Abstract
Stinging nettle (Urtica dioica L.) is a common perennial herb well known for its therapeutic, cosmetic and food use. Despite the popularity of nettle hydrolate, there is currently no literature describing its composition; likewise, there is still a lack of research describing in detail the parameters of hydrolates in general. U. dioica hydrolate fractions were obtained by industrial steam distillation of fresh herb. Total stinging nettle hydrolate was prepared by mixing an equal volume of each fraction. The volatiles were isolated from hydrolate samples by liquid–liquid extraction with diethyl ether, and analysed using GC-FID-MS. Over eighty volatile compounds were identified in U. dioica hydrolate. The main group of constituents were oxygenated compounds, mainly alcohols (e.g., (E)- and (Z)-hex-3-en-1-ol, carvacrol) and oxides (e.g., caryophyllene oxide). The content of volatiles in the representative sample of total hydrolate amounted to 58.2 mg/L. Some qualitative and quantitative changes in the composition of U. dioica hydrolate were observed during the progress of distillation. The content of low chain aliphatic alcohols ((E)- and (Z)-hex-3-en-1-ol) decreased, whereas the percentage of some monoterpene alcohols (carvacrol and α-terpineol) increased. The total content of volatiles in hydrolate also changed and decreased (128.0–6.2 mg/L) during distillation progress. According to our results, to produce stinging nettle hydrolate of good quality, the proper relationship between the amount of hydrolate and raw plant material should result in obtaining 0.74 L hydrolate from 1 kg of fresh stinging nettle herb. Therefore, it may be assumed that the high alcohol content may increase the microbiological stability of the product.
Keywords: stinging nettle, Urtica doica L., Urticaceae, hydrolate, volatile compounds, secondary metabolites
1. Introduction
Hydrolates are products of distillation that create pleasant scents and many biological activities. In the past, hydrolates were a waste product of the production of essential oils. Currently, in this age of green chemistry and the trend of zero waste, they are considered a very valuable raw material for many industries, especially perfumery and toiletries, as well as food and cosmetics. More and more often they are also one of the only products of distillation of plant raw materials, but due to plant biodiversity, the production process of hydrolates has not been standardized to date. For this reason, it was considered necessary to determine the hydrodistillation parameters (product volume to column batch ratio) needed to obtain the hydrolate fraction with the highest volatiles content.
Stinging nettle (Urtica dioica L., Urticaceae) is a plant that is commonly found in Europe, Asia, and Northern America. It grows wild in meadows, backyard gardens, forests, fields and roadside ditches. It is a highly competitive ruderal species that often forms monospecific stands. U. dioica is a quite tall (1–2 m), usually dioecious, rhizomatous, perennial herb best known for its stems and leaves being densely covered with stinging hairs, which release potential pain-inducing toxins when contact with them is made. This plant is best known for its medicinal properties [1]. U. dioica root and leaf extracts are used as a remedy for prostatic hypertrophy and urinary tract diseases, as well as eczema, menstrual hemorrhage, rheumatism, or anemia [2,3]. Many different classes of organic compounds of medical importance including phytosterols, saponins, flavonoids, tannins, sterols, fatty acids, carotenoids, chlorophylls, proteins, amino acids, and vitamins are produced by stinging nettle [2,3,4]. Dried U. dioica leaves are considered a pharmacopoeia raw material [5].
Stinging nettle releases a very low content of volatiles during hydrodistillation, thus it is classified as a non essential oil bearing plant. Nevertheless, some investigations concerning volatile constituents of U. dioica herb were recently conducted. According to Ilies et al. [6] hexahydrofarnesylacetone (31.2%), phytol (11.2%), and β-ionone (11.9%) were the main constituents identified in U. dioica essential oil. However, essential oil investigated by Gül et al. [7] revealed a different composition. The main constituents were carvacrol (38.2%), naphthalene (8.9%), and carvone (9.0%), although hexahydrofarnesylacetone and phytol were also detected, but in much lower amounts (3.0% and 2.7%, respectively). The unknown composition of stinging nettle’s volatile secondary metabolites, its well-known therapeutic properties, and the availability of this pharmacopoeial material [5] were the reasons that stinging nettle was chosen as the plant material for this study. In the literature, there are currently no studies on the composition, production and use of U. dioica hydrolate, therefore, this is the first study showing a composition of the hydrolate obtained industrially from stinging nettle. An additional advantage of this work is the indication of the dependence of the composition of volatile compounds on the volume of fraction collection.
2. Results and Discussion
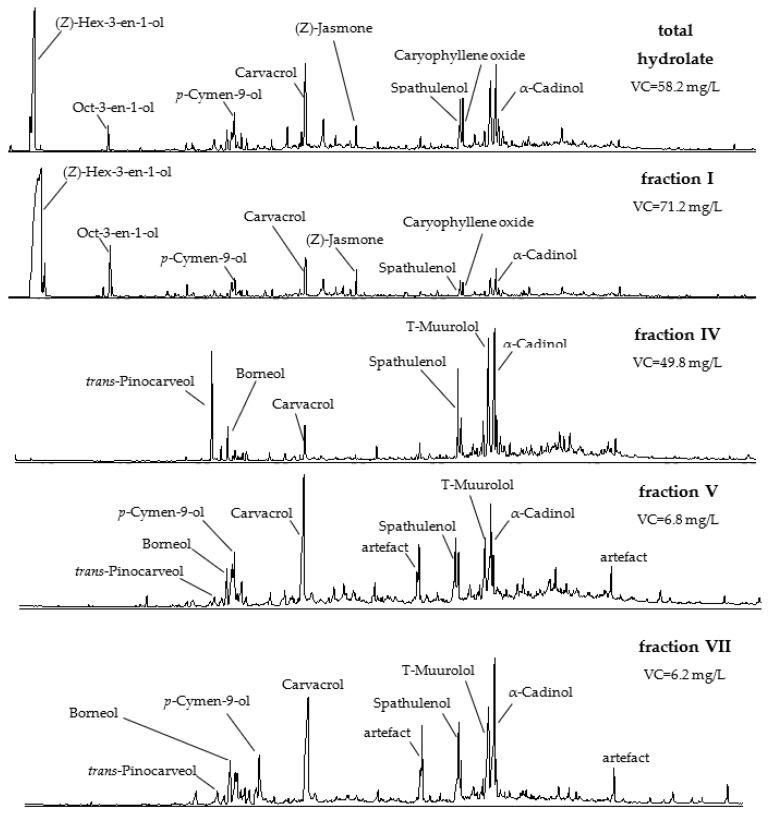
Seven fractions of stinging nettle hydrolate collected during distillation, and representative total hydrolate prepared by mixing equal volumes of each fraction, were analysed. Every fraction of U. dioica hydrolate was colourless liquid with a pleasant, green, herbal scent characteristic of nettle. The pH value (presented in Figure 1) was acidic and ranged from 6.00 to 6.54. The pH values of the analysed samples were quite high in comparison with other hydrolates [8]. The content and composition of volatiles are presented in Table 1. Significant changes in U. dioica hydrolate composition during distillation were observed, similarly for other plant materials, e.g., Rosa rugosa Thunb [9].
Figure 1.
pH value (green bullet line) and content of volatile compounds (green bars) in hydrolate fractions and total hydrolate of the U. dioica L.
Table 1.
Composition of stinging nettle (Urtica dioica L.) hydrolate.
| No | Compound | RIlit | RIexp | Total Hydrolate | Hydrolate Fraction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | |||||
| [%] | |||||||||||
| 1. | (E)-Hex-3-en-1-ol | 836 | 833 | 5.7 | 0.5 | 7.1 | - | - | - | 0.2 | - |
| 2. | (Z)-Hex-3-en-1-ol | 841 | 841 | 27.9 | 73.7 | 14.3 | - | - | - | - | - |
| 3. | Hexan-1-ol | 854 | 851 | 0.5 | 1.8 | 0.1 | - | - | - | - | - |
| 4. | (Z)-Hex-2-en-1-ol | 951 | 857 | t | 0.4 | t | - | - | - | - | t |
| 5. | α-Thujene | 926 | 920 | - | - | - | - | t | t | - | 0.1 |
| 6. | α-Pinene | 934 | 928 | - | - | - | - | t | t | - | t |
| 7. | Camphene | 950 | 944 | - | - | - | - | t | t | - | - |
| 8. | 6-Methyl-2-heptanol | 950 | 952 | 0.1 | 0.1 | 0.4 | - | - | - | - | - |
| 9. | Heptan-1-ol | 957 | 954 | - | 0.5 | - | - | - | - | - | - |
| 10. | Oct-1-en-3-ol | 962 | 962 | 1.6 | 3.4 | 0.5 | - | - | - | - | - |
| 11. | Allyl isovalerate | 920 a | 966 | 0.2 | 0.7 | - | - | - | - | - | - |
| 12. | Octan-4-ol | 975 | 974 | t | 0.1 | - | - | - | - | - | - |
| 13. | Dehydro-1,8-cineol | 979 | 974 | - | - | - | - | - | - | 0.4 | - |
| 14. | 2,6-Dimethylhept-6-en-2-ol * | - b | 1009 | 0.1 | t | - | - | - | - | - | - |
| 15. | p-Cymene | 1014 | 1010 | t | t | 0.1 | - | t | t | - | t |
| 16. | Limonene | 1025 | 1017 | t | 0.1 | t | - | t | 0.8 | - | t |
| 17. | (Z)-Oct-5-en-1-ol | 1048 | 1052 | t | 0.1 | - | - | - | - | - | - |
| 18. | Octan-1-ol | 1057 | 1053 | t | 0.3 | 0.1 | - | - | - | - | - |
| 19. | trans-Linalool oxide (f) | 1058 | 1056 | t | 0.1 | t | t | t | t | 0.2 | t |
| 20. | Heptanoic acid | 1068 | 1068 | t | 0.1 | - | - | - | - | - | - |
| 21. | cis-Linalool oxide (f) | 1072 | 1071 | t | 0.2 | t | - | t | t | - | - |
| 22. | Linalool | 1086 | 1085 | 0.2 | 0.2 | 0.1 | 0.1 | 0.4 | 0.4 | 1.0 | 0.2 |
| 23. | β-Phenyloethanol | 1088 | 1085 | 0.2 | 0.2 | 0.4 | - | t | - | t | - |
| 24. | Benzeneacetonitrile | 1089 | 1092 | 0.1 | 0.1 | - | - | - | 0.3 | - | - |
| 25. | cis-Rose oxide | 1096 | 1096 | 0.1 | 0.1 | - | - | - | - | 0.8 | - |
| 26. | trans-Rose oxide | 1116 | 1121 | t | t | 0.1 | - | t | 0.2 | - | - |
| 27. | trans-Pinocarveol | 1126 | 1123 | 0.1 | t | 0.1 | - | 0.1 | 0.2 | - | - |
| 28. | trans-Verbenol | 1134 | 1129 | 0.7 | 0.3 | 0.6 | - | t | 0.7 | t | 1.6 |
| 29. | Isopulegol | 1135 | 1130 | 0.6 | 0.1 | 0.6 | - | - | - | 3.2 | - |
| 30. | Citronellal | 1135 | 1130 | t | t | t | - | - | t | - | - |
| 31. | Camphene hydrate | 1137 | 1131 | - | - | - | - | 0.2 | - | t | t |
| 32. | Pinocarvone | 1144 | 1138 | t | 0.2 | - | - | 1.6 | t | - | - |
| 33. | p-Mentha-1,5-dien-8-ol | 1148 | 1140 | 0.4 | t | t | - | - | 0.8 | - | 1.0 |
| 34. | Isomenthone | 1146 | 1141 | - | - | - | - | t | 0.2 | - | - |
| 35. | Isoborneol | 1148 | 1142 | - | - | - | - | t | 2.0 | - | - |
| 36. | Isoisopulegol | 1150 | 1143 | t | t | 0.1 | - | t | - | - | |
| 37. | Borneol | 1155 | 1149 | 1.1 | 0.1 | 0.4 | 0.5 | 3.0 | 2.0 | 0.7 | 4.5 |
| 38. | Nonan-1-ol | 1163 | 1157 | t | 0.6 | - | - | - | - | - | |
| 39. | p-Cymen-8-ol | 1158 | 1158 | 1.6 | 0.2 | 2.0 | 1.6 | 0.5 | 5.4 | 3.3 | 3.4 |
| 40. | trans-Car-2-en-4-ol | - b | 1159 | t | 0.2 | 0.9 | 1.5 | 0.1 | - | - | 1.7 |
| 41. | p -Cymen-9-ol | 1168 | 1162 | 3.2 | 0.7 | 3.4 | 3.2 | 1.4 | 6.5 | 3.2 | 1.7 |
| 42. | 2-Methylisoborneol | 1164 | 1167 | 0.3 | 0.1 | 0.7 | 1.0 | 0.3 | 0.3 | t | 0.5 |
| 43. | α-Terpineol | 1176 | 1173 | 0.9 | 0.2 | 0.9 | 1.4 | 0.6 | 2.4 | 1.7 | 1.3 |
| 44. | Myrtenal | 1174 | 1174 | t | t | t | t | 0.2 | t | t | t |
| 45. | Verbenone | 1183 | 1181 | 0.7 | 0.2 | 1.3 | 1.4 | t | 0.8 | 0.3 | 1.5 |
| 46. | Myrtenol | 1184 | 1184 | t | t | t | t | 1.4 | t | t | t |
| 47. | Coumaran | 1191 | 1198 | 0.4 | 0.5 | t | 0.2 | t | 0.1 | 2.3 | 8.2 |
| 48. | Citronellol | 1210 | 1211 | 0.2 | 0.3 | 0.2 | 1.7 | - | t | - | - |
| 49. | Neral | 1218 | 1214 | - | - | - | 1.2 | - | - | - | - |
| 50. | Carvone | 1218 | 1215 | t | - | - | - | t | 0.2 | - | 0.1 |
| 51. | 2,2-Dimethyloct-4-enal * | - b | 1216 | - | - | - | - | 0.7 | - | - | - |
| 52. | Car-3-en-2-one | 1245 | 1222 | 0.7 | - | 1.0 | 1.1 | t | 1.3 | - | 0.6 |
| 53. | Piperitone | 1232 | 1227 | t | t | - | - | - | - | 0.3 | - |
| 54. | Geraniol | 1238 | 1237 | t | 0.1 | - | - | - | - | - | - |
| 55. | Geranial | 1247 | 1242 | 0.2 | t | - | 2.5 | t | - | - | 0.3 |
| 56. | trans-Ascaridol glycol | 1266 a | 1244 | 0.1 | 0.1 | 0.7 | - | 0.6 | 1.6 | - | 0.1 |
| 57. | p-Cymen-7-ol | 1269 | 1264 | - | - | 0.5 | - | t | t | - | t |
| 58. | Nonanoic acid | 1262 | 1264 | 0.1 | 0.1 | - | - | - | - | - | - |
| 59. | Bornyl acetate | 1273 | 1269 | 0.2 | 0.2 | 0.6 | 0.5 | 0.3 | 0.1 | t | t |
| 60. | Carvacrol | 1282 | 1280 | 10.5 | 2.5 | 16.9 | 22.0 | 4.5 | 22.0 | 10.4 | 23.4 |
| 61. | Citronellic acid | 1300 | 1302 | 0.1 | t | 0.3 | - | t | - | - | - |
| 62. | 8-Hydroxyneomentol | 1310 | 1310 | 2.7 | 0.2 | 1.2 | 0.4 | 0.4 | 0.2 | 0.4 | 0.2 |
| 63. | 8-Hydroxymenthol (isomer) * | - b | 1325 | 0.1 | 0.3 | 0.1 | - | 0.1 | t | 0.4 | t |
| 64. | 8-Hydroxymenthol (izomer) * | - b | 1331 | 0.7 | - | - | - | 0.1 | - | 2.1 | t |
| 65. | α-Terpinyl acetate | 1334 | 1332 | 0.5 | 0.1 | 0.4 | 0.4 | 0.1 | 0.8 | t | t |
| 66. | Ethyl nerolate | 1335 | 1338 | 0.3 | 0.1 | - | - | t | - | 0.3 | - |
| 67. | 5,9-Dimethyldeca-4,8-dienal * | - b | 1339 | - | 0.2 | - | - | - | - | - | - |
| 68. | (E)-Jasmone | 1359 | 1358 | 0.3 | 0.2 | 1.6 | t | 0.1 | t | t | - |
| 69. | (Z)-Jasmone | 1369 | 1367 | 1.3 | 1.0 | 1.2 | 0.3 | 0.3 | 0.9 | 0.2 | 0.3 |
| 70. | Methyleugenol | 1375 | 1370 | 0.1 | t | t | t | t | 0.3 | 0.1 | 0.1 |
| 71. | p-Menth-2-ene-1,7-diol monoacetate | - b | 1407 | 0.4 | t | 0.6 | 0.8 | 1.0 | 1.1 | 0.2 | 1.0 |
| 72. | β-Ionone epoxide | 1460 | 1461 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | t | 0.1 | - |
| 73. | Dihydroactinidiolide | 1495 | 1489 | 0.8 | 0.2 | 1.4 | 1.6 | 1.6 | 2.0 | 2.3 | 2.4 |
| 74. | γ-Cadinene | 1512 | 1508 | 0.4 | 0.1 | 0.4 | 0.2 | 0.7 | t | t | 0.4 |
| 75. | α-Calacorene | 1534 | 1528 | 0.2 | t | 0.2 | 0.2 | 0.3 | 0.4 | 0.2 | 0.3 |
| 76. | β-Caryophyllene oxide | 1546 | 1541 | 0.1 | t | 0.1 | t | 0.2 | 0.1 | 0.5 | t |
| 77. | γ-Calacorene | 1551 | 1547 | t | t | t | t | t | t | t | t |
| 78. | Spathulenol | 1569 | 1566 | 2.8 | 0.5 | 3.8 | 7.6 | 8.2 | 4.8 | 7.2 | 5.9 |
| 79. | Caryophylene oxide | 1578 | 1572 | 3.1 | 0.4 | 2.8 | 3.6 | 3.9 | 3.6 | 8.2 | 2.6 |
| 80. | Aromadendrene epoxide | 1590 | 1592 | 0.1 | - | - | - | - | - | - | - |
| 81. | 6-epi-Cubenol | 1602 | 1603 | 0.2 | t | 0.2 | 1.0 | t | 0.1 | t | 0.2 |
| 82. | 1,10-diepi-Cubenol | 1615 | 1616 | 0.7 | 0.1 | 0.8 | 2.0 | 2.4 | 0.6 | 0.3 | 0.8 |
| 83. | T-Muurolol | 1628 | 1626 | 2.4 | 0.4 | 2.6 | 6.4 | 7.1 | 3.4 | 4.0 | 4.5 |
| 84. | α-Muurolol | 1628 | 1628 | 1.2 | 0.2 | 1.4 | 3.5 | 3.6 | 0.9 | 2.1 | 4.2 |
| 85. | T-Cadinol | 1633 | 1629 | 1.1 | 0.2 | 1.3 | 3.2 | 3.6 | 1.1 | 1.3 | t |
| 86. | α-Cadinol | 1643 | 1640 | 4.7 | 0.7 | 5.6 | 12.7 | 13.0 | 6.8 | 3.9 | 3.5 |
| 87. | trans-Calamen-10-ol | 1669 a | 1645 | 1.1 | 0.2 | 1.2 | 1.4 | 2.9 | 1.8 | 0.6 | 8.7 |
| 88. | Eudesma-4(15),7-dien-1β-ol | 1688 a | 1661 | t | 0.1 | 0.2 | t | 0.9 | 0.2 | 0.3 | t |
| 89. | cis-14-nor-Muurol-5-en-4-one | 1684 a | 1664 | 0.2 | t | 0.3 | 0.3 | 0.7 | 0.1 | 0.1 | 0.2 |
| 90. | 10-nor-Calamen-10-one | 1684 | 1673 | 0.3 | t | 0.4 | 0.5 | 1.1 | 0.4 | 0.1 | 0.3 |
| 91. | Oplopanone | 1715 | 1710 | 0.6 | 0.3 | 0.7 | 0.7 | 0.7 | 0.6 | 0.3 | 0.3 |
| 92. | Oplopanonyl acetate | 1791 | 1791 | 0.1 | - | - | - | 1.2 | - | 0.2 | - |
| 93. | Isopropyl tetradecanoate | 1827 a | 1808 | 0.2 | t | 0.6 | 0.6 | 1.3 | 0.4 | 0.2 | 0.4 |
| 94. | Platambin | 1867 a | 1833 | 0.4 | 0.1 | 0.3 | t | 0.6 | 0.3 | 0.1 | 0.2 |
| Total content of oxygenated compounds | 85.5 | 94.9 | 83.2 | 87.2 | 71.2 | 78.1 | 65.9 | 86.4 | |||
| Alcohols: | 73.9 | 89.6 | 69.3 | 71.2 | 55.9 | 64.5 | 48.7 | 67.6 | |||
| Aliphatic alcohols | 35.9 | 81.5 | 22.5 | 0.0 | 0.0 | 0.0 | 2.5 | 0.0 | |||
| Monoterpene alcohols | 23.4 | 5.6 | 29.4 | 33.4 | 13.6 | 44.5 | 26.4 | 39.6 | |||
| Sesquiterpene alcohols | 14.6 | 2.5 | 17.4 | 37.8 | 42.3 | 20.0 | 19.8 | 28.0 | |||
| Hydrocarbons | 0.7 | 0.3 | 0.7 | 0.4 | 1.0 | 1.5 | 0.2 | 0.8 | |||
| Total indentified [%] | 86.2 | 95.2 | 83.9 | 87.6 | 72.2 | 79.6 | 66.1 | 87.2 | |||
| Content of volatiles in hydrolate (VC) [mg/L] | 58.2 | 71.2 | 94.0 | 128.0 | 49.8 | 6.8 | 10.0 | 6.2 | |||
RIlit, literature retention index; RIexp, experimental retention index; t, trace (<0.05%). * isomer not identified. a RI on column of other polarity. b RI not available.
The total content of volatiles changed during the progress of the distillation process. At the beginning it increased from 71.2 mg/L in the I fraction to 128.0 mg/L in the III fraction, and then decreased considerably to 49.8 mg/L in the IV and 6.2–10.0 mg/L in other fractions. A similar correlation between the volume of produced hydrolate and the content of volatiles was observed for R. rugosa hydrolate [9]. The total content of volatiles in the total hydrolate amounted to 58.2 mg/L and was a little higher than the calculated mean value (52.3 mg/L). It has been reported that the content of organic compounds in hydrolate is around 100–200 mg/L [10]. Conversely, it is obvious that the amount of hydrolate should be in proper relationship to the quantity of plant material. Between 1 and 4 L of hydrolate can be obtained from 1 kg of biomass depending on the particular plant material. However, it is often said that the weight of hydrolate should be equal to that of material [10]. Taking this into account, it could be assumed that hydrolate with quite a high amount of volatiles equal to 86 mg/L could be obtained by mixing the first four fractions. This means that from 1 kg of stinging nettle herb, 0.74 L of hydrolate might be produced.
Over eighty volatile compounds were identified in representative stinging nettle hydrolate. Oxygenated compounds (85.5%) were the principal group of volatile constituents, and among them, alcohols were dominant (73.9%), e.g., (Z)-hex-3-en-1-ol (27.9%), carvacrol (10.5%), (E)-hex-3-en-1-ol (5.7%), and α-cadinol (4.7%). However, other compounds such as oxides (e.g., cis-rose oxide and caryophyllene oxide), ketones (e.g., (Z)- and (E)-jasmone, verbenone, and car-3-en-2-one), aldehydes (e.g., geranial), esters (e.g., α-terpinyl acetate, ethyl nerolate, bornyl acetate and verbanyl acetate), acids (e.g., citronellic acid, nonanoic acid) were also detected. Some sesquiterpene hydrocarbons, e.g., γ-cadinene (0.4%) and α-calacorene (0.2%), as well as trace amounts of monoterpene hydrocarbons were identified in this product. Total content of hydrocarbons accounted to 0.7%, which corresponded with literature data. Obviously, due to their polarity, oxygenated compounds were the main constituents of every hydrolate [9,10,11,12].
The composition of volatiles in stinging nettle hydrolate changed regularly during the time of hydrodistillation (Figure 2). Short-chain aliphatic alcohols C6-C10 (mainly (E)- and (Z)-hex-3-en-1-ol) were the main group of volatiles identified in the I fraction (81.5%).
Figure 2.
Changes in the profile of the U. dioica L. hydrolate volatile compounds, in total and by fraction (I, IV, V and VII).
Short-chain aliphatic alcohols constituted 22.5% in the II fraction and were scarcely visible in the remaining fractions. On the contrary, the content of monoterpene alcohols (e.g., carvacrol, p-cymen-8-ol, p-cymen-9-ol, α-terpineol, borneol) revealed a rising tendency. Among sesquiterpene compounds, a slightly different dependence between the content of constituents and volume of produced hydrolate was determined. The content of T-muurolol, α-muurolol, T-cadinol, α-cadinol, spathulenol, and caryophyllene oxide increased in fractions I to III, and then decreased.
Summarizing the obtained results concerning both the total volatiles content and their composition, it was concluded that to produce stinging nettle hydrolate of good quality, the proper relationship between the amount of hydrolate and raw plant material should result in obtaining 0.74 L hydrolate from 1 kg fresh stinging nettle herb. Such products would contain mainly (E)- and (Z)-hex-3-en-1-ol that are produced in small amounts by most plants [13]. These are important hydrolate ingredients because of their pleasant grassy-green flavour profile. According to Kalemba and Kunicka [14], carvacrol, as well as low chain alcohols, generated the highest microbial activity, therefore, we suspect that the high content of these constituents could positively affect the microbiological stability of hydrolate. Other terpenoid constituents present in hydrolate are also known for their beneficial effect on the human organism [15].
It is worth mentioning that the chemical composition of U. dioica hydrolate was significantly different to that of previously published research on essential oil. Two available previous reports presented two different essential oils that showed that the profile of stinging nettle volatiles was dependent on the location of growth [6,7]. Understandably, high molecular compounds such as hexahydrofarnesylacetone and phytol, that were present in both previously reported essential oil studies, were not detected in the hydrolate. Nevertheless, some hydrolate volatile compounds identified in this study were previously found in stinging nettle essential oil, e.g., carvacrol was the main constituent in the essential oil of the Turkish herb (32.8%) [7]. The differences between hydrolate volatiles and essential oil composition were likely due to differences in water solubility of oxygenated compounds, especially alcohols and hydrocarbons, than to the location of growth.
3. Materials and Methods
3.1. Plant Material
Stinging nettle herb (Urtica dioica L.) was harvested in May from natural stands in the Northern region of Poland (54°41′59″ N, 18°21′1″ E).
3.2. Hydrolate Production
The fresh herb, 14.6 kg, was subjected to two-column distillation apparatus, Innotec-Tetekov TWE 250–2000 VA. During the distillation process that lasted 100 min, seven hydrolate fractions were collected (2.7 L each, 18.9 L in total). A representative sample of hydrolate called “total hydrolate” was also prepared by mixing an equal volume (250 mL) of each fraction. The essential oil was not obtained during the distillation process.
3.3. pH Value
The pH value of every hydrolate fraction and total hydrolate was determined using a CP-511 pH meter with IJ44A electrode (Elmetron Company, Zabrze, Poland). The measurements were performed at 20 °C.
3.4. Isolation of Volatile Compounds from Hydrolate
Volatile compounds were isolated from each hydrolate fraction and total hydrolate by liquid–liquid extraction with diethyl ether. The hydrolate sample (500 mL) was salted with NaCl (180 g) to reduce the solubility of the volatile compounds in water, and extracted with 100 mL of solvent. The extraction process was repeated five times, each time using a fresh amount of diethyl ether. Extracts were merged to obtain a final 500 mL of diethyl ether extract from each fraction of the hydrolate (I–VII) and total hydrolate. Extracts were dried over anhydrous sodium sulfate and filtered. The solvent was removed using a rotary vacuum evaporator at 36 °C and under the pressure of 25 mmHg. The remaining mixture of volatiles was weighed and the content of volatiles was reported as mg/L. The composition of isolated volatiles was analysed by GC-FID-MS method.
3.5. Analysis of Volatile Compounds
Volatile compounds isolated from hydrolate fractions were analysed by gas chromatography coupled with mass spectrometry (GC-FID-MS).
Apparatus: Trace GC Ultra gas chromatograph coupled with DSQ II mass spectrometer (Thermo Electron Corporation), non-polar capillary column Rtx-1 ms (60 m × 0.25 mm, 0.25 m film thickness), programmed temperature: 50 (3 min)–300 °C, 4 °C/min, injector (SSL) temperature 280 °C, detector (FID) temperature 300 °C, transfer line temperature 250 °C, carrier gas—helium, flow with constant pressure 200 kPa, split ratio 1:20. The mass spectrometer parameters: ion source temperature 200 °C, ionization energy 70 eV (EI), scan mode: full scan, mass range 33–420. The percentages of constituents were computed from the GC peak area without using a correction factor.
Identification of the components was based on a comparison of their mass spectra with literature data as well as linear retention indices (RI, non-polar column), determined with reference to a series of n-alkanes C8–C24, by comparing with those in Adams [16] as well as in computer libraries: NIST 2011 (USA) and MassFinder 4.1 (Germany).
4. Conclusions
Over eighty types of volatiles were identified in stinging nettle hydrolate. The main group of constituents was alcohols: (Z)- and (E)-hex-3-en-1-ol, carvacrol, α-cadinol (4.7%), p-cymen-9-ol, and T-muurolol. The content of total volatiles and their quantitative composition was related to the volume of produced hydrolate and changed with the following fractions. Some regular changes in the first four fractions (I–IV) were observed. The content of short-chain aliphatic alcohols decreased, while the content of mono- and sesquiterpene alcohols increased.
According to the content and chemical composition of volatiles, the first four fractions (I–IV) of stinging nettle hydrolate were considered as the most valuable. Due to this statement, to obtain a product with good quality, from 1 kg of fresh plant material, 0.74 L of U. dioica hydrolate might be produced. It is worth noting that this is one of the few studies showing the dependence of the content of volatile components on the amount of the obtained hydrolate, and the first one to our knowledge, showing the qualitative and quantitative composition of the volatile components of the stinging nettle hydrolate. Continuous interest in using this raw material, especially in the food and cosmetic industries, indicates the need for further research in this field.
Acknowledgments
The authors wish to thank Robert Gwiazdecki for providing test material, and Agata Waleriańczyk for technical assistance.
Author Contributions
Conceptualization, A.K. methodology, A.K.; investigation, A.K. and K.M.; resources, A.K.; data curation, A.K.; writing—original draft preparation, A.K. and K.M.; writing—review and editing, A.K. and K.M.; visualization, K.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the hydrolate are not available.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Taylor K. Biological Flora of the British Isles: Urtica dioica L. J. Ecol. 2009;97:1436–1458. doi: 10.1111/j.1365-2745.2009.01575.x. [DOI] [Google Scholar]
- 2.Joshi B.C., Mukhija M., Kalia A.N. Pharmacognostical review of Urtica dioica L. Int. J. Green Pharm. 2014;8:201–209. doi: 10.22377/ijgp.v8i4.414. [DOI] [Google Scholar]
- 3.Chrubasik J.E., Roufogalis B.D., Wagner H., Chrubasik S.A. A comprehensive review on nettle effect and efficacy profiles, Part I: Herba urticae. Phytomedicine. 2007;14:423–435. doi: 10.1016/j.phymed.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Asgarpanah J., Mohajerani R. Phytochemistry and pharmacologic properties of Urtica dioica L. J. Med. Plants Res. 2012;6:5714–5719. doi: 10.5897/JMPR12.540. [DOI] [Google Scholar]
- 5.EDQM—Council of Europe . European Pharmacopoeia. EDQM; Strasbourg, France: 2020. [Google Scholar]
- 6.Ilies D.-C., Tudor I., Radulescu V. Chemical composition of the essential oil of Urtica dioica. Chem. Nat. Compd. 2012;48:506–507. doi: 10.1007/s10600-012-0291-4. [DOI] [Google Scholar]
- 7.Gül S., Demirci B., Can Başer K.H., Akpulat H.A., Akşu P. Chemical Composition and In Vitro Cytotoxic, Genotoxic Effects of Essential Oil from Urtica dioica L. Bull. Environ. Contam. Toxicol. 2012;88:666–671. doi: 10.1007/s00128-012-0535-9. [DOI] [PubMed] [Google Scholar]
- 8.Catty S. Hydrosols the Next Aromatherapy. 1st ed. Healing Arts Press; Rochester, Vermont: 2001. [Google Scholar]
- 9.Maciąg A., Kalemba D. Composition of rugosa rose (Rosa rugosa Thunb.) hydrolate according to the time of distillation. Phytochem. Lett. 2015;11:373–377. doi: 10.1016/j.phytol.2014.10.024. [DOI] [Google Scholar]
- 10.Price L., Price S. Understanding Hydrolats: The Specific Hydrosol from Aromatherapy. Churchill Livingstone; London, UK: 2004. [Google Scholar]
- 11.Wajs-Bonikowska A., Sienkiewicz M., Stobiecka A., Maciąg A. Chemical Composition and Biological Activity of Abies alba and A. koreana Seed and Cone Essential Oils and Characterization of Their Seed Hydrolates. Chem. Biodivers. 2015;12:407–418. doi: 10.1002/cbdv.201400167. [DOI] [PubMed] [Google Scholar]
- 12.Wawrzyńczak K., Więckowska M., Sadowska B., Kalemba D. Composition and Antimicrobial Activity of Myrica gale L. Leaf and Flower Essential Oils and Hydrolates. Rec. Nat. Prod. 2021;15:35–45. doi: 10.25135/rnp.190.20.04.1628. [DOI] [Google Scholar]
- 13.McRae J.F., Mainland J.D., Jaeger S.R., Adipietro K.A., Matsunami H., Newcomb R.D. Genetic Variation in the Odorant Receptor OR2J3 Is Associated with the Ability to Detect the ’’Grassy’’ Smelling Odor, cis-3-hexen-1-ol. Chem. Senses. 2012;37:585–593. doi: 10.1093/chemse/bjs049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalemba D., Kunicka A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003;10:813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- 15.Georgian D., Ramadoss N., Dona C., Basu C. Medicinal Plants. Springer; Berlin/Heidelberg, Germany: 2019. Therapeutic and Medicinal Uses of Terpenes. [Google Scholar]
- 16.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. 4th ed. Allured Publishing Corp; Carol Stream, IL, USA: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.