Abstract
The ubiquitous oomycete Pythium oligandrum is a potential biocontrol agent for use against a wide range of pathogenic fungi and an inducer of plant disease resistance. The ability of P. oligandrum to compete with root pathogens for saprophytic colonization of substrates may be critical for pathogen increase in soil, but other mechanisms, including antibiosis and enzyme production, also may play a role in the antagonistic process. We used transmission electron microscopy and gold cytochemistry to analyze the intercellular interaction between P. oligandrum and Phytophthora parasitica. Growth of P. oligandrum towards Phytophthora cells correlated with changes in the host, including retraction of the plasma membrane and cytoplasmic disorganization. These changes were associated with the deposition onto the inner host cell surface of a cellulose-enriched material. P. oligandrum hyphae could penetrate the thickened host cell wall and the cellulose-enriched material, suggesting that large amounts of cellulolytic enzymes were produced. Labeling of cellulose with gold-complexed exoglucanase showed that the integrity of the cellulose was greatly affected both along the channel of fungal penetration and also at a distance from it. We measured cellulolytic activity of P. oligandrum in substrate-free liquid medium. The enzymes present were almost as effective as those from Trichoderma viride in degrading both carboxymethyl cellulose and Phytophthora wall-bound cellulose. P. oligandrum and its cellulolytic enzymes may be useful for biological control of oomycete pathogens, including Phytophthora and Pythium spp., which are frequently encountered in field and greenhouse production.
The potential of fungal antagonists for biological control of plant pathogens has been recognized for many years (11), and our understanding of the coordinated series of events leading to successful parasitism has increased recently (12, 13). Antagonistic fungi vary greatly in their modes of action, virulence, and degrees of host specificity (6, 7, 9) and may use mechanisms such as competition (31), antibiosis (20), production of hydrolytic enzymes (21), or a combination of all these processes (12, 28, 30) to attack their target hosts.
The ubiquitous oomycete Pythium oligandrum Dreschler is a potential biocontrol agent for use against a wide range of economically important soilborne plant pathogens (1, 10, 15, 26). Detailed information regarding the mechanisms that this fungus uses to induce plant protection is not available, although, in addition to its antimicrobial properties, it may be able to induce resistance in host plants (8). The antagonism exerted by P. oligandrum is thought to involve the action of cell wall hydrolytic enzymes and/or antibiotics and to depend upon the target host species (9).
The role played by hydrolytic enzymes in the antifungal activity attributed to P. oligandrum needs clarification, although a role for these lytic enzymes in cell wall penetration and degradation often has been suggested (26). The correlation between lytic enzyme synthesis and biocontrol activity by P. oligandrum remains controversial. For example, isolates of several parasitic Pythium spp. (17) cannot produce cellulases in culture media containing cellulose or carboxymethyl cellulose (MC) (28). Recently, Benhamou et al. (9) showed that cellulose hydrolysis was a key component of the mycoparasitism of two pathogenic Pythium spp. by P. oligandrum and that the isolate of P. oligandrum which they used could synthesize cellulases which facilitated the mycoparasitic process.
These observations raise several important questions. For example, are cellulases involved in antagonism of other oomycetes (e.g., Phytophthora parasitica)? What is the relative importance of cellulases and antibiotics? How does the activity of the P. oligandrum-produced cellulases compare with that of similar enzymes from other sources?
We studied the in vitro interaction between P. oligandrum 1010 and P. parasitica ultrastructurally and cytochemically. The objectives of the present study were (i) to determine the sequence of events involved in the P. oligandrum-P. parasitica interaction, (ii) to confirm the involvement of cellulases in the process resulting in internal colonization of the host, and (iii) to determine if the activity of these enzymes in terms of Phytophthora cell wall degradation, was similar to the activity of the living antagonist. We found that in vitro attack by P. oligandrum induces a set of events that adversely affects Phytophthora growth and development. Knowledge of these events can be used to identify cropping systems and disease management strategies in which P. oligandrum is likely to function as an effective biological control agent.
MATERIALS AND METHODS
Fungal culture and growth conditions.
P. oligandrum Drechsler isolate 1010 was recovered from pea roots in Denmark, and P. parasitica 149 was selected for its virulence on tomato. Both fungi were grown either on potato dextrose agar (PDA) (Difco Laboratories, Detroit, Mich.) or on V8 agar (200 ml of V8 juice [Campbell Soup Company Ltd., Toronto, Ontario, Canada), 2.5 g of CaCO3, 0.1 g of β-sitosterol, 15 g of agar, 800 ml of deionized water). Plates were incubated at 24°C in the dark and were subcultured every week. V8 agar was used for dual-culture test experiments.
For cellulase activity analysis, P. oligandrum was grown in Erlenmeyer flasks containing 100 ml of Difco potato dextrose broth (PDB) at 24°C for 6 days at 150 rpm. Mycelium was harvested by centrifugation at 7,000 × g for 20 min. The supernatant was filtered through a 0.2-μm-pore-size filter (Millipore Corporation, Bedford, Mass.) and stored at −20°C until it was used for enzyme analysis.
Dual-culture tests.
The P. parasitica-P. oligandrum interaction was studied as previously described (6). Briefly, a PDA disk (diameter, 5 mm) from the margin of an actively growing P. parasitica culture was transferred to fresh agar medium. Forty-eight hours later, a plug (diameter, 5 mm) of P. oligandrum mycelium was placed 3 cm from the P. parasitica disk, and the plates were incubated at 25°C under continuous light. P. parasitica mycelium began to be overgrown by hyphae of P. oligandrum 3 days after inoculation. Samples from the interaction regions taken 1, 2, 3, 4, and 5 days after inoculation of P. oligandrum were processed for electron microscopy.
Tissue processing for ultrastructural observations.
Mycelial samples (2 mm3), collected from the region of interaction between P. oligandrum and P. parasitica, were fixed by immersion in glutaraldehyde and osmium tetroxide, dehydrated in a graded ethanol series (25 to 100% ethanol), and embedded in Epon 812 (JBEM Chemical Co., Pointe-Claire, Québec, Canada) as previously described (6). Ultrathin sections (thickness, 0.1 μm), collected on nickel grids by using a diamond knife, were either contrasted with uranyl acetate and lead citrate for immediate examination with a 1200 EX transmission electron microscope (JEOL, Tokyo, Japan) operating at 80 kV or processed further for cytochemical labeling. An average of five samples from five different plates for each sampling time were examined. For each sample, 10 to 15 ultrathin sections were observed.
Cytochemical labeling.
Colloidal gold with particles averaging 12 nm in diameter was prepared as described by Frens (18) by using sodium citrate as a reducing agent. The beta-1,4-exoglucanase–gold complex used for localization of the cell wall-bound cellulose was prepared as described by Benhamou et al. (5) by using a beta-1,4-d-glucan cellobiohydrolase (EC 3.2.1.21) complexed to colloidal gold at pH 9.0.
Ultrathin sections were floated for 5 min on a drop of 10 mM sodium phosphate-buffered saline (pH 6.0) containing 0.02% (wt/vol) polyethylene glycol 20,000 (Fisher Scientific, Nepean, Ontario, Canada), transferred to a drop of the exoglucanase-gold-complex, and incubated for 30 min at room temperature in a moist chamber. Grids carrying sections were washed thoroughly with phosphate-buffered saline (pH 7.4), rinsed with distilled water, and allowed to dry before staining with uranyl acetate and lead citrate.
Labeling specificity was assessed with the following controls: (i) addition of beta-1,4-glucan from barley (1 mg/ml) prior to section labeling for a competition experiment; (ii) replacement of the enzyme-gold complex being studied by a bovine serum albumin-gold complex to assess nonspecific adsorption of a protein-gold complex to the tissue sections; (iii) incubation of the tissue sections with the enzyme-gold complex under nonoptimal conditions for biological activity; and (iv) incubation of the sections with colloidal gold alone to assess nonspecific adsorption of the gold particles.
Detection of cellulase activity.
We filled petri dishes with PDA amended with 0.5% (wt/vol) low-viscosity CMC (Sigma, St. Louis, Mo.) in 50 mM sodium acetate buffer (pH 5.0). Cellulysin, a cellulolytic complex from Trichoderma viride (Calbiochem-Novabiochem, La Jolla, Calif.), was used as a positive control at a concentration of 1 mg/ml in the same buffer. Petri dishes were divided into four sections, and drops (20 μl) of either cellulysin, P. oligandrum supernatant, PDB alone, or sodium acetate buffer (pH 5.0) were deposited on the CMC-enriched PDA. The plates were incubated at 25°C in the dark for 24 h. Enzymatically active areas were visualized with a 2UVTM transilluminator (model IM 26E; UVP, San Gabriel, Calif.) operating at 365 nm after incubation of the plates with Calcofluor fluorescent brightener 28 (Sigma). The amounts of cellulolytic activity were estimated by the sizes of the enzymatically digested areas.
Cellulolytic effect of culture supernatant on P. parasitica.
Disks (2 mm3) of P. parasitica growing on PDA were placed on microscope slides, covered with a thin layer of 2% (wt/vol) water agar, and allowed to grow overnight at 25°C in a moist chamber. One drop (10 μl) of either P. oligandrum culture supernatant, cellulysin, or sodium acetate buffer was placed on the hyphal filaments emerging from each disk. Inoculated slides were incubated for 2 to 6 h at 25°C in a moist chamber prior to examination with a light microscope (Zeiss Canada Ltd., North York, Ontario, Canada) equipped with differential interference contrast (Nomarski). Mycelial samples also were taken from the inoculated slides and processed for electron microscope investigation as described above.
RESULTS
Mycelial interactions between P. oligandrum and P. parasitica.
In petri dish dual cultures, the first apparent contact between hyphae of the two fungi occurred 2 days after inoculation. At that time, the P. oligandrum hyphae were intertwined with those of P. parasitica. In the subsequent days, the P. oligandrum mycelium continued to grow and to colonize the agar. When transferred to fresh medium after 6 days of coculture, P. parasitica hyphae did not grow.
Time course investigation of the cellular events involved in the P. oligandrum-P. parasitica interaction. (i) Events preceding host cell penetration.
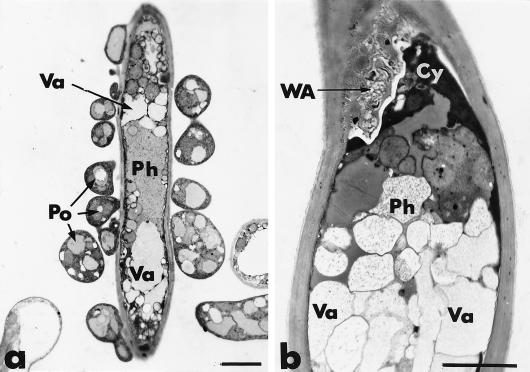
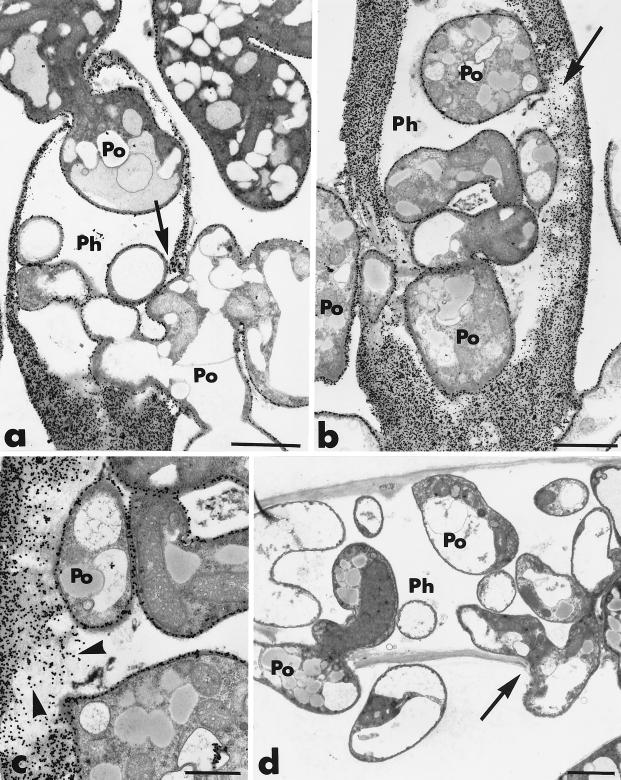
After 1 day of coculture, the hyphae of P. oligandrum could be recognized by the greater electron density of their protoplasm, and they encircled and/or were closely appressed against hyphae of P. parasitica (Fig. 1a). The host cells were not obviously altered, although some slight cytoplasmic disorganization, primarily associated with increased vacuolation, was noticed. After 2 days of dual culture, host cell disorganization increased, as shown by the frequent retraction of the plasma membrane, which is usually correlated with an early stage of cytoplasmic aggregation (Fig. 1b). Plasma membrane retraction often was accompanied by deposition of a fibrillo-granular network in the paramural space (Fig. 1b). These wall appositions were heterogeneous in size, shape, and texture and could appear either as small hemispherical protuberances or as elongated deposits on a large portion of the host cell wall.
FIG. 1.
Transmission electron micrographs of mycelial samples, collected after 1 and 2 days of coculture of P. parasitica (Ph) and P. oligandrum (Po), in the region where the two fungi are interacting. (a) Hypha of P. parasitica encircled by hyphae of P. oligandrum. Structural changes are mainly characterized by an increase in the number of vacuoles (Va). Bar, 2 μm. (b) After 2 days, host cell disorganization is characterized by local retraction of the plasma membrane from the cell wall and by an early stage of cytoplasm (Cy) aggregation. A heterogeneous wall apposition (WA) is formed in the paramural space. Bar, 2 μm.
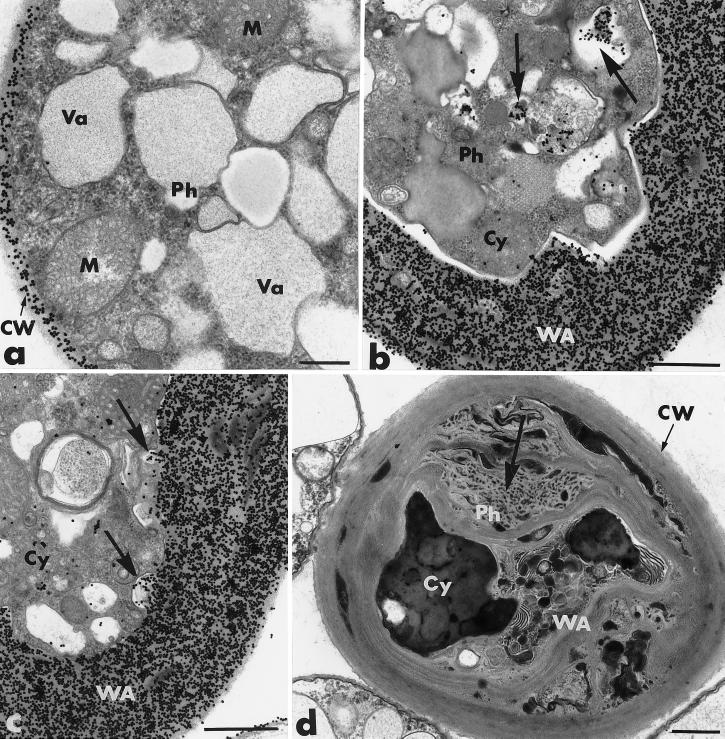
After 3 days of coculture, the extent and magnitude of the host cellular changes increased (Fig. 2b through d). Retraction of the plasma membrane was complete, and the wall appositions were significantly larger. Section labeling with gold-complexed exoglucanase resulted in massive deposition of gold particles in the host cell wall and the wall appositions (Fig. 2b and c). The amount of wall-bound labeling appeared to be 10-fold greater than that observed for Phytophthora hyphae grown in single culture (Fig. 2a). Specifically labeled, electron-opaque vesicles, frequently seen in the condensed cytoplasm (Fig. 2b), could be enclosed in invaginations of the plasma membrane, where they apparently released their labeled contents (Fig. 2c).
FIG. 2.
Transmission electron micrographs of mycelial samples, collected after 3 to 4 days of coculture of P. parasitica (Ph) and P. oligandrum (Po), in the region where the two fungi are interacting. Cellulosic beta-1,4-glucans are labeled with the exoglucanase-gold complex. (a) P. parasitica grown in single culture. The cell wall (CW) is specifically labeled, while the cytoplasm and the organelles, including mitochondria (M) and vacuoles (Va), are not labeled. Bar, 0.375 μm. (b and c) After 3 days, the host structural changes include complete retraction of the plasma membrane, condensation of the cytoplasm (Cy) and enlargement of the wall apposition (WA). Gold labeling occurs in the host cell wall, the wall appositions, and the electron-opaque vesicles (panel b, arrows), which can also be enclosed in invaginations of the plasma membrane (panel c, arrows). Bars, 0.75 μm. (d) After 4 days of coculture, the space between the thickened cell wall (CW) and the small aggregated cytoplasmic remnants (Cy) is filled with a material forming a tight network (large arrow). Osmiophilic inclusions are embedded in the network, giving the Phytophthora cell a honeycomb appearance. Bar, 0.75 μm.
By 4 days, the space between the thickened cell wall and the small aggregated cytoplasmic remnants could be filled with a heterogeneous material forming a tight network (Fig. 2d) surrounded by distorted wall-like strands. Osmiophilic inclusions were embedded in this network, giving the entire system a honeycomb appearance (Fig. 2d).
(ii) Host cell binding and penetration.
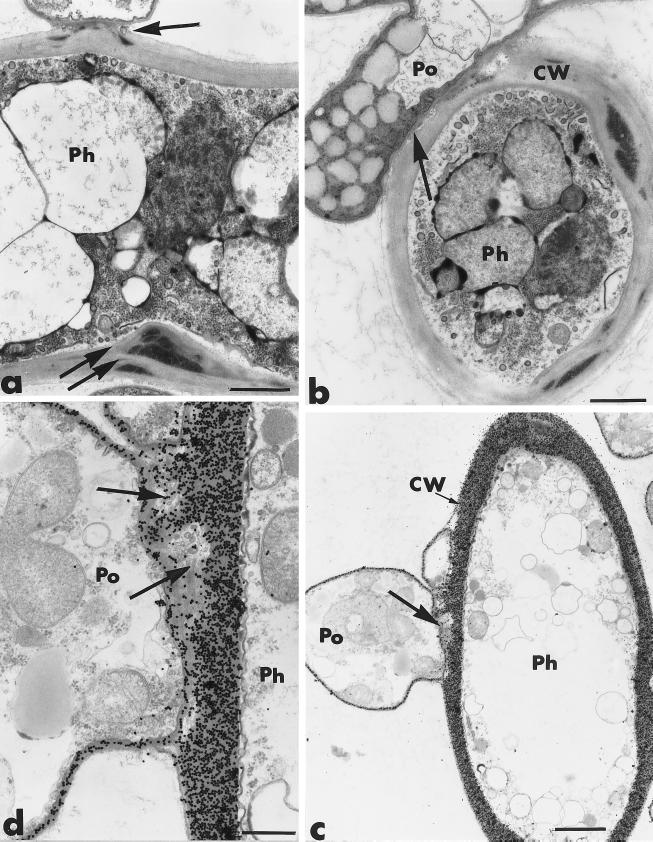
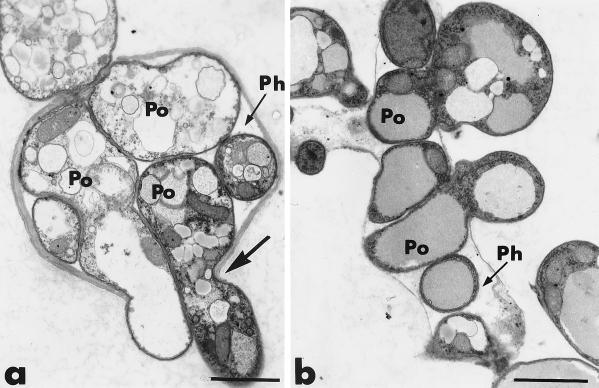
After 3 to 4 days of coculture, the hyphae of P. oligandrum were tightly bound to those of P. parasitica (Fig. 3a and b). Cell walls of both fungi appeared to be diffuse when they were in close contact (Fig. 3b), and it often was difficult to distinguish them, although the thin cell wall of P. oligandrum usually was more electron dense than the thickened wall of P. parasitica (Fig. 3b). Adhesion of P. oligandrum hyphae was usually accompanied by little wall displacement (Fig. 3a). Host cell reactions, including the formation of wall appositions, also were detected at sites of potential penetration (Fig. 3a). Firm binding of P. oligandrum to its host preceded host cell wall degradation and host penetration (Fig. 3c and d). The lytic activity of P. oligandrum was confirmed by the degradation of the thickened host cell wall and the enlarged wall appositions (Fig. 4a and b). Lysis zones were always free of exoglucanase-gold labeling (Fig. 4b).
FIG. 3.
Transmission electron micrographs of mycelial samples, collected after 3 to 4 days of coculture of P. parasitica (Ph) and P. oligandrum (Po), in the region where the two fungi are interacting. Cellulosic beta-1,4-glucans are labeled with the exoglucanase-gold complex. (a and b) Hyphae of P. oligandrum establish tight binding with the host cells (arrows). Adhesion of P. oligandrum hyphae to the host cells is accompanied by little wall displacement (panel a, arrow). Wall thickening occurs at sites of potential antagonist penetration (panel a, double arrows). CW, cell wall. Bars, 0.75 μm. (c and d) Firm binding of P. oligandrum to its host is associated with local cell wall degradation (arrows). (c) Bar, 1.5 μm; (d) bar, 0.75 μm.
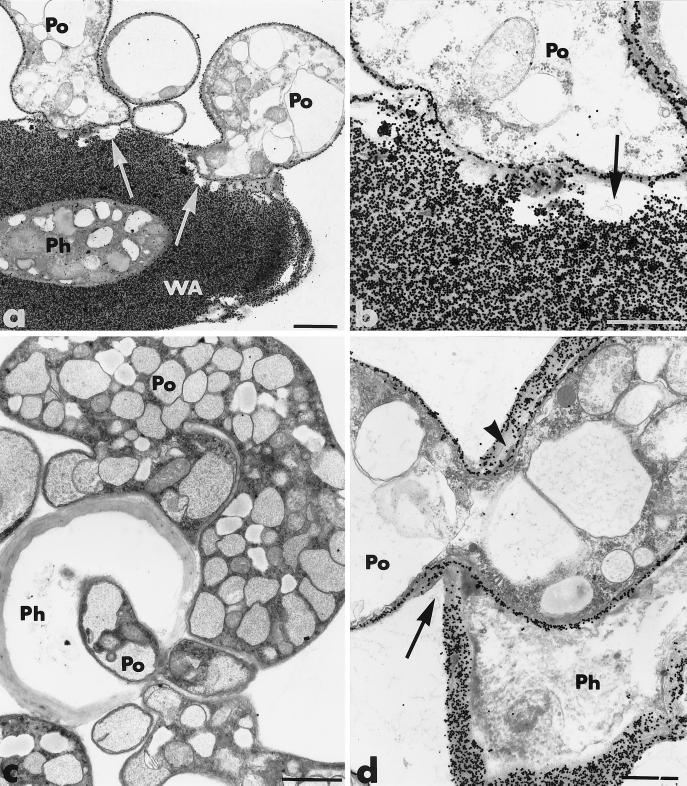
FIG. 4.
Transmission electron micrographs of mycelial samples, collected after 3 to 4 days of coculture of P. parasitica (Ph) and P. oligandrum (Po), in the region where the two fungi are interacting. (a and b) Lytic zones are free of exoglucanase-gold labeling (arrows). (a) WA, wall apposition. Bar, 1.5 μm. (b) Bar, 0.75 μm. (c and d) Host cell penetration is achieved by means of constricted hyphae of the antagonist (panel d, arrow). There is a decrease in gold labeling some distance from the fungal pathway (panel d, arrowhead). (c) Bar, 1.5 μm; (d) bar, 0.75 μm.
Constricted hyphae of P. oligandrum penetrated the host cells (Fig. 4c and d). Channels of penetration often were much narrower than the average hyphal diameter and usually were associated with little wall displacement (Fig. 4d). Gold labeling decreased not only along the fungal antagonist pathway but also at some distance from it (Fig. 4d).
P. oligandrum ingress into the pathogen protoplasm coincided with extensive cell alterations leading to complete dissolution of the host cytoplasm (Fig. 5a). At this stage, P. parasitica hyphae appeared to be little more than empty shells (Fig. 5a and d). Cells of P. oligandrum grew abundantly in the host hyphae and invaded the area that was originally occupied by the host cytoplasm (Fig. 5a and b). This colonization was usually accompanied by a generalized lytic activity that resulted in host wall alterations (Fig. 5b). The release of fibrillar fragments, which were specifically labeled by the gold-complexed exoglucanase, occurred in areas of host cell wall degradation (Fig. 5c). Extensive digestion of the host cell walls resulted in cell perforation in many places (Fig. 5d).
FIG. 5.
Transmission electron micrographs of mycelial samples, collected after 4 to 5 days of coculture of P. parasitica (Ph) and P. oligandrum (Po), in the region where the two fungi are interacting. (a through c) Cells of the antagonist proliferate in the host hyphae, resulting in host wall alterations (panels a and b, arrows). Labeled wall fragments are released (panel c, arrowheads). (a and b) Bars, 1.5 μm; (c) bar, 0.75 μm. (d) Host cell is perforated in many places (arrow). Bar, 1.5 μm.
Eventually, cells of P. oligandrum multiplied so extensively in the host hyphae that it was difficult to identify free space in the area that was originally occupied by the host cytoplasm (Fig. 6). Under such pressure, the P. parasitica hyphae apparently burst, leaving only some tiny wall fragments to indicate the former presence of Phytophthora cells (Fig. 6).
FIG. 6.
Transmission electron micrographs of mycelial samples, collected after 4 to 5 days of coculture of P. parasitica (Ph) and P. oligandrum (Po), in the region where the two fungi are interacting. Active multiplication of the antagonist results in apparent bursting of the host hyphae (panel a, large arrow) and in release of the actively multiplying P. oligandrum hyphae. Bars, 2 μm.
All control tests, including previous adsorption of the enzyme-gold complexes with their corresponding substrate molecules, yielded negative results (data not shown).
Cellulase activity in the culture supernatant of P. oligandrum.
The culture supernatant of P. oligandrum was applied to PDA plates amended with the substrate CMC, and its activity was compared to that of a pure cellulolytic complex from T. viride. Following calcofluor staining of the cellulosic polymer, negative zones, corresponding to the areas where drops were deposited, were easily seen for both the cellulysin from T. viride and the culture supernatant of P. oligandrum (data not shown). By contrast, no signal was obtained with either PDB or sodium acetate buffer alone (data not shown).
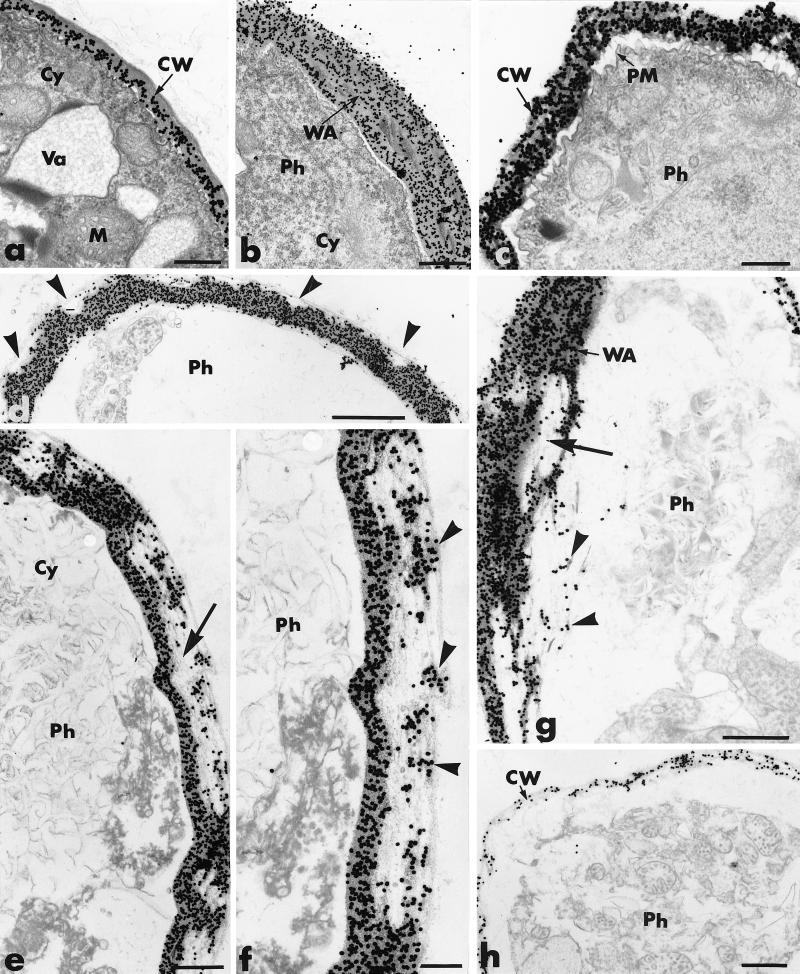
We observed mycelium of P. parasitica growing in water agar on microscope slides at 1-h intervals. Mycelia exposed to either PDB or sodium acetate buffer had hyphae delimited by a thin cell wall that contained dense polyribosome-enriched cytoplasm in which a large number of organelles, including mitochondria and small vacuoles, were found (Fig. 7a). Incubation of sections with the beta-1,4-exoglucanase–gold complex resulted in regular deposition of gold particles over the cell wall, with some preferential labeling of the internal wall (Fig. 7a).
FIG. 7.
Transmission electron micrographs of mycelial samples collected from agar-coated slides 2 to 6 h after inoculation of P. parasitica (Ph): cellulolytic effect of P. oligandrum (Po) culture supernatant on P. parasitica hyphae. (a) P. parasitica exposed to PDB (control). Hyphae are delimited by a regularly labeled cell wall (CW) and contain a dense cytoplasm (Cy) in which mitochondria (M) and vacuoles (Va) are visible. Bar, 0.25 μm. (b) After 2 h of exposure to the culture supernatant of P. oligandrum, local retraction of the plasma membrane is accompanied by formation of wall appositions (WA). Bar, 0.5 μm. (c) After 3 h of exposure, complete retraction of the plasma membrane (PM), condensation of the cytoplasm, and loss of cell wall rigidity are detected. Bar, 0.25 μm. (d through g) Upon prolonged exposure (4 h or more), a large number of unlabeled areas are detected in the outermost wall layers of Phytophthora hyphae (panel d, arrowheads). These areas can extend to large portions of the cell wall (panel e, arrow) in which labeling is restricted to small wall fragments (panel f, arrowheads). Alteration of the wall appositions (panel g, large arrow) leads to release of labeled fragments (panel g, arrowheads). (d) Bar, 1 μm; (e) bar, 0.5 μm; (f) bar, 0.25 μm; (g) bar, 0.5 μm. (h) After 6 h of exposure, Phytophthora hyphae are surrounded by a slightly labeled cell wall. Bar, 0.5 μm.
Mycelia exposed to P. oligandrum culture supernatant had morphological and structural alterations that could be detected as soon as 2 h after exposure. These changes, primarily local retraction of the plasma membrane and the formation of wall appositions, usually were restricted to well-delineated wall areas (Fig. 7b). By 3 h after exposure, the plasma membrane was completely retracted, the cytoplasm had condensed, and the cell wall was no longer rigid (Fig. 7c).
Following 4 to 5 h of exposure to the culture supernatant, most P. parasitica cells were severely damaged (Fig. 7c through g). When cells were incubated with the gold-complexed exoglucanase, numerous unlabeled areas were seen in the outermost wall layers of Phytophthora hyphae (Fig. 7d). These areas often included large portions of the cell wall (Fig. 7e) in which labeling was restricted to relatively small wall fragments (Fig. 7f). In some cases, the wall appositions also were altered and labeled fragments were released (Fig. 7g).
By 6 h after exposure to the culture filtrate, most Phytophthora hyphae (∼80%) were surrounded by a thin, wavy cell wall which was labeled by a few scattered gold particles (Fig. 7h).
Changes similar to those induced by the culture supernatant of P. oligandrum also were observed after exposure to cellulysin from T. viride (data not shown). However, the alterations in response to cellulysin occurred earlier (within 2 h) and were more severe than those induced by the P. oligandrum culture filtrate.
DISCUSSION
In this study, we showed that the oomycete fungus P. parasitica is highly vulnerable to attack by the antagonistic fungus P. oligandrum. Our results provide ultrastructural evidence that P. oligandrum-mediated antagonism is a multifaceted process that requires the synergistic contribution of several mechanisms, including cell surface attachment and production of hydrolytic enzymes (e.g., cellulases). According to our observations, the process of Phytophthora colonization by P. oligandrum 1010 involves a chronological sequence of events, including (i) attachment and local penetration of the antagonist into the pathogen hyphae, (ii) induction of a host structural response, (iii) alteration of the host protoplasm, and (iv) active multiplication of the antagonistic cells in the pathogen hyphae, leading to host cell breakdown.
One of the earliest events of the antagonistic process was the apparent affinity of P. oligandrum hyphae for cells of the pathogenic fungus (Fig. 1a). The antagonist is attracted to the host cells by an unknown mechanism that probably involves specific chemical stimuli (32) or chemotropic growth (11). Support for the hypothesis that molecular signals are exchanged between the fungi includes the host cell changes initiated prior to adhesion of P. oligandrum hyphae and the similarities between these reactions and those seen in other fungal cells exposed to antibiotics and/or fungicides (3, 19, 23). In particular, alterations in membrane permeability could result in internal osmotic imbalances (25), leading to cytoplasm disorganization and aggregation such as that which precedes parasitism and subsequent internal colonization of P. parasitica cells.
Host cell changes were correlated with abnormal deposition of a cellulose-enriched material between the invaginated plasma membrane and the host cell wall. We think that the deposits are a defense reaction to restrict ingress of the antagonist and to shield the cell wall from hydrolytic enzymes and toxins. The formation of structural barriers as a response by resistant plant cultivars to fungal attack (4, 8, 16) is well documented, but relatively little is known about defense-related structural responses in fungi. There are at least two hypotheses that could explain the response of the Phytophthora cells. First, the production of low levels of hydrolytic enzymes, e.g., beta-1,3-glucanases, by P. oligandrum may have allowed the release of elicitor-active molecules, e.g., beta-1,3-glucans, from the host cell wall that ultimately increased the activity of cellulose synthase. In plants, glucan preparations derived from fungal cell walls can stimulate a resistance response (14). Alternatively, the signal molecule could be a toxin or an antibiotic secreted by the antagonist. Alteration of the lipid composition of the plasma membrane of Phytophthora hyphae, possibly resulting from the binding of such molecules, may have induced deregulation of membrane-bound enzymes, resulting in areas of abnormal wall-like deposition.
Whatever the origin and role of the deposited material, the antagonist can penetrate this barrier by altering its structure (Fig. 5). Degradation of the host cell wall and the wall appositions always was preceded by firm binding of P. oligandrum to its host. Cell surface molecules play an important role in cell-to-cell interactions in many biological systems (22, 29), and early recognition events, mediated by molecules with sugar-binding affinity, are known to be important determinants in establishing the mycoparasitic relationship between Trichoderma spp. and their target hosts (2, 6). We think that the tight binding observed between hyphae of the antagonist and cells of P. parasitica is mediated by a specific cell surface recognition process which, in turn, triggers a series of events that includes host wall penetration and cell invasion.
The successful penetration of the thickened host cell wall and the enlarged wall appositions by P. oligandrum hyphae suggests that large amounts of cellulolytic enzymes were produced. The labeling pattern of cellulose showed that the integrity of this compound was affected in wall areas adjacent to P. oligandrum cells and also at a distance from the sites of antagonist entry, suggesting that cellulases may have diffused extracellularly. Production of extracellular lytic enzymes, e.g., beta-1,3-glucanases, lipases, and proteases, by P. oligandrum may be involved in antagonism against an array of pathogenic fungi (17, 24, 26, 27). Our results show that at least under our experimental conditions, P. oligandrum could produce large amounts of cellulases in substrate-free liquid medium and that these enzymes were nearly as effective as the cellulolytic complex from T. viride in degrading both CMC and Phytophthora wall-bound cellulose.
In vitro demonstration that cellulases produced by P. oligandrum may play a major role in biological control of P. parasitica provides an incentive to develop this organism and these enzymes as a biological control agent for commercial use. However, the most important problem to be solved prior to large-scale application of biocontrol strains is the ability to predict the behavior of these strains in the field based on laboratory results. The possibility of improving and maintaining the biocontrol activities of fungal antagonists by genetic manipulation techniques is promising. Future research should focus on development of transgenic P. oligandrum strains capable of producing large quantities of cellulases while the intrinsic vigor and the ecological competence of the fungus are preserved. Manipulated biocontrol fungi need to become more predictable and reliable for use in the field and could potentially reduce the quantity of fungicides required to produce disease-free plants.
ACKNOWLEDGMENTS
We thank J. Hockenhull (The Royal Veterinary and Agricultural University, Copenhagen, Denmark) and M. Ponchet (INRA, Antibes, France) for providing the isolates of P. oligandrum and P. parasitica, respectively, and C. Garand, A. Goulet, and H. Chamberland (Laval University, Québec, Canada) for technical assistance.
This work was supported by grants from the Fonds Québécois pour la Formation de Chercheurs et l'Aide à la Recherche (FCAR), the Natural Sciences and Engineering Council of Canada (NSERC), the GIS-LBIO Program (ONIFLHOR), and the Brittany Regional Council (France).
REFERENCES
- 1.Al-Rawahi A K, Hancock J G. Parasitism and biological control of Verticillium dahliae by Pythium oligandrum. Plant Dis. 1998;82:1100–1106. doi: 10.1094/PDIS.1998.82.10.1100. [DOI] [PubMed] [Google Scholar]
- 2.Barak R, Elad Y, Mirelman D, Chet I. Lectins: a possible basis for specific recognition in the interaction of Trichoderma and Sclerotium rolfsii. Phytopathology. 1985;75:458–462. [Google Scholar]
- 3.Bélanger R R, Dufour N, Caron J, Benhamou N. Chronological events associated with the antagonistic properties of Trichoderma harzianum against Botrytis cinerea: indirect evidence for sequential role of antibiosis and parasitism. Biocontrol Sci Technol. 1995;5:41–53. [Google Scholar]
- 4.Benhamou N. Elicitor-induced plant defense pathways. Trends Plant Sci. 1996;1:233–240. [Google Scholar]
- 5.Benhamou N, Chamberland H, Ouellette G B, Pauzé F J. Ultrastructural localization of β-1,4-d-glucans in two pathogenic fungi and in their host tissues by means of an exoglucanase-gold complex. Can J Microbiol. 1987;33:405–417. [Google Scholar]
- 6.Benhamou N, Chet I. Hyphal interactions between Trichoderma harzianum and Rhizoctonia solani: ultrastructure and gold cytochemistry of the mycoparasitic process. Phytopathology. 1993;83:1062–1071. [Google Scholar]
- 7.Benhamou N, Chet I. Cellular and molecular mechanisms involved in the interaction between Trichoderma harzianum and Pythium ultimum. Appl Environ Microbiol. 1997;63:2095–2099. doi: 10.1128/aem.63.5.2095-2099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benhamou N, Rey P, Chérif M, Hockenhull J, Tirilly Y. Treatment with the mycoparasite, Pythium oligandrum, triggers induction of defense-related reactions in tomato roots when challenged with Fusarium oxysporum f. sp. radicis-lycopersici. Phytopathology. 1997;87:108–122. doi: 10.1094/PHYTO.1997.87.1.108. [DOI] [PubMed] [Google Scholar]
- 9.Benhamou N, Rey P, Picard K, Tirilly Y. Ultrastructural and cytochemical aspects of the interaction between the mycoparasite Pythium oligandrum and soilborne plant pathogens. Phytopathology. 1999;89:506–517. doi: 10.1094/PHYTO.1999.89.6.506. [DOI] [PubMed] [Google Scholar]
- 10.Berry L A, Jones E E, Deacon J W. Interaction of the mycoparasite Pythium oligandrum with other Pythium species. Biocontrol Sci Technol. 1993;3:247–260. [Google Scholar]
- 11.Chet I. Trichoderma—applications, mode of action and potential as a biocontrol agent of soilborne plant pathogenic fungi. In: Chet I, editor. Innovative approaches to plant disease control. New York, N.Y: John Wiley & Sons; 1987. pp. 137–160. [Google Scholar]
- 12.Chet I, Benhamou N, Haran S. Mycoparasitism and lytic enzymes. In: Harman G E, Kubicek C P, editors. Trichoderma and Gliocladium. London, England: Taylor and Francis Ltd.; 1998. pp. 153–173. [Google Scholar]
- 13.Chet I, Inbar J. Biological control of fungal pathogens. Appl Biochem Biotechnol. 1994;48:37–43. doi: 10.1007/BF02825358. [DOI] [PubMed] [Google Scholar]
- 14.Coté F, Hahn M. Oligosaccharins: structures and signal transduction. Plant Mol Biol. 1994;26:1379–1411. doi: 10.1007/BF00016481. [DOI] [PubMed] [Google Scholar]
- 15.Deacon J W. Studies on Pythium oligandrum, an aggressive parasite of other fungi. Trans Br Mycol Soc. 1976;66:383–391. [Google Scholar]
- 16.Dixon R A, Harrison M J, Lamb C J. Early events in the activation of plant defense responses. Annu Rev Phytopathol. 1994;32:479–501. [Google Scholar]
- 17.Foley M F, Deacon J W. Susceptibility of Pythium species and other fungi to antagonism by the mycoparasite Pythium oligandrum. Soil Biol Biochem. 1986;18:91–95. [Google Scholar]
- 18.Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold solution. Nature Phys Sci. 1973;241:20–22. [Google Scholar]
- 19.Fuller M S, Robertson R W, Gisi U. Effects of the sterol demethylase inhibitor, cyproconazole, on hyphal tip cells of Sclerotium rolfsii. III. Cell wall chemistry. Pestic Biochem Physiol. 1990;36:115–126. [Google Scholar]
- 20.Ghisalberti E L, Rowland C Y. Antifungal metabolites from Trichoderma harzianum. J Nat Prod. 1993;56:1799–1804. doi: 10.1021/np50100a020. [DOI] [PubMed] [Google Scholar]
- 21.Haran S, Schickler H, Chet I. Molecular mechanisms of lytic enzymes involved in the biocontrol activity of Trichoderma harzianum. Microbiology. 1996;142:2321–2331. [Google Scholar]
- 22.Inbar J, Chet I. A newly isolated lectin from the plant pathogenic fungus Sclerotium rolfsii: purification, characterization and role in mycoparasitism. Microbiology. 1994;140:651–657. doi: 10.1099/00221287-140-3-651. [DOI] [PubMed] [Google Scholar]
- 23.Klecan A L, Hippe S, Lumsden R D. Reduced growth of Erysiphe graminis f. sp. hordei induced by Tilletiopsis pallescens. Phytopathology. 1990;80:325–331. [Google Scholar]
- 24.Laing S A K, Deacon J W. Video microscopical comparison of mycoparasitism by Pythium oligandrum, Pythium nunn, and an unnamed Pythium species. Mycol Res. 1991;95:469–479. [Google Scholar]
- 25.Lewis J A, Papavizas G C. Permeability changes in hyphae of Rhizoctonia solani induced by germling preparations of Trichoderma and Gliocladium. Phytopathology. 1987;77:699–703. [Google Scholar]
- 26.Lewis K, Whipps J M, Cooke R C. Mechanisms of biological disease control with special reference to the case study of Pythium oligandrum as an antagonist. In: Whipps J M, Lumdsen R D, editors. Biotechnology of fungi for improving plant growth. Cambridge, England: Cambridge University Press; 1989. pp. 191–217. [Google Scholar]
- 27.Madsen A M, de Neergaard E. Interactions between the mycoparasite Pythium oligandrum and sclerotia of the plant pathogen Sclerotinia sclerotiorum. Eur J Plant Pathol. 1999;105:761–768. [Google Scholar]
- 28.Martin F N, Loper J E. Soilborne plant diseases caused by Pythium spp.: ecology, epidemiology, and prospects for biological control. Crit Rev Plant Sci. 1999;18:111–181. [Google Scholar]
- 29.Sequeira L. Surface components involved in bacterial pathogen-plant host recognition. J Cell Sci Suppl. 1985;2:301–316. doi: 10.1242/jcs.1985.supplement_2.16. [DOI] [PubMed] [Google Scholar]
- 30.Shirmböck M, Lorito M, Wang Y L, Hayes C K, Arisan-Atac I, Scala F, Harman G E, Kubicek C P. Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Appl Environ Microbiol. 1994;60:4364–4370. doi: 10.1128/aem.60.12.4364-4370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sivan A, Chet I. The possible role of competition between Trichoderma harzianum and Fusarium oxysporum on rhizosphere colonization. Phytopathology. 1989;79:198–203. [Google Scholar]
- 32.Whipps J M, Lewis K, Cooke R C. Mycoparasitism and plant disease control. In: Burge M N, editor. Fungi in biological control systems. Manchester, United Kingdom: Manchester University Press; 1988. pp. 161–187. [Google Scholar]