Abstract
Exposure to bisphenols is related to negative effects on male reproduction. The bisphenols exposure is associated with several modes of action including negative impact on the blood–testis barrier (BTB) in testes or direct effect on spermatozoa. Bisphenols have been detected in human seminal plasma, but the possible mechanism of seminal transfer of bisphenols is not clear. Some authors consider the transfer through the blood–testis barrier to be crucial. Therefore, in this work, we compared normozoospermic men and men after vasectomy who have interrupted vas deferens and their ejaculate does not contain testicular products. We measured the concentration of bisphenol A (BPA), bisphenol S (BPS) and bisphenol F (BPF) in the urine and seminal plasma of these men using liquid chromatography tandem mass spectrometry (LC/MSMS). We found that the ratio of urinary and seminal plasma content of bisphenols did not differ in normozoospermic men or men after vasectomy. From the obtained data, it can be concluded that the pathways of transport of bisphenols into seminal plasma are not primarily through the testicular tissue, but this pathway is applied similarly to other routes of transmission by a corresponding ejaculate volume ratio. To a much greater extent than through testicular tissue, bisphenols enter the seminal plasma mainly as part of the secretions of the accessory glands.
Keywords: bisphenol A, bisphenol S, bisphenol F, endocrine disruptors, human, spermatozoa, IVF, vasectomy, biotransformation
1. Introduction
Bisphenols are industrial chemicals widely used in consumer products (food packing materials, pipe linings, etc.) in order to increase their thickness and durability [1,2,3]. Because of their widespread consumer and commercial use, bisphenols are frequently detected in human samples including seminal plasma [4,5]. Their potential negative impact on male infertility [6], human oocyte development [7] or differentiation of foetal testicular tissue [8] have been described in several studies.
The most common pathway of their entry into organism is with food and drinks [9] that were stored in containers or bottles made of plastics containing for example bisphenol A (BPA) in its polymerized form. Mainly after exposure to high temperatures, its monomers can be released into the content and can be ingested into the body [10].
Regarding development of spermatozoa, there are several theories of negative effect of bisphenols on spermatogenesis. It is partially caused by binding to estrogen and androgen receptors in testicles [2] and partially by direct effect on spermatozoa and germinal cells [11]. Its effect on germinal epithelium through production of oxygen species and subsequent oxidative stress also is not irrelevant.
Despite rather a lot being known about potential effects of these substances on spermatogenesis, the way how bisphenols get into seminal plasma, where they directly affect sperm cells, is still not clear. Bisphenols were detected several times in blood, urine or seminal plasma [5]. Its content in seminal plasma is the most important in terms of spermatogenesis and is the main subject of this study.
Bisphenols that were not metabolized after entry to the organism, enter circulation in their unbound form and their lipophilic character enables their distribution to various tissues, such as adipose [12], brain [13], breast tissue [14], reproductive organs [15], prostate [16], maternal milk [17] or their transmembraneous transfer to foetal bodies [18]. From the tissues, they can be released to the systemic circulation again and be excreted with urine [15]. Besides urine, they can be detected in seminal plasma which is primarily a product of accessory sex glands. Therefore, it can be assumed that the source of bisphenols in seminal plasma is transport from blood circulation to accessory glands. However, it is not clear how transport through BTB in testicles contributes to the concentration of bisphenols in seminal plasma [19].
So far, most studies focused on the effect of bisphenols on spermatozoa evaluated bisphenols detected in urine. Results of the studies that detected bisphenols in urine and searched for correlations with deteriorated ejaculate quality, do not have consistent conclusions. One possible reason is that urine is not an optimal matrix for studies of this type. Metabolism of bisphenols is rather fast; most of the ingested bisphenols are excluded with urine within 24 h. However, urinary BPA concentrations do not directly inform about bioactive concentrations in specific tissues as mainly the conjugated forms, which are biologically inactive, are excluded with urine, while the unbound forms, that show high biological activity were detected in the seminal plasma.
For the evaluation of the effect of these substances on spermiogenesis, it is suitable to measure their concentration in seminal plasma directly since the concentration of bisphenols differs among blood, urine and seminal plasma. In addition, a negative correlation between concentration of BPA and sperm concentration, sperm count and morphology were observed only in samples of seminal plasma, not in blood plasma [5].
Although the transport of these substances to the ejaculate has not been studied thoroughly, their transport through the BTB in testicles has been mentioned frequently [19]. In men with vasectomy—artificial sterilization via surgical cross-section of vas deferens—products of testicular tissue are not present in the ejaculate. It lacks for example proteins specific for testicular tissue, such as testes specific protein 101 [20] or ACRV1 [21]. For this reason, these patients were selected as control for bisphenol biotransformation in organism and verification of hypothesis that bisphenols are not transferred to ejaculate from testicular tissue.
In this study, we have decided to verify the content of selected bisphenols in seminal plasma of non-operated men and men after surgical vasectomy. Subsequently, we performed comparison with concentration of these compounds in urine.
The aim of the work was to evaluate the importance of the transport of bisphenols through testicular tissue, since there are no products of testes in the ejaculate of men after vasectomy, including the products that pass the BTB.
2. Materials and Methods
2.1. Study Design
This prospective study included a total of 8 patients with normozoospermia and 8 men after surgical vasectomy with azoospermia at average age 32.4 years (from 22 to 41). The criteria for normozoospermia and azoospermia were defined according to the WHO manual (2010) [22]. Samples were collected between January 2020 and December 2021. The project was approved by the Ethics Committee of University Hospital Brno (Approval No. 10-170221/EK). All the patients agreed with their participation in the study and signed an informed consent. Semen sample was collected immediately after spermiogram analyses. The concentration of bisphenol A (BPA), bisphenol S (BPS) and bisphenol F (BPF) in the urine and seminal plasma of these men was detected by using liquid chromatography tandem mass spectrometry (LC/MSMS).
2.2. Semen and Urine Collection
Patients first time urinate to glass tube and after that semen samples were collected by masturbation after 3–8 days of sexual abstinence. Patients were asked to wash hands before masturbation. All samples were incubated at room temperature for 46–60 min until total liquefaction. Semen analyses was carried out in liquefied samples according to WHO guidelines [22]. Immediately after the spermiogram analyses, samples were centrifugated and seminal plasma was frozen in glass tube (−20 °C).
2.3. Vasectomy
All patients in the vasectomy group underwent classic open-ended vasectomy. The procedure is performed under general anaesthesia by an incision on each side of the scrotum. The cut is a maximum of 2 cm long. Following the preparation of the individual layers, the vas deferens is exposed and interrupted. A small portion of the vas deferens is removed. The ends of the vas deferens are ligated with a non-absorbable suture. The lumen is closed by coagulation. Finally, fascial interposition of the stumps is performed, i.e., the ends are immersed to different depths to increase the effect.
2.4. Bisphenol Detection in Urine
For the determination of total BPA, BPS and BPF, the urine samples were processed according to the methods described by Ye et al. [23,24]. Briefly, 500 μL of urine sample were mixed with 10 μL of 2 μg/mL internal standards solution, 20 μL of a β-glucuronidase/sulfatase solution (Helix pomatia, H1, 10,000 units/mL) and 100 μL of 1 M ammonium acetate buffer (pH 6.5). Samples were subsequently incubated for 1 h at 37 °C and sonicated. Thereafter, samples were extracted using solid phase extraction with Oasis WAX cartridges (Waters, Milford, MA, USA) that were previously washed and conditioned with water. Elution of bisphenols was carried out using methanol. The elution solvent was evaporated to dryness under a gentle stream of nitrogen gas. Prior to the analysis the residue was redissolved in 50% methanol (v/v). LC separation wad performed with an Agilent 1290 instrument (Agilent Technologies, Waldbronn, Germany) using Waters ™ ACQUITY ™ UPLC ™BEH C18 (100 × 2.1 mm, 1.7 µm) column and gradient elution with a mixture of 0.1 mM ammonium fluoride (A) and methanol (B) as mobile phases. The gradient profile was 50% B (0–1 min), then was linearly increased to 90% B (1–5 min), maintained at 90% B (5–7 min) and dropped to 50% B (7.01–10 min). The flow rate was 0.25 mL/min, and the injection volume was 5 μL. The elution time was 10 min. The column compartment was maintained at 30 °C. Bisphenols were measured with an Agilent 6495 Triple Quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) operating in the ESI-negative mode. Two MRM transitions were used for quantitative LC/MSMS analyses.
2.5. Bisphenol Detection in Seminal Plasma
For the determination of free BPA, BPS and BPF from seminal fluid, a mixture of toluene and ethyl acetate (50/50; v/v) was used. In general, 0.5 mL of seminal plasma was placed into a 5 mL Eppendorf tube and spiked with the mixture of isotopically labelled standards at level of 1 ng/mL. Then, 1 mL of cold acetonitrile was added for deproteination followed by vortexing and then sonicating for 10 min. Afterwards, the mixture was centrifuged at 2880× g for 10 min at 4 °C and the supernatant was removed and further evaporated to dryness under the gentle stream of nitrogen at 50 °C. The residue was reconstituted in a deionized water for subsequent extraction of bisphenols. For toluene/ethyl acetate extraction, 0.5 mL of toluene/ethyl acetate mixture (1 (thin space (1/6-em]]–(thin space (1/6-em))1, v/v) was added to 0.5 mL of redissolved residue in deionized water and shaken for 10 min. After the phase separation, 300 μL of the organic fraction was quantitatively transferred to HPLC glass vial and evaporated to dryness under gentle stream of nitrogen at 50 °C. The residue was redissolved in 50% methanol for bisphenol analysis. Chromatographic separation was performed on Waters ™ ACQUITY ™ UPLC ™ BEH C18 (100 × 2.1 mm, 1.7 µm) column using gradient elution with a mixture of 0.1 mM ammonium fluoride and methanol as mobile phases. Samples were analysed on Agilent 6495 Triple Quadrupole (Agilent Technologies, Santa Clara, CA, USA) operating in the ESI-negative mode. Two MS/MS transitions were used for quantitative LC-MS/MS analyses. Because it was impossible to obtain matrix that is blank for ever-present contaminants, non-spiked matrix was analysed simultaneously, and the measured concentration was subtracted from the concentration calculated in the samples.
3. Results
All the sample sets were analysed and concentrations of bisphenol A, bisphenol S and bisphenol F were determined in urine and seminal plasma. The results of bisphenol content in urine were standardized by conversion based on specific weight of urine according to [25]. Results of bisphenol content ratio in urine and seminal plasma are presented in Table 1. The levels detected in urine range from 0.24 to 5.15 ng/mL in case of BPA (Table 2), 0.03 to 6.7 ng/mL for BPS (Table 3) and 0.8–1.94 ng/mL for BPF (Table 4). The levels of bisphenols detected in seminal plasma are at similar level in the control patients and in the patients after vasectomy, ranging from 0.01 to 0.75 for BPA and from 0.01 to 0.85 for BPS. The detection frequency for BPF was low (18%, see Table 2) and these samples were removed from the further investigation. One value of BPS in urine below the limit of quantification (LOQ = 0.017 ng/mL) was replaced by LOQ/√2.
Table 1.
Relative ratio of bisphenol content in urine and seminal plasma in men with normozoospermia and in men after vasectomy.
| Normozoospermic Men | Vasectomized Men | |
|---|---|---|
| Ratio of urinary BPA/seminal plasma BPA | 7.64 | 5.64 |
| Ratio of urinary BPS/seminal plasma BPS | 6.11 | 2.95 |
| Ratio of urinary bisphenols /seminal plasma bisphenols | 7.21 | 4.5 |
Table 2.
Detection of bisphenol S (BPS) in all the observed patients in urine and in seminal plasma.
| Urine (ng/mL) | Seminal Plasma (ng/mL) | ||
|---|---|---|---|
| Normozoospermic men | patient 1 | 0.037 | 0.038 |
| patient 2 | 0.101 | 0.074 | |
| patient 3 | 0.997 | 0.140 | |
| patient 4 | 0.012 | 0.011 | |
| patient 5 | 1.190 | 0.110 | |
| patient 6 | 0.099 | 0.010 | |
| patient 7 | 0.512 | 0.064 | |
| patient 8 | 1.786 | 0.083 | |
| Vasectomized men | patient 1 | 5.105 | 0.280 |
| patient 2 | 0.418 | 0.089 | |
| patient 3 | 1.010 | 0.204 | |
| patient 4 | 0.039 | 0.507 | |
| patient 5 | 0.530 | 0.749 | |
| patient 6 | 0.268 | 0.132 | |
| patient 7 | 3.230 | 0.226 | |
| patient 8 | 0.120 | 0.749 |
Table 3.
Detection of bisphenol A (BPA) in all the observed patients in urine and in seminal plasma.
| Urine (ng/mL) | Seminal Plasma (ng/mL) | ||
|---|---|---|---|
| Normozoospermic men | patient 1 | 0.085 | 0.017 |
| patient 2 | 0.247 | 0.041 | |
| patient 3 | 0.030 | 0.013 | |
| patient 4 | 0.134 | 0.009 | |
| patient 5 | 0.078 | 0.006 | |
| patient 6 | 0.022 | 0.008 | |
| patient 7 | 0.077 | 0.037 | |
| patient 8 | 0.335 | 0.090 | |
| Vasectomized men | patient 1 | 0.031 | 0.007 |
| patient 2 | 2.503 | 0.202 | |
| patient 3 | 0.202 | 0.060 | |
| patient 4 | 0.369 | 0.850 | |
| patient 5 | 0.079 | 0.500 | |
| patient 6 | <0.017 | 33.683 | |
| patient 7 | 0.430 | 0.329 | |
| patient 8 | 0.302 | 0.516 |
Table 4.
Detection of bisphenol F (BPF) in all the observed patients in urine and in seminal plasma. LOD = limit of detection.
| Urine (ng/mL) | Seminal Plasma (ng/mL) | ||
|---|---|---|---|
| Normozoospermic men | patient 1 | <0.040 | <LOD |
| patient 2 | <0.040 | <LOD | |
| patient 3 | <0.040 | <LOD | |
| patient 4 | <0.040 | <LOD | |
| patient 5 | <0.130 | <LOD | |
| patient 6 | <0.040 | <LOD | |
| patient 7 | <0.040 | <LOD | |
| patient 8 | 0.810 | <LOD | |
| Vasectomized men | patient 1 | <0.040 | <LOD |
| patient 2 | 1.94 | <LOD | |
| patient 3 | <0.040 | <LOD | |
| patient 4 | <0.040 | <LOD | |
| patient 5 | <0.040 | <LOD | |
| patient 6 | <0.040 | 0.991 | |
| patient 7 | <0.040 | <LOD | |
| patient 8 | <0.040 | <LOD |
Since both the urine and seminal fluid concentrations did not differ from log-normal statistical distribution (Shapiro–Wilk’s test, p-value > 0.050), the ratio of urine–seminal plasma was calculated and log-transformed to obtain a normal distribution (again tested with Shapiro–Wilk’s test with p-value > 0.050). Mean of that ratio in the control group was 7.64 for BPA and 6.11 for BPS, mean in the patient with vasectomy was 5.64 for BPA and 3.39 for BPS. Finally, the differences in ratios between the control group and patients with vasectomy were tested using Welch’s t-test with p-values of 0.268 for BPA and 0.083 for BPS. These data were verified using Mann–Whitney’s test with p = 0.382 for BPA and p = 0.121 for BPS (see Table 1). None of the methods found any significant difference in ratios of urinary to seminal fluid bisphenols concentrations between the control group and patients with vasectomy.
4. Discussion
Bisphenols are ubiquitous substances, common in our environment. Their presence can affect a variety of physiological processes and they are often considered gametogenesis disruptors. Their effect is either direct on spermatozoa [11] or on germinal epithelium [26] or they can disrupt the BTB [27]. Until recently, many authors considered crossing the BTB to be the way for bisphenols to enter the ejaculate [19]. After ingestion in the organism, most BPA is metabolized by glucuronidation or sulfonation [28]. The greatest amount of BPA is metabolized in the liver, by UDP-glucuronosyltransferase 2B15 to inactive form BPA-glucuronide [29]. This form is water-soluble and is excreted in urine with a 5.4–6.4 h elimination half-life [30].
Unlike in plastic containers and plastic films in cans, where bisphenols are in polymerized forms, thermal paper contains up to milligrams of free BPA per gram [31,32]. Transdermal entry to the organism seems to be very important because it bypasses the metabolization of bisphenols in the liver. This route of transmission may significantly contribute to the total amount of bisphenols detected in urine or seminal plasma.
In addition, this transfer is accelerated by frequent use of hand disinfectants that can disrupt dermal barrier [31]. Because transdermal transfer bypasses the metabolization of bisphenols in the liver, this route of transmission may be significant for the total amount of bisphenols detected in urine or seminal plasma.
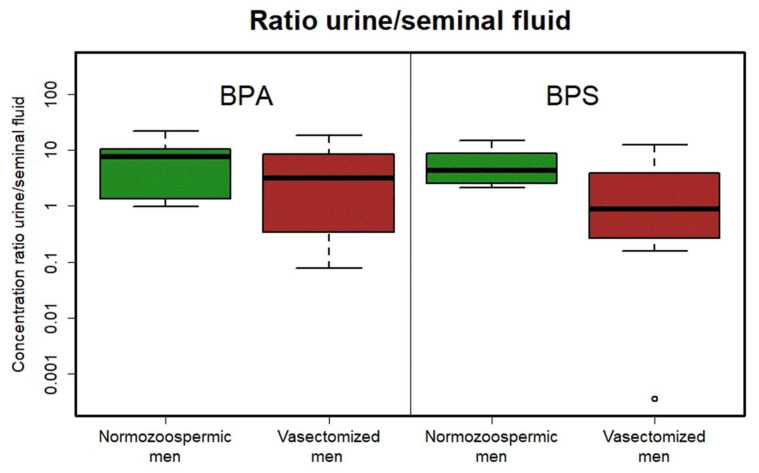
Several authors have mentioned bisphenol transport primary via the blood–testis barrier in the testes [33]. In our study we did not find any differences in bisphenol urinal/seminal ratio in normozoospermic men and men after vasectomy (which ejaculate is without testicular products). This study, focused on detection of bisphenols in urine and seminal plasma, clearly demonstrated that the pathway for bisphenols to seminal plasma is not primarily via testicular tissue and that these substances are much more transported together with secrets of accessory glands (prostate, seminal vesicles and bulbourethral glands) that produce more than 95% of the human ejaculate volume. Our results suggest even the opposite trend, i.e., higher levels of bisphenols in seminal plasma than in urine in men after vasectomy in comparison with the control group. This is probably caused by a small set of patients with high individual differences. Indeed, in urine, total BPA was detected (i.e., also after metabolization), while only free BPA was detected in seminal plasma. Detection of multiple extreme values of the ratio of urinal/seminal bisphenols is observed in the group after vasectomy more often than in normozoospermic patients (Figure 1). This is probably due to the large dispersion of values and partly the role of chance. In this vasectomized men, we would expect there to be less bisphenols in ejaculate than urine and the urinal/seminal ratio would increase. However, we observe extreme values only in the opposite direction, i.e., higher concentrations of bisphenols in seminal plasma compared to urine. The final proportion of bisphenols contents in urine and seminal plasma can be affected by time of exposition or entrance of bisphenols to the organism. As mentioned before, there are two different biotransformation ways after per oral or transdermal exposition.
Figure 1.
Boxplots of ratios between urinary and seminal plasma BPA (left) and BPS (right) concentrations in normozoospermic (green) and vasectomized (red) patients. ° indicates patient outside the main groupe.
Seminal plasma is a mixture of secrets produced primarily by accessory glands, with 65–75% of volume produced by seminal vesicles, 20–30% by prostate and epididymis, 1% by bulbourethral and periurethral glands and 2–5% by testes [34]. Since the ratio of concentrations detected in seminal plasma and urine did not differ in normozoospermic men and men after vasectomy, it is highly probable that the transport of bisphenols to seminal plasma is mediated by accessory glands that contribute to the final composition of the ejaculate. This statement is supported by the finding that bisphenol F was detected only in three samples, but independently on the sample type and vasectomy performed. It is evident, that if a higher dose of bisphenols enters the organism, they reach the ejaculate no matter if there is a functional connection with testicular tissue or not.
Our results even did not show a significant decrease of bisphenol concentrations in vasectomized men compared to normozoospermic men. It suggests that transfer via testicular tissue is rather minor and that these substances enter seminal plasma via other transport pathways, from seminal vesicles, prostate or bulbourethral glands.
5. Conclusions
The importance of bisphenol transport from testicles via the BTB is negligible and its contribution to overall bisphenol content in seminal plasma is minimal. In conclusion, the transport of bisphenols to seminal plasma is mediated primarily via the accessory glands. The transport via testes is rather minor and its share on the total amount of bisphenols in seminal plasma is only small and corresponds to the share of seminal plasma produced in testicles.
Author Contributions
Conceptualization, M.J. and J.N.; methodology, M.J., J.K. (Jiří Kohoutek) and J.N.; validation, J.K. (Jiří Kohoutek) and J.N.; formal analysis, M.J., P.V., I.C., J.Ž., B.K., K.F. and J.N.; investigation, S.M., L.M. and M.K.; data curation, M.J., K.F. and J.K. (Jiří Kalina); writing—original draft preparation, M.J. and J.N.; writing—review and editing, M.J., P.V., I.C., B.K., L.M., K.F., J.K. (Jiří Kalina) and J.N.; supervision, J.N.; project administration, M.J.; funding acquisition, M.J. and J.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institution Ethics Committee of University Hospital Brno (protocol code 10-170221, 17 February 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the MH CZ—DRO (FNBr, 65269705) and the project AZV NV18-01-00544. J.N. was supported from project MSCAfellow3@MUNI (No. CZ.02.2.69/0.0/0.0/19_074/0012727). The authors also thank the Research Infrastructure RECETOX RI (No LM2018121) financed by the Ministry of Education, Youth and Sports, and Operational Programme Research, Development and Innovation—project CETOCOEN EXCELENCE (No CZ.02.1.01/0.0/0.0/17_043/0009632) for the supportive background.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Almeida S., Raposo A., Almeida-González M., Carrascosa C. Bisphenol A: Food Exposure and Impact on Human Health. Compr. Rev. Food Sci. Food Saf. 2018;17:1503–1517. doi: 10.1111/1541-4337.12388. [DOI] [PubMed] [Google Scholar]
- 2.Ješeta M., Navrátilová J., Franzová K., Fialková S., Kempisty B., Ventruba P., Žáková J., Crha I. Overview of the Mechanisms of Action of Selected Bisphenols and Perfluoroalkyl Chemicals on the Male Reproductive Axes. Front. Genet. 2021;12:692897. doi: 10.3389/fgene.2021.692897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rochester J.R., Bolden A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015;123:643–650. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palak E., Lebiedzińska W., Anisimowicz S., Sztachelska M., Pierzyński P., Wiczkowski W., Żelazowska-Rutkowska B., Niklińska G.N., Ponikwicka-Tyszko D., Wołczyński S. The Association between Bisphenol A, Steroid Hormones, and Selected MicroRNAs Levels in Seminal Plasma of Men with Infertility. J. Clin. Med. 2021;10:5945. doi: 10.3390/jcm10245945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitku J., Heracek J., Sosvorova L., Hampl R., Chlupacova T., Hill M., Sobotka V., Bicikova M., Stárka L. Associations of bisphenol A and polychlorinated biphenyls with spermatogenesis and steroidogenesis in two biological fluids from men attending an infertility clinic. Environ. Int. 2016;89:166–173. doi: 10.1016/j.envint.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Ješeta M., Crha T., Žáková J., Ventruba P. Bisphenols in the pathology of reproduction. Ceska. Gynekol. 2019;84:161–165. [PubMed] [Google Scholar]
- 7.Machtinger R., Combelles C.M., Missmer S.A., Correia K.F., Williams P., Hauser R., Racowsky C. Bisphenol-A and human oocyte maturation in vitro. Hum. Reprod. 2013;28:2735–2745. doi: 10.1093/humrep/det312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong J., Chen F., Wang X., Bai Y., Zhou R., Li Y., Chen L. Exposure of preimplantation embryos to low-dose bisphenol A impairs testes development and suppresses histone acetylation of StAR promoter to reduce production of testosterone in mice. Mol. Cell. Endocrinol. 2016;427:101–111. doi: 10.1016/j.mce.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Geens T., Aerts D., Berthot C., Bourguignon J.-P., Goeyens L., Lecomte P., Maghuin-Rogister G., Pironnet A.-M., Pussemier L., Scippo M.-L., et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012;50:3725–3740. doi: 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- 10.Le H.H., Carlson E.M., Chua J.P., Belcher S.M. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol. Lett. 2008;176:149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiffer C., Müller A., Egeberg D.L., Alvarez L., Brenker C., Rehfeld A., Frederiksen H., Wäschle B., Kaupp U.B., Balbach M., et al. Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep. 2014;15:758–765. doi: 10.15252/embr.201438869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venisse N., Cambien G., Robin J., Rouillon S., Nadeau C., Charles T., Rabouan S., Migeot V., Dupuis A. Development and validation of an LC–MS/MS method for the simultaneous determination of bisphenol A and its chlorinated derivatives in adipose tissue. Talanta. 2019;204:145–152. doi: 10.1016/j.talanta.2019.05.103. [DOI] [PubMed] [Google Scholar]
- 13.Van Der Meer T.P., Artacho-Cordón F., Swaab D.F., Struik D., Makris K.C., Wolffenbuttel B.H.R., Frederiksen H., Van Vliet-Ostaptchouk J.V. Distribution of Non-Persistent Endocrine Disruptors in Two Different Regions of the Human Brain. Int. J. Environ. Res. Public Health. 2017;14:1059. doi: 10.3390/ijerph14091059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dualde P., Pardo O., Corpas-Burgos F., Kuligowski J., Gormaz M., Vento M., Pastor A., Yusà V. Biomonitoring of bisphenols A, F, S in human milk and probabilistic risk assessment for breastfed infants. Sci. Total Environ. 2019;668:797–805. doi: 10.1016/j.scitotenv.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Karrer C., Roiss T., Von Goetz N., Skledar D.G., Mašič L.P., Hungerbühler K. Physiologically Based Pharmacokinetic (PBPK) Modeling of the Bisphenols BPA, BPS, BPF, and BPAF with New Experimental Metabolic Parameters: Comparing the Pharmacokinetic Behavior of BPA with Its Substitutes. Environ. Health Perspect. 2018;126:077002. doi: 10.1289/EHP2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prins G.S., Hu W.-Y., Shi G.-B., Hu D.-P., Majumdar S., Li G., Huang K., Nelles J.L., Ho S.-M., Walker C.L., et al. Bisphenol A Promotes Human Prostate Stem-Progenitor Cell Self-Renewal and Increases In Vivo Carcinogenesis in Human Prostate Epithelium. Endocrinology. 2014;155:805–817. doi: 10.1210/en.2013-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez M., Arrebola J.P., Taoufiki J., Navalón A., Ballesteros O., Pulgar R., Vilchez J., Olea N. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod. Toxicol. 2007;24:259–264. doi: 10.1016/j.reprotox.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Domoradzki J.Y., Pottenger L.H., Thornton C.M., Hansen S.C., Card T.L., Markham D.A., Dryzga M.D., Shiotsuka R.N., Waechter J.M. Metabolism and Pharmacokinetics of Bisphenol A (BPA) and the Embryo-Fetal Distribution of BPA and BPA-Monoglucuronide in CD Sprague-Dawley Rats at Three Gestational Stages. Toxicol. Sci. 2003;76:21–34. doi: 10.1093/toxsci/kfg206. [DOI] [PubMed] [Google Scholar]
- 19.Cheng C.Y., Wong E.W., Lie P.P., Li M.W., Mruk D.D., Yan H.H., Mok K.-W., Mannu J., Mathur P.P., Lui W.-Y., et al. Regulation of blood-testis barrier dynamics by desmosome, gap junction, hemidesmosome and polarity proteins. Spermatogenesis. 2011;1:105–115. doi: 10.4161/spmg.1.2.15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korbakis D., Schiza C., Brinc D., Soosaipillai A., Karakosta T.D., Légaré C., Sullivan R., Mullen B., Jarvi K., Diamandis E.P., et al. Preclinical evaluation of a TEX101 protein ELISA test for the differential diagnosis of male infertility. BMC Med. 2017;15:60. doi: 10.1186/s12916-017-0817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gashti N.G., Gilani M.A.S., Jabari A., Qasemi M., Feizollahi N., Abbasi M. The Germ Cell–Specific Markers ZPBP2 and PGK2 in Testicular Biopsies Can Predict the Presence as well as the Quality of Sperm in Non-obstructive Azoospermia Patients. Reprod. Sci. 2021;28:1466–1475. doi: 10.1007/s43032-020-00427-9. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization . WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. WHO Press; Geneva, Switzerland: 2010. [Google Scholar]
- 23.Ye X., Kuklenyik Z., Needham L.L., Calafat A.M. Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2005;383:638–644. doi: 10.1007/s00216-005-0019-4. [DOI] [PubMed] [Google Scholar]
- 24.Ye X., Kuklenyik Z., Needham L.L., Calafat A.M. Measuring environmental phenols and chlorinated organic chemicals in breast milk using automated on-line column-switching–high performance liquid chromatography–isotope dilution tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life. 2006;831:110–115. doi: 10.1016/j.jchromb.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 25.Kuiper J.R., O’Brien K.M., Ferguson K.K., Buckley J.P. Urinary specific gravity measures in the U.S. population: Implications for the adjustment of non-persistent chemical urinary biomarker data. Environ. Int. 2021;156:106656. doi: 10.1016/j.envint.2021.106656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiorini C., Tilloy-Ellul A., Chevalier S., Charuel C., Pointis G. Sertoli cell junctional proteins as early targets for different classes of reproductive toxicants. Reprod. Toxicol. 2004;18:413–421. doi: 10.1016/j.reprotox.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Li M.W., Mruk D.D., Lee W.M., Cheng C.Y. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: Is this a suitable model for studying blood-testis barrier dynamics? Int. J. Biochem. Cell Biol. 2009;41:2302–2314. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thayer K.A., Doerge D.R., Hunt D., Schurman S.H., Twaddle N.C., Churchwell M.I., Garantziotis S., Kissling G.E., Easterling M.R., Bucher J.R., et al. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int. 2015;83:107–115. doi: 10.1016/j.envint.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanioka N., Naito T., Narimatsu S. Human UDP-glucuronosyltransferase isoforms involved in bisphenol A glucuronidation. Chemosphere. 2008;74:33–36. doi: 10.1016/j.chemosphere.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 30.Castellini C., Totaro M., Parisi A., D’Andrea S., Lucente L., Cordeschi G., Francavilla S., Francavilla F., Barbonetti A. Bisphenol A and Male Fertility: Myths and Realities. Front. Endocrinol. 2020;11:353. doi: 10.3389/fendo.2020.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hormann A.M., Vom Saal F.S., Nagel S.C., Stahlhut R.W., Moyer C.L., Ellersieck M.R., Welshons W.V., Toutain P.-L., Taylor J.A. Holding Thermal Receipt Paper and Eating Food after Using Hand Sanitizer Results in High Serum Bioactive and Urine Total Levels of Bisphenol A (BPA) PLoS ONE. 2014;9:e110509. doi: 10.1371/journal.pone.0110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao C., Kannan K. Widespread Occurrence of Bisphenol A in Paper and Paper Products: Implications for Human Exposure. Environ. Sci. Technol. 2011;45:9372–9379. doi: 10.1021/es202507f. [DOI] [PubMed] [Google Scholar]
- 33.Cariati F., D’Uonno N., Borrillo F., Iervolino S., Galdiero G., Tomaiuolo R. Bisphenol a: An emerging threat to male fertility. Reprod. Biol. Endocrinol. 2019;17:6. doi: 10.1186/s12958-018-0447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samanta L., Parida R., Dias T.R., Agarwal A. The enigmatic seminal plasma: A proteomics insight from ejaculation to fertilization. Reprod. Biol. Endocrinol. 2018;16:41. doi: 10.1186/s12958-018-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.