Abstract
Widespread COVID-19 vaccination is crucial for limiting the spread of SARS-CoV-2 and minimizing the risk of novel variants arising in the general population, especially in pregnant women. According to the publicly available research data, vaccination intentions vary significantly by country, with Romania among the European countries with the lowest vaccination rates. Thus, we sought to determine the scale of acceptance of the COVID-19 vaccination campaign among pregnant women in Romania, as well as the variables affecting their choices. A cross-sectional study was conducted on pregnant women referred to the Obstetrics and Gynecology Clinic of the Timisoara Municipal Emergency Hospital in Romania, where participants were asked to complete an online survey including standardized and unstandardized questionnaires indicating their willingness to receive a COVID-19 vaccine and the reasons for their willingness. Out of the 500 women who were requested to participate, there was a total of 345 validated questionnaires, with 184 vaccinated and 161 unvaccinated pregnant women. The statistically significant determinant factors for COVID-19 vaccination acceptance were the urban area of residence (OR = 0.86), having a higher level of education (OR = 0.81), the third trimester of pregnancy (OR = 0.54), trusting the government (OR = 0.83), being a frequent traveler (OR = 0.76), fearing the severity of COVID-19 (OR = 0.68), the higher availability of COVID-19 vaccines nearby (OR = 0.87), and seeing more people getting vaccinated (OR = 0.75). As there are no increased risks associated with SARS-CoV-2 immunization in pregnant women, the variables identified in this research are crucial in determining the acceptability of COVID-19 vaccines that should be addressed in this vulnerable group to increase vaccination rates.
Keywords: SARS-CoV-2, COVID-19, pregnancy vaccination, vaccination acceptance
1. Introduction
Coronavirus disease 2019 (COVID-19) is continuously spreading around the globe, despite the fact that exhaustive steps have been taken, including a universal vaccination campaign against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, which is anticipated to be the most effective preventative strategy for restricting the pandemic’s spread [1,2]. The pandemic is likely to continue to cause an important rise in mortality [3,4] and disrupt global communities and economies while generating a worldwide public health disaster that continues to spread and cause severe economic and social consequences [5,6]. The increase in incidence has prompted the adoption of new treatment methods, but with little effectiveness [7,8,9]. The development of a vaccine, in particular, was pursued as a more practical and effective means to prevent and terminate the spread of SARS-CoV-2 and its detrimental effects on healthcare systems and patients with special needs [10,11,12].
In the past year, the public’s focus has been concentrated on the improvement and adoption of a vaccine that can serve as a reliable and cost-effective preventive tool against infections and severe disease forms, and a variety of COVID-19 vaccines are either in the clinical trials phase or have been approved for emergency use in many countries [13,14]. While some laboratories and private businesses have manufactured vaccines against SARS-CoV-2 using well-known methods, others have pursued the development of genetically engineered vaccines [15]. Among the most popular ones, Pfizer/BioNTech (Reinbek, Germany), AstraZeneca (Oxford, UK), Moderna (Norwood, MA, USA), and Johnson & Johnson’s Janssen (New Brunswick, NJ, USA) COVID-19 vaccines are now approved and undergoing trials while being distributed all around the globe. Despite this, corporations were under immense pressure to accelerate the manufacturing process, which led to widespread skepticism over their effectiveness and safety [16,17,18]. However, the urgency of the situation demanded the quick implementation of global immunization procedures.
With the development of effective COVID-19 vaccines, herd immunity must be achieved by the widespread vaccination of the entire population. In addition to vaccination safety, efficacy, and cost-efficiency, public acceptability is a major factor in determining overall effectiveness [19]. Since the beginning of the vaccination campaign in late 2020, there have been more than 5 billion people fully vaccinated worldwide, accounting for a total of roughly 60% of the global population [20]. In spite of strong warnings, the acceptability of the COVID-19 vaccination among the remaining 40% differs significantly across nations and groups, with distinct sociodemographic factors [21]. Even among persons with chronic medical illnesses who are at a higher risk of negative consequences associated with SARS-CoV-2 infection, COVID-19 vaccination rates remain inadequate [22,23,24]. Despite the availability of vaccination facilities, the phrase “vaccine reluctant” is widely used to characterize those who are uncertain about or unwilling to follow the guidelines [25].
A spectrum of vaccine acceptability exists, ranging from a minority that vehemently opposes all vaccinations to a majority that is prepared to take all necessary immunizations [26]. Vaccine-hesitant individuals are a heterogeneous group with varying levels of uncertainty and worries [27,28]. This group is of particular interest to public health services, as many vaccine-hesitant individuals may be influenced to change their attitudes and behaviors if their fears are handled effectively and systemic barriers to accessing health services are removed. Individuals who are not addressed about their vaccination refusal are not likely to adjust their beliefs. In order to restore society to its pre-pandemic state, it is essential to comprehend the factors that impact vaccination acceptance among various socioeconomic groups—particularly pregnant women, with their specific vulnerabilities [29,30,31]. Therefore, the goal of this study was to investigate pregnant women’s perspectives on COVID-19 vaccination, with a particular focus on the variables behind COVID-19 vaccine acceptance, with the aim of addressing these factors to improve their acceptance.
2. Materials and Methods
2.1. Study Design and Participants
From 1 January 2022 to 1 May 2022, a cross-sectional study was conducted on pregnant outpatients at the Obstetrics and Gynecology Clinic of the Timisoara Municipal Emergency Hospital, associated with the University of Medicine and Pharmacy in Timisoara, Romania. Patients were told of the purpose and consequences of the research, and each patient signed a written informed consent form to be included in the present study. The surveys were delivered online, and data collection was performed based on complete answers received in parallel with paper records of the pregnant women followed at our clinic. All patients with a history of SARS-CoV-2 infection were excluded from the study, as well as incomplete questionnaires. Our research was conducted in accordance with the Helsinki Declaration’s guidelines for scientific studies involving human participants, and it was authorized by the Scientific Ethics Committee of the Timisoara Municipal Hospital on 23 December 2021 (code I-32467/23.12.2021).
2.2. Surveys and Variables
A convenience sampling strategy to calculate the appropriate sample size was employed, which was estimated to comprise at least 385 pregnant women, with a margin of error of 5% at a confidence level of 95%, and a vaccination rate estimate of 50% at the time of the research [32]. Out of the 500 women who were requested to participate, 412 consented to participate in the research and fill out our questionnaires, of whom 67 failed to provide consistent and complete answers, leaving a total of 345 validated questionnaires. Out of the remaining pregnant women included in the study, 184 were vaccinated, and 161 were unvaccinated.
There were three standardized questionnaires that were validated and translated into Romanian before being given to the participants, including the HADS (Hospital Anxiety and Depression Scale), SF-12 (Short-Form Health Survey), and CORE-OM (CORE Outcome Measure Questionnaire). The HADS test is a 14-item [33] tool used to assess depression and anxiety, including 7 questions designed to measure sadness and 7 questions designed to measure anxiety. Increased scores suggest a rise in anxiety and depressive symptoms, and a score of 11 or above indicates a clinical diagnosis. The SF-12 is a regularly used tool for assessing general health and health outcomes [34], and was originally used to assess health-related quality of life. The SF-12 is a 12-item physical and mental health assessment questionnaire. The summary scores for the physical and mental components were generated in line with predetermined criteria. A low score implies poor mental and physical health. The CORE-OM is a 34-item validated self-report questionnaire with a five-point scale ranging from “never” to “most of/always” [35]. All four aspects of women’s wellbeing, concerns and symptoms, everyday functioning, and risk/harm are documented. Mean and total scores were calculated to determine the level of global psychological discomfort. A higher score suggests improved health and less global suffering in terms of wellbeing, problems and symptoms, and everyday functioning. A high risk or harm score is indicative of increased psychological distress. Another set of unstandardized questions was created by the researchers with the aim of evaluating the perceptions of pregnant women towards accepting the COVID-19 vaccines. All surveyed questions were categorical “yes” or “no”.
2.3. Statistical Analysis
To perform descriptive and inferential statistics, the IBM SPSS Statistics for Windows, Version 26.0 (Armonk, NY, USA, IBM Corp), was utilized. Means and standard deviations were employed to describe continuous data, while absolute values and percentages were utilized to represent categorical variables. Student’s t-test was used to compare the mean values of the data examined in this investigation. In a multivariate backward stepwise logistic regression analysis, all variables with statistically significant differences between groups were included. Chi-squared and Fisher’s tests were used for proportional comparisons between the two research groups. The significance threshold was established at alpha = 0.05.
3. Results
3.1. Background and Obstetrical Characteristics
At the end of the study period, a total of 184 vaccinated and 161 unvaccinated pregnant women completed the given questionnaires, and were eligible for inclusion in the study. Table 1 describes a comparison between the two study groups in their baseline characteristics. It was observed that most of the patients were in the 25–34-year-old range (51.6% vaccinated and 47.8% unvaccinated; p-value = 0.772). A statistically significant difference in proportions was observed in the area of residence of participants, where 42.9% of unvaccinated participants were residing in rural areas of Romania, compared with only 31% in the vaccinated group (p-value = 0.022). In consequence, there was a higher frequency of low-income participants in the unvaccinated group (41.6% vs. 28.8%; p-value = 0.042). Other statistically significant differences were observed in the level of education of the participants, where 92 (50.0%) had higher education in the vaccinated group, compared with 50 (31.1%) in the unvaccinated group (p-value = 0.001). Moreover, frequent alcohol consumption was more common in the unvaccinated group (5.0% vs. 3.3%; p-value = 0.026). Lastly, it was observed that a majority of 163 (88.6%) were vaccinated with the BNT162b2 (Pfizer/BioNTech) mRNA vaccine.
Table 1.
Comparison in baseline characteristics between vaccinated and unvaccinated pregnant women.
| Variables * | Vaccinated (n = 184) | Unvaccinated (n = 161) | p |
|---|---|---|---|
| Age range (years) | 0.772 | ||
| <25 | 38 (20.7%) | 35 (21.7%) | |
| 25–34 | 95 (51.6%) | 77 (47.8%) | |
| >34 | 51 (27.7%) | 49 (30.4%) | |
| Area of Residence | 0.022 | ||
| Rural | 57 (31.0%) | 69 (42.9%) | |
| Urban | 127 (69.0%) | 92 (57.1%) | |
| Relationship Status | 0.182 | ||
| Married/concubinage | 171 (92.9%) | 143 (88.8%) | |
| Single/divorced/widowed | 13 (7.1%) | 18 (11.2%) | |
| Income | 0.042 | ||
| Low | 53 (28.8%) | 67 (41.6%) | |
| Medium | 98 (53.3%) | 72 (44.7%) | |
| High | 33 (17.9%) | 22 (13.7%) | |
| Education | 0.001 | ||
| Primary education | 10 (5.4%) | 16 (9.9%) | |
| High school | 82 (44.6%) | 95 (59.0%) | |
| Higher education | 92 (50.0%) | 50 (31.1%) | |
| Occupation | 0.740 | ||
| Employed/self-employed | 153 (83.2%) | 136 (84.5%) | |
| Unemployed | 31 (16.8%) | 25 (15.5%) | |
| Behavior | 0.026 | ||
| Frequent alcohol consumption | 6 (3.3%) | 8 (5.0%) | |
| Frequent smoker | 18 (9.8%) | 29 (18.0%) | |
| COVID-19 vaccine | |||
| BNT162b2 | 163 (88.6%) | - | - |
| mRNA-1273 | 18 (9.8%) | - | - |
| Ad26.COV2.S | 3 (1.6%) | - | - |
* Data reported as n (frequency) and calculated using the chi-squared test and Fisher’s exact test unless otherwise specified; BNT162b2—Pfizer/BioNTech; mRNA-1273—Moderna; Ad26.COV2.S—Johnson & Johnson.
A comparison of the obstetrical and medical history of vaccinated and unvaccinated pregnant women is described in Table 2. The majority of participants were in their first pregnancy (>50% in both groups), without noteworthy differences in gravidity and parity. A statistically significant difference was observed in the trimester of pregnancy of the studied women, where 88 (47.8%) in the third trimester were vaccinated, compared to 48 (29.8%) unvaccinated in the third trimester of pregnancy (p-value = 0.002). Other variables—such as pregnancy-associated complications, body mass index, history of pregnancy loss, and comorbidities—did not have significant differences in proportions between the two study groups. The most common complaints of the studied women were respiratory and cardiovascular, including asthma and high blood pressure. Patients were also screened for a history of depression, without significant differences (3.3% in the vaccinated group, compared with 3.7% in the unvaccinated group; p-value = 0.813).
Table 2.
Comparison of obstetrical and medical history between vaccinated and unvaccinated pregnant women.
| Variables * | Vaccinated (n = 184) | Unvaccinated (n = 161) | p |
|---|---|---|---|
| Gravidity | 0.754 | ||
| 1 | 94 (51.1%) | 88 (54.7%) | |
| 2 | 72 (39.1%) | 60 (37.3%) | |
| ≥3 | 18 (9.8%) | 13 (8.1%) | |
| Parity | 0.881 | ||
| 1 | 99 (53.8%) | 90 (55.9%) | |
| 2 | 74 (40.2%) | 63 (39.1%) | |
| ≥3 | 11 (6.0%) | 8 (5.0%) | |
| Trimester of pregnancy | 0.002 | ||
| 1 | 32 (17.4%) | 40 (24.8%) | |
| 2 | 64 (34.8%) | 73 (45.3%) | |
| 3 | 88 (47.8%) | 48 (29.8%) | |
| Pregnancy-associated complications ** | 0.362 | ||
| 0 | 147 (79.9%) | 138 (85.7%) | |
| 1 | 32 (17.4%) | 20 (12.4%) | |
| ≥ 2 | 5 (2.7%) | 3 (1.9%) | |
| Body mass index *** | 0.780 | ||
| Normal weight | 136 (73.9%) | 124 (77.0%) | |
| Overweight | 30 (16.3%) | 24 (14.9%) | |
| Obese | 18 (9.8%) | 13 (8.1%) | |
| History of pregnancy loss | 0.371 | ||
| None | 146 (79.3%) | 135 (83.9%) | |
| Medical abortion | 6 (3.3%) | 8 (5.0%) | |
| Stillbirth (<20 weeks) | 13 (7.1%) | 7 (4.3%) | |
| Miscarriage (>20 weeks) | 19 (10.3%) | 11 (6.8%) | |
| Comorbidities | |||
| Cardiovascular | 7 (3.8%) | 4 (2.5%) | 0.486 |
| Metabolic | 4 (2.2%) | 4 (2.5%) | 0.848 |
| Autoimmune | 3 (1.6%) | 1 (0.6%) | 0.382 |
| Respiratory | 9 (4.9%) | 7 (4.3%) | 0.810 |
| Other | 4 (2.2%) | 2 (1.2%) | 0.508 |
| History of depression | 6 (3.3%) | 6 (3.7%) | 0.813 |
* Data reported as n (frequency) and calculated using the chi-squared test and Fisher’s exact test unless otherwise specified. ** Including high blood pressure, gestational diabetes, infections, and preeclampsia. *** Adjusted for the month of pregnancy.
3.2. Standardized and Unstandardized Questionnaires
The studied participants were asked to complete a series of standardized and unstandardized surveys. Based on the hypothesis that higher levels of anxiety and depression are associated with COVID-19, hospitals are likely to determine COVID-19 vaccination. It was observed that vaccinated pregnant women scored statistically significantly higher average HADS anxiety scores (7.3 vs. 6.2; p-value = 0.012), HADS depression scores (6.4 vs. 5.1; p-value < 0.001), and HADS total scores (12.5 vs. 10.2; p-value < 0.001). However, there were no significant differences in completed answers for the SF-12 and CORE-OM surveys (Table 3).
Table 3.
Comparison of standardized questionnaires between vaccinated and unvaccinated pregnant women.
| Variables * | Vaccinated (n = 184) | Unvaccinated (n = 161) | p |
|---|---|---|---|
| HADS | |||
| Anxiety | 7.3 ± 4.1 | 6.2 ± 4.0 | 0.012 |
| Depression | 6.4 ± 3.5 | 5.1 ± 2.9 | <0.001 |
| Total score | 12.5 ± 5.4 | 10.2 ± 4.6 | <0.001 |
| SF-12 | |||
| Physical | 56.3 ± 7.5 | 55.1 ± 7.0 | 0.127 |
| Mental | 53.8 ± 9.1 | 52.4 ± 8.8 | 0.148 |
| Total score | 55.2 ± 8.3 | 53.7 ± 7.6 | 0.082 |
| CORE-OM | |||
| CORE-W | 0.89 ± 0.58 | 0.93 ± 0.62 | 0.536 |
| CORE-P | 0.71 ± 0.57 | 0.77 ± 0.58 | 0.334 |
| CORE-F | 0.63 ± 0.53 | 0.71 ± 0.56 | 0.174 |
| CORE-R | 0.26 ± 0.14 | 0.28 ± 0.11 | 0.145 |
| Total score | 0.80 ± 0.52 | 0.84 ± 0.55 | 0.488 |
* Data reported as n (frequency) and calculated using the chi-squared test and Fisher’s exact test unless otherwise specified; HADS—Hospital Anxiety and Depression Scale; SF-12—Short-Form Health Survey; CORE-OM—CORE Outcome Measure Questionnaire.
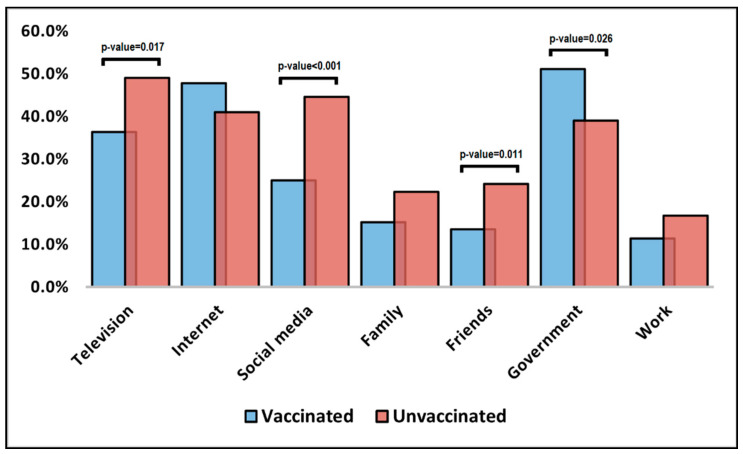
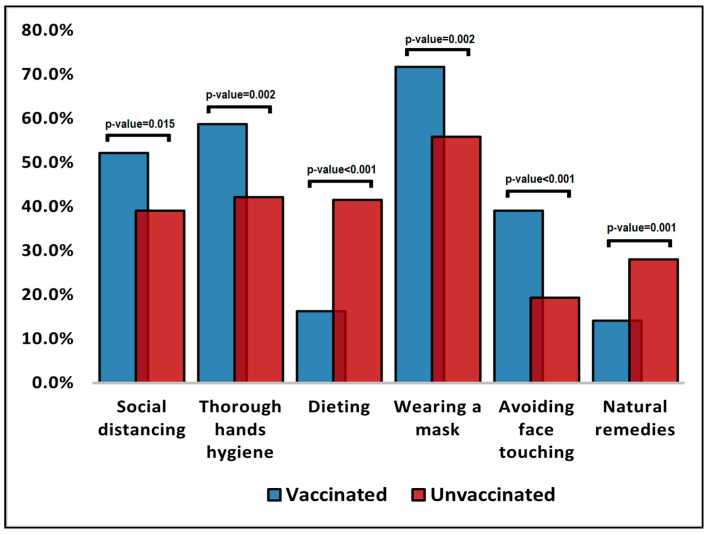
The comparison of unstandardized surveyed questions for pregnant women and their willingness to get vaccinated against COVID-19 is presented in Table 4 and in Figure 1 and Figure 2, respectively. It was observed that unvaccinated pregnant women were significantly more likely to choose television (49.1% vs. 36.4%), social media (44.7% vs. 25.0%), and friends (24.2% vs. 13.6%) as trustworthy decision factors (Figure 1). Conversely, vaccinated pregnant women were statistically significantly more likely to trust the government (51.1% vs. 39.1%; p-value = 0.026). The patients’ beliefs over factors that can prevent COVID-19 were significantly different in proportions between all surveyed questions (Figure 2). It was observed that vaccinated pregnant women were more likely to believe that social distancing, thorough hand hygiene, wearing a mask, and avoiding face touching are important in preventing COVID-19.
Table 4.
Comparison of unstandardized questionnaires between vaccinated and unvaccinated pregnant women.
| Variables * | Vaccinated (n = 184) | Unvaccinated (n = 161) | p |
|---|---|---|---|
| Influencing factors | |||
| Television | 67 (36.4%) | 79 (49.1%) | 0.017 |
| Internet | 88 (47.8%) | 66 (41.0%) | 0.202 |
| Social media | 46 (25.0%) | 72 (44.7%) | <0.001 |
| Family | 28 (15.2%) | 36 (22.4%) | 0.088 |
| Friends | 25 (13.6%) | 39 (24.2%) | 0.011 |
| Government | 94 (51.1%) | 63 (39.1%) | 0.026 |
| Work | 21 (11.4%) | 27 (16.8%) | 0.151 |
| Beliefs over factors that can prevent COVID-19 | |||
| Social distancing | 96 (52.2%) | 63 (39.1%) | 0.015 |
| Thorough hand hygiene | 108 (58.7%) | 68 (42.2%) | 0.002 |
| Dieting | 30 (16.3%) | 67 (41.6%) | <0.001 |
| Wearing a mask | 132 (71.7%) | 90 (55.9%) | 0.002 |
| Avoiding face touching | 72 (39.1%) | 31 (19.3%) | <0.001 |
| Natural remedies | 26 (14.1%) | 45 (28.0%) | 0.001 |
* Data reported as n (frequency) and calculated using the chi-squared test and Fisher’s exact test unless otherwise specified.
Figure 1.
Influencing factors for COVID-19 vaccination.
Figure 2.
Beliefs over factors that can prevent COVID-19.
3.3. Factors for Acceptance
Another set of questions meant to determine COVID-19 vaccination acceptance is presented in Table 5, where patients were asked what they care about, what their concerns are, and what they believe their decision was based on. Vaccinated pregnant women were more likely to care about putting an end to the pandemic, traveling without restrictions, the severity of SARS-CoV-2 infection, COVID-19 vaccination becoming mandatory, and what doctors recommend. On the other hand, unvaccinated pregnant women were more concerned about vaccination side effects, efficacy, and the quality of vaccines delivered to Romania, while they also believed in a significantly higher proportion that COVID-19 is a conspiracy (23.0% vs. 6.0%; p-value < 0.001). Furthermore, the group of unvaccinated pregnant women answered that they might be influenced by the higher availability of COVID-19 vaccines nearby (42.2% vs. 22.3%; p-value < 0.001) and seeing more people getting vaccinated (35.4% vs. 6.5%; p-value < 0.001).
Table 5.
Assessment of reasons for COVID-19 vaccine acceptance.
| Questions | Vaccinated (n = 184) | Unvaccinated (n = 161) | p |
|---|---|---|---|
| “I care about” | |||
| Putting an end to the pandemic | 166 (90.2%) | 129 (80.1%) | 0.007 |
| Allowing life to return to normal | 124 (67.4%) | 97 (60.2%) | 0.167 |
| Travelling without restrictions | 109 (59.2%) | 62 (38.5%) | <0.001 |
| The severity and complications of COVID-19 | 127 (69.0%) | 56 (34.8%) | <0.001 |
| COVID-19 vaccines becoming mandatory | 88 (47.8%) | 50 (31.1%) | 0.001 |
| What doctors recommend | 70 (38.0%) | 44 (27.3%) | 0.034 |
| “I am concerned about” | |||
| Vaccination side effects | 51 (27.7%) | 84 (52.2%) | <0.001 |
| Vaccination efficacy | 48 (26.1%) | 74 (46.0%) | <0.001 |
| COVID-19 being a conspiracy | 11 (6.0%) | 37 (23.0%) | <0.001 |
| Vaccination being against my religion | 8 (4.3%) | 12 (7.5%) | 0.218 |
| The quality of vaccines received by my country | 15 (8.2%) | 31 (19.3%) | 0.002 |
| Vaccine efficacy against new SARS-CoV-2 strains | 19 (10.3%) | 24 (14.9%) | 0.198 |
| The technology of COVID-19 vaccines | 60 (32.6%) | 87 (54.0%) | |
| “I might be influenced by” | |||
| Availability of vaccines near me | 41 (22.3%) | 68 (42.2%) | <0.001 |
| Seeing better results against new SARS-CoV-2 infections | 45 (24.5%) | 53 (32.9%) | 0.082 |
| Seeing more people getting vaccinated | 26 (14.1%) | 57 (35.4%) | <0.001 |
| Clinical trials’ results | 12 (6.5%) | 9 (5.6%) | 0.718 |
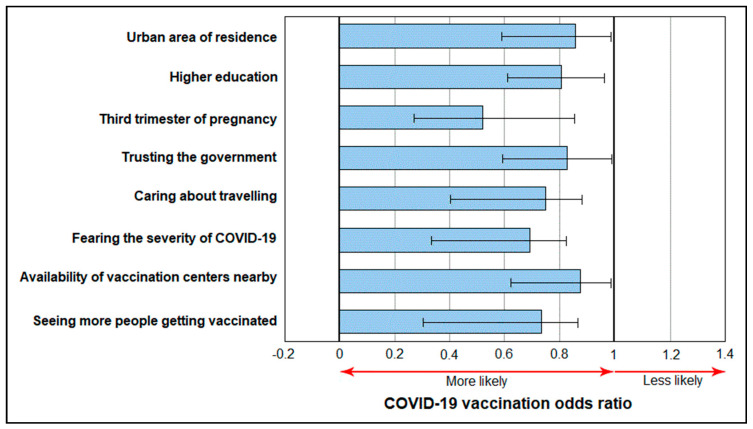
The analysis of determinants for COVID-19 vaccine acceptance among pregnant women is described in Table 6, where a series of independent factors were observed as determining a higher likelihood of vaccination acceptance (0 > OR < 1). Among these statistically significant factors were the urban area of residence (OR = 0.86), having a higher education (OR = 0.81), the third trimester of pregnancy (OR = 0.54), trusting the government (OR = 0.83), caring about traveling (OR = 0.76), fearing the severity of COVID-19 (OR = 0.68), the higher availability of COVID-19 vaccines nearby (OR = 0.87), and seeing more people getting vaccinated (OR = 0.75), as presented in Figure 3.
Table 6.
Analysis of determinants for COVID-19 vaccine acceptance among pregnant women.
| Determinants | OR * | 95% CI | p-Value |
|---|---|---|---|
| Urban area of residence | 0.86 | 0.59–0.98 | 0.043 |
| Higher education | 0.81 | 0.62–0.95 | 0.030 |
| Third trimester of pregnancy | 0.54 | 0.28–0.86 | <0.001 |
| Trusting the government | 0.83 | 0.59–0.99 | 0.047 |
| Caring about travelling | 0.76 | 0.40–0.87 | 0.005 |
| Fearing the severity of COVID-19 | 0.68 | 0.34–0.82 | 0.001 |
| Availability of vaccination centers nearby | 0.87 | 0.63–0.99 | 0.045 |
| Seeing more people getting vaccinated | 0.75 | 0.33–0.88 | <0.001 |
* OR—odds ratio (a value between 0 and 1 indicating a protective effect).
Figure 3.
Determinant factors of the likelihood of COVID-19 vaccination.
4. Discussion
The present study managed to identify several factors that are believed to determine the acceptance of COVID-19 vaccines among pregnant women or to be associated with women who choose to vaccinate. To date, there have been several studies researching COVID-19 vaccination hesitancy and acceptance in the general population or among certain categories of people, while fewer had the purpose of studying the determining factors for acceptance among pregnant women in Romania, as a country with a COVID-19 vaccination campaign that did not develop adequately compared with the other countries in the European Union community. Therefore, as of May 2022, Malta had the highest COVID-19 immunization rate in Europe, having provided 248 doses per 100 persons, while Romania was the second-least vaccinated country in Europe, behind Bulgaria, with just 87 doses per 100 persons [36].
Although several studies reported a higher vaccination acceptance among women compared to the opposite gender, pregnant women are more reluctant due to their pregnancy status and fears with regard to the implications vaccines might have for the unborn child. As such, a recent study by Citu et al. [16] using the Vaccination Attitudes Examination (VAX) scale found that the pregnant women who completed the survey had much more reluctant responses than the non-pregnant group, with pregnant women recording significantly more hesitant responses (52% vs. 40%). They exhibited considerably higher average scores on all subscales of the VAX scale, and 78.1% attributed their COVID-19 vaccination choice to social media, compared to 64.0% of non-pregnant women. The independent risk variables for reluctance were found to be the lack of fear of COVID-19, a below-average level of income, trusting social media, a lack of belief in the existence of SARS-CoV-2, and a general lack of belief in vaccines and how they work.
Another research determined that numerous pregnant women understand the means of being safe and healthy but do not take the necessary precautions. The decision of a pregnant woman to participate in the vaccination campaign or take it seriously depends on her perceptions of the dangers caused by SARS-CoV-2 infection and the efficacy of immunization. Regardless of whether they were vulnerable, pregnant women who declined COVID-19 vaccines were observed to deny the danger of the virus or seeing any advantages from the immunizations currently in use. Acceptance was determined to be positively correlated with belief in vaccination’s advantages and self-reported patient outcomes [37].
Since pregnancy is considered to be a medical condition, pregnant women cannot participate in clinical studies of COVID-19 vaccination [38]. Thus, parents worry about the health of their unborn children due to the paucity of information about the safety and efficacy of the vaccine during pregnancy. The unknowns of the new mRNA vaccines have not been thoroughly evaluated during pregnancy in large cohorts of patients, and preliminary studies are insufficient to provide guidance for pregnant women, even though the medical advice is currently promoting vaccination [39]. Similar findings were observed in our study, where unvaccinated respondents were more likely to answer that they do not trust the efficacy of these vaccines, and that they are more concerned about the side effects.
In order to overcome these obstacles, awareness initiatives must be strengthened in order to increase the possibility of acceptance among pregnant women. It is vital to increase the perception of risk and severity among both pregnant and non-pregnant women [40]. It is vital that awareness campaigns address the concerns of pregnant women about the safety and efficacy of the vaccination during pregnancy, in order to reduce their perception of these obstacles [41]. Concerning safety, the obstetric population must be reassured that COVID-19 vaccines are not live vaccines, which are generally avoided during pregnancy because they can harm the developing fetus, and that there should be no increased reaction beyond what is anticipated [42].
The current research is restricted to a population-based analysis, so the findings of the questionnaires for pregnant women in Romania may not be relevant to other locations with different perceptions about SARS-CoV-2, as COVID-19 vaccination rates might vary significantly. Other country-specific factors that seemed to influence vaccination included rural origin, below-average income, and low levels of education, all of which are more frequent in Romania than in the rest of the EU. Other limitations include the study’s online design, as well as its failure to fulfill the estimated ideal sample size.
5. Conclusions
There are no increased dangers of immunization in pregnant women beyond what is anticipated. Additionally, no harmful maternal or fetal abnormalities were seen after the vaccination of pregnant women with COVID-19 vaccines. The vaccines now available are equally effective for pregnant and non-pregnant women. The claims that SARS-CoV-2 vaccines may cause infertility or impair embryonic development are unfounded, and there is no need for pregnant women to be concerned about their safety. Therefore, the factors identified in this study as important for determining the acceptance of COVID-19 vaccination should be addressed in this sensitive population to improve vaccination rates.
Author Contributions
Conceptualization, C.C. and V.D.C.; methodology, C.C. and V.D.C.; software, I.M.C. and O.M.G.; validation, I.M.C. and O.M.G.; formal analysis, F.B. and B.B.; investigation, F.B. and B.B.; resources, D.-E.P.; data curation, D.-E.P.; writing—original draft preparation, C.C. and V.D.C.; writing—review and editing, A.R. and O.B.; visualization, A.R. and O.B.; supervision, F.G.; project administration, F.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the University of Medicine and Pharmacy “Victor Babes” Timisoara (No. 7110/15.12.2021) and the Ethics Committee of the Timisoara Municipal Hospital (No. I-32467/23.12.2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhao Z.-Y., Niu Y., Luo L., Hu Q.-Q., Yang T.-L., Chu M.-J., Chen Q.-P., Lei Z., Rui J., Song C.-L., et al. The optimal vaccination strategy to control COVID-19: A modeling study in Wuhan City, China. Infect. Dis. Poverty. 2021;10:140. doi: 10.1186/s40249-021-00922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayouni I., Maatoug J., Dhouib W., Zammit N., Fredj S.B., Ghammam R., Ghannem H. Effective public health measures to mitigate the spread of COVID-19: A systematic review. BMC Public Health. 2021;21:1015. doi: 10.1186/s12889-021-11111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., Agha M., Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Citu I.M., Citu C., Gorun F., Sas I., Tomescu L., Neamtu R., Motoc A., Gorun O.M., Burlea B., Bratosin F., et al. Immunogenicity Following Administration of BNT162b2 and Ad26.COV2.S COVID-19 Vaccines in the Pregnant Population during the Third Trimester. Viruses. 2022;14:307. doi: 10.3390/v14020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryson H., Mensah F., Price A., Gold L., Mudiyanselage S.B., Kenny B., Dakin P., Bruce T., Noble K., Kemp L., et al. Clinical, financial and social impacts of COVID-19 and their associations with mental health for mothers and children experiencing adversity in Australia. PLoS ONE. 2021;16:e0257357. doi: 10.1371/journal.pone.0257357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogdan I., Citu C., Bratosin F., Malita D., Romosan I., Gurban C.V., Bota A.V., Turaiche M., Bratu M.L., Pilut C.N., et al. The Impact of Multiplex PCR in Diagnosing and Managing Bacterial Infections in COVID-19 Patients Self-Medicated with Antibiotics. Antibiotics. 2022;11:437. doi: 10.3390/antibiotics11040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garegnani L.I., Madrid E., Meza N. Misleading clinical evidence and systematic reviews on ivermectin for COVID-19. BMJ Evid. Based Med. 2021;27:156–158. doi: 10.1136/bmjebm-2021-111678. [DOI] [PubMed] [Google Scholar]
- 8.Tirnea L., Bratosin F., Vidican I., Cerbu B., Turaiche M., Timircan M., Margan M.-M., Marincu I. The Efficacy of Convalescent Plasma Use in Critically Ill COVID-19 Patients. Medicina. 2021;57:257. doi: 10.3390/medicina57030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han F., Liu Y., Mo M., Chen J., Wang C., Yang Y., Wu J. Current treatment strategies for COVID-19 (Review) Mol. Med. Rep. 2021;24:858. doi: 10.3892/mmr.2021.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang E.A.L., Chai P., Yu J., Fan X. Effects of cancer on patients with COVID-19: A systematic review and meta-analysis of 63,019 participants. Cancer Biol. Med. 2021;18:298–307. doi: 10.20892/j.issn.2095-3941.2020.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marincu I., Bratosin F., Vidican I., Bostanaru A.-C., Frent S., Cerbu B., Turaiche M., Tirnea L., Timircan M. Predictive Value of Comorbid Conditions for COVID-19 Mortality. J. Clin. Med. 2021;10:2652. doi: 10.3390/jcm10122652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerbu B., Pantea S., Bratosin F., Vidican I., Turaiche M., Frent S., Borsi E., Marincu I. Liver Impairment and Hematological Changes in Patients with Chronic Hepatitis C and COVID-19: A Retrospective Study after One Year of Pandemic. Medicina. 2021;57:597. doi: 10.3390/medicina57060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Citu I.M., Citu C., Margan M.-M., Craina M., Neamtu R., Gorun O.M., Burlea B., Bratosin F., Rosca O., Grigoras M.L., et al. Calcium, Magnesium, and Zinc Supplementation during Pregnancy: The Additive Value of Micronutrients on Maternal Immune Response after SARS-CoV-2 Infection. Nutrients. 2022;14:1445. doi: 10.3390/nu14071445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Z., Wang L., Pandey A., Lim W.W., Chinazzi M., Piontti A.P.Y., Lau E.H.Y., Wu P., Malani A., Cobey S., et al. Modeling comparative cost-effectiveness of SARS-CoV-2 vaccine dose fractionation in India. Nat. Med. 2022;28:934–938. doi: 10.1038/s41591-022-01736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinz F.X., Stiasny K. Distinguishing features of current COVID-19 vaccines: Knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Citu I.M., Citu C., Gorun F., Motoc A., Gorun O.M., Burlea B., Bratosin F., Tudorache E., Margan M.-M., Hosin S., et al. Determinants of COVID-19 Vaccination Hesitancy among Romanian Pregnant Women. Vaccines. 2022;10:275. doi: 10.3390/vaccines10020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honora A., Wang K.-Y., Chih W.-H. How does information overload about COVID-19 vaccines influence individuals’ vaccination intentions? The roles of cyberchondria, perceived risk, and vaccine skepticism. Comput. Hum. Behav. 2022;130:107176. doi: 10.1016/j.chb.2021.107176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Citu I.M., Citu C., Gorun F., Sas I., Bratosin F., Motoc A., Burlea B., Rosca O., Malita D., Gorun O.M. The Risk of Spontaneous Abortion Does Not Increase Following First Trimester mRNA COVID-19 Vaccination. J. Clin. Med. 2022;11:1698. doi: 10.3390/jcm11061698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolia V., Singh R.R., Deshpande S., Dave A., Rathod R.M. Understanding Factors to COVID-19 Vaccine Adoption in Gujarat, India. Int. J. Environ. Res. Public Health. 2022;19:2707. doi: 10.3390/ijerph19052707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritchie H., Mathieu E., Rodés-Guirao L., Appel C., Giattino C., Ortiz-Ospina E., Hasell J., Macdonald B., Beltekian D., Roser M. Coronavirus Pandemic (COVID-19). Published Online at OurWorldInData.org. 2020. [(accessed on 20 April 2022)]. Available online: https://ourworldindata.org/coronavirus.
- 21.Litaker J.R., Tamez N., Bray C.L., Durkalski W., Taylor R. Sociodemographic Factors Associated with Vaccine Hesitancy in Central Texas Immediately Prior to COVID-19 Vaccine Availability. Int. J. Environ. Res. Public Health. 2021;19:368. doi: 10.3390/ijerph19010368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fekadu G., Bekele F., Tolossa T., Fetensa G., Turi E., Getachew M., Abdisa E., Assefa L., Afeta M., Demisew W., et al. Impact of COVID-19 pandemic on chronic diseases care follow-up and current perspectives in low resource settings: A narrative review. Int. J. Physiol. Pathophysiol. Pharm. 2021;13:86–93. [PMC free article] [PubMed] [Google Scholar]
- 23.Khairat S., Zou B., Adler-Milstein J. Factors and reasons associated with low COVID-19 vaccine uptake among highly hesitant communities in the US. Am. J. Infect. Control. 2022;50:262–267. doi: 10.1016/j.ajic.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Citu I.M., Citu C., Gorun F., Neamtu R., Motoc A., Burlea B., Rosca O., Bratosin F., Hosin S., Manolescu D., et al. Using the NYHA Classification as Forecasting Tool for Hospital Readmission and Mortality in Heart Failure Patients with COVID-19. J. Clin. Med. 2022;11:1382. doi: 10.3390/jcm11051382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen J.D., Feng W., Corlin L., Porteny T., Acevedo A., Schildkraut D., King E., Ladin K., Fu Q., Stopka T.J. Why are some people reluctant to be vaccinated for COVID-19? A cross-sectional survey among U.S. Adults in May–June 2020. Prev. Med. Rep. 2021;24:101494. doi: 10.1016/j.pmedr.2021.101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alqudeimat Y., Alenezi D., AlHajri B., Alfouzan H., Almokhaizeem Z., Altamimi S., Almansouri W., Alzalzalah S., Ziyab A.H. Acceptance of a COVID-19 Vaccine and Its Related Determinants among the General Adult Population in Kuwait. Med. Princ. Pract. 2021;30:262–271. doi: 10.1159/000514636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saied S.M., Saied E.M., Kabbash I.A., Abdo S.A.E. Vaccine hesitancy: Beliefs and barriers associated with COVID-19 vaccination among Egyptian medical students. J. Med. Virol. 2021;93:4280–4291. doi: 10.1002/jmv.26910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekizawa Y., Hashimoto S., Denda K., Ochi S., So M. Association between COVID-19 vaccine hesitancy and generalized trust, depression, generalized anxiety, and fear of COVID-19. BMC Public Health. 2022;22:126. doi: 10.1186/s12889-021-12479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timircan M., Bratosin F., Vidican I., Suciu O., Turaiche M., Bota A.V., Mitrescu S., Marincu I. Coping Strategies and Health-Related Quality of Life in Pregnant Women with SARS-CoV-2 Infection. Medicina. 2021;57:1113. doi: 10.3390/medicina57101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galanis P., Vraka I., Siskou O., Konstantakopoulou O., Katsiroumpa A., Kaitelidou D. Uptake of COVID-19 Vaccines among Pregnant Women: A Systematic Review and Meta-Analysis. Vaccines. 2022;10:766. doi: 10.3390/vaccines10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timircan M., Bratosin F., Vidican I., Suciu O., Tirnea L., Avram V., Marincu I. Exploring Pregnancy Outcomes Associated with SARS-CoV-2 Infection. Medicina. 2021;57:796. doi: 10.3390/medicina57080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romanian Government Daily Update (13/03)—Records of People Vaccinated against COVID-19. [(accessed on 12 June 2022)];2022 Available online: https://vaccinare-covid.gov.ro/actualizare-zilnica-13-03-evidenta-persoanelor-vaccinate-impotriva-covid-19-3/
- 33.Zigmond A.S., Snaith R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 34.Ware J.E., Kosinski M., Keller S.D. A 12-item short-form health survey. Med. Care. 1996;34:220–223. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Skre I., Friborg O., Elgarøy S., Evans C., Myklebust L.H., Lillevoll K., Sørgaard K., Hansen V. The factor structure and psychometric properties of the Clinical Outcomes in Routine Evaluation—Outcome Measure (CORE-OM) in Norwegian clin-ical and non-clinical samples. BMC Psychiatry. 2013;13:99. doi: 10.1186/1471-244X-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Statista Number of COVID-19 Vaccine Doses Administered in Europe as of 12 May 2022, by Country. 2022. [(accessed on 12 June 2022)]. Available online: https://www.statista.com/statistics/1196071/covid-19-vaccination-rate-in-europe-by-country/
- 37.Lamptey E. Overcoming barriers to COVID-19 vaccination of pregnant women. Gynecol. Obstet. Clin. Med. 2022;2:29–33. doi: 10.1016/j.gocm.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith D.D., Pippen J.L., Adesomo A.A., Rood K.M., Landon M.B., Costantine M.M. Exclusion of Pregnant Women from Clinical Trials during the Coronavirus Disease 2019 Pandemic: A Review of International Registries. Am. J. Perinatol. 2020;37:792–799. doi: 10.1055/s-0040-1712103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beigi R.H., Krubiner C., Jamieson D.J., Lyerly A.D., Hughes B., Riley L., Faden R., Karron R. The need for inclusion of pregnant women in COVID-19 vaccine trials. Vaccine. 2021;39:868–870. doi: 10.1016/j.vaccine.2020.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skjefte M., Ngirbabul M., Akeju O., Escudero D., Hernandez-Diaz S., Wyszynski D.F., Wu J.W. COVID-19 vaccine acceptance among pregnant women and mothers of young children: Results of a survey in 16 countries. Eur. J. Epidemiol. 2021;36:197–211. doi: 10.1007/s10654-021-00728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iacobucci G. COVID-19 and pregnancy: Vaccine hesitancy and how to overcome it. BMJ. 2021;375:n2862. doi: 10.1136/bmj.n2862. [DOI] [PubMed] [Google Scholar]
- 42.Kachikis A., Englund J.A., Singleton M., Covelli I., Drake A.L., Eckert L.O. Short-term Reactions Among Pregnant and Lactating Individuals in the First Wave of the COVID-19 Vaccine Rollout. JAMA Netw. Open. 2021;4:e2121310. doi: 10.1001/jamanetworkopen.2021.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.