Abstract
Since it has been reported that extracellular vesicles (EVs) carry cargo using cell-to-cell comminication according to various in vivo situations, they are exprected to be applied as new drug-delivery systems (DDSs). In addition, non-coding RNAs, such as microRNAs (miRNAs), have attracted much attention as potential biomarkers in the encapsulated extracellular-vesicle (EV) form. EVs are bilayer-based lipids with heterogeneous populations of varying sizes and compositions. The EV-mediated transport of contents, which includes proteins, lipids, and nucleic acids, has attracted attention as a DDS through intracellular communication. Many reports have been made on the development of methods for introducing molecules into EVs and efficient methods for introducing them into target vesicles. In this review, we outline the possible molecular mechanisms by which miRNAs in exosomes participate in the post-transcriptional regulation of signaling pathways via cell–cell communication as novel DDSs, especially small EVs.
Keywords: adeno-associated virus, extracellular vesicles, intracellular communication, membrane vesicle production, non-coding RNAs
1. Introduction
Recently, because the safety and efficacy of the drug and its proper transmission to the target organ are important factors, the development of various drug-delivery systems (DDSs) has been energetically promoted (Table 1). Expections are especially growing for new DDSs using extracellular vesicles (EVs) via cell-to-cell communications. In addition, emerging evidence from the modulatory role of non-coding-RNA (ncRNA) fetuses has attracted attention to their potential as diagnostic biomarkers for liquid biopsies and therapeutic applications [1,2,3]. Based on their length, ncRNAs can be subdivided into two main categories: short ncRNAs (<200 nucleotides) and long ncRNAs (>200 nucleotides) [4,5,6]. Within short ncRNAs, circulating microRNAs, which are single-stranded RNAs that are approximately 22 nucleotides long, can be used as potential biomarkers for diagnosis, prognosis, and therapeutics in various diseases, but are also delivered for intracellular communications through EVs-encapsulated forms [7,8].
Table 1.
Drug-delivery-system strategies.
| Pharmaceutical Technology | Advantage |
|---|---|
| Liposome | Improved transduction and safety |
| Microcapsule | Extension in dosing interval |
| PEG modification * | Extension in dosing interval |
| Transdermal administration | Improved convenience and safety |
| Sublingual administration | Immediate effect improvement |
| Antibody drug conjugate | Improved transduction and safety |
* PEG: polyethylene glycol.
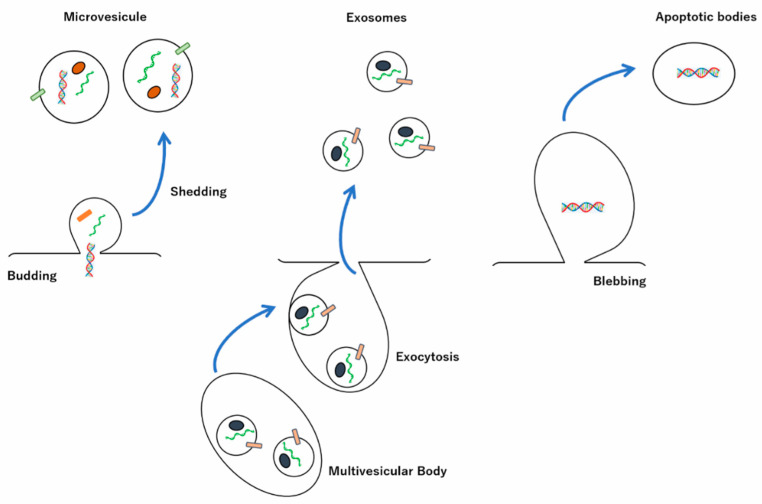
EVs are a heterogeneous group of membrane-enclosed structures of varying sizes and different origins that are released from almost every cell type [9,10]. EVs, classified by size, are categorized as such: exosomes (30–150 nm diameter, 100,000× g sedimentation speed), which are released from the fusion of multivesicular bodies (MVBs) with the plasma membrane; large EVs, such as microvesicles, also called outer membrane vesicles, ectosomes, or shedding vesicles (100 to 1000 nm diameter, 10,000× g sedimentation speed), which are derived from the outward budding of the cell membrane when the curtain is torn off; apoptotic bodies, which fall off during the process of cell apoptosis (50 to 5000 nm diameter, 2000× g sedimentation speed) [11,12,13,14,15,16,17]. EVs are also categorized based on their content, biogenesis, functions, sedimentation speed during stepwise centrifugation of the sample, as well as by their secretory origins, such as the plasma membrane and endosomes (Figure 1) [18,19,20].
Figure 1.
Secretions of extracellular vesicles (EVs). EVs are divided into three main populations according to their diameters: exosomes at 30 to 150 nm, microvesicles (MVs) at 100 to 1000 nm, and apoptotic bodies at 50 to 5000 nm. The exosome biogenesis involves the formation of multivesicular bodies and transfer to the plasma membrane, where the exosomal contents, proteins, and miRNAs are released via their fusion. The formation of both MVs and apoptotic bodies is accompanied by budding and blebbing of the cell membrane to pinch off new vesicles, respectively.
Furthermore, it has recently become clear that there are subtypes with different contents, even specific EV subtypes [21,22]. However, it remains unclear how the heterogeneity of EVs affects their functions. Additionally, because the markers that clearly distinguish exosomes and microvesicles are not specified, they have recently been fractionated into large, intermediate-sized, and small EVs according to the sedimentation rate [23,24,25]. In fact, recently, a novel type of small nonmembranous nanoparticle (<35 nm), called an exomere, was identified [23,24,25]. Small EVs exhibit a cup-shaped morphology, whereas the exomeres show a dot-shaped morphology, ranging in size from 39 to 71 nm, which were found to be enriched with Argonaute proteins (Ago1, Ago2, and Ago3), esterified cholesterol (4:1 esterified/unesterified), and more small RNAs compared with the small EVs, but an approximately four-fold reduction in total lipid content relative to small EVs was also found [23,24,25]. Furthermore, a new population of nanoparticles, referred to as supermeres (supernatant of exomeres), which are morphologically and structurally distinct from exomeres and exhibit smaller heights and diameters than exomeres, have recently been reported [26,27]. The supermere, which is highly enriched with glycolytic enzymes, transforming growth factor beta-induced (TGFBI), miR-1246, cellular–mesenchymal–epithelial transition factor (MET), and Ago2, showed different compositions of proteins and ribonucleic acid (RNA) compared to EVs and exosomes. Surprisingly, the majority of extracellular RNA is associated with supermeres rather than with small EVs and exomeres. Additionally, the supermere exhibits markedly greater uptake in vivo than small EVs and exomeres. Furthermore, supermeres and exomeres display significantly slower cellular uptake than small EVs.
First, the Greek word “exosomes” comes from “Exo”(“outside”) and “soma”(“body”) [28]. Recently, while investigating the maturation of sheep reticulocytes into erythrocytes by horizontally transferring proteins, lipids and nucleic acids, exosomes, which were assumed to be cellular debris or garbage released from reticulocytes and considered as signs of cell death, were observed to influence crucial regulatory roles as intracellular communicators as well as in accelerating interorgan crosstalk in various pathophysiological processes, including cancer, immune responses, inflammation, infection, neurodegenerative diseases, metabolic diseases, and cardiovascular diseases, in both parent and recipient cells [29,30,31,32,33]. These EV-encapsulated contents could be potential biomarkers and therapeutic targets as alternatives to liposome-mediated drug-delivery systems for diverse diseases.
2. Biogenesis and Release of Exosomes
2.1. Endosomal Sorting Complexes Required for Transport (ESCRT)-Dependent and -Independent Pathways
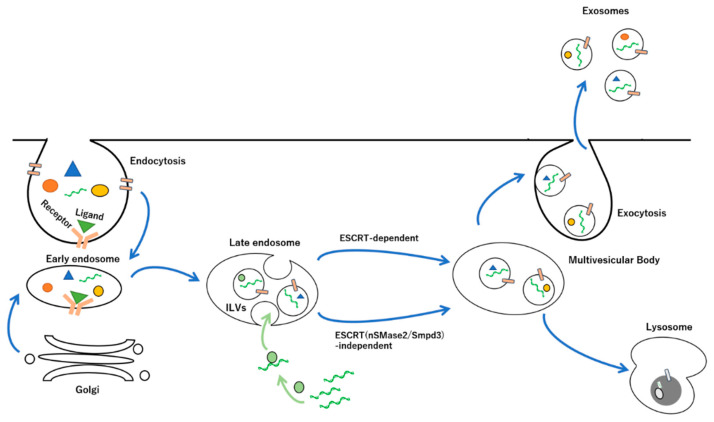
Biogenesis of exosomes begins with the inward budding of the plasma membrane by endocytosis of the plasma membrane to form multivesicular endosomes (MVEs), which mature into MVBs that incorporate different cytosolic components such as proteins, nucleic acids, and lipids by the intraluminal vesicles (ILVs) through the limiting membrane of late endosomes, where the invagination process of the ILVs determines the specific compositions of proteins and lipids on the vesicle membrane, leading to distinctive molecular profiles [34,35,36]. Furthermore, ESCRT-dependent and ESCRT-independent (neutral sphingomyelinase 2/Sphingomyelin phosphodiesterase 3, nSMase2/Smpd3) pathways have been identified as the pathway for protein sorting into exosomes and exosome biogenesis via hydrolysis of sphingolipids to ceramides on the cell membrane [37,38]. The ESCRT family can regulate cargo loading into exosomes through post-translational modifications of protein ubiquitination [39]. It has been reported that suppression of the ESCRT-dependent pathway inhibits the release of small-exosome-encapsulated ADAM metallopeptidase domain 10 (Adam10) and MET Proto-Oncogene, Receptor Tyrosine Kinase (Met), while secretion is not affected by nSMase2/Smpd3 [40]. This suggests that different small exosomes with different compositions of protein cargos may be produced by the ESCRT complex and the nSMase2/Smpd3 protein owing to the specificity and affinity of these membrane proteins to specific membrane domains. In addition, the ESCRT-dependent pathway works in concert with heparan sulfate proteoglycan, syndecan, and its adapter protein, syntenin-1, and is responsible for the formation of syntenin-1-containing exosomes [41,42,43,44,45]. Syntenin directly interacts with ALG-2-interacting protein X (ALIX), an auxiliary component of the ESCRT machinery [41,45,46]. The syntenin–ALIX complex links syndecans and syndecan cargo to the ESCRT budding machinery at the MVBs. Interestingly, this pathway is activated by heparanase, which degrades heparan sulfate, indicating that glycan modifications modulate exosome biogenesis [44]. Some tetraspanins in the biogenesis of exosomes determine the abundance of ESCRT components and associated proteins, such as tumor susceptibility gene 101 (TGS101) and ALIX on the exosome membrane [47]. The MVEs fuse with lysosomes to degrade their cargo, including their ILVs, by hydrolases [48,49,50]. However, after the fusion of late endosomes and MVBs with cell membranes, their ILVs are released into the extracellular space as exosomes, which are detected in different biological fluids, such as cerebrospinal fluid, saliva, blood, breast milk, amniotic fluid, seminal fluid, and urea (Figure 2) [51,52,53,54,55,56,57].
Figure 2.
Mechanisms of exosome biogenesis and secretion. Upon the binding of ligand with exosome, the endocytosis process involves inward budding of the cell membrane, which occurs at lipid rafts containing common membrane proteins, such as tetraspanins (e.g., CD9, CD81, CD63, etc.), MHC class I and class II, and adhesion molecules (e.g., integrins, cadherins, etc.). The internalized cargoes, including proteins, nucleic acids, and lipids are sorted into early endosomes, which mature into late endosomes. The intraluminal vesicles (ILVs) of the late endosomes are formed through budding from the perimeter membrane into the endosome lumen following the encompassing the bioactive molecules by ESCRT-dependent or -independent pathways. Multivesicular bodies (MVBs) containing some ILVs are selectively led into two different pathways, i.e., lysosomal degradation or secretion of exosomes toward the extracellular space through the fusion of lysosomes, or the plasma membrane in the case of exocytosis, which are regulated by the Rab GTPase family. miRNAs transcribed from nuclei are incorporated into the ILVs via interactions with RNA-binding proteins.
2.2. Proteins, Peptides, Lipid, and Nucleic Acids in Exosomes
In addition, the composition of proteins, peptides, and nucleic acids in exosomes is independent of donor cell type [58]. Lipid compositions in exosomes, which are commonly enriched with cholesterol and sphingomyelin, and minimal amounts of lecithin and phosphatidylethanolamine, primarily depend on exosome-producing cells [58,59,60,61,62]. Furthermore, it has been revealed that lean mice become insulin resistant due to the administration of exosomes isolated from the feces of obese mice that were fed a high-fat diet (HFD) and of type II diabetic patients [63,64,65]. An HFD alters the lipid composition of exosomes [63]. In addition, phosphatidylethanolamine is abundant in the exosomes of lean animals [63]. In contrast, phosphatidylcholine (PC) is abundant in the exosomes of obese animals [63]. Mechanistically, it was suggested that exosomes in the intestine of obese animals are taken up by macrophages and hepatocytes, leading to the inhibition of the insulin-signaling pathway. Exosome-derived PCs bind to and activate aromatic-hydrocarbon receptors (AhRs) and suppress the expression of genes essential for the activation of insulin-signaling pathways, such as insulin receptor substrate 2 (IRS-2) and its downstream genes, phosphatidylinositol-3 kinase (PI3K) and Protein Kinase B/thymoma viral proto-oncogene 1 (PKB/AKT) [63]. These reports reveal that exosomes produced by an HFD may contribute to the development of insulin resistance.
Transmission electron microscopy (TEM) with negative staining and cryo-TEM indicated that exosomes have cup-shaped and perfectly spherical structures, respectively [66]. The selective enrichment of miRNAs, which is abundant among small RNA families that are encapsulated in exosomes, depends on their size, origin, and transcription by RNA polymerase III [67,68,69,70]. miRNAs are transcribed by RNA polymerase (Pol) II into primary miRNAs, which Drosha/DGCR8 processes into precursor miRNAs [71,72,73,74]. The pre-miRNA is transported from the nucleus into the cytoplasm by exportin 5 and then processed by Dicer/TRBP into an miRNA duplex [75,76,77]. After the formation of a duplex complex with the RNA-induced silencing complex (RISC), mature microRNAs are packed into exosomes in different manners: I) the miRISC-related pathway, II) miRNA motif-sumoylated heterogeneous nuclear ribonucleoproteins (hnRNPs)- and the synaptotagmin-binding cytoplasmic-RNA-interacting-protein (SYNCRIP)-dependent pathway, which recognizes the short sequence motifs GGAG and GCUG in the 3′-miRNA sequence, and III) the ceramide-dependent pathway [78,79,80,81,82,83,84]. In addition, RNA-binding protein (heterogeneous nuclear ribonucleoprotein A2B1: hnRNPA2B1) and Y-box protein are involved in the sorting of miRNAs in the EVs [85,86].
3. EV-Mediated DDS
3.1. microRNAs in EV-Mediated DDS
The expansion of drug-discovery modalities has been remarkable in recent years. In particular, nucleic-acid drugs can directly act on gene regulation [87,88,89]. Compared with conventional small-molecule drugs, biopharmacology makes it easier to design drugs that specifically act on various molecules. For example, short double-stranded RNA (siRNAs) can degrade RNA with complementary sequences [90,91]. Furthermore, the design of a double-stranded RNA with a base sequence corresponding to the target gene makes it possible to specifically suppress the expression in the target gene [92]. Since there is no difference in the basic physiochemical properties of different siRNAs in terms of biopharmacology, it is unlikely that some problems related to physicochemical properties, such as solubility, stability, and pharmacokinetics—including absorption and metabolism—caused by differences in physical properties between compounds will occur. Therefore, they have attracted attention as a next-generation modality that sets them apart from other pharmaceutical products. However, nucleic-acid drugs have problems such as low intracellular-delivery efficiency due to low cell-membrane permeability, their large molecular weight, and elimination by the cellular immune system [93,94,95,96,97]. To solve these problems, the development of a DDS using the chemical modification of nucleic acids and liquid nanoparticles is underway. [98,99] In addition, liposome-based drugs have been approved, and research and development of novel DDS strategies for nucleic-acid drugs is accelerating [100,101,102]. However, because liposomes are non-natural substances in the human body, problems such as immunogenicity and biotoxicity remain. On the other hand, EVs are natural substances with low immunogenicity and biotoxicity [103,104,105,106,107,108,109,110,111,112,113,114,115,116]. Cancer-cell-derived EVs include cancer antigens according to individual cancer types, and the anti-tumor immune response is induced by the uptake of dendritic cells, which are the main antigen-presenting cells [117,118,119,120]. In particular, they have the potential to be used as a new immunotherapy for cancers for which cancer antigens have not yet been identified. However, even if cancer-cell-derived EVs are used alone, activation of DCs, which are essential for inducing an anti-tumor immune response, cannot be expected.
3.2. Modification of EVs as DDS Methods
When trying to develop DDSs with EVs, which are natural drug carriers, there are two methods: loading the drug directly into the recovered EVs [121,122,123], and loading drugs into EVs by modifying the function of secretory cells [124,125,126]. In particular, the method of imparting functions to EVs that are extracted by manipulating the cells described above is significantly different from the DDS technology that is applied to conventional artificial nanoparticle preparations. It was also revealed that CpG DNA-modified EVs showed a strong anti-tumor immune response by reacting with biotin derivatives of CpG DNA, which is an immunostimulatory nucleic acid, with the EVs modified by the fusion of protein with streptavidin showing binding to biotin and lactadherin, which is an EV-migrating protein [117,127].
In addition, the secretion of EVs is not limited to mammals, and has already been found in plants and microorganisms [128,129,130]. It is thought that there is a mechanism by which molecules with immunomodulatory ability are secreted from microorganisms that have beneficial effects to the outside of the cell and delivered to the target cell of the living body [131]. When various EVs derived from bifizus, butyric-acid, and lactic-acid bacteria were added to two types of immune cells, mouse macrophage-like-cell line RAW264.7 and dendritic-cell line DC2.4, the production of inflammatory cytokines, tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) was increased [132,133,134,135]. This report confirmed that EVs derived from probiotics have immunostimulatory abilities.
3.3. Recovery of EVs as DDSs
In order to use EVs as DDSs, recovery-system operations are required because EVs are contained in the supernatant of the culture medium in cultured cells and serum in various concentrations and forms [136,137]. Although several EV-recovery methods have been reported, they have both advantages and disadvantages in terms of yield and use [20,138]. (1) The ultracentrifugation method removes impurities from cell-culture supernatants and blood samples by stepwise centrifugation [139,140]. Generally, debris such as cells are removed by centrifuging the culture supernatant and serum at 10,000× g for 30 min. They are then centrifuged at 100,000× g for 70 min to precipitate EVs and proteins. PBS is then added to the pellet, which is washed and centrifuged at 100,000× g for 70 min to precipitate the EVs. The ultracentrifugation method is easy to operate and suitable for large-scale recovery, but it is inferior in terms of the recovery rate. (2) The polyethylene-glycol (PEG) precipitation method uses PEG as a precipitating agent [141,142]. The PEG reagent with a molecular weight of 6000 or 8000 is mixed with a biological sample to a final concentration of 10% (w/v), allowed to stand at 4 °C, and then centrifuged at 1500 to 3000× g for 300 to 60 min. The resulting precipitate contains the EVs. The advantages of this method are that it does not require an ultracentrifuge or expensive and special reagents, and its operation is as simple as the ultracentrifugation method. However, the particle-size distribution of the obtained EVs is wide, and it cannot be ruled out that particles larger than EVs may be included. (3) The immunoprecipitation method is a recovery method that uses an antibody that recognizes EV membrane proteins [141,143]. Magnetic beads, to which an antibody is bound, can be added to a sample, and exosomes can be easily separated and recovered using a magnet. Because the IP method is based on the antigen–antibody reaction, it is possible to recover high-purity and specific EVs. This method can be used for unknown samples; however, it is difficult to recover EVs for which the target antigens have not been found. (4) Density-gradient centrifugation is a method of separating relatively low-density EVs from other vesicles and particles by combining a sucrose density gradient and a sucrose cushion [144]. Although this separation method can isolate high-purity EVs, it is time-consuming and requires sophisticated techniques.
3.4. Preparation of EVs as DDSs
Furthermore, drug delivery using the substance-transport capacity of EVs can be broadly divided into two types. One is the pre-loading method, which modifies EV-producing cells, and the other is the post-loading method, in which the recovered EVs are chemically or physically loaded with a drug [145,146,147,148,149]. In the post-loading method, at first, the electroporation method is used to introduce a substance using an electric current [126]. The electric field causes partial membrane disruption and improves membrane permeability, allowing substances to enter the EVs. While this method can be applied to any EV, there is a concern that RNA and EVs will aggregate due to electric-field treatment. Second, sonication is a method of introducing a drug into the membrane by temporarily impairing its integrity by ultrasonic treatment [126]. However, this method also detects non-spherical EVs owing to changes in the membrane structure. Third, saponin, a surfactant molecule, can form a complex with cholesterol in the lipid membrane to form pores and improve the permeability of hydrophilic molecules [150]. However, saponins have been reported to have hemolytic activity in vivo and should be carefully considered before use.
In the above-mentioned method of loading a drug into EVs, due to the necessity of isolating and purifying the EVs using both methods, it is difficult to avoid a significant decrease in the recovery rate and degeneration of the EV membrane during the process [151,152]. Yamayoshi et al. reported a new DDS strategy for intracellularly delivering drugs using EVs without isolation and purification [153]. This new method indicates that a complex of an antibody-bound nucleic acid consisting of a monoclonal antibody targeting CD63, which is an EV membrane surface protein, and anti-miR is taken up into cells by being associated with EVs and inhibits the function of miRNA in the cytoplasm. Furthermore, in cancer-bearing mice that were subcutaneously transplanted with cells treated with this antibody–anti-miR complex, the tumor volume was significantly smaller than that in the non-treated group, suggesting that a tumor-forming-inhibition ability was observed. In addition, it has been observed that tumor volume is reduced when this complex is administered to cancer-bearing mice via the tail vein. This report demonstrated the effectiveness of functional molecules targeting EV–miRNAs, which exert advanced functions in trace amounts and play a major role in bioregulatory systems. Moreover, this method can introduce not only anti-miRs but also antisense nucleic acids, siRNAs, aptamer-targeting mRNA, small molecule compounds, and nucleic acids. Therefore, the development of new molecular-targeted drugs targeting not only EV–miRNA but also various molecules contained in EVs is expected.
Furthermore, proteinase K was observed to reduce uptake efficiency by removing proteins from the surface of exosomes or uptake cells [154,155]. The recognition of molecules on the surface of exosomes by uptake cells is important for the mechanism, in which the surface molecule of an exosome depends on the type of producing cell, and the type of molecule on the uptake-cell side also depends on the type of uptake cell. It has been shown that the combination of producing cells and uptake cells is an important factor that determines the cellular-uptake characteristics of exosomes [156,157]. Further, it has been shown that uptake occurs by fusion of the lipid membrane between the membrane of exosomes and the cell membrane of uptake cells [156]. Moreover, the lipid composition is important because the addition of filipin reduces the efficiency of membrane fusion by changing the lipid composition of the uptake-cell membrane [156]. In addition, it has also been reported that uptake changes depending on the state of exosome-producing or uptake cells and the surrounding environment, in addition to the types of exosome-producing cells and uptake cells [34]. Exosomes recovered under acidic conditions change their lipid composition and are more easily taken up by cells [158]. It has been reported that exosome uptake efficiency increases under acidic conditions. Moreover, since treatment with TNF-α increases the expression level of the integrin-recognition molecule ICAM on the fibroblast surface of uptake cells, the uptake of B-cell-derived exosomes by fibroblasts is increased due to the expression of integrin β1 and β2 on the surface of the exosome [159]. Regarding the uptake mechanism of exosomes by human plasmacytoid dendritic cells (pDC) and conventional dendritic cells (cDC), it became clear that the uptake rate in cDCs was faster than that in pDC. The uptake by cDC has been shown to be due to endocytosis, whereas the small contribution of endocytosis to the uptake of exosomes by pDC indicates that this uptake may be slow [160]. It has also been shown that phagocytic cells such as mouse macrophage-like-cell line RAW264.7 cells and human-leukemia-T-cell line Jurkat cells have high exosome uptake efficiency, but non-phagocytic cells such as mouse fibroblast-like-cell line NIH3T3 cells and fibroblast-like-cell lines derived from monkey kidney-tissue COS-7 cells take up few exosomes, and this uptake is reduced by inhibitors of actin polymerization and PI3K [161]. Endocytosis is the main mechanism of exosome uptake, and inhibitors of clathrin-dependent endocytosis and macropinocytosis reduce uptake [162,163].
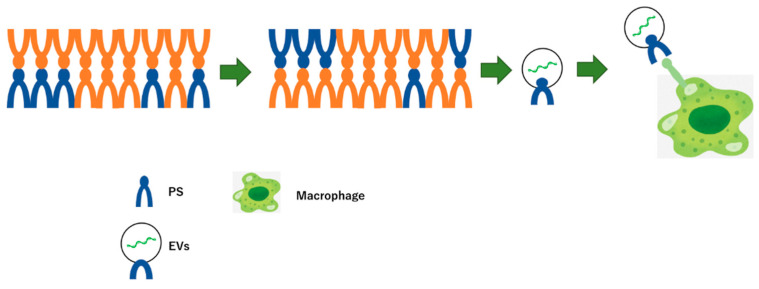
Additionally, macrophages phagocytose apoptotic cells through recognition of the externalization of the phosphatidylserine (PS)-induced cleavage of intracellular proteins by activated caspase as “eat me signals”. The PS on the surface of the exosome may modulate the “eat me signal” in the apoptotic pathway through molecular regulation via the cargo (Figure 3) [164,165].
Figure 3.
Macrophage phagocytoses apoptotic cells via externalization of phosphatidylserine (PS) on EVs induced apoptosis as “eat me signal”. In apoptotic conditions, PS in the plasma membranes of cells externalizes, which is recognize by macrophages. Further, like the plasma membrane, the surface of the EVs has the PS, leading to uptake into macrophages.
4. EV-Encapsulated Adeno-Associated Virus
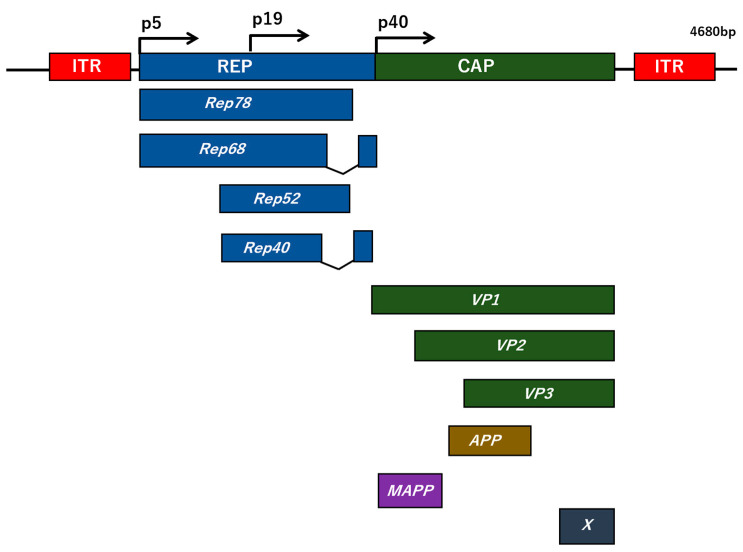
Adeno-associated virus (AAV) is a virus with a diameter of approximately 20–26 nm, which is extremely physicochemically stable with no envelope and is classified in the genus Dependoparvovirus of the family Parvoviridae [166,167]. More than 100 types of AAV are known, but the most advanced AAV is the type 2 AAV (AAV2) [168]. The AAV2 capsid structure is composed of three types of proteins, VP1 (82 kDa), VP2 (65 kDa), and VP3 (60 kDa), assembled at a ratio of 1:1:10 (Figure 4) [169]. The genome is a single-stranded DNA consisting of approximately 4.7 nucleotides, with plus and minus strands mixed in approximately the same population, where the 145 nucleotides at both ends of the genome form a T-shaped hairpin structure referred to as inverted terminal repeats (ITRs), which becomes the starting point of duplication [170]. The AAV genome contains two genes, rep and cap, which encode nonstructural proteins and capsid proteins, respectively. AAV can only grow in the presence of helper viruses such as adenovirus and herpesvirus. When a cell is infected with AAV alone, its genome is specifically integrated into the adeno-associated-virus-integration-site-1 (AAVS1) region of chromosome 19, 19q13.42, resulting in latent infection [171]. However, it is extremely unlikely that the transgene loaded on the viral genome of the recombinant AAV, referred to as the AAV vector, will be integrated into the chromosome by the AAV vector, and it is believed that the transgene is basically extrachromosomal. The transgene is a single-stranded DNA in the AAV vector, but it is converted to double-stranded DNA in the cell, where the transgene is expressed. The expression of genes introduced into neurons by the AAV vector has been shown to persist for long periods of time [172]. Furthermore, no specific disease associated with AAV2 infection has been reported, and its non-pathogenicity has attracted attention as a useful and safe gene-therapy vector for various diseases. Moreover, AAV has a wide host range within humans and primates, and infects various proliferating and non-proliferating cells [171]. It has been reported that heparan sulfate, fibroblast-growth-factor (FGF) receptor, and αVβ5 integrin are known as receptors or co-receptors for AAV2 [173,174,175]. In addition, AAV4 and AAV5 have been shown to bind to sialic acid, and the platelet-derived-growth-factor (PDGF) receptor acts as a receptor for AAV5 [176].
Figure 4.
The AAV2 genome structure. The genome of AVV consists of a linear single-stranded DNA spanning approximately 4.7 kilobases (kb), flanking with two 145 nucleotides of long inverted terminal repeats (ITRs) at their terminals. Within the genome, the two viral genes rep and cap code for non-structural and structural proteins, respectively. The non-structural (rep) coding region is regulated by two promoters, p5 and p19, resulting in the generation of a set of four overlapping proteins, Rep78, Rep68, and Rep52, Rep40, respectively. The cap gene encodes three structural capsids (VP1 to VP3) by promoter p40 and the assembly-activating protein (AAP), which promotes virion assembly and the membrane-associated accessory protein (MAAP) from an alternative open reading frame (ORF). X ORF at the 3′ end of the cap gene has a specific role in AAV DNA replication.
On the other hand, the safety and efficacy of AAV vectors need to be improved due to the occurrence of deaths in clinical trials caused by single high-dose administration, imperfect organ orientation, or the presence of neutralizing antibodies of AAV vectors that bind to the AAV capsid and inhibit the interaction with target cells, resulting in a reduction of AAV-transduction efficacy, which is a major clinical barrier. Previously, it was reported that AAV is naturally secreted via the interior or surface of EVs into the extracellular space in two types of forms, EV-encapsulated or EV-associated AAVs (EV–AAVs), which can be taken up with high transduction efficacy by target cells [177,178,179,180,181,182,183,184]. Furthermore, the EV–AAV vector can be protected from neutralizing antibodies in vivo and in vitro compared to non-encapsulated or associated AAVs [185,186]. Taken together, the EV–AAV will be a possible new therapeutic strategy in various diseases.
5. Conclusions
EVs are membrane-enclosed structures with heterogeneous sizes and origins. EVs have attracted much attention as novel potent DDSs owing to the incorporation of molecular information such as proteins, lipids, and nucleic acids via intracellular communications. The diversity of EV functions is dependent on their composition, such as the type and amount of proteins and lipids on the membrane or nucleic acids, including miRNAs, within the EVs. Therefore, the isolation and purification standards of EVs are important for the establishment of therapeutic methods using the EV-mediated modification of target genes. Furthermore, a virus-vector-encapsulated EV showed very high transduction efficacy and protection from neutralizing antibodies compared to free virus vectors. Thus, EV-mediated transduction of the contents, including virus vectors or miRNAs, will be a powerful tool for various disease treatments as a novel DDS.
Author Contributions
Writing—review and editing, Y.M.; supervision, R.Y.; funding acquisition, Y.M. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This review was funded by Fukuda Foundation for Medical Technology 2021, and “The APC was funded by Fukuda Foundation for Medical Technology 2021.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Panvongsa W., Pegtel D.M., Voortman J. More than a Bubble: Extracellular Vesicle microRNAs in Head and Neck Squamous Cell Carcinoma. Cancers. 2022;14:1160. doi: 10.3390/cancers14051160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toden S., Goel A. Non-coding RNAs as liquid biopsy biomarkers in cancer. Br. J. Cancer. 2022;126:351–360. doi: 10.1038/s41416-021-01672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosseinalizadeh H., Mahmoodpour M., Ebrahimi A. Circulating non-coding RNAs as a diagnostic and management biomarker for breast cancer: Current insights. Mol. Biol. Rep. 2022;49:705–715. doi: 10.1007/s11033-021-06847-3. [DOI] [PubMed] [Google Scholar]
- 4.Dolati S., Shakouri S.K., Dolatkhah N., Yousefi M., Jadidi-Niaragh F., Sanaie S. The role of exosomal non-coding RNAs in aging-related diseases. Biofactors. 2021;47:292–310. doi: 10.1002/biof.1715. [DOI] [PubMed] [Google Scholar]
- 5.Cannavicci A., Zhang Q., Kutryk M.J.B. Non-Coding RNAs and Hereditary Hemorrhagic Telangiectasia. J. Clin. Med. 2020;9:3333. doi: 10.3390/jcm9103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao J., Zhang M., Zhang L., Lou J., Zhou F., Fang M. Non-coding RNA in thyroid cancer-Functions and mechanisms. Cancer Lett. 2021;496:117–126. doi: 10.1016/j.canlet.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Hwang S., Yang Y.M. Exosomal microRNAs as diagnostic and therapeutic biomarkers in non-malignant liver diseases. Arch. Pharm. Res. 2021;44:574–587. doi: 10.1007/s12272-021-01338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou R., Wang L., Zhao G., Chen D., Song X., Momtazi-Borojeni A.A., Yuan H. Circulating exosomal microRNAs as emerging non-invasive clinical biomarkers in heart failure: Mega bio-roles of a nano bio-particle. IUBMB Life. 2020;72:2546–2562. doi: 10.1002/iub.2396. [DOI] [PubMed] [Google Scholar]
- 9.Cavallaro S., Pevere F., Stridfeldt F., Görgens A., Paba C., Sahu S.S., Mamand D.R., Gupta D., El Andaloussi S., Linnros J., et al. Multiparametric Profiling of Single Nanoscale Extracellular Vesicles by Combined Atomic Force and Fluorescence Microscopy: Correlation and Heterogeneity in Their Molecular and Biophysical Features. Small. 2021;17:e2008155. doi: 10.1002/smll.202008155. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J., Li H., Fan B., Xu W., Zhang X. Extracellular vesicles in normal pregnancy and pregnancy-related diseases. J. Cell Mol. Med. 2020;24:4377–4388. doi: 10.1111/jcmm.15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y., Hu Y., Xu S., Liu F., Gao Y. Exosomal microRNAs as Potential Biomarkers and Therapeutic Agents for Acute Ischemic Stroke: New Expectations. Front. Neurol. 2022;12:747380. doi: 10.3389/fneur.2021.747380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y., Zhao Y., Yin Y., Jia X., Mao L. Mechanism of cargo sorting into small extracellular vesicles. Bioengineered. 2021;12:8186–8201. doi: 10.1080/21655979.2021.1977767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiteside T.L., Diergaarde B., Hong C.S. Tumor-Derived Exosomes (TEX) and Their Role in Immuno-Oncology. Int. J. Mol. Sci. 2021;22:6234. doi: 10.3390/ijms22126234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stentz R., Carvalho A.L., Jones E.J., Carding S.R. Fantastic voyage: The journey of intestinal microbiota-derived microvesicles through the body. Biochem. Soc. Trans. 2018;46:1021–1027. doi: 10.1042/BST20180114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez N., James V., Onion D., Fairclough L.C. Extracellular vesicles and chronic obstructive pulmonary disease (COPD): A systematic review. Respir. Res. 2022;23:82. doi: 10.1186/s12931-022-01984-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clancy J.W., Schmidtmann M., D’Souza-Schorey C. The ins and outs of microvesicles. FASEB Bioadv. 2021;3:399–406. doi: 10.1096/fba.2020-00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W., Huang P., Lin J., Zeng H. The Role of Extracellular Vesicles in Osteoporosis: A Scoping Review. Membranes. 2022;12:324. doi: 10.3390/membranes12030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lima T.S., Souza W., Geaquinto L.R., Sanches P.L., Stepień E.L., Meneses J., Fernández-de Gortari E., Meisner-Kober N., Himly M., Granjeiro J.M., et al. Nanomaterial Exposure, Extracellular Vesicle Biogenesis and Adverse Cellular Outcomes: A Scoping Review. Nanomaterials. 2022;12:1231. doi: 10.3390/nano12071231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang X., You L., Zhang Z., Cui X., Zhong H., Sun X., Ji C., Chi X. Biological Properties of Milk-Derived Extracellular Vesicles and Their Physiological Functions in Infant. Front. Cell Dev. Biol. 2021;9:693534. doi: 10.3389/fcell.2021.693534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stam J., Bartel S., Bischoff R., Wolters J.C. Isolation of extracellular vesicles with combined enrichment methods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021;1169:122604. doi: 10.1016/j.jchromb.2021.122604. [DOI] [PubMed] [Google Scholar]
- 21.Nehrbas J., Butler J.T., Chen D.W., Kurre P. Extracellular Vesicles and Chemotherapy Resistance in the AML Microenvironment. Front. Oncol. 2020;10:90. doi: 10.3389/fonc.2020.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokoi A., Ochiya T. Exosomes and extracellular vesicles: Rethinking the essential values in cancer biology. Semin. Cancer Biol. 2021;74:79–91. doi: 10.1016/j.semcancer.2021.03.032. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H., Freitas D., Kim H.S., Fabijanic K., Li Z., Chen H., Mark M.T., Molina H., Martin A.B., Bojmar L., et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018;20:332–343. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H., Lyden D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019;14:1027–1053. doi: 10.1038/s41596-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Q., Higginbotham J.N., Jeppesen D.K., Yang Y.P., Li W., McKinley E.T., Graves-Deal R., Ping J., Britain C.M., Dorsett K.A., et al. Transfer of Functional Cargo in Exomeres. Cell Rep. 2019;27:940–954.e6. doi: 10.1016/j.celrep.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clancy J.W., Boomgarden A.C., D’Souza-Schorey C. Profiling and promise of supermeres. Nat. Cell Biol. 2021;23:1217–1219. doi: 10.1038/s41556-021-00808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q., Jeppesen D.K., Higginbotham J.N., Graves-Deal R., Trinh V.Q., Ramirez M.A., Sohn Y., Neininger A.C., Taneja N., McKinley E.T., et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat. Cell Biol. 2021;23:1240–1254. doi: 10.1038/s41556-021-00805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witwer K.W., Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles. 2019;8:1648167. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thangaraju K., Neerukonda S.N., Katneni U., Buehler P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2020;22:153. doi: 10.3390/ijms22010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hade M.D., Suire C.N., Suo Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells. 2021;10:1959. doi: 10.3390/cells10081959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi D., Lee T.H., Spinelli C., Chennakrishnaiah S., D’Asti E., Rak J. Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. Semin. Cell Dev. Biol. 2017;67:11–22. doi: 10.1016/j.semcdb.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Schneider A., Simons M. Exosomes: Vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 2013;352:33–47. doi: 10.1007/s00441-012-1428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y., Hu Y.W., Zheng L., Wang Q. Characteristics and Roles of Exosomes in Cardiovascular Disease. DNA Cell Biol. 2017;36:202–211. doi: 10.1089/dna.2016.3496. [DOI] [PubMed] [Google Scholar]
- 34.Abels E.R., Breakefield X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol. Neurobiol. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han Y., Jia L., Zheng Y., Li W. Salivary Exosomes: Emerging Roles in Systemic Disease. Int. J. Biol. Sci. 2018;14:633–643. doi: 10.7150/ijbs.25018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guay C., Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes. Metab. 2017;19:137–146. doi: 10.1111/dom.13027. [DOI] [PubMed] [Google Scholar]
- 37.Li S.P., Lin Z.X., Jiang X.Y., Yu X.Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta. Pharmacol. Sin. 2018;39:542–551. doi: 10.1038/aps.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo B.B., Bellingham S.A., Hill A.F. The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J. Biol. Chem. 2015;290:3455–3467. doi: 10.1074/jbc.M114.605253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atukorala I., Mathivanan S. The Role of Post-Translational Modifications in Targeting Protein Cargo to Extracellular Vesicles. Subcell. Biochem. 2021;97:45–60. doi: 10.1007/978-3-030-67171-6_3. [DOI] [PubMed] [Google Scholar]
- 40.Harada Y., Suzuki T., Fukushige T., Kizuka Y., Yagi H., Yamamoto M., Kondo K., Inoue H., Kato K., Taniguchi N., et al. Generation of the heterogeneity of extracellular vesicles by membrane organization and sorting machineries. Biochim. Biophys. Acta Gen. Subj. 2019;1863:681–691. doi: 10.1016/j.bbagen.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Baietti M.F., Zhang Z., Mortier E., Melchior A., Degeest G., Geeraerts A., Ivarsson Y., Depoortere F., Coomans C., Vermeiren E., et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 42.Hurley J.H., Odorizzi G. Get on the exosome bus with ALIX. Nat. Cell Biol. 2012;14:654–655. doi: 10.1038/ncb2530. [DOI] [PubMed] [Google Scholar]
- 43.Stoorvogel W. Resolving sorting mechanisms into exosomes. Cell Res. 2015;25:531–532. doi: 10.1038/cr.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.David G., Zimmermann P. Heparanase Involvement in Exosome Formation. Adv. Exp. Med. Biol. 2020;1221:285–307. doi: 10.1007/978-3-030-34521-1_10. [DOI] [PubMed] [Google Scholar]
- 45.Addi C., Presle A., Frémont S., Cuvelier F., Rocancourt M., Milin F., Schmutz S., Chamot-Rooke J., Douche T., Duchateau M., et al. The Flemmingsome reveals an ESCRT-to-membrane coupling via ALIX/syntenin/syndecan-4 required for completion of cytokinesis. Nat. Commun. 2020;11:1941. doi: 10.1038/s41467-020-15205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cone A.S., Hurwitz S.N., Lee G.S., Yuan X., Zhou Y., Li Y., Meckes D.G., Jr. Alix and Syntenin-1 direct amyloid precursor protein trafficking into extracellular vesicles. BMC Mol. Cell Biol. 2020;21:58. doi: 10.1186/s12860-020-00302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colombo M., Moita C., van Niel G., Kowal J., Vigneron J., Benaroch P., Manel N., Moita L.F., Théry C., Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 48.Tanno H., Komada M. The ubiquitin code and its decoding machinery in the endocytic pathway. J. Biochem. 2013;153:497–504. doi: 10.1093/jb/mvt028. [DOI] [PubMed] [Google Scholar]
- 49.Kim M.S., Muallem S., Kim S.H., Kwon K.B., Kim M.S. Exosomal release through TRPML1-mediated lysosomal exocytosis is required for adipogenesis. Biochem. Biophys. Res. Commun. 2019;510:409–415. doi: 10.1016/j.bbrc.2019.01.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nag S., Rani S., Mahanty S., Bissig C., Arora P., Azevedo C., Saiardi A., van der Sluijs P., Delevoye C., van Niel G., et al. Rab4A organizes endosomal domains for sorting cargo to lysosome-related organelles. J. Cell Sci. 2018;131:jcs216226. doi: 10.1242/jcs.216226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rome S., Forterre A., Mizgier M.L., Bouzakri K. Skeletal Muscle-Released Extracellular Vesicles: State of the Art. Skeletal Muscle-Released Extracellular Vesicles: State of the Art. Front. Physiol. 2019;10:929. doi: 10.3389/fphys.2019.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aheget H., Tristán-Manzano M., Mazini L., Cortijo-Gutierrez M., Galindo-Moreno P., Herrera C., Martin F., Marchal J.A., Benabdellah K. Exosome: A New Player in Translational Nanomedicine. J. Clin. Med. 2020;9:2380. doi: 10.3390/jcm9082380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jan A.T., Rahman S., Khan S., Tasduq S.A., Choi I. Biology, Pathophysiological Role, and Clinical Implications of Exosomes: A Critical Appraisal. Cells. 2019;8:99. doi: 10.3390/cells8020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar D.N., Chaudhuri A., Aqil F., Dehari D., Munagala R., Singh S., Gupta R.C., Agrawal A.K. Exosomes as Emerging Drug Delivery and Diagnostic Modality for Breast Cancer: Recent Advances in Isolation and Application. Cancers. 2022;14:1435. doi: 10.3390/cancers14061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jayaseelan V.P. Emerging role of exosomes as promising diagnostic tool for cancer. Cancer Gene. Ther. 2020;27:395–398. doi: 10.1038/s41417-019-0136-4. [DOI] [PubMed] [Google Scholar]
- 56.Ghafourian M., Mahdavi R., Akbari Jonoush Z., Sadeghi M., Ghadiri N., Farzaneh M., Mousavi Salehi A. The implications of exosomes in pregnancy: Emerging as new diagnostic markers and therapeutics targets. Cell Commun. Signal. 2022;20:51. doi: 10.1186/s12964-022-00853-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aleksejeva E., Zarovni N., Dissanayake K., Godakumara K., Vigano P., Fazeli A., Jaakma Ü., Salumets A. Extracellular vesicle research in reproductive science: Paving the way for clinical achievements. Biol. Reprod. 2022;106:408–424. doi: 10.1093/biolre/ioab245. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y., Liu Y., Liu H., Tang W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang W., Zhu N., Yan T., Shi Y.N., Chen J., Zhang C.J., Xie X.J., Liao D.F., Qin L. The crosstalk: Exosomes and lipid metabolism. Cell Commun. Signal. 2020;18:119. doi: 10.1186/s12964-020-00581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Donoso-Quezada J., Ayala-Mar S., González-Valdez J. The role of lipids in exosome biology and intercellular communication: Function, analytics and applications. Traffic. 2021;22:204–220. doi: 10.1111/tra.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skotland T., Hessvik N.P., Sandvig K., Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019;60:9–18. doi: 10.1194/jlr.R084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skotland T., Sagini K., Sandvig K., Llorente A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020;159:308–321. doi: 10.1016/j.addr.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Kumar A., Sundaram K., Mu J., Dryden G.W., Sriwastva M.K., Lei C., Zhang L., Qiu X., Xu F., Yan J., et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat. Commun. 2021;12:213. doi: 10.1038/s41467-020-20500-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar A., Ren Y., Sundaram K., Mu J., Sriwastva M.K., Dryden G.W., Lei C., Zhang L., Yan J., Zhang X., et al. miR-375 prevents high-fat diet-induced insulin resistance and obesity by targeting the aryl hydrocarbon receptor and bacterial tryptophanase (tnaA) gene. Theranostics. 2021;11:4061–4077. doi: 10.7150/thno.52558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodrigues A.C., Spagnol A.R., Frias F.T., de Mendonça M., Araújo H.N., Guimarães D., Silva W.J., Bolin A.P., Murata G.M., Silveira L. Intramuscular Injection of miR-1 Reduces Insulin Resistance in Obese Mice. Front. Physiol. 2021;12:676265. doi: 10.3389/fphys.2021.676265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jung M.K., Mun J.Y. Sample Preparation and Imaging of Exosomes by Transmission Electron Microscopy. J. Vis. Exp. 2018;131:56482. doi: 10.3791/56482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corrado C., Barreca M.M., Zichittella C., Alessandro R., Conigliaro A. Molecular Mediators of RNA Loading into Extracellular Vesicles. Cells. 2021;10:3355. doi: 10.3390/cells10123355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Groot M., Lee H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells. 2020;9:1044. doi: 10.3390/cells9041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geng X., Lin X., Zhang Y., Li Q., Guo Y., Fang C., Wang H. Exosomal circular RNA sorting mechanisms and their function in promoting or inhibiting cancer. Oncol. Lett. 2020;19:3369–3380. doi: 10.3892/ol.2020.11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J., Li S., Li L., Li M., Guo C., Yao J., Mi S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee D., Shin C. Emerging roles of DROSHA beyond primary microRNA processing. RNA Biol. 2018;15:186–193. doi: 10.1080/15476286.2017.1405210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshida T., Asano Y., Ui-Tei K. Modulation of MicroRNA Processing by Dicer via Its Associated dsRNA Binding Proteins. Noncoding RNA. 2021;7:57. doi: 10.3390/ncrna7030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michlewski G., Cáceres J.F. Post-transcriptional control of miRNA biogenesis. RNA. 2019;25:1–16. doi: 10.1261/rna.068692.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davis B.N., Hata A. Regulation of MicroRNA Biogenesis: A miRiad of mechanisms. Cell Commun. Signal. 2009;7:18. doi: 10.1186/1478-811X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheng P., Fields C., Aadland K., Wei T., Kolaczkowski O., Gu T., Kolaczkowski B., Xie M. Dicer cleaves 5’-extended microRNA precursors originating from RNA polymerase II transcription start sites. Nucleic Acids Res. 2018;46:5737–5752. doi: 10.1093/nar/gky306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shapiro J.S., Langlois R.A., Pham A.M., Tenoever B.R. Evidence for a cytoplasmic microprocessor of pri-miRNAs. RNA. 2012;18:1338–1346. doi: 10.1261/rna.032268.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fabbiano F., Corsi J., Gurrieri E., Trevisan C., Notarangelo M., D’Agostino V.G. RNA packaging into extracellular vesicles: An orchestra of RNA-binding proteins? J. Extracell. Vesicles. 2020;10:e12043. doi: 10.1002/jev2.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tan P.P.S., Hall D., Chilian W.M., Chia Y.C., Mohd Zain S., Lim H.M., Kumar D.N., Ching S.M., Low T.Y., Md Noh M.F., et al. Exosomal microRNAs in the development of essential hypertension and its potential as biomarkers. Am. J. Physiol. Heart Circ. Physiol. 2021;320:H1486–H1497. doi: 10.1152/ajpheart.00888.2020. [DOI] [PubMed] [Google Scholar]
- 80.Wei H., Chen Q., Lin L., Sha C., Li T., Liu Y., Yin X., Xu Y., Chen L., Gao W., et al. Regulation of exosome production and cargo sorting. Int. J. Biol. Sci. 2021;17:163–177. doi: 10.7150/ijbs.53671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akgül B., Erdoğan İ. Intracytoplasmic Re-localization of miRISC Complexes. Front. Genet. 2018;9:403. doi: 10.3389/fgene.2018.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mizutani A., Fukuda M., Ibata K., Shiraishi Y., Mikoshiba K. SYNCRIP, a cytoplasmic counterpart of heterogeneous nuclear ribonucleoprotein R, interacts with ubiquitous synaptotagmin isoforms. J. Biol. Chem. 2000;275:9823–9831. doi: 10.1074/jbc.275.13.9823. [DOI] [PubMed] [Google Scholar]
- 83.Shurtleff M.J., Yao J., Qin Y., Nottingham R.M., Temoche-Diaz M.M., Schekman R., Lambowitz A.M. Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc. Natl. Acad Sci. USA. 2017;114:E8987–E8995. doi: 10.1073/pnas.1712108114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cha D.J., Franklin J.L., Dou Y., Liu Q., Higginbotham J.N., Demory Beckler M., Weaver A.M., Vickers K., Prasad N., Levy S., et al. KRAS-dependent sorting of miRNA to exosomes. Elife. 2015;4:e07197. doi: 10.7554/eLife.07197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Villarroya-Beltri C., Gutiérrez-Vázquez C., Sánchez-Cabo F., Pérez-Hernández D., Vázquez J., Martin-Cofreces N., Martinez-Herrera D.J., Pascual-Montano A., Mittelbrunn M., Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shurtleff M.J., Temoche-Diaz M.M., Karfilis K.V., Ri S., Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Life. 2016;5:e19276. doi: 10.7554/eLife.19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kulkarni J.A., Witzigmann D., Thomson S.B., Chen S., Leavitt B.R., Cullis P.R., van der Meel R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021;16:630–643. doi: 10.1038/s41565-021-00898-0. [DOI] [PubMed] [Google Scholar]
- 88.Yamada Y. Nucleic Acid Drugs-Current Status, Issues, and Expectations for Exosomes. Cancers. 2021;13:5002. doi: 10.3390/cancers13195002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Damase T.R., Sukhovershin R., Boada C., Taraballi F., Pettigrew R.I., Cooke J.P. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021;9:628137. doi: 10.3389/fbioe.2021.628137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neumeier J., Meister G. siRNA Specificity: RNAi Mechanisms and Strategies to Reduce Off-Target Effects. Front. Plant Sci. 2021;11:526455. doi: 10.3389/fpls.2020.526455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dana H., Chalbatani G.M., Mahmoodzadeh H., Karimloo R., Rezaiean O., Moradzadeh A., Mehmandoost N., Moazzen F., Mazraeh A., Marmari V., et al. Molecular Mechanisms and Biological Functions of siRNA. Int. J. Biomed. Sci. 2017;13:48–57. [PMC free article] [PubMed] [Google Scholar]
- 92.Choudhary S., Lee H.C., Maiti M., He Q., Cheng P., Liu Q., Liu Y. A double-stranded-RNA response program important for RNA interference efficiency. Mol. Cell Biol. 2007;27:3995–4005. doi: 10.1128/MCB.00186-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stewart M.P., Langer R., Jensen K.F. Intracellular Delivery by Membrane Disruption: Mechanisms, Strategies, and Concepts. Chem. Rev. 2018;118:7409–7531. doi: 10.1021/acs.chemrev.7b00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Durymanov M., Reineke J. Non-viral Delivery of Nucleic Acids: Insight Into Mechanisms of Overcoming Intracellular Barriers. Front. Pharmacol. 2018;9:971. doi: 10.3389/fphar.2018.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang M.Z., Xu Y., Xie J.F., Jiang Z.H., Peng L.H. Ginsenoside as a new stabilizer enhances the transfection efficiency and biocompatibility of cationic liposome. Biomater. Sci. 2021;9:8373–8385. doi: 10.1039/D1BM01353J. [DOI] [PubMed] [Google Scholar]
- 96.Deshayes S., Simeoni F., Morris M.C., Divita G., Heitz F. Peptide-mediated delivery of nucleic acids into mammalian cells. Methods Mol. Biol. 2007;386:299–308. doi: 10.1007/978-1-59745-430-8_11. [DOI] [PubMed] [Google Scholar]
- 97.Della Pelle G., Kostevšek N. Nucleic Acid Delivery with Red-Blood-Cell-Based Carriers. Int. J. Mol. Sci. 2021;22:5264. doi: 10.3390/ijms22105264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hammond S.M., Aartsma-Rus A., Alves S., Borgos S.E., Buijsen R.A.M., Collin R.W.J., Covello G., Denti M.A., Desviat L.R., Echevarría L., et al. Delivery of oligonucleotide-based therapeutics: Challenges and opportunities. EMBO Mol. Med. 2021;13:e13243. doi: 10.15252/emmm.202013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kimura N., Maeki M., Sato Y., Note Y., Ishida A., Tani H., Harashima H., Tokeshi M. Development of the iLiNP Device: Fine Tuning the Lipid Nanoparticle Size within 10 nm for Drug Delivery. ACS Omega. 2018;3:5044–5051. doi: 10.1021/acsomega.8b00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ewert K.K., Scodeller P., Simón-Gracia L., Steffes V.M., Wonder E.A., Teesalu T., Safinya C.R. Cationic Liposomes as Vectors for Nucleic Acid and Hydrophobic Drug Therapeutics. Pharmaceutics. 2021;13:1365. doi: 10.3390/pharmaceutics13091365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fan Y., Marioli M., Zhang K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021;192:113642. doi: 10.1016/j.jpba.2020.113642. [DOI] [PubMed] [Google Scholar]
- 102.Filipczak N., Pan J., Yalamarty S.S.K., Torchilin V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020;156:4–22. doi: 10.1016/j.addr.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 103.Goutas D., Pergaris A., Goutas N., Theocharis S. Utilizing Exosomal-EPHs/Ephrins as Biomarkers and as a Potential Platform for Targeted Delivery of Therapeutic Exosomes. Int. J. Mol. Sci. 2022;23:3551. doi: 10.3390/ijms23073551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rao D., Huang D., Sang C., Zhong T., Zhang Z., Tang Z. Advances in Mesenchymal Stem Cell-Derived Exosomes as Drug Delivery Vehicles. Front. Bioeng. Biotechnol. 2022;9:797359. doi: 10.3389/fbioe.2021.797359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Q.C., Li C., Zhang W., Pi W., Han N. Potential Effects of Exosomes and Their MicroRNA Carrier on Osteoporosis. Curr. Pharm. Des. 2022 doi: 10.2174/1381612828666220128104206. in press . [DOI] [PubMed] [Google Scholar]
- 106.Jiang L., Chen W., Ye J., Wang Y. Potential Role of Exosomes in Ischemic Stroke Treatment. Biomolecules. 2022;12:115. doi: 10.3390/biom12010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang W., Yue C., Gao S., Li S., Zhou J., Chen J., Fu J., Sun W., Hua C. Promising Roles of Exosomal microRNAs in Systemic Lupus Erythematosus. Front. Immunol. 2021;12:757096. doi: 10.3389/fimmu.2021.757096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jurgielewicz B., Stice S., Yao Y. Therapeutic Potential of Nucleic Acids when Combined with Extracellular Vesicles. Aging Dis. 2021;12:1476–1493. doi: 10.14336/AD.2021.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu M., Feng T., Liu B., Qiu F., Xu Y., Zhao Y., Zheng Y. Engineered exosomes: Desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11:8926–8944. doi: 10.7150/thno.62330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao Y., Liu P., Tan H., Chen X., Wang Q., Chen T. Exosomes as Smart Nanoplatforms for Diagnosis and Therapy of Cancer. Front. Oncol. 2021;11:743189. doi: 10.3389/fonc.2021.743189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiang L., Gu Y., Du Y., Tang X., Wu X., Liu J. Engineering Exosomes Endowed with Targeted Delivery of Triptolide for Malignant Melanoma Therapy. ACS Appl. Mater. Interfaces. 2021;13:42411–42428. doi: 10.1021/acsami.1c10325. [DOI] [PubMed] [Google Scholar]
- 112.Jayaraman S., Gnanasampanthapandian D., Rajasingh J., Palaniyandi K. Stem Cell-Derived Exosomes Potential Therapeutic Roles in Cardiovascular Diseases. Front. Cardiovasc. Med. 2021;8:723236. doi: 10.3389/fcvm.2021.723236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moghadasi S., Elveny M., Rahman H.S., Suksatan W., Jalil A.T., Abdelbasset W.K., Yumashev A.V., Shariatzadeh S., Motavalli R., Behzad F., et al. A paradigm shift in cell-free approach: The emerging role of MSCs-derived exosomes in regenerative medicine. J. Transl. Med. 2021;19:302. doi: 10.1186/s12967-021-02980-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Shi J., Zhao Y.C., Niu Z.F., Fan H.J., Hou S.K., Guo X.Q., Sang L., Lv Q. Mesenchymal stem cell-derived small extracellular vesicles in the treatment of human diseases: Progress and prospect. World J. Stem Cells. 2021;13:49–63. doi: 10.4252/wjsc.v13.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liang Y., Duan L., Lu J., Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang Y., Li R., Ye S., Lin S., Yin G., Xie Q. Recent Advances in the Use of Exosomes in Sjögren’s Syndrome. Front. Immunol. 2020;11:1509. doi: 10.3389/fimmu.2020.01509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Markov O., Oshchepkova A., Mironova N. Immunotherapy Based on Dendritic Cell-Targeted/-Derived Extracellular Vesicles-A Novel Strategy for Enhancement of the Anti-tumor Immune Response. Front. Pharmacol. 2019;10:1152. doi: 10.3389/fphar.2019.01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang P., Peng Y., Feng Y., Xu Z., Feng P., Cao J., Chen Y., Chen X., Cao X., Yang Y., et al. Immune Cell-Derived Extracellular Vesicles-New Strategies in Cancer Immunotherapy. Front. Immunol. 2021;12:771551. doi: 10.3389/fimmu.2021.771551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hosseini R., Asef-Kabiri L., Yousefi H., Sarvnaz H., Salehi M., Akbari M.E., Eskandari N. The roles of tumor-derived exosomes in altered differentiation, maturation and function of dendritic cells. Mol. Cancer. 2021;20:83. doi: 10.1186/s12943-021-01376-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Y., Xiang Y., Xin V.W., Wang X.W., Peng X.C., Liu X.Q., Wang D., Li N., Cheng J.T., Lyv Y.N., et al. Dendritic cell biology and its role in tumor immunotherapy. J. Hematol. Oncol. 2020;13:107. doi: 10.1186/s13045-020-00939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Han Y., Jones T.W., Dutta S., Zhu Y., Wang X., Narayanan S.P., Fagan S.C., Zhang D. Overview and Update on Methods for Cargo Loading into Extracellular Vesicles. Processes. 2021;9:356. doi: 10.3390/pr9020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen C., Sun M., Wang J., Su L., Lin J., Yan X. Active cargo loading into extracellular vesicles: Highlights the heterogeneous encapsulation behaviour. J. Extracell. Vesicles. 2021;10:e12163. doi: 10.1002/jev2.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rankin-Turner S., Vader P., O’Driscoll L., Giebel B., Heaney L.M., Davies O.G. A call for the standardised reporting of factors affecting the exogenous loading of extracellular vesicles with therapeutic cargos. Adv. Drug Deliv. Rev. 2021;173:479–491. doi: 10.1016/j.addr.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Walker S., Busatto S., Pham A., Tian M., Suh A., Carson K., Quintero A., Lafrence M., Malik H., Santana M.X., et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9:8001–8017. doi: 10.7150/thno.37097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Raghav A., Jeong G.B. A systematic review on the modifications of extracellular vesicles: A revolutionized tool of nano-biotechnology. J. Nanobiotechnol. 2021;19:459. doi: 10.1186/s12951-021-01219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.N’Diaye E.R., Orefice N.S., Ghezzi C., Boumendjel A. Chemically Modified Extracellular Vesicles and Applications in Radiolabeling and Drug Delivery. Pharmaceutics. 2022;14:653. doi: 10.3390/pharmaceutics14030653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cao X.H., Liang M.X., Wu Y., Yang K., Tang J.H., Zhang W. Extracellular vesicles as drug vectors for precise cancer treatment. Nanomedicine. 2021;16:1519–1537. doi: 10.2217/nnm-2021-0123. [DOI] [PubMed] [Google Scholar]
- 128.Urzì O., Gasparro R., Ganji N.R., Alessandro R., Raimondo S. Plant-RNA in Extracellular Vesicles: The Secret of Cross-Kingdom Communication. Membranes. 2022;12:352. doi: 10.3390/membranes12040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.He B., Hamby R., Jin H. Plant extracellular vesicles: Trojan horses of cross-kingdom warfare. FASEB Bioadv. 2021;3:657–664. doi: 10.1096/fba.2021-00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cuesta C.M., Guerri C., Ureña J., Pacual M. Role of Microbiota-Derived Extracellular Vesicles in Gut-Brain Communication. Int. J. Mol. Sci. 2021;22:4235. doi: 10.3390/ijms22084235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pérez-Cabezas B., Santarém N., Cecílio P., Silva C., Silvestre R., AM Catita J., Cordeiro da Silva A. More than just exosomes: Distinct Leishmania infantum extracellular products potentiate the establishment of infection. J. Extracell. Vesicles. 2018;8:1541708. doi: 10.1080/20013078.2018.1541708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morishita M., Horita M., Higuchi A., Marui M., Katsumi H., Yamamoto A. Characterizing Different Probiotic-Derived Extracellular Vesicles as a Novel Adjuvant for Immunotherapy. Mol. Pharm. 2021;18:1080–1092. doi: 10.1021/acs.molpharmaceut.0c01011. [DOI] [PubMed] [Google Scholar]
- 133.Morishita M., Sagayama R., Yamawaki Y., Yamaguchi M., Katsumi H., Yamamoto A. Activation of Host Immune Cells by Probiotic-Derived Extracellular Vesicles via TLR2-Mediated Signaling Pathways. Biol. Pharm. Bull. 2022;45:354–359. doi: 10.1248/bpb.b21-00924. [DOI] [PubMed] [Google Scholar]
- 134.Kim S.H., Lee J.H., Kim E.H., Reaney M.J.T., Shim Y.Y., Chung M.J. Immunomodulatory Activity of Extracellular Vesicles of Kimchi-Derived Lactic Acid Bacteria (Leuconostoc mesenteroides, Latilactobacillus curvatus, and Lactiplantibacillus plantarum) Foods. 2022;11:313. doi: 10.3390/foods11030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hu R., Lin H., Wang M., Zhao Y., Liu H., Min Y., Yang X., Gao Y., Yang M. Lactobacillus reuteri-derived extracellular vesicles maintain intestinal immune homeostasis against lipopolysaccharide-induced inflammatory responses in broilers. J. Anim. Sci. Biotechnol. 2021;12:25. doi: 10.1186/s40104-020-00532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang F., Cerione R.A., Antonyak M.A. Isolation and characterization of extracellular vesicles produced by cell lines. STAR Protoc. 2021;2:100295. doi: 10.1016/j.xpro.2021.100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tang Y.T., Huang Y.Y., Zheng L., Qin S.H., Xu X.P., An T.X., Xu Y., Wu Y.S., Hu X.M., Ping B.H., et al. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int. J. Mol. Med. 2017;40:834–844. doi: 10.3892/ijmm.2017.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao Z., Wijerathne H., Godwin A.K., Soper S.A. Isolation and analysis methods of extracellular vesicles (EVs) Extracell. Vesicles Circ. Nucl. Acids. 2021;2:80–103. doi: 10.20517/evcna.2021.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kurian T.K., Banik S., Gopal D., Chakrabarti S., Mazumder N. Elucidating Methods for Isolation and Quantification of Exosomes: A Review. Mol. Biotechnol. 2021;63:249–266. doi: 10.1007/s12033-021-00300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Clos-Sansalvador M., Monguió-Tortajada M., Roura S., Franquesa M., Borràs F.E. Commonly used methods for extracellular vesicles’ enrichment: Implications in downstream analyses and use. Eur. J. Cell Biol. 2022;101:151227. doi: 10.1016/j.ejcb.2022.151227. [DOI] [PubMed] [Google Scholar]
- 141.Tiwari S., Kumar V., Randhawa S., Verma S.K. Preparation and characterization of extracellular vesicles. Preparation and characterization of extracellular vesicles. Am. J. Reprod. Immunol. 2021;85:e13367. doi: 10.1111/aji.13367. [DOI] [PubMed] [Google Scholar]
- 142.Zhou Y., McNamara R.P., Dittmer D.P. Purification Methods and the Presence of RNA in Virus Particles and Extracellular Vesicles. Viruses. 2020;12:917. doi: 10.3390/v12090917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Y., Li Y., Zhou H., Qian X. Syntaxin 2 promotes colorectal cancer growth by increasing the secretion of exosomes. J. Cancer. 2021;12:2050–2058. doi: 10.7150/jca.51494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen B.Y., Sung C.W., Chen C., Cheng C.M., Lin D.P., Huang C.T., Hsu M.Y. Advances in exosomes technology. Clin. Chim. Acta. 2019;493:14–19. doi: 10.1016/j.cca.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 145.Zhang F., Guo J., Zhang Z., Duan M., Wang G., Qian Y., Zhao H., Yang Z., Jiang X. Application of engineered extracellular vesicles for targeted tumor therapy. J. Biomed. Sci. 2022;29:14. doi: 10.1186/s12929-022-00798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu M., Yang Q., Sun X., Wang Y. Recent Advancements in the Loading and Modification of Therapeutic Exosomes. Front. Bioeng. Biotechnol. 2020;8:586130. doi: 10.3389/fbioe.2020.586130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Busatto S., Iannotta D., Walker S.A., Di Marzio L., Wolfram J. A Simple and Quick Method for Loading Proteins in Extracellular Vesicles. Pharmaceuticals. 2021;14:356. doi: 10.3390/ph14040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Herrmann I.K., Wood M.J.A., Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 149.Luan X., Sansanaphongpricha K., Myers I., Chen H., Yuan H., Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta. Pharmacol. Sin. 2017;38:754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gangadaran P., Ahn B.C. Extracellular Vesicle- and Extracellular Vesicle Mimetics-Based Drug Delivery Systems: New Perspectives, Challenges, and Clinical Developments. Pharmaceutics. 2020;12:442. doi: 10.3390/pharmaceutics12050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu X., Li L., Iliuk A., Tao W.A. Highly Efficient Phosphoproteome Capture and Analysis from Urinary Extracellular Vesicles. J. Proteome Res. 2018;17:3308–3316. doi: 10.1021/acs.jproteome.8b00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Coumans F.A.W., Brisson A.R., Buzas E.I., Dignat-George F., Drees E.E.E., El-Andaloussi S., Emanueli C., Gasecka A., Hendrix A., Hill A.F., et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017;120:1632–1648. doi: 10.1161/CIRCRESAHA.117.309417. [DOI] [PubMed] [Google Scholar]
- 153.Soma E., Yamayoshi A., Toda Y., Mishima Y., Hosogi S., Ashihara E. Successful Incorporation of Exosome-Capturing Antibody-siRNA Complexes into Multiple Myeloma Cells and Suppression of Targeted mRNA Transcripts. Cancers. 2022;14:566. doi: 10.3390/cancers14030566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Escrevente C., Keller S., Altevogt P., Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108. doi: 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lundberg V., Berglund M., Skogberg G., Lindgren S., Lundqvist C., Gudmundsdottir J., Thörn K., Telemo E., Ekwall O. Thymic exosomes promote the final maturation of thymocytes. Sci. Rep. 2016;6:36479. doi: 10.1038/srep36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gurung S., Perocheau D., Touramanidou L., Baruteau J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021;19:47. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bunggulawa E.J., Wang W., Yin T., Wang N., Durkan C., Wang Y., Wang G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnol. 2018;16:81. doi: 10.1186/s12951-018-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A., Coscia C., Iessi E., Logozzi M., Molinari A., et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Clayton A., Turkes A., Dewitt S., Steadman R., Mason M.D., Hallett M.B. Adhesion and signaling by B cell-derived exosomes: The role of integrins. FASEB J. 2004;18:977–979. doi: 10.1096/fj.03-1094fje. [DOI] [PubMed] [Google Scholar]
- 160.Bastos-Amador P., Pérez-Cabezas B., Izquierdo-Useros N., Puertas M.C., Martinez-Picado J., Pujol-Borrell R., Naranjo-Gómez M., Borràs F.E. Capture of cell-derived microvesicles (exosomes and apoptotic bodies) by human plasmacytoid dendritic cells. J. Leukoc. Biol. 2012;91:751–758. doi: 10.1189/jlb.0111054. [DOI] [PubMed] [Google Scholar]
- 161.Feng D., Zhao W.L., Ye Y.Y., Bai X.C., Liu R.Q., Chang L.F., Zhou Q., Sui S.F. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 162.Tian T., Zhu Y.L., Zhou Y.Y., Liang G.F., Wang Y.Y., Hu F.H., Xiao Z.D. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J. Biol. Chem. 2014;289:22258–22267. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Horibe S., Tanahashi T., Kawauchi S., Murakami Y., Rikitake Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer. 2018;18:47. doi: 10.1186/s12885-017-3958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wei X., Liu C., Wang H., Wang L., Xiao F., Guo Z., Zhang H. Surface Phosphatidylserine Is Responsible for the Internalization on Microvesicles Derived from Hypoxia-Induced Human Bone Marrow Mesenchymal Stem Cells into Human Endothelial Cells. PLoS ONE. 2016;11:e0147360. doi: 10.1371/journal.pone.0147360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bhatta M., Shenoy G.N., Loyall J.L., Gray B.D., Bapardekar M., Conway A., Minderman H., Kelleher R.J., Jr., Carreno B.M., Linette G., et al. Novel phosphatidylserine-binding molecule enhances antitumor T-cell responses by targeting immunosuppressive exosomes in human tumor microenvironments. J. Immunother. Cancer. 2021;9:e003148. doi: 10.1136/jitc-2021-003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Meier A.F., Fraefel C., Seyffert M. The Interplay between Adeno-Associated Virus and its Helper Viruses. Viruses. 2020;12:662. doi: 10.3390/v12060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zinn E., Vandenberghe L.H. Adeno-associated virus: Fit to serve. Curr. Opin. Virol. 2014;8:90–97. doi: 10.1016/j.coviro.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wu Z., Asokan A., Samulski R.J. Adeno-associated virus serotypes: Vector toolkit for human gene therapy. Mol. Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 169.Wörner T.P., Bennett A., Habka S., Snijder J., Friese O., Powers T., Agbandje-McKenna M., Heck A.J.R. Adeno-associated virus capsid assembly is divergent and stochastic. Nat. Commun. 2021;12:1642. doi: 10.1038/s41467-021-21935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gonçalves M.A. Adeno-associated virus: From defective virus to effective vector. Virol. J. 2005;2:43. doi: 10.1186/1743-422X-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Owens R.A. Second generation adeno-associated virus type 2-based gene therapy systems with the potential for preferential integration into AAVS1. Curr. Gene Ther. 2002;2:145–159. doi: 10.2174/1566523024605627. [DOI] [PubMed] [Google Scholar]
- 172.Rivière C., Danos O., Douar A.M. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13:1300–1308. doi: 10.1038/sj.gt.3302766. [DOI] [PubMed] [Google Scholar]
- 173.Blackburn S.D., Steadman R.A., Johnson F.B. Attachment of adeno-associated virus type 3H to fibroblast growth factor receptor 1. Arch. Virol. 2006;151:617–623. doi: 10.1007/s00705-005-0650-6. [DOI] [PubMed] [Google Scholar]
- 174.Asokan A., Hamra J.B., Govindasamy L., Agbandje-McKenna M., Samulski R.J. Adeno-associated virus type 2 contains an integrin alpha5beta1 binding domain essential for viral cell entry. J. Virol. 2006;80:8961–8969. doi: 10.1128/JVI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Woodard K.T., Liang K.J., Bennett W.C., Samulski R.J. Heparan Sulfate Binding Promotes Accumulation of Intravitreally Delivered Adeno-associated Viral Vectors at the Retina for Enhanced Transduction but Weakly Influences Tropism. J. Virol. 2016;90:9878–9888. doi: 10.1128/JVI.01568-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Walters R.W., Pilewski J.M., Chiorini J.A., Zabner J. Secreted and transmembrane mucins inhibit gene transfer with AAV4 more efficiently than AAV5. J. Biol. Chem. 2002;277:23709–23713. doi: 10.1074/jbc.M200292200. [DOI] [PubMed] [Google Scholar]
- 177.Liu B., Li Z., Huang S., Yan B., He S., Chen F., Liang Y. AAV-Containing Exosomes as a Novel Vector for Improved Gene Delivery to Lung Cancer Cells. Front. Cell Dev. Biol. 2021;9:707607. doi: 10.3389/fcell.2021.707607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang W., Liu J., Yang M., Qiu R., Li Y., Bian S., Hao B., Lei B. Intravitreal Injection of an Exosome-Associated Adeno-Associated Viral Vector Enhances Retinoschisin 1 Gene Transduction in the Mouse Retina. Hum. Gene Ther. 2021;32:707–716. doi: 10.1089/hum.2020.328. [DOI] [PubMed] [Google Scholar]
- 179.Maurya S., Jayandharan G.R. Exosome-associated SUMOylation mutant AAV demonstrates improved ocular gene transfer efficiency in vivo. Virus Res. 2020;283:197966. doi: 10.1016/j.virusres.2020.197966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Khan N., Maurya S., Bammidi S., Jayandharan G.R. AAV6 Vexosomes Mediate Robust Suicide Gene Delivery in a Murine Model of Hepatocellular Carcinoma. Mol. Ther. Methods Clin. Dev. 2020;17:497–504. doi: 10.1016/j.omtm.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Meliani A., Boisgerault F., Fitzpatrick Z., Marmier S., Leborgne C., Collaud F., Simon Sola M., Charles S., Ronzitti G., Vignaud A., et al. Enhanced liver gene transfer and evasion of preexisting humoral immunity with exosome-enveloped AAV vectors. Blood Adv. 2017;1:2019–2031. doi: 10.1182/bloodadvances.2017010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wassmer S.J., Carvalho L.S., György B., Vandenberghe L.H., Maguire C.A. Exosome-associated AAV2 vector mediates robust gene delivery into the murine retina upon intravitreal injection. Sci. Rep. 2017;7:45329. doi: 10.1038/srep45329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hudry E., Martin C., Gandhi S., György B., Scheffer D.I., Mu D., Merkel S.F., Mingozzi F., Fitzpatrick Z., Dimant H., et al. Exosome-associated AAV vector as a robust and convenient neuroscience tool. Gene Ther. 2016;23:819. doi: 10.1038/gt.2016.65. [DOI] [PubMed] [Google Scholar]
- 184.Maguire C.A., Balaj L., Sivaraman S., Crommentuijn M.H., Ericsson M., Mincheva-Nilsson L., Baranov V., Gianni D., Tannous B.A., Sena-Esteves M., et al. Microvesicle-associated AAV vector as a novel gene delivery system. Mol. Ther. 2012;20:960–971. doi: 10.1038/mt.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cheng M., Dietz L., Gong Y., Eichler F., Nammour J., Ng C., Grimm D., Maguire C.A. Neutralizing Antibody Evasion and Transduction with Purified Extracellular Vesicle-Enveloped Adeno-Associated Virus Vectors. Hum. Gene Ther. 2021;32:1457–1470. doi: 10.1089/hum.2021.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.György B., Fitzpatrick Z., Crommentuijn M.H., Mu D., Maguire C.A. Naturally enveloped AAV vectors for shielding neutralizing antibodies and robust gene delivery in vivo. Biomaterials. 2014;35:7598–7609. doi: 10.1016/j.biomaterials.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]