Abstract
Downstream from the ptsHI operon of Lactobacillus sakei, the genes atkY and atkB, organized in an operon, were observed. The two putative proteins, AtkB and AtkY, show sequence similarity to the Enterococcus hirae copper P-type ATPase, responsible for copper efflux, and its negative regulator. Characterization of AtkB as a copper P-type ATPase could not be demonstrated since an atkB mutant did not show any phenotype. Thus, another strategy was followed in order to investigate the transcriptional regulation of the atkYB locus, leading to the development of new genetic tools for L. sakei. A plasmid was constructed, the use of which allowed gene replacement at the lacLM locus in L. sakei by two successive crossovers. A strain deleted of the lacLM operon encoding the β-galactosidase of L. sakei was constructed by this method, and the Escherichia coli lacZ gene could then be used as a reporter gene to investigate the regulation of atkYB. Results show that the atkYB operon is induced by small concentrations of CuSO4 (30 to 40 μM) but not when CuSO4 is omitted or added at higher concentrations.
The P-type ATPases are a large family of enzymes so named because of a phospho-aspartate intermediate in the ATP-driven cation transport cycle. A wide range of different cations has been demonstrated to serve as substrates for P-type ATPases of procaryotes (39). All P-type ATPases function as cation pumps, either for uptake, efflux, or exchange. Bacteria have developed specific genes for resistance to the toxic ions of heavy metal elements (see reference 40 for a review). In the chromosome of Lactobacillus sakei, downstream from the 3′ end of ptsI encoding enzyme I of the phosphotransferase system, two open reading frames (ORFs) were observed (41). They showed sequence similarity to a copper efflux P-type ATPase and its negative regulator, which have been cloned from Enterococcus hirae (30, 31). Expression of these proteins is regulated by copper in E. hirae (29, 42). L. sakei is naturally found on meat and meat products, but little information is known about the requirement and sensitivity of this species for metal ions. The requirement for manganese by some bacteria, including lactic acid bacteria, is known (34). It was previously shown that the l-lactate dehydrogenase of L. sakei is activated by manganese and cadmium salts (15), and a dipeptidase activated by cobalt and manganese has been purified (25).
Although several genes have now been cloned from L. sakei (14) and some genetic tools are emerging (2, 3, 18, 22), the molecular biology techniques specific for this species are still poorly developed. For example, no straightforward reporter gene system has been developed that would help in the analysis of gene regulation. The aim of the present work was to use the promoter of the atkYB locus to identify its possible regulation by heavy metals in order to develop a reporter gene system for L. sakei. Several genes have been used as reporter genes in lactic acid bacteria, such as the luciferase genes in Lactococcus lactis (5); the gusA gene in L. lactis, Leuconostoc lactis, Lactobacillus plantarum, and Lactobacillus casei (33); the nuc gene in L. lactis (21); or the gfp gene in L. plantarum (12) and L. lactis (38). The lacZ gene of Escherichia coli, encoding β-galactosidase, and the chloramphenicol acetyltransferase gene have also been widely used in bacteria. Among these genes nuc, gusA, and lacZ are very convenient as reporter genes since their expression can be very easily monitored on plates with a chromogenic substrate (17, 21, 24). The activity of β-glucuronidase (gusA) and β-galactosidase (lacZ) can also be very easily and rapidly quantified on bacterial extracts. lux genes encoding luciferase or gfp encoding the green fluorescent protein require more expensive equipment, and the luciferase activity might depend on the energetic state of bacteria. The small size of gusA might render its use easier than lacZ; however, the difficulty of expressing gusA in some species of lactic acid bacteria has been reported (33). In L. sakei, the presence of a β-galactosidase encoding operon (lacLM) in the chromosome (28) enabled the use of lacZ since a β-galactosidase activity is already present in this species. For these reasons, several genes could be expressed experimentally in L. sakei, but no reporter gene was obviously ideal. Preliminary experiments showed that the luciferase genes could not be expressed at a satisfactory level, and the expression of gusA could not be detected. Finally, we decided to use the most widely used reporter gene lacZ. First, a vector was constructed that allows gene replacement by two successive crossovers. The lacLM operon, encoding L. sakei β-galactosidase, was deleted by the use of this vector. Second, we used lacZ to construct a chromosomal fusion with the promoter of the atkYB operon in the ΔlacLM mutant. The transcription level of the atkY::lacZ fusion in different conditions suggested that AtkB is a copper ATPase operating at low levels of Cu.
MATERIALS AND METHODS
Bacterial strains and plasmids.
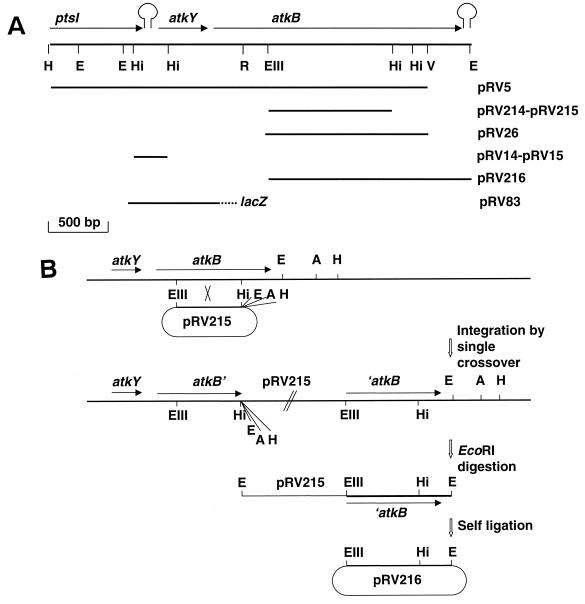
E. coli strains TG1 (37), TG90 (13), and DH5α (37) were used for cloning and subcloning experiments and for plasmid propagation. The E. coli strain GM2929 carrying a dcm mutation was used for the propagation of plasmids prior to digestions with the BalI restriction enzyme. E. coli XL1 Blue (Stratagene) was used for single-strand DNA preparation. L. sakei 23K, a laboratory strain originally isolated from sausage and cured of plasmids, was used as the recipient strain for cloning experiments and for the construction and analysis of mutants. Fragments cloned into plasmids and their subsequent names are shown in Fig. 1A. The HincII fragment from pRV5 (41) was cloned in both orientations in the phagemid pBluescript (Stratagene), yielding to pRV14 and pRV15. The Eco47III/HincII fragment was cloned in both orientations in the integrative vector pRV300 (22), yielding pRV214 and pRV215. The plasmid pRV216 was obtained by chromosome walking (see the text and Fig. 1B), and pRV27 contains an internal fragment of atkB resulting from a deletion in pRV5 generated by exonuclease III, followed by the cloning of the erythromycin resistance gene of pAMβ1 in the pBluescript moiety. The plasmid pRV80 was obtained by the cloning in pRV300 of two PCR-amplified DNA fragments containing, respectively, the 5′ and 3′ ends of lacLM with a large internal deletion. pRV81 was obtained by the cloning of the atkYB promoter region upstream from lacZ in pJM783 (32). pRV83 was obtained by cloning the EcoRI/BalI fragment of pRV81, containing the atkY operon and the lacZ gene, into the integrative plasmid pRV300.
FIG. 1.
Organization and restriction map and cloning of the atkYB region. (A) Restriction map of the atkYB region and plasmids. The arrows represent the genes. The transcription terminators of ptsI and atkB are shown. Restriction sites for EcoRI (E), EcoRV (V), Eco47III (EIII), HindIII (H), and HincII (Hi) are shown. The RsaI site (R), upstream from which the DNA sequence was previously determined, is shown. The inserts cloned in various plasmids used in this study are presented on the lower part of the figure. pRV5, pRV14, and pRV15 contain inserts cloned in pBluescript. pRV214, pRV215, pRV27, and pRV83 contain inserts cloned in the integrative vector pRV300. In pRV83, the lacZ gene is fused downstream from the atkYB promoter. (B) Cloning of the 3′ end of atkB in pRV216 by chromosome walking. Plasmid pRV215 was introduced into the chromosome of L. sakei 23K by single crossover. In the resulting transformant, the 3′ end of atkB plus the part of plasmid which is replicative in E. coli and including selective markers, were surrounded by two EcoRI (E), AccI (A), and HindIII (H) sites. After digestion of chromosomal DNA, self-ligation, and transformation of E. coli to ampicillin resistance, plasmid pVR216 carrying the 3′ end of atkB was isolated.
Media, growth conditions, and transformations.
E. coli strains were grown in Luria-Bertani medium (37) at 37°C. L. sakei strains were grown at 30°C in the complex medium MRS (6) or in the defined medium MCD (19). E. coli and L. sakei electrocompetent cells were prepared and transformed by the methods of Dower et al. (8) and Berthier et al. (3), respectively. E. coli transformants were selected on ampicillin at 100 μg · ml−1 or erythromycin at 200 μg · ml−1. L. sakei transformants were selected on erythromycin at 5 μg · ml−1.
Oligonucleotides and PCR.
Primers lac1 (5′-GATCAAGCTTATGCTTTAAGGGTACTGG), lac2 (5′-ACGTGAATTCTTGTCATCGGACGTTGAA), lac4 (5′-GATCGAGCTCGCGCTTTGAACAATAGCT), and lac6 (5′-ACGTGAATTCCGGTGCTGGATAATTGTT) were used for the PCR amplification of two DNA fragments corresponding, respectively, to the 5′ and 3′ ends of the L. sakei 23K lacLM operon. Restriction sites added at the 5′ end of each primer are underlined. Primers ATK1 (5′-GATCGAATTCTAGTCGAAGATTTTATGA) and ATK3 (5′-GATCGGATCCCATCGTGTTTCATCGTTA) were used to generate a fragment containing the promoter and regulatory region of atkYB, plus the upstream transcription terminator of ptsI. PCR experiments were performed on a Perkin-Elmer 9600 apparatus, with Taq DNA polymerase from Boehringer. Reactions were carried out in 100-μl mixtures containing 0.2 mM concentrations of each deoxynucleoside triphosphate, 1 μg of chromosomal DNA template, and 2.5 μM concentrations of each primer. Amplification was performed for 30 cycles (94°C for 1 min, 55°C for 2 min, and 72°C for 3 min). The PCR reactions on chromosomal DNA of mutants, to verify the integration of recombinant plasmids, were performed in the same conditions, but the volume of the reaction mixtures was 10 μl.
Nucleic acid manipulation.
DNA and RNA were prepared by standard methods as previously described (41).
DNA sequence analysis.
Subclones were obtained by generating overlapping deletions on the initial clones with an exonuclease III-mung bean nuclease kit (Stratagene) or with restriction enzymes. Single- and double-strand DNAs were sequenced according to the instructions of the manufacturer (Perkin-Elmer) for cycle sequencing reactions on a GenAmp PCR system 9600. Dideoxynucleotide reaction chain termination sequencing reactions were performed with Taq DNA polymerase. Dye-coupled dideoxynucleotides (Applied Biosystems) and synthetic primers were used. Sequencing was determined on both strands. DNA sequences were analyzed with programs using the University of Wisconsin Genetics Computer Group software package.
β-Galactosidase activity measurement.
Bacteria were grown at 30°C in MCD medium until the optical density at 600 nm (OD600) reached 0.3. The atkYB::lacZ fusion was then induced by the addition of CuSO4 and incubated at room temperature for 90 min. Bacteria from 10-ml culture aliquots were collected by centrifugation and resuspended in 1 ml of Z buffer (sodium phosphate, 100 mM, pH 7.0; KCl, 10 mM; MgSO4, 1 mM; β-mercaptoethanol, 50 mM) containing 20% glycerol. Bacteria were broken with zirconium beads in a Fast-Prep bead beater (Bio 101) two times for 20 s each time at maximum speed with a 5-min pause on ice. Cellular debris were removed by centrifugation. The β-galactosidase activity was measured in 1 ml of Z buffer at 28°C. The reaction was started by the addition of 200 μl of ONPG (o-nitrophenyl-β-d-galactopyranoside; 4 mg · ml−1) and stopped with 500 μl of Na2CO3 (1 M). Absorbance was measured at 420 nm. The activity was expressed in Miller Units (24). The results are the means of at least three independent assays.
Detection of β-galactosidase activity on plates was performed as follows: fresh cultures of L. sakei strains were either plated or streaked on MCD plates containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) at 0.0032%; then, 50 μl of metal ion or salt solutions was deposited in the center of the MCD plates and allowed to dry. The plates were then incubated for 24 h to allow bacterial growth and ion diffusion.
Nucleotide sequence accession number.
The atkYB sequence has been deposited under GenBank accession no. AF172726.
RESULTS AND DISCUSSION
Molecular cloning of the atkY and atkB genes.
The HindIII/EcoRV insert harboring atkY and the 5′ end of atkB (Fig. 1A) was previously cloned (41), and the HindIII/RsaI part was sequenced (GenBank accession no. U82366). In order to clone the 3′ end of the atkB gene, a 1,092-bp Eco47III/HindII fragment was subcloned into the integrative vector pRV300 (pRV215, Fig. 1B). Plasmid pRV215 was used to transform the strain L. sakei 23K by single crossover integration as shown Fig. 1B. One transformant, RV1018, was used for further experiments. Restriction mapping of the atkB 3′-terminal region allowed the localization of three restriction sites downstream from the EcoRV site. The three corresponding restriction enzymes EcoRI, AccI, and HindIII were used to digest RV1018 chromosomal DNA. After digestion and self-ligation, the DNA was used to transform E. coli. E. coli DH5α strain transformed with DNA derived from the EcoRI digestion allowed the cloning of a plasmid corresponding to pRV300 plus the expected Eco47III/EcoRI insert, with the 250-bp additional fragment located at the 3′ end of atkB (pRV216, Fig. 1B).
Nucleotide sequence of the atkB gene.
The nucleotide sequence of the 1,172-bp RsaI/EcoRI fragment was determined and allowed the complete sequence of atkB to be obtained. Two nucleotides downstream from the TAA stop codon of atkY, atkB starts with an ATG and ends 2,040 bp downstream by a TAA stop codon. The deduced protein sequence of 680 amino acids shares 56.2% identity with CopB, the copper ATPase of E. hirae responsible for copper efflux. A 30-nucleotide palindromic region resembling a transcription termination signal followed by a stretch of six T's was observed 19 nucleotides downstream from the atkB stop codon (ΔG0′, −9,23 kcal · mol−1). Because of the short distance between the atkB stop codon and the EcoRI restriction site, no significant ORF could be observed downstream from atkB.
Analysis of the predicted polypeptides AtkY and AtkB.
The 146-amino-acid peptide AtkY is 42% identical to CopY, the negative regulator of the copYZAB operon of E. hirae. As was observed with CopY (42), the comparison of AtkY with other proteins in the database revealed sequence similarity to the β-lactamase repressor proteins MecI of Staphylococcus epidermidis (43), PenI of Bacillus licheniformis (16), and BlaI of Staphylococcus aureus (36). All of these proteins are similar in their N-terminal part. Indeed, the N-terminal region of AtkY fits with the alignment proposed by Odermatt and Solioz (30) for these different proteins (Fig. 2). The C-terminal region of AtkY contains the heavy metal binding motif CXC(X4)CXC located between the amino acid residues 133 and 142, as in CopY (30). In the nucleotide sequence, 50 nucleotides upstream from the ATG start codon of atkY, a TTGTAA(N13)TTACAA motif was observed. This motif is similar to part of the CopY operator, TTGTAA(N19)TTACAA, located 57 nucleotides upstream from the ATG of copY. As shown in E. hirae, this inverted repeat motif might be the operator DNA sequence with which AtkY interacts for its transcription repressor function. A single inverted repeat sequence was observed upstream from atkY, whereas two copies of such DNA binding sites were found by footprinting experiments in the copYZAB operon of E. hirae (42).
FIG. 2.
Alignment of the deduced amino acid sequence of AtkY with CopY and β-lactamase repressor proteins. Eh CopY, copper resistance regulatory protein CopY of E. hirae (30); Sa BlaI, BlaI of S. aureus (36); Se MecI, methicillin resistance regulatory protein MecI of S. epidermidis (43); Bl PenI, PenI of B. licheniformis (16). Identical amino acids in AtkY and the other proteins are shaded. Two deduced regions with high identity are boxed.
The known proteins having the highest identity score with AtkB are the CopB ATPase of E. hirae (56.2% identity) and the E. coli histidine-rich heavy metal-associated P-type ATPases Hra1 and Hra2 (42.7 and 41.7% identities, respectively) (44). Hra1 and Hra2 are two putative copper transporters like CopB. Homology was also found with other P-type ATPases involved in the cadmium transport: AtkB is 27.9% identical to CadA of L. lactis (GenBank accession no. U78967) and 25.3% identical to CadA of S. aureus (26). All of the characteristic patterns of the known P-type ATPases were also observed in AtkB. AtkB has an N-terminal sequence resembling the N-terminal sequences of CopB from E. hirae, Hra1 and Hra2 from E. coli, and CopA and CopC from the copper resistance operon of Pseudomonas syringae (23). The N-terminal part of these proteins has a region which contains several M, H, and D residues implicated in the binding of copper atoms in CopA and CopC of P. syringae (5). The characteristic TGE motif, which is supposed to be included in the phosphatase region, was also observed in AtkB (positions 225 to 227). A CPH motif was also found in AtkB (positions 328 to 330). Among the known P-type ATPases, only CopB, Hra1, and Hra2 contain a CPH instead of the CPC motif usually found in other P-type ATPases and which might be part of the cation channel. Another feature of P-type ATPases is the DKTGTLT motif, with the D residue being phosphorylated and dephosphorylated during transport (1). This motif is conserved in AtkB (positions 372 to 378). The GDGVND motif, which is believed to be part of the nucleotide-binding domain (4), was also observed in AtkB from positions 569 to 574. The putative transmembrane helices of AtkB were investigated by the TM-pred of BCM launcher and the SAPS program. The compilation of the two methods predicted eight transmembrane helices for AtkB.
Construction and analysis of atkB mutants.
The sequence of atkB suggests that it encodes a P-type ATPase. Since the identity of AtkB is higher to the CopB of E. hirae, responsible for copper efflux, than to the CopA responsible for copper uptake in E. hirae, one could expect that AtkB encodes a protein also responsible for copper efflux. Mutation of such efflux P-type ATPases might lead to a higher sensitivity to the transported ion salt, which should be accumulated in the mutant, as has been described in E. hirae, Synechococcus sp., and Helicobacter pylori (11, 20, 31). Two atkB mutants were constructed by insertional mutagenesis in L. sakei 23K. For this purpose, two plasmids were used. First, a clone derived from pRV5 was obtained by exonuclease III deletion. It contained an internal part of atkB missing its 5′ and 3′ ends. The erythromycin resistance gene of pAMβ1 was then cloned at the SspI site of the pBluescript moiety, leading to pRV27 (Fig. 1A). Second, the plasmid pRV215 was constructed, which contained an internal fragment of atkB cloned in pRV300. These plasmids were used to transform the strain 23K for erythromycin resistance. Transformants obtained by single crossover integration of pRV27 or pRV215 were grown in MCD medium containing increasing concentrations of various heavy metal ions [CuSO4, 0.1 to 1.2 mM; Cd(CH3COO)2, 1 to 7 μM; CoCl2, 0.125 to 1.5 mM] or NaCl (3 to 5%). The wild-type strain was grown under the same conditions, and the OD was measured after 16 h of growth. No significant difference was observed between the parent strain and the two atkB mutants (data not shown). The copper sensitivity of the mutants was also tested either by streaking or by deposing a drop of a fresh culture on MCD plates containing 10 to 500 μM CuSO4. No difference was observed between the parent strain and the two atkB mutants. Exponential growth was also followed on cultures in MCD medium containing CuSO4, Cd(CH3COO)2, CoCl2, or NaCl. Concentrations affecting the growth of the wild-type strain were chosen, but no significant differences could be observed between the three strains 23K (data not shown). Thus, although sequence similarities suggested that AtkB was a copper P-type ATPase, the analysis of two atkB mutants could not confirm the function of AtkB. In order to obtain more information on the putative role of AtkB, we investigated its regulation. For that purpose, an approach using a reporter gene was chosen.
Construction of a lacLM mutant by double crossover for use as recipient strain of lacZ fusions.
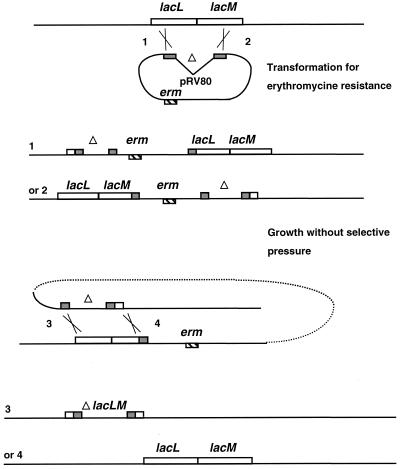
No reporter gene is yet available for L. sakei. The plasmid described by Platteeuw et al. (33) carrying the β-glucuronidase encoding gene (gusA) designed for lactic acid bacteria was used to construct an atkYB::gusA fusion. However, as was previously mentioned by these authors for other lactobacilli, gusA was not expressed in L. sakei. Although the fusion was expressed in E. coli, no activity could be detected in L. sakei. This prompted us to try other genes. Among several genes usually used as reporter genes, the lacZ gene of E. coli has several advantages since the β-galactosidase activity can easily be detected both on plates and in bacterial extracts. Some L. sakei strains possess a β-galactosidase or a phospho-β-galactosidase activity (27). The strain 23K, used as the recipient strain, has a β-galactosidase. In order to use lacZ as a reporter gene, it was therefore necessary to first delete the lacLM operon of L. sakei 23K encoding the L. sakei β-galactosidase. The lacLM operon of L. sakei DSM 20017 was previously cloned and sequenced (28). Oligonucleotides deduced from this sequence were designed in order to amplify two fragments corresponding to the 5′ end and the 3′ end of lacLM. A first PCR fragment, obtained with the lac1 and lac2 primers, was 513 bp long and contained the lacLM promoter and the 5′ end of lacL. The second fragment, obtained with the lac4 and lac6 primers, was 522 bp long and contained the 3′ end of lacM and the downstream region. A restriction site was present at the 5′ extremity of each primer. The two resulting PCR fragments were cloned in the pRV300 integrative vector. In the resulting plasmid pRV80, the lacLM operon contains a 2,193-bp internal deletion. pRV80 was then used to transform L. sakei 23K for erythromycin resistance (Fig. 3). The correct insertion of pRV80, by a Campbell-like recombination, at the lacLM locus was checked by a PCR experiment using the lac1 and lac4 primers. One transformant, RV2001, was then kept for further experiments. Since pRV80 contains the 5′ end and the 3′ end of the lacLM operon, its insertion in the chromosome by a single crossover in lacLM restored one copy of the wild-type operon and was therefore not mutagenic. Additionally, the insertion of pRV80 led to the duplication of part of the lacLM operon, a structure which is unstable unless selective pressure is maintained by the addition of erythromycin. In the absence of selective pressure, the plasmid can excise, by a second crossover, and is then lost since pRV300 cannot replicate in L. sakei. Since one copy of the wild-type lacLM operon and one copy of the mutated lacLM operon are present after the first recombination, the excision of the plasmid could lead to the excision either of the wild-type lacLM copy or of the ΔlacLM copy. The excision of the mutated copy would restore a wild-type genotype, whereas the excision of the wild-type copy would lead to a copy of the lacLM operon with the internal deletion (Fig. 3). In order to generate the second crossover, the RV2001 transformant was grown in MRS without erythromycin. After 100 generations, diluted culture aliquots were plated on MRS containing X-Gal and no erythromycin. Of 300 clones, two were white. The structure of the lacLM operon was verified by PCR on the chromosomal DNA extracted from these two clones, with primers complementary to various parts of lacLM. The expected 2.2-kb deletion of the internal part of lacLM was demonstrated. PCR experiments performed with combinations of primers complementary to lacLM and reverse or universal primers complementary to pRV300 were negative. Moreover, these two clones were erythromycin sensitive and had thus lost the pRV300 moiety. This confirmed that the two strains were resulting from the expected recombination. One of the two clones was named RV2002 and was used for further constructions.
FIG. 3.
Construction of an internal deletion in the lacLM operon. Plasmid pRV80 contains the 5′ end of lacL and the 3′ end of lacM, with a deletion of an internal part of lacLM. pRV80 was introduced in the chromosome of L. sakei. Transformants, selected on erythromycin, can result from a crossover at position 1 or position 2. In each case, one copy of the WT lacLM operon and one copy of the ΔlacLM operon are present. The duplication of part of the lacLM operon is unstable. By growing one transformant without erythromycin, strains resulting from a second crossover can be isolated. The plasmid can excise at position 3, leading to replacement of lacLM by ΔlacLM, or at position 4, restoring a wild-type genotype.
Construction of an atkY::lacZ transcriptional fusion.
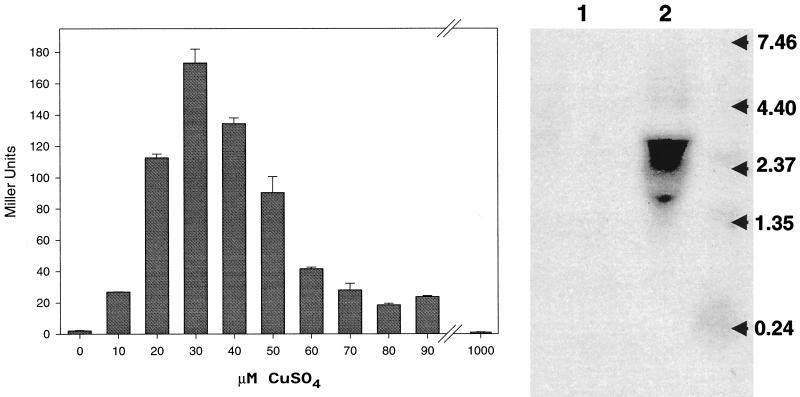
Plasmid pJM783 contains the lacZ gene of E. coli and a chloramphenicol resistance gene (32). This plasmid has already been largely used to construct transcriptional or translational fusions in Bacillus subtilis. A 634-bp DNA fragment, containing the transcription terminator of ptsI, the promoter region of atkYB, and the repressor encoding gene atkY, was amplified by PCR with primers ATK1 and ATK3 and cloned in pJM783, in front of lacZ. Then, the lacZ gene, fused to the atkYB promoter region described above, was cloned in the integrative plasmid pRV300. The resulting plasmid, pRV83 (Fig. 1A) was used to transform RV2002, which is deficient for β-galactosidase activity. The resulting strain, RV1023, contains an insertion of the atkYB::lacZ fusion and an intact copy of the atkYB operon. The β-galactosidase activity of RV1023 was tested on MCD plates containing X-Gal. Fifty microliters of various solutions of metals or salts ions was added in the center of the plates and allowed to diffuse from the center to the periphery of the plates. Only copper induced the expression of the atkYB::lacZ fusion, as seen from the blue color of the clones. However, the color was observed only at a certain distance from the center of the plates, suggesting that the fusion was induced by a precise concentration of CuSO4. Furthermore, the activity was higher when the plates were incubated at room temperature than at 30°C. RV1023 was grown in liquid MCD medium to an OD600 of 0.3, and then various concentrations of CuSO4 were added and the cultures were incubated for 90 min at room temperature. The β-galactosidase activities measured on crude extracts obtained from these cultures are shown in Fig. 4 (left panel). As expected from the observation made on the plates, an optimal concentration of CuSO4 which induced expression of the atkYB::lacZ fusion was found (30 to 40 μM), which is much lower than the concentration required to inhibit the growth of L. sakei.
FIG. 4.
Expression of the atkYB operon. (Left) Induction of β-galactosidase activity of the atkYB::lacZ fusion in RV1023 by increasing CuSO4 concentration in the medium. (Right) Northern blot of RNA isolated from 23K induced with 40 μM CuSO4 (lane 2) or noninduced (lane 1) and hybridized with atkB. The size of the molecular weight ladder is indicated.
In order to confirm copper induction of the atkB gene, Northern blot experiments were performed with RNAs isolated from bacteria grown under the same conditions as described for the measurement of β-galactosidase activity. The probe used was the internal part of atkB in pRV214. A 2.7-kb mRNA was detected in the 23K strain induced by 40 μM CuSO4, which was absent in the noninduced bacteria (Fig. 4, right panel). This size correlates with a transcript initiated at the putative promoter located upstream from atkY and ending at the palindromic sequence, downstream from atkB.
Conclusion.
The two genes found downstream from the ptsHI operon of L. sakei are homologous to a copper P-type ATPase responsible for copper efflux and its negative regulator. The construction of mutants in the gene encoding the putative copper ATPase did not lead to clear identification of its function, since no sensitivity to copper nor to other ions could be observed. It is possible that a second gene, coding for a protein able to export copper, is present on the L. sakei chromosome. The use of lacZ as a reporter gene and Northern blot experiments showed that the atkYB operon is induced when small concentrations of CuSO4 were added to the medium. Although the function of AtkB could not be demonstrated, this study suggests that it is indeed a copper ATPase. Among other known copper P-type ATPases, CopA and CopB from E. hirae have been extensively studied, and it was shown that these two ATPases, which are part of the same operon, are induced by high concentrations of copper (1 mM) or when copper is depleted from the medium (29). In E. hirae, copZ and copY, encoding a positive and a negative regulator, respectively, are also present in the operon. In L. sakei, only genes similar to copY and copB are present in the operon. Whether copZ and copA are present somewhere else on the chromosome is not yet known. The gene organization in L. sakei, which is different from what was described for E. hirae, might result in a different regulation. Finally, since AtkB was not expressed at a high (1 mM) copper concentration, this suggests that it is not required at this concentration and might explain why no hypersensitivity to a high concentration of copper was observed in the atkB mutants.
The strain RV2002 was obtained by two successive crossovers. The construction of such a stable deletion mutation represents a new method for the construction of mutants in L. sakei. The use of pRV300 to generate the first crossover, followed by growth without selective pressure to generate the second, mutagenic crossover will allow the construction of a new generation of L. sakei mutants with the possibility of generating point mutations, large deletions, or stable insertions. The resulting mutants, containing point mutations or deletions, will not contain any trace of heterologous DNA and might thus be considered as food-grade strains. Plasmid pVR80, derived from pRV300, was used to generate a deletion in lacLM. Plasmids derived from pRV80 containing foreign genes should allow gene replacement at the lacLM locus by using X-Gal as an indicator to select recombinant clones. The strain RV2002 (ΔlacLM) was then used as a recipient strain to introduce a atkYB::lacZ fusion that allowed investigation of the atkYB regulation. This study shows that lacZ can be used as reporter gene in L. sakei. Since genetic tools based on pRV300 designed for L. sakei have been successfully used in other lactic acid bacteria, such as L. casei and L. lactis (7, 9, 35), new pRV300 derived vectors containing lacZ will be helpful in studying gene regulation in these species. Furthermore, the PCR-amplified fragment containing the transcription terminator of ptsI, the copper-inducible promoter of atkYB, atkY, and a few codons of atkB, might be used to expressed foreign genes in L. sakei, with an expression controlled by the addition of small amounts of CuSO4.
REFERENCES
- 1.Addison R, Scarborough G A. Conformational changes of the Neurospora plasma membrane H+ ATPase during its catalytic cycle. J Biol Chem. 1982;257:10421–10426. [PubMed] [Google Scholar]
- 2.Axelsson L, Katla T, Bjornslett M, Eijsink V G H, Holck A. A system for heterologous expression of bacteriocins in Lactobacillus sake. FEMS Microbiol Lett. 1998;168:137–143. doi: 10.1111/j.1574-6968.1998.tb13266.x. [DOI] [PubMed] [Google Scholar]
- 3.Berthier F, Zagorec M, Champomier-Vergès M, Ehrlich S D, Morel-Deville F. Efficient transformation of Lactobacillus sake by electroporation. Microbiology. 1996;142:1273–1279. doi: 10.1099/13500872-142-5-1273. [DOI] [PubMed] [Google Scholar]
- 4.Brandl C J, Green N M, Korcczak B, Mac Lennan D H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986;44:597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- 5.Cha J-S, Cooksey D A. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc Natl Acad Sci USA. 1991;88:8915–8919. doi: 10.1073/pnas.88.20.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Man J C, Rogosa M, Sharpe M E. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 7.Dossonnet V, Monedero V, Zagorec M, Galinier A, Pérez-Martinez G, Deutscher J. Phosphorylation of HPr by the bifunctional HPr kinase/P-Ser-HPr phosphatase from Lactobacillus casei controls catabolite repression and inducer exclusion, but not inducer expulsion. J Bacteriol. 2000;182:2582–2590. doi: 10.1128/jb.182.9.2582-2590.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dower W J, Miller J F, Ragsdale C W. High-efficiency transformation of Escherichia coli by high-voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duwat P, Ehrlich S D, Gruss A. Effects of metabolic flux on stress response pathways in Lactococcus lactis. Mol Microbiol. 1999;31:845–858. doi: 10.1046/j.1365-2958.1999.01222.x. [DOI] [PubMed] [Google Scholar]
- 10.Eaton T J, Shearman C, Gasson M J. The use of bacterial luciferase genes as reporter genes in Lactococcus: regulation of the Lactococcus lactis subsp. lactis lactose genes. J Gen Microbiol. 1993;139:1495–1501. doi: 10.1099/00221287-139-7-1495. [DOI] [PubMed] [Google Scholar]
- 11.Ge Z, Hiratsuka K, Taylor D E. Nucleotide sequence and mutational analysis indicate that two Helicobacter pylori genes encode a P-type ATPase and a cation-binding protein associated with copper transport. Mol Microbiol. 1995;15:97–106. doi: 10.1111/j.1365-2958.1995.tb02224.x. [DOI] [PubMed] [Google Scholar]
- 12.Geoffroy M-C, Guyard C, Quatannens B, Pavan S, Lange M, Mercenier A. Use of green fluorescent protein to tag lactic acid bacterium strains under development as live vaccine vectors. Appl Environ Microbiol. 2000;66:383–391. doi: 10.1128/aem.66.1.383-391.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzy-Tréboul G, Karmzyn-Campelli C, Stragier P. Developmental regulation of transcription of the Bacillus subtilis ftsAZ operon. J Mol Biol. 1992;224:967–979. doi: 10.1016/0022-2836(92)90463-t. [DOI] [PubMed] [Google Scholar]
- 14.Hammes W P, Hertel C. New developments in meat starter cultures. Meat Sci. 1998;49:125–138. [PubMed] [Google Scholar]
- 15.Hensel R, Mayr U, Stetter K O, Kandler O. Comparative studies of lactic acid dehydrogenase in lactic acid bacteria. Arch Microbiol. 1977;112:81–93. doi: 10.1007/BF00446658. [DOI] [PubMed] [Google Scholar]
- 16.Himeno T, Imanaka T, Aiba S. Nucleotide sequence of the penicillase repressor gene penI of Bacillus licheniformis and regulation of penP and penI by the repressor. J Bacteriol. 1986;168:1128–1132. doi: 10.1128/jb.168.3.1128-1132.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jefferson R A, Burgess S M, Hirsh D. β-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci USA. 1986;83:8447–8451. doi: 10.1073/pnas.83.22.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langella P, Zagorec M, Ehrlich S D, Morel-Deville F. Intergeneric and intrageneric conjugal transfer of plasmids pAMb1, pIL205, and pIP501 in Lactobacillus sake. FEMS Microbiol Lett. 1996;139:51–56. [Google Scholar]
- 19.Lauret R, Morel-Deville F, Berthier F, Champomier-Vergès M, Postma P W, Ehrlich S D, Zagorec M. Carbohydrate utilization in Lactobacillus sake. Appl Environ Microbiol. 1996;62:1922–1927. doi: 10.1128/aem.62.6.1922-1927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanamaru K, Kashiwagi S, Mizuno T. The cyanobacterium Synechococcus sp. PCC7942, possesses two distinct genes encoding cation-transporting P-type ATPases. FEBS Lett. 1993;330:99–104. doi: 10.1016/0014-5793(93)80928-n. [DOI] [PubMed] [Google Scholar]
- 21.Le Loir Y, Gruss A, Ehrlich S D, Langella P. Direct screening of recombinants in gram-positive bacteria using the secreted staphylococcal nuclease as a reporter. J Bacteriol. 1994;176:5135–5139. doi: 10.1128/jb.176.16.5135-5139.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leloup L, Ehrlich S D, Zagorec M, Morel-Deville F. Single-crossover integration in the Lactobacillus sake chromosome and insertional inactivation of the ptsI and lacL genes. Appl Environ Microbiol. 1997;63:2117–2123. doi: 10.1128/aem.63.6.2117-2123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellano M, Cooksey D A. Nucleotide sequence and organization of copper resistance genes from Pseudomonas syringae pv. tomato. J Bacteriol. 1988;170:2879–2883. doi: 10.1128/jb.170.6.2879-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller J. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 25.Montel M C, Seronie M P, Talon R, Hébraud M. Purification and characterization of a dipeptidase from Lactobacillus sake. Appl Environ Microbiol. 1995;61:837–839. doi: 10.1128/aem.61.2.837-839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nucifora G, Chu L, Mirsa T K, Silver S. Cadmium resistance from Staphylococcus aureus plasmid pI258 cadA gene results from a cadmium-efflux ATPase. Proc Natl Acad Sci USA. 1989;86:3544–3548. doi: 10.1073/pnas.86.10.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obst M, Hehn R, Vogel R F, Hammes W P. Lactose metabolism in Lactobacillus curvatus and Lactobacillus sake. FEMS Microbiol Lett. 1992;97:209–214. [Google Scholar]
- 28.Obst M, Meding E R, Vogel R F, Hammes W P. Two genes encoding the β-galactosidase of Lactobacillus sake. Microbiology. 1995;141:3059–3066. doi: 10.1099/13500872-141-12-3059. [DOI] [PubMed] [Google Scholar]
- 29.Odermatt A, Krapf R, Solioz M. Induction of the putative copper ATPases, CopA and CopB, of Enterococcus hirae by Ag+ and Cu2+, and Ag+ extrusion by CopB. Biochem Biophys Res Commun. 1994;202:44–48. doi: 10.1006/bbrc.1994.1891. [DOI] [PubMed] [Google Scholar]
- 30.Odermatt M, Solioz M. Two trans-acting metalloregulatory proteins controlling expression of the copper-ATPases of Enterococcus hirae. J Biol Chem. 1995;270:4349–4354. doi: 10.1074/jbc.270.9.4349. [DOI] [PubMed] [Google Scholar]
- 31.Odermatt A, Suter H, Krapf R, Solioz M. Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J Biol Chem. 1993;17:12775–12779. [PubMed] [Google Scholar]
- 32.Perego M, Spiegelman G B, Hoch J A. Structure of the gene of the transition state regulator abrB: regulator synthesis is controlled by the spoOA sporulation gene in Bacillus subtilis. Mol Microbiol. 1988;2:689–699. doi: 10.1111/j.1365-2958.1988.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 33.Platteeuw C, Simons G, de Vos W M. Use of the Escherichia coli β-glucoronidase (gusA) gene as a reporter gene for analyzing promoters in lactic acid bacteria. Appl Environ Microbiol. 1994;60:587–593. doi: 10.1128/aem.60.2.587-593.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raccach M. Manganese and lactic acid bacteria. J Food Prot. 1985;48:595–598. doi: 10.4315/0362-028X-48.10.895. [DOI] [PubMed] [Google Scholar]
- 35.Rallu F, Gruss A, Ehrlich S D, Maguin E. Acid- and multi-stress-resistant mutants of Lactococcus lactis: identification of intracellular stress signals. Mol Microbiol. 2000;35:517–528. doi: 10.1046/j.1365-2958.2000.01711.x. [DOI] [PubMed] [Google Scholar]
- 36.Rowland S J, Dyke K G. Tn552, a novel transposable element from Staphylococcus aureus. Mol Microbiol. 1990;4:961–975. doi: 10.1111/j.1365-2958.1990.tb00669.x. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 38.Scott K P, Mercer D K, Richardson A J, Melville C M, Glover L A, Flint H J. Chromosomal integration of the green fluorescent protein gene in lactic acid bacteria and the survival of marked strains in human gut simulations. FEMS Microbiol Lett. 2000;182:23–27. doi: 10.1111/j.1574-6968.2000.tb08867.x. [DOI] [PubMed] [Google Scholar]
- 39.Silver S, Ji G, Broer S, Dey S, Dou D, Rosen B P. Orphan enzyme or patriarch of a new tribe: the arsenic resistance ATPase of bacterial plasmids. Mol Microbiol. 1993;8:637–642. doi: 10.1111/j.1365-2958.1993.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 40.Silver S. Bacterial resistances to toxic metal ions—a review. Gene. 1996;179:9–19. doi: 10.1016/s0378-1119(96)00323-x. [DOI] [PubMed] [Google Scholar]
- 41.Stentz R, Lauret R, Ehrlich S D, Morel-Deville F, Zagorec M. Molecular cloning and analysis of the ptsHI operon in Lactobacillus sake. Appl Environ Microbiol. 1997;63:2111–2116. doi: 10.1128/aem.63.6.2111-2116.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strausak D, Solioz M. CopY is a copper-inducible repressor of the Enterococcus hirae copper ATPases. J Biol Chem. 1997;272:8932–8936. doi: 10.1074/jbc.272.14.8932. [DOI] [PubMed] [Google Scholar]
- 43.Traub W H. Simple screening method for gram-positive bacterial beta-lactam antibiotic tolerance on routine laboratory Bauer-Kirby antibiogram plates. Chemotherapy. 1982;28:110–118. doi: 10.1159/000238065. [DOI] [PubMed] [Google Scholar]
- 44.Trenor C, III, Lin W, Andrews N C. Novel bacteria P-type ATPases with histidine-rich heavy metal-associated sequences. Biochem Biophys Res Commun. 1994;205:1644–1650. doi: 10.1006/bbrc.1994.2856. [DOI] [PubMed] [Google Scholar]