Abstract
Nocardiosis, a rare infectious disease in dogs and cats, is caused by Gram-positive aerobic actinomycetes of the genus Nocardia. A one-year-old castrated male Great Dane was presented with clinical signs of an ulcerated nodule on the right ear, which was observed after two weeks of treatment with cyclosporine and prednisolone due to idiopathic hepatitis. Cytological examination revealed pyogranulomatous inflammatory cells and blanched filamentous rods. To detect infectious agents, serosanguinous discharge of the nodule was subjected to bacterial and fungal cultures. For phenotyping of the infectious agents, colonies on blood agar culture plates were further analyzed by matrix-assisted laser desorption ionization (MALDI)-time-of-flight (TOF) mass spectrometry (VITEK MS). The MALDI-TOF spectra were identified as N. africana. Thus, the present case was diagnosed as cutaneous nocardiosis. The skin lesions of ulcerated nodules with fistulous tracts were gradually resolved by the administration of meropenem (8 mg/kg TID, IV) and doxycycline (5 mg/kg BID, PO). Although complete resolution of the skin lesions was observed on day 91 after the initial presentation, single administration of doxycycline was continued until day 198 after the initial presentation to prevent recurrence. To the best of our knowledge, this is the first report of Nocardia africana infection in a dog. In addition, our results show that MALDI-TOF mass spectrometry analysis could be a useful tool for the detection of Nocardia. spps.
Keywords: dog, skin, nocardiosis, Nocardia africana, MALDI-TOF analysis
1. Introduction
Nocardiosis is a rare infectious disease in dogs and cats that is caused by Gram-positive, nonmotile, aerobic actinomycetes in the genus Nocardia. Nocardia spp. are common in organic materials, soil, water, and plants, and usually induce pyogranulomatous inflammation in immunocompromised humans and animals [1,2,3]. Accordingly, inhalation or subcutaneous transmission of Nocardia spp. induces pyogranulomatous to suppurative inflammatory reactions either in localized organs (skin or lungs), or disseminates to more than one organ. Therefore, it can be classified into three clinical presentations, cutaneous, pulmonary, and systemic forms [1,2,3]. Among these three presentations, cutaneous nocardiosis is the most common and manifests as mycetoma, regional lymphadenitis, ulceration, and abscesses with fistulous tracts that drained a serosanguineous discharge. Lesions are usually observed on the extremities, facial area, and neck [4]. In animals, the majority of reported cases are caused by species of the Nocardia (N.) asteroides complex. Other common pathogenic species include N. brasiliensis, N. nova and N. otitidiscaviarum [5,6]. N. africana, N. elegans, and N. tenerifensis have rarely been reported in cats and N. abscessus has rarely been reported in dogs [5,6,7,8]. However, to the best of our knowledge, there have been no reports of N. africana infection in dogs.
The routine diagnosis of nocardiosis in veterinary medicine is based on histopathological analysis and microbiological culture [5]. Molecular methods, such as 16S rRNA, heat shock protein and essential secretory protein–based polymerase chain reaction (PCR), have been used to confirm the phenotypic diagnosis of Nocardia spp. [6]. Recently, mass spectrometry-based proteomic analysis, including matrix-assisted laser desorption ionization (MALDI)-time-of-flight (TOF), has emerged as a rapid and reliable methodology that allows the phenotypic diagnosis of a great variety of microorganisms of clinical interest [9]. Routine extraction methods for detection of Nocardia spp. by MALDI-TOF analysis can be used to reliably identify Nocardia spp. [10,11].
2. Case Description
A one-year-old castrated male Great Dane living outdoors was presented with history of nodules on his right ear for one week. The dog had a prior medical history that included recent diagnosis with idiopathic hepatitis, for which he had been receiving cyclosporine (10 mg/kg SID, PO, Neoral; Novartis, Switzerland) and prednisone (1 mg/kg BID, PO, Sorondo; Yuhan Co. Ltd., Seoul, Korea). The nodules were first observed after about two weeks of immunosuppressive therapy. At the initial presentation, a single button-like nodule was observed on the right pinna (Figure 1A). The nodule was firm, and the entire surface was ulcerated. The next day, the lesions became swollen, and another ulcer with a fistulous tract appeared on the right pinna (Figure 1B). The fistulous tract drained the serosanguinous discharge. On cytological examination of the ulcerated lesions, the main inflammatory cells were degenerative neutrophils, macrophages, and lymphocytes. In addition, branched filamentous rods were observed (data not shown). Based on the cytological findings, deep bacterial infection, deep fungal infection, foreign body reaction and sterile pyogranuloma/granuloma syndrome were included as differential diagnosis. To investigate systemic condition of the patient, we performed complete blood count and serum chemistry that revealed neutrophilia (38.88; reference range: 6–17 K/uL), hypoalbuminemia (2.1; reference range: 2.3–3.9 g/dL), increased ALT(122; reference range: 3–50 U/L), increased AST (63; reference range: 10–37 U/L), increased total bilirubin (2.6; reference range: 0.1–0.7 mg), increased amylase (1156; reference ranges: 388–1007 U/L), decreased total calcium (8.8; reference range: 9.1–11.7 mg). Hypoalbuminemia and increased liver enzymes and increased total bilirubin was thought be associated chronic hepatitis of the patients. The serosanguinous discharge of ulcerated lesions was collected by swabbing fistulous tract on ulcerative lesions and subjected to fungal and bacterial cultures and PCR for detection of mycobacteria (POPANILAB, Gyeonggi, Korea). Cefazolin (20 mg/kg TID, IV, Cefazolin injection; Yuhan Co. Ltd., Seoul, Korea) and enrofloxacin (10 mg/kg, SID, IV, Baytril; Bayer AG, Barmen, Germany) were administered during the waiting period.
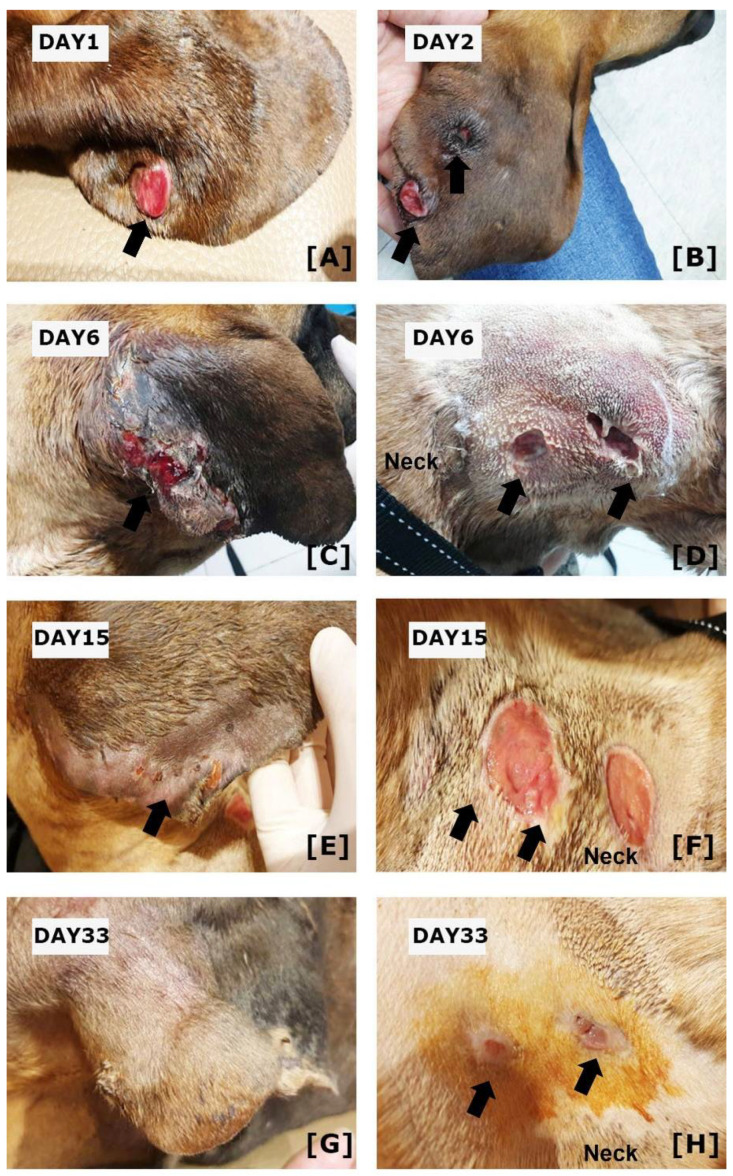
Figure 1.
Clinical Features of the Present Case. (A) At the initial presentation, ulcerated nodules on the right ear were seen. (B) On day two, another lesion with ulceration was observed on the right pinna. (C) On day 6, the ulcerative lesions on right pinna have enlarged, (D) and ulcerations with fistulous tract were observed on the neck. (E) After administration of meropenem and doxycycline, the ulcerations on right pinna have gradually resolved on day 15. (F) However, the ulcerations on the neck were still observed. (G) On day 33, the skin lesions on the right pinna were resolved and show hair regeneration. (H) Ulcerative lesions on the neck also show skin regeneration. Arrows indicate skin lesions.
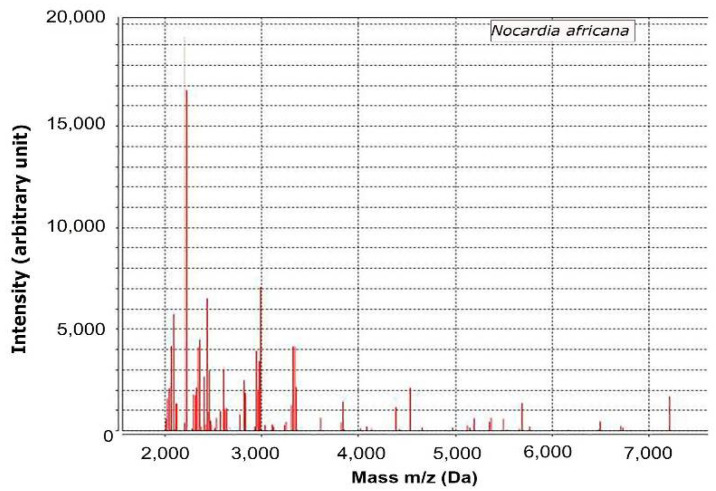
The culture colonies of serosanguinous discharge of ulcerated lesions were further investigated by MALDI-TOF mass spectrometry. The intensity of the specimen showed 99% of confidence value with N. africana.
The specimens were cultured on blood agar plates and incubated at 37 °C. On day two of incubation, chalky white and cotton appearance colonies were observed on blood agar plates. The colonites were further subjected to MALDI-TOF mass spectrometry (VITEK MS v.3 bioMérieux, Inc., Durham, NC, USA) for the phenotyping of infectious agents. Briefly, colonies were picked with a sterile loop and then prepared for analysis using a Mycobacterium/Nocardia reagent kit (Vitek MS; bioMérieux, Inc., Durham, NC, USA) according to the manufacturer’s instructions. Mechanical disruption using 0.5 mm-diameter glass beads and bead beating for 5 min were performed, and then the samples was incubated with 70% ethanol for 10-min at room temperature and then protein was extracted using 70% formic acid. One microliter of the protein extract was then applied to a single spot on the MALDI target plate (Vitek MS-DS; bioMérieux, Inc., Durham, NC, USA), allowed to dry, and 1 μL of alpha-cyano-4-hydroxycinnamic acid (Vitek MS-CHCA matrix; bioMérieux, Inc., Durham, NC, USA) was added. After the plate was dried, spectra were acquired using MALDI-TOF mass spectrometry (VITEK MS v.3 bioMérieux, Inc., Durham, NC, USA) and compared with the VITEK MS database, which includes more than 15,000 distinct microorganisms, including Nocardia spp. The spectra of the specimen were identified as N. africana (Figure 2) showing a 99% of confidence value. Thus, the present case was diagnosed as nocardiosis. In contrast, no infectious agents were detected by fungal culture or PCR to detect mycobacteria (data not shown). Further antibiotic sensitivity tests were performed using automated minimum inhibitory concentration testing (VITEK 2 AST, bioMérieux, Inc., Durham, NC, USA). While waiting for the results of antibiotic sensitivity tests, they were changed to meropenem (8 mg/kg TID, IV, MEROPENEM Daewoong; Daewoong Pharmaceutical, Seoul, Korea) and trimethoprim-sulfamethoxazole (21 mg/kg BID, PO, Septrin; Samil Pharmaceutical Co., Seoul, Korea) on day four, as meropenem in addition to trimethoprim-sulfonamide are known to be effective in nocardiosis. However, the skin lesions did not resolve, and another ulcerative lesion with a fistulous tract appeared on the neck 6 days after initial presentation (Figure 1C,D). Antibiotic sensitivity tests revealed that N. africana isolated from this dog were resistant to trimethoprim-sulfonamides, and the antibiotics were changed to meropenem and minocycline (5 mg/kg BID, PO, Minocin; SK chemical, Gyeonggi-do, Korea)) on day nine. The results of antibiotic sensitivity tests are shown in Table 1. Although ulceration and serosanguinous discharge gradually resolved after treatment with meropenem and minocycline, the antibiotics were changed to meropenem and doxycycline (5 m/kg BID, PO, Doxycycline tab; Youngpoong pharmaceuticals, Korea) on day 14. On day 15, the ulceration of the right ear was almost resolved, and also indicated by regrowth of hair (Figure 1E). Ulcerations of the neck were still present (Figure 1F). On day 33, complete resolution of the skin lesion on the right ear was observed (Figure 1G), and skin regeneration was observed in the neck (Figure 1H). On day 45, the administration of meropenem was discontinued and single administration of doxycycline was used. On day 91, complete resolution of the skin lesions in the right ear and neck was observed. Administration of doxycycline was continued until day 198 to prevent the recurrence of clinical signs. No relapse was observed as of 12 months after completion of antibiotic therapy, at which time the patient was lost to follow-up.
Figure 2.
Analysis of MALDI-TOF mass spectrometry in the present case.
Table 1.
Antibiotic sensitivity results of Nocardia africana in this case.
| Antibiotics | Sensitivity | Antibiotics | Sensitivity |
|---|---|---|---|
| Amikacin | S | Cefalothin | R |
| Cefazolin | S | Cefotaxime | R |
| Cefotetan | S | Cefoxitin | R |
| Gentamicin | S | Ciprofloxacin | R |
| Imipenem | S | Doxycycline | R |
| Kanamycin | S | Penicillin | R |
| Minocycline | S | Sulphamethox/Trimethoprim | R |
| Amoxycillin/Clavulanic acid | R | Vancomycin | R |
| Ampicillin | R |
3. Discussion
In this report, we describe the first case report confirming cutaneous nocardiosis caused by N. africana in the dog. The present case showed ulcerated nodules and a fistulous tract with serosanguinous discharge after two weeks of immunosuppressive drug administration. In this report, we performed MALDI-TOF spectra analysis using VITEK MS instrument revealed that the culture colonies of the skin specimen were N. africana. The skin lesions gradually resolved with administration of meropenem and doxycycline. Therefore, the present case was diagnosed as cutaneous nocardiosis caused by N. africana infection.
N. africana, which was first identified in human patients with chronic lung disease, has rarely been reported in humans [12]. In veterinary medicine, two cases of N. africana have been reported in cats [7,8]. It was first identified in cats with mycetoma, which showed white-yellow nodules with a fistulous tract on the tail and abdomen. The nucleotide sequence of the 16S ribosomal DNA of the clinical isolate corresponded to that of a reference strain of N. africana [7]. Another report of feline nocardiosis caused by N. africana described fever, submandibular lymphadenitis, and an ulcerated mass with serosanguineous discharge in the left mandible. Radiography of the mandible also revealed osteomyelitis with intense bone proliferation and osteolysis. Sequencing analysis of the 16S rDNA identified the organism as N. africana based on 99% sequence similarity with the reference sequence [8]. In this case of N. africana infection in a dog, the skin lesions of ulcerated nodules with a fistulous tract were similar to those in previous reports of N. africana infections in cats.
As nocardiosis can induce fatal systemic infections, rapid diagnosis and appropriate treatment are required. Furthermore, differentiating Nocardia spp. at the species level provides more information on the predicted response to antibiotic therapy and patient prognosis [13]. Recently, mass spectrometry-based proteomic analysis, including MALDI-TOF, has been reported to provide rapid and reliable diagnosis of disease including canine nocardiosis [14,15]. Nocardia spp. detected by MALDI-TOF showed high correspondence at the species level with those detected by PCR targeting 16S ribosomal DNA [10,11]. The time to perform MALDI-TOF analysis was shorter than that of classical molecular testing [10,11]. In this case, MALDI-TOF analysis using VITEK MS instrument was conducted within 1 day, after observing colonies on plates. Similarly, a previous report [14] of canine nocardiosis caused by N. veterana successful treatment could be achieved by early diagnosis of nocardiosis by MALDI-TOF analysis. Therefore, MALDI-TOF analysis might be useful for rapid diagnosis nocardiosis and identification of the specific levels of Nocardia.
Treatment of nocardiosis usually requires long-term antibiotic administration. Trimethoprim-sulfamethoxazole has long been used as first-line antibiotic therapy for nocardiosis, but resistance to this treatment has recently emerged [16]. In two previous reports of feline nocardiosis caused by N. africana, clinical signs were not controlled by treatment with trimethoprim-sulfamethoxazole [7,8]. Similarly, N. africana isolated from this dog were resistant to trimethoprim-sulfonamides, and administration of trimethoprim-sulfamethoxazole did not resolve the skin lesions. Other first-choice drugs for the treatment of nocardiosis include amoxicillin-clavulanate, imipenem, and some cephalosporins. It has also been reported that clinical signs can improve by combining other drugs such as ampicillin, linezolid, doxycycline, erythromycin, and minocycline [13]. The present case also used a combination therapy of meropenem and doxycycline, and complete resolution of skin lesions was observed with these treatments. Furthermore, even after clinical signs have resolved, long-term antibiotic administration is required due to the fact that clinical relapses can be observed after short-term protocols [1,2]. In the present case, even though clinical signs already resolved on day 91, antibiotic therapy was continued until day 198, and no relapse of nocardiosis was observed as of 12 months after stopping treatments.
Nocardia infection is more likely in immunosuppressed individuals, as previously reported [1,2]. In the present case, Nocardia infection was also observed in an immunocompromised dog treated with prednisolone and cyclosporine. Nocardia infection should be considered when cutaneous nodules and ulcers are observed in dogs during ongoing administration of immunosuppressive drugs, and culture and phenotyping of infectious agents need to be performed.
One of the potential limitations of this case report is that other diagnostic tests such as histopathological analysis were not performed. In addition, other tests to investigate systemic condition such as x-ray, ultrasonography, and CT scan were not performed and thus concurrent systemic signs of nocardiosis were not completely ruled out. Further tests such as histopathological analysis and CT scan would provide further information to diagnose cutaneous nocardiosis in this case.
4. Conclusions
In this report, we describe diagnosis of cutaneous nocardiosis caused by N. africana infection in a dog using MALDI-TOF of VITEK MS instrument. To the best of our knowledge, this is the first report of an N. africana infection in a dog. MALDI-TOF mass spectrometry was a useful tool for the detection of Nocardia spp.
Author Contributions
J.-S.Y. contributed to review of the case, writing and editing of the manuscript and review of final submission. H.S. contributed to writing the manuscript, management of case and figure preparation. B.J., J.P. (Jihong Park) and I.-S.J. contributed to management of case and collection of data. G.-J.L. contributed to management of case, collection of data and writing and editing of the manuscript. J.P. (Jinho Park) contributed to supervision, writing and editing of the manuscript and review of finial submission. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the owner to publish this paper.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Project No. PJ01690702 from the Rural Development Administration, Republic of Korea.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kirpensteijn J., Fingland R.B. Cutaneous actinomycosis and nocardiosis in dogs: 48 cases (1980–1990) J. Am. Vet. Med. Assoc. 1992;201:917–920. [PubMed] [Google Scholar]
- 2.Malik R., Krockenberger M.B., O’Brien C.R., White J.D., Foster D., Tisdall P.L., Gunew M., Carr P.D., Bodell L., McCowan C., et al. Nocardia infections in cats: A retrospective multi-institutional study of 17 cases. Aust. Vet. J. 2006;84:235–245. doi: 10.1111/j.1751-0813.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- 3.Wilson J.W. Nocardiosis: Updates and clinical overview. Mayo Clin. Proc. 2012;87:403–407. doi: 10.1016/j.mayocp.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipovan I., Burlacu M.R., Mareš M., Vulpe V. Multiple dermal ulcers in a case of feline nocardial mycetoma. Sci. Work. Ser. C Vet. Med. 2015;61:139–142. [Google Scholar]
- 5.Ribeiro M.G., Salerno T., Mattos-Guaraldi A.L., Camello T.C., Langoni H., Siqueira A.K., Paes A.C., Fernandes M.C., Lara G.H. Nocardiosis: An overview and additional report of 28 cases in cattle and dogs. Rev. Inst. Med. Trop. Sao Paulo. 2008;50:177–185. doi: 10.1590/S0036-46652008005000004. [DOI] [PubMed] [Google Scholar]
- 6.Brown-Elliott B.A., Brown J.M., Conville P.S., Wallace R.J., Jr. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin. Microbiol. Rev. 2006;19:259–282. doi: 10.1128/CMR.19.2.259-282.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hattori Y., Kano R., Kunitani Y., Yanai T., Hasegawa Y. Nocardia africana isolated from a feline mycetoma. J. Clin. Microbiol. 2003;41:908–910. doi: 10.1128/JCM.41.2.908-910.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Farias M.R., Werner J., Ribeiro M.G., Rodigheri S.M., Cavalcante C.Z., Chi K.D., Condas L.A., Gonoi T., Matsuzama T., Yazama K. Uncommon mandibular osteomyelitis in a cat caused by Nocardia africana. BMC Vet. Res. 2012;8:239. doi: 10.1186/1746-6148-8-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singhal N., Kumar M., Kanaujia P.K., Virdi J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015;6:791. doi: 10.3389/fmicb.2015.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girard V., Mailler S., Polsinelli S., Jacob D., Saccomani M.C., Celliere B., Monnin V., van Belkum A., Hagen F., Meis J.F., et al. Routine identification of Nocardia species by MALDI-TOF mass spectrometry. Diagn. Microbiol. Infect. Dis. 2017;87:7–10. doi: 10.1016/j.diagmicrobio.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Durand T., Vautrin F., Bergeron E., Girard V., Polsinelli S., Monnin V., Durand G., Dauwalder O., Dumitrescu O., Laurent F., et al. Assessment of VITEK® MS IVD database V3.0 for identification of Nocardia spp. using two culture media and comparing direct smear and protein extraction procedures. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:559–567. doi: 10.1007/s10096-019-03758-x. [DOI] [PubMed] [Google Scholar]
- 12.Hamid M.E., Maldonado L., Sharaf Eldin G.S., Mohamed M.F., Saeed N.S., Goodfellow M. Nocardia africana sp. nov., a new pathogen isolated from patients with pulmonary infections. J. Clin. Microbiol. 2001;39:625–630. doi: 10.1128/JCM.39.2.625-630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao P., Zhang X., Du P., Li G., Li L., Li Z. Susceptibility profiles of Nocardia spp. to antimicrobial and antituberculotic agents detected by a microplate Alamar Blue assay. Sci. Rep. 2017;7:43660. doi: 10.1038/srep43660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaemsiri S., Sykes J.E. Sykes Successful Treatment of Disseminated Nocardiosis Caused by Nocardia veterana in a Dog. J. Vet. Intern. Med. 2018;32:418–422. doi: 10.1111/jvim.14855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uhde A.K., Kilwinski J., Peters M., Verspohl J., Feßler A.T., Schwarz S., Wohlsein P. Fatal nocardiosis in a dog caused by multiresistant Nocardia veterana. Vet. Microbiol. 2016;183:78–84. doi: 10.1016/j.vetmic.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Saksena R., Rynga D., Rajan S., Gaind R., Dawar R., Sardana R., Sen M.K., Suri J.C. Fatal pulmonary infection by trimethoprim-sulfamethoxazole resistant Nocardia otitidiscaviarum: Report of two cases and review. J. Infect. Dev. Ctries. 2020;14:214–222. doi: 10.3855/jidc.10169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.