Abstract
The century-old, well-known odd–even effect phenomenon is still a very attractive and intriguing topic in supramolecular and nano-scale organic chemistry. As a part of our continuous efforts in the study of supramolecular chemistry, we have prepared three novel aromatic alcohols (1,2-bis[2-(hydroxymethyl)phenoxy]butylene (Do4OH), 1,2-bis[2-(hydroxymethyl)phenoxy]pentylene (Do5OH) and 1,2-bis[2-(hydroxymethyl)phenoxy]hexylene (Do6OH)) and determined their crystal and molecular structures by single-crystal X-ray diffraction. In all compounds, two benzyl alcohol groups are linked by an aliphatic chain of different lengths (CH2)n; n = 4, 5 and 6. The major differences in the molecular structures were found in the overall planarity of the molecules and the conformation of the aliphatic chain. Molecules with an even number of CH2 groups tend to be planar with an all-trans conformation of the aliphatic chain, while the odd-numbered molecule is non-planar, with partial gauche conformation. A direct consequence of these structural differences is visible in the melting points—odd-numbered compounds of a particular series display systematically lower melting points. Crystal and molecular structures were additionally studied by the theoretical calculations and the melting points were correlated with packing density and the number of CH2 groups. The results have shown that the generally accepted rule, higher density = higher stability = higher melting point, could not be applied to these compounds. It was found that the denser packaging causes an increase in the percentage of repulsive H‧‧‧H interactions, thereby reducing the stability of the crystal, and consequently, the melting points. Another interesting consequence of different molecular structures is their electrochemical and antioxidative properties—a non-planar structure displays the highest oxidation peak of hydroxyl groups and moderate antioxidant activity.
Keywords: aromatic alcohols, aliphatic chain, odd–even effect, melting point deviation, crystal structures
1. Introduction
Aromatic dialcohols containing a different number of methylene groups in the aliphatic chain are well known as a precursor in the synthesis of a vast number of azaoxa and thiaoxa macrocycles [1]. It is also important to emphasize that the change in chain length can be used to manipulate the size of the binding space in the macrocyclic system, and thus, to modify the binding affinity towards different chemical species. As previously described by our group in the case of dialdehydes, these subtle changes in the chain length can lead to the different extraction properties of macrocycles [2,3]. Further on, such changes result in diverse conformations of macrocycles, with an important impact on the dimensionality and supramolecular assembly of coordination compounds [4].
It was recognized almost 120 years ago [5] that n-alkyl carboxylic acids do not undergo a constant increase in melting point depending on the length of the aliphatic chain. Instead of such behavior, compounds with an even number of carbons have higher melting points in comparison to molecules with an odd number of carbon atoms of the same series. Such behavior is known as the odd–even effect [6]. The origin of this effect was attributed to the optimal intermolecular and interlayer interactions between molecules in the solid-state [7] and usually correlated to packing densities. This effect was also observed in a series of n-alkanes [8], α,ω-alkendiamines [8], α,ω-alkendiols [8] and α,ω-alkendithiols [9]. In the case of n-alkanes, the melting point is directly correlated to the packing density—the odd-numbered n-alkanes having lower density, while in the case of α, ω-dicarboxylic acids, the effect is just the opposite. This contradiction was explained by the intermolecular arrangement of molecules in crystalline state that maximize van der Waals interactions between adjacent molecules and layers of molecules [8]. Of particular interest to our investigation is the study of the odd–even effect in α,ω-alkendiols conducted by Thalladi et al. [8]. This systematic investigation has shown three important conclusions: (i) the OH group acts both as a hydrogen bond donor and acceptor and represents the primary mode of intermolecular connection; (ii) the conformation of the aliphatic chain in even-membered molecules is all-trans, leading to offset packing of aliphatic chain and more dense packing with favorable hydrophobic interactions; (iii) odd-membered diols display a partial gauche conformation of the aliphatic chain, leading towards the formation of 3D supramolecular networks with less-effective hydrophobic interactions. Certainly, the most interesting consequence of the odd–even effect was found in n-alkenthiolates. These compounds were used as self-assembled monolayers (SAMs) in Ag/GaOx/EGaIn electrode systems. It was demonstrated in this work that odd-numbered molecules impede the charge transfer process in the investigated system [10].
In order to further contribute to the clarification of the odd–even effect phenomenon, we have prepared and structurally characterized three novel n-alkylene aromatic alcohols. The compounds were characterized by means of elemental analysis, IR and NMR spectroscopy. The molecular and crystal structures were determined by the single-crystal X-ray diffraction method. The structure–melting point–electrochemical property correlation was conducted by comparison with previously published chain analogs ((CH2)n; n = 1, 2, 3) using theoretical calculations (Hirshfeld surface analysis and interaction energy calculations), thermal analysis and cyclic voltammetry measurements.
2. Materials and Methods
All chemicals were procured from commercial sources and were of reagent grade. IR spectra were recorded on a Shimadzu FTIR 8400S spectrophotometer using a DRS 8000 attachment, in the 4000–400 cm−1 region. Thermogravimetric analyses were performed using a simultaneous Mettler Toledo TGA/DSC 1. The samples were heated in a nitrogen atmosphere (200 mL min−1) in 100 μL aluminum pans up to 500 °C at a rate of 5 °C min−1. Before measurements, a blank curve measurement under the same experimental conditions was run, and the blank curve was subtracted. The data collection and analyses were performed using the program package STARe Software 10.0 [11]. A DSC analysis of Do6OH polymorphic forms I and II was performed using a Setaram MicroSC from 25 °C to 150 °C at a rate of 1.2 °C min−1 in Hastelloy C crucibles. The 1H NMR spectra were recorded on a Bruker Avance III HD NMR 500 MHz instrument at 500 MHz, using deuterated DMSO as solvent. All NMR experiments were performed at 298 K. All coupling constants are reported in hertz (Hz). Chemical shifts are reported in ppm and are referenced to residual solvent peak DMSO (1H 2.5 ppm). Stock solutions of investigated compounds (Do4OH, Do5OH and Do6OH) were prepared in DMF. Before each measurement, an appropriate amount of each compound was diluted in 0.1 M KOH. Electrochemical experiments were performed on a PalmSens potentiostat/galvanostat (PalmSens BV, Utrecht, The Netherlands) driven by PSTrace 4.2 software. A conventional three-electrode cell was used with a gold electrode as a working electrode, Ag/AgCl as a reference electrode and a platinum wire as a counter electrode. The gold working electrode was polished with polishing α-Al2O3 (0.05 µm, ALS, Japan) before each measurement. The cyclic voltammetry scan rate varied from 50 mV s−1 to 400 mV s–1. Fresh DPPH (c(2,2-diphenyl-1-picrylhydrazyl) = 3 × 10−4 mol dm−3) solution was prepared in methanol. The reaction of radical scavenging was carried out by mixing the 100 µL of the investigated sample with 500 µL of the DPPH solution, and samples were kept in the dark and covered with foil for 15 min. The UV-Vis measurements were performed using a Shimadzu UV-2600 Spectrophotometer at λmax = 518 nm. The antioxidant activity of samples was evaluated by changes in colors, from dark purple to pale yellow. The measurements were performed in triplicate and expressed as % scavenging activity (% DPPH).
2.1. X-ray Crystallography
The single-crystal X-ray diffraction data were collected at 150 K on an Oxford Diffraction SuperNova CCD diffractometer equipped with an Atlas detector and with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å) using ω–scans. The data reduction was performed using CrysAlis Pro [12]. Structures were solved by ShelXT [13] using intrinsic phasing and refined by a full-matrix least-squares procedure based on F2 with SHELXL-2018/3 [14]. All non-hydrogen atoms were refined anisotropically, and hydrogen atoms in the structures were initially located in difference Fourier maps; those attached to C-atoms were afterward placed in calculated positions and refined using the riding model, while those residing on oxygen atoms were refined freely. Geometrical calculations and Newman projections were made using PLATON [15,16], and structure drawings were made with the MERCURY [17] program. The crystallographic data are summarized in Table S1.
2.2. Interaction Energy Calculation and Hirshfeld Surface Analysis
CrystalExplorer 17.5 was used to analyze Hirshfeld surfaces and evaluate the interaction energies and Hirshfeld surface analysis, using CIF files as input [18]. The energy calculations were performed with a B3LYP/6-31+G(d,p) computational model [18]. Scale factors used for calculations were set according to the used level of theory. Crystal lattice energies were calculated as one-half of the product of the two columns labeled N and Etot, where N is the number of molecule pairs in the cluster with that particular interaction energy using data obtained from interaction energy calculation (Tables S4–S7). In these calculations, a symmetry-independent molecule was selected, and energy interactions with adjacent molecules in the 3.8 Å cluster radius were calculated. For compounds containing more than 1 molecule in the asymmetric unit, calculations were made for both conformer molecules independently. Energies with the same value of distance (R) were counted only once. Crystal lattice energies were also calculated using the PixelC [19] program implemented in the Oscail software package. The obtained CIF files were imported in the Oscail program and PixelC was run using the Oscail interface (Run Job) to convert files into appropriate format. Using PixelC, the centroids and centroid contacts were calculated with a maximum contact distance of 20 Å. Thus, prepared files were opened in PixelC and lattice energy was calculated using Orca [20] with MultiWFN [21] and with a B3LYP computational model [18]. The computations were performed following the previously described procedure [22]. The Hirshfeld surfaces were plotted over normalized contact distances (dnorm) with standard color settings: regions highlighted in red represent short contacts while longer contacts are shown in blue. White areas represent contacts at the distance equal to the sum of the van der Waals radii.
Additionally, the full and resolved 2D fingerprint plots that show distances from each point on the Hirshfeld surface to the nearest atom inside (di) and outside (de) of it were calculated for all four structures.
2.3. Synthesis
Compounds were prepared by the reduction of appropriate aldehyde groups with NaBH4, following a previously reported method [23], according to Scheme S1. Dialdehyde precursors 1, 2, 3 were synthesized by previously reported methods [3,24,25]. Details regarding the synthesis procedure can be found in ESI. The recrystallization of the Do6OH from two different solvent systems (DMF/H2O and DMSO/H2O) resulted in the formation of two polymorphs (Forms I and II, respectively).
3. Results
3.1. Description of Crystal Structures
Investigated compounds crystallize in the orthorhombic (Do5OH and Do6OH II) and monoclinic (Do4OH and Do6OH I) crystal systems. The molecular structures of compounds are shown in Figure 1 and Figure 2. The selected bond lengths and angles are presented in Table S2. In all four compounds two benzyl alcohol units are connected by an aliphatic chain of different lengths, (CH2)n, with n = 4, 5 and 6. The primary interest of this work is to investigate the impact of aliphatic chain length on molecular and crystal structure. In all four crystal structures, the benzyl alcohol moieties are oriented opposite to each other (anti-orientation). The influence of aliphatic chain length on the molecular structure of prepared compounds (and consequently, on the crystal packing arrangement and thermal properties) can be determined by using two parameters: the deviation of benzene rings from planarity and the conformation of the aliphatic chain.
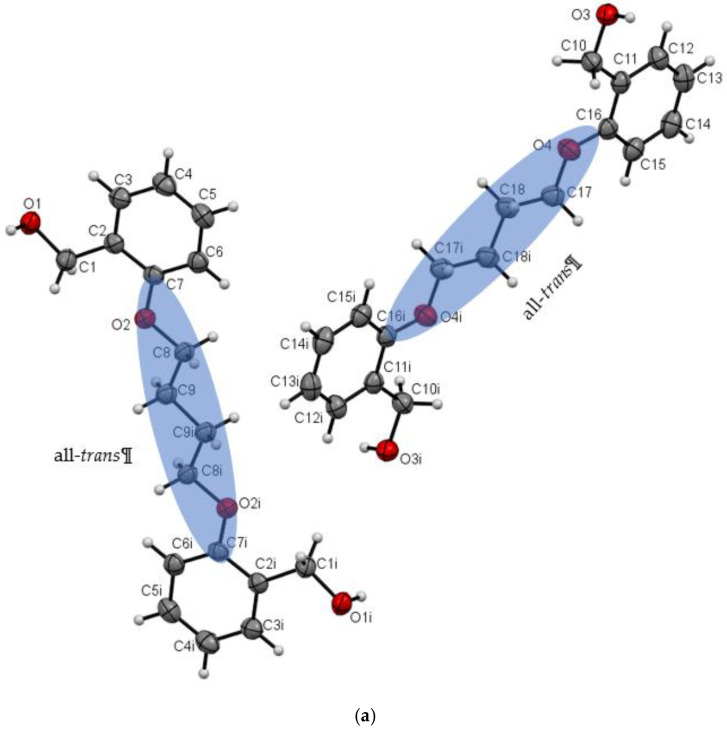
Figure 1.
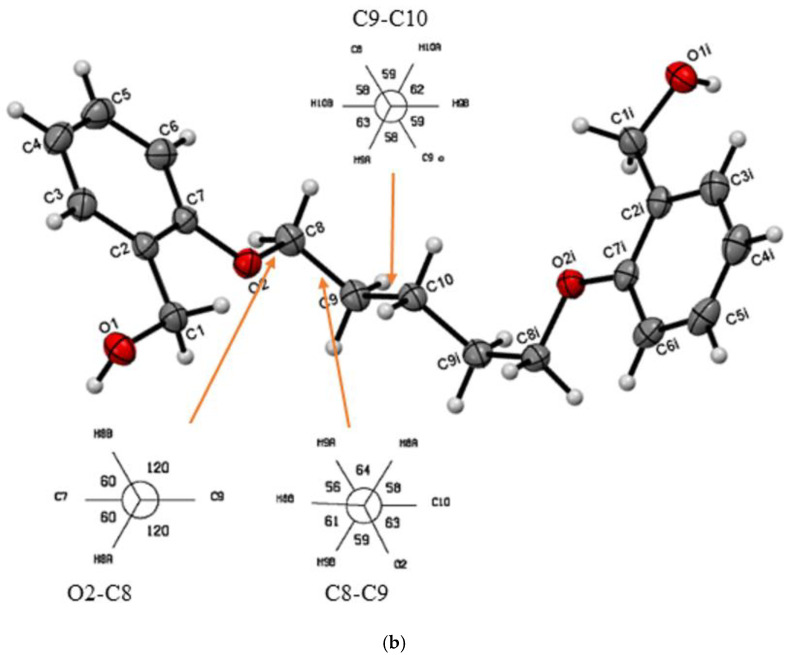
(a) ORTEP plot of Do4OH with displacement ellipsoids of non-hydrogen atoms drawn at the 50% probability level. The conformation of the aliphatic chain is highlighted by the transparent blue ellipse. (b) ORTEP plot of Do5OH with displacement ellipsoids of non-hydrogen atoms drawn at the 50% probability level. Representation of Newman projections looking down the O2–C8, C8–C9 and C9–C10 bonds indicating a partial gauche conformation of the aliphatic chain in Do5OH. I = -x, -y, -z.
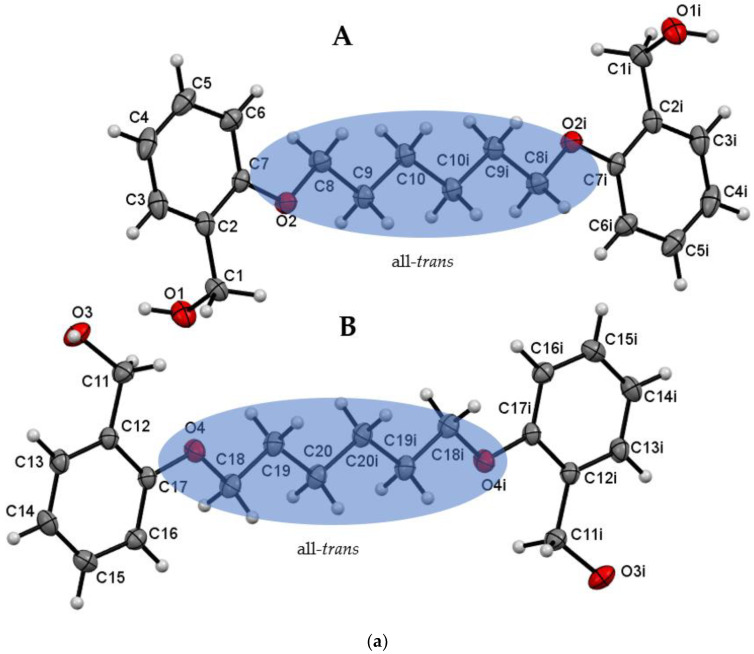
Figure 2.
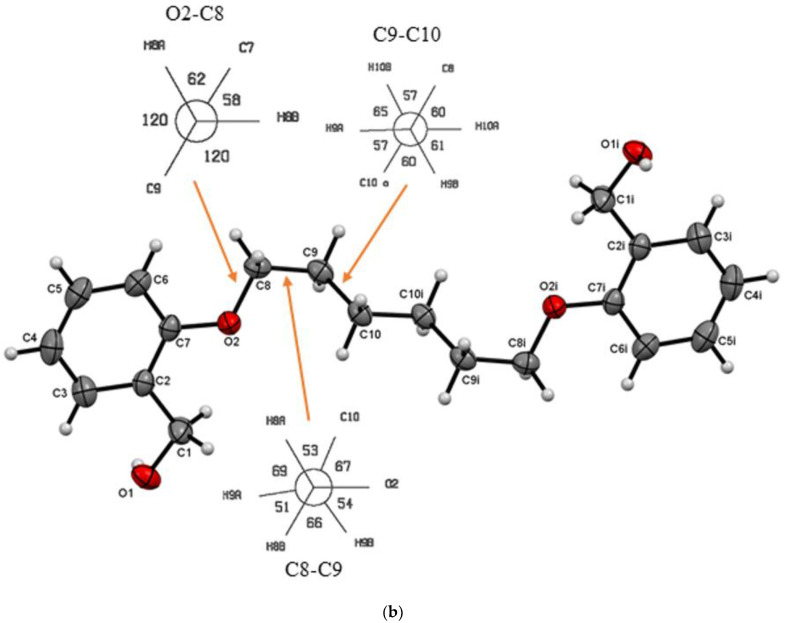
(a) ORTEP plot of Do6OH I with displacement ellipsoids of non-hydrogen atoms drawn at the 50% probability level (i = -x, -y, -z). The conformation of the aliphatic chain is highlighted by the transparent blue ellipse. (b) ORTEP plot of Do6OH II with displacement ellipsoids of non-hydrogen atoms drawn at the 50% probability level. The representation of the Newman projections of O2–C8, C8–C9 and C9–C10 bonds indicating a partial gauche conformation of the aliphatic chain in Do6OH II (i = -x, -y, -z).
The asymmetric unit of Do4OH is composed of two symmetrically independent half molecules. In each molecule, the crystallographic center of inversion lies between two central atoms of the aliphatic chain (C9–C9i and C18–C18i). The aliphatic chain is in an all-trans conformation (Table 1 and Table S2) and molecules are essentially planar, with negligible deviations of atoms from the plane calculated through the molecule. Unlike Do4OH, the compound Do5OH is a non-planar molecule with a dihedral angle between the benzene rings of 84.2(1)°. The torsion angle O2–C8––C9–C10 (−62.67(11)°) indicates a partial gauche conformation of the aliphatic chain (trans–gauche–trans–gauche–trans conformation of aliphatic chain starting from C7 atom). It is interesting to note that the OH∙∙∙OH distance of alcohol groups is significantly shorter in compounds with 5 CH2 groups (11.133(2) Å) in comparison to Do4OH (13.754(2) Å).
Table 1.
Selected parameters for investigated compounds.
| Compound | The Dihedral Angle between Benzene Rings/° | Aliphatic Chain Conformation | OH‧‧‧OH Distance /Å | Density/g cm−3 | Melting Point/°C | CSD Code |
|---|---|---|---|---|---|---|
| 1,2-bis[2-(hydroxyformyl)phenoxy]methylene | 59.43 | gauche | 9.815(2) | 1.332 | 121–123 ref. [26] | FASCUY [27] |
| 1,2-bis[2-(hydroxyformyl)phenoxy]ethylene | 60.89 | trans–gauche–trans | 6.446(4) | 1.242 | 122–124 ref. [23] | SAZCIF [28] |
| 1,2-bis[2-(hydroxyformyl)phenoxy]propylene | 80.73 84.59 |
trans–gauche-gauche–trans | 9.659(2) 9.725(2) |
1.274 | 96 ref. [23] | FASDAF [27] |
| Do4OH | 0 | all-trans | 13.754(2) | 1.236 | 98 | This work |
| Do5OH | 84.2(1) | trans–gauche–trans–gauche–trans | 11.133(2) | 1.268 | 64 | This work |
| Do6OH I | 0 | all-trans | 13.954(2)–conformer A 15.799(2)–conformer B |
1.188 | 63 | This work |
| Do6OH II | 0 | trans–gauche–trans–trans–trans–gauche-trans | 13.461(2) | 1.242 | 60 | This work |
The recrystallization of the Do6OH resulted in the formation of two polymorphs (Forms I and II). Both forms were obtained as transparent needle-like crystals. Form I crystallized in the monoclinic crystal system (P21/c) and II in the orthorhombic crystal system (Pbca). The asymmetric unit of form I is composed of two molecules (conformer A and B—Figure 2a). As in Do4OH, in each molecule, the crystallographic center of inversion lies between two central atoms of the aliphatic chain (C10–C10i and C20–C20i). The aliphatic chain is in an all-trans conformation (Table S2) and molecules are essentially planar. However, in this case (form I), there is another difference in the conformation of molecules—in conformer A, alcohol groups are oriented away from the plane of the molecule, while in B, alcohol groups reside in the plane of the molecule (structure overlay can be found in ESI—Figure S1). These differences are also observable (Table S2) in values of torsion angles (C7–C2–C1–O1 and C17–C12–C11–O3). Considering the molecular structure of form II, the largest difference is in the aliphatic chain conformation in comparison to form I. In form II, the aliphatic chain is in a partial gauche conformation (trans–gauche–trans–trans–trans–gauche–trans conformation of aliphatic chain starting from C7 atom). Due to this conformation, the molecule has the shape of a ladder (structure overlay with form I, conformer A can be found in ESI—Figure S2).
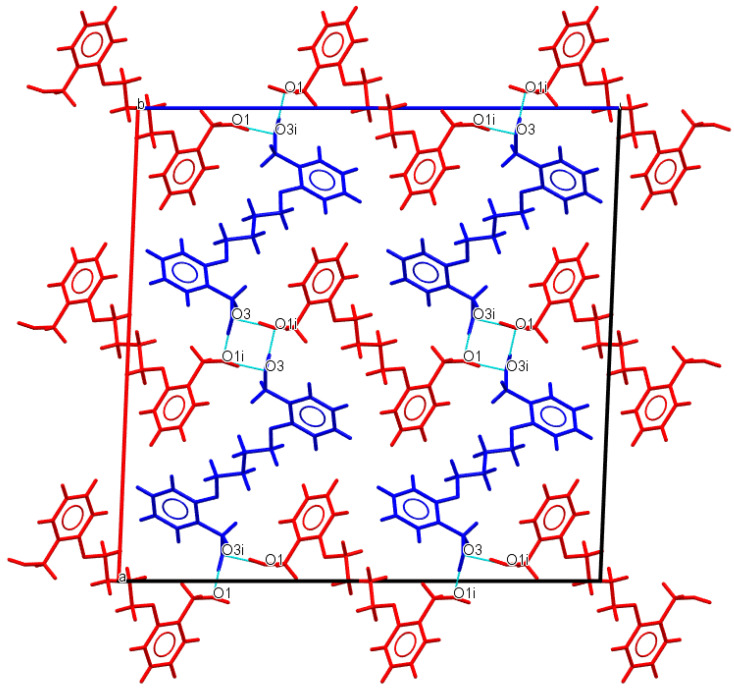
As expected, in all crystal structures, molecules are primarily connected by strong O−H∙∙∙O hydrogen bonds through terminal alcohol groups. Although rather similar compounds, there are some major differences in their crystal packing arrangements. In Do4OH, molecules are connected via O−H∙∙∙O hydrogen bonds (Figure 3). Additional stabilization of the crystal structure is achieved by a series of weak C−H∙∙∙O van der Waals interactions and C−H∙∙∙ π interactions of adjacent symmetrically independent molecules (Table 2).
Figure 3.
Representation of O−H∙∙∙O hydrogen bond interactions in Do4OH (blue dashed lines) along b-axis. Two symmetrically independent molecules are represented by blue and red colors.
Table 2.
Hydrogen bond geometry (Å, °) for Do4OH, Do5OH, Do6OH I and Do6OH II.
| D−H∙∙∙A | d(D—H) | d(H⋅⋅⋅A) | d(D⋅⋅⋅A) | ∠(D—H⋅⋅⋅A) | Symmetry code |
| Do4OH | |||||
| O1—H1···O3 | 0.976(3) | 1.678(3) | 2.648(3) | 172(2) | -x+1,+y-1/2,-z+1/2 |
| O3—H3A···O1 | 0.946(3) | 1.708(3) | 2.653(2) | 176(3) | x,+y+1,+z |
| Do5OH | |||||
| O1—H1···O1 | 0.883(2) | 1.850(2) | 2.710(1) | 164(2) | -x+1/2,+y-1/2,+z |
| Do6OH I | |||||
| O3—H3A···O1 | 0.875(2) | 1.802(2) | 2.669(1) | 170(2) | x,+y,+z-1 |
| O1—H1···O3 | 0.884(2) | 1.813(2) | 2.687(1) | 169(2) | x,-y+1/2,+z+1/2 |
| Do6OH II | |||||
| O3—H1···O1 | 0.880(2) | 1.790(2) | 2.658(1) | 168(2) | x-1/2,+y,-z+1/2+1 |
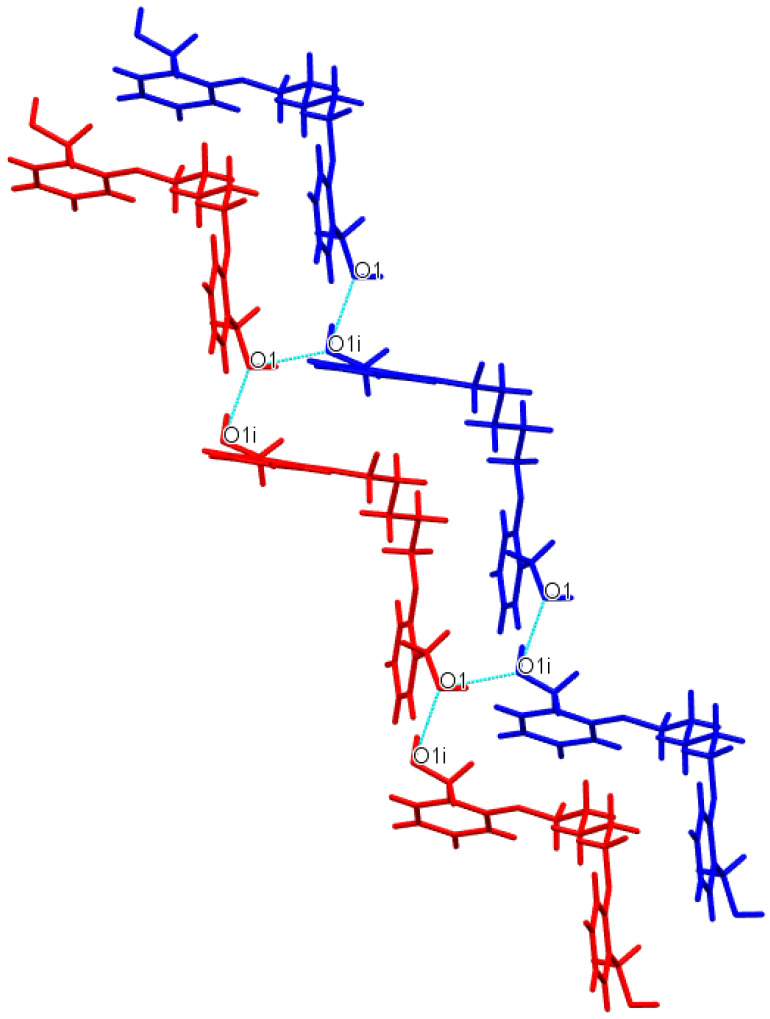
In contrast to the above-described structure, Do5OH molecules are connected by strong O−H∙∙∙O hydrogen bond interactions into infinite chains approximately along an a-axis. These interactions form a staircase-like motif (Figure 4). Two adjacent chains (staircases) are connected by O−H∙∙∙O hydrogen bonds along the b-axis. The final arrangement of molecules in the crystal structure is achieved by a series of weak C−H∙∙∙O van der Waals interactions along the c-axis.
Figure 4.
Representation of staircase-like motif in Do5OH. O−H∙∙∙O hydrogen bond interactions are represented by blue dashed lines and adjacent chains by blue and red colors.
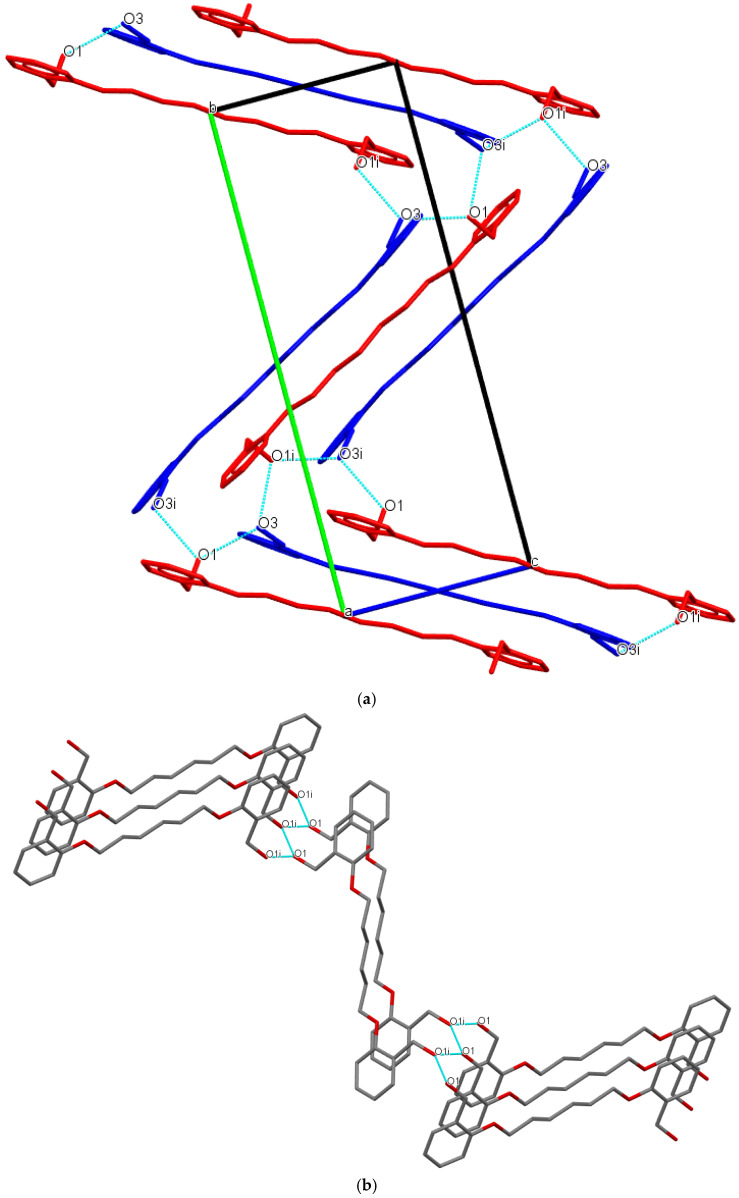
Differences in the molecular structure of Do6OH polymorphs caused variations in packing arrangements and intermolecular interactions. In the structure of form I, conformer molecules (A and B) are linked via strong O−H∙∙∙O hydrogen bond interactions (Table 2). In both conformers, terminal oxygen atoms (O1, O1i, O3 and O3i) form two hydrogen bonds (bifurcated) with the adjacent conformer molecules’ interactions forming a supramolecular staircase motif (Figure 5a).
Figure 5.
(a) View of the staircase packing arrangement in Do6OH I. O−H∙∙∙O hydrogen bond interactions is represented by blue dashed lines. Conformer molecules are red (A) and blue (B) colored and hydrogen atoms are omitted due to clarity. (b) Representation of 2D sheet motif in Do6OH II. O−H∙∙∙O hydrogen bond interactions are represented by blue dashed lines and hydrogen atoms are omitted due to clarity.
A rather similar packing arrangement is observed in the crystal structure of form II—bifurcated terminal oxygen atoms (O1 and O1i) connected by strong O−H∙∙∙O hydrogen bonds. Such interactions lead to the formation of 2D sheets, connected by weak C−H∙∙∙O van der Waals interactions along the b-axis (Figure 5b).
To further correlate the properties of the investigated compounds, a Cambridge Structural Database (CSD) [29] search was performed for compounds containing 2-(hydroxyformyl)phenoxy units linked by a different number of methylene groups in the aliphatic chain. The search resulted in three relevant hits, and the query results are presented in Table 1. Some common features of the compounds can be summarized: (i) pendant alcohol groups are anti-oriented in all compounds except in SAZCIF, resulting in an unusually short OH‧‧‧OH distance (6.446(4)) in this compound; (ii) molecules with an even number of methylene groups in the aliphatic chain are planar (except SAZCIF), while molecules with an odd number display significant deviation from planarity (see dihedral angles in Table 1); (iii) the conformation of the aliphatic chain is all-trans for molecules with an even number of methylene groups (except in SAZCIF), while in odd-numbered molecules, a different pattern of chain conformation is observed. The impact of the molecular and crystal structures of compounds on their thermal properties is discussed in the thermal analysis section (vide infra).
3.2. Hirshfeld Surface (HS) Analysis and Interaction Energy Calculation
Hirshfeld surfaces of Do4OH, Do5OH, Do6OH I and Do6OH II plotted over dnorm are shown in Figure S3. The most characteristic feature in all four crystal structures are short O‧‧‧H intermolecular contacts, depicted by red dots in the vicinity of hydroxylic oxygens, belonging to the O-H‧‧‧O hydrogen bonds as the most important interaction between the neighboring molecules. Interestingly, in two representatives of the even series, i.e., in Do4OH and Do6OH I, there are two symmetry-independent molecules in each crystal structure. In the former, the Hirshfeld surfaces of both molecules are very similar, with one close interaction around hydroxylic oxygen on each end of the Do4OH molecules. The same holds true also for Do5OH. However, the Hirshfeld surfaces of symmetry-independent molecules in Do6OH I do differ: one mimics the close contacts, as are in Do4OH and Do5OH, but in the other molecule of Do6OH I, two close contacts appear at each end of the molecule. The same pattern is present also in the Do6OH II polymorph (unluckily, it is impossible to orientate the molecule in a way that would enable both pairs of short contacts to be spotted simultaneously).
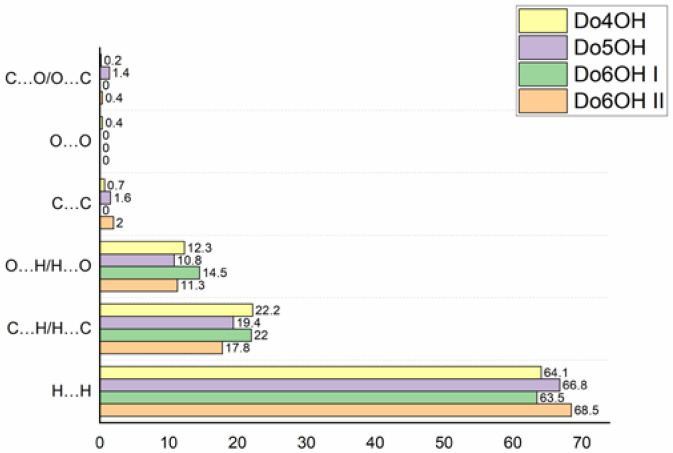
The full 2D fingerprint plots are depicted in Figure S4. Despite differences in the packing arrangements of molecules in the studied crystal structures, the 2D fingerprint plots show that the dominant contributions to the Hirshfeld surface are H‧‧‧H, C‧‧‧H/H‧‧‧C and O‧‧‧H/H‧‧‧O interactions in all four crystal structures. These interactions contribute at least 97% to the total Hirshfeld surface. In all four cases, the largest contribution to the total Hirshfeld surface is by H‧‧‧H contacts, from 63.5% in Do6OH I to 68.5% in Do6OH II. These contacts are graphically represented mostly by turquoise points with the central characteristic wide spike at (di + de) ~ 2.2 Å. Two additional characteristic sharp spikes that are close to symmetrical at (di + de) ~ 1.65 Å correspond to H‧‧‧O/O‧‧‧H interactions originating either from O-H‧‧‧O or C-H‧‧‧O hydrogen bonds (the former are represented as intense red spots in Figure S3). However, C‧‧‧H/H‧‧‧C interactions that contribute more to the total HS, in comparison with the H‧‧‧O/O‧‧‧H interactions, are more dispersed in 2D fingerprint plots and appear at higher (di + de) sums. The percentage contributions of different interactions to the total HS are shown in Figure 6.
Figure 6.
The percentage contributions of different interactions to the total Hirshfeld surface in four studied compounds.
The total interaction energies for compounds are separated into different energy contributions—Coulomb, polarization, dispersion and repulsion (Table 3). These partitioned energies are particularly useful to determine the type of intramolecular interactions (for example, π∙∙∙π stacking is characteristic of large dispersive energy interactions and large values of electrostatic energy can be attributed to hydrogen bonding) [30]. In all four investigated compounds, the largest contribution to the total energy value comes from dispersive interactions, indicating the importance of weak C−H∙∙∙C, C−H∙∙∙π and π∙∙∙π interactions in the overall stability of these compounds. Another important contribution to total energy comes from electrostatic interactions, which can be attributed to O−H∙∙∙O hydrogen bond interactions of terminal hydroxide moieties. Considering the individual contribution of specific energies to total energy, there is a discrepancy in the calculated individual energy in the Do6OH II compound in comparison to other compounds. This is especially true for dispersion energy with a value of −129.08 kJ/mol. This could be explained by a weaker contact of molecules in the crystal due to a deviation from planarity, which decreases the number of aromatic interactions. From the percentage contributions of individual energies, it can be concluded that overall stability in planar compounds (Do4OH and Do6OH I) is balanced between dispersive and electrostatic interactions, while in non-planar (Do5OH and Do6OH II), the largest contribution to stability comes from dispersion interactions. The values of the total calculated energies for Do6OH I and II are −188.29 kJ mol−1 and −128.53 kJ mol−1. Lattice energies are estimated to be −208 kJ mol−1 and −194 kJ mol−1, and −205.2 kJ mol−1 and −200.7 kJ mol−1 from Pixel calculations. These results show that the more stable form of the Do6OH is form I, which was also observed in the DSC experiments (vide infra). Considering the lattice energy calculations, it is interesting to note that the energies calculated by Pixel and CrystalExplorer correlate quite well, with the largest difference calculated for Do5OH. Similar results with other computational methods and different physical characteristics were previously reported [31]. Unfortunately, the crystal structures of the n = 1 and 3 chain analogs were determined without hydrogen atoms, and the structure of the chain analog n = 2 is disordered; therefore, a detailed analysis of intermolecular interactions was not conducted.
Table 3.
Molecular pair interaction total energy (Etot) and energies partitioned into electrostatic (Eele), polarization (Epol), dispersion (Edis) and repulsion (Erep) contributions (kJ/mol) for individual compounds. Percentage contributions of individual attractive interaction energies to total energies are given in brackets.
| Compound | Eele | Epol | Edis | Erep | Etot | Elatt | PixelC |
|---|---|---|---|---|---|---|---|
| Do4OH | −151.99 (44.5%) | −24.79 (7.3%) | −164.97 (48.2%) | 148.81 | −192.94 | −192 | −188.7 |
| Do5OH | −80.01 (31.5%) | −13.39 (5.3%) | −161.39 (63.2%) | 97.39 | −157.40 | −193 | −215.2 |
| Do6OH I | −150.09 (44.9%) | −23.45 (7.2%) | −160.96 (47.9%) | 146.22 | −188.29 | −208 | −205.2 |
| Do6OH II | −77.48 (35.3%) | −12.13 (5.2%) | −129.08 (59.5%) | 90.16 | −128.53 | −194 | −200.7 |
3.3. Thermal Analysis (TG/DSC)
The TG curves of the Do4OH and Do5OH compounds indicate one-step thermal decomposition close to 300 °C with an accompanying exothermic maximum on the DSC curve (black curves on Figures S5 and S6). The endothermic events at 98.23 °C (ΔHfus = 42.6 kJ mol–1) and 63.77 °C (ΔHfus = 37.33 kJ mol–1) are attributed to the melting points of Do4OH and Do5OH, respectively (Table S3).
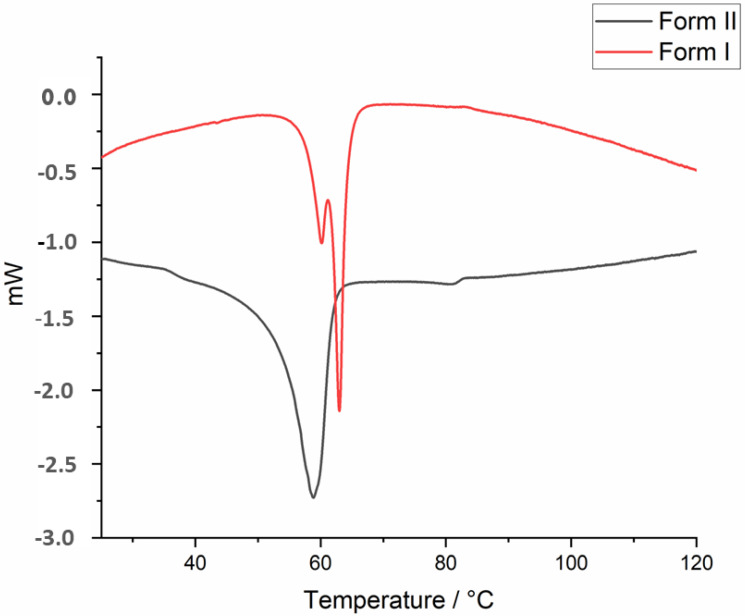
On the DSC curve of Do6OH II (Figure 7), a distinct endothermic peak is observed at 60 °C, ΔHfus = 27.09 kJ mol–1), which is attributed to the melting point. Two endothermic thermal events are observable on the DSC curve of form I (Figure 7). The results of the DSC analysis indicate the presence of both forms in the investigated sample. The first thermal event can be attributed to the melting point of II, while the second thermal event is the melting point of I. The deconvolution was used to separate these two thermal events and calculate the fusion enthalpy for I (ΔHfus = 17.84 kJ mol–1). The calculated melting entropies for I and II are 0.053 kJ mol−1 K−1 and 0.081 kJ mol−1 K−1, respectively. The Gibbs free energy diagram (Figure S7) was constructed over the temperature range of 100 K–400 K. The results indicate that two Gibbs free energy curves intersect at approximately 150 K. The heat of fusion rule, entropy of fusion rule [30] and Gibbs energy diagram indicate the enantiotropic relationship between two polymorphs [31,32,33].
Figure 7.
DSC curves of Do6OH forms I and II.
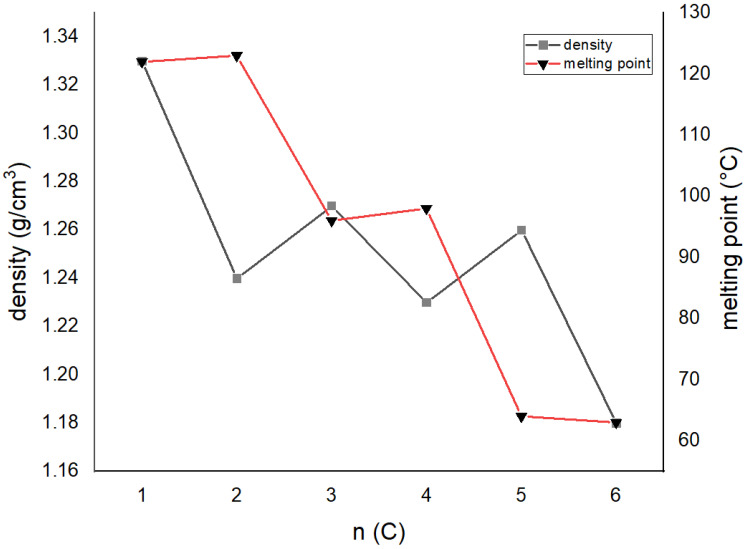
To further explain the odd–even effect in these compounds, a correlation of densities obtained by diffraction experiments, melting points and total energies with a number of methylene groups was carried out (Figure 8, Table 1 and Table 3). Although the data are not complete, some conclusions may be drawn: (i) the melting point of compounds decreases with a larger number of methylene groups; (ii) odd-numbered compounds display systematically lower melting points than even-numbered analogs, (iii) compounds with higher densities display lower melting points, and even-membered compounds of a particular series (i.e., n = 3 and 4) display lower densities and higher melting points; (iv) the total interaction energy is larger in even-membered analogs; (v) lattice energy calculations do not correlate to melting points. These conclusions are rather difficult to reconcile with the previously mentioned investigations of the odd–even effect. For example, in α,ω-alkendiols [8], the melting point increases with the number of methylene groups and decreases with lower densities. In terms of the relationship between molecular structure and melting point, it is obvious that planar systems have higher melting points (i.e., n = 3 (non-planar) and 4 (planar)), but at the same time have lower densities, which indicates less-effective crystal packing.
Figure 8.
Melting point and density alteration with an increasing number of methylene groups in the aliphatic chain (only data for Do6OH I are presented).
The question is, why do less-effectively (lower density) packed molecules in the crystal structure display higher melting points, when it is usually just the opposite? A plausible explanation of this phenomenon can be found in the percentage contribution of specific contacts calculated by the Hirschfeld analysis (Figure 6). In more densely packed molecules (Do5OH and Do6OH II), the contribution of close H‧‧‧H contacts is higher, and for O‧‧‧H and C‧‧‧H contacts it is lower. This indicates that close H‧‧‧H contacts act repulsively and reduce the overall stability of the crystal structure. The influence of O‧‧‧H and C‧‧‧H contacts is just the opposite. If correlated with molecular structure, it can be concluded that planar molecules (Do4OH and Do6OH I) are forming more favorable attractive (O‧‧‧H) contacts, and at the same time diminishing unfavorable repulsive H‧‧‧H contacts. The cost of such interplay is a lower density, but higher melting points. This research also indicates that the generally accepted rule, higher density = higher stability = higher melting point, is not always valid, especially in the case of systems more complex than the simple α, ω-substituted aliphatic chains. A quite nice example of this deviation from the rule is two polymorphic forms of Do6OH–Form I, with a higher melting point and lower density than II.
3.4. Spectroscopy
IR spectra of compounds are given in ESI, Figures S8–S11. The broad OH stretching vibrations are located in the range from 3130 cm−1 to 3380 cm−1 in all compounds. Vibrations close to 1050 cm−1 in all spectra can be attributed to the C–OH group and vibrations close to 1250 cm−1 are due to Caromatic–O–Caliphatic stretching vibration. Maxima typical for the ortho-substituted benzene ring at 750 cm−1 and the stretching vibrations of the benzene ring at 1600 cm−1 can be observed in the spectrum. Some minor differences are observable in the intensity and position of OH stretching vibration in all compounds and spectra of two polymorphs (Figure S12). These differences can be explained by different hydrogen bond interaction patterns in compounds.
3.5. Cyclic Voltammetry
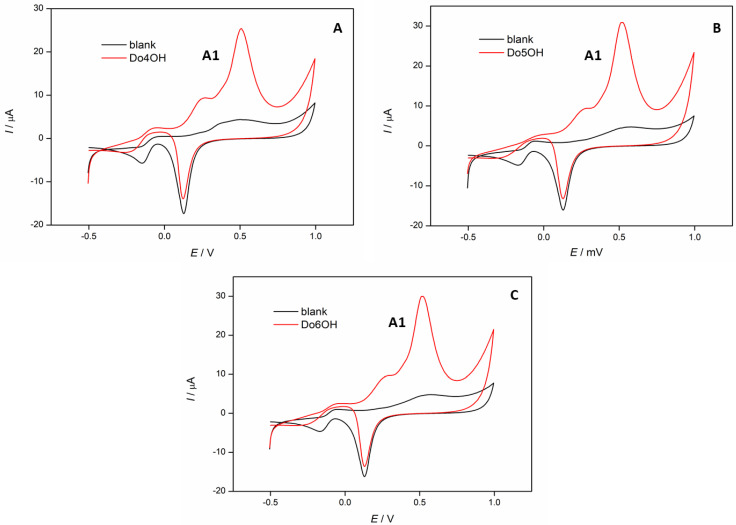
Cyclic voltammetry was used to study the electrochemical properties of the synthesized compounds and to obtain information regarding the charge transfer and structure of the investigated compounds. Earlier studies have shown that odd-numbered molecules impede the charge transfer in n-alkenthiolates [10]. Dhiman et al. have also found that the radical scavenging and antioxidant activity of different isomers of hydroxybenzyl alcohols are structure-dependent [34]. In Figure 9, cyclic voltammograms of Do4OH (Figure 9A), Do5OH (Figure 9B) and Do6OH (Figure 9C) are shown. In all voltammograms, one oxidation peak current (A1) at the potential Ep,a = 0.52 V can be observed, which corresponds to the oxidation of hydroxyl groups in the investigated compounds [35]. This oxidation peak was the most pronounced for the odd-numbered compound Do5OH, where the oxidation peak current Ipa = 30.6 μA, which could be explained by an enhanced charge transfer in this compound. This effect was less visible in even-numbered Do4OH and Do6OH compounds, where the oxidation peak current was Ip,a = 24.4 μA and 29.2 μA, respectively.
Figure 9.
Cyclic voltammograms of investigated compounds (c = 1 × 10−4 M): (A) Do4OH, (B) Do5OH and (C) Do6OH, recorded in 0.1 M NaOH. Scan rate 100 mV/s.
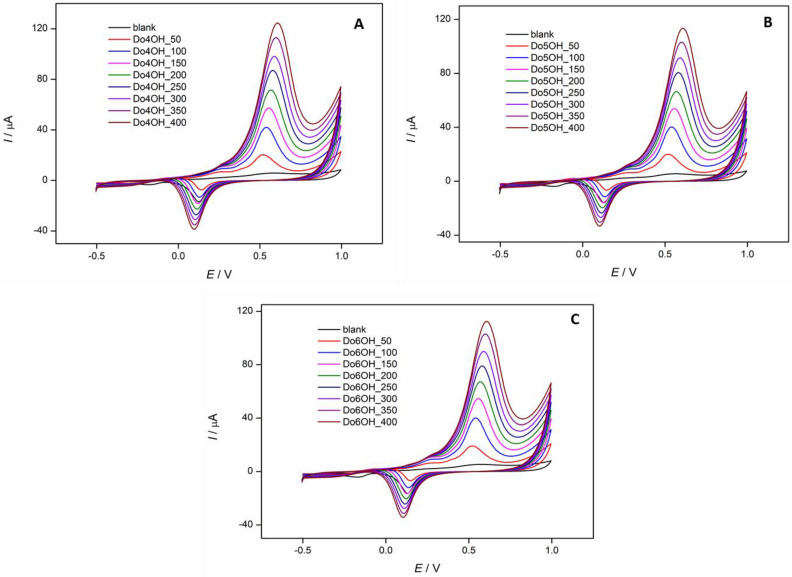
In Figure 10, the effect of scan rate on the oxidation peak potential and oxidation peak current of all investigated compounds was examined. It was determined that the oxidation peak current and the oxidation peak potential increase with the increase of scan rate for all investigated compounds.
Figure 10.
Cyclic voltammograms of investigated compounds (c = 1 × 10−4 M): (A) Do4OH, (B) Do5OH and (C) Do6OH, recorded in 0.1 M NaOH at different scan rates (v = 50–400 mV/s).
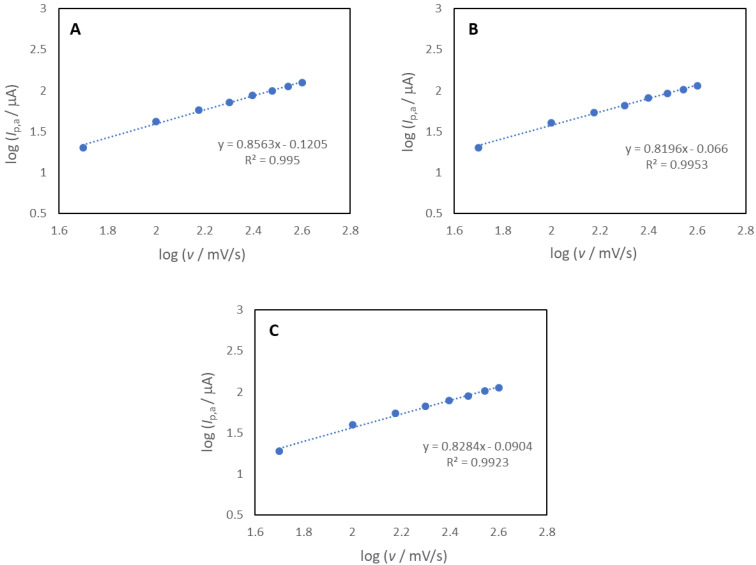
In Figure 11, the logarithms of the anodic peak currents (log Ip,a) as a function of the logarithms of the scan rates (log v) for the Do4OH (Figure 11A), Do5OH (Figure 11B) and Do6OH (Figure 11C) compounds are shown. The linear correlation was obtained for all four compounds (R2 = 0.995 for the Do4OH, 0.9953 for the Do5OH and 0.9923 for the Do6OH compound) with a slope around 0.83, which confirmed that the oxidation process in all investigated compounds is under mixed adsorption and diffusion control [36].
Figure 11.
The logarithm of peak current (log Ip) as a function of the logarithm of scan rate (log v) for the (A) Do4OH, (B) Do5OH and (C) Do6OH compounds.
3.6. DPPH Assay
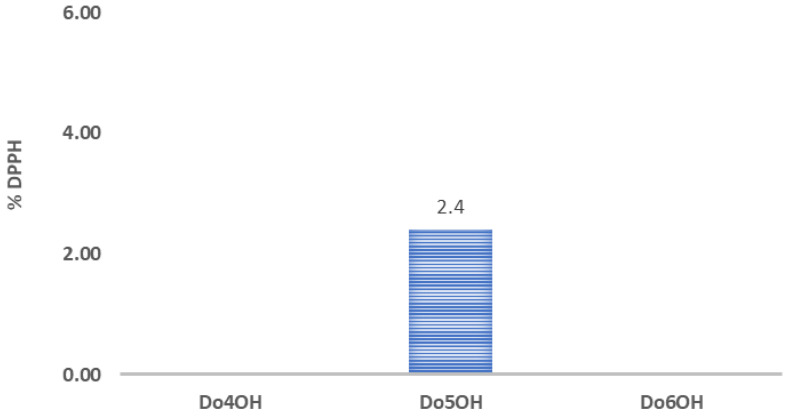
The antioxidant activities of synthesized compounds (Do4OH, Do5OH and Do6OH) were investigated with a DPPH assay in order to study the correlation of the chain length of the investigated compounds with their antioxidant activity. The obtained results are shown in Figure 12. It can be concluded that only compound Do5OH showed small antioxidant activity (% DPPH = 2.4 ± 0.2), while other compounds showed no antioxidant activity. This result agrees with the result of cyclic voltammetry since the odd-numbered compound Do5OH, which showed the most-pronounced oxidation peak due to the enhanced charge transfer, showed antioxidant activity, while the even-numbered Do4OH and Do6OH compounds showed no antioxidant activity.
Figure 12.
Column graphs showing % DPPH inhibition of all investigated compounds.
4. Conclusions
Three novel aromatic alcohol chain isomers were prepared and structurally characterized. In all compounds, two benzyl alcohol units are connected by an aliphatic chain of different lengths, ((CH2)n; n = 4, 5 and 6). The major differences in the molecular structures were found in the overall planarity of the molecules and in the conformation of the aliphatic chain. Molecules with an even number of CH2 groups are planar with the all-trans conformation of the aliphatic chain, and odd-numbered molecules are non-planar, with partial gauche conformation (with the exception of Do6OH II). As a consequence of these structural modifications, odd-numbered compounds display systematically lower melting points. The comparison with previously reported chain analogs (n = 1, 2 and 3) indicates a decrease in melting point with an increase in the number of CH2 groups, but with higher melting points of particular even-numbered molecules. Interestingly, higher melting point compounds of particular odd–even pairs display lower density, which is in contradiction to previously studied cases of the odd–even effect. It was found that more densely packed molecules (even-membered) show a higher percentage of repulsive H‧‧‧H contacts, which is a plausible explanation for the lowering of the melting point. The electrochemical study has shown one oxidation peak which corresponds to the oxidation of hydroxyl groups in all compounds. The highest oxidation peak current and moderate antioxidant activity were found for the non-planar compound (Do5OH), indicating the importance of the conformational properties of molecules in antioxidant activity.
Acknowledgments
A. Széchenyi and M. Medvidović-Kosanović thank the European Structural and Investment Funds grant for the Croatian National Scientific Center of Excellence for Personalized Health Care, University of Josip Juraj Strossmayer Osijek (grant #KK.01.1.1.01.0010), for support. T. Balić and M. Paurević thank the Josip Juraj Strossmayer University of Osijek (Project: UNIOS-ZUP 2018-112) for support. M. Počkaj thanks the Slovenian Research Agency (ARRS) through project P1-0175 for support and the EN FIST Centre of Excellence for the use of SuperNova diffractometer. The research in Hungary was funded by the NKFI-137793 and the TKP2021-EGA-17 projects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27123781/s1, CCDC 2161717–2161720 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
Author Contributions
T.B.: Conceptualization, Methodology, Writing—Original draft preparation, Data curation, Writing—Reviewing and Editing, Supervision. M.P. (Marija Paurević): Formal analysis, Data curation, Writing—Reviewing and Editing. M.P. (Marta Počkaj): Investigation, Formal analysis, Writing—Original draft preparation, Writing—Reviewing and Editing. M.M.-K.: Investigation, Data curation, Writing—Reviewing and Editing, Writing—Original draft preparation. D.G.: Investigation, Formal analysis. A.S.: Investigation, Formal analysis, Writing—Reviewing and Editing. Z.P.: Investigation, Data curation, Writing—Reviewing and Editing. S.K.-M.: Investigation, Data curation, Writing—Reviewing and Editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not aplicable.
Informed Consent Statement
Not aplicable.
Data Availability Statement
Not aplicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Funding Statement
A. Széchenyi and M. Medvidović-Kosanović thank the European Structural and Invest-ment Funds grant for the Croatian National Scientific Center of Excellence for Personalized Health Care, University of Josip Juraj Strossmayer Osijek (grant #KK.01.1.1.01.0010) for support. T. Balić and M. Paurević thank the Josip Juraj Strossmayer University of Osijek (Project: UNIOS-ZUP 2018-112) for support. M. Počkaj thanks the Slovenian Research Agency (ARRS) through project P1-0175 for support and EN FIST Centre of Excellence for the use of SuperNova diffractometer. The research in Hungary was funded by the NKFI-137793 and the TKP2021-EGA-17 projects.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baldwin D.S., Bowden B.F., Duckworth P.A., Lindoy L.F., McCool B.J., Meehan G.V., Vasilescu I.M., Wild S.B. New Macrocyclic Ligands. XIV. Synthesis and X-Ray Structures of Potentially Pentadentate Ligands Incorporating Non-Symmetrically Arranged N4S-, N3OS-, N2O2S- and N2S2O-Heteroatoms. Aust. J. Chem. 2002;55:597–603. doi: 10.1071/CH02121. [DOI] [Google Scholar]
- 2.Paurević M., Dandić A., Šrajer Gajdošik M., Vidović B., Perdih F., Balić T. Efficient Synthesis of New 17-, 18-, 19- and 20-Membered N2O2-Donor Macrocycles by NaBH4 Reduction and Metal Picrate Extraction Studies. J. Incl. Phenom. Macro. 2020;97:87–98. doi: 10.1007/s10847-020-00987-y. [DOI] [Google Scholar]
- 3.Balić T., Matasović B., Marković B., Šter A., Štivojević M., Matković-Čalogović D. Synthesis, Structural Characterization and Extraction Studies of 17-, 18-, 19- and 20-Membered N2O2-Donor Macrocyclic Schiff Bases. J. Incl. Phenom. Macro. 2016;85:217–226. doi: 10.1007/s10847-016-0621-4. [DOI] [Google Scholar]
- 4.Balić T., Perdih F., Mršo T., Balić I. Ligand Influence on the Formation of Exo-Coordinated Silver(I) Complexes with N2O2 Schiff Base Macrocycles and the Role of Anion in Supramolecular Aggregation. Polyhedron. 2020;190:114774. doi: 10.1016/j.poly.2020.114774. [DOI] [Google Scholar]
- 5.Baeyer A. Ueber Regelmässigkeiten Im Schmelzpunkt Homologer Verbindungen. Ber. Dtsch. Chem. Ges. 1877;10:1286–1288. doi: 10.1002/cber.18770100204. [DOI] [Google Scholar]
- 6.Pradeilles J.A., Zhong S., Baglyas M., Tarczay G., Butts C.P., Myers E.L., Aggarwal V.K. Odd–Even Alternations in Helical Propensity of a Homologous Series of Hydrocarbons. Nat. Chem. 2020;12:475–480. doi: 10.1038/s41557-020-0429-0. [DOI] [PubMed] [Google Scholar]
- 7.Bond A.D. On the Crystal Structures and Melting Point Alternation of the N-Alkyl Carboxylic Acids. New J. Chem. 2004;28:104–114. doi: 10.1039/B307208H. [DOI] [Google Scholar]
- 8.Thalladi V.R., Boese R., Weiss H.-C. The Melting Point Alternation in α,ω-Alkanediols and α,ω-Alkanediamines: Interplay between Hydrogen Bonding and Hydrophobic Interactions. Angew. Chem. Int. Edit. 2000;39:918–922. doi: 10.1002/(SICI)1521-3773(20000303)39:5<918::AID-ANIE918>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Thalladi V.R., Boese R., Weiss H.-C. The Melting Point Alternation in α,ω-Alkanedithiols. J. Am. Chem. Soc. 2000;122:1186–1190. doi: 10.1021/ja993422l. [DOI] [Google Scholar]
- 10.Jiang L., Sangeeth C.S.S., Nijhuis C.A. The Origin of the Odd–Even Effect in the Tunneling Rates across EGaIn Junctions with Self-Assembled Monolayers (SAMs) of n-Alkanethiolates. J. Am. Chem. Soc. 2015;137:10659–10667. doi: 10.1021/jacs.5b05761. [DOI] [PubMed] [Google Scholar]
- 11.STARe. Mettler-Toledo Inc.; Greifensee, Switzerland: 2009. Software 10.0; Mettler-Toledo GmbH; [Google Scholar]
- 12.Rigaku Oxford Diffraction . CrystAlis PRO. Rigaku Corporation; Tokyo, Japan: 2018. [Google Scholar]
- 13.Sheldrick G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A. 2015;71:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheldrick G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C. 2015;71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spek A.L. PLATON: An Integrated Tool for the Analysis of the Results of a Single Crystal Structure Determination. Acta Crystallogr. A. 1990;46:34. [Google Scholar]
- 16.Spek A.L. PLATON: A Multipupose Crystallographic Tool. Utrecht University; Utrecht, The Netherlands: 1998. [Google Scholar]
- 17.Macrae C.F., Edgington P.R., McCabe P., Pidcock E., Shields G.P., Taylor R., Towler M., Van de Streek J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Crystallogr. 2006;39:453–457. doi: 10.1107/S002188980600731X. [DOI] [Google Scholar]
- 18.Turner M.J., McKinnon J.J., Wolff S.K., Grimwood D.J., Spackman P.R., Jayatilaka D., Spackman M.A. CrystalExplorer17. University of Western Australia; Crawley, WA, Australia: 2017. [Google Scholar]
- 19.Gavezzotti A. Calculation of Lattice Energies of Organic Crystals: The PIXEL Integration Method in Comparison with More Traditional Methods. Z. Kristallogr. 2005;220:499–510. doi: 10.1524/zkri.220.5.499.65063. [DOI] [Google Scholar]
- 20.Neese F. WIREs. Comput. Mol. Sci. 2018;8:e1327. [Google Scholar]
- 21.Lu T., Chen F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012;33:580–592. doi: 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- 22.McArdle P. Pixel Calculations Using Orca or GAUSSIAN for Electron Density Automated within the Oscail Package. J. Appl. Crystallogr. 2021;54:1535–1541. doi: 10.1107/S1600576721008529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q.-X., Zhao Z.-X., Zhao X.-J., Wei Q., Chen A.-H., Li H.-L., Wang X.-G. Structures of NHC Hg(II) and Ag(I) Complexes and Selective Recognition of Nitrate Anion. CrystEngComm. 2015;17:1358–1373. doi: 10.1039/C4CE01519C. [DOI] [Google Scholar]
- 24.Balić T., Marković B. Crystal Structure of 2,2′-[Pentane-1,5-Diylbis(Oxy)]Dibenzaldehyde, C19H20O4. Z. Krist.—New Cryst. St. 2016;231:619–621. doi: 10.1515/ncrs-2015-0213. [DOI] [Google Scholar]
- 25.Balić T., Marković B., Jaźwiński J., Matković-Čalogović D. Synthesis and Structural Characterization of New N2O2-Donor Schiff Base Macrocycles and Their Silver(I) Coordination Polymers. Inorg. Chim. Acta. 2015;435:283–291. doi: 10.1016/j.ica.2015.07.016. [DOI] [Google Scholar]
- 26.Darabi H.R., Sobhani L., Rastgar S., Aghapoor K., Amini S.K., Zadmard R., Jadidi K., Notash B. Synthesis, Characterization and Selective Cu2+ Recognition of Novel E– and Z–Stilbenophanes. Supramol. Chem. 2019;31:45–51. doi: 10.1080/10610278.2018.1528010. [DOI] [Google Scholar]
- 27.Seo J., Jin Y., Lee J.-E., Park K.-M., Lee B.Y., Lee S.S. Crystal Structures of 1,3-Bis(2-Benzyl Alcohol)-1,3-Dioxapropane and 1,5-Bis(2-Benzyl Alcohol)-1,5-Dioxapentane. Anal. Sci. 2004;20:x15–x16. doi: 10.2116/analscix.20.x15. [DOI] [Google Scholar]
- 28.Bailey N.A., Fenton D.E., Williams M.G., Winter D.J. The Structure of 2,2’-[1,2-Ethanediylbis(Oxy)]Bis(Benzenemethanol) Acta Crystallogr. C. 1989;45:1778–1780. doi: 10.1107/S0108270189003288. [DOI] [Google Scholar]
- 29.Groom C.R., Bruno I.J., Lightfoot M.P., Ward S.C. The Cambridge Structural Database. Acta Crystallogr. B. 2016;72:171–179. doi: 10.1107/S2052520616003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burger A., Ramberger R. On the Polymorphism of Pharmaceuticals and Other Molecular Crystals. II. Microchim. Acta. 1979;72:273–316. doi: 10.1007/BF01197380. [DOI] [Google Scholar]
- 31.Marti E. Applied Chemical Thermodynamics and Kinetics on Pharmaceutical Compounds. J. Therm. Anal. 1988;33:37–45. doi: 10.1007/BF01914582. [DOI] [Google Scholar]
- 32.Balić T., Perdih F., Počkaj M., Molnar M., Komar M., Balić I. Polymorphism of Coumarin Thione-Triazole—4-Methyl-7-[(4-Phenyl-5-Thioxo-4,5-Dihydro-1H-1,2,4-Triazol-3-Yl)Methoxy]-2H-Chromen-2-One. J. Mol. Struct. 2021;1231:129957. doi: 10.1016/j.molstruc.2021.129957. [DOI] [Google Scholar]
- 33.Othman M.I.A., Said S., Marin M. A novel model of plane waves of two-temperature fiber-reinforced thermoelastic medium under the effect of gravity with three-phase-lag model. Int. J. Numer. Method. H. 2019;29:4788–4806. doi: 10.1108/HFF-04-2019-0359. [DOI] [Google Scholar]
- 34.Dhiman S.B., Kamat J.P., Naik D.B. Antioxidant activity and free radical scavenging reactions of hydroxybenzyl alcohols. Chem.-Biol. Interact. 2009;182:119–127. doi: 10.1016/j.cbi.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Wang B., Tao L., Cheng Y., Yang F., Jin Y., Zhou C., Yu H., Yang Y. Electrocatalytic Oxidation of Small Molecule Alcohols over Pt, Pd, and Au Catalysts: The Effect of Alcohol’s Hydrogen Bond Donation Ability and Molecular Structure Properties. Catalysts. 2019;9:387. doi: 10.3390/catal9040387. [DOI] [Google Scholar]
- 36.Wu J. Electrochemical Behavior and Direct Quantitative Determination of Tanshinone IIA in Micro-Emulsion. Int. J. Electrochem. Sc. 2016;11:5165–5179. doi: 10.20964/2016.06.55. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not aplicable.