Abstract
Lactobacillus reuteri LTH2584 exhibits antimicrobial activity that can be attributed neither to bacteriocins nor to the production of reuterin or organic acids. We have purified the active compound, named reutericyclin, to homogeneity and characterized its antimicrobial activity. Reutericyclin exhibited a broad inhibitory spectrum including Lactobacillus spp., Bacillus subtilis, B. cereus, Enterococcus faecalis, Staphylococcus aureus, and Listeria innocua. It did not affect the growth of gram-negative bacteria; however, the growth of lipopolysaccharide mutant strains of Escherichia coli was inhibited. Reutericyclin exhibited a bactericidal mode of action against Lactobacillus sanfranciscensis, Staphylococcus aureus, and B. subtilis and triggered the lysis of cells of L. sanfranciscensis in a dose-dependent manner. Germination of spores of B. subtilis was inhibited, but the spores remained unaffected under conditions that do not permit germination. The fatty acid supply of the growth media had a strong effect on reutericyclin production and its distribution between producer cells and the culture supernatant. Reutericyclin was purified from cell extracts and culture supernatant of L. reuteri LTH2584 cultures grown in mMRS by solvent extraction, gel filtration, RP-C8 chromatography, and anion-exchange chromatography, followed by rechromatography by reversed-phase high-pressure liquid chromatography. Reutericyclin was characterized as a negatively charged, highly hydrophobic molecule with a molecular mass of 349 Da. Structural characterization (A. Höltzel, M. G. Gänzle, G. J. Nicholson, W. P. Hammes, and G. Jung, Angew. Chem. Int. Ed. 39:2766–2768, 2000) revealed that reutericyclin is a novel tetramic acid derivative. The inhibitory activity of culture supernatant of L. reuteri LTH2584 corresponded to that of purified as well as synthetic reutericyclin.
Lactic acid bacteria (LAB) are the biological basis for the production of a great multitude of fermented foods. Their metabolic activity during these fermentative processes determines and maintains food quality. Food preservation by lactic fermentations relies mainly on the accumulation of organic acids and the acidification of the substrate. Metabolites such as acetaldehyde, diacetyl, hydrogen peroxide, and carbon dioxide contribute to this preservative effect (15). Niku-Paavola et al. (40) have identified low-molecular-weight compounds from cultures of Lactobacillus plantarum that contribute to the inhibitory effect of lactic acid. Certain strains of Lactobacillus reuteri produce a unique antagonistic activity, reuterin (1). This antimicrobial activity against a broad range of microorganisms was attributed to monomers, hydrated monomers, and cyclic dimers of β-hydroxypropionic aldehyde formed during anaerobic catabolism of glycerol. Furthermore, a great number of strains of LAB produce bacteriocins, ribosomally synthesized peptides that exhibit antagonistic activity against closely related species (32, 54). These compounds have received increasing attention since they have the potential to inhibit food pathogens (24, 51). Furthermore, lactobacilli of intestinal origin exhibit antimicrobial activity that could not be attributed to either bacteriocins or organic acids (10, 49). However, to date, no nonbacteriocin antibiotic of lactobacilli has been purified and characterized on the molecular level.
The applications of antagonistic compounds produced by lactobacilli are not limited to food preservation. Antimicrobials of LAB have been employed successfully to prevent the formation of biogenic amines (30), to inhibit pathogens causing mastitis (46), and to inhibit enteropathogens in the small intestines of animals (3). Furthermore, bacteriocin formation by meat starter cultures contributes to the competitiveness of the producer strain during sausage fermentation (59).
The majority of bacteriocins and antagonistic compounds characterized to date are produced by lactobacilli originating from meat or milk fermentations. Few data are available on antimicrobials produced by the lactobacilli employed in cereal fermentations. The metabolism and the physiological properties of lactobacilli from sourdoughs are highly adapted to their natural substrate (19, 26), and several studies suggest that the production of antagonists may further account for their dominance in the dough environment (11, 35, 41). Gänzle et al. (21) screened 65 strains of lactobacilli previously isolated from wheat and rye sourdoughs. Two of these 65 strains, L. mucosae LTH3566 and L. reuteri LTH2854, produced inhibitory activity against L. sanfranciscensis ATCC 27651. This study was undertaken to characterize the active compound produced by L. reuteri LTH2584, reutericyclin, on the molecular level and to determine a possible role for this antagonistic compound in the microecology of sourdough.
MATERIALS AND METHODS
Media and culture conditions.
Modified MRS medium containing the following components per liter was used unless otherwise stated: 10 g of tryptone, 5 g of meat extract, 5 g of yeast extract, 10 g of maltose, 5 g of fructose, 5 g of glucose, 2.6 g of KH2PO4, 4 g of K2HPO4 · 3H2O, 3 g of diammonium citrate, 3 g of NH4Cl, 0.5 g of cysteine · HCl, 1 g of Tween 80, 0.2 mg of MgSO4 · 7H2O, 0.05 g of MnSO4 · H2O, and 0.5 μg each of cobalamine, folic acid, niacin, panthotheic acid, pyridoxal, and thiamine (mMRS4) (52). The pH was adjusted to 6.2 prior to autoclaving. The sugars were autoclaved separately, and the vitamins were sterilized by filtration. For preparation of mMRS4 (oleic acid), mMRS4 (linoleic acid), and mMRS4 (wheat germ oil), Tween 80 was replaced by 1 g of either oleic acid (Sigma, Deisenhofen, Germany), linoleic acid technical grade (Fluka, Buchs, Switzerland), or wheat germ oil (obtained at a local supermarket) per liter. Lactobacilli were incubated anaerobically at 30°C, and bacilli, staphylococci, listeriae, and enterococci were incubated in a rotary shaker (200 rpm) at 37°C unless otherwise stated. To obtain a spore suspension of Bacillus subtilis FAD109, cells of an overnight culture on mMRS4 agar were suspended in saline, heated to 80°C for 30 min, and stored in aliquots at −85°C. Vegetative cells of Bacillus were obtained by harvesting cells of a culture grown to early logarithmic growth phase. Cell counts were determined by plating appropriate dilutions on mMRS4 agar.
Quantification of inhibitory activity.
The antimicrobial activity was determined by using a critical-dilution assay on microtiter plates as described previously (20). In short, twofold serial dilutions of the analyte were prepared with mMRS4, inoculated with the indicator strain L. sanfranciscensis ATCC 27651 to a cell count of about 107 CFU ml−1, and incubated overnight at 30°C. Growth of the indicator strain was judged by measuring the optical density at 595 nm (OD595). The amount of analyte resulting in 50% growth inhibition was defined as d50, and the antimicrobial activity was calculated as 1/d50 and expressed as arbitrary units (AU) per milliliter. This protocol was used for all determinations of inhibitory activity and was modified with respect to the preparation of precultures and incubation times for determination of the inhibitory spectrum of reutericyclin (see below).
Bactericidal activity of L. reuteri LTH2584 culture supernatant.
Neutralized culture supernatant (NCS) of L. reuteri LTH2584 was prepared from cultures grown for 16 h at 37°C in mMRS4. Cells were removed by centrifugation, the pH of the supernatant was adjusted to 6.2, and the NCS was sterilized by filtration. The NCS had an inhibitory activity of 75 ± 15 AU ml−1. The bactericidal activity of NCS against L. sanfranciscensis ATCC 27651, B. subtilis FAD109, and Staphylococcus aureus LTH1493 was assessed in mMRS4. Cells of these organisms were harvested from overnight cultures, washed once in mMRS4, and diluted to a cell count of 5 × 106 to 1 × 107 CFU ml−1. The activity of NCS against germinating spores of B. subtilis FAD109 was compared with the activity against vegetative cells. Spores or vegetative cells were incubated in mMRS4 under conditions permitting or not permitting growth of the organism (37°C with 200 rpm agitation and 20°C with 5% NaCl, respectively). NCS of L. reuteri LTH2584 was added to an activity of 20 AU ml−1; addition of mMRS4 served as control. The cell counts were determined after 16 h.
The lytic activity of NCS was determined using L. sanfranciscensis ATCC 27651 as the target organism. Cells of an overnight culture were suspended in mMRS4 (pH 5.0, containing 4% NaCl) and mixed with various amounts of culture supernatant of L. reuteri adjusted to pH 5.0 and 4% NaCl. Lysis of L. sanfranciscensis was monitored by measuring the OD578. The OD data were fitted to the Fermi equation to calculate the maximum lysis rate (κmax) and the time required for 50% lysis of the population (τ50).
Adsorption of reutericyclin to the producer cell wall.
The adsorption and desorption of reutericyclin to the producer cell walls was assessed in mMRS4, mMRS4 (oleic acid), mMRS4 (linoleic acid), and mMRS4 (wheat germ oil) containing the indicated sources of unsaturated fatty acids instead of Tween 80. L. reuteri LTH2584 was incubated in the various media for 16 h at 37°C and harvested by centrifugation (3,000 × g for 15 min). Cell extracts were prepared by resuspending the cells in equal volumes of 50 mM phosphate buffer (pH 6.5) containing 30% (wt/wt) isopropanol, incubation for 1 h, and removal of cells by centrifugation. The NCS and the cell extracts were analyzed for their inhibitory activity, taking into account the background inhibitory effect of the extraction buffer.
Effect of emulsifiers on reutericyclin production by L. reuteri LTH2584.
The effect of emulsifiers on the production of reutericyclin was determined in mMRS4 and in mMRS4 in which Tween 80 (polyoxyethylene sorbitol monooleate) was replaced by Lamesorb SMO (sorbitol monooleate), Lamegin GLO30 (polyoxyethylene monooleate), Lamegin ZE 609 O18 (oleoylmonoglycerol citrate), or sodium oleate at 1 g liter−1 (all emulsifiers were kindly provided by Grünau Illertissen GmbH, Illertissen, Germany). L. reuteri LTH2584 was incubated in the various media for 16 h at 37°C, and the inhibitory activity of the NCS was evaluated.
In a second set of experiments, L. reuteri LTH2584 was grown overnight at 37°C in mMRS4 (oleic acid). After this incubation period, the emulsifiers Tween 80, Lamesorb SMO, Lamegin GLO30, Lamegin ZE 609 O18, or sodium oleate were added to a final concentration of 1 g liter and the culture was further incubated for 1 h at 20°C in a rotary shaker (200 rpm) before the NCS was collected. It was verified that none of the emulsifiers exhibited inhibitory activity against L. sanfranciscensis ATCC 27651 or affected reutericyclin activity against this indicator strain (data not shown).
Effect of pH and NaCl concentration on the inhibitory activity of NCS against L. sanfranciscensis.
The effect of pH and NaCl concentration on the inhibitory activity of L. reuteri culture supernatant was evaluated. The NaCl concentration of mMRS4 was adjusted to 0, 1, and 2%; the pH at each NaCl concentration was adjusted to 5.5, 5.0, and 4.5; and the media were sterilized by filtration. The inhibitory activity of L. reuteri NCS was determined in each of the media as described above.
Purification of reutericyclin.
Reutericyclin was purified from a 2-liter culture of L. reuteri LTH2584 in buffered mMRS4 (20 g of maltose per liter, 10 g each of glucose and fructose per liter, 5 g of sodium acetate · 3H2O) per liter, and 4 g of diammonium citrate per liter; other components as described above). Cells were harvested by centrifugation (200 × g for 30 min) and washed once with 50 mM phosphate buffer (pH 2.5), and reutericyclin was extracted from the cells with 500 ml of 50 mM phosphate buffer (pH 6.5) containing 30% (wt/wt) isopropanol. NaCl was added to the cell extract to saturation, and the organic phase was removed. The remaining aqueous phase was extracted twice with 100 ml of isopropanol. The organic phases were pooled and evaporated to dryness in a rotary evaporator, and the pellet was suspended in 10 ml of isopropanol-water (80:20). The suspension was mixed, and the organic phase was recovered, evaporated to dryness, and resuspended in 2 ml of isopropanol-water (80:20).
The organic phase was loaded on a gel filtration column (Superdex 30 prep grade; all fast protein liquid chromatography (FPLC) columns and equipment from Amersham Pharmacia, Uppsala, Sweden) and eluted with 50 mM triethylamine buffer (pH adjusted to 7.2 with CO2) containing 25% (wt/wt) isopropanol (flow rate, 0.4 ml min−1). The active fractions were pooled, evaporated to dryness, dissolved in 1 ml of isopropanol-H2O (80:20), and loaded on a reversed-phase (RP) FPLC column (ProRPC, 15 μm, HR16/10). The sample was eluted with a gradient of 0.1% trifluoroacetic acid (TFA) in H2O against 0.1% TFA in isopropanol at a flow rate of 2 ml min−1. The active fractions were pooled, evaporated to dryness, dissolved in acetonitrile-H2O (80:20), and rechromatographed on an RP-C18 polymeric high-pressure liquid chromatography (HPLC) column (250 by 6 mm, 5 μm; Advanced Separation Technologies, Whippany, N.J.). The HPLC elution was carried out with acetonitrile-H2O-TFA (85:15:0.1) at a flow rate of 1 ml min−1.
The purification protocol was also applied for cells grown in mMRS4 containing wheat germ oil or oleic acid instead of Tween 80. The following modifications were used to purify reutericyclin from culture supernatants of L. reuteri LTH2584: 60% (wt/wt) ammonium sulfate was added to 1 liter of culture supernatant, and the mixture was stored at 0°C for 1 h and centrifuged for 30 min at 3,000 × g. The pellet and the surface pellicle were recovered and dissolved in 200 ml of H2O. This solution was further purified as described for the cell extract. Material prepared from the culture supernatant required an additional chromatography step using an ion-exchange column (MonoQ HR 5/5). The sample was eluted from the ion-exchange column using a gradient of 25 mM Tris-HCl (pH 8.00) against 25 mM Tris-HCl and 1.5 M NaCl. Either solvent contained 25% (wt/wt) isopropanol. The active fractions were pooled, dissolved in isopropanol-H2O (80:20), and desalted by gel filtration prior to the final HPLC purification step.
MIC and inhibitory spectrum of reutericyclin.
The inhibitory spectrum of reutericyclin was determined using a stock solution of purified reutericyclin in isopropanol-water (80:20) at a concentration of 3 mg ml−1. The inhibitory activity of reutericyclin was determined in mMRS4 essentially as described above, using the strains listed in Table 4. Strains of the genera Bacillus, Escherichia, Enterococcus, and Staphylococcus were incubated for 16 to 18 h at 37°C (200 rpm agitation), the cultures were subcultured by using 5% inoculum with fresh medium and were grown to early logarithmic growth phase (OD595, 0.1 to 0.4). The microtiter plates were inoculated with these indicator strains to an OD595 of 0.006 to 0.01 and incubated without agitation at 37°C. Strains of the genera Lactobacillus, Weissella, and Listeria were incubated overnight at 30°C and subcultured for 16 to 18 h. The microtiter plates were inoculated to an OD595 of 0.03 to 0.05 and incubated at 30°C without agitation. Precultures of yeasts were prepared in essentially the same way as those of lactobacilli; the incubation conditions were 27°C and 250 rpm agitation. Growth of the indicator strains was monitored by measuring OD595 at 30-min intervals over 24 h. The data for OD versus dose recorded at the time when the control culture (no addition of reutericyclin) had reached the midlogarithmic growth phase (OD595, 0.4 to 0.5) were used to calculate the MIC of reutericyclin.
TABLE 4.
Inhibitory spectrum of reutericyclin
| Organism | Strain designation | Strain properties (reference) | MIC (mg/liter) (n)a |
|---|---|---|---|
| B. cereus | DSM345 | 0.19 ± 0.04 (2) | |
| B. subtilis | DSM347 | 0.13 ± 0.003 (2) | |
| B. subtilis | DSM10 | 0.26 ± 0.02 (2) | |
| B. subtilis | FAD109 | Rope formation in bread (43) | 0.14 ± 0.02 (6) |
| B. subtilis | FAD109 | Spore formation | 0.23 ± 0.02 (4) |
| B. subtilis | FAD11/2 | Rope formation in bread (43) | 0.28 (1) |
| B. subtilis | FAD77 | Rope formation in bread (43) | 0.26 (1) |
| B. subtilis | FAD94 | Rope formation in bread (43) | 0.22 (1) |
| B. subtilis | FAD97 | Rope formation in bread (43) | 0.30 (1) |
| B. subtilis | FAD99 | Rope formation in bread (43) | 0.31 (1) |
| E. coli | LTH1600 | >100 (4) | |
| E. coli | LTH4346 | O157:H7b EHEC | >100 (4) |
| E. coli | ATCC27325 | K-12b | >100 (1) |
| E. coli | WBB06 | Reb (7) | 12 (1) |
| E. coli | F492 | O8b (47) | >100 (1) |
| E. coli | F470 | R1b (47) | 13 (1) |
| E. coli | F515 | Reb (47) | 17 (1) |
| E. faecalis | DSM20409 | 0.06 ± 0.01 (3) | |
| E. faecium | DSM20477 | 0.10 ± 0.02 (3) | |
| E. faecium | 70/90 | Antibiotic-resistant clinical isolatec | 0.16 (1) |
| E. faecium | 1528 | Antibiotic-resistant clinical isolatec | 0.08 (1) |
| L. brevis | DSM0054 | 0.36 (1) | |
| L. buchneri | LTH2162 | 0.91 (1) | |
| L. buchneri | DSM20057 | 0.20 (1) | |
| L. casei | LTH2600 | 0.63 (1) | |
| L. curvatus | LTH1174 | Curvacin A producer (55) | 0.12 (1) |
| L. curvatus | LTH1432 | 0.15 (1) | |
| L. curvatus | DSM20019 | 0.12 (1) | |
| L. paracasei subsp. paracasei | DSM20312 | 0.50 (1) | |
| L. plantarum | LTH3904 | 0.29 (1) | |
| L. plantarum | DSM20174 | 0.14 (1) | |
| L. pontis | DSM8475 | Sourdough (58) | 0.10 (1) |
| L. reuteri | DSM20016 | 0.09 (1) | |
| L. sakei | LTH673 | Sakacin P producer (55) | 0.09 (1) |
| L. sakei | DSM20017 | 0.11 (1) | |
| L. sanfranciscensis | ATCC27651 | Sourdough (34) | 0.10 ± 0.02 (6) |
| L. sanfranciscensis | LTH1729 | Sourdough (6) | 0.16 (1) |
| L. sanfranciscensis | LTH4469 | Sourdough | 0.12 (1) |
| L. sanfranciscensis | LTH4801 | Sourdough | 0.18 (1) |
| Weissella confusa | DSM20196 | 0.13 (1) | |
| L. reuteri | LTH3569 | SERd (5) | 0.26 (1) |
| L. mucosae | LTH3566 | SER (5) | 0.36 (1) |
| Lactobacillus sp. | LTH3568 | SER (5) | 0.59 (1) |
| Lactobacillus sp. | LTH3578 | SER (5) | 0.67 (1) |
| Lactobacillus sp. | LTH3579 | SER (5) | 0.25 (1) |
| L. reuteri | LTH2584 | SER (5), reutericyclin producer | 6.5 ± 1.2 (6) |
| Listeria innocua | DSM20649 | 0.19 (1) | |
| Listeria ivanovii | DSM20750 | 0.12 (1) | |
| S. aureus | DSM20231 | 0.20 (1) | |
| S. aureus | BB255 | Metse | 0.26 (1) |
| S. aureus | RN450 | Mets | 0.24 (1) |
| S. aureus | BB270 | Metre, derivative of strain BB255 | 0.21 (1) |
| S. aureus | SG 511 | Mets | 0.13 (1) |
| S. aureus | 581/93 | Metr | 0.21 (1) |
| S. aureus | COL | Metr | 0.20 (1) |
| Brettanomyces sp. | LTH036 | >100 (1) | |
| Candida krusei | LTH062 | >100 (1) | |
| Saccharomyces cerevisiae | LTH218 | >100 (1) | |
| Saccharomyces cerevisiae | LTH072 | >100 (1) |
Mean ± standard deviation of n determinations. The coefficient of variation of the assay system was generally 20% or less.
LPS chemotype. EHEC, enterohemorrhagic E. coli.
Resistant to cephalothin, erythromycin, clindamycin, gentamicin, tetracyclin, and vancomycin (G. Reuter, personal communication).
SER, sourdough extract rye (in-house sourdough used for production of a commercial baking aid).
Mets and Metr, methicillin sensitive and resistant, respectively. S. aureus data from H. Maidhoff (personal communication).
Inhibitory activity of synthetic reutericyclin.
The inhibitory activity of reutericyclin purified from cultures of L. reuteri LTH2584 was compared to that of synthetic reutericyclin, kindly provided by Udo Marquardt (EMC microcollections GmbH, Tübingen, Germany), using L. sanfranciscensis ATCC 27651 and L. reuteri LTH2584 as indicator strains.
Determination of the molecular mass of reutericyclin.
The molecular mass of purified reutericyclin was determined by electrospray ionization mass spectrometry on a API III triple-quadrupole mass spectrometer (Sciex, Thornhill, Canada) equipped with a nebulizer-assisted electrospray source.
RESULTS
Preliminary characterization of the antimicrobial compound produced by L. reuteri LTH2584.
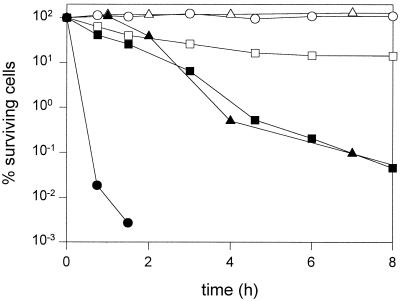
We previously reported the production of antimicrobial activity by L. reuteri LTH2584 (21). The inhibitory activity of NCS was not abolished upon incubation with proteinase K or trypsin, excluding any possibility that bacteriocins are the antimicrobially active components of L. reuteri NCS. The bactericidal activity of L. reuteri LTH2584 NCS was evaluated using L. sanfranciscensis ATCC 27651, B. subtilis FAD109, and S. aureus LTH1493 as target organisms. As shown in Fig. 1, the NCS strongly reduced the cell counts of all target organisms. L. sanfranciscensis was the most sensitive indicator strain; the cell counts were reduced by 5 orders of magnitude within 1.5 h. The activity of NCS of L. reuteri was determined against vegetative cells and spores of B. subtilis FAD109 under conditions permitting or not permitting growth. NCS reduced the numbers of vegetative cells of B. subtilis in mMRS4 and mMRS4 (5% NaCl) by 3 and 5 log units, respectively within 16 h. NCS inhibited the germination of spores of B. subtilis in mMRS4; however, the spores remained unaffected by NCS under conditions not permitting spore outgrowth (data not shown).
FIG. 1.
Killing of L. sanfranciscensis ATCC 27651 (●), B. subtilis FAD109 (■), and S. aureus LTH1493 (▴) by NCS of L. reuteri LTH2584. Open symbols indicate controls (addition of mMRS4). Media were inoculated with 5 × 106 to 1 × 107 CFU ml−1, and the detection limit was 120 CFU ml−1.
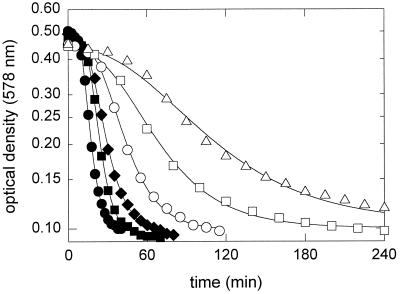
Cells of L. sanfranciscensis ATCC 27651 incubated in mMRS4 (pH 5.0) (4% NaCl) were lysed by NCS in a dose-dependent manner (Fig. 2). The lysis of the target organism was well described by fitting the OD data to the Fermi equation (42) with the parameters lysis rate (κmax) and time required for 50% reduction of the OD (time at κmax, τ50). κmax and τ50 correlated well with the amount of NCS used in the assay (r2 = 0.984 and 0.989 for κmax and τ50, respectively). Lysis of L. sanfranciscensis was not observed when the target cells were incubated with NCS in phosphate buffer, indicating that NCS triggered lysis but was not the lytic principle.
FIG. 2.
Lysis of L. sanfranciscensis ATCC 27651 in mMRS4 (pH 5.0) (4% NaCl) in the presence of 91% (●), 45% (■), 23% (⧫), 11% (○), 5.7% (□), or 2.8% (▵) NCS of L. reuteri LTH2584. The lines represent the regression curves obtained by fitting the data to the logistic growth curve.
Effect of the fatty acid source in the growth medium on the adsorption of reutericyclin to the producer cell walls.
Tween 80 (polyethoxy sorbitol monooleate) added to MRS at 1 g liter−1 is the main source of oleic acid, which is essential for the growth of L. sanfranciscensis (53). Likewise, no growth of L. reuteri LTH2584 was observed in mMRS4 without Tween 80 but this organism grew to the same cell counts in media where Tween 80 was replaced by oleic acid, linoleic acid, or wheat germ oil (data not shown). However, in addition to its role as growth factor for LAB, Tween 80 affects the solubility and activity of hydrophobic antimicrobials due to its emulsifying properties (29). To determine the effect of Tween 80 on the inhibitory activity of NCS of L. reuteri LTH2584, this compound was replaced by oleic acid, linoleic acid, or wheat germ oil. The inhibitory activity of the NCS is shown in Table 1. It is striking that a high inhibitory activity was observed only in medium containing Tween 80 whereas little or no inhibitory activity was detected in NCS of cultures containing free fatty acids or wheat germ oil. Inhibitory activity could be extracted from the producer cells when these were treated with 50 mM phosphate buffer (pH 6.5) containing 30% isopropanol. Table 1 further shows the activity of cell extracts prepared from cells grown with the various sources of unsaturated fatty acids. Remarkably, the extracts from Tween 80- and oleic acid-grown cells exhibited the same inhibitory activity. The activity recovered from wheat germ oil-grown cells was higher, whereas only low activity was associated with cells grown in the presence of linoleic acid. These results indicate that the fatty acid source in the growth medium affects the production of inhibitory activity by L. reuteri LTH2584. Wheat germ oil, the natural source of unsaturated fatty acid in wheat sourdoughs, resulted in the highest inhibitory activities in cell extracts.
TABLE 1.
Effect of fatty acid source on the inhibitory activity of L. reuteri LTH2584 culture supernatants and cell extracts
| Source of fatty acids in mMRS4 | Inhibitory activity (AU/ml) recovered ina:
|
|
|---|---|---|
| NCS | Cell extracts | |
| Tween 80 | 77 ± 5 | 33 ± 3 |
| Oleic acid | 2 ± 1 | 34 ± 4 |
| Linoleic acid | 1 ± 1 | 20 ± 2 |
| Wheat germ oil | 1 ± 2 | 52 ± 4 |
Values are means ± standard deviation of triplicate determinations.
Effect of emulsifiers on inhibitory activity of L. reuteri LTH2584.
The experiments described above indicated that the type of fatty acid affected the inhibitory activity associated with the producer cells but provided no explanation for the effect of the source of fatty acids on the inhibitory activity of the culture supernatant. To determine the functional properties of the oleic acid source responsible for this effect, i.e., its addition as free fatty acid or as part of a more complex molecule with emulsifying properties, Tween 80 was replaced by other emulsifiers, all of which contained an oleoyl moiety and thus met the growth requirement of L. reuteri LTH2584 with respect to oleic acid. The inhibitory activities of culture supernatants of L. reuteri grown in these media were compared to that of mMRS4 (oleate)-grown cultures. In a second set of experiments, the ability of these emulsifiers to solubilize inhibitory activity from mMRS4 (oleate)-grown cells was evaluated. The antimicrobial activity of the NCS prepared from these cultures is shown in Table 2. The presence of Tween 80 or Lamegin GLO30 in the growth medium resulted in high inhibitory activities of the respective NCS. Correspondingly, desorption of inhibitory activity from mMRS4 (oleate)-grown cells was achieved by addition of Tween 80 or Lamegin GLO30 to mMRS4 (oleate)-grown cultures. Desorption of inhibitory activity from oleate-grown L. reuteri by these emulsifiers amounted to about 50% of the activity recovered with the isopropanol-phosphate buffer extraction (34 AU ml−1 [Table 1]). Addition of Lamesorb SMO or Lamegin ZE 609 O18 to the growth medium resulted in low inhibitory activities of NCS, comparable to that of oleate-grown cultures, and these compounds furthermore failed to solubilize inhibitory activity from oleate-grown cells. These data are strongly in support of the hypothesis that (i) the inhibitory activity attached to producer cells depends on the type of fatty acids in the growth medium and (ii) the inhibitory activity of the culture supernatant is determined mainly by the solubility of the active compound, which is apparently greatly enhanced by emulsifiers such as Tween 80.
TABLE 2.
Effect of emulsifiers on reutericyclin production
| Source of fatty acids added before or after growth | Inhibitory activity (AU ml−1) of NCS prepared froma:
|
|
|---|---|---|
| Culture in mMRS4 with Tween 80 replaced by emulsifiers | Culture in mMRS4 (oleate), emulsifiers added after growth | |
| Oleate (control) | 4.5 ± 0.9 | 6.3 ± 2.3 |
| Lamesorb SMO | 10 ± 7.3 | 5.2 ± 0.7 |
| Lamegin GLO30 | 85 ± 38 | 16 ± 5.7 |
| Lamegin ZE 609 O18 | 7.8 ± 1.1 | 6.2 ± 2.6 |
| Tween 80 | 86 ± 21 | 14 ± 10 |
| Wheat germ oil | 7.8 ± 2.8 | NDb |
Shown is the mean ± standard deviation of two independent experiments.
ND, not determined.
Effect of pH and NaCl concentration on the inhibitory activity of L. reuteri LTH2584.
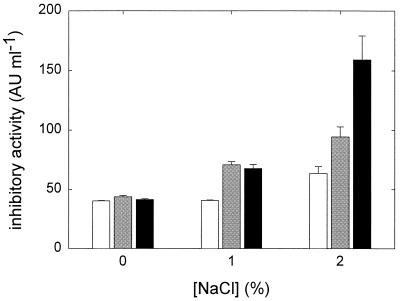
It is well established that bacteriocins of LAB may act synergistically with other preservative principles in fermented food, e.g., low pH and high NaCl concentrations (4, 18). The pH drop during cereal fermentations with L. reuteri thus may strongly affect its inhibitory activity in dough. The effect of pH and NaCl concentration on the inhibitory activity of L. reuteri NCS was evaluated using L. sanfranciscensis ATCC 27651 as an indicator organism. Acidity and NaCl concentrations in the ranges used in this work have no major effect on the growth of this strain (34). Figure 3 shows the effect of pH and NaCl concentration on the inhibitory activity of L. reuteri NCS. Synergistic effects were observed both at low pH and high NaCl concentrations, indicating that the inhibitory activity of L. reuteri LTH2584 should increase in fermented foods.
FIG. 3.
Effect of pH and NaCl concentration on the inhibitory activity of NCS. Shown are the activity at pH 4.5 (▪), 5.0 ( ) and 5.5 (□). The means of two experiments are shown; error bars indicate standard deviation.
) and 5.5 (□). The means of two experiments are shown; error bars indicate standard deviation.
Purification of reutericyclin.
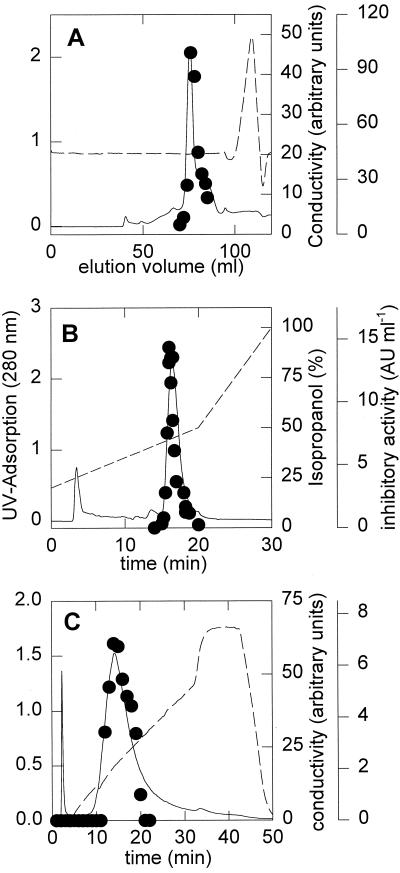
Reutericyclin was purified from cell extracts of Tween 80-, oleic acid-, and wheat germ oil-grown cells of L. reuteri LTH2584. The purification protocol for cell extracts included gel filtration, chromatography on a preparative RP-C8 column, and rechromatography on an RP-C18 HPLC column. The chromatograms for the gel filtration and the RP-C8 column are shown in Fig. 4A and B, respectively. Reutericyclin was furthermore purified from culture supernatant of the producer organism in mMRS4 with a yield of 12.3%. The purification from culture supernatant required an additional purification step using a MonoQ anion-exchange column (Fig. 4C), followed by desalting on the gel filtration column.
FIG. 4.
Chromatograms of reutericyclin on a Superdex 30 gel filtration column (A), an RP-C8 column (B), and a MonoQ anion-exchange column (C). Shown are the UV absorption at 280 nm (solid line), the conductivity (dotted line), and the inhibitory activity (●).
The activity recovered from the cell extracts of Tween 80- and oleic acid-grown cells is shown in Table 3. The active peak in the cell extract accounted for >90% of the UV-absorbing material (Fig. 4A), and the ratio of UV absorption to inhibitory activity did not change during the purification protocol. Contaminants present in the active fractions eluting from the gel filtration, RP chromatography, and anion-exchange columns were detectable only by mass spectrometry. Depending on whether the purification protocol was based on the cell extract or the culture supernatant, the yield was calculated on the basis of the total inhibitory activity of the cell extracts and the culture supernatants, respectively. Taking into account the accuracy of the assay system for determination of inhibitory activity (coefficient of variation = 30%) and inevitable losses during liquid handling, the purification protocol was performed with a virtually quantitative yield of inhibitory activity.
TABLE 3.
Purification protocol of reutericyclin
| Purification step | mMRS4 (oleic acid)
|
mMRS4
|
||||
|---|---|---|---|---|---|---|
| Vol (ml) | Total activity (103 AU) | Yield (%) | Vol (ml) | Total activity (103 AU) | Yield (%) | |
| Cell extract | 500 | 150 | 100 | 500 | 100 | 100 |
| Gel filtration | 1 | 120 | 80 | 1 | 70 | 70 |
| RP-FPLC | 1 | 90 | 60 | 1 | 72 | 72 |
| RP-HPLC | 0.5 | 70 | 50 | 0.5 | 23 | 23 |
The molecular mass of the purified active compound was determined by electrospray ionization mass spectrometry using material purified from cell extracts of mMRS4 (oleic acid)-, mMRS4 (wheat germ oil)-, and mMRS4-grown cells as well as culture supernatant of mMRS4 grown cells. These preparations yielded identical mass spectra. The spectra obtained in the negative mode showed a single peak at m/z 348, which was interpreted as [M − H]−, corresponding to a molecular mass of reutericyclin of 349 Da. The [M + H]+ peak at m/z 350, as well as the Na+ and K+ adducts of reutericyclin, were observed when electrospray ionization mass spectrometry was used in the positive mode (data not shown). Thus, the antimicrobial activity of L. reuteri LTH2584 can be attributed to a single compound, reutericyclin.
MIC and inhibitory spectrum of reutericyclin.
The MIC of reutericyclin purified to homogeneity was determined against L. sanfranciscensis ATCC 27651 as a target strain in three independent experiments performed in duplicate and found to be 0.1 ± 0.02 mg l−1. The inhibitory spectrum of reutericyclin was determined using the strains listed in Table 4. Virtually all gram-positive indicator strains were sensitive to reutericyclin; the sensitivity was in the range of 0.05 to 1 mg liter−1. It was verified for 15 of the 63 strains that the inhibitory spectrum of purified reutericyclin did not differ appreciably from that of the NCS of the producer organism (data not shown). Remarkably, several organisms recognized as food pathogens or opportunistic pathogens, as well as toxigenic organisms, are included in the inhibitory spectrum, i.e., B. cereus, S. aureus, Enterococcus faecalis, and Listeria ivanovii. The sensitivity of B. subtilis strains known to cause ropiness of bread (43) was evaluated, and the sensitivity of these spoilage organism to reutericyclin was in the same range as that of the DSM reference strains (0.14 to 0.3 mg liter−1). Germinating spores of B. subtilis FAD109 were less sensitive than were vegetative cells of the same strain. No cross-resistance was observed with clinical isolates of Enterococcus faecium resistant to β-lactam antibiotics, erythromycin, and vancomycin or with methicillin-resistant staphylococci. S. aureus BB270, a methicillin-resistant derivative of S. aureus BB255, exhibited the same sensitivity as the parent strain. The reutericyclin producer strain, L. reuteri LTH2584, tolerated concentrations of up to 6.4 mg liter−1. The inhibitory-spectrum screening included lactobacilli isolated from the same batch of SER sourdough as the reutericyclin producer strain (5). Remarkably, these strains were rather resistant to reutericyclin (MIC, 0.3 to 0.7 mg liter) compared to other sourdough isolates, for which the MICs ranged from 0.1 to 0.2 mg liter−1. This observation does conform with the assumption that reutericyclin may be produced in sourdough to exert selective pressure on competitors of the producer strain.
The gram-negative organisms including the pathogenic Escherichia coli O157:H7 were resistant to reutericyclin. However, growth inhibition of E. coli LTH1600 by reutericyclin was observed at a medium pH of 4.5 (data not shown). To evaluate a possible role of the outer membrane of gram-negative bacteria in resistance to the hydrophobic reutericyclin, the sensitivity of lipopolysaccharide (LPS) mutant strains was compared to that of wild-type strains with smooth LPS. The deep rough mutants E. coli F515 and WBB06 (LPS chemotype Re), as well as E. coli F470 (LPS chemotype R1, core without O antigen), were sensitive to reutericyclin. The sensitivity of the LPS mutant strains was more than 1 order of magnitude lower than that of the gram-positive bacteria. Growth of yeasts was not inhibited by reutericyclin.
Inhibitory activity of synthetic reutericyclin.
Höltzel et al. (27) recently elucidated the structure of reutericyclin [3-acetyl-1-(2-trans-decenoyl)-2-hydroxy-(5R)-isobutyl-Δ2-pyrroline-4-one], which allowed the preparation of synthetic reutericyclin as racemic mixture of (5R, 5S) reutericyclin (36). This racemic mixture was found to inhibit the growth of L. sanfranciscensis ATCC 27651 with an MIC of 0.75 ± 0.14 mg liter−1, whereas the reutericyclin producer strain, L. reuteri LTH2584, was highly resistant (MIC > 20 mg liter−1). This finding is further proof that reutericyclin is indeed the active inhibitory compound of NCS of L. reuteri LTH2584 and furthermore indicates that the stereochemistry of reutericyclin is important for its antimicrobial activity.
DISCUSSION
We have purified an antimicrobial compound, reutericyclin, from cultures of L. reuteri LTH2584. Reutericyclin is a novel tetramic acid derivative structurally related to tenuazonic acid (27) and differs in its chemical structure and biological activity from any other known compound produced by LAB. The majority of naturally occurring tetramic acids isolated to date exhibit biological activity. Much research interest has been focused on tenuazonic acid produced by Alternaria species because of its broad spectrum of biological activity, which includes antibacterial, cytotoxic, and antitumor activities (23). The toxicity of tenuazonic acid has impeded its clinical application. Provided that reutericyclin does not resemble tenuazonic acid regarding its cytotoxicity, its inhibitory spectrum shows potential for use as antibiotic in clinical and food applications. The characterization of reutericyclin and the physiological properties of L. reuteri LTH2584 presented in this study will allow an evaluation of a possible role of antimicrobials in the sourdough environment. Furthermore, the potential for application of reutericyclin in bread and related products can be assessed.
The ability of strains of L. reuteri to produce a large variety of inhibitory compounds, including reutericin (31) and reuterin (1), raises the question whether such compounds, in addition to reutericyclin, contribute to the inhibitory activity of L. reuteri NCS. The following observations argue in favor of the assumption that reutericyclin is the major, if not the only, antimicrobially active compound produced by L. reuteri LTH2584. (i) NCS inhibitory activity was not affected by proteases, thus excluding a contribution by bacteriocins. (ii) Reuterin production did not occur in media with maltose, glucose, or fructose as the sole source of carbon but required the presence of glycerol (1). Furthermore, gram-negative organisms have been found to be the most sensitive indicator strains for reuterin (1), whereas these organisms were not inhibited by L. reuteri LTH2584. (iii) Reutericyclin purification from cultures of L. reuteri LTH2584 was achieved with a virtually quantitative yield of inhibitory activity, thus excluding any possibility that compounds other than reutericyclin played a major role in the inhibitory activity of L. reuteri NCS.
The production of antimicrobial compounds by LAB may provide the producer organisms with an ecological advantage in their habitat (59). Olsen et al. (41) characterized the microflora of kenkey, a fermented maize produced by the spontaneous fermentation of the raw material, and concluded that both acid production and specific antagonistic activities play an important role in obtaining a stable population of LAB. Bacteriocin production was suggested to contribute to the dominance of strains of L. plantarum and L. bavaricus in rye sourdoughs (35).
L. reuteri has frequently been isolated from the intestines of humans and animals (8) and is a predominant organism in sourdoughs as well as cereal fermentations in tropical climates (57). L. reuteri LTH2584 was isolated from SER, a type II sourdough, by Böcker et al. (5). This in-house, rye-based sourdough is fermented at temperatures above 35°C and after drying is used for the production of a baking aid. The parameters required for optimum production of reutericyclin by L. reuteri LTH2584 match those encountered in sourdough fermentation, i.e., pH values of 3.5 to 5.5 and temperatures above 30°C (21). This study has shown that the natural source of fatty acids in wheat doughs favored reutericyclin production compared to other fatty acids. Furthermore, reutericyclin activity against L. sanfranciscensis was increased at the low pH of sourdough. The five strains of LAB isolated from the same batch of SER as the reutericyclin producer, L. reuteri LTH2584, exhibited higher resistance to reutericyclin than did other sourdough isolates, indicating that reutericyclin may exert selective pressure in situ. Reutericyclin production may thus contribute to the competitiveness of L. reuteri in the dough environment.
Reutericyclin shares characteristic properties with bacteriocins from LAB, although its chemical structure is different. Comparable to bacteriocins, it is an amphiphilic molecule with a tendency to form aggregates in aqueous solution. Similar to the kinetics of bacteriocin production by LAB, reutericyclin production by L. reuteri LTH2584 is described by primary-metabolite kinetics (14, 21), and the compound adsorbs to the producer cell walls (12, 60). In accordance with the behavior of bacteriocins, the addition of acid and NaCl increased its inhibitory activity (4, 18). Most remarkably, the MIC of reutericyclin against the most sensitive indicator strain, E. faecalis (0.05 mg liter−1), is about 50 times higher than that reported for bacteriocins. Curvacin A and sakacin P were shown to inhibit the most sensitive indicator strains, L. sakei and Carnobacterium piscicola, respectively, at levels of 0.001 mg liter−1 (16), and nisin inhibits strains of L. sakei at 0.003 mg liter−1 (2). However, whereas 100- to 1,000-fold differences in sensitivity to bacteriocins were observed within one species (2, 16), virtually all gram-positive indicator strains with the exception of the reutericyclin producer were inhibited by reutericyclin at concentrations of 0.05 to 0.9 mg liter−1; i.e., the differences in sensitivity were less than 20-fold.
Reutericyclin triggered the lysis of L. sanfranciscensis; however, this lytic activity was not observed under all assay conditions. Therefore, autolysins of L. sanfranciscensis (13) rather than reutericyclin itself appear to be the lytic principle. It was reported previously that lactococcins A, B, and M, which exhibit no lytic activity per se, trigger a series of events that eventually result in the lysis of sensitive indicator strains (38). Cheese-making trials employing lactococcin A-, B-, and M-producing starter cultures in combination with sensitive cultures were shown to accelerate cell lysis in the cheese-making process. Lysis of lactic starter cultures liberated intracellular peptidases and increased proteolysis and the generation of aroma compounds during cheese ripening (37). Aroma development in baked cereal goods depends on the proteolytic liberation of amino acids during dough fermentation, and therefore reutericyclin-induced autolysis of cereal starters may positively affect aroma development in bread.
The sensitivity of rope-forming bacilli to reutericyclin suggests the application of reutericyclin to prevent ropy spoilage of bread. B. subtilis is recognized as the causative agent of this spoilage problem (43, 45). The ability of the spores to survive during baking (100°C for 15 to 60 min) and high amylase and protease activities have been identified as characteristics of strains of rope-forming bacilli (43, 45). The acidification and production of organic acids by heterofermentative lactobacilli in sourdough prevents ropy spoilage of bread (43, 44). However, the levels of organic acids in most bread varieties are too low to inhibit B. subtilis. The use of antimicrobials produced in situ by LAB as preservatives in bread has been proposed by Rosenquist and Hansen (44). Nisin failed to inhibit B. subtilis and B. licheniformis in bread despite its in vitro inhibitory activity. Since reutericyclin resists proteolytic degradation during dough fermentation, it may contribute to the antagonistic effect of sourdough on rope-forming bacilli.
The inhibitory spectrum of reutericyclin does not include Escherichia coli and Salmonella. The chemical composition and biophysical structure of the LPS located in the outer membrane of gram-negative bacteria confers a high degree of resistance of these organisms to hydrophobic antibiotics (39). Evidence that the outer membrane of gram-negative bacteria confers resistance to bacteriocins of LAB was initially provided by Stevens et al. (50), who observed that EDTA-treated cells of Salmonella lost their nisin resistance. LPS mutant strains with a well-defined composition of the outer membrane further allow the assessment of resistance mechanisms of gram-negative organisms to antibiotics (56). The observation that LPS mutant strains of Salmonella enterica and E. coli were highly sensitive to nisin, curvacin A, and other bacteriocins of LAB whereas the wild-type strains were resistant further emphasized the prime importance of the gram-negative outer membrane for bacteriocin resistance (17, 22). Remarkably, factors that increase the sensitivity of gram-negative bacteria to nisin and curvacin A, i.e., truncated LPS, low pH, and high salt concentrations, also increase the sensitivity of these organisms to reutericyclin, indicating that reutericyclin resistance is based on similar mechanisms. Thus, reutericyclin-mediated killing of gram-negative pathogens is possible by appropriate processes or storage conditions that disrupt the outer membrane and allow the bacteriocin-mediated inactivation of Salmonella and E. coli (48).
The intestinal microflora of humans and animals exerts a strong effect on the health of the hosts (28). Strains of L. reuteri colonize the intestines of humans and animals and enhance host resistance to bacterial and viral infections (8, 33). The production of antimicrobials by lactobacilli used as dietary adjuncts contribute to these protective effects (3, 9). Although the scarce data provided on the chemical properties of antimicrobials produced by lactobacilli of intestinal origin do not allow us to determine whether these compounds are structurally related to reutericyclin, reutericyclin represents a new class of compounds produced by lactobacilli and reutericyclin or related compounds may be important in intestinal microbiology. However, it remains questionable whether L. reuteri LTH2584 is a suitable probiotic strain. The organism was found by Hammes et al. (25) to have a low tolerance to hydrochloric acid and bile compared to other food-fermenting lactobacilli and to a strain of L. johnsonii of intestinal origin. Adaptation of strains of L. reuteri to the sourdough environment apparently requires the acquisition of properties different from those allowing the stable establishment of strains of the same species in the intestinal tract.
ACKNOWLEDGMENTS
We thank G. Reuter (Berlin), H. Maidhof (Berlin), W. Röcken (Detmold), and W. Brabetz (Borstel) for providing bacterial strains; Dagmar Glenewinkel for excellent technical assistance; and Christian Hertel and Gudrun Wolf for helpful discussions during the work. We are further indebted to Udo Marquardt, EMC microcollections GmbH, Tübingen, Germany, for kindly providing synthetic reutericyclin.
REFERENCES
- 1.Axelsson L T, Chung T C, Dobrogosz W J, Lindgren S E. Production of a broad spectrum antimicrobial substance by Lactobacillus reuteri. Microb Ecol Health Dis. 1989;2:131–136. [Google Scholar]
- 2.Bennik M H J, Verheul A, Abee T, Naaktgeboren-Stoffels G, Gorris L G M, Smid E J. Interactions of nisin and pediocin PA-1 with closely related lactic acid bacteria that manifest over 100-fold differences in bacteriocin sensitivity. Appl Environ Microbiol. 1997;63:3628–3639. doi: 10.1128/aem.63.9.3628-3636.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernet-Camard M-F, Lievin V, Brassart D, Neeser J-R, Servin A L, Hudault S. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl Environ Microbiol. 1997;63:2747–2753. doi: 10.1128/aem.63.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blom H, Katla T, Hagen B F, Axelsson L. A model assay to demonstrate how intrinsic factors affect diffusion of bacteriocins. Int J Food Microbiol. 1997;38:103–109. doi: 10.1016/s0168-1605(97)00098-6. [DOI] [PubMed] [Google Scholar]
- 5.Böcker G, Stolz P, Hammes W P. Neue Erkenntnisse zum Ökosystem Sauerteig und zur Physiologie der sauerteig-typischen Stämme Lactobacillus sanfrancisco und Lactobacillus pontis. Getreide Mehl Brot. 1995;49:370–374. [Google Scholar]
- 6.Böcker G, Vogel R F, Hammes W P. Lactobacillus sanfrancisco als stabiles Element in einem Reinzucht-Sauerteig Präparat. Getreide Mehl Brot. 1990;44:269–271. [Google Scholar]
- 7.Brabetz W, Müller-Loennies S, Holst O, Brade H. Deletion of the heptosyltransferase genes rfaC and rfaF in Escherichia coli K-12 results in an Re-type lipopolysaccharide with a high degree of 2-aminoethanol phosphate substitution. Eur J Biochem. 1997;247:716–724. doi: 10.1111/j.1432-1033.1997.00716.x. [DOI] [PubMed] [Google Scholar]
- 8.Casas I A, Dobrogosz W J. Lactobacillus reuteri: overview of a new probiotic for humans and animals. Microecol Ther. 1997;26:221–231. [Google Scholar]
- 9.Coconnier M-H, Lievin V, Hemery E, Servin A L. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl Environ Microbiol. 1998;64:4573–4580. doi: 10.1128/aem.64.11.4573-4580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coconnier M-H, Lievin V, Bernet-Camard M-F, Hudault S, Servin A L. Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob Agents Chemother. 1997;41:1046–1052. doi: 10.1128/aac.41.5.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corsetti A, Gobbetti M, Smacchi E. Antibacterial activity of sourdough lactic acid bacteria: isolation of a bacteriocin-like inhibitory substance from Lactobacillus sanfrancisco C57. Food Microbiol. 1996;13:447–456. [Google Scholar]
- 12.Daba H, Lacroix C, Huang J, Simard R E, Lemieux L. Simple method of purification and sequencing of a bacteriocin produced by Pediococcus acidilactici UL5. J Appl Bacteriol. 1994;77:682–688. doi: 10.1111/j.1365-2672.1994.tb02819.x. [DOI] [PubMed] [Google Scholar]
- 13.De Angelis M, Pollacci P, Gobbetti M. Autolysis of Lactobacillus sanfranciscensis. Eur Food Res Technol. 1999;210:57–61. [Google Scholar]
- 14.De Vuyst L, Callewaert R, Crabbé K. Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology. 1996;142:817–827. doi: 10.1099/00221287-142-4-817. [DOI] [PubMed] [Google Scholar]
- 15.De Vuyst L, Vandamme E J. Antimicrobial potential of lactic acid bacteria. In: De Vuyst L, Vandamme E J, editors. Bacteriocins of lactic acid bacteria. London, United Kingdom: Chapman & Hall; 1994. pp. 91–142. [Google Scholar]
- 16.Eijsink V G, Skeie M, Middelhoven P H, Brurberg M-B, Nes I F. Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl Environ Microbiol. 1998;64:3275–3281. doi: 10.1128/aem.64.9.3275-3281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gänzle M G, Hertel C, Hammes W P. Resistance of Escherichia coli and Salmonella against nisin and curvacin A. Int J Food Microbiol. 1999;48:37–50. doi: 10.1016/s0168-1605(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 18.Gänzle M G, Weber S, Hammes W P. Effect of ecological factors on the inhibitory spectrum and activity of bacteriocins. Int J Food Microbiol. 1999;48:207–217. doi: 10.1016/s0168-1605(98)00205-0. [DOI] [PubMed] [Google Scholar]
- 19.Gänzle M G, Ehmann M, Hammes W P. Modeling of growth of Lactobacillus sanfranciscensis and Candida milleri in response to process parameters of the sourdough fermentation. Appl Environ Microbiol. 1998;64:2616–2623. doi: 10.1128/aem.64.7.2616-2623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gänzle M G, Hertel C, Hammes W P. Antimicrobial activity of bacteriocin-producing cultures in meat products. Modelling of the effect of pH, NaCl, and nitrite concentrations on the antimicrobial activity of sakacin P against Listeria ivanovii DSM20750. Fleischwirtschaft. 1996;76:409–412. [Google Scholar]
- 21.Gänzle M G, Hertel C, Hammes W P. Antimicrobial activity in lactobacilli from sourdough. In: Scheffers H W A, van Dijken J P, editors. Beijerinck Centennial. Microbial physiology and gene regulation: emerging principles and applications. Delft, The Netherlands: Delft University Press; 1995. pp. 380–381. [Google Scholar]
- 22.Gao Y, van Belkum M J, Stiles M E. The outer membrane of gram-negative bacteria inhibits antibacterial activity of brochocin-C. Appl Environ Microbiol. 1999;65:4329–4333. doi: 10.1128/aem.65.10.4329-4333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gittermann C O. Antitumor, cytotoxic, and antibacterial activities of tenuazonic acid and congeneric tetramic acids. J Med Chem. 1965;8:483–486. doi: 10.1021/jm00328a015. [DOI] [PubMed] [Google Scholar]
- 24.Hammes W P, Hertel C. New developments in meat starter cultures. Meat Sci. 1998;49:S125–S138. [PubMed] [Google Scholar]
- 25.Hammes W P, Haller D, Brassart D, Bode C. Traditional starter cultures as probiotics. Microecol Ther. 1997;26:97–114. [Google Scholar]
- 26.Hammes W P, Stolz P, Gänzle M G. Metabolism of lactobacilli in traditional sourdoughs. Adv Food Sci. 1996;18:176–184. [Google Scholar]
- 27.Höltzel A, Gänzle M G, Nicholson G J, Hammes W P, Jung G. The first low-molecular-weight antibiotic from lactic acid bacteria: reutericyclin, a new tetramic acid. Angew Chem Int Ed. 2000;39:2766–2768. [PubMed] [Google Scholar]
- 28.Huis in't Veld J H J, Havenaar R. Selection criteria and applications of probiotic microorganisms in man and animal. Microecol Ther. 1997;26:43–57. [Google Scholar]
- 29.Huot E, Barrena-Bonzalez C, Petitdemange H. Tween 80 effect on bacteriocin synthesis by Lactococcus lactic subsp. cremoris J46. Lett Appl Microbiol. 1996;22:307–310. doi: 10.1111/j.1472-765x.1996.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 30.Joosten H M L J, Nunez M. Prevention of histamine formation in cheese by bacteriocin-producing lactic acid bacteria. Appl Environ Microbiol. 1996;62:1178–1181. doi: 10.1128/aem.62.4.1178-1181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kabuchi T, Saito T, Kawai Y, Uemura J, Itoh T. Production, purification and characterization of reutericin 6, a bacteriocin with lytic activity produced by Lactobacillus reuteri LA6. Int J Food Microbiol. 1997;34:145–156. doi: 10.1016/s0168-1605(96)01180-4. [DOI] [PubMed] [Google Scholar]
- 32.Klaenhammer T R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–86. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 33.Klein G, Eichberg J, Grund S, Reuter G. Characterisation of a potential probiotic strain of Lactobacillus reuteri isolated from pigeon crop. Microecol Ther. 1997;26:233–241. [Google Scholar]
- 34.Kline L, Sugihara T F. Microorganisms of the San Francisco sour dough bread process. II. Isolation and characterization of undescribed bacterial species responsible for the souring activity. Appl Microbiol. 1971;21:456–465. doi: 10.1128/am.21.3.459-465.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsen A G, Vogensen F K, Josephsen J. Antimicrobial activity of lactic acid bacteria isolated from sour doughs: purification and characterization of bavaricin A, a bacteriocin produced by Lactobacillus bavaricus MI401. J Appl Bacteriol. 1993;75:113–122. doi: 10.1111/j.1365-2672.1993.tb02755.x. [DOI] [PubMed] [Google Scholar]
- 36.Marquardt U, Schmid D, Jung G. Racemic synthesis of the new antibiotic tetramic acid reutericyclin. Synlett. 2000;2000:1131–1132. [Google Scholar]
- 37.Morgan S, Ross R P, Hill C. Increasing starter cell lysis in cheddar cheese using a bacteriocin-producing adjunct. J Dairy Sci. 1997;80:1–10. [Google Scholar]
- 38.Morgan S, Ross R P, Hill C. Bacteriolytic activity caused by the presence of a novel lactococcal plasmid encoding lactococcins A, B, and M. Appl Environ Microbiol. 1995;61:2995–3001. doi: 10.1128/aem.61.8.2995-3001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikaido H. Outer membrane. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 29–47. [Google Scholar]
- 40.Niku-Paavola M-L, Laitila A, Mattila-Sandholm T, Haikara A. New types of antimicrobial compounds produced by Lactobacillus plantarum. J Appl Microbiol. 1999;86:29–36. doi: 10.1046/j.1365-2672.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- 41.Olsen A, Halm M, Jakobsen M. The antimicrobial activity of lactic acid bacteria from fermented maize (kenkey) and their interactions during fermentation. J Appl Bacteriol. 1995;79:506–512. doi: 10.1111/j.1365-2672.1995.tb03170.x. [DOI] [PubMed] [Google Scholar]
- 42.Peleg M. A model of microbial growth and decay in a closed habitat based on combined Fermi's and the logistic equations. J Sci Food Agric. 1996;71:225–231. [Google Scholar]
- 43.Röcken W, Spicher G. Fadenziehende Bakterien—Vorkommen, Bedeutung, Gegenmaßnahmen. Getreide Mehl Brot. 1993;47:30–35. [Google Scholar]
- 44.Rosenquist H, Hansen A. The antimicrobial effect of organic acids, sour dough and nisin against Bacillus subtilis and B. licheniformis isolated from wheat bread. J Appl Microbiol. 1998;85:621–631. [Google Scholar]
- 45.Rosenkvist H, Hansen A. Contamination profiles and characterisation of Bacillus species in wheat bread and raw materials for bread production. Int J Food Microbiol. 1995;26:353–363. doi: 10.1016/0168-1605(94)00147-x. [DOI] [PubMed] [Google Scholar]
- 46.Ryan M P, Meaney W J, Ross R P, Hill C. Evaluation of lacticin 3147 and a teat seal containing this bacteriocin for inhibition of mastitis pathogens. Appl Environ Microbiol. 1998;64:2287–2290. doi: 10.1128/aem.64.6.2287-2290.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt G, Jann B, Jann K. Immunochemistry of R lipopolysaccharides of Escherichia coli. Eur J Biochem. 1970;16:382–392. doi: 10.1111/j.1432-1033.1970.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 48.Shefet S M, Sheldon B W, Klaenhammer T R. Efficacy of optimized nisin-based treatments to inhibit Salmonella typhimurium and extend shelf life of broiler carcasses. J Food Prot. 1995;58:1077–1082. doi: 10.4315/0362-028X-58.10.1077. [DOI] [PubMed] [Google Scholar]
- 49.Silva M, Jacobus N V, Deneke C, Gorbach S L. Antimicrobial substance from a human Lactobacillus strain. Antimicrob Agents Chemother. 1987;31:1231–1233. doi: 10.1128/aac.31.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens K A, Sheldon B W, Klapes N A, Klaenhammer T R. Nisin treatment for inactivation of Salmonella species and other gram-negative bacteria. Appl Environ Microbiol. 1991;57:3613–3615. doi: 10.1128/aem.57.12.3613-3615.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stiles M E. Biopreservation by lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:331–345. doi: 10.1007/BF00395940. [DOI] [PubMed] [Google Scholar]
- 52.Stolz P, Böcker G, Vogel R F, Hammes W P. Utilisation of maltose and glucose by lactobacilli isolated from sourdough. FEMS Microbiol Lett. 1993;109:237–242. [Google Scholar]
- 53.Sugihara T F, Kline L. Further studies on a growth medium for Lactobacillus sanfrancisco. J Milk Food Technol. 1975;38:667–672. [Google Scholar]
- 54.Tagg J R, Dajani A S, Wannamaker L W. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1976;40:722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tichaczek P S, Nissen-Meyer J, Nes I F, Vogel R F, Hammes W P. Characterization of the bacteriocins curvacin A from Lactobacillus curvatus LTH1174 and sakacin P from L. sake LTH673. Syst Appl Microbiol. 1992;15:460–468. [Google Scholar]
- 56.Vaara M. Antibiotic supersusceptible mutants of Escherichia coli and Salmonella typhimurium. Antimicrob Agents Chemother. 1993;37:2255–2260. doi: 10.1128/aac.37.11.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogel R F, Knorr R, Müller M R A, Steudel U, Gänzle M G, Ehrmann M A. Non-dairy lactic fermentations: the cereal world. Antonie Leeuwenhoek. 1999;76:403–411. [PubMed] [Google Scholar]
- 58.Vogel R F, Böcker G, Stolz P, Ehrmann M, Fanta D, Ludwig W, Pot B, Kersters K, Schleifer K H, Hammes W P. Identification of lactobacilli from sourdough and description of Lactobacillus pontis sp. nov. Int J Syst Bacteriol. 1994;44:223–229. doi: 10.1099/00207713-44-2-223. [DOI] [PubMed] [Google Scholar]
- 59.Vogel R F, Pohle B S, Tichaczek P S, Hammes W P. The competitive advantage of Lactobacillus curvatus LTH1174 in sausage fermentations is caused by formation of curvacin A. Syst Appl Microbiol. 1993;16:457–462. [Google Scholar]
- 60.Yang R, Johnson M C, Ray B. Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Appl Environ Microbiol. 1992;58:3355–3359. doi: 10.1128/aem.58.10.3355-3359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]