Abstract
A collection of propionibacteria was screened for bacteriocin production. A new bacteriocin named propionicin T1 was isolated from two strains of Propionibacterium thoenii. This bacteriocin shows no sequence similarity to other bacteriocins. Propionicin T1 was active against all strains of Propionibacterium acidipropionici, Propionibacterium thoenii, and Propionibacterium jensenii tested and also against Lactobacillus sake NCDO 2714 but showed no activity against Propionibacterium freudenreichii. The bacteriocin was purified, and the N-terminal part of the peptide was determined with amino acid sequencing. The corresponding gene pctA was sequenced, and this revealed that propionicin T1 is produced as a prebacteriocin of 96 amino acids with a typical sec leader, which is processed to give a mature bacteriocin of 65 amino acids. An open reading frame encoding a protein of 424 amino acids was found 68 nucleotides downstream the stop codon of pctA. The N-terminal part of this putative protein shows strong similarity with the ATP-binding cassette of prokaryotic and eukaryotic ABC transporters, and this protein may be involved in self-protection against propionicin T1. Propionicin T1 is the first bacteriocin from propionibacteria that has been isolated and further characterized at the molecular level.
In the last few decades great emphasis has been placed on the isolation, characterization, and mode of action of bacteriocins (10, 11, 16, 17). Bacteriocins are antibacterial peptides or proteins produced by a wide range of microorganisms. Bacteriocins produced by food-grade organisms such as lactic acid bacteria and propionibacteria are of special interest due to their potential application in food preservation (29). Although a number of bacteriocins from gram-positive bacteria have shown relatively broad inhibitory spectra, most bacteriocins characterized thus far have a generally narrow spectrum of activity.
Numerous bacteriocins from lactic acid bacteria have been isolated and characterized. The best-studied bacteriocin so far is nisin, which is produced by strains of Lactococcus lactis and is approved as a food additive in many countries. The efficiency of nisin in preventing the growth of spoilage bacteria has been proven in a number of food systems (5). Another important bacteriocin is pediocin PA1 produced by Pediococcus acidilactici PAC1.0 (6), which is very active against the food-borne pathogen Listeria monocytogenes (22), an organism not usually very sensitive to nisin (30).
The dairy or classical propionibacteria have a long history of use in dairy fermentations. These bacteria are especially important in starter cultures in the production of Swiss-type cheeses, where they are responsible for the formation of flavor and of the characteristic eyes. Microgard is skim milk fermented by a strain of Propionibacterium freudenreichii subsp. shermanii. This product inhibits molds, gram-negative species, and heterofermentative lactic acid bacteria and is used in approximately 30% of the cottage cheese made in the United States (3). A 700-Da peptide has been implicated in the action of Microgard, but it has not been unequivocally demonstrated that the active ingredient in Microgard is a bacteriocin (7).
Among the dairy propionibacteria, only two bacteriocins have been described: propionicin PLG-1 from Propionibacterium thoenii P127 (13, 14) and jenseniin G from P. thoenii (former P. jensenii) P126 (7, 24). Propionicin PLG-1 is active against a variety of microorganisms such as propionibacteria, as well as many other gram-positive and gram-negative bacteria and even fungi (13). This bacteriocin has been purified to homogeneity and, according to amino acid composition analysis, it contains 99 amino acid residues and has a calculated molecular weight of 9,328 (20). Propionicin PLG-1 is stable after long-term storage in a dry or frozen state and rapidly kills sensitive cells upon exposure in culture medium or skim milk (9). Jenseniin G is a heat-stable bacteriocin that inhibits several propionibacteria and lactic acid bacteria (24), such as Lactobacillus delbruecki subsp. bulgaricus and Streptococcus thermophilus. This bacteriocin therefore has a potential role in preventing overacidification of yogurt (31). However, none of the bacteriocins from propionibacteria mentioned above have been thoroughly described, and the primary structures are not known.
Bacteriocin-producing starter cultures produce bacteriocins during food fermentations. The use of bacteriocin producers as starter cultures may therefore be of advantage in safeguarding fermented foods from the transmission of food-borne pathogens compared to starter cultures lacking the ability to produce bacteriocins. Bacteriocins from secondary starter cultures may also contribute to accelerated ripening of dairy products by killing primary starter bacteria. These dead bacteria will gradually lyse and release cell compounds such as lipids, peptides, and amino acids that are important for the characteristics of the product.
Several bacteriocin producers have already been isolated from foods. It is therefore likely that a battery of different bacteriocins, although in variable amounts, is already present in a lot of the food products. These bacteria and their products are considered safe since they have been an important part of the human diet for centuries.
One limitation to the use of bacteriocin-producing propionibacteria is the slow growth and relatively late bacteriocin production. An alternative and more effective approach could be to use purified and concentrated bacteriocins directly as food additives.
If bacteriocins are going to be used in food manufacturing, there are some important requirements, such as stability, broad activity spectrum, no effects on food properties, lack of toxicity, and a thorough understanding of their biochemical and genetic properties. A detailed knowledge of the isolated bacteriocin structures and properties are therefore needed. In this work we describe a new bacteriocin from propionibacteria called propionicin T1. This is the first bacteriocin from propionibacteria to be characterized at the amino acid and DNA sequence level.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains used are shown in Table 1. The propionibacteria were propagated in sodium lactate broth (SLB) at 22 or 30°C. The propionibacteria used as indicator strains were propagated in SLB or M17 (Oxoid) with glucose (5 g/liter) at 30°C. The indicator strain Lactobacillus sake NCDO 2714 was propagated in MRS (Difco) at 30°C.
TABLE 1.
Inhibition of strains sensitive to propionicin T1
| Indicator species | Straina | Inhibition (radius of inhibition zone in mm) by:
|
|
|---|---|---|---|
| P. thoenii 419 | P. thoenii LMG 2792 | ||
| Propionibacterium acidipropionici | ATCC 4965 | 5.0 | 3.0 |
| Propionibacterium acidipropionici | ATCC 4875 | 2.0 | 1.5 |
| Propionibacterium jensenii | ATCC 4868 | 3.2 | 5.0 |
| Propionibacterium jensenii | ATCC 9614 | 4.0 | 3.5 |
| Propionibacterium jensenii | ATCC 4964 | 6.0 | 5.5 |
| Propionibacterium jensenii | ATCC 14072 | 1.5 | 0.3 |
| Propionibacterium jensenii | P17 | 4.0 | 3.0 |
| Propionibacterium jensenii | P52 | 3.2 | 2.0 |
| Propionibacterium thoenii | LMG 2792 | 3.0 | 2.0 |
| Propionibacterium thoenii | 419 | 3.0 | 2.0 |
| Propionibacterium thoenii | TL 221 | 4.0 | 2.0 |
| Propionibacterium thoenii | ATCC 4871 | 5.0 | 2.5 |
| Propionibacterium thoenii | ATCC 4872 | 3.5 | 2.5 |
| Lactobacillus sake | NCDO 2714 | 3.5 | 4.5 |
Abbreviations: ATCC, American Type Culture Collection (Rockville, Md.); NCDO, National Collection of Food Bacteria (Reading, United Kingdom); LMG and P, our strain collection. P. thoenii 419 comes from the Environmental Bacteriology Culture Collection, University of the Orange Free State, Bloemfontein, South Africa. P. thoenii TL 221 comes from l'Institut National de la Recherche Agronomique (INRA).
Screening for antimicrobial activity.
Colonies of strains of propionibacteria were grown on agar plates for 72 to 120 h. A lawn of 5 ml of GM17 soft agar containing 500 μl of a fresh culture of the indicator organisms was then poured over the plates. After incubation for 24 to 48 h at 30°C, the colonies were examined for zones of growth inhibition.
Bacteriocin assay.
Antimicrobial activity was determined by a microtiter plate assay (8). Each well of the microtiter plate contained 50 μl of twofold serial dilutions in SLB or GM17 of the bacteriocin samples and 150 μl of a 100-fold-diluted fresh overnight culture of the indicator strain. The plates were incubated at 30°C for 24 to 48 h, and growth inhibition of the indicator organisms was measured spectrophotometrically at 620 nm with a microtiter plate reader. One bacteriocin unit (BU) was defined as the amount of bacteriocin that inhibited the growth of the indicator organism by 50% compared with a control culture without bacteriocin.
Effect of proteinase K.
Proteinase K (10 mg/ml) was spotted around colonies of potential bacteriocin-producing bacteria. After an incubation period of 1 h, soft agar with indicator strains was poured over the colonies. Lack of inhibition zones when sensitive bacteria were used as indicators indicated that the antimicrobial compound was proteinlike.
Bacteriocin purification.
The bacteriocin was purified from 1-liter cultures of P. thoenii 419. The culture was grown in SLB broth at 30°C until early logarithmic phase (ca. 72 h). The cells were removed from the supernatant by centrifugation at 12,000 × g for 20 min at 4°C. The bacteriocin was precipitated from the culture supernatant by the addition of ammonium sulfate to 40%. The sample was kept at 4°C for at least 1 h. After centrifugation at 12,000 × g for 20 min, the pellet was dissolved in 50 ml of water, and the pH of the sample was adjusted to pH 3.0 by the addition of concentrated HCl. This solution was applied to a 3-ml SP-Sepharose Fast-Flow cation-exchange column (Pharmacia-LKB, Uppsala, Sweden) equilibrated with 10 mM acetic acid. The column was washed with 10 ml of 30 mM sodium phosphate buffer (pH 6.0), 10 ml of 30 mM sodium phosphate buffer (pH 7.0), and 10 ml of 0.1 M NaCl before the bacteriocin was eluted in 9 ml of 0.3 M NaCl. The active fraction was further purified by reversed-phase chromatography (PepRPC HR5/5) using a fast-performance liquid chromatography (FPLC) system (Pharmacia-LKB). The bacteriocin was eluted from the reversed-phase column with a linear 2-propanol gradient in 0.1% trifluoroacetic acid at a flow rate of 0.5 ml min−1. Fractions with high bacteriocin activity were then applied on a cation-exchange FPLC column (RESOURCETMS; Pharmacia-LKB) equilibrated with 5.0 mM sodium phosphate (pH 6.0) in 50% methanol. The bacteriocin was eluted from this column with a linear gradient of 0 to 1.0 M NaCl in 5.0 mM sodium phosphate (pH 6.0) in 50% methanol buffer at a flow rate of 0.5 ml min−1. The fractions with the highest activity eluted from this column were then mixed and rechromatographed on the reversed-phase column to obtain pure bacteriocin.
The culture of P. thoenii LMG 2792 was grown at 22°C until the late logarithmic phase (ca. 120 h). The cells were removed from the supernatant by centrifugation at 12,000 × g for 20 min at 4°C. The supernatant was adjusted to pH 4.0 with HCl and applied to a 4-ml SP-Sepharose Fast-Flow cation-exchange column (Pharmacia-LKB) equilibrated with 10 mM acetic acid. The bacteriocin from this culture was then further purified by the same procedure as the bacteriocin from P. thoenii 419.
Effect of the bacteriocin on the viability of sensitive cells.
Partially purified bacteriocin from P. thoenii 419 eluted in 0.3 M NaCl from an ion-exchange column was added in variable amounts to a 48-h culture of P. acidipropionici grown in GM17, diluted 100-fold. The optical density at 620 nm and the viable count (by dilution and plate counting) were determined at various time intervals.
N-terminal amino acid sequencing.
The N-terminal amino acid sequence was determined by automated Edman degradation using an Applied Biosystems (Foster City, Calif.) 447A automatic sequence analyzer with an on-line 120A amino acid phenylthiohydantoin analyzer as described by Cornwell et al. (2).
DNA sequence analysis.
Total DNA from the bacteriocin-producing bacteria was obtained using Advamax beads (Advanced Genetic Technologies Corp., Gaithersburg, Md.) according to the procedure described by the manufacturer. Restriction enzymes, Taq polymerase, and other DNA-modifying enzymes were used as recommended by the manufacturers (Promega, Madison, Wis.; New England BioLabs, Inc., Hertfordshire, United Kingdom; Advanced Biotechnologies, Ltd., London, United Kingdom).
PCR reactions were carried out in a DNA-Thermal Cycler (Perkin-Elmer Cetus, Norwalk, Conn.). The reactions (100 μl) were run with 2.5 U of Taq polymerase (Advanced Biotechnologies, Ltd.) and 100 pmol of each primer. The PCR conditions used for amplifying of small DNA fragments (>200 bp) included a hot start at 97°C (3 min), annealing at 55°C (30 s), polymerization at 72°C (10 s), and denaturation at 94°C (10 s). The PCR condition used for primer walking included a hot start at 94°C (3 min), followed by 40 cycles of denaturation at 94°C (30 s), annealing at 60°C (30 s), and polymerization at 72°C (3 min).
PCR fragments were isolated by agarose gel electrophoresis and extracted using Wizard Plus SV Minipreps columns (Promega). The isolated PCR products were sequenced with the ABI Prism Dye terminator Cycle Sequencing Ready reaction kit and an ABI Prism 377 DNA Sequencer (Perkin-Elmer).
Two degenerate primers, 419P1 (5′ GTN CCN GGN GGN TGY AC 3′) and 419P2 (5′ TCN GGN GCR TTR TTR CA 3′), were designed from the amino acid sequence (N-terminal part) of the bacteriocin obtained by Edman degradation and used in the PCR. New specific primers were designed from the sequence of the primary PCR product. Samples of total DNA were cut with different restriction enzymes (BamHI, SalI, and SmaI) and ligated to the plasmid pBluescript II SK(+) (Stratagene, La Jolla, Calif.) cut with the same restriction enzymes. These ligation mixtures were used as templates in PCR reactions using combinations of bacteriocin specific primers and the vector-specific primer T7. New primers were constructed from the sequences of the PCR products obtained, and this procedure (primer walking) was repeated until the sequence was obtained (see Fig. 3).
FIG. 3.
DNA sequence of 2210 contiguous nucleotides, including the ORFs pctA and orf2. Potential −35, −10 sites and ribosome-binding sites (RBS) are indicated in boldface. The amino acid sequence of propionicin T1 showing the cleavage site of the leader sequence (↑) and the amino acid sequence of the putative ABC transporter, with three conserved sequence motifs in the ATP-hydrolyzing domain, are also noted.
Analyses of DNA and protein sequences were performed using the OMIGA 1.1 DNA and Protein Sequence Analysis Software (Oxford Molecular). Identification of signal peptide and cleavage site was performed with SignalP V1.1 (18).
Nucleotide sequence accession number.
The DNA sequence described here has been deposited in the GenBank database under accession number AF294258.
RESULTS
Characterization of a bacteriocin from propionibacteria.
A collection of dairy propionibacteria was screened for antimicrobial activity. P. thoenii 419 showed inhibitory activity against all strains tested of P. acidipropionici, P. thoenii, and Propionibacterium jensenii when grown on agar plates (Table 1). Even the producer strain itself and P. thoenii LMG 2792, which produces the same bacteriocin, were sensitive against the antimicrobial activity produced by colonies of P. thoenii 419 (Table 1). Treatment with proteinase K inactivated the antimicrobial activity, indicating that the inhibitory compound was proteinaceous.
Ten strains of P. freudenreichii were tested. None of them were inhibited by P. thoenii 419 in this overlay assay (results not shown). Lactococcus, Lactobacillus, Enterococcus, Carnobacterium, and Listeria strains were also tested. None of these strains were inhibited except L. sake NCDO 2714.
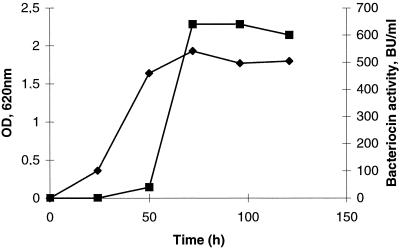
The inhibitory activity was detected in liquid culture, and maximum antimicrobial activity was found in the early stationary growth phase (Fig. 1). Among the indicator strains tested, P. acidipropionici ATCC 4965 was one of the most sensitive on agar plates and in the microtiter plate assay and so was used as the standard indicator.
FIG. 1.
Growth kinetics and bacteriocin production of P. thoenii 419. Bacteriocin activity was determined from cell-free culture supernatants using a microtiter plate assay. The sensitive indicator was P. acidipropionici ATCC 4965. Symbols: ⧫, optical density at 620 nm; ■, BU per milliliter.
The bacteriocin was isolated from P. thoenii 419 by a procedure involving ammonium sulfate precipitation and ion-exchange and reversed-phase chromatography. The new bacteriocin was named propionicin T1.
The first 38 amino acid residues of the N-terminal amino acid sequence of the bacteriocin were determined by Edman degradation. The following sequence was obtained: VPGGCTYTRSNRDVIGTCKTGSGQFRIRLDCNNAPDKT. The same bacteriocin was also isolated and purified from stationary cultures of P. thoenii LMG 2792. This strain showed the same inhibition spectrum as P. thoenii 419. The N-terminal amino acid sequence of the bacteriocin isolated from P. thoenii LMG 2792 was identical to the sequence of the bacteriocin from P. thoenii 419. We assume that P. thoenii 419 and P. thoenii LMG 2792 are different strains of P. thoenii because of morphological differences such as color and slime production and because of different growth optima. The two strains showed great differences with respect to bacteriocin production. While P. thoenii 419 produced the bacteriocin in late logarithmic growth phase, bacteriocin production in P. thoenii LMG 2792 could only be detected late in the stationary phase (after 120 h; results not shown). Bacteriocin production of P. thoenii LMG 2792 was observed at 22°C but not at 30°C, while P. thoenii 419 produced the bacteriocin at 30°C.
Stability of propionicin T1.
The stability of the peptide was tested by exposing the bacteriocin (0.3 M NaCl eluate from the cation-exchange column) to temperatures of 60 and 100°C for 15 min and to pH 2.5 for 1 h. No reduction in antimicrobial activity was observed. Freezing, thawing, and storage of the bacteriocin fraction at 4 or −20°C for up to 6 months or at room temperature for 24 h showed no effect on the bacteriocin activity.
Effect of propionicin T1 on the viability of sensitive cells.
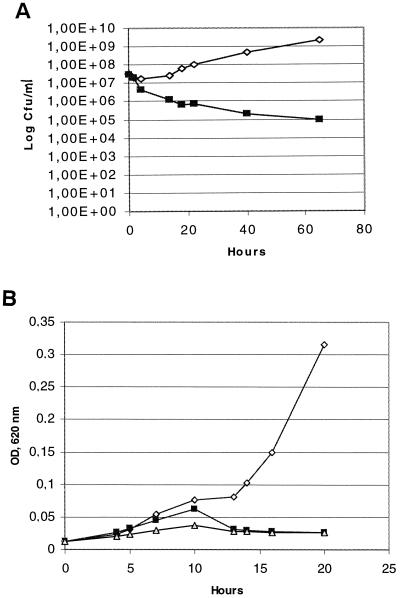
Several experiments were performed to show the effect of propionicin T1 on the viability of the indicator cells. Cultures of P. acidipropionici ATCC 4965 were exposed to different amounts of partially purified propionicin T1. Figure 2 shows the results of a typical experiment. The results from the plate counting demonstrated that propionicin T1 had a bactericidal effect on the indicator cells. A reduction in viable count was observed after 4 h. More than 99% of the cells were killed after 40 h (Fig. 2A). The same result was also obtained using 100 or 4,000 BU/ml. Despite the reduction in cell number, bacteriocin-treated cultures showed an increase in the optical density for several hours after the addition of bacteriocin. This increase was followed by a decrease in the optical density (Fig. 2B).
FIG. 2.
(A) Growth of the sensitive indicator P. acidipropionici ATCC 4965 with (1,000 BU/ml of culture) or without propionicin T1. Symbols: ◊, without bacteriocin; ■, with bacteriocin. (b) Optical density (620 nm) of growing cultures of P. acidipropionici ATCC 4965 with or without propionicin T1. Symbols: ◊, without bacteriocin; ■, with 100 BU/ml of culture; ▵, with 1,000 BU/ml of culture.
Genetic analyses of propionicin T1.
Total DNA was isolated from P. thoenii LMG 2792. The sequence of the structural bacteriocin gene pctA was obtained by PCR using degenerate primers based on the N-terminal amino acid sequence, followed by a primer-walking strategy. Figure 3 shows the DNA sequence of 2,210 contiguous nucleotides, including the open reading frame (ORF) pctA. The DNA sequencing confirmed the results of the amino acid sequencing and revealed that the bacteriocin is translated as a 96-amino-acid prebacteriocin, which is processed to give a mature propionicin T1 of 65 amino acids (Fig. 3). The prebacteriocin contains a typical sec leader peptide (18), and this leader peptide is cleaved off immediately after the Ala-Met-Ala residues. The calculated molecular mass of the mature propionicin T1 was 7,130.20 Da, and the pI was calculated to be 9.50. A putative promoter area was found upstream the start codon of the bacteriocin structural gene pctA (Fig. 3). No amino acid or DNA sequence in various sequence databases show significant homology to the sequences presented here.
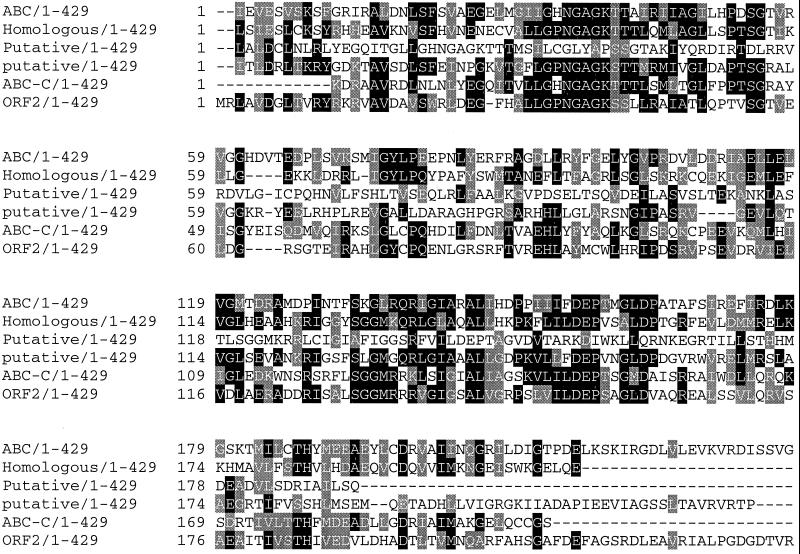
A second ORF, orf2, encoding a protein of 424 amino acids with a calculated molecular weight of 45,163.95, was found 68 nucleotides downstream the stop codon of pctA (Fig. 3). The ATG start codon is preceded seven nucleotides upstream by a potential ribosome-binding site (Fig. 3). No potential promoter region was found upstream of the start codon. The N-terminal part of this putative protein shows strong similarity with the ATP-binding cassette of prokaryotic and eukaryotic ABC transporters (Fig. 4).
FIG. 4.
Alignment of prokaryotic and eukaryotic ABC transporters and the N-terminal part of the putative protein encoded by orf2.
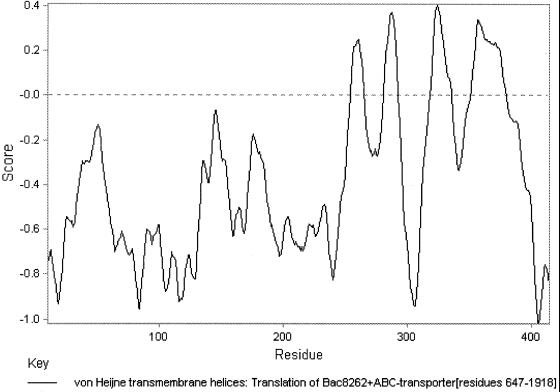
The ATP-hydrolyzing domains of the ABC transporters are characterized by three short sequence motifs in their primary structure: Walker A, GXXGXGKS/T, where X can be varied, and the linker peptide, LSGGQQ/R/KQR, which precedes the third motif, Walker B motif hhhhD, where “h” stands for hydrophobic (27). These three motifs were found in the sequence encoded by orf2 (Fig. 3). Furthermore, the amino acid sequence indicates the presence of a membrane-integral domain consisting of four potential transmembrane helices in the C-terminal part of the protein (Fig. 5).
FIG. 5.
Protein profile analyses of ORF2 indicate the presence of a membrane-integral domain consisting of four potential transmembrane helices in the C-terminal part of the protein.
DISCUSSION
Propionicin T1 is the first bacteriocin from propionibacteria that has been isolated and further characterized at the molecular level. Propionibacteria play an important role in the production and preservation of various dairy products, especially Swiss-type cheeses. In addition to the production of propionic acid and other antagonistic compounds such as acetic acid and carbon dioxide, bacteriocins from propionibacteria could contribute to this preservation.
The majority of bacteriocins characterized thus far have fairly narrow inhibition spectra, only killing bacteria closely related to the producer (10). Propionicin T1 falls into this category since it only inhibits a narrow group of propionibacteria. Classification studies based on 16S ribosomal DNA sequences have shown that the classical propionibacteria form two phylogenetic clusters: one containing P. acidipropionici, P. thoenii, and P. jensenii and one containing P. freudenreichii subspecies and the new species Propionibacterium cyclohexanicum (4). Propionicin T1 seems to inhibit only the members in one of these two clusters of propionibacteria, since no strains of P. freudenreichii were inhibited by the bacteriocin. This may be of practical importance in cheese-making. Propionicin T1 might be used to prevent the formation of red spots caused by pigment-forming strains of P. thoenii and P. jensenii, a common problem in the production of Swiss-type cheeses, without harming the starter culture that contains strains of P. freudenreichii.
Sensitive bacteria were killed after exposure to the bacteriocin for several hours, demonstrating that propionicin T1 has a slow bactericidal mode of action. It was rather surprising to observe an increase in the optical density in cultures with added bacteriocin. During the first few hours after bacteriocin addition, this increase was almost identical to the increase in the control culture (Fig. 2B). Thus, the optical density in the bacteriocin-treated culture increased at the same time that the numbers of viable cells were reduced (Fig. 2). A similar phenomenon was observed for lactococcin 972 (15), a bacteriocin only active against lactococci. The addition of lactococcin 972 to sensitive cultures resulted in a sharp reduction in viable counts, although the cells remained metabolically active. It was suggested that lactococcin 972 inhibited septum formation (15). Propionicin T1 may also interfere with the biosynthesis of essential macromolecules. Continued production of cellular compounds may explain the increase in the optical density during the first hours after bacteriocin addition.
The sequence of propionicin T1 showed no sequence similarity to other bacteriocins. On the other hand, propionicin T1 has several features in common with most bacteriocins isolated from lactic acid bacteria. These bacteriocins are relatively small (30 to 100 amino acids), thermostable, and cationic peptides (17), features they share with propionicin T1.
Like most bacteriocins from lactic acid bacteria, propionicin T1 is synthesized as a precursor with an N-terminal leader peptide (10, 17). The deduced leader sequence of propionicin T1 conforms to the pattern of a typical signal peptide, as described by von Heijne (18), for proteins processed and secreted by the sec-dependent pathway (23). The signal peptides have a positively charged N terminus, a hydrophobic core, and a specific cleavage region, features found in the leader sequence of propionicin T1.
An ORF encoding a peptide with the characteristics of an ABC transporter was found 68 nucleotides downstream the stop codon of the structural gene of propionicin T1. A potential ribosome-binding site but no obvious promoter region was found upstream of orf2. This gene can therefore be considered a part of the propionicin T1 operon. The putative ABC transporter is not likely to be involved in the regular transport of propionicin T1 out of the cell because, as already mentioned, the bacteriocin shows the features of proteins that are exported by the general secretory pathway.
Immunity factors protect the bacteria against self-toxicity, and the corresponding genes are usually located on the same operon as the structural bacteriocin genes (17). In most cases, the structural gene of the bacteriocin and the gene encoding the immunity protein are located adjacent to one another (1, 17). Several antibiotic resistance mechanisms involve ABC transporters (19). These systems export antibiotics out of the cells. It has been shown that dedicated ABC transporter systems also make an important contribution to producer protection against some bacteriocins. ABC transporters have thus far been identified to be involved in self-protection of producers of nisin (26, 28), subtilin (12), epidermin (19, 21), and lacticin 481 (25).
Both producers of propionicin T1 were sensitive to their own bacteriocin in the overlay assay (Table 1). An explanation for this may be that the immunity factors are not constitutively expressed but are connected to the production of the bacteriocin. Propionicin T1 is produced in late logarithmic stage by P. thoenii 419 and in stationary phase by P. thoenii LMG 2792. Apparently, cells in the early exponential growth phase are sensitive to the bacteriocin. This indicates that bacteriocin production and immunity are coregulated.
The putative ABC transporter encoded by orf2 may be involved in the immunity of the producer bacteria against propionicin T1 since this ORF is probably transcriptionally linked to the bacteriocin structural gene. The immunity could be mediated by active transport of bacteriocin molecules out of the producer cells or by the import and degradation of bacteriocin inside the cells.
ACKNOWLEDGMENTS
We thank K. Sletten for performing the amino acid sequencing analyses.
T. Faye was funded by a grant from the Norwegian Research Council. H. Holo was supported by grants from Norwegian Dairies Association, Oslo, Norway.
REFERENCES
- 1.Allison G E, Klaenhammer T R. Functional analysis of the gene encoding immunity to lactacin F, and its use as a Lactobacillus-specific, food-grade genetic marker. Appl Environ Microbiol. 1996;62:4450–4460. doi: 10.1128/aem.62.12.4450-4460.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornwell G G, Sletten K, Johansson B, Westemark P. Evidence that the amyloid fibril protein in senile systemic amyloidosis is derived from normal prealbumin. Biochem Biophys Res Commun. 1988;154:648–653. doi: 10.1016/0006-291x(88)90188-x. [DOI] [PubMed] [Google Scholar]
- 3.Daeschel M A. Antimicrobial substances from lactic acid bacteria for use as food preservatives. Food Technol. 1989;43:164–166. [Google Scholar]
- 4.Dasen G, Smutny J, Teuber M, Meile L. Classification and identification of propionibacteria based on ribosomal RNA genes and PCR. Syst Appl Microbiol. 1998;21:251–259. doi: 10.1016/S0723-2020(98)80030-1. [DOI] [PubMed] [Google Scholar]
- 5.Delves-Broughton J, Blackburn P, Evans R J, Hugenholtz J. Applications of the bacteriocin nisin. Antonie Leeuwenhoek. 1996;69:193–202. doi: 10.1007/BF00399424. [DOI] [PubMed] [Google Scholar]
- 6.González C, Kunka B S. Plasmid-associated bacteriocin production and sucrose fermentation in Pediococcus acidilactici. Appl Environ Microbiol. 1987;53:2534–2538. doi: 10.1128/aem.53.10.2534-2538.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grinstead D A, Barefoot S F. Jenseniin G, a heat-stable bacteriocin produced by Propionibacterium jensenii P126. Appl Environ Microbiol. 1992;58:215–220. doi: 10.1128/aem.58.1.215-220.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holo H, Nilsen Å, Nes I F. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J Bacteriol. 1991;173:3879–3887. doi: 10.1128/jb.173.12.3879-3887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh H-Y, Glatz B A. Long-term storage stability of the bacteriocin propionicin PLG-1 produced by Propionibacterium thoenii and potential as a food preservative. J Food Prot. 1996;59:481–486. doi: 10.4315/0362-028X-59.5.481. [DOI] [PubMed] [Google Scholar]
- 10.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klaenhammer T R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–86. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 12.Klein C, Entian K-D. Genes involved in self-protection against the lantibiotic subtilin produced by Bacillus subtilis ATCC 6633. Appl Environ Microbiol. 1994;60:2793–2801. doi: 10.1128/aem.60.8.2793-2801.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyon W J, Glatz B A. Partial purification and characterization of a bacteriocin produced by Propionibacterium thoenii. Appl Environ Microbiol. 1991;57:701–706. doi: 10.1128/aem.57.3.701-706.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyon W J, Glatz B A. Isolation and purification of propionicin PLG-1, a bacteriocin produced by a strain of Propionibacterium thoenii. Appl Environ Microbiol. 1993;59:83–88. doi: 10.1128/aem.59.1.83-88.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez B, Rodríguez A, Suárez J E. Lactococcin 972, a bacteriocin that inhibits septum formation in lactococci. Microbiology. 2000;146:949–955. doi: 10.1099/00221287-146-4-949. [DOI] [PubMed] [Google Scholar]
- 16.Moll G N, Konings W N, Driessen A J M. Bacteriocins: mechanism of membrane insertion and pore formation. Antonie Leeuwenhoek. 1999;76:185–198. [PubMed] [Google Scholar]
- 17.Nes I F, Diep D B, Håvarstein L S, Brurberg M B, Eijsink V, Holo H. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:113–138. doi: 10.1007/BF00395929. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of procaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Otto M, Peschel A, Götz F. Producer self-protection against the lantibiotic epidermin by the ABC transporter EpiFEG of Staphylococcus epidermis Tü3298. FEMS Microbiol Lett. 1998;166:203–211. doi: 10.1111/j.1574-6968.1998.tb13891.x. [DOI] [PubMed] [Google Scholar]
- 20.Paik H-D, Glatz B A. Purification and partial amino acid sequence of propionicin PLG-1, a bacteriocin produced by Propionibacterium thoenii P127. Lait. 1995;75:367–377. [Google Scholar]
- 21.Peschel A, Götz F. Analysis of the Staphylococcus epidermidis genes epiF, -E, and -G involved in epidermin immunity. J Bacteriol. 1996;178:531–536. doi: 10.1128/jb.178.2.531-536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pucci M J, Vedamuthu E R, Kunka B S, Vandenbergh P A. Inhibition of Listeria monocytogenes by using bacteriocin PA-1 produced by Pediococcus acidilactici PAC1.0. Appl Environ Microbiol. 1988;54:2349–2353. doi: 10.1128/aem.54.10.2349-2353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratnam P, Barefoot S F, Price L D, Bodine A B, McCaskill L H. Partial purification and characterization of the bacteriocin produced by Propionibacterium jensenii B1264. Lait. 1999;79:125–136. [Google Scholar]
- 25.Rince A, Dufour A, Uguen P, Le Pennec J-P, Haras D. Characterization of the lactacin 481 operon: the Lactococcus lactis genes lctF, lctE, and lctG encode a putative ABC transporter involved in bacteriocin immunity. Appl Environ Microbiol. 1997;63:4252–4260. doi: 10.1128/aem.63.11.4252-4260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saris P E J, Immonen T, Reis M, Sahl H-G. Immunity to lantibiotics. Antonie Leeuwenhoek. 1996;69:151–159. doi: 10.1007/BF00399420. [DOI] [PubMed] [Google Scholar]
- 27.Schneider E, Hunke S. ATP-binding-cassette (ABC) transport systems: Functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiol Rev. 1998;22:1–20. doi: 10.1111/j.1574-6976.1998.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 28.Siegers K, Entian K-D. Genes involved in immunity to the lantibiotic nisin produced by Lactococcus lactis 6F3. Appl Environ Microbiol. 1995;61:1082–1089. doi: 10.1128/aem.61.3.1082-1089.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stiles M E. Biopreservation by lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:331–345. doi: 10.1007/BF00395940. [DOI] [PubMed] [Google Scholar]
- 30.de Vos W M, Mulders J W M, Siezen R J, Hugenholtz R J, Kuipers O P. Properties of nisin Z and distribution of its gene, nisZ, in Lactococcus lactis. Appl Environ Microbiol. 1993;59:213–218. doi: 10.1128/aem.59.1.213-218.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinbrenner D R, Barefoot S F, Grinstead D A. Inhibition of yogurt starter cultures by jenseniin G, a Propionibacterium bacteriocin. J Dairy Sci. 1997;80:1246–1253. [Google Scholar]