Abstract
Treatment of advanced ovarian cancer using PD-1/PD-L1 immune checkpoint blockade shows promise, however current clinical trials are limited by modest response rates. Radiation therapy has been shown to synergize with PD-1/PD-L1 blockade in some cancers but has not been utilized in advanced ovarian cancer due to toxicity associated with conventional abdominopelvic irradiation. While ultra-high dose rate (FLASH) irradiation has emerged as a strategy to reduce radiation-induced toxicity, the immunomodulatory properties of FLASH irradiation remain unknown. Here we demonstrate that single high dose abdominopelvic FLASH irradiation promoted intestinal regeneration and maintained tumor control in a preclinical mouse model of ovarian cancer. Reduced tumor burden in conventional and FLASH treated mice was associated with an early decrease in intratumoral regulatory T cells and a late increase in cytolytic CD8+ T cells. Compared to conventional irradiation, FLASH irradiation increased intratumoral T cell infiltration at early timepoints. Moreover, FLASH irradiation maintained the ability to increase intratumoral CD8+ T cell infiltration and enhance the efficacy of αPD-1 therapy in preclinical models of ovarian cancer. These data highlight the potential for FLASH irradiation to improve the therapeutic efficacy of checkpoint inhibition in the treatment of ovarian cancer.
Introduction
Ovarian cancer is the deadliest gynecologic malignancy with 295,000 new cases and 185,000 deaths worldwide in 2018 (1). The majority of women present with advanced stage disease, and due to high recurrence rates (70–80%), prognosis remains poor with 5-year overall survival at approximately 46.5% (2). Standard treatment for ovarian cancer consists of surgical debulking followed by platinum-taxane chemotherapy (3). In recent years, there has been an influx of novel therapies including biologics and targeted therapies for the treatment of advanced ovarian cancer.
Immunotherapies, including anti-programmed cell death-1 (αPD-1) immune checkpoint inhibitors, are a promising therapeutic strategy for advanced stage ovarian cancer (4). However, many tumors are either resistant or relapse following immune checkpoint inhibition, with clinical trials demonstrating at most a 15% response rate (5). T cell infiltration into the tumor microenvironment is associated with improved immunotherapy efficacy, and is correlated with antitumor activity and prolonged survival in ovarian cancer (6,7). Therefore, there is a critical need for treatment modalities that increase T cell infiltration and reduce immunosuppressive features of the tumor microenvironment to improve immunotherapy responses in ovarian cancer.
Radiotherapy is an attractive strategy to optimize the efficacy of immune checkpoint blockade due to its ability to induce a cell death that is immunogenic and convert the tumor into an in situ vaccine (8). Preclinical and clinical studies demonstrate that radiotherapy can synergize with immune checkpoint blockade in multiple tumor types and can sensitize resistant tumors to immune checkpoint inhibition (8). Importantly, radiotherapy in combination with checkpoint blockade results in improved local control of the irradiated tumor, enhanced systemic tumor control, and durable responses due to the induction of immunologic memory (8). Thus, radiotherapy is a promising strategy to enhance the effects of immune checkpoint blockade in multiple tumor types. While ovarian cancer is known to be radiosensitive, the use of abdominopelvic radiotherapy in metastatic peritoneal disease is limited by gastrointestinal and hematopoietic toxicities (9–14).
Over the past decade, major technological advances in radiation oncology have enabled the use of ultra-high dose rate irradiation (FLASH) to deliver dose rates of >40 Gy/s. Preclinical studies of FLASH irradiation in multiple tissue sites including lung, brain, and skin have shown reduced normal tissue toxicity while maintaining tumor control mice, minipigs and cats (15–23). Clinically, FLASH irradiation has been used in a human study of cutaneous T cell lymphoma (24). While preclinical and clinical studies have demonstrated immunostimulatory effects with conventional radiotherapy, the immunomodulatory properties of FLASH irradiation remain unknown (25,26).
We previously reported a preclinical FLASH irradiation platform using a clinical linear accelerator (LINAC) for electron FLASH irradiation of small animals (27). In addition, we demonstrated that abdominal FLASH irradiation reduces gastrointestinal morbidity and mortality while maintaining tumor control in a preclinical ovarian cancer model (15). In this study, we investigate the immunomodulatory role of FLASH radiotherapy in combination with αPD-1 immune checkpoint blockade in sensitive and resistant preclinical models of ovarian cancer. We demonstrate efficacy and tolerability of FLASH and αPD-1 combination therapy in preclinical models of advanced ovarian cancer.
Materials and Methods
Mice
All animal experiments and procedures were approved by the Institutional Animal Care and Use Committee of Stanford University in accordance with institutional and NIH guidelines. Six to eight-week-old female C57BL/6 mice were obtained from Jackson Laboratory (Bar Harbour, ME). Standard animal care and housing was provided by the Stanford University School of Medicine and is under the care and supervision of the Department of Comparative Medicine’s Veterinary Service Center (VSC). Mice were maintained on the irradiated Envigo Tekland diet containing 18% protein and 6% fat.
All mice in this study were age matched, sex matched and irradiated at the same time of day. All mice were irradiated 10 days following ID8 or UPK10 tumor cell intraperitoneal injection. During irradiation, mice were anaesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) injected into the peritoneum. Control mice were treated with the same dose of ketamine/xylazine but were not exposed to radiation. Immediately after irradiation, mice were placed on a warming blanket until they woke up from the anesthesia. Mice were then placed back into their standard housing environment and were monitored daily for body weight, appearance, respiratory rate, general behavior, and provoked behavior using the Mouse Intervention Scoring System (MISS 3) (28).
Mouse irradiation
As previously described, we developed a custom mouse stereotactic positioning frame made of PLA plastic using 3-D printing (15). Reproducible positioning within the frame was achieved by registering the front teeth on a nylon filament at a fixed location at the cranial end of the frame with extension of the hindlimbs and tail through designated slots at the caudal end of the frame. Positioning reproducibility to within 1 mm was confirmed by micro CT imaging in a representative cohort of mice. An abdominal irradiation shield was made by 3-D printing a PLA plastic shell with a central opening of 4 cm (lateral) by 4 cm (craniocaudal). Internally, a 3 cm thick layer of aluminum oxide powder in tandem with a 1 cm thick layer of tungsten spheres (2 mm diameter) was placed, a combination designed to minimize leakage dose from bremsstrahlung radiation produced in the shield materials. The stereotactic frame was registered to the shield such that the opening extended from the tenth rib at the cranial border to 4 cm caudally, encompassing all of the small intestine. EBT3 Gafchromic film (Ashland Advanced Materials, Bridgewater NJ) dosimetry confirmed that leakage dose to the shielded portions of the body when irradiating with 16 MeV electrons was <3.5% of the central dose at 10 mm from the field edge and further.
The shield and positioning frame were loaded into a polystyrene cradle registered to specified locations relative to the LINAC treatment head for FLASH and CONV irradiation. For both FLASH and CONV setups, we placed EBT3 Gafchromic films between layers of polystyrene to measure transverse and depth dose profiles to characterize dose homogeneity throughout the treatment volume. In addition, entrance dose for every individual mouse irradiation was recorded by EBT3 Gafchromic films (1×2 inch) placed inside the positioning frame. For both FLASH and CONV irradiation, the dose was prescribed at the entrance surface of the mouse.
CONV irradiation setup
We used a Varian Trilogy radiotherapy system (Varian Medical Systems, Palo Alto, CA) to perform both CONV and FLASH irradiation. For CONV irradiation, the gantry was rotated to 180 degree (beam direction from floor to ceiling) and the collimator was rotated to 0 degree. The cradle with the shield and mouse jig was placed on top of a 15 cm electron applicator such that the distance from the electron scattering foil to the shield was 77.6 cm. Under service mode, irradiation was delivered using a clinical 16 MeV electron beam in the 400 MU/minute dose rate mode (pulse repetition rate 72 Hz). Calibration by film dosimetry determined that the entrance dose after the shield was 1.91 cGy/MU, with a resulting average dose rate of 0.126 Gy/s.
FLASH irradiation setup
We configured the Varian Trilogy radiotherapy system to perform FLASH irradiation as previously described (27). The gantry was rotated to 180 degree, the treatment head cover was removed, and the jaws were fully opened (40 × 40 cm). The cradle with radiation shield and mouse stereotactic frame was loaded and registered to fixed points on the face of the gantry, such that the distance from the electron scattering foil to the shield was 14.6 cm. Beam parameters were configured on a dedicated electron beam control board. We used an electron beam energy of approximately 16 MeV with the 16 MeV scattering foil (confirmed by depth dose measurements) and adjusted the radiofrequency power and gun current settings to produce a dose per pulse of 2.0 Gy at the entrance surface of the mouse. We controlled pulse delivery using a programmable controller board (STEMlab 125–14, Red Pitaya, Solkan, Slovenia) and relay circuit to count the number of delivered pulses detected by the internal monitor chamber and impose beam hold and release through the respiratory gating system of the LINAC. We used an external ion chamber positioned after the mouse and 10 cm of solid water (where the dose rate did not saturate the chamber), calibrated to film measurements of entrance dose, to provide immediate dose readout per mouse. The pulse repetition rate was set to 90 Hz for an average dose rate of 210 Gy/sec at 2 Gy/pulse at the entrance surface of the mouse.
Tissue processing and histological analysis
Animals were euthanized via CO2 asphyxiation and secondary cardiac exsanguination. Soft tissues were harvested and immersion-fixed in 10% neutral buffered formalin for 24 h, followed by PBS for 24 h and then stored in 70% ethanol. Three transverse sections of the small intestine from the jejunum (mid-segment) were collected. Formalin-fixed tissues were processed, embedded in paraffin, sectioned at 5 μm and stained with hematoxylin and eosin.
Regenerating crypt counts
A total of three transverse sections of jejunum were analyzed per mouse for the number of regenerating crypts by the crypt microcolony assay (29). Transverse sections were analyzed if they met the following criteria: 1) a complete jejunal circumference was present; and 2) the mucosa was oriented perpendicular to the long axis of the intestine. Crypts were considered regenerating if they comprised >10 basophilic crypt epithelial cells.
Ovarian cancer tumor models
The murine ID8 ovarian cancer cell line was obtained from Dr. Katherine F. Roby, University of Kansas Medical Center, Kansas City, KS (30). The murine UPK10 ovarian cancer cell line was obtained from Dr. Jose Conejo-Garcia, The Wistar Institute, Philadelphia, PA (31). Cell lines were authenticated from the original source and used within early passage numbers. Additionally, cells were tested upon receipt for viability, cell morphology, and the presence of Mycoplasma and viruses (Charles River Laboratories). ID8 cells were passaged in DMEM supplemented with 10% FCS and Pen/Strep. UPK10 cells were passaged in RPMI supplemented with 10% FCS, Pen/Step, and 50 μM beta-mercaptoethanol. ID8 and UPK10 cells (5×106 cells in 200 μl PBS) were intraperitoneally injected using a 27-gauge needle on day 0.
Injections
For immunotherapy treatments, 200 μg (ID8 model) or 20 μg (UPK10 model) of αPD-1 (clone 29F.1A12, Bio X Cell) or rat IgG2α isotype control antibody (clone 2A3, Bio X Cell) was suspended in 200 μl PBS and intraperitoneally injected on days 7, 10, and 13 post tumor cell injection.
Tumor response and intestinal function
Tumor response was determined by counting tumor nodule numbers, measuring tumor weight and measuring ascites volume (ID8 model) on necropsy on day 22 (UPK10 model) or 27 (ID8 model) following tumor cell injection. The number of formed stool pellets made over 24 hours at specified time points in the study were collected to determine intestinal function in live animals.
Flow cytometry
For flow cytometric analysis of tumor bearing omentum and/or mesenteric lymph nodes were harvested on day 22 or 27 post-tumor inoculation. Gating and analysis were performed using FlowJo software (TreeStar). Single cell suspensions were prepared in PBS with 2% BSA, and red blood cells were lysed using ACK Lysis Buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA). Live/dead cell discrimination was performed using Zombie NIR Fixable Viability Kit (BioLegend). Cells were treated with Fc block (Miltenyi Biotech). Cell surface staining was done for 20 minutes. Immune cells were identified as CD45+ (eBioscience, 64–0451-82). B cells were identified as B220+ (eBioscience, 17–0452-81), macrophages as CD11b+ (eBioscience, 56–0112-82), F4/80+ (BioLegend, 123132), M-MDSCs as CD11b+Ly6C+ (BD Biosciences, 560593), PMN-MDSCs as CD11b+Ly6CloLy6G+ (BD Biosciences, 746448), M2 macrophage marker CD206 (Biolegend 141727), M1 macrophage marker iNOS (eBioscience, CXNFT). T cells were identified as TCRβ+ (eBioscience, 45–5961-82) and divided as CD8+ (eBioscience, 25–0081-82) or CD4+ (eBioscience, 11–0041-82). T cells were stained intracellularly after using a fixation/permeabilization kit (eBioscience) for FoxP3 (eBioscience, 50–5773-80) and Ki-67 (BD Biosciences, 561227) and CD107a (Biolegend, 121631). Samples were run on an LSR Fortessa X-20 (BD) and analyzed using FlowJo software (TreeStar).
Complete blood count (CBC) analysis
At the time of sacrifice, blood was collected via cardiac puncture and processed using a Hemavet 950FS (Drew Scientific). Complete CBC analysis was performed on the IgG, FLASH, αPD-1, and aPD-1 + FLASH treated mice harboring ID8 or UPK10 tumors. Data for the hematocrit, hemoglobin, white blood cells and platelets are shown.
Statistical analysis
Statistical significance was conducted using GraphPad Prism. Results were reported as mean ± standard deviation (SD). Analysis of two groups were performed using a Student’s t-test. Analyses of three groups or more were performed using an ANOVA and pair-wise comparisons were performed in a post hoc analysis with a Tukey or Sidak adjustment for multiple comparisons. All statistical tests were two-sided with an alpha level of 0.05.
Results
Conventional and FLASH irradiation reduce regulatory T cells and increase T cells with cytolytic potential in the ovarian cancer tumor microenvironment.
FLASH irradiation has emerged as a new treatment modality to reduce radiation-induced intestinal injury. Moreover, FLASH irradiation maintains similar tumor control to conventional irradiation in multiple tumor models (15–23). However, the immunomodulatory effects of FLASH irradiation remain unknown. To compare the immunomodulatory effects of total abdominopelvic conventional (CONV) and FLASH irradiation in preclinical models of ovarian cancer, we used a clinical linear accelerator modified to generate a 16 MeV electron beam to deliver both uniform treatment across the mouse and a homogenous depth dose (within <10% heterogeneity) throughout the mouse abdominal cavity (1.3 cm maximum depth) in both FLASH (210 Gy/s) and conventional mode (0.126 Gy/s) (Fig. 1A-B, Fig. S1). We developed a mouse stereotactic positioning frame with an irradiation field that extends 4 cm in the cranial/caudal direction starting at the 10th rib allowing for total abdominopelvic treatment (Fig. 1A). In agreement with our previous study, a sublethal dose of 14 Gy FLASH irradiation enhanced intestinal regeneration and maintained tumor control in the ID8 model of ovarian cancer metastasis (Fig. 1C-D, (15)). The ID8 ovarian cancer model is syngeneic in C57BL/6 mice, metastasizes throughout the peritoneal cavity and forms tumor nodules along the small and large intestine (30). Ten days after ID8 cancer cell injection, the mice were randomized into sham irradiated control, 14 Gy CONV, or 14 Gy FLASH irradiation treatment groups. At 96 hours post irradiation, ID8 tumor bearing mice treated with FLASH irradiation showed increased numbers of regenerating crypts in the jejunum compared to mice treated with CONV irradiation (Fig. 1C). Analysis of tumor burden at day 27 post irradiation revealed a decrease in the total tumor weight and ascites volume in CONV and FLASH irradiated mice compared to the sham irradiated control mice (Fig. 1D). No significant differences in tumor weight or ascites volume were observed when comparing CONV and FLASH irradiated mice, indicating that FLASH and CONV irradiation have similar efficacy in the treatment of ovarian cancer peritoneal tumors (Fig. 1D).
Figure 1. Abdominopelvic FLASH irradiation promotes intestinal regeneration and has similar tumor control compared to CONV irradiation in the ID8 ovarian cancer mouse model.
(A) CT images (coronal and sagittal slices) showing reproducible mouse positioning within the stereotactic frame. The yellow shaded rectangle (4 cm × 4 cm) highlights the irradiated region in the abdomen; the 4 cm length with the cranial border at the 10th rib encompasses the entire small intestine and pelvic region. The distance from the abdominal wall to the ventral surface of the spine, representing the most posterior extent of the abdominal cavity, is 1.3 cm (yellow arrow). (B) Depth dose, craniocaudal and lateral profiles at the entrance surface for FLASH and CONV setups using EBT3 Gafchromic films between layers of polystyrene. The doses are uniformly distributed (within <10% heterogeneity) within the abdominopelvic region. (C) Quantification of the average number of regenerating crypts per jejunal circumference 96 hours after 14 Gy abdominopelvic irradiation, demonstrating over double the number of regenerating crypts after FLASH vs. CONV irradiation (n=10 mice in CONV; n=7 in FLASH; 3 circumferences per mouse were analyzed). **p<0.01, CONV vs. FLASH compared by unpaired 2-tailed Student’s t-test. Scale bar shows 200 μm (top) and 100 μm (bottom). (D) Representative images showing metastatic tumor burden in mice that were intraperitoneally injected with ID8 ovarian tumor cells (left) and the quantification of total tumor weight and ascites volume in sham (Control), 14 Gy CONV, or 14 Gy FLASH irradiated mice demonstrating similar tumor control efficacy with both FLASH and CONV irradiation (n=8 mice per group). *p<0.05, **p<0.01 compared by one-way ANOVA followed by Tukey’s multiple comparisons test. Error bars represent standard deviation of the mean.
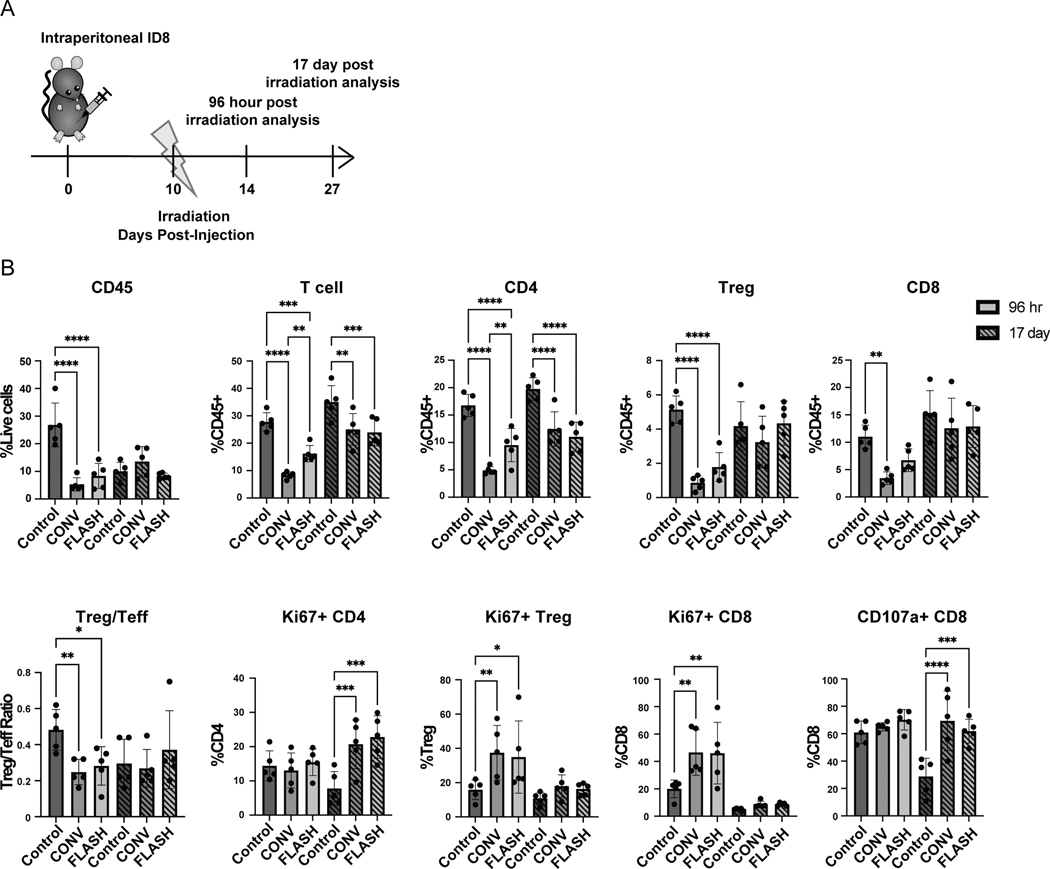
Next we compared the effects of 14 Gy CONV and FLASH irradiation in modulating the ovarian tumor immune microenvironment at both early (96 hours post irradiation) and late (17 days post irradiation) timepoints. ID8 tumor bearing mice were treated with sham irradiation, 14 Gy conventional (CONV) irradiation or 14 Gy FLASH irradiation at 10 days post cancer cell injection to allow for tumor seeding throughout the peritoneal cavity. At 96 hours and 17 days post irradiation immune cell populations were analyzed in the tumor-bearing omentum and the draining mesenteric lymph nodes (Fig. 2A, Fig. S2). At early timepoints, CONV and FLASH irradiation reduced the frequency of CD45+ leukocytes, T and B cells in the tumor microenvironment compared to the sham irradiated control (Fig. 2B, Fig. S3A). Notably, CONV and FLASH irradiation showed a shift in the Treg to T effector ratio with a decrease in regulatory T cells and an increase in CD8+ T cell proliferation compared to the sham irradiated control (Fig. 2B). When comparing CONV and FLASH irradiation, there was an increase in CD4+ cells in FLASH treated tumors compared to CONV treated tumors (Fig. 2B). At later timepoints, CONV and FLASH irradiation showed an enhancement in CD107a+ CD8+ T cells with cytolytic potential compared to the sham irradiated control (Fig. 2B). There were no differences found in the frequency of PMN-MDSCs, M-MDSCs or the M2/M1 macrophage ratio within the tumor microenvironment with comparing sham irradiated to CONV or FLASH irradiated mice (Fig. S3A). Within the mesenteric lymph node, CONV and FLASH irradiation increased CD8+ T cell proliferation consistent with a priming T cell response in the local draining lymph node (Fig. S3B). Overall, these data demonstrate that abdominopelvic FLASH irradiation maintains the ability to decrease regulatory T cells and increase cytolytic CD8+ T cells in the tumor microenvironment, suggesting an enhanced antitumor immune response with both treatment modalities in the ovarian cancer microenvironment.
Figure 2. Abdominopelvic CONV and FLASH irradiation decrease intratumoral immunosuppressive regulatory T cells and increase cytolytic CD8+ T cells in ID8 ovarian tumors.
(A) Experimental design where C57BL/6 mice were injected with 5×106 ID8 cancer cells. Ten days post-injection, mice were irradiated with either 14 Gy CONV or FLASH radiotherapy. Mice were analyzed at early (96 hours post irradiation) and late (17 days post irradiation). (B) Flow cytometry analysis for lymphocytes (CD45+), T cells (TCRβ+), CD4+, CD8+, and Treg (CD4+ FoxP3+) infiltration, Ki-67+ proliferation, and CD107a+ staining in the tumor bearing omentum at 96 hours and 17 days post irradiation (n= 5 mice per group). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared by one-way ANOVA followed by Tukey’s multiple comparisons post-hoc test. Error bars represent standard deviation of the mean. At least 2 independent experiments showed similar results.
CONV and FLASH irradiation enhance the efficacy of αPD-1 therapy in the ID8 ovarian cancer model.
Given the immunomodulatory properties of abdominopelvic CONV and FLASH irradiation, we hypothesized that both treatment modalities may enhance the efficacy of αPD-1 immune checkpoint blockade in preclinical models of ovarian cancer. We first used the ID8 syngeneic model of ovarian cancer, which is resistant to αPD-1 therapy even though the tumors express PD-L1 (Fig. S4A, (32)). Mice were intraperitoneally injected with ID8 ovarian cancer cells and randomized into 6 cohorts: isotype control antibody (IgG), IgG + 14 Gy CONV, IgG + 14 Gy FLASH, αPD-1, αPD-1 + 14 Gy CONV, or αPD-1 + 14 Gy FLASH using the treatment scheme shown in Fig. 3A. On day 27 post-injection, mice were euthanized to analyze tumor burden and immune infiltration. CONV and FLASH irradiation reduced tumor weight and ascites volume in both the IgG and αPD-1 treatment arms (Fig. 3B-C). However, the FLASH + αPD-1 combination therapy had superior efficacy compared to FLASH + IgG with a significant reduction in tumor weight and ascites volume (Fig. 3C). These data indicate that CONV and FLASH irradiation enhance the efficacy of αPD-1 in a resistant ovarian cancer model.
Figure 3. Abdominopelvic CONV and FLASH irradiation enhance the efficacy of αPD-1 therapy in the ID8 ovarian cancer tumor model.
(A) Experimental design where C57BL/6 mice were injected with 5×106 ID8 cancer cells. On days 7, 10, and 13 post-injection, mice were treated with αPD-1 or IgG control. On day 10 post-injection, mice were treated with 14 Gy CONV or 14 Gy FLASH irradiation. On day 27, mice were analyzed. (B) Macroscopic images of the tumor-bearing mice in each treatment arm. (C) Intraperitoneal tumor weight and volume of ascites fluid (n= 5 mice per group). *p<0.05, **p<0.01, ***p<0.001 compared by one-way ANOVA followed by Tukey’s multiple comparisons post-hoc test. Error bars represent standard deviation of the mean.
CONV and FLASH irradiation combined with αPD-1 decreases the Treg to T effector ratio and increases intratumoral CD8+ T cell infiltration in ovarian cancer peritoneal metastases.
We next investigated the effects of each treatment on the ovarian tumor immune microenvironment. In the ID8 model, the combination of CONV or FLASH irradiation with αPD-1 were the only treatment groups that decreased the Treg to T effector ratio that was associated with an increase in tumor infiltrating CD8+ T cells (Fig. 4). In addition, CONV or FLASH irradiation in combination with αPD-1 reduced the immunosuppressive PMN-MDSC and M2 to M1 macrophage ratios in the tumor microenvironment (Fig. S4B). These findings demonstrate that similar to CONV irradiation, FLASH irradiation in combination with αPD-1 is an effective strategy to reduce tumor progression and simultaneously enhance intratumoral CD8+ T cell infiltration and reduce immunosuppressive monocyte populations in the αPD-1 resistant ID8 model of ovarian cancer.
Figure 4. Abdominopelvic CONV and FLASH irradiation in combination with αPD-1 therapy enhance intratumoral CD8+ infiltration and decrease the Treg to T effector ratio in the ID8 ovarian tumor microenvironment.
Flow cytometry analysis for lymphocytes (CD45+), T cells (TCRβ+), CD4+, CD8+, and Treg (CD4+ FoxP3+) infiltration, Ki-67+ proliferation, and CD107a+ staining in the tumor bearing omentum at 96 hours and 17 days post irradiation (n= 5 mice per group). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared by one-way ANOVA followed by Tukey’s multiple comparisons post-hoc test. Error bars represent standard deviation of the mean. At least 2 independent experiments showed similar results.
Next we sought to determine if CONV or FLASH irradiation would enhance immune cell responses to αPD-1 treatment in the UPK10, αPD-1 sensitive model of ovarian cancer (33). UPK10 cells were intraperitoneally injected into C57BL/6 mice and mice were randomized into treatment groups as described above (Fig. 5A). Mice were sacrificed at 22 days post-injection for analysis. In this αPD-1 sensitive model of ovarian cancer, mice treated with αPD-1, 14 Gy CONV, 14 Gy FLASH, 14Gy CONV + αPD-1 and 14Gy FLASH + αPD-1 showed a similar decrease in tumor weight compared to mice treated with the IgG control (Fig. 5B-C). Despite similar tumor control, the combination CONV + αPD-1 and FLASH + αPD-1 treatments were the only treatment groups that increased intratumoral CD8+ T cell infiltration compared to the IgG control (Fig. 5D, Fig. S5). These findings demonstrate that similar to CONV irradiation, FLASH irradiation in combination with PD-1 blockade therapy improves intratumoral CD8+ T cell infiltration in a αPD-1 sensitive ovarian cancer models.
Figure 5. Abdominopelvic CONV and FLASH irradiation in combination with αPD-1 therapy enhance intratumoral CD8+ infiltration and decrease the Treg to T effector ratio in the UPK10 ovarian tumor microenvironment.
(A) Experimental design where C57BL/6 mice were injected with 5 million UPK10 cancer cells. On days 7, 10, and 13 post-injection, mice were treated with αPD-1 or IgG control. On day 10 post-injection, mice received 14 Gy CONV or 14 Gy FLASH irradiation. On day 22, mice were analyzed. (B) Macroscopic images of the tumor-bearing mice. (C) Intraperitoneal tumor nodule weight. (D) Flow cytometry analysis for lymphocytes (CD45+), T cells (TCRβ+), CD4+, CD8+, and Treg (CD4+ FoxP3+) infiltration, Ki-67+ proliferation, and CD107a+ staining in the tumor bearing omentum at 96 hours and 17 days post irradiation (n= 5 mice per group) *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared by one-way ANOVA followed by Tukey’s multiple comparisons post-hoc test. Error bars represent standard deviation of the mean. At least 2 independent experiments showed similar results.
FLASH irradiation and αPD-1 combination therapy does not increase toxicity compared to FLASH irradiation alone.
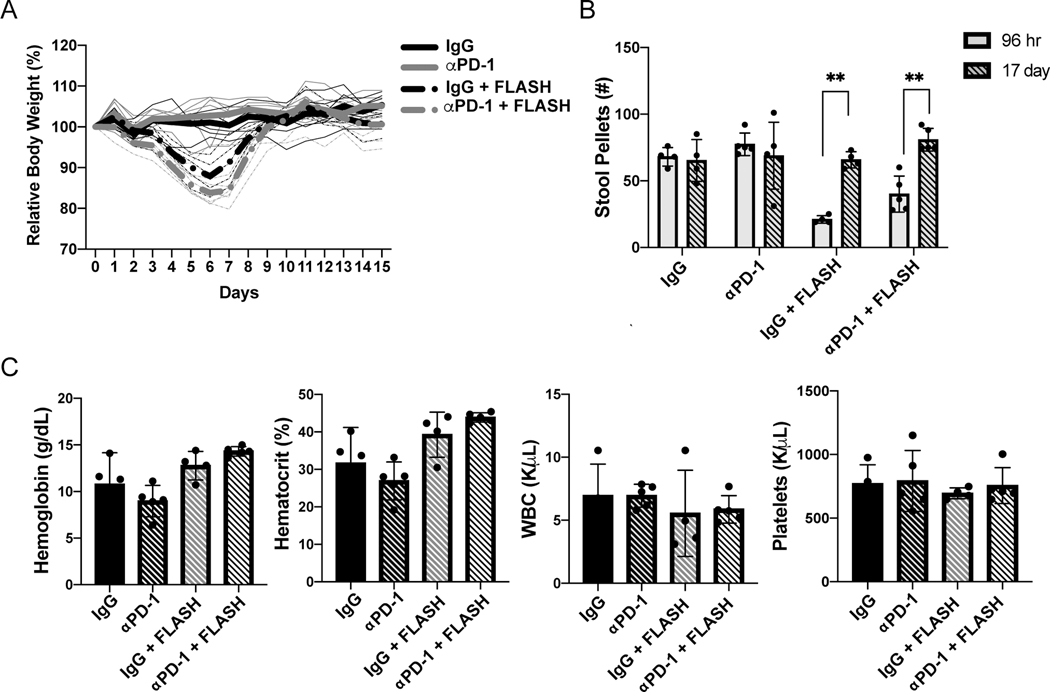
Collectively, our data demonstrate that FLASH irradiation reduces radiation-induced intestinal injury, while maintaining similar tumor control and immuomodulation compared to CONV irradiation. These data raise the intriguing possibility that FLASH irradiation may be an effective strategy to enhance immune checkpoint blockade responses in ovarian cancer. However, both radiation and checkpoint blockade therapy are known to cause gastrointestinal toxicity (26,34). Therefore, we determined the safety and tolerability of the FLASH irradiation and αPD-1 combination therapy. As we have previously observed, 14 Gy abdominal FLASH irradiation in the FLASH + IgG and the FLASH + αPD-1 treatment groups reduced body weight over 7 days post irradiation that returned to baseline levels by 10 days post irradiation (Fig. 6A, (15)). Reduced body weight in the FLASH + IgG and FLASH + αPD-1 treated mice was associated with a decrease in formed stool pellets at 96 hours post-irradiation that recovered to control levels by day 17 (Fig. 6B, S6). Importantly, there were no significant differences in body weight or stool production between the FLASH + IgG and FLASH + αPD-1 treated mice (Fig. 6A-B). Similar results were observed in the UPK10 model (Fig. S7). Complete blood count (CBC) analysis at 14 days post-treatment revealed comparable hemoglobin, hematocrit, white blood cell counts (WBC), and platelets among all treatment groups indicating that abdominal FLASH and αPD-1 treatments are not associated with hematologic toxicity (Fig. 6C). These findings demonstrate that combination of FLASH irradiation with αPD-1 inhibition does not increase gastrointestinal or hematological toxicities in mice compared to FLASH treatment alone.
Figure 6. Abdominopelvic FLASH irradiation combination with αPD-1 therapy does not increase toxicity compared to FLASH irradiation alone in the ID8 ovarian cancer model.
(A) Body weight of mice following treatment with IgG, 14 Gy abdominal FLASH irradiation, αPD-1, or the FLASH and αPD-1 combination treatment. (B) Stool pellet counts (24 hr) from mice in treatment groups at 96 hours or 17 days post irradiation. (C) Complete blood cell (CBC) analysis of blood collected at day 27 of the experiment after tumor injection.
Discussion
As immunotherapy becomes an increasingly utilized tool in our armamentarium against cancer, efforts to understand its interaction with traditional therapies are underway. In ovarian cancer, checkpoint inhibition is a promising therapeutic strategy. However, many tumors are either resistant or relapse following immune checkpoint monotherapy. Thus, optimization of immunotherapies through the identification of safe and effective immunomodulatory combination therapies is needed. Here we demonstrate that combining abdominal FLASH radiotherapy with PD-1 blockade enhanced tumor control associated with increased immunomodulation, while demonstrating safety and tolerability.
FLASH irradiation is emerging as a new treatment paradigm that has the potential enhance the therapeutic index of radiotherapy through its ability to reduce normal tissue toxicity. FLASH irradiation has been shown to reduce radiation-induced injury in multiple tissues (skin, lung, brain and intestine) and in multiple species (mice, minipig and human) (15–23). In the intestine, preclinical studies have demonstrated that FLASH irradiation reduces intestinal injury and promotes regeneration in both naïve and tumor bearing mice (15–18). Here we provide further data to support that FLASH irradiation promotes intestinal regeneration following radiation injury in ovarian tumor bearing mice.
While preclinical and clinical studies have demonstrated the immunostimulatory effects of conventional radiotherapy, the immunomodulatory properties of FLASH remain largely unknown. Kim and colleagues recently reported increased intratumoral CD8+ T cells at 6 hours post irradiation in a lewis lung carcinoma model (35). Our study reveals that at 96 hours post irradiation, FLASH irradiation increases intratumoral T cells, most notably CD4+ T cells, compared to CONV irradiation in a preclinical model of ovarian cancer. Both FLASH and CONV irradiation, increased CD4+ and CD8+ T cell proliferation and increased CD8+ T cells with cytolytic potential in the ovarian cancer tumor microenvironment and mesenteric lymph node. In addition, FLASH and CONV irradiation decreased the frequency of immunosuppressive regulatory T cells and reduced the regulatory to effector T cell ratio both in the tumor and the mesenteric lymph node. Overall, our findings indicate that while FLASH irradiation reduces radiation-induced intestinal injury, it maintains the ability to increase T cell infiltration and reduce immunosuppressive cells in the tumor microenvironment.
Conventional radiotherapy combined with checkpoint blockade has demonstrated improved tumor control in preclinical and clinical studies. However, the efficacy and safety of abdominal FLASH irradiation combined with immunotherapy is not known. We show that similar to conventional irradiation, combining FLASH and αPD-1 inhibition enhanced tumor control in an αPD-1 resistant model of ovarian cancer. Though the precise mechanism of therapeutic efficacy is unknown, the enhanced intratumoral effector to regulatory T cell ratio following FLASH irradiation and αPD-1 therapy likely contributes to improved tumor control. Studies have shown the ovarian cancer microenvironment correlates with patient outcomes. Increased levels of CD3+ tumor-infiltrating lymphocytes and decreased levels of regulatory T cells in ovarian cancer correspond with improved prognosis and survival (36,37).
Our findings have important clinical implications for the treatment of ovarian cancer and other abdominal/pelvic tumors. The overall low success of immunotherapy highlights the need to find alternate therapies that can modulate and improve immune treatment therapies. We have shown that combining FLASH irradiation with PD-1 blockade therapy is an effective and safe strategy in a preclinical models of ovarian cancer. Future studies are needed to further elucidate mechanisms and long-term effects of FLASH exposure combined with immunotherapy use.
Supplementary Material
Acknowledgements
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Department of Defense Ovarian Cancer Research Program under Award No. W81XWH-17-1-0042; the National Science Foundation Graduate Research Fellowship under DGE-1656518 (JTE); 1R01CA23395801 (EE and BWL); Carol and Doug Kimmelman Scholarship (EBR) and the My Blue Dots fund (EBR). The authors would like to thank Miguel Jimenez, Daniel Pawlak, and James Clayton from Varian Medical Systems for their technical assistance on the FLASH irradiation system.
Abbreviations:
- Anti-PD-1
Antibody targeting programmed cell death protein 1
- FLASH
ultra-high dose rate irradiation
- RT
radiation therapy
- LINAC
linear accelerator
- TCR
T cell receptor
- Treg
regulatory T cell
- CONV
conventional irradiation
- MDSC
myeloid derived suppressor cell
- PMN-MDSC
polymorphonuclear myeloid derived suppressor cell
Footnotes
Conflict of Interest Statement: The authors declare that Billy W. Loo is a co-founder and board member of TibaRay.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941–53. [DOI] [PubMed] [Google Scholar]
- 2.Matulonis UA, Sood AK, Fallowfield L, Howitt BE, Sehouli J, Karlan BY. Ovarian cancer. Nat Rev Dis Primers 2016;2:16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan RJ Jr., Alvarez RD, Armstrong DK, Boston B, Burger RA, Chen LM, et al. Epithelial ovarian cancer. J Natl Compr Canc Netw 2011;9:82–113. [DOI] [PubMed] [Google Scholar]
- 4.Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol 2019;30:1080–7. [DOI] [PubMed] [Google Scholar]
- 5.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J Clin Oncol 2015;33:4015–22. [DOI] [PubMed] [Google Scholar]
- 6.Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer 2018;18:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England journal of medicine 2003;348:203–13. [DOI] [PubMed] [Google Scholar]
- 8.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015;16:e498–509. [DOI] [PubMed] [Google Scholar]
- 9.Klaassen D, Shelley W, Starreveld A, Kirk M, Boyes D, Gerulath A, et al. Early stage ovarian cancer: a randomized clinical trial comparing whole abdominal radiotherapy, melphalan, and intraperitoneal chromic phosphate: a National Cancer Institute of Canada Clinical Trials Group report. J Clin Oncol 1988;6:1254–63. [DOI] [PubMed] [Google Scholar]
- 10.Sell A, Bertelsen K, Andersen JE, Stroyer I, Panduro J. Randomized study of whole-abdomen irradiation versus pelvic irradiation plus cyclophosphamide in treatment of early ovarian cancer. Gynecol Oncol 1990;37:367–73. [DOI] [PubMed] [Google Scholar]
- 11.Chiara S, Conte P, Franzone P, Orsatti M, Bruzzone M, Rubagotti A, et al. High-risk early-stage ovarian cancer. Randomized clinical trial comparing cisplatin plus cyclophosphamide versus whole abdominal radiotherapy. Am J Clin Oncol 1994;17:72–6. [DOI] [PubMed] [Google Scholar]
- 12.Dembo AJ. Abdominopelvic radiotherapy in ovarian cancer. A 10-year experience. Cancer 1985;55:2285–90. [DOI] [PubMed] [Google Scholar]
- 13.Hepp R, Baeza MR, Olfos P, Suarez E. Adjuvant whole abdominal radiotherapy in epithelial cancer of the ovary. Int J Radiat Oncol Biol Phys 2002;53:360–5. [DOI] [PubMed] [Google Scholar]
- 14.Cmelak AJ, Kapp DS. Long-term survival with whole abdominopelvic irradiation in platinum-refractory persistent or recurrent ovarian cancer. Gynecologic oncology 1997;65:453–60. [DOI] [PubMed] [Google Scholar]
- 15.Levy K, Natarajan S, Wang J, Chow S, Eggold JT, Loo PE, et al. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Sci Rep 2020;10:21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan JL, Lee C, Wouters S, Tullis ID, Verslegers M, Mysara M, et al. Irradiation at ultra-high (FLASH) dose rates reduces acute normal tissue toxicity in the mouse gastrointestinal system. Int J Radiat Oncol Biol Phys 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diffenderfer ES, Verginadis II, Kim MM, Shoniyozov K, Velalopoulou A, Goia D, et al. Design, Implementation, and in Vivo Validation of a Novel Proton FLASH Radiation Therapy System. Int J Radiat Oncol Biol Phys 2020;106:440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MM, Verginadis II, Goia D, Haertter A, Shoniyozov K, Zou W, et al. Comparison of FLASH Proton Entrance and the Spread-Out Bragg Peak Dose Regions in the Sparing of Mouse Intestinal Crypts and in a Pancreatic Tumor Model. Cancers (Basel) 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin Cancer Res 2019;25:35–42. [DOI] [PubMed] [Google Scholar]
- 20.Soto LA, Casey KM, Wang J, Blaney A, Manjappa R, Breitkreutz D, et al. FLASH Irradiation Results in Reduced Severe Skin Toxicity Compared to Conventional-Dose-Rate Irradiation. Radiat Res 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Science translational medicine 2014;6:245ra93. [DOI] [PubMed] [Google Scholar]
- 22.Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond JF, Petit B, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2017;124:365–9. [DOI] [PubMed] [Google Scholar]
- 23.Simmons DA, Lartey FM, Schuler E, Rafat M, King G, Kim A, et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother Oncol 2019;139:4–10. [DOI] [PubMed] [Google Scholar]
- 24.Bourhis J, Sozzi WJ, Jorge PG, Gaide O, Bailat C, Duclos F, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol 2019;139:18–22. [DOI] [PubMed] [Google Scholar]
- 25.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrera FG, Irving M, Kandalaft LE, Coukos G. Rational combinations of immunotherapy with radiotherapy in ovarian cancer. Lancet Oncol 2019;20:e417–e33. [DOI] [PubMed] [Google Scholar]
- 27.Schuler E, Trovati S, King G, Lartey F, Rafat M, Villegas M, et al. Experimental Platform for Ultra-high Dose Rate FLASH Irradiation of Small Animals Using a Clinical Linear Accelerator. International journal of radiation oncology, biology, physics 2017;97:195–203. [DOI] [PubMed] [Google Scholar]
- 28.Koch A, Gulani J, King G, Hieber K, Chappell M, Ossetrova N. Establishment of Early Endpoints in Mouse Total-Body Irradiation Model. PLoS One 2016;11:e0161079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Withers HR, Elkind MM. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med 1970;17:261–7. [DOI] [PubMed] [Google Scholar]
- 30.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 2000;21:585–91. [DOI] [PubMed] [Google Scholar]
- 31.Rutkowski MR, Stephen TL, Svoronos N, Allegrezza MJ, Tesone AJ, Perales-Puchalt A, et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 2015;27:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 2013;73:3591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miao YR, Thakkar KN, Qian J, Kariolis MS, Huang W, Nandagopal S, et al. Neutralization of PD-L2 is Essential for Overcoming Immune Checkpoint Blockade Resistance in Ovarian Cancer. Clin Cancer Res 2021;27:4435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Malet A, Antoni G, Collins M, Soularue E, Marthey L, Vaysse T, et al. Evolution and recurrence of gastrointestinal immune-related adverse events induced by immune checkpoint inhibitors. Eur J Cancer 2019;106:106–14. [DOI] [PubMed] [Google Scholar]
- 35.Kim YE, Gwak SH, Hong BJ, Oh JM, Choi HS, Kim MS, et al. Effects of Ultra-high doserate FLASH Irradiation on the Tumor Microenvironment in Lewis Lung Carcinoma: Role of Myosin Light Chain. Int J Radiat Oncol Biol Phys 2021;109:1440–53. [DOI] [PubMed] [Google Scholar]
- 36.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005;102:18538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.