Abstract
The importance of waterborne transmission of Cryptosporidium parvum to humans has been highlighted by recent outbreaks of cryptosporidiosis. The first step in a survey of contaminated water currently consists of counting C. parvum oocysts. Data suggest that an accurate risk evaluation should include a determination of viability and infectivity of counted oocysts in water. In this study, oocyst infectivity was addressed by using a suckling mouse model. Four-day-old NMRI (Naval Medical Research Institute) mice were inoculated per os with 1 to 1,000 oocysts in saline. Seven days later, the number of oocysts present in the entire small intestine was counted by flow cytometry using a fluorescent, oocyst-specific monoclonal antibody. The number of intestinal oocysts was directly related to the number of inoculated oocysts. For each dose group, infectivity of oocysts, expressed as the percentage of infected animals, was 100% for challenge doses between 25 and 1,000 oocysts and about 70% for doses ranging from 1 to 10 oocysts/animal. Immunofluorescent flow cytometry was useful in enhancing the detection sensitivity in the highly susceptible NMRI suckling mouse model and so was determined to be suitable for the evaluation of maximal infectivity risk.
Cryptosporidium parvum is presently identified as a common cause of diarrhea in immunocompetent individuals. In immunodeficient individuals, cryptosporidiosis may lead to life-threatening chronic diarrhea, and, because of the incidence of AIDS, the disease poses a significant public health problem in developing countries where AIDS is endemic (8, 14, 17, 22).
Recent outbreaks of cryptosporidiosis support the concern about C. parvum oocyst contamination of treated and surface water (15). In Sydney, Australia, from July to September 1998, contamination of the drinking water supply involved over 3,000,000 residents (16).
Water oocyst count is a commonly used parameter to evaluate the infectious risk. However, the significance of oocyst numbers is questionable, since storage duration and environmental conditions, such as pH, temperature, and/or the presence of oxidants, are likely to influence oocyst viability (5, 10, 18). Moreover, factors such as salinity, temperature, or storage duration may not decrease the infectivity enough to prevent infection in susceptible individuals (11, 12).
Oocyst viability is currently estimated by the quantitation of in vitro excystation rates or by incorporation of nucleic acid dyes (7). However, dyeing is influenced by the degree of oocyst permeabilization and may not reflect parasite infectivity (3, 21). Oocyst infectivity can be evaluated by monitoring in vitro parasite development in highly permissive cells (9, 13, 24). In vivo, C. parvum infection is usually investigated using susceptible animal models such as immunocompromised or neonatal mice (19).
The aim of this work was to assess C. parvum oocyst infectivity using a suckling mouse model (6). Flow cytometry, a rapid and simple alternative to microscopy, was used to detect viable oocysts and to document experimental parasite loads (2, 25, 26). In this model, high-yield parasite amplification was useful for evaluation of the maximal infectivity risk, since the estimated sensitivity was as low as 1 to 10 viable oocysts.
MATERIALS AND METHODS
C. parvum oocysts.
Oocysts were purified from feces obtained from calves experimentally infected with an isolate maintained by M. Naciri (Laboratoire de Pathologie Aviaire, Institut National de la Recherche Agronomique, Nouzilly, France). Oocysts were purified using density separation (1). Briefly, feces stored in a 2.5% K2Cr2O7 solution for less than 2 months were layered on a discontinuous sucrose gradient (densities, 1.045 and 1.090), and spun at 1,800 × g for 30 min. After three washings in 0.1 M saline, oocysts were suspended in a 10% sodium hypochlorite (fresh commercial bleach) aqueous solution for 10 min at −20°C and washed twice before either further characterization or use in animal infectivity assays.
Animal infectivity assays.
Four-day-old NMRI (Naval Medical Research Institute) suckling mice (Iffa-Credo, Lyon, France) were used to evaluate C. parvum infectivity. All liters and their dams were maintained free of Cryptosporidium by the breeding facility, held separately in plastic cages, and given food and water ad libitum. Oocysts were prepared from stock suspensions (106/ml), and doses were prepared by serial dilutions (1,000, 500, 100, 50, 25, 10, 5, and 1 oocyst(s) in 100 μl of saline). Suckling mice were orally inoculated using a 24-gauge needle. Uninfected control mice were inoculated with 100 μl of saline under the same conditions. Seven days after inoculation, mice were killed by cervical dislocation and the entire small intestine was removed, cut into small pieces, and individually homogenized vigorously for 60 s in 1.5 ml of deionized water. Two-hundred-microliter samples were used for further immunofluorescent flow cytometry analysis (IFCM). For each suckling mouse, infection was expressed as the number of oocysts in the entire small intestine (i.e., in 1.5 ml of homogenate). Intestinal homogenates from 12 control mice never exposed to the parasite were similarly processed and the threshold of background fluorescence (nonspecific binding and autofluorescence) was determined as the mean background fluorescence plus 2 standard deviations.
Immunofluorescent staining.
Samples (intestinal homogenates or purified oocysts used as a control) were incubated for 30 min at 37°C with a 1:10 final dilution of a fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody (MAb) directed against a Cryptosporidium wall antigen, which was selected for its lack of cross-reactivity with other microorganisms (FITC-COW MAb, Monofluo kit; Diagnostic Pasteur, Marnes-la-Coquette, France).
Flow cytometry.
IFCM was performed using a Facscalibur flow cytometer Becton Dickinson Immunocytometry Systems, [BDIS], San Jose, Calif.), aligned as described in the manufacturer's protocol (Calibrite beads; BDIS). Detection of C. parvum oocysts was done using the following settings: (i) forward-angle light scatter detector (FSC) at E00, (ii) side-angle light scatter detector (SSC) at 304 V, and (iii) green fluorescence detector (530 ± 30 nm band-pass filter) at 430 V. The FSC parameter was used as the threshold and set at a value of 200. For each series of experiments, a control oocyst suspension was used to verify the cytometer settings. Absolute oocyst counts were performed using fluorescent calibrated beads which were highly uniform with respect to granularity and fluorescence intensity (True-count tube; BDIS).
Statistical analysis.
The significance of differences between groups of experiments was assessed by an unpaired Student's t test, assuming a normal distribution of data. Linear correlation was evaluated by estimating the significance of r coefficient values.
RESULTS
Quantitative IFCM detection of C. parvum oocysts.
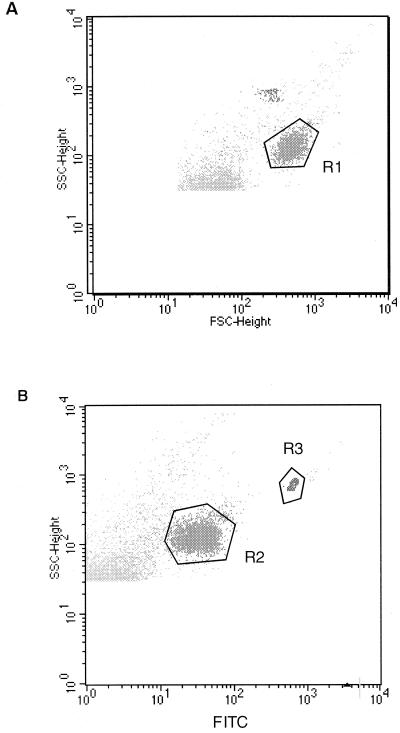
Purified oocyst suspensions were used to define the light scatter region for oocysts. These suspensions exhibited a high degree of homogeneity, since region R1 was determined to contain >99% of events with microscopically controlled oocyst morphology (Fig. 1). In three independent series of experiments with oocyst numbers ranging from 30 to 150 × 103/ml, more than 99% of oocysts appearing in region R1 were labeled with the FITC-COW MAb (Fig. 1, region R2), and debris particles were clearly excluded. Under these conditions, IFCM detection was quantitative from a minimum of 45 oocysts/ml.
FIG. 1.
Flow cytometric detection of C. parvum oocysts. Graphs show profiles from a representative experiment with a purified oocyst suspension. (A) A flow cytometric region (R1) was defined according to the parameters of size (FSC-height) and internal complexity (SSC-height). (B) Region R2 represents the corresponding fluorescence profile after labeling with FITC-conjugated MAb for Cryptosporidium oocyst wall. Region R3 includes fluorescent beads used as an internal standard for oocyst counting.
Quantitation of C. parvum infectivity in NMRI suckling mice.
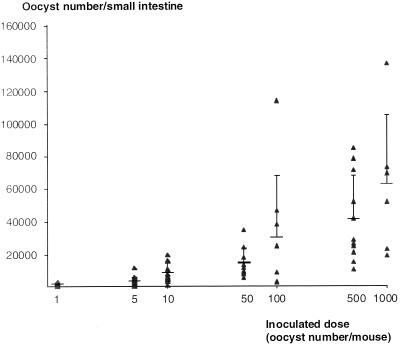
The IFCM method described above was applied for the quantitation of oocysts in the whole small intestines of suckling mice after oocyst ingestion. From a series of experiments, the dose-response effect is depicted in Fig. 2. Seven days after ingestion, the mean number of intestinal oocysts was directly related to the number of inoculated oocysts and it exhibited an exponential trend at higher oocyst doses.
FIG. 2.
Dose-response effect of ingested oocysts on day 7 intestinal C. parvum infection of suckling mice. The number of small intestine oocysts per animal is presented on a logarithmic scale. Under these conditions, the linear correlation r value was 0.87 (P < 0.01). Bar, 1 standard deviation from the mean.
As shown in Table 1, the number of experimental animals ranged from 7 to 27 for the different oocyst doses. In groups that ingested from 1,000 to 25 oocysts/animal, all mice exhibited intestinal oocysts. A proportion of about 70% of animals had detectable oocysts after ingestion of lower doses (estimated to be 1 to 10 oocysts/animal). No infection was detected in control C. parvum-free animals. From these data, the corresponding detection sensitivity of infective oocysts could be estimated as being within the range of 1 to 10 ingested oocysts.
TABLE 1.
Infectivity of inoculated C. parvum oocysts in NMRI suckling mice
| Ingested inoculum size (no. of oocysts/mouse) |
Infectivity (no. infected animals/ no. challenged)a |
|---|---|
| 0 | 0/12 |
| 1 | 11/15 |
| 5 | 18/27 |
| 10 | 13/18 |
| 25 | 11/11 |
| 50 | 11/11 |
| 100 | 9/10 |
| 500 | 12/12 |
| 1,000 | 7/7 |
Number of mice with small intestine oocysts on day 7 after oocyst ingestion.
DISCUSSION
In this work, maximal infective risk of C. parvum oocysts was estimated by measuring infectivity in a suckling mouse model. C. parvum oocyst counting was performed using IFCM.
Flow cytometry was accurate and reliable for rapid quantitative analysis and characterization of large numbers of oocysts. Moreover, sensitivity of IFCM counting was reported at a level 10-fold higher than that reported for microscopy, and a lower variability was achieved at low element concentrations (2, 4). In this work, oocyst counting was based on simultaneous quantitation of an internal standard of fluorescent calibrated beads for maximal accuracy.
In this work, oocyst oxidation was used for achieving maximal infectivity. Oxidation did not reduce oocyst viability as determined by excystation rate and nucleic acid dyeing (viability, >90%), and the present data show that detectable infection can be obtained in suckling mice by ingestion of oocysts prepared as above. Although subsequent to oxidation a lack of identification may occur when other MAbs are used, our data suggest that no epitope damage interfering with detection by the FITC-COW MAb used in this study was caused by this procedure (20).
A modified NMRI suckling mouse model was used to quantify oocyst infectivity (6). In this highly susceptible host, oral oocyst inoculation leads to amplification of intestinal parasite burden. To our knowledge, the total number of oocysts present in the entire small intestine was not taken into account in previous studies. Compared with purified oocyst preparations, the sensitivity of IFCM detection for intestinal samples was decreased due to background signals of intestinal particulate debris, which may adsorb MAb nonspecifically and thus require appropriate controls with uninfected intestinal preparations. In other suckling mouse models, the 50% infectious dose reportedly ranges between 60 and 1,000 oocysts/mouse, while most authors use 105 to 107 oocysts/animal to induce experimental infections (19).
Previous studies have shown that in seronegative healthy adult volunteers, the 50% infectious dose ranged from 9 to 1,042 ingested oocysts, depending on the isolate (23). For this reason, maximal infectivity was assessed in the present study by preoxidizing oocysts for maximal excystation. In the NMRI suckling mouse model, this results in a detection sensitivity of 1 to 10 oocysts, i.e., a range suitable for the evaluation of maximal infectivity risk in humans.
ACKNOWLEDGMENTS
This work was supported by a grant from Ministère de l'Environnement, 1999.
We thank M. Naciri and R. Mancassola for the kind gift of C. parvum oocysts.
REFERENCES
- 1.Arrowood M J, Sterling C R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987;73:314–319. [PubMed] [Google Scholar]
- 2.Arrowood M J, Hurd M R, Mead J R. A new method for evaluating experimental cryptosporidial parasite loads using immunofluorescent flow cytometry. J Parasitol. 1995;81:404–409. [PubMed] [Google Scholar]
- 3.Belosevic M, Guy R A, Taghikilani R, Neumann N F, Gyurek L L, Liyanage L R J, Millard P J, Finch G R. Nucleic acid stains as indicators of Cryptosporidium parvum oocyst viability. Int J Parasitol. 1997;27:787–798. doi: 10.1016/s0020-7519(97)00033-7. [DOI] [PubMed] [Google Scholar]
- 4.Bennett J W, Gauci M R, Le Moënic S, Schaefer III F W, Lindquist H D A. A comparison of enumeration techniques for Cryptosporidium parvum oocysts. J Parasitol. 1999;85:1165–1168. [PubMed] [Google Scholar]
- 5.Brasseur P, Uguen C, Moreno-Sabater A, Favennec L, Ballet J J. Viability of Cryptosporidium parvum oocysts in natural waters. Folia Parasitol. 1998;45:113–116. [PubMed] [Google Scholar]
- 6.Buraud M, Kapel N, Benhamou Y, Savel J, Gobert J G. A high-yield outbred suckling mouse model of cryptosporidiosis. Parasite. 1995;2:81–84. doi: 10.1051/parasite/1995021081. [DOI] [PubMed] [Google Scholar]
- 7.Campbell A T, Robertson L J, Smith H V. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with inclusion or exclusion of fluorogenic vital dyes. Appl Environ Microbiol. 1992;58:3488–3493. doi: 10.1128/aem.58.11.3488-3493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casemore D P, Wright S E, Coop R L. Cryptosporidiosis—human and animal epidemiology. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 65–92. [Google Scholar]
- 9.Di Giovanni G D, Hashemi F H, Shaw N J, Abrams F A, LeChevallier M W, Abbaszadegan M. Detection of infectious Cryptosporidium parvum oocysts in surface and filter backwash water samples by immunomagnetic separation and integrated cell culture-PCR. Appl Environ Microbiol. 1999;65:3427–3432. doi: 10.1128/aem.65.8.3427-3432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fayer R, Trout J M, Jenkins M C. Infectivity of Cryptosporidium parvum oocysts stored in water at environmental temperatures. J Parasitol. 1998;84:1165–1169. [PubMed] [Google Scholar]
- 11.Freire-Santos F, Oteiza-Lopez A M, Vergara-Castiblanco C A, Ares-Mazas M E. Effect of salinity, temperature and storage time on mouse experimental infection by Cryptosporidium parvum. Vet Parasitol. 1999;87:1–7. doi: 10.1016/s0304-4017(99)00160-0. [DOI] [PubMed] [Google Scholar]
- 12.Fricker C R, Crabbs J H. Water-borne cryptosporidiosis: detection methods and treatment options. Adv Parasitol. 1998;40:241–278. doi: 10.1016/s0065-308x(08)60123-2. [DOI] [PubMed] [Google Scholar]
- 13.Gargala G, Delaunay A, Favennec L, Brasseur P, Ballet J J. Enzyme immunoassay detection of Cryptosporidium parvum inhibition by sinefungin in sporozoite infected HCT-8 enterocytic cells. Int J Parasitol. 1999;29:703–709. doi: 10.1016/s0020-7519(99)00031-4. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths J K. Human cryptosporidiosis: epidemiology, transmission, clinical disease, treatment and diagnosis. Adv Parasitol. 1998;40:37–85. doi: 10.1016/s0065-308x(08)60117-7. [DOI] [PubMed] [Google Scholar]
- 15.Guerrant R L. Cryptosporidiosis: an emerging, highly infectious threat. Emerg Infect Dis. 1997;3:51–57. doi: 10.3201/eid0301.970106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson G, Alla P. Minimising the risk from Cryptosporidium and other waterborne particles. International Conference of the International Association of Water Quality; 1999. Protection of public health—Sydney Cryptosporidium problem. [Google Scholar]
- 17.Kelly P, Davies S E, Mandanda B, Veitch A, McPhail G, Zulu I, Drobniewsky F, Fuchs D, Summerbell C, Luo N P, Pobee J O M, Farthing M J G. Enteropathy in Zambians with HIV related diarrhoea: regression modelling of potential determinants of mucosal damage. Gut. 1997;41:811–816. doi: 10.1136/gut.41.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korich D G, Mead J R, Madore M S, Sinclair N A, Sterling C R. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl Environ Microbiol. 1990;56:1423–1428. doi: 10.1128/aem.56.5.1423-1428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindsay D S. Laboratory models of cryptosporidiosis. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 209–223. [Google Scholar]
- 20.Moore A G, Vesey G, Champion A, Scandizzo P, Deere D, Val D, Williams K L. Viable Cryptosporidium parvum oocysts exposed to chlorine or other oxidising conditions may lack identifying epitopes. Int J Parasitol. 1998;28:1205–1212. doi: 10.1016/s0020-7519(98)00070-8. [DOI] [PubMed] [Google Scholar]
- 21.Neumann N F, Gyürék L L, Finch G R, Belosevic M. Intact Cryptosporidium parvum oocysts isolated after in vitro excystation are infectious to neonatal mice. FEMS Microbiol Lett. 2000;183:331–336. doi: 10.1111/j.1574-6968.2000.tb08980.x. [DOI] [PubMed] [Google Scholar]
- 22.O'Donoghue P J. Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol. 1995;25:139–195. doi: 10.1016/0020-7519(94)e0059-v. [DOI] [PubMed] [Google Scholar]
- 23.Okhuysen P C, Chappell C L, Crabb J H, Sterling C R, Du Pont H L. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J Infect Dis. 1999;180:1275–1281. doi: 10.1086/315033. [DOI] [PubMed] [Google Scholar]
- 24.Slifko T R, Friedman D, Rose J B, Jakubowski W. An in vitro method for detecting infectious Cryptosporidium oocysts with cell culture. Appl Environ Microbiol. 1997;63:3669–3675. doi: 10.1128/aem.63.9.3669-3675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vesey G, Griffiths K R, Gauci M R, Deere D, Williams K L, Veal D A. Simple and rapid measurement of Cryptosporidium excystation using flow cytometry. Int J Parasitol. 1997;27:1353–1359. doi: 10.1016/s0020-7519(97)00085-4. [DOI] [PubMed] [Google Scholar]
- 26.Vesey G, Hutton P, Champion A, Ashbolt N, Williams K L, Warton A, Veal D. Application of flow cytometric methods for the routine detection of Cryptosporidium and Giardia in water. Cytometry. 1994;16:1–6. doi: 10.1002/cyto.990160102. [DOI] [PubMed] [Google Scholar]