Summary
Background
COVID-19 vaccination is recommended for patients with multiple sclerosis (pwMS), while disease-modifying therapies (DMTs) may influence the efficacy of SARS-CoV-2 vaccines in this population. Thus, we conducted a meta-analysis to evaluate the impact of DMTs on immune response to COVID-19 vaccines in pwMS.
Methods
Literature search from December 1, 2019 to March 31, 2022 was performed in PubMed, MedRxiv, Embase and Cochrane Library. The risk of impaired response to vaccination in pwMS receiving DMTs was estimated in odds ratios (ORs) using random-effects method.
Findings
A total of 48 studies comprising 6860 pwMS were included. Overall, pwMS with anti-CD20 (OR=0.02, 95% CI: 0.01-0.03) and sphingosine-1-phosphate receptor modulator (S1PRM) (OR=0.03, 95% CI: 0.01-0.06) treatments had attenuated serologic response after full vaccination compared with those without DMTs. Additionally, pwMS vaccinated within six months since last anti-CD20 therapy were at significantly higher risk of blunted response compared with those receiving anti-CD20 therapy more than six months prior to vaccination (P = 0.001). We found no significant associations between other treatments (including IFN-β, GA, DMF, TERI, NTZ, CLAD, and ALE) and humoral response to SARS-CoV-2 vaccines in pwMS. As for T-cell response, no significant difference was found between pwMS on anti-CD20 and those without DMTs after vaccination, while S1PRM was marginally associated with impaired cellular response (P = 0.03).
Interpretation
Our findings suggested that routine serological monitoring may be required for pwMS on anti-CD20 and S1PRMs after SARS-CoV-2 vaccination and highlighted the benefits of a booster dose. The effect of cellular response and optimal interval from last anti-CD20 treatment to vaccination should be further addressed.
Funding
This study was supported by Natural Science Foundation of Shanghai (21ZR1433000).
Keywords: COVID-19 vaccines, Multiple sclerosis, Disease-modifying therapies, Immune response, Meta-analysis
Research in context.
Evidence before this study
Under the context of the COVID-19 pandemic, vaccination against SARS-CoV-2 is the primary strategy to stop this global public health emergency. Although the safety and efficacy of COVID-19 vaccines have been well-established in the general population, concerns about immunogenicity have been raised in patients with multiple sclerosis (pwMS). Previous studies suggested that specific disease-modifying therapies (DMTs), such as anti-CD20 monoclonal antibodies, may decrease immune response to COVID-19 vaccination. Despite the increasing immunogenicity data in MS patients, the impact of a wide spectrum of DMTs, which are often immunosuppressive or immunomodulatory, on response to COVID-19 vaccination is not fully understood. To narrow this knowledge gap, we conducted a comprehensive meta-analysis. Based on literature from PubMed, MedRxiv, Embase and Cochrane Library, the risk of decreased humoral and cellular response to COVID-19 vaccination in pwMS receiving various DMTs was estimated as odds ratios (ORs) with corresponding 95% confidence intervals (CIs) using the random-effects method. In addition, sensitivity analysis and assessment of publication bias and heterogeneity were performed.
Added value of this study
Based on 48 studies with 6860 pwMS, the impacts of various DMTs including anti-CD20, S1PRM, IFN-β, GA, DMF, TERI, NTZ, CLAD, and ALE treatments on response to COVID-19 vaccines were investigated. We found pwMS on anti-CD20 and S1PRM therapies were at higher risk of seronegative response after vaccination compared with untreated MS patients, while there was no significant evidence indicating impaired humoral response in pwMS with other DMTs. Additionally, our finding suggested that the interval (less or more than six months) between last anti-CD20 therapy and vaccination also significantly affected the humoral response in pwMS. The analyses of T-cell response showed no significant difference between pwMS on anti-CD20 therapy and those without DMTs, while S1PRM treatment was marginally associated with an attenuated cellular response.
Implications of all the available evidence
The present study indicated the effectiveness of COVID-19 vaccination might be suboptimal for pwMS with anti-CD20 and S1PRM treatments compared to patients without DMTs. Therefore, routine serological monitoring may be needed, especially for those receiving anti-CD20-directed therapy within 6 months before vaccination. Since pwMS with anti-CD20 therapy have been identified to be at an increased risk of severe COVID-19 and adverse outcomes, an adapted vaccination strategy, such as a third dose, heterologous vaccine regimens and temporary adjustment of therapy, may be required. Current evidence regarding to T-cell response of pwMS on DMTs after vaccination against SARS-CoV-2 was still limited. Thus, the role of post-vaccination cellular response, long-term efficacy of vaccination, and effect of new oral antiviral agents should be further addressed for pwMS on DMTs in the future.
Alt-text: Unlabelled box
Introduction
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to global mortality of over 6 million deaths, with a broad spectrum of clinical manifestations from asymptomatic or mild-to-moderate symptoms, to severe illness with both pulmonary and extrapulmonary manifestations. Safety and efficacy of vaccines developed against SARS-CoV-2 have been well established in the general population, but their performance is still undetermined among certain cohorts with a higher risk of infection. Multiple sclerosis (MS) is an immune-mediated central nervous system (CNS) disorder that requires disease-modifying therapies (DMTs). Although DMTs have been shown to reduce the incidence of relapses and long-term disability,1,2 specific DMTs are reported to elevate the risk of serious infections in patients with MS (pwMS).3 Recent evidence also indicated that MS patients on certain DMTs, such as anti-CD20 monoclonal antibodies, were at an increased risk of severe COVID-19 course and adverse outcomes,4, 5, 6, 7, 8 which made vaccination of prominent importance in this vulnerable population. Although COVID-19 vaccination has been recommended in all pwMS,9 the impact of various DMTs, which are often immunosuppressive or immunomodulatory, on vaccine response is still unclear. Several lines of evidence indicate anti-CD20 therapy commonly used to treat MS as well as other autoimmune disease and hematologic malignancies, may lead to blunted humoral response after vaccination against SARS-CoV-2.10, 11, 12 Attenuated post-vaccination seroconversion of pwMS treated with sphingosine-1-phosphate receptor modulator (S1PRM), such as fingolimod, has also been observed,13,14 while there are inconsistent results.15 Despite accumulating data on immunosuppressive DMTs, the effects of the broad spectrum of MS treatment regimens on the humoral and cellular immune responses after COVID-19 vaccination have not yet been fully understood. Thus, we performed a comprehensive meta-analysis to evaluate the influence of DMTs on immune response to vaccines against SARS-CoV-2 in pwMS.
Methods
Identification and eligibility of relevant studies
This study followed the PRISMA guidelines16 and the protocol was registered with PROSPERO (CRD42022326302). We performed a comprehensive literature search in PubMed, MedRxiv, Embase and Cochrane Library without language restriction from December 1, 2019 to March 31, 2022. The search term included keywords relevant to COVID-19 (“coronavirus disease 2019” OR “covid-19” OR “2019-nCoV” OR “severe acute respiratory syndrome coronavirus 2” OR “sars-cov-2” OR “2019 novel coronavirus”) AND vaccine in combination with words related to multiple sclerosis and DMTs, including interferons (IFN), glatiramer acetate (GA), dimethyl fumarate (DMF), teriflunomide (TERI), S1PRM, natalizumab (NTZ), cladribine (CLAD), alemtuzumab (ALE), and anti-CD20 therapies.
Eligible studies were required to meet the following criteria: clinical studies detecting humoral response based on anti-SARS-CoV-2 [anti-S1 or anti-receptor-binding domain (RBD)] IgG or T-cell response in patients with MS receiving DMTs compared with patients without DMTs; original articles reporting independent data; original articles reporting relative risks with 95% confidence intervals (CIs) or sufficient information for effect size calculation. The titles and abstracts of potential articles were screened by two authors independently, and the full-texts of potentially relevant articles were assessed. The references of included studies were scrutinized and hand-searched for additional eligible studies. The exclusion criteria were commentaries, reviews, non-research letters, case reports, and studies with insufficient data. Studies containing less than five pwMS were also excluded.
Quality assessment and data extraction
To assess the risk of introducing bias, the 9-point Newcastle-Ottawa Scale (NOS) was used to assess the methodological quality of included studies (0-3 as low; 4-6 as moderate and 7-9 as high quality).17 Data extraction from all the included studies was performed by two authors independently. Extracted information includes authorship, country, study design, number of patients, age, sex, number of patients with response to vaccination, type and number of COVID-19 vaccine doses, type of anti-S/RBD IgG immunoassay and cut-off value used to define serologic response, time from the last vaccine dose to the serologic test, type of immunoassay for T-cell response, time from last DMT to vaccination and duration of disease or treatment. Any disagreements were resolved by discussion.
Statistical analysis
The primary result was the risk of suboptimal humoral response to COVID-19 vaccines in pwMS receiving DMTs compared with those without DMTs, estimated in odds ratio (OR) with corresponding 95% CIs. The significance of the overall OR was determined by the Z-test. The DerSimonian-Laird random effects model was used to calculate pooled effect estimates.18 Response rates were measured by assessing the proportion of pwMS classified as vaccine responders (number of pwMS with positive antibody or T-cell response versus all patients). Serologic responses were separately assessed after first and second dose of COVID-19 vaccine. Cochran's Q test and I2 index which describes the percentage of total variation across studies that are due to heterogeneity rather than chance (cut-offs of 25% as low, 50% as medium, and 75% as high heterogeneity) were calculated to explore heterogeneity across included studies.19 To assess the stability of the results, a sensitivity analysis was conducted by removing each individual study in turn from the total and reanalyzing the remainder. In meta-regression analysis, study design and sample size were analyzed as covariates. Furthermore, Galbraith plot was also used to assess the potential sources of heterogeneity. Begg's funnel plot and Egger's tests were used to identify potential publication bias.20,21 The non-parametric Mann-Whitney U independent-samples test was used for continuous variables. Type I error rate was set at 0.05 for two-sided analysis. All statistical analyses were performed using the STATA software (version 11.0).
Role of Funders
The funders had no role in study design, data collection, analysis, interpretation, or writing of the manuscript.
Results
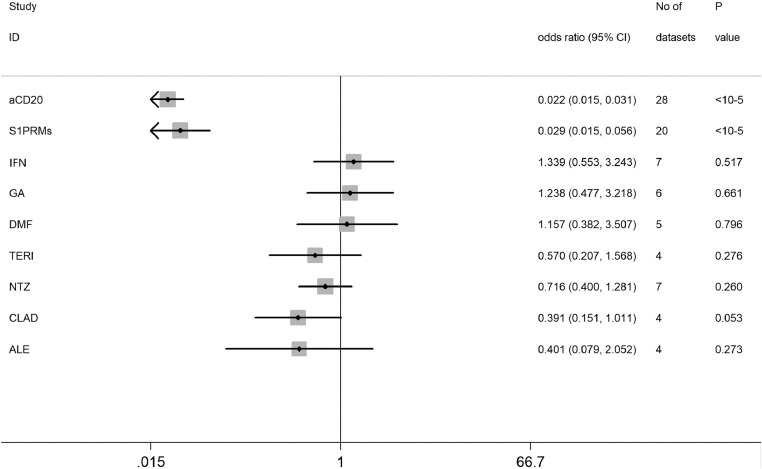
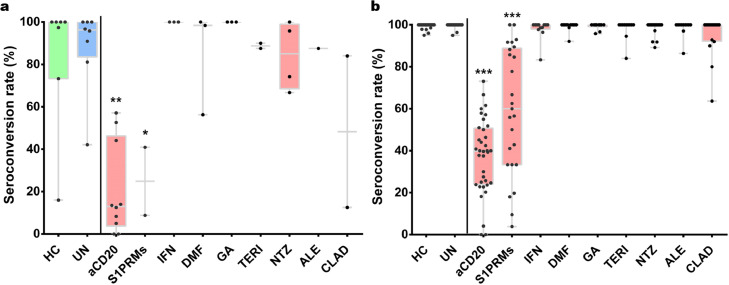
A total of 48 studies involving 6860 pwMS and 2033 healthy controls were finally included in the present study (Supplementary Figure 1). The main characteristics of included studies are shown in Supplementary Table 1. As for methodological quality assessment, 41 studies were awarded ≥7 points, and 7 studies were awarded 5 to 6 points, indicating that included studies were of median-to-high quality (Supplementary Table 2). The main results of humoral response in pwMS treated with a variety of DMTs following vaccination against SARS-CoV-2 were listed in Table 1. Overall, we found the humoral response was significantly decreased in pwMS receiving therapies with anti-CD20 antibodies (OR=0.02, 95% CI: 0.02-0.03, P < 10−5; I2=0%) and S1PRMs (OR=0.03, 95% CI: 0.02-0.06, P < 10−5; I2= 40.6%) following both partial and completed vaccination (Figures 1 and 2). There was no significant difference in seroconversion rate after COVID-19 vaccination between untreated pwMS and healthy controls (Figure 2). And we found no significant evidence of decreased humoral responses in pwMS with other DMTs after vaccination compared with that of pwMS without DMTs (Table 1).
Table 1.
Overall and stratified analyses of serological response in pwMS with DMTs after COVID-19 vaccination.
| DMT | Dosage | No. of datasets | OR (95% CI) | P(Z) | P(Q) | I2 |
|---|---|---|---|---|---|---|
| Anti-CD20 | Partial | 5 | 0.029 (0.015-0.057) | <10−5 | 0.478 | 0% |
| Full | 23 | 0.019 (0.013-0.030) | <10−5 | 0.953 | 0% | |
| Overall | 28 | 0.022 (0.015-0.031) | <10−5 | 0.936 | 0% | |
| S1PRMs | Partial | 3 | 0.046 (0.018-0.115) | <10−5 | 0.319 | 12.6% |
| Full | 17 | 0.027 (0.013-0.059) | <10−5 | 0.035 | 42.0% | |
| Overall | 20 | 0.029 (0.015-0.056) | <10−5 | 0.031 | 40.6% | |
| IFN-β | Partial | 3 | 1.762 (0.293-10.581) | 0.536 | 0.999 | 0% |
| Full | 4 | 1.226 (0.444-3.389) | 0.694 | 0.480 | 0% | |
| Overall | 7 | 1.339 (0.553-3.243) | 0.517 | 0.858 | 0% | |
| GA | Partial | 3 | 1.518 (0.249-9.259) | 0.651 | 0.981 | 0% |
| Full | 3 | 1.145 (0.372-3.524) | 0.814 | 0.693 | 0% | |
| Overall | 6 | 1.238 (0.477-3.218) | 0.661 | 0.974 | 0% | |
| DMF | Partial | 3 | 0.731 (0.162-3.293) | 0.683 | 0.195 | 38.9% |
| Full | 2 | 1.883 (0.467-7.595) | 0.374 | 0.180 | 44.4% | |
| Overall | 5 | 1.157 (0.382-3.507) | 0.796 | 0.078 | 52.4% | |
| TERI | Partial | 2 | 0.424 (0.067-2.677) | 0.362 | 0.663 | 0.0% |
| Full | 2 | 0.484 (0.068-3.424) | 0.467 | 0.192 | 41.2% | |
| Overall | 4 | 0.570 (0.207-1.568) | 0.276 | 0.579 | 0.0% | |
| NTZ | Partial | 3 | 0.629 (0.269-1.470) | 0.284 | 0.756 | 0.0% |
| Full | 4 | 0.803 (0.361-1.787) | 0.591 | 0.401 | 0.0% | |
| Overall | 7 | 0.716 (0.400-1.281) | 0.260 | 0.722 | 0.0% | |
| CLAD | Overall | 4 | 0.391 (0.151-1.011) | 0.053 | 0.956 | 0.0% |
| ALE | Partial | 1 | 0.239 (0.022-2.606) | 0.240 | NA | NA |
| Full | 3 | 0.475 (0.046-4.913) | 0.533 | 0.118 | 53.2% | |
| Overall | 4 | 0.401 (0.079-2.052) | 0.273 | 0.210 | 33.7% |
NA: not available, OR: odds ratio, CI: 95% confidence interval.
Figure 1.
Risk of impaired serological response after receiving SARS-CoV-2 vaccine in pwMS on different DMTs by Z-test.
Figure 2.
Boxplots of seroconversion rates (%) in pwMS treated with different DMTs after the first dose (a) and second dose (b) of SARS-CoV-2 vaccine. Each point indicates a study cohort where data were available. Pairwise comparisons are based on the non-parametric Mann-Whitney U independent-samples test (patients without DMTs as reference group). HC: healthy controls, UN: untreated pwMS, *: P = 0.03, **: P = 0.001 and ***: P < 10−5).
Post-vaccination response in pwMS on anti-CD20
There are 40 studies investigating post-vaccination response in pwMS on anti-CD20 therapy, most of which evaluated the serologic response after two doses of COVID-19 vaccines. Among these studies, 30 studies included 1154 patients on ocrelizumab, while there were 7 studies involving 99 patients on rituximab and limited data on ofatumumab (6 studies involving 10 patients). The pooled analysis indicated that humoral response of these patients was significantly lower than patients without DMTs following completed vaccination (OR=0.02, 95% CI: 0.01-0.03, P < 10−5; I2= 0%). Similar results were observed after one dose of COVID-19 vaccine (OR=0.03, 95% CI: 0.02-0.06, P < 10−5, I2= 0%; Supplementary Figure 2) without between-study heterogeneity.
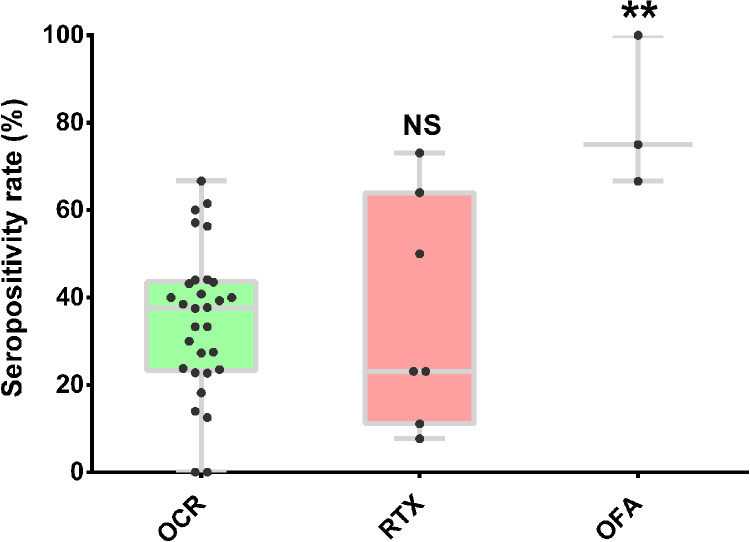
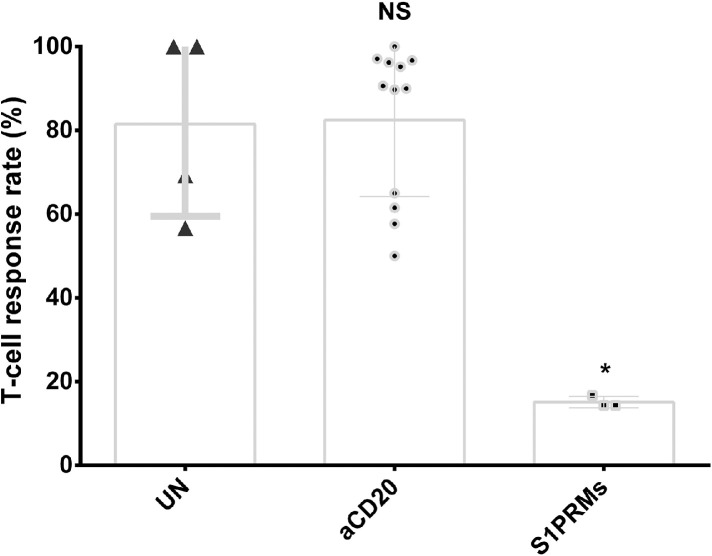
The seroconversion rate was significantly reduced in patients on anti-CD20 treatment after partial or completed vaccination (36.0%, 95% CI: 31-42, P < 10−5; Supplementary Figure 3). Significant heterogeneity was observed among the included studies (I2=79.0%), while meta-regression (Supplementary Table 3) and Galbraith plot analysis (Supplementary Figure 4) failed to identify potential source of heterogeneity. When stratified by different anti-CD20 monoclonal antibodies, we found there was no statistical difference in seroconversion rate in MS patients treated with rituximab and ocrelizumab (P = 0.91), while we observed a trend of higher seroconversion in MS patients treated with ofatumumab (only 8 patients from 3 studies) compared to ocrelizumab (P = 0.0058, Figure 3). However, significantly decreased seroconversion rate sustained after excluding data on ofatumumab treatment from anti-CD20 therapy group. As for T-cell response, the analysis based on 12 datasets demonstrated that there was no significant difference between pwMS on anti-CD20 treatment and untreated patients (P = 0.63; Figure 4).
Figure 3.
Boxplots of seropositivity rates (%) in pwMS stratified by different anti-CD20 monoclonal antibodies after receiving SARS-CoV-2 vaccine. Each point indicates a study cohort where data were available. Pairwise comparisons are based on the non-parametric Mann-Whitney U independent-samples test (patients with ocrelizumab as reference group). OCR: ocrelizumab, RTX: rituximab, OFA: ofatumumab, NS: not significant (P = 0.91), **: P = 0.0058.
Figure 4.
Boxplots of T-cell response rate (%) in pwMS on Anti-CD20, S1PRMs and without DMTs after receiving SARS-CoV-2 vaccine. Each point indicates a study cohort where data were available. Pairwise comparisons are based on the non-parametric Mann-Whitney U independent-samples test (patients without DMTs as reference group). UN: untreated, NS: not significant (P = 0.63), *: P = 0.03.
In the subgroup analysis according to the interval between last anti-CD20 treatment and vaccination, the humoral response of pwMS vaccinated within six months since last anti-CD20 therapy was significantly lower than those receiving anti-CD20 therapy more than six months (OR=0.21, 95% CI: 0.08-0.52, P = 0.001; I2=42.5%, Supplementary Figure 5).
Post-vaccination response in pwMS on S1PRM
A total of 28 studies assessing response after COVID-19 vaccination for S1PRM therapy were included. Included studies were dominated by investigation on fingolimod (28 studies involving 764 patients), while there were 4 studies involving 8 patients on ozanimod, and 4 studies involving 13 patients on siponimod. When compared with untreated patients, serologic response was significantly reduced in pwMS on S1PRMs (OR=0.03, 95% CI: 0.02-0.06, P < 10−5; I2= 40.6%, Supplementary Figure 6) with moderate heterogeneity. Consistently, the rate of serological positivity after two doses of SARS-CoV-2 vaccines was significantly decreased in patients on S1PRM treatment (60.2%, 95% CI: 46-74, P < 10−5; Supplementary Figure 7) than that among untreated patients with significant heterogeneity (I2=92.5%). We then conducted meta-regression analysis (Supplementary Table 3) and Galbraith plot analysis which identified 5 studies as potential source of heterogeneity (Supplementary Figure 8). Moreover, T-cell response after SARS-CoV-2 vaccination was also significantly impaired compared to pwMS without DMTs (P = 0.03; Figure 4).
Post-vaccination response in pwMS on IFN-β
There were 13 reports assessing serological response in pwMS on IFN-β. Our analysis demonstrated no evidence of reduced IgG response to SARS-CoV-2 vaccination (OR=1.34, 95% CI: 0.55-3.24, P = 0.517, I2= 0%; Supplementary Figure 9) in pwMS with IFN-β therapy and post-vaccination response rate did not significantly differ from that of untreated MS patients (P = 0.095 for dose 1, P = 0.050 for dose 2; Figure 2).
Post-vaccination response in pwMS on GA
The pooled analysis based on 11 studies found no significant difference of seroconversion following COVID-19 vaccination between GA-treated pwMS and untreated group (OR=1.24, 95% CI: 0.48 - 3.22, P = 0.661; I2=0%, Supplementary Figure 10) with no heterogeneity. There was no pwMS on GA with negative response reported except one study,22 and the response rate was not significantly different from pwMS without DMTs (P = 0.095 for dose 1, P = 0.437 for dose 2; Figure 2).
Post-vaccination response in pwMS on DMF
There were 14 studies evaluating the efficacy of COVID-19 vaccine in pwMS on DMF, and the combined analysis indicated that the serological response was comparable with untreated group (OR=1.16, 95% CI: 0.38-3.51, P = 0.80; I2=52.4%, Supplementary Figure 11) with moderate heterogeneity detected. All studies reported 100% seroconversion rate, but one study reported a seroconversion rate of 98.7%.22 The pooled analysis found no significant difference in post-vaccination seroconversion between pwMS on DMF and untreated patients (P = 0.917 for dose 1, P = 0.520 for dose 2; Figure 2).
Post-vaccination response in pwMS on TERI
A total of 12 studies investigated the post-vaccination response in pwMS on TERI. And the results showed no evidence of decreased humoral response (OR=0.57, 95% CI: 0.21-1.57, P = 0.276; I2=0%, Supplementary Figure 12), or lower seroconversion rate (P = 0.290 for dose 1, P = 0.381 for dose 2; Figure 2).
Post-vaccination response in pwMS on NTZ
We did not observe a statistically significant correlation between NTZ treatment and blunted antibody response following vaccination based on data from 22 studies (OR=0.72, 95% CI: 0.40-1.28, P = 0.26; I2= 0%, Supplementary Figure 13), while the response rate was comparable with that of untreated patients (P = 0.545 for dose 1, P = 0.251 for dose 2; Figure 2).
Post-vaccination response in pwMS on CLAD
There were 17 studies investigating the effect of CLAD on response to COVID-19 vaccines. We found no evidence of significant difference in responses between pwMS with CLAD and those without DMTs after completed vaccination (OR=0.39, 95% CI: 0.15-1.01, P = 0.05; I2= 0%, Supplementary Figure 14) with no heterogeneity. Accordantly, the pooled response rate of this group after vaccination did not differ significantly from that of untreated group (P = 0.113 for dose 1, P = 0.054 for dose 2; Figure 2).
Post-vaccination response in pwMS on ALE
There were 13 studies evaluating the IgG response in pwMS on ALE. The combined analysis showed that the ALE treatment may not affect humoral response following vaccination against SARS-CoV-2 (OR=0.40, 95% CI: 0.08-2.05, P = 0.273, I2=33.7%; Supplementary Figure 15) and seroconversion rate of pwMS with ALE therapy was comparable with untreated MS patients (P = 0.431 for dose 1, P = 0.459 for dose 2; Figure 2).
Safety
Owing to the heterogeneity in definition and different forms of data in the 8 studies performing safety evaluation on SARS-CoV-2 vaccines, the meta-analysis could not be performed. Overall, mild to moderate vaccine-related adverse reactions (local pain, fatigue, myalgia, headache and low-grade fever) were commonly reported in pwMS. There was no evidence of increased incidence of MS worsening or relapses after receiving COVID-19 vaccination based on included studies.
Sensitivity analysis and publication bias
Sensitivity analysis indicated that no single study influenced the pooled results, and significant associations sustained for anti-CD20 and S1PRM therapies after excluding articles with moderate quality (Supplementary Table 4). The shape of the funnel plots was symmetrical (Supplementary Figure 16-17) and Egger's test showed no publication bias for the overall and subgroup analyses (Supplementary Table 5).
Discussion
In the present meta-analysis, we found significantly reduced humoral response in patients treated with anti-CD20 and S1PRM after receiving COVID-19 vaccination, while there were no significant associations between other seven types of DMTs and impaired antibody response in MS patients.
Anti-CD20 treatment was most strongly associated with an increased risk of seronegative response following COVID-19 vaccination among all the DMTs. Emerging evidence from patients with hematologic malignancies and other immune-mediated inflammatory disorders also indicated that anti-CD20 therapy was correlated with the risk of serological negative responses after completed vaccination against SARS-CoV-2, which were in line with our finding.23,24 In consideration of potential influences of molecular structure of the substances, immunological effects and pharmacokinetics of different drugs, we performed subgroup analysis by the types of anti-CD20 antibodies. We found no statistical difference in seroconversion rate between MS patients treated with rituximab and ocrelizumab. Although higher seroconversion rate in pwMS treated with ofatumumab was observed compared to those with ocrelizumab, the results must be interpreted with caution. As the analysis was performed on the basis of limited data available, selection bias may have occurred and our results may be overinflated. Thus, additional trials are warranted to further evaluate the potential impact of ofatumumab on serological response to SARS-CoV-2 vaccination in pwMS. The time interval since the last anti-CD20 treatment prior to vaccination was found to be correlated with humoral response among pwMS in our study. Recent international recommendations have suggested a 4-to-6-month interval between the last dose of anti-CD20 treatment and administration of vaccination in pwMS.25,26 Our result indicated that anti-CD20 therapy less than 6 months before SARS-CoV-2 vaccination may strongly interfere with the development of a protective humoral response in pwMS, which agreed with previous observation that slow B-cell repopulation began about 6 months after the last anti-CD20 treatment.27 Prior studies suggested that postponing anti-CD20 treatment by 3-to-6 months before vaccination in selected stable MS patients could increase the probability of mounting adequate humoral response against SARS-CoV-2 infection.28 However, extending dosage interval may associate with significant risk of potential relapse and worsening disease. Thus, further evidence is needed to define the optimal interval between anti-CD20 therapy and COVID-19 vaccination in pwMS, finding a balance between the risk of MS disease progression and protection against SARS-CoV-2 infection.
Despite the attenuated antibody response, our result illustrated that T-cell response was preserved among pwMS on anti-CD20 treatment, in accordance with previous reports.29, 30, 31 It has been shown that exposure to SARS-COV-2 can induce a cellular immune response without seroconversion,32 and T-cell responses are associated with disease severity of COVID-19.33,34 Animal study demonstrated that cellular immune response contributed to protection against SARS-CoV-2 when antibody response was insufficient in rhesus macaques.35 Moreover, T-cell response following vaccination has been suggested to cross-recognize SARS-CoV-2 variants including the newly emerging Omicron.36 Thus, although the role of T-cell immunity against SARS-CoV-2 has not been fully elucidated, it may provide some extent of protection to pwMS under anti-CD20 treatment after receiving COVID-19 vaccination.
Of note, previous limited investigations on the third dose of SARS-CoV-2 vaccine demonstrated that the humoral or cellular response was not significantly increased compared to that following the second dose in pwMS with anti-CD20 treatment.37, 38, 39 However, one study reported that T-cell response decreased 6 months after the second vaccination, but restored after the administration of a third vaccine.39 Moreover, there was no significant difference in cellular response between pwMS on anti-CD20 and healthy controls after receiving two or three doses of vaccines,39 supporting our finding that anti-CD20 may not affect the T-cell response to vaccination against SARS-CoV-2. Interestingly, another study identified robust T-cell responses recognizing the newly Delta and Omicron variants 6 months following the second vaccination in pwMS on anti-CD20 treatment, and found increased T-cell response rate after the third dose of COVID-19 vaccine.40 Although the duration of T-cell response after completed vaccination is still not fully clarified, these evidences highlighted the importance of a booster dose for patients with anti-CD20 therapy.
S1PRMs act by preventing lymphocytes from leaving the lymph nodes and reducing the circulating lymphocyte count.41 In agreement with previous evidence in other vaccines,42 our result showed pwMS on S1PRM treatment, mainly with fingolimod, had both attenuated antibody and T-cell responses after COVID-19 vaccination. Although there were some studies reporting a relatively high rate of positive response in pwMS with S1PRMs, a low antibody titre was commonly detected in these patients. Intriguingly, unlike anti-CD20 treatment, S1PRMs may not increase the risk for COVID-19 infection or severity.43,44 It has been reported that one patient (a 34-years old woman) taking fingolimod with COVID-19 infection showed reduced IgG response, but did not develop a severe form of the disease.45 Available data on the effect of a third vaccination in patients with S1PRM treatment are still limited. A cohort study in patients with a wide spectrum of immune-mediated inflammatory disorders demonstrated that seroconversion rates in those on S1PRMs did not significantly increase after a third dose.23 Therefore, although accumulating evidence and our results confirmed that S1PRM therapy blunted responses to SARS-CoV-2 vaccination, the impact of this treatment on COVID-19 outcomes in pwMS remains to be elucidated. Moreover, several studies identified a negative correlation between humoral response after COVID-19 vaccination and treatment duration of S1PRMs,46,47 which deserves further verification.
As for other DMTs, pwMS on DMF, NTZ and TERI were reported to have low peripheral blood lymphocyte counts, which may result in increased risk of infections or suboptimal response to vaccinations.48,49 The immunomodulation mechanism of DMF is believed to be depend on inhibition of Nfr-2 protein, which ultimately limits inflammatory cascades.41 Previous studies on various vaccines in pwMS have illustrated that DMF-treated patients developed an adequate immune response.50 Similarly, our study found pwMS on DMF achieved antibody responses comparable to those without DMTs after vaccination against SARS-CoV-2. NTZ is a non-depleting immunomodulator, which acts by blocking the interaction between α4-integrin and vascular cell adhesion molecules, and preventing the leukocyte migration to the central nervous system. We found NTZ treatment had no negative impact on the immune response following vaccination against SARS-CoV-2, which is consistent with published research on influenza vaccine.51 TERI is a dihydroorotate dehydrogenase inhibitor with modest efficacy in preventing MS relapses and lesion accumulation. It is reported that a TERI-treated MS patient with SARS-CoV-2 infection was able to mount an effective antibody level comparable to immunocompetent COVID-19 patients.45 A recent review also indicated that pwMS with TERI did not have attenuated response after vaccination,52 which is in accordance with our result.
IFN-β is an immunomodulating agent which provides a modest disease-modifying effect against MS relapses and accumulation of brain lesions. As IFN-β does not act primarily by depleting lymphocytes, it is unlikely to interfere with the immune response against SARS-CoV-2 or increase the risk of COVID-19 infection.53 GA is another immunomodulator, which competes with myelin antigens to interact with major histocompatibility complex molecules on antigen-presenting cells. Like IFN-β, lymphopenia is rarely reported in GA therapy, and there is no evidence indicating an elevated infection risk of COVID-19 during this treatment.54 Our study further demonstrated that the serological responses after COVID-19 vaccination in pwMS under IFN-β and GA treatments did not differ from that among those without DMTs.
CLAD and ALE are two high efficacy DMTs with different mechanisms. CLAD acts by inhibiting DNA synthesis and repair in highly dividing cells including B- and T-cells, which leads to significant myelosuppression by cell apoptosis.43 It has been reported that CLAD can induce B-cell depletion by about 85-90%, and T-cell depletion by average of 50%.55 But in the present study, we found sufficient serological response in pwMS receiving CLAD treatment. As for cellular response, only one study reported a lower frequency of T-cell response found in CLAD-treated patients (70%) compared to healthy controls (100%).56 ALE is a humanized monoclonal antibody targeting CD52 receptors on the surface of mature lymphocytes, which can cause generalized lymphopenia.41 Nevertheless, our analysis of humoral response after COVID-19 vaccination indicated no difference between pwMS on ALE and untreated MS patients.
COVID-19 vaccination is generally safe for MS patients. Adverse events following SARS-CoV-2 vaccines were commonly mild to moderate in pwMS with rates comparable with that in general population.57 Although MS worsening or relapses were rarely observed after vaccination, the incidence of relapses was similar to the rate in non-vaccinated patients.58 There was no clear evidence suggesting a potential risk of disease reactivation in pwMS on active treatment after vaccination against SARS-CoV-2 while more investigations with detailed safety data are needed. More importantly, increasing opportunity of information and concerns sharing among health-care workers and MS patients remain the key to decrease patients’ hesitancy and thus increase adherence to vaccination schemes.
There are several limitations in our study. Firstly, only humoral responses were measured in most studies, more researches on cellular responses following COVID-19 vaccination are needed. Secondly, the sampling time after vaccination was not standardized in a few studies, which may lead to some discrepancy. Beyond that, interval from receiving SARS-CoV-2 vaccine to sampling in most included studies for the current analysis was 2-6 weeks. Thirdly, some studies did not provide information about previous COVID-19 symptoms in pwMS, which made it unable to screen out patients previously infected with SARS-CoV-2. Additionally, different immunoassays and lack of unified standards of immune response might also have impact on our analyses. Different commercial kits for detecting SARS-CoV-2 IgG may differ in sensitivity, however, earlier findings indicated that different kits provide acceptable performance and are consistent in results.59
In summary, we provided a comprehensive meta-analysis to evaluate the response after COVID-19 vaccination in pwMS with various DMTs. There was no or only moderate heterogeneity detected in the main results. Our study suggested that MS patients treated with anti-CD20 and S1PRM exhibited reduced seroconversion compared to those without DMTs after receiving SARS-CoV-2 vaccines. The T-cell response was preserved in pwMS on anti-CD20, but significantly decreased in S1PRM-treated pwMS compared with untreated patients. Thus, routine serological monitoring after COVID-19 vaccination may be required for patients on anti-CD20 and S1PRM treatments to assess whether an adequate immune response was mounted or a booster dose was needed. The effect of post-vaccination cellular response, time interval from last treatment to vaccination, and new oral antiviral agents should be further addressed for pwMS with DMTs in the future.
Contributors
Conceptualization: KF. T. Data curation: L.W., L.S., KF.T. and X.W. Investigation, methodology and project administration: X.W. and KF.T. Writing – original draft: X.W. and KF.T. Writing – review & editing: L.W., L.S., X.W. and KF.T. Funding acquisition: X.W.
Data sharing statement
The data supporting the findings in this study might be requested via the corresponding author of this article upon reasonable request.
Declaration of interests
The authors declare that they have no conflicts of interest.
Acknowledgements
This study was supported by Natural Science Foundation of Shanghai (21ZR1433000).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104102.
Appendix. Supplementary materials
References
- 1.Hauser SL, Cree BAC. Treatment of multiple sclerosis: a review. Am J Med. 2020;133(12) doi: 10.1016/j.amjmed.2020.05.049. 1380-90.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321(2):175–187. doi: 10.1001/jama.2018.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luna G, Alping P, Burman J, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. 2020;77(2):184–191. doi: 10.1001/jamaneurol.2019.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safavi F, Nourbakhsh B, Azimi AR. B-cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Mult Scler Relat Disord. 2020;43 doi: 10.1016/j.msard.2020.102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahraian MA, Azimi A, Navardi S, Ala S, Naser Moghadasi A. Evaluation of the rate of COVID-19 infection, hospitalization and death among Iranian patients with multiple sclerosis. Mult Scler Relat Disord. 2020;46 doi: 10.1016/j.msard.2020.102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.REDONE.br – Neuroimmunology Brazilian Study Group Focused on COVID-19 and MS. Incidence and clinical outcome of Coronavirus disease 2019 in a cohort of 11,560 Brazilian patients with multiple sclerosis. Multi Scler. 2021;27(10):1615–1619. doi: 10.1177/1352458520978354. [DOI] [PubMed] [Google Scholar]
- 8.Langer-Gould A, Smith JB, Li BH. Multiple sclerosis, rituximab, and COVID-19. Ann Clinic Transl Neurol. 2021;8(4):938–943. doi: 10.1002/acn3.51342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reyes S, Cunningham AL, Kalincik T, et al. Update on the management of multiple sclerosis during the COVID-19 pandemic and post pandemic: An international consensus statement. J Neuroimmunol. 2021;357 doi: 10.1016/j.jneuroim.2021.577627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waldman RA, Creed M, Sharp K, et al. Toward a COVID-19 vaccine strategy for patients with pemphigus on rituximab. J Am Acad Dermatol. 2021;84(4) doi: 10.1016/j.jaad.2020.10.075. e197-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conte WL. B-cell depleters attenuate the humoral response to SARS-CoV-2 vaccines in multiple sclerosis patients: a case-control study. Mult Scler Relat Disord. 2022;57 doi: 10.1016/j.msard.2021.103413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tallantyre EC, Vickaryous N, Anderson V, et al. COVID-19 vaccine response in people with multiple sclerosis. Ann Neurol. 2022;91(1):89–100. doi: 10.1002/ana.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Therap Adv Neurol Disord. 2021;14 doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bsteh G, Dürauer S, Assar H, et al. Humoral immune response after COVID-19 in multiple sclerosis: a nation-wide Austrian study. Mult Scler. 2021;27(14):2209–2218. doi: 10.1177/13524585211049391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 22.Pitzalis M, Idda ML, Lodde V, et al. Effect of different disease-modifying therapies on humoral response to BNT162b2 vaccine in sardinian multiple sclerosis patients. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.781843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wieske L, van Dam KPJ, Steenhuis M, et al. Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol. 2022;4(5):e338–e350. doi: 10.1016/S2665-9913(22)00034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang A, Akhtar A, Linderman SL, et al. Humoral responses against SARS-CoV-2 and variants of concern after mRNA vaccines in patients with non-hodgkin lymphoma and chronic lymphocytic leukemia. J Clinic Oncol. 2022 doi: 10.1200/JCO.22.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf A, Alvarez E. COVID-19 vaccination in patients with multiple sclerosis on disease-modifying therapy. Neurol Clinic Pract. 2021;11(4):358–361. doi: 10.1212/CPJ.0000000000001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamout BI, Zakaria M, Inshasi J, et al. MENACTRIMS practice guideline for COVID-19 vaccination in patients with multiple sclerosis. Mult Scler Relat Disord. 2021;56 doi: 10.1016/j.msard.2021.103225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker D, Pryce G, James LK, Marta M, Schmierer K. The ocrelizumab phase II extension trial suggests the potential to improve the risk: benefit balance in multiple sclerosis. Mult Scler Relat Disord. 2020;44 doi: 10.1016/j.msard.2020.102279. [DOI] [PubMed] [Google Scholar]
- 28.Disanto G, Sacco R, Bernasconi E, et al. Association of disease-modifying treatment and anti-CD20 infusion timing with humoral response to 2 SARS-CoV-2 vaccines in patients with multiple sclerosis. JAMA Neurol. 2021;78(12):1529–1531. doi: 10.1001/jamaneurol.2021.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brill L, Rechtman A, Zveik O, et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2021;78(12):1510–1514. doi: 10.1001/jamaneurol.2021.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iannetta M, Landi D, Cola G, et al. B- and T-Cell Responses After SARS-CoV-2 vaccination in patients with multiple sclerosis receiving disease modifying therapies: immunological patterns and clinical implications. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.796482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallais F, Velay A, Nazon C, et al. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without Seroconversion, France. Emerg Infect Dis. 2021;27(1):113–121. doi: 10.3201/eid2701.203611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venet F, Cour M, Rimmelé T, et al. Longitudinal assessment of IFN-I activity and immune profile in critically ill COVID-19 patients with acute respiratory distress syndrome. Crit Care. 2021;25(1):140. doi: 10.1186/s13054-021-03558-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4) doi: 10.1016/j.cell.2020.09.038. 996-1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarke A, Coelho CH, Zhang Z, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185(5) doi: 10.1016/j.cell.2022.01.015. 847-59.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Achtnichts L, Jakopp B, Oberle M, et al. Humoral immune response after the third SARS-CoV-2 mRNA vaccination in CD20 depleted people with multiple sclerosis. Vaccines. 2021;9(12):1470. doi: 10.3390/vaccines9121470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bajwa HM, Novak F, Nilsson AC, et al. Persistently reduced humoral and cellular immune response following third SARS-CoV-2 mRNA vaccination in anti-CD20-treated multiple sclerosis patients. Mult Scler Relat Disord. 2022;60:103729. doi: 10.1016/j.msard.2022.103729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brill L, Raposo C, Rechtman A, et al. SARS-CoV-2 third vaccine immune response in MS patients treated with ocrelizumab. Ann Neurol. 2022;91(6):796–800. doi: 10.1002/ana.26343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madelon N, Heikkilä N, Sabater Royo I, et al. Omicron-specific cytotoxic T-cell responses after a third dose of mRNA COVID-19 vaccine among patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2022;79(4):399–404. doi: 10.1001/jamaneurol.2022.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cross AH, Naismith RT. Established and novel disease-modifying treatments in multiple sclerosis. J Intern Med. 2014;275(4):350–363. doi: 10.1111/joim.12203. [DOI] [PubMed] [Google Scholar]
- 42.Kappos L, Mehling M, Arroyo R, et al. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology. 2015;84(9):872–879. doi: 10.1212/WNL.0000000000001302. [DOI] [PubMed] [Google Scholar]
- 43.Giovannoni G, Hawkes C, Lechner-Scott J, Levy M, Waubant E, Gold J. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult Scler Relat Disord. 2020;39 doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barzegar M, Mirmosayyeb O, Gajarzadeh M, et al. COVID-19 among patients with multiple sclerosis: a systematic review. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1001. doi: 10.1212/NXI.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bollo L, Guerra T, Bavaro DF, et al. Seroconversion and indolent course of COVID-19 in patients with multiple sclerosis treated with fingolimod and teriflunomide. J Neurol Sci. 2020;416 doi: 10.1016/j.jns.2020.117011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Capuano R, Bisecco A, Conte M, et al. Six-month humoral response to mRNA SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab and fingolimod. Mult Scler Relat Disord. 2022;60 doi: 10.1016/j.msard.2022.103724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Türkoğlu R, Baliç N, Kızılay T, et al. Fingolimod impairs inactivated vaccine (CoronaVac)-induced antibody response to SARS-CoV-2 spike protein in persons with multiple sclerosis. Mult Scler Relat Disord. 2022;58 doi: 10.1016/j.msard.2022.103524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schweitzer F, Laurent S, Fink GR, Barnett MH, Hartung HP, Warnke C. Effects of disease-modifying therapy on peripheral leukocytes in patients with multiple sclerosis. J Neurol. 2021;268(7):2379–2389. doi: 10.1007/s00415-019-09690-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fox EJ, Buckle GJ, Singer B, Singh V, Boster A. Lymphopenia and DMTs for relapsing forms of MS: considerations for the treating neurologist. Neurol Clinic Pract. 2019;9(1):53–63. doi: 10.1212/CPJ.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Hehn C, Howard J, Liu S, et al. Immune response to vaccines is maintained in patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm. 2018;5(1):e409. doi: 10.1212/NXI.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vågberg M, Kumlin U, Svenningsson A. Humoral immune response to influenza vaccine in natalizumab-treated MS patients. Neurol Res. 2012;34(7):730–733. doi: 10.1179/1743132812Y.0000000059. [DOI] [PubMed] [Google Scholar]
- 52.Tornatore C, Wiendl H, Lublin AL, et al. Vaccine response in patients with multiple sclerosis receiving teriflunomide. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.828616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cabreira V, Abreu P, Soares-Dos-Reis R, Guimarães J, Sá MJ. Multiple sclerosis, disease-modifying therapies and COVID-19: a systematic review on immune response and vaccination recommendations. Vaccines. 2021;9(7):773. doi: 10.3390/vaccines9070773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng C, Kar I, Chen CK, et al. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs. 2020;34(9):879–896. doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drulovic J, Ivanovic J, Martinovic V, et al. Humoral response to SARS-CoV-2 COVID-19 vaccines in patients with multiple sclerosis treated with immune reconstitution therapies. Mult Scler Relat Disord. 2021;54 doi: 10.1016/j.msard.2021.103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with ms using different disease-modifying therapies. Neurology. 2022;98(5):e541–ee54. doi: 10.1212/WNL.0000000000013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Capone F, Lucchini M, Ferraro E, et al. Immunogenicity and safety of mRNA COVID-19 vaccines in people with multiple sclerosis treated with different disease-modifying therapies. Neurotherapeutics. 2022;19(1):325–333. doi: 10.1007/s13311-021-01165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trabaud MA, Icard V, Milon MP, Bal A, Lina B, Escuret V. Comparison of eight commercial, high-throughput, automated or ELISA assays detecting SARS-CoV-2 IgG or total antibody. J Clinic Virol. 2020;132 doi: 10.1016/j.jcv.2020.104613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.