Abstract
Bacillus clausii is a probiotic that benefits human health. Its key characteristics include the ability to form spores; the resulting tolerance to heat, acid, and salt ensures safe passage through the human gastrointestinal tract with no loss of cells. Although B. clausii has been widely used for many decades, the beneficial properties of other probiotics, such as Lactobacillus spp. and Bifidobacterium spp., are better disseminated in the literature. In this review, we summarize the physiological, antimicrobial, and immunomodulatory properties of probiotic B. clausii strains. We also describe findings from studies that have investigated B. clausii probiotics from the perspective of quality and safety. We highlight innovative properties based on biochemical investigations of non-probiotic strains of B. clausii, revealing that B. clausii may have further health benefits in other therapeutic areas.
Keywords: Alkalihalobacillus clausii, Bacillus clausii, Bacillus subtilis, Bacillus spores, dysbiosis, gut barrier, gut microbiota, immunomodulation, probiotics, spore probiotic
1. Introduction
The reduced incidence of infectious diseases in the last century has coincided with an increase in allergic and autoimmune diseases, including asthma, allergic rhinitis, atopic dermatitis, multiple sclerosis, type I diabetes, and Crohn’s disease [1]. Apart from contributing environmental factors such as lifestyle, hygiene, physical activity, and exposure to antibiotics, the microbiome plays a crucial role in the development of these diseases [2].
Dysbiosis is a change in the microbiome structure that affects its composition or function and is associated with modern diseases that are affected by many factors [2]. In dysbiosis, potentially pathogenic microorganisms may dominate the intestinal environment over potentially beneficial microbes [3]. There has been increasing interest in attempts to restore the gut microbiota to a eubiotic state—a healthy and balanced state—using functional foods such as probiotics, prebiotics, and synbiotics [3].
In the consensus statement published in 2014, the International Scientific Association for Probiotics and Prebiotics defines probiotics as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [4]. Probiotics exert their beneficial effects through several modes of action [5,6] and have found wide use in preventing or treating many diseases [7,8,9,10,11,12,13,14,15,16,17,18]. Probiotics belonging to Lactobacillus spp., Bifidobacterium spp., Saccharomyces spp., Bacillus spp., Enterococcus spp., and Streptococcus spp. are consumed around the world for their health benefits [19].
Bacillus is a genus of spore-forming bacteria found in the air, water, food, soil, and the human gut [19]. When environmental conditions are harsh, spore-forming bacteria undergo a complex developmental process in which the bacterial cell differentiates into a spore that can indefinitely survive in the absence of water, nutrients, extremes of temperature, pH, ultraviolet radiation, and noxious chemicals [20]. When favorable environmental conditions return, the spores germinate into vegetative cells that can grow and reproduce [20]. Bacillus spores are metabolically inactive and can tolerate bile salts, survive the acidic environment of the gastrointestinal tract, and are more stable than vegetative bacteria during processing and storage of pharmaceutical or food-based probiotic formulations [19,21,22].
Probiotics that can naturally be isolated from the human gut are likely to have the ability to survive passage through the gut [23]. Bacillus clausii and Bacillus licheniformis have been isolated from healthy human adult feces, indicating their ability to survive passage through the gastrointestinal tract [23,24].
Due to their inherent antibiotic resistance [25] and the excellent compositional quality of some probiotic formulations [26], B. clausii strains have been concomitantly used with antibiotics to reduce the gastrointestinal side effects of antibiotic treatment [27,28]. As an example, the probiotic strains Bacillus clausii, O/C (CNCM I-276), N/R (CNCM I-274), SIN (CNCM I-275), and T (CNCM I-273), marketed as Enterogermina® by Sanofi, are well-tolerated and have been efficaciously used in humans for several decades [24,29]. They have been available as an over-the-counter medicine since 1999 [30]. These four strains derived from a single penicillin-resistant strain, B. subtilis ATCC 9799 [31], were initially classified as B. subtilis until their reclassification as B. clausii in 2001 [25].
Although B. clausii probiotics have been widely available and consumed, probiotics of other species, such as Lactobacillus spp. and Bifidobacterium spp., have been more reported and better disseminated in the literature [19]. In this review, we summarize the findings from biochemical, preclinical, and clinical studies on B. clausii probiotics.
2. Physiological Properties of Bacillus clausii
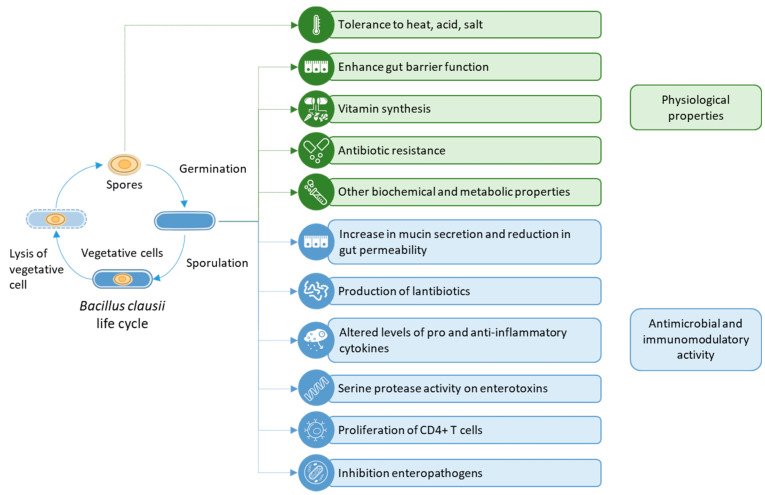
B. clausii strains have been used in a range of studies that highlight their useful physiological properties, such as heat-, acid-, and bile salt-tolerance; enhancement of gut barrier function; broad spectrum antibiotic resistance that cannot be genetically transferred to other species; and vitamin synthesis (Figure 1) [24,32]. These properties and their relevance to clinical practice are described in the sections below.
Figure 1.
A summary of the physiological, antimicrobial, and immunomodulatory properties of Bacillus clausii.
2.1. Tolerance to Heat, Acid, and Salt
To exert a measurable beneficial effect, probiotics need to survive the hostile environment of the gastrointestinal tract and have the ability to multiply and colonize the intestine [33]. In the clinical context, strains that do not display this tolerance are unlikely to be viable and/or colonize the gastrointestinal tract and will therefore have reduced or no efficacy [34]. In this regard, several probiotics have recently been investigated for their potential ability to tolerate gastric and intestinal environments over different time durations ranging from 0 min to 360 min. Spores of the B. clausii strains (O/C, N/R, SIN, and T) were found to have the ability to survive for at least 120 min in simulated gastric fluids in contrast to the other probiotics included in this study, the majority of which experienced a reduction in viability after 30 min of exposure to gastric juice [34]. Of note, the B. clausii strains (O/C, N/R, SIN, and T) were the only ones that displayed the ability to survive and reproduce after 240 min of exposure to the simulated intestinal fluid, at which time point the majority of other tested probiotics experienced significant reductions in viability [34]. Thus, the B. clausii strains (O/C, N/R, SIN, and T) have the ability to colonize the gastrointestinal tract.
2.2. Vitamin Synthesis
Humans and animals do not produce riboflavin (vitamin B2), even though it is essential for proper cellular functioning and growth [35]. Bacteria that produce and secrete riboflavin are more attractive for use as probiotics than those that do not, as they are able to compensate for host deficits in riboflavin levels [35]. This is especially important in the clinical context of vitamin deficiencies induced by chemotherapy in patients with colon cancer. The vegetative cells of the B. clausii strains, O/C, N/R, SIN, and T, produce enough riboflavin to support their own growth on riboflavin-depleted media [35]. Additionally, the B. clausii strains SIN and T release high levels of riboflavin, enabling the growth of other bacteria that depend on absorbing riboflavin from the growth medium [35]. The B. clausii strains O/C, N/R, and 17A1 also secrete riboflavin, albeit to lower levels [35]. Thus, B. clausii probiotics may aid the proper functioning and growth of cells in patients.
2.3. Antibiotic Resistance
The contamination of aquatic environments by tetracycline antibiotics (TCs) is an increasingly pressing issue. The antibiotic resistance of B. clausii strain T has been leveraged to remove antibiotics tetracycline, oxytetracycline, and chlortetracycline from aquatic environments [36]. Vegetative cells of the B. clausii strains T and O/C remove a mix of antibiotics cefuroxime, cefotaxime, and cefpirome from the culture medium [37].
Antibiotic resistance coupled with the proven inability for this resistance to be transferred to other bacteria is a positive safety attribute of a probiotic [38]. It enables the probiotic to be used concomitantly with antibiotic treatment—one of the contexts in which the gut is likely to be stripped of its natural flora and in need of being re-populated with beneficial bacteria. Therefore, clinicians need to be aware of the antimicrobial resistance profiles of commercially available probiotics.
The vegetative cells of the B. clausii strains, O/C, N/R, SIN, and T, are resistant to different degrees to different antibiotics. All strains are fully resistant to erythromycin, azithromycin, clarithromycin, spiramycin, clindamycin, lincomycin, and metronidazole; each strain displays a slightly different resistance profile to some of the other tested antibiotics [29] and Table 1 below.
Table 1.
Antibiotic resistance profiles of the B. clausii strains O/C, SIN, N/R, and T to some of the antibiotics tested in [29].
| Inhibition Zone Diameter (mm) | ||||
|---|---|---|---|---|
| Antibiotic | B. clausii O/C | B. clausii SIN | B. clausii N/R | B. clausii T |
| Oxacillin | 8 | 0 | 0 | 9 ± 1.1 |
| Cefuroxime | 10 ± 0.7 | 0 | 0 | 12 ± 0.8 |
| Cefepime | 8 ± 1 | 0 | 0 | 11 ± 0.5 |
| Streptomycin | 28 ± 0.4 | 0 | 26 ± 0.6 | 30 ± 0.5 |
| Chloramphenicol | 0 | 16 ± 0.6 | 13 | 15 ± 0.6 |
| Rifampicin | 24 ± 0.5 | 26 ± 0.5 | 0 | 27 ± 0.6 |
| Metronidazole | 0 | 0 | 0 | 0 |
From Ref. [29], used under Creative Commons CC-BY 4.0 license.
Several studies have shown a potentially low risk of the subsequent transfer of antibiotic resistance from B. clausii to pathogenic microorganisms. The N/R strain of B. clausii contains a chromosomally-encoded β-lactamase gene, blaBCL-1, which confers resistance to penicillins [39]. The gene conferring resistance to macrolides (erm) is also chromosomally-located [40], as is the gene conferring resistance to aminoglycosides (aadD2; [41]). The chloramphenicol resistance gene, catBcl, has been acquired by B. clausii and is present as a chromosomal copy [42]. Attempts to transfer this gene to other bacterial species, such as E. faecalis JH202, E. faecium HM107, and B. subtilis UCN19, by conjugation have been unsuccessful [42], suggesting that the antibiotic resistance genes of B. clausii are confined to this species.
3. Preclinical Studies on the Probiotic Effects of B. clausii
The genomes of several B. clausii strains have been sequenced and annotated. Within the clade of B. clausii strains, the O/C, N/R, SIN, and T strains are most closely related to the B106 strain, which is in turn similar to the UBBC07 strain [43]. All of these strains share a common ancestor, the KSM-K16 strain used in industrial applications [43]. The genome of B. clausii strain B106 reveals the presence of several genes that support its role as a probiotic: acid tolerance, bile tolerance, fibronectin-binding proteins, enolase, bacteriocins, synthesis of vitamins, and antibiotic resistance [44]. The genomes of the UBBC07 strain [45] and the AKU0647 strain [46] of B. clausii have also been sequenced. The UBBC07 strain possesses antimicrobial properties, i.e., it produces chemicals that kill or prevent the growth of other microorganisms [47]. The AKU0647 strain produces a glycosyl hydrolase, an enzyme that breaks down glycoproteins [46] and may play an important role as a component of lysozyme. This may allow the strain to prevent other, possibly pathogenic, microorganisms from growing. The composite genome of B. clausii (O/C, N/R, SIN, and T) also includes genes conferring antibiotic resistance, genes encoding bacteriocins (peptides or proteins that are toxic to other bacterial species), and stress- and adhesion-related proteins [43].
Preclinical studies have identified several modes of action for B. clausii. These include enhancement of barrier function and gut homoeostasis, and, conversely, antimicrobial activity, inhibition of enterotoxins, and immunomodulatory activity (Table 2). These are described in the sections below.
Table 2.
Mechanisms of action of B. clausii probiotics identified through preclinical studies.
| Mechanism of Action | Tested Strain | Host Environment | Effect of Probiotic | Reference |
|---|---|---|---|---|
| Enhancing gut immune function | B. clausii (O/C, N/R, SIN, and T) live cells | Caco-2 cell line | Production of antimicrobial peptides, mucin, and tight junction proteins; increase of cell proliferation; release of pro-inflammatory cytokines | [48] |
| B. clausii (O/C, N/R, SIN, and T) live cells | Duodenal cells of esophagitis patients | Modulation of gene expression related to immunity, cell growth and death, cell signaling, and cell adhesion | [49] | |
| B. clausii as part of gut community in fecal microbiota transfer | Mice with pancreatic cancer | Alteration of tumor microbiome composition, tumor growth, and immune infiltration | [50] | |
| B. clausii SC-109 spores as part of a synbiotic formulation | Simulator of Human Intestinal Microbial Ecosystem (SHIME®) | Increased production of butyrate, alteration of gut microbiota | [51] | |
| B. clausii UBBC07 spores | Rat model of uremia | Reduction of acetaminophen-induced nephrotoxicity | [52] | |
| Antimicrobial and immunomodulatory activity | B. clausii Sinuberase® live cells | In vitro fermentation | Production of antimicrobial peptides | [53] |
| B. clausii UBBC07 live cells | SHIME® | Production of the lantibiotic clausin | [47] | |
| B. clausii O/C | In vitro culture medium | Production of the lantibiotic clausin | [54] | |
| Live cells of B. clausii isolate #KCTC 10,277 BP from tidal mudflats of the Korean Yellow Sea | Mouse model of allergic asthma | Reduction of inflammation | [55] | |
| B. clausii MTCC-8326 live cells | RAW264.7 murine macrophage cell line | Balance expression of pro- and anti-inflammatory cytokines, protection from S. typhimurium infections and related toxicity | [56,57] | |
| B. clausii O/C live cells | Caco-2 cell line | Protection from cytotoxic effects of Clostridium difficile and Bacillus cereus | [58] | |
| B. clausii (O/C, N/R, SIN, and T) live cells | Swiss murine peritoneal cells | Increased expression of pro-inflammatory cytokines and stimulation of nitrite production and proliferation of CD4+ T cells | [59] | |
| B. clausii O/C live cells | RAW 264.7 murine macrophage cell line | Induction of nitric oxide production | [60] | |
| B. clausii (O/C, N/R, SIN, and T) spores | Mouse model of ulcerative colitis | Slight improvement in symptoms of mild colitis | [61] | |
| B. clausii (O/C, N/R, SIN, and T) spores | Mouse model of schistosomiasis | Reduction of parasitic load and egg load, reduction of inflammation | [62] | |
| B. clausii (O/C, N/R, SIN, and T) spores | Mice with enteropathogenic E. coli infection | Reduction in intestinal lesions, debris and immune cell infiltration, increase in mucus-secreting goblet cells | [63] |
3.1. Gut Immune Function
3.1.1. Enhancing Gut Barrier Function
As well as in vitro studies of its physiological properties that support gut barrier function, B. clausii have been shown to enhance the gut barrier in preclinical studies using cell lines. A recent study has shown how B. clausii strains protect the gut from a rotavirus infection by multiple modes of action. In a human pediatric enterocyte model of rotavirus infection, the vegetative cells of B. clausii (O/C, N/R, SIN, and T) strains induce synthesis of human beta defensin 2 and cathelicidin, which are antimicrobial peptides. The strains also rescue cell proliferation that has been slowed by rotavirus infection. Treatment with B. clausii strains or their supernatant also reduces the proportion of necrotic or apoptotic enterocytes as well as increases mucin production and synthesis of tight junction proteins, increasing the gut barrier integrity. In addition, they inhibit ROS production by rotavirus and the release of pro-inflammatory cytokines, such as IL-8, IFN-β, and TLR-3 pathway genes [48]. Thus, this study shows the mechanistic basis for the clinical efficacy of B. clausii in pediatric viral acute gastroenteritis [48].
Apart from the context of clinical disorders, vegetative cells of B. clausii affect the global reprogramming of gene expression in the gastrointestinal tract of relatively healthy individuals. In duodenal cells derived from patients with mild esophagitis, B. clausii affect the expression of genes involved in immunity and inflammation, apoptosis, cell growth and differentiation, cell–cell signaling, cell adhesion, signal transcription, and transduction [49].
3.1.2. Contributing to Gut Homoeostasis
Patients undergoing chemotherapy often suffer from a dysbiotic gut microbiome, which leads to several general side effects of chemotherapy, such as nausea, vomiting, abdominal pain, and diarrhea [64]. Patients with pancreatic adenocarcinoma who survive longer than five years harbor tumors with a microbiome signature that includes B. clausii, Pseudoxanthomonas spp., Streptomyces spp., and Saccharopolyspora spp. [50]. Specifically, the presence of B. clausii is associated with longer survival times [50]. In mice with pancreatic cancer, transfer of long-term survivors’ gut microbiomes can alter tumor microbiome composition, tumor growth, and tumor immune infiltration [50]. Thus, use of fecal microbiota transfer may represent an attractive clinical option for increasing the life expectancy of patients with pancreatic adenocarcinoma.
In an in vitro simulation of the human gastrointestinal tract, a synbiotic formulation consisting of B. clausii SC-109 spores along with other probiotic bacteria and prebiotic ingredients increased butyrate production by the microbiome and the diversity of gut microbiota, especially the levels of Faecalibacterium prausnitzii, Bifidobacterium spp., and Lactobacillus spp. [51], which exert anti-inflammatory effects in the gut, contributing to gut homeostasis.
Uremia is a major syndrome of chronic kidney disease and presents with high levels of urea in the blood of patients. In a rat model of uremia, administration of B. clausii UBBC07 spores reduced serum urea, creatinine, and malondialdehyde levels that were induced by acetaminophen treatment [52]. The authors of this study attributed this observation to an anti-oxidant effect exerted by B. clausii. Other studies have also shown a decrease in serum urea levels in patients with chronic renal failure administered probiotics [65]. Therefore, this may represent a novel clinical use of probiotics in chronic kidney disease.
3.2. Antimicrobial and Immunomodulatory Activity
3.2.1. Antimicrobial Activity
The Bacillales are an order of Gram-positive bacteria, which include the genera Bacillus, Listeria, and Staphylococcus. Based on genome mining, Bacillales are predicted to be a rich source of novel antimicrobials. These antimicrobials comprise three classes of bacteriocins, amounting to 583 bacteriocin gene clusters from 57 species [66]. Bacteria belonging to the genus Bacillus produce a wide range of antimicrobial substances, including lantibiotics, which are post-translationally modified peptides [67]. The production of antimicrobials such as the lantibiotic clausin is a key route by which probiotics prevent the growth of pathogenic bacteria in the gastrointestinal tract; this is clinically relevant when administering probiotics alongside antibiotic therapy.
When cultured in whey, vegetative cells of B. clausii produce antimicrobial peptides that inhibit the growth of Salmonella typhimurium, Escherichia coli, Shigella flexneri, Staphylococcus aureus, Listeria monocytogenes, and Enterococcus faecalis [53]. These bacterial species are also inhibited by spent coffee grounds fermented with B. clausii Sinuberase® [68], indicating that this B. clausii strain secretes the antimicrobial peptides into the growth or fermentation medium.
The vegetative cells from two strains of B. clausii—UBBC07 and O/C—have been shown to produce clausin [47,54,69]. The clausin from B. clausii UBBC07 exhibits antimicrobial activity against some Gram-positive bacteria [47]. The O/C strain of B. clausii produces clausin that exhibits antimicrobial activity against some Gram-positive bacteria and inhibits the cytotoxic effects of Clostridioides difficile [58,59]. The clausin from O/C has also been shown to target lipid intermediates of bacterial peptidoglycan synthesis [54].
3.2.2. Immunomodulatory Activity
Whereas the antimicrobial activity of probiotics has direct effects on other microorganisms in the gut, the immunomodulatory activity of probiotics rebalances the host immune system, enabling long-term health effects for the host. The following studies point to the potent immunomodulatory mechanisms by which B. clausii probiotics exert their effects.
Chronic inflammation, due to an aberrant immune response involving Th2 cells, can lead to asthma [70], which is characterized by airway inflammation involving eosinophils, and structural changes to the airways, termed airway remodeling [71]. When administered to mice with ovalbumin-induced asthma, B. clausii isolated from tidal mudflats have been shown to reduce the numbers of eosinophils, neutrophils, and lymphocytes and reduce the thickening of the airway epithelium [55]. B. clausii also reduce IL-4 and IL-5 levels and the expression of hypoxia-related genes in these mice [55], pointing to their potential use in reducing airway inflammation in clinical settings.
Macrophages in the intestine play a key role in either increased inflammation following an infection, or in decreased inflammation to enable wound repair [72]. Vegetative cells of B. clausii MTCC-8326 induce a controlled inflammatory response in RAW264.7 murine macrophage cells by increasing pro-inflammatory cytokines at earlier time points and anti-inflammatory cytokines at later time points [56]. This strain also protects murine macrophages from S. typhimurium-induced cytotoxicity [56]. It colonizes the mouse gut and protects BALB/c mice, but not C57BL/6 mice from S. typhimurium infections [57].
Infection with C. difficile causes diarrhea, pseudomembranous colitis, and septicemia, and it may also be fatal. It is often transmitted as a nosocomial infection and following antibiotic therapy. Equally, other pathogens, such as Bacillus cereus, secrete enterotoxins, such as hemolysin BL and cytotoxin K, which damage the intestinal epithelium, causing diarrhea, emesis, or hemorrhage. In vitro, the vegetative cells of the O/C strain of B. clausii secrete a serine protease, which protects intestinal cells from the cytotoxic effects of C. difficile and Bacillus cereus [58]. Two hours of co-incubation with B. clausii O/C can rescue the low viability, low proportion of cell attachment, and decreased mitochondrial activity induced by C. difficile or B. cereus infection [58]. These studies highlight the clinical relevance of B. clausii probiotics in protecting patients at risk of C. difficile-associated diarrhea.
Following an infection, macrophages stimulate nitrite production, which leads to destruction of the pathogen. Pro-inflammatory cytokines and CD4+ T cells also play a role in mounting a coordinated response. Vegetative cells of B. clausii (O/C, N/R, SIN, and T) have been shown to stimulate nitrite production in Swiss murine peritoneal cells and induce the pro-inflammatory cytokine, IFN-γ, and increase the proliferation of CD4+ T cells in murine BL/6j spleen cells [59]. In addition, lipoteichoic acid from the O/C strain of B. clausii induces nitric oxide production in RAW 264.7 macrophages and may underlie the immunomodulatory ability of B. clausii [60].
Ulcerative colitis is another disorder characterized by chronic inflammation due to immune dysregulation [61,73,74]. Mouse models in which colitis is induced by treatment with dextran sodium sulfate, can be used to study the gut microbiota alterations involved in colitis [61]. Administration of B. clausii O/C, N/R, SIN, and T spores over a two-week period slightly reduces the symptoms of colitis, as measured by the disease activity index [61]. It also results in significant changes to the prevalence of various bacterial species in the mouse gut [61].
Schistosomiasis is an infection caused by the parasites Schistosoma mansoni, Schistosoma japonicum, and Schistosoma haematobium, found in contaminated freshwater in the tropics and sub-tropics [75]. Eggs shed by the worms in the intestine or bladder can cause inflammation, leading to anemia, malnutrition, and learning difficulties in children; prolonged infection can damage the intestine, bladder, liver, spleen, and lungs [75]. In mice infected with this parasitic worm, administration of B. clausii O/C, N/R, SIN, and T spores reduces total worm load and the load of eggs deposited in the liver and intestine [62]. B. clausii increases the levels of the anti-inflammatory cytokine IL-10 and decreases the levels of the pro-inflammatory cytokines IFN-γ, TNF-α, and IL-6 [62]. They also increase the levels of Treg and Th17 cells, which contribute to a reduction of inflammation [62].
3.2.3. Inhibition of Enteropathogens
B. clausii have the potential to prevent enteropathogenic infections. In mice infected with enteropathogenic E. coli O127:H21, intestinal villi slough off and lesions and lymphocytic infiltration are observed. Pre-treatment with spores of the B. clausii strains O/C, N/R, SIN, and T reduces lesions, lymphocytic infiltration, and intestinal debris and increases mucus-secreting goblet cells. The resultant more intact mucosa and increased mucin levels exert a protective and immunomodulatory effect in the spleen and in the mesenteric lymph nodes [63].
4. Clinical Studies on Probiotic Effects of B. clausii
In addition to the preclinical studies that indicate a variety of modes of action, B. clausii probiotics have been efficaciously and safely used in humans for several decades [24] and Table 3.
Table 3.
Clinical benefits of B. clausii administration.
| Strain (Dose) | Study Design | Disease | Efficacy | Reference |
|---|---|---|---|---|
| O/C, N/R, SIN, T (2 × 109 to 4 × 109 CFU/day) | Prospective, open-label, multi-center, observational study; n = 3178 | Acute pediatric diarrhea | Reduced duration of diarrhea | [76] |
| O/C, SIN, N/R, T (2 × 109 to 4 × 109 CFU/day) |
Meta-analysis; n = 898 from 6 studies | Rotavirus infection | Reduced frequency and duration of diarrhea Shortened hospital stay |
[77] |
| O/C, SIN, N/R, T (6 × 109 spores/day) |
Single-center, double blind, randomized, placebo-controlled prospective study; n = 120 Randomized, double blind, single-center, placebo-controlled, parallel group, phase 3b study; n = 130 |
Helicobacter pylori treatment | Reduced nausea, diarrhea, and epigastric pain Fewer days of diarrhea Lower incidence of diarrhea |
[27,28] |
| O/C, SIN, N/R, T | Randomized, double-blind, placebo-controlled trial; n = 244 | Necrotizing enterocolitis and late-onset sepsis | Faster attainment of full feeds | [78] |
| O/C, SIN, N/R, T | Randomized, single-blind, multi-center, two arm parallel group study; n = 80 | Upper respiratory tract infections | Fewer and shorter duration of infections | [79] |
| O/C, SIN, N/R, T | Single-blind, non-controlled study; n = 10 | Allergic rhinitis | Reduction in pro-inflammatory cytokines, higher levels of anti-inflammatory cytokines | [80,81] |
| UBBC07 (4 × 109 spores/day) | Randomized, double-blind, placebo-controlled trial; n = 153 | Acute pediatric diarrhea | Reduced stool frequency and duration of diarrhea | [82] |
| Unknown | Observational study; n = 65 | Rotavirus infection | Normalization of IgA and IgM to pre-infection levels Reduction in general weakness, swelling, and/or abdominal pain, fever, vomiting, and duration of diarrhea |
[83,84] |
| Mix of Bacillus species, strain of B. clausii unavailable | Randomized, double-blind, placebo-controlled study with pre-screening for responders; n = 28 | Endotoxemia | Reduction in serum endotoxin and serum triglycerides, reduction in levels of pro-inflammatory markers | [85] |
| Unknown | Randomized controlled study; n = 80 | Recurrent aphthous stomatitis | Reduction in erythema, pain, burning sensation, and oral thrush | [86] |
Spores of B. clausii (O/C, SIN, N/R, T) have been shown to germinate in the human gut and have been detected from day one to day twelve after administration [30]. A recent study has shown that different formulations of B. clausii (O/C, SIN, N/R, T) have similar kinetic profiles and presence/persistence patterns in the intestines of healthy adults, allowing for flexibility in choosing a treatment regimen or dose that is likely to have high adherence [87].
B. clausii exert a beneficial effect in several gastrointestinal disorders [27,28,76,77,78,82,88,89,90,91,92,93,94,95], allergic rhinitis [80,81,96], and upper respiratory tract infections in children [79].
The excessive consumption of calorie-dense, highly processed foods has led to an increase in the incidence of gastrointestinal distress and permeability [85]. Such a disruption to gut permeability, or the gut microbiota profile, or both, caused by diet, is termed as dietary or metabolic endotoxemia [85]. It leads to a transient increase in systemic inflammation, which in turn increases an individual’s risk of developing metabolic or cardiovascular disease [85]. Administration of a mix of spore-based probiotic strains—including B. clausii—is associated with a 42% reduction in serum endotoxin at 5 h after a meal, whereas consumption of a placebo (rice flour) is associated with a 36% increase in serum endotoxin at the same time point. The probiotic is also associated with a 24% reduction in serum triglycerides at 3 h after a meal compared with a 5% reduction with placebo at the same time point. In addition, the probiotic mix is associated with lower levels of pro-inflammatory markers IL-12p70, IL-1β, and ghrelin [85]. Of interest is the observation that similar reductions in inflammatory biomarkers require a 4-fold longer timespan in long-term (>12 weeks) weight-loss interventions [85]. Thus, B. clausii and other spore-forming Bacillus probiotics may represent an attractive therapeutic opportunity for transiently reducing systemic inflammation in patients at risk of metabolic endotoxemia and related cardiovascular disease.
Recurrent aphthous stomatitis is a frequently-occurring disease of the oral mucosa [97]. It is characterized by round or elliptical ulcers in the oral cavity, which can cause severe pain and affect chewing and swallowing, thus reducing the patient’s quality of life [97]. Equally, a disruption to the oral microbiota, caused by the use of immunosuppressive drugs or broad-spectrum antimicrobials, can allow the overgrowth of normal commensals such as Candida albicans, leading to oral candidiasis [98]. Symptoms include pain, lesions, burning sensations, and bleeding, resulting in lowered food intake [98]. If the infection enters the bloodstream, invading the rest of the body, it often leads to hospitalization and in some cases can be fatal [98]. Available treatments include antifungal drugs, which can cause frequent side effects and importantly lead to antifungal resistance [98]. In patients suffering from recurrent aphthous ulcers and oral candidiasis, the local adjunct application of B. clausii, alongside triamcinolone treatment, reduces erythema, pain, oral thrush, and burning sensation in the mouth, compared with triamcinolone treatment alone [86]. This may be due to the formation of a biofilm in the oral cavity, which prevents the growth of other microorganisms and protects the oral mucosa [86]. Recent meta-analyses and systematic reviews have found a beneficial effect of probiotics in reducing oral pain from recurrent aphthous stomatitis [97] and reduced oral Candida spp. counts [98].
Thus, B. clausii probiotics show promise in a variety of clinical contexts, apart from their well-known role in intestinal health and restoring dysbiotic gut microbiota.
5. Compositional Quality and Safety
Because the efficacy of probiotics hinges on the species used and the number of viable cells/spores, it is crucial that commercially marketed probiotics stand up to the claims on their labels. In different products marketed in different countries, there may be discrepancies in terms of the strains present, their viability, or count, leading to the possibility of reduced efficacy or toxicity upon administration [26,99]. Several studies in recent years have investigated the compositional quality of commercially available probiotics, including that of B. clausii (O/C, SIN, N/R, T). Enterogermina® has been shown to be homogenous for B. clausii, whereas other commercial probiotics either contain bacterial species not indicated on the label or have a poor correlation between quantitative label indications and bacterial plate counts [100].
In a study of ten products marketed in Italy as containing Bacillus spores, only two (Biogermin® and Enterogermina) have been shown to respect the label indications of quality and quantity, as measured by MALDI-TOF mass spectrometry, biochemical analysis, 16S rRNA sequencing, and plate counts [26]. Contaminant bacterial species, such as Bacillus cereus, B. licheniformis, B. badius, Brevibacillus choshinensis, Lysinibacillus fusiformis, and Acinetobacter baumannii, have been detected in other products [26]. The viability of several of the other probiotic formulations have also been shown to be lower than that indicated on the label [26].
From a clinical standpoint, only those probiotics that have undergone a stringent process of quality control can be administered to patients, as the beneficial effects of probiotics are strain-specific and dosage-dependent [101]. Equally, different formulations—vial, capsule, oral powder for suspension, and oral powder with no need for suspension—of B. clausii O/C, N/R, SIN, and T have been shown to be equivalent with regard to their kinetic profiles and presence/persistence in the gastrointestinal tract [87]. Thus, as long as the dosage and route of intake are the same, different formulations of B. clausii probiotics may exert similar effects.
Although B. clausii administration is generally considered to be safe, there have been two reports of sepsis under very specific conditions [102,103]. These remain exceptions; the overall safety of its use has been proven by the billions of doses administered over several decades [28]. In addition, acute toxicity studies indicate that B. clausii UBBC07 are safe for use in humans [104]. B. clausii (O/C, N/R, SIN, and T) are presumed to be safe by the European Food Safety Authority and have been added to the Qualified Presumption of Safety (QPS) list [105]. A different strain of B. clausii (088AE) has been notified as “Generally Recognized As Safe” with the U.S. Food and Drug Administration [106].
6. Other Biochemical and Metabolic Properties
In addition to the physiological properties that are directly relevant to their use as probiotics, B. clausii strains display a range of other properties that enable their wide use in different industries. These properties highlight the potential of different strains in different environments.
Laccases are multi-copper oxidases that oxidize a wide range of substrates. They are used in industrial applications such as delignification, chlorophenol- and dye-degradation, beverage stabilization, biosensors and fuel cells, and in fine biochemical and pharmaceutical industries [107]. B. clausii laccase-like multi-copper oxidases have a high activity yield in comparison to those from Streptomyces and Gram-negative bacteria [108], warranting further research into the possibility of its use in the industrial production of laccases.
Terpenes are a class of hydrocarbons produced by certain plant and animal species, which have been found to be useful as natural insecticides and have a wide variety of health benefits [109]. Thus, there has been considerable interest in their production from a pharmacological perspective. Carotenes, lycopenes, and natural rubber are examples of biologically important terpenes [109]. Based on their size, they are classified into hemi-, mono-, sesqui-, di-, tri-, tetra-, and poly-terpenes [109]. B. clausii shows considerable promise for use in terpene production. An (all-E)-isoprenyl diphosphate synthase homologue from B. clausii functions as a geranylfarnesyl diphosphate (GFPP)/hexaprenyl diphosphate (HexPP)/heptaprenyl diphosphate (HepPP) synthase during the biosynthesis of sesterterpenes, head-to-tail triterpenes, and sesquarterpenes [110]. In a functional analysis of isoprenoid metabolites and recombinant enzymes, the B. clausii homolog of tetraprenyl-β-curcumene synthase catalyzes the conversion of a geranylfarnesyl diphosphate and a hexaprenyl diphosphate into novel acyclic sesterterpene and triterpene [111].
β-1,3-Glucanases are plant proteins that have an antifungal effect and play roles in growth and development [112]. One of the by-products of the reaction is β-1,3-glucan, which induces TNF-α production in human monocytes in vitro [113]. B. clausii NM-1 produces an extracellular alkaline-stable β-1,3-glucanase that depolymerizes laminarin (a storage carbohydrate) into β-1,3-glucan [114].
Acetoin is a flavor additive important to the food industry and is also a metabolite produced by microorganisms [115]. Although it can be produced through chemical synthesis and enzymatic conversion, microbial production is more environment-friendly and cost-effective [115]. A butane-2,3-diol dehydrogenase from B. clausii DSM 8716 has been shown to catalyze the oxidation of meso-butane-2,3-diol to acetoin [116].
Cyclodextrins are cyclic oligosaccharides with the ability to form water-soluble inclusion complexes; they are used in the pharmaceutical industry to increase the solubility and bioavailability of active ingredients that are poorly water-soluble [117]. Cyclomaltodextrin glucanotransferase converts starch into cyclodextrin [118]. B. clausii E16 produces a CGTase that is efficient at this conversion [118]. The optimal culture conditions that enable high yields of this enzyme have been reported [119].
Although the four strains of Enterogermina have very similar genomes [25], they display minor phenotypic differences, such as the different bioenergetics of the respiratory chain enzymes [120], and differences in the secretomes of each strain [121]. Secretomes are particularly important in the clinical context as they describe the set of proteins that are secreted by a cell into its environment. These may include proteases that act on other microorganisms or their toxins and confer a protective effect on the host.
7. Conclusions
The beneficial effects of B. clausii (O/C, N/R, SIN, and T) on intestinal health are well known, including their capacity to relieve gastrointestinal distress and their immunomodulatory effects. However, there are likely to be further benefits in other therapeutic areas, which are only now beginning to be discovered. Biochemical investigations have revealed several innovative properties for different strains of B. clausii that may be salient to their function as probiotics. Further research using pre-clinical simulations, real-world evidence, and clinical trials may reveal further modes of action for B. clausii and the ideal dosage and treatment duration to derive optimal benefit from its consumption.
Acknowledgments
The authors would like to thank Dorothea Maren Greifenberg, and Daniel Marquez of Sanofi for support in the development of this article. The authors acknowledge Subhashini Muralidharan, and Ella Palmer, CMPP, of inScience Communications, Springer Healthcare Ltd., UK, for medical writing support funded by Sanofi.
Author Contributions
Writing—original draft preparation, G.Á.C., A.P.M.C., A.T.A.y.A., C.B.M., E.G. and M.P.III; writing—review and editing, G.Á.C., A.P.M.C., A.T.A.y.A., C.B.M., E.G. and M.P.III. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This study does not report any data.
Conflicts of Interest
G.Á.C. declares the following conflict of interest: medical writing support for this manuscript funded by Sanofi. A.P.M.C. declares the following conflict of interests: participation in advisory boards for Danone Nutricia and GSK; membership in the ASBAI (Associação Brasileira de Alergia e Imunologia) and Food Allergy Committee; and medical writing support for this manuscript funded by Sanofi. A.T.A.A. declares the following conflicts of interest: payment for presentations in educational events conducted by Sanofi; and medical writing support for this manuscript funded by Sanofi. C.B.M. declares the following conflicts of interest: consulting fees from Sanofi, Nutricia, and Mead Johnson; honoraria from Sanofi, Nutricia, and Mead Johnson; support for attending meetings and/or travel from Sanofi and Nutricia; participation in advisory boards or data safety monitoring boards for Sanofi, Nutricia, and Mead Johnson; leadership or fiduciary role in SIAMPyP and LASPGHAN; and medical writing support for this manuscript funded by Sanofi. E.G. declares the following conflicts of interest: research grants from Sanofi, OFF Health, and Abiogen Pharma; speaker honorarium from Sanofi; meeting and travel support from Sanofi; and medical writing support for this manuscript funded by Sanofi. M.P. declares the following conflict of interest: employee of Sanofi, issued vested shares as part of employment benefits.
Funding Statement
The preparation of this review was funded by Sanofi.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bach J.F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 2.Levy M., Kolodziejczyk A.A., Thaiss C.A., Elinav E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017;17:219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 3.Gagliardi A., Totino V., Cacciotti F., Iebba V., Neroni B., Bonfiglio G., Trancassini M., Passariello C., Pantanella F., Schippa S. Rebuilding the Gut Microbiota Ecosystem. Int. J. Environ. Res. Public Health. 2018;15:1679. doi: 10.3390/ijerph15081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 5.Wieers G., Belkhir L., Enaud R., Leclercq S., De Foy P.J.M., Dequenne I., de Timary P., Cani P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2019;9:454. doi: 10.3389/fcimb.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders M.E., Merenstein D.J., Reid G., Gibson G.R., Rastall R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019;16:605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- 7.Navarro-Lopez V., Martinez-Andres A., Ramirez-Bosca A., Ruzafa-Costas B., Nunez-Delegido E., Carrion-Gutierrez M.A., Prieto-Merino D., Codoner-Cortes F., Ramon-Vidal D., Genoves-Martinez S., et al. Efficacy and Safety of Oral Administration of a Mixture of Probiotic Strains in Patients with Psoriasis: A Randomized Controlled Clinical Trial. Acta Derm. Venereol. 2019;99:1078–1084. doi: 10.2340/00015555-3305. [DOI] [PubMed] [Google Scholar]
- 8.Michail S.K., Stolfi A., Johnson T., Onady G.M. Efficacy of probiotics in the treatment of pediatric atopic dermatitis: A meta-analysis of randomized controlled trials. Ann. Allergy Asthma Immunol. 2008;101:508–516. doi: 10.1016/S1081-1206(10)60290-6. [DOI] [PubMed] [Google Scholar]
- 9.Kim S.O., Ah Y.M., Yu Y.M., Choi K.H., Shin W.G., Lee J.Y. Effects of probiotics for the treatment of atopic dermatitis: A meta-analysis of randomized controlled trials. Ann. Allergy Asthma Immunol. 2014;113:217–226. doi: 10.1016/j.anai.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Leblhuber F., Steiner K., Schuetz B., Fuchs D., Gostner J.M. Probiotic Supplementation in Patients with Alzheimer’s Dementia-An Explorative Intervention Study. Curr. Alzheimer Res. 2018;15:1106–1113. doi: 10.2174/1389200219666180813144834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouchaki E., Tamtaji O.R., Salami M., Bahmani F., Kakhaki R.D., Akbari E., Tajabadi-Ebrahimi M., Jafari P., Asemi Z. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2017;36:1245–1249. doi: 10.1016/j.clnu.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Groeger D., O’Mahony L., Murphy E.F., Bourke J.F., Dinan T.G., Kiely B., Shanahan F., Quigley E.M. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4:325–339. doi: 10.4161/gmic.25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emre I.E., Eroglu Y., Kara A., Dinleyici E.C., Ozen M. The effect of probiotics on prevention of upper respiratory tract infections in the paediatric community-A systematic review. Benef. Microbes. 2020;11:201–211. doi: 10.3920/BM2019.0119. [DOI] [PubMed] [Google Scholar]
- 14.McFarland L.V., Ozen M., Dinleyici E.C., Goh S. Comparison of pediatric and adult antibiotic-associated diarrhea and Clostridium difficile infections. World J. Gastroenterol. 2016;22:3078–3104. doi: 10.3748/wjg.v22.i11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szajewska H., Horvath A., Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii supplementation and eradication of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2015;41:1237–1245. doi: 10.1111/apt.13214. [DOI] [PubMed] [Google Scholar]
- 16.Miller L.E., Lehtoranta L., Lehtinen M.J. Short-term probiotic supplementation enhances cellular immune function in healthy elderly: Systematic review and meta-analysis of controlled studies. Nutr. Res. 2019;64:1–8. doi: 10.1016/j.nutres.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann P., Curtis N. The influence of probiotics on vaccine responses—A systematic review. Vaccine. 2018;36:207–213. doi: 10.1016/j.vaccine.2017.08.069. [DOI] [PubMed] [Google Scholar]
- 18.Lei W.T., Shih P.C., Liu S.J., Lin C.Y., Yeh T.L. Effect of Probiotics and Prebiotics on Immune Response to Influenza Vaccination in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2017;9:1175. doi: 10.3390/nu9111175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elshaghabee F.M.F., Rokana N., Gulhane R.D., Sharma C., Panwar H. Bacillus As Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017;8:1490. doi: 10.3389/fmicb.2017.01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cutting S.M., Ricca E. Bacterial spore-formers: Friends and foes. FEMS Microbiol. Lett. 2014;358:107–109. doi: 10.1111/1574-6968.12572. [DOI] [PubMed] [Google Scholar]
- 21.Hong H.A., Duc L.H., Cutting S.M. The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 2005;29:813–835. doi: 10.1016/j.femsre.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Sanders M.E., Morelli L., Tompkins T.A. Sporeformers as Human Probiotics: Bacillus, Sporolactobacillus, and Brevibacillus. Compr. Rev. Food Sci. Food Saf. 2003;2:101–110. doi: 10.1111/j.1541-4337.2003.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 23.Hoyles L., Honda H., Logan N.A., Halket G., La Ragione R.M., McCartney A.L. Recognition of greater diversity of Bacillus species and related bacteria in human faeces. Res. Microbiol. 2012;163:3–13. doi: 10.1016/j.resmic.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Lopetuso L.R., Scaldaferri F., Franceschi F., Gasbarrini A. Bacillus clausii and gut homeostasis: State of the art and future perspectives. Expert Rev. Gastroenterol. Hepatol. 2016;10:943–948. doi: 10.1080/17474124.2016.1200465. [DOI] [PubMed] [Google Scholar]
- 25.Senesi S., Celandroni F., Tavanti A., Ghelardi E. Molecular characterization and identification of Bacillus clausii Strains marketed for use in oral bacteriotherapy. Appl. Environ. Microbiol. 2001;67:834–839. doi: 10.1128/AEM.67.2.834-839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celandroni F., Vecchione A., Cara A., Mazzantini D., Lupetti A., Ghelardi E. Identification of Bacillus species: Implication on the quality of probiotic formulations. PLoS ONE. 2019;14:e0217021. doi: 10.1371/journal.pone.0217021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plomer M., Perez M.I., Greifenberg D.M. Effect of Bacillus clausii Capsules in Reducing Adverse Effects Associated with Helicobacter pylori Eradication Therapy: A Randomized, Double-Blind, Controlled Trial. Infect. Dis. Ther. 2020;9:867–878. doi: 10.1007/s40121-020-00333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nista E.C., Candelli M., Cremonini F., Cazzato I.A., Zocco M.A., Franceschi F., Cammarota G., Gasbarrini G., Gasbarrini A. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: Randomized, double-blind, placebo controlled trial. Aliment. Pharmacol. Ther. 2004;20:1181–1188. doi: 10.1111/j.1365-2036.2004.02274.x. [DOI] [PubMed] [Google Scholar]
- 29.Abbrescia A.P., Palese L.L., Papa S., Gaballo A., Alifano P., Sardanelli A.M. Antibiotic Sensitivity of Bacillus clausii Strains in Commercial Preparation. Clin. Immunol. Endocr. Metab. Drugs. 2014;1:102–110. doi: 10.2174/2212707002666150128195631. [DOI] [Google Scholar]
- 30.Ghelardi E., Celandroni F., Salvetti S., Gueye S.A., Lupetti A., Senesi S. Survival and persistence of Bacillus clausii in the human gastrointestinal tract following oral administration as spore-based probiotic formulation. J. Appl. Microbiol. 2015;119:552–559. doi: 10.1111/jam.12848. [DOI] [PubMed] [Google Scholar]
- 31.Ciffo F. Determination of the spectrum of antibiotic resistance of the “Bacillus subtilis” strains of Enterogermina. Chemioterapia. 1984;3:45–52. [PubMed] [Google Scholar]
- 32.dos Santos D.F.A., dos Santos S.S.F. Bacillus clausii: Review of characteristics and applications in medicine, biotechnology and the food industry. Rev. Biocienc. 2019;25:29–38. [Google Scholar]
- 33.Kolacek S., Hojsak I., Berni Canani R., Guarino A., Indrio F., Orel R., Pot B., Shamir R., Szajewska H., Vandenplas Y., et al. Commercial Probiotic Products: A Call for Improved Quality Control. A Position Paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2017;65:117–124. doi: 10.1097/MPG.0000000000001603. [DOI] [PubMed] [Google Scholar]
- 34.Vecchione A., Celandroni F., Mazzantini D., Senesi S., Lupetti A., Ghelardi E. Compositional Quality and Potential Gastrointestinal Behavior of Probiotic Products Commercialized in Italy. Front. Med. 2018;5:59. doi: 10.3389/fmed.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvetti S., Celandroni F., Ghelardi E., Baggiani A., Senesi S. Rapid determination of vitamin B2 secretion by bacteria growing on solid media. J. Appl. Microbiol. 2003;95:1255–1260. doi: 10.1046/j.1365-2672.2003.02095.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu C.X., Xu Q.M., Yu S.C., Cheng J.S., Yuan Y.J. Bio-removal of tetracycline antibiotics under the consortium with probiotics Bacillus clausii T and Bacillus amyloliquefaciens producing biosurfactants. Sci. Total Environ. 2020;710:136329. doi: 10.1016/j.scitotenv.2019.136329. [DOI] [PubMed] [Google Scholar]
- 37.Kong X.X., Jiang J.L., Qiao B., Liu H., Cheng J.S., Yuan Y.J. The biodegradation of cefuroxime, cefotaxime and cefpirome by the synthetic consortium with probiotic Bacillus clausii and investigation of their potential biodegradation pathways. Sci. Total Environ. 2019;651:271–280. doi: 10.1016/j.scitotenv.2018.09.187. [DOI] [PubMed] [Google Scholar]
- 38.Food and Agriculture Organization of the United Nations. World Health Organization . Guidelines for the Evaluation of Probiotics in Food. Food and Agriculture Organization of the United Nations; Rome, Italy: World Health Organization; Geneva, Switzerland: 2002. [Google Scholar]
- 39.Girlich D., Leclercq R., Naas T., Nordmann P. Molecular and biochemical characterization of the chromosome-encoded class A β-lactamase BCL-1 from Bacillus clausii. Antimicrob. Agents Chemother. 2007;51:4009–4014. doi: 10.1128/AAC.00537-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bozdogan B., Galopin S., Leclercq R. Characterization of a new erm-related macrolide resistance gene present in probiotic strains of Bacillus clausii. Appl. Environ. Microbiol. 2004;70:280–284. doi: 10.1128/AEM.70.1.280-284.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bozdogan B., Galopin S., Gerbaud G., Courvalin P., Leclercq R. Chromosomal aadD2 encodes an aminoglycoside nucleotidyltransferase in Bacillus clausii. Antimicrob. Agents Chemother. 2003;47:1343–1346. doi: 10.1128/AAC.47.4.1343-1346.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galopin S., Cattoir V., Leclercq R. A chromosomal chloramphenicol acetyltransferase determinant from a probiotic strain of Bacillus clausii. FEMS Microbiol. Lett. 2009;296:185–189. doi: 10.1111/j.1574-6968.2009.01633.x. [DOI] [PubMed] [Google Scholar]
- 43.Khatri I., Sharma G., Subramanian S. Composite genome sequence of Bacillus clausii, a probiotic commercially available as Enterogermina®, and insights into its probiotic properties. BMC Microbiol. 2019;19:307. doi: 10.1186/s12866-019-1680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapse N.G., Engineer A.S., Gowdaman V., Wagh S., Dhakephalkar P.K. Genome Profiling for Health Promoting and Disease Preventing Traits Unraveled Probiotic Potential of Bacillus clausii B106. Microbiol. Biotechnol. Lett. 2018;46:334–345. doi: 10.4014/mbl.1804.04001. [DOI] [Google Scholar]
- 45.Upadrasta A., Pitta S., Madempudi R.S. Draft Genome Sequence of Bacillus clausii UBBC07, a Spore-Forming Probiotic Strain. Genome Announc. 2016;4:e00235-16. doi: 10.1128/genomeA.00235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higuchi Y., Mori K., Suyama A., Huang Y., Tashiro K., Kuhara S., Takegawa K. Draft Genome Sequence of Bacillus clausii AKU0647, a Strain That Produces Endo-β-N-Acetylglucosaminidase A. Genome Announc. 2016;4:e00310-16. doi: 10.1128/genomeA.00310-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahire J.J., Kashikar M.S., Madempudi R.S. Survival and Germination of Bacillus clausii UBBC07 Spores in in vitro Human Gastrointestinal Tract Simulation Model and Evaluation of Clausin Production. Front. Microbiol. 2020;11:1010. doi: 10.3389/fmicb.2020.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paparo L., Tripodi L., Bruno C., Pisapia L., Damiano C., Pastore L., Canani R.B. Protective action of Bacillus clausii probiotic strains in an in vitro model of Rotavirus infection. Sci. Rep. 2020;10:12636. doi: 10.1038/s41598-020-69533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Caro S., Tao H., Grillo A., Franceschi F., Elia C., Zocco M.A., Gasbarrini G., Sepulveda A.R., Gasbarrini A. Bacillus clausii effect on gene expression pattern in small bowel mucosa using DNA microarray analysis. Eur. J. Gastroenterol. Hepatol. 2005;17:951–960. doi: 10.1097/00042737-200509000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Riquelme E., Zhang Y., Zhang L., Montiel M., Zoltan M., Dong W., Quesada P., Sahin I., Chandra V., Lucas A.S., et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell. 2019;178:795–806.e12. doi: 10.1016/j.cell.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duysburgh C., Van den Abbeele P., Krishnan K., Bayne T.F., Marzorati M. A synbiotic concept containing spore-forming Bacillus strains and a prebiotic fiber blend consistently enhanced metabolic activity by modulation of the gut microbiome in vitro. Int. J. Pharm. X. 2019;1:100021. doi: 10.1016/j.ijpx.2019.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel C., Patel P., Acharya S. Therapeutic Prospective of a Spore-Forming Probiotic-Bacillus clausii UBBC07 Against Acetaminophen-Induced Uremia in Rats. Probiotics Antimicrob. Proteins. 2020;12:253–258. doi: 10.1007/s12602-019-09540-x. [DOI] [PubMed] [Google Scholar]
- 53.Rochin-Medina J.J., Ramirez-Medina H.K., Rangel-Peraza J.G., Pineda-Hidalgo K.V., Iribe-Arellano P. Use of whey as a culture medium for Bacillus clausii for the production of protein hydrolysates with antimicrobial and antioxidant activity. Food Sci. Technol. Int. 2018;24:35–42. doi: 10.1177/1082013217724705. [DOI] [PubMed] [Google Scholar]
- 54.Bouhss A., Al-Dabbagh B., Vincent M., Odaert B., Aumont-Nicaise M., Bressolier P., Desmadril M., Mengin-Lecreulx D., Urdaci M.C., Gallay J. Specific interactions of clausin, a new lantibiotic, with lipid precursors of the bacterial cell wall. Biophys. J. 2009;97:1390–1397. doi: 10.1016/j.bpj.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park H., Jung A.-Y., Chang C.-S., Kim Y.H. Bacillus clausii, a Foreshore-Derived Probiotic, Attenuates Allergic Airway Inflammation through Downregulation of Hypoxia Signaling. J. Rhinol. 2020;27:108–116. doi: 10.18787/jr.2020.00325. [DOI] [Google Scholar]
- 56.Pradhan B., Guha D., Ray P., Das D., Aich P. Comparative Analysis of the Effects of Two Probiotic Bacterial Strains on Metabolism and Innate Immunity in the RAW 264.7 Murine Macrophage Cell Line. Probiotics Antimicrob. Proteins. 2016;8:73–84. doi: 10.1007/s12602-016-9211-4. [DOI] [PubMed] [Google Scholar]
- 57.Pradhan B., Guha D., Naik A.K., Banerjee A., Tambat S., Chawla S., Senapati S., Aich P. Probiotics L. acidophilus and B. clausii Modulate Gut Microbiota in Th1- and Th2-Biased Mice to Ameliorate Salmonella Typhimurium-Induced Diarrhea. Probiotics Antimicrob. Proteins. 2019;11:887–904. doi: 10.1007/s12602-018-9436-5. [DOI] [PubMed] [Google Scholar]
- 58.Ripert G., Racedo S.M., Elie A.M., Jacquot C., Bressollier P., Urdaci M.C. Secreted Compounds of the Probiotic Bacillus clausii Strain O/C Inhibit the Cytotoxic Effects Induced by Clostridium difficile and Bacillus cereus Toxins. Antimicrob. Agents Chemother. 2016;60:3445–3454. doi: 10.1128/AAC.02815-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urdaci M.C., Bressollier P., Pinchuk I. Bacillus clausii probiotic strains: Antimicrobial and immunomodulatory activities. J. Clin. Gastroenterol. 2004;38:S86–S90. doi: 10.1097/01.mcg.0000128925.06662.69. [DOI] [PubMed] [Google Scholar]
- 60.Villeger R., Saad N., Grenier K., Falourd X., Foucat L., Urdaci M.C., Bressollier P., Ouk T.S. Characterization of lipoteichoic acid structures from three probiotic Bacillus strains: Involvement of D-alanine in their biological activity. Antonie Van Leeuwenhoek. 2014;106:693–706. doi: 10.1007/s10482-014-0239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scaldaferri F.G.C., Mora V., Petito V., Lopetuso L.R., Puca P., Ianiro G., Napolitano D., Quaranta G., Masucci L., Sanguinetti M., et al. Bacillus Clausii (O/C, SIN, N/R, T) Improves Acute Mild Colitis in Mice while in vivo Modulating Gut Microbiota. Ann. Gastroenterol. Dig. Syst. 2018;4:1035. [Google Scholar]
- 62.Cruz C.S. Estudo do Potencial Imunomodulatório do Bacillus Clausii (O/C, N/R, T e SIN) Sobre a Esquistossomose Mansoni Experimental. Universidade Federal de Pernambuco; Recife, Brazil: 2020. [Google Scholar]
- 63.Yu M.G.T.R., Tuano D.F., Tud R.M., Umali A., Umandap C.H., Ver M.L., Villalobos R.E., Villanueva A.P., Villarante K.L., Villasenor L., et al. Histomorphologic Effects of Bacillus clausii spores in Enteropathogenic E. coli O127:H21-infected Mice: A Pilot Study. Philipp. J. Intern. Med. 2016;54:wpr-633362. [Google Scholar]
- 64.Montassier E., Gastinne T., Vangay P., Al-Ghalith G.A., Bruley des Varannes S., Massart S., Moreau P., Potel G., de La Cochetiere M.F., Batard E., et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015;42:515–528. doi: 10.1111/apt.13302. [DOI] [PubMed] [Google Scholar]
- 65.Miranda Alatriste P.V., Urbina Arronte R., Gomez Espinosa C.O., Espinosa Cuevas Mde L. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr. Hosp. 2014;29:582–590. doi: 10.3305/nh.2014.29.3.7179. [DOI] [PubMed] [Google Scholar]
- 66.Zhao X., Kuipers O.P. Identification and classification of known and putative antimicrobial compounds produced by a wide variety of Bacillales species. BMC Genom. 2016;17:882. doi: 10.1186/s12864-016-3224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abriouel H., Franz C.M., Ben Omar N., Galvez A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 2011;35:201–232. doi: 10.1111/j.1574-6976.2010.00244.x. [DOI] [PubMed] [Google Scholar]
- 68.Rochin-Medina J.J., Ramirez K., Rangel-Peraza J.G., Bustos-Terrones Y.A. Increase of content and bioactivity of total phenolic compounds from spent coffee grounds through solid state fermentation by Bacillus clausii. J. Food Sci. Technol. 2018;55:915–923. doi: 10.1007/s13197-017-2998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bressollier P., Brugo M.A., Robineau P., Schmitter J.M., Sofeir M., Urdaci M.C., Verneuil B. Peptide Compound with Biological Activity, Its Preparation and Its Application. No. 8,691,773. U.S. Patent. 2007 April 6;
- 70.Holgate S.T. Pathophysiology of asthma: What has our current understanding taught us about new therapeutic approaches? J. Allergy Clin. Immunol. 2011;128:495–505. doi: 10.1016/j.jaci.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 71.Saglani S., Lloyd C.M. Novel concepts in airway inflammation and remodelling in asthma. Eur. Respir. J. 2015;46:1796–1804. doi: 10.1183/13993003.01196-2014. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y., Liu H., Zhao J. Macrophage Polarization Induced by Probiotic Bacteria: A Concise Review. Probiotics Antimicrob. Proteins. 2019;12:798–808. doi: 10.1007/s12602-019-09612-y. [DOI] [PubMed] [Google Scholar]
- 73.Boros É., Prontvai B., Kellermayer Z., Balogh P., Sarlós P., Vincze Á., Varga C., Maróti Z., Bálint B., Nagy I. Transcriptome Based Profiling of the Immune Cell Gene Signature in Rat Experimental Colitis and Human IBD Tissue Samples. Biomolecules. 2020;10:974. doi: 10.3390/biom10070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pavlidis S., Monast C., Loza M.J., Branigan P., Chung K.F., Adcock I.M., Guo Y., Rowe A., Baribaud F. I_MDS: An inflammatory bowel disease molecular activity score to classify patients with differing disease-driving pathways and therapeutic response to anti-TNF treatment. PLoS Comput. Biol. 2019;15:e1006951. doi: 10.1371/journal.pcbi.1006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.CDC About Schistosomiasis. [(accessed on 19 October 2021)]; Available online: https://www.cdc.gov/parasites/schistosomiasis/gen_info/faqs.html.
- 76.de Castro J.A., Guno M.J.V., Perez M.O. Bacillus clausii as adjunctive treatment for acute community-acquired diarrhea among Filipino children: A large-scale, multicenter, open-label study (CODDLE) Trop. Dis. Travel Med. Vaccines. 2019;5:14. doi: 10.1186/s40794-019-0089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ianiro G., Rizzatti G., Plomer M., Lopetuso L., Scaldaferri F., Franceschi F., Cammarota G., Gasbarrini A. Bacillus clausii for the Treatment of Acute Diarrhea in Children: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2018;10:1074. doi: 10.3390/nu10081074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tewari V.V., Dubey S.K., Gupta G. Bacillus clausii for Prevention of Late-onset Sepsis in Preterm Infants: A Randomized Controlled Trial. J. Trop. Pediatr. 2015;61:377–385. doi: 10.1093/tropej/fmv050. [DOI] [PubMed] [Google Scholar]
- 79.Marseglia G.L., Tosca M., Cirillo I., Licari A., Leone M., Marseglia A., Castellazzi A.M., Ciprandi G. Efficacy of Bacillus clausii spores in the prevention of recurrent respiratory infections in children: A pilot study. Ther. Clin. Risk Manag. 2007;3:13–17. doi: 10.2147/tcrm.2007.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ciprandi G., Tosca M.A., Milanese M., Caligo G., Ricca V. Cytokines evaluation in nasal lavage of allergic children after Bacillus clausii administration: A pilot study. Pediatr. Allergy Immunol. 2004;15:148–151. doi: 10.1046/j.1399-3038.2003.00102.x. [DOI] [PubMed] [Google Scholar]
- 81.Ciprandi G., Vizzaccaro A., Cirillo I., Tosca M.A. Bacillus clausii effects in children with allergic rhinitis. Allergy. 2005;60:702–703. doi: 10.1111/j.1398-9995.2005.00722.x. [DOI] [PubMed] [Google Scholar]
- 82.Sudha M.R., Jayanthi N., Pandey D.C., Verma A.K. Bacillus clausii UBBC-07 reduces severity of diarrhoea in children under 5 years of age: A double blind placebo controlled study. Benef. Microbes. 2019;10:149–154. doi: 10.3920/BM2018.0094. [DOI] [PubMed] [Google Scholar]
- 83.Smiyan O.I., Smiian-Horbunova K.O., Bynda T.P., Loboda A.M., Popov S.V., Vysotsky I.Y., Moshchych O.P., Vasylieva O.G., Manko Y.A., Ovsianko O.L., et al. Optimization of the Treatment of Rotavirus Infection in Children by Using Bacillus Clausii. Wiadomości Lek. 2019;72:1320–1323. doi: 10.36740/WLek201907117. [DOI] [PubMed] [Google Scholar]
- 84.Smiian K.S.O., Bynda T., Loboda A. Bacillus clausii in treatment of rotavirus infection in children; Proceedings of the International Scientific Conference on Medicine; Riga, Latvia. 20 March 2020; p. 225. [Google Scholar]
- 85.McFarlin B.K., Henning A.L., Bowman E.M., Gary M.A., Carbajal K.M. Oral spore-based probiotic supplementation was associated with reduced incidence of post-prandial dietary endotoxin, triglycerides, and disease risk biomarkers. World J. Gastrointest. Pathophysiol. 2017;8:117–126. doi: 10.4291/wjgp.v8.i3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nirmala M., Smitha S.G., Kamath G.J. A Study to Assess The Efficacy of Local Application of Oral Probiotic in Treating Recurrent Aphthous Ulcer and Oral Candidiasis. Indian J. Otolaryngol. Head Neck Surg. 2019;71:113–117. doi: 10.1007/s12070-017-1139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Navarra P., Milleri S., Perez I.M., Uboldi M.C., Pellegrino P., De Fer B.B., Morelli L. Kinetics of Intestinal Presence of Spores Following Oral Administration of Bacillus clausii Formulations: Three Single-Centre, Crossover, Randomised, Open-Label Studies. Eur. J. Drug Metab. Pharmacokinet. 2021;46:375–384. doi: 10.1007/s13318-021-00676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Castro J.A., Kesavelu D., Lahiri K.R., Chaijitraruch N., Chongsrisawat V., Jog P.P., Liaw Y.H., Nguyen G.K., Nguyen T.V.H., Pai U.A., et al. Recommendations for the adjuvant use of the poly-antibiotic-resistant probiotic Bacillus clausii (O/C, SIN, N/R, T) in acute, chronic, and antibiotic-associated diarrhea in children: Consensus from Asian experts. Trop. Dis. Travel Med. Vaccines. 2020;6:21. doi: 10.1186/s40794-020-00120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sudha M.R., Bhonagiri S., Kumar M.A. Efficacy of Bacillus clausii strain UBBC-07 in the treatment of patients suffering from acute diarrhoea. Benef. Microbes. 2013;4:211–216. doi: 10.3920/BM2012.0034. [DOI] [PubMed] [Google Scholar]
- 90.Soman R.J., Swamy M.V. A prospective, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of SNZ TriBac, a three-strain Bacillus probiotic blend for undiagnosed gastrointestinal discomfort. Int. J. Color. Dis. 2019;34:1971–1978. doi: 10.1007/s00384-019-03416-w. [DOI] [PubMed] [Google Scholar]
- 91.Hungin A.P., Mulligan C., Pot B., Whorwell P., Agreus L., Fracasso P., Lionis C., Mendive J., de Foy J.M.P., Rubin G., et al. Systematic review: Probiotics in the management of lower gastrointestinal symptoms in clinical practice-an evidence-based international guide. Aliment. Pharmacol. Ther. 2013;38:864–886. doi: 10.1111/apt.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Catinean A., Neag A.M., Nita A., Buzea M., Buzoianu A.D. Bacillus spp. Spores-A Promising Treatment Option for Patients with Irritable Bowel Syndrome. Nutrients. 2019;11:1968. doi: 10.3390/nu11091968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lahiri K.J.K., Gahlowt P., Najmuddin F. Bacillus clausii as an adjuvant therapy in acute childhood diarrhea. IOSR J. Dent. Med. Sci. 2015;14:74–76. doi: 10.9790/0853-14517476. [DOI] [Google Scholar]
- 94.Bueno C.P.A.R.F.R.V., Sanchez A.C., Reyes M.L.U. Effectiveness of Bacillus clausii as adjutant treatment for paediatric irritable bowel syndrome (IBS); Proceedings of the 6th World Congress of PGHAN: Abstracts; Vienna, Austria. 2–5 June 2021; pp. 1–1313. [Google Scholar]
- 95.Guarner F., Khan A.G., Garisch J., Eliakim R., Gangl A., Thomson A., Krabshuis J., Lemair T., Kaufmann P., De Paula J.A., et al. World gastroenterology organisation global guidelines: Probiotics and prebiotics october 2011. J. Clin. Gastroenterol. 2012;46:468–481. doi: 10.1097/MCG.0b013e3182549092. [DOI] [PubMed] [Google Scholar]
- 96.Ciprandi G., Vizzaccaro A., Cirillo I., Tosca M.A. Bacillus clausii exerts immuno-modulatory activity in allergic subjects: A pilot study. Eur. Ann. Allergy Clin. Immunol. 2005;37:129–134. [PubMed] [Google Scholar]
- 97.Cheng B., Zeng X., Liu S., Zou J., Wang Y. The efficacy of probiotics in management of recurrent aphthous stomatitis: A systematic review and meta-analysis. Sci. Rep. 2020;10:21181. doi: 10.1038/s41598-020-78281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mundula T., Ricci F., Barbetta B., Baccini M., Amedei A. Effect of Probiotics on Oral Candidiasis: A Systematic Review and Meta-Analysis. Nutrients. 2019;11:2449. doi: 10.3390/nu11102449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mazzantini D., Calvigioni M., Celandroni F., Lupetti A., Ghelardi E. Spotlight on the Compositional Quality of Probiotic Formulations Marketed Worldwide. Front. Microbiol. 2021;12:693973. doi: 10.3389/fmicb.2021.693973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Patrone V., Molinari P., Morelli L. Microbiological and molecular characterization of commercially available probiotics containing Bacillus clausii from India and Pakistan. Int. J. Food Microbiol. 2016;237:92–97. doi: 10.1016/j.ijfoodmicro.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 101.Morelli L., Pellegrino P. A critical evaluation of the factors affecting the survival and persistence of beneficial bacteria in healthy adults. Benef. Microbes. 2021;12:15–25. doi: 10.3920/BM2021.0017. [DOI] [PubMed] [Google Scholar]
- 102.Princess I., Natarajan T., Ghosh S. When good bacteria behave badly: A case report of Bacillus clausii sepsis in an immunocompetant adult. Access Microbiol. 2020;2:e000097. doi: 10.1099/acmi.0.000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Joshi S., Udani S., Sen S., Kirolikar S., Shetty A. Bacillus Clausii Septicemia in a Pediatric Patient After Treatment With Probiotics. Pediatr. Infect. Dis. J. 2019;38:e228–e230. doi: 10.1097/INF.0000000000002350. [DOI] [PubMed] [Google Scholar]
- 104.Lakshmi S.G., Jayanthi N., Saravanan M., Ratna M.S. Safety assesment of Bacillus clausii UBBC07, a spore forming probiotic. Toxicol. Rep. 2017;4:62–71. doi: 10.1016/j.toxrep.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.European Food Safety Authority Qualified Presumption of Safety (QPS) [(accessed on 6 May 2022)]. Available online: https://www.efsa.europa.eu/en/topics/topic/qualified-presumption-safety-qps.
- 106.United States Food and Drug Administration GRN No. 971. Bacillus clausii Strain 088AE Spore Preparation. [(accessed on 23 May 2022)]; Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=971&sort=GRN_No&order=DESC&startrow=1&type=basic&search=clausii.
- 107.Brander S., Mikkelsen J.D., Kepp K.P. Characterization of an alkali- and halide-resistant laccase expressed in E. coli: CotA from Bacillus clausii. PLoS ONE. 2014;9:e99402. doi: 10.1371/journal.pone.0099402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ihssen J., Reiss R., Luchsinger R., Thony-Meyer L., Richter M. Biochemical properties and yields of diverse bacterial laccase-like multicopper oxidases expressed in Escherichia coli. Sci. Rep. 2015;5:10465. doi: 10.1038/srep10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Paduch R., Kandefer-Szerszeń M., Trytek M., Fiedurek J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007;55:315–327. doi: 10.1007/s00005-007-0039-1. [DOI] [PubMed] [Google Scholar]
- 110.Ueda D., Yamaga H., Murakami M., Totsuka Y., Shinada T., Sato T. Biosynthesis of Sesterterpenes, Head-to-Tail Triterpenes, and Sesquarterpenes in Bacillus clausii: Identification of Multifunctional Enzymes and Analysis of Isoprenoid Metabolites. ChemBioChem. 2015;16:1371–1377. doi: 10.1002/cbic.201500138. [DOI] [PubMed] [Google Scholar]
- 111.Sato T., Yamaga H., Kashima S., Murata Y., Shinada T., Nakano C., Hoshino T. Identification of novel sesterterpene/triterpene synthase from Bacillus clausii. Chembiochem. 2013;14:822–825. doi: 10.1002/cbic.201300035. [DOI] [PubMed] [Google Scholar]
- 112.Balasubramanian V., Vashisht D., Cletus J., Sakthivel N. Plant β-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012;34:1983–1990. doi: 10.1007/s10529-012-1012-6. [DOI] [PubMed] [Google Scholar]
- 113.Miyanishi N., Iwamoto Y., Watanabe E., Odaz T. Induction of TNF-α production from human peripheral blood monocytes with β-1,3-glucan oligomer prepared from laminarin with β-1,3-glucanase from Bacillus clausii NM-1. J. Biosci. Bioeng. 2003;95:192–195. doi: 10.1016/S1389-1723(03)80128-7. [DOI] [PubMed] [Google Scholar]
- 114.Miyanishi N., Hamada N., Kobayashi T., Imada C., Watanabe E. Purification and characterization of a novel extracellular β-1,3-glucanase produced by Bacillus clausii NM-1 isolated from ezo abalone Haliotis discus hannai. J. Biosci. Bioeng. 2003;95:45–51. doi: 10.1016/S1389-1723(03)80147-0. [DOI] [PubMed] [Google Scholar]
- 115.Wang M., Fu J., Zhang X., Chen T. Metabolic engineering of Bacillus subtilis for enhanced production of acetoin. Biotechnol. Lett. 2012;34:1877–1885. doi: 10.1007/s10529-012-0981-9. [DOI] [PubMed] [Google Scholar]
- 116.Muschallik L., Molinnus D., Bongaerts J., Pohl M., Wagner T., Schoning M.J., Siegert P., Selmer T. (R,R)-Butane-2,3-diol dehydrogenase from Bacillus clausii DSM 8716(T): Cloning and expression of the bdhA-gene, and initial characterization of enzyme. J. Biotechnol. 2017;258:41–50. doi: 10.1016/j.jbiotec.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 117.Davis M.E., Brewster M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 118.Alves-Prado H.F., Carneiro A.A., Pavezzi F.C., Gomes E., Boscolo M., Franco C.M., da Silva R. Production of cyclodextrins by CGTase from Bacillus clausii using different starches as substrates. Appl. Biochem. Biotechnol. 2008;146:3–13. doi: 10.1007/s12010-007-8093-z. [DOI] [PubMed] [Google Scholar]
- 119.Alves-Prado H.F., Bocchini D.A., Gomes E., Baida L.C., Contiero J., Roberto I.C., Da Silva R. Optimization of cyclodextrin glucanotransferase production from Bacillus clausii E16 in submerged fermentation using response surface methodology. Appl. Biochem. Biotechnol. 2007;137–140:27–40. doi: 10.1007/s12010-007-9037-3. [DOI] [PubMed] [Google Scholar]
- 120.Abbrescia A., Martino P.L., Panelli D., Sardanelli A.M., Papa S., Alifano P., Palese L.L., Gaballo A. The respiratory chains of four strains of the alkaliphilic Bacillus clausii. FEBS Open Bio. 2014;4:714–721. doi: 10.1016/j.fob.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lippolis R., Siciliano R.A., Mazzeo M.F., Abbrescia A., Gnoni A., Sardanelli A.M., Papa S. Comparative secretome analysis of four isogenic Bacillus clausii probiotic strains. Proteome Sci. 2013;11:28. doi: 10.1186/1477-5956-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study does not report any data.