Abstract
A series of copper-based photocatalysts of the type Cu(NN)(BINAP)BF4 were synthesized bearing π-extended diimine ligands. Their behavior in several photocatalytic processes were evaluated and revealed acceptable levels of activity in an SET process, but negligible activity in PCET or ET processes. Suitable activity in ET processes could be restored through modification of the ligand. The BINAP-derived complexes were then evaluated for activity against triple-negative breast cancer cell lines. Controls indicated that copper complexes, and not their ligands, were responsible for activity. Encouraging activity was displayed by a homoleptic complex Cu(dppz)2BF4.
Keywords: copper, photochemistry, medicinal chemistry
1. Introduction
Copper-based complexes have demonstrated their potential across photocatalysis [1]. As an alternative to precious metal complexes [2,3,4], discreet copper-based complexes can be exploited as photocatalysts under UV [5,6,7] and visible-light irradiation [8,9,10,11,12]. In turn, a number of copper complexes can be formed in situ and used in metallaphotoredox processes, which are particularly advantageous for asymmetric photocatalysis [13]. In addition to synthetic applications, copper-based complexes have found interest in solar energy sciences [14], photocatalytic water splitting [15] and organic light-emitting diodes [16].
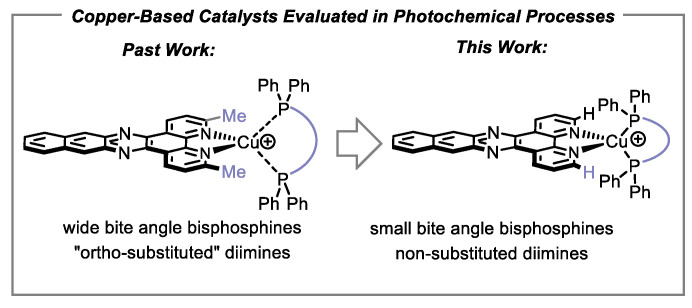
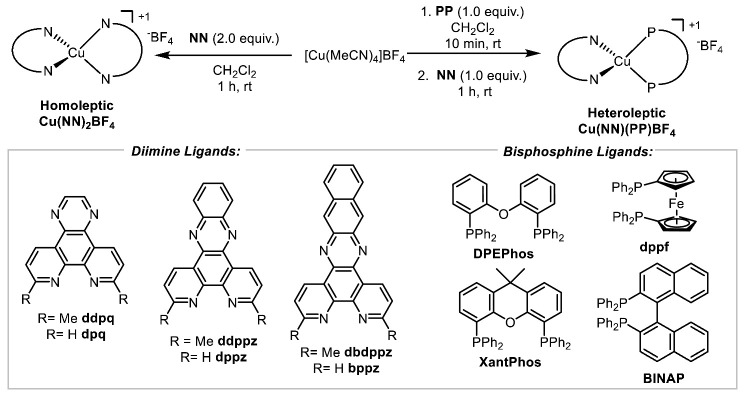
McMillin and co-workers first reported that heteroleptic copper-based complexes (Cu(NN)(PP)X) bearing the wide-bite-angle bisphosphine DPEPhos possessed unusually long excited-state lifetimes [17]. Following our initial discovery that such complexes exhibited significant potential for synthetic photocatalysis [18], we reported a structure/activity study library of 50 copper complexes that were evaluated in single-electron transfer (SET) [19,20], energy transfer (ET) [21] and proton-coupled electron transfer (PCET) reactions [22]. Although most photocatalysis using heteroleptic complexes continue to employ wide-bite-angle bisphosphines [23,24], the aforementioned library study revealed that the small-bite-angle bisphosphine BINAP could form copper complexes that afforded high yields in all three of the mechanistic processes evaluated. Our bank of available diimines and bisphosphines has since expanded to allow for development of improved complexes for energy transfer processes [25]. Among the diimine structures evaluated were those that possessed extended π-surfaces, which unfortunately did not afford heteroleptic complexes with remarkable activities in photocatalysis [26]. However, previous studies were limited to wide-bite-angle bisphosphines, as the preparation of the corresponding complexes with BINAP was problematic (Figure 1). Herein, we describe the synthesis of the “missing” copper-based complexes of the type Cu(NN)(BINAP)BF4, their evaluation in photochemical processes and preliminary biological testing against triple-negative breast cancer cell lines.
Figure 1.
Small-bite-angle bisphosphine for heteroleptic copper-based complexes.
2. Results and Discussion
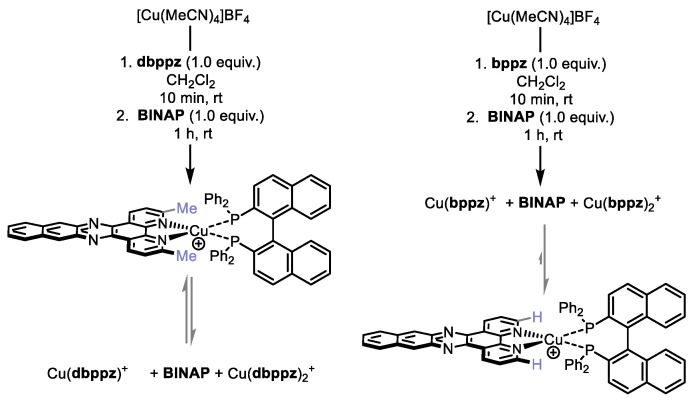
Heteroleptic complexes are typically formed by sequential addition of the diimine and bisphosphine ligands to a copper salt in a solvent, followed by precipitation. When the synthesis of heteroleptic Cu(I)-based photocatalysts using BINAP and the ligands ddpq, ddppz, or dbdppz− [27] was attempted, the resulting solids were mixtures of the corresponding hetero- and homoleptic complexes (1H-NMR and mass spectrometry, see supplementary materials) (Figure 2). Our hypothesis was that the small-bite-angle oriented the phenyl groups of the phosphine over the copper center, which is already encumbered by the methyl groups found on the diimine ligands. Attempts at conducting the synthesis in other solvents (PhME, THF, and mixtures thereof with CH2Cl2) did not result in a shift in the equilibrium between heteroleptic and homoleptic complexes. Experiments involving lower temperatures and/or slow addition of the bisphosphine were also non-productive. Repeated crystallization of crude reaction mixtures did improve the ratio of heteroleptic versus homoleptic complexes, but did not approach selectivities or yields that were synthetically useful.
Figure 2.
Synthesis of heteroleptic copper-based complexes using BINAP.
Consequently, the BINAP-containing complexes were prepared with the analogous diimines dpq, dppz, and bdppz (Table 1). Gratifyingly, all three complexes were isolated in good yields (53–77%). When examining the photophysical data, the UV-vis absorption characteristics of the BINAP-containing photocatalysts did not change significantly with respect to the diimine. The absorption maxima are all within a narrow window (424–462 nm), although the emission maxima are more spread out (560–625 nm) with lower-wavelength emissions observed for the larger diimine ligands. Extinction coefficients and excited-state lifetimes are again all relatively similar across the series. The short excited-state lifetimes are to be expected, as the absence of both ortho-substitution on the diimines and the small bite angle of the bisphosphine will not stabilize the geometry of the excited state. Excited state reduction potentials all were in the range of ~1.0 eV which corresponds to what was observed with the complexes derived from ortho-substituted analogues having ddpq, ddppz, and dbdppz ligands.
Table 1.
Synthesis and Properties of Cu(I)-Based Photocatalysts of the Type Cu(NN)(BINAP)BF4.
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | NN | PP | Yield (%) a | λ max (nm) | ε (L/mol·cm) | τ (ns) |
λ emm (nm) | ET (eV) | E (* CuI/CuII) |
| 1 | ddpq | 78 | 458 | 6760 | 3 | 680 | 1.82 | −1.36 | |
| 2 | ddpq | DPEPhos | 84 | 382 | 4485 | 5 | 565 | 2.19 | −1.26 |
| 3 | ddpq | XantPhos | 85 | 386 | 3444 | 3 | 560 | 2.21 | −1.72 |
| 4 | ddpq | dppf | 91 | 380 | 3346 | 73 | 530 | 2.34 | −1.15 |
| 5 | dpq | BINAP | 75 | 424 | 5752 | 1.4 | 625 | 2.38 | −1.02 |
| 6 | ddppz | 99 | 453 | 14428 | 4 | 762 | 1.63 | −0.90 | |
| 7 | ddppz | DPEPhos | 78 | 380 | 17508 | 44 | 664 | 1.87 | −1.12 |
| 8 | ddppz | XantPhos | 91 | 380 | 12489 | 71 | 634 | 1.95 | −0.82 |
| 9 | ddppz | dppf | 79 | 380 | 17508 | 61 | 510 | 2.43 | −1.59 |
| 10 | dppz | BINAP | 77 | 433 | 6759 | 1.8 | 545 | 2.27 | −1.26 |
| 11 | dbdppz | 82 | 412 | 25891 | 78 | 567 | 2.19 | −1.34 | |
| 12 | dbdppz | DPEPhos | 77 | 409 | 16663 | 69 | 489 | 2.53 | −1.82 |
| 13 | dbdppz | XantPhos | 50 | 408 | 13754 | 75 | 565 | 2.19 | −1.29 |
| 14 | dbdppz | dppf | 79 | 413 | 11711 | 69 | 597 | 2.08 | −0.80 |
| 15 | bdppz | BINAP | 53 | 462 | 5930 | 2.3 | 560 | 2.21 | −1.20 |
a Isolated yields following precipitation with Et2O; * The astericks demotes the excited state of Cu(I).
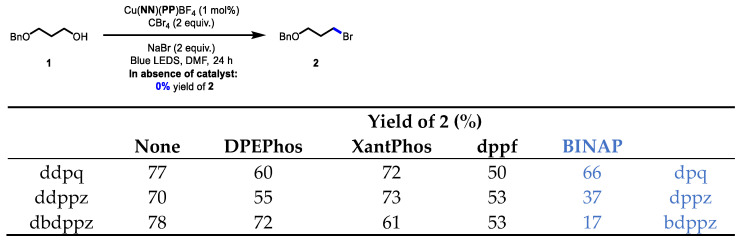
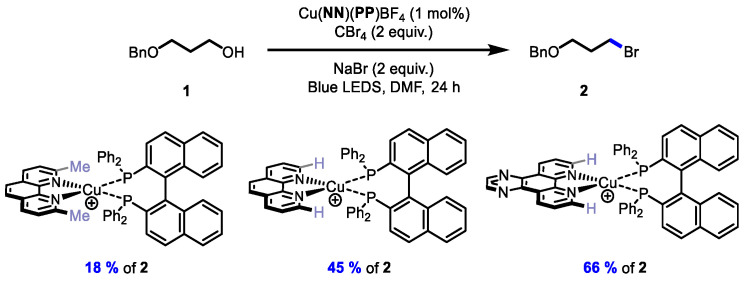
With the new BINAP-containing copper-based complexes, their evaluation in photocatalysis was performed and compared to analogous catalysts. Three mechanistically distinct photocatalytic transformations were pursued. In a visible-light Appel-type reaction (Figure 3) [28,29], the new BINAP-containing copper-based catalyst Cu(dpq)(BINAP)BF4 provided similar yields to other complexes having large-bite-angle bisphosphines. Note that control reactions performed in the absence of light or in the absence of catalyst at 450 nm did not afford any significant conversion to the alkyl bromide 2. However, as the π-surface of the ligands grew, the BINAP-containing complexes of dppz and bdppz were all inferior to analogous complexes having wide-bite-angle bisphosphines. Although the complexes of dppz and bdppz had larger excited-state reduction potentials, it is possible that the complexes with ligands with larger π-surfaces could be more unstable in solution. The stability of various copper complexes with diimines having large π-surfaces was previously shown to decrease with the size of the ligand in other photocatalytic processes [26]. However, it should be noted that amongst other BINAP-derived complexes, the Cu(dpq)(BINAP)BF4 (66% of 2) was superior in the Appel-type reaction to other structurally similar complexes such as Cu(dmp)(BINAP)BF4 (18% of 2) and Cu(phen)(BINAP)BF4 (45% of 2), suggesting that the dpq offered some beneficial reactivity (Figure 4).
Figure 3.
Comparison of the BINAP-containing copper complexes bearing π-extended ligands in a photochemical Appel-type process.
Figure 4.
Effects of the diimine ligand of heteroleptic copper complexes in a photochemical Appel-type process.
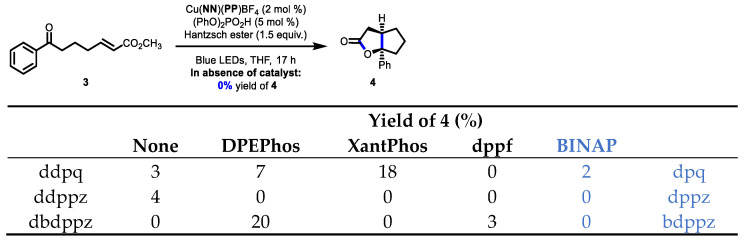
The BINAP-containing catalysts were then compared to the previous series being the ortho-substituted diimines in a reductive proton-coupled electron transfer (PCET) reaction. Our group has previously used the homolytic activation of ketones to benchmark complexes for their efficiency in a PCET process (Figure 5) [30] Previous evaluation with the ortho-substituted series revealed very poor reactivity and low yields (0–20% yield). Unfortunately, the screening with the new BINAP-containing complexes was equally disappointing. Recent work suggests that the process is in fact a reductive quenching of the Cu-based photocatalysts in the excited state [31,32] The electron-rich π-extended ligands would not be favorable in such a mechanism. Furthermore, given the results from the oxidative quenching in the Appel process, it is clear that the bisphosphine is not playing a significant role in altering the excited-state redox potentials of the resulting complexes.
Figure 5.
Comparison of the BINAP-containing copper complexes bearing π-extended ligands in a photochemical PCET process.
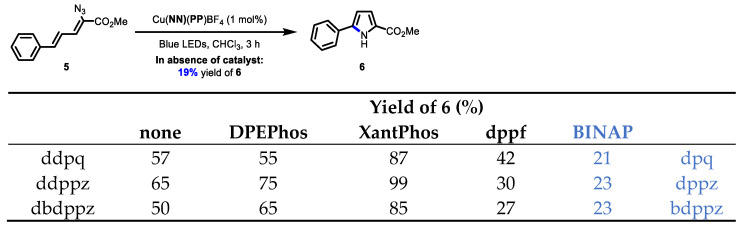
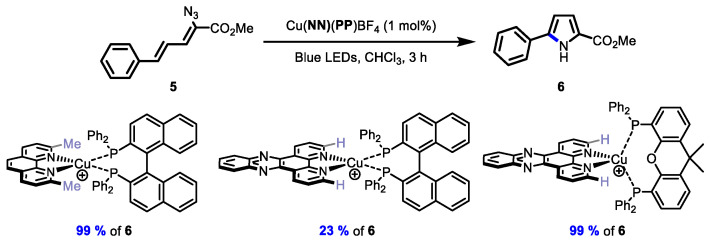
The last evaluation of the new BINAP-containing Cu-based photocatalysts was via energy transfer for the transformation of vinyl azides to the corresponding pyrrole (Figure 6) [33]. Given that the new complexes had neither wide-bite-angle phosphines or ortho-substituted diimines to stabilize the excited state, the yields of the pyrrole were expected to drop. Note that the excited-state lifetimes of the new BINAP-containing complexes were all approximately an order of magnitude less than analogous complexes (e.g., Cu(bdppz)(XantPhos)BF4 τ = 71 ns; Cu(dppz)(BINAP)BF4 τ = 1.8 ns). Indeed, the yields of the pyrrole 6 with the BINAP-containing complexes (21–23% of 6) were barely above the observed background reaction in the absence of any catalyst at 450 nm (19% of 6). A further comparison of Cu(phen)(BINAP)BF4 (38% of 6) and Cu(dpq)(BINAP)BF4 (21% of 6) showed that the dpq ligand had a deleterious effect on the energy transfer process. It should be noted that good yields of the pyrrole are possible when switching to any ligand known to extend the excited-state lifetimes (Figure 7). For example, using an ortho-substituted diimine ligand in a complex with BINAP affords quantitative yields of the product (Cu(dmp)(BINAP)BF4, 99% of 6). In addition, using a wide-bite-angle bisphosphine also affords a quantitative yield of 6 (Cu(dppz)(XantPhos)BF4, 99% of 6).
Figure 6.
Comparison of the BINAP-containing copper complexes bearing π-extended ligands in an energy transfer process.
Figure 7.
Ligand effects in heteroleptic copper complexes in an energy transfer process.
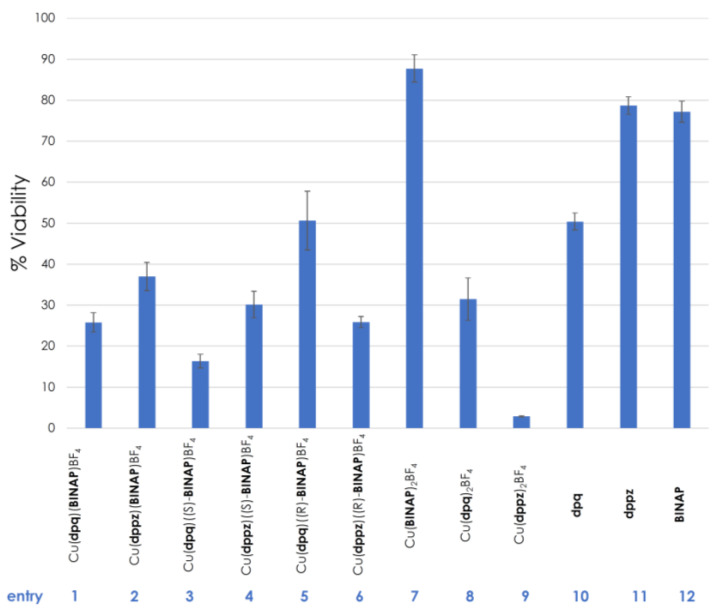
Given the recent interest in copper-containing complexes for medicinal chemistry [34,35,36], it was decided to test the most soluble of the new BINAP-containing complexes against triple-negative breast cancer cell lines (MBA-MB-231) (Figure 8). The viability of the cell lines was evaluated with the Cu(dpq)(BINAP)BF4 and Cu(dppz)(BINAP)BF4 complexes (5 μM, entries 1 and 2, respectively) and both displayed approximately 25–35% viability. Controls performed from the dpq, dppz, and BINAP ligands (entries 10–12) demonstrated that biological activity was originating from the metal complexes themselves. While a homoleptic complex Cu(BINAP)2BF4 was poorly active, the homoleptic complexes derived from the diimines showed low cell viabilities, with Cu(dppz)2BF4 being the most active of all complexes tested. Finally, given that the BINAP used in the above photocatalysis and biological evaluations was racemic, we prepared and evaluated the enantiomer variants of the dpq- and dppz-containing complexes. Interestingly, for the dpq complexes, the (S)-BINAP-containing complex Cu(dpq)((S)-BINAP)BF4 was approximately twice as active as the analogous (R)-BINAP complex. The copper complexes of dppz bearing either (S)- or (R)-BINAP did not show any difference in activity. The complex [Cu(MeCN)4]BF4 (10 µM) had negligible effects on cell viability (>75%), indicating that the complexes, rather than free copper, were responsible for biological activity.
Figure 8.
Viability of MDA-MB-231 cells at 5 µM. MDA-MB-231 cells were seeded at the density of 7000 cells per well in 96-well plates; plates were incubated overnight. The cells were then treated with growth media containing 5 µM of the copper complexes (entries 1–9) or controls (entries 10–12) and allowed to incubate at 37 °C for 72 h. The viabilities of cells were finally determined by MTT assay and converted to percentages. Data are an average of three different experiments.
In summary, a series of copper-based photocatalysts of the type Cu(NN)(BINAP)BF4 were synthesized bearing π-extended diimine ligands. Their behavior in several photocatalytic processes was evaluated and revealed the following:
Copper-based complexes derived from BINAP with π-extended diimine ligands without ortho-substitution did not show significant different photophysical properties when compared to analogous complexes with the exception of the excited state lifetime, which decreased by approximately an order of magnitude.
The new BINAP-containing complexes were active in the visible-light Appel-type process, with the Cu(dpq)(BINAP)BF4 complex having slightly better activity than analogous complexes derived from phen of dmp ligands.
The new BINAP-derived complexes did not afford complexes active for a PCET process.
In an energy transfer process, high yields of the desired product could be obtained with either BINAP or the dpq, dppz, and ddppz diimines through judicious choice of the accompanying ligand. For example, Cu(dmp)(BINAP)BF4 and Cu(dppz)(XantPhos)BF4 afforded quantitative yields of the product.
In addition to the photocatalysis, the copper complexes were evaluated for the first time in a medicinal chemistry context against triple-negative breast cancer cell lines. Controls indicated that copper complexes, and not their ligands, were responsible for activity. Encouraging activity was displayed by a homoleptic complex Cu(dppz)2BF4.
Acknowledgments
This manuscript is dedicated to Steve Hanessian for his outstanding contributions to synthesis and medicinal chemistry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27123745/s1, experimental procedures, photophysical data and NMR and mass spectra [37,38,39,40,41].
Author Contributions
Conceptualization, S.K.C. and J.K.; biotesting, S.G.; synthesis and catalysis, C.C.; writing and draft preparation, S.K.C., J.K., S.G. and C.C. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Samples of the compounds are not available from the authors.
Conflicts of Interest
No conflict of interest.
Funding Statement
The research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC, Discovery 1043344 and RGPIN-2020-05006), Université de Montréal, Concordia University and the Fonds de recherche Nature et technologie via the Centre in Green Chemistry and Catalysis (FRQNT-2020-RS4-265155-CCVC). JJK and SG gratefully acknowledge the National Science Foundation (CHE 1800395, CHE 1764235) for funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hossain A., Bhattacharyya A., Reiser O. Copper’s rapid ascent in visible-light photoredox catalysis. Science. 2019;364:6439–6450. doi: 10.1126/science.aav9713. [DOI] [PubMed] [Google Scholar]
- 2.Albini A., Fagnoni M., editors. Handbook of Synthetic Photochemistry. Wiley-VCH; Weinheim, Germany: 2010. [Google Scholar]
- 3.Teply F. Chemical Photocatalysis. Volume 111 De Gruyter; Berlin, Germany: 2013. The twentieth century roots. [Google Scholar]
- 4.Nagib D.A., Scott M.E., MacMillan D.W.C. Enantioselective α-Trifluoromethylation of Aldehydes via Photoredox Organocatalysis. J. Am. Chem. Soc. 2009;131:10875. doi: 10.1021/ja9053338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwendiman D.P., Kutal C. Catalytic role of copper(I) in the photoassisted valence isomerization of norbornadiene. J. Am. Chem. Soc. 1977;99:5677. doi: 10.1021/ja00459a025. [DOI] [Google Scholar]
- 6.Hertel R., Mattay J., Runsink J. Cycloadditions. 30. Copper(I)-catalyzed intramolecular diene-diene cycloaddition reactions and rearrangements. J. Am. Chem. Soc. 1991;113:657. doi: 10.1021/ja00002a039. [DOI] [Google Scholar]
- 7.Mitani M., Nakayama M., Koyama K. The cuprous chloride catalyzed addition of halogen compounds to olefins under photo-irradiation. Tetrahedron Lett. 1980;21:4457. doi: 10.1016/S0040-4039(00)92199-3. [DOI] [Google Scholar]
- 8.Do H.-Q., Bachman S., Bissember A.C., Peters J.C., Fu G.C. Photoinduced, copper-catalyzed alkylation of amides with unactivated secondary alkyl halides at room temperature. J. Am. Chem. Soc. 2014;136:2162. doi: 10.1021/ja4126609. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler D.T., Choi J., Muçoz-Molina J.M., Bissember A.C., Peters J.C., Fu G.C. A Versatile Approach to Ullmann C–N Couplings at Room Temperature: New Families of Nucleophiles and Electrophiles for Photoinduced, Copper-Catalyzed Processes. J. Am. Chem. Soc. 2013;135:13107. doi: 10.1021/ja4060806. [DOI] [PubMed] [Google Scholar]
- 10.Paria S., Reiser O. Copper in Photocatalysis. ChemCatChem. 2014;6:2477. doi: 10.1002/cctc.201402237. [DOI] [Google Scholar]
- 11.Kern J.-M., Sauvage J.-P. Photoassisted C–C coupling via electron transfer to benzylic halides by a bis(di-imine) copper(I) complex. J. Chem. Soc. Chem. Commun. 1987;546:546–548. doi: 10.1039/C39870000546. [DOI] [Google Scholar]
- 12.Pirtsch M., Paria S., Matsuno T., Isobe H., Reiser O. [Cu(dap)2Cl] As an Efficient Visible-Light-Driven Photoredox Catalyst in Carbon–Carbon Bond-Forming Reactions. Chem. Eur. J. 2012;18:7336–7340. doi: 10.1002/chem.201200967. [DOI] [PubMed] [Google Scholar]
- 13.Prentice C., Morrisson J., Smith A.D., Zysman-Colman E. Recent developments in enantioselective photocatalysis. Beilstein J. Org. Chem. 2020;16:2363–2441. doi: 10.3762/bjoc.16.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandroni M., Pellegrin Y., Odobel F.C.R. Heteroleptic bis-diimine copper(I) complexes for applications in solar energy conversion. Comptes Rendus Chim. 2016;19:79. doi: 10.1016/j.crci.2015.06.008. [DOI] [Google Scholar]
- 15.Luo S.-P., Meja E., Friedrich A., Pazidis A., Junge H., Surkus A.-E., Jackstell R., Denurra S., Gladiali S., Lochbrunner S., et al. Photocatalytic Water Reduction with Copper-Based Photosensitizers: A Noble-Metal-Free System. Angew. Chem. Int. Ed. 2013;52:419. doi: 10.1002/anie.201205915. [DOI] [PubMed] [Google Scholar]
- 16.Armaroli N., Accorsi G., Holler M., Moudam O., Nierengarten J.F., Zhou Z., Wegh R.T., Welter R., Armaroli N., Accorsi G., et al. Highly Luminescent CuI Complexes for Light-Emitting Electrochemical Cells. Adv. Mater. 2006;18:1313. doi: 10.1002/adma.200502365. [DOI] [Google Scholar]
- 17.Cuttell D.G., Kuang S.-M., Fanwick P.E., McMillin D.R., Walton R.A. Simple Cu(I) Complexes with Unprecedented Excited-State Lifetimes. J. Am. Chem. Soc. 2002;124:6. doi: 10.1021/ja012247h. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Perez A.C., Collins S.K. A visible-light-mediated synthesis of carbazoles. Angew. Chem. Int. Ed. 2013;52:12696. doi: 10.1002/anie.201306920. [DOI] [PubMed] [Google Scholar]
- 19.Beatty J.W., Stephenson C.R.J. Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis. Acc. Chem. Res. 2015;48:1474. doi: 10.1021/acs.accounts.5b00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prier C.K., Rankic D.A., MacMillan D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013;113:5322. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arias-Rotondo D.M., McCusker J.K. The photophysics of photoredox catalysis: A roadmap for catalyst design. Chem. Soc. Rev. 2016;45:5803. doi: 10.1039/C6CS00526H. [DOI] [PubMed] [Google Scholar]
- 22.Gentry E.C., Knowles R.R. Synthetic Applications of Proton-Coupled Electron Transfer. Acc. Chem. Res. 2016;49:1546. doi: 10.1021/acs.accounts.6b00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knorn M., Rawner T., Czerwieniec R., Reiser O. [Copper(phenanthroline)(bisisonitrile)]+-Complexes for the Visible-Light-Mediated Atom Transfer Radical Addition and Allylation Reactions. ACS Catal. 2015;5:5186. doi: 10.1021/acscatal.5b01071. [DOI] [Google Scholar]
- 24.Hernandez-Perez A.C., Collins S.K. Heteroleptic Cu-Based Sensitizers in Photoredox Catalysis. Acc. Chem. Res. 2016;49:1557. doi: 10.1021/acs.accounts.6b00250. [DOI] [PubMed] [Google Scholar]
- 25.Cruché C., Neiderer W., Collins S.K. Heteroleptic Copper-Based Complexes for Energy-Transfer Processes: E → Z Isomerization and Tandem Photocatalytic Sequences. ACS Catal. 2021;11:8829–8836. doi: 10.1021/acscatal.1c01983. [DOI] [Google Scholar]
- 26.Sosoe J., Cruché C., Morin É., Collins S.K. Evaluating Heteroleptic Copper(I)-Based Complexes Bearing π-Extended Diimines in Different Photocatalytic Processes. Can. J. Chem. 2020;98:461–465. doi: 10.1139/cjc-2020-0014. [DOI] [Google Scholar]
- 27.Guo W., Engelman B.J., Haywood T.L., Blok N.B., Beaudoin D.S., Obare S.O. Dual fluorescence and electrochemical detection of the organophosphorus pesticides-ethion, malathion and fenthion. Talanta. 2011;87:276–283. doi: 10.1016/j.talanta.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Chunhui Dai C., Narayanam J.M.R., Stephenson C.R.J. Visible-light-mediated conversion of alcohols to halides. Nat. Chem. 2011;3:140. doi: 10.1038/nchem.949. [DOI] [PubMed] [Google Scholar]
- 29.Minozzi C., Grenier-Petel J.-C., Parisien-Collette S., Collins S.K. Photocatalyic Appel reaction enabled by copper-based complexes in continuous flow. Beilstein J. Org. Chem. 2018;14:2730. doi: 10.3762/bjoc.14.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarantino K.T., Liu P., Knowles R.R. Catalytic Ketyl-Olefin Cyclizations Enabled by Proton-Coupled Electron Transfer. J. Am. Chem. Soc. 2013;135:10022–10025. doi: 10.1021/ja404342j. [DOI] [PubMed] [Google Scholar]
- 31.Caron A., Morin É., Collins S.K. Bifunctional Copper-Based Photocatalyst for Reductive Pinacol-Type Couplings. ACS Catal. 2019;9:9458. doi: 10.1021/acscatal.9b01718. [DOI] [Google Scholar]
- 32.Michelet B., Deldaele C., Kajouj S., Moucheron C., Evano G. A General Copper Catalyst for Photoredox Transformations of Organic Halides. Org. Lett. 2017;19:3576. doi: 10.1021/acs.orglett.7b01518. [DOI] [PubMed] [Google Scholar]
- 33.Farney E.P., Yoon T.P. Visible-Light Sensitization of Vinyl Azides by Transition-Metal Photocatalysis. Angew. Chem. Int. Ed. 2014;53:793–797. doi: 10.1002/anie.201308820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith C.B., Days L.C., Alajroush D.R., Faye K., Khodour Y., Beebe S.J., Holder A.A. Photodynamic Therapy of Inorganic Complexes for the Treatment of Cancer. Photochem. Photobiol. 2022;98:17–41. doi: 10.1111/php.13467. [DOI] [PubMed] [Google Scholar]
- 35.Ruan Y., Jia X., Wang C., Zhen W., Jiang X. Methylene Blue Loaded Cu-Tryptone Complex Nanoparticles: A New Glutathione-Reduced Enhanced Photodynamic Therapy Nanoplatform. ACS Biomater. Sci. Eng. 2019;5:1016–1022. doi: 10.1021/acsbiomaterials.8b01398. [DOI] [PubMed] [Google Scholar]
- 36.Devi L.R., Raza M.K., Musib D., Roy M. Selenonaphthaquinone-Based Copper (II) Complexes as the Next-Generation Photochemotherapeutic Agents. Anti-Cancer Agents Med. Chem. 2021;21:33–41. doi: 10.2174/1871520620999200727204237. [DOI] [PubMed] [Google Scholar]
- 37.Wu W., Ji S., Wu W., Shao J., Guo H., James T.D., Zhao J. Ruthenium(II)–Polyimine–Coumarin Light-Harvesting Molecular Arrays: Design Rationale and Application for Triplet–Triplet-Annihilation-Based Upconversion. Chem. Eur. J. 2012;18:4953–4964. doi: 10.1002/chem.201101377. [DOI] [PubMed] [Google Scholar]
- 38.Kitagawa Y., Kumagai M., Nakanishi T., Fushimi K., Hasegawa Y. First aggregation-induced emission of a Tb(iii) luminophore based on modulation of ligand–ligand charge transfer bands. Dalton Trans. 2020;49:2431–2436. doi: 10.1039/D0DT00094A. [DOI] [PubMed] [Google Scholar]
- 39.Roy N., Sen U., Ray Chaudhuri S., Muthukumar V., Moharana P., Paira P., Bose B., Gauthaman A., Moorthy A. Mitochondria specific highly cytoselective iridium(iii)–Cp* dipyridophenazine (dppz) complexes as cancer cell imaging agents. Dalton Trans. 2021;50:2268–2283. doi: 10.1039/D0DT03586F. [DOI] [PubMed] [Google Scholar]
- 40.Barrientos L., Araneda C., Loeb B., Crivelli I.G. Synthesis, spectroscopic and electrochemical characterization of copper(I) complexes with functionalized pyrazino[2,3-f]-1,10-phenanthroline. Polyhedron. 2008;27:1287–1295. doi: 10.1016/j.poly.2007.12.014. [DOI] [Google Scholar]
- 41.Plutschack M.B., Seeberger P.H., Gilmore K. Visible-Light-Mediated Achmatowicz Rearrangement. Org. Lett. 2017;19:30–33. doi: 10.1021/acs.orglett.6b03237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Samples of the compounds are not available from the authors.