Abstract
Immunotherapy has redefined the treatment of cancer patients and it is constantly generating new advances and approaches. Among the multiple options of immunotherapy, bispecific antibodies (bsAbs) represent a novel thoughtful approach. These drugs integrate the action of the immune system in a strategy to redirect the activation of innate and adaptive immunity toward specific antigens and specific tumor locations. Here we discussed some basic aspects of the design and function of bsAbs, their main challenges and the state-of-the-art of these molecules in the treatment of hematological and solid malignancies and future perspectives.
Keywords: bispecific antibodies, immunotherapy, immune restoration, cancer therapy
1. Introduction
Regardless of efforts from the scientific community, options to treat cancer patients in advanced stages achieving complete response with low recurrence are limited. For that reason, the search of effective alternatives to treat cancer has increase in the last years. Currently, some alternatives that are effective in the treatment of other conditions are now being studied as an alternative to treat cancer patients. Monoclonal antibodies are known to have a positive impact on many conditions such as autoimmune disorders, cardiovascular, pulmonary, and even infectious diseases [1]. Even though monoclonal antibodies are usually specific to one epitope, genetic and cell engineering have allowed the biosynthesis of bispecific antibodies (bsAbs). BsAbs were first described by Nisonoff et al. over 60 years ago; however, they gained clinical relevance after the first approval by the Food and Drugs Administration (FDA) [2] of blinatumomab, a bsAb approved for the treatment of acute myeloid leukemia. Since then, these molecules have become an attractive choice to treat cancer, due to their efficacy and safety profile (Figure 1) [3]. The original concept of bsAbs was a molecule that can bind to two different epitopes [2].
Figure 1.
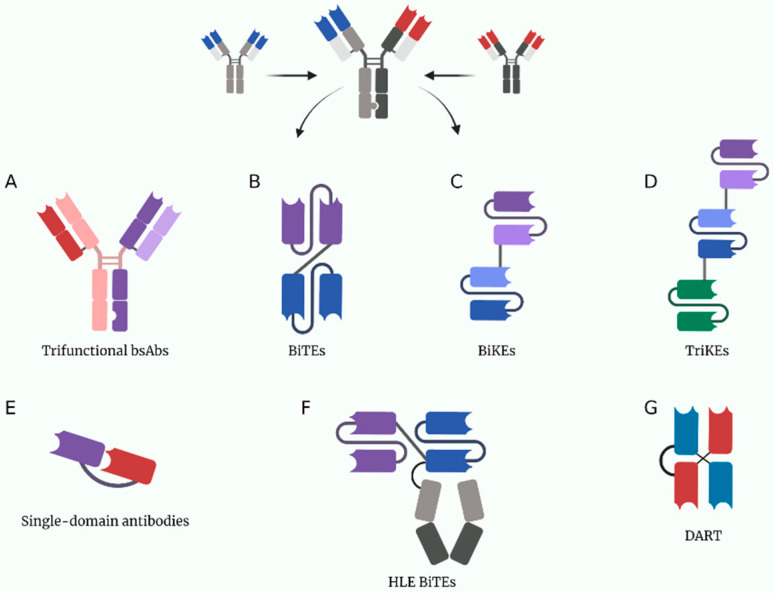
A depiction of some current multivalent antibody structures under study. (A) Trifuctional antibodies conserved their Fc domain to be able to bind to cells expressing Fc receptors. (B) BiTEs (bispecific T-cell engagers). (C) BiKEs (bispecific NK-cell engagers). (D) TriKEs (trispecific NK-cell engagers). (E) Single-domain antibodies only have one variable chain per target, they are usually made from heavy chain nanobodies derived from the structure of heavy-chain only camelid antibodies. (F) HLE BiTEs (half-life extended bispecific T-cell engagers) are BiTEs with an Fc portion that increases its half-life. (G) DARTs (dual affinity retargeting antibodies). Created with BioRender.com.
The first application of bsAbs in cancer immunotherapy was focused on leading T cells toward tumor cells by the interaction between the extracellular subunit of CD3 on T cells and cancer-related antigens. The bsAbs ease the interaction of the major histocompatibility complex (MHC) with its cognate T-cell receptor (TCR) resulting in a proper T-cell priming and activation. Despite this, some adverse effects of these drugs such as cytokine release syndrome or liver toxicity and other limitations such as a short half-life have been reported. For that matter a vast quantity of clinical trials with these molecules is being conducted [4].
Nevertheless, bsAbs still represent a novel and effective approach to treat cancer patients because they target molecules expressed on the surface of cancer cells (tumor-associated antigens [TAAs]) and bind to specific receptors that are located on effector cells of the immune system (Figure 2) [5,6]. Furthermore, there have been other smart approaches for the use of bsAbs. Fournier et al. used the Newcastle Disease Virus to specifically infect cancer cells and make them express viral antigens such as hemagglutinin-neuraminidase and fusion molecules. By expressing these viral antigens, bsAbs can be engineered to engage immune effector cells to cancer cells, decreasing the risk of on-target/off-tumor toxicity seen by targeting TAAs that are also expressed in healthy cells such as EGFR or VEGFR [7].
Figure 2.
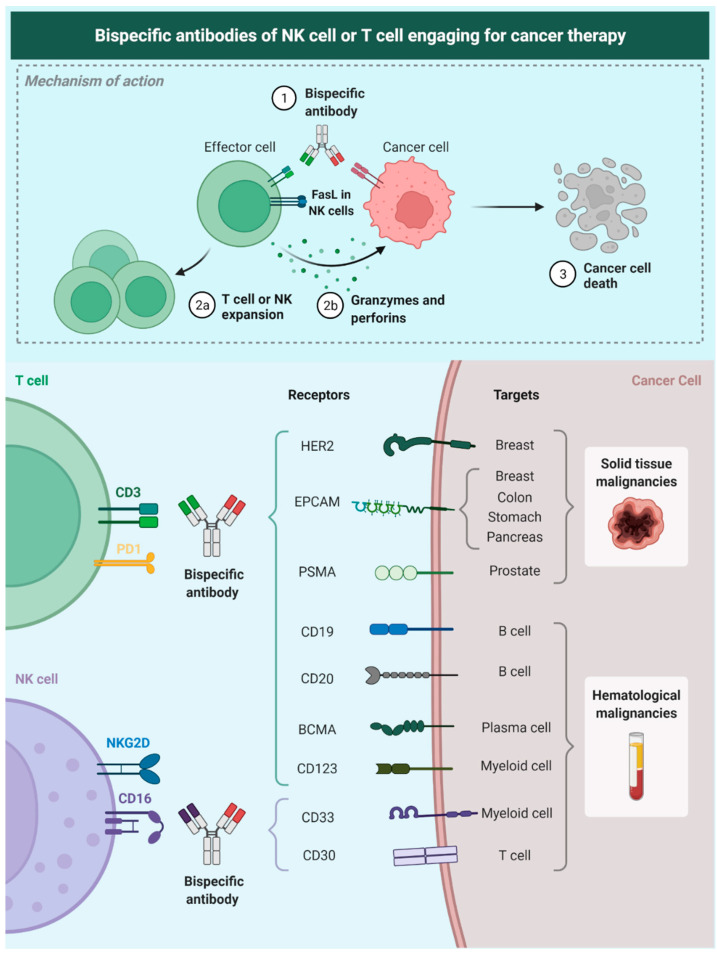
Description of the mechanism of NK-Bias; these antibodies target a tumor-related antigen and bind membrane receptors on NK cells allowing a spatial and molecular immune-mediated cell-killing process. We also show some of the tumor-associated targets that currently have been studied for therapy. Epithelial cell adhesion molecule (EpCAM), epidermal growth factor receptor 2 (HER2), prostate specific membrane antigen (PSMA), B-cell maturation antigen (BCMA), CD19, CD20, CD123, CD33, CD30 (cluster of differentiation [CD]).
Currently, an important number of bsAbs are being studied in many clinical trials, showing positive results in a specific group of tumoral cells and a prolonged antitumoral response. Particularly, some malignancies such as lymphomas seem to have a better antitumoral response with bsAbs, in comparison with myeloid neoplasias or solid tumors [8]. For solid tumors, an optimal antibody impregnation to the tumor has been reported; however, a short half-life and concerns about their safety are still subjects of study [9].
Despite the breakthrough that these bsAbs are represented in the field of cancer immunotherapy, there are still many questions to be answered and challenges to be solved about the safety, efficacy, and range of possible treatable tumors. Here, we review some basic aspects of the bsAbs and their functions, along with their common use in the current clinical practice.
2. Pharmacology of Bispecific Antibodies
Antibodies are molecules made of different structural and functional parts. These parts are combined to create molecules with unique affinity, specificity, and interaction properties. The special structure-function of these macromolecules is beyond the scope of this review; however, understanding some basic principles is essential to comprehend the pharmacology behind bsAbs [10].
From a structural point of view, a bsAbs is made of two identical light chains (LCs) and two identical heavy chains (HCs). Each domain, between the LC and HC has disulfide bonds. The structural conformation creates three zones: two antigen-binding fragments (Fab) and one crystallizable fragment or Fc. Both Fab regions bind to molecular targets, the same epitope. On the other hand, the Fc region attaches to receptors such as Fcγ receptors (FcγRs), C1q, and neonatal Fc receptor (FcRn), mediating its effector functions [10,11].
Previously, it was mentioned that although bsAbs contain two Fab regions, these only bind to the same epitope in an antigen, defining monospecificity. As the name suggests, bsAbs are bispecific, because they bind to two antigens. Structurally, there are two principal types of bsAbs: (1) single-chain variable fragment (scFv) antibodies and (2) full-length IgG-based antibody. In the past time, three techniques were used for their creation bispecific T-cell engager (BiTE), dual-affinity retargeting proteins (DARTs) and tandem diabodies (TandAbs) [12]. Currently, they are created by orthogonal Fab interface, DuoBody, XmAb, CrossMab, and knobs-into-holes (KiH) [3].
Mechanisms of action are diverse. First, the process of binding immune cells with tumor cells, leads to suppression of malignant cells’ ability to escape the immune response. Second, bsAbs decrease the expression of certain molecules and the release of immune suppression mediators. Additionally, bsAbs also block targets such as PD1, CTLA-4, LAG-3, IL-23, TNF-a, and others and they also stimulate immune cells, those mechanisms act synergistically [13,14].
From a pharmacokinetic (PK) view the properties of bsAbs oscillate due to their different compositions. The Fc region plays a key role in bsAbs PK. It is reported that bioavailability is lower by oral administration, so other ways of administration are better choices.
With respect of the distribution, three parameters affect this variable: extravasation, distribution within a tissue, and elimination/clearance. Extravasation occurs when macromolecules have a high volume of distribution (Vd) and tend to remain in the tissues. Additionally, it is known that intracellular catabolism and renal clearance, both influenced by Fc region, increase the molecular weight of some molecules leading to slow clearance. After that, those molecules bind to FcRn, escape acidic endosomes, and return to the circulation or to the interstitial space. In the case of specific elimination, bsAbs bind to specific antigens on cell surfaces mediating its clearance in a process named target-mediated drug disposition (TMDD). In an attempt to integrate some of the previous concepts, scFvs vs. full-length IgG-based bsAbs have different molecular weights; this distinction determines the route of clearance and the time in circulation and route of administration [15,16].
BsAbs pharmacology is complicated, many situations and variables affect its pharmacodynamics and pharmacokinetics. Understanding the design, development, and properties of these molecules is fundamental for the development of new molecules [14].
3. T-Cell Engaging Bispecific Antibodies
Among all the antibody-centered cancer therapy, T-cell engaging bispecific antibodies (T-biAbs) have a promising role in future cancer therapeutics. These antibodies use the main principle of two different binding arms of the bispecific antibodies. One of the binding sites recognizes the invariable CD3 subunits of cytotoxic T lymphocytes (CTLs) and the other one recognizes certain tumor antigens. Therefore, the T-biAb can activate CTLs bypassing the MHC pathway and redirecting this activation to attack specific cancer cells [17]. Currently, there are two FDA-approved T-biAbs blinatumomab (for the management of acute lymphoblastic leukemia) [18,19].
4. T-biAbs Action on Immune Effector Cells
In 1989 Gross and Eshhar et al. were the first ones to approach the concept of T-cell reactivation against tumor cells. At that time, they combined monoclonal antibodies and T-cell receptors to promote immune activation [20]. However, this early model had a lack of costimulatory signals, so T-cell redirection against tumor cells was not possible. Currently, a lot of options of T-bsAbs with costimulatory properties are available and are approved to treat cancer patients [5].
For a better understanding of the T-biAbs mechanism of action on immune effector cells, it is important to have in mind the usual adaptive immune response. T-cell activation depends on the linkage between the T-cell receptor (TCR)/CD3 complex and the major histocompatibility complex (MHC)/peptide complex and on the interaction of co-stimulatory receptors and their ligands [21]. In contrast, because of the strong affinity between T-BsAbs and TAA/CD3, a significant quantity of activation receptors (TCR/CD3 complexes) accumulate between cells, leading to an efficient T-cell activation with the need of only one receptor–ligand interaction [22,23]. However, some studies showed that those T cells activated by T-BsAbs are less effective over time because they experience more rapid exhaustion [24]. It is also reported that T-bsAbs activate memory T cells, including central memory and effector memory phenotypes, instead of naïve T cells that do not induce tumor lysis. Those memory T cells have highly cytotoxic activity on CD8-positive peripheral T cells because of the high gene expression associated with CD8+ T-cell function, resulting in a major anti-tumor activity [25].
Furthermore, some studies reported that T-bsAb increased T-cell proliferation in the tumor and recruitment from the periphery to tumor tissues, leading to a significant amount of tumor-infiltrating T cells after treatment [26,27,28]. It is known that CXCR3 receptor–ligand interaction regulates T-cell migration. Some studies have reported that T-bsAb treatment induces expression of pro-inflammatory cytokines and chemokines such as CXCR3 ligands in tumors and upregulation of CXCR3 expression on T cells, leading to a major T-cell migration [29].
It is important to highlight that our understanding of the exact mechanism of the activity of effector T cells engaged by T-bsAbs is still insufficient. For example, it is not fully understood if the presence of preexisting T cells inside the tumor is necessary for peripheral T-cells recruitment by T-bsAbs [30].
5. T-biAbs for Redirecting Cells of the Innate Immune System
As previously mentioned, bispecific antibodies are an intelligent strategy to put close IECs and their intended target cell. Antitumor effects can be provided directly or indirectly by innate or adaptive immune cells. Immune effector cells comprise an arsenal of supportive and cytotoxic cells that can arrest a threat from its beginning, clearing out infections or cells with early malignant properties, or at least slowing down the process while the adaptive immune system is appropriately recruited [31].
Effector mechanisms of the innate immune system can be enhanced by using biAbs [32]. Most data regarding biAbs in the immune system approach dendritic cells (DCs) or natural killer (NK) cells. DCs are professional antigen-presenting cells (APCs), with the highest efficacy in T-cell priming (initial activation of naïve T cells with its cognate MHC–epitope complex). For proper T-cell activation, TCR binding with MHC-Epitope complex is necessary; however, co-stimulatory signals also need to be present, namely the CD28/CD80-86 binding [33]. Primed DCs express high levels of CD80/86. BiAbs can be used to maximize the likelihood of encounter of DCs and T-cells when preserving the antibody Fc region.
In vitro experiments have shown that trifunctional biAbs were effective in inducing specific cytotoxicity of melanoma cells, with proper T-cell priming measured by the upregulation of CD69 and downregulation of CD62L (naïve T-cell marker). The rationale of trifunctional biAbs is to preserve the Fc region in the antibody structure [34]. When the Fc region is present, it can be used by innate immune cells to attach to the drug via FcγR and engage in the effector response. DCs will attach to the Fc region and get close to T-cells and their respective targets, inducing a more potent activation and cytotoxicity. However, these results have only been positive in vitro, where conditions can be easily controlled and isolated scenarios can be tested.
On the other hand, in the in vivo setting, biAbs with preserved Fc regions have shown to be counterproductive, as FcγR is also expressed by other innate immune system cells such as macrophages and NK cells, inducing overactivation of the immune system with further severe adverse events such as cytokine release syndrome (CRS) [35]. Moreover, by preserving Fc regions, the half-life of the antibody will be considerably decreased due to undesired attachment to other FcγR expressing cells. Silencing of Fc domains by mutating key peptides or using alternative scFv structures are current strategies used to bypass these effects [32,33,34,35].
NK cells have also been addressed in biAbs research. NKs are highly cytotoxic, less prone to exhaustion, and do not require binding to an MHC-Epitope complex. The standard effector mechanism exploited in monoclonal antibody therapy for cancer is antibody-dependent cellular cytotoxicity (ADCC). This exact mechanism has been shown to be critical in using biAbs, even in the absence of an Fc domain. NK cells are one of the most efficient IECs in surveying and executing tumor cell cytotoxicity. Engaging NK cells to malignant cells has been proven to be effective in reducing tumor size in animal models at a greater extent than regular mAb therapy [36]. Natural killer cell engagers (NKCEs), biAbs with a shortened and silenced Fc domain that engages NKp46 in NK cells to the desired target, are subjects of study. NKp46 is a vital membrane protein that triggers NK cytotoxicity in HLA class I-unprotected cells, a common phenomenon in cancer, where HLA class I is downregulated. The evidence suggests that treatment with these antibodies could increase the NK tumor infiltrating cells and generate a good tumor response in animal models [37].
Novel approaches with NK cells include engagers targeting CD16, especially the CD16A isoform (FcγRIIIA). Currently, most NK cell engagers are directed against antigens expressed in hematological malignancies (CD19, CD20, CD30, and CD33), a trend that is also seen in other immunotherapies such as CAR-T cells and CAR-NK cells [38,39,40,41]. An essential turn onto solid malignancies is necessary.
6. T-biAbs for the Restoration and Enhancement of Antitumor Immunity
Antitumor immunity is one the main mechanisms of cancer control under micro-homeostatic conditions. A wide array of cells, signaling peptides, and membrane-bound proteins are involved in the building machinery of surveillance for malignancy in healthy cells. CD8+ T cells and NK cells are the main orchestrators of this mechanism. Nevertheless, evolutionary events inside potentially malignant cells can induce transcriptomic regulatory mechanisms that might allow them to evade immune surveillance [42,43].
Some of these regulatory pathways include molecules such as CD80/86 and PD-L1, which respectively bind to CTLA-4 and PD1, two of the primary immune checkpoints. When attached to their receptors, these molecules can induce a switch off in T cells, making them dormant and further modifying the tumor microenvironment (TME), creating the perfect place for continued growth and progression. By restoring and enhancing these dormant cells, dramatic treatment responses can be achieved. These have been shown in the past decade with the introduction of multiple mAbs immune checkpoint inhibitors that target PD1, PD-L1, and CTLA-4 [42]. Important advancements in overall and progression-free survival have been achieved in lung cancer, renal cell carcinoma, urothelial carcinoma, and melanoma, among others [44].
The tumor microenvironment also induces important changes in other tumor-infiltrating cells, including macrophages, DCs, myeloid-derived suppressor cells, and plasma cells. From these cells, DCs are particularly important. It has been shown that reactivating DCs inside the tumor can restore CD8+ T-cell function and induce other anticancer changes in the TME [43].
Approaching immune restoration using biAbs is a relatively new field; most literature is recent and experimental, with promising results potentially translational into the clinic. Liu et al. performed an animal in vivo study using a biAb directed against CD3e (T-cells), and PD-L1. Analysis of their data showed that dramatic responses obtained in multiple syngeneic tumor models were due to restoring CD8+ T-cell activity when bsAbs were targeting PD-L1 in the membrane of DCs, inhibiting negative regulation and enhancing the stimulation via cytokines and co-stimulatory molecules. These data are intriguing as no tumor-specific antibody was used, but instead, a “generalist” approach of immune checkpoint blockade was done [45].
A similar approach was used by Kraman et al., using a biAb directed against LAG3 and PD-L1. LAG3 is another critical immune checkpoint and maker of T-cell exhaustion. Results showed high antitumoral activity in murine syngeneic models [46]. Cui et al. developed a biAb for PD-L1 and VEGF; this innovative approach showed high efficacy in preclinical models and exploited the association between high PD-L1 expression by tumor cells and the addiction to angiogenic pathways [47]. Ramaswamy et al. developed an anti-CD47/anti-PD-L1 biAb active against hematological malignancies [48]. Other researchers have tried not only to block PD-L1 but also to stimulate the effector cell at the same time by targeting a co-stimulatory molecule such as CD28 [49] or 4-1BB [50]; positive results were obtained in preclinical studies. All these new structures are made without a functional Fc domain, avoiding possible adverse events that did not allow previous biAbs to be approved for therapy in solid tumors.
7. Delivery Strategies for T-biAbs
Currently, T-biAbs are used as immunotherapy in cancer treatment. There are two approved delivery strategies for T-biAbs-based therapies: delivery of Y-biAbs as recombinant proteins or in vivo production.
7.1. Delivery of T-biAbs as Recombinant Proteins
T-biAbs as recombinant proteins are composed of two linked antibody-binding regions that simultaneously recognize two antigens [12]. One of the antigens recruits and activates T cells because it binds to the CD3ε chain of the T-cell receptor. The other antigen redirects the cytotoxicity of the T cells to tumor cells, because this antigen is on the surface of the tumor cells [51,52]. Additionally, a new strategy that has been developed eases tumor-specific antigen (TSA) and tumor-associated antigen (TAA) targeting by linking T-biAbs and chimeric antigen receptor T cells (CAR-T). These are known as switchable CAR-Ts and are activated by a bispecific protein [53,54].
In this delivery strategy, recombinant proteins with a short circulatory half-life (T-biAbs in tandem single-chain variable fragment recombinant format) and long circulatory half-life (T-biAbs with Fc domain format) are administered as continuous intravenous (IV) infusion or as repeated high- dose bolus injections respectively [52].
On this topic, it is worth highlighting that some limitations to this type of delivery have been reported. First of all, the high cost of therapy, is derived from the production process and the usually long treatment. Furthermore, it the difficulty to achieve sustained plasma levels, high enough to be effective and low enough to be not toxic [55]. Moreover, due to the different expression technologies to develop T-biAbs, the diverse glycosylation patterns might affect the efficacy of the T-biAbs [56]. Additionally, the poor stability during long-term storage and tendency to aggregate over time are also reported. Finally, some limitations related to systemic administration are noted. In some cases, an infusion pump is required for continuous delivery. In other cases, some purified antibodies need to be administered through slow IV infusions to limit infusion reactions [57].
7.2. Delivery of T-biAbs In Vivo Production
To seek the balance between efficacy and safety, in vivo gene therapy was developed. It is known that modified oncolytic viruses eliminate tumor cells and stimulate systemic immune response without harming healthy cells and tissues [52]. It is known that those viruses can be modified to encode therapeutic transgenes, such as a functional T-biAb leading to an in situ expression of T-biAb by tumor cells [58]. Currently, there are two ways to lead to in vivo secretion of T-biAbs. The first one is transduction by using vectors. The oncolytic viruses that have been engineered as vectors for T-biAb expression are adenovirus and measles [59]. The second one, is the in vivo inoculation of synthetic nucleic acid-encoded T-biAb by using messenger RNA and plasmid DNA [60]. Some studies have demonstrated that the in vivo delivery strategy has an effective antitumor activity (reducing tumor growth and metastasis) and leads to delayed cancer progression in mice models [56,57,61].
The mechanism of action of the latter delivery system has shown advantages over the former. One of the advantages is that it is a cheaper alternative because it obviates the need for manufacturing and administering purified T-biAbs. Additionally, in some cases the in vivo production maintains an effective antibody concentration, so no concerns about long-term storage and rapid renal clearance arise [51,61].
8. T-biAbs in Hematologic Malignancies
There is solid and growing evidence about the use of T-biAbs in hematologic malignancies. This is possible because most hematologic neoplasms fulfill two important features for an effective biAbs therapy. The first one is that antigens from hematologic malignancies are mainly (or only) expressed on malignant cells, leading to a reduction of on-target/off cancer toxicity. The second one is that the antigen is strongly associated with the malignant phenotype, leading to a reduction of antigen loss variants [62].
According to the anterior principle, the approach of T-biAbs focuses on targeting CD3 on T cells (CD3ε fragment) and an antigen commonly expressed in tumor cells. For example, for B-cell malignancies, some T-BiAbs target CD19 and CD20. CD19 persists the entire course of the B-cell development. This overexpressed target allows the lysis of malignant cells and avoids the attack of the normal lymphocytes [63].
The most remarkable drug of this kind is blinatumomab. This antibody is made of two scFv combined in one protein chain [8]. The history of this novel drug began in 2014 when it was approved for Philadelphia chromosome (Ph)-negative relapsed or refractory (R/R) B-cell precursor acute lymphoblastic leukemia (ALL) in adults. Later, in 2016, FDA approved blinatumomab for pediatric patients with Ph- R/R B-cell precursor ALL [8]. After that, the TOWER trial and the ALCANTARA trial gave support for the full approval of the drug for R/R B-cell precursor ALL in adults and children in 2017, and for minimal residual disease-positive B-cell precursor ALL in 2018 [64,65,66,67].
Besides the patients’ positive outcomes under blinatumomab treatment, some concerns related to the main adverse effects (AE) are still present. Among the most dangerous AE, neurological affections are the most common cause of interruption of the drug. Neurotoxicity symptoms include headache, tremor, confusion, disorientation, and other more life-threatening symptoms such as seizures or stupor. Other significant AEs include CRS, produced because of T cell and macrophages activation, and infectious and hematologic toxicity [68,69]. In addition to AEs, blinatumomab has a short half-life and a significant but limited response among R/R ALL patients, and this has exposed the need for newer therapeutic alternatives.
However, many other hematologic neoplasms could benefit from this kind of immunotherapy. Once again, by targeting a common antigen on the tumor cell’s surface, the therapeutic effort focuses on malignant cells with little or no on-target off-tumor toxicity. For example, for acute myeloid leukemia (AML), CD123 is one of the over-expressed antigens targeted by novel drugs such as flotetuzumab (MGD006) or vibecotamab (XmAb1405) with positive results in phase I/II and phase I studies respectively [70,71]. There is also growing evidence that molecules that block CD33, an acid-binding sialo-adhesins receptor expressed in nearly 90% of AML, such as gemtuzumab ozogamicin are safe and effective [72]. Among the molecules AMG330 (NCT02520427), AMG673 (NCT03224819), and AMV564 (NCT03144245) are being evaluated under clinical trials. Only to mention other possible targets currently under study for AML include FLT3, CLEC12A, and WT1 [73].
For other neoplasms as multiple myeloma (MM), some possible targets for immunotherapy include B-cell maturation antigen (BCMA), G protein-coupled resector 5D (GPR5D), CD38, and Fc receptor-like 5 (FCRL5) [73]. Anti-BCMA AMG 420 has demonstrated a response rate near 70% in patients with R/R MM in phase I trials with a favorable security profile [74]. Some molecules have been proposed for the other targets and are currently under evaluation [75,76]. For non-Hodgkin lymphoma ideal targets include CD19, CD20, and CD47, and for Hodking lymphoma CD30 targets seems promising, and for myelodysplasias syndromes CD123 could be a suitable targe but is still under discussion [8,73,77].
9. T-biAbs in Solid Malignancies
As it was mentioned earlier, since the approval of blinatumomab in 2014, T-biAbs have become an essential section in the investigation of hematologic malignancies. However, it is not the case for solid malignancies. There are some challenges in developing T-biAbs for solid tumors, one of them the hypoxia-induced immunosuppression in the tumor microenvironment, as a successful T-biAb depends on activation of CTL targeting CD3 [52].
Nevertheless, a large number of molecules that target antigens expressed on solid tumor malignant cells are under investigation (see Table 1). Targets under examination include epithelial cell adhesion molecule (EpCAM) in non-small cell lung carcinoma, glycan 3 (GPC3) in live cancer, HER2 in breast cancer, and prostate-specific membrane antigen (PSMA) in prostate cancer [9].
Table 1.
Some of the clinical trials of different molecules that remain under evaluation for use, we present the code of the trial (ClinicalTrials.gov accessed on 16 March 2022), the name of the trial, the type of cancer-related to the molecule, and the current phase of the study.
| ClinicalTrials.gov Identifier | Title | Conditions | Interventions | Phase |
|---|---|---|---|---|
| Recruiting | ||||
| NCT03146637 | Study of Activated CIK Armed With Bispecific Antibody for Advanced Liver Cancer | Advanced Liver Cancer | Biological: Activated CIK|Biological: CIK | Phase 2 |
| NCT05125016 | REGN4336 (a PSMAXCD3 Bispecific Antibody) Administered Alone or in Combination With Cemiplimab in Adult Male Patients With Metastatic Castration-Resistant Prostate Cancer | Metastatic Castration-resistant Prostate Cancer | Drug: REGN4336|Drug: Cemiplimab|Other: 18F-DCFPyL | Phase 2 |
| NCT04868877 | A Phase 1/2 Study Evaluating MCLA-129, a Human Anti-EGFR and Anti-c-MET Bispecific Antibody, in Patients With Advanced NSCLC and Other Solid Tumors | Non-Small Cell Lung Cancer Metastatic|Gastric Cancer|Head and Neck Cancer | Drug: MCLA-129 | Phase 2 |
| NCT05090566 | MagnetisMM-4: Umbrella Study of Elranatamab (PF-06863135) in Combination With Anti-Cancer Treatments in Multiple Myeloma | Multiple Myeloma | Drug: Elranatamab + Nirogacestat|Drug: Elranatamab + lenalidomide + dexamethasone | Phase 2 |
| NCT03860207 | Study of the Safety and Efficacy of Humanized 3F8 Bispecific Antibody (Hu3F8-BsAb) in Patients With Relapsed/Refractory Neuroblastoma, Osteosarcoma and Other Solid Tumor Cancers | Neuroblastoma|Osteosarcoma|Other Solid Tumor Cancers | Biological: Humanized 3F8 Bispecific Antibody|Other: Blood draw | Phase 2 |
| NCT04380805 | A Study of AK104, a PD-1/CTLA-4 Bispecific Antibody in Subjects With Recurrent/Metastatic Cervical Cancer | Recurrent Cervical Cancer|Metastatic Cervical Cancer | Biological: AK104 | Phase 2 |
| NCT04886271 | Recombinant Humanized Anti-CD47/PD-1 Bifunctional Antibody HX009 Injection in the Treatment of Advanced Solid Tumors | Advanced Solid Tumor | Drug: HX009 | Phase 2 |
| NCT04276493 | Anti-HER2 Bispecific Antibody ZW25 Activity in Combination With Chemotherapy With/Without Tislelizumab | Breast Cancer|Gastric Cancer|Gastroesophageal Junction Cancer | Biological: ZW25|Drug: Docetaxel|Biological: Tislelizumab|Drug: Capecitabine|Drug: Oxaliplatin | Phase 2 |
| NCT04999605 | A Study of AK112 Combined With PARP Inhibitor in the Treatment of Recurrent Ovarian Cancer | Ovarian Neoplasms|Recurrent Ovarian Carcinoma|Relapsed Ovarian Cancer|Ovarian Cancer | Drug: AK112 low dose|Drug: AK112 medium dose|Drug: AK112 high dose | Phase 2 |
| NCT04618393 | A Study of EMB-02 in Participants With Advanced Solid Tumors | Advanced Solid Tumors | Biological: EMB-02 | Phase 2 |
| NCT05214482 | A Study of AK112 in Advanced Malignant Tumors | Advanced Malignant Tumors | Drug: AK112|Drug: AK117|Drug: Chemotherapy | Phase 2 |
| NCT03406858 | Pembrolizumab and HER2Bi-Armed Activated T Cells in Treating Patients With Metastatic Castration Resistant Prostate Cancer | Castration Levels of Testosterone|Castration-Resistant Prostate Carcinoma|Prostate Carcinoma Metastatic in the Bone|PSA Progression|Stage IV Prostate Adenocarcinoma AJCC v7 | Biological: HER2Bi-Armed Activated T Cells|Other: Laboratory Biomarker Analysis|Biological: Pembrolizumab | Phase 2 |
| NCT02912949 | A Study of Zenocutuzumab (MCLA-128) in Patients With Solid Tumors Harboring an NRG1 Fusion | Solid Tumours Harboring NRG1 Fusion|NSCLC Harboring NRG1 Fusion|Pancreatic Cancer Harboring NRG1 Fusion|NRG1 Fusion | Drug: zenocutuzumab (MCLA-128) | Phase 2 |
| NCT04995523 | A Study to Assess the Safety and Efficacy of AZD2936 in Participants With Advanced or Metastatic Non-small Cell Lung Cancer (NSCLC) | Non-Small-Cell Lung Carcinoma | Drug: AZD2936 | Phase 2 |
| NCT05102214 | HLX301 (TIGIT × PDL1 Bispecific) in Patients With Locally Advanced or Metastatic Solid Tumors | Locally Advanced or Metastatic Solid Tumors|Non-small Cell Lung Cancer | Drug: HLX301 | Phase 2 |
| NCT03269526 | BATs Treatment for Pancreatic Cancer, Phase Ib/II | Locally Advanced Pancreatic Adenocarcinoma|Metastatic Pancreatic Adenocarcinoma | Drug: EGFR BATs after standard of care chemo | Phase 2 |
| NCT04547101 | A Study of AK104 in Subjects With Locally Advanced Unresectable or Metastatic MSI-H/dMMR Solid Tumors | MSI-H/dMMR Solid Tumor | Drug: AK104 | Phase 2 |
| NCT03564340 | Study of REGN4018 Administered Alone or in Combination With Cemiplimab in Adult Patients With Recurrent Ovarian Cancer | Recurrent Ovarian Cancer|Recurrent Fallopian Tube Cancer|Recurrent Primary Peritoneal Cancer | Drug: REGN4018|Drug: cemiplimab | Phase 2 |
| NCT05159388 | A Study of PRS-344/S095012 (PD-L1 × 4-1BB Bispecific Antibody-Anticalin Fusion) in Patients With Solid Tumors | Solid Tumor | Drug: PRS-344/S095012 | Phase 2 |
| NCT04626635 | REGN7075 in Combination With Cemiplimab in Adult Participants With Advanced Solid Tumors | Advanced Solid Tumors | Drug: REGN7075|Drug: cemiplimab | Phase 2 |
| NCT04930432 | Study of MCLA-129, a Human Bispecific EGFR and cMet Antibody, in Patients With Advanced NSCLC and Other Solid Tumors | Solid Tumor|Non-Small Cell Lung Cancer|Head and Neck Cancer|Colorectal Cancer | Drug: MCLA-129 | Phase 2 |
| NCT04750239 | Safety and Clinical Activity of Nivatrotamab in Relapsed/Recurrent Metastatic Small-cell Lung Cancer | SCLC | Drug: Nivatrotamab | Phase 2 |
| NCT04931654 | A Study to Assess the Safety and Efficacy of AZD7789 in Participants With Advanced or Metastatic Solid Cancer | Carcinoma, Non-Small-Cell Lung | Drug: AZD7789 | Phase 2 |
| NCT03440437 | FS118 First in Human Study in Patients With Advanced Malignancies | Advanced Cancer|Metastatic Cancer|Squamous Cell Carcinoma of Head and Neck | Drug: FS118 | Phase 2 |
| NCT04900363 | A Trial of AK112 (PD-1/VEGF Bispecific Antibody) in Patients With NSCLC | Non-small Cell Lung Cancer | Drug: AK112 | Phase 2 |
| NCT04870177 | Study of AK112 in the Treatment of Advanced Gynecological Tumors | Gynecologic Cancer|Cancer Metastatic|Ovarian Neoplasms|Cervical Neoplasm|Endometrial Neoplasms | Drug: AK112 | Phase 2 |
| NCT04557098 | A Study of Teclistamab, in Participants With Relapsed or Refractory Multiple Myeloma | Hematological Malignancies | Drug: Teclistamab | Phase 2 |
| NCT04634552 | A Study of Talquetamab in Participants With Relapsed or Refractory Multiple Myeloma | Hematological Malignancies | Drug: Talquetamab | Phase 2 |
| NCT05180474 | Research Trial to Study Safety of GEN1047 (DuoBody®-CD3xB7H4) in Participants With Malignant Solid Tumors | Breast Cancer|Uterine Cancer|Ovarian Cancer|Non Small Cell Lung Cancer (NSCLC)|Cervical Cancer|Head and Neck Squamous Cell Carcinoma (HNSCC), Except for Nasopharyngeal Carcinoma|Urothelial Cancer|Cholangiocarcinoma (CCA) | Biological: GEN1047 is a bispecific antibody that induces T-cell-mediated cytotoxicity of B7H4-positive cells. | Phase 2 |
| NCT03888105 | Assess the Anti-Tumor Activity and Safety of Odronextamab in Patients With Relapsed or Refractory B-cell Non-Hodgkin Lymphoma | B-cell Non-Hodgkin Lymphoma (NHL) | Drug: Odronextamab | Phase 2 |
| NCT04696809 | A Study of Teclistamab in Japanese Participants With Relapsed or Refractory Multiple Myeloma | Hematologic Malignancies | Drug: Teclistamab | Phase 2 |
| NCT04496674 | Bispecific PSMAxCD3 Antibody CC-1 in Patients With Squamous Cell Carcinoma of the Lung | Lung Cancer Squamous Cell | Drug: CC-1 and Toczilizumab | Phase 2 |
| NCT05228470 | MagnetisMM-8: Study Of Elranatamab (PF-06863135) Monotherapy in Chinese Participants With Refractory Multiple Myeloma | Elranatamab|Myeloma|Multiple Myeloma|Relapsed Multiple Myeloma|Refractory Multiple Myeloma|PF-06863135|BCMA|Bispecific|Bispecific Antibody|BCMA-CD3 Bispecific|MagnetisMM-8 | Drug: Elranatamab | Phase 2 |
| NCT04590781 | Safety and Efficacy of XmAb18087 ± Pembrolizumab in Advanced Merkel Cell Carcinoma or Extensive-stage Small Cell Lung Cancer | Merkel Cell Carcinoma|Small Cell Lung Cancer | Biological: XmAb18087|Drug: XmAb18087 ± Pembrolizumab | Phase 2 |
| NCT03272334 | Her2-BATS and Pembrolizumab in Metastatic Breast Cancer | Metastatic Breast Cancer | Drug: HER2 BATs with Pembrolizumab | Phase 2 |
| NCT04492033 | A Study of ABL001 in Combination With Irinotecan or Paclitaxel in Patients With Advanced or Metastatic Solid Tumors | P1b: Advanced Solid Tumors|P2: Biliary Tract Cancer | Drug: ABL001|Drug: Paclitaxel|Drug: Irinotecan | Phase 2 |
| NCT04466891 | A Study of ZW25 (Zanidatamab) in Subjects With Advanced or Metastatic HER2-Amplified Biliary Tract Cancers | HER2-amplified Biliary Tract Cancers | Drug: ZW25 (Zanidatamab)|Diagnostic Test: In situ hybridization (ISH)-based companion diagnostic assay|Diagnostic Test: Immunohistochemistry (IHC)-based companion diagnostic assay | Phase 2 |
| NCT03929666 | A Safety and Efficacy Study of ZW25 (Zanidatamab) Plus Combination Chemotherapy in HER2-expressing Gastrointestinal Cancers, Including Gastroesophageal Adenocarcinoma, Biliary Tract Cancer, and Colorectal Cancer | HER2-expressing Gastrointestinal Cancers, Including Gastroesophageal Adenocarcinoma, Biliary Tract Cancer, and Colorectal Cancer | Drug: ZW25 (Zanidatamab)|Drug: Capecitabine|Drug: Cisplatin|Drug: Fluorouracil|Drug: Leucovorin|Drug: Oxaliplatin|Drug: Bevacizumab|Drug: Gemcitabine | Phase 2 |
| NCT03761108 | First in Human (FIH) Study of REGN5458 in Patients With Relapsed or Refractory Multiple Myeloma | Multiple Myeloma | Drug: REGN5458 | Phase 2 |
| NCT04224272 | A Study of ZW25 (Zanidatamab) With Palbociclib Plus Fulvestrant in Patients With HER2+/HR+ Advanced Breast Cancer | HER2+/HR+ Breast Cancer | Drug: ZW25 (Zanidatamab)|Drug: Palbociclib|Drug: Fulvestrant | Phase 2 |
| NCT05176665 | EMB-01 in Patients With Advanced/Metastatic Gastrointestinal Cancers | Neoplasms|Neoplasm Metastasis|Metastatic Gastrointestinal Carcinoid Tumor | Drug: EMB-01 | Phase 2 |
| NCT03797391 | A Dose Escalation With Expansion Study of EMB-01 in Participants With Advanced/Metastatic Solid Tumors | Neoplasms|Neoplasm Metastasis|Non-Small-Cell Lung Cancer | Drug: EMB-01 | Phase 2 |
| NCT04785820 | A Study of RO7121661 and RO7247669 Compared With Nivolumab in Participants With Advanced or Metastatic Squamous Cell Carcinoma of the Esophagus | Advanced or Metastatic Esophageal Squamous Cell Carcinoma | Drug: RO7121661|Drug: RO7247669|Drug: Nivolumab | Phase 2 |
| NCT04735575 | A Ph1/2 Study of EMB-06 in Participants With Recurrent or Refractory Myeloma | Relapsed or Refractory Multiple Myeloma | Biological: EMB-06 | Phase 2 |
| NCT05014412 | A Study to Learn About the Study Medicine (Elranatamab) in Participants With Multiple Myeloma That Has Come Back After Responding to Treatment or Has Not Responded to Treatment | Multiple Myeloma | Drug: Elranatamab | Phase 2 |
| NCT05189093 | Recombinant Humanized Anti-CD47/PD-1 Bifunctional Antibody HX009 in Patients With Relapsed/Refractory Lymphoma | Relapsed/Refractory Lymphoma | Drug: Recombinant humanized anti-CD47/PD-1 bifunctional antibody HX009 injection | Phase 2 |
| NCT04728321 | A Study of Anti-PD-1/CTLA-4 Bispecific AK104 Alone or in Combination With Lenvatinib in Advanced Hepatocellular Carcinoma | Hepatocellular Carcinoma | Biological: AK104 lenvatinib|Biological: AK104 | Phase 2 |
| NCT04889716 | CAR-T Followed by Bispecific Antibodies | Large B-cell Lymphoma | Drug: mosunetuzumab|Drug: glofitamab|Drug: obinutuzumab | Phase 2 |
| NCT04444167 | A Study of Anti-PD-1/CTLA-4 Bispecific AK104 Plus Lenvatinib in First-line Advanced Hepatocellular Carcinoma | Hepatocellular Carcinoma | Biological: AK104|Drug: Lenvatinib | Phase 2 |
| NCT04444141 | A Study of PD-1/CTLA-4 Bispecific AK104 in Relapsed or Refractory Peripheral T-cell Lymphoma | Peripheral T-cell Lymphoma | Biological: AK104 | Phase 2 |
| NCT04602065 | Evaluation of Safety and Efficacy of IBI318 Monotherapy for Relapsed/Refractory Extranodal NK/T Cell Lymphoma (Nasal Type) Trial | Extranodal NK/T Cell Lymphoma, Nasal Type | Drug: IBI318(Recombinant human anti-PD1/PD-L1 bispecific antibody) | Phase 2 |
| NCT05044897 | A Clinical Study to Evaluate the Efficacy and Safety of SI-B001 in the Treatment of Recurrent and Metastatic HNSCC | Head and Neck Squamous Cell Carcinoma | Drug: SI-B001 | Phase 2 |
| NCT04763083 | First in Human Study of NVG-111 in Chronic Lymphocytic Leukaemia and Mantle Cell Lymphoma | Chronic Lymphocytic Leukaemia|Small Lymphocytic Lymphoma|Mantle Cell Lymphoma | Drug: NVG-111|Drug: NVG-111 (RP2D) | Phase 2 |
| NCT04703686 | Treatment by a Bispecific CD3xCD20 Antibody for Relapse/Refractory Lymphomas After CAR T-cells Therapy | Diffuse Large B-Cell Lymphoma Refractory|Refractory Indolent Adult Non-Hodgkin Lymphoma|Refractory Transformed B-cell Non-Hodgkin Lymphoma|Refractory Primary Mediastinal Large B-Cell Cell Lymphoma|Refractory Mantle Cell Lymphoma | Drug: Obinutuzumab|Drug: RO7082859 | Phase 2 |
| NCT04469725 | KN046 (a Humanized PD-L1/CTLA4 Bispecific Single Domain Fc Fusion Protein Antibody) in Subjects With Thymic Carcinoma | Thymic Carcinoma | Drug: KN046 | Phase 2 |
| NCT05020236 | MagnetisMM-5: Study of Elranatamab (PF-06863135) Monotherapy and Elranatamab + Daratumumab Versus Daratumumab + Pomalidomide + Dexamethasone in Participants With Relapsed/Refractory Multiple Myeloma | Multiple Myeloma | Drug: Elranatamab|Drug: Daratumumab|Drug: Pomalidomide|Drug: Dexamethasone | Phase 3 |
| NCT05152147 | A Study of Zanidatamab in Combination With Chemotherapy Plus or Minus Tislelizumab in Patients With HER2-positive Advanced or Metastatic Gastric and Esophageal Cancers | Gastric Neoplasms|Gastroesophageal Adenocarcinoma|Esophageal Adenocarcinoma | Drug: Zanidatamab|Drug: Tislelizumab|Drug: Trastuzumab|Drug: Capecitabine|Drug: Oxaliplatin|Drug: Cisplatin|Drug: 5-Fluorouracil|Diagnostic Test: In situ hybridization (ISH)-based companion diagnostic assay|Diagnostic Test: Immunohistochemistry (IHC)-based companion diagnostic assay | Phase 3 |
| NCT03643276 | Treatment Protocol for Children and Adolescents With Acute Lymphoblastic Leukemia—AIEOP-BFM ALL 2017 | Acute Lymphoblastic Leukemia, Pediatric | Drug: Blinatumomab|Drug: Bortezomib|Drug: Cyclophosphamide|Drug: Cytarabine|Drug: Daunorubicin|Drug: Myocet|Drug: Dexamethasone|Drug: Doxorubicin|Drug: Etoposide|Drug: Fludarabine Phosphate|Drug: Ifosfamide|Drug: 6-Mercaptopurine|Drug: Methotrexate|Drug: Pegaspargase|Drug: Prednisolone|Drug: Tioguanin|Drug: Vincristine|Drug: Vindesine|Drug: Erwinase | Phase 3 |
| NCT04722848 | Sequential Treatment With Ponatinib and Blinatumomab vs. Chemotherapy and Imatinib in Newly Diagnosed Adult Ph+ ALL | Acute Lymphoblastic Leukemia (Philadelphia Chromosome Positive)|ALL, Adult|Philadelphia-Positive ALL | Drug: Ponatinib + Blinatumomab|Drug: Chemotherapy + Imatinib | Phase 3 |
| Active, not recruiting | ||||
| NCT02620865 | Bispecific Antibody Armed Activated T-cells With Aldesleukin and Sargramostim in Treating Patients With Locally Advanced or Metastatic Pancreatic Cancer | Metastatic Pancreatic Adenocarcinoma|Recurrent Pancreatic Carcinoma|Stage III Pancreatic Cancer|Stage IV Pancreatic Cancer | Biological: Aldesleukin|Biological: Antibody Therapy|Drug: Fluorouracil|Drug: Gemcitabine Hydrochloride|Drug: Irinotecan Hydrochloride|Other: Laboratory Biomarker Analysis|Drug: Leucovorin Calcium|Drug: Oxaliplatin|Drug: Paclitaxel Albumin-Stabilized Nanoparticle Formulation|Biological: Sargramostim | Phase 2 |
| NCT03321981 | MCLA-128 With Trastuzumab/Chemotherapy in HER2+ and With Endocrine Therapy in ER+ and Low HER2 Breast Cancer | Breast Cancer Metastatic | Drug: MCLA-128|Drug: Trastuzumab|Drug: Vinorelbine|Drug: Endocrine therapy | Phase 2 |
| NCT04649359 | MagnetisMM-3: Study Of Elranatamab (PF-06863135) Monotherapy in Participants With Multiple Myeloma Who Are Refractory to at Least One PI, One IMiD and One Anti-CD38 mAb | Multiple Myeloma | Drug: Elranatamab (PF-06863135) | Phase 2 |
| NCT04083534 | First In Human (FIH) Study of REGN5459 in Adult Patients With Relapsed or Refractory Multiple Myeloma (MM) | Relapsed Multiple Myeloma|Refractory Multiple Myeloma | Drug: REGN5459 | Phase 2 |
| Not yet recruiting | ||||
| NCT04868708 | A Study of AK104 (an Anti-PD-1 and Anti-CTLA-4 Bispecific Antibody) in Recurrent or Metastatic Cervical Cancer | Recurrent or Metastatic Cervical Cancer | Biological: AK104|Biological: Bevacizumab|Drug: Paclitaxel|Drug: Cisplatin or Carboplatin | Phase 2 |
| NCT04556253 | AK104 in Locally Advanced MSI-H/dMMR Gastric Carcinoma and Colorectal Cancer | MSI-H/dMMR Gastric Carcinoma and Colorectal Cancer | Drug: AK104 | Phase 2 |
| NCT04172454 | Safety and Efficacy of AK104, a PD-1/CTLA-4 Bispecific Antibody, in Selected Advanced Solid Tumors | Advanced Solid Tumors|Melanoma | Biological: AK104 | Phase 2 |
| NCT04597541 | A Study of AK112, a PD-1/VEGF Bispecific Antibody, for Advanced Solid Tumors | Solid Tumor, Adult | Drug: AK112 | Phase 2 |
| NCT05229497 | A Phase Ib/II Study of AK112 in Combination With AK117 in Advanced Malignant Tumors | Advanced Malignant Tumors | Drug: AK112|Drug: AK117|Drug: Carboplatin|Drug: Cisplatin|Drug: 5-Fluorouracil | Phase 2 |
| NCT05235542 | A Phase Ib/II Study of AK104 and AK117 in Combination With or Without Chemotherapy in Advanced Malignant Tumors | Advanced Malignant Tumors | Drug: AK104|Drug: AK117|Drug: Capecitabine tablets|Drug: Oxaliplatin|Drug: Cisplatin|Drug: Paclitaxel|Drug: Irinotecan|Drug: Docetaxel|Drug: 5-FU | Phase 2 |
| NCT05247684 | AK112 Neoadjuvant/Adjuvant Treatment for Resectable NSCLC | Resectable Non-small Cell Lung Cancer | Drug: AK112|Drug: Carboplatin|Drug: Cisplatin|Drug: Paclitaxel | Phase 2 |
| NCT04841538 | A Study of ES101 (PD-L1x4-1BB Bispecific Antibody) in Patients With Advanced Malignant Thoracic Tumors | Thoracic Tumors|Non-small Cell Lung Cancer|Small Cell Lung Cancer | Drug: ES101 | Phase 2 |
| NCT05227651 | AK104 in Neoadjuvant Treatment of Cervical Cancer | Cervical Cancer | Drug: AK104 | Phase 2 |
| NCT05215067 | A Phase II Trial of AK104 in Advanced Non-Small Cell Lung Cancer | Advanced Non-small Cell Lung Cancer | Drug: AK104|Drug: Docetaxel | Phase 2 |
| NCT05032040 | A Study of XmAb20717 in Patients With Selected Advanced Gynecologic and Genitourinary Malignancies | Ovarian Cancer|Clear Cell Carcinoma|Endometrial Cancer|Cervical Carcinoma|Metastatic Castration-Resistant Prostate Cancer (mCRPC) | Biological: XmAb20717 | Phase 2 |
| NCT05216835 | Safety and Preliminary Efficacy Assessment of AZD7789 in Patients With Relapsed or Refractory Classical Hodgkin Lymphoma | Relapsed or Refractory Classical Hodgkin Lymphoma | Drug: AZD7789 | Phase 2 |
| NCT04542837 | The Study of KN046 in Combination With Lenvatinib in Advanced Hepatocellular Carcinoma | HCC | Biological: KN046|Drug: Lenvatinib | Phase 2 |
| NCT05256472 | A Study of AK104 Plus Axitinib in Advanced/Metastatic Clear Cell Renal Cell Carcinoma | Clear Cell Renal Cell Carcinoma|First-line Treatment | Drug: AK104|Drug: axitinib | Phase 2 |
| Unknown status | ||||
| NCT03852251 | A Study of AK104, a PD-1/CTLA-4 Bispecific Antibody, for Advanced Solid Tumors or With mXELOX as First-line Therapy for Advanced Gastric or GEJ Adenocarcinoma | Gastric Adenocarcinoma|Advanced Solid Tumors|Gastroesophageal Junction Adenocarcinoma | Biological: AK104|Drug: Oxaliplatin|Drug: Capecitabine | Phase 2 |
| NCT02173093 | Activated T Cells Armed With GD2 Bispecific Antibody in Children and Young Adults With Neuroblastoma and Osteosarcoma | Disseminated Neuroblastoma|Recurrent Neuroblastoma | Biological: IL-2|Biological: GD2Bi-aATC|Biological: GM-CSF|Other: laboratory evaluations of immune responses | Phase 2 |
| NCT04220307 | A Study of a PD-1/CTLA-4 Bispecific Antibody AK104 in Patients With Metastatic Nasopharyngeal Carcinoma | Nasopharyngeal Carcinoma | Biological: AK-104 | Phase 2 |
| NCT02744768 | D-ALBA Frontline Sequential Dasatinib and Blinatumomab in Adult Philadelphia Positive Acute Lymphoblastic Leukemia | Acute Lymphoblastic Leukemia | Drug: Dasatinib|Drug: Blinatumomab | Phase 2 |
EpCAM is a conserved type I transmembrane protein, and it is highly expressed in some malignancies, lung cancer included [78]. Molecules such as catumaxomab demonstrated in phase I/II clinical trials a good response in treating malignant-related ascites. However, its production was stopped due to severe AEs (CRS and hepatotoxicity) [79,80]. Currently, MT110 (AMG110) demonstrated safety and tolerability, however, its clinical effect is still to be dilucidated [81].
In regards to GPC3, a heparan-sulfate proteoglycan expressed over the 65% of hepatocellular carcinoma and related to poor prognosis [82], is an important target as well. ERY74, a humanized IgG antibody, has demonstrated suppression of tumor growth that expresses GPC3 [83]. For HER2 in advanced breast cancer, the evidence is more solid. Options include ertumaxomab (HER2/CD3), amivantamab (JNJ-61186372), and MM-111 (HER2/HER3) have been proved to be safe and effective in human studies [9]. PSMA, expressed in androgen-independent prostate cancers, has several targeting antibodies. HPN424 (NCT03577028) and AMG160 (NCT3792841) also have shown positive responses in animal models and clinical scenarios but the complete understanding of the molecules is still to be shown in the future [84,85].
10. CAR-T Switches
Among other redirecting T-cell activation therapies against cancer cells, chimeric antigen receptors (CAR) expressed by T cells (CAR-T) represent a revolution in immunotherapy. The approval of tisagenlecleucel (tisa-cel) and axicabtagene ciloleucel (axi-cel) in 2017 by FDA marked a turning point in cancer treatment [86]. These cells are genetically medicated to express chimeric antigen receptor that enables them to target tumor cells.
CAR-Ts put together two of the three necessary signals to T-cell priming, the TCR/CD3 signals, and the costimulatory receptors/mediators. This allows the cell to recognize user-defined cell surface tumor-associated antigens. However, similar to other immune therapies, CAR-T cells have some AEs. Among them we can mention the off-target toxicity (attack of normal cells that express some common antigens as cancer cells), tumor lysis syndrome, and CRS.
A novel strategy called CAR-T switches has been developed to avoid these undesirable side effects that could be quite harmful. These switches focus on recruiting and activation of immune cells by a bispecific adapter protein. The adaptor, conformed as a monoclonal antibody, can target the tumor antigen and activate the dormant CAR-T with a protein located in one of its N- or C-terminal region [87].
These switches allow control of the CAR-T cells, improve their specificity, and avoid unpredictable reactivity. There are three perspectives to switch design: suicide, endogenous, and exogenous switches. The suicide switches are molecules exposed to CAR-T cells; they are activated by administering an external drug or molecular mediator (iC9, EGFR, HSV-TK, CD20). The endogenous switches comprehend molecular strategies of the immune synapsis such as target-antigen recognition or expression of immune regulation singles such as PD-1 or CTL-4 (i.e SynNotch switches). The exogenous switches include antibodies or derived proteins with multiple binding sites that could lead to a more specific union of the CRA-T cell with its target [88].
11. Conclusions
BiTAbs have been demonstrated to be a groundbreaking alternative in immune therapy and a vanguard option that combines the engineering of molecular biology and the biological activity of the immune system. These drugs represent a more individual and specific treatment for cancer patients providing a “personalized” attack on malignant cells. However, there is still a lot of information to be elucidated in relation to this kind of immunotherapy. The complexity of the pharmacokinetics and pharmacodynamics of these drugs represents a change for future application. Finally, the possible adverse effects related to the nature of its mechanism of action should be addressed in incoming studies. Nevertheless, the evidence is promising and growing, and maybe these pharmacological agents represent the next and safer generation for cancer treatment.
Author Contributions
A.F.C., C.O.-R., J.E.G.-R., A.M., L.S.: Study design, Manuscript Writting, Preparation and Acceptance. D.F.C., A.R.-P., O.A., L.Z.-B., L.R., A.R., D.d.M.-P., C.R.: Manuscript Preparation and Acceptance. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Andrés F. Cardona discloses financial research support from Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb and The Foundation for Clinical and Applied Cancer Research—FICMAC. Additionally, he was linked with and received honoraria as advisor, participate in speakers’ bureau and gave expert testimony to Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Pfizer, Novartis, Celldex Therapeutics, Foundation Medicine, Eli Lilly and Foundation for Clinical and Applied Cancer Research—FICMAC. Oscar Arrieta reports personal fees from Pfizer, grants and personal fees from Astra zeneca, grants and personal fees from Boehringer Ingelheim, personal fees from Lilly, personal fees from Merck, personal fees from Bristol Myers Squibb, grants and personal fees from Roche, outside the submitted work. Christian Rolfo is a current employee of the Center for Thoracic Oncology at Tisch Cancer Institute, Mount Sinai Health System and Icahn School of Medicine. He reports receiving supported research funding from Lung Cancer Research Foundation-Pfizer; receiving non-financial research support from Guardant Health; providing advisory services to ARCHER, Inivata, EMD Serono, BMS, Novartis, Boston Pharmaceuticals, Pfizer, Mirati, and Eisai; providing speaker services to MSD, AstraZeneca, Roche, and Guardant Health; and participating in the Safety Monitoring Board for EMD Serono.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nuñez-Prado N., Compte M., Harwood S., Álvarez-Méndez A., Lykkemark S., Sanz L., Álvarez-Vallina L. The Coming of Age of Engineered Multivalent Antibodies. Drug Discov. Today. 2015;20:588–594. doi: 10.1016/j.drudis.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Labrijn A., Janmaat M., Reichert J., Parren P. Bispecific Antibodies: A Mechanistic Review of the Pipeline. Nat. Rev. Drug Discov. 2019;18:585–608. doi: 10.1038/s41573-019-0028-1. [DOI] [PubMed] [Google Scholar]
- 3.Viardot A., Bargou R. Bispecific Antibodies in Haematological Malignancies. Cancer Treat. Rev. 2018;65:87–95. doi: 10.1016/j.ctrv.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Waldman A.D., Fritz J.M., Lenardo M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trabolsi A., Arumov A., Schatz J.H., Alerts E. T Cell—Activating Bispecific Antibodies in Cancer Therapy. J. Immunol. 2022;203:585–592. doi: 10.4049/jimmunol.1900496. [DOI] [PubMed] [Google Scholar]
- 6.Blanco B., Compte M., Lykkemark S., Sanz L., Alvarez-Vallina L. T Cell-Redirecting Strategies to ‘sTAb’ Tumors: Beyond CARs and Bispecific Antibodies. Trends Immunol. 2019;40:243–257. doi: 10.1016/j.it.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Fournier P., Schirrmacher V. Bispecific Antibodies and Trispecific Immunocytokines for Targeting the Immune System Against Cancer. BioDrugs. 2013;27:35–53. doi: 10.1007/s40259-012-0008-z. [DOI] [PubMed] [Google Scholar]
- 8.Duell J., Lammers P.E., Djuretic I., Chunyk A.G., Alekar S., Jacobs I., Gill S. Bispecific Antibodies in the Treatment of Hematologic Malignancies. Clin. Pharmacol. Ther. 2019;106:781–791. doi: 10.1002/cpt.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y., Yi M., Zhu S., Wang H., Wu K. Recent Advances and Challenges of Bispecific Antibodies in Solid Tumors. Exp. Hematol. Oncol. 2021;10:56. doi: 10.1186/s40164-021-00250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goulet D.R., Atkins W.M. Considerations for the Design of Antibody-Based Therapeutics. J. Pharm. Sci. 2021;109:74–103. doi: 10.1016/j.xphs.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu M., Goulet D., Teplyakov A., Gilliland G. A Review of Bispecific Antibodies and Antibody Constructs in Oncology and Clinical Challenges. Pharmacol. Ther. 2019;201:103–119. doi: 10.1016/j.pharmthera.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q., Chen Y., Park J., Liu X., Hu Y., Wang T., Mcfarland K., Betenbaugh M.J. Design and Production of Bispecific Antibodies.Pdf. Antibodies. 2019;8:43. doi: 10.3390/antib8030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S., Chen K., Lei Q., Ma P., Yuan A.Q., Zhao Y., Jiang Y., Fang H., Xing S., Fang Y., et al. The State of the Art of Bispecific Antibodies for Treating Human Malignancies. EMBO Mol. Med. 2021;13:e14291. doi: 10.15252/emmm.202114291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J., Mo Y., Tang M., Shen J., Qi Y., Zhao W., Huang Y., Xu Y., Qian C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021;12:626616. doi: 10.3389/fimmu.2021.626616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobo E.D., Hansen R.J., Balthasar J.P. Antibody Pharmacokinetics and Pharmacodynamics. J. Pharm. Sci. 2004;93:2645–2668. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y. Pharmacokinetics of Bispecific Antibody. Curr. Pharmacol. Rep. 2017;3:126–137. doi: 10.1007/s40495-017-0090-5. [DOI] [Google Scholar]
- 17.Wu Z., Cheung N. T Cell Engaging Bispecific Antibody (T-BsAb): From Technology to Therapeutics. Pharmacol. Ther. 2018;182:161–175. doi: 10.1016/j.pharmthera.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linke R., Klein A., Seimetz D. Clinical Development and Future Directions Catumaxomab. Mabs. 2010;2:129–136. doi: 10.4161/mabs.2.2.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chow V. Clinical Use of Blinatumomab for B-Cell Acute Lymphoblastic Leukemia in Adults. Ther. Clin. Risk Manag. 2016;12:1301–1310. doi: 10.2147/TCRM.S84261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross G., Waks T., Eshhar Z. Expression of Immunoglobulin-T-Cell Receptor Chimeric Molecules as Functional Receptors with Antibody-Type Specificity. Proc. Natl. Acad. Sci. USA. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamakura D., Asano R. T Cell Bispecific Antibodies: An Antibody-Based Delivery System for Inducing Antitumor Immunity. Pharmaceuticals. 2021;14:1172. doi: 10.3390/ph14111172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holland C.J., Crean R.M., Pentier J.M., De Wet B., Lloyd A., Srikannathasan V., Lissin N., Lloyd K.A., Blicher T.H., Conroy P.J., et al. Specificity of Bispecific T Cell Receptors and Antibodies Targeting Peptide-HLA. J. Clin. Investig. 2020;130:2673–2688. doi: 10.1172/JCI130562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huehls A.M., Coupet T.A., Sentman C.L. Bispecific T Cell Engagers for Cancer Immunotherapy. Immunol. Cell Biol. 2015;93:290–296. doi: 10.1038/icb.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meermeier E.W., Welsh S.J., Sharik M.E., Du M.T., Garbitt V.M., Riggs D.L., Shi C., Stein C.K., Bergsagel M., Chau B., et al. Tumor Burden Limits Bispecific Antibody Efficacy through T-Cell Exhaustion Averted by Concurrent Cytotoxic Therapy. Blood Cancer Discov. 2021;2:354–396. doi: 10.1158/2643-3230.BCD-21-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim A., Han C.-J., Driver I., Olow A., Sewell A.K., Zhang Z., Ouyang W., Egen J.G., Yu X. LILRB1 Blockade Enhances Bispecific T Cell Engager Antibody–Induced Tumor Cell Killing by Effector CD8+ T Cells. J. Immunol. 2019;203:1076–1087. doi: 10.4049/jimmunol.1801472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benonisson H., Labrijn A.F., Schuurhuis D.H., Houtkamp M.A., Verbeek J.S., Schuurman J., Hall T. Van CD3-Bispeci Fi c Antibody Therapy Turns Solid Tumors into Inflammatory Sites but Does Not Install Protective Memory. Mol. Cancer Ther. 2019;18:16–18. doi: 10.1158/1535-7163.MCT-18-0679. [DOI] [PubMed] [Google Scholar]
- 27.Gupta V.R., Root A., Fisher T., Norberg R., David J., Cohen J., May C., Giddabasappa A. Molecular Imaging Reveals Biodistribution of P-Cadherin LP- DART Bispecific and Trafficking of Adoptively Transferred T Cells in Mouse Xenograft Model. Oncotarget. 2020;11:1344–1357. doi: 10.18632/oncotarget.27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J., Ybarra R., Mak J., Herault A., De Almeida P., Arrazate A., Ziai J., Totpal K., Junttila M.R., Walsh K.B., et al. IFN g -Induced Chemokines Are Required for CXCR3-Mediated T-Cell Recruitment and Antitumor Ef Fi Cacy of Anti-HER2/CD3 Bispeci Fi c Antibody. Clin. Cancer Res. 2018;24:6447–6458. doi: 10.1158/1078-0432.CCR-18-1139. [DOI] [PubMed] [Google Scholar]
- 29.Franciszkiewicz K., Boissonnas A., Boutet M., Mami-chouaib F. Role of Chemokines and Chemokine Receptors in Shaping the Effector Phase of the Antitumor Immune Response. Cancer Res. 2012;72:6325–6332. doi: 10.1158/0008-5472.CAN-12-2027. [DOI] [PubMed] [Google Scholar]
- 30.Cremasco F., Menietti E., Speziale D., Sam J., Sammicheli S., Richard M., Varol A., Klein C., Umana P., Bacac M., et al. Cross-Linking of T Cell to B Cell Lymphoma by the T Cell Bispecific Antibody CD20-TCB Induces IFN γ/CXCL10-Dependent Peripheral T Cell Recruitment in Humanized Murine Model. PLoS ONE. 2021;16:e0241091. doi: 10.1371/journal.pone.0241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gasteiger G., Osualdo D., Schubert D.A., Bruscia E.M. Cellular Innate Immunity: An Old Game with New Players. J. Innate Immun. 2017;9:111–125. doi: 10.1159/000453397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Bano J., Chames P., Baty D., Kerfelec B. Taking up Cancer Immunotherapy Challenges: Bispecific Antibodies, the Path Forward? Antibodies. 2016;5:1. doi: 10.3390/antib5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciabattini A., Pettini E., Medaglini D., Dunkley M.L. CD4 + T Cell Priming as Biomarker to Study Immune Response to Preventive Vaccines. Front. Immunol. 2013;4:421. doi: 10.3389/fimmu.2013.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eissler N., Mysliwietz J., Deppisch N., Ruf P., Lindhofer H., Mocikat R. Potential of the Trifunctional Bispecific Antibody Surek Depends on Dendritic Cells: Rationale for a New Approach Of Tumor Immunotherapy. Mol. Med. 2013;19:54–61. doi: 10.2119/molmed.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L., Hoseini S., Xu H., Ponomarev V., Cheung N. Silencing Fc Domains in T Cell–Engaging Bispecific Antibodies Improves T-Cell Trafficking and Antitumor Potency. Cancer Immunol. Res. 2019;7:2013–2024. doi: 10.1158/2326-6066.CIR-19-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demaria O., Gauthier L., Debroas G., Vivier E. Natural Killer Cell Engagers in Cancer Immunotherapy: Next Generation of Immuno-Oncology Treatments. Eur. J. Immunol. 2021;81:1934–3942. doi: 10.1002/eji.202048953. [DOI] [PubMed] [Google Scholar]
- 37.Gauthier L., Morel A., Anceriz N., Morel Y., Narni-mancinelli E., Vivier E., Gauthier L., Morel A., Anceriz N., Rossi B., et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity Article Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell. 2019;177:1701–1713. doi: 10.1016/j.cell.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 38.Ellwanger K., Reusch U., Fucek I., Wingert S., Ross T., Müller T., Schniegler-mattox U., Haneke T., Rajkovic E., Koch J., et al. Redirected Optimized Cell Killing (ROCK®): A Highly Versatile Multispecific Fit-for- Purpose Antibody Platform for Engaging Innate Immunity. mAbs. 2019;11:899–918. doi: 10.1080/19420862.2019.1616506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han D., Xu Z., Zhuang Y., Ye Z., Qian Q. Current Progress in CAR-T Cell Therapy for Hematological Malignancies. J. Cancer. 2021;12:326–334. doi: 10.7150/jca.48976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreno Cortes E., Garcia Robledo J., Booth N., Forero J., Castro J. Optimization of Third Generation Chimeric Antigen Receptor T Cells Targeting ROR1 for Hematological Malignancies. Blood. 2021;138:4804. doi: 10.1182/blood-2021-154127. [DOI] [Google Scholar]
- 41.Forero J., Moreno Cortes E., Garcia Robledo J., Booth N., Castro J. Preclinical NK Cell Platform for CAR Directed Therapies: Functional and Phenotypic Comparison Using a Rechallenge Cytotoxicity Assay. Blood. 2021;138:4805. doi: 10.1182/blood-2021-154176. [DOI] [Google Scholar]
- 42.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-rosenberg S., Hedrick C.C., et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galo J., Bruni D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity. 2020;52:55–81. doi: 10.1016/j.immuni.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 44.He X., Xu C. Immune Checkpoint Signaling and Cancer Immunotherapy. Cell Res. 2020;30:660–669. doi: 10.1038/s41422-020-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L., Chen J., Bae J., Li H., Sun Z., Moore C., Hsu E., Han C., Qiao J., Fu Y. Rejuvenation of Tumour-Specific T Cells through Bispecific Antibodies Targeting PD-L1 on Dendritic Cells. Nat. Biomed. Eng. 2021;5:1261–1273. doi: 10.1038/s41551-021-00800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kraman M., Faroudi M., Allen N.L., Kmiecik K., Gliddon D., Seal C., Koers A., Wydro M.M., Batey S., Winnewisser J., et al. FS118, a Bispeci Fi c Antibody Targeting LAG-3 and PD-L1, Enhances T-Cell Activation Resulting in Potent Antitumor Activity. Clin. Cancer Res. 2020;44:3333–3344. doi: 10.1158/1078-0432.CCR-19-3548. [DOI] [PubMed] [Google Scholar]
- 47.Cui X., Jia H., Xin H., Zhang L., Chen S., Xia S., Li X. A Novel Bispecific Antibody Targeting PD-L1 and VEGF With Combined Anti-Tumor Activities. Front. Immunol. 2021;12:1–13. doi: 10.3389/fimmu.2021.778978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen S.-H., Dominik P.K., Stanfield J., Ding S., Yang W., Kurd N., Llewellyn R., Heyen J., Wang C., Melton Z., et al. Dual Checkpoint Blockade of CD47 and L1 Using an Affinity-Tuned Bispecific Antibody Maximizes Antitumor Immunity. J. Immunother. Cancer. 2021;9:e003464. doi: 10.1136/jitc-2021-003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramaswamy M., Taeil K., Jones D.C., Ghadially H., Mahmoud T., Garcia A., Browne G., Zenonos Z., Puplampu-Dove Y., Riggs J.M., et al. Immunomodulation of T- and NK-Cell Responses by a Bispecific Antibody Targeting CD28 Homolog and PD-L1. Cancer Immunol. Res. 2022;10:200–214. doi: 10.1158/2326-6066.CIR-21-0218. [DOI] [PubMed] [Google Scholar]
- 50.Peper-gabriel J.K., Pavlidou M., Pattarini L., Morales-kastresana A., Jaquin J., Gallou C., Hansbauer E., Richter M., Lelievre H., Bossenmaier B., et al. The PD-L1/4-1BB Bispecific Antibody-Anticalin Fusion Protein PRS-344/S095012 Elicits Strong T_cell Stimulation in a Tumor-Localized Manne. Clin. Cancer Resear. 2022:1078–1082. doi: 10.1158/1078-0432.CCR-21-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Miguel M., Umana P., Luiza A., De Morais G., Moreno V., Calvo E. T-Cell—Engaging Therapy for Solid Tumors. Clin. Cancer Res. 2021;27:1595–1603. doi: 10.1158/1078-0432.CCR-20-2448. [DOI] [PubMed] [Google Scholar]
- 52.Rader C. Bispecific Antibodies in Cancer Immunotherapy. Curr. Opin. Biotechnol. 2020;65:9–16. doi: 10.1016/j.copbio.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minutolo N.G., Hollander E.E., Powell D.J., Jr. The Emergence of Universal Immune Receptor T Cell Therapy for Cancer. Front. Oncol. 2019;9:176. doi: 10.3389/fonc.2019.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feldmann A., Arndt C., Koristka S., Berndt N., Bergmann R., Bachmann M.P. Conventional CARs versus Modular CARs. Cancer Immunol. Immunother. 2019;68:1713–1719. doi: 10.1007/s00262-019-02399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samaranayake H., Wirth T., Schenkwein D., Räty J.K., Ylä-herttuala S. Challenges in Monoclonal Antibody-Based Therapies. Ann. Med. 2009;41:3890. doi: 10.1080/07853890802698842. [DOI] [PubMed] [Google Scholar]
- 56.Sanz L., Luis A., Sa D. Engineering Human Cells for in Vivo Secretion of Antibody and Non-Antibody Therapeutic Proteins. Curr. Opin. Biotechnol. 2011;22:924–930. doi: 10.1016/j.copbio.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Blanco B., Dominguez-Alonso C., Alvarez-Vallina L. Bispecific Immunomodulatory Antibodies for Cancer Immunotherapy. Clin. Cancer Res. 2021;27:5457–5464. doi: 10.1158/1078-0432.CCR-20-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaw A.R., Suzuki M. Oncolytic Viruses Partner With T-Cell Therapy for Solid Tumor Treatment. Front. Immunol. 2018;9:2103 . doi: 10.3389/fimmu.2018.02103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fajardo C.A., Guedan S., Rojas L.A., Moreno R., Arias-badia M., De Sostoa J., June C.H., Alemany R. Oncolytic Adenoviral Delivery of an EGFR-Targeting T-Cell Engager Improves Antitumor Ef Fi Cacy. Cancer Res. 2017;77:2052–2063. doi: 10.1158/0008-5472.CAN-16-1708. [DOI] [PubMed] [Google Scholar]
- 60.Stadler C.R., Bähr-mahmud H., Celik L., Hebich B., Roth A.S., Roth R.P., Karikó K., Türeci Ö., Sahin U. Elimination of Large Tumors in Mice by MRNA-Encoded Bispecific Antibodies. Nat. Med. 2017;23:815–817. doi: 10.1038/nm.4356. [DOI] [PubMed] [Google Scholar]
- 61.De Sostoa J., Fajardo C.A., Moreno R., Ramos M.D., Farrera-sal M., Alemany R. Targeting the Tumor Stroma with an Oncolytic Adenovirus Secreting a Fibroblast Activation Protein-Targeted Bispecific T-Cell Engager. J. Immunother. Cancer. 2019;7:19. doi: 10.1186/s40425-019-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang F., Wen W., Qin W. Bispecific Antibodies as a Development Platform for New Concepts and Treatment Strategies. Int. J. Mol. Sci. 2016;18:48. doi: 10.3390/ijms18010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Velasquez M.P., Bonifant C.L., Gottschalk S., Gottschalk S., Place D.T. Redirecting T Cells to Hematological Malignancies with Bispecific Antibodies. Blood. 2017;131:30–38. doi: 10.1182/blood-2017-06-741058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu L., Lam C.K., Long V., Widjaja L., Yang Y., Li H., Jin L., Burke S., Gorlatov S., Brown J., et al. MGD011, A CD19 x CD3 Dual-Af Fi Nity Retargeting Bi-Speci Fi c Molecule Incorporating Extended Circulating Half-Life for the Treatment of B-Cell Malignancies. Clin. Cancer Res. 2017;23:1506–1518. doi: 10.1158/1078-0432.CCR-16-0666. [DOI] [PubMed] [Google Scholar]
- 65.Fielding A.K., Ph D., Schuh A.C., Dombret H., Foà R., Bassan R., Arslan Ö., Sanz M.A., Ph D., Bergeron J., et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017;376:836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinelli G., Boissel N., Chevallier P., Ottmann O., Go N., Rambaldi A., Ritchie E.K., Papayannidis C., Tuglus C.A., Morris J.D., et al. Long-Term Follow-up of Blinatumomab in Patients with Relapsed/Refractory Philadelphia Chromosome e Positive B-Cell Precursor Acute Lymphoblastic Leukaemia: Final Analysis of ALCANTARA Study. Eur. J. Cancer. 2021;146:107–114. doi: 10.1016/j.ejca.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 67.DRUGS Blincyo FDA Approval History. [(accessed on 17 March 2022)]. Available online: https://www.drugs.com/history/blincyto.html.
- 68.Frey N.V., Porter D.L. Cytokine Release Syndrome with Novel Therapeutics for Acute Lymphoblastic Leukemia. Hematology. 2016;2016:567–572. doi: 10.1182/asheducation-2016.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Conde-royo D., Juárez-salcedo L.M., Dalia S. Management of Adverse Effects of New Monoclonal Antibody Treatments in Acute Lymphoblastic Leukemia Blinatumomab. Drugs Context. 2020;9:1–15. doi: 10.7573/dic.2020-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uy G., Stewart S., Baughman J., Rettig M., Chichili G., Bonvini E., Wigginton J., Lechleider R., Dipersio J. A Phase I Trial of MGD006 in Patients with Relapsed Acute Myeloid Leukemia (AML) J. Immunother. Cancer. 2014;2:P87. doi: 10.1186/2051-1426-2-S3-P87. [DOI] [Google Scholar]
- 71.Ravandi F., Bashey A., Stock W., Egan D. Complete Responses in Relapsed/Refractory Acute Myeloid Leukemia (AML) Patients on a Weekly Dosing Schedule of Vibecotamab (XmAb14045), a CD123 x CD3 T Cell-Engaging Bispecific Antibody; Initial Results of a Phase 1 Study. Blood. 2020;136:4–5. doi: 10.1182/blood-2020-134746. [DOI] [Google Scholar]
- 72.Lambert J., Pautas C., Terré C., Raffoux E., Turlure P., Caillot D., Legrand O., Thomas X., Gardin C., Gogat-marchant K., et al. Gemtuzumab Ozogamicin for de Novo Acute Myeloid Leukemia: Final Efficacy and Safety Updates from the Open-Label, Phase III ALFA-0701 Trial. Hematologica. 2019;104:113–119. doi: 10.3324/haematol.2018.188888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian Z., Liu M., Zhang Y., Wang X. Bispecific T Cell Engagers: An Emerging Therapy for Management of Hematologic Malignancies. J. Hematol. Oncol. 2021;14:75. doi: 10.1186/s13045-021-01084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Topp M.S., Duell J., Zugmaier G., Attal M., Moreau P., Langer C. Anti—B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma Abstract. J. Clin. Oncol. 2022;38:775–783. doi: 10.1200/JCO.19.02657. [DOI] [PubMed] [Google Scholar]
- 75.De Zafra C.L.Z., Fajardo F., Zhong W., Bernett M.J., Muchhal U.S., Moore G.L., Stevens J., Case R., Pearson J.T., Liu S., et al. Targeting Multiple Myeloma with AMG 424, a Novel Anti-CD38/CD3 Bispeci Fi c T-Cell—Recruiting Antibody Optimized for Cytotoxicity and Cytokine Release. Clin. Cancer Res. 2019;25:3921–3933. doi: 10.1158/1078-0432.CCR-18-2752. [DOI] [PubMed] [Google Scholar]
- 76.Kodama T., Kochi Y., Nakai W., Mizuno H., Baba T., Habu K., Sawada N., Tsunoda H., Shima T., Miyawaki K., et al. Anti-GPRC5D/CD3 Bispeci Fi c T-Cell -- Redirecting Antibody for the Treatment of Multiple Myeloma. Mol. Cancer Ther. 2019;18:1555–1564. doi: 10.1158/1535-7163.MCT-18-1216. [DOI] [PubMed] [Google Scholar]
- 77.Thakur A., Huang M., Lum L.G. Bispecific Antibody Based Therapeutics: Strengths and Challenges. Blood Rev. 2018;32:339–347. doi: 10.1016/j.blre.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 78.Kim Y., Kim H.Y.O.S., Cui Z.Y.U.N., Lee H., Ahn J.I.N.S., Park C.K., Park K., Ahn M. Clinicopathological Implications of EpCAM Expression in Adenocarcinoma of the Lung. Anticancer Res. 2009;1822:1817–1822. [PubMed] [Google Scholar]
- 79.Dudnichenko A.S., Aleknaviciene B., Razbadauskas A., Gore M., Ganea-motan E. The Trifunctional Antibody Catumaxomab for the Treatment of Malignant Ascites Due to Epithelial Cancer: Results of a Prospective Randomized Phase II/III Trial. Int. J. Cancer. 2010;2221:2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brinkmann U., Kontermann R.E. Bispecific Antibodies. Science. 2021;372:916–917. doi: 10.1126/science.abg1209. [DOI] [PubMed] [Google Scholar]
- 81.Kebenko M., Goebeler M., Wolf M., Hasenburg A., Seggewiss-bernhardt R. A Multicenter Phase 1 Study of Solitomab (MT110, AMG 110), a Bispecific EpCAM/CD3 T-Cell Engager (BiTE Ò) Antibody Construct, in Patients with Refractory Solid Tumors. Oncoimmunology. 2018;7:e1450710. doi: 10.1080/2162402X.2018.1450710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haruyama Y., Kataoka H. Glypican-3 Is a Prognostic Factor and an Immunotherapeutic Target in Hepatocellular Carcinoma. World J. Gastroenterol. 2016;22:275–283. doi: 10.3748/wjg.v22.i1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu L., Yang X., Huang N., Lang Q., He Q., Jian-hua W., Liang-peng G. A Novel Targeted GPC3/CD3 Bispecific Antibody for the Treatment Hepatocellular Carcinoma. Cancer Biol. Ther. 2020;21:597–603. doi: 10.1080/15384047.2020.1743158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Bono J., Fong L., Beer T., Gao X., Geynisman D., Burris H. Results of an Ongoing Phase 1/2a Dose Escalation Study of HPN424, a Tri-Specific Half-Life Extended PSMA-Targeting T-Cell Engager, in Patients with Metastatic Castration-Resistant Prostate Cancer (MCRPC) J. Clin. Oncol. 2021;39:5013. doi: 10.1200/JCO.2021.39.15_suppl.5013. [DOI] [Google Scholar]
- 85.Petra D., Thomas O., Nolan-Stevaux O., Li S., Wahl J., Bogner P., Aeffner F., Friedrich M., Liao M., Matthes K., et al. The PSMA-Targeting Half-Life Extended BiTE Therapy AMG 160 Has Potent Antitumor Activity in Preclinical Models of Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2021;27:2928–2937. doi: 10.1158/1078-0432.CCR-20-3725. [DOI] [PubMed] [Google Scholar]
- 86.Geyer M. First CAR to Pass the Road Test: Tisagenlecleucel’s Drive to FDA Approval. Clin. Cancer Res. 2020;25:1133–1135. doi: 10.1158/1078-0432.CCR-18-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rodgers D.T., Mazagova M., Hampton E.N., Cao Y., Ramadoss N.S., Hardy I.R., Schulman A., Du J., Wang F., Singer O., et al. Switch-Mediated Activation and Retargeting of CAR-T Cells for B-Cell Malignancies. Proc. Natl. Acad. Sci. USA. 2016;113:E459–E468. doi: 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moghanloo E., Mollanoori H., Talebi M., Pashangzadeh S. Translational Oncology Remote Controlling of CAR-T Cells and Toxicity Management: Molecular Switches and next Generation CARs. Transl. Oncol. 2021;14:101070. doi: 10.1016/j.tranon.2021.101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.