Abstract
Background: Histological changes induced by gluten in the duodenal mucosa of patients with non-coeliac gluten sensitivity (NCGS) are poorly defined. Objectives: To evaluate the structural and inflammatory features of NCGS compared to controls and coeliac disease (CeD) with milder enteropathy (Marsh I-II). Methods: Well-oriented biopsies of 262 control cases with normal gastroscopy and histologic findings, 261 CeD, and 175 NCGS biopsies from 9 contributing countries were examined. Villus height (VH, in μm), crypt depth (CrD, in μm), villus-to-crypt ratios (VCR), IELs (intraepithelial lymphocytes/100 enterocytes), and other relevant histological, serologic, and demographic parameters were quantified. Results: The median VH in NCGS was significantly shorter (600, IQR: 400–705) than controls (900, IQR: 667–1112) (p < 0.001). NCGS patients with Marsh I-II had similar VH and VCR to CeD [465 µm (IQR: 390–620) vs. 427 µm (IQR: 348–569, p = 0·176)]. The VCR in NCGS with Marsh 0 was lower than controls (p < 0.001). The median IEL in NCGS with Marsh 0 was higher than controls (23.0 vs. 13.7, p < 0.001). To distinguish Marsh 0 NCGS from controls, an IEL cut-off of 14 showed 79% sensitivity and 55% specificity. IEL densities in Marsh I-II NCGS and CeD groups were similar. Conclusion: NCGS duodenal mucosa exhibits distinctive changes consistent with an intestinal response to luminal antigens, even at the Marsh 0 stage of villus architecture.
Keywords: non-coeliac gluten sensitivity, histology, normal mucosa, coeliac disease
1. Introduction
Wheat, especially for its gluten component, is well-known to be a worldwide major food antigen. As such, its role has been implicated in several disorders, including celiac disease, dermatitis Herpetiformis, wheat allergy, gluten-induced ataxia and, more recently, in non-coeliac gluten sensitivity (NCGS). The expanding spectrum of gluten related disorders, from typical CeD to atypical CeD presentation, has now advanced into a new dimension with emerging recognition of the condition known as “non-coeliac gluten sensitivity” [1,2,3]. ‘Non-coeliac’ implies the absence of autoantibodies in the presence of gluten-induced symptoms in susceptible individuals. Non-coeliac has also been classified as gluten sensitivity with normal or nearly normal histology. However, possible microscopic and sub-microscopic [4] enteropathies that may overlap with the NCGS group have not, so far, been systematically evaluated. In addition, evidence to support the view that NCGS could exclusively present with normal histology or a milder enteropathy is lacking.
Systemic immune activation is reported to damage the intestinal epithelium [5,6,7] in NCGS. The genetic and host immune responses are recognised by increased intraepithelial CD3(+) T cells and increased cytokines as a response to a gluten challenge [8].
The factors contributing to the variability of mucosal changes in CeD are insufficiently explored and there are no acceptable explanations in the current literature as to why the immune reactions involved in NCGS and some CeD patients [6] induce only mild or minimal mucosal changes.
In NCGS, similar to CeD, gluten and other factors may cause alterations in villus morphology [9,10] and remodelling of intestinal mucosa [11,12,13]. Nevertheless, severe gluten-induced mucosal damages in antibody-negative patients [14,15] is not recognized to be a feature of NCGS despite similar clinical presentation and identical HLA class II haplotype frequency [16]. When the initial gluten-sensitive seronegative individuals were reported [17], NCGS was not recognised. Therefore, the severity of small bowel mucosal damages in NCGS is still contentious and has become even more complicated since histology is not even a mandatory part of the diagnostic work-up for NCGS [18]. Indeed, the exclusion of CeD is frequently assumed through negative serology. Erroneous reports of mucosal “atrophy” also preclude accurate understandings of the mucosal histopathogenesis. In fact, neither CeD nor NCGS mucosal changes could be due to an atrophic process simply because structural recovery of villi ensues in most cases following removal of the immunogenic antigens [19].
New data on NCGS histology presented in this report quantified the morphological changes in CeD with milder enteropathies (Marsh I-II) in comparison with NCGS, with the aim of delineating the histological spectrum of NCGS and its distinction from normal intestinal mucosa. In addition, we aimed to (i) quantify the eosinophils as an additional cause of NCGS and (ii) delineate clustering or linear distributions of IELs in villus epithelium or deeper lamina propria [20,21,22].
Furthermore, we assessed the double-blind placebo-controlled (DBPC) gluten challenge diagnostic strategy compared to an open gluten challenge policy for the diagnosis of NCGS.
2. Methods and Materials
This multicentre study was designed by clinicians and expert pathologists at the International Meeting on Digestive Pathology, Bucharest 2017. The study is based on a harmonised methodology for histological evaluation as conducted between investigators with interobserver agreement. Participants consisted of 23 tertiary centres with clinical and pathological expertise in CeD, NCGS, DH, and gluten or wheat allergies from 9 countries on 4 continents. Archived histology slides of 698 patients were randomly recruited from Australia (n = 20), Finland (n = 20), India (n = 25), Iran (n = 37), Italy (n = 239), Romania (n = 10), Turkey (n = 30), UK (n = 166), and USA (n = 151) (Supplementary Table S1). Those slides were from patients diagnosed with CeD Marsh I-II histology (n = 261), NCGS (n = 175), and 262 controls fulfilled our inclusion and exclusion criteria as formulized in a single agreed protocol. The inclusion and exclusion criteria for controls were similar to our previous study [9], as follows: The control group were included only if they had negative tTG and/or EMA. We included patients with anaemia and weight loss unrelated to the upper gastrointestinal tract with normal biopsies. Patients with infection (e.g., H pylori), bacterial overgrowth, functional dyspepsia, history of major abdominal surgery, liver disease, food intolerance, decompensated diabetes mellitus, or thyroid disease, patients using non-steroidal anti-inflammatory drugs (NSAIDs), and patients with any other liver or gastrointestinal disorders or diagnosis were excluded.
Patients were diagnosed in leading tertiary centres and biopsies were performed based on presentation with symptoms compatible with CeD or NCGS. All patients included in this study (CeD and NCGS) were untreated and the intestinal biopsies were taken before starting a gluten free diet. Details of inclusion and exclusion criteria are delineated in supplementary documents.
Biopsies were taken both from the duodenal bulb (D1) and distal duodenum (D2) as recommended by the International Guidelines. All selected cases had concordant pathology in terms of Marsh I or II histology in D1 and D2 biopsies. However, histopathological measurements were limited to the well-oriented D2 biopsies as villi are shorter and architecturally distorted due to Brunner’s glands in the duodenal bulb.
Our morphometric and quantitative examinations provided objective measurement of enteropathy by using villus height (VH), crypt depth (CrD), VD:CrD ratio and intraepithelial lymphocyte count (per 100 enterocytes). Intraepithelial lymphocytes were counted per 100 enterocytes covering the villous epithelium of the entire villus for the total IEL count of each case. IEls per individual villus were calculated by counting 10 consecutive well-oriented villi and then averaging, while IELs per individual crypt were calculated by counting 10 consecutive well-oriented crypts and obtaining the average.
Some studies suggest a continuous and even distribution of IELs is indicative of CeD [23] and a non-specific increase in IELs is more likely to decrease along the villus tip. Therefore, we assessed the percentage of villi with increased IELs (PVIIEL) and also the percentage of villi with even distribution of IELs (PVEIEL) and their distribution in the crypt epithelial mucosa.
Further evaluation was made of the inflammatory cell infiltrate in the lamina propria with a particular focus on eosinophil count (>5 eosinophils per high-power field (HPF) at ×40 magnification) [20,22,24]. A supplementary micrograph summarizes the steps included in the morphometric analysis of the duodenal mucosae.
Interclass correlation coefficient: see supplementary material.
2.1. Ethical Considerations
This study involved the rescoring of archived histology slides and the removal of all identifiable medical information, and analyses were performed using anonymized data. The data collection was in line with clinical best practice policies with approval by research and development departments of countries involved under the Reg.No 565 at Milton Keynes University Hospital, Milton Keynes, UK. The study was also approved by the ethical committee of the Research Institute for Gastroenterology and Liver Disease, Shahid Beheshti University of Medical Science, Tehran under the Ref.No: IR.SBMU.RETECH.REC.1399.1003.
2.2. Statistical Analysis
The distribution pattern of all continuous variables across the three main groups of NCGS, CeD and control biopsies are presented as median and interquartile range (IQR). Differences between NCGS and other groups were tested using both adjusted and non-adjusted models. In models, various general linear models were used, in which the histological parameters in a continuous form (or their log-transformed equivalent when data were obviously asymmetric, defined by skewness and kurtosis beyond the range of −1 to 1) were the dependent variables and histological groups were independent variables. Country, age, and sex were used as adjusting factors and covariates. For receiver operating curve (ROC) analyses of IEL density in the overall cohort and subgroups, area under the curve area (AUC) was calculated under non-parametric assumption. All tests of significance were set to p < 0.05 in two-sided tests.
3. Results
3.1. A. General Characteristics of Groups
In this multicentre cross-sectional study, endoscopic biopsy specimens from a total of 698 participants were evaluated, including 175 NCGS patients, 261 CeD patients, and 262 control subjects. Details of recruited subjects by group, centre, and country are presented in the Supplementary Table S1. The median age of the NCGS group (37·0, IQR: 27·0–47·0) was similar to the CeD patients (38·0, IQR: 23·0–51·0) (p = 0·839) but younger than the control group (43·0, IQR: 30·5–57·0), (p < 0·001). Only 4·0% of NCGS patients were under 18 years, similar to the control group (5·3%, p = 0·650) but significantly different from the CeD group, where 16.5% were under 18 years (p < 0·001). A total of 82·9% of NCGS patients were women, which was higher than the proportion of women in the control (60·9%, p < 0.001) and CeD groups (67·0%, p < 0·001).
There was a range of gastrointestinal and non-specific symptoms recorded as the predominant symptom at the time of diagnosis (Supplementary Table S3). When the structural changes in the histology were classified by Marsh’s grading system [25], 53% of NCGS patients were Marsh 0, and 47% were Marsh I/II. All control participants were Marsh 0 and all in the CeD group were Marsh I-II histology.
3.2. B. Mucosal Characteristics in NCGS, Coeliac, and Normal Mucosa
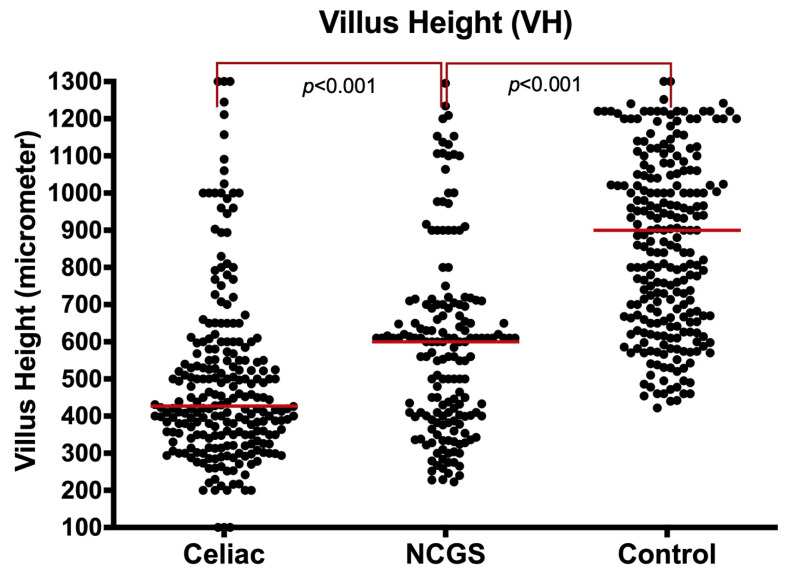
B1. Villus Height (VH): Villus heights were significantly shorter in NCGS patients than in the controls [600 µm (IQR: 400–705) vs. 900 µm (IQR: 667–1112), p < 0.001] and significantly longer than the CeD patients [600 µm (IQR: 400–705) vs. 427 µm (IQR: 348–569), p < 0.001] (Figure 1 and Table S4).
Figure 1.
Villus height (μm) in NCGS compared to CeD and controls, entire cohort.
The significance of the shorter villous height in NCGS patients compared to controls persisted, even after limiting the analysis to Marsh 0 specimens [617 µm (IQR: 549–863) vs. 900 µm (IQR: 667–1112), p < 0.001]. When comparing villus height in Marsh I/II NCGS with the same grade CeD specimens, NCGS patients had a similar villus height to the CeD group [465 µm (IQR: 390–620) vs. 427 µm (IQR: 348–569, p = 0.176). The villus height in Marsh I-II were significantly shorter than controls (p < 0·001), accounting for the country, age, and gender of the patients (Supplementary Figure S1).
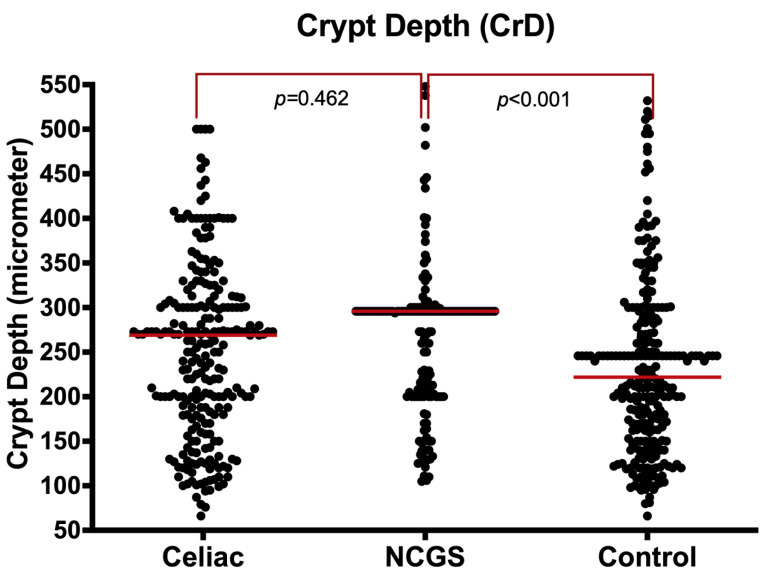
B2. Crypt Depth (CrD): The median crypt depth (CrD) was significantly longer (deeper) in the NCGS group than the controls [296 µm (IQR: 205–300) vs. 222 µm (IQR: 158–294), p < 0.001) but similar to the CeD group [296 µm (IQR: 205–300) vs. 269 µm (IQR: 182–323), p = 0.462] (Figure 2 and Supplementary Table S4).
Figure 2.
Crypt depth (μm) in NCGS compared to CeD and controls, entire cohort.
The longer crypts in NCGS cases compared to controls remained significant even after limiting analysis to Marsh 0 specimens [296 µm (IQR: 261–300) vs. 222 µm (IQR: 158–294), p < 0.001]. The CrD values were similar in Marsh I-II for both NCGS and CeD groups [260µm (IQR: 200–296) vs. 269 µm (IQR: 182–323), p = 0.409)].
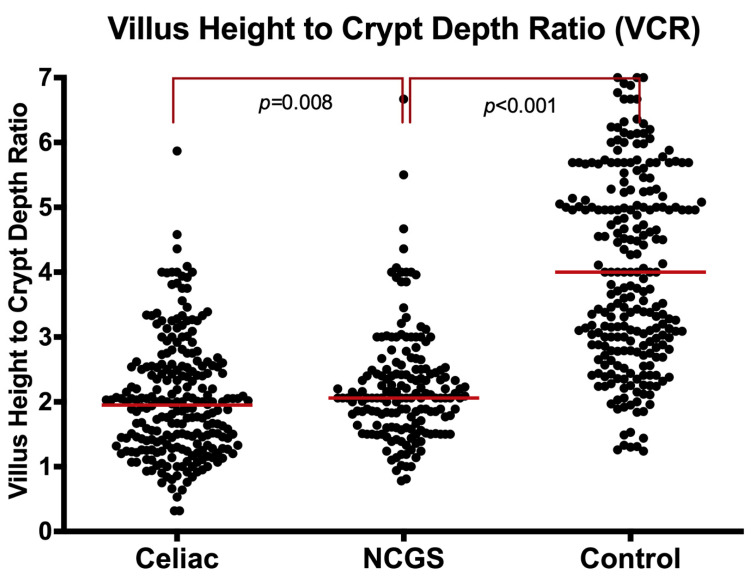
B3. Villus Height to Crypt Depth Ratio (VCR): The villus to crypt length ratio (VCR) was significantly lower in NCGS patients compared to controls [2.1 (IQR: 1.6–2.5) vs. 4.0 (IQR: 2.9–5.6), p < 0.001] (Figure 3, and Supplementary Table S4). This was significant even when the analysis was limited to Marsh 0 [2.1 (IQR: 1.8–2.4) vs. 4.0 (IQR: 2.9–5.6), p < 0·001]. In contrast, the VCR in NCGS was significantly higher than the CeD group [2·1 (IQR: 1.6–2.5) vs. 1.9 (IQR: 1.3–2.5), p = 0.008] in the entire cohort. The VCR was also higher in NCGS than CeD, when the analysis was limited to Marsh I-II [2.1 (IQR: 1.5–2.6) vs. 1·9 (IQR: 1.3–2.5), p = 0.019].
Figure 3.
Villus height to crypt depth ratio (VCR) in NCGS compared to CeD and controls, entire cohort.
3.3. C. Intraepithelial Lymphocytes (IELs)
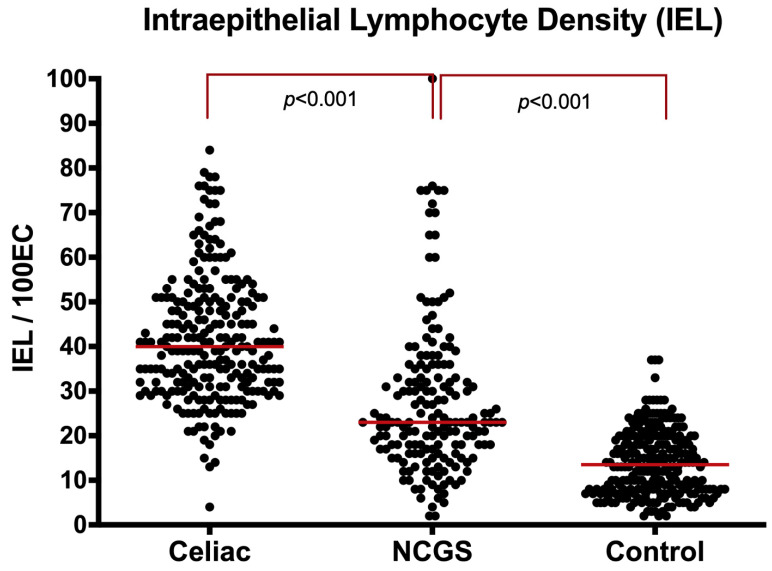
C1. IELs in Villi: The median IEL density for NCGS patients [23 (IQR: 17–34)] was significantly higher than controls [14 (IQR: 8–20), p < 0.001] and significantly lower than the CeD group [40 (IQR: 31–50) p < 0.001] (Figure 4, Supplementary Figure S2 and Table S4). Marsh 0 NCGS patients had significantly higher IEL densities than controls [18 (IQR: 14–23) vs. 14 (IQR: 8–20), p = 0.010]. Comparing IEL densities between the Marsh I/II NGGS and the Marsh I/II CeD group showed slightly lower density in the NCGS mucosa, but the difference did not reach statistical significance [33 (IQR: 27–44) vs. 40 (IQR: 31–50), p = 0.224].
Figure 4.
Intraepithelial lymphocytes (IELs) of villi in NCGS compared to CeD and controls, entire cohort.
To differentiate the NCGS from the control group, the ROC analysis, limited to Marsh 0, revealed 79% sensitivity and 55% specificity at a cut-off of 14/100 enterocytes for NCGS. The area under the curve was 0.70 (95%CI: 0.63–0.76, p < 0.001) (Supplementary Figure S2A). IEL densities in Marsh I-II NCGS and CeD groups were similar and the ROC analysis failed to differentiate NCGS from CeD with an area under the curve of 0·43 (95%CI: 0.35–0.65, p = 0.065) (Supplementary Figure S2B).
C2. IELs in Crypts: The median IEL density in crypts in the NCGS group was similar to the controls [4.0 (IQR: 2.0–7.3) vs. 3.0 (IQR: 2.0–6.0), p = 0.121] but lower than the CeD group [6.0 (IQR: 3.0–11.7), p = 0.001] (Supplementary Figure S3). When the comparison was restricted to Marsh 0, NCGS had a crypt IEL density similar to the controls [2.7 (IQR: (1.0–5.0) vs. 3.0 (IQR: 2.0–6.0), p = 0.135]. Comparison between crypt IEL densities in Marsh I/II NCGS and the CeD group showed similar results [6.0 (IQR: 2.9–12.0) vs. 6.0 (IQR: 3.0–11.7), p = 0.160].
C3. Percentage of Villi with Increased IEL (PVIIEL): Data of PVIIEL were available for 550 (79%) cases of the entire cohort. The median PVEIEL in the NCGS group [50% (IQR: 19–89)] was significantly higher than the normal controls [0% (IQR: 0–10), p < 0.001] but significantly lower than the CeD group [80% (IQR: 50–100), p < 0.001]. When restricted to Marsh 0, PVIIEL in NCGS cases remained higher than the normal controls [20% (IQR: 0–42) vs. 0% (IQR: 0–10), p < 0.001]. Comparing PVIIEL in the Marsh I/II NCGS to the Marsh I/II CeD group showed similar percentages in both groups [80% (IQR: 50–100) vs. 80% (IQR: 50–100), p= 0.462] (Supplementary Figure S4).
C4. Percentage of Villi with even distribution of IEL (PVEIEL): Data for PVEIEL were available for 513 (73.5%) cases of the entire cohort. The median PVEIEL in the NCGS group was similar to the normal controls [66% (IQR: 38–100) vs. 80% (IQR: 24–100), p= 0.631] and the CeD group [66% (IQR: 38–100) vs. 62% (IQR: 40–83), p= 0·655]. When the comparison was restricted to Marsh 0, there were no significant differences between PVEIEL in the NCGS and the normal control group [50% (IQR: 26–100) vs. 80% (IQR: 24–100), p= 0.637]. Similarly, when the analysis was restricted to Marsh I-II, PVEIEL was similar in NCGS and CeD groups [67% (IQR: 48–90) vs. 62% (IQR: 40–83), p= 0·937] (Supplementary Figure S4).
3.4. D. Eosinophils in Lamina Propria
The density of eosinophils in the lamina propria (LPEO) was assessed in 294 (42%) cases. The median LPEO density in NCGS [10 (IQR: 5–15) was similar to the normal controls [15 (IQR: 10–18), p= 0.844] but significantly lower than CeD patients [16 (IQR: 7–29), p < 0.001]. Patients with NCGS diagnosed by the open challenge approach had similar eosinophil counts to NCGS cases diagnosed by the DBPC method [10 (IQR: 5–19) vs. 10 (IQR: 4–15), respectively p= 0.497].
3.5. E. Serological Assessment
Quantitative serological data was available for a total of 627 (89.8%) cases of the entire cohort. A positive result on either TTG or EMA measurement was considered a positive CeD serology result. By definition, none of the NCGS patients or normal controls but all CeD patients had a positive TTG or EMA test or positive for tests both. None of the NCGS patients or normal controls were positive but 118 (91.5%) of CeD patients were positive for EMA serology.
Serum TTG in NCGS was negative and significantly lower than that in CeD patients [(2.0, IQR: 0.6–3.5) vs. (46.0, IQR: 24.0–95.0), p < 0.001] but was similar to the normal control group [(2.0, IQR: 0.6–3.5) vs. (1·3, IQR: 0.6–2.4), p= 0.289]. Additionally, when we compared TTG titre in Marsh 0 NCGS cases with normal controls (all Marsh 0), results were similar in both groups, being 1.2 (IQR: 0.1–3.1) in NCGS and 1.3 in controls (IQR: 0.6–2.4), p= 0.430. When the analysis was restricted to Marsh I and II histological grades, the NCGS group had significantly lower TTG titre than the CeD patients [2.2 (IQR: 1.2–3.7) vs. 46.0 (IQR: 24.0–94.0), p = 0.010).
3.6. F. Histological Differences of NCGS Patients Diagnosed by DBPC versus OC
Among 175 NCGS patients, 110 (63%) patients were diagnosed by the open challenge technique and the remaining 65 (37%) with the DBPC test. The median ages in the OC and DBPC subgroups were similar [(38, IQR: 30–49) vs. (35, IQR: 25–43), p = 0.087]. Female to male ratios were similar in the OC and DBPC subgroups (4.8:1 vs. 4.9:1, p = 1.000). Patients in OC subgroup had significantly higher Marsh grades (Marsh above 0) compared to DBPC (58% vs. 30%, p = 0.001). Additionally, the OC subgroup had shorter villus heights than patients diagnosed with DBPC tests [500 (IQR: 376–635) vs. 695 (IQR: 600–900) p < 0.001. However, their VCR were similar [2.06 (IQR: 1.60–2.51) vs. 2·18 (IQR: 1.80–2.85) p= 0.350. The OC subgroup also had higher IEL densities than the DBPC subgroup [27.0 (IQR: 17.0–40.0) vs. 21.0 (IQR: 15.5–30.0) p = 0.006, but the two subgroups had a similar eosinophil count, being [10.0 (IQR: 5.0–19.0) vs. 10.0 (IQR: 4.0–15.0), p= 0.497], respectively.
4. Discussion
This work is a unique global study using conventional histological criteria to elucidate the major mucosal changes associated with non-coeliac gluten sensitivity (NCGS). Our findings show that the intestinal mucosae in NCGS patients exhibited changes consistent with mucosal immune response [4,5,6,26,27] to luminal antigenic stimulation. The NCGS histologic alterations were characterized in 175 patients with the diagnosis, based on the expert Salerno criteria [18], including both double-blind placebo-controlled (DBPC) and open gluten challenge methods. However, no differences were identified between cases diagnosed with the DBPC or open gluten challenge approach.
Our findings indicate that NCGS mucosae are associated with (i) reduced villus height, (ii) increased crypt depth, (iii) increased lymphocyte infiltration of either villi or crypts and corresponding alterations in villus/crypt ratios even at Marsh 0 stage. On quantification, the TTG titres in NCGS cases were similar to the normal control population but markedly lower than the CeD patients matched for Marsh I/II histology.
Morphological changes identified in NCGS patients with Marsh 0 challenges the current definition of a normal mucosa, as noted previously [11]. These findings therefore clearly suggest a need for greater precision in delineating normal mucosae from minimal mucosal changes, such as microenteropathy [4]. Our further analysis suggests that intestinal villi in Marsh I-II might be shorter than controls when measured in a morphometric manner, despite their pseudo-normal appearance at microscopy (Supplementary Figure S1).
A consensus on the extent of enteropathy in NCGS and any potential histological difference between NCGS and CeD is lacking in the current literature. In addition, there are only limited studies [21] that compare the IEL densities and the cut-off between milder enteropathy like Marsh I-II in comparison with Marsh III like pathology.
We initially believed that IELs are overestimated by a factor of two in patients with severe architectural distortion due to severely damaged epithelial cells [9]. However, a similar IEL cut-off value of 25/100EC for both CeD and NCGS with Marsh I-II lesions in this study brought evidence for the lack of credibility of this point of view.
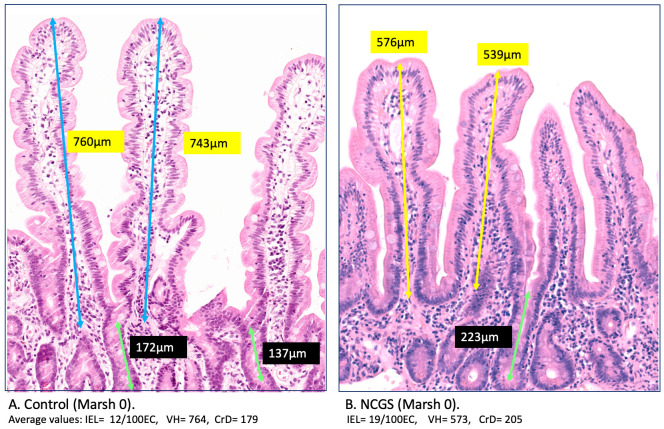
In addition, the Marsh 0 stage seems to have a different meaning in NCGS in comparison with normal controls [24], including differences in IEL counts [28] and measurable microstructural abnormalities affecting the villus [29] and crypt architecture (Figure 5). The IELs in the control group mucosae were 13.7/100EC with the majority between 5–10 per 100 epithelial cells. Before the current study, the gap between 5–10 IEL/100EC and the pathological threshold 25 IEL/100EC had not been adequately investigated. Architectural aberrations seem to start from the Marsh 0 stage [25,26,27,28,29] as evident from the significant villus/crypt ratio reduction [30] at this stage in NCGS patients.
Figure 5.
Architectural distortion at Marsh 0 stage. The measurable subtle changes that have been considered as a component of the spectrum of normal mucosa represent a considerable part of architectural distortion signifying the NCGS phenotype (B). This reflects in VH, VH/CrD ratio, and the IEL infiltration that were significantly different in NCGS Marsh 0 (B) compared to healthy control (A).
Our morphometric measurements as carried out in highly experienced leading tertiary centres indicate progressive remodelling, including changes in villi and crypts, starting from Marsh 0 stage and thus well before the Marsh II stage [29]. The truth seems to be that the expansion of the crypt epithelium and quantifiable changes in villus structure [12,19,31] start as early as the Marsh 0 stage, as demonstrated in our findings (Supplementary Figure S2). Our study suggests that we need to revisit what can only be described as minimal or subtle alterations, previously defined as “microscopic enteropathies” [4,32,33,34,35]. These alterations must not be dismissed as “non-specific”, as often stated, since these changes nevertheless appear to be a clear response to the stimulating antigen. Instead, they should, perhaps, be regarded as the more subtle changes in the rather wider-scale spectrum of “gluten sensitive enteropathy”, wisely defined by Marsh three decades ago. Nevertheless, despite the progress in our understanding of morphological changes in the small intestinal mucosa, we emphasize that a diagnosis of NCGS based solely on histology would have a poor accuracy or performance, and that there is a need for more specific and sensitive diagnostic markers. Spanish studies report that the intraepithelial lymphocyte (IEL) subpopulations evaluated by flow cytometry are useful to identify a profile named ‘coeliac lymphogram’, consisting of an increase in CD3+ T-cell receptor gamma-delta+ IEL plus a concomitant decrease in CD3- IEL, which is considered highly accurate for CeD irrespective of the degrees of enteropathies [36].
A number of studies recently appeared in the literature concerning lamina propria eosinophils, not only in eosinophilic gastroenteritis [24] but also as a diagnostic aid in NCGS. In one study [20] counts of eosinophils >5 per high-power field (viewed through ×40 objective) in 18 NCGS patients were reported to be a marker of NCGS. In the second study, [22] an increase in eosinophils was also reported, including in rectal mucosa. Therefore, we specifically decided to focus on eosinophils, but as our large number of comparative counts shows, we were unable to verify these findings and the alleged diagnostic utility of this finding reported in these other studies. Indeed, our counts of eosinophils were lower in NCGS patients compared to both the CeD group and the normal controls. Considering the conflicting data from the literature, in addition to the environmental and immunologic factors that can influence the eosinophils in the intestinal mucosa, future studies would be needed to explore the role and cell functional aspects in gluten-induced mucosal inflammation.
In summary, based on the validated quantitative histologic analysis [37] and our interobserver agreement (IOA), we identified mucosal alterations associated with NCGS and provided evidence that architectural distortion starts at the Marsh 0 stage. This suggests that the spectrum of what is considered to be normal mucosa needs to be refined further so it can be reliably distinguished from the minimal and subtle alterations of NCGS and CeD. We endorse the IEL cut-off value of 25/100EC in CeD with microenteropathy in addition to demonstrating the existence of mucosal changes that coexist between 5–10 IEL/100EC normal and the pathological threshold of 25 IEL/100EC in NCGS. Lamina propria eosinophils do not appear to be useful for a diagnosis of NCGS.
Finally, our analysis showed no significant histological differences between those patients subjected to a double-blind placebo-controlled diagnostic strategy compared to patients who were included following a simple open challenge approach.
Acknowledgments
We are grateful for the technical assistance of Clare Orange, Jennifer Hay, and Hannah Morgan who tirelessly contributed to Derakhshan’s team in the Digital Histology Section at Queen Elizabeth University Hospital, Glasgow, UK. Many thanks to David Rowlands, histopathologist at the Royal Wolverhampton NHS Trust, New Cross Hospital for his dedication in guiding Laura Lu in morphometric assessment. We also would like to thank Jafar Ali, gastroenterologist at Milton Keynes University Hospital, UK for his contribution to data collection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14122487/s1, Figure S1: This analysis shows not only the diversity of villus height in different countries but also demonstrate that intestinal villi’s are significantly shorter in coeliac disease Marsh I-II compared to controls despite their normal looking appearance; Figure S2: Differential performance of villus IEL density to separate NCGS from CeD and controls groups; Panel A: NCGS versus controls, all are Marsh 0; Panel B: NCGS versus CeD, all are Marsh I or II; Figure S3: Intraepithelial lymphocytes (IEL) of crypts in NCGS compared with CeD and controls, entire cohort; Figure S4: Histomicrograph: This figure summarizes the histopathologic evaluation Table S1: Contributing centres and countries by number of subjects in each study group; Table S2: Predominant symptoms in three main groups; Table S3: Main histology and serology parameters in NCGS compared to celiac and controls; Table S4: Results of inter-observer agreement (IOA) study.
Author Contributions
Conceptualization and Methodology: K.R., A.E., M.N.M., A.S. (Amitabh Srivastava), V.V., A.C., M.D. (Mohammad Derakhshan); Data analysis: M.D. (Mohammad Derakhshan), K.R., D.T.S.H., H.M.-S.; software, formal analysis and validation: M.D. (Mohammad Derakhshan), D.T.S.H., A.E., V.V., A.S. (Amitabh Srivastava), G.B. (Gabriel Becheanu), P.D., K.R. Original drafting, review: K.R., A.S. (Amitabh Srivastava), M.N.M., A.E., M.W., V.V., M.D. (Mohammad Derakhshan), A.S. (Anna Sapone), A.C., D.S.S. Visualisation and supervision: C.C. (Carlo Catassi), A.E., C.C. (Carolina Ciacci), A.C., M.W., A.F., D.S.S., S.G., A.S. (Amitabh Srivastava) V.V. Investigation, data curation: A.E., A.S. (Anna Sapone), V.V., A.C., H.A.A., A.M.B., J.C.B., G.B. (Gabrio Bassotti), C.C. (Carlo Catassi), C.C. (Carolina Ciacci), G.C., S.S.C., M.D. (Mihai Danciu), M.D. (Michael Drage), P.D., R.D.S., L.E., A.F., A.M.F., N.F., J.J.G., S.G., C.E.H., L.L., M.C., D.T.S.H., C.D.B., S.I., H.J., M.J. (Melanie Johncilla), M.J. (Matt Johnson), K.K., A.L., S.L., G.K.M., S.M., G.M., R.M., P.B., K.L.W.M., H.M.-S., A.M., C.J.J.M., R.R., C.R., A.M.B., M.R.-N., D.G.A.C., A.S. (Amitabh Srivastava), D.S.S., U.V., J.T., M.W., M.D. (Mohammad Derakhshan), K.R. Manuscript review and editing and final approval: All authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study involved the rescoring of archived histology slides and the removal of all identifiable medical information, and analyses were performed using anonymized data. The data collection was in line with clinical best practice policies with approval by research and development departments of countries involved under the Reg.No 565 at Milton Keynes University Hospital, Milton Keynes, UK. The study was also approved by the ethical committee of the Research Institute for Gastroenterology and Liver Disease, Shahid Beheshti University of Medical Science, Tehran under the Ref.No: IR.SBMU.RETECH.REC.1399.1003.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Dedication
It is with genuine gratitude and honour that we dedicate this manuscript in memory of our distinguished colleague, Professor Michael N. Marsh, who passed away while we finalized this manuscript. Professor Marsh believed that the complex three-dimensional intestinal mucosa is still poorly understood because it cannot be easily assessed given the limited three-dimensional perspective imposed by conventional histology under a light microscope. His ground-breaking work in recent years, using scanning electron microscopy to highlight large surface “wells” over an apparently flat mucosal surface, introduced a new dimension to small intestinal immunopathology. Dr. Marsh inspired many to follow his footsteps during his lifetime (including many authors in this manuscript) and his insightful comments always showed a path forward to those of us who were fortunate enough to work with him until the very end.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sapone A., Bai J.C., Ciacci C., Dolinsek J., Green P.H.R., Hadjivassiliou M., Kaukinen K., Rostami K., Sanders D.S., Schumann M., et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012;10:13. doi: 10.1186/1741-7015-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villanacci V., Lanzini A., Lanzarotto F., Ricci C. Observations on the paper of Carroccio et al. “Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: Exploring a new clinical entity”. Am. J. Gastroenterol. 2013;108:619–620. doi: 10.1038/ajg.2013.22. [DOI] [PubMed] [Google Scholar]
- 3.Carroccio A., Mansueto P., Iacono G., Soresi M., D’Alcamo A., Cavataio F. Non Celiac Wheat Sensitivity diagnosed by double-blind placebo controlled challenge exploring a new entity. Am. J. Gastroenterol. 2012;107:1898–1906. doi: 10.1038/ajg.2012.236. [DOI] [PubMed] [Google Scholar]
- 4.Rostami K., Aldulaimi D., Holmes G., Johnson M.W., Robert M., Srivastava A., Fléjou J.-F., Sanders D.S., Volta U., Derakhshan M.H., et al. Microscopic enteritis: Bucharest consensus. World J. Gastroenterol. 2015;21:2593–2604. doi: 10.3748/wjg.v21.i9.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uhde M., Ajamian M., Caio G., De Giorgio R., Indart A., Green P.H., Verna E.C., Volta U., Alaedini A. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut. 2016;65:1930–1937. doi: 10.1136/gutjnl-2016-311964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rotondi Aufiero V., Fasano A., Mazzarella G. Non-Celiac Gluten Sensitivity: How Its Gut Immune Activation and Potential Dietary Management Differ from Celiac Disease. Mol. Nutr. Food. Res. 2018;62:e1700854. doi: 10.1002/mnfr.201700854. [DOI] [PubMed] [Google Scholar]
- 7.Brottveit M., Beitnes A.C., Tollefsen S., Bratlie J.E., Jahnsen F.L., Johansen F.E., Sollid L.M., Lundin K.E. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am. J. Gastroenterol. 2013;108:842–850. doi: 10.1038/ajg.2013.91. [DOI] [PubMed] [Google Scholar]
- 8.Asri N., Rostami-Nejad M., Anderson R.P., Rostami K. The Gluten Gene: Unlocking the Understanding of Gluten Sensitivity and Intolerance. Appl. Clin. Genet. 2021;14:37–50. doi: 10.2147/TACG.S276596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rostami K., Marsh M.N., Johnson M.W., Mohaghegh H., Heal C., Holmes G., Ensari A., Aldulaimi D., Bancel B., Bassotti G., et al. ROC-king onwards: Intraepithelial lymphocyte counts, distribution & role in coeliac disease mucosal interpretation. Gut. 2017;66:2080–2086. doi: 10.1136/gutjnl-2017-314297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludvigsson J.F., Bai J.C., Biagi F., Card T.R., Ciacci C., Ciclitira P.J., Green P.H.R., Hadjivassiliou M., Holdoway A., van Heel D.A., et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210–1228. doi: 10.1136/gutjnl-2013-306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsh M.N., Rostami K. What Is a Normal Intestinal Mucosa? Gastroenterology. 2016;151:784–788. doi: 10.1053/j.gastro.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Adelman D.C., Murray J., Wu T.T., Maki M., Green P.H., Kelly C.P. Measuring Change in Small Intestinal Histology in Patients with Celiac Disease. Am. J. Gastroenterol. 2018;113:339–347. doi: 10.1038/ajg.2017.480. [DOI] [PubMed] [Google Scholar]
- 13.Gibiino G., Lopetuso L., Ricci R., Gasbarrini A., Cammarota G. Coeliac disease under a microscope: Histological diagnostic features and confounding factors. Comput. Biol. Med. 2019;104:335–338. doi: 10.1016/j.compbiomed.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Volta U., Caio G., Boschetti E., Giancola F., Rhoden K.J., Ruggeri E., Paterini P., De Giorgio R. Seronegative celiac disease: Shedding light on an obscure clinical entity. Dig. Liver Dis. 2016;48:1018–1022. doi: 10.1016/j.dld.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Aziz I., Peerally M.F., Barnes J.H., Kandasamy V., Whiteley J.C., Partridge D., Vergani P., Cross S.S., Green P.H., Sanders D.S. The clinical and phenotypical assessment of seronegative villous atrophy; a prospective UK centre experience evaluating 200 adult cases over a 15-year period (2000–2015) Gut. 2017;66:1563–1572. doi: 10.1136/gutjnl-2016-312271. [DOI] [PubMed] [Google Scholar]
- 16.Dore M.P., Pes G.M., Dettori I., Villanacci V., Manca A., Realdi G. Clinical and genetic profile of patients with seronegative coeliac disease: The natural history and response to gluten-free diet. BMJ Open Gastroenterol. 2017;4:e000159. doi: 10.1136/bmjgast-2017-000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rostami K., Kerckhaert J., von Blomberg B.M., Meijer J.W., Wahab P., Mulder C.J. SAT and serology in adult coeliacs, seronegative coeliac disease seems a reality. Neth. J. Med. 1998;53:15–19. doi: 10.1016/S0300-2977(98)00050-3. [DOI] [PubMed] [Google Scholar]
- 18.Catassi C., Elli L., Bonaz B., Bouma G., Carroccio A., Castillejo G., Cellier C., Cristofori F., De Magistris L., Dolinsek J., et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients. 2015;7:4966–4977. doi: 10.3390/nu7064966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ensari A., Marsh M.N. Exploring the villus. Gastroenterol. Hepatol. Bed Bench. 2018;11:181–190. [PMC free article] [PubMed] [Google Scholar]
- 20.Zanini B., Villanacci V., Marullo M., Cadei M., Lanzarotto F., Bozzola A., Ricci C. Duodenal histological features in suspected non-celiac gluten sensitivity: New insights into a still undefined condition. Virchows Arch. 2018;473:229–234. doi: 10.1007/s00428-018-2346-9. [DOI] [PubMed] [Google Scholar]
- 21.Kirmizi A., Kalkan C., Yuksel S., Gencturk Z., Savas B., Soykan I., Cetinkaya H., Ensari A. Discriminant value of IEL counts and distribution pattern through the spectrum of gluten sensitivity: A simple diagnostic approach. Virchows Arch. 2018;473:551–558. doi: 10.1007/s00428-018-2430-1. [DOI] [PubMed] [Google Scholar]
- 22.Carroccio A., Giannone G., Mansueto P., Soresi M., La Blasca F., Fayer F., Iacobucci R., Porcasi R., Catalano T., Geraci G., et al. Duodenal and Rectal Mucosa Inflammation in patients with Non-celiac Wheat Sensitivity. Clin. Gastroenterol. Hepatol. 2019;17:682–690.e3. doi: 10.1016/j.cgh.2018.08.043. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein N.S., Underhill J. Morphologic features suggestive of gluten sensitivity in architecturally normal duodenal biopsy specimens. Am. J. Clin. Pathol. 2001;116:63–71. doi: 10.1309/5PRJ-CM0U-6KLD-6KCM. [DOI] [PubMed] [Google Scholar]
- 24.Jarbrink-Sehgal M.E., Talley N.J. Duodenal and Rectal Eosinophilia Are New Biomarkers of Nonceliac Gluten Sensitivity. Clin. Gastroenterol. Hepatol. 2019;17:613–615. doi: 10.1016/j.cgh.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 25.Marsh M.N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–354. doi: 10.1016/0016-5085(92)91819-P. [DOI] [PubMed] [Google Scholar]
- 26.Uhde M., Caio G., De Giorgio R., Green P.H., Volta U., Alaedini A. Subclass Profile of IgG Antibody Response to Gluten Differentiates Non-Celiac Gluten Sensitivity from Celiac Disease. Gastroenterology. 2020;159:1965–1967.e2. doi: 10.1053/j.gastro.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen L.B.S., Roager H.M., Sondertoft N.B., Gøbel R.J., Kristensen M., Vallès-Colomer M., Vieira-Silva S., Ibrügger S., Lind M.V., Mærkedahl R.B., et al. A low-gluten diet induces changes in the intestinal microbiome of healthy Danish adults. Nat. Commun. 2018;9:4630. doi: 10.1038/s41467-018-07019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Losurdo G., Piscitelli D., Pezzuto F., Fortarezza F., Covelli C., Marra A., Iannone A., Amoruso A., Principi M., Ierardi E., et al. T Helper Lymphocyte and Mast Cell Immunohistochemical Pattern in Nonceliac Gluten Sensitivity. Gastroenterol. Res. Pract. 2017;2017:5023680. doi: 10.1155/2017/5023680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh M.N., Johnson M.W., Rostami K. Mucosal histopathology in celiac disease: A rebuttal of Oberhuber’s sub-division of Marsh III. Gastroenterol. Hepatol. Bed Bench. 2015;8:99–109. [PMC free article] [PubMed] [Google Scholar]
- 30.Das P., Gahlot G.P., Singh A., Baloda V., Rawat R., Verma A.K., Khanna G., Roy M., George A., Singh A., et al. Quantitative histology-based classification system for assessment of the intestinal mucosal histological changes in patients with celiac disease. Intest. Res. 2019;17:387–397. doi: 10.5217/ir.2018.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taavela J., Koskinen O., Huhtala H., Lähdeaho M.-L., Popp A., Laurila K., Collin P., Kaukinen K., Kurppa K., Mäki M. Validation of morphometric analyses of small-intestinal biopsy readouts in celiac disease. PLoS ONE. 2013;8:e76163. doi: 10.1371/journal.pone.0076163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludvigsson J.F., Leffler D.A., Bai J.C., Biagi F., Fasano A., Green P.H.R., Hadjivassiliou M., Kaukinen K., Kelly C.P., Leonard J.N., et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sbarbati A., Valletta E., Bertini M., Cipolli M., Morroni M., Pinelli L., Tatò L. Gluten sensitivity and ‘normal’ histology: Is the intestinal mucosa really normal? Dig. Liver Dis. 2003;35:768–773. doi: 10.1016/S1590-8658(03)00457-2. [DOI] [PubMed] [Google Scholar]
- 34.Pellegrino S., Villanacci V., Sansotta N., Scarfì R., Bassotti G., Vieni G., Princiotta A., Sferlazzas C., Magazzù G., Tuccari G. Redefining the intraepithelial lymphocytes threshold to diagnose gluten sensitivity in patients with architecturally normal duodenal histology. Aliment. Pharmacol. Ther. 2011;33:697–706. doi: 10.1111/j.1365-2036.2011.04578.x. [DOI] [PubMed] [Google Scholar]
- 35.Al-Toma A., Volta U., Auricchio R., Castillejo G., Sanders D.S., Cellier C., Mulder C.J., Lundin K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019;7:583–613. doi: 10.1177/2050640619844125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz-Ramírez P., Carreras G., Fajardo I., Tristán E., Carrasco A., Salvador I., Zabana Y., Andújar X., Ferrer C., Horta D., et al. Intraepithelial Lymphocyte Cytometric Pattern Is a Useful Diagnostic Tool for Coeliac Disease Diagnosis Irrespective of Degree of Mucosal Damage and Age-A Validation Cohort. Nutrients. 2021;13:1684. doi: 10.3390/nu13051684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bujang M.A. A simplified guide to determination of sample size requirements for estimating the value of intraclass correlation coefficient: A review. Arch. Orofac. Sci. 2017;12:1–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.