Abstract
In situ poly(hydroxyalkanoate) (PHA) levels and repeating-unit compositions were examined in stratified photosynthetic microbial mats from Great Sippewissett Salt Marsh, Mass., and Ebro Delta, Spain. Unlike what has been observed in pure cultures of phototrophic bacteria, the prevalence of hydroxyvalerate (HV) repeating units relative to hydroxybutyrate (HB) repeating units was striking. In the cyanobacteria-dominated green material of Sippewissett mats, the mole percent ratio of repeating units was generally 1HB:1HV. In the purple sulfur bacteria-dominated pink material the relationship was typically 1HB:2HV. In Sippewissett mats, PHA contributed about 0.5 to 1% of the organic carbon in the green layer and up to 6% in the pink layer. In Ebro Delta mats, PHA of approximately 1HB:2HV-repeating-unit distribution contributed about 2% of the organic carbon of the composite photosynthetic layers (the green and pink layers were not separated). Great Sippewissett Salt Marsh mats were utilized for more extensive investigation of seasonal, diel, and exogenous carbon effects. When the total PHA content was normalized to organic carbon, there was little seasonal variation in PHA levels. However, routine daily variation was evident at all sites and seasons. In every case, PHA levels increased during the night and decreased during the day. This phenomenon was conspicuous in the pink layer, where PHA levels doubled overnight. The daytime declines could be inhibited by artificial shading. Addition of exogenous acetate, lactate, and propionate induced two- to fivefold increases in the total PHA levels when applied in the daylight but had no effect when applied at night. The distinct diel pattern of in situ PHA accumulation at night appears to be related, in some phototrophs, to routine dark energy metabolism and is not influenced by the availability of organic nutrients.
Poly(hydroxyalkanoates) (PHAs) are intracellular lipid storage compounds accumulated by many types of bacteria. PHAs are of technological and commercial interest because the extracted materials are thermoplastics which can be processed into a variety of consumer goods and medical devices. In contrast to petroleum-based plastics, these biologically produced polymers are synthesized from renewable resources and are completely biodegradable. Much work has been done toward understanding and enhancing the production, material properties, and biodegradability of PHAs (14, 28). In conjunction with these efforts, the enzymology and genetics of PHA-producing and -degrading organisms have been extensively studied (25, 55), so that a considerable body of knowledge about the laboratory production of PHA has accumulated.
However, little is known about the ecological role of PHAs in the indigenous bacterial populations of natural ecosystems. PHAs have been detected in many natural environments, e.g., estuarine and intertidal sediments (4, 19, 24), groundwater aquifers (65), gypsum-rich sands in New Mexico (4), sewage sludge (11, 64), rivers (21, 33), lakes (17, 22, 38, 48, 59), root nodules (26, 43), microbial mats (7, 34, 37), and deep-sea hydrothermal mound sediments (23). Pure cultures have been studied to better understand the involvement of PHA in environmentally significant physiological processes of prokaryotes such as sporulation (15), starvation resistance and stress response (35, 39), nitrogen fixation and the associated modulation of reducing power (10, 16), and dark metabolism of purple sulfur bacteria (57). In complex natural ecosystems, however, experimental evidence to confirm such functions is very difficult to obtain and therefore is quite scarce.
The composition of PHAs found in nature is another question of considerable interest. It had long been thought that poly(3-hydroxybutyrate) (PHB) was the ubiquitous polymer in nature and that the heteropolymers of more commercially useful composition such as poly(3-hydroxybutyrate/3-hydroxyvalerate) (PHB/V) and poly(hydroxyoctonoate) (PHO) could be produced in the laboratory only by feeding bacteria particular substrates (27, 29). While most earlier studies reported that PHB was the routinely detected lipid storage polymer in nature, more contemporary analysis techniques employing gas chromatography (GC), high-pressure liquid chromatography (HPLC), and mass spectroscopy have generally revealed more complex naturally occurring PHAs (4, 7, 11, 19, 64). Findlay and White (19) detected 11 different β-hydroxy fatty acids in estuarine sediment; sewage sludge analyzed by Odham et al. (46) contained significant amounts of β-hydroxybutyric, β-hydroxyhexanoic, and β-hydroxyoctanoic acids; and deep-sea hydrothermal mound sediment has been shown to contain PHA dominated by β-hydroxyoctanoic and β-hydroxy-decanoic acid repeating units (23). In environmental samples such as sewage sludge (11, 64), cyanobacterial biomass (7), gypsum-rich sediments (4), and estuarine sediments (19), 3-hydroxyvalerate (HV) repeating units were more prevalent than 3-hydroxybutyrate (HB) repeating units.
Here we report (i) the detection of considerable amounts of PHA (consisting of HB and HV repeating units) in the photosynthetic layers of the stratified microbial mats of Great Sippewissett Salt Marsh on Cape Cod, and the Ebro Delta, Spain, and (ii) an in situ light-dependent diel pattern of PHA levels in these complex mat environments.
MATERIALS AND METHODS
Mat samples.
Stratified microbial mats from the Great Sippewissett Salt Marsh on the western coast of Cape Cod, Mass. (Fig. 1), and from the La Banya spit of the Ebro Delta in Catalonia, northeastern Spain (Fig. 2), were utilized for the studies reported here. These mat assemblages are stratified communities of photoautotrophs (diatoms, cyanobacteria, purple sulfur bacteria [PSB], and green sulfur bacteria), chemoautotrophs (colorless sulfur bacteria [CSB] such as Thiobacillus and Beggiatoa), and heterotrophs (sulfate-reducing bacteria [SRB] fermentative bacteria, and various aerobic and anaerobic respiratory bacteria) that develop, typically, on the sand flats of intertidal and/or frequently inundated zones of marine coastal environments. The fine microzonation of the various strata of these mats has been described by other workers (40, 45). For this study we made only three gross layer distinctions: (i) the upper green layer, which is usually 1 to 3 mm thick and, while inhabited by every type of organism described above, is physically dominated by a dense mesh of filamentous cyanobacteria (this “cyano/green” layer is the site of oxygenic photosynthesis); (ii) the underlying pink layer, which is the primary site of anoxygenic photosynthesis by PSB (this 1- to 3 mm-thick “PSB/pink” layer is so heavily populated by pink- and red-pigmented purple sulfur bacteria that most of the year it has an intense bright pink color); and (iii) the bottom, nonphotosynthetic zone, which is anoxic and blackened by the presence of metal sulfides.
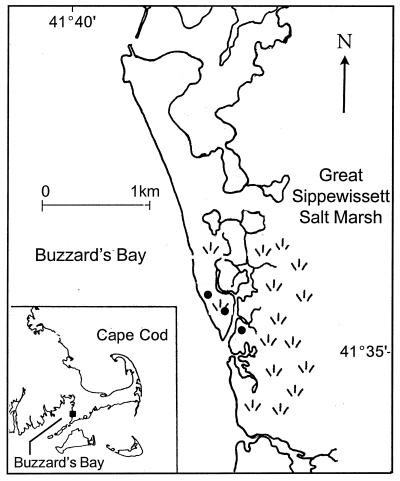
FIG. 1.
Location map of the Great Sippewissett Salt Marsh, Cape Cod, Mass. The salt marsh is located on the eastern coast of Buzzard's Bay between West Falmouth and Woods Hole. The study sites, near the main channel of the tidal river, are marked with dots.
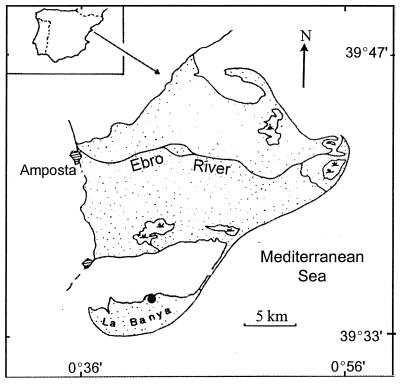
FIG. 2.
Location map of the Ebro Delta in Catalonia, northeastern Spain. The study site, on the northern shore of the La Banya spit, is marked with a dot. The map in the inset corresponds to the Iberian peninsula.
Sampling for the seasonal and diel studies involved cutting sections of the mats, approximately 2 cm wide by 10 cm long by 1.5 cm deep, and rapidly freezing them in plastic containers on dry ice in the field. After transportation to the laboratory, the samples were stored at −80°C until they were prepared for PHA and organic carbon analysis. The Sippewissett Salt Marsh mat samples were thawed for a brief period for dissection into green material and pink material, and the black layer material and the interface between the photosynthetic layers were discarded. The separated mat material was refrozen and lyophilized. It was not possible to dissect the two photosynthetic layers of the more undulated mats from the Ebro Delta. After removal of the black layer, these Ebro samples were lyophilized and analyzed as a green-pink composite. All mat samples from Sippewissett that were used for the seasonal studies were harvested between noon and 2:00 p.m.
Finally, the diel studies also included examination of a laminated but barely cohesive material from the Massachusetts site referred to as pink sand. This material is made up of a rosy-pink upper layer and a peach lower layer and forms on tidal-stream banks in the Great Sippewissett Salt Marsh in July and August of each year due to extensive blooms of PSB. These blooms have neither an overlying cyanobacterial layer nor a black layer underneath. This pink-sand material was sampled with the barrel of a 60-ml syringe, and the cores were frozen, lyophilized, and processed for PHA and organic matter determination in the same manner as the layers of the stratified mat.
The PHA contents of soil, compost, and sewage sludge were analyzed for the sake of comparison to the mat materials. The soil sample was Hadley, Mass., garden soil; the compost was backyard garden compost from Hadley, Mass.; and the sewage sludge was obtained from the Chicopee, Mass., municipal water treatment plant.
PHA characterization and quantification.
We had previously determined by the flame ionization detector-GC method described by Comeau et al. (11) that the repeating-unit composition of the PHA from the photosynthetic layers of the mat involved solely HB and HV repeating units (data not shown). We were therefore able to utilize an adaptation of the much more convenient Karr et al. (26) HPLC method for detection of short-side-chain PHAs described by Brandl (5). In this procedure, the PHAs are depolymerized by base hydrolysis and the soluble monomeric units are characterized and quantified by HPLC analysis. This simple method is particularly advantageous for precise quantification studies, such as the diel fluctuations reported here, for several reasons. First, because the total biomass is hydrolyzed, there is no danger of inefficient polymer extraction by solvents. Second, because this is an aqueous reaction, there is no loss of analyte due to sequestration at an organic/aqueous interface as there might be after an acid methanolysis in chloroform. Finally, there is no danger of loss of material during transfer to other containers. Base hydrolysis was accomplished by heating 1 g of pulverized, lyophilized mat material in 3.0 ml of 2.5 N NaOH in polytetrafluoroethylene (PTFE)-taped screw-top 25-ml Corex tubes, sealed with Teflon-lined caps, at 100°C in a silicon oil bath for 1 h with brief intermittent removal of tubes for vortexing. After removal from the oil bath, the tubes were rapidly cooled in room temperature water, and 1 ml of 0.8 M Na2HPO4–KH2PO4 (pH 6.9) buffer and 0.5 ml of 10 N HCl were added. The mixture was centrifuged in the Corex tubes for 12 min at 6,800 × g, and the supernatants were filtered through 0.45-μm-pore-size filters. The PHA repeating units in the filtered hydrolysates (which are converted to their respective 2-alkenoic acids by the reaction) were separated on a 7.8- by 100-mm HPX-87 “fast-acid” column (Bio-Rad Laboratories, Hercules, Calif.) with 0.005 M sulfuric acid as the eluent at a flow rate of 0.6 ml/min. The repeating-unit derivatives were detected at 210 nm and identified by comparison of their retention times to the retention times of the hydrolysis products of commercially available purified PHB/V polymer from Aldrich Chemical Co., Milwaukee, Wis. (24% HV as indicated by the manufacturer and confirmed by nuclear magnetic resonance analysis). We also analyzed the PHA content of sewage sludge, soil, and compost by HPLC to compare their HB and HV contents to those of the photosynthetic mats. The soil and composts required different processing because of the presence of residual interfering materials after the base hydrolysis and neutralization steps described above. To produce an adequately clarified solution of sewage sludge hydrolysate, an alteration of the proportions of the mixture was required. A 200-mg portion of sewage sludge was hydrolyzed in 6.0 ml of 2.5 N NaOH and subsequently neutralized with 2.0 ml of 0.8 M Na2HPO4–KH2PO4 buffer and 1.0 ml of 10 N HCl. For the soil and compost, viscous and chromogenic material (presumably humic acids) remained in the hydrolysate after the usual preparation procedure. This problem was resolved by initially oxidizing 2 g of each sample of soil or compost with 5% hypochlorite solution in screw-cap Corex tubes at 37°C for 50 min with shaking. This digestion was followed by centrifugation at 5,900 × g for 15 min, a water wash of the pellet, a second centrifugation, and, finally, lyophilization of the remaining pellet. The pellets were then hydrolyzed in 4.5 ml of 2.5 N NaOH and neutralized with 1.5 ml of 0.8 M Na2HPO4–KH2PO4 buffer and 0.75 ml of 10 N HCl under the conditions described above. The putative humic acids were precipitated by acidification of the hydrolysate with HCl to about pH 3 and removed by filtration through a 0.45-μm syringe filter, resulting in a highly clarified solution suitable for HPLC.
Organic carbon determination.
The biomass content of the cyano/green layer was quite different from that of the PSB/pink layer. In addition, within a given layer there was considerable spatial and seasonal variation in biomass content. Therefore, the PHA levels were normalized to organic carbon. The amount of organic carbon was determined by the modified Mebius potassium dichromate “wet-combustion” procedure described by Nelson and Sommers (44).
Field manipulations.
Artificial in situ shading of the stratified mat and the pink sand of Sippewissett was accomplished by positioning white Styrofoam slabs a few millimeters above the surface so as to maintain the same temperature and gas exchange as the normal sunlight-exposed sites while blocking out light. The organic carbon amendments were made to blocks of stratified mat, approximately 10 cm long by 10 cm wide by 4 cm deep, that were cut and fit snugly into plastic containers with perforated sides. These containers of mat were submerged in larger plastic containers (about 20 by 20 by 10 cm) containing seawater amended with either 40 mM acetate, 40 mM lactate, 40 mM propionate, or nothing. The pH of all amended seawater baths was adjusted to 7.4 with NaOH. For the light assimilation test, samples were incubated (submerged) in the containers in full, bright sunlight for 6 h; for the dark assimilation test, samples were incubated (submerged) in the containers in the dark at nighttime for 6 h. After the incubation period, the samples were drained, frozen on dry ice, and subsequently handled and processed in the same manner as the samples for the seasonal and diel measurements.
Micrographs of Nile Blue A-stained cells.
Cell suspensions for smears were prepared from freshly collected mats. Green and pink layer material was prepared separately. Mat material was placed in a 0.1% pyrophosphate solution (approximately 5 g of mat/30 ml) and shaken for 30 to 60 min. After shaking, the sand grains were allowed to settle and the cell suspension was decanted into centrifuge tubes. After a 10-min centrifugation at 3,000 × g, the 30 ml of supernatant was discarded and the cell pellet was resuspended in 5 to 10 ml of water. Smears of these cell suspensions were heat fixed to glass microscope slides. The heat-fixed smears were stained with a 1% Nile Blue A solution by the method of Ostle and Holt (47) and observed under fluorescent light centered at 436 nm. Nile Blue A-stained PHA granules in the cells fluoresce orange.
RESULTS
Repeating-unit characterization.
HV repeating units were detected in the bulk PHA hydrolysate from every environment that was examined. The HV content of total PHA ranged from a low of 4 mol% in soil to a high of 73 mol% in pink sand (Table 1). In all of the phototroph-dominated environments, i.e., Great Sippewissett Salt Marsh stratified microbial mats, Great Sippewissett Salt Marsh pink sand, and Ebro Delta stratified mat, HV was a major, if not the predominant, component.
TABLE 1.
HV component of naturally occurring, in situ PHAa
| Location | Sampling date | Mol% of HVb |
|---|---|---|
| Sippewissett cyano/green layer | Oct 1995 | 54 |
| Jan 1996 | 27 | |
| May 1996 | 41 | |
| July 1996 | 42 | |
| Apr 1997 | 45 | |
| July 1997 | 33 | |
| July 1998 | 45 | |
| Oct 1998 | 49 | |
| Nov 1998 | 23 | |
| Sippewissett PSB/pink layer | Oct 1995 | 54 |
| Jan 1996 | 25 | |
| May 1996 | 59 | |
| July 1996 | 62 | |
| Apr 1997 | 64 | |
| July 1997 | 63 | |
| July 1998 | 60 | |
| Oct 1998 | 57 | |
| Nov 1998 | 50 | |
| Sippewissett pink sand | 73 | |
| Ebro Delta, composite mat (green plus pink layers) | 61 | |
| Sewage sludge | 59 | |
| Garden soil | 4 | |
| Compost | 7 |
In this study, PHA is the sum of the HB and HV components because no other repeating units were detected. Therefore, mol% HB = 100% − mol% HV.
The moles percent of the HV (and HB) repeating units were determined by computation from the weights of the respective compounds in the hydrolysate solutions. The HV unit is 16.3% heavier than the HB unit (molecular weights of 100 and 86, respectively).
In the purple sulfur bacteria-dominated pink materials (PSB/pink) of Great Sippewissett stratified mat, HV was the distinctly predominant repeating unit in PHA. In the spring, summer, and fall months the HV component made up approximately 55 to 65 mol% of the PHA in the pink layer of the stratified mat. The of HV in the pink sand was present at 73 mol% in midsummer. The only exception to HV predominance in PSB/pink material PHA occurred in the winter, when HV was present at only 25 to 50 mol%. In contrast, HB repeating units usually outnumbered HV units in the cyanobacteria-dominated green layer (cyano/green) of Great Sippewissett stratified mat (October 1995 was the only exception). In the mild or warm seasons of spring, summer, and fall, the HV content in the cyano/green layer ranged from 37 to 49 mol%, whereas on the coldest sampling dates (January 1996, −2°C; and November 1998, 11°C) the HV content was closer to 25 mol%. In the green/pink composite material of the Ebro Delta mats, the HV content was an average of 61 mol%, with some variation depending on the time of day.
The HB and HV components of PHA in sewage sludge, soil, and compost were also quantified (no attempt was made to detect any other repeating units in these environments). A predominance of HV repeating units, 59 mol%, was evident in sewage sludge. In distinct contrast to sewage sludge and microbial mats, HV was only a minor constituent of soil and compost PHA, being present at 4 and 7 mol%, respectively.
Note that in this study as well as other environmental investigations cited herein, the repeating-unit quantities reported are those of the bulk pool of PHA. The repeating units detected by HPLC or GC analysis are the gross hydrolysis products of all PHA polymers in the sample. These bulk quantities of repeating units do not offer any information about the repeating-unit composition of the individual hetero- and/or homopolymers from which they were derived. Also note that in this study, PHA is defined as the sum of the HB and HV components because no other repeating units were detected.
Seasonal PHA levels.
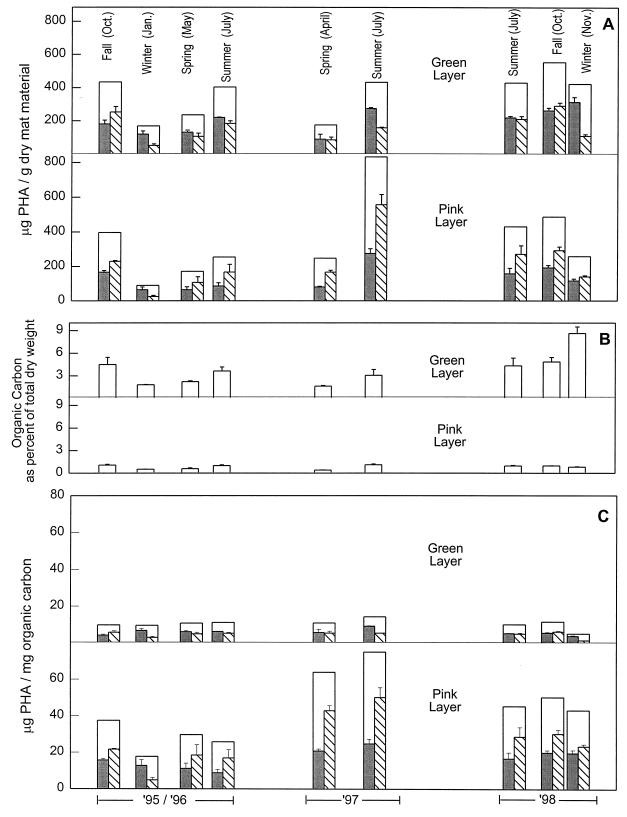
Midday levels of PHA in one of the stratified microbial mats of the Great Sippewissett Salt Marsh (Fig. 1) were monitored over a 3-year period (Fig. 3). When the PHA levels were normalized to total dry weight (Fig. 3A), they were highest in the summer and fall months. The total dry weight consisted primarily of bacterial biomass and quartz sand particles. The summer and fall PHA levels ranged from about 250 to 500 μg of PHA/g of dry mat (with the exception of an unusually high level of 830 μg/g in the pink layer material in July 1997), whereas the winter and spring PHA levels were only about 90 to 250 μg/g. The total dry-weight-normalized levels of PHA in the cyano/green layer were very similar to the levels in the PSB/pink layer.
FIG. 3.
Seasonal PHA and organic carbon content of the cyano/green layer, and the PSB/pink layer of stratified microbial mat in Great Sippewissett Salt Marsh. PHA levels (□) are reported as the sum of measured HB ( ) and HV (▧) repeating units. (A) PHA relative to the total dry weight of microbial mat material consisting of sand, biomass, and debris. (B) Organic carbon content of the mat layers. (C) PHA levels normalized to organic carbon. The histograms show means for three replicates and standard deviations.
) and HV (▧) repeating units. (A) PHA relative to the total dry weight of microbial mat material consisting of sand, biomass, and debris. (B) Organic carbon content of the mat layers. (C) PHA levels normalized to organic carbon. The histograms show means for three replicates and standard deviations.
The organic matter content of the mats varied with the seasons; it was greatest in the summer and the fall (Fig. 3B). The seasonal PHA levels relative to dry weight were directly related to the organic carbon content (compare Fig. 3A and B). The organic carbon content of the cyano/green layer mat material was typically near 4% of the total dry weight of the mat in the summer and fall but only about 2% in the winter and early spring months (with the conspicuous exception of November 1998, when the organic carbon content of that unusually dark and leathery green layer was 8.5%). The organic carbon content of the PSB/pink layer was considerably lower: only about 1% of the total dry weight in the summer and fall and about 0.5% in the cold months. Normalizing the PHA concentrations to organic carbon levels (Fig. 3C) revealed several facts. (i) PSB/pink layer organisms produced markedly more PHA than did cyano/green layer organisms. Levels of PHA in the PSB/pink layer ranged from 18 to 75 μg per mg of total organic carbon, whereas the PHA level in the cyano/green layer was typically about 10 μg per mg of organic carbon. (ii) Within a particular year, there was little seasonal variation in PHA content normalized to organic carbon. (iii) While the PHA content of the cyano/green layer remained fairly constant over the 3-year period, there were marked differences from year to year in the PSB/pink layer. (We have visually observed differences in the mats from year to year; e.g., the April 1997 pink layer was notably less colored than typical pink material, and the July 1997 pink layer was exceptionally thin [<1 mm].)
Diel fluctuation in PHA content.
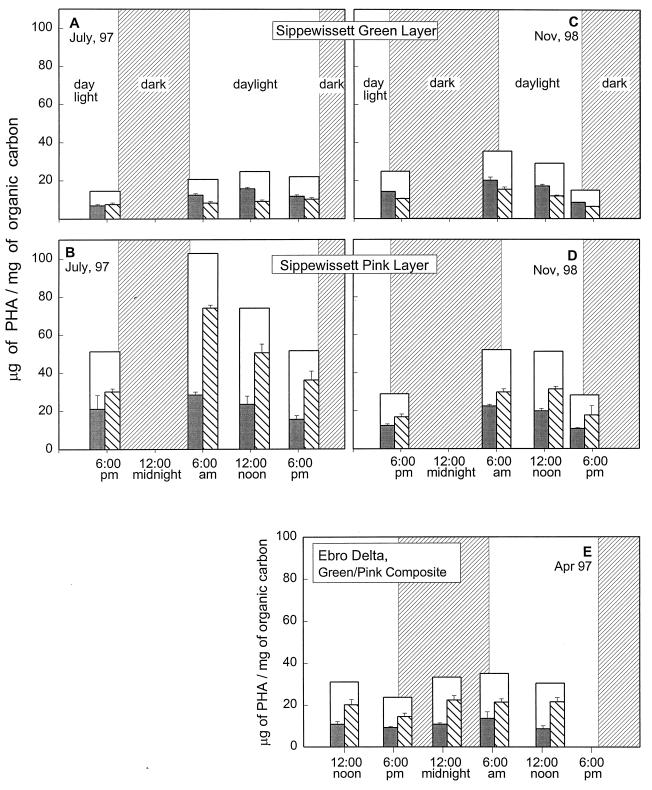
A distinct diel fluctuation of PHA levels was evident over all of the sampling periods in all of the phototroph-dominated environments examined in this study (Fig. 4 and 5). In each of these environments, PHA levels increased overnight and decreased over the course of the day. This pattern was most distinct in the PSB-dominated materials: the PHA content routinely doubled overnight, and declined by about half over the day (Fig. 4B and D and Fig. 5B and C). A similar but less pronounced diel pattern was also evident in the cyano/green materials over all of the sampling periods. The microbial mat material from Ebro Delta (Fig. 4E), which was a composite of cyano/green material and PSB/pink material, showed a diel pattern similar to that seen in the pink materials of Sippewissett Salt Marsh but with less pronounced differences between evening and dawn levels.
FIG. 4.
Diel fluctuations in stratified microbial mats. (A and B) Cyano/green (A) and PSB/pink (B) layers from Field Site 1 of Great Sippewissett Salt Marsh sampled over a dusk-to-dusk 24-h period in July 1997. (C and D) Cyano/green (C) and PSB/pink (D) layers from Great Sippewissett sampled over a 24-h dusk-to-dusk period in November 1998. (E) Composite of the photosynthetic layers of mat from the Ebro Delta, Spain, sampled over a noon-to-noon period. Bars are as in the legend to Fig. 3. All measurements were normalized to organic carbon.
FIG. 5.
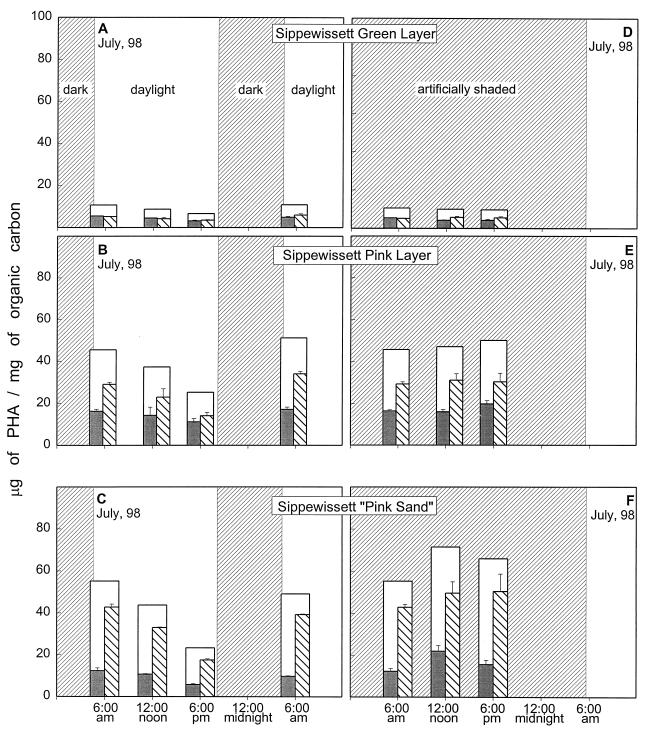
Diel patterns of PHA accumulation over a dawn-to-dawn 24-h period under normal light conditions (left panels) and artificially shaded conditions (right panels). (A and D) Cyano/green layer of stratified mat; (B and E) PSB/pink layer of stratified mat; (C and F) pink sand from the tidal stream bank. Bars are as in the legend to Fig. 3. All measurements were normalized to organic carbon.
The dependence of the PHA decline on light was tested by artificially shading areas of the mat that were immediately adjacent to the primary sites. In the shaded samples, there was no decline in PHA concentration over the course of the day; for the pink material, there was instead a slight additional increase (Fig. 5, right panels).
Differences in overnight accumulation of HB and HV in Sippewissett materials.
The net overnight increases in the PHA levels during each of the monitoring periods described above generally occurred with proportional increases in the HB and HV components. However, there was a marked increase in the relative amount of HV accumulation in comparison to HB accumulation in the pink materials during the midsummer blooms of PSBs in the Great Sippewissett Salt Marsh (Table 2). In both July 1997 and July 1998, the overnight increases in HV units were approximately twice as great as the overnight increases in HB units. These large differences in accumulation rates shifted the repeating-unit composition of the polymers toward a notably higher HV content. For the pink sand, the distinction between the rates of overnight accumulation of the two types of repeating units was not so dramatic but the PHA in that material already had a very high HV content (72 mol%) at dusk.
TABLE 2.
Dusk-to-dawn changes in HB and HV repeating unit concentrations, and the mole percent of HV in the total PHA in PSB/pink material from Great Sippewissett Salt Marsh
| Sample | Sampling date | Amt of repeating unit at dawn relative to amt at previous dusk (%)
|
Mol% of HV in total PHA at:
|
||
|---|---|---|---|---|---|
| HB | HV | Dusk | Dawn | ||
| PSB/pink layer | July 1997 | 135 | 246 | 55 | 69 |
| July 1998 | 153 | 242 | 52 | 63 | |
| Pink sand | July 1998 | 160 | 215 | 72 | 78 |
Carbon amendments.
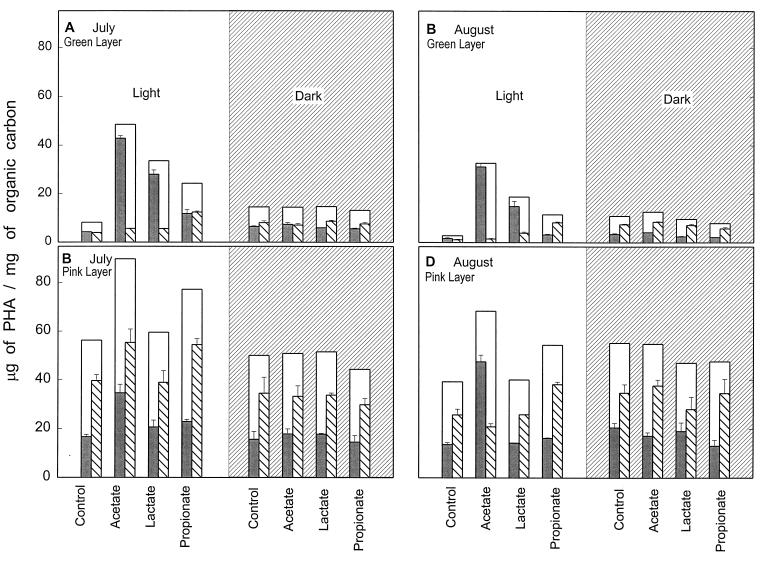
When Great Sippewissett microbial mats were amended by the addition of acetate, lactate, or propionate in full sunlight, PHA levels increased and the HB/HV distribution was affected (Fig. 6, light panels). This phenomenon was quite dramatic in the cyano/green layer, where acetate amendment led to a 10- to 18-fold increase in the HB component (July and August treatments, respectively) with no effect on the HV component. Lactate amendment produced similar effects in the green layer, although the increase in the HB component was more moderate (sixfold in July and ninefold in August). The HV component of the green layer was affected by propionate amendment: it increased threefold in the July treatment and sevenfold in the August treatment. The PSB/pink layer PHA was also affected by the organic amendments that were applied in the daylight, but to a much lesser degree. Acetate amendment led to a two- to threefold increase in the HB component, but lactate amendment had minimal effect. A slight increase in the HV component, about 1.5 times greater than the control, occurred with propionate amendment.
FIG. 6.
Effect of organic carbon amendment on PHA levels in stratified mats of Great Sippewissett Salt Marsh. The white panels represent the daylight exposure of mats to amended seawater, and the grey panels represent the night time exposure of mats to amended seawater. Bars are as in the legend to Fig. 3. All measurements were normalized to organic carbon.
In contrast, when mats were exposed to the organic carbon amendments in the dark, at night, there was no effect on either the HB or the HV component of PHA in either layer (Fig. 6, dark panels). PHA quantity and composition were not changed by the nighttime applications of acetate, lactate, or propionate.
Micrographs of PHA-accumulating organisms.
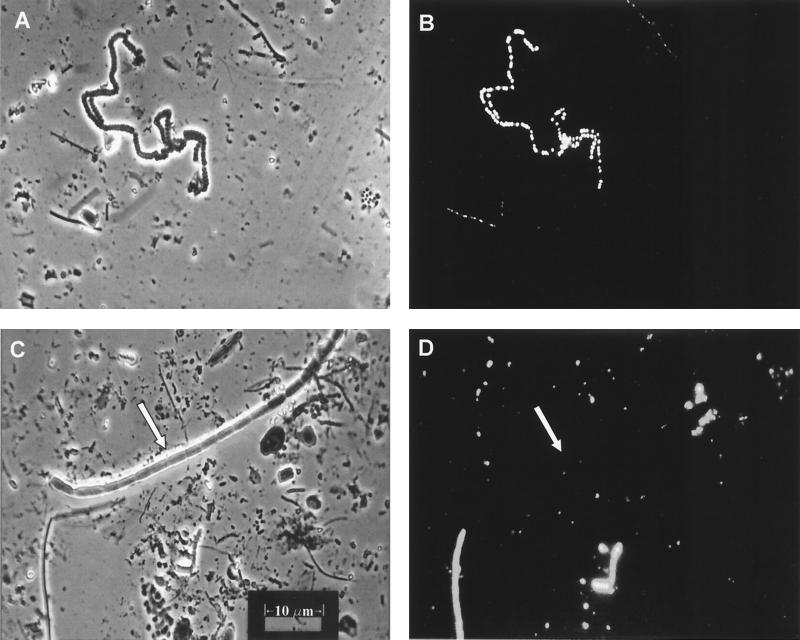
Large accumulations of fluorescing PHA granules were found in many types of cells in the microbial mat suspensions (Fig. 7 and 8). In cyano/green layer Nile Blue A slide preparations, PHA granules were routinely observed in some of the medium-sized, distinctly cyanobacterial, filaments (Fig. 7B, upper left, and Fig. 7D, lower left). PHA granules were never observed in the large Oscillatoria-type filaments such as the one visible across the upper half of Fig. 7C.
FIG. 7.
Micrographs of Nile Blue A-stained cells prepared directly from cyano/green layer mat material. (A) Exposure of a stained slide under incandescent light. (B) Same field as in panel A but exposed under fluorescent light. The fluorescing PHA granules of the cyanobacterium morphotype in the upper left of the field are distinctly visible. (C and D) Incandescent and fluorescent exposures, respectively, of another slide of the green layer material. Note the very large cyanobacterial filament marked by the arrow in panel C. This organism is not visible in panel D, indicating that it did not contain PHA granules.
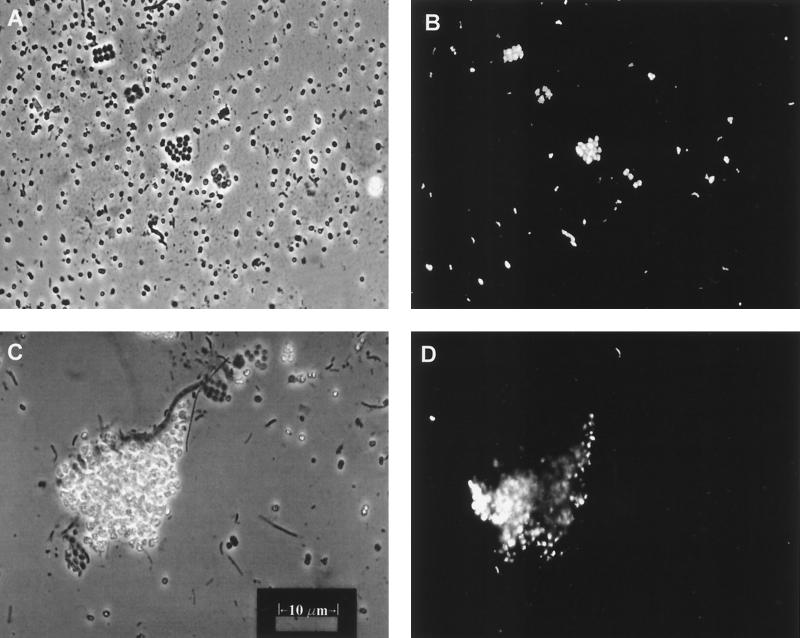
FIG. 8.
Micrographs of Nile Blue A-stained cells prepared directly from PSB/pink mat material. (A) Exposure of the stained slide under incandescent light. (B) Same field as in panel A but exposed under fluorescent light. The PHA granules in the packet morphotypes of PSB are quite visible, but none are apparent in the large, single-celled Chromatium-type sp. that is distributed evenly across the field. (C) Incandescent exposure showing a large clump of Thiocapsa-type cells glowing from phase-bright sulfur inclusions. (D) Fluorescent exposure showing that the cells in this clump also contain large inclusions of PHA (sulfur globules do not fluoresce).
In the slide preparations made from PSB/pink layer material, many PSB morphotypes contained PHA inclusions. These inclusions were most prevalent in the packet morphotypes (Fig. 8B) and the large masses of clumped PSB (Fig. 8D). While large, single-celled PSB such as those dispersed across the fields of Fig. 8A and C were commonly observed on the slides, these organisms rarely contained PHA granules. Note that while both sulfur globules and PHA granules are phase-bright and appear similar when observed under white light, sulfur globules do not absorb Nile Blue A stain and do not fluoresce.
DISCUSSION
We report here the natural, in situ, occurrence of PHA in materials obtained from a variety of photosynthetic benthic microbial mats as well as soil, compost, and sewage sludge. A striking prevalence of the HV repeating unit was observed in all of the phototrophic environments, as was a marked impact of the diel light-dark cycle on PHA concentrations.
Our 3-year seasonal study of multilayer microbial mat from the Great Sippewissett Salt Marsh showed little seasonal variability in PHA levels (normalized to organic carbon) in spite of considerable seasonal variations in mat biomass. Other studies of marine microbial mat systems (20, 49) have described a marked impact of seasonal variables (e.g., length of daylight, temperature, and nitrogen and phosphorus limitation) on mat biomass and community composition. The relatively stable ratio of PHA to organic carbon content observed in our study suggests that seasonal variables are not particularly influential factors in overall PHA levels. There were, however, significant differences in PHA content from year to year in the PSB/pink layer; this may have been due to the effects of random events such as storms and shifting sands that affect the development and maturity of the mats at Sippewissett.
In contrast to the relatively stable seasonal PHA content, there was distinct variation in PHA levels in the mats depending on the time of day. The diel pattern that we observed—overnight increase in PHA and daytime decrease—was remarkably similar throughout all the sites and types of mats that we monitored and at all seasons in the Sippewissett multilayered mat. Diel fluctuations of PHA levels in phototrophic environments have been reported by others. The PHA content of the planktonic cyanobacterium Trichodesmium thiebautii harvested from the Caribbean and Sargasso Seas peaked in the early morning and declined by 31% by nightfall (51). In 1985, van Gemerden et al. reported a decline in PHA levels from 3 to 1 μmol/liter over a daylight period in PSB-rich Lake Cisó water, followed by an increase during the night to almost 8 μmol/liter (59). Esteve et al. (17) determined the PHA content of PSB from Lake Cisó by electron microscopic examination of ultrathin sections of these morphologically distinctive cells and observed that the surface area of the PHA inclusion bodies of cells harvested at noon was only about half that of cells collected at 6:00 a.m.
The activities of the various functional groups of organisms (photosynthetic, heterotrophic, sulfur oxidizing, sulfate reducing) present in a microbial mat fluctuate dramatically with the light and dark periods of the diel cycle, resulting in steep fluctuating gradients of oxygen and sulfide (56). Redox conditions change from oxygen saturated during the day to sulfide rich and highly reduced at night. Also, obviously, the radiant energy that drives the system is unavailable at night. The extreme fluctuations of physical and chemical parameters (e.g., light, pH, anoxia, and electron donor availability) have been correlated with the accumulation and utilization of storage compounds such as glycogen, polyphosphate, zero-valent sulfur, and PHB in phototrophic bacteria in a number of studies (3, 13, 36, 52, 53, 57, 58, 60). Glycogen biosynthesis even competes with growth for Calvin cycle intermediates (3, 13, 58, 60). It appears likely that storage compounds contribute to nighttime energy production and competitive advantage in a complex environment (3, 36, 54). The necessity for organisms to adapt to extreme environmental fluctuations by alternate production and utilization of storage compounds provides a possible explanation for the diel pattern of PHA accumulation in the in situ microbial mats that we examined.
The indigenous populations of photosynthetic bacteria in natural environments may be carrying out a PHA-producing dark metabolism similar to that described for Chromatium vinosum by van Gemerden in 1968 (57). van Gemerden proposed that to generate ATP in the dark, C. vinosum (referred to at that time as strain 6412) fermented glycogen that had been accumulated photosynthetically during the previous light period. van Gemerden found that C. vinosum did not excrete by-products such as acetate, as might be expected from the fermentation of glycosyl units (54), but instead formed PHB. The PHB would have presumably been formed via the condensation of acetyl coenzyme A molecules (acetyl-CoA) derived from glycolytically produced pyruvate into the PHB precursor molecule, acetoacetyl-CoA. NAD+ was regenerated in part by reduction of acetoacetyl-CoA to β-hydroxybutyryl-CoA and in part by the associated reduction of elemental sulfur to H2S. van Gemerden's dark metabolism experiments with C. vinosum (57) produced molar ratios very close to 1 glucosyl moiety:3S0:1 PHB monomer:3H2S. The stoichiometry for this scheme is (C6H10O5)n + n H2O + 3n S0 → (C4H6O2)n + 2n CO2 + 3n H2S
When PHB is produced in the dark in the manner described above, only three ATP molecules are conserved when one glucosyl moiety is utilized. It may, at first consideration, appear that this PHB-producing scheme is not as efficient a way to conserve energy as the excretion of acetate; i.e., if the acetyl-CoA molecules produced after pyruvate decarboxylation are phosphorylated instead of condensed as described above, two more ATP molecules can be generated from the acetyl phosphates (with acetate then excreted from the cell). However, in the conservation of five ATP molecules by acetate excretion, six carbon atoms would be lost (two as CO2 and four as acetate). These six carbon atoms initially cost 18 ATP molecules to fix photosynthetically, and there would also be eight reducing equivalents produced, which must then be utilized via S0 respiration or some other form of deposition. However, for PHB production, only two carbon atoms are lost (as CO2) and only six reducing equivalents must be utilized.
While this PHB-producing metabolism appears favorable for nighttime energy conservation in S0-containing phototrophs, our data clearly show that more HV than HB is produced in the microbial mats during the nighttime. As shown in Table 3, this is also the case in all reports of PHA occurrence in phototroph-dominated environments where the repeating-unit composition was precisely characterized. The “algal mats” of Shark Bay are formed by cyanobacteria, and the PHA from this environment has a large HV component (7). Autotrophically grown Oscillatoria limosa and Spirulina subsalsa isolated from a North Sea microbial mat accumulate an HV-containing PHA polymer which is atypical for pure cultures of cyanobacteria (53). The red sand zone of the White Sands National Monument sediment measured by Brandl (4) is populated by PSB, and the PHA in this material showed a 2HB:3HV ratio. Other studies of the Ebro Delta mats have reported an HV predominance (34, 37). Also, although the composition of PHA in the PSB from Lake Cisó has not been delineated in the literature, it has been observed that these organisms also tend to produce a 2HB:3HV polymer. These characterizations of PHA from natural environments are in marked contrast to the results of an extensive study by Liebergesell et al. (29) of the formation of PHA from a variety of organic substrates by pure cultures of photo- and chemolithotrophic organisms. Of the 15 strains of PSB that were screened, most did not produce any HV repeating units; only 3 strains produced HV, and this occurred only when cells were grown with fatty acids of odd-numbered carbons (propionate, valerate, or heptanoate). It is also interesting that while Wallen and Rohwedder (64) detected a distinct HV predominance in the PHA of sewage sludge (as did we) (Table 3), they could not produce any PHA containing HV in isolates they cultured from the same sewage sludge they had analyzed.
TABLE 3.
Naturally occurring PHA in various environments
| Environmental material | Reference | Amt of PHA in environment | Approx PHA repeating-unit ratios (mol%)
|
||
|---|---|---|---|---|---|
| HB | HV | Other HAs | |||
| GSa cyano/green layer, microbial mat | This study | 0.17–0.55 mg/g of dry mat material | 1 | 1 | NDb |
| GSa PSB/pink layer, microbial mat | This study | 0.09–0.83 mg/g of dry mat material | 2 | 3 | ND |
| Garden soil (Hadley, Mass.) | This study | 0.026 mg/g of dry soil | 1 | Trace | ND |
| Garden compost (Hadley, Mass.) | This study | 0.049 mg/g of dry compost | 1 | Trace | ND |
| Sewage sludge (Chicopee, Mass.) | This study | 22.3 mg/g of dry sludge | 2 | 3 | ND |
| Ebro Delta, microbial mat | This study | 5.0 mg/g of dry mat material | 2 | 3 | ND |
| Sewage Sludge | 64 | 1.3% of dry sludge | 1 | 5 | Trace |
| Intertidal sediment, mangrove area | 4 | 0.14 mg/g of dry sediment | 4 | 1 | ND |
| Gypsum-rich sand, red/green area | 4 | 0.095 mg/g of dry sediment | 2 | 3 | ND |
| “Algal” mat, Shark Bay, Australia | 7 | 0.02–0.03% (dry wt) of algal mat | 1 | 1 | |
| 1 | 4 | Trace | |||
| Plankton, Sargasso and Caribbean Seas | 51 | 1.8 mg/g of dry plankton | —c | — | — |
| River biofilm | 21 | 0.05–1 μg/cm2 of biofilm | — | — | — |
| Estuarine sediment | 19 | 31 μmol/g of dry sediment | 1 | 1 | 1 |
| Pond, lake, and lagoon water (Spain) | 48 | 50–500 μg/liter of water | NKd | NK | NK |
| Water, Lake Cisó, Spain | 59 | ≤8 μmol/liter of water | NK | NK | NK |
| Microbial mat, Ebro Delta, Spain | 37 | ≤1.456 mg/g of dry mat material | 2 | 3 | ND |
GS, Great Sippewissett Salt Marsh.
ND, not determined.
—, only PHB analyzed.
NK, not definitively known.
We considered the possibility that assimilation of exogenous organic compounds of odd-numbered carbons was the source of HV units in the microbial mat, as had been the case with PSB fed propionate, valerate, and heptanoate by Liebergesell et al. (29). Many cyanobacteria are known to ferment glycogen under anoxic conditions in the dark in order to produce maintenance energy and, in the process, to excrete typical fermentation products such as acetate, lactate, propionate, CO2, and ethanol (54) that could serve as substrates for other organisms. We did see apparent assimilation of acetate, lactate, and propionate into the PHA of the cyano/green layer and assimilation of acetate and propionate into the PHA of the PSB/pink layer; however, as described above, this occurred only in the light. Net accumulation of PHA, especially HV units in PSB/pink material (Table 2), occurred only at nighttime, and exogenous organic carbon was not incorporated at nighttime. Considering that natural, unamended, PHA accumulation occurs at night, it is not likely that the HV repeating units that accumulate overnight are formed from the uptake of exogenous compounds with odd numbers of carbon atoms. Apparently the nighttime production of naturally occurring PHA in phototrophs involves endogenous production of the five-carbon HV units by a pathway as yet undescribed in phototrophs. We suggest that HV production by phototrophs in the dark may occur in a manner similar to that proposed for HV production in two other quite different systems: in Rhodococcus rubrum grown on glucose (2) and in activated sewage sludge bacteria in aerobic/anaerobic digesters (50). According to these schemes, the production of HV repeating units occurs when one of the pyruvate molecules produced by glycolysis is metabolized to acetyl-CoA by the familiar route but the other pyruvate molecule is metabolized to propionyl-CoA by a route similar to the succinate-propionate pathway of propionate-producing bacteria. The acetyl-CoA and propionyl-CoA condense to 3-oxovaleryl-CoA, are then reduced to 3-hydroxyvaleryl-CoA, and are ultimately polymerized into PHA. No reducing equivalents are left over after PHV is produced. Such a pathway is reasonable for microbial mat phototrophs during anoxic dark periods. The advantage to these phototrophs would be in conserving one ATP molecule (or possibly two) for nighttime maintenance energy while retaining five of the six carbons of each glycosyl moiety utilized and preserving redox balance in the reduced, sulfide-rich nighttime environment of marine microbial mats.
Although we analyzed only two gross components of the microbial mats, i.e., the cyano/green layer and the PSB/pink layer, microbial mat ecosystems are in fact extremely complex. Cyanobacteria, CSB, PSB, and SRB are dominant contributors to the structure and function of the mats, while aerobic heterotrophs and fermentative organisms play important roles in oxygen utilization and organic carbon flow (56). The large, filamentous cyanobacteria markedly dominate the biomass of the cyano/green layer, but large numbers of CSB, PSB, and SRB as well as other heterotrophs inhabit this zone; and although the PSB are so numerous in the PSB/pink layer that it is colored intensely pink by their pigments, this zone is also inhabited by CSB, SRB, and other heterotrophs as well as some cyanobacteria (9, 45, 62, 63).
Several of our observations implicate the cyanobacteria, rather than heterotrophs or PSB, as the primary producers of PHA in the green material of the Sippewissett mat. First, PHA accumulation appears to be the result of phototrophic activity: natural PHA levels in the green layer rise and fall with the dark-light cycle, and artificially induced PHA changes as a result of organic carbon supplements occur only in the light. If heterotrophic production of PHA from excess carbon was a significant factor in overall PHA levels in the microbial mat, it would be reasonable to assume that incorporation of acetate, lactate, and propionate would occur in the dark as well as in the light or, indeed, especially in the dark, when oxygen is depleted in the mats, since limitation of oxygen can be a trigger for PHA production by heterotrophs (1). Second, different types of phototrophs appear to be responsible for the apparent photoassimilation of the supplemented organic carbon because the amount and the HB/HV characterization of the artificially induced PHA were quite different between the green and pink layers. If PSB occurring in the green layer were responsible for the PHA changes observed there, it would be reasonable to expect those changes to be similar to the changes observed in the pink layer, but they are strikingly different. Finally, cyanobacterial filamentous morphotypes from the Sippewissett mat green layer clearly do accumulate large PHA inclusions in situ, as illustrated in Fig. 7.
The occurrence of PHA in cyanobacteria-dominated natural environments is interesting, since very little is known about the function of PHA in cyanobacteria. These organisms do not have a complete tricarboxylic acid cycle; it is therefore unlikely that PHA could serve as an energy storage compound for them. Several possible roles for PHA in cyanobacteria have been suggested, including its use as a source of acetyl-CoA for biosynthesis or as a reduced compound that functions as an electron sink, but there is little experimental evidence demonstrating or confirming such functions (52). In spite of this, reports of autotrophically and mixotrophically produced PHA in laboratory cultures of cyanobacteria have been appearing in the literature since 1966 (6, 8, 41, 52, 61).
In contrast to the relatively few reports of PHA occurrence in cyanobacteria, PHA production by PSB has been frequently investigated in both pure culture (29, 57) and natural environments (17, 18, 34, 36–38, 59). PHA synthase genes from a variety of PSB have been cloned and sequenced (31, 32), and, in some cases the gene product has been isolated and characterized (30, 42). PSB grown in pure culture with acetate or other fatty acids can accumulate enough PHA to account for as much as 83% of the cell dry weight, although the amount is more commonly less than 25% (29). The in situ PHA from the Sippewissett PSB/pink layer constituted about 1.5 to 6% of the organic carbon in that layer (HB is 55.8% C, and HV is 60% C). Several factors suggest that it was the PSB in the pink material environment of Sippewissett mats, rather than heterotrophs or CSB, that were the producers of the PHA we detected. First, as indicated above, many pure-culture studies have demonstrated the ability of PSB to accumulate large stores of PHA. Second, the biomass of PSB can be distinctly dominant in the pink layer of mats. Although the actual numbers of CSB and SRB may equal or exceed the number of PSB in the pink layer (63), the biovolume of PSB may very well exceed that of the other organisms in that stratum. For example, Thiocapsa roseopersicina cells are 10 times bigger than Thiobacillus cells (62) and their biomass has been determined to be at least equal to (62) or possibly as much as 17 times greater than (12) that of the CSB. Third, PHA levels in the Great Sippewissett Salt Marsh pink materials are markedly affected by the light-dark cycle. Finally, PHA inclusions can be seen in a variety of PSB morphotypes from the Sippewissett microbial mat pink layer (Fig. 8).
In summary, we have determined that about 0.5 to 6% of the organic carbon in the photosynthetic layers of microbial mats is found in PHA. This is almost certainly an underestimate of the actual PHA in viable prokaryotic cells because our total organic carbon determinations would have included the carbon in detritus, extracellular slime, and eukaryotes. Second, the HV repeating unit is the major component of the microbial mat PHA, and this five-carbon unit does not appear to be synthesized from an exogenous organic substrate. Finally, the distinct diel pattern of in situ PHA accumulation at night and utilization in the light—evident in all the mat sites and seasons examined in this study—suggests that PHA production by phototrophs in natural environments could be a routine mode of energy metabolism that is not influenced by the availability of organic nutrients.
ACKNOWLEDGMENTS
We acknowledge the support of the National Science Foundation (MCB-9202419), the New Energy and Industrial Technology Development Organization of Japan, and the Institute of Catalan Studies of Spain.
We thank the Parc National of the Ebro Delta for access to the study site. We thank Sheila Browne of Mt. Holyoke College, South Hadley, Mass., for NMR analysis of the purified PHB/V polymer used for standards in this study. Finally, we are grateful to Ugo d'Ambrosio, Laia Calaf, and Alex Künzel for their tireless assistance in the processing and measurements of the mat materials.
REFERENCES
- 1.Anderson A J, Dawes E A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990;54:450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson A J, Williams D R, Dawes E A, Ewing D F. Biosynthesis of poly(3-hydroxybutyrate-co-3-valerate) in Rhodococcus ruber. Can J Microbiol. 1995;41(Suppl. 1):4–13. [Google Scholar]
- 3.Beeftink H H, van Gemerden H. Actual and potential rates of substrate oxidation and product formation in continuous cultures of Chromatium vinosum. Arch Microbiol. 1979;121:161–167. [Google Scholar]
- 4.Brandl H. The occurrence of poly(3-hydroxyalkanoates) in samples from natural ecosystems. In: Schlegel H, Steinbüchel A, editors. Proceedings of the International Symposium on Bacterial Polyhydroxyalkanoates. 1993. pp. 415–416. [Google Scholar]
- 5.Brandl H. A sensitive HPLC method for the detection of short-side-chain poly(3-hydroxyalkanoates) In: Schlegel H, Steinbüchel A, editors. Proceedings of the International Symposium on Bacterial Polyhydroxyalkanoates. 1993. pp. 441–442. [Google Scholar]
- 6.Campbell J, Stevens S E J, Balkwill D L. Accumulation of poly-β-hydroxybutyrate in Spirulina platensis. J Bacteriol. 1982;149:361–363. doi: 10.1128/jb.149.1.361-363.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capon R J, Dunlop R W, Ghisalberti E L, Jefferies P R. Poly-3-hydroxyalkanoates from marine and freshwater cyanobacteria. Phytochemistry. 1983;22:1181–1184. [Google Scholar]
- 8.Carr N G. The occurrence of poly-β-hydroxybutyrate in the blue-green alga, Chlorogloea fritschii. Biochim Biophys Acta. 1966;120:308–310. doi: 10.1016/0926-6585(66)90353-0. [DOI] [PubMed] [Google Scholar]
- 9.Castenholz R W. Microbial mat research: The recent past and new perspectives. NATO ASI Ser. 1994;G35:3–18. [Google Scholar]
- 10.Cevallos M A, Encarnación S, Leija A, Mora Y, Mora J. Genetic and physiological characterization of a Rhizobium etli mutant strain unable to synthesize poly-β-hydroxybutyrate. J Bacteriol. 1996;178:1646–1654. doi: 10.1128/jb.178.6.1646-1654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comeau Y, Hall K J, Oldham W K. Determination of poly-β-hydroxybutyrate and poly-β-hydroxyvalerate in activated sludge by gas-liquid chromatography. Appl Environ Microbiol. 1988;54:2325–2327. doi: 10.1128/aem.54.9.2325-2327.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Wit R, van den Ende F P, van Gemerden H. Mathematical simulation of the interactions among cyanobacteria, purple sulfur bacteria, and chemotrophic sulfur bacteria in microbial mat communities. FEMS Microbiol Ecol. 1995;17:117–136. [Google Scholar]
- 13.de Wit R, Van Gemerden H. Growth and metabolism of the purple sulfur bacterium Thiocapsa roseopersicina under combined light/dark and oxic/anoxic regimens. Arch Microbiol. 1990;154:459–464. [Google Scholar]
- 14.Doi Y. Microbial synthesis, physical properties, and biodegradability of polyhydroxyalkanoates. Macromol Symp. 1995;98:585–599. [Google Scholar]
- 15.Emeruwa A C, Hawirko R Z. Poly-β-hydroxybutyrate metabolism during growth and sporulation of Clostridium botulinum. J Bacteriol. 1973;116:989–993. doi: 10.1128/jb.116.2.989-993.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Encarnación S, Dunn M, Willms K, Mora J. Fermentative and aerobic metabolism in Rhizobium etli. J Bacteriol. 1995;177:3058–3066. doi: 10.1128/jb.177.11.3058-3066.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esteve I, Mas J, Gaju N, Guerrero R. Cellular content of storage inclusions in purple sulfur bacteria determined by ultrathin sections. Microbiología. 1996;12:563–570. [PubMed] [Google Scholar]
- 18.Esteve I, Montesinos E, Mitchell J G, Guerrero R. A quantitative ultrastructural study of Chromatium minus in the bacterial layer of Lake Cisó (Spain) Arch Microbiol. 1990;153:316–323. [Google Scholar]
- 19.Findlay R H, White D C. Polymeric beta-hydroxyalkanoates from environmental samples and Bacillus megaterium. Appl Environ Microbiol. 1983;45:71–78. doi: 10.1128/aem.45.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fong P, Zedler J, Donohoe R. Nitrogen vs. phosphorus limitation of algal biomass in shallow coastal lagoons. Limnol Oceanogr. 1993;38:906–923. [Google Scholar]
- 21.Freeman C, Lock M A, Marxsen J. Poly-beta-hydroxy alkanoate and the support of river biofilm metabolism following radical changes in environmental conditions. Hydrobiologia. 1993;271:159–164. [Google Scholar]
- 22.Guerrero R, Montesinos E, Pedrós-Alió C, Esteve I, Mas J, van Gemerden H, Hofman P A G, Bakker J F. Phototrophic sulfur bacteria in two Spanish Lakes: vertical distribution and limiting factors. Limnol Oceanogr. 1985;30:919–931. [Google Scholar]
- 23.Guezennec J, Rocchiccioli F, Maccaron-Gomez B, Khelifa N, Dussauze J, Rimbault A. Occurrence of 3-hydroxyalkanoic acids in sediments from the Guaymas basin (Gulf of California) FEMS Microbiol Ecol. 1998;26:335–344. [Google Scholar]
- 24.Herron J S, King J D, White D C. Recovery of poly-β-hydroxybutyrate from estuarine microflora. Appl Environ Microbiol. 1978;35:251–257. doi: 10.1128/aem.35.2.251-257.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jendrossek D. Microbial degradation of polyesters: a review on the extracellular poly(hydroxyalkanoic acid) depolymerases. Polymer Degrad Stabil. 1998;59:317–325. [Google Scholar]
- 26.Karr D B, Waters J K, Emerich D W. Analysis of poly-β-hydroxybutyrate in Rhizobium japonicum bacteroids by ion-exclusion high-pressure liquid chromatography and UV detection. Appl Environ Microbiol. 1983;46:1339–1344. doi: 10.1128/aem.46.6.1339-1344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lageveen R G, Huisman G W, Preusting H, Ketelaar P, Eggink G, Witholt B. Formation of polyesters by Pseudomonas oleovorans: effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-3-hydroxyalkenoates. Appl Environ Microbiol. 1988;54:2924–2932. doi: 10.1128/aem.54.12.2924-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenz R W. Poly-β-hydroxyalkanoates: polyesters of commerce produced by bacteria as reserve materials. SIM News. 1995;45:15–21. [Google Scholar]
- 29.Liebergesell M, Hustede E, Timm A, Steinbüchel A, Fuller R C, Lenz R W. Formation of poly(3-hydroxyalkanoates) by phototrophic and chemolithotrophic bacteria. Arch Microbiol. 1991;155:415–421. [Google Scholar]
- 30.Liebergesell M, Sonomoto K, Madkour M, Mayer R, Steinbüchel A. Purification and characterization of the poly(hydroxyalkanoic acid) synthase from Chromatium vinosum and localization of the enzyme at the surface of poly(hydroxyalkanoic acid) granules. Eur J Biochem. 1994;226:71–80. doi: 10.1111/j.1432-1033.1994.tb20027.x. [DOI] [PubMed] [Google Scholar]
- 31.Liebergesell M, Steinbüchel A. Cloning and molecular analysis of the poly(3-hydroxybutyric acid) biosynthesis genes of Thiocystis violacea. Appl Microbiol Biotechnol. 1993;38:493–501. doi: 10.1007/BF00242944. [DOI] [PubMed] [Google Scholar]
- 32.Liebergesell M, Steinbüchel A. Cloning and nucleotide sequences of genes relevant for biosynthesis of poly(3-hydroxybutyric acid) in Chromatium vinosum strain D. Eur J Biochem. 1992;209:135–150. doi: 10.1111/j.1432-1033.1992.tb17270.x. [DOI] [PubMed] [Google Scholar]
- 33.López N I, Floccari M E, Steinbüchel A, García A F, Méndez B S. Effect of poly(3-hydroxybutyrate)(PHB) content on the starvation-survival of bacteria in natural waters. FEMS Microbiol Ecol. 1995;16:95–102. [Google Scholar]
- 34.Macarrón B. Utilización de marcadores lipídicos en el estudio de la biomasa, la estructura, y el estado nutricional de las comunidades de los tapices microbianos del Delta del Ebro. Ph.D. thesis. Barcelona, Spain: Universitat Autònoma de Barcelona; 1998. [Google Scholar]
- 35.Malmcrona-Friberg K, Tunlid A, Mårdén P, Kjelleberg S, Odham G. Chemical changes in cell envelope and poly-β-hydroxybutyrate during short term starvation of a marine bacterial isolate. Arch Microbiol. 1986;144:340–345. [Google Scholar]
- 36.Mas J, Van Gemerden H. Storage products in purple and green sulfur bacteria. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. 2nd ed. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 973–990. [Google Scholar]
- 37.Mas-Castellà J. Acumulación de poli-β-hidroxialcanoatos por bacterias. Distribución en la naturaleza y biotecnología. Ph.D. thesis. Barcelona, Spain: Universidad de Barcelona; 1991. [Google Scholar]
- 38.Mas-Castellà J, Guerrero R. Poly(β-hydroxyalkanoate) accumulation in bacterioplankton from Lake Cisó (Spain) Can J Microbiol. 1995;41(Suppl. 1):80–83. [Google Scholar]
- 39.Matin A, Veldhuis C, Stegeman V, Veenhuis M. Selective advantage of a Spirillum sp. in a carbon-limited environment. Accumulation of poly-β-hydroxybutyric acid and its role in starvation. J Gen Microbiol. 1979;112:349–355. doi: 10.1099/00221287-112-2-349. [DOI] [PubMed] [Google Scholar]
- 40.Mir J, Martínez-Alonso M, Esteve I, Guerrero R. Vertical stratification and microbial assemblage of a microbial mat in the Ebro Delta (Spain) FEMS Microbiol Ecol. 1991;86:59–68. [Google Scholar]
- 41.Miyake M, Erata M, Asada Y. A thermophilic cyanobacterium, Synechococcus sp. MA19, highly accumulating poly-β-hydroxybutyrate. J Ferment Bioeng. 1996;82:516–581. [Google Scholar]
- 42.Muh U, Sinskey A J, Kirby D P, Lane W S, Stubbe J. PHA synthase from Chromatium vinosum: cysteine 149 is involved in covalent catalysis. Biochemistry. 1999;38:826–837. doi: 10.1021/bi9818319. [DOI] [PubMed] [Google Scholar]
- 43.Ndoye I, Debilly S F, Vasse J, Dreyfus B, Truchet G. Root nodulation of Sesbania rostrata. J Bacteriol. 1994;176:1060–1068. doi: 10.1128/jb.176.4.1060-1068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson D W, Sommers L E. Total carbon, organic carbon, and organic matter, p. 539–580. In Methods of soil analysis, part 2. Chemical and microbiological properties. (Agronomy monograph no. 9, 2nd ed.). Madison, Wis: American Society of Agronomy, Inc., and Soil Science Society of America; 1982. [Google Scholar]
- 45.Nicholson J A, Stolz J F, Pierson B K. Structure of a microbial mat at Great Sippewissett Marsh, Cape Cod, Massachusetts. FEMS Microbiol Ecol. 1987;45:343–364. [Google Scholar]
- 46.Odham G, Tunlid A, Westerdahl G, Mårdén P. Combined determination of poly-β-hydroxyalkanoate and cellular fatty acids in starved marine bacteria and sewage sludge by gas chromatography with flame ionization or mass spectrometry detection. Appl Environ Microbiol. 1986;52:905–910. doi: 10.1128/aem.52.4.905-910.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostle A G, Holt J G. Nile blue A as a fluorescent stain for poly-beta-hydroxybutyrate. Appl Environ Microbiol. 1982;44:238–241. doi: 10.1128/aem.44.1.238-241.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pedrós-Alió C, Mas-Castellà J, Mas J, Guerrero R. Polyhydroxyalkanoate accumulation in planktonic and anaerobic environments. In: Dawes E A, editor. Novel biodegradable microbial polymers. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 263–274. [Google Scholar]
- 49.Pinckney J, Paerl H, Fitzpatrick M. Impacts of seasonality and nutrients on microbial mat community structure and function. Mar Ecol Prog Ser. 1995;123:207–216. [Google Scholar]
- 50.Satoh H, Mino T, Matsuo T. PHA production by activated sludge. Internation J Biol Macromol. 1999;25:105–109. doi: 10.1016/s0141-8130(99)00021-5. [DOI] [PubMed] [Google Scholar]
- 51.Siddiqui P J A, Bergman B, Björkman P-O, Carpenter E J. Ultrastructural and chemical assessment of poly-β-hydroxybutyric acid in the marine cyanobacterium Trichodesmium thiebautii. FEMS Microbiol Lett. 1992;94:143–148. doi: 10.1016/0378-1097(92)90598-i. [DOI] [PubMed] [Google Scholar]
- 52.Stal L J. Poly(hydroxyalkanoate) in cyanobacteria: an overview. FEMS Microbiol Rev. 1992;103:169–180. [Google Scholar]
- 53.Stal L J, Heyer H, Jacobs G. Occurrence and role of poly-hydroxy-alkanoate in the cyanobacterium Oscillatoria limosa. In: Dawes E A, editor. Novel biodegradable microbial polymers. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 435–438. [Google Scholar]
- 54.Stal L J, Moezelaar R. Fermentation in cyanobacteria. FEMS Microbiol Rev. 1997;21:179–211. [Google Scholar]
- 55.Steinbüchel A, Hustede E, Leibergesell M, Pieper U, Timm A, Valentin H. Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol Rev. 1992;103:217–230. doi: 10.1111/j.1574-6968.1992.tb05841.x. [DOI] [PubMed] [Google Scholar]
- 56.van Gemerden H. Microbial mats: a joint venture. Mar Geol. 1993;113:3–25. [Google Scholar]
- 57.van Gemerden H. On the ATP generation by Chromatium in darkness. Arch Mikrobiol. 1968;64:118–124. doi: 10.1007/BF00406970. [DOI] [PubMed] [Google Scholar]
- 58.van Gemerden H, Beeftink H H. Specific rates of substrate oxidation and product formation in autotrophically growing Chromatium vinosum cultures. Arch Microbiol. 1978;119:135–143. [Google Scholar]
- 59.van Gemerden H, Montesinos E, Mas J, Guerrero R. Diel cycle of metabolism of phototrophic purple sulfur bacteria in Lake Cisó (Spain) Limnol Oceanogr. 1985;30:932–943. [Google Scholar]
- 60.van Gemerden H, Pieter, Visscher T, Jordi Mas. Environmental control of sulfur deposition in anoxygenic purple and green sulfur bacteria. In: Dawes E A, editor. Novel biodegradable microbial polymers. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 247–262. [Google Scholar]
- 61.Vincenzini M, Sili C, de Philippis R, Ena A, Materassi R. Occurrence of poly-β-hydroxybutyrate in Spirulina species. J Bacteriol. 1990;172:2791–2792. doi: 10.1128/jb.172.5.2791-2792.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Visscher P, van den Ende F P, Schaub B E M, van Gemerden H. Competition between anoxygenic phototrophic bacteria and colorless sulfur bacteria in a microbial mat. FEMS Microbiol Ecol. 1992;101:51–58. [Google Scholar]
- 63.Visscher P T, van Gemerden H. Sulfur cycling in laminated marine microbial ecosystems. In: Oremland R S, editor. Biogeochemistry of global change: radiatively active trace gases. New York, N.Y: Chapman & Hall; 1993. pp. 672–690. [Google Scholar]
- 64.Wallen L L, Rohwedder W K. Poly-β-hydroxyalkanoate from activated sludge. Environ Sci Technol. 1974;8:576–579. [Google Scholar]
- 65.White D C, Smith G A, Gehron M J, Parker J H, Findlay R H, Martz R F, Fredrickson H L. The ground-water aquifer microbiota: biomass, community structure, and nutritional status. Dev Ind Microbiol. 1983;24:210–211. [Google Scholar]