res. 2019;15:331. doi: 10.1186/s12917-019-2083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Azzag N., Petit E., Gandoin C., Bouillin C., Ghalmi F., Haddad N., Boulouis H.-J. Prevalence of select vector-borne pathogens in stray and client-owned dogs from Algiers. Comp. Immunol. Microbiol. Infect. Dis. 2015;38:1–7. doi: 10.1016/j.cimid.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 145.De La Fuente J., Torina A., Naranjo V., Nicosia S., Alongi A., La Mantia F., Kocan K.M. Molecular characterization of Anaplasma platys strains from dogs in Sicily, Italy. BMC Vet. Res. 2006;2:24. doi: 10.1186/1746-6148-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hofmann-Lehmann R., Wagmann N., Meli M.L., Riond B., NOvacco M., JOeket D., Centilini F., Marsilio F., Pennisi M.G., Lloret A., et al. Detection of “Candidatus Neoehrlichia mikurensis” and other Anaplasmataceae and Rickettsiaceae in Canidae in Switzerland and Mediterranean countries. Schweiz. Arch. Tierh. 2016;158:691–700. doi: 10.17236/sat00087. [DOI] [PubMed] [Google Scholar]
- 147.Selmi R., Dhibi M., Ben Said M., Ben Yahia H., Abdelaali H., Ameur H., Baccouche S., Gritli A., Mhabdi M. Evidence of natural infections with Trypanosoma, Anaplasma and Babesia spp. in military livestock from Tunisia. Trop Biomed. 2019;36:742–757. [PubMed] [Google Scholar]
- 148.Sparagano O.A.E., de Vos A.P., Paoletti B., Cammà C., de Santis P., Otranto D., Giangaspero A. Molecular Detection of Anaplasma Platys in Dogs Using Polymerase Chain Reaction and Reverse Line Blot Hybridization. J. VET Diagn. Investig. 2003;15:527–534. doi: 10.1177/104063870301500604. [DOI] [PubMed] [Google Scholar]
- 149.Mancini F., Vescio M.F., Toma L., Di Luca M., Severini F., Cacciò S.M., Mariano C., Nicolai G., Laghezza Masci V., Fausto A.M., et al. Detection of tick-borne pathogens in ticks collected in the suburban area of Monte Romano, Lazio Region, Central Italy. Ann. Ist Super Sanita. 2019;55:143–150. doi: 10.4415/ANN_19_02_06. [DOI] [PubMed] [Google Scholar]
- 150.Otranto D., Napoli E., Latrofa M.S., Annoscia G., Tarallo V.D., Greco G., Lorusso E., Gulotta L., Falsone L., Basano F.S., et al. Feline and canine leishmaniosis and other vector-borne diseases in the Aeolian Islands: Pathogen and vector circulation in a confined environment. Vet. Parasitol. 2017;236:144–151. doi: 10.1016/j.vetpar.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 151.Pennisi M.G., La Camera E., Giacobbe L., Orlandella B.M., Lentini V., Zummo S., Fera M.T. Molecular detection of Bartonella henselae and Bartonella clarridgeiae in clinical samples of pet cats from Southern Italy. Res. Vet. Sci. 2009;88:379–384. doi: 10.1016/j.rvsc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 152.Azzag N., Haddad N., Durand B., Petit E., Ammouche A., Chomel B., Boulouis H.-J. Population Structure of Bartonella henselae in Algerian Urban Stray Cats. PLoS ONE. 2012;7:e43621. doi: 10.1371/journal.pone.0043621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bessas A., Leulmi H., Bitam I., Zaidi S., Ait-Oudhia K., Raoult D., Parola P. Molecular evidence of vector-borne pathogens in dogs and cats and their ectoparasites in Algiers, Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2016;45:23–28. doi: 10.1016/j.cimid.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 154.Chisu V., Foxi C., Masu G., D’Amaddio B., Masala G. Detection of potentially pathogenic bacteria from Ixodes ricinus carried by pets in Tuscany, Italy. Vet. Rec. Open. 2020;7:e000395. doi: 10.1136/vetreco-2020-000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Diakou A., Di Cesare A., Accettura P.M., Barros L., Iorio R., Paoletti B., Frangipane di Regalbono A., Halos L., Beugnet F., Traverse D. Intestinal parasites and vector-borne pathogens in stray and free-roaming cats living in continental and insular Greece. PLoS Negl. Trop. Dis. 2017;11:e0005335. doi: 10.1371/journal.pntd.0005335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pinna Parpaglia M.L., Masu G., Masala G., Porcu R., Zobba R., Pintori G., Cocco R. Seroprevalence of Bartonella henselae in Dogs and Cats in Sassari. Vet. Res. Commun. 2007;31:317. doi: 10.1007/s11259-007-0056-x. [DOI] [PubMed] [Google Scholar]
- 157.Chochlakis D., Cutler S., Giadinis N.D., Psaroulaki A. Bartonella vinsonii subsp. arupensis infection in animals of veterinary importance, ticks and biopsy samples. New Microbes New Infect. 2020;34:100652. doi: 10.1016/j.nmni.2020.100652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Henn J.B., Vanhorn B.A., Kasten R.W., Kachani M., Chomel B.B. Antibodies to Bartonella vinsonii subsp. berkhoffii in Moroccan dogs. Am. J. Trop. Med. Hyg. 2006;74:222–223. doi: 10.4269/ajtmh.2006.74.222. [DOI] [PubMed] [Google Scholar]
- 159.Brinkmann A., Hekimoğlu O., Dinçer E., Hagedorn P., Nitsche A., Ergünay K. A cross-sectional screening by next-generation sequencing reveals Rickettsia, Coxiella, Francisella, Borrelia, Babesia, Theileria and Hemolivia species in ticks from Anatolia. Parasites Vectors. 2019;12:26. doi: 10.1186/s13071-018-3277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chisu V., Foxi C., Tanda A., Masala G. Molecular evidence of Chlamydiales in ticks from wild and domestic hosts in Sardinia, Italy. Parasitol. Res. 2018;117:981–987. doi: 10.1007/s00436-018-5772-3. [DOI] [PubMed] [Google Scholar]
- 161.Abdullah H.H.A.M., El-Shanawany E.E., Abdel-Shafy S., Abou-Zeina H.A.A., Abdel-Rahman E.H. Molecular and immunological characterization of Hyalomma dromedarii and Hyalomma excavatum (Acari: Ixodidae) vectors of Q fever in camels. Vet. World. 2018;11:1109–1119. doi: 10.14202/vetworld.2018.1109-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Barkallah M., Gharbi Y., Hmani M., Mallek Z., Gautier M., Gdoura R., Fendri I. Serological and molecular evidence of coxiellosis and risk factors in sheep flocks in central-eastern Tunisia. Comp. Immunol. Microbiol. Infect. Dis. 2018;57:15–21. doi: 10.1016/j.cimid.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 163.Benaissa M.H., Ansel S., Mohamed-Cherif A., Benfodil K., Khelef D., Youngs C.R., Kaidi R., Ait-Oudhia K. Seroprevalence and risk factors for Coxiella burnetii, the causative agent of Q fever in the dromedary camel (Camelus dromedarius) population in Algeria. Onderstepoort J. Vet. Res. 2017;84:1461. doi: 10.4102/ojvr.v84i1.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chochlakis D., Santos A.S., Giadinis N.D., Papadopoulos D., Boubaris L., Kalaitzakis E., Psaroulaki A., Kritas S.K., Petridou E.I. Genotyping of Coxiella burnetii in sheep and goat abortion samples. BMC Microbiol. 2018;18:204. doi: 10.1186/s12866-018-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Di Domenico M., Curini V., De Massis F., Di Provvido A., Scacchia M., Cammà C. Coxiella burnetii in Central Italy: Novel Genotypes Are Circulating in Cattle and Goats. Vector-Borne Zoonotic Dis. 2014;14:710–715. doi: 10.1089/vbz.2014.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ebani V.V. Retrospective Study on the Occurrence of Antibodies against Coxiella burnetii in Dogs from Central Italy. Pathogens. 2020;9:1068. doi: 10.3390/pathogens9121068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Filioussis G., Theodoridis A., Papadopoulos D., Gelasakis A.I., Vouraki S., Bramis G., Arsenos G. Serological prevalence of Coxiella burnetii in dairy goats and ewes diagnosed with adverse pregnancy outcomes in Greece. Ann. Agric. Environ. Med. 2017;24:702–705. doi: 10.26444/aaem/80706. [DOI] [PubMed] [Google Scholar]
- 168.Knap N., Žele D., Glinšek Biškup U., Avšič-Županc T., Vengušt G. The prevalence of Coxiella burnetii in ticks and animals in Slovenia. BMC Vet. Res. 2019;15:368. doi: 10.1186/s12917-019-2130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lausevic D. Prevalence of Coxiella burnetii antibodies in sheep in the territory of Montenegro. Acta Vet. Beo. 2001;51:149–156. [Google Scholar]
- 170.Loftis A., Reeves W., Szumlas D., Abbassy M., Helmy I., Moriarity J., Dash G. Rickettsial agents in Egyptian ticks collected from domestic animals. Exp. Appl. Acarol. 2006;40:67–81. doi: 10.1007/s10493-006-9025-2. [DOI] [PubMed] [Google Scholar]
- 171.Masala G., Porcu R., Sanna G., Chessa G., Cillara G., Chisu V., Tola S. Occurrence, distribution, and role in abortion of Coxiella burnetii in sheep and goats in Sardinia, Italy. Vet. Microbiol. 2004;99:301–305. doi: 10.1016/j.vetmic.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 172.Pape M., Bouzalas E.G., Koptopoulos G.S., Mandraveli K., Arvanitidou-Vagiona M., Nikolaidis P., Alexou-Daniel S. The serological prevalence of Coxiella burnetii antibodies in sheep and goats in northern Greece. Clin. Microbiol. Infect. 2009;15:146–147. doi: 10.1111/j.1469-0691.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 173.Psaroulaki A., Ragiadakou D., Kouris G., Papadopoulos B., Chaniotis B., Tselentis Y. Ticks, Tick-Borne Rickettsiae, and Coxiella burnetii in the Greek Island of Cephalonia. Ann. N. Y. Acad. Sci. 2006;1078:389–399. doi: 10.1196/annals.1374.077. [DOI] [PubMed] [Google Scholar]
- 174.Ruiz-Fons F., Rodríguez Ó., Torina A., Naranjo V., Gortázar C., de la Fuente J. Prevalence of Coxiella burnetti infection in wild and farmed ungulates. Vet. Microbiol. 2008;126:282–286. doi: 10.1016/j.vetmic.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 175.Selmi R., Ben Said M., Mamlouk A., Ben Yahia H., Messadi L. Molecular detection and genetic characterization of the potentially pathogenic Coxiella burnetii and the endosymbiotic Candidatus Midichloria mitochondrii in ticks infesting camels (Camelus dromedarius) from Tunisia. Microb. Pathog. 2019;136:103655. doi: 10.1016/j.micpath.2019.103655. [DOI] [PubMed] [Google Scholar]
- 176.Selmi R., Mamlouk A., Ben Yahia H., Abdelaali H., Ben Said M., Sellami K., Daaloul-Jedidi M., Jemli M.H., Messadi L. Coxiella burnetii in Tunisian dromedary camels (Camelus dromedarius): Seroprevalence, associated risk factors and seasonal dynamics. Acta Trop. 2018;188:234–239. doi: 10.1016/j.actatropica.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 177.Spyridaki I., Psaroulaki A., Loukaides F., Antoniou M., Hadjichristodolou C., Tselentis Y. Isolation of Coxiella burnetii by a centrifugation shell-vial assay from ticks collected in Cyprus: Detection by nested polymerase chain reaction (PCR) and by PCR-restriction fragment length polymorphism analyses. Am. J. Trop. Med. Hyg. 2002;66:86–90. doi: 10.4269/ajtmh.2002.66.86. [DOI] [PubMed] [Google Scholar]
- 178.Attipa C., Hicks C.A.E., Barker E.N., Christodoulou V., Neofytou K., Mylonakis M.E., Siarkou V.I., Vingopoulou E.I., Soutter F., Chochlakis D. Canine tick-borne pathogens in Cyprus and a unique canine case of multiple co-infections. Ticks Tick Borne Dis. 2017;8:341–346. doi: 10.1016/j.ttbdis.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Migliore S., Gargano V., De Maria C., Gambino D., Gentile A., Vitale Badaco V., Shirò G., Mira F., Galluzzo P., Vicari D. A Cross Sectional Study on Serological Prevalence of Ehrlichia canis and Rickettsia conorii in Different Canine Population of Sicily (South-Italy) during 2017–2019. Animals. 2020;10:2444. doi: 10.3390/ani10122444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Miro G., Montoya A., Roura X., Galvez R., Sainz A. Seropositivity rates for agents of canine vector-borne diseases in Spain: A multicentre study. Parasites Vectors. 2013;6:117. doi: 10.1186/1756-3305-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Piantedosi D., Neola B., D’Alessio N., Di Prisco F., Santoro M., Pacifico L., Sgroi G., Auletta L., Buch J., Chandrashekar R. Seroprevalence and risk factors associated with Ehrlichia canis, Anaplasma spp., Borrelia burgdorferi sensu lato, and D. immitis in hunting dogs from southern Italy. Parasitol. Res. 2017;116:2651–2660. doi: 10.1007/s00436-017-5574-z. [DOI] [PubMed] [Google Scholar]
- 182.Solano-Gallego L., Llull J., Osso M., Hegarty B., Breitschwerdt E. A serological study of exposure to arthropod-borne pathogens in dogs from northeastern Spain. Vet. Res. 2006;37:231–244. doi: 10.1051/vetres:2005054. [DOI] [PubMed] [Google Scholar]
- 183.Cicculli V., Capai L., Quilichini Y., Masse S., Fernández-Alvarez A., Minodier L., Bompard P., Charrel R., Falchi A. Molecular investigation of tick-borne pathogens in ixodid ticks infesting domestic animals (cattle and sheep) and small rodents (black rats) of Corsica, France. Ticks Tick-Borne Dis. 2019;10:606–613. doi: 10.1016/j.ttbdis.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 184.Horton K., Wasfy M., Samaha H., Abdel-Rahman B., Safwat S., Fadeel M., Mohareb E., Dueger E. Serosurvey for Zoonotic Viral and Bacterial Pathogens Among Slaughtered Livestock in Egypt. Vector-Borne Zoonotic Dis. 2014;14:633–639. doi: 10.1089/vbz.2013.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Aktas M., Ozubek S. Molecular survey of haemoplasmas in shelter dogs and associations with Rhipicephalus sanguineus sensu lato. Med. Vet. Entomol. 2017;31:457–461. doi: 10.1111/mve.12244. [DOI] [PubMed] [Google Scholar]
- 186.Tennant K.V., Barker E.N., Polizopoulou Z., Helps C.R., Tasker S. Real-time quantitative polymerase chain reaction detection of haemoplasmas in healthy and unhealthy dogs from Central Macedonia, Greece. J. Small Anim. Pract. 2011;52:645–649. doi: 10.1111/j.1748-5827.2011.01126.x. [DOI] [PubMed] [Google Scholar]
- 187.Abdullah H.H.A.M., El-Molla A., Salib F.A., Allam N.A.T., Ghazy A.A., Abdel-Shafy S. Morphological and molecular identification of the brown dog tick Rhipicephalus sanguineus and the camel tick Hyalomma dromedarii (Acari: Ixodidae) vectors of Rickettsioses in Egypt. Vet. World. 2016;9:1087–1101. doi: 10.14202/vetworld.2016.1087-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Azagi T., Klement E., Perlman G., Lustig Y., Mumcuoglu K.Y., Apanaskevich D.A., Gottlieb Y. Francisella-Like Endosymbionts and Rickettsia Species in Local and Imported Hyalomma Ticks. Appl. Environ. Microbiol. 2017;83:e01302-17. doi: 10.1128/AEM.01302-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Beninati T., Genchi C., Torina A., Caracappa S., Bandi C., Lo N. Rickettsiae in Ixodid Ticks, Sicily. Emerg. Infect. Dis. 2005;11:509–511. doi: 10.3201/eid1103.040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ereqat S., Nasereddin A., Al-Jawabreh A., Azmi K., Harrus S., Mumcuoglo K., Apanaskevich D., Abdeen Z. Molecular Detection and Identification of Spotted Fever Group Rickettsiae in Ticks Collected from the West Bank, Palestinian Territories. PLoS Negl. Trop. Dis. 2016;10:e0004348. doi: 10.1371/journal.pntd.0004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Khrouf F., M’Ghirbi Y., Znazen A., Ben Jemaa M., Hammami A., Bouattour A. Detection of Rickettsia in Rhipicephalus sanguineus Ticks and Ctenocephalides felis Fleas from Southeastern Tunisia by Reverse Line Blot Assay. J. Clin. Microbiol. 2014;52:268–274. doi: 10.1128/JCM.01925-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Márquez F.J. Spotted fever group Rickettsia in ticks from southeastern Spain natural parks. Exp. Appl. Acarol. 2008;45:185–194. doi: 10.1007/s10493-008-9181-7. [DOI] [PubMed] [Google Scholar]
- 193.Oteo J.A., Portillo A., Santibáñez S., Pérez-Martínez L., Blanco J.R., Jiménez S., Ibarra V., Pérez-Palacios A., Sanz M. Prevalence of spotted fever group Rickettsia species detected in ticks in La Rioja, Spain. Ann. N. Y. Acad. Sci. 2006;1078:320–323. doi: 10.1196/annals.1374.060. [DOI] [PubMed] [Google Scholar]
- 194.Abdel-Shafy S., Allam N., Mediannikov O., Parola P., Raoult D. Molecular Detection of Spotted Fever Group Rickettsiae Associated with Ixodid Ticks in Egypt. Vector-Borne Zoonotic Dis. 2012;12:346–359. doi: 10.1089/vbz.2010.0241. [DOI] [PubMed] [Google Scholar]
- 195.Cicculli V., Oscar M., Casabianca F., Villechenaud N., Charrel R., de Lamballerie X., Falchi A. Molecular Detection of Spotted-Fever Group Rickettsiae in Ticks Collected from Domestic and Wild Animals in Corsica, France. Pathogens. 2019;8:138. doi: 10.3390/pathogens8030138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Demoncheaux J.P., Socolovschi C., Davoust B., Haddad S., Raoult D., Parola P. First detection of Rickettsia aeschlimannii in Hyalomma dromedarii ticks from Tunisia. Ticks Tick-Borne Dis. 2012;3:398–402. doi: 10.1016/j.ttbdis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 197.Kleinerman G., Baneth G., Mumcuoglu K., Van Straten M., Berlin D., Apanaskevich D., Abdeen Z., Nasereddin A., Harrus S. Molecular Detection of Rickettsia africae, Rickettsia aeschlimannii, and Rickettsia sibirica mongolitimonae in Camels and Hyalomma spp. Ticks from Israel. Vector Borne Zoonotic Dis. 2013;13:851–856. doi: 10.1089/vbz.2013.1330. [DOI] [PubMed] [Google Scholar]
- 198.Matsumoto K., Parola P., Brouqui P., Raoult D. Rickettsia aeschlimannii in Hyalomma ticks from Corsica. Eur. J. Clin. Microbiol. Infect. Dis. 2004;23:732–734. doi: 10.1007/s10096-004-1190-9. [DOI] [PubMed] [Google Scholar]
- 199.Cicculli V., de Lamballerie X., Charrel R., Falchi A. First molecular detection of Rickettsia africae in a tropical bont tick, Amblyomma variegatum, collected in Corsica, France. Exp. Appl. Acarol. 2019;77:207–214. doi: 10.1007/s10493-018-00336-2. [DOI] [PubMed] [Google Scholar]
- 200.Orkun Ö., Karaer Z., Çakmak A., Nalbantoğlu S. Spotted fever group rickettsiae in ticks in Turkey. Ticks Tick-Borne Dis. 2014;5:213–218. doi: 10.1016/j.ttbdis.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 201.Selmi R., Ben Said M., Ben Yahia H., Abdelaali H., Messadi L. Molecular epidemiology and phylogeny of spotted fever group Rickettsia in camels (Camelus dromedarius) and their infesting ticks from Tunisia. Transbound. Emerg. Dis. 2020;67:733–744. doi: 10.1111/tbed.13392. [DOI] [PubMed] [Google Scholar]
- 202.Espejo E., Andrés M., Pérez J., Prat J., Guerrero C., Muñoz M.T., Alegre M.D., Lite J., Bella F. Prevalence of antibodies to Rickettsia conorii in human beings and dogs from Catalonia: A 20-year perspective. Epidemiol. Infect. 2016;144:1889–1894. doi: 10.1017/S0950268816000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Morganti G., Veronesi F., Stefanetti V., Di Murcio T., Fiorentino E., Diaferia M., Santoro A., Passamonti F., Gramiccia M. Emerging feline vector-borne pathogens in Italy. Parasites Vectors. 2019;12:193. doi: 10.1186/s13071-019-3409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ortuño A., Pons I., Nogueras M.M., Castellà J., Segura F. The dog as an epidemiological marker of Rickettsia conorii infection. Clin. Microbiol. Infect. 2009;15:241–242. doi: 10.1111/j.1469-0691.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 205.Psaroulaki A., Spyridaki I., Ioannidis A., Babalis T., Gikas A., Tselentis Y. First isolation and identification of Rickettsia conorii from ticks collected in the region of Fokida in Central Greece. J. Clin. Microbiol. 2003;41:3317–3319. doi: 10.1128/JCM.41.7.3317-3319.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Solano-Gallego L., Caprì A., Pennisi M.G., Caldin M., Furlanello T., Trotta M. Acute febrile illness is associated with Rickettsia spp infection in dogs. Parasites Vectors. 2015;8:216. doi: 10.1186/s13071-015-0824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chisu V., Masala G., Foxi C., Socolovschi C., Raoult D., Parola P. Rickettsia conorii israelensis in Rhipicephalus sanguineus ticks, Sardinia, Italy. Ticks Tick-Borne Dis. 2014;5:446–448. doi: 10.1016/j.ttbdis.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 208.Raele D.A., Galante D., Pugliese N., Salandra G.L., Cafiero M.A. Spotted fever group rickettsiae associated with ixodid ticks in wild environment in Southern Italy. Microbiologyopen. 2018;7:e00527. doi: 10.1002/mbo3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Toledo A., Olmeda A.S., Escudero R., Jado I., Valcárcel F., Casado-Nistal M.A., Rodríguez-Vargas M., Gil H., Anda P. Tick-borne zoonotic bacteria in ticks collected from central Spain. Am. J. Trop. Med. Hyg. 2009;81:67–74. doi: 10.4269/ajtmh.2009.81.67. [DOI] [PubMed] [Google Scholar]
- 210.Dib L., Lafri I., Boucheikhchoukh M., Dendani Z., Bitam I., Benakhla A. Seasonal distribution of Rickettsia spp. in ticks in northeast Algeria. New Microbes New Infect. 2018;27:48–52. doi: 10.1016/j.nmni.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bitam I., Parola P., Matsumoto K., Rolain J.M., Baziz B., Boudidi S.C., Harrat Z., Belkaid M., Raoult D. First Molecular Detection of R. conorii, R. aeschlimannii, and R. massiliae in Ticks from Algeria. Ann. N. Y. Acad. Sci. 2006;1078:368–372. doi: 10.1196/annals.1374.073. [DOI] [PubMed] [Google Scholar]
- 212.Chochlakis D., Ioannou I., Sandalakis V., Dimitriou T., Kassinis N., Papadopoulos B., Tselentis Y., Psaroulaki A. Spotted Fever Group Rickettsiae in Ticks in Cyprus. Microb. Ecol. 2012;63:314–323. doi: 10.1007/s00248-011-9926-4. [DOI] [PubMed] [Google Scholar]
- 213.Leulmi H., Aouadi A., Bitam I., Bessas A., Benakhla A., Raoult D., Parola P. Detection of Bartonella tamiae, Coxiella burnetii and rickettsiae in arthropods and tissues from wild and domestic animals in northeastern Algeria. Parasites Vectors. 2016;9:27. doi: 10.1186/s13071-016-1316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Márquez F.J., Millán J. Rickettsiae in ticks from wild and domestic carnivores of Doñana National Park (Spain) and surrounding area. Clin Microbiol Infect. 2009;15((Suppl. 2)):224–226. doi: 10.1111/j.1469-0691.2008.02147.x. [DOI] [PubMed] [Google Scholar]
- 215.Mura A., Masala G., Tola S., Satta G., Fois F., Piras P., Rolain J.-M., Raoult D., Parola P. First direct detection of rickettsial pathogens and a new rickettsia, “Candidatus Rickettsia barbariae”, in ticks from Sardinia, Italy. Clin. Microbiol. Infect. 2008;14:1028–1033. doi: 10.1111/j.1469-0691.2008.02082.x. [DOI] [PubMed] [Google Scholar]
- 216.Scarpulla M., Barlozzari G., Marcario A., Salvato L., Blanda V., De Liberato C., D’Agostini C., Torina A., Macri G. Molecular detection and characterization of spotted fever group rickettsiae in ticks from Central Italy. Ticks Tick-Borne Dis. 2016;7:1052–1056. doi: 10.1016/j.ttbdis.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 217.Waner T., Keysary A., Eremeeva M.E., Mumcuoglu K.Y., Atiya-Nasagi Y. Rickettsia africae and Candidatus Rickettsia barbariae in Ticks in Israel. Am. J. Trop. Med. Hyg. 2014;90:920–922. doi: 10.4269/ajtmh.13-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dib L., Bitam I., Bensouilah M., Parola P., Raoult D. First description of Rickettsia monacensis in Ixodes ricinus in Algeria. Clin. Microbiol. Infect. 2009;15:261–262. doi: 10.1111/j.1469-0691.2008.02277.x. [DOI] [PubMed] [Google Scholar]
- 219.Pascucci I., Di Domenico M., Curini V., Cocco A., Averaimo D., D’Alterio N., Cammà C. Diversity of Rickettsia in Ticks Collected in Abruzzi and Molise Regions (Central Italy) Microorganisms. 2019;7:696. doi: 10.3390/microorganisms7120696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Vila A., Estrada-Peña A., Altet L., Cusco A., Dandreano S., Francino O., Halos L., Roura X. Endosymbionts carried by ticks feeding on dogs in Spain. Ticks Tick Borne Dis. 2019;10:848–852. doi: 10.1016/j.ttbdis.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 221.Chisu V., Leulmi H., Masala G., Piredda M., Foxi C., Parola P. Detection of Rickettsia hoogstraalii, Rickettsia helvetica, Rickettsia massiliae, Rickettsia slovaca and Rickettsia aeschlimannii in ticks from Sardinia, Italy. Ticks Tick-Borne Dis. 2017;8:347–352. doi: 10.1016/j.ttbdis.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 222.Ortuño A., Pons I., Quesada M., Lario S., Anton E., Gil A., Castellà J., Segura F. Evaluation of the Presence of Rickettsia slovaca Infection in Domestic Ruminants in Catalonia, Northeastern Spain. Vector-Borne Zoonotic Dis. 2012;12:1019–1022. doi: 10.1089/vbz.2012.0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tuppurainen E.S.M., Venter E.H., Coetzer J.A.W., Bell-Sakyi L. Lumpy skin disease: Attempted propagation in tick cell lines and presence of viral DNA in field ticks collected from naturally-infected cattle. Ticks Tick Borne Dis. 2015;6:134–140. doi: 10.1016/j.ttbdis.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Alfano N., Tagliapietra V., Rosso F., Ziegler U., Arnoldi D., Rizzoli A. Tick-borne encephalitis foci in northeast Italy revealed by combined virus detection in ticks, serosurvey on goats and human cases. Emerg. Microbes Infect. 2020;9:474–484. doi: 10.1080/22221751.2020.1730246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Papa A., Pavlidou V., Antoniadis A. Greek Goat Encephalitis Virus Strain Isolated from Ixodes ricinus, Greece. Emerg. Infect. Dis. 2008;14:330–332. doi: 10.3201/eid1402.070889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Albayrak H., Ozan E., Kurt M. Molecular Detection of Crimean-Congo Haemorrhagic Fever Virus (CCHFV) but not West Nile Virus (WNV) in Hard Ticks from Provinces in Northern Turkey. Zoonoses Public Health. 2010;57:e156–e160. doi: 10.1111/j.1863-2378.2009.01316.x. [DOI] [PubMed] [Google Scholar]
- 227.Chisholm K., Dueger E., Fahmy N.T., Samaha H.A.T., Zayed A., Abdel-Dayem M., Villinski J.T. Crimean-Congo Hemorrhagic Fever Virus in Ticks from Imported Livestock, Egypt. Emerg. Infect. Dis. 2012;18:181–182. doi: 10.3201/eid1801.111071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dinçer E., Brinkmann A., Hekimoğlu O., Hacioğlu S., Földes K., Karapinar Z., Polat P.F., Oğuz B., Orunç Kilinç Ö., Hagedorn P., et al. Generic amplification and next generation sequencing reveal Crimean-Congo hemorrhagic fever virus AP92-like strain and distinct tick phleboviruses in Anatolia, Turkey. Parasites Vectors. 2017;10:335. doi: 10.1186/s13071-017-2279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lugaj A., Koni M., Mertens M., Groschup M.H., Bërxholi K. Serological survey of Crimean-Congo hemorrhagic fever virus in cattle in Berat and Kolonje, Albania. Alb. J. Agric. 2014;13:325–328. [Google Scholar]
- 230.Lugaj A., Mynyr K., Isolde S., Kristaq B. A Seroepidemiological Survey of Crimean Congo Hemorrhagic Fever among Goats and Sheep in Lezhe Torovica Province, Albania. [(accessed on 12 March 2021)]. Available online: https://agris.fao.org/agris-search/search.do?recordID=AL2015102705.
- 231.Lugaj A., Mertens M., Groschup M., Berxholi K. Serological survey of CCHFV in cattle in 10 regions of Albania. Int. J. Res. Appl. Nat. Soc. Sci. 2014;2:55–60. [Google Scholar]
- 232.Mohamed M., Said A.-R., Murad A., Graham R. A serological survey of Crimean-Congo haemorrhagic fever in animals in the Sharkia Governorate of Egypt. Vet. Ital. 2010;44:513–517. [PubMed] [Google Scholar]
- 233.Negredo A., Habela M.Á., Ramírez de Arellano E., Diez F., Lasala F., López P., Sarriá A., Labiod N., Calero-Bernal R., Arenas M., et al. Survey of Crimean-Congo Hemorrhagic Fever Enzootic Focus, Spain, 2011–2015. Emerg. Infect. Dis. 2019;25:1177–1184. doi: 10.3201/eid2506.180877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Schuster I., Mertens M., Mrenoshki S., Staubach C., Mertens C., Brüning F., Wernike K., Hechinger S., Berxholi K., Mitrov D., et al. Sheep and goats as indicator animals for the circulation of CCHFV in the environment. Exp. Appl. Acarol. 2016;68:337–346. doi: 10.1007/s10493-015-9996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tonbak S., Aktas M., Altay K., Azkur A.K., Kalkan A., Bolat Y., Dumanli N., Ozdarendeli A. Crimean-Congo hemorrhagic fever virus: Genetic analysis and tick survey in Turkey. J. Clin. Microbiol. 2006;44:4120–4124. doi: 10.1128/JCM.00644-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tahir D., Davoust B., Parola P. Vector-borne nematode diseases in pets and humans in the Mediterranean Basin: An update. Vet. World. 2019;12:1630–1643. doi: 10.14202/vetworld.2019.1630-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ewing S.A., Panciera R.J. American canine hepatozoonosis. Clin. Microbiol. Rev. 2003;16:688–697. doi: 10.1128/CMR.16.4.688-697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Aktas M., Özübek S., Altay K., Balkaya I., Utuk A.E., Kirbas A., Şimsek S., Dumanli N. A molecular and parasitological survey of Hepatozoon canis in domestic dogs in Turkey. Vet. Parasitol. 2015;209:264–267. doi: 10.1016/j.vetpar.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 239.Mans B.J., Pienaar R., Latif A.A. A review of Theileria diagnostics and epidemiology. Int. J. Parasitol. Parasites Wildl. 2015;4:104–118. doi: 10.1016/j.ijppaw.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Altay K., Aydin M.F., Dumanli N., Aktas M. Molecular detection of Theileria and Babesia infections in cattle. Vet. Parasitol. 2008;158:295–301. doi: 10.1016/j.vetpar.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 241.Ben Said M., Belkahia H., Messadi L. Anaplasma spp. in North Africa: A review on molecular epidemiology, associated risk factors and genetic characteristics. Ticks Tick Borne Dis. 2018;9:543–555. doi: 10.1016/j.ttbdis.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 242.Kocan K.M., De La Fuente J., Cabezas-Cruz A. The genus Anaplasma: New challenges after reclassification. Rev. Sci. Tech. OIE. 2015;34:577. doi: 10.20506/rst.34.2.2381. [DOI] [PubMed] [Google Scholar]
- 243.Houpikian P., Raoult D. Molecular phylogeny of the genus Bartonella: What is the current knowledge? FEMS Microbiol. Lett. 2001;200:1–7. doi: 10.1111/j.1574-6968.2001.tb10684.x. [DOI] [PubMed] [Google Scholar]
- 244.Álvarez-Fernández A., Breitschwerdt E.B., Solano-Gallego L. Bartonella infections in cats and dogs including zoonotic aspects. Parasites Vectors. 2018;11:624. doi: 10.1186/s13071-018-3152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Margos G., Gofton A., Wibberg D., Dangel A., Marosevic D., Loh L.-M., Oskam C., Fingerle V. The genus Borrelia reloaded. PLoS ONE. 2018;13:e0208432. doi: 10.1371/journal.pone.0208432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kernif T., Leulmi H., Raoult D., Parola P. Emerging Tick-Borne Bacterial Pathogens. Microbiol. Spectr. 2016;4:295–310. doi: 10.1128/microbiolspec.EI10-0012-2016. [DOI] [PubMed] [Google Scholar]
- 247.Angelakis E., Raoult D. Q fever. Vet. Microbiol. 2010;140:297–309. doi: 10.1016/j.vetmic.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 248.Ismail N., Bloch K.C., McBride J.W. Human Ehrlichiosis and Anaplasmosis. Clin. Lab. Med. 2010;30:261–292. doi: 10.1016/j.cll.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Portillo A., Santibáñez P., Palomar A.M., Santibáñez S., Oteo J.A. ‘Candidatus Neoehrlichia mikurensis’ in Europe. New Microbes New Infect. 2018;22:30–36. doi: 10.1016/j.nmni.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rohani M., Shahraki A.H., Ghasemi A., Esmaeili S., Karadenizli A., Mostafavi E. The prevalence of Francisella spp. in different natural surface water samples collected from northwest of Iran. Iran. J. Microbiol. 2019;11:19–24. doi: 10.18502/ijm.v11i1.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Levett P.N. Leptospirosis. Clin. Microbiol. Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wójcik-Fatla A., Zając V., Cisak E., Sroka J., Sawczyn A., Dutkiewicz J. Leptospirosis as a tick-borne disease? Detection of Leptospira spp. in Ixodes ricinus ticks in eastern Poland. Ann. Agric. Environ. Med. 2012;19:656–659. [PubMed] [Google Scholar]
- 253.Jambhekar A., Robin E., Le Boedec K. A systematic review and meta-analyses of the association between 4 mycoplasma species and lower respiratory tract disease in dogs. J. Vet. Intern. Med. 2019;33:1880–1891. doi: 10.1111/jvim.15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mascarelli P.E., Keel M.K., Yabsley M., Last L.A., Breitschwerdt E.B., Maggi R.G. Hemotropic mycoplasmas in little brown bats (Myotis lucifugus) Parasites Vectors. 2015;7:117. doi: 10.1186/1756-3305-7-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Parker A.M., Sheehy P.A., Hazelton M.S., Bosward K.L., House J.K. A review of mycoplasma diagnostics in cattle. J. Vet. Intern. Med. 2018;32:1241–1252. doi: 10.1111/jvim.15135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Abdad M.Y., Abou Abdallah R., Fournier P.E., Stenos J., Vasoo S. A Concise Review of the Epidemiology and Diagnostics of Rickettsioses: Rickettsia and Orientia spp. J. Clin. Microbiol. 2018;56:e01728-17. doi: 10.1128/JCM.01728-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tomassone L., Berriatua E., De Sousa R., Duscher G.G., Mihalca A.D., Silaghi C., Sprong H., Zintl A. Neglected vector-borne zoonoses in Europe: Into the wild. Vet. Parasitol. 2018;251:17–26. doi: 10.1016/j.vetpar.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 258.Tuppurainen E.S.M., Venter E.H., Shisler J.L., Gari G., Mekonnen G.A., Juleff N., Lyons N.A., De Clercq K., Upton C., Bowden T.R., et al. Review: Capripoxvirus Diseases: Current Status and Opportunities for Control. Transbound. Emerg. Dis. 2017;64:729–745. doi: 10.1111/tbed.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Holbrook M.R. Historical Perspectives on Flavivirus Research. Viruses. 2017;9:97. doi: 10.3390/v9050097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Portillo A., Palomar A., Santibáñez P., Oteo J. Epidemiological Aspects of Crimean-Congo Hemorrhagic Fever in Western Europe: What about the Future? Microorganisms. 2021;9:649. doi: 10.3390/microorganisms9030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Estrada-Peña A. Ticks of Domestic Animals in the Mediterranean Region: A Guide to Identification of Species. University of Zaragoza; Zaragoza, Spain: 2004. [Google Scholar]
- 262.Vayssier-Taussat M., Cosson J.F., Degeilh B., Eloit M., Fontanet A., Moutailler S., Raoult D., Sellal E., Ungehuer M.-N., Zylbermann P. How a multidisciplinary ‘One Health’ approach can combat the tick-borne pathogen threat in Europe. Futur. Microbiol. 2015;10:809–818. doi: 10.2217/fmb.15.15. [DOI] [PubMed] [Google Scholar]
- 263.Pérez-Eid C. Les Tiques : Identification, Biologie, Importance Médicale et Vétérinaire. Lavoisier; Cachan, France: 2007. [Google Scholar]
- 264.Vatansever Z. Haemaphysalis parva (Neumann, 1897) (Figs. 100–102) In: Estrada-Peña A., Mihalca A.D., Petney T.N., editors. Ticks of Europe and North Africa: A Guide to Species Identification. Springer International Publishing; Berlin/Heidelberg, Germany: 2017. pp. 259–263. [DOI] [Google Scholar]
- 265.Yonow T. The life-cycle of Amblyomma variegatum (Acari: Ixodidae): A literature synthesis with a view to modelling. Int. J. Parasitol. 1995;25:1023–1060. doi: 10.1016/0020-7519(95)00020-3. [DOI] [PubMed] [Google Scholar]
- 266.Hornok S., Kontschán J., Takács N., Chaber A.-L., Halajian A., Abichu G., Kamani J., Szekeres S., Plantard O. Molecular phylogeny of Amblyomma exornatum and Amblyomma transversale, with reinstatement of the genus Africaniella (Acari: Ixodidae) for the latter. Ticks Tick-Borne Dis. 2020;11:101494. doi: 10.1016/j.ttbdis.2020.101494. [DOI] [PubMed] [Google Scholar]
- 267.Hasan M. Detection of ectoparasites in different birds. Iraqi J. Vet. Sci. 2019;33:37–41. doi: 10.33899/ijvs.2019.162896. [DOI] [Google Scholar]
- 268.de Rancourt M., Mottet A. Mediterranean animal production: Development or decline? Options Mediterr. 2006;78:13–22. [Google Scholar]
- 269.Capek M., Literak I., Kocianova E., Sychra O., Najer T., Trnka A., Kverek P. Ticks of the Hyalomma marginatum complex transported by migratory birds into Central Europe. Ticks Tick-Borne Dis. 2014;5:489–493. doi: 10.1016/j.ttbdis.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 270.De Liberato C., Frontoso R., Magliano A., Montemaggiori A., Antorino G.L., Sala M., Bosworth A., Scicluna M.T. Monitoring for the possible introduction of Crimean-Congo haemorrhagic fever virus in Italy based on tick sampling on migratory birds and serological survey of sheep flocks. Prev. Vet. Med. 2018;149:47–52. doi: 10.1016/j.prevetmed.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 271.Bruderer B., Liechti F. Bird migration across the Mediterranean. Proc. Int. Ornithol. Congr. 1999;22:1983–1999. [Google Scholar]
- 272.Grech-Angelini S., Stachurski F., Vayssier-Taussat M., Devillers E., Casabianca F., Lancelot R., Uilenberg G., Moutailler S. Tick-borne pathogens in ticks (Acari: Ixodidae) collected from various domestic and wild hosts in Corsica (France), a Mediterranean island environment. Transbound. Emerg. Dis. 2020;67:745–757. doi: 10.1111/tbed.13393. [DOI] [PubMed] [Google Scholar]
- 273.Galluzzo P., Grippi F., Di Bella S., Santangelo F., Sciortino S., Castiglia A., Sciacca C., Arnone M., Alduina R., Chiarenza G. Seroprevalence of Borrelia burgdorferi in Stray Dogs from Southern Italy. Microorganisms. 2020;8:1688. doi: 10.3390/microorganisms8111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Otranto D., Brianti E., Latrofa M.S., Annoscia M.S., Weigl S., Lia R.P., Gaglio G., Napoli E., Giannetto S., Papadopoulos E., et al. On a Cercopithifilaria sp. transmitted by Rhipicephalus sanguineus: A neglected, but widespread filarioid of dogs. Parasites Vectors. 2012;5:1. doi: 10.1186/1756-3305-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.