Abstract
Necrotic enteritis (NE) is a multifactorial and important enteric infectious disease etiologically caused by pathogenic C. perfringens infection, accounting for the estimated loss of around USD 6 billion in the global poultry industry. The increasing incidence of NE was found to be associated with the voluntary reduction or withdrawal of antibiotic growth promoters from animal feed during recent years. Therefore, the development of effective vaccines specific to NE assumes a priority for the poultry industry. This study aimed to identify the potential C. perfringens proteins as vaccine targets for NE. Three recombinant C. perfringens proteins targeting five antigens were prepared: two chimeric proteins (alpha-toxin and NetB, fructose-1,6-bisphosphate aldolase (FBA) and a zinc metalloprotease (Zm)), and one single collagen adhesion protein (Cna). Their protection efficacies were evaluated with a potent challenge model of Eimeria maxima/C. perfringens dual infections using a netB+tpeL+ C. perfringens strain. Young chicks were immunized twice subcutaneously with adjuvanted C. perfringens proteins on Days 4 and 15. At six days after the second immunization, the chickens immunized with Cna, FBA, and Zm antigens, and alpha-toxin had much higher serum antibody titers than unvaccinated controls prior to the challenge. Following the challenge, the pooled antigen-immunized group demonstrated no mortality and the least lesion scores against virulent challenge. The results indicate that the immunization with multicomponent antigens, including C. perfringens housekeeping protein Cna, may confer partial protection.
Keywords: Clostridium perfringens; vaccine candidates; NetB; phospholipase C; fructose-1,6-bisphosphate aldolase; zinc metalloprotease; collagen adhesion protein
1. Introduction
Necrotic enteritis (NE) is an important enteric disease, responsible for the annual loss of around 6 billion US dollars to global poultry producers [1]. NE is a complex and multifactorial disease etiologically caused by the pathogenic C. perfringens, the isolates of which are classified into six types (A-G) based on the major toxins produced [2,3,4]. Other key risk factors favoring the growth of C. perfringens may also enhance the development of NE, such as the pre- or co-infection with coccidiosis, nutritional factors (diets enriching animal proteins, high energy, wheat- or barley-based components), husbandry mismanagement, or bird immunosuppression [5,6]. NE has been well-controlled by the application of antibiotic growth promoters (AGP) and ionophore coccidiostats in the feed in the past few decades [5]. However, a resurgence in NE incidence has been found to be associated with voluntary reduction or complete withdrawal of these AGPs from feed [7]. There are increasing pressures from public health concerns and regulatory agencies over the emergence of multidrug-resistant bacteria or the presence of AGP residual pollution in the food products or environment. Ideally, vaccination as an alternative approach to antibiotics would assume high priority. Only one live Salmonella-vector vaccine (RASV) expressing C. perfringens genes coding for an a-toxin fragment and NetB toxin (AVERTR NE) has recently been commercially available (http://www.huvepharma.us/product/avert-ne/) (accessed on 27 May 2022)).
To control the NE, scientists have made great efforts toward understanding the pathogenesis of C. perfringens and developing vaccines against necrotic enteritis in broiler chickens. Multiple vaccine platforms have been tested, including toxoids, whole inactivated vaccines, live-attenuated vaccines, subunit vaccines, recombinant vectored vaccines, or attenuated Salmonella-vectored vaccines [8,9,10,11]. Among them, some vaccine targets have been reported for recombinant subunit vaccines. Alpha-toxin (CPA or phospholipase C fragment of CPA, Plc) is an essential protein proposed for disease processes [12], but the role of CPA may be limited since the engineered cpa mutants are found to still maintain full virulence in vivo [13]. Later, the presence of necrotic enteritis B-like toxin (netB) gene in Type G (plc+netB+) strains has broadly been shown to be essential for NE pathogenicity [2,14]. In addition to NetB toxin, other virulence factors or virulence-associated factors have also been studied. Fructose-1,6-bisphosphate aldolase (FBA) is an enzyme involved in the Embden–Meyerhof–Parnas glycolytic pathway and in gluconeogenesis [15]. Zinc metalloproteases (Zm) are zinc-binding proteases shown to degrade mucin, the primary constituent glycoprotein of the mucosa, and have been implicated to be involved in the pathogenesis of necrotic enteritis [16]. Collagen-binding protein A (Cna) and fibrinogen-binding proteins FbpA and FbpB are implied to function as adhesins during infection. They are highly associated with the production of a sortase-dependent pilus by C. perfringens and play critical roles in collagen binding and NE pathogenesis [17]. Among these recombinant subunit vaccine targets, various levels of protection against NE have been reported in vaccine studies using the single subunit versions of CPA toxin, NetB toxin, FBA, pyruvate ferredoxin oxidoreductase (PFOR), and elongation factor Tu [18,19,20,21]. Understanding the pathogenesis of C. perfringens is vital for full NE prevention and control. It appears that several factors, such as NetB, Zm, and Cna, are more likely expressed in virulent C. perfringens strains causing necrotic enteritis than avirulent strains based on preliminary findings [16,17,21,22]. Interestingly, NetB, Plc, and FBA are suggested to be probably expressed on the C. perfringens surfaces, and FBA may serve as an adhesin function [10,15]. Immunization with mucosally vectored Salmonella expressing these triple antigens (NetB, Plc, and FBA) conferred partial protection [10]. Zinc metalloprotease was initially reported to be immunogenic as a hypothetic protein [21], and was found to contribute to the virulence of C. perfringens strains that cause avian NE [16]. Mutant generation via disruption of the zm gene using mutagenesis was shown to significantly reduce the virulence of C. perfringens, suggesting its role in disease pathogenesis [16]. Collagen adhesion protein as a pilus component of C. perfringens has been shown to be associated with the adherence of C. perfringens to the host in pathogenesis study, and inactivation of the pilus genes resulted in inhibition of pilus production, highly reducing the capability of C perfringens to bind collagen and initiate disease [17,23,24]. We hypothesized that the pooled recombinant immunogens from these key targets could offer better protection against NE challenges. The objective of this study was to prepare recombinant proteins targeting five virulence factors or virulence-associated factors of C. perfringens in either single (Cna) or chimeric forms (NetB-CPA and FBA-Zm), and compare the vaccine efficacies via subcutaneous immunization in a well-established NE challenge model, a dual infection model with Eimeria maxima pre-infection, followed by pathogenic C. perfringens co-infection.
2. Materials and Methods
2.1. Design and Construction of Recombinant Chimeric NetB-CPA (NA) and FBA-Zm (FZ), and Cna Expression Vector
The specific fragment gene sequences from NetB toxin and Plc portion of alpha-toxin (CPA) were fused as chimeric recombinant NA using alpha-helix forming linkers A(EAAAK)4A, as similarly described elsewhere [25], while the fba gene and zinc metallopeptidase zm gene were used to construct chimeric fz gene using the same alpha-helix forming linker, as previously described [26]. The cnaA gene was used as a single construct using the same sequence as described elsewhere [27]. All these genes were codon-optimized and synthesized by Synbio Inc. (Synbio Technologies LLC, Monmouth Junction, NJ, USA). EcoRI and HindIII restriction sites were inserted at 5′ and 3′ ends of each construct, which were later subcloned into the EcoRI and HindIII restriction sites of the pET-20b(+) vector (Novagen, Madison, WI, USA).
2.2. Expression and Purification of Recombinant C. perfringens Proteins
The synthesized plasmids were transformed into BL21-AI E. coli competent cells (Thermo Fisher Scientific, Waltham, MA, USA) on LB agar plates (Research Products International Inc., Mt Prospect, IL, USA) supplied with 100µg/mL of ampicillin (Sigma-Aldrich, St. Louis, MO, USA). The positive bacterial strains containing the recombinant plasmids were characterized. Bacterial culture was incubated at 37 °C with shaking at 225 rpm overnight under the induction of 0.25 mM isopropyl-β-D-thiogalactopyranoside (IPTG, Sigma-Aldrich, St. Louis, MO, USA) in LB media containing 100 µg/mL of ampicillin (Sigma-Aldrich, St. Louis, MO, USA). The expressed recombinant proteins were purified with the Ni-NTA Agarose based on the manufacturer’s product instruction (QIAGEN Inc., South San Francisco, CA, USA). Quality control was carried out with an anti-His tag antibody (Thermo Fisher Scientific, Waltham, MA, USA) using Western blot analysis. Briefly, bacteria were harvested by centrifugation at 15,000× g for 10 min at 4 °C. The supernatants were removed, and cell pellets were resuspended with 1× PBS. Bacterial samples were sonicated for 10 min and centrifugated at 15,000× g for 20 min at 4 °C, and the pellets were collected and resuspended in 8M Urea at room temperature for over 5 h or overnight. Inclusion body samples containing the recombinant proteins were centrifugated at 15,000× g for 30 min at 15 °C. Ni-NTA resin/1× PBS was added to the supernatants, and tubes were incubated on a shaker for 1 hour (hr). Samples were then centrifugated at 1100× g for 5 min, and resins were collected and washed with 1× PBS, 15 mL of 0.1 M Tris-HCl (pH 7.4), and 15 mL of 0.1 M Tris-HCl (pH 8.0), respectively. The recombinant protein was eluted from the column with 0.25 M Imidazole in PBS and dialyzed against three changes in PBS with stirring at 4 °C. The purified protein was passed through the Detoxi-Gel Endotoxin Removing Columns (ThermoFisher Scientific, Waltham, MA, USA) to remove endotoxin, and protein concentration was measured using Bradford Reagent (Sigma-Aldrich, St. Louis, MO, USA). The molecular weight and purity of the purified proteins were determined with SDS–PAGE and Western blot analysis.
2.3. Bacterial Strain
The LLN_Tpel17 C. perfringens isolate (simplified as Tpel17) were prepared as described elsewhere [28,29]. Briefly, the Tpel17 isolate was first cultured in chopped meat glucose medium for 24 hr in anaerobic chambers that utilized a gas packet (Mitsubishi Gas Chemical Company, New York, NY, USA) to generate anaerobic conditions (O2 < 2%, CO2 = 9–13%). The bacteria culture was then inoculated in 150 mL of BYC medium (1:100 dilution) containing BactoTM Brain–Heart Infusion broth (BHI; Becton Dickinson and Company, Sparks, MD, USA), yeast extract, and Cysteine (Sigma-Aldrich, St. Louis, MO, USA), and cultured for 18 hr at the same anaerobic conditions.
2.4. Broiler Chick Husbandry and Experimental Design
One-day-old Ross 708 broiler chicks obtained from Longenecker’s Hatchery (Elizabethtown, PA) were housed in Petersime starter brooder units and provided with feed and water ad libitum. A total of 91 one-day-old chicks were randomly assigned to 7 groups, with 13 birds per group: naïve sham control (N), E. maximum/C. perfringens (EMCP) challenge control, adjuvant control (Adj), Cna-immunized group (Cna), FZ-immunized group (FZ), NA-immunized group (NA), and pooled antigen group (Pooled) (Table 1). Chickens were transferred into large hanging cages at the age of 2 weeks and were fed a nonmedicated starter diet containing 16% crude protein and 61% carbohydrate prior to C. perfringens infection, and then a standard grower diet containing 24% crude protein and 54% carbohydrate after C. perfringens infection, as described elsewhere [19,30,31]. All diets contained 2.4% fiber, 4.7% fat, and 15% vitamin and mineral mixture and were prepared at USDA-Feed Mill (Beltsville, MD). All experiments were approved by the local Institutional Animal Care and Use Committee and performed in animal facilities at Beltsville Agricultural Research Center (Animal Use Protocol No 17-027).
Table 1.
Experimental design of vaccination studies *.
| Group No. | Group Name | Bird Number |
Vaccination (D4, D15) |
Challenge | Sacrifice (D28) |
|
|---|---|---|---|---|---|---|
| EM(D22) | CP (D26) | |||||
| 1 | Naive | 13 | PBS | PBS | BYC | S |
| 2 | Challenge Control (EMCP) | 13 | PBS | EM | CP | S |
| 3 | Adjuvant control (Adj) |
13 | Adj + PBS | EM | CP | S |
| 4 | Cna | 13 | Adj + Cna | EM | CP | S |
| 5 | FZ | 13 | Adj + FZ | EM | CP | S |
| 6 | NA | 13 | Adj + NA | EM | CP | S |
| 7 | Pooled (NA + FZ + Cna) |
13 | Adj + NA + FZ + Cna | EM | CP | S |
* Vaccinated chickens were challenged with E. maxima (EM, 5 × 103 oocysts/bird orally on Day 22), followed by oral gavage of C. perfringens (CP, 1 × 109 CFU at D26). BYC = BYC medium; PBS = phosphate-buffered saline; S = sacrificed; EMCP = positive challenge control; Adj = adjuvant + EMCP challenge; NA= chimeric NetB and Plc; FZ = chimeric FBA and Zm; Cna = collagen-binding protein; Pooled = pooled NA, FZ, and Cna antigens.
2.5. In Vivo Evaluation of Vaccine Efficacy of Recombinant C. perfringens Proteins
Chickens were vaccinated subcutaneously with recombinant proteins adjuvanted with Montanide ISA VG 71 (Seppic Inc., Puteaux, France) on Day 4 (100 µg proteins for the first immunization) and D15 (boost with 50 µg proteins) according to the manufacturer’s product instruction [19,32,33] (Table 1). The pooled antigen groups were immunized with a total of 75 µg of pooled antigens (mixture of NA/FZ/Cna 25 µg each for the first dosing) and boosted with 37.5 µg (12.5 µg of each antigen for the second dosing). Six days after the last immunization, birds were bled from the wings for antibody determination via ELISA. Seven days after the second immunization, chickens were challenged with 5 × 103 sporulated oocysts of E. maxima (EM) per bird by oral gavage, then followed by inoculation of C. perfringens Tpel17 of around 1 × 109 colony forming units per bird (CFU, all bacteria from the same overnight broth culture in a large flask for the entire trial) by oral gavage at 4 days after E. maxima inoculation (Table 1). Two days after the C. perfringens infection, birds were euthanized by cervical dislocation (Day 28). The jejunum lesion scores were determined with a section of around 10 cm length flanking Meckel’s diverticulum region, as described elsewhere [19,30]. The lesion scores were evaluated on a scale from 0 (no lesions) to 4 (severe lesions), and those in dead chickens were considered as 4 points in the weighted lesion scoring [34].
2.6. Determination of Antibody Titers in Vaccinated Birds by ELISA
Purified Cna and Plc, or chimeric FZ proteins (1 μg/mL in 1 × PBS), respectively, as coating antigens, were added to 96-well ELISA plates and incubated at 4 °C overnight. Plates were washed once and blocked with 200 μL of blocking buffer (1 × PBS/0.1% Tween-20 detergent (Sigma-Aldrich, St. Louis, MO, USA)) at 37 °C for 1 hr. After blocking, 100 μL of serially diluted chicken serum was added to each well of the plate for incubation at 37 °C for 1 hr. Plates were then washed four times, and 100 μL of diluted HRP-conjugated goat anti-chicken IgY antibody (Sigma-Aldrich, St. Louis, MO, USA) was added to each well for incubation at 37 °C for 30 min. Following four washes, 100 μL of TMB Reagent (GenScript, Piscataway, NJ, USA) was added to each well and incubated at room temperature for 15–20 min. Lastly, 100 μL of stop solution (2N H2SO4) was added to the wells and the OD450 value was read using a microplate reader. Antibody titers were determined as the reciprocals of dilution folds of serum samples, in which OD450 reached around 0.5 with ELISA.
2.7. Statistical Analysis
The mortality rate in each group was estimated using sample proportion with the 95% confidence interval (95% CI), computed using a numerical method based on the likelihood function of the number of deaths (modeled as a realization of a binomial random variable). The difference in mortality rates among the seven groups was tested using the Fisher’s exact test (developed by Ronald A. Fisher, London, England, UK) in R (version 4.1.2). Since the number of groups was larger than two, the p-value computed via Monte Carlo simulation was compared with the p-value calculated using the analytical approach. The antibody titers were analyzed using a one-way ANOVA in SAS 9.4 for Windows followed by Duncan’s multiple range tests (Cary, NC, USA). All of the data are expressed as mean ± SEM for each treatment. The lesion scores were analyzed using a Kruskal–Wallis Test among the groups and the Mann–Whitney U test between two groups [10,11]. Differences were considered statistically significant at p ≤ 0.05.
3. Results
3.1. Mortality Post-Challenge in Vaccinated Groups
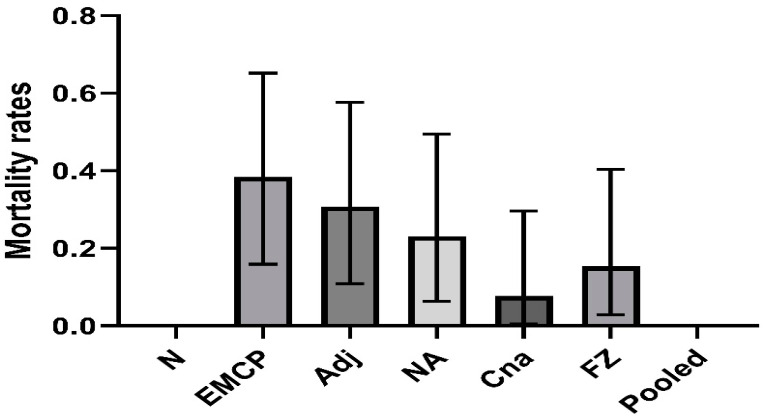
Figure 1 shows the mortality rates in control and vaccinated groups. Fisher’s exact test suggested that the mortality rates were significantly different among the groups (p = 0.036 with Monte Carlo simulation). In the E. maxima/C. perfringens challenge control group, 0.385 mortality (5 dead out of 13) was observed. Meanwhile, 0.308 mortality was found in the adjuvant control group (4 dead out of 13). There were lower mortalities in vaccinated groups: 0.231 mortality for the NA-immunized group (3 dead out of 13), 0.154 mortality for the FZ-immunized group (2 dead out of 13), and 0.077 mortality for the Cna-immunized group (1 dead out of 13) (Figure 1). No mortality was seen in the pooled antigens-immunized group, indicating that these antigens were likely to provide good protection against the lethal challenge of E. maxima and C. perfringens.
Figure 1.
Mortality rates with 95% confidence intervals of vaccinated broiler chickens following necrotic enteritis challenges. Birds were immunized with recombinant proteins on Day 4 and Day 15: EMCP = positive challenge control; Adj = adjuvant + EMCP challenge; NA= chimeric NetB and Plc; FZ = chimeric FBA and Zm; Cna = collagen-binding protein; Pooled = pooled NA, FZ, and Cna antigens. The birds with 13 birds per group were then challenged with Eimeria maxima (EM, 5 × 103 oocysts/bird, oral gavage) on Day 22, followed by CP netB+tpel+ LLY_Tpel 17 (1 × 109 cfu /bird, oral gavage) on Day 26. Mortalities were recorded after C. perfringens challenge.
3.2. Jejunum Lesion Scores
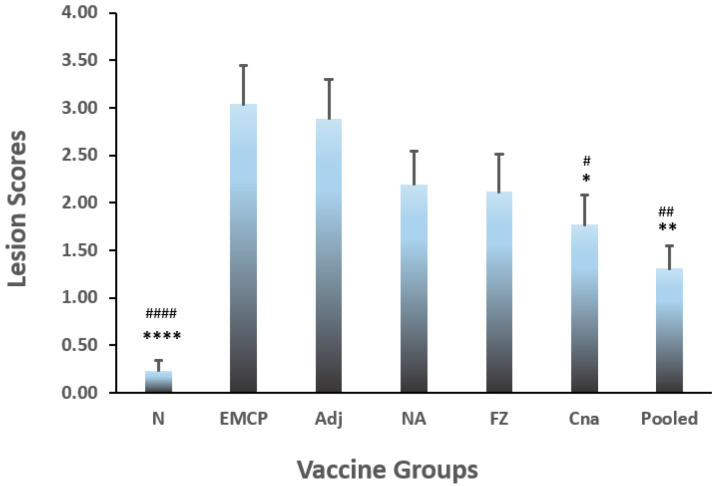
Intestine lesion scores were also determined on Day 2 post-C. perfringens infection. The Kruskal–Wallis test indicated there was a significant difference among the groups (p < 0.0001). Post hoc analysis was further conducted with the Mann–Whitney U test between independent groups. Figure 2 showed the lesion scores in all the experimental groups. Compared with the control groups, all of the vaccination groups had lower lesion scores. However, a significant statistical difference in lesion scores was observed between pooled antigen group and controls groups (both EMCP and adjuvant control) (p < 0.01), and between the Cna group and either control group (adjuvant or EMCP) and (p < 0.05), while no significant difference was detected between either control (EMCP or Adjuvant) and other vaccinated groups (p > 0.05). No significant difference was found between EMCP and adjuvant control groups.
Figure 2.
Lesion scores in vaccinated broiler chickens following necrotic enteritis challenge. The statistical differences were analyzed by the Kruskal–Wallis test and the Mann–Whitney U test. The chicks with 13 birds per group were immunized with recombinant proteins on Day 4 and Day 15: EMCP = positive challenge control; Adj = adjuvant + EMCP challenge; NA = chimeric NetB and Plc; FZ= chimeric FBA and Zm; Cna= collagen-binding protein; Pooled = pooled NA, FZ, and Cna antigens. The vaccinated birds were then administrated with Eimeria maxima (EM, 5 × 103 oocysts/bird, oral gavage) on Day 22, followed by C. perfringens (CP) netB+tpel+LLY_Tpel 17 strain (1 × 109 cfu /bird, oral gavage) on Day 26. Birds were sacrificed on Day 2 post-CP infection, and jejunum lesion scores were determined. The * or # marks show statistically significant difference (* p ≤ 0.05; ** p ≤ 0.01; **** p ≤ 0.0001) when compared with the value in Adj group, while # p ≤ 0.05; ## p ≤ 0.01; #### p ≤ 0.0001 when compared with the value in EMCP group, respectively).
3.3. Determination of Antibody Titers
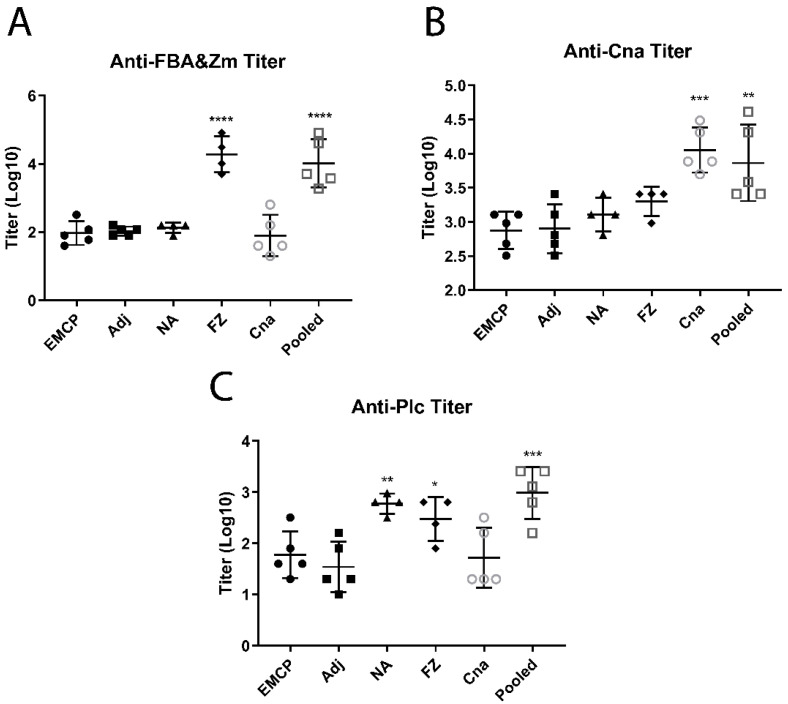
After immunization with recombinant proteins, specific antibodies were produced in these chickens. On Day 6 after the second immunization (Day 21), chickens were bled to determine antibodies prior to the NE challenge. As indicated by Figure 3, large amounts of anti-FZ (chimeric FBA-Zm) antibodies were generated in the single FBA-Zm antigen and pooled antigen groups, which were both immunized with chimeric FBA-Zm (Figure 3A), where 2 log10 difference in titers existed between the adjuvant control group and group with FBA-Zm immunization (p ≤ 0.0001 for either single or pooled antigens), while higher antibodies were also produced in single Cna and the pooled antigen groups, which were immunized with Cna antigens, where about less than 1 log10 difference existed between the adjuvant group and Cna group (p ≤ 0.001) or between the adjuvant group and pooled antigen group (p ≤ 0.01) (Figure 3B). There were also higher anti-CPA (Plc) antibodies in the single NA immunized group or pooled antigen group with a significant difference (p ≤ 0.01 for NA vs. Adj group; p ≤ 0.001 for pooled antigens vs. Adj group) (Figure 3C). Interestingly, the FZ-immunized group was found to have some binding activities with the Plc antigens coated on the ELISA plate, which was most possibly caused by the nonspecific binding with common His-tag (other than with FBA or Zm epitopes). All the antigens were His-tagged, so that a high level of anti-FZ antibodies may interfere with the anti-Plc readings when the plate was coated with His-tagged Plc.
Figure 3.
The antibody titers in control and vaccination groups determined via ELISA: (A) anti-FZ (FBA-Zm) titers; (B) anti-Cna titers; (C) anti-Plc titers. Antibody titers were determined using log10 (reciprocals of dilution folds of serum samples), which OD450 reached around 0.5 with ELISA. The recombinant FZ (chimeric FBA-Zm), Cna, or Plc proteins were used as coating antigens. EMCP = positive challenge control; Adj = adjuvant + EMCP challenge; NA = chimeric NetB and Plc; FZ= chimeric FBA and Zm; Cna = collagen-binding protein; Pooled = pooled NA, FBA, and Cna antigens. The * marks show statistically significant differences (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; **** p ≤ 0.0001) when compared with the value in adjuvant (Adj) control group.
4. Discussion
Necrotic enteritis is a serious enteric infectious disease for chickens that may cause sudden death with up to 50% mortality (acute form) or may result in mucosal damage to intestines, leading to necrosis, ulceration, inflammation, reduced weight loss, and carcass condemnation (the most common subclinical form) [5,6]. Therefore, NE is an economically important disease that has a significant adverse impact on bird welfare and profitability for the poultry producer with an estimated economic loss of around USD 6 billion annually to the global poultry industry [1]. Vaccination is a good antibiotic alternative strategy as a preventive measure. The availability of an effective NE vaccine could improve poultry well-being and profitability for the poultry producers.
NetB, Plc, Cna, FBA, and Zm are important virulence factors or virulence-associated factors, which play critical roles in bacterial adhesion to the host, localization, energy utilization, and spread in the early infection. Vaccination with these recombinant proteins may help ameliorate the severity of the NE challenge in chickens. The potent challenge model was utilized with E. maxima pre-infection, followed by pathogenic C. perfringens Tpel17 inoculation. This study was carried out to evaluate the protection efficacies of three types of recombinant C. perfringens proteins adjuvanted with ISA71VG using a well-established experimental NE model. Understanding the roles of these virulence factors and their associated factors in pathogenesis may help design optimal vaccines to efficiently disrupt the phases of transmission, colonization, and proliferation of C. perfringens during the early infection stage.
In this study, several encouraging results were found: (1) Chickens immunized with all adjuvanted proteins post potent challenge had reduced mortalities with no mortality in the pooled antigen group; (2) chickens immunized with the adjuvanted pooled proteins post potent challenge had significantly reduced lesion scores, compared with the challenge control or infected and adjuvant groups; (3) much higher anti-FZ antibody titers were observed in the FZ-immunized and pooled antigens groups, and higher anti-Cna antibody titer in Cna-immunized and pooled antigen groups, when compared with adjuvant control or challenge control group; (4) mortality rates were very close between the adjuvant control and challenge control groups after potent challenge, implying that immunization with only ISA71VG adjuvant may not appear to have a protective effect against NE, in agreement with previous observation [19].
In this study, it seems that robust immune responses were observed by the immunizations with chimeric FZ protein or with Cna at a magnitude level. The molecular sizes are as follows: in chimeric FBA-Zm and Cna, the size was around 70 kD, while in chimeric NA, it was around 50 kD. More epitopes on the large FBA-Zm or Cna protein molecules may induce the host to generate more antibodies. On the other hand, FBA and Zm were identified via mass spectrophotometry to be two out of six secreted proteins unique to virulent strains that were highly immunoreactive to serum antibodies from immune birds by Prescott’s group [21]. As the gut mucosa integrity is disrupted by the predisposing factors (such as Eimeria spp. infection), C. perfringens outgrowth and penetration of the intestinal mucosa may lead to either mild mucosal damage or even extensive necrosis by accompanying secretion of a series of mucolytic enzymes, pore-forming toxins such as NetB, and tissue-degrading toxins (such as α-toxin and TpeL) [35]. Induction of robust neutralization antibodies against these toxins, other virulence factors and virulence-associated factors resulting from the systemic vaccination via a subcutaneous route may help establish a regulatory environment to reduce bacteria spreading and ameliorate these factors’ negative effects. The ideal vaccines should elicit both mucosal and systemic immune responses against mucosal pathogens such as C. perfringens [5,10,11,35].
In this study, the immunization with Cna protein resulted in less mortality (7.7%) post-NE challenge compared with higher mortality in the positive challenge control group (38.5%) and adjuvant/infection control (30.8%) and had less intestinal lesion score than the adjuvant control group. The additive contribution effect of the Cna factor was evident in the mortality rate. Gong’s group demonstrated that subcutaneous triple immunization with CnaA or another pilus subunit FimB conferred partial protection against the onset of NE by significantly reducing the NE lesions when compared with the adjuvant control [27]. Of course, multiple vaccinations by laborious intramuscular injection are not practical in the field of the poultry industry. The ideal immunogen delivery route in mass vaccination should be mucosal one or in ovo one in broiler chickens. The pooled antigen group had no mortality in this study, which is supportive of the notion that vaccination with a single immunogenic component does not provide full protection against a complex NE, fully echoing other reports [20,36,37]. Immunizations with multiple immunogens and other practical mass vaccination means should be considered in the design of the NE vaccine. As discussed earlier, FBA, Zm, Cna, NetB, and Plc are good vaccine candidates against NE in chickens. In fact, several chimeric proteins containing multiple immunogenic components have been reported in design, such as chimeric proteins NetB-Plc-Tpel [38], NetB-Plc-Zm [39], and Plc-FBA-NetB [10], and some may confer partial protection in chickens. Future studies will focus on a better vector or route to deliver these multiple immunogenic targets. It should be mentioned that the combination of these antigens may need repetition before future studies are performed.
In summary, three C. perfringens recombinant proteins were founded to elicit varying levels of protective immunity in an experimental NE challenge model. Multiple immunogenic components should be considered for the development of an effective vaccine to control NE. Future studies merit further investigation into these critical proteins to define their molecular mechanisms for the control of NE.
Acknowledgments
The authors would like to sincerely acknowledge Stacy O’Donnell for ordering animals, Seppic Inc. for providing the adjuvant, and Yunfei Wang for professional advice in statistics. This research was supported by the funds from Agricultural Research Service, USDA. The sponsors had no role in the writing process of the article.
Abbreviations
| NE | Necrotic enteritis |
| CPA | Alpha-toxin |
| Plc | Phospholipase C (PLC) enzyme of alpha-toxin |
| NetB | Necrotic enteritis B-like toxin |
| FBA | Fructose-1,6-bisphosphate aldolase (FBA) |
| Zm | Zinc metalloprotease (Zm) |
| Cna | Collagen adhesion protein |
| AGP | Antibiotic growth promoters |
| EM | Eimeria maxima |
| CFU | Colony-forming units |
Author Contributions
Conceptualization: C.L.; Data curation: B.Y., Z.S., M.L., Y.L., L.L., and C.L.; Formal analysis: B.Y., M.L, X.Y., D.A.Y., and C.L.; Software: M.L. and D.A.Y.; Funding acquisition: H.L. and C.L.; Supervised the project, Project administration: C.L.; Writing–original draft: B.Y., M.L., and C.L.; Writing–review & editing: M.L., H.L., D.A.Y., and C.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocols were approved by the local Institutional Animal Care and Use Committee at Beltsville Agricultural Research Center (Animal Use Protocol No 17-027).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by ARS Animal Health project number 8042-32000-107-00D.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wade B., Keyburn A. The true cost of necrotic enteritis. World Poult. 2015;31:16–17. [Google Scholar]
- 2.Uzal F.A., Navarro M.A., Li J., Freedman J.C., Shrestha A., McClane B.A. Comparative pathogenesis of enteric clostridial infections in humans and animals. Anaerobe. 2018;53:11–20. doi: 10.1016/j.anaerobe.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rood J.I., Adams V., Lacey J., Lyras D., McClane B.A., Melville S.B., Moore R.J., Popoff M.R., Sarker M.R., Songer J.G., et al. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe. 2018;53:5–10. doi: 10.1016/j.anaerobe.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gohari I.M., Navarro M.A., Li J., Shrestha A., Uzal F., McClane B.A. Pathogenicity and virulence of Clostridium perfringens. Virulence. 2021;12:723–753. doi: 10.1080/21505594.2021.1886777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott J.F., Smyth J.A., Shojadoost B., Vince A. Experimental reproduction of necrotic enteritis in chickens: A review. Avian Pathol. 2016;45:317–322. doi: 10.1080/03079457.2016.1141345. [DOI] [PubMed] [Google Scholar]
- 6.Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: A critical review. Vet. Res. 2012;43:74. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper K.K., Songer J.G. Necrotic enteritis in chickens: A paradigm of enteric infection by Clostridium perfringens type A. Anaerobe. 2009;15:55–60. doi: 10.1016/j.anaerobe.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Thompson D.R., Parreira V.R., Kulkarni R.R., Prescott J.F. Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Vet. Microbiol. 2006;113:25–34. doi: 10.1016/j.vetmic.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W., Wang P., Wang B., Ma B., Wang J. A combined Clostridium perfringens/Trueperella pyogenes inactivated vaccine induces complete immunoprotection in a mouse model. Biologicals. 2017;47:1–10. doi: 10.1016/j.biologicals.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Wilde S., Jiang Y., Tafoya A.M., Horsman J., Yousif M., Vazquez L.A., Roland K.L. Salmonella-vectored vaccine delivering three Clostridium perfringens antigens protects poultry against necrotic enteritis. PLoS ONE. 2019;14:e0197721. doi: 10.1371/journal.pone.0197721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra N., Smyth J.A. Oral vaccination of broiler chickens against necrotic enteritis using a non-virulent NetB positive strain of Clostridium perfringens type A. Vaccine. 2017;35 Pt B:6858–6865. doi: 10.1016/j.vaccine.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Awad M.M., Bryant A.E., Stevens D.L., Rood J.I. Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 1995;15:191–202. doi: 10.1111/j.1365-2958.1995.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 13.Coursodon C.F., Trinh H.T., Mallozzi M., Vedantam G., Glock R., Songer J. Clostridium perfringens alpha toxin is produced in the intestines of broiler chicks inoculated with an alpha toxin mutant. Anaerobe. 2010;16:614–617. doi: 10.1016/j.anaerobe.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Timbermont L., Lanckriet A., Gholamiandehkordi A.R., Pasmans F., Martel A., Haesebrouck F., Ducatelle R., Van Immerseel F. Origin of Clostridium perfringens isolates determines the ability to induce necrotic enteritis in broilers. Comp. Immunol. Microbiol. Infect. Dis. 2009;32:503–512. doi: 10.1016/j.cimid.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Shams F., Oldfield N.J., Wooldridge K.G., Turner D.P. Fructose-1,6-bisphosphate aldolase (FBA)-a conserved glycolytic enzyme with virulence functions in bacteria: ‘ill met by moonlight’. Biochem. Soc. Trans. 2014;42:1792–1795. doi: 10.1042/BST20140203. [DOI] [PubMed] [Google Scholar]
- 16.Wade B., Keyburn A.L., Haring V., Ford M., Rood J.I., Moore R.J. Two putative zinc metalloproteases contribute to the virulence of Clostridium perfringens strains that cause avian necrotic enteritis. J. Vet. Diagn. Investig. 2020;32:259–267. doi: 10.1177/1040638719898689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lepp D., Zhou Y., Ojha S., Gohari I.M., Carere J., Yang C., Prescott J.F., Gong J. Clostridium perfringens Produces an Adhesive Pilus Required for the Pathogenesis of Necrotic Enteritis in Poultry. J. Bacteriol. 2021;203:e00578-20. doi: 10.1128/JB.00578-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Y., Kulkarni R., Parreira V.R., Prescott J.F. Immunization of broiler chickens against clostridium perfringens–Induced necrotic enteritis using purified recombinant immunogenic proteins. Avian Dis. 2009;53:409–415. doi: 10.1637/8656-021109-Reg.1. [DOI] [PubMed] [Google Scholar]
- 19.Jang S.I., Lillehoj H.S., Lee S.-H., Lee K.-W., Lillehoj E.P., Hong Y.H., An D.-J., Jeong W., Chun J.-E., Bertrand F., et al. Vaccination with Clostridium perfringens recombinant proteins in combination with Montanide™ ISA 71 VG adjuvant increases protection against experimental necrotic enteritis in commercial broiler chickens. Vaccine. 2012;30:5401–5406. doi: 10.1016/j.vaccine.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni R.R., Parreira V.R., Sharif S., Prescott J.F. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis. Clin. Vaccine Immunol. 2007;14:1070–1077. doi: 10.1128/CVI.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulkarni R.R., Parreira V.R., Sharif S., Prescott J.F. Clostridium perfringens antigens recognized by broiler chickens immune to necrotic enteritis. Clin. Vaccine Immunol. 2006;13:1358–1362. doi: 10.1128/CVI.00292-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keyburn A.L., Boyce J.D., Vaz P., Bannam T.L., Ford M.E., Parker D., Di Rubbo A., Rood J.I., Moore R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;4:e26. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wade B., Keyburn A.L., Haring V., Ford M., Rood J.I., Moore R.J. The adherent abilities of Clostridium perfringens strains are critical for the pathogenesis of avian necrotic enteritis. Vet. Microbiol. 2016;197:53–61. doi: 10.1016/j.vetmic.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Wade B., Keyburn A., Seemann T., Rood J., Moore R. Binding of Clostridium perfringens to collagen correlates with the ability to cause necrotic enteritis in chickens. Vet. Microbiol. 2015;180:299–303. doi: 10.1016/j.vetmic.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y., Mo H., Willingham C., Wang S., Park J.-Y., Kong W., Roland K.L., Curtiss R. Protection Against Necrotic Enteritis in Broiler Chickens by Regulated Delayed Lysis Salmonella Vaccines. Avian Dis. 2015;59:475–485. doi: 10.1637/11094-041715-Reg. [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni R.R., Parreira V.R., Sharif S., Prescott J.F. Oral immunization of broiler chickens against necrotic enteritis with an attenuated Salmonella vaccine vector expressing Clostridium perfringens antigens. Vaccine. 2008;26:4194–4203. doi: 10.1016/j.vaccine.2008.05.079. [DOI] [PubMed] [Google Scholar]
- 27.Lepp D., Ojha S., Gohari I.M., Chakravarty B., Prescott J., Gong J. Immunization with subunits of a novel pilus produced by virulent Clostridium perfringens strains confers partial protection against necrotic enteritis in chickens. Vet. Microbiol. 2019;230:7–13. doi: 10.1016/j.vetmic.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Gu C., Lillehoj H.S., Sun Z., Lee Y., Zhao H., Xianyu Z., Yan X., Wang Y., Lin S., Li C., et al. Characterization of Virulent netB(+)/tpeL(+) Clostridium perfringens Strains from Necrotic Enteritis-Affected Broiler Chicken Farms. Avian Dis. 2019;63:461–467. doi: 10.1637/11973-092018-Reg.1. [DOI] [PubMed] [Google Scholar]
- 29.Liu L., Yan X., Lillehoj H., Sun Z., Zhao H., Xianyu Z., Lee Y., Melville S., Gu C., Wang Y., et al. Comparison of the Pathogenicity of Five Clostridium perfringens Isolates Using an Eimeria maxima Coinfection Necrotic Enteritis Disease Model in Commercial Broiler Chickens. Avian Dis. 2020;64:386–392. doi: 10.1637/aviandiseases-D-19-00098. [DOI] [PubMed] [Google Scholar]
- 30.Li C., Lillehoj H.S., Gadde U.D., Ritter D., Oh S. Characterization of Clostridium perfringens Strains Isolated from Healthy and Necrotic Enteritis-Afflicted Broiler Chickens. Avian Dis. 2017;61:178–185. doi: 10.1637/11507-093016-Reg.1. [DOI] [PubMed] [Google Scholar]
- 31.Lee K.-W., Lillehoj H.S., Jeong W., Jeoung H.Y., An D.J. Avian necrotic enteritis: Experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poult. Sci. 2011;90:1381–1390. doi: 10.3382/ps.2010-01319. [DOI] [PubMed] [Google Scholar]
- 32.Lillehoj H.S., Jang S.I., Panebra A., Lillehoj E.P., Dupuis L., Ben Arous J., Lee S.K., Oh S.T. In ovo vaccination using Eimeria profilin and Clostridium perfringens NetB proteins in Montanide IMS adjuvant increases protective immunity against experimentally-induced necrotic enteritis. Asian-Australas. J. Anim. Sci. 2017;30:1478–1485. doi: 10.5713/ajas.17.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee K.-W., Lillehoj H.-S., Jang S.-I., Lee S.-H., Bautista D.A., Ritter G.D., Lillehoj E.P., Siragusa G.R. Comparison of live Eimeria vaccination with in-feed salinomycin on growth and immune status in broiler chickens. Res. Vet. Sci. 2013;95:110–114. doi: 10.1016/j.rvsc.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Prescott J.F. The prevention of experimentally induced necrotic enteritis in chickens by avoparcin. Avian Dis. 1979;23:1072. doi: 10.2307/1589625. [DOI] [PubMed] [Google Scholar]
- 35.Alizadeh M., Shojadoost B., Boodhoo N., Astill J., Taha-Abdelaziz K., Hodgins D.C., Kulkarni R.R., Sharif S. Necrotic enteritis in chickens: A review of pathogenesis, immune responses and prevention, focusing on probiotics and vaccination. Anim. Health Res. Rev. 2021;22:147–162. doi: 10.1017/S146625232100013X. [DOI] [PubMed] [Google Scholar]
- 36.Sarmah H., Hazarika R., Tamuly S., Deka P., Manoharan S., Sharma R.K. Evaluation of different antigenic preparations against necrotic enteritis in broiler birds using a novel Clostridium perfringens type G strain. Anaerobe. 2021;70:102377. doi: 10.1016/j.anaerobe.2021.102377. [DOI] [PubMed] [Google Scholar]
- 37.Mot D., Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Progress and problems in vaccination against necrotic enteritis in broiler chickens. Avian Pathol. 2014;43:290–300. doi: 10.1080/03079457.2014.939942. [DOI] [PubMed] [Google Scholar]
- 38.Goshadrou F., Langroudi R.P., Riazi A., Rostami A., Bathaie S.Z., Amani J., Ahmadian G. Design and expression of a chimeric vaccine candidate for avian necrotic enteritis. Protein Eng. Des. Sel. 2017;30:39–45. doi: 10.1093/protein/gzw060. [DOI] [PubMed] [Google Scholar]
- 39.Katalani C., Ahmadian G., Nematzadeh G., Amani J., Ehsani P., Razmyar J., Kiani G. Immunization with oral and parenteral subunit chimeric vaccine candidate confers protection against Necrotic Enteritis in chickens. Vaccine. 2020;38:7284–7291. doi: 10.1016/j.vaccine.2020.09.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available within the article.