Abstract
The optimal treatment for osteoarticular infection due to multidrug-resistant tuberculosis strains (MDR-OATB) remains unclear. This study aims to evaluate the diagnosis, management and outcome of MDR-OATB in France. We present a case series of MDR-OATB patients reviewed at the French National Reference Center for Mycobacteria between 2007 and 2018. Medical history and clinical, microbiological, treatment and outcome data were collected. Twenty-three MDR-OATB cases were reported, representing 3% of all concurrent MDR-TB cases in France. Overall, 17 were male, and the median age was 32 years. Six patients were previously treated for TB, including four with first-line drugs. The most frequently affected site was the spine (n = 16). Bone and joint surgery were required in 12 patients. Twenty-one patients (91%) successfully completed the treatment with a regimen containing a mean of four drugs (range, 2–6) for a mean duration of 20 months (range, 13–27). Overall, high rates of treatment success were achieved following WHO MDR-TB treatment guidelines and individualized patient management recommendations by the French National TB Consilium. However, the optimal combination of drugs, duration of treatment and role of surgery in the management of MDR-OATB remains to be determined.
Keywords: MDR-TB, XDR-TB, bone, spinal
1. Introduction
Tuberculosis (TB) remains a major public health concern worldwide [1]. Extrapulmonary TB accounts for about one quarter of the TB cases, whereas osteoarticular TB (OATB) represents 10 to 15% of all extrapulmonary TB cases in Europe and the USA [2]. Sadly, there are no specific data for France. OATB onset is usually insidious and a long delay in diagnosis is common [2]. Spinal TB, which accounts for about 50% of OATB patients in most series, may lead to neurologic deficits and spinal deformity in up to 50% of cases [2,3]. In spinal TB, the thoracic and lumbar vertebrae are mostly involved, and multifocal non-contiguous bone destruction with relative disc preservation and paravertebral abscesses are common [3]. Extraspinal TB localizations are diverse, and prosthetic joint infections can also occur [4]. Overall, microbiologic confirmation of the diagnosis is rare, as samples are difficult to obtain [2].
According to the World Health Organization (WHO), in 2020, 150,000 people developed rifampin-resistant TB or multidrug-resistant TB (MDR-TB), defined as resistant to rifampin and isoniazid [1]. Due to disruptions in TB diagnosis and treatment during the COVID-19 outbreak, TB and MDR-TB cases are expected to increase globally [1]. Overall, MDR-TB-causing strains are estimated to be implicated in approximately 2% of all OATB cases [1,5].
Guidelines for the treatment of MDR-TB are based on those for pulmonary TB, as no specific recommendations for osteoarticular forms are available [6,7]. In this study, we present a 12-year national case series of MDR-OATB with the aim of describing the diagnosis, treatment, and outcome of these rare forms.
2. Materials and Methods
2.1. Study Population
We retrospectively included all consecutive bacteriologically-confirmed MDR-OATB cases reported to the French National Reference Center for Mycobacteria (NRC) from 1 January 2007 to 31 December 2018. The following data were retrieved from medical files, anonymized and collated in a database at the NRC: demographics, history of previous TB treatment, comorbidities, clinical presentation, TB localization, drug susceptibility testing results, treatment, adverse events, surgery and treatment outcome.
2.2. Definitions
Standard definitions of MDR- and XDR-TB were used (MDR-TB is a TB resistant to rifampin and isoniazid; XDR-TB is a MDR-TB also resistant to any fluoroquinolone and to at least one of three second-line injectable drugs (capreomycin, kanamycin and amikacin) [8]. The revised definition of XDR-TB released in 2021 was not used since the study includes cases of TB occurring before 2021 [9]. MDR-OATB cases refers to bacteriologically-confirmed cases of MDR-TB involving joints, bones or soft tissue adjacent to the affected bone or joint [8]. A bacteriologically-confirmed TB case is one from whom a biological specimen is positive by smear microscopy, culture or WHO-approved rapid diagnostics, i.e., genotypic methods.
Treatment outcome was assigned according to WHO definitions [8] and the proposal of Schwoebel et al. [10] in cases of treatment failure (Supplementary Table S1).
2.3. Drug Susceptibility Testing
Phenotypic drug susceptibility testing was performed using the proportion method [11]. Mutations involved in resistance to anti-TB drugs were identified by line probe assays (Genotype MTBDRplus and MTBDRsl, Hain Lifescience, Nehren, Germany) or in-house PCR combined with Sanger sequencing [12].
2.4. Statistical Analysis
Continuous variables are presented as mean (range) or median (interquartile range, IQR), and categorical variables as proportion. The rate of drug resistance was compared between MDR-OATB and overall MDR-TB strains using Fisher’s exact test. Statistical analysis was performed with Stata version 11.0 (StataCorp, College Station, TX, USA). Significance was determined as p < 0.05.
2.5. Ethical Considerations
The study protocol was approved by the Comité d’Ethique de la Recherche de Sorbonne Université (Approval Number: CER-2021-005) and registered on ClinicalTrials.gov (ID: APHP210051).
3. Results
3.1. Patient Characteristics
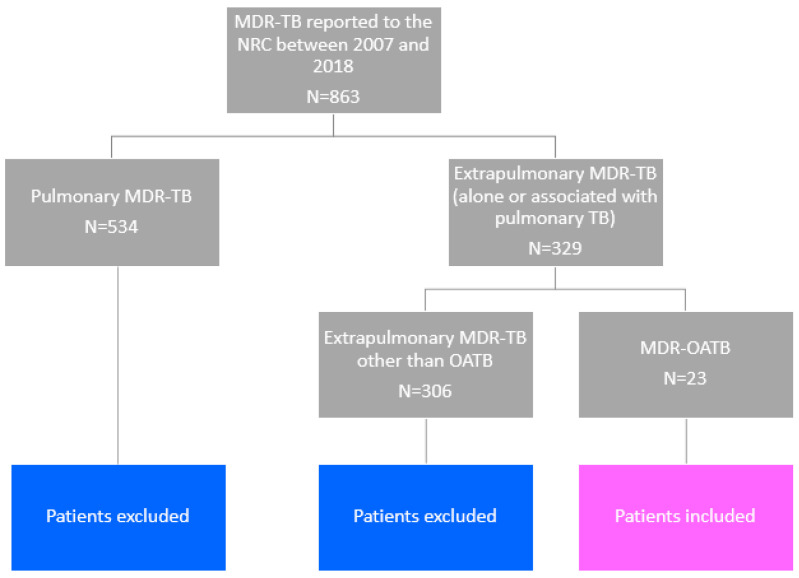
Overall, 863 MDR-TB cases were reported to the NRC between 2007 and 2018; among these, 329 were extrapulmonary (alone or associated with pulmonary TB) and 23 were MDR-OATB cases (2.7% and 7.0% of all MDR-TB and of extrapulmonary MDR-TB cases, respectively) (Figure 1).
Figure 1.
Flowchart for the study.
Among the 23 patients, the male/female sex ratio was 2.8/1 and the median age was 32 years (IQR, 25–40) (Table 1 and Table 2). Overall, six patients were previously treated for TB: four with first-line drugs, one with second-line drugs and one with an unknown treatment regimen. For the 23 patients, the median time from symptom onset to diagnosis was 3.5 months (IQR, 2–6). The most common clinical presentation was local pain (78.3%) and weight loss (52.2%). In most cases (n = 16), OATB affected the spine. In 14 patients, evidence of active TB was also found elsewhere, predominantly in the lung and lymph nodes (Table 1 and Table 2).
Table 1.
Characteristics of 23 patients with multidrug-resistant osteoarticular TB in France from 2007 to 2018.
| n | % | |
|---|---|---|
| Sex | ||
| Female | 6 | 26.1 |
| Male | 17 | 73.9 |
| Age (years) | ||
| 0–14 | 1 | 4.3 |
| 15–24 | 4 | 17.4 |
| 25–44 | 16 | 69.7 |
| 45–64 | 1 | 4.3 |
| ≥65 | 1 | 4.3 |
| Country of birth | ||
| Western Europe | 3 | 13.1 |
| Africa | 11 | 47.8 |
| Eastern Europe | 4 | 17.4 |
| Asia | 5 | 21.7 |
| Duration of stay in France for foreign-born patients (years) | ||
| <5 | 16 | |
| 5–10 | 4 | |
| >10 | 1 | |
| Previous TB treatment [8] | ||
| New patients | 17 | 73.9 |
| Previously treated patients | 6 | 26.1 |
| Relapse | 4 | |
| Treatment after failure | 1 | |
| Other previously treated patients | 1 | |
| Comorbidities 1 | ||
| HIV infection | 4 | 17.4 |
| Hepatitis B infection | 1 | 4.3 |
| Hepatitis C infection | 1 | 4.3 |
| Chronic renal failure | 1 | 4.3 |
| Immunosuppressive therapy | 1 | 4.3 |
| Body mass index < 18.5 | 7 | 30.4 |
| Clinical signs/symptoms | ||
| Pain | 18 | 78.3 |
| Weight loss | 12 | 52.2 |
| Cough | 6 | 26.1 |
| Neurological deficit | 5 | 21.7 |
| Fever | 5 | 21.7 |
| Median (IQR) C-reactive protein at diagnosis (mg/L) | 66 (21.4–114.3) | |
| Median (IQR) serum vitamin D at diagnosis (ng/L) | 6 (5–14.5) | |
| Delay between first symptoms and TB diagnosis (months) | ||
| <6 | 15 | 65.2 |
| 6–12 | 5 | 21.7 |
| ≥12 | 2 | 8.8 |
| Unknown | 1 | 4.3 |
| Tuberculin skin test | ||
| Positive (>10 mm) | 7 | 30.4 |
| Not done | 16 | 69.6 |
| Interferon-gamma release assay | ||
| Positive | 2 | 8.8 |
| Undetermined | 1 | 4.3 |
| Not done | 20 | 86.9 |
| Osteoarticular localization 2 | ||
| Spine | 16 | 69.6 |
| Hip joint | 4 | 17.4 |
| Knee joint | 2 | 8.8 |
| Ribs | 2 | 8.8 |
| Sacro-iliac joint | 3 | 13.1 |
| Calcaneum | 1 | 4.3 |
| Pulmonary TB associated | 9 | 39.1 |
1 Diabetes mellitus was not added to the table since no patient had diabetes. 2 Six patients had TB with multiple localizations.
Table 2.
Clinical features of multidrug-resistant osteoarticular tuberculosis in France from 2007 to 2018.
| Patient | Sex, Age (Years) | Comorbidities | Previous TB Treatment | Year of TB Diagnosis | Bone/Joint Localization | Other TB Localizations | Sample Used for Diagnosis | Histology of the Sample Used for Diagnosis | Treatment * | Surgical Treatment | Outcome (Post-Treatment Follow-Up) [8] § |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M, 42 | None | None | 2009 | Left hip joint | - | Joint aspirate | ND |
1–2[Am-Emb †-Lzd †-Mfx-PAS ‡]/ 3[Am-Mfx-PAS ‡]/ 4–6[Am †-Cs -Mfx-PAS ‡]/ 7–27[Cs-Mfx-PAS ‡] |
Debridement | Success § (5 years) |
| 2 | F, 65 | HIV and chronic renal failure | Isoniazid-monoresistant TB 4 years previously | 2009 | Right knee joint | - | Joint aspirate | Inflammatory joint fluid | 1–16[Emb-Mfx-Pza] | No surgery | Success (no post-treatment follow-up) |
| 3 | M, 41 | None | None | 2010 | C5–C6, prevertebral abscess | Lymph nodes, liver, spleen, lung | Prevertebral abscess | ND |
1–2[Am-Emb-Eto-Lzd-Mfx-Pza]/ 3[Am-Emb-Lzd-Mfx-Pza]/ 4–5[Emb-Lzd †-Mfx-Pza]/ 6–20[Emb-Mfx-Pza] |
Anterior vertebrectomy | Success (no post-treatment follow-up) |
| 4 | M, 48 | None | None | 2011 | T2 until the conus of spinal cord, left iliac bone | Kidneys, meningitis, lung | Urine | ND |
1–3[Am-Emb-Lzd †-Mfx-Pza]/ 4–6[Am-Emb-Mfx-PAS †-Pza]/ 7–19[Emb-Cs-Mfx-Pza] |
No surgery | Success § (no post-treatment follow-up) |
| 5 | F, 21 | None | None | 2012 | T3-T5 | Lymph nodes | Lymph node | Epithelioid granuloma with caseous necrosis |
1[Am-Lzd †-Mfx]/ 2–4[Am-Bdq-Mfx †-PAS]/ 5[Am-Lfx-PAS]/ 6–8[Am-Lfx-PAS †]/ 9–11[Am-Lfx]/ 12–19[Cfz-Lfx] |
No surgery | Success § (no post-treatment follow-up) |
| 6 | F, 40 | Hepatitis C infection | Several episodes of TB the last 20 years | 2013 | Ribs at T5–T6 level | Lymph nodes, lung | Pulmonary sample | ND |
1[Bdq-Cs-Emb-Lzd-Mpm/Clv-PAS]/ 2–7[Bdq †-Cs-Emb-Lzd-Mpm/Clv]/ 8–10[Cs-Emb-Lzd-Mpm/Clv-Pza]/ 11–25[Cs-Emb-Lzd-Pza] |
Lobectomy | Success (3 months) |
| 7 | M, 27 | None | None | 2013 | C6, T8, L3, S1 | Lung | Bone tissue | ND |
1[Am-Cs-Lzd-Mpm/Clv-Mfx-PAS]/ 2–6[Am-Cs-Lzd-Mfx-PAS †]/ 7–25[Cs-Lzd-Mfx-Pza] |
Laminectomy | Success (2 years) |
| 8 | M, 30 | None | Isoniazid-monoresistant TB 2 years previously | 2013 | T9–T12, prevertebral abscesses | - | Prevertebral abscess | ND |
1–4[Am-Cs-Emb-Lzd-Mfx-Pza]/ 5–10[Cs-Emb-Lzd-Mfx-Pza †]/ 11–20[Cs-Emb-Lzd-Mfx] |
No surgery | Success (no post-treatment follow-up) |
| 9 | M, 29 | None | None | 2014 | T12–L4, paravertebral abscess | - | Bone tissue | ND |
1–3[Am-Lzd-Mfx-PAS]/ 4–?[Lzd-Mfx-PAS] (duration not known due to loss of follow-up) |
Abscess drainage, stabilization | Not evaluated (loss of follow-up after 9 months of treatment) |
| 10 | F, 39 | None | None | 2014 | Left hip prosthetic joint, psoas abscess | - | Synovial tissue | Epithelioid granuloma with giant cells and necrosis |
1–2[Am-Cs-Lzd-Mfx-PAS]/ 3–7[Am-Lzd‡-Mfx-Pza-Rif]/ 8–24[Lzd‡-Mfx-Pza-Rif] |
Revision arthroplasty (two-stage exchange) | Success (2 years) |
| 11 | M, 32 | None | None | 2014 | L4–L5, L5–S1, right sacro-iliac joint | Lymph nodes, lung | Bone tissue | Granuloma and necrosis |
1–2[Am-Lfx-Lzd-Pza]/ 3–6[Am-Lzd-Mfx-Pza-Rif]/ 7–18[Lzd-Mfx-Pza-Rif] |
Vertebral cementoplasty | Success (6 years) |
| 12 | F, 39 | None | Isoniazid-monoresistant TB 2 years before | 2014 | Right calcaneum | - | Bone tissue | ND |
1[Am-Emb-Lzd-Mfx-Pza]/ 2[Am-Emb-Lzd-Mfx-Pza-Rif †]/ 3–6[Am-Emb-Lzd-Mfx-Pza]/ 7–12[Emb †Lzd †-Mfx-Pza]/ 13–19[Cs-Mfx-PAS-Pza] |
Resection then bone graft (after 1 year of antibiotic therapy) | Success § (3 years) |
| 13 | M, 15 | None | None | 2014 | T10–L1, pre- and paravertebral abscesses | - | Paravertebral abscess | ND |
1–5[Am-Bdq-Cs-Lfx-Lzd-Pza]/ 6–7[Bdq-Cs-Lfx-Lzd-Pza]/ 8–9[Cs-Lfx-Lzd-Pza]/ 10–20[Cs-Lfx-Lzd-Pza-Rif]/ 21–24[Lfx-Lzd-Pza] |
No surgery | Success (no post-treatment follow-up) |
| 14 | M, 27 | None | None | 2015 | T5, dorsal abscess | Lung | Paravertebral abscess | ND |
1–12[Emb †-Mfx-Pza-Rif]/ 13–15[Mfx-Pza-Rif] |
No surgery | Success despite diffuse arthralgia (2 years) |
| 15 | M, 20 | Rheumatoid arthritis (corticosteroids and methotrexate) | None | 2015 | L3–L4, paravertebral abscess | Lymph nodes | Bone tissue | Epithelioid granuloma with giant cells and necrosis |
1–6[Am-Cs-Lzd-Mfx-PAS-Pza †]/7[Am-Cs-Lzd-Mfx-PAS]/ 8–19[Cs-Lzd-Mfx-PAS] |
No surgery | Success despite back pain (2 months) |
| 16 | F, 39 | HIV | Drug-susceptible TB 3 years previously | 2015 | T11–T12, paravertebral abscess, left knee joint | - | Bone tissue | ND |
1–7[Am-Emb-Lzd-Mfx-Pza]/ 8–26[Emb-Lzd-Mfx-Pza] |
Resection arthroplasty | Success despite knee pain (3 years) |
| 17 | M, 31 | None | None | 2017 | C6–C7, paravertebral abscess | Spleen | Bone tissue | Epithelioid granuloma with giant cells and caseous necrosis |
1–4[Am-Cfx-Emb-Lzd-Mfx-Pza]/ 5–19[Cfx-Emb-Lzd-Mfx-Pza] |
No surgery | Success despite left sciatica S1, cervical paresthesia (1 year) |
| 18 | M, 40 | None | None | 2018 | C5, T10, T11, L1–L4, S1, pre- and paravertebral abscesses | Lung, lymph nodes, pleura, liver, spleen, small intestine | Bone tissue | Granuloma |
1[Am-Emb-Eto-Lzd-Mfx-Pza]/ 2–3[Am †-Bdq-Cfz-Lzd-Mfx-Pza]/ 4–19[Bdq-Cfz-Lzd-Mfx-Pza] |
Corpectomy and anterior cervical arthrodesis | Success (6 months) |
| 19 | M, 25 | None | None | 2018 | T4, T5, L1, L4, paravertebral and psoas abscesses | Lung, pleura | Bone tissue | Epithelioid granuloma with giant cells without necrosis |
1–2[Am-Bdq-Cfz-Cs-Lzd-Mfx]/3[Bdq-Cfz-Cs-Lzd-Mfx]/ 4–6[Bdq-Cfz-Dlm-Lzd-Mfx]/ 7–13[Bdq-Cfz-Dlm-Mfx] |
No surgery | Success (3 months) |
| 20 | M, 16 | Hepatitis B infection | None | 2018 | L3, S3, right hip joint | Lymph nodes, pleura, liver, peritoneum | Joint aspirate | Epithelioid granuloma with giant cells without necrosis |
1–2[Am-Eto-Lzd-Mfx-Pza]/ 3[Am-Emb-Eto-Lzd-Mfx-Pza]/ 4–5[Am †-Bdq-Emb-Eto †-Lzd-Mfx-Pza]/ 6–23[Bdq-Cfx-Emb †-Lzd-Mfx-Pza] |
Joint washing | Success § (1 year) |
| 21 | M, 35 | HIV | Ongoing pulmonary MDRTB treatment for two years | 2018 | L2–S1, psoas abscess | - | Psoas abscess | ND |
1–5[Bdq-Cfz-Lzd-PAS]/ 6–7[Bdq-Cfz-Dlm-Lzd-Mpm/Clv]/ 8–14[Bdq-Cfz-Cm-Dlm-Lzd-Mpm/Clv] 15–?[Bdq-Cm-Dlm-Lzd]-(duration not known due to loss of follow-up) |
Laminectomy | Not evaluated (loss of follow-up after 15 months of treatment) |
| 22 | M, 8 | None | None | 2018 | Right hip joint, gluteus abscess | Lymph nodes | Peri-joint abscess | ND | 1–18[Cs-Dlm-Eto-Lfx-Lzd] | Peri-joint and gluteus abscess drainage | Success (no post-treatment follow-up) |
| 23 | M, 39 | HIV | None | 2018 | Left seventh rib, both iliac bones | Lung, lymph nodes, pleura, liver, spleen | Pulmonary sample | ND |
1[Am-Emb-Lzd-Mfx-Pza]/ 2–3[Am-Dlm-Emb-Lzd †-Mfx-Pza]/ 4–19[Dlm-Mfx-Pza] |
No surgery | Success (6 months) |
F, female; M, male; TB, TB; Am, amikacin; Bdq, bedaquiline; Cfz, clofazimine; Cm, capreomycin; Cs, cycloserine; Dlm, delamanid; Emb, ethambutol; Eto, ethionamide; Inh, isoniazid; Km, kanamycin; Lfx, levofloxacin; Lzd, linezolid; Mpm/Clv, meropenem/clavulanate; Mfx, moxifloxacin; Ofx, ofloxacin; PAS, para-aminosalicyclic acid; Pto, protionamide; Rif, rifampin; Pza, pyrazinamide. * Treatments are shown as number of months followed by drugs administered in each phase. Different phases are divided by “/”. † Discontinued due to toxicity. Myelosuppression led to the withdrawal of linezolid (n = 2) and PAS (n = 1). Peripheral neuropathy was observed with linezolid in 2 cases. Gastro-intestinal symptoms were encountered with PAS (n = 2), ethionamide (n = 1) and pyrazinamide (n = 1). Ethambutol was withdrawn after retrobulbar optic neuritis in 2 cases, and both ethambutol and linezolid were withdrawn for the same reason in 2 cases. Two cases of renal insufficiency and one of ototoxicity occurred with amikacin. Bedaquiline was discontinued because of QT prolongation (n = 1). Hepatic disorders occurred with pyrazinamide, rifampin and moxifloxacin (n = 1 in each case). ‡ Toxicity without discontinuation. § Patients who would have been classified as treatment failure if we had applied the fourth criterion (discontinuation of ≥2 drugs) during the consolidation phase (Table S1).
3.2. Methods Used for the Diagnosis of OATB
All TB cases were bacteriologically-confirmed. OATB diagnosis was proven microbiologically for 19 patients and based on radiologic findings in four patients (Table 2). Positive cultures were obtained from bone or joint samples in 19 cases, and from other samples in four cases (Table 2). Histology was performed in less than half of the cases, even in cases of spinal TB, and was suggestive of TB (giant cell epithelioid granuloma with caseous necrosis) in eight out of nine patients (Table 2).
3.3. Drug Susceptibility Testing
Overall, resistance to antibiotics was significantly lower (p < 0.05) among MDR-OATB patients than among all MDR-TB patients diagnosed in France during the same period for streptomycin, ethambutol, pyrazinamide, kanamycin, cycloserine, fluoroquinolones, capreomycin and PAS (Table 3).
Table 3.
Resistance to anti-TB drugs (%) among strains isolated from patients with multidrug-resistant osteoarticular TB in France (n = 23) and among all strains isolated from MDR-TB patients (n = 863), from 2007 to 2018.
| Drug | MDR-OATB 1 n (%) |
MDR-TB n (%) |
p-Value |
|---|---|---|---|
| Rifampin | 18 (78) high level/5 (22) low level 2 | 837 (97) high level/26 (3) low level | <0.01 |
| Protionamide | 15 (65) | 673 (78) | 0.06 |
| Streptomycin | 15 (65) | 811 (94) | <0.01 |
| Ethambutol | 12 (52) | 681 (79) | <0.01 |
| Pyrazinamide | 6 (26) | 379 (44) | 0.01 |
| Kanamycin | 3 (13) | 267 (31) | <0.01 |
| Cycloserine | 2 (9) | 215 (25) | <0.01 |
| Amikacin | 2 (9) | 121 (14) | 0.38 |
| Ofloxacin | 2 (9) | 259 (30) | <0.01 |
| Moxifloxacin | 2 (9) | 259 (30) | <0.01 |
| Bedaquiline 3 | 1 (4) | 34 (4) | 1 |
| Capreomycin | 1 (4) | 138 (16) | <0.01 |
| PAS | 1 (4) | 155 (18) | <0.01 |
| Linezolid | 0 (0) | 0 (0) | 1 |
3.4. Medical and Surgical Treatment
Seventeen patients were presented in the frame of the French TB Consilium [14]; the initial treatment regimen was based on genotypic resistance results, and then adapted according to phenotypic drug susceptibility testing results. For the last six patients, treatment was initiated in their setting and also based on genotypic and phenotypic resistance results. Patients received an average of four drugs in the intensive (range, 3–6) and the continuation phase of treatment (range, 2–5). All except two patients received two of the three drugs belonging to group A (fluoroquinolones (n = 21), linezolid (n = 21) and bedaquiline (n = 7)). Twenty-one patients completed the treatment with a mean duration of 20 months (range, 13–27 months), whereas two patients were not evaluated as they were lost to follow-up after 9 and 15 months of treatment, respectively. In 22 cases, a drug was withdrawn because of toxicity (Table 2).
Bone and joint surgery were performed for six patients with spinal TB and for six patients with other osteoarticular localizations. Surgical procedures were performed at the time of MDR-OATB diagnosis, except for the bone graft, the vertebral cementoplasty and one laminectomy that were performed 6 months after the initiation of treatment.
3.5. Patient Follow-Up and Treatment Outcome
Overall, 21 out of 23 (91%) patients achieved treatment success. As previously proposed [10], we did not take into account the WHO criterion concerning discontinuation of ≥2 drugs in the case of adverse events. Interestingly, the five patients in whose case ≥2 drugs were discontinued because of adverse events achieved treatment success (Table S1). Post-treatment follow-up was available for 14 patients, none of whom experienced failure or relapse. Sequelae, namely arthralgia, pain, sciatica and paresthesia, were reported in four patients during post-treatment follow-up. Fifteen patients performed imaging at the end of treatment: in 12 cases, persisting osteoarticular abnormalities were observed (Table 2).
4. Discussion
In our study, we report high rates of treatment success in MDR-OATB cases treated with individualized regimens in France. To date, our knowledge about the epidemiology and treatment outcome of MDR-OATB is limited, and mostly based on case reports and small retrospective series lacking comprehensive data on drug regimen and treatment outcome [15,16,17]. Moreover, WHO guidelines for the treatment of MDR-TB do not distinguish among different localizations of TB [6,7].
In our study, we have shown that MDR-OATB is rare among all MDR-TB cases (2.7%) and that overall high treatment success rates can be achieved by using individualized treatment following international recommendations and with the support of the French national TB Consilium [6,7,14]. Between 2007 and 2018, 2.7% and 7.0% of the MDR-TB patients diagnosed in France had an OATB among all MDR-TB cases and among the extrapulmonary cases, respectively. These proportions are similar to those previously reported [2,5]. Among our patients, the spine was affected in about 70% of all cases, while, at the time of MDR-OATB diagnosis, 61% of patients had active TB in at least one other localization, predominantly the lungs and lymph nodes. Similar findings have been reported in the literature [2,3,18].
Diagnosing OATB is challenging for several reasons: (i) the detection relies on specific tests, (ii) it is clinically indistinguishable from other chronic infections caused by more common bacterial pathogens, and (iii) the expertise in diagnosing TB has decreased in low-burden countries [18,19]. In the present study, the median time from symptom onset to OATB diagnosis was 3.5 months, which is longer than for overall TB cases in France (2 months) [19] but similar to what was reported recently for bone and joint TB in France [18].
The diagnosis of MDR-OATB was based mainly on microbiological findings. However, imaging was shown to be indispensable to establish the diagnosis of OATB in 17% of patients, highlighting the necessity of imaging for diagnosing extrapulmonary TB (Table 2). At the end of treatment, persisting osteoarticular abnormalities are usual; therefore, systematic imaging should be avoided. Indeed, using imaging to define treatment duration could lead clinicians to unnecessarily prolonged treatment with a higher risk of toxicity [20].
In our study, the rates of drug resistance in MDR-OATB strains were much lower than those of other MDR-TB strains in France (Table 3). An explanation might be that the majority of the 30% of patients with MDR-OATB with a previous active or latent TB were treated with the standard first-line TB regimen, whereas the majority of the 40% of the MDR-TB patients with a previous active TB were treated with second-line drugs (NRC data, not presented). As previously described, in the former cases, drug susceptibility profiles could be related to acquired drug resistance when patients were treated with first- or second-line drug regimens [7,21].
A daunting question concerning all osteoarticular infections is whether surgery is required, especially in cases of prosthetic infections. Although some evidence suggests that M. tuberculosis can develop biofilms [22], there are studies that document favorable outcomes of OATB without prosthesis removal [23,24,25]. MDR-TB guidelines are only available for lung surgery [6,7]. According to ATS/CDC/IDSA guidelines, surgery may be indicated in the case of failure to respond to chemotherapy with evidence of ongoing infection, relief of cord compression in patients with persistence or recurrence of neurologic deficits, or instability of the spine [20]. Such criteria could be reasonably applied to MDR-OATB cases. In our series, bone and joint surgery was performed for six patients with spinal TB, of whom four had neurological impairment, in accordance with the above indications [20], and for six patients with another osteoarticular localization, including one with a prosthesis and one with articular damage requiring a knee joint prosthesis.
Treatment regimens used in our study followed WHO MDR-TB treatment recommendations used before 2018, namely, the use of injectables as part of longer (18–20 months) individualized regimens [6,7]. Regimen design was supported by the national TB Consilium for 74% of patients [14]. Since 2016, WHO guidelines have recommended a shorter (9–11 months) regimen for patients with MDR-TB without resistance to drugs included in the regimen [6]; subsequently, the second-line injectable in the shorter regimen was replaced by bedaquiline, resulting in an all-oral regimen [26]. Retrospectively, five patients among our cohort would have been eligible for this shorter regimen. However, more studies are needed before applying the shorter regimen to MDR-OATB since (i) there is limited evidence on the activity of the shorter regimen in extrapulmonary TB in general, and in OATB in particular, and (ii) overall, while it is well-known that some drugs such as rifampin, fluoroquinolones and linezolid penetrate well into bone tissue [27], there is a lack of information regarding concentrations of new anti-TB drugs, such as bedaquiline.
Strong points of this study are the bacteriological confirmation of all cases and representative number of national MDR-TB patients included through the French National Reference Center [28]. This study also has some limitations. First, although one of the largest reported case series of MDR-OATB, the number of cases remains small. Second, post-treatment follow-up was not available for many patients, which makes it difficult to draw conclusions regarding patient management.
Overall, however, our study provides promising results in the treatment of MDR-OATB with longer, individualized regimens.
Acknowledgments
The authors thank the physicians, Marie Garnotel, Anne-Laure Herisse, Anne Joubert, Nicole Le Flour and Tamar Togonidze, and the technicians working at the National Reference Center for Mycobacteria. The authors also thank Ekkehard Collatz for English editing. Members of the CRIOAC Pitié-Salpêtrière: Alexandre Bleibtreu, Nicolas Barrut, Nicolas Bocahut, Ruxandra Calin, Frédéric Clarençon, Georges Daas, Bruno Fautrel, Anne Fustier, Frédérique Gandjbakhch, Nagisa Godefroy, Helga Junot, Frédéric Khiami, Sandra Kossi, Emilie Lafeuille, Jean-Yves Lazennec, Maxime Marchant, Cyril Meloni, Guillaume Mercy, Carole Metz, Mihaela Miu, Stéphane Mitrovic, Yann Mohsinaly, Gentiane Monsel, Quentin Monzani, Olivier Paccoud, Rachid Redjati, Vanessa Reubrecht, Jérôme Robert, Nicolas Rosine, Julie Smati, Pauline Vidal, Sylvain Viltard, Noël Zahr. Members of the TB Consilium of the National Reference Center for Mycobacteria: Claire Andrejak, Christine Bernard, Katarina Chadelat, Bertrand Dautzenberg, Najoua Helali, Benoît Henry, Jérémy Jaffré, Vincent Jarlier, Marie Jaspard, Damien Le Dû, Bénédicte Rivoire, Jérôme Robert, Guillaume Thouvenin.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10061215/s1, Table S1: Outcomes definitions for RR-TB/MDR-TB/XDR-TB patients treated with second-line treatment used in the study, according to the WHO guidelines.
Author Contributions
Conceptualization, A.A. and S.J.; investigation, I.B., E.H., P.B., L.B., A.B., R.B., G.C., E.C., L.E., E.F., M.F.-J., A.G., H.G., B.L.-D., J.-P.L., P.L., A.L., B.L., N.L., C.M., D.M.-O., P.M., F.M., S.P.-D., T.P., C.P., V.P., V.Z. (Virginie Zarrouk) and V.Z. (Valérie Zeller); writing—original draft preparation, I.B. and E.H.; writing—review and editing, A.A., I.B., L.G., E.H., S.J. and N.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee “Comité d’éthique de la Recherche de Sorbonne Université” of Sorbonne Université (CER-2021-005X approved the 14/05/2021).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This work was supported by annual grants from Santé Publique France (Paris, France).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . Global TB Report 2021. World Health Organization; Geneva, Switzerland: 2021. [(accessed on 14 October 2021)]. Available online: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Pigrau-Serrallach C., Rodríguez-Pardo D. Bone and joint TB. Eur. Spine J. 2013;22:556–566. doi: 10.1007/s00586-012-2331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansen I.S., Nielsen S.L., Hove M., Kehrer M., Shakar S., Woyen A.V.T., Andersen P.H., Bjerrum S., Wejse C., Andersen A.B. Characteristics and Clinical Outcome of Bone and Joint TB from 1994 to 2011: A Retrospective Register-based Study in Denmark. Clin. Infect. Dis. 2015;61:554–562. doi: 10.1093/cid/civ326. [DOI] [PubMed] [Google Scholar]
- 4.Meyssonnier V., Zeller V., Malbos S., Heym B., Lhotellier L., Desplaces N., Marmor S., Ziza J.-M. Prosthetic joint infections due to Mycobacterium TB: A retrospective study. Jt. Bone Spine. 2019;86:239–243. doi: 10.1016/j.jbspin.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Tuli S.M. General principles of osteoarticular TB. Clin. Orthop. Relat. Res. 2002;398:11–19. doi: 10.1097/00003086-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 6.WHO . WHO Consolidated Guidelines on Drug-Resistant TB Treatment. WHO; Geneva, Switzerland: [(accessed on 20 March 2019)]. Available online: https://apps.who.int/iris/handle/10665/311389. [Google Scholar]
- 7.Nahid P., Mase S.R., Migliori G.B., Sotgiu G., Bothamley G.H., Brozek J.L., Cattamanchi A., Cegielski J.P., Chen L., Daley C.L. Treatment of Drug-Resistant TB. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2019;200:e93–e142. doi: 10.1164/rccm.201909-1874ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO . Definitions and Reporting Framework for TB. World Health Organization; Geneva, Switzerland: [(accessed on 30 December 2020)]. Available online: https://apps.who.int/iris/handle/10665/79199. [Google Scholar]
- 9.WHO . WHO Announces Updated Definitions of Extensively Drug-Resistant Tuberculosis. WHO; Geneva, Switzerland: 2021. [(accessed on 27 January 2021)]. Available online: www.who.int/news/item/27-01-2021-who-announces-updated-definitions-of-extensively-drug-resistant-tuberculosis. [Google Scholar]
- 10.Schwoebel V., Chiang C.-Y., Trébucq A., Piubello A., Aït-Khaled N., Koura K.G., Heldal E., Van Deun A., Rieder H.L. Outcome definitions for multidrug-resistant TB treated with shorter treatment regimens. Int. J. Tuberc. Lung Dis. 2019;23:619–624. doi: 10.5588/ijtld.18.0798. [DOI] [PubMed] [Google Scholar]
- 11.Canetti G., Rist N., Grosset J. Measurement of sensitivity of the tuberculous bacillus to antibacillary drugs by the method of proportions. Methodology, resistance criteria, results and interpretation. Rev. Tuberc. Pneumol. 1963;27:217–272. [PubMed] [Google Scholar]
- 12.Brossier F., Sougakoff W., French National Reference Center for Mycobacteria Molecular detection methods of resistance to antiTB drugs in Mycobacterium tuberculosis. Med. Mal. Infect. 2017;47:340–348. doi: 10.1016/j.medmal.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Andre E., Goeminne L., Cabibbe A., Beckert P., Kabamba Mukadi B., Mathys V., Gagneux S., Niemann S., Van Ingen J., Cambau E. Consensus numbering system for the rifampicin resistance-associated rpoB gene mutations in pathogenic mycobacteria. Clin. Microbiol. Infect. 2017;23:167–172. doi: 10.1016/j.cmi.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Guglielmetti L., Jaffré J., Bernard C., Brossier F., El Helali N., Chadelat K., Thouvenin G., Dautzenberg B., Henry B., Jaspard M. Multidisciplinary advisory teams to manage multidrug-resistant TB: The example of the French Consilium. Int. J. Tuberc. Lung Dis. 2019;23:1050–1054. doi: 10.5588/ijtld.18.0779. [DOI] [PubMed] [Google Scholar]
- 15.Suárez-García I., Noguerado A. Drug treatment of multidrug-resistant osteoarticular TB: A systematic literature review. Int. J. Infect. Dis. 2012;16:e774–e778. doi: 10.1016/j.ijid.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Seddon J.A., Donald P.R., Vlok G.J., Schaaf H.S. Multidrug-resistant TB of the spine in children--characteristics from a high burden setting. J. Trop. Pediatr. 2012;58:341–347. doi: 10.1093/tropej/fmr104. [DOI] [PubMed] [Google Scholar]
- 17.Kumar V., Neradi D., Sherry B., Gaurav A., Dhatt S.S. Tuberculosis of the spine and drug resistance: A review article. Neurosurg. Rev. 2022;45:217–219. doi: 10.1007/s10143-021-01595-1. [DOI] [PubMed] [Google Scholar]
- 18.Guillouzouic A., Andrejak C., Peuchant O., Hery-Arnaud G., Hamdad F., Lanotte P., Gaborit B., Bernard L., Bémer P. Treatment of Bone and Joint TB in France: A Multicentre Retrospective Study. J. Clin. Med. 2020;9:2529. doi: 10.3390/jcm9082529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tattevin P., Che D., Fraisse P., Gatey C., Guichard C., Antoine D., Paty M.C., Bouvet E. Factors associated with patient and health care system delay in the diagnosis of TB in France. Int. J. Tuberc Lung Dis. 2012;16:510–515. doi: 10.5588/ijtld.11.0420. [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society CDC, Infectious Diseases Society of America. Treatment of TB. MMWR Recomm. Rep. 2003;52:1–77. doi: 10.1164/rccm.2508001. [DOI] [PubMed] [Google Scholar]
- 21.Gegia M., Winters N., Benedetti A., van Soolingen D., Menzies D. Treatment of isoniazid-resistant TB with first-line drugs: A systematic review and meta-analysis. Lancet Infect. Dis. 2017;17:223–234. doi: 10.1016/S1473-3099(16)30407-8. [DOI] [PubMed] [Google Scholar]
- 22.Kulka K., Hatfull G., Ojha A.K. Growth of Mycobacterium TB biofilms. J. Vis. Exp. 2012;15:3820. doi: 10.3791/3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uhel F., Corvaisier G., Poinsignon Y., Chirouze C., Beraud G., Grossi O., Varache N., Arvieux C., Le Berre R., Tattevin P. Mycobacterium TB prosthetic joint infections: A case series and literature review. J. Infect. 2019;78:27–34. doi: 10.1016/j.jinf.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Veloci S., Mencarini J., Lagi F., Beltrami G., Campanacci D.A., Bartoloni A., Bartalesi F. Tubercular prosthetic joint infection: Two case reports and literature review. Infection. 2018;46:55–68. doi: 10.1007/s15010-017-1085-1. [DOI] [PubMed] [Google Scholar]
- 25.Shanbhag V., Kotwal R., Gaitonde A., Singhal K. Total hip replacement infected with Mycobacterium TB. A case report with review of literature. Acta Orthop. Belg. 2007;73:268–274. [PubMed] [Google Scholar]
- 26.WHO . WHO Consolidated Guidelines on Tuberculosis, Module 4: Treatment—Drug-Resistant Tuberculosis Treatment. WHO; Geneva, Switzerland: 2020. [(accessed on 15 January 2020)]. Available online: https://www.who.int/publications/i/item/9789240007048. [PubMed] [Google Scholar]
- 27.Fitzgerald D.W., Sterling T.R., Haas D.W. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Elsevier; Amsterdam, The Netherlands: 2015. Mycobacterium tuberculosis. [DOI] [Google Scholar]
- 28.Guerrin-Tran E., Thiolet J.M., Rousseau C., Henry S., Poirier C., Che D., Vinas J.-M., Jarlier V., Robert J. An evaluation of data quality in a network for surveillance of Mycobacterium TB resistance to antiTB drugs in Ile-de-France region-2001–2002. Eur. J. Epidemiol. 2006;21:783–785. doi: 10.1007/s10654-006-9069-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.