Abstract
Differential scanning calorimetry (DSC) and fatty acid analysis were used to determine how cold shocking reduces the thermal stability of Listeria monocytogenes. Additionally, antibiotics that can elicit production of cold or heat shock proteins were used to determine the effect of translation blockage on ribosome thermal stability. Fatty acid profiles showed no significant variations as a result of cold shock, indicating that changes in membrane fatty acids were not responsible for the cold shock-induced reduction in thermal tolerance. Following a 3-h cold shock from 37 to 0°C, the maximum denaturation temperature of the 50S ribosomal subunit and 70S ribosomal particle peak was reduced from 73.4 ± 0.1°C (mean ± standard deviation) to 72.1 ± 0.5°C (P ≤ 0.05), indicating that cold shock induced instability in the associated ribosome structure. The maximum denaturation temperature of the 30S ribosomal subunit peak did not show a significant shift in temperature (from 67.5 ± 0.4°C to 66.8 ± 0.5°C) as a result of cold shock, suggesting that either 50S subunit or 70S particle sensitivity was responsible for the intact ribosome fragility. Antibiotics that elicited changes in maximum denaturation temperature in ribosomal components also elicited reductions in thermotolerance. Together, these data suggest that ribosomal changes resulting from cold shock may be responsible for the decrease in D value observed when L. monocytogenes is cold shocked.
Listeria monocytogenes is known for its ability to grow in reduced-water-activity environments and at refrigeration temperatures, making this organism a continuing public health problem in ready-to-eat foods. The ability of L. monocytogenes to thrive under conditions frequently used to control microbial growth in ready-to-eat foods has been the subject of numerous studies, yet the organism continues to be a significant problem accounting for a large number of voluntary recalls. L. monocytogenes is the cause of listeriosis, a food-borne disease that results in an estimated 2,518 cases annually in the United States (15). The high fatality rate associated with listeriosis results in L. monocytogenes being responsible for 27.6% of all deaths due to food-borne pathogens in the United States (15). Various stress responses have been shown to increase the resistance of L. monocytogenes and other bacteria to subsequent processing steps (5, 9, 13). Inadvertent exposure of microorganisms to conditions that initiate adaptive stress responses may make elimination of the microorganisms from food more difficult. We have been studying the response of L. monocytogenes to various conditions of osmolarity and temperature in model and food systems in order to gain a better understanding of how this organism responds to stress. During the course of our investigations, we have determined that L. monocytogenes shows a decreased thermal tolerance following exposure to a cold shock (17).
Microorganisms respond to cold stress in a variety of ways. Typically, microorganisms exposed to a temperature downshift near or below the minimum growth temperature alter protein synthesis, cell membranes, and a variety of other cellular structures in an attempt to adapt to the new environmental conditions (7). L. monocytogenes has been shown to induce preferential synthesis of between 12 and 32 proteins upon exposure to cold stress (3, 19). Additionally, L. monocytogenes has been shown to undergo changes in its membrane fatty acid profile upon long-term exposure to reduced temperature (2). One proposed prokaryotic sensor of both cold shock and heat shock is the ribosomes (26). A number of antibiotics that bind to ribosomes have been used to mimic both heat-shock and cold-shock responses (8, 26). This has led to a model that seeks to explain the observed effects of various antibiotics in eliciting production of either heat-stress or cold-stress proteins (8). In this study, we used differential scanning calorimetry (DSC) to determine whether the cold shock-induced reduction in thermal sensitivity seen in L. monocytogenes was a result of ribosome sensing.
MATERIALS AND METHODS
Strains and media.
L. monocytogenes Scott A, from the Eastern Regional Research Center (ERRC) culture collection, was permanently maintained at −70°C. For each experiment, one frozen tube from a working stock was thawed at room temperature and 200 μl was transferred into 20 ml of brain heart infusion broth (Difco) and incubated at 37°C with agitation (100 rpm) for 6 h. After 6 h, fresh brain heart infusion broth was inoculated at 1:100 with the exponential-phase culture, and the culture was incubated overnight for 16 h at 37°C with agitation (100 rpm). Where noted, defined medium used was that of Pine et al. (20) with 0.5% (wt/vol) glucose but without choline and proline.
Lipid extraction and methanolysis.
Lipids present in dried biomass were extracted and converted to fatty acid methyl esters (FAME) by using a modification of the procedure described by Juneja et al. (10). Approximately 20 to 40 mg of lyophilized L. monocytogenes cells was placed into a 10-ml glass centrifuge tube, and 3 ml of dry methanol-toluene-methanesulfonic acid (30:15:1, by volume) mixture was added. The mixture was heated at 60°C for 12 to 14 h and cooled.
Fatty acid analysis.
FAME were quantitated on a Hewlett Packard (HP; Wilmington, Del.) 5890 Series II Plus gas chromatograph equipped with an Innowax capillary column (30 m by 0.53 mm by 0.25 μm), flame ionization detector, and capillary split-splitless injector. The injector and detector temperatures were both 260°C. A 2-μl sample volume was analyzed with split injection (10:1). Helium was used as the carrier gas at a constant flow of 10 ml min−1 (electronic pressure control, 9 lb/in2). FAME separations were obtained using an oven temperature profile: initial temperature of 120°C, hold for 2 min, then increase to 230°C at 5°C min−1; hold at 230°C for 16 min. FAME assignments were made by comparison with standards (bacterial acid methyl esters CPTM mix; Matreya, Inc., Pleasant Gap, Pa.). Unknown FAME were identified by gas chromatography-mass spectrometry (GC-MS) on an HP 5890 Series II Plus gas chromatograph and an HP 6972 mass-selective detector set to scan from 10 to 600 at 1.2 scans s−1. A capillary column (HP-5MS, 30 m by 0.25 mm by 0.25 μm) was used to separate the FAME. Oven temperature was programmed to be from 80 to 230°C at 10°C per min. The injector port temperature was 230°C and the detector transfer line temperature was 240°C.
Calorimetry.
DSC was performed in a DSC-7 calorimeter (Perkin-Elmer, Norwalk, Conn.). Cells from a 25-ml overnight stationary-phase culture were harvested by centrifugation at 8,000 × g for 15 min and resuspended in an equal volume of cold ribosome buffer (10 mM Tris-HCl, pH 7.5; 6 mM MgCl2; 30 mM NH4Cl [21]). The cells were repelleted, and 5 to 15 mg of cell paste was transferred into a volatile DSC pan and hermetically sealed. Scanning was from 0 to 100°C at 10°C min−1. Reference pans contained 10 μl of ribosome buffer.
Thermal inactivation.
Thermal inactivations were performed as described by Cole and Jones (4) by using a Colworth House submerged-coil heating apparatus (Protrol Limited, Surrey, United Kingdom). Sampling frequency and volume were computer controlled. Unless noted differently, the basic procedure consisted of diluting cultures, before injection, 10-fold in ribosome buffer (pH 7.5). Samples were collected in 15-by-45-mm glass vials (Kimble Glass Co., Vineland, N.J.) and immediately cooled in an ice bath. Samples were appropriately diluted into 0.1% peptone blanks (pH 6.85; Difco) and plated using an Autoplate 4000 spiral plater (Spiral Systems, Cincinnati, Ohio) onto duplicate brain heart infusion agar plates. Inoculated plates from the submerged-coil experiments were incubated for 48 h at 37°C and counted either manually or using an automated system (Model 500A; Spiral Systems). Thermal inactivations of cold-shocked cell cultures were performed similarly except that a 3-h cold incubation at the specified temperatures was carried out prior to dilution, heat treatment, plating, and enumeration.
Thermal inactivations involving antibiotic-treated cultures were performed by adding antibiotics to a 16-h stationary-phase culture of L. monocytogenes to a final concentration determined to be the MIC for L. monocytogenes Scott A or at a final concentration of 310 μM. The MICs for the antibiotics tested were determined using the spiral gradient endpoint method (18), and the values were rounded up to the nearest twofold dilution equivalent. All antibiotics were obtained from Sigma Chemical Company (St. Louis, Mo.). The cells were exposed to the antibiotics for 30 min at 37°C. Antibiotic-treated cells were diluted 10-fold in buffer containing the same concentration of antibiotic as was in the culture and then were heat challenged at 60°C. A portion of the sample collected after heat treatment was transferred to a microcentrifuge tube, and the cells were washed to remove residual antibiotic. Washing consisted of pelleting the cells for 20 s (13,000 × g), removal of the supernatant, and resuspension of cells in an equal volume of buffer without antibiotic. Cells were diluted and plated as described earlier.
RESULTS
Fatty acid analysis.
Fatty acid analysis revealed that there are no significant changes in the fatty acid profile of the stationary-phase cells following a 3-h 0°C cold shock when compared to those of stationary-phase controls (data not shown).
Calorimetry.
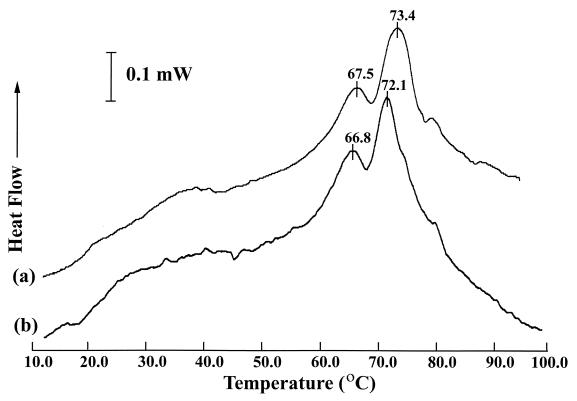
DSC of L. monocytogenes whole cells resulted in a characteristic thermogram profile which was predominated by the melting transitions of associated ribosomes and their dissociated subunits (Fig. 1). Stationary-phase L. monocytogenes whole-cell thermograms characteristically had two major peaks at 67.5 ± 0.4°C (mean ± standard deviation [SD]) and 73.4 ± 0.1°C, which corresponded to the thermal denaturation of the 30S ribosome subunit and the combined 50S subunit and 70S particle, respectively (14, 16). Exposing L. monocytogenes cells to a cold shock from 37 to 0°C resulted in a statistically significant (P ≤ 0.05) shift in the peak denaturation temperature, from 73.4 ± 0.1°C to 72.1 ± 0.5°C, for the portion of the thermogram corresponding to the 50S subunit and the 70S particle. Cold shock did not produce a significant change in the peak denaturation temperature (from 67.5 ± 0.4°C to 66.8 ± 0.5°C) for the portion of the thermogram corresponding to the 30S subunit. Similar results were obtained with cold shocks from 37 to 5°C, but not with cold shocks from 37 to 10°C (Table 1).
FIG. 1.
DSC of L. monocytogenes cells grown at 37°C (a) or grown at 37°C and cold shocked to 0°C for 3 h (b) prior to DSC analysis.
TABLE 1.
Maximum denaturation temperature for DSC thermogram peaks corresponding to ribosomesa
| Treatment group | Maximum denaturation temp (mean ± SD)
|
n | |
|---|---|---|---|
| 30S subunit peak | 50S subunit + 70S particle peak | ||
| Control | 67.5 ± 0.4 | 73.4 ± 0.1 | 5 |
| Cold shock to 0°C | 66.8 ± 0.5 | 72.1 ± 0.3** | 8 |
| Cold shock to 5°C | 66.5 ± 0.2 | 72.1 ± 0.3** | 3 |
| Cold shock to 10°C | 67.2 ± 0.3 | 73.1 ± 0.5 | 4 |
Results are from cold-shocked and non-cold-shocked L. monocytogenes cells. **, P ≤ 0.05.
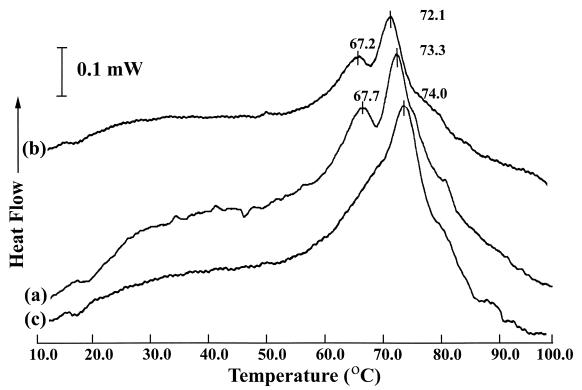
We also examined the effect of seven antibiotics (six with activity at the ribosome and one with activity at the level of RNA polymerase) on L. monocytogenes to determine if antibiotic treatment resulted in alterations of peak denaturation temperature in thermogram peaks corresponding to ribosomes or their subunits. The MICs determined for chloroamphenicol, erythromycin, kanamycin, rifampin, and tetracycline were 2.0, 0.03, 2.0, 0.03, and 0.125 mg liter−1, respectively. The MIC could not be determined for streptomycin, and puromycin was used at 310 μM. Antibiotic treatment of L. monocytogenes with tetracycline or kanamycin resulted in shifts in the peaks associated with L. monocytogenes ribosomes (Fig. 2). Kanamycin treatment (reported to induce synthesis of heat stress proteins [26]) resulted in a decreased peak temperature of denaturation, from 73.4 ± 0.1°C (mean ± SD) to 72.1 ± 0.7°C, in the thermogram peak corresponding to the 50S ribosomal subunit and the 70S particle. This reduction in peak denaturation temperature was analogous to that seen in cells cold shocked for 3 h at 0°C. Tetracycline treatment, reported to induce synthesis of cold-stress proteins (26), produced a striking alteration of the thermogram whereby the peak corresponding to the 30S ribosomal subunit either did not appear or was possibly shifted to a much higher temperature. This interpretation was based on the absence of a defined 30S subunit peak and the appearance of a peak shoulder on the 50S/70S peak. It was not possible to determine whether the peak shoulder in question was comprised of 30S subunits or whether changes in either the 50S subunits or 70S particles caused the observed shouldering. Treating L. monocytogenes with streptomycin, puromycin, chloramphenicol, erythromycin, or rifampin produced thermograms that were not statistically different from those of the controls (data not shown). Experiments to determine the MICs of these antibiotics for L. monocytogenes Scott A showed that our strain was streptomycin resistant. Since the organism is resistant to streptomycin, the antibiotic would not be expected to have effects on the ribosome or thermal tolerance.
FIG. 2.
DSC of L. monocytogenes cells grown at 37°C and treated for 30 min with antibiotic at 37°C prior to washing in buffer and DSC analysis. a, control cells; b, kanamycin-treated cells; c, tetracycline-treated cells.
Thermal inactivations.
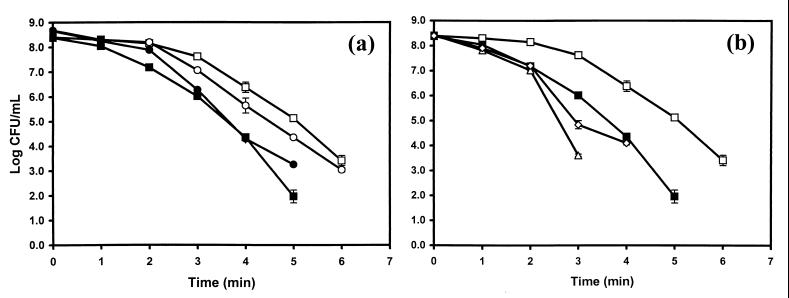
L. monocytogenes stationary-phase cells that were cold shocked from 37 to 0°C and then thermally challenged at 60°C had a reduced thermal tolerance compared to those of the controls. This same effect was observed for cells grown in complex or defined medium (Fig. 3a). Antibiotics that measurably altered the thermograms of L. monocytogenes cells, tetracycline and kanamycin, were the antibiotics that caused the greatest reduction in thermal tolerance. Multiple D60-value determinations for kanamycin- or tetracycline-treated L. monocytogenes cultures showed that these antibiotics reduced the D60 of the organism, on average, 27 and 26% compared to those of the controls, respectively. These reductions in D60 value are approximately equivalent to that seen with L. monocytogenes following a 37-to-0°C cold shock (17). Representative results from one experiment are shown in Fig. 3b. Similar cold shock-induced reductions in thermotolerance were seen when L. monocytogenes was cold shocked from 37 to 5°C (data not shown).
FIG. 3.
Survivor curves for thermal inactivation of L. monocytogenes cells at 60°C. All cells were grown and inactivated as described in Materials and Methods. (a) L. monocytogenes cells grown in brain heart infusion at 37°C (□), L. monocytogenes cells grown in brain heart infusion at 37°C and cold shocked to 0°C for 3 h (■), L. monocytogenes cells grown in Pine's medium at 37°C (○), L. monocytogenes grown in Pine's medium at 37°C and cold shocked to 0°C for 3 h (●). (b) L. monocytogenes cells grown in brain heart infusion at 37°C (□), L. monocytogenes cells grown in brain heart infusion at 37°C and cold shocked to 0°C for 3 h (■), L. monocytogenes cells grown in brain heart infusion at 37°C and treated with kanamycin (310 μM) (▵), L. monocytogenes cells grown in brain heart infusion at 37°C and treated with tetracycline (310 μM) (◊).
DISCUSSION
The effect that cold shock has in reducing the thermal tolerance of L. monocytogenes illustrates the use of an imposed stress in order to make the microorganism more sensitive to a subsequent processing step. We began investigating the nature of cold shock-induced reduction of thermal tolerance in order to better understand the mechanism of this phenomenon. Several cellular targets have been proposed as sites where thermal stress might cause cell damage. Membranes have been implicated as a site important in the thermal destruction of microorganisms; however, the membrane does not appear to be the site of action for the cold shock-induced reduction in thermal tolerance. First, our analysis indicates the membrane fatty acid profiles do not undergo any significant changes during the course of the 3-h cold shock. This is in agreement with other studies that showed a similar result with exponentially growing L. monocytogenes cultures (2). Secondly, cells that have been exposed to a cold shock continue to show a reduction in thermal tolerance even after the cells are returned to 28°C for 30 min prior to thermal challenge (17). We have focused our initial investigation on the ribosome as a primary source of the cold shock. VanBogelen and Neidhardt (26) proposed that the ribosome might be the major sensor of heat shock and cold shock in Escherichia coli. This concept has been reviewed recently (8). Katsui et al. (12) presented data that showed E. coli had reduced thermal tolerance after exposure to a cold shock. More recently, Gay and Cerf (6) presented data that indicate that cold shock decreased the D value of L. monocytogenes at reduced pH. This suggests that cold shock may also sensitize L. monocytogenes to subsequent exposure to low pH treatments.
Our initial work utilized DSC to study the mechanisms responsible for the cold shock-induced reduction in thermal resistance of L. monocytogenes. The use of DSC to study thermal inactivation mechanisms has been previously described (1). Stephens and Jones (24) have correlated the thermal tolerance of L. monocytogenes with the state of the 30S ribosomal subunit. This is in agreement with earlier ribosome studies that indicated that the 30S ribosomal subunit was more thermally sensitive than the 50S subunit or the associated 70S particle (22, 25). We propose that changes occurring during cold shock are acting at the level of the ribosome and may explain the reduction in thermal sensitivity seen in L. monocytogenes.
Cold shocking stationary-phase L. monocytogenes cells from 37 to 5 or 0°C for 3 h resulted in a statistically significant decrease in the peak denaturation temperature, from 73.4 ± 0.1°C (mean ± SD) to 72.1 ± 0.5°C, for the thermogram peak corresponding to the 50S ribosomal subunit and the 70S particle but not in the thermogram peak corresponding to the 30S ribosomal subunit. The cold shock-induced changes in DSC profiles appear to be temperature dependent since a cold shock from 37 to 10°C did not result in a significant maximum denaturation temperature shift for the peak corresponding to the 50S ribosomal subunit and the 70S particle. It is worthy to note that while no shifts in ribosomal maximum denaturation temperature were seen in cells shifted from 37 to 10°C, cold shocks of this magnitude were able to induce reductions in L. monocytogenes thermal sensitivity (17). The difference between DSC and thermal inactivation results likely stems from the lower sensitivity of the DSC analyses and the concomitant inability of DSC to differentiate the less dramatic changes in ribosome state occurring with milder cold shocks. Interestingly, DSC analysis of exponential-phase cells cultured at 5°C as well as stationary-phase cells cultured at 5°C did not show a change in the maximum denaturation temperatures for either ribosomal peak (data not shown). This suggests that the effect is a cold shock-associated phenomenon.
The observed changes in maximum denaturation temperatures indicate intracellular changes that impact ribosomes. One explanation that would correlate the changes observed in DSC thermograms with changes in the thermal tolerance of L. monocytogenes would be a change in the association status of the 70S ribosomal particles. Changes that result in dissociation of the 70S particle would tend to make the ribosomes more thermally labile since the dissociated subunits, and in particular the 30S subunit, are more thermally labile than the associated 70S particle (22, 24). Dissociation might occur due to increased mRNA structure that results at low temperature. This dissociation might also occur as translating ribosomes complete protein synthesis in progress at the time of cold shock and then fail to initiate subsequent translation events due to unfavorable thermodynamics. An alternative hypothesis revolves around the presence of 70S-70S dimers, which arise in stationary-phase E. coli and Bacillus subtilis cells (23). These dimers do not appear to be complexed with mRNA. The thermal stability as well as the tendency of these dimers to dissociate under conditions of cold shock are unknown.
Recently, Trigger factor (TF), a molecular chaperone, has been shown to play a role in maintaining E. coli cell viability at low temperatures (11). TF increased at low temperatures in a fashion similar to those of other cold shock proteins. Overexpression of TF was found to reduce the viability of exponential-phase cells when challenged with a 50°C heat shock. Interestingly, TF is closely associated with the 50S ribosomal subunit. Whether TF is present in L. monocytogenes and whether it is induced in stationary phase cells in response to a temperature downshift is not known.
Various antibiotics that act on ribosomes have been noted to preferentially induce synthesis of either the heat shock or cold shock proteins (8, 26). Our results indicate that the antibiotics tetracycline and kanamycin that caused prominent shifts in DSC thermogram peaks corresponding to ribosomes and their subunits also were the antibiotics that caused reductions in thermal tolerance. The other antibiotics tested did not cause DSC profile peak shifts and did not cause reductions in thermal tolerance, with the exception of chloramphenicol, which did cause a reduction in thermal tolerance, but no change in DSC profile. This difference may also relate to the sensitivity of the DSC analysis and the effect of chloramphenicol on the ribosomes. Overall, the antibiotic treatment data support the concept that changes at the level of the ribosome have a significant impact on the thermal resistance of L. monocytogenes. The ability of antibiotics to reduce thermal tolerance did not appear to be dependent upon whether the antibiotic was able to preferentially induce synthesis of heat or cold shock proteins (8). Rather, antibiotics that reduced the thermal tolerance of L. monocytogenes correlated to antibiotics that altered DSC thermograms. Initially, we were surprised that streptomycin, which is reported to increase the stability of the 70S particle (27), did not yield changes in the DSC thermogram compared to those of the controls. Correspondingly, there was no alteration in thermal resistance as a result of streptomycin treatment. Experiments to determine the MIC of the various antibiotics on L. monocytogenes revealed that the test strain of L. monocytogenes was streptomycin resistant. Accordingly, the antibiotic did not bind to the ribosomes and treated cells behaved identically to the untreated controls. The unifying theme between the DSC results obtained from cold-shocked or antibiotic-treated cells and thermal inactivation studies is that cold shock and certain antibiotics that can alter ribosome state (as reflected by changes in DSC profile) also effect a reduction in thermal tolerance upon heat challenge due to changes in the ribosomes. This likely reflects the changes in the unassociated proportion of cellular 30S subunits, which are more thermally labile and more effectively denatured by a heat treatment. Enriching the cells for unassociated 30S subunits sets up a situation in which the protein-synthesizing machinery is more efficiently destroyed upon a heat treatment.
An enhanced awareness and understanding of microbial physiology, such as the response of cells to various stresses, opens the door for exploitation of those responses in order to increase food safety. In this regard, the effects of cold shock can reduce the thermal tolerance of L. monocytogenes, ostensibly through the involvement of the ribosomes.
REFERENCES
- 1.Anderson W A, Hedges N D, Jones M V, Cole M B. Thermal inactivation of Listeria monocytogenes studied by differential scanning calorimetry. J Gen Microbiol. 1991;137:1419–1424. doi: 10.1099/00221287-137-6-1419. [DOI] [PubMed] [Google Scholar]
- 2.Annous B A, Becker L A, Bayles D O, Labeda D P, Wilkinson B J. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl Environ Microbiol. 1997;63:3887–3894. doi: 10.1128/aem.63.10.3887-3894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayles D O, Annous B A, Wilkinson B J. Cold stress proteins induced in Listeria monocytogenes in response to temperature downshock and growth at low temperatures. Appl Environ Microbiol. 1996;62:1116–1119. doi: 10.1128/aem.62.3.1116-1119.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole M B, Jones M V. A submerged-coil heating apparatus for investigating thermal inactivation of micro-organisms. Lett Appl Microbiol. 1990;11:233–235. [Google Scholar]
- 5.Farber J M, Brown B E. Effect of prior heat shock on heat resistance of Listeria monocytogenes in meat. Appl Environ Microbiol. 1990;56:1584–1587. doi: 10.1128/aem.56.6.1584-1587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gay M, Cerf O. Significance of temperature and preincubation temperature on survival of Listeria monocytogenes at pH 4.8. Lett Appl Microbiol. 1997;25:257–260. doi: 10.1046/j.1472-765x.1997.00216.x. [DOI] [PubMed] [Google Scholar]
- 7.Gounot A-M. Bacterial life at low temperatures: physiological aspects and biotechnological implications. J Appl Bacteriol. 1991;71:386–397. doi: 10.1111/j.1365-2672.1991.tb03806.x. [DOI] [PubMed] [Google Scholar]
- 8.Graumann P, Marahiel M A. Some like it cold: response of microorganisms to cold shock. Arch Microbiol. 1996;166:293–300. doi: 10.1007/s002030050386. [DOI] [PubMed] [Google Scholar]
- 9.Jørgensen F, Stephens P J, Knøchel S. The effect of osmotic shock and subsequent adaptation on the thermotolerance and cell morphology of Listeria monocytogenes. J Appl Bacteriol. 1995;79:274–281. [Google Scholar]
- 10.Juneja V J, Foglia T A, Marmer B S. Heat resistance and fatty acid composition of Listeria monocytogenes: effect of pH, acidulant, and growth temperature. J Food Prot. 1998;61:683–687. doi: 10.4315/0362-028x-61.6.683. [DOI] [PubMed] [Google Scholar]
- 11.Kandror O, Goldberg A L. Trigger factor is induced upon cold shock and enhances viability of Escherichia coli at low temperatures. Proc Natl Acad Sci USA. 1997;94:4978–4981. doi: 10.1073/pnas.94.10.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsui N, Tsuchido T, Takano M, Shibasaki I. Viability of heat-stressed cells of micro-organisms as influenced by pre-incubation and post-incubation temperatures. J Appl Bacteriol. 1982;53:103–108. doi: 10.1111/j.1365-2672.1982.tb04739.x. [DOI] [PubMed] [Google Scholar]
- 13.Lou Y Q, Yousef A E. Adaptation to sublethal environmental stresses protects Listeria monocytogenes against lethal preservation factors. Appl Environ Microbiol. 1997;63:1252–1255. doi: 10.1128/aem.63.4.1252-1255.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackey B M, Miles C A, Parsons S E, Seymour D A. Thermal denaturation of whole cells and cell components of Escherichia coli examined by differential scanning calorimetry. J Gen Microbiol. 1991;137:2361–2374. doi: 10.1099/00221287-137-10-2361. [DOI] [PubMed] [Google Scholar]
- 15.Mead P S, Slutsker L, Dietz V, McCaig L F, Bresee J S, Shapiro C, Griffin P M, Tauxe R V. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miles C A, Mackey B M, Parsons S E. Differential scanning calorimetry of bacteria. J Gen Microbiol. 1986;132:939–952. doi: 10.1099/00221287-132-4-939. [DOI] [PubMed] [Google Scholar]
- 17.Miller A J, Bayles D O, Eblen B S. Cold shock induction of thermal sensitivity in Listeria monocytogenes. Appl Environ Microbiol. 2000;66:4345–4350. doi: 10.1128/aem.66.10.4345-4350.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paton J H, Holt H A, Bywater M J. Measurement of MICs of antibacterial agents by spiral gradient endpoint compared with conventional dilution method. Int J Exp Clin Chemother. 1990;3:31–38. [Google Scholar]
- 19.Phan-Thanh L, Gormon T. Analysis of heat and cold shock proteins in Listeria by two-dimensional electrophoresis. Electrophoresis. 1995;16:444–450. doi: 10.1002/elps.1150160172. [DOI] [PubMed] [Google Scholar]
- 20.Pine L, Malcolm G B, Brooks J B, Daneshvar M I. Physiological studies on the growth and utilization of sugars by Listeria species. Can J Microbiol. 1989;35:245–254. doi: 10.1139/m89-037. [DOI] [PubMed] [Google Scholar]
- 21.Rheinberger H, Geigenmuller U, Wedde M, Neirhaus K H. Parameters for preparation of E. coli ribosomes and ribosomal sub-units active in tRNA binding. Methods Enzymol. 1988;164:658–662. doi: 10.1016/s0076-6879(88)64076-6. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal L J, Iandolo J J. Thermally induced intracellular alteration of ribosomal ribonucleic acid. J Bacteriol. 1970;103:833–835. doi: 10.1128/jb.103.3.833-835.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharrock W J, Rabinowitz J C. Fractionation of ribosomal particles from Bacillus subtilis. Methods Enzymol. 1979;59:371–382. doi: 10.1016/0076-6879(79)59098-3. [DOI] [PubMed] [Google Scholar]
- 24.Stephens P J, Jones M V. Reduced ribosomal thermal denaturation in Listeria monocytogenes following osmotic and heat shocks. FEMS Microbiol Lett. 1993;106:177–182. doi: 10.1111/j.1574-6968.1993.tb05955.x. [DOI] [PubMed] [Google Scholar]
- 25.Tal M. Thermal denaturation of ribosomes. Biochemistry. 1969;8:424–435. doi: 10.1021/bi00829a058. [DOI] [PubMed] [Google Scholar]
- 26.VanBogelen R A, Neidhardt F C. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:5589–5593. doi: 10.1073/pnas.87.15.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfe A D, Hahn F I. Stability of ribosomes from streptomycin-exposed Escherichia coli. Biochem Biophys Res Commun. 1968;31:945–949. doi: 10.1016/0006-291x(68)90544-5. [DOI] [PubMed] [Google Scholar]