Abstract
The use of bacteriocins from food-grade lactic acid bacteria to fight against the food-borne pathogen Listeria monocytogenes has been gaining interest. However, the emergence of resistant cells is frequently reported when Listeria is exposed to such antibacterials. A two-dimensional electrophoresis study of whole-cell protein expression of Listeria monocytogenes variants sensitive or resistant to the action of a bacteriocin produced by Carnobacterium divergens V41, divercin V41, is reported in this paper. The resistant variant obtained from the sensitive strain of L. monocytogenes P was also resistant to piscicocins V1 and SF668, but remained sensitive to nisin. Its growth rate was 50% less than the sensitive strain, and the MIC for it was 104 times higher. No reversion of the resistance was observed after 20 successive cultures in the absence of divercin V41. Comparison of the protein patterns by two-dimensional gel electrophoresis analysis showed clear differences. In the resistant variant pattern, at least nine spots had disappeared and eight new ones were observed. One of the newly synthesized proteins was identified as a flagellin of L. monocytogenes. Direct interaction between flagellin and divercin V41 was not evidenced. Intracellular synthesis of flagellin is probably an indirect effect of a modification in transcriptional regulation with widespread effects through a sigma factor. An intense protein, only present in the sensitive strain, was identified as a non-heme iron-binding ferritin displaying strong similarities to Dps proteins. Common modifications in the transcriptional regulation for these two proteins are discussed.
During the past decade, Listeria monocytogenes has been incriminated in numerous food-borne outbreaks and several sporadic episodes of listeric illness (25). The emergence and persistence of L. monocytogenes on a large variety of dairy, ready-to-eat, and processed foods has led to enhanced interest in antimicrobials for its control. In addition to conventional antimicrobials (organic acids, radiation, packaging, etc.), interest in the use of bacteriocins from food-grade lactic acid bacteria (LAB) has increased. Bacteriocins were defined as ribosomally produced precursor polypeptides or proteins that, in their mature (active) form, exert an antibacterial effect against a narrow spectrum of closely related bacteria. Most of the reported bacteriocins are produced by LAB, which are naturally present in a lot of food products or are added for their technological and preserving characteristics (40).
However, in most studies, when Listeria is exposed to such antibacterial activity, emergence of resistant cells is frequently reported (35). The mechanisms underlying the bacteriocin resistance phenomenon are largely unknown. Because bacteriocin acts mainly in the cytoplasmic membrane, potential modifications of bilayer lipid content and quality have been investigated. Resistance to nisin has been correlated with both modified fatty acid and phospholipid composition (27).
Even if differences in protein expression between sensitive target cells and resistant cells are potentially numerous, the roles of proteins in bacteriocin resistance are unclear. In some target cell species, specific membrane-located bacteriocin receptors of a proteic nature have been identified (42). Modifications or the absence of such receptors could lead to resistance. Some killer toxin-resistant mutants of Saccharomyces cerevisiae expressed much smaller amounts of a protein which acts as a docking protein, facilitating toxin binding to the membrane, where it forms lethal ion channels, like bacteriocins do (38). Synthesis of new membrane proteins could interfere with bacteriocin anchorage on the receptor or in the membrane. In Lactococcus lactis subsp. lactis biovar diacetylactis, the nisin resistance gene nsr encodes a putative protein with a molecular mass of 35 kDa. A strongly hydrophobic region supports the prediction that this protein is an integral membrane protein which could decrease bacteriocin activity. Decreased bacteriocin penetration could also appear to result from membrane protein oversynthesis, as observed by Koch et al. (22) in multidrug-resistant mouse and hamster cells. Synthesis of an enzyme able to degrade the bacteriocin is also a potential efficient resistance mechanism. Jarvis (20) described a nisinase which inactivated nisin. Moreover, cell wall proteins could play a crucial role in resistance, as clearly demonstrated by Dielbandhoesing et al. (10) for two cell wall proteins in nisin resistance of yeast cells. Knowledge of the involvement of proteins in bacteriocin resistance, even if studied in gram-positive bacteria, could also highlight the role of outer membrane proteins in gram-negative resistance, which is probably essential, as demonstrated for Omp4 for the bacteriocin 28b resistance phenotype in Escherichia coli (18).
Two-dimensional electrophoresis (2DE) of proteins is currently the highest-resolution analytical technique available for the study of protein expression patterns. This technique has already been used for studying minocycline-susceptible and -resistant Mycobacterium smegmatis (44). Comparative proteome analysis of Mycobacterium tuberculosis virulent and nonvirulent vaccine strains was carried out with the help of 2DE (21). 2DE can be an important resource in identifying proteins involved in bacteriocin resistance. Thus, 2DE is a powerful tool to highlight the biochemical mechanisms governing development of cell resistance and then will help in the design of new efficient molecules or mixing of molecules with different cell targets.
In this paper, we report physiological and metabolic differences between Listeria monocytogenes variants sensitive (wild type) and resistant to the action of divercin V41, a bacteriocin produced by the LAB Carnobacterium divergens V41. Moreover, 2DE was carried out to study differential protein expression in these two characterized variants.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and bacteriocin production.
Experiments were carried out with Listeria monocytogenes P (serotype 4b), a wild-type sensitive strain isolated from vacuum-packed cold-smoked salmon (Escola Superior de Biotechnologia, Porto, Portugal) and its bacteriocin-resistant variant (RV41) obtained as described below. The bacteriocin used for resistance studies was divercin V41, the bacteriocin produced by Carnobacterium divergens V41 (34).
Bacteria were subcultured and cultured overnight aerobically at 37°C in Elliker broth (Biokar, Bauvais, France). Growth was determined by optical density at 550 nm (OD550) measurements and by enumeration on Elliker agar after incubation for 24 h at 37°C.
Divercin V41 was purified as described by Métivier et al. (29).
Divercin MIC determination.
The MIC of divercin V41 was determined after growth on Elliker broth or the simulated cold smoked fish system (SCSFS) (12) at 37°C in microtiter plates containing 100 μl of 1% glucose-supplemented Muller-Hinton broth (Biokar) in each well. The total protein content of the purified bacteriocin stock solution was found to be 700 μg/ml as determined by the bicinchoninic acid assay (Pierce, Rockford, Ill.). The divercin V41 was serially diluted in Mueller-Hinton broth (1:1). Each well received 100 μl of inoculum suspension (105 cells per well), and the microtiter plate was incubated overnight at 37°C. After incubation, yellow wells were positive for growth and red wells were negative. The MIC was arbitrarily defined as the amount of total bacteriocin in the highest dilution which inhibited L. monocytogenes growth.
Isolation of a divercin V41-resistant variant of L. monocytogenes.
After MIC experiments, L. monocytogenes P cells were picked from the well containing 87.5 μg of divercin per ml and grown in divercin-free Elliker medium. One of the resistant variants, Listeria monocytogenes RV41, was isolated on a Palcam plate (selective agar and supplement; Merck, Nogent sur Marne, France), and divercin sensitivity and resistance were verified according to the spotting method described by Pilet et al. (34). The frequency of isolation of resistant variants was determined according to the method of Dykes and Hastings (13), in the presence of divercin V41 at five times the MIC.
Antibiogram of the two L. monocytogenes variants.
To determine if the resistance to divercin confers a particular antibiotic phenotype to the resistant variant, 19 antibiotics (Table 1) acting on different cell targets were tested. The method used was based on the disk technique using Mueller-Hinton medium (3).
TABLE 1.
Antibiogram spectrum of L. monocytogenes sensitive and resistant to divercin V41
| Antibiotic | Target |
L. monocytogenes response to divercin V41a
|
|
|---|---|---|---|
| Sensitive | Resistant | ||
| Penicillin | Peptidoglycan | S | S |
| Amoxicillin | Peptidoglycan | S | S |
| Imipenem | Peptidoglycan | S | S |
| Oxacillin | Peptidoglycan | R | R |
| Ceftriaxone | Peptidoglycan | R | R |
| Cefoxitin | Peptidoglycan | R | R |
| Aztreonam | Peptidoglycan | R | R |
| Vancomycin | Peptidoglycan | S | S |
| Teicoplanin | Peptidoglycan | S | S |
| Fosfomycin | Peptidoglycan | S | S |
| Colistin | Outer membrane of gram-negative isolate | R | R |
| Pefloxacin | Gyrase inhibitor | S | S |
| Rifampin | ARN polymerase inhibitor | S | S |
| Chloramphenicol | Protein synthesis inhibitor | S | S |
| Erythromycin | Protein synthesis inhibitor | S | S |
| Kanamycin | Protein synthesis inhibitor | S | S |
| Gentamicin | Protein synthesis inhibitor | S | S |
| Tetracycline | Protein synthesis inhibitor | S | S |
| Minocycline | Protein synthesis inhibitor | S | S |
R, resistant; S, sensitive.
Serotyping and lysotyping of the two L. monocytogenes variants.
Serotyping of Listeria monocytogenes strains was performed as described previously (39). Phage typing was carried out according to reference 37 with 29 well-characterized phages isolated from lysogenic strains.
Two-dimensional PAGE.
2D polyacrylamide gel electrophoresis (PAGE) experiments were performed essentially according to the method of Gormond and Phan-Thanh (16). When not indicated, chemicals and materials were from Pharmacia-Biotech (Orsay, France).
Sample preparation.
Sensitive Listeria monocytogenes and RV41 cells were grown at 37°C until the middle exponential phase (OD550 = 0.5, which corresponds to 109 CFU/ml) in 10 ml of Elliker broth. Cells were harvested by centrifugation (4,000 × g, at 25°C for 30 min). Pellets were washed three times in 10 ml of physiological water (8.5 g of NaCl per liter) and recovered in 0.2 ml of Tris (2-amino-2-hydroxymethyl-1,3-propanediol) buffer (10 mM, pH 7.2) containing 5 mM Mg2+, 5 μl of a cocktail of protease inhibitors containing leupeptin, pepstatin, and PEFABLOC [4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride; Boehringer, Mannheim, Germany] at 2 mg/ml, 5 μl of deoxyribonuclease and ribonuclease (100 mg/ml; Boehringer), and 20 μl of a solubilization solution containing 20% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]propane sulfonate}, 10% DTT (dithiothreitol) and 20% IPG (immobilized pH gradient) 4/7 buffer. Cells were sonicated (Vibra cell; Bioblock, Illkirch, France) three times for 1 min at a power setting of 5 and 50% pulse at 4°C. The mixture was incubated at room temperature for 30 min. Urea (7 M) was added, samples were vigorously agitated (Vortex level 5) at room temperature for 15 min, and the mixture was finally centrifuged (20,000 × g at room temperature for 20 min). Supernatants could be stored at −20°C. Proteins were quantified by the Bradford method (5).
IEF.
The 1D separation was carried out on immobilized pH gradients (4/7 Immobilin dry strips of 18 cm) as described by Görg et al. (15) with Multiphor II apparatus. The following voltage gradient was applied: from 0 to 50 V in 0.02 h; 50 V for 1 h; from 50 to 150 V in 0.02 h; 150 V for 1 h; from 150 to 300 V in 0.02 h; 300 V for 2 h; from 300 to 3,500 V in 5 h; and 3,500 V for 11 h. Protein samples (100 μg) were loaded into cups at the anode end. After isoelectric focusing (IEF), strips were equilibrated in a solution containing 50 mM Tris (pH 6.2), 6 M urea, 30% glycerol (vol/vol), 2% (wt/vol) sodium dodecyl sulfate (SDS), and 0.3% (wt/vol) DTT followed by a second bath with the same solution, but with 4.5% (wt/vol) iodoacetamide in place of DTT.
SDS-PAGE.
The 2D separation was performed, essentially according to the method of Laemmli (24), in an IsoDalt apparatus (Hoeffer, San Francisco, Calif.) with a 14% acrylamide separating gel, but without a stacking gel and at constant voltage (below 180 V). Large plate gels (200 by 250 by 1 mm) were used to improve resolution. Ten gels were run simultaneously in one tank to improve reproducibility. A low-molecular-weight electrophoresis calibration kit (Amersham-Pharmacia-Biotech, Buckinghamshire, England) was used for protein molecular mass (daltons) reference standards (phosphorylase b, 94,000; bovine serum albumin, 67,000; ovalbumin, 43,000; carbonic anhydrase, 30,000; soybean trypsin inhibitor, 20,100; and α-lactalbumin, 14,400).
1D SDS-PAGE was also performed (Protean II or Mini-Protean; Bio-Rad, Paris, France). Samples were diluted twice in a mixture of 62.5 M Tris-HCl (pH 6.8), 10% glycerol, 2% SDS, and 5% β-mercaptoethanol and heat treated at 100°C for 2 min before loading (20 μl) on wells of SDS-PAGE gels at a 14% polyacrylamide concentration.
In the same way, delay gels were performed with flagellin extracted from cells grown at 20 and 37°C (see below) and purified divercin V41. Interaction studies were performed with flagellin extract of RV41 (200 μg) and purified divercin V41 (3.5 μg) in phosphate-buffered saline (PBS) (0.01 M sodium phosphate with 0.15 M sodium chloride [pH 7.6]) for 10 min at room temperature. Separations were carried out in either the presence or absence of SDS to visualize interactions between the two proteins.
Gels were stained either with silver (41) or with Coomassie brilliant blue (PhastGel Blue R; Amersham-Pharmacia-Biotech) and stored in a 20% ethanol solution at 4°C for several weeks.
Analysis of protein spots on 2D gels.
Images were scanned (CanoScan FB620 P, Canon, France). Protein gel analysis was performed with Melanie II 2D PAGE software (release 2.2; Bio-Rad, Ivry sur Seine, France). Reference points (landmarks) were marked on images to align and match gels. After gel alignment and matching, pairs (spots present in several gels) could be highlighted. The reverse function evidenced the differences between each gel. Ten gels per sample were analyzed and compared. Spots present in at least nine gels were considered to be consistent spots and were taken into account.
Protein identification.
Coomassie blue-stained proteins which had been separated by 2D gel electrophoresis were excised and washed five times with 30 μl each of 40% n-propanol, followed by five washes with 30 μl each of 0.2 M ammonium carbonate containing 50% acetonitrile. The gel pieces were completely dried under reduced pressure (Speed-Vac; Savant). To the dried gel pieces, 0.5 μg of trypsin in 10 μl of 100 mM ammonium carbonate (digestion buffer) was added to allow reswelling of the gel pieces. About 15 μl of digestion buffer was added to completely immerse the gel pieces. Digestion was carried out at 37°C for 2 h. The supernatant was collected, and the gel pieces were extracted with 15 μl of 0.1% formic acid, followed by 15 μl of acetonitrile. Extraction was repeated twice, and all supernatants were pooled and dried in a Speed Vac. To be able to remove supernatants from the gel pieces, two 500-μl Eppendorf tubes arranged concentrically were used. The tube containing gel pieces was pierced with a hypodermic needle to generate a hole large enough to allow the liquid to be centrifuged into the lower tube, but to retain the gel pieces. Also in order to avoid possible cross-contamination, all buffers and wash solutions were pipetted with clean Hamilton syringes, which were reserved solely for the handling of the same solutions, making sure that the needle never touched the gel pieces.
For mass spectral analysis, the peptides were dissolved in 10 μl of 0.1% trifluoroacetic acid, and 5 μl was used for identification. Separation of the digests was carried out on 100-μm capillary columns which were packed with POROS R2 material (PerSeptive Biosystems, Framingham, Mass.). In short, fused silica capillaries (100 by 280-μm LC Packings; Polymer Laboratories, Marseille, France) were drawn to an aperture of 1 to 2 μm on a laser puller (Sutter Instruments, Science Products AG, Geneva, Switzerland). A column frit was constructed by introducing a few grains of 5-μm-diameter silica beads. The POROS material was then packed into the capillary with the aid of a stainless steel reservoir connected to a high-performance liquid chromatography pump capable of delivering pressures up to 6,000 lb/in2. After the packing process, the columns were cut to approximately 2.5 cm and inserted into a microsource. The microcolumns were developed with a linear gradient of 0.02% acetonitrile to 80% methanol containing 0.02% acetic acid in 15 min at a flow rate of approximately 200 nl/min. Mass spectral data were acquired on a TSQ7000 triple quadrupole instrument (Finnigan, San José, Calif.) with data-controlled switching between precursor ions and daughter ions during a single chromatographic run. For precursor ion scanning, the resolution of the instrument was set to 1 Da. For operation in the MS/MS mode, the resolution of Q1 was set to transmit a window of 4 Da, and the resolution of Q3 was adjusted to 1.5 Da. Daughter ion scanning was performed between 50 and 2,000 Da in 3.5 s. Argon was used as the collision gas at a pressure of 3.0 Torr. The collision energy was kept constant at −32 eV during individual runs. The sequences of trypsic peptides were compared to known Listeria peptides on the sequence database SwissProt.
Preparation of crude flagella.
One liter of culture was harvested by centrifugation at 5,000 × g, washed twice in a 0.01 M sodium phosphate–0.15 M sodium chloride (pH 7.6) (PBS) buffer, and resuspended at a ratio of 5 ml of PBS/liter of broth culture. Samples, in bottles containing glass beads, were shaken vigorously for 30 min at 20°C. The suspension was centrifuged at 5,000 × g for 15 min, and the supernatant was retained. The pellet was washed twice with PBS by vigorous pipetting to remove sheared flagella trapped within the cell mass. The supernatant and cell washing were pooled and centrifuged at 14,000 × g for 40 min to clear the remaining bacteria, and the resulting supernatant was centrifuged at 200,000 × g for 90 min to harvest crude flagella (33). One milligram of crude flagellar protein was recovered in each experiment and solubilized in 50 μl of PBS.
Sample preparation for scanning electron microscopy.
Five milliliters of the cultures was filtered over membranes (0.2-μm-pore-diameter GTTP 01300; Millipore Polycarbonate, Molsheim, France). Membranes were loaded on the surface of a 0.1 M sodium cacodylate (pH 7.2) buffer containing 2.5% glutaraldehyde. Cells or flagella were fixed for at least 48 h at room temperature. Membranes were dehydrated in successive baths of ethanol (10, 25, 50, 75, 95, and 100%) for 10 to 20 min. Samples were desiccated by introducing them into a pressurized enclosure where ethanol was replaced by liquid CO2 (10°C). Samples were desiccated by heating until the critical point was reached (31°C and 73.8 bars) without deterioration and then were metalized with gold and observed.
RESULTS AND DISCUSSION
Physiological comparison of Listeria monocytogenes sensitive and RV41 variants.
Sensitive and resistant variants present the same phenotypic characters, the same serotype (4b), and the same lysotype (1444, 1317, 3274, 2671, and 340) (data not shown). Thus, no drastic surface modifications could be postulated on these bases. The MICs for the sensitive and RV41 strains were determined after growth on Elliker and SCSFS broths. In Elliker broth, the MIC for the resistant variant was at least 104 times higher than that for the sensitive strain (higher than 104 and 0.01 μg/ml−1, respectively). This result was in accordance with that obtained by Métivier (28) with L. monocytogenes Scott A. In the SCSFS medium, the MIC for RV41 was 106 times higher than that for the sensitive strain (higher than 104 and 5.7 10−5 μg/ml−1, respectively). For the latter strain, the MIC was 175 times higher on Elliker broth than on SCSFS. These results could be explained by two facts. (i) SCSFS is a less nutritive medium in which L. monocytogenes had difficulty in growing, and thus it was more sensitive to bacteriocin activity. (ii) Elliker broth contains many more molecules which are able to interfere with the bacteriocin and artificially decrease the number of molecules free to interact with the target cells. These observations underlined the impact of environmental conditions on bacterial growth and bacterial sensitivity to antibacterial agents.
The frequency of appearance of resistance was 3.5 × 10−5 at five times the MIC. The stability of the resistant variant was tested against divercin V41, and no reversion of the resistance was observed after 20 successive cultures in the absence of divercin V41 as observed by Rekhif et al. (35) for mesentericin 52, curvaticin 13, and plantaricin C19. On the contrary, Dykes and Hastings (13) found a reversion frequency within the range of 10−4 to 10−5 with leucocins A, B, and E and sakacin A. The stability or instability of the resistant phenotype remained unexplained, but it is reasonable to argue for several resistance mechanisms among the different species, and possibly within the same species, leading to different mechanisms and thus different frequencies of reversibility.
The resistant variant obtained from the wild-type strain of L. monocytogenes P was resistant to divercin V41, piscicocin V1, and piscicocin SF668, but kept its sensitivity to nisin (data not shown). The cross-resistance between nonlantibiotic bacteriocins has been already observed by Rekhif et al. (35) and Métivier (28). The difference in sensitivity of RV41 to divercin V41 and nisin has been correlated with a potential difference in the mode of action of lantibiotics and nonlantibiotic bacteriocins.
The resistance or sensitivity of both strains to several antibiotics, acting on different cell targets was investigated to observe potential differences or similarities between bacteriocin and antibiotic resistance. The results are presented in Table 1 and show, first, that there are no differences between the two spectra and, second, that the resistances or sensitivities measured correspond to the common phenotype of L. monocytogenes 4b. These results show that resistance to divercin does not confer any resistance to the antibiotics tested. This point had already been observed for the nisin resistance phenotype (8).
No clear morphological difference was observed between the sensitive and RV41 variants either by optical or by scanning electron microscopy (data not shown). The absence of morphological differences between sensitive and divercin-resistant cells was in accordance with the results of Crandall and Montville (8) on L. monocytogenes ATCC 700302 resistant to nisin.
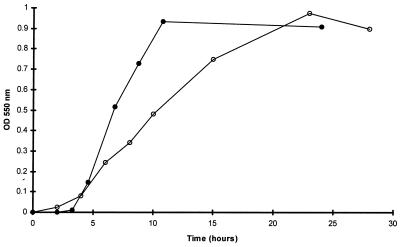
Comparative growth of the L. monocytogenes sensitive and resistant variants in Elliker broth at 37°C is represented in Fig. 1. The sensitive strain had a 4-h lag phase, followed by a rapid exponential phase (μ = 0.13 h−1) up to the 10 hours of growth. For the resistant variant, the lag phase was shorter (2 h), but the growth rate was twofold lower (μ = 0.07 h−1). The decrease in the growth rate could be explained by the energy cost of the potential resistance metabolic pathway(s) which reduces the fitness for growth (13). However, this rather important difference in growth efficiency could be the result of a more wide alteration of the cell metabolism than only a difference in bacteriocin resistance, which is the unique phenotypic characteristic observed here.
FIG. 1.
Growth curves of the L. monocytogenes wild type (solid symbols) and the resistant variant (open symbols) on Elliker broth at 37°C.
Comparison of the protein patterns of L. monocytogenes sensitive and RV41 variants.
No significant difference was observed between the two 1DE patterns of the total proteins from the two variants of L. monocytogenes P (data not shown).
The protein extractability was similar for both, as demonstrated by the protein content of preparations (2.10 ± 0.12 and 2.14 ± 0.11 mg/ml for RV41 and the sensitive strain, respectively). When minor differences between sample preparations occurred, standardization of the amount of protein of the samples was performed before 2DE experiments in order to allow direct comparison of the patterns.
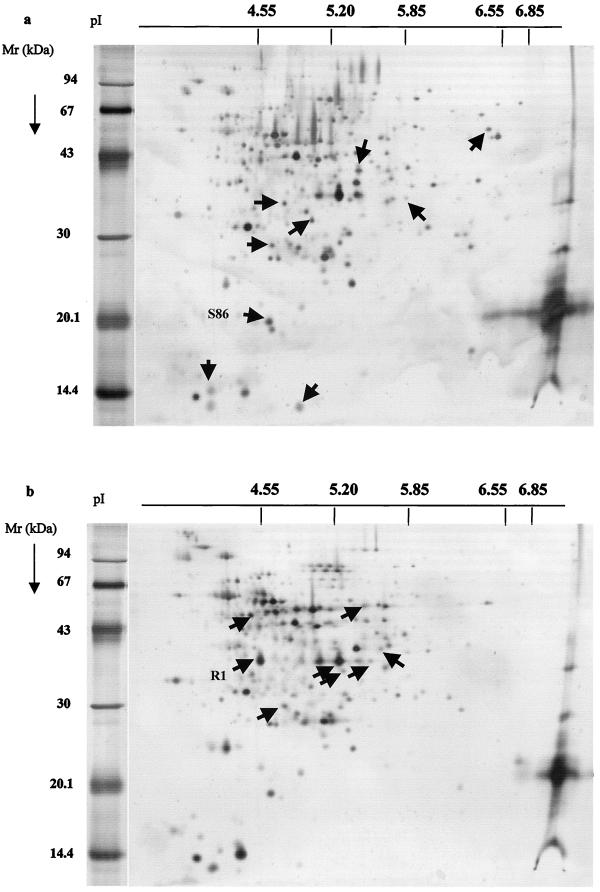
Ten 2D gels were run for each L. monocytogenes variant. Figure 2 shows two typical patterns of the L. monocytogenes strain sensitive to divercin V41 (Fig. 2a) and the resistant variant (Fig. 2b). 211 and 278 spots could be detected on each gel, respectively. Silver staining is not quantitative; only proteins present or absent on nine of the two patterns were considered. Arrows indicate the main differences between the two patterns and were determined after computer-assisted analysis with Melanie II software.
FIG. 2.
Silver-stained 2D gels of 100 μg of total proteins of L. monocytogenes strains sensitive (a) and resistant (b) to the divercin V41. Arrows indicate noncommon proteins. Mr, molecular mass.
Nine intense spots, lacking in the resistant pattern, were chosen in the sensitive strain pattern (Fig. 2a). Three of them are small (<20 kDa) and acidic (pI of <5.20) proteins; four have average size (about 30 kDa) proteins with a pI ranging from 4.6 to 5.8; two are bigger proteins (<35 kDa) with pIs higher than 5.4 and 5.85. In parallel, in the protein pattern derived from the resistant strain, we selected eight new and intense protein spots absent in the sensitive one. All of these proteins have a molecular mass ranging from 25 to 65 kDa and a pI between 4.5 and 5.8 (Fig. 2b).
Among the spots analyzed by mass spectroscopy, only spots R1 and S86 were identified as L. monocytogenes or L. innocua proteins, respectively (Table 2). The masses and the segment sequences of the other spots did not share any homology with identified proteins of Listeria spp. (available in the SWISS PROT database). The protein R1, present only in the RV41 variant, has been identified as a flagellin of L. monocytogenes characterized by Dons et al. (11). Flagellin is the main component of the flagellar filament. This is the engine of mobile bacteria which allows movement to high-nutrient-concentrated zones or away from toxic substances. How could flagellin be involved in the bacteriocin resistance phenomenon? Two hypotheses are presented: the flagella could act directly in the resistance phenomenon. It could play the role of a biological magnet, attracting, by electrostatic forces (14), molecules of divercin which then become unavailable to interact with L. monocytogenes membrane. A second hypothesis is that in RV41 cells, flagellin synthesis is indirectly affected by modification of gene control expression with widespread effects.
TABLE 2.
Characterization of R1 and S86 proteic spots
| Spot | Mol mass (Da) | Amino acid sequencea |
|---|---|---|
| R1 | 30,445 | MKVNTNIISLKTQEYLRKNNEGMTQAQERLASGKRINSSLDDAAGLAVVTRMNVKSTGLDAASKNSSMGIDLLQTADSALSSMSSILQRMRQLAVQSSNGSFSDEDRKQYTAEFGSLIKELDHVADTTNYNNIKLLDQTATGAATQVSIQASDKANDLINIDLFNAKGLSAGTITLGSGSTVAGYSALSVADADSSQEATEAIDELINNISNGRALLGAGMSRLSYNVSNVNNQSIATKASASSIEDADMAAEMSEMTKYKILTQTSISMLSQANQTPQMLTQLINS |
| S86 | 18,048 | MKTINSVDTKEFLNHQVANLNVFTVKIHQIHWYMRGHNFFTLHEKMDDLYSEFGEQMDEVAERLLAIGGSPFSTLKEFLENASVEEAPYTKPKTMDQLMEDLVGTLELLRDEYKQGIELTDKEGDDVTNDMLIAFKASIDKHIWMFKAFLGKAPLE |
Boldface letters represent sequence obtained by mass sequence analysis of tryptic peptides.
In order to assess if flagella were directly implicated in bacteriocin resistance, the MICs for sensitive, RV41, and RV41 flagellum-free (obtained by vigorous shaking and centrifugation) strains were determined at 20 and 37°C, temperatures at which cells are mobile and immobile, respectively (33). No change in MICs was evidenced between the two temperatures and between RV41 and flagellum-free RV41 (data not shown).
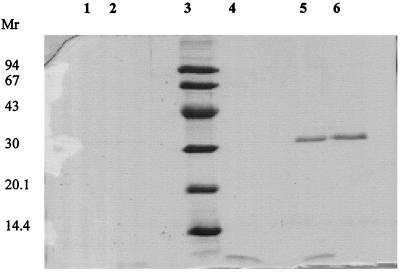
The physical interaction between flagellin and divercin V41 was explored. Figure 3 shows that no migration delay was observed when flagellin and divercin V41 were comigrated in electrophoresis experiments under native and SDS conditions. This suggests that, under our experimental conditions, there is no interaction between these two molecules. The presence of flagella on the RV41 cell surface was investigated. Optical microscopy showed that, for whichever variant considered, L. monocytogenes cells were mobile at 20°C and not at 37°C. Scanning electron microscopy confirmed that both L. monocytogenes sensitive and RV41 cells possessed flagella on their surface at 20°C and not at 37°C. Thus it could be postulated that flagellin is present in a large amount in the intracellular fraction of the RV41 cells, cultivated at 37°C, but flagellin export at the cell surface does not occur. Thus direct involvement of flagellin in the resistance phenomenon is doubtful, except, maybe, it plays a potential unknown role at the internal face of the cytoplasmic membrane.
FIG. 3.
Flagellin expression and interaction with divercin V41 of sensitive and RV41 samples cultivated at 37°C. Lanes: 1, flagellin extract of the sensitive variant; 2, flagellin extract of sensitive strain plus divercin V41 (3.5 μg); 3, molecular mass standards (kilodaltons); 4, divercin V41 (3.5 μg); 5, flagellin extract of RV41 (200 μg) plus divercin V41 (3.5 μg); 6, flagellin extract (200 μg) of RV41. Mr, molecular mass.
Variation in the level of flagellin synthesis in RV41 is probably the result of modification(s) in the transcriptional regulation of this protein. This hypothesis is based first on the fact that flagellin synthesis is thermoregulated at the transcriptional level (35). In our experiments, flagellin synthesis is repressed at 37°C in the sensitive variant and seems derepressed in the resistant one. A second element of response is based on the results obtained by Robichon et al. (36). They found a mesentericin Y105-resistant phenotype of L. monocytogenes obtained by transposition insertion. The insertion of the transposon was in the rpoN gene encoding an alternative transcriptional ς54 factor. ς54 is known to be involved in the control of many genes, including some genes of flagellar synthesis (36).
The flagellin role is of particular interest, because this protein has been hypothesized to increase the virulence of L. monocytogenes (9). PrfA, the transcriptional activator of virulence genes, which is maximally expressed at 37°C, down regulates motility genes in Listeria (31). If alteration(s) occurred in the transcriptional regulation of these genes, leading to flagellin synthesis as observed in our variant, we suppose that other genes encoding proteins directly involved in sensitivity and resistance phenomena are also deregulated. Moreover, such genes encoding entry and belonging to a multigene family have been observed near the virulence genes, on the same notA fragment of the physical map of L. monocytogenes (30). The expression of the gene encoding flagellin is frequently reported as being not only temperature regulated, but also influenced by stresses such as osmotic stress (19). In E. coli, the RNA polymerase ςF subunit, involved in the transcription of the flagellar and chemotaxis genes, possesses a strict promoter recognition property as found for minor sigma subunits involved in stress response. The transcription efficiency is salt dependent (23). Could we postulate that bacteriocin is considered by the target cell as a stress, like heat or osmotic stress, and that the cell response then uses the same kinds of mechanisms? This is an open question. This comparison was also suggested by O'Connor et al. (32), studying the response of Salmonella enterica serovar Typhimurium to deleterious conditions, including, besides oxidative and osmotic stresses, exposure to toxic cationic peptides. The regulation of the proteins involved in these resistance mechanisms is complex and overlapping. Moreover, a recent report (43) demonstrated that acid-adapted L. monocytogenes cells exhibit increased tolerance toward nisin and lacticin 3147. These results suggest common cell responses toward both types of attack.
Spot S86, only present in the sensitive strain, has been identified as a DNA-binding protein already described by Bozzi et al. (4) in Listeria innocua. This non-heme iron-binding ferritin is able to sequester many iron atoms inside the protein cage. Bacteriocin activity leads to intracellular ion leakage through the altered membrane. The absence of such iron-chelating intracellular systems, as observed in the resistant variant, could be a major problem for the cell attacked by bacteriocin molecules, but the mechanisms are unknown. Divalent cations (Mg2+, Ca2+, Mn2+, and Ba2+) increased the resistance of a nisin-resistant strain of L. monocytogenes Scott A in a concentration-dependent manner (8). Iron was not tested. In their discussion, the authors described a model in which cations may interfere with the lipids of the membrane and the cell wall. However the decreased bactericidal activity of lactostrepcin 5 on Streptococcus cremoris in the presence of Mg2+ and Ca2+ was attributed to their stimulative role on membrane-bound ATPase (45).
Moreover, this ferritin shows strong similarities to Dps proteins. These stress-induced widespread conserved polypeptides, present in diverse groups of bacteria, are involved in DNA protection during oxidative stress (26). Proteins induced by stress are considered to be members of global regulatory networks which comprise multiple unlinked genes and operons coordinately controlled by a common regulatory signal. In Escherichia coli, mutant cells lacking Dps show dramatic changes in the pattern of proteins synthesized during starvation. This result prompted Almiron et al. (1) to postulate that Dps plays a role in gene expression.
Altuvia et al. (2) found that Dps mRNA levels were controlled by RpoS and ς70 factors. These data had to be linked with the conclusions of Robichon et al. (36) on ς54 involvement in mesentericin resistance and confirm that genes responsible for divercin resistance are controlled by sigma factors. The role of Dps in bacteriocin sensitivity remains unexplored, but is of special concern because of the importance of iron in the infection process caused in human cells by L. monocytogenes (17). In Bacillus subtilis, mrgA, encoding a Dps protein, is a gene repressed by metal irons (6). Expression of virulence genes (inl) is positively iron regulated at the transcriptional level in L. monocytogenes (7). It could be interesting to test the influence of iron on L. monocytogenes sensitivity or resistance to bacteriocins and strain virulence.
We are currently trying to establish a relationship between variants exhibiting different levels of resistance and a quantitative evolution of the observed differences between sensitive and resistant variants. Experiments to determine the role of transcriptional regulation with respect to resistance acquisition are under way, and the results will be reported later.
We will also investigate the resistance phenotype through, on one hand, identification of all the proteins detected as highly repressed or oversynthesized in the resistant clone, and, on the other hand, 2DE of specially extracted membrane proteins from purified membrane fractions of wild and resistant variants.
ACKNOWLEDGMENTS
We thank Jane Hall for improving the English of this paper; J. Rocourt for serotyping and lysotyping of strains; G. Jan, M. Bossis, and L. Phan-Than for help with 2D gel electrophoresis training; Thierry Mini for mass spectroscopy analyses; and J. Berrier for expertise in electron microscope studies.
F.D. was given a grant by the European Community (FAIR CT95-1207).
REFERENCES
- 1.Almiron M, Link A J, Furlong D, Kolter R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 1992;6:2646–2654. doi: 10.1101/gad.6.12b.2646. [DOI] [PubMed] [Google Scholar]
- 2.Altuvia S, Almiron M, Huisman G, Kolter R, Storz G. The dps promoter is activated by OxyR during growth and by IHF and sigma S in stationary phase. Mol Microbiol. 1994;13:265–272. doi: 10.1111/j.1365-2958.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 3.Antibiogram Committee of the French Society for Microbiology. Statement. Pathol Biol. 1998;46:1–16. [Google Scholar]
- 4.Bozzi M, Mignogna G, Stefanini S, Barra D, Longhi C, Valenti P, Chiancone E. A novel non-heme iron-binding ferritin related to the DNA-binding proteins of the Dps family in Listeria innocua. J Biol Chem. 1997;272:3259–3265. doi: 10.1074/jbc.272.6.3259. [DOI] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Helmann J D. Bacillus subtilis Mrg A is a Dps (Pex B) homologue: evidence for metalloregulation of an oxidative-stress gene. Mol Microbiol. 1995;18:295–300. doi: 10.1111/j.1365-2958.1995.mmi_18020295.x. [DOI] [PubMed] [Google Scholar]
- 7.Conte M P, Longhi C, Polidoro M, Petrone G, Buonfiglio V, Di Santo S, Papi E, Seganti L, Visca P, Valenti P. Iron availability affects entry of Listeria monocytogenes into the enterocytelike cell line Caco-2. Infect Immun. 1996;64:3925–3929. doi: 10.1128/iai.64.9.3925-3929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crandall A D, Montville T J. Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl Environ Microbiol. 1998;64:231–237. doi: 10.1128/aem.64.1.231-237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czuprynski C J, Brown J F, Roll J T. Growth at reduced temperatures increases the virulence of Listeria monocytogenes for intravenously but not intragastrically inoculated mice. Microb Pathog. 1989;7:213–223. doi: 10.1016/0882-4010(89)90057-0. [DOI] [PubMed] [Google Scholar]
- 10.Dielbandhoesing S K, Zhang H, Caro L H P, van der Vaart J M, Klis F M, Verrips C T, Brul S. Specific cell wall proteins confer resistance to nisin upon yeast cells. Appl Environ Microbiol. 1998;64:4047–4052. doi: 10.1128/aem.64.10.4047-4052.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dons L, Rasmussen O F, Olsen J E. Cloning and characterization of a gene encoding flagellin of Listeria monocytogenes. Mol Microbiol. 1992;6:2919–2929. doi: 10.1111/j.1365-2958.1992.tb01751.x. [DOI] [PubMed] [Google Scholar]
- 12.Duffes F, Leroi F, Boyaval P, Dousset X. Inhibition of Listeria monocytogenes by Carnobacterium spp. strains in a simulated cold-smoked fish system stored at 4°C. Int J Food Microbiol. 1999;47:33–42. doi: 10.1016/s0168-1605(98)00206-2. [DOI] [PubMed] [Google Scholar]
- 13.Dykes G A, Hastings W N. Fitness costs associated with class II a bacteriocin resistance in Listeria monocytogenes B73. Lett Appl Microbiol. 1998;26:5–8. doi: 10.1046/j.1472-765x.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- 14.Gerber B R, Routledge L M. Self-assembly of bacterial flagellar protein: dielectric behavior of monomers and polymers. J Mol Biol. 1972;71:317–337. doi: 10.1016/0022-2836(72)90354-3. [DOI] [PubMed] [Google Scholar]
- 15.Görg A, Postel W, Weser J, Gunther S, Strahler S M, Hanash S M, Sommerlot L. Elimination of point streaking on silver stained two-dimensional gels by addition of iodoacetamide to the equilibration buffer. Electrophoresis. 1987;8:122–124. [Google Scholar]
- 16.Gormond T, Phan-Thanh L. Identification and classification of Listeria by two-dimensional protein mapping. Res Microbiol. 1995;146:143–154. doi: 10.1016/0923-2508(96)80892-8. [DOI] [PubMed] [Google Scholar]
- 17.Gray M L, Killinger A H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966;30:309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guash J F, Ferrer S, Enfedaque J, Viejo M B, Regue M. A 17 kDa outer-membrane protein (Omp4) from Serratia marcescens confers partial resistance to bacteriocin 28b when expressed in Escherichia coli. Microbiology. 1995;141:2535–2542. doi: 10.1099/13500872-141-10-2535. [DOI] [PubMed] [Google Scholar]
- 19.Heuner K, Brand B C, Hacker J. The expression of the flagellum of Legionella pneumophila is modulated by different environmental factors. FEMS Microbiol Lett. 1999;175:69–77. doi: 10.1111/j.1574-6968.1999.tb13603.x. [DOI] [PubMed] [Google Scholar]
- 20.Jarvis B. Resistance to nisin and production of nisin-inactivating enzymes by several Bacillus species. J Gen Microbiol. 1967;47:33–48. doi: 10.1099/00221287-47-1-33. [DOI] [PubMed] [Google Scholar]
- 21.Jungblut P R, Schaible U E, Mollenkopf H-J, Zimny-Arndt U, Raupach B, Mattow J, Halada P, Lamer S, Hagens K, Kaufmann S H E. Comparative proteome analysis of Mycobacterium tuberculosis and Mycobacterium bovis BCG strains: towards functional genomics of microbial pathogens. Mol Microbiol. 1999;33:1103–1117. doi: 10.1046/j.1365-2958.1999.01549.x. [DOI] [PubMed] [Google Scholar]
- 22.Koch G, Smith M, Twentyman P, Wright K. Identification of a novel calcium-binding protein (CP22) in multidrug-resistant murine and hamster cells. FEBS Lett. 1986;195:275–279. doi: 10.1016/0014-5793(86)80176-4. [DOI] [PubMed] [Google Scholar]
- 23.Kundu T K, Kusano S, Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase ςF holoenzyme involved in transcription of flagellar and chemotaxis genes. J Bacteriol. 1997;179:4264–4269. doi: 10.1128/jb.179.13.4264-4269.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Lorber B. Listeriosis. Clin Infect Dis. 1997;24:1–11. doi: 10.1093/clinids/24.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Martinez A, Kolter R. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J Bacteriol. 1997;179:5188–5194. doi: 10.1128/jb.179.16.5188-5194.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazotta A S, Montville T J. Nisin induces changes in membrane fatty acid composition of Listeria monocytogenes nisin-resistant strains at 10°C and 30°C. J Appl Microbiol. 1997;82:32–38. doi: 10.1111/j.1365-2672.1997.tb03294.x. [DOI] [PubMed] [Google Scholar]
- 28.Métivier A. Study on divercine V41, bacteriocin produce by Carnobacterium divergens V41. Purification, physico-chimical characterisation and genetics. Ph.D. thesis. Nantes, France: Nantes University; 1997. [Google Scholar]
- 29.Métivier A, Boyaval P, Duffes F, Dousset X, Compoint J P, Marion D. Triton X-114 phase partitioning for the isolation of a pediocin-like bacteriocin from Carnobacterium divergens. Lett Appl Microbiol. 2000;30:42–46. doi: 10.1046/j.1472-765x.2000.00655.x. [DOI] [PubMed] [Google Scholar]
- 30.Michel E, Cossart P. Physical map of the Listeria monocytogenes chromosome. J Bacteriol. 1992;174:7098–7103. doi: 10.1128/jb.174.22.7098-7103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michel E, Mengaud J, Galsworthy S, Cossart P. Characterization of a large motility gene cluster containing the cheR, motAB genes of Listeria monocytogenes and evidence that Prfa downregulates motility genes. FEMS Microbiol Lett. 1998;169:341–347. doi: 10.1111/j.1574-6968.1998.tb13338.x. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor C D, Farris M, Fowler R, Qi S-Y. The proteome of Salmonella enteritica serovar typhimurium: current progress on its determination and some applications. Electrophoresis. 1997;18:1483–1490. doi: 10.1002/elps.1150180823. [DOI] [PubMed] [Google Scholar]
- 33.Peel M, Donachie W, Shaw A. Temperature-dependent expression of flagella of Listeria monocytogenes studied by electron microscopy, SDS-PAGE and western blotting. J Gen Microbiol. 1988;134:2171–2178. doi: 10.1099/00221287-134-8-2171. [DOI] [PubMed] [Google Scholar]
- 34.Pilet M F, Dousset X, Barré R, Novel G, Desmazeaud M, Piard J C. Evidence for two bacteriocins produced by Carnobacterium piscicola and Carnobacterium divergens isolated from fish and active against Listeria monocytogenes. J Food Prot. 1995;58:256–262. doi: 10.4315/0362-028X-58.3.256. [DOI] [PubMed] [Google Scholar]
- 35.Rekhif N, Atrih A, Lefebvre G. Selection and properties of spontaneous mutants of Listeria monocytogenes ATCC15313 resistant to different bacteriocins produced by lactic acid bacteria strains. Curr Microbiol. 1994;28:237–241. [Google Scholar]
- 36.Robichon D, Gouin E, Débarbouillé M, Cossart P, Cenatiempo Y, Héchard Y. The rpoN (ς54) gene from Listeria monocytogenes is involved in resistance to mesentericin Y105, an antibacterial peptide from Leuconostoc mesenteroides. J Bacteriol. 1997;179:7591–7594. doi: 10.1128/jb.179.23.7591-7594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocourt J, Audurier A, Courtieu A L, Durst J, Ortel S, Schrettenbrunner A, Taylor A G. A multi-centre study on the phage typing of Listeria monocytogenes. Zbl Bakteriol Hyg A. 1985;259:489–497. doi: 10.1016/s0176-6724(85)80081-x. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt M J, Compain P. Killer-toxin-resistant kre12 mutants of Saccharomyces cerevisiae: genetic and biochemical evidence for a secondary K1 membrane receptor. Arch Microbiol. 1995;164:435–443. doi: 10.1007/BF02529742. [DOI] [PubMed] [Google Scholar]
- 39.Seelinger H P R, Höhne K. Serotyping of Listeria monocytogenes and related species. Methods Microbiol. 1979;13:31–49. [Google Scholar]
- 40.Stiles M E. Biopreservation by lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:331–345. doi: 10.1007/BF00395940. [DOI] [PubMed] [Google Scholar]
- 41.Tunon P, Johansson K E. Yet another improved staining method for the detection of the proteins in polyacrylamide gels. J Biochem Biophys Methods. 1984;9:171–179. doi: 10.1016/0165-022x(84)90008-3. [DOI] [PubMed] [Google Scholar]
- 42.van Belkum M J, Kok J, Venema G, Holo H, Nes I F, Konings W N, Abee T. The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent protein-mediated manner. J Bacteriol. 1991;173:7934–7941. doi: 10.1128/jb.173.24.7934-7941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Schaik W, Gahan C G, Hill C. Acid-adapted Listeria monocytogenes displays enhanced tolerance against the lantibiotics nisin and lacticin 3147. J Food Prot. 1999;62:536–539. doi: 10.4315/0362-028x-62.5.536. [DOI] [PubMed] [Google Scholar]
- 44.Yamada T, Mizuguchi Y, Isono S, Isono K. Genetic and biochemical analysis of ribosomal proteins of minocycline-susceptible and -resistant Mycobacterium smegmatis. Microbiol Immunol. 1992;36:139–148. doi: 10.1111/j.1348-0421.1992.tb01651.x. [DOI] [PubMed] [Google Scholar]
- 45.Zajdel J K, Ceglowski P, Dobrzański W T. Mechanism of action of lactostrepcin 5, a bacteriocin produced by Streptococcus cremoris 202. Appl Environ Microbiol. 1985;49:969–974. doi: 10.1128/aem.49.4.969-974.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]