Abstract
Plasmids containing an antisense fragment of the ς32 gene were constructed and introduced into Escherichia coli cells. Downregulation of the ς32-mediated stress response was evaluated under heat shock and ethanol stress and during the production of organophosphorus hydrolase (OPH). Northern blot analyses revealed that ς32 sense mRNA was virtually undetected in antisense-producing cultures from 5 to 20 min after antisense induction. However, lower-molecular-weight bands were found, presumably due to partial degradation of ς32 mRNA. While a >10-fold increase in ς32 protein level was found under ethanol stress in the control cultures, antisense producing cultures resulted in a <3-fold increase, indicating downregulation of ς32. Correspondingly, antisense synthesis resulted in a decreased level of a ς32 regulated chaperone (GroEL) for the first 2 h after induction relative to control cultures without ς32 antisense mRNA. The total yield of OPH in the presence of ς32 antisense was, on average, 62% of the yield without antisense. However, during ς32 antisense production, a sixfold-higher specific OPH activity was observed compared to non-antisense-producing cultures.
The ς32-mediated stress response in Escherichia coli is induced by a variety of factors, including ethanol and heat shock, as well as the overexpression of recombinant protein (16, 17, 21, 22, 25, 27). The hallmark of this response is a rapid increase in the concentration of the ς32 sigma factor (3, 16, 17, 21, 26, 27, 30). For both heat shock and ethanol stress, ς32 accumulation is mediated through control of transcription and translation, as well as ς32 protein stabilization (3, 7, 8, 14, 26, 30, 31). Conversely, the ς32 accumulation following production of recombinant protein is due to stabilization (16, 21). When bound to RNA polymerase, forming the holoenzyme Eς32, ς32 directs the production of a number of chaperone proteins (e.g., GroEL, GroES, DnaK, DnaJ, and GrpE) and proteases (e.g., Lon, ClpB, and FtsH) (7, 8, 12–15, 19, 21, 22, 26, 31, 32). Chaperones often help to fold proteins into their proper configuration, while they and other proteins with unfoldase activity also facilitate the degradation of proteins by folding them into protease-susceptible configurations. The stress proteases then degrade the targeted proteins.
Under ethanol stress or heat shock conditions, it is well known that the synthesis of ς32 increases (16, 17, 21, 22, 25, 27). Additionally, the ς32 protein that is already present in the cytoplasm is stabilized (3, 16, 17, 21, 26, 27, 30). Under nonstress conditions, ς32 has a high turnover rate with a half-life on the order of 1 min (21, 26, 27). Under stress conditions, the half-life of ς32 protein has been reported to increase by as much as a factor of 10 (27). FtsH degrades ς32 only after ς32 has bound to DnaK, DnaJ, and GrpE, creating a multiprotein complex (7, 8). All of these proteins are heat shock chaperone proteins except for FtsH, which is a heat shock protease (31). Under stress conditions, the chaperones bind to misfolded proteins that arise due to the imposed stress (7, 8, 26, 29). The result is a sequestering of the ς32 binding these proteases and chaperones and increased stability of ς32. This, in turn, further increases production of stress proteins. Then, as chaperone proteins accumulate, ς32 is degraded more swiftly.
To facilitate the expression of recombinant proteins in E. coli, it may be convenient at times to downregulate the activity and/or concentration of chaperone proteins and/or proteases (29). This is especially true since increased proteolytic activity accompanying the overexpression of recombinant proteins in E. coli can be detrimental to product yield (15). One strategy to overcome proteolytic degradation has been to use knockout mutations (18). However, multiple knockouts can be detrimental to cell growth, and, additionally, some mutations are lethal (14, 31, 32). Hypothetically, in the event that a global regulator such as the ς32 sigma factor was downregulated, the level of all ς32 activated proteases, including those not currently characterized, could be simultaneously reduced. Since ς32 mutations are lethal at temperatures greater than 20°C (21, 32), a method that transiently downregulates the ς32 stress response in vivo could be advantageous.
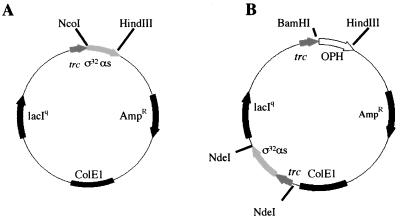
Recently, antisense RNA was introduced as a mechanism for manipulating biosynthesis pathways in prokaryotes for the synthesis of commercially relevant products, specifically acetone and butanol (6). However, there have been no reports demonstrating antisense RNA as a transient and potentially tunable mechanism for enhancing production of such biologicals, including proteins. Moreover, there have been no reports demonstrating control of a regulatory network using antisense RNA. Both naturally occurring and artificial antisense transcripts accomplish downregulation by either blocking ribosome binding or reducing mRNA stability (2, 5, 6, 10, 20). In the present work, an antisense sequence targeting a 284-bp segment of ς32, including the ribosomal binding site, was cloned into a plasmid under the control of the trc promoter as shown in Fig. 1A. This vector and a subsequent vector for coexpression of organophosphorus hydrolase (OPH) were evaluated to examine whether plasmid-encoded ς32 antisense RNA could influence the levels of ς32 sense RNA, ς32 protein, and GroEL (normally upregulated by ς32 under stress) and both the level and activity of OPH.
FIG. 1.
Maps of ς32 antisense expression plasmid pSE420αs (A) and OPH-ς32 antisense expression plasmid pTOas (B). Antisense was inserted into pSE420αs between NcoI and HindIII restriction sites under control of the trc promoter. For the pTOas plasmid, trc-ς32 antisense fragment from pSE420αs was engineered with NdeI restriction enzyme sites and inserted into a plasmid containing the OPH gene (pTO), also under trc control.
MATERIALS AND METHODS
Bacterial strains.
E. coli TOP10 [F− mcrA Δ(mrr-hsdRMS-mcrBC) φ801acZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu) 7697 galU galK rpsL (Strr) endA1 nupG] (Invitrogen, Carlsbad, Calif.) was used for plasmid construction. E. coli strain JM105 [supE endA sbcB15 hsdR4 rpsL thi Δ(lac-proAB) F′ (traD36 proAB+ lacIq lacZΔM15)] was used for expression (ATCC).
Construction of antisense plasmid.
Primers were designed to amplify a 284-bp segment of the ς32 gene from the E. coli K-12 genome using PCR. A naturally occurring HindIII site was found at the 5′ end of the segment of interest, while a naturally occurring NcoI site was found ∼284 bp downstream. This enabled flanking restriction sites to be incorporated into the homologous sequence of interest by appropriate primer design. The 5′ primer sequence used was 5′-CCG AAG CTT GCA TTG AAC TTG TGG-3′, while the 3′ primer was 5′-GCT GCT TCC AGA TCG CCA TGG-3′. After PCR the isolated segment (purified via a Bio-Rad Prep-A-Gene kit) was inserted in the antisense orientation between the NcoI site and the HindIII site of the pSE420 plasmid (Invitrogen) as shown in Fig. 1A. PCR was further used to amplify the promoter, antisense, and termination sequence from pSE420αs and also incorporate NdeI restriction enzyme sites at either end of the fragment. The resulting PCR-amplified (5′ primer, 5′-TTC ATT CAT ATG CGA CAT CAT AAC GGT TCT GGC AAA TATTC-3′; 3′ primer, 5′-TAA GCT CAT ATG GCG GAT TTG TCC TAC TCA AGG AGA GCG-3′) and purified fragment was then inserted into a unique NdeI site in the pTO vector as shown in Fig. 1B. The resulting vector coexpresses ς32 antisense RNA and OPH upon IPTG (isopropyl-β-d-thiogalactopyranoside) addition.
Media, chemicals, and culture conditions.
Experiments were performed in which cell cultures were exposed to either heat stress or ethanol stress and induced to produce ς32 antisense RNA. The effects of the antisense ς32 mRNA on sense ς32 mRNA, ς32 protein, and GroEL protein (as a ς32-regulated model) were subsequently monitored. That is, GroEL was monitored to examine whether ς32 antisense could influence the level of a protein normally upregulated by ς32 under stress conditions. OPH was coexpressed to see whether the ς32 antisense RNA could influence both the level and activity of a model recombinant protein product. Minimal M9 medium supplemented with thiamine (0.17 μg/ml) and ampicillin (50 μg/ml) was used for all experiments (28). One vial (1.0 ml) of −80°C E. coli freezer stock was grown overnight at 30°C in 50 ml of medium in 250-ml Erlenmeyer flasks in an air incubator-shaker. The shaker speed was set at 250 rpm. Fresh culture was inoculated with 5% (vol/vol) overnight culture for a final working volume of 210 ml. Nonstressed, ethanol shocked, and OPH producing cultures were all grown at 37°C. Cultures were grown to an optical density at 600 nm (OD600) of 0.3; at this point, they were induced (1 mM IPTG) and/or stressed (4% [vol/vol] ethanol). Cultures that were heat shocked and their controls were grown at 30°C using a 100-ml working volume in 250-ml Erlenmeyer flasks. For heat shock, the cultures were moved to a 42°C incubator-shaker water bath at an OD600 of 0.3, where the elevated temperature (42°C) was reached in less than 3 min (determined experimentally). The temperature was maintained at 42°C for the duration of the experiment.
RNA extraction, detection, and analysis.
Twenty-five-milliliter samples from each culture were harvested periodically as noted. Total RNA was isolated using an RNAqueous kit (Ambion, Austin, Tex.). Total RNA concentration was determined using the OD260 method (23, 24). Expression of sense and antisense ς32 mRNA was analyzed via Northern analysis. RNA (10 μg/well) was run on a formaldehyde denaturing gel with 1% agarose (wt/vol). Total RNA per sample was analyzed using ethidium bromide to ensure legitimate comparison between lanes. The gel was blotted to a nylon membrane (Boehringer-Mannheim, Indianapolis, Ind.) using the capillary action method (24). The membrane was probed for either sense or antisense ς32 mRNA. Several probes ranging in size from 20 to 60 bp were tested for their specificity and binding sensitivity; ultimately 40-bp probes were selected (sense probe, 5′-CAGACCATGGTAATGCAGCTTTTCAGCCAGCGCCCGCTTT-3′; antisense probe, 5′-CGACTCTAGATCGATTGAGAGGATTTGAATGACTGACAAA-3′). All probes were labeled at the 3′ end with digoxigenin (Boehringer-Mannheim) for fluorescence detection. Membranes were developed using a wash and blocking kit (Boehringer-Mannheim). For ς32 sense mRNA detection, 5 μl of the chemiluminescence solution (Boehringer-Mannheim) was diluted in 495 μl of 1× detection buffer (Boehringer-Mannheim). The solution was applied to the membrane, and the membrane was incubated at 37°C for 1 h. The membrane was exposed to Fuji X-ray film for 10 min, after which the film was developed. For the ς32 antisense mRNA detection, 300 μl of Vistra ECF substrate solution (Amersham Life Sciences, Princeton, N.J.) was applied in place of the chemiluminescent/detection substrate. The membrane was then scanned using a STORM860 fluorescence imaging system (Molecular Dynamics, Sunnyvale, Calif.) and quantified using ImageQuant software (Molecular Dynamics).
Protein extraction, detection, and analysis.
Culture volumes equivalent to 1 ml at an OD600 of 2 were withdrawn. The samples were centrifuged at 7,500 × g for 5 min at 4°C and decanted, and the pellets were stored at −80°C until needed. The pellets were then resuspended in gel running buffer (0.5 M Tris-HCl [pH 6.8], 10% glycerol, 5% sodium dodecyl sulfate, 5% β-mercaptoethanol, 0.25% bromophenol blue), heated to 100°C for 5 min, and vortexed again. The samples were loaded onto a sodium dodecyl sulfate–12.5% polyacrylamide gel for electrophoresis. The gels were blotted onto supported nitrocellulose membranes (Bio-Rad) using a mini-trans blot cell (Bio-Rad) and Bjerrum and Schafer-Nielsen transfer buffer (48 mM Tris, 29 mM glycine, 20% methanol) for 20 min at 10 V and another 20 min at 20 V. Anti-GroEL mouse monoclonal antibody (StressGen, Vancouver, Canada) was diluted 1:2,000 in antibody buffer (0.5% Tween 20 [vol/vol], Tris-buffered saline with 1% [wt/vol] nonfat dry milk) and used to probe for GroEL. Anti-ς32 mouse monoclonal antibody, kindly shared by the laboratory of Richard Burgess (University of Wisconsin), was diluted 1:1,000 in antibody buffer and used to probe for ς32. Antihistidine mouse monoclonal antibody was diluted 1:3,000 in antibody buffer and used to probe for the N-terminal hexahistidine tag on OPH (Sigma, St. Louis, Mo.). The membranes were then transferred to a 1:4,000 diluted goat anti-mouse antibody (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) solution. The membranes were washed and developed colorometrically with 5-bromo-4-chloro-indolyl phosphate–nitro blue tetrazolium tablets (Sigma). The membranes were then scanned and their images were analyzed using NIH Image software. Images depicted in figures are from representative experiments. Data depicted in bar charts are averaged from duplicate or triplicate assays for each. Thus, there might not be a direct visual correspondence between the depicted image and bar chart in all cases.
OPH activity assays.
Two milliliters of cell culture was collected, frozen with liquid nitrogen, and stored at −80°C until needed. Thawed samples were centrifuged at 7,500 × g for 5 min at 4°C. The samples were then decanted and suspended in 2 ml of phosphate-buffered saline (20 mM sodium phosphate and 500 mM sodium chloride) at a pH of 8.5. They were then sonicated for 1 min using a 0.5-s pulse with a Fisher Scientific 550 Sonic Dismembrator. After sonication, the samples were spun down as before, and the supernatant was collected. After the supernatant had come to room temperature, 75-μl were added to 900 μl of phosphate-buffered saline and to 25 μl of 1 mM paraoxon. The absorbance of each sample was measured at 400 nm, for which the extinction coefficient is 17,000 M−1 cm−1. Activities were expressed in micromoles of paraoxon hydrolyzed per minute per OD600 of whole cells.
RESULTS
Plasmid-mediated expression of antisense.
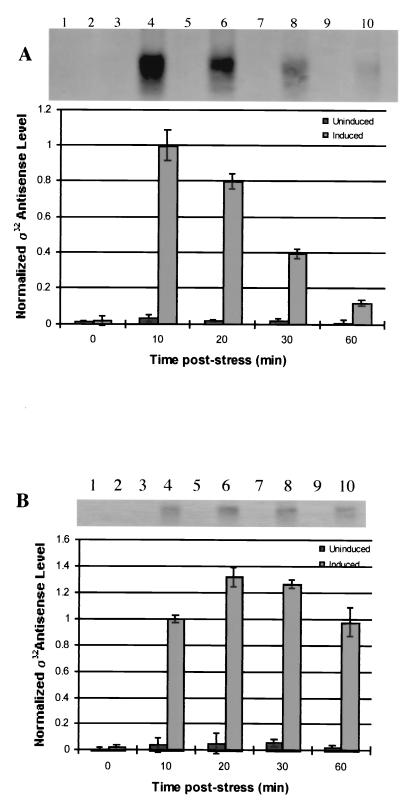
E. coli JM105 (pSE420αs) was induced and probed for antisense ς32 mRNA under nonstressed (Fig. 2A) and ethanol-stressed (Fig. 2B) conditions. In all cases preinduction samples had virtually no detectable antisense ς32 mRNA; the antisense ς32 mRNA was, however, detected in the samples from 10 min postinduction. Subsequent time points were thus normalized to the 10-min level. Antisense ς32 mRNA cultures that were induced but not stressed had maximal antisense RNA 10 min following induction. Cultures that were stressed showed an increase in antisense ς32 mRNA levels up to 20 min. After 20 min, the level decreased steadily. There was no detectable ς32 mRNA in cultures that were not induced. There was also no noticeable change in cell growth rate after induction (data not shown).
FIG. 2.
Northern blot analyses of ς32 antisense mRNA. (A) Lanes 1, 3, 5, 7, and 9 are time course samples from unstressed cultures to which no IPTG has been added (no induction of ς32 antisense mRNA). Lanes 2, 4, 6, 8, and 10 are time course samples from unstressed cultures that were induced for ς32 antisense mRNA synthesis by addition of 1 mM IPTG at 0 min. All quantified values were normalized to the value of lane 4 (10 min). (B) Lanes 1, 3, 5, 7, and 9 are time course samples from cultures that were ethanol stressed (4%, vol/vol) at 0 min but to which no IPTG has been added (Uninduced). Lanes 2, 4, 6, 8, and 10 are time course samples from cultures that were both ethanol stressed and induced for antisense synthesis (Induced). All quantified values were normalized to the value of lane 4. Error bars in both panels represent standard error from multiple blots from multiple experiments.
Antisense effect on sense ς32 mRNA.
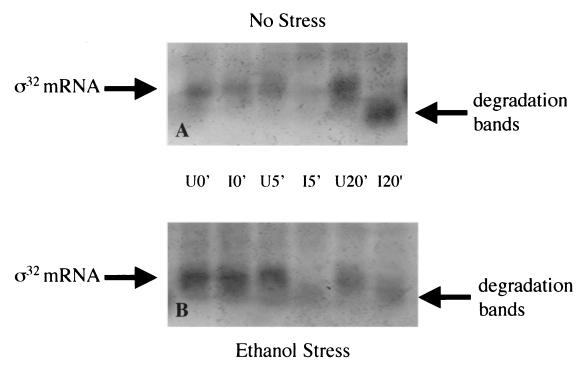
To evaluate regulation of ς32 mRNA, ς32 sense mRNA was probed using Northern blotting analysis. Cultures were examined under ethanol shock and nonstress conditions (as described in Materials and Methods). As seen in Fig. 3, ς32 mRNA was present in each culture before induction of the antisense as expected. In all antisense-induced cultures, ς32 mRNA decreased within 5 min and remained low up to 20 min after induction (Fig. 3). Interestingly our 5′-targeted probe detected putative ς32 mRNA degradation bands at lower molecular weights in these same cultures. Also, there was no rapid accumulation of ς32 mRNA in uninduced but ethanol-stressed cultures (Fig. 3B), which is consistent with ς32 protein stabilization as a principal means of ς32 upregulation under stress (16, 21).
FIG. 3.
Northern analyses of ς32 sense mRNA. All samples were taken at 0, 5, and 20 min postinduction. (A) Northern blot of nonstressed cell culture over a 20-min time course. Uninduced 0-, 5-, and 20-min samples (U0′, U5′, and U20′, respectively) were taken from a culture that was not induced; induced 0-, 5-, and 20-min samples (I0′, I5′, and I20′, respectively) were taken from a culture that had 1 mM IPTG added at 0 min. (B) Northern blot of cell cultures stressed with ethanol (4%, vol/vol) at 0 min. Lanes are as described for panel A.
Antisense effect on ς32 protein levels.
ς32 protein levels were monitored by Western blotting. The cells without ς32 antisense mRNA showed a 10-fold increase in ς32 after ethanol stress, which was sustained for over 30 min. In the cultures producing antisense, ς32 increased threefold initially and then dropped to twice the initial value after 30 min and remained there for the duration of the experiment.
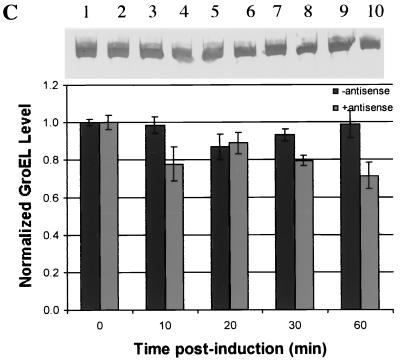
Antisense effect on GroEL.
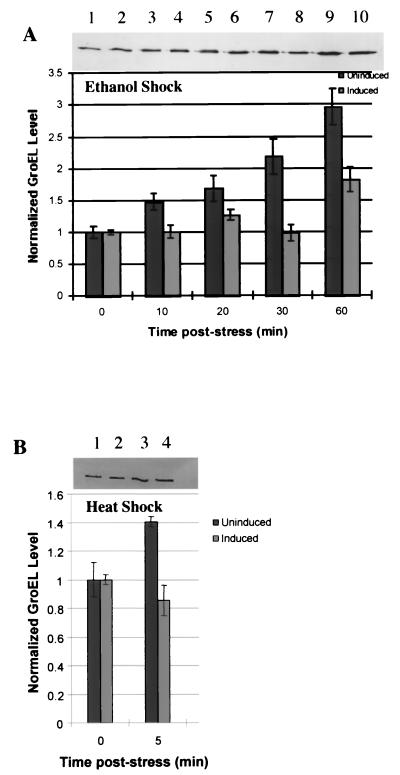
GroEL was used as a model target protein to evaluate ς32 antisense downregulation of ς32-regulated proteins (cascade effect). For the first hour after stressing the cultures with ethanol, ς32 antisense mRNA-producing cultures showed significantly lower levels of GroEL than those cultures not producing antisense (Fig. 5A). In samples taken beyond 2 h, the levels of GroEL in both cultures were comparable (data not shown). In cultures that were not stressed and not induced, GroEL concentration was constant throughout (also not shown). Also, in unstressed but induced cultures, there was a 33% reduction in GroEL during the first 10 min postinduction. In the remaining 50 min, the GroEL level was typically one-half to two-thirds that in control cultures without ς32 antisense. Similarly, the effect of antisense on GroEL was also evaluated under heat shock conditions (Fig. 5B), where there was a 30% decrease in GroEL in the antisense-producing cultures after 5 min.
FIG. 5.
Time course analysis of GroEL determined from Western blot quantification. (A) Cultures were stressed at 0 min with ethanol (4%, vol/vol). Uninduced cultures had no IPTG added. Induced cultures had 1 mM IPTG added at 0 min. Uninduced samples were normalized to the 0-min uninduced sample value, and induced samples were normalized to the 0-min induced sample value. Samples were taken at 0, 10, 20, 30, and 60 min. (B) Analysis of GroEL level after heat shock via Western quantification. Samples were normalized to their pre-heat shock values. Uninduced cultures had no IPTG added. Induced cultures had 1 mM IPTG added at 0 min. Samples were taken at 0 min (30°C) and again after cultures had been at 42°C for 5 min. Error bars on both panels represent standard error from multiple blots from multiple experiments.
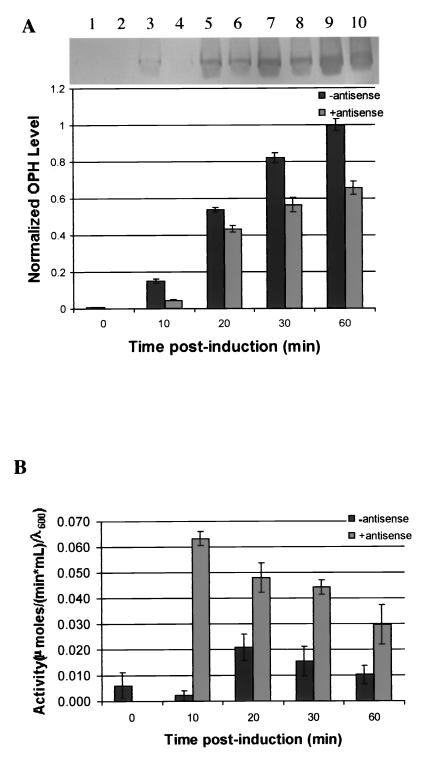
Antisense effect on OPH levels and activity.
The effect of the coexpression of ς32 antisense on OPH production was investigated by comparing the induction of pTO to the induction of pTOas (Fig. 6). There was no OPH detected from pTO prior to induction, and there was a very low level of OPH detected for pTOas at the zero time point; hence, values were normalized to the final nonantisense OPH-producing culture samples. Throughout the postinduction period, the pTOas culture produced less OPH than the pTO cultures and the absolute difference increased monotonically. Ultimately, at 60 min, the ς32 antisense-producing cultures yielded roughly two-thirds the OPH yielded by the controls.
FIG. 6.
(A) Time course analysis of OPH determined from Western blot quantification. Cultures were induced at 0 min with 1 mM IPTG. Cultures contained either the pTO plasmid (lanes 1, 3, 5, 7, and 9) and produced only OPH, or contained pTOαs plasmid (2, 4, 6, 8, and 10) and produced OPH and ς32 antisense RNA. (B) OPH activity assay results for non-antisense- and for antisense-producing cells. (C) Time course analysis of GroEL determined from Western blot quantification during OPH production. Cultures were induced as described for panel A. Error bars shown represent multiple assays from one experiment, which was duplicated with the same result.
OPH activity results are depicted in Fig. 6B. In this case, results were not normalized to a specific time point although they were indicated on a per cell or OD basis. After induction, the specific activity of the OPH produced by the pTO was maximal (0.02 U) after 20 min and then dropped to a final specific activity of 0.01 U at the end of 1 h. The specific activity of OPH in cultures making ς32 antisense mRNA was over 0.06 U within the first 10 min and then dropped monotonically for the remaining 50 min. At the end of the hour, the antisense-containing cultures exhibited a threefold-greater activity than cultures not producing antisense.
Finally, GroEL levels were determined in these experiments (Fig. 6C), and they generally decreased over time in the ς32 antisense RNA producing cultures. Moreover, they generally decreased relative to the nonantisense controls, which is consistent with the previous experiments with no OPH synthesis.
DISCUSSION
Importantly, we demonstrated that pSE420αs produced ς32 mRNA in antisense configuration and that this rapidly accumulated within 10 min and was still prevalent at 60 min postinduction. We also found that sense ς32 mRNA was lost within the first 5 to 20 min following stress and ς32 antisense production. Significantly less ς32 protein in antisense-producing cultures was found (Fig. 4), demonstrating a correlation between ς32 mRNA and ς32 protein levels. The ς32 protein in the antisense-induced and ethanol-stressed cultures increased by a factor of 3 within 10 min. This is substantially less than the 10-fold increase seen in the non-antisense-producing cultures. Therefore, in ς32 antisense mRNA-producing cells, the capacity for the synthesis of ς32 protein was reduced.
FIG. 4.
Time course analysis of ς32 determined from Western blot quantification. Cultures were stressed at 0 min with ethanol (4%, vol/vol). Uninduced cultures had no IPTG added. Induced cultures had 1 mM IPTG added at 0 min. Samples were taken at 0, 10, 20, 30, and 60 min. Uninduced samples were normalized to the 0-min uninduced sample value, and induced samples were normalized to the 0-min induced sample value. Error bars represent standard error from duplicate experiments.
Importantly, we found the amount of OPH produced in the presence of ς32 antisense mRNA was roughly two-thirds that produced without antisense. This could in part be rationalized by a transcriptional limitiation. Specifically, there is one trc promoter on the pTO vector and two trc promoters on the pTOas plasmid (Fig. 1); hence, for the antisense producing cultures there are two sites enabled for RNA transcription, with only one leading to OPH protein. The resulting competition for RNA polymerase could therefore be the reason that less OPH was found in the antisense-producing cultures. An additional and consistent observation was made in that the cells containing the antisense vector produced no detectable OPH prior to induction, while the other vector (pTO) resulted in low but detectable OPH. This makes the pTOas vector potentially useful for the production of toxic proteins in E. coli. Most importantly, however, the pTOas vector was found to enable a threefold increase in biologically active OPH. It was also interesting that the level of ς32 antisense mRNA tracked almost exactly the level of active OPH (highest 10 min after induction followed by a gradual decrease until 60 min), suggesting a temporal relationship between cause and effect. We do not know the specific molecular mechanisms for both the reduced OPH level and increased OPH activity, however. There are many ς32-regulated proteins in E. coli, each of which may have contributed to these phenomena. Perhaps OPH undergoes a chaperone-assisted proteolytic degradation (e.g., DnaK-, DnaJ-, or GrpE-mediated FtsH degradation of ς32), and while the antisense acts to suppress the chaperone level (which would otherwise increase), the available chaperones are sequestered away from an OPH degradation pathway. This would be consistent with the increased loss of OPH activity and decreased accumulation rate observed as the antisense RNA disappeared (compare Fig. 6B and 2A). We are presently evaluating the transcriptional response to ς32 antisense production on a global basis using reverse transcription-PCR to begin to elucidate these mechanisms (10).
While the use of antisense RNA has been shown to increase the yield of biologicals by manipulating pathway enzymes in prokaryotes, this work is the first to demonstrate an effect on heterologous protein. Importantly, as a temporal metabolic control mechanism, the potential for affecting a downstream product, GroEL, through downregulation of a global regulatory protein, ς32, was demonstrated here. Results indicated that GroEL production was significantly reduced compared to cells not producing ς32 antisense when exposed to either ethanol or heat shock. It was interesting that for the OPH-producing cultures, GroEL levels were roughly constant in cells not producing antisense. However, GroEL was found to decrease in the presence of antisense (Fig. 6C), though not as dramatically as in the ethanol stress or heat shock cases. This may be due to the differences in the mechanisms by which ς32 is accumulated in the various cases. During recombinant protein expression, ς32 protein is stabilized. For heat shock and ethanol stress, not only is ς32 stabilized, but the synthesis of ς32 is increased. Since our ς32 antisense mRNA targets the synthesis mechanism, it may have had more of an impact in heat and ethanol shock where ς32 synthesis normally increases.
In summary an in vivo antisense system for effective delivery and downregulation of ς32 was clearly demonstrated. Additionally, downregulation of a ς32-regulated protein, GroEL, was shown. One unique attribute of our result was to demonstrate that antisense RNA could be used to downregulate expression of a protein that, if unavailable or nonfunctional, would be lethal (ς32 knockouts are lethal above 20°C). We have also shown that by downregulating a sigma factor, we were able to influence the level of a downstream gene product for which the sigma factor is responsible under stress, GroEL. Through manipulation of a global regulatory unit, the ability to potentially affect the expression of an entire regulatory system can potentially be achieved. The transient nature of the process provides a further advantage by not causing any permanent change to the cell system being employed. Finally, we were able to show that by using antisense we were able to increase specific activity of OPH.
ACKNOWLEDGMENTS
This work was supported by the Division of Bioengineering and Environmental Systems grant BES 9319366-001 from the National Science Foundation.
The gene for OPH was graciously provided by J. Wild.
REFERENCES
- 1.Amann E, Brosius J, Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983;25:167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- 2.Belasco J G, Brawerman G, editors. Control of messenger RNA stability. San Diego, Calif: Academic Press Inc.; 1993. [Google Scholar]
- 3.Blaszczak A, Zylicz M, Georgopoulos C, Liberek K. Both ambient temperature and the DnaK chaperone machine modulate the heat shock response in Escherichia coli by regulating the switch between ς70 and ς32 factors assembled with RNA polymerase. EMBO J. 1995;14:5085–5093. doi: 10.1002/j.1460-2075.1995.tb00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brosius J, Erfle M, Storella J. Spacing of the −10 and −35 regions in the tac promoter. Effect on its in vivo activity. J Biol Chem. 1985;260:3539–3541. [PubMed] [Google Scholar]
- 5.Daugherty B L, Hotta K, Kumar C, Ahn Y H, Zhu J D, Pestka S. Antisense RNA: effect of ribosome binding sites, target location, size, and concentration on the translation of specific mRNA molecules. Gene Anal Techniques. 1989;6:1–16. doi: 10.1016/0735-0651(89)90007-1. [DOI] [PubMed] [Google Scholar]
- 6.Desai R P, Papoutsakis E T. Antisense RNA strategies for metabolic engineering of Clostridium acetobutylicum. Appl Environ Microbiol. 1999;65:936–945. doi: 10.1128/aem.65.3.936-945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamer J, Bujard H, Bukau B. Physical interaction between heat shock proteins DnaK, DnaJ, and GrpE and the bacterial heat shock transcription factor ς32. Cell. 1992;69:833–842. doi: 10.1016/0092-8674(92)90294-m. [DOI] [PubMed] [Google Scholar]
- 8.Gamer J, Multhaup G, Tomoyasu T, McCarty J S, Rudiger S, Schonfeld H, Schirra C, Bujard H, Bukau B. A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulates activity of the Escherichia coli heat shock transcription factor ς32. EMBO J. 1996;15:607–617. [PMC free article] [PubMed] [Google Scholar]
- 9.Georgiou G. Optimizing the production of recombinant proteins in microorganisms. AIChE J. 1988;34:1233–1248. [Google Scholar]
- 10.Gill R T, Valdes J J, Bentley W E. Reverse transcription-PCR differential display analysis of Escherichia coli global gene regulation in response to heat shock. Appl Environ Microbiol. 1999;65:5386–5393. doi: 10.1128/aem.65.12.5386-5393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Good L, Nielsen P E. Antisense inhibition gene expression in bacteria by PNA targeted to mRNA. Nat Biotechnol. 1998;16:355–358. doi: 10.1038/nbt0498-355. [DOI] [PubMed] [Google Scholar]
- 12.Gottesman S. Genetics of proteolysis in Escherichia coli. Annu Rev Genet. 1989;23:163–198. doi: 10.1146/annurev.ge.23.120189.001115. [DOI] [PubMed] [Google Scholar]
- 13.Gottesman S. Proteases and their targets in Escherichia coli. Annu Rev Genet. 1996;30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- 14.Herman C, Thevenet D, D'Ari R, Bouloc P. Degradation of sigma 32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc Natl Acad Sci USA. 1995;92:3516–3520. doi: 10.1073/pnas.92.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jubete Y, Maurizi M R, Gottesman S. Role of the heat shock protein DnaJ in the Lon-dependent degradation of naturally unstable proteins. J Biol Chem. 1996;217:30798–30803. doi: 10.1074/jbc.271.48.30798. [DOI] [PubMed] [Google Scholar]
- 16.Kanemori M, Mori H, Yura T. Induction of heat shock proteins by abnormal proteins results from stabilization and not increased synthesis of ς32 in Escherichia coli. J Bacteriol. 1994;176:5648–5653. doi: 10.1128/jb.176.18.5648-5653.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesley S A, Thompson N E, Burgess R R. Studies of the role of the Escherichia coli heat shock regulatory protein ς32 by the use of monoclonal antibodies. J Biol Chem. 1987;262:5404–5407. [PubMed] [Google Scholar]
- 18.Meerman H J, Georgiou G. Construction and characterization of a set of E. coli strains deficient in all known loci affecting the proteolytic stability of secreted recombinant proteins. Bio/Technology. 1994;12:1107–1110. doi: 10.1038/nbt1194-1107. [DOI] [PubMed] [Google Scholar]
- 19.Muffler A, Barth M, Marschall C, Hengge-Aronis R. Heat shock regulation of ςs turnover: a role for DnaK and relationship between stress responses mediated by ςs and ς32Escherichia coli. J Bacteriol. 1997;179:445–452. doi: 10.1128/jb.179.2.445-452.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray J A H, editor. Antisense RNA and DNA. New York, N.Y: Wiley-Liss; 1992. [Google Scholar]
- 21.Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magansik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. [Google Scholar]
- 22.Parsell D A, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damage proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- 23.Rapley R, Mannings D L, editors. RNA isolation and characterization protocols. Totowa, N.J: Humana Press; 1998. [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Strauss D B, Walter W A, Gross C A. The activity of ς32 is reduced under conditions of excess heat shock protein production in Escherichia coli. Genes Dev. 1989;3:2003–2010. doi: 10.1101/gad.3.12a.2003. [DOI] [PubMed] [Google Scholar]
- 26.Strauss D B, Walter W A, Gross C A. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of ς32. Genes Dev. 1989;4:2202–2209. doi: 10.1101/gad.4.12a.2202. [DOI] [PubMed] [Google Scholar]
- 27.Strauss D B, Walter W A, Gross C A. The heat shock response of E. coli is regulated by changes in the concentration of ς32. Nature. 1987;329:348–391. doi: 10.1038/329348a0. [DOI] [PubMed] [Google Scholar]
- 28.Tait R L, Tait R C. Recombinant DNA techniques: an introduction. Reading, Mass: Addison-Wesley; 1983. [Google Scholar]
- 29.Thomas J G, Baneyx F. Protein misfolding and inclusion body formation in recombinant Escherichia coli cells overexpressing heat-shock proteins. J Biol Chem. 1996;271:11141–11147. doi: 10.1074/jbc.271.19.11141. [DOI] [PubMed] [Google Scholar]
- 30.Tilly K, Spence J, Georgopoulos C. Modulation of the stability of Escherichia coli heat shock regulatory factor ς32. J Bacteriol. 1989;171:1585–1589. doi: 10.1128/jb.171.3.1585-1589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomoyasu T, Gamer J, Bukau B, Kanemori M, Mori H, Rtman A J, Oppenheim A B, Yura T, Yamanaka K, Niki H, Hiraa S, Ogura T. Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor ς32. EMBO J. 1995;14:2551–2560. doi: 10.1002/j.1460-2075.1995.tb07253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Kusukawa N, Erickson J W, Gross C A, Yura T. Isolation and characterization of Escherichia coli mutants that lack the heat shock sigma factor ς32. J Bacteriol. 1988;170:3640–3649. doi: 10.1128/jb.170.8.3640-3649.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]