Abstract
Carpesium abrotanoides L. (Asteraceae) is a medicinal plant with immense therapeutic importance and bioactivities. It is commonly encountered in various Asian regions. It has numerous ethnomedicinal uses for curing diverse ailments such as toothache, stomach ulcer, boils, tonsillitis, bronchitis, bacterial infection, bruises, swelling, virus infection, fever, and amygdalitis, as well as an anthelmintic versus round-, tape-, hook-, and pinworms. Different classes of phytoconstituents such as sesquiterpenes, sesquiterpene dimers, monoterpenes, and nitrogenous compounds have been reported from this plant. These phytoconstituents have proved to possess anti-inflammatory, cytotoxic, antimicrobial, and insecticidal capacities. The present review aims to summarize all published data on C. abrotanoides including traditional uses, phytoconstituents, bioactivities, and toxicological aspects, as well as the synthesis and biosynthesis of its metabolites through an extensive survey on various databases and various publishers. These reported data could draw the attention of various natural-metabolite-interested researchers and medicinal chemists towards the development of this plant and/or its metabolites into medicine for the prevention and treatment of certain illnesses. Despite the diverse traditional uses of C. abrotanoides, there is a need for scientific evidence to support these claims. Clinical trials are also required to further assure these data and validate this plant utilization in treating several diseases.
Keywords: Carpesium abrotanoides, Asteraceae, sesquiterpenes, sesquiterpene dimers, bioactivities, biosynthesis
1. Introduction
Medicinal plants have remarkable participation in the separation and discovery of new drugs. Obviously, there is increasing interest in using medicinal plants as part of various traditional medicines for treating various ailments [1,2,3]. The side effects, high cost, and therapeutic limitations of available medications are principal factors that encourage the revivification of herbal treatment [4]. It was also reported that more than 80% of people worldwide rely on traditional medicines as a primary source of healthcare, involving the utilization of plant extracts or their phytoconstituents [4]. Recently, plant-derived metabolites have become greatly important due to their structural diversity and promising bioactivities [5,6,7].
Carpesium genus (Asteraceae) comprises about 21 species worldwide [8]. Its species are found in Europe and Asia, particularly in Southwest China mountainous [9]. Its plants are utilized as a traditional remedy in some Asian regions for various complaints such as colds, fevers, insects, bruises, inflammation, and snake bites [9]. Its frequent phytochemically investigated species are C. cernuum L., C. abrotanoides L., C. rosulatum Miq., C. lipskyi C.Winkl., C. triste Maxim, C. macrocephalum Franch. & Sav., C. rosulatum Miq., and C. divaricatum Sieb. & Zucc.. They have been stated to biosynthesize structurally varied metabolites, including mono-, sesqui-, and di-terpenoids, flavonoids, and phenolics [9]. These metabolites displayed cytotoxicity, anti-parasitic, insecticidal, anti-tumor, anti-inflammatory, antimicrobial, and antioxidant properties [9]. C. abrotanoides L. is widely encountered in eastern Asia, Korea, China, Vietnam, Japan, India, Iran, Myanmar, and Russia [10,11]. C. abrotanoides has different synonyms; Amphirhapis wightiana Hook.f., C. abrotanoides var. thunbergianum (Siebold & Zucc.) Makino, C. racemosum Wall., C. wulfenianum Bertol, C. thunbergianum Siebold & Zucc., and C. wulfenianum Schreb. ex DC. [12]. The plant is a perennial highly branched herb having simple, elliptic, and alternate leaves. It possesses small branched clusters of flower-heads (capitula) that arise from the leaf axils [10]. Each capitulum has yellow 130–300 tiny, densely packed florets that develop into longitudinal and cylindrical ribbed achenes (fruit) (≈1 mm diam. × 3.5 mm long) [10]. C. abrotanoides has been used in various traditional medicines for treating various health concerns and had an array of bioactivities including cytotoxic, antiparasitic, insecticidal, antidiabetic, antioxidant, and anti-inflammatory. Furthermore, it was found to yield diverse metabolites such as sesquiterpenes, dimeric sesquiterpenes, monoterpenes, sterols, and aliphatic and nitrogenous compounds with promising bioactivities. Although, the substantial role that C. abrotanoides plays in Asian countries’ traditional medicine practice, there is no current review article that deals specifically with this plant. As a result, the present review summarized all published data regarding its phytoconstituents, traditional uses, bioactivities, and toxicity, as well as the reported biosynthesis and synthesis of its metabolites. This review can supply the researchers with knowledge about the recent research developments on C. abrotanoides that can help them for quick identification of secondary metabolites and their possible biological activity evaluation. Additionally, we intend to shed light on one of the important medicinal plants and to attract the interest of natural products researchers for more development and utilization of the reported metabolites from it as potential drug leads. Additionally, this work may reflect the existing gaps and highlight the need for C. abrotanoides’ traditional uses in scientific validation. Moreover, summarizing the reported bioactivities may be significant in identifying the potential therapeutic applications that have not yet been explored for C. abrotanoides or its constituents.
2. Methodology
To carry out an extensive literature review on C. abrotanoides, the data were collected from various scientific databases (e.g., Scopus, Science-Direct, Pub-Med), Publishers (Springer, Taylor & Francis, MDPI, Bentham, Wiley Online Library, and Thieme), and Google Scholar, as well as books and abstracts of articles published in non-English languages. A total of 84 articles were listed in this work.
3. Traditional Uses and Clinical Trials
This plant has been utilized in various Asian regions for treating diverse complaints. In Chinese traditional medicine, its fruit (Carpesii Fructus, He Shi, Heshi, Nan-He-Shi, and Bei-He-Shi) have been utilized for treating enterobiasis, ancylastomiasis, cestodiasis, and ascariasis particularly in children, in addition to parasitic infection-associated abdominal pain [13,14]. Additionally, it is very efficient in killing tapeworms and roundworms. In Korea, Japan, and China, its aerial parts were employed orally for various ailments such as toothache, stomach ulcer, boils, tonsillitis, bronchitis, bacterial infection, bruises, swelling, virus infection, fever, and amygdalitis, as well as against snake bites and insects [9,15]. In Southwest China, especially in Miao and Tujia minorities, it is called Tianmingjing and was utilized for bruises, bronchitis, and hepatitis [16]. The herb’s oral decoction is utilized for treating chronic inflammatory disorders [17]. It is called Tenmyoseishi in Japan. In India, it is called “Ban sario” and its root, leaf, and seed infusions are utilized for intestinal worm killing by locals of the Kishtwar plateau [18]. The whole plant was traditionally utilized as an insecticide to control the vegetable insect pests such as Euxoa segetum and Pieris rapae. Moreover, the whole plants for controlling insect pests in the stored product were placed into the grain depot such as Tribolium castaneum and Sitophilus zeamais in China [10]. Furthermore, in Korea, C. abrotanoides was traditionally consumed as a wild vegetable [19] and the shoots are employed for treating fever and inflammation [20]. A water decoction prepared using C. abrotanoides, Quispualis indica, and Areca catechu (9 g, each) is utilized orally for hook-, round-, pin-, and tapeworm. A handwash (1.5 kg C. abrotanoides + 4 kg H2O, decoction for 2 h until a volume of 1500 mL, then filtered) was utilized as a skin disinfectant and for handwashing before operation [11].
It was reported that the orally administrated Chinese medicine contains Fructus Carpesii, in addition to Semen Arecae, Fructus Ulmus preparatium, Fructus Quisqualis meat, Radix Glycyrrhizae, and Fructus Toosendan in the form of decoction for two days in the empty stomach caused 89% cure rate in 18 belly worm infected patients [21]. The plant root has been utilized for treating trigeminal neuralgia, oral cavity erosion, folliculitis, herpes zoster, and flat wart in the clinic [9].
4. Pharmacological Activities of Crude Extracts
4.1. Cytotoxic Activity
Shen et al. stated that the total sesquiterpene lactone and ethanol extracts of the entire plant displayed cytotoxicity capacities versus HepG-2 cells (IC50 4.2 and 28.3 µg/mL, respectively) [16]. Additionally, the aerial part benzene extract was reported to exhibit cytotoxic effectiveness versus HL60 and L1210 (ED50 < 5 µg/mL) [22]. The plant EtOH extract was found to induce NQO1(NAD(P)H:quinone oxidoreductase) activity via Nrf2 (NF-E2-related factor 2) nuclear accumulation in HCT116. Interestingly, It had antiproliferative and apoptotic potential as observed by G2/M cell cycle arrest, phosphatidylserine externalization, chromatin condensation, elevated sub-G0/G1 content, PARP (poly(ADPribose) polymerase) cleavage, and p53 upregulation. Therefore, the plant produced chemo-preventive potential via inducing apoptosis and phase II detoxification enzymes [19]. The fruits n-BuOH, EtOAc, and aqueous fractions had no influence on the RAW 264.7 murine macrophage cell viability (IC50 > 100 µg/mL), whereas the CHCl3 (conc. 50 µg/mL, IC50 83.65 µg/mL) and hexane-fraction (Conc. > 6.25 µg/mL, IC50 16.14 µg/mL) displayed cytotoxic potential on these cells [20].
Breast cancer represents one of the most prevalent malignant tumors in women with low survival rates. Chai et al. evaluated the anticancer capacity of root pet. ether fraction (Conc. 50 and 100 µg/mL) versus MDA-MB-231 and MCF-7 using MTT assay. The fraction was found to noticeably suppress proliferation and migration and cause apoptosis in both cells [17]. C. abrotanoides exerted its potential via prohibiting glycolysis-linked genes, GLUT1 (glucose transporter-1), LDHA (lactate dehydrogenase A), and HK2 (hexokinase-2) and down-regulating PKM2 (pyruvate kinase M2). PKM2 is closely linked to the angiogenesis, migration, and proliferation of cancer cells, as well as autophagy inhibition [23]. Therefore, the plant was established as PKM2/HIF-1α (hypoxia-inducible factor-1α) axis inhibitor, revealing its potential versus breast cancer [17]. Additionally, the cytotoxic capacity of the essential oil on various hepatic cancer cells (Hep3B, HepG2, Huh7, and SMMC-7721) and LO-2 normal liver cell line was investigated by Wang et al. in the MTT assay. The oil prohibited the proliferation of all cancer cells (IC50s from 41.28 to 130.36 μg/mL), with little cytotoxicity of L-O2 cells (IC50 > 300 µg/mL). Furthermore, it caused apoptotic morphological changes and G2/M and S phases cell cycle arrest in HepG2 cells. Additionally, it reduced Bcl-2/Bax ratio and raised caspase-3 and -9 activation, suggesting the involvement of mitochondria-induced apoptosis in this effect [15].
4.2. Antiparasitic Activity
Paralichthys olivaceus (Olive flounder) is an important South Korean fish species; however, there are serious industrial losses owing to diseases influencing this fish [24]. Scuticociliatosis is one of the prevalent parasitic diseases caused by Miamiensis avidus, resulting in high fish mortality [25]. Additionally, this parasite infects various fish species such as turbot, sharks, adult kingfish, and black rockfish [24]. Hydrogen peroxide and formalin are the approved antiparasitic agents for scuticociliatosis in P. olivaceus [26]. However, this treatment is non-effective as they cannot enter the internal organs and formalin over-use is carcinogenic. Therefore, effective and safe natural antiparasitic agents versus M. avidus are needed.
The fruit EtOH extract (conc. 50, 70, and 100% v/v) demonstrated antiparasitic potential versus M. avidus as evident by the induced morphological alterations, including cell shrinkage, reduced motility, and lysis, whereas 100% cell lysis was noticed after 2 h treating with 100% extract [24]. Additionally, the 70% and 100% extracts following concanavalin A pretreatment increased CD8-α, IL-8, IL-1β, TNF-α, UGT2B19, CYP-1A, CYP-1B, and CYP-3A4 expression; however, their expression reduced with 50% extract. Furthermore, they reduced HINAE (hirame natural embryo) cell viability with % between 67 and 80% and IC50s from 38.39 to 102.3 mg/L, revealing the less cytotoxic potential of 50% EtOH extract than 70% or 100% extract, while still exhibiting antiparasitic activity against M. avidus [24]. Therefore, C. abrotanoides had potential as an antiparasitic agent versus M. avidu,s supporting its utilization as an anti-parasite in the aquaculture industry [24]. However, the main components accountable for this influence need to be specified and in vivo investigations should be carried out to assess its usage in aquaculture.
Taenia asiatica cysticerci was in vitro cultured with C. abrotanoides H2O decoction (Conc. 20, 40, and 60 mg/mL). It was found that the decoction had a prominent killing capacity on the cysticerci with the highest %mortality (95.0%) at conc. 60 mg/mL. The dead cysticercus had shrunk with degenerated sucker tissue and enlarged scolex [27].
4.3. Insecticidal Activity
Plants are a substantial pool for discovering novel and safe insect repellents, especially to protect from blood-sucking insect pests such as bed bugs and mosquitoes. Spodoptera exigua Hübner is one of the major pests that occurs in fields, vegetables, and flower crops [28]. The repeated use of chemical pesticides resulted in the development of S. exigua resistance to different insecticides, leading to difficulty in controlling this pest [29]. A growing interest has been focused on the discovery of phytochemicals with insecticidal potential [30]. Since C. abrotanoides has been traditionally utilized as an insecticide in Chinese and Korean medicines. Feng et al. assessed the insecticidal potential of the fruit EtOH extract versus S. exigua. The results revealed that the fruit extract displayed a promising antifeedant potential versus the S. exigua larvae with FDI (Feeding-deterrence index) of 48.32% when the larvae fed on treated leaves with 10 mg/mL extract [30].
Aedes aegypti (L.) is one of the most threatening pests for humans globally [31]. Ae. aegypti was reported to have a potential in the transmission of dengue and zika viruses in humans [32]. Various insecticides such as organophosphate (pirimiphos-methyl and malathion), organochlorines (DDT), carbamate (bendicarb and propoxur), and pyrethroids (lambda cyhalothrin, permethrin, and deltamethrin) were used for controlling Ae. Aegypti. [33]. However, their repeated use results in increasing resistance to Ae. aegypti versus these insecticides in addition to their hazardous effect on humans that necessitates the discovery of natural alternative agents [34].
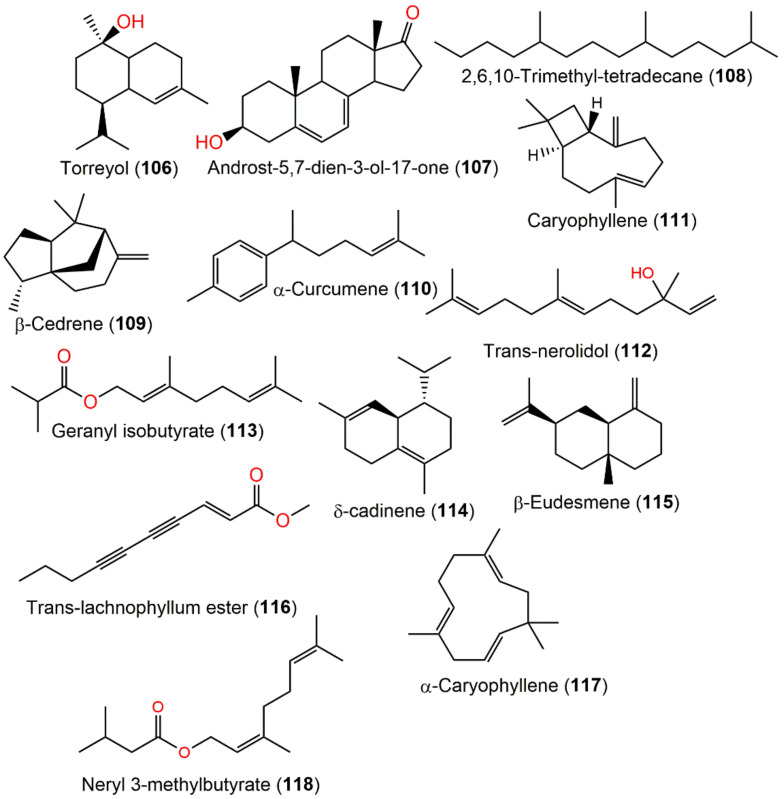
In 2022, Haris et al. evaluated the herbs’ essential oil repellent potential versus Ae. aegypti adult females utilizing the human bait technique before the scotophase period. C. abrotanoides oil (doses 330, 165, and 33 μg/cm2) possessed immediate and significant repellency as DEET (N,N-diethyl-meta-toluamide) with maximum repellency periods of 315 and 720 min, respectively, at a dose of 330 μg/cm2. Furthermore, the repellent potential of caryophyllene (111) and trans-nerolidol (112) (doses 33, 165, and 330 μg/cm2), the major constituents of essential oil, was assessed versus Ae. aegypti. Interestingly, Ae. aegypti landing was completely prohibited for 45 min by 112 when tested at 330 μg/cm2, while 111 did not completely prohibit Ae. aegypti landing. On the other side, 111/112 mixture (dose of 330 μg/cm2) completely prohibited Ae. egypti landing for 60 min. Therefore, C. abrotanoides or trans-nerolidol could be formulated as safer, commercial mosquito repellent products [32].
4.4. Antidiabetic Activity
Diabetes is a chronic worldwide disease that influences millions of people [35]. It was estimated to be the ninth reason for death with 1.5 million deaths in 2019 [35]. One of its therapeutic approaches for treatment is the carbohydrate absorption suppression after food intake which is aided by the prohibition of α-glucosidase and α-amylase enzymes [36,37]. The main goal of its treatment is to keep the blood glucose levels near normal in both the postprandial and fasting conditions [38]. The aerial parts’ 80% MeOH extract were found to contain remarkable amounts of total phenolics (88 mg gallic acid/g extract) and flavonoids (2 mg quercetin/g extract). It also revealed non-competitive α-glucosidase inhibitory effectiveness (IC50 44.22 µg/mL), compared to acarbose (IC50 2.5 µg/mL) [39].
4.5. Antioxidant Activity
The aerial parts’ 80% MeOH extract demonstrated antioxidant potential in various assays: ABTS (2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid), DPPH (1,1-diphenyl-2-picrylhydrazyl), NOS (nitric oxide scavenging), and FIC (ferrous ion chelating) (IC50s 15.6, 111.2, 798.5, and 150.2 µg/mL, respectively) compared to ascorbic acid (IC50s 6.99 µg/mL for ABTS, 7.55 µg/mL for DPPH, and 220 µg/mL for NOS) and citric acid (IC50 9764 µg/mL for FIC). It also prohibited lipid peroxidation. This influence was attributed to its high phenolic contents [39]. The fruit hexane-fraction (IC50 12.95 mg/mL) was found to have more powerful antioxidant capacity than n-BuOH and EtOAc-fractions (IC50s 31.17 and 21.43 mg/mL, respectively) in the DPPH method [20]. The 80% EtOH extract of the aerial parts displayed scavenging potential in the DPPH and ABTS assays, as well as reducing power such as butylated hydroxyanisole. It significantly repressed RBC oxidative hemolysis and AAPH (2,2′-azobis (2-methylpropionamidine) dihydrochloride)-induced MDA (malondialdehyde) formation, and GSH depletion in RBCs, indicating its anti-hemolytic potential [40].
4.6. Anti-Inflammatory Activity
Jeong et al. assessed the influence of the aerial parts’ EtOH extract on the COX-2 (cyclooxygenase-2) expression by various TLRs (Toll-like receptors) agonists in murine macrophages. TLRs and their signaling components could be a potential therapeutic target for chronic inflammation disorders. C. abrotanoides repressed COX-2 expression caused by LPS (lipopolysaccharide, TLR4 agonist), macrophage-activating lipopeptide 2-kDa (TLR6 and TLR2 agonist), and polyriboinosinic polyribocytidylic acid (TLR3 agonist). This indicated its capacity to regulate the TLRs TRIF (Toll-interleukin-1 receptor domain-containing adapter inducing interferon-β)- and MyD-88 (myeloid-differential-factor-88)-dependent pathways, resulting in reduced expression of inflammatory genes; therefore, it could be beneficial for treating chronic inflammatory disorders [41]. In another study, Lee et al. also revealed that the 70% EtOH extract of aerial parts suppressed iNOS (inducible nitric oxide synthase) expression boosted by these TLRs agonists in murine macrophages [42]. Additionally, the blooms’ MeOH extract (dose 10 mg/kg BW) exhibited anti-inflammatory capacity by reducing the elevated IL-6 and IFNγ levels induced by LPS in mice. It also attenuated IL-6, IFNγ, and IL-4 levels in a Con-A inflammation model [43].
The anti-inflammation capacity of various fruit fractions was assessed by Lee et al. [20]. The CHCl3 (89.1%, IC50 37.1 µg/mL) and EtOAc (74.4%, IC50 78.35 µg/mL) fractions remarkably prohibited NO production in macrophage cells caused by LPS via NO chemical scavenging and iNOS transcription repression. Moreover, all fractions potently prohibited PGE2 biosynthesis, whereas CHCl3 (Conc. 50 µg/mL, 94.5%) and hexane (conc. 6.25 µg/mL, 89.1%) had more potent effectiveness than celecoxib (Conc. 20 µg/mL, 87.3%). This effect was due to the attenuation of LPS-boosted COX-2 expression. Additionally, the EtOAc, hexane, and aqueous fractions repressed IL-1β levels significantly almost to the basal (IC50s 5.13, 0.26, and 3.53 µg/mL and %inhibition 77.5–92.8%) [20]. Hence, the plant could have a cartilage protective influence and retard the inflammation process in rheumatoid arthritis [20].
5. Phytochemicals and Their Pharmacological Activities
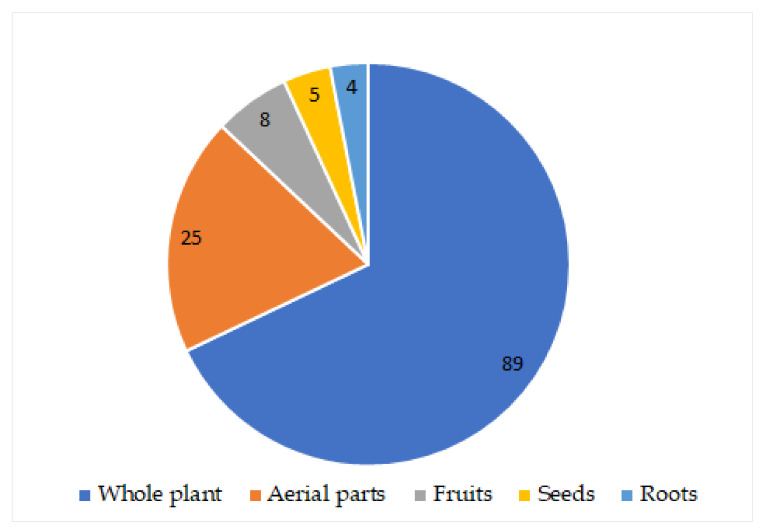
In total, 118 metabolites have been separated from various parts of C. abrotanoides using various chromatographic tools, including various terpenoids, sterols, and aliphatic and nitrogenous compounds (Figure 1).
Figure 1.
Number of metabolites reported from different parts of C. abrotanoides. 89 Compounds from whole plant; 25 compounds from aerial parts; 8 compounds from fruits; 5 compounds from seeds; 4 compounds from roots.
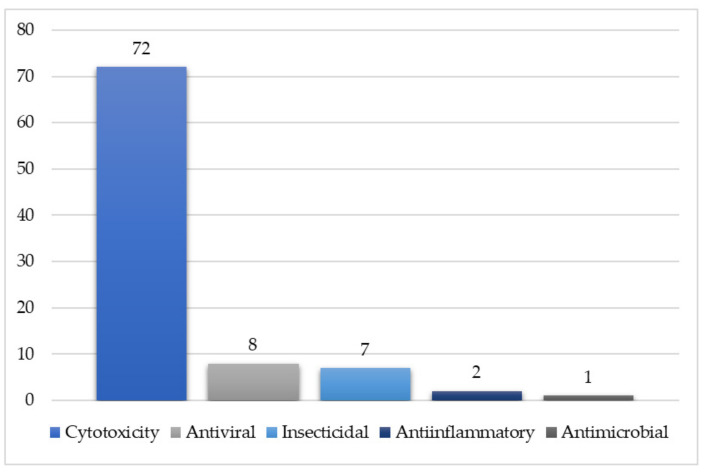
Additionally, some volatile compounds have been characterized from the plant essential oil by GCMS. These metabolites have been assessed for diverse bioactivities (Figure 2). It is obvious that most of the reported metabolites have been evaluated for their cytotoxic potential.
Figure 2.
Tested metabolites for each biological activity.
Furthermore, the reported compounds along with their molecular weights/formulae, part of the plant/fraction from which they were isolated, and the place of location are listed in Table 1.
Table 1.
List of reported metabolites from Carpesium abrotanoides.
| Compound Name | Plant Part | Extract/Fraction | Mol. Wt | Mol. Formula | City, Country | Ref. |
|---|---|---|---|---|---|---|
| Monoterpenes | ||||||
| 2,5-Dihydroxy-p-menthane (1) | Fruits | PE/acetone fraction of MeOH extract | 172 | C10H20O2 | Tianquan, Sichuan, China | [44] |
| 9-Hydroxy-thymol (2) | Whole plant | PE fraction of 95% EtOH extract | 166 | C10H14O2 | China | [45] |
| 8-Hydroxy-9,10-diisobutyryloxy-thymol (3) | Whole plant | PE fraction of 95% EtOH extract | 338 | C18H26O6 | China | [45] |
| 8,10-Dihydroxy-9-isobutyryloxy-thymol (4) | Whole plant | PE fraction of 95% EtOH extract | 268 | C14H20O5 | China | [45] |
| 10-Hydroxy-8,9-dioxy-isopropylidene-thymol (5) | Whole plant | PE fraction of 95% EtOH extract | 238 | C13H18O4 | China | [45] |
| 8-Hydroxy-9,10-dioxy-isopropylidene-thymol (6) | Whole plant | PE fraction of 95% EtOH extract | 238 | C13H18O4 | China | [45] |
| Guaianolide sesquiterpenes | ||||||
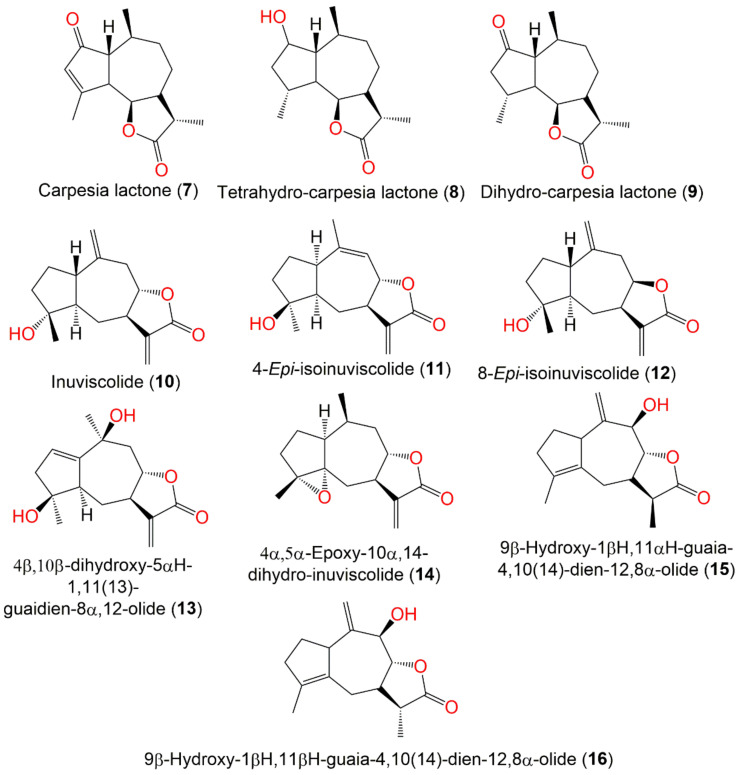
| Carpesia lactone (7) | Ripe seeds | Ether extract | 248 | C15H20O3 | Japan | [46,47,48,49,50] |
| Tetrahydro-carpesia lactone (8) | Ripe seeds | Ether extract | 252 | C15H24O3 | Japan | [46] |
| Dihydro-carpesia lactone (9) | Ripe seeds | Ether extract | 250 | C15H22O3 | Japan | [48] |
| Inuviscolide (10) | Whole plant | EtOAc fraction of 95% EtOH extract | 248 | C15H20O3 | Changyang, Hubei, China | [51] |
| Whole plant | EtOAc fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| 4-Epi-isoinuviscolide (11) | Aerial parts | PE/acetone fraction of 95% EtOH extract | 248 | C15H20O3 | Tiger Leaping Gorge, Yunnan, China | [13] |
| 8-Epi-isoinuviscolide (12) | Whole plant | EtOAc fraction of 95% EtOH extract | 248 | C15H20O3 | Changyang, Hubei, China | [51] |
| 4β,10β-dihydroxy-5αH-1,11(13)-guaidien-8α,12-olide (13) | Whole plant | EtOAc fraction of 95% EtOH extract | 264 | C15H20O4 | Hebei, China | [53] |
| 4α,5α-Epoxy-10α,14-dihydro-inuviscolide (14) | Aerial parts | CHCl3-soluble fraction of MeOH extract | 248 | C15H20O3 | Korea | [51] |
| 9β-Hydroxy-1βH,11αH-guaia-4,10(14)-dien-12,8α-olide (15) | Fruits | PE/acetone fraction of MeOH extract | 248 | C15H20O3 | Tianquan, Sichuan, China | [44] |
| 9β-Hydroxy-1βH,11βH-guaia-4,10(14)-dien-12,8α-olide (16) | Fruits | PE/acetone fraction of MeOH extract | 248 | C15H20O3 | Tianquan, Sichuan, China | [44] |
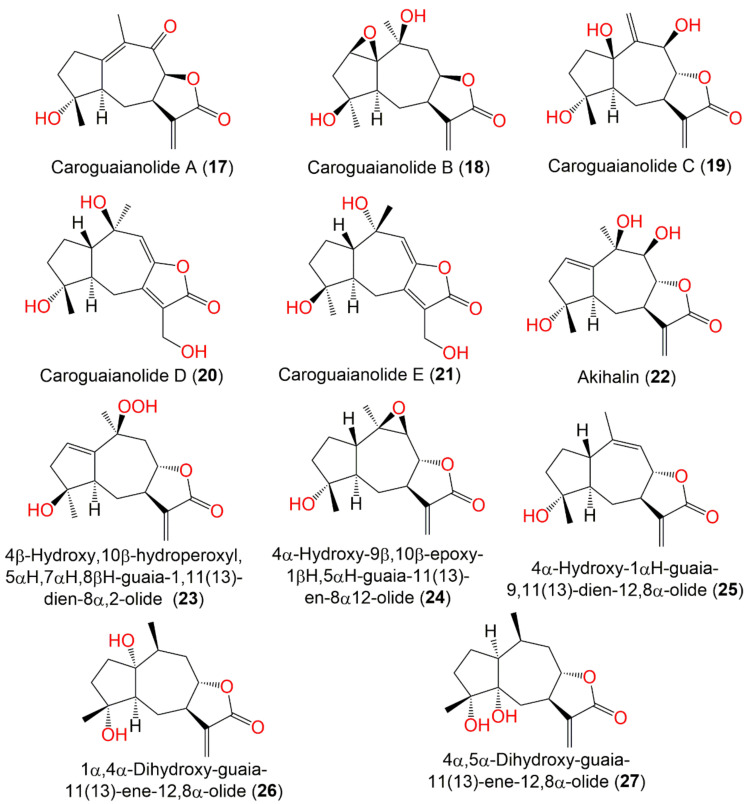
| Caroguaianolide A (17) | Whole plant | EtOAc fraction of 95% EtOH extract | 262 | C15H18O4 | Changyang, Hubei, China | [51] |
| Caroguaianolide B (18) | Whole plant | EtOAc fraction of 95% EtOH extract | 280 | C15H20O5 | Changyang, Hubei, China | [51] |
| Caroguaianolide C (19) | Whole plant | EtOAc fraction of 95% EtOH extract | 280 | C15H20O5 | Changyang, Hubei, China | [51] |
| Caroguaianolide D (20) | Whole plant | EtOAc fraction of 95% EtOH extract | 280 | C15H20O5 | Changyang, Hubei, China | [51] |
| Caroguaianolide E (21) | Whole plant | EtOAc fraction of 95% EtOH extract | 280 | C15H20O5 | Changyang, Hubei, China | [51] |
| Akihalin (22) | Whole plant | EtOAc fraction of 95% EtOH extract | 280 | C15H20O5 | Changyang, Hubei, China | [51] |
| 4β-Hydroxy,10β-hydroperoxyl,5αH,7αH,8βH-guaia-1,11(13)- dien-8α,12-olide (23) | Whole plant | EtOAc fraction of 95% EtOH extract | 280 | C15H20O5 | Changyang, Hubei, China | [51] |
| 4α-Hydroxy-9β,10β-epoxy-1βH,5αH-guaia-11(13)-en-8α,12-olide (24) | Whole plant | EtOAc fraction of 95% EtOH extract | 264 | C15H20O4 | Changyang, Hubei, China | [51] |
| 4α-Hydroxy-1βH-guaia-9,11(13)-dien-12,8α-olide (25) | Whole plant | EtOAc fraction of 95% EtOH extract | 248 | C15H20O3 | Changyang, Hubei, China | [51] |
| 1α,4α-Dihydroxy-guaia-11(13)-ene-12,8α-olide (26) | Whole plant | EtOAc fraction of 70% EtOH extract | 266 | C15H22O4 | Changsha, Hunan, China | [54] |
| 4α,5α-Dihydroxy-guaia-11(13)-en-12,8α-lactone (27) | Whole plant | EtOAc fraction of 70% EtOH extract | 266 | C15H22O4 | Changsha, Hunan, China | [54] |
| Pseudo-guaianolide sesquiterpenes | ||||||
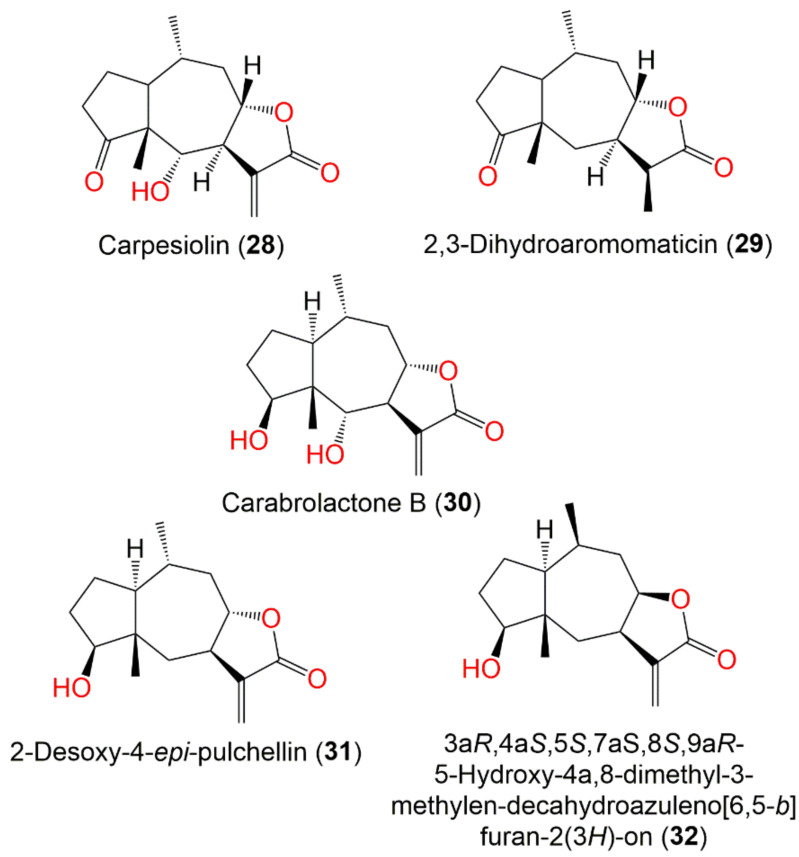
| Carpesiolin (28) | Whole plant | CHCl3-soluble fraction of MeOH extract | 264 | C15H20O4 | Japan | [55] |
| Aerial parts | CHCl3-soluble fraction of MeOH extract | - | - | Korea | [51] | |
| 2,3-Dihydroaromomaticin (29) | Aerial parts | CHCl3-soluble fraction of MeOH extract | 250 | C15H22O3 | Korea | [51] |
| Whole plant | PE fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| Carabrolactone B (30) | Aerial parts | PE/acetone fraction of 95% EtOH extract | 266 | C15H22O4 | Tiger Leaping Gorge, Yunnan, China | [13] |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Changyang, Hubei, China | [51] | |
| Whole plant | EtOAc fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| 2-Desoxy-4-epi-pulchellin (31) | Aerial parts | PE/acetone fraction of 95% EtOH extract | 250 | C15H22O3 | Tiger Leaping Gorge, Yunnan, China | [13] |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Changsha County of Hunan, China | [56] | |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Changyang, Hubei, China | [51] | |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Hebei, China | [53] | |
| Whole plant | EtOAc fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| 3aR,4aS,5S,7aS,8S,9aR)-5-Hydroxy-4a,8-dimethyl-3-methylen-decahydroa-zuleno [6,5-b]furan-2(3H)-on (32) | Whole plant | EtOAc fraction of 95% EtOH extract | 250 | C15H22O3 | Changyang, Hubei, China | [51] |
| Eudesmanolide sesquiterpenes | ||||||
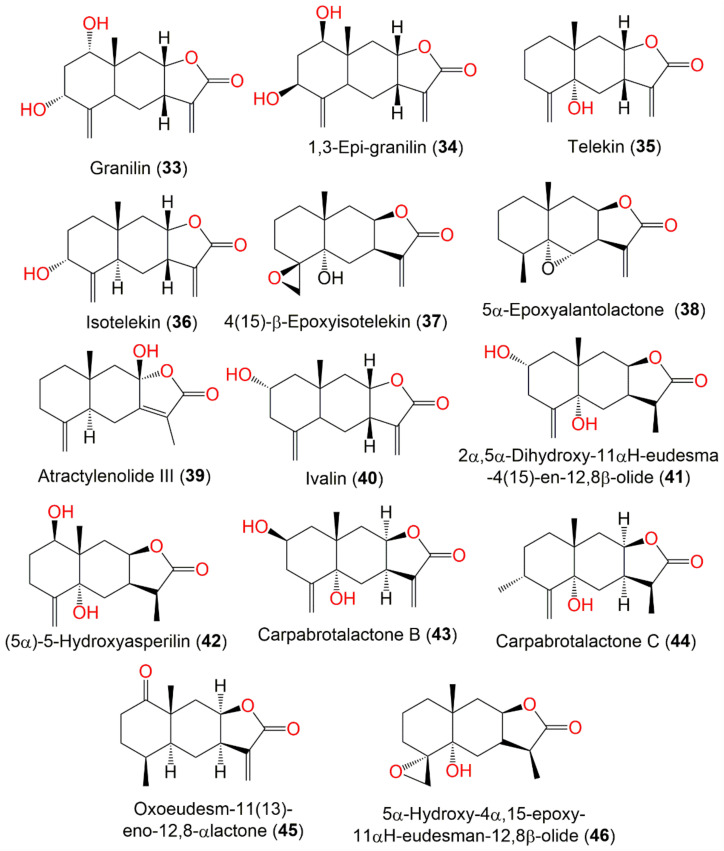
| Granilin (33) | Whole plant | CHCl3-soluble fraction of MeOH extract | 264 | C15H20O4 | Japan | [57] |
| 1.3-Epi-granilin (34) | Whole plant | EtOAc fraction of 95% MeOH extract | 264 | C15H20O4 | Bozhou, Anhui, China | [52] |
| Fruits | PE/acetone fraction of MeOH extract | - | - | Tianquan, Sichuan, China | [44] | |
| Telekin (35) | Aerial parts | CHCl3-soluble fraction of MeOH extract | 248 | C15H20O3 | Korea | [51] |
| Whole plant | EtOAc fraction of 70% EtOH extract | - | - | Dao County, Hunan, China | [16] | |
| Whole plant | PE fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| Isotelekin (36) | Whole plant | EtOAc fraction of 95% MeOH extract | 248 | C15H20O3 | Bozhou, Anhui, China | [52] |
| 4(15)-β-Epoxyisotelekin (37) | Whole plant | EtOAc fraction of 95% EtOH extract | 264 | C15H20O4 | Hebei, China | [53] |
| Whole plant | EtOAc fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| 5α-Epoxyalantolactone (38) | Whole plant | EtOAc fraction of 95% MeOH extract | 248 | C15H20O3 | Bozhou, Anhui, China | [52] |
| Atractylenolide III (39) | Whole plant | EtOAc fraction of 95% MeOH extract | 248 | C15H20O3 | Bozhou, Anhui, China | [52] |
| Ivalin (40) | Aerial parts | CHCl3-soluble fraction of MeOH extract | 248 | C15H20O3 | Korea | [51] |
| Whole plant | EtOAc fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| 2α,5α-Dihydroxy-11αH-eudesma-4(15)-en-12,8β-olide (41) | Whole plant | EtOAc fraction of 70% EtOH extract | 266 | C15H22O4 | Changsha, Hunan, China | [54] |
| Whole plant | EtOAc fraction of 70% EtOH extract | - | - | Dao County, Hunan, China | [16] | |
| (5α)-5-Hydroxyasperilin (42) | Whole plant | EtOAc fraction of 70% EtOH extract | 266 | C15H22O4 | Changsha, Hunan, China | [54] |
| Whole plant | PE fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| Carpabrotalactone B (43) | Whole plant | EtOAc fraction of 70% EtOH extract | 264 | C15H20O4 | Changsha, Hunan, China | [54] |
| Carpabrotalactone C (44) | Whole plant | EtOAc fraction of 70% EtOH extract | 264 | C15H20O4 | Changsha, Hunan, China | [58] |
| Oxoeudesm-11(13)-eno-12,8α-lactone (45) | Whole plant | EtOAc fraction of 70% EtOH extract | 248 | C15H20O3 | Dao County, Hunan, China | [16] |
| 5α-Hydroxy-4α,15-epoxy-11αH-eudesman-12,8β-olide (46) | Whole plant | EtOAc fraction of 95% EtOH extract | 266 | C15H22O4 | Hebei, China | [53] |
| Germacranolide sesquiterpenes | ||||||
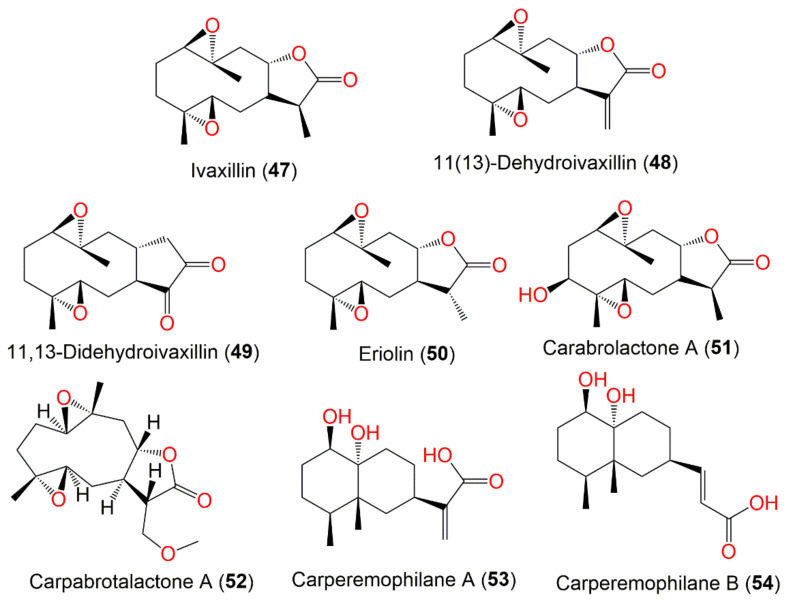
| Ivaxillin (47) | Whole plant | CHCl3 fraction of MeOH extract | 266 | C15H22O4 | Japan | [59] |
| Aerial parts | PE/acetone fraction of 95% EtOH extract | - | - | Tiger Leaping Gorge, Yunnan, China | [13] | |
| Fruits | PE/acetone fraction of MeOH extract | - | - | Tianquan, Sichuan, China | [44] | |
| 11(13)-Dehydroivaxillin (48) | Whole plant | CHCl3 fraction of MeOH extract | 264 | C15H20O4 | Japan | [59] |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Changsha, Hunan, China | [56] | |
| Whole plant | EtOAc fraction of 70% EtOH extract | - | - | Changsha, Hunan, China | [54] | |
| Whole plant | PE fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| 11,13-Didehydroivaxillin (49) | Aerial parts | CHCl3 fraction of MeOH extract | 264 | C15H20O4 | Korea | [51] |
| Eriolin (50) | Whole plant | CHCl3-soluble fraction of MeOH extract | 266 | C15H22O4 | Japan | [59] |
| Aerial parts | PE/acetone fraction of 95% EtOH extract | - | - | Tiger Leaping Gorge, Yunnan, China | [13] | |
| Fruits | PE/acetone fraction of MeOH extract | - | - | Tianquan, Sichuan, China | [44] | |
| Whole plant | EtOAc fraction of 70% EtOH extract | - | - | Changsha, Hunan, China | [54] | |
| Carabrolactone A (51) | Aerial parts | PE/acetone fraction of 95% EtOH extract | 282 | C15H22O5 | Tiger Leaping Gorge, Yunnan, China | [13] |
| Carpabrotalactone A (52) | Whole plant | EtOAc fraction of 70% EtOH extract | 296 | C16H24O5 | Changsha, Hunan, China | [54] |
| Eremophilanoide sesquiterpenes | ||||||
| Carperemophilane A (53) | Whole plant | EtOAc fraction of 95% EtOH extract | 268 | C15H24O4 | Changyang, Hubei, China | [60] |
| Carperemophilane B (54) | Whole plant | EtOAc fraction of 95% EtOH extract | 268 | C15H24O4 | Changyang, Hubei, China | [60] |
| Sesquiterpenes with cyclopropane ring | ||||||
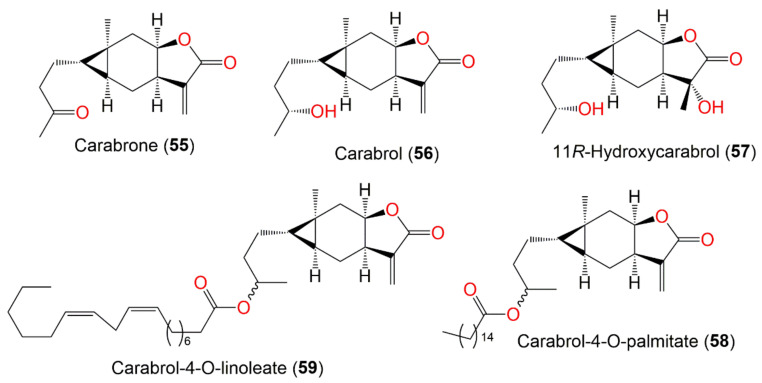
| Carabrone (55) | Fruits | Ether extract | 248 | C15H20O3 | Japan | [61] |
| Whole plant | CHCl3-soluble fraction of MeOH extract | - | - | Japan | [59] | |
| Aerial parts | PE/acetone fraction of 95% EtOH extract | - | - | Tiger Leaping Gorge, Yunnan, China | [13] | |
| Aerial parts | CHCl3 fraction of MeOH extract | - | - | Korea | [51] | |
| Fruits | PE/acetone fraction of MeOH extract | - | - | Tianquan, Sichuan, China | [44] | |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Hebei, China | [53] | |
| Carabrol (56) | Whole plant | CHCl3-soluble fraction of MeOH extract | 250 | C15H22O3 | Japan | [59] |
| Aerial parts | CHCl3-soluble fraction of MeOH extract | - | - | Korea | [51] | |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Changsha, Hunan, China | [56] | |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Hebei, China | [53] | |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Changyang, Hubei, China | [60] | |
| Whole plant | EtOAc fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| 11R-Hydroxycarabrol (57) | Whole plant | CHCl3-soluble fraction of 95% EtOH | 268 | C15H24O4 | Henan, China | [62] |
| Carabrol-4-O-palmitate (58) | Whole plant | EtOAc fraction of 95% EtOH extract | 488 | C31H52O4 | Hebei, China | [53] |
| Carabrol-4-O-linoleate (59) | Whole plant | EtOAc fraction of 95% EtOH extract | 516 | C33H56O4 | Hebei, China | [53] |
| Dimeric sesquiterpenes | ||||||
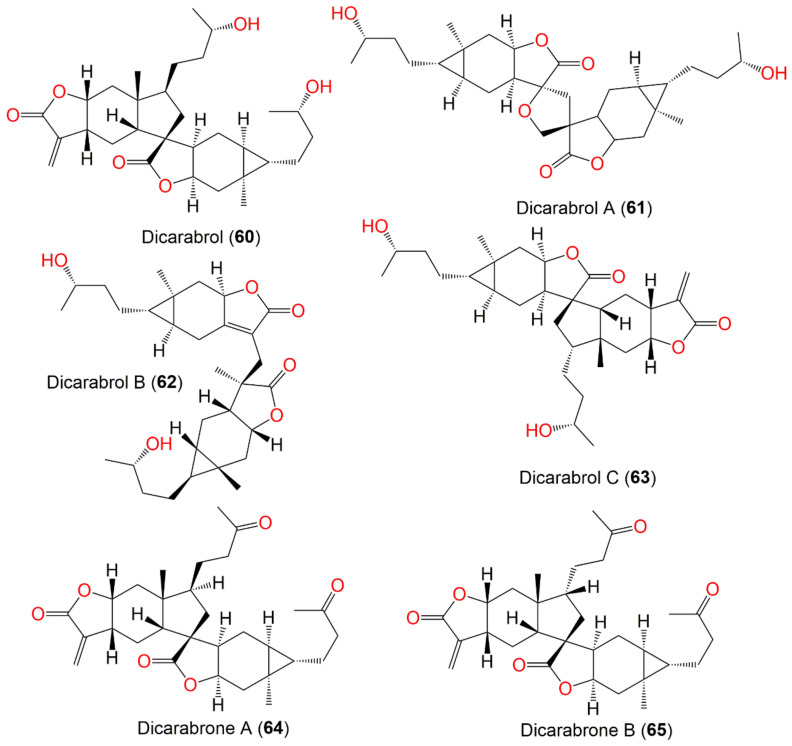
| Dicarabrol (60) | Whole plant | EtOAc fraction of EtOH extract | 500 | C30H44O6 | Changsha, Hunan, China | [56] |
| Dicarabrol A (61) | Whole plant | CH2Cl2-soluble fraction of 95% EtOH extract | 516 | C30H44O7 | Henan, China | [63] |
| Dicarabrol B (62) | Whole plant | CHCl3-soluble fraction of 95% EtOH | 500 | C30H44O6 | Henan, China | [62] |
| Dicarabrol C (63) | Whole plant | CHCl3-soluble fraction of 95% EtOH | 500 | C30H44O6 | Henan, China | [62] |
| Dicarabrone A (64) | Whole plant | CHCl3-soluble fraction of 95% EtOH extract | 496 | C30H40O6 | Henan, China | [64] |
| Dicarabrone B (65) | Whole plant | CHCl3-soluble fraction of 95% EtOH extract | 496 | C30H40O6 | Henan, China | [64] |
| Dicarabrone C (66) | Whole plant | CH2Cl2-soluble fraction of 95% EtOH extract | 512 | C30H40O7 | Henan, China | [63] |
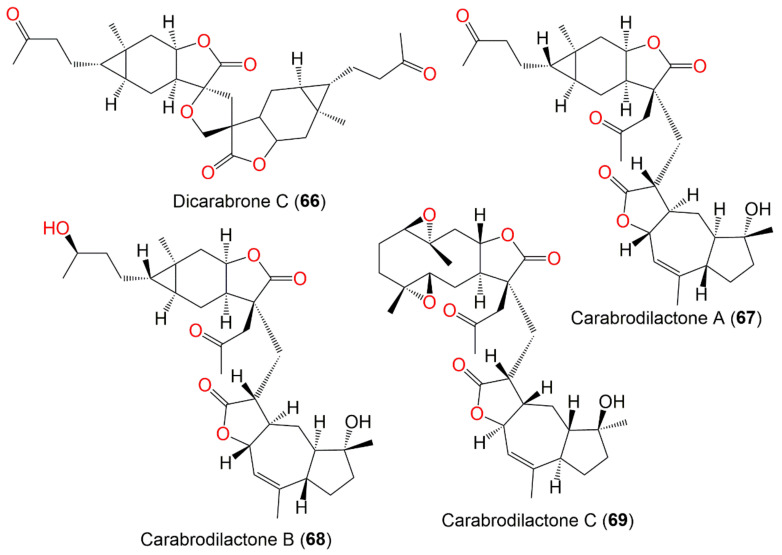
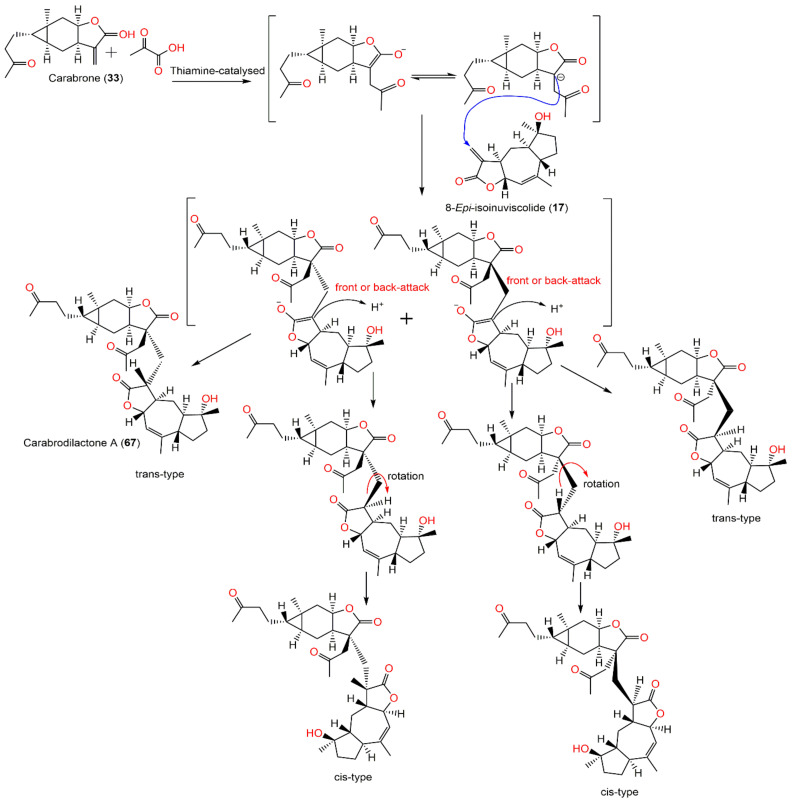
| Carabrodilactone A (67) | Whole plant | EtOAc fraction of 95% EtOH extract | 540 | C32H44O7 | Pu’an, GuiZhou, China | [65] |
| Carabrodilactone B (68) | Whole plant | EtOAc fraction of 95% EtOH extract | 542 | C32H46O7 | Pu’an, GuiZhou, China | [65] |
| Carabrodilactone C (69) | Whole plant | EtOAc fraction of 95% EtOH extract | 556 | C32H44O8 | Pu’an, GuiZhou, China | [65] |
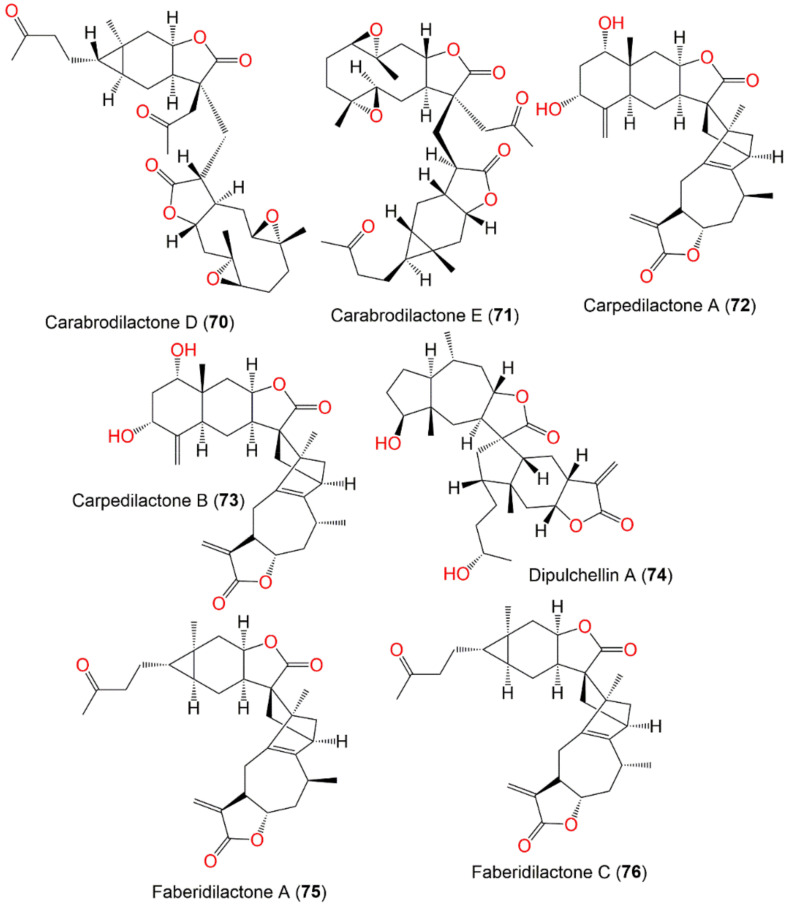
| Carabrodilactone D (70) | Whole plant | EtOAc fraction of 95% EtOH extract | 556 | C32H44O8 | Pu’an, GuiZhou, China | [65] |
| Carabrodilactone E (71) | Whole plant | EtOAc fraction of 95% EtOH extract | 556 | C32H44O8 | Pu’an, GuiZhou, China | [65] |
| Carpedilactone A (72) | Whole plant | EtOAc fraction of 95% EtOH extract | 494 | C30H38O6 | Pu’an, GuiZhou, China | [65] |
| Carpedilactone B (73) | Whole plant | EtOAc fraction of 95% EtOH extract | 494 | C30H38O6 | Pu’an, GuiZhou, China | [65] |
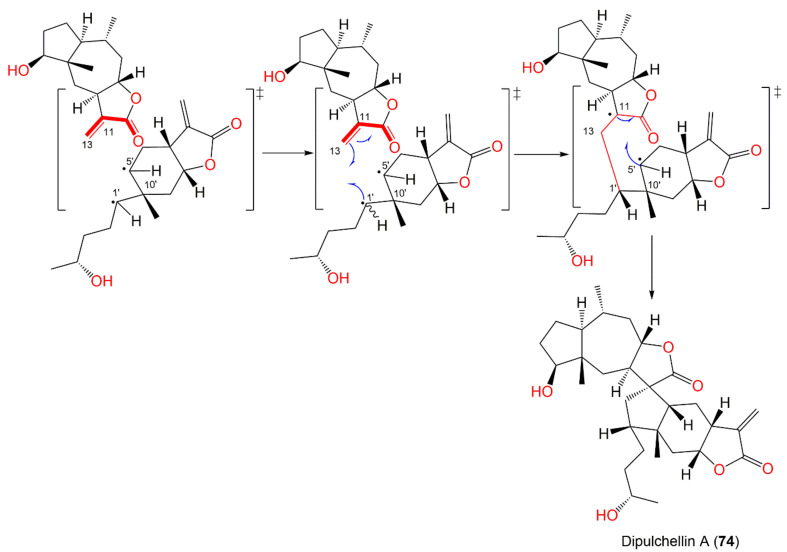
| Dipulchellin A (74) | Whole plant | CH2Cl2-soluble fraction of 95% EtOH extract | 500 | C30H44O6 | Henan, China | [63] |
| Faberidilactone A (75) | Whole plant | EtOAc fraction of 95% EtOH extract | 478 | C30H38O5 | Pu’an, GuiZhou, China | [65] |
| Faberidilactone C (76) | Whole plant | EtOAc fraction of 95% EtOH extract | 478 | C30H38O5 | Pu’an, GuiZhou, China | [65] |
| Other metabolites | ||||||
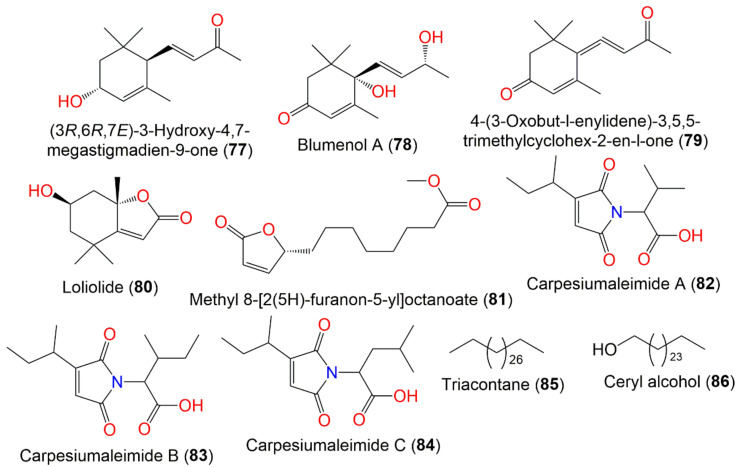
| (3R,6R,7E)-3-Hydroxy-4,7-megastigmadien-9-one (77) | Whole plant | PE fraction of 95% EtOH extract | 208 | C13H20O2 | China | [45] |
| Blumenol A (78) | Whole plant | PE fraction of 95% EtOH extract | 224 | C13H20O3 | China | [45] |
| 4-(3-Oxobut-l-enylidene)-3,5,5-trimethylcyclohex-2-en-l-one (79) | Whole plant | PE fraction of 95% EtOH extract | 208 | C13H16O2 | China | [45] |
| Loliolide (80) | Whole plant | PE fraction of 95% EtOH extract | 196 | C11H16O3 | China | [45] |
| Methyl 8-[2(5H)-furanon-5-yl]octanoate (81) | Whole plant | EtOAc fraction of 70% EtOH extract | 240 | C13H20O4 | Changsha, Hunan, China | [58] |
| Carpesiumaleimide A (82) | Whole plant | EtOAc fraction of 95% EtOH extract | 253 | C13H19NO4 | Changyang, Hubei, China | [60] |
| Carpesiumaleimide C (83) | Whole plant | EtOAc fraction of 95% EtOH extract | 267 | C14H21NO4 | Changyang, Hubei, China | [60] |
| Carpesiumaleimide B (84) | Whole plant | EtOAc fraction of 95% EtOH extract | 267 | C14H21NO4 | Changyang, Hubei, China | [60] |
| Triacontane (85) | Ripe seeds | Ether extract | 422 | C30H62 | Japan | [46] |
| Ceryl alcohol (86) | Ripe seeds | Ether extract | 382 | C26H54O | Japan | [66] |
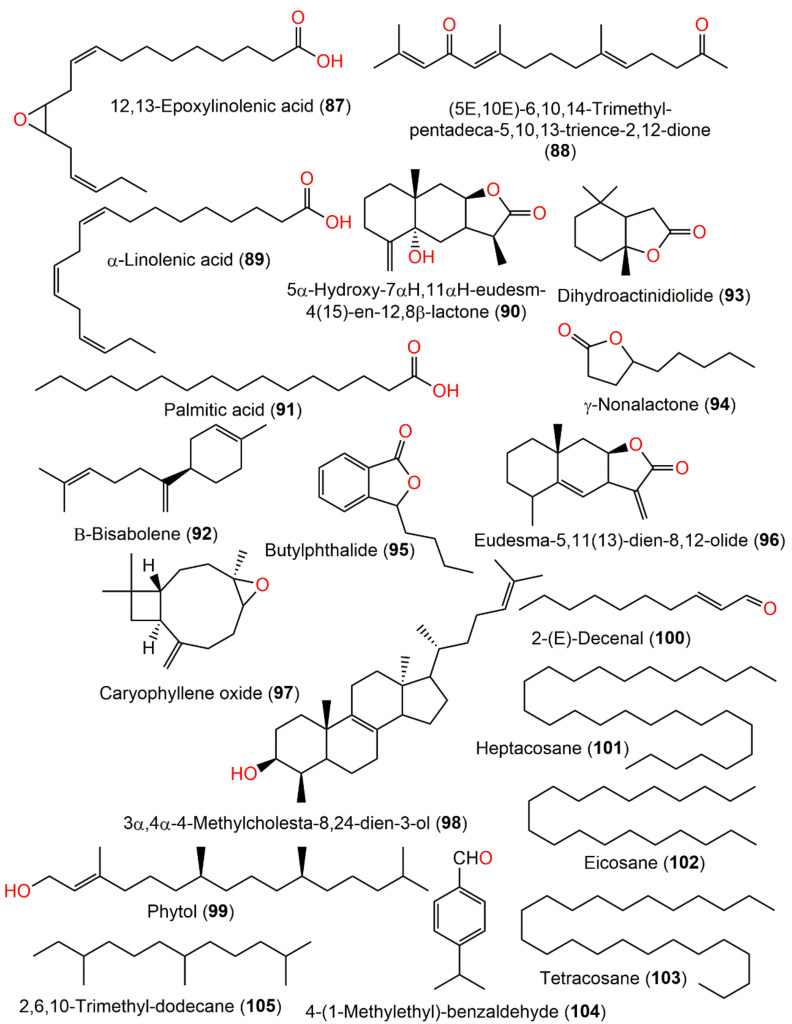
| 12,13-Epoxylinolenic acid (87) | Roots | PE fraction of 80% EtOH extract | 294 | C18H30O3 | Fuyang, Zhejinag, China | [17] |
| (5E,10E)-6,10,14-trimethyl-pentadeca-5,10,13-trience-2,12-dione (88) | Roots | PE fraction of 80% EtOH extract | 276 | C18H28O2 | Fuyang, Zhejinag, China | [17] |
| 5α-hydroxy-7αH,11αH-eudesm-4(15)-en-12,8β-lactone (90) | Roots | PE fraction of 80% EtOH extract | 250 | C15H22O3 | Fuyang, Zhejinag, China | [17] |
| Palmitic acid (91) | Roots | PE fraction of 80% EtOH extract | 256 | C16H32O2 | Fuyang, Zhejinag, China | [17] |
| β-Bisabolene (92) | Whole plant | Essential oil | 204 | C15H24 | Japan | [67] |
| Whole plant | Essential oil | - | - | Hubei, China | [15] | |
| Dihydroactinidiolide (93) | Whole plant | Essential oil | 182 | C11H18 | Japan | [67] |
| γ-Nonalactone (94) | Whole plant | Essential oil | 156 | C9H16O2 | Japan | [67] |
| Butylphthalide (95) | Whole plant | Essential oil | 190 | C9H16O2 | Japan | [67] |
| Eudesma-5,11(13)-dien-8,12-olide (96) | Whole plant | Essential oil | 232 | C15H20O2 | Hubei, China | [15] |
| Caryophyllene oxide (97) | Whole plant | Essential oil | 220 | C15H24O | Hubei, China | [15] |
| Aerial parts | Essential oil | Abbottabad, Pakistan | [32] | |||
| 3α,4α-4-Methylcholesta-8,24-dien-3-ol (98) | Whole plant | Essential oil | 398 | C28H46O | Hubei, China | [15] |
| Phytol (99) | Whole plant | Essential oil | 296 | C20H40O | Hubei, China | [15] |
| 2-(E)-Decenal (100) | Whole plant | Essential oil | 154 | C10H18O | Hubei, China | [15] |
| Heptacosane (101) | Whole plant | Essential oil | 380 | C27H56 | Hubei, China | [15] |
| Eicosane (102) | Whole plant | Essential oil | 280 | C20H42 | Hubei, China | [15] |
| Tetracosane (103) | Whole plant | Essential oil | 338 | C24H50 | Hubei, China | [15] |
| 4-(1-Methylethyl)-benzaldehyde (104) | Whole plant | Essential oil | 148 | C10H12O | Hubei, China | [15] |
| 2,6,10-Trimethyl-dodecane (105) | Whole plant | Essential oil | 212 | β-cedrene | Hubei, China | [15] |
| Torreyol (106) | Whole plant | Essential oil | 222 | C15H26O | Hubei, China | [15] |
| Androst-5,7-dien-3-ol-17-one (107) | Whole plant | Essential oil | 286 | C19H26O2 | Hubei, China | [15] |
| β-Cedrene (108) | Whole plant | Essential oil | 204 | C15H24 | Hubei, China | [15] |
| α-Curcumene (109) | Whole plant | Essential oil | 202 | C15H22 | Hubei, China | [15] |
| 2,6,10-Trimethyl-tetradecane (110) | Whole plant | Essential oil | 240 | C17H36 | Hubei, China | [15] |
| Caryophyllene (111) | Aerial parts | Essential oil | 204 | C15H24 | Abbottabad, Pakistan | [32] |
| Trans-nerolidol (112) | Aerial parts | Essential oil | 222 | C15H26O | Abbottabad, Pakistan | [32] |
| Geranyl isobutyrate (113) | Aerial parts | Essential oil | 224 | C14H24O2 | Abbottabad, Pakistan | [32] |
| δ-cadinene (114) | Aerial parts | Essential oil | 204 | C15H24 | Abbottabad, Pakistan | [32] |
| β-Eudesmene (115) | Aerial parts | Essential oil | 204 | C15H24 | Abbottabad, Pakistan | [32] |
| Trans-lachnophyllum ester (116) | Aerial parts | Essential oil | 176 | C11H12O2 | Abbottabad, Pakistan | [32] |
| α-Caryophyllene (117) | Aerial parts | Essential oil | 204 | C15H24 | Abbottabad, Pakistan | [32] |
| Neryl 3-methylbutyrate (118) | Aerial parts | Essential oil | 238 | C15H26O2 | Abbottabad, Pakistan | [32] |
5.1. Monoterpenes
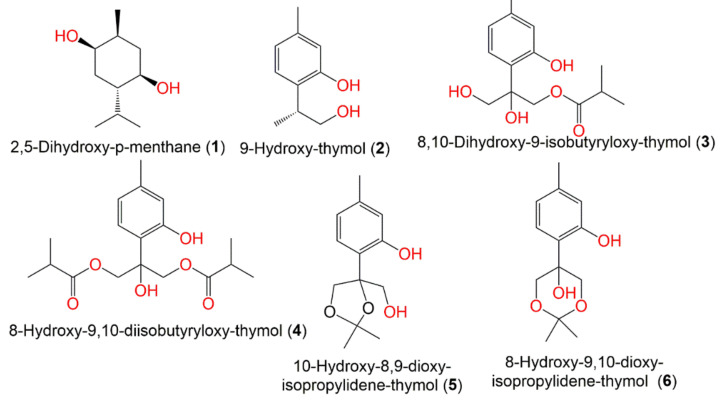
In 2021, Bao-jia et al. separated thymol-related metabolites from the petroleum ether extract of the whole plant: 8-hydroxy-9,10-diisobutyryloxy-thymol (3), 8,10-dihydroxy-9-isobutyryloxy-thymol (4), 9-hydroxy-thymol (2), 10-hydroxy-8,9-dioxy-isopropylidene-thymol (5), and 8-hydroxy-9,10-dioxy-isopropylidene-thymol (6) were elucidated through the comparison of their spectral data with the literature. These compounds were assessed for their cytotoxicity (Conc. 40 μM) versus HL60, SMMC7721, A549, SW480, and MCF7 cell lines. Compound 4 revealed a remarkable potential versus MCF-7 and SW480 (%inhibition 72 and 81%, respectively) [45]. In 2016, from the C. abrotanoides fruit MeOH extract, Wu et al. separated and characterized a monoterpene, 2,5-dihydroxy-p-menthane (1) using Sephadex LH-20 and SiO2 CC and spectral analyses, respectively (Figure 3). Compound 1 had antifeedant effectiveness versus Plutella xylostella third-instar larvae (EC50 43.99 mg/L). Furthermore, it displayed stomach–contact combination toxicity versus B. odoriphaga fourth-instar larvae (LD50 68.47 mg/L) [44].
Figure 3.
Structures of monoterpenes (1–6) reported from C. abrotanoides.
5.2. Sesquiterpene Lactones (STLs)
STLs Isolation and Identification
Various sesquiterpenes including guaianolide, pseudo-guaianolide, eudesmanolide, germacranolide, eremophilanoide, and STLs with cyclopropane ring in addition to dimeric sesquiterpenes were separated from C. abrotanoides using various chromatographic tools. For isolation of STLs, the aerial parts, blooms, fruits, or whole plant was extracted using various solvents (e.g., MeOH, 95% MeOH, 95% EtOH, 70% EtOH) [10,43,68]. The EtOAc or CHCl3 fraction of the different extracts was chromatographed using SiO2 CH2Cl2/MeOH, pertroleum ether/EtOAc, petroleum ether/acetone, petroleum ether/CHCl3, CHCl3/MeOH, petroleum ether/EtOAc/MeOH, cyclohexane/EtOAc), Sephadex LH-20 (CHCl3/MeOH 2:3, hexane/CHCl3/MeOH 1:2:3, MeOH, MeOH:CH2Cl2 1:1, MeOH:CHCl3 1:1), MCI gel CHP20P (EtOH/H2O or MeOH/H2O gradient), and RP-18 (MeOH/H2O gradient) CC [16,54,60,62]. Purification of the isolated STLs was achieved using HPLC (CH3CN/H2O or MeOH-H2O gradient), preparative-TLC (CHCl3/acetone 30:1, n-hexane/acetone 5:1, n-hexane/EtOAc/acetone 3:1:1), or HPLC-ESI-QTOF-MS/MS combined with HSCCC (high-speed counter-current chromatography, n-hexane/EtOAc/MeOH/H2O, 1:1:1:1, 1:9:9:1, 3:1:1:1 v/v/v/v), n-hexane/ MeOH/H2O (5:4:1, v/v/v), and CHCl3/MeOH/H2O (4:3:2, v/v/v) strategy [16,54,60,62]. Their structures and stereo-configurations were verified by various spectral tools (e.g., IR, NMR, HRMS), as well as ECD, X ray, and TDDFT-ECD (time-dependent density-functional-theory electronic circular dichroism) calculations [51,54,63]. These metabolites and their activities are highlighted in this work.
The sesquiterpene lactones, 14, 28, 29, 35, 40, 49, 55, and 56 were assessed for their cytotoxic effectiveness versus A549, L1210, SK-MEL-2, SK-OV-3, HCT-15, and XF-498 cell lines. They demonstrated (ED50 < 20 μM) notable cytotoxic potential versus all cell lines, whereas 14, 29, 35, and 40 displayed potent effectiveness (ED50 < 10 μM) compared to cisplatin. It was established that γ-lactone-α-methylene moiety was substantial for activity and the ketone or epoxide group in the cyclopentane enhanced the activity, while oxidation in the cycloheptane reduced activity [68] (Table 2).
Table 2.
Biological activity of reported metabolites from Carpesium abrotanoides.
| Compound Name | Biological Activity | Assay/Organism/ Cell Line |
Biological Results | Ref. | |
|---|---|---|---|---|---|
| Compound | Positive Control | ||||
| Inuviscolide (10) | Cytotoxicity | MTT/MDA-MB-231 | 17.26 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| 8-Epi-isoinuviscolide (12) | Cytotoxicity | MTT/MDA-MB-231 | 16.87 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| 4α,5α-Epoxy-10α,14-dihydro-inuviscolide (14) | Cytotoxicity | SRB/L1210 | 1.8 μM (ED50) | Cisplatin 0.07 μM (ED50) | [51] |
| SRB/A549 | 5.5 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 3.4 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 3.3 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 4.4 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 5.8 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| Caroguaianolide A (17) | Cytotoxicity | MTT/MDA-MB-231 | 7.96 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| MTT/HGC-27 | 10.47 μM (IC50) | Mitomycin C 6.68 μM (IC50) | [51] | ||
| Caroguaianolide B (18) | Cytotoxicity | MTT/MDA-MB-231 | 4.25 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| MTT/HGC-27 | 6.47 μM (IC50) | Mitomycin C 6.68 μM (IC50) | [51] | ||
| Caroguaianolide C (19) | Cytotoxicity | MTT/MDA-MB-231 | 2.67 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| MTT/HGC-27 | 4.83 μM (IC50) | Mitomycin C 6.68 μM (IC50) | [51] | ||
| Caroguaianolide D (20) | Cytotoxicity | MTT/MDA-MB-231 | 17.21 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| Caroguaianolide E (21) | Cytotoxicity | MTT/MDA-MB-231 | 18.37 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| Akihalin (22) | Cytotoxicity | MTT/MDA-MB-231 | 4.83 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| MTT/HGC-27 | 7.35 μM (IC50) | Mitomycin C 6.68 μM (IC50) | [51] | ||
| 4β-Hydroxy,10β-hydroperoxyl,5αH,7αH,8βH-guaia-1,11(13)- dien-8α,12-olide (23) | Cytotoxicity | MTT/MDA-MB-231 | 5.79 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| MTT/HGC-27 | 12.34 μM (IC50) | Mitomycin C 6.68 μM (IC50) | [51] | ||
| 4α-Hydroxy-1βH-guaia-9,11(13)-dien-12,8α-olide (25) | Cytotoxicity | MTT/MDA-MB-231 | 4.07 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| MTT/HGC-27 | 8.95 μM (IC50) | Mitomycin C 6.68 μM (IC50) | [51] | ||
| 1α,4α-Dihydroxy-guaia-11(13)-ene-12,8α-olide (26) | Cytotoxicity | CCK-8/HeLa | 15.6 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] |
| CCK-8/Caco-2 | 28.5 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| Anti-influenza A | CCK-8/H1N1 | 1.3 μM (IC50) | Oseltamivir 0.05 μM (IC50) | [54] | |
| 4α,5α-Dihydroxy-guaia-11(13)-en-12,8α-lactone (27) | Cytotoxicity | CCK-8/ISK | 30.3 μM (IC50) | Cisplatin 31.2 μM (IC50) | [54] |
| CCK-8/HeLa | 9.9 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] | ||
| CCK-8/A549 | 47.4 μM (IC50) | Cisplatin 40.1 μM (IC50) | [54] | ||
| CCK-8/Caco-2 | 16.4 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| Anti-influenza A | CCK-8/H1N1 | 0.4 μM (IC50) | Oseltamivir 0.05 μM (IC50) | [54] | |
| Carpesiolin (28) | Cytotoxicity | SRB/L1210 | 9.6 μM (ED50) | Cisplatin 0.07 μM (ED50) | [51] |
| SRB/A549 | 13.2 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 9.8 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 7.7 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 9.3 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 6.7 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| 2,3-Dihydroaromomaticin (29) | Cytotoxicity | SRB/L1210 | 5.0 μM (ED50) | Cisplatin 0.07 μM (ED50) | [51] |
| SRB/A549 | 7.1 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 3.9 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 4.0 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 3.3 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 8.1 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| MTT/A549 | 7.09 μM (IC50) | Vinblastine 18.5 μM (IC50) | [52] | ||
| MTT/HepG2 | 9.03 μM (IC50) | Vinblastine 9.29 μM (IC50) | [52] | ||
| MTT/MDA-MB-231 | 5.16 μM (IC50) | Vinblastine 42.51 μM (IC50) | [52] | ||
| MTT/HCT116 | 10.15 μM (IC50) | Vinblastine 30.62 μM (IC50) | [52] | ||
| MTT/CNE2 | 12.99 μM (IC50) | Vinblastine 1.16 μM (IC50) | [52] | ||
| 2-Desoxy-4-epi-pulchellin (31) | Cytotoxicity | CCK-8/K562 | 17.0 μM (IC50) | Taxol 3.8 μM (IC50) | [56] |
| CCK-8/MCF-7 | 11.0 μM (IC50) | Taxol 2.3 μM (IC50) | [56] | ||
| CCK-8/HeLa | 6.0 μM (IC50) | Taxol 2.9 μM (IC50) | [56] | ||
| CCK-8/DU145 | 3.1 μM (IC50) | Taxol 3.1 μM (IC50) | [56] | ||
| CCK-8/U937 | 2.2 μM (IC50) | Taxol 2.1 μM (IC50) | [56] | ||
| CCK-8/H1975 | 13.0 μM (IC50) | Taxol 2.8 μM (IC50) | [56] | ||
| CCK-8/SGC-7901 | 22.0 μM (IC50) | Taxol 9.8 μM (IC50) | [56] | ||
| CCK-8/A549 | 6.2 μM (IC50) | Taxol 2.1 μM (IC50) | [56] | ||
| CCK-8/MOLT-4 | 5.5 μM (IC50) | Taxol 2.7 μM (IC50) | [56] | ||
| CCK-8/HL60 | 2.4 μM (IC50) | Taxol 3.8 μM (IC50) | [56] | ||
| MTT/MDA-MB-231 | 18.67 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] | ||
| MTT/A549 | 6.04 μM (IC50) | Vinblastine 18.5 μM (IC50) | [52] | ||
| MTT/HepG2 | 18.25 μM (IC50) | Vinblastine 9.29 μM (IC50) | [52] | ||
| MTT/MDA-MB-231 | 21.55 μM (IC50) | Vinblastine 42.51 μM (IC50) | [52] | ||
| Antiviral | CPE inhibition/H1N1 | 29.3 μM (IC50) | Osehamivir 0.025 μM (IC50) | [56] | |
| CPE inhibition/H3N2 | 47.3 μM (IC50) | Osehamivir 0.015 μM (IC50) | [56] | ||
| Antimycobacterial | GFPMA/Mycobacterium tuberculosis strains H37Rv, H37Ra | 7.6 μM (MIC) | Isoniazid, 2.0 μM (MIC) | [56] | |
| 3aR,4aS,5S,7aS,8S,9aR)-5-Hydroxy-4a,8-dimethyl-3-methylen-decahydroa-zuleno [6,5-b]furan-2(3H)-on (32) | Cytotoxicity | MTT/MDA-MB-231 | 5.32 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| MTT/HGC-27 | 11.44 μM (IC50) | Mitomycin C 6.68 μM (IC50) | [51] | ||
| Telekin (35) | Cytotoxicity | SRB/L1210 | 7.5 μM (ED50) | Cisplatin 0.07 μM ED50) | [51] |
| SRB/A549 | 7.5 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 4.1 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 4.9 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 6.6 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 6.7 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| HepG2/CCK-8 | 2.95 μM (IC50) | Paclitaxel | [16] | ||
| MTT/A549 | 11.08 μM (IC50) | Vinblastine 18.5 μM (IC50) | [52] | ||
| MTT/HepG2 | 7.89 μM (IC50) | Vinblastine 9.29 μM (IC50) | [52] | ||
| MTT/MDA-MB-231 | 9.46 μM (IC50) | Vinblastine 42.51 μM (IC50) | [52] | ||
| MTT/HCT116 | 9.69 μM (IC50) | Vinblastine 30.62 μM (IC50) | [52] | ||
| MTT/CNE2 | 13.66 μM (IC50) | Vinblastine 1.16 μM (IC50) | [52] | ||
| 5α-Epoxyalantolactone (38) | Cytotoxicity | MTT/A549 | 3.51 μM (IC50) | Vinblastine 18.5 μM (IC50) | [52] |
| MTT/HepG2 | 2.73 μM (IC50) | Vinblastine 9.29 μM (IC50) | [52] | ||
| MTT/MDA-MB-231 | 4.18 μM (IC50) | Vinblastine 42.51 μM (IC50) | [52] | ||
| MTT/HCT116 | 3.75 μM (IC50) | Vinblastine 30.62 μM (IC50) | [52] | ||
| MTT/CNE2 | 7.21 μM (IC50) | Vinblastine 1.16 μM (IC50) | [52] | ||
| MTT/NCM460 | 13.66 μM (IC50) | Vinblastine | [52] | ||
| Ivalin (40) | Cytotoxicity | SRB/L1210 | 7.5 μM (ED50) | Cisplatin 0.07 μM ED50) | [51] |
| SRB/A549 | 9.9 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 3.5 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 3.0 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 2.9 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 2.6 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| MTT/A549 | 7.77 μM (IC50) | Vinblastine 18.5 μM (IC50) | [52] | ||
| MTT/HepG2 | 6.1 μM (IC50) | Vinblastine 9.29 μM (IC50) | [52] | ||
| MTT/MDA-MB-231 | 7.42 μM (IC50) | Vinblastine 42.51 μM (IC50) | [52] | ||
| MTT/HCT116 | 13.12 μM (IC50) | Vinblastine 30.62 μM (IC50) | [52] | ||
| MTT/CNE2 | 23.6 μM (IC50) | Vinblastine 1.16 μM (IC50) | [52] | ||
| 2α,5α-Dihydroxy-11αH-eudesma-4(15)-en-12,8β-olide (41) | Cytotoxicity | CCK-8/ISK | 21.8 μM (IC50) | Cisplatin 31.2 μM (IC50) | [54] |
| CCK-8/HeLa | 8.5 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] | ||
| CCK-8/Sw620 | 42.3 μM (IC50) | Cisplatin 37.9 μM (IC50) | [54] | ||
| CCK-8/RBE | 45.1 μM (IC50) | Cisplatin 42.5 μM (IC50) | [54] | ||
| CCK-8/Caco-2 | 42.6 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| CCK-8/HepG2 | 9.83 μM (IC50) | Paclitaxel | [16] | ||
| (5α)-5-Hydroxyasperilin (42) | Cytotoxicity | CCK-8/ISK | 25.8 μM (IC50) | Cisplatin 31.2 μM (IC50) | [54] |
| CCK-8/HeLa | 10.7 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] | ||
| CCK-8/Sw620 | 28.1 μM (IC50) | Cisplatin 37.9 μM (IC50) | [54] | ||
| CCK-8/RBE | 28.4 μM (IC50) | Cisplatin 42.5 μM (IC50) | [54] | ||
| CCK-8/Caco-2 | 21.5 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| Carpabrotalactone B (43) | Cytotoxicity | CCK-8/ISK | 33.8 μM (IC50) | Cisplatin 31.2 μM (IC50) | [54] |
| CCK-8/HeLa | 25.6 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] | ||
| CCK-8/Caco-2 | 45.4 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| Anti-influenza A | CCK-8/H1N1 | 6.2 μM (IC50) | Oseltamivir 0.05 μM (IC50) | [54] | |
| Carpabrotalactone C (44) | Cytotoxicity | CCK-8/A549 | 19.1 μM (IC50) | Cisplatin 12.77 μM (IC50) | [58] |
| CCK-8/SMMC-7721 | 23.68 μM (IC50) | Cisplatin 5.1 μM (IC50) | [58] | ||
| CCK-8/MCF-7 | 20.32 μM (IC50) | Cisplatin 12.6 μM (IC50) | [58] | ||
| CCK-8/SW480 | 11.46 μM (IC50) | Cisplatin 6.67 μM (IC50) | [58] | ||
| Oxoeudesm-11(13)-eno-12,8α-lactone (45) | Cytotoxicity | CCK-8/HepG2 | 4.15 μM (IC50) | Paclitaxel | [16] |
| Anti-inflammatory | NO inhibition/LPS | 63.23 μM (IC50) | Dexamethasone 3.61 μM (IC50) | [58] | |
| 11(13)-Dehydroivaxillin (48) |
Cytotoxicity | CCK-8/K562 | 1.7 μM (IC50) | Taxol 3.8 μM (IC50) | [56] |
| CCK-8/MCF-7 | 4.1 μM (IC50) | Taxol 2.3 μM (IC50) | [56] | ||
| CCK-8/HeLa | 1.0 μM (IC50) | Taxol 2.9 μM (IC50) | [56] | ||
| CCK-8/DU145 | 0.38 μM (IC50) | Taxol 3.1 μM (IC50) | [56] | ||
| CCK-8/U937 | 0.21 μM (IC50) | Taxol 2.1 μM (IC50) | [56] | ||
| CCK-8/H1975 | 5.4 μM (IC50) | Taxol 2.8 μM (IC50) | [56] | ||
| CCK-8/SGC-7901 | 6.4 μM (IC50) | Taxol 9.8 μM (IC50) | [56] | ||
| CCK-8/A549 | 2.0 μM (IC50) | Taxol 2.1 μM (IC50) | [56] | ||
| CCK-8/MOLT-4 | 1.2 μM (IC50) | Taxol 2.7 μM (IC50) | [56] | ||
| CCK-8/ISK | 13.7 μM (IC50) | Cisplatin 31.2 μM (IC50) | [54] | ||
| CCK-8/HeLa | 12.3 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] | ||
| CCK-8/Sw620 | 12.1 μM (IC50) | Cisplatin 37.9 μM (IC50) | [54] | ||
| CCK-8/RBE | 7.8 μM (IC50) | Cisplatin 42.5 μM (IC50) | [54] | ||
| CCK-8/Caco-2 | 20.4 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| MTT/A549 | 17.6 μM (IC50) | Vinblastine 18.5 μM (IC50) | [52] | ||
| MTT/HepG2 | 14.59 μM (IC50) | Vinblastine 9.29 μM (IC50) | [52] | ||
| MTT/MDA-MB-231 | 10.88 μM (IC50) | Vinblastine 42.51 μM (IC50) | [52] | ||
| CCK-8/HL60 | 0.18 μM (IC50) | Taxol 3.8 μM (IC50) | [60] | ||
| Anti-influenza A | CCK-8/H1N1 | 11.6 μM (IC50) | Oseltamivir 0.05 μM (IC50) | [54] | |
| Antiviral | CPE inhibition/H1N1 | 10.8 μM (IC50) | Osehamivir 0.025 μM (IC50) | [56] | |
| CPE inhibition/H3N2 | 11.6 μM (IC50) | Osehamivir 0.015 μM (IC50) | [56] | ||
| Antimycobacterial | GFPMA/Mycobacterium tuberculosis strains H37Rv, H37Ra | 6.0 μM (MIC) | Isoniazid, 2.0 μM (MIC) | [56] | |
| 11,13-Didehydroivaxillin (49) | Cytotoxicity | SRB/L1210 | 11.4 μM (ED50) | Cisplatin 0.07 μM ED50) | [51] |
| SRB/A549 | 18.4 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 9.7 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 9.5 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 10.6 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 7.4 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| Eriolin (50) | Cytotoxicity | CCK-8/ISK | 41.6 μM (IC50) | Cisplatin 31.2 μM (IC50) | [54] |
| CCK-8/HeLa | 14.8 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] | ||
| CCK-8/Sw620 | 46.4 μM (IC50) | Cisplatin 37.9 μM (IC50) | [54] | ||
| CCK-8/Caco-2 | 12.7 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| Anti-influenza A | CCK-8/H1N1 | 16.4 μM (IC50) | Oseltamivir 0.05 μM (IC50) | [54] | |
| Carpabrotalactone A (52) | Cytotoxicity | CCK-8/ISK | 21.0 μM (IC50) | Cisplatin 31.2 μM (IC50) | [54] |
| CCK-8/HeLa | 9.6 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] | ||
| CCK-8/Sw620 | 44.5 μM (IC50) | Cisplatin 37.9 μM (IC50) | [54] | ||
| CCK-8/Caco-2 | 13.4 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| Carperemophilane A (53) | Cytotoxicity | MTT/MDA-MB-231 | 22.67 μM (IC50) | Mitomycin C 4.89 μM (IC50) | [60] |
| MTT/HGC-27 | 24.83 μM (IC50) | Mitomycin C 6.73 μM (IC50) | [60] | ||
| Carperemophilane B (54) | Cytotoxicity | MTT/MDA-MB-231 | 34.83 μM (IC50) | Mitomycin C 4.89 μM (IC50) | [60] |
| MTT/HGC-27 | 37.35 μM (IC50) | Mitomycin C 6.73 μM (IC50) | [60] | ||
| Carabrone (55) | Antifungal | Spore germination/Colletotrichum lagenarium | 7.1 μg/mL (EC50) | Chlorothalonil 0.75 μg/mL (EC50) | [69] |
| Cytotoxicity | SRB/L1210 | 6.2 μM (ED50) | Cisplatin 0.07 μM ED50) | [51] | |
| SRB/A549 | 17.1 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 11.3 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 8.5 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 19.8 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 7.4 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| MTT/HCT117 | 17.13 μM (IC50) | Doxorubicin 0.20 μM (IC50) | [70] | ||
| MTT/CCRF-CEM | 43.66 μM (IC50) | Doxorubicin 0.003 μM (IC50) | [70] | ||
| MTT/K562 | 19.42 μM (IC50) | Doxorubicin 0.04 μM (IC50) | [70] | ||
| MTT/HL60 | 23.29 μM (IC50) | Doxorubicin < 0.001 μM (IC50) | [70] | ||
| Carabrol (56) | Cytotoxicity | SRB/L1210 | 11.4 μM (ED50) | Cisplatin 0.07 μM ED50) | [51] |
| SRB/A549 | 13.1 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 10.6 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 11.2 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 13.5 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 10.8 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| CCK-8/K562 | 4.7 μM (IC50) | Taxol 3.8 μM (IC50) | [56] | ||
| CCK-8/MCF-7 | 47.0 μM (IC50) | Taxol 2.3 μM (IC50) | [56] | ||
| CCK-8/HeLa | 6.6 μM (IC50) | Taxol 2.9 μM (IC50) | [56] | ||
| CCK-8/DU145 | 2.6 μM (IC50) | Taxol 3.1 μM (IC50) | [56] | ||
| CCK-8/U937 | 0.94 μM (IC50) | Taxol 2.1 μM (IC50) | [56] | ||
| CCK-8/H1975 | 4.0 μM (IC50) | Taxol 2.8 μM (IC50) | [56] | ||
| CCK-8/MOLT-4 | 8.9 μM (IC50) | Taxol 2.7 μM (IC50) | [56] | ||
| CCK-8/HL60 | 0.36 μM (IC50) | Taxol 3.8 μM (IC50) | [56] | ||
| MTT/MDA-MB-231 | 7.45 μM (IC50) | Mitomycin C 4.89 μM (IC50) | [60] | ||
| MTT/HGC-27 | 10.27 μM (IC50) | Mitomycin C 6.73 μM (IC50) | [60] | ||
| MTT/HCT117 | 29.95 μM (IC50) | Doxorubicin 0.20 μM (IC50) | [70] | ||
| MTT/CCRF-CEM | 32.26 μM (IC50) | Doxorubicin 0.003 μM (IC50) | [70] | ||
| MTT/K562 | 9.1 μM (IC50) | Doxorubicin 0.04 μM (IC50) | [70] | ||
| MTT/HL60 | 23.73 μM (IC50) | Doxorubicin < 0.001 μM (IC50) | [70] | ||
| Antiviral | CPE inhibition/H1N1 | 45.5 μM (IC50) | Osehamivir 0.025 μM (IC50) | [56] | |
| Carabrol-4-O-palmitate (58) | Cytotoxicity | MTA/HL60 | 45.85 μM (IC50) | Cisplatin 2.32 μM (IC50) | [53] |
| Dicarabrol (60) | Cytotoxicity | CCK-8/K562 | 1.2 μM (IC50) | Taxol 3.8 μM (IC50) | [56] |
| CCK-8/MCF-7 | 3.3 μM (IC50) | Taxol 2.3 μM (IC50) | [56] | ||
| CCK-8/HeLa | 0.61 μM (IC50) | Taxol 2.9 μM (IC50) | [56] | ||
| CCK-8/DU145 | 0.31 μM (IC50) | Taxol 3.1 μM (IC50) | [56] | ||
| CCK-8/U937 | 0.15 μM (IC50) | Taxol 2.1 μM (IC50) | [56] | ||
| CCK-8/H1975 | 1.4 μM (IC50) | Taxol 2.8 μM (IC50) | [56] | ||
| CCK-8/SGC-7901 | 0.71 μM (IC50) | Taxol 9.8 μM (IC50) | [56] | ||
| CCK-8/A549 | 2.7 μM (IC50) | Taxol 2.1 μM (IC50) | [56] | ||
| CCK-8/MOLT-4 | 1.3 μM (IC50) | Taxol 2.7 μM (IC50) | [56] | ||
| CCK-8/HL60 | 0.10 μM (IC50) | Taxol 3.8 μM (IC50) | [56] | ||
| Antiviral | CPE inhibition/H1N1 | 15.9 μM (IC50) | Osehamivir 0.025 μM (IC50) | [56] | |
| CPE inhibition/H3N2 | 30.0 μM (IC50) | Osehamivir 0.015 μM (IC50) | [56] | ||
| Antimycobacterial | GFPMA/Mycobacterium tuberculosis strains H37Rv, H37Ra | 3.7 μM (MIC) | Isoniazid, 2.0 μM (MIC) | [56] | |
| Dicarabrol A (61) | Cytotoxicity | MTT/HL60 | 8.7 μM (IC50) | Doxorubicin | [63] |
| Dicarabrol B (62) | Cytotoxicity | MTT/HL60 | 20.0 μM (IC50) | Doxorubicin | [62] |
| MTT/A549 | 20.0 μM (IC50)) | Doxorubicin | [62] | ||
| Dicarabrol C (63) | Cytotoxicity | MTT/HL60 | 3.6 μM (IC50) | Doxorubicin | [62] |
| Dicarabrone A (64) | Cytotoxicity | MTT/HL60 | 9.1 μM (IC50) | Doxorubicin | [64] |
| Dicarabrone B (65) | Cytotoxicity | MTT/HL60 | 8.2 μM (IC50) | Doxorubicin | [64] |
| Dicarabrone C (66) | Cytotoxicity | MTT/HL60 | 8.2 μM (IC50) | Doxorubicin | [63] |
| Carabrodilactone A (67) | Cytotoxicity | MTT/A549 | 4.34 μM (IC50) | Doxorubicin 0.05 μM (IC50) | [65] |
| MTT/HCT117 | 3.08 μM (IC50) | Doxorubicin 0.05 μM (IC50) | [65] | ||
| MTT/MDA-MB 231 | 8.05 μM (IC50) | Doxorubicin 0.05 μM (IC50) | [65] | ||
| MTT/BEL 7404 | 3.2 μM (IC50) | Doxorubicin 0.05 μM (IC50) | [65] | ||
| Carpedilactone A (72) | Cytotoxicity | MTT/A549 | 2.63 μM (IC50) | Doxorubicin 0.05 μM (IC50) | [71] |
| MTT/BEL 7404 | 5.53 μM (IC50) | Doxorubicin 0.22 μM (IC50) | [71] | ||
| MTT/HLF | 1.45 μM (IC50) | Doxorubicin 0.07 μM (IC50) | [71] | ||
| MTT/CCRF-CEM | 0.14 μM (IC50) | Doxorubicin 0.01 μM (IC50) | [71] | ||
| Carpedilactone B (73) | Cytotoxicity | MTT/A549 | 9.53 μM (IC50) | Doxorubicin 0.05 μM (IC50) | [71] |
| MTT/BEL 7404 | 14.19 μM (IC50) | Doxorubicin 0.22 μM (IC50) | [71] | ||
| MTT/HLF | 3.31 μM (IC50) | Doxorubicin 0.07 μM (IC50) | [71] | ||
| MTT/CCRF-CEM | 0.32 μM (IC50) | Doxorubicin 0.01 μM (IC50) | [71] | ||
| Dipulchellin A (74) | Cytotoxicity | MTT/HL60 | 8.9 μM (IC50) | Doxorubicin | [63] |
| Faberidilactone A (75) | Cytotoxicity | MTT/HCT117 | 3.44 μM (IC50) | Doxorubicin 0.20 μM (IC50) | [70] |
| MTT/CCRF-CEM | 2.71 μM (IC50) | Doxorubicin 0.003 μM (IC50) | [70] | ||
| MTT/K562 | 2.63 μM (IC50) | Doxorubicin 0.04 μM (IC50) | [70] | ||
| MTT/HL60 | 4.95 μM (IC50) | Doxorubicin < 0.001 μM (IC50) | [70] | ||
| Faberidilactone C (76) | Cytotoxicity | MTT/HCT117 | 4.00 μM (IC50) | Doxorubicin 0.20 μM (IC50) | [70] |
| MTT/CCRF-CEM | 2.66 μM (IC50) | Doxorubicin 0.003 μM (IC50) | [70] | ||
| MTT/K562 | 4.92 μM (IC50) | Doxorubicin 0.04 μM (IC50) | [70] | ||
| MTT/HL60 | 8.12 μM (IC50) | Doxorubicin < 0.001 μM (IC50) | [70] | ||
Abbreviations: A375: Human malignant melanoma cell line; A549: Lung adenocarcinoma epithelial cell line; BEL 7404: Human liver carcinoma cell line; Caco-2: Human colorectal adenocarcinoma cells line; Caski: Human cervical carcinoma; CCRF-CEM: Human leukemic cell line; CNE2: Human nasopharyngeal carcinoma cell; DU145: Human prostate cancer cell line; GES-1: Human gastric cell line; GP2-293:Human embryonic kidney line; H1975: Human non-small lung cancer cell line; HCC: Human hepatocellular carcinoma cell line; HCT116: Human colon cancer cell line; HCT-15: Human colon adenocarcinoma colorectal adenocarcinoma; HCT117: Colon carcinoma cell line; Hep3B: Human hepatoma cell line; HepG2: Human liver cancer cell line; HeLa: Human cervical cancer cell line; HGC-27: Human gastric cancer cell line; HL60: Human leukemia cell line; HLF: Human hepatoma cell line; HTC-15: Human colorectal carcinoma cell line; Huh7: Human hepato-cellular carcinoma cell line; ISK: Human endometrial epithelial cell line; K562: Human immortalized myelogenous leukemia cell line; L1210: Mouse lymphocytic leukemia cell line; L-O2: Normal human hepatic cell line; MCF-7: Human breast cancer cell line; MDA-MB-231: Human breast cancer cell line; MOLT-4: Human T lymphoblast cell line; MDCK: Madin-Darby canine kidney; NCM460: Human normal colonic epithelial cell line; RBE: Human esophageal cancer cell line; SGC-7901: Human gastric cancer cell line; SH-SY5Y: Human neuroblastoma cell line; SK-MEL-2: Human melanoma cell line; SK-OV-3: Human ovarian cancer cell line; SMMC-7721: Human hepatocarcinoma cell line; SW620, SW480: Human colorectal cancer cell line; U937: Human leukemia cell line; XF-498: Human central nervous system solid tumor cell line; CCK-8: Cell counting Kit-8; CPE: Cytopathic effect; EC50: Half maximal effective concentration; H3N2: Influenza A Virus subtype H3N2; H1N1: Influenza A Virus subtype H1N1; LPS: lipopolysaccharide; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; MIC: Minimum inhibitory concentration; NO: Nitric oxide; GFPMA: Green fluorescent protein microplate assay; SRB: Sulforhodamine B; MTS: (3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazoliuminner salt).
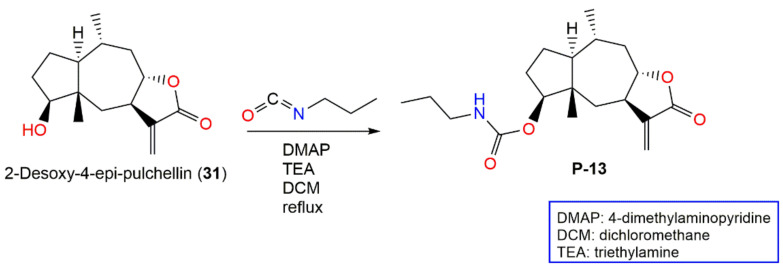
In 2019, Huang et al. synthesized P-13 (2-desoxy-4β-propylcarbamate-pulchellin), a 2-desoxy-4-epi-pulchellin (31) derivative [72] (Scheme 1). This compound (IC50 1.5 μM) had 10 times more potent STAT3 signaling inhibitory potential than 31 in the STAT3-responsive luciferase-gene-reporter assay. P-13 suppressed IL-6-caused STAT3 activation. Additionally, it prohibited JAK2 phosphorylation and JAK2 kinase activity that was blocked by reducing agents, DTT (dithiothreitol) or GSH (glutathione), indicating its thiol-reactive α-β-unsaturated carbonyl functionality involvement. Further molecular docking displayed its covalent binding with the C452 (cysteine-452) in the JAK2 SH2 domain. It also suppressed growth and produced apoptosis on various cancer cell lines, especially those with activated STAT3 and prohibited in vivo tumor growth in mice bearing the HT29 xenografts. Therefore, this derivative represented a novel JAK2-covalent inhibitor and provided a promising candidate for anticancer drugs [72].
Scheme 1.
Synthetic pathway of P-13 [72].
Tian et al. investigated the cytotoxic potential of 11 (Figure 4) versus different cancer cells, MDA-MB-231, CaCo-2, A549, CNE-2, SH-SY5Y, HepG-2, and HGC-27, and normal cell lines, Marc-145, GES-1, and MDCK, in the MTT assay. It was found that the MDA-MB-231 cell was the most affected cell (IC50 4.07 µM), whereas 11 had a lower effect on the normal cells, revealing its selectivity. It induced apoptosis and pro-death autophagy in MDA-MB-231 cells through elevating Bax, LC3-II, Bad, and p-ULK1 expression, as well as down-regulation of p-Akt, p-PI3K, Bcl-2, p-mTOR, LC3-I, p62, and Bcl-xl. Hence, it produced its effect through the Akt/PI3K/ mTOR signaling pathway [73].
Figure 4.
Guaianolide sesquiterpenes (7–16) reported from C. abrotanoides.
4α,5α-Epoxy-10α,14-dihydro-inuviscolide (14) was separated from the blooms’ MeOH extract [43]. This compound noticeably lessened IL-6, -4, -10, and -13, IFNγ without affecting TNFα in LPS-treated RAW264.7 cells and PMA-treated NK-YS and EL-4 cells. Furthermore, it prohibited PMA-induced IL-10 promoter activity and NF-κB activation as Bay11. It is noteworthy that this compound did not influence PMA-mediated IκB and MAPK kinase phosphorylation, IκB degradation, and NF-κB-p65 nuclear translocation [43]. Therefore, it blocked the cytokine production accompanied with the inflammation by suppressing NF-κB activation. It could be potentially utilized as an immunosuppressant for attenuating inflammation-induced disorders [43].
Bradysia odoriphaga is one of the main insect pests influencing Northern China’s Chinese chive that results in 30–80% loss in production and attacks 20–30% of Chinese chives [74]. It also feeds on other plant species such as garlic, Welsh onion, cucumber, Chinese cabbage, and lettuce, as well as mushroom sheds causing production losses. Its larvae gather in the stems and roots of the plant making its control hard with common strategies. Generally, organophosphates are the principal tool for controlling Chinese chives pests [75].
Wu et al. separated and characterized two new sesquiterpenes, 9β-hydroxy-1βH,11αH-guaia-4,10(14)-dien-12,8α-olide (15) and 9β-hydroxy-1βH, 11βH-guaia-4,10(14)-dien-12,8α-olide (16), along with four known related analogs, 33, 47, 50, and 55, from the C. abrotanoides fruits’ MeOH extract. These metabolites had antifeedant effectiveness versus Plutella xylostella third-instar larvae (EC50 ranged from 19.84 to 97.94 mg/L). Furthermore, they displayed stomach–contact combination toxicity versus B. odoriphaga fourth-instar larvae (LD50s 18.71–319.67 mg/L), whereas 15 had the potent activity, revealing its potential use as a natural insecticide. The results indicated that the β-C-11 methyl group in 15 and 47 made them more active than 16 and 50 that have α-C-11 methyl group [44].
Wang et al. purified five new guaiane-type sesquiterpene lactones, caroguaianolides A–E (17–21), along with formerly reported 10, 12, 22–25, 30 and 32 from the EtOAc fraction of 95% EtOH extract (Figure 5).
Figure 5.
Guaianolide sesquiterpenes (17–27) reported from C. abrotanoides.
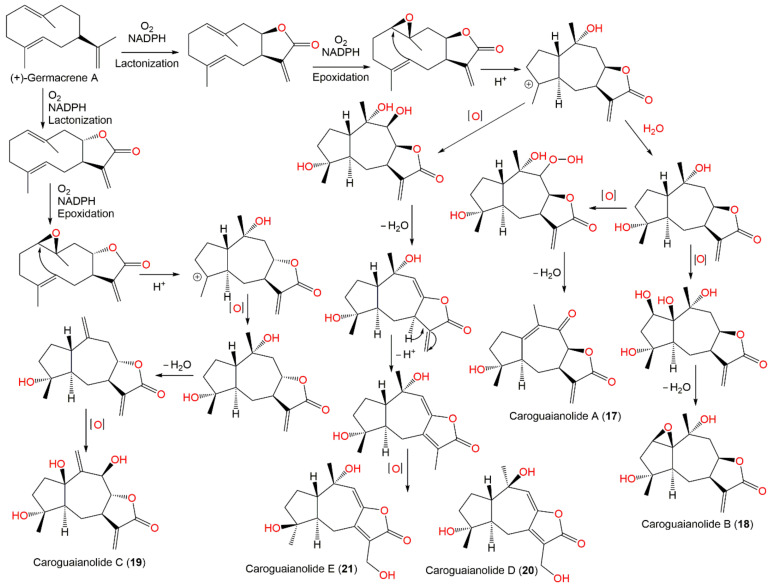
In the MTT assay, 17–19, 22, 23, 31, and 32 demonstrated marked cytotoxic potential (IC50 ranged from 2.67 to 12.34 μM) versus HGC-27 and MDA-MB-231 cell lines, compared to mitomycin C (IC50 6.68 and 4.56, respectively). It was found that 32, 17–19, 22, 23, and 25 with α-methylene γ-lactone moiety had strong effectiveness; however, 20 and 21 without this moiety displayed weak activity, indicating that α-methylene γ-lactone moiety was substantial for guaiane sesquiterpenes’ cytotoxic effectiveness [51]. These metabolites were postulated to be derived from (+)-germacrene A as illustrated in Scheme 2.
Scheme 2.
Biosynthetic pathway of caroguaianolides A–E (17–21) [51].
In 2018, Hu et al. characterized new sesquiterpene lactones, 46, 58, and 59, along with 13, 31, 37, 55, and 56 from the whole plant EtOAc fraction (Figure 6). Compound 46 was similar to 37 except for the existence of a C-12 methyl group instead of the exocyclic methylene in 37. On the other side, 58 and 59 were analogs of 56 with a C-4 palmitoyloxy and linoleoyl group, respectively, instead of C-4 OH in 56. Only 58 possessed cytotoxic influence versus the HL60 cells (IC50 45.85 μM) in the MTS method [53].
Figure 6.
Pseudo-guaianolide sesquiterpenes (28–32) reported from C. abrotanoides.
Telekin (35), 2α,5α-dihydroxy-11αH-eudesma-4(15)-en-12,8β-olide (41), and oxoeudesm-11(13)-eno-12,8α-lactone (45) (Figure 7) demonstrated cytotoxic potential versus HepG-2, where 35 and 45 (IC50s 2.95 and 4.15 μM, respectively) with γ-lactone-α-methylene moiety had more potent effects than 41 (IC50 9.83 μM). These metabolites noticeably reduced the STAT3 and JAK2 mRNA expression as well as prohibited the p-STAT3 and p-JAK2 expression in the HepG-2 cells. Hence, their antiproliferative potential versus HepG-2 was due to STAT3/JAK2 signaling pathway suppression [16].
Figure 7.
Eudesmanolide sesquiterpenes (33–46) reported from C. abrotanoides.
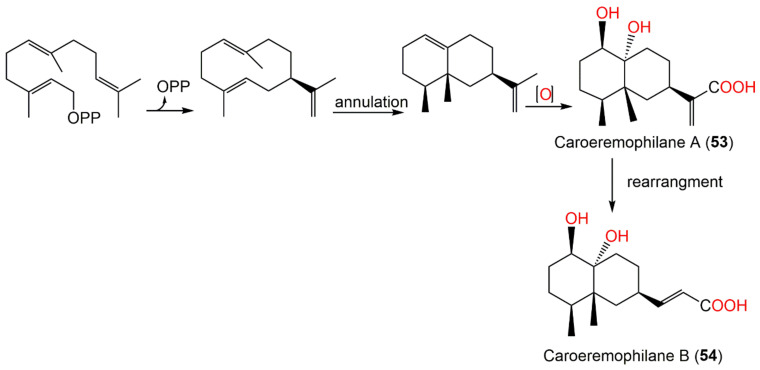
In 2019, Wang et al. reported the separation of new eremophilane sesquiterpenoids, carperemophilanes A (53) and B (54), along with 56 from the EtOAc fraction of the whole plant 95% EtOH extract [60]
Compounds 53 and 54 had 1R/4S/5R/7S/10R configuration. These metabolites displayed cytotoxic capacities (IC50 ranging from 7.45 to 37.35 μM) versus MDA-MB-231 and HGC-27 in the MTT method, whereas 56 displayed potent effectiveness (IC50s 7.45 and 10.27 μM, respectively). Biosynthetically, 53 and 54 are assumed to be produced from farnesylpyrophosphate (FPP) through the mevalonate pathway. Caroeremophilane A (53) is generated from FPP by annulation and a series of oxidation; however, a rare rearrangement and turning of the isopropyl group into n-propyl result in the formation of caroeremophilane B (54) (Scheme 3) [60].
Scheme 3.
Biosynthetic pathway of carperemophilanes A and B (53 and 54) [60].
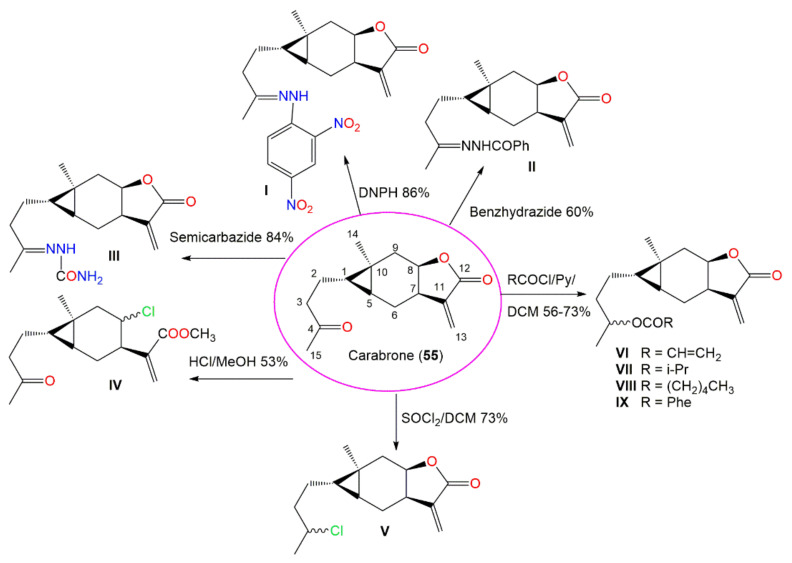
Carabrone (55), a sesquiterpene lactone with cyclopropane moiety possessed noticeable in vitro antifungal potential (EC50 7.1 μg/mL) versus Colletotrichum lagenarium Ell. et. Halst compared with chlorothalonil (EC50 0.75 μg/mL) in the spore germination method. It was found that C-4 carbonyl group and Δ11,13 double bond are the active sites as evident by the synthesis of new (I–IX) derivatives of 55 (Scheme 4). It was demonstrated that the γ-lactone was substantial for the effectiveness and introduction of hydrazone substituents at C-4, resulting in more potent derivatives [69].
Scheme 4.
Synthetic pathway of carabrone derivatives I–IX from carabrone (55) [69].
Two new sesquiterpenes, named carpabrotalactones A (52) and B (43), together with 26, 27, 41, 42, 48, and 50 were purified from the EtOAc fraction of the herb’s 70% EtOH extract [54]. Compounds 43 and 52 had 2R/5R/7S/8R/10R and 1R/4R/5R/7R/8S/10R/11S configuration based on X-ray analysis (Figure 8). These sesquiterpenes possessed cytotoxic potential (IC50 ranged from 7.8 to 47.4 μM) versus ISK, A549, Sw620, HeLa, Caco-2, and RBE cell lines in the CCK-8 assay. Moreover, 26, 27, 43, 48, and 50 displayed notable anti-H1N1 effectiveness (IC50 ranged from 0.4 to 16.4 μM), whereas 26 and 27 revealed the potent effect compared to oseltamivir (IC50 0.05 μM), suggesting their potential as anti-H1N1 agents [54].
Figure 8.
Germacranolide (47–52) and eremophilanoide (53 and 54) sesquiterpenes reported from C. abrotanoides.
Carpabrotalactone C (44) revealed two alicyclic ring-containing sesquiterpene lactones and had 3R/5S/7R/8R/10R/11S configuration. It exhibited cytotoxic potential versus MCF-7, A549, SW480, and SMMC-7721 cell lines (IC50s 11.46–23.68 μM) in the CCK-8 assay compared to cisplatin (IC50s 5.1–12.77 μM). Meantime, it had weak inhibition potential (IC50 63.23 μM) on NO release induced by LPS in RAW 264.7 cells relative to dexamethasone (IC50 3.61 μM) [58].
Yang et al. separated 10, 29–31, 34–40, 42, 48, and 56 from the PE and EtOAc fractions of the whole plant’s 95% MeOH extract (Figure 9) that were assessed for cytotoxic potential towards A549, MDA-MB-231, HepG2, CNE2, HCT116, and NCM460 in the MTT assay. Compound 38 (IC50 2.73–7.21 μM) demonstrated the powerful effectiveness versus all cell lines. It was found to effectively induce G2/M cell cycle arrest and ROS accumulation, leading to cancer cell apoptosis. Interestingly, 35 and 38 activated protective autophagy and induced lysosomal biogenesis (173.2% and 163.7%, respectively) [52].
Figure 9.
Sesquiterpenes with cyclopropane ring (55–58) reported from C. abrotanoides.
Additionally, sesquiterpenes dimers are reported from C. abrotanoides. Biosynthetically, they possess uncommon carbon skeletons that are derived via the coupling of two monomeric sesquiterpene moieties. These compounds have attracted remarkable research interest for their diversified and peculiar structural features with complicated linking patterns and multiple chiral centers.
A new dimeric sesquiterpene, dicarabrol (60), in addition to 31, 48, and 56 were purified from C. abrotanoides whole plant EtOAc fraction (Figure 10). Compound 60 was 2R,3R,4R,6R,7S,10S,11R,12R,14R,15R,20S,23S,28S-configured and featured two sesquiterpene lactone units connecting through a cyclopentane ring. These metabolites revealed potent in vitro cytotoxic capacity versus the K562, MCF-7, Hela, DU145, U937, H1975, SGC-7901, A549, MOLT4, and HL60 cell lines (IC50 ranged from 0.10 to 46.7 μM), compared with taxol (IC50s 2.1–9.8 μM), whereas 60 had powerful effectiveness than taxol versus all cell lines. Moreover, compounds 31, 48, and 60 had antiviral potential versus H1N1 (IC50s 10.8–45.5 μM) and H3N2 (IC50s 11.6–47.3 μM) compared to osehamivir (IC50s 0.025 and 0.015 μM, respectively) in the CPE inhibition assay and marked antimycobacterial capacity (MICs 3.7, 6.0, and 7.6 μM, respectively) versus M. tuberculosis, relative to INH (isoniazid, MIC 2.0 μM) in the GFPMA (green fluorescent protein microplate assay. All compounds possessed no COX-1 and COX-2 inhibition [56].
Figure 10.
Dimeric sesquiterpenes (60–65) reported from C. abrotanoides.
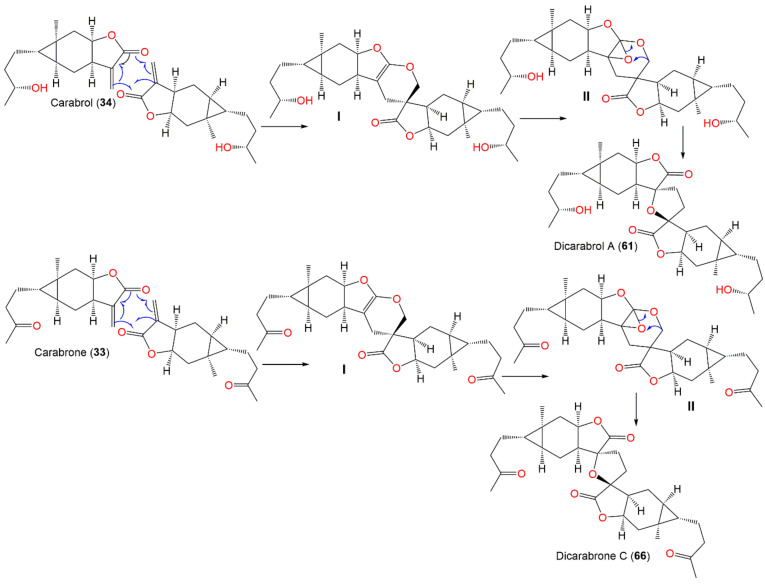
Jie-Wei et al. reported the separation of two new dimeric sesquiterpenes of carabrane class, dicarabrols B (62) and C (63), and a new carabrane sesquiterpene, 11R-hydroxycarabrol (57) (Figure 8) [62]. Compound 62 has a C30 novel dimeric skeleton, possessing a methylene-bridged two sesquiterpene moieties, whereas 63 displayed unique cyclopentane linkage. Compound 63 revealed selective cytotoxic effectiveness versus HL-60 (IC50 3.7 μM) and had a weak influence on A549 cells (IC50 > 20 μM), while 62 and 57 were inactive, suggesting that γ-lactone-α-methylene moiety had a role in activity [62].
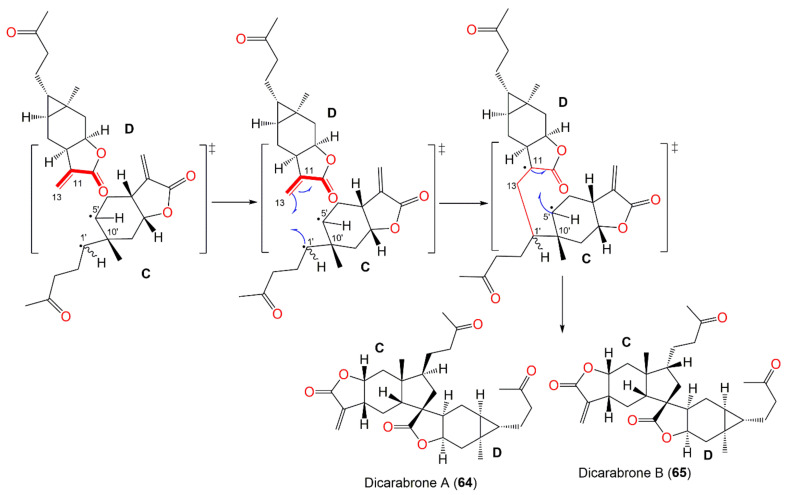
Wu et al. characterized a pair of dimeric sesquiterpene epimers, dicarabrones A and B (64 and 65), from the whole plant CHCl3 fraction. Their skeletons are characterized by two sesquiterpene-lactone moieties, connected via a cyclopentane ring. They are epimers at C-1′ with 1S,5S,7R,8R,10R,11R,1′S,5′R,7′R,8′R,10′R and 1S,5S,7R,8R,10R,11R,1′R,5′R,7′R,8′R,10′R configurations, respectively, relying on ROESY and X-ray results. Compounds 64 and 65 (IC50s 9.1 and 8.2 μM, respectively) displayed selective cytotoxic potential versus HL-60 cells and weak effectiveness (IC50 > 10 μM) versus A549 cells in the MTT and SRB assays, respectively [64].
It was postulated that the cyclopentane unit in 64 and 65 could be generated from a [3+2] cyclo-addition reaction beginning from a three-membered ring in one molecule and a double bond in the other (Scheme 5) through radical intermediates. The cleavage of the bond between C-5′ and C-1′ forms a radical-intermediate C having C-5′ already radical that is then trapped with the double bond in D. Due to steric hindrance in the structure, C-1′ firstly forms a new bond with C-13, and then C-5′ forms another bond with C-11. Hence, a five-membered ring fuses the two monomers C and D. Additionally, the free rotation of the side chain around C-1′ resulted in two possible configurations at C-1′ [64].
Scheme 5.
Biosynthetic pathway of dicarabrones A (64) and B (65) [64].
The investigation of the whole plant’s 95% EtOH extract afforded rare dimeric sesquiterpenes, dicarabrol A (61), dicarabrone C (66), and dipulchellin A (74). Compounds 61 and 66 featured an unwonted skeleton with two linked carabranolide moieties via a spiro-tetrahydrofuran ring that was assumed to be generated by a [4+2] cycloaddition; however, 74 is a product of [3+2] cycloaddition of a carabranolide moiety and guaianolide moiety connecting via a cyclopentane ring. Their configurations were established as 1S/5S/7S/8R/10R/11S/1′S/5′S/7′R/8′R/10′R/11′R for 61 and 66 and 1S/4S/5S/7R/8S/10R/11S/1′R/4′S/5′R/7′R/8′R/10′R for 74. These metabolites revealed selective cytotoxic potential versus HL-60 cells (IC50s 8.7, 8.2, and 8.9 μM, respectively), while they had no activity versus A549 cells (IC50 > 10 μM) in the MTT assay [63]. Biosynthetically, it was proposed that [4+2] Diels–Alder cycloaddition is the main step for 61 and 66 biosyntheses (Scheme 6). Carabrone (55) and carabrol (56) could be the parent compounds for their biosynthesis. The double bond (C11′=C13′) of one carabrone/carabrol undergoes [4+2] Diels–Alder cycloaddition with the α,β-unsaturated carbonyl (C13=C11–C12=O) of another carabrone (55)/carabrol (56) to yield an intermediate I with a dihydropyran ring. Then, the enzymatic oxygenation of the dihydropyran double bond produces an oxirane ring in II. Lastly, two C–O bonds displace each other to form the tetrahydrofuran ring in dicarabrone C (66)/dicarabrol A (61) [63].
Scheme 6.
Biosynthetic pathways of dicarabrol A (61) and dicarabrone C (66) [63].
In 2020, Yang et al. purified five uncommon C15/C17 dimeric sesquiterpenes, carabrodilactones A–E (67–71), along with formerly reported C15/C15 dimeric analogs, 72, 73, 75, and 76, from the EtOAc fraction of 70% EtOH of the whole plant (Figure 11 and Figure 12). Compounds 67–71 displayed tailed acetyl linked to the C-13 position and flexible C-13′/C-11-linked single bond among the two sesquiterpene moieties [65]. They were evaluated for their cytotoxic potential versus MDA-MB, A549, BEL7404, and HCT116 cells using the MTT assay. Among them, 67 had potent effectiveness versus these cell lines (IC50 ranged from 3.08–8.05 μM); however, the others possessed weak potential (IC50 27.70–101.34 μM). It was demonstrated that the α-ethylene-γ-lactone group’s exo-ethylene, C-4 carbonyl of carabrone unit, and two sesquiterpene units’ spatial orientation were crucial for the activity. Moreover, 75 and 76 were found to have cytotoxic potential versus K562, CCRF-CEM, HCT116, and HL-60 cells (IC50 ranged from 2.63 to 8.50 µM) in the MTT assay [70].
Figure 11.
Dimeric sesquiterpenes (66–69) reported from C. abrotanoides.
Figure 12.
Structures of dimeric sesquiterpenes (70–76) reported from C. abrotanoides.
Biosynthetically, these C15/C17 metabolites were assumed to be generated through Stetter and Michael’s addition reactions. Firstly, the acetaldehyde anion is formed by umpolung of pyruvic acid through catalysis by thiamine. After that, it attacks the first C15 sesquiterpene lactone exoethylene to generate the C17-enolate anion at C-11 by Stetter reaction. Thenceforward, the Michael addition reaction of enolate with the α,β-unsaturated bond of the other lactone moiety yields four possible C17/C15 stereo-isomeric dimers with a C-13′/C-11 C-C bond among the two sesquiterpene moieties [65] (Scheme 7).
Scheme 7.
Biosynthetic pathway of carabrodilactones A–E (67–71) [65].
It was proposed that Dipulchellin A (74) be biosynthesized from [3+2] cycloaddition as reported in 64 and 65 C-5′-C4′ bond. The cyclopentane ring possibly originated from a [3+2] cycloaddition of a cyclopropane ring of carabrone (55) monomer and the double bond of 2-desoxy-4-epi-pulchellin (31) (Scheme 8) via radical intermediates. The C-5′- C-1′ bond is cleaved to form a radical intermediate, and the C-5′ radical is then trapped with the double bond. Due to steric hindrance in the structure, C-1′ forms a new bond with C-13 first, and subsequently C-5′ forms another bond with C-11. Thus, a cyclopentane ring is introduced to link the two monomers [63].
Scheme 8.
Biosynthetic pathways of dipulchellin A (74) [63].
5.3. Other Metabolites and Volatile Constituents
Compounds 77–80 were separated from petroleum ether extract of the whole plant and evaluated for their cytotoxicity (Conc. 40 μM) versus HL60, SMMC7721, A549, SW480, and MCF7 cell lines. Only 79 had moderate effectiveness (inhibitory rates 47, 51, and 57%, respectively) versus A549, MCF-7, and SW480 [45].
Methyl 8-[2(5H)-furanon-5-yl]octanoate (81) was 5R-configured with 2(5H)-furanone connecting with the methyl octanoate moiety at C-5. It possessed the same features as ethyl-8-[2(5H)-furanon-5-yl]octanoate formerly separated from Diospyros maritima with less methylene [76]. It had no cytotoxic influence versus SMMC-7721, A549, SW480, and MCF-7, as well as no inhibitory potential on LPS-caused NO release in RAW 264.7 cell lines [58].
Wang et al. characterized carpesiumaleimides A–C (82–84), rare maleimide-bearing metabolites from the whole plant’s EtOAc fraction (Figure 13). Compound 83 was similar to 82 with isoleucine residue instead of a valine residue in 82, while 84 exhibited a leucine residue instead of isoleucine residue in 83. They showed weak activity (IC50 > 50 μM) versus MDA-MB-231 and HGC-27 in the MTT method [60].
Figure 13.
Other metabolites (77–86) reported from C. abrotanoides.
The UPLC-QTOF-MS analysis of the roots’ petroleum ether fraction led to the characterization of 12,13-epoxylinolenic acid (87), (5E,10E)-6,10,14-trimethyl-pentadeca-5,10,13-trience-2,12-dione, γ-linolenic acid (88), α-linolenic acid (89), 5α-hydroxy-7αH,11αH-eudesm-4(15)-en-12,8β-lactone (90), and palmitic acid (91) [17]. In 1989, Kameoka et al. analyzed the essential oil of Japanese C. abrotanoides herb obtained by steam distillation using GCMS, IR, GC, and 1H NMR. The oil had a fatty acid odor and contained β-bisabolene (92) and C2~C16 carboxylic and C18 fatty acids as major components. Additionally, dihydroactinidiolide (93), γ-nonalactone (94), C3~C14 aliphatic alcohols, butylphthalide (95), and aldehydes were identified [67] (Figure 14).
Figure 14.
Structures of some volatile metabolites (87–105) reported from C. abrotanoides.
In another study, GC/MS analysis of the essential oil of the whole plant collected from Hubei province, China, that was prepared by hydro-distillation (yield 0.39% w/w) revealed the existence of sixteen compounds that represented 97.53% of the total oil, including eudesma-5,11(13)-dien-8,12-olide (96) (21.92%), caryophyllene oxide (97, 13.01%), β-bisabolene (92, 7.26%), 3α,4α-4-methylcholesta-8,24-dien-3-ol (98, 6.15%), phytol (99, 5.57%), 2-(E)-decenal (100, 5.56%), heptacosane (101, 4.84%), eicosane (102, 4.66%), tetracosane (103, 4.56%), 4-(1-methylethyl)-benzaldehyde (104, 4.52%), 2,6,10-trimethyl-dodecane (105, 4.05%), torreyol (106, 3.82%), androst-5,7-dien-3-ol-17-one (107, 3.24%), β-cedrene (108, 2.83%), α-curcumene (109), and 2,6,10-trimethyl-tetradecane (3.15%, 110) (2.43%) [15]. On the other side, the fresh aerial parts of C. abrotanoides collected from the Abbottabad, Pakistan, yielded 0.04% essential oil. Its GC/MS analysis displayed 39 compounds, including caryophyllene (111, 24%), trans-nerolidol (112, 2%), geranyl isobutyrate (113, 10.63%), δ-cadinene (114, 8.83%), β-eudesmene (115, 4.67%), caryophyllene oxide (97, 3.89%), trans-lachnophyllum ester (116, 3.87%), α-caryophyllene (117, 3.5%), and neryl 3-methylbutyrate (118, 4.67%) (Figure 15) [32].
Figure 15.
Structures of some volatile metabolites (106–118) reported from C. abrotanoides.
6. Toxicity Studies
Toxicity evaluation is substantial to assess the untoward effects of plants on biological systems. It is noteworthy that systemic toxicity and safety assessments are not extensively performed on this plant. Additionally, C. abrotanoides is formally reported to have slight human toxicity [77]. Thus, there is little evidence of this plant’s toxicity. In Chinese pharmacopeia, this plant was recorded as a mild toxic medicine [14]. C. abrotanoides is safe for health and its commercial preparations are commonly used for years [10]. It was reported that carpesia lactone is the main poisonous ingredient that has irritant properties and a bitter taste [78]. Its ingredients could produce certain symptoms [78] such as asdizziness, tinnitus, nausea, diarrhea, abdominal pain, colonic spasm, and weakness [79]. In Western medicine, the reported methods of its poisoning treatment include promoting vomiting, stomach washing out, grape sugar supplies, and symptomatic treatment. In Korean medicine, the herbs such as Glycyrrhizae Radix and Phaseoli Radiati Semen are used to counteract its poison [78]. Furthermore, Shi et al. reported a 96 h acute toxicity (LC50 2.60 g/L) of C. abrotanoides extracts in goldfish [80]. The fruit extract also had a 24 h LC50 0.76 g/L versus Penaeus monodon [81]. However, a study by Xia et al. proved that the fruit (Conc. 140 to 230 mg/L) had a developmental toxic influence on zebrafish larvae/embryos. It noticeably altered the embryos’ hatching rate and time and raised malformations, where the heart was the target organ. Moreover, it reduced caspase-3 potential, changed the defense enzymes (CAT, SOD, and GPX), and elevated MDA content, indicating that apoptosis and oxidative stress might be accountable for its toxic effects [82]. In clinical practice, Carpesii Fructus is mainly used as antiparasitic drugs and is usually combined with other drugs since it displayed poor efficacy as a single drug [78]. Therefore, this plant is contraindicated during pregnancy, not suitable for long time usage, and should be taken with caution for debilitated patients [79]. However, further safety studies must be carried out on C. abrotanoides. Future investigations are required to explore the mechanism of toxicity and to analyze and specify the extract components relevant to toxicity.
7. Conclusions
In this review, the traditional uses, phytoconstituents, and pharmacological activities of C. abrotanoides have been highlighted. The current work revealed that 118 metabolites have been purified from different parts of the plant, among them monoterpenes and sesquiterpenes are the main constituents. It is obvious that more research studies have been carried out on the aerial parts; therefore, the other plant parts should be further investigated.
It was found that the synthesis and structure modifications of certain metabolites produced more bioactive derivatives as in 13 and 33. Hence, these constituents could be prominent candidates for developing effective and novel pharmaceutical agents. Additionally, this research direction should be further investigated by medicinal and organic chemists. These metabolites and plant extracts have been assessed for various bioactivities. Some metabolites demonstrated potent cytotoxic capacities (e.g., 11, 13, 19, 23, 24, 47, 53, 57, and 60) similar or more than positive controls. Furthermore, the main reported mechanisms of their cytotoxic capacity included the induction of autophagy and apoptosis. These studies suggested that the plant and its sesquiterpenes could serve as promising chemotherapeutic agents for various cancer types. Additionally, compound 31 exhibited marked antiH1N1 potential. However, future in vitro and in vivo studies for chemo-preventive effects are essential to confirm this efficacy and to identify the compounds responsible for this chemo-preventive potential. Moreover, studies on the structure–activity relationships and/or derivatization of these metabolites should be carried out.
The anti-inflammatory potential of the plant/metabolites could support its usage as an alternative medicine for relieving and retarding inflammatory responses. Based on its powerful antioxidant and α-glucosidase inhibitory capacities, the plant could have remarkable potential for diabetes treatment. However, further studies for separating the active phytoconstituents accountable for these activities along with in vivo experiments and mechanistic studies are needed. Most of the reported activities of the plants have been attributed to its sesquiterpene contents. C. abrotanoides extracts and some metabolites have been established to display insecticidal capacity versus various pest species verifying their potential use in developing botanical insecticides. Comparing the results of biological studies with the plant traditional uses, it is obvious that only a few of the traditional uses have been scientifically verified. Moreover, the study of bioactivities of aqueous extracts is substantially needed for confirming the data of traditional use of decoctions and infusions. Some of the reported constituents are either not tested or possessed no notable effectiveness in the evaluated activities; therefore, an in silico screening for other potential bioactivities could be a clearly goal for future research studies. Most of the natural metabolites are frequently produced by the plants in small amounts; therefore, securing sufficient quantities of them for further clinical applications has become a persisting concern [83]. The technological progress, including genome sequencing and development of molecular biology and genetic techniques engineer the biosynthesis of many valuable natural metabolites [84]. In this review, highlighting the biosynthesis of these metabolites could draw the attention of molecular biologist- and genetic-interested researchers for isolating the genes accountable for the biosynthesis of these interesting metabolites and discovering the detailed mechanisms of their formation by various enzymes. This could allow the preparation of these metabolites and their analogs by engineering their biosynthetic pathways. Although the reported studies of C. abrotanoides revealed great promise, most of its activities have only been assessed in vitro. Accordingly, it is of the uttermost importance that the investigation of C. abrotanoides extracts and its constituents’ safety and efficacy testing should be carried out in in vivo studies. Lastly, clinical trials must be implemented to prove if the prominent activities of C. abrotanoides can result in clinical usability in an effective and safe manner and to completely certify its renowned use in the traditional medicines of various countries.
Abbreviations
| ABTS | 2,2′-Azinobis-3-ethylbenzothiazoline-6-sulfonic acid |
| C452 | Cysteine 452 |
| CAT | Catalase |
| CH2Cl2 | Dichloromethane |
| CHCl3 | Chloroform |
| COX-2 | Cyclooxygenase |
| DEET | N,N-Diethyl-meta-toluamide |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| DTT | Dithiothreitol |
| ECD | Electronic circular dichroism |
| EtOH | Ethanol |
| EtOAc | Ethyl acetate |
| FDI | Feeding-deterrence index |
| FIC | Ferrous ion chelating |
| GC-MS | Gas chromatography–mass spectrometry |
| GLUT1 | Glucose transporter-1 |
| Gpx | Glutathione peroxidase |
| GSH | Glutathione |
| HIF-1α | Hypoxia-inducible factor-1α |
| HINAE | Hirame natural embryo |
| HK2 | Hexokinase-2 |
| HPLC-ESI-Q-TOF-MS/MS | Electrospray ionization-quadrupole time-of-flight mass spectrometry |
| HSCCC | Highspeed counter-current chromatography |
| IC50 | Half-maximal inhibitory concentration |
| IL-6 | Interleukin-6 |
| IL-4 | Interleukin-4 |
| IL-13 | Interleukin-13 |
| iNOS | Inducible nitric oxide synthase |
| IR | Infrared |
| LD50 | Half maximal lethal concentration |
| LDHA | Lactate dehydrogenase A |
| MDA | Malondialdehyde |
| MeOH | Methanol |
| MyD-88 | Myeloid-differential-factor-88 |
| NMR | Nuclear magnetic resonance |
| NOS | Nitric oxide scavenging |
| NQO1 | NAD(P)H:quinone oxidoreductase |
| Nrf2 | NF-E2-related factor 2 |
| PARP | Poly(ADPribose) polymerase |
| PE | Petroleum ether |
| PGE2 | Prostaglandin E2 |
| PKM2 | Pyruvate kinase M2 |
| RP-18 | Reversed phase-18 |
| SiO2 CC | Silica gel column chromatography |
| SOD | Superoxide dismutase |
| TDDFT-ECD | Time-dependent density-functional-theory electronic circular dichroism |
| TLC | Thin layer chromatography |
| TLRs | Toll-like receptors |
| TRIF | Toll-interleukin-1 receptor domain-containing adapter inducing interferon-β |
Author Contributions
Conceptualization, S.R.M.I., H.M.A. and G.A.M.; resources, S.A.F., H.A.F. and R.H.H.; writing—original draft preparation, S.R.M.I., H.M.A. and G.A.M.; writing—review and editing, S.R.M.I. and G.A.M.; funding acquisition, S.A.F., H.A.F. and R.H.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chaachouay N., Douira A., Zidane L. Herbal medicine used in the treatment of human diseases in the rif, Northern Morocco. Arab. J. Sci. Eng. 2022;47:131–153. doi: 10.1007/s13369-021-05501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosseini S.H., Sadeghi Z., Hosseini S.V., Bussmann R.W. Ethnopharmacological study of medicinal plants in Sarvabad, Kurdistan, Iran. J. Ethnopharmacol. 2022;288:114985. doi: 10.1016/j.jep.2022.114985. [DOI] [PubMed] [Google Scholar]
- 3.Jahangir M.A., Muheem A., Imam S.S., Gilani S.J., Zafar A., Alshehri S., Jafar M. Edible Plants in Health and Diseases. Springer; Berlin/Heidelberg, Germany: 2022. High altitude edible plants: A great resource for human health and their socio-economic significance; pp. 161–180. [Google Scholar]
- 4.Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibrahim S.R.M., Bagalagel A.A., Diri R.M., Noor A.O., Bakhsh H.T., Mohamed G.A. Phytoconstituents and pharmacological activities of Indian Camphorweed (Pluchea Indica): A multi-potential medicinal plant of nutritional and ethnomedicinal importance. Molecules. 2022;27:2383. doi: 10.3390/molecules27082383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdallah H.M., Mohamed G.A., Ibrahim S.R.M. Lansium domesticum—A fruit with multi-benefits: Traditional uses, phytochemicals, nutritional value, and bioactivities. Nutrients. 2022;14:1531. doi: 10.3390/nu14071531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibrahim S.R.M., Omar A.M., Bagalagel A.A., Diri R.M., Noor A.O., Almasri D.M., Mohamed S.G.A., Mohamed G.A. Thiophenes—naturally occurring plant metabolites: Biological activities and in silico evaluation of their potential as cathepsin D inhibitors. Plants. 2022;11:539. doi: 10.3390/plants11040539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Y., Lu Y. Noteworthy plants in Asteraceae from China. J. Zhejiang Univ. 2019;46:209–214. [Google Scholar]
- 9.Zhang J.P., Wang G.W., Tian X.H., Yang Y.X., Liu Q.X., Chen L.P., Li H.L., Zhang W.D. The genus Carpesium: A review of its ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2015;163:173–191. doi: 10.1016/j.jep.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Abid R., Zehra N. Micromorphology of cypsela and its taxonomic significance of some genera in the tribe Inuleae (Asteraceae) from Pakistan. Pak. J. Bot. 2007;39:1407–1416. [Google Scholar]
- 11.Ye H., Li C., Ye W., Zeng F., Liu F., Liu Y., Wang F., Ye Y., Fu L., Li J. Common Chinese Materia Medica. Springer; Berlin/Heidelberg, Germany: 2022. Medicinal angiosperms of Compositae (cont. I) pp. 287–362. [Google Scholar]
- 12.The Plant List Retrived 10 March 2022. [(accessed on 3 March 2022)]. Available online: http://www.theplantlist.org/
- 13.Wang F., Yang K., Ren F., Liu J. Sesquiterpene lactones from Carpesium abrotanoides. Fitoterapia. 2009;80:21–24. doi: 10.1016/j.fitote.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Editorial Board of Chinese Materia Medica . Chinese Materia Medica. Shanghai Science & Technology Press; Shanghai, China: 1999. pp. 756–763. [Google Scholar]
- 15.Wang Q., Pan L., Lin L., Zhang R., Du Y., Chen H., Huang M., Guo K., Yang X. Essential oil from Carpesium abrotanoides L. induces apoptosis via activating mitochondrial pathway in hepatocellular carcinoma cells. Curr. Med. Sci. 2018;38:1045–1053. doi: 10.1007/s11596-018-1982-2. [DOI] [PubMed] [Google Scholar]
- 16.Shen B., Yang Y., Wan D., Yu H., Cai Y., Wang X., Tang D., Tang C., Zhang S. Sesquiterpene lactones isolated from Carpesium abrotanoides L. by LC–MS combined with HSCCC inhibit liver cancer through suppression of the JAK2/STAT3 signaling pathway. Med. Chem. Res. 2022;31:436–445. doi: 10.1007/s00044-021-02838-y. [DOI] [Google Scholar]
- 17.Chai X.X., Le Y.F., Wang J.C., Mei C.X., Feng J.F., Zhao H., Wang C., Lu D.Z. Carpesium abrotanoides (L.) root as a potential source of natural anticancer compounds: Targeting glucose metabolism and PKM2/HIF-1α axis of breast cancer cells. J. Food Sci. 2019;84:3825–3832. doi: 10.1111/1750-3841.14953. [DOI] [PubMed] [Google Scholar]
- 18.Thakur S., Tashi N., Singh B., Dutt H.C. Ethnobotanical plants used for gastrointestinal ailments by the inhabitants of Kishtwar plateau in Northwestern Himalaya. Indian J. Tradit. Knowl. 2020;19:288–298. [Google Scholar]
- 19.Lee S.B., Kang K., Lee H.J., Yun J.H., Jho E.H., Kim C.Y., Nho C.W. The chemopreventive effects of carpesium abrotanoides are mediated by induction of phase II detoxification enzymes and apoptosis in human colorectal cancer cells. J. Med. Food. 2010;13:39–46. doi: 10.1089/jmf.2009.1157. [DOI] [PubMed] [Google Scholar]
- 20.Lee J.H., Hwang K.H., Kim G.H. In vitro evaluation of anti-inflammatory activity for salad-food material Carpesium abrotanoides. J. Food Biochem. 2013;37:18–25. doi: 10.1111/j.1745-4514.2011.00591.x. [DOI] [Google Scholar]
- 21.Guochao J., Wy M., Tshingh T.W. Oral Traditional Chinese Medicine for Treating Intestinal Ascariasis. CN 200610172207. 2006 December 25;
- 22.Bae K.H., Lee J.S., Min B.S. Screening on the cytotoxicity of medicinal plants against L1210 and HL60 cancer Cells. Korean J. Pharmacogn. 1996;27:173–177. [Google Scholar]
- 23.Wang C., Jiang J., Ji J., Cai Q., Chen X., Yu Y., Zhu Z., Zhang J. PKM2 promotes cell migration and inhibits autophagy by mediating PI3K/AKT activation and contributes to the malignant development of gastric cancer. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-03031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo S.J., Jeong M.G., Jeon E.J., Do M.Y., Kim N.Y. Antiparasitic potential of ethanolic extracts of carpesii fructus against Miamiensis avidus in Hirame natural embryo cell line and their effects on immune response-and biotransformation-related genes. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2022;251:109214. doi: 10.1016/j.cbpc.2021.109214. [DOI] [PubMed] [Google Scholar]
- 25.Moustafa E.M.M., Naota M., Morita T., Tange N., Shimada A. Pathological study on the scuticociliatosis affecting farmed Japanese flounder (Paralichthys olivaceus) in Japan. J. Vet. Med. Sci. 2010;72:1359–1362. doi: 10.1292/jvms.10-0026. [DOI] [PubMed] [Google Scholar]
- 26.Jung S.H., Sim D.S., Park M.S., Jo Q., Kim Y. Effects of formalin on haematological and blood chemistry in olive flounder, Paralichthys olivaceus (Temminck et Schlegel) Aquacult. Res. 2003;34:1269–1275. doi: 10.1046/j.1365-2109.2003.00936.x. [DOI] [Google Scholar]
- 27.Liu X.Y., Guo G.W., Wang H. KillingeEffect of Carpesium abrotanoides on Taenia asiatica cysticercus. Chin. J. Parasitol. Parasit. Dis. 2015;33:18. [PubMed] [Google Scholar]
- 28.Ahmad M., Farid A., Saeed M. Resistance to new insecticides and their synergism in Spodoptera exigua (Lepidoptera: Noctuidae) from Pakistan. Crop. Prot. 2018;107:79–86. doi: 10.1016/j.cropro.2017.12.028. [DOI] [Google Scholar]
- 29.De Castro A.A., Legaspi J.C., Tavares W.D.S., Meagher R.L., Jr., Miller N., Kanga L., Haseeb M., Serrao J.E., Wilcken C.F., Zanuncio J.C. Lethal and behavioral effects of synthetic and organic insecticides on Spodoptera exigua and its Predator Podisus maculiventris. PLoS ONE. 2018;13:e0206789. doi: 10.1371/journal.pone.0206789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng X., Jiang H., Zhang Y., He W., Zhang L. Insecticidal activities of ethanol extracts from thirty Chinese medicinal plants against Spodoptera Exigua (Lepidoptera: Noctuidae) J. Med. Plants Res. 2012;6:1263–1267. [Google Scholar]
- 31.Jansen C.C., Beebe N.W. The dengue vector Aedes aegypti: What comes next. Microb. Infect. 2010;12:272–279. doi: 10.1016/j.micinf.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Haris A., Azeem M., Binyameen M. Mosquito repellent potential of Carpesium abrotanoides essential oil and its main components against a Dengue vector, Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 2022;59:801–809. doi: 10.1093/jme/tjac009. [DOI] [PubMed] [Google Scholar]
- 33.Mazzarri M.B., Georghiou G.P. Characterization of resistance to organophosphate, carbamate, and pyrethroid insecticides in field populations of Aedes aegypti from Venezuela. J. Am. Mosq. Control Assoc. 1995;11:315–322. [PubMed] [Google Scholar]
- 34.Arslan A., Rathor H.R., Mukhtar M.U., Mushtaq S., Bhatti A., Asif M., Arshad I., Ahmad J.F. Spatial Distribution and insecticide susceptibility status of Aedes aegypti and Aedes albopictus in dengue affected Urban areas of Rawalpindi, Pakistan. J. Vector Borne Dis. 2016;53:136. [PubMed] [Google Scholar]
- 35.World Health Organization Diabetes 2021. Retrived 15 March 2022. [(accessed on 3 March 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes.
- 36.Ibrahim S.R.M., Mohamed G.A., Zayed M.F., Ross S.A. 8-Hydroxyirilone 5-methyl ether and 8-hydroxyirilone, new antioxidant and α-amylase inhibitors isoflavonoids from Iris germanica rhizomes. Bioorg. Chem. 2017;70:192–198. doi: 10.1016/j.bioorg.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Ibrahim S.R.M., Mohamed G.A., Elfaky M.A., Al Haidari R.A., Zayed M.F., El-Kholy A.A., Khedr A.I.M. Garcixanthone A, a new cytotoxic xanthone from the pericarps of Garcinia mangostana. J. Asian Nat. Prod. Res. 2019;21:291–297. doi: 10.1080/10286020.2017.1423058. [DOI] [PubMed] [Google Scholar]
- 38.Alhakamy N.A., Mohamed G.A., Fahmy U.A., Eid B.G., Ahmed O.A.A., Al-Rabia M.W., Khedr A.I.M., Nasrullah M.Z., Ibrahim S.R.M. New alpha-amylase inhibitory metabolites from pericarps of Garcinia mangostana. Life. 2022;12:384. doi: 10.3390/life12030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayur B., Sancheti S., Shruti S., Sung-Yum S. Antioxidant and alpha-glucosidase inhibitory properties of Carpesium Abrotanoides L. J. Med. Plants Res. 2010;4:1547–1553. [Google Scholar]
- 40.Kang H.J., Kim H.J., Jeong S.I., Kim H.S., Jeon I.H., Mok J.Y., Shim J.S., Jang S.I. Antioxidant and antihemolytic activities of ethanol extracts of Carpesii Fructus and Farfarae Flos. Korea J. Herbol. 2013;28:25–31. doi: 10.6116/kjh.2013.28.3.25. [DOI] [Google Scholar]
- 41.Jeong D.W., Lee E.K., Lee C.H., Lim S.J., Gu G.J., Paek J.H., Kim S., Lim S.S., Youn H.S. Carpesium Abrotanoides extract inhibits cyclooxygenase-2 Expression Induced by Toll-Like Receptor Agonists. Toxicol. Environ. Health Sci. 2013;5:92–96. doi: 10.1007/s13530-013-0161-x. [DOI] [Google Scholar]
- 42.Lee E.K., Jeong D.W., Lim S.J., Gu G.J., Ahn S.I., Kim J.S., Paek J.H., Kim S., Hong J.S., Lim S.S. Carpesium Abrotanoides extract inhibits inducible nitric oxide synthase expression induced by Toll-like receptor agonists. Food Sci. Biotechnol. 2014;23:1637–1641. doi: 10.1007/s10068-014-0223-0. [DOI] [Google Scholar]
- 43.Ko Y.E., Oh S.R., Song H.H., Ryu H.W., Ly S.Y., Kim J.W. The effect of 4α, 5α-epoxy-10α, 14-dihydro-inuviscolide, a novel immunosuppressant isolated from Carpesium abrotanoides, on the cytokine profile in vitro and in vivo. Int. Immunopharmacol. 2015;25:121–129. doi: 10.1016/j.intimp.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Wu H., Wu H., Wang W., Liu T., Qi M., Feng J., Li X., Liu Y. Insecticidal activity of sesquiterpene lactones and monoterpenoid from the fruits of Carpesium Abrotanoides. Ind. Crop. Prod. 2016;92:77–83. doi: 10.1016/j.indcrop.2016.07.046. [DOI] [Google Scholar]
- 45.Yang B.J., Zeng Z.Q., Song Y., Hao X.J., Li S.L. Terpenes from Carpesium abrotanoides L. and their anti-tumor activity. Nat. Prod. Res. Develop. 2021;33:951. [Google Scholar]
- 46.Kariyone T., Kawano N. Components of Carpesium abrotanoides. I. Yakugaku Zasshi. 1949;69:317–318. doi: 10.1248/yakushi1947.69.6-10_317. [DOI] [Google Scholar]
- 47.Kariyone T., Naito S., Chatani J. Studies on the component of Carpesium abrotanoides. II.: Chemical constitution of Carpesia Lactone.(1) Pharm. Bull. 1954;2:339–341. doi: 10.1248/cpb1953.2.339. [DOI] [PubMed] [Google Scholar]
- 48.Kariyone T., Naito S. Studies on the components of Carpesium abrotanoides. III. Chemical structure of carpesia lactone. (2) Yakugaku Zasshi. 1955;75:39–43. doi: 10.1248/yakushi1947.75.1_39. [DOI] [PubMed] [Google Scholar]
- 49.Naito S. Studies on the components of Carpesium abrotanoides. IV. Chemical structure of carpesia lactone. (3) Yakugaku Zasshi. 1955;75:93–97. doi: 10.1248/yakushi1947.75.1_93. [DOI] [Google Scholar]
- 50.Naito S. Studies on the components of Carpesium abrotanoides. V. Chemical structure of carpesia lactone. (4) Yakugaku Zasshi. 1955;75:325–329. doi: 10.1248/yakushi1947.75.3_325. [DOI] [Google Scholar]
- 51.Wang L., Qin W., Tian L., Zhang X., Lin F., Cheng F., Chen J., Liu C., Guo Z., Proksch P. Caroguaianolide A–E, five new cytotoxic sesquiterpene lactones from Carpesium abrotanoides L. Fitoterapia. 2018;127:349–355. doi: 10.1016/j.fitote.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 52.Yang B., Wang J., Zeng Z., Yang X., Huang A., Hao X., Ding X., Li S. Sesquiterpene lactones from Carpesium abrotanoides L. and their activity in inducing protective autophagy. Nat. Prod. Res. 2021:1–4. doi: 10.1080/14786419.2021.1955881. [DOI] [PubMed] [Google Scholar]
- 53.Hu Q., Wu P., Liu Y., Qi F., Yu C., Zhao Y., Yu Y., Fei D., Zhang Z. Three new sesquiterpene lactones from Carpesium abrotanoides. Phytochem. Lett. 2018;27:154–159. doi: 10.1016/j.phytol.2018.07.010. [DOI] [Google Scholar]
- 54.He Y., Cai L., Qian Q., Yang S., Chen D., Zhao B., Zhong Z., Zhou X. Anti-influenza A (H1N1) viral and cytotoxic sesquiterpenes from Carpesium abrotanoides. Phytochem. Lett. 2020;35:41–45. doi: 10.1016/j.phytol.2019.10.013. [DOI] [Google Scholar]
- 55.Maruyama M., Omura S. Carpesiolin from Carpesium Abrotanoides. Phytochemistry. 1977;16:782–783. doi: 10.1016/S0031-9422(00)89261-4. [DOI] [Google Scholar]
- 56.Wang J., He W., Zhang X., Zhao B., Liu Y., Zhou X. Dicarabrol, a new dimeric sesquiterpene from Carpesium abrotanoides L. Bioorg. Med. Chem. Lett. 2015;25:4082–4084. doi: 10.1016/j.bmcl.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 57.Maruyama M., Shibata F. Stereochemistry of granilin isolated from Carpesium abrotanoides. Phytochemistry. 1975;14:2247–2248. doi: 10.1016/S0031-9422(00)91108-7. [DOI] [Google Scholar]
- 58.Qian Q., Gong L., Yang S., Zhao B., Cai J., Zhang Z., Wang Y., Zhou X. Two new compounds from Carpesium abrotanoides. Phytochem. Lett. 2020;40:5–9. doi: 10.1016/j.phytol.2020.08.012. [DOI] [Google Scholar]
- 59.Maruyama M., Karube A., Sato K. Sesquiterpene lactones from Carpesium abrotanoides. Phytochemistry. 1983;22:2773–2774. doi: 10.1016/S0031-9422(00)97693-3. [DOI] [Google Scholar]
- 60.Wang L., Jin G., Tian L., Ebrahim W., Höfert S., Janiak C., Chen J., Guo Z., Schäberle T.F., Liu Z. New eremophilane-type sesquiterpenes and maleimide-bearing compounds from Carpesium abrotanoides L. Fitoterapia. 2019;138:104294. doi: 10.1016/j.fitote.2019.104294. [DOI] [PubMed] [Google Scholar]
- 61.Minato H., Nosaka S., Horibe I. 1057. Studies on Sesquiterpenoids. Part VIII. The Structure of Carabrone, a New Component of Carpesium abrotanoides, Linn. J. Chem. Soc. 1964. [(accessed on 3 March 2022)]. pp. 5503–5510. Available online: https://pubs.rsc.org/en/content/articlelanding/1964/jr/jr9640005503.
- 62.Jie-Wei W., Chun-Ping T., Sheng Y., Chang-Qiang K.E., Yang Y.E. Three new carabrane sesquiterpenoid derivatives from the whole plant of Carpesium abrotanoides L. Chin. J. Nat. Med. 2021;19:868–873. doi: 10.1016/S1875-5364(21)60091-2. [DOI] [PubMed] [Google Scholar]
- 63.Wu J., Tang C., Ke C., Yao S., Liu H., Lin L., Ye Y. Dicarabrol A, dicarabrone c and dipulchellin a, unique sesquiterpene lactone dimers from Carpesium abrotanoides. RSC Adv. 2017;7:4639–4644. doi: 10.1039/C6RA27626A. [DOI] [Google Scholar]
- 64.Wu J., Tang C., Chen L., Qiao Y., Geng M., Ye Y. Dicarabrones A and B, a pair of new epimers dimerized from sesquiterpene lactones via a [3+2] cycloaddition from Carpesium abrotanoides. Org. Lett. 2015;17:1656–1659. doi: 10.1021/acs.orglett.5b00371. [DOI] [PubMed] [Google Scholar]
- 65.Yang Y., Wang J., Li H., Mo Q., Huang H., Tao M., Luo Q., Liu H. Five new C17/C15 sesquiterpene lactone dimers from Carpesium abrotanoides. Fitoterapia. 2020;145:104630. doi: 10.1016/j.fitote.2020.104630. [DOI] [PubMed] [Google Scholar]
- 66.Naito S. Studies on the components of Carpesium abrotanoides. VI Non-acidic components. Yakugaku Zasshi. 1955;75:355–356. doi: 10.1248/yakushi1947.75.3_355. [DOI] [Google Scholar]
- 67.Kameoka H., Sagara K., Miyazawa M. Components of essential oils of kakushitsu (Daucus carota L. and Carpesium abrotanoides L.) J. Agric. Chem. Soc. JPN. 1989;63:185–188. [Google Scholar]
- 68.Lee J.S., Min B.S., Lee S.M., Na M.K., Kwon B.M., Lee C.O., Kim Y.H., Bae K.H. Cytotoxic sesquiterpene lactones from Carpesium abrotanoides. Planta Med. 2002;68:745–747. doi: 10.1055/s-2002-33789. [DOI] [PubMed] [Google Scholar]
- 69.Feng J., Ma Z., Li J., He J., Xu H., Zhang X. Synthesis and antifungal activity of carabrone derivatives. Molecules. 2010;15:6485–6492. doi: 10.3390/molecules15096485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Y., Gao S., Zu X., Shen Y., Shan L., Li H., Zhang W. Cytotoxic 2, 4-linked sesquiterpene lactone dimers from Carpesium faberi exhibiting NF-κB inhibitory activity. RSC Adv. 2015;5:55285–55289. doi: 10.1039/C5RA09243D. [DOI] [Google Scholar]
- 71.Yang Y., Shan L., Liu Q., Shen Y., Zhang J., Ye J., Xu X., Li H., Zhang W. Carpedilactones A–D, four new isomeric sesquiterpene lactone dimers with potent cytotoxicity from Carpesium faberi. Org. Lett. 2014;16:4216–4219. doi: 10.1021/ol501923k. [DOI] [PubMed] [Google Scholar]
- 72.Huang H., Niu J., Wang F., Hu L., Yu Q. A natural compound derivative P-13 inhibits STAT3 signaling by covalently inhibiting janus kinase 2. Invest New Drugs. 2019;37:452–460. doi: 10.1007/s10637-018-0637-2. [DOI] [PubMed] [Google Scholar]
- 73.Tian L., Cheng F., Wang L., Qin W., Zou K., Chen J. CLE-10 from Carpesium abrotanoides L. suppresses the growth of human breast cancer cells (MDA-MB-231) in vitro by inducing apoptosis and pro-death autophagy via the PI3K/Akt/mTOR signaling pathway. Molecules. 2019;24:1091. doi: 10.3390/molecules24061091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang P., Liu F., Mu W., Wang Q., Li H., Chen C. Life table study of the effects of sublethal concentrations of thiamethoxam on Bradysia Odoriphaga Yang and Zhang. Pestic. Biochem. Physiol. 2014;111:31–37. doi: 10.1016/j.pestbp.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 75.Liu J., Wang W., Xu K. G1san improved detection of pesticide residues in sulfur-containing vegetables based on acetylcholineesterase inhibition effects. Agro Food Ind. Hi-Tech. 2013;24:47–51. [Google Scholar]
- 76.Kuo Y.H., Huang S.L., Chang C.I. A Phenolic and an aliphatic lactone from Diospyros maritima. Phytochemistry. 1998;49:2505–2507. doi: 10.1016/S0031-9422(98)00360-4. [DOI] [Google Scholar]
- 77.Liu X., Wang Q., Song G., Zhang G., Ye Z., Williamson E.M. The classification and application of toxic Chinese materia medica. Phytother. Res. 2014;28:334–347. doi: 10.1002/ptr.5006. [DOI] [PubMed] [Google Scholar]
- 78.Park J.H., Kim J.J., Seo B. A philological study on poisoning and side effects of Carpesii Fructus. J. East West Med. 2014;39:17–22. [Google Scholar]
- 79.Maclean W. Clinical Handbook of Chinese Herbs: Desk Reference, Revised Edition. Singing Dragon; Philadelphia, PA, USA: 2017. [Google Scholar]
- 80.Shi H.W., Wang Y.X., Wu J., Lin M.C. Effects of four chinese medicinal herbs on adenosine deaminase (ADA) in blood-serum of goldfish. Shandong Fish. 2008;25:4–6. [Google Scholar]
- 81.Zhou L., Chen X., Zheng T. Study on the ecological safety of algacides: A comprehensive strategy for their screening. J. Appl. Phycol. 2010;22:803–811. doi: 10.1007/s10811-010-9522-x. [DOI] [Google Scholar]
- 82.Xia Q., Luo J., Mei X., Wang Y., Huang W., Wang J., Yang R., Ma Z., Lin R. A developmental toxicity assay of Carpesii Fructus on zebrafish embryos/larvae. Toxicol. Res. 2017;6:460–467. doi: 10.1039/C7TX00005G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu X., Liu X., Liu T., Wang Y., Ahmed N., Li Z., Jiang H. Synthetic biology of plant natural products: From pathway elucidation to engineered biosynthesis in plant cells. Plant Commun. 2021;2:100229. doi: 10.1016/j.xplc.2021.100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kishimoto S., Tsunematsu Y., Sato M., Watanabe K. Elucidation of biosynthetic pathways of natural products. Chem. Rec. 2017;17:1095–1108. doi: 10.1002/tcr.201700015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.