Abstract
Lepidopteran insects are one of the most widespread and speciose lineages on Earth, with many common pests and beneficial insect species. The evolutionary success of their diversification depends on the essential functions of gut microorganisms. This diverse gut microbiota of lepidopteran insects provides benefits in nutrition and reproductive regulation and plays an important role in the defence against pathogens, enhancing host immune homeostasis. In addition, gut symbionts have shown promising applications in the development of novel tools for biological control, biodegradation of waste, and blocking the transmission of insect-borne diseases. Even though most microbial symbionts are unculturable, the rapidly expanding catalogue of microbial genomes and the application of modern genetic techniques offer a viable alternative for studying these microbes. Here, we discuss the gut structure and microbial diversity of lepidopteran insects, as well as advances in the understanding of symbiotic relationships and interactions between hosts and symbionts. Furthermore, we provide an overview of the function of the gut microbiota, including in host nutrition and metabolism, immune defence, and potential mechanisms of detoxification. Due to the relevance of lepidopteran pests in agricultural production, it can be expected that the research on the interactions between lepidopteran insects and their gut microbiota will be used for biological pest control and protection of beneficial insects in the future.
Keywords: lepidopteran insect, gut microbiota, diversity, function
1. Introduction
Lepidoptera is one of the most widespread and diverse insect clades in terms of the number of species and biomass [1,2]. Approximately 180,000 species of lepidopteran insects have been described all over the world, some of which act as forestry and agricultural pests, such as butterflies, moths, and skippers. They cause severe damage to major crops, and hence, an efficient strategy is required for management; however, adults of many species play a vital role in ecosystems as pollinators and as prey in the food chain. Others, such as Bombyx mori, can be regarded as economically important insects [3,4].
The microbiota of insects comprises bacteria, fungi, viruses, archaea, and protozoa, of which bacteria are found in the gut of almost all insects and are often the most abundant microbes [5,6,7]. Additionally, these microbial symbionts can be divided into endosymbionts and ectosymbionts based on whether they live within insect tissue cells or colonize the lumen or lining of insect body surface cavity walls [8]. Microbial inhabitants are pervasive in hosts and have ubiquitous impacts on multiple aspects of insect biology [9,10]. The evolutionary and diversification success of insects into large-scale ecological niches depends on the beneficial microbiome, which is known to promote insect fitness, protect hosts against parasites and pathogens, detoxify insecticidal defence chemicals, and stimulate host immune responses, in addition to its biotechnological applications [11,12,13,14]. For example, gut microbial communities enhance digestive efficiency by providing enzymatic functions and facilitating vitamin synthesis, helping their hosts optimize nutrient absorption and energy extraction [15]. Liang et al. reported that Enterococcus mundtii, isolated from the B. mori gut, efficiently produces lactic acid under extremely alkaline conditions and is an important metabolite for industrial bioplastic polylactic acid production [16]. In contrast, the lepidopteran microbiome is affected by many factors, including the environment, diet, gut physiology, and developmental stage. Recent studies have found that the microbial community changes significantly between early (1st and 2nd) and late (3rd to 5th) instar silkworms, consistent with host developmental changes [5].
With recent developments in Next-generation sequencing (NGS) technology, a growing number of studies have catalogued and characterized microbial communities [17,18,19]. To date, several studies have examined their role via approaches ranging from community functional diversity surveys to the examination of gut bacterial interaction mechanisms with hosts. However, many of the studies are mostly descriptive and have focused on larvae, while only a few have addressed the potential impact on host traits. Here, we highlight the structure, function, and host relationships of the lepidopteran insect gut microbiota. Based on previous studies of the gut microbiota, we provide some promising new insights into the fundamental molecular mechanisms of insect immunity and integrated pest management applications (i.e., utilizing symbionts to control devastating pests) [20].
2. Gut Structure of Lepidopterans
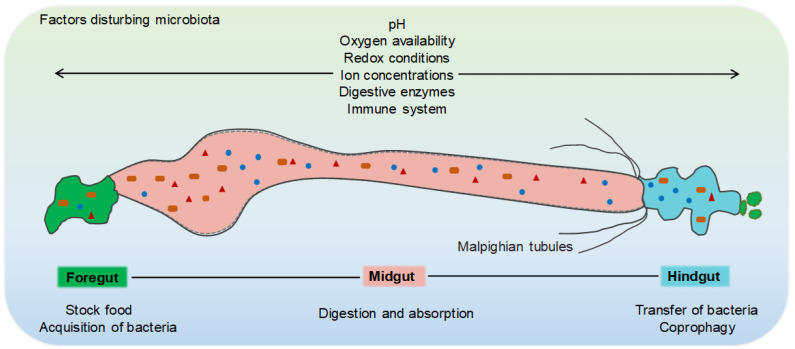
The alimentary canal of the lepidopteran larva is divided into three primary regions: The foregut and hindgut, which arise from the embryonic ectoderm and are lined with the chitin exoskeleton, and the midgut, which originates from the ectoderm [21,22,23]. The demarcations of each part are distinguished by the cardiac valve (between the foregut and midgut) and pylorus (between the midgut and hindgut) (Figure 1).
Figure 1.
Gut structure of lepidopteran insects. Take the B. mori as an example. The foregut and hindgut are lined by a cuticle layer (thick black line), and the midgut secretes a peritrophic matrix (dashed line). Factors influencing the composition of the gut microbiota of lepidopteran insects include host development, pH, oxygen availability, redox conditions, ion concentrations, digestive enzymes and the immune system in different gut compartments, available sources for bacterial acquisition, and the capability to transfer bacteria to progeny. Green indicates the foregut, red indicates the midgut, and blue indicates the hindgut.
The foregut of many lepidopteran insects comprises the crop and oesophagus, where food fragments are stored [24,25]. For most lepidopteran larvae, including silkworms, the midgut is observably larger than the foregut and hindgut, secreting the vast majority of enzymes and small molecules for food digestion, such as proteases and carbohydrases. Thus, the midgut is the primary site of digestion and absorption. In addition, the midgut lines the peritrophic matrix, which includes three different cell types: goblet cells, columnar cells, and stem cells [26,27,28]. Goblet cells are considered to differentiate from stem cells, and there is evidence that lepidopteran insect goblet cells have a critical role in the gut immune defence [29,30,31]. The peritrophic matrix distinguishes the midgut into two spaces: endo-peritrophic and ecto-peritrophic. Normally, gut microorganisms cannot cross the endo-peritrophic space, which prevents them from coming in direct contact with midgut epithelial cells [32,33,34]. The hindgut of lepidopteran insects consists of three regions: the ileum, the rectum, and the posterior rectum. They mediate the uptake of uric acid, water, and salts derived from Malpighian tubules, which are part of the excretory system of insects [30,35]. All these structures are beneficial to the caterpillar, leading to very high feeding and food digestion rates [36,37].
Gut physicochemical conditions can influence the metabolic activity of gut microbes, including pH, oxygen availability, redox conditions, ion concentrations, and digestive enzymes [30,38,39]. The pH of the digestive tract is energetically regulated and often departs from that of the haemolymph, which is generally approximately 7 [40,41,42]. The gut of lepidopteran larvae generally exhibits extreme alkalinity, with a midgut pH as high as 7–12 [43]. Compared with that of the midgut, the alkalinity of the foregut and hindgut is relatively weak. Therefore, digestive enzymes in the lepidopteran gut are accustomed to alkaline conditions [44,45]. For example, the activity of intestinal phosphatase as a mucosal defence factor requires an alkaline environment, which is achieved by the proton pump activity of V-ATPase [22,46].
3. Diversity of the Lepidopteran Gut Microbiota
Lepidopteran insects harbor a large number of microbiota in their midgut, which includes both pathogenic and nonpathogenic bacteria [47]. Insights into the composition of the species-specific gut microbiota are principally obtained from culture-independent techniques and NGS approaches [48,49]. Similar to other invertebrate and vertebrate species, highly active microorganisms live inside the lepidopteran gut, including bacteria, fungi, and archaea. Paniagua Voirol et al. surveyed 30 lepidopteran species and found that gut bacteria of the Enterobacteriaceae, Pseudomonadaceae, and Bacillaceae families were the most widespread [50]. For example, Enterobacter, Pantoea, Pseudomonas, and Acinetobacter were present in Acronicta major larvae [5]. Compared to that in A. major, the bacterial diversity observed in Diaphania pyloalis was observably simple, principally composed of Wolbachia (40.60%) [5]. In addition, bacteria detected in the gut microbiota of B. mori larvae were distinguished based on high-throughput sequencing. The results show that Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes are dominant species [5]. In the intestines of other lepidopteran insects, such as Plutella xylostella, high-throughput sequencing revealed that 97% of the bacteria were from Enterobacteriales (45.17%), Vibrionales (22.51%), and Lactobacillales (29.49%) [51]. A core gut community consisting of Enterococci (42.3%) and Clostridium (42.2%) were revealed in S. littoralis larvae, Helicoverpa armigera larvae, and S. littoralis larvae [52].
Notably, the type of diet, host plant, season, population density, and geographic position influence gut bacterial diversity [53]. Feeding can change the gut microbiota community of lepidopteran insects [52,54]. For instance, mulberry leaves are primarily composed of xylan (10–40%) and cellulose (19–25%), which shows the importance of intestinal microbes for food digestion in silkworms [55]. In 5th-instar larvae of B. mori fed on mulberry leaves (the traditional rearing method), the gut microbiota is dominated by Rhodococcus, Escherichia, and Enterococcus [3,56]. When the diet was changed to lettuce leaves, Bacteroides and Acinetobacter were the predominant species [57]. In addition, the species diversity and richness of the gut microbial communities showed a significant relationship with the Agrilus planipennis Fairmaire population size [58]. Furthermore, lepidopteran insects are holometabolic, and few studies have reflected the gut microbiota composition throughout development from egg to adult, especially in monophagous species. Francisco et al. showed that the bacterial composition of Brithys crini was stage-specific, and Rosenbergiella and Serratia were highly abundant in the eggs. Twenty-seven genera (Empedobacter, 23%; Enterococcus, 10%) were statistically more abundant in larvae, while only one genus (Serratia, 75%) was significantly more abundant in adults [59]. More surprisingly, recent work has shown that DNA extraction methodology has the largest effect on the outcome of the metagenomic analysis in B. mori gut microbiome studies based on high-throughput 16S rRNA gene sequencing and computational analysis [60]. A taxonomic analysis revealed that the most common phylum was Proteobacteria, which, together with Firmicutes and Actinobacteria, was detected in lepidopteran insects. At the genus level, the dominant bacteria were mainly Enterococcus, Enterobacter, Clostridium, Acinetobacter, Pseudomonas, Pantoea, and Bacillus. The composition of the dominant gut microbiota of other insects was different. These differences depend on the diet source and behavioral characteristics of the host insects, which show the relationship between gut symbiotic bacteria and the coevolution of the host from another perspective [61,62].
Although a few sequencing-based studies have confirmed the composition of gut bacteria, lepidopteran fungal communities have been largely ignored. However, endosymbiotic fungi are also ubiquitous among lepidopteran insects. Here, we review the reported fungal gut microbiota of lepidopteran insects, including Lycaeides melissa, A. planipennis, A. major, D. pyloalis, and B. mori. Basidiomycota and Ascomycota predominated the gut fungal communities, as determined by sequencing of the fungal internal transcribed spacer (ITS). Most fungal sequences were assigned to the genera Ascomycota and Basidiomycota. At the genus level, most fungal sequences were assigned to the genera Cladosporium, Hannaella, Kabatiella, Pyrenochaeta, Pyrenochaeta, Malassezia, and Rhodosporidium [5,58,63].
4. Functional Roles of the Lepidopteran Gut Microbiota
The success of lepidopteran insects in diversity and evolution depends on various beneficial gut symbiotic bacteria, especially for upgrading nutritionally deficient diets [64]. The limited metabolic networks of most insects have been enhanced by symbiotic relationships. The insect gut is colonized by multitudinous communities of resident bacteria, and such microbes are considered to be essential for the fecundity, development, and growth of the hosts [65,66]. They not only play important roles in food digestion and the production of vitamins but also contribute positively by protecting the host against pathogens, detoxifying insecticidal defence chemicals and stimulating the host immune response [67,68] (Table 1).
Table 1.
The category, function and reference for some important symbiotic bacteria and fungi of lepidopteran insects.
| Genus Level | Category | Function | |
|---|---|---|---|
| Bacteria | Bacillus | Firmicutes | Counteract anti-herbivore plant defences [69] |
| Staphylococcus | Firmicutes | Against plant-derived protease inhibitor; pest control [70] | |
| Enterococcus | Firmicutes | Increase anti-herbivore defence; insecticidal activities [71] | |
| Methylobacterium | Proteobacteria | Nitrogen fixation [72] | |
| Sphingomonas | Proteobacteria | Microbe-mediated detoxification of phytotoxins and pesticides [73] | |
| Propionibacterium | Actinobacteria | Produce antimicrobial peptides [74] | |
| Microbacterium | Actinobacteria | Antibiotic-resistant [75] | |
| Pseudomonas | Pseudomonas | Anti-phytopathogenic fungi [76] | |
| Pantoea | Proteobacteria | Affect oviposition behavior, morphogenesis and development [77,78] | |
| Acinetobacter | Proteobacteria | Metabolize insecticides [79] | |
| Enterobacter | Proteobacteria | Anti-phytopathogenic fungi activity; growth and development [80] | |
| Wolbachia | Proteobacteria | Participate in reproductive regulations, increase host resistance [81,82] | |
| Fungi | Stenotrophomonas | Pseudomonadaceae | Insecticide resistance [83] |
| Cladosporium | Ascomycota | Produce many antimicrobial agents [84] | |
| Botrytis | Ascomycota | Development and oviposition behavior [85] | |
| Fusarium | Ascomycota | Secretory defence [86] | |
| Cryptococcus | Basidiomycota | Immune Response [86] | |
| Clonostachys | Ascomycota | Potential biocontrol agents [87] | |
| Erythrobasidium | Basidiesvampar | Potential biocontrol agents [88] |
4.1. Host Nutrition and Metabolism
Insects provide stable environments and nutrition for symbionts, and in return, symbionts can offer the host necessary enzymes for food digestion, thereby expanding the host’s diet options and even changing the host’s eating habits [89]. The symbionts of the gut can contribute to the nitrogen cycle and can also produce nutrients that are essential to the development of the host organisms but are lacking in natural food, including amino acids, B vitamins, and sterols [90]. For instance, 118 culturable bacterial strains were isolated from the intestine of Diatraea saccharalis larvae. Among them, Klebsiella, Stenotrophomonas, Microbacterium, Bacillus, and Enterococcus were found to possess cellulolytic activity. All bacterial strains were cultured using soluble carboxymethyl cellulose (CMC) for degradation assays, and Bacillus and Klebsiella showed the highest degradation activity [91]. In addition, ten gut bacteria were isolated from the lepidopteran insect gut by in vitro culture, including gram-positive and gram-negative bacteria. Klebsiella can hydrolyse starch, whereas Proteus vulgaris, Erwinia sp., and Serratia liquefaciens can utilize xylanolytic, pectinolytic, and polysaccharides, respectively [56]. The main components of mulberry leaves are cellulose (19% to 25%) and xylan (10% to 40%), which shows the importance of the intestinal microbes of silkworms for food digestion [92,93].
Gut symbionts (Bacillus cereus, Enterococcus gallinarum, E. mundtii, Staphylococcus xylosus) are the pivotal species in soybean pests and are abundant in the caterpillar host. They exhibit a high tolerance for serine-proteinase inhibitors [70]. Enterobacter asburiae YT1 and Bacillus YP1 from the larvae of Plodia interpunctella were capable of degrading polyethylene films [94]. In addition, vitamins are the fundamental micronutrients that are normally found as precursors of various enzymes that are necessary for vital biochemical reactions during insect growth and development [95]. Hassan et al. tested the hypothesis that two actinobacterial gut symbionts provide Dysdercus fasciatus with B vitamins [37]. Insects actively harvest vitamins from bacterial symbionts by using specific enzymes that burst open the bacterial cell walls and thereby ensure host metabolic homeostasis [47].
4.2. Pathogen and Immune Defences
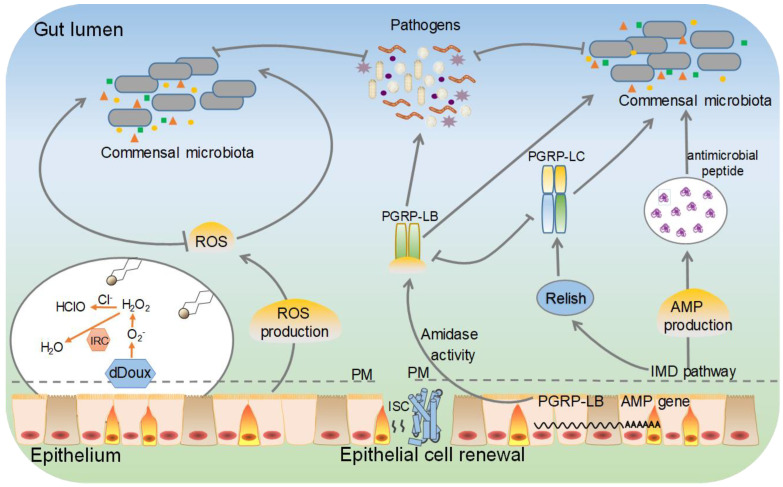
Under normal living conditions, the gut is the first line of defence because ingestion is the most likely route by which organisms come in contact with pathogens, including bacteria, fungi, viruses, and parasites [96,97]. Insects lack adaptive immune components, such as B cells and T cells, and rely on innate immune responses against infection. To combat infection, insects rely on multiple innate defence mechanisms, including the use of immune responses together with resource competition [98]. Lepidopteran insect guts use a battery of strategies, such as the generation of reactive oxygen species (ROS), to defend against harmful bacteria through cellular immune responses. Moreover, antimicrobial peptides (AMPs) and other immune effector molecules exhibit a broader spectrum of antimicrobial activity (Figure 2). In addition, the peritrophic membrane is a semipermeable barrier that can prevent most pathogens from damaging gut tissue via per os infection [99,100,101].
Figure 2.
Putative immune signalling pathways are involved in the defences against pathogenic microbial infections in the gut of lepidopteran insects. This model is based on the local production of reactive oxygen species (ROS), and antimicrobial peptide (AMP) of Drosophila and findings in lepidopteran insects. The immune deficiency (IMD) includes the major signalling pathways inducing AMP production, and AMP genes provide inducible defense mechanisms in the gut. PM, peritrophic matrix.
Bacteria are important pathogens of lepidopteran insects [102]. Bacterial peptidoglycans (PGs) and proteases may disrupt the host’s cellular and biochemical processes [103,104]. The recognition of pathogens by lepidopteran insects relies on the interaction between pathogen-associated molecular patterns (PAMPs) and pattern-recognition receptors (PRRs) [105,106]. Lys (lysine) and lipopolysaccharide (LPS), as immune stimulators in insects, are major components of bacterial cell walls. They can trigger strong host immune responses in multitudinous insects [107,108,109]. In the midgut of lepidopteran insects, the immune reaction is primarily mediated by regulating the expression level of key immune components in the dual oxidase (DUOX) system and the immune deficiency (IMD) pathway, thus obtaining immune tolerance to beneficial gut microorganisms. Larvae carrying a Duox deletion are more susceptible to bacterial infection. Similar to the immune response, the local systemic response is regulated via the recognition of gram-negative proteoglycans (PGNs) by peptidoglycan recognition protein LC (PGRP-LC). Injection of pathogenic bacteria induces transient expression of AMP genes, suggesting the existence of a mechanism to downregulate the host immune response (Figure 2) [110,111]. For example, the expression of BmDuox was significantly upregulated in the midgut of B. mori fed Escherichia coli. Microbial proliferation in the midgut was increased after BmDuox knockout, suggesting that BmDuox has an important role in maintaining gut microbial homeostasis [112]. Peroxiredoxins (Prxs), as antioxidant enzymes in the lepidopteran insect gut, are notably enriched upon Pseudomonas aeruginosa and Bacillus bombyseptieus infection, and increased ROS levels can be induced by bacterial infection and proliferation [103,113]. In addition, the immune system of Hyalophora cecropia and Galleria mellonella was found to contain P9A and P9B antibacterial proteins, which are active against several Gram-negative bacteria (i.e., Escherichia coli and P. aeruginosa) [114]. In some lepidopteran insects, such as Choristoneura fumiferana, depletion of the gut microbiota increases the susceptibility of hosts to pathogenic infection [115]. Intriguingly, some lepidopteran gut microbes are universal opportunistic pathogens [50]. A commensal-to-pathogen switch is observed under multifactorial conditions, which depends on the pathogens and immune status of the host. This poses the question of how the immune system in the gut distinguishes between symbiotic microorganisms and pathogenic bacteria [116].
Fungi, such as Beauveria bassiana, Metarhizium anisopliae, and Microsporidia, are another group of important pathogens of lepidopteran insects [117]. Transcriptomic analyses revealed that infection by the B. bassiana strain upregulated the expression of immunity-related genes in G. mellonella, including hydrolytic enzymes, β-1,3-glucan recognition proteins, and spätzle genes [118]. A significant increase in the expression pattern of prophenoloxidase cascade (PPO) genes was found in Chilo suppressalis after treatment with B. bassiana, M. anisopliae, Isaria fumosoroseus and Lecanicilium lecanii, suggesting that host immune responses are critical against fungal infections [119]. Another study predicted serine proteases (SPs) and pattern recognition receptors (PRRs) as upstream components of the Toll pathway in Manduca sexta and Spodoptera exigua infected with Metarhizium rileyi [120]. In addition, Microsporidia, which are pathogens of lepidopteran insects, are a group of obligate intracellular parasites related to fungi. N. bombycis mainly infects B. mori through oral infection, and cuticle infection occasionally occurs [117]. Virulence studies showed that per os infection of silkworm larvae by microsporidia led to stimulation of the JAK/STAT and Toll signalling pathways in the midgut, which possibly induced the upregulation of AMPs to defend against the invading N. bombycis. The subtilisin-like serine protease NbSLP1 was activated after infection of N. bombycis in the midgut [121]. NbSLP1 is localized at the two poles of the spore and is likely involved in the polar tube extrusion process [122]. Two studies have also shown that feeding with Enterococcus faecalis LX10 or Lactobacillus could reduce the spore germination rate or increase the survival rate of silkworm larvae challenged by N. bombycis [3,123].
Viruses are significant natural pathogens of lepidopteran insects, and horizontal transmission of viruses is common in these species [124]. In addition, viruses infecting beneficial insects such as silkworms or bees can cause significant economic losses [125]. Host responses to viral infections include immunoreactions as well as mechanical barriers that prevent viruses from establishing infection [126]. Agata et al. observed that baculovirus infection leads to decreased expression of immune genes in the S. exigua larval gut. The expression of immune genes affects the diversity of gut microorganisms, many of which are responsible for growth and development functions [126]. In addition, several immune-related genes were found to be implicated in the midgut’s response against BmCPV infection of B. mori larval, including proteolytic enzymes, hormonal signaling, and heat-shock proteins [127]. In Drosophila, RNAi is a powerful method for defending against viruses, and activation of the Toll pathway inhibited Drosophila virus growth [128]. In the midgut of B. mori, alkaline trypsin protein and serine protease-2 showed strong antiviral activity, while immunoglobulin proteins, including Hemolin, a lepidopteran plasma protein produced after viral injection, demonstrated antiviral activity in oak silkworm, M. sexta and the Samia cecropia [129,130]. These studies indicate that lepidopterans circulate key proteins that serve as potent antiviral factors in the midgut.
Recent studies have shown that gut microorganisms can protect insects from propagating pathogens by accommodating host metabolism and repairing gut wall integrity, stimulating the host immune system and serving as essential probiotics for insect growth and development [131,132].
4.3. Potential Mechanism of Detoxificationby Lepidopteran Gut Bacteria
Gut symbiotic bacteria can also assist the host in degrading toxic or harmful substances, including insecticides, secondary plant compounds, and microplastics.
It has been reported that bacteria can directly degrade organic insecticides, such as ethoprophos, dimethoate, and chlorpyrifos, and these bacteria are often ingested from sources in the environment and food sources by agricultural pests [133,134,135]. Moreover, the gut microbiota may also enhance detoxification by influencing host fitness and the immune system [136]. For example, some soil Burkholderia strains degrading fenitrothion establish symbiosis with Riptortus pedestris and enhance host resistance to fenitrothion [137]. Indoxacarb is a highly effective insecticide widely used in the production of fruits and vegetables. B. cereus from the P. xylostella (Linnaeus) gut microbiota degraded indoxacarb by up to 20% and could use insecticides as an energy substance for growth and metabolism [138]. In addition, monoassociation of B. mori with gut bacteria of the genus Stenotrophomonas enhanced host resistance to organophosphate insecticides (chlorpyrifos), as confirmed by gut metabolomic analysis [83].
The majority of plants produce a wide variety of secondary metabolites that are toxic to pathogens and herbivores [139]. Recent studies have shown that gut microorganisms can assist the host in degrading toxic secondary metabolites. For instance, the gut bacteria Acinetobacter sp. R7-1 of Lymantria dispar has already been confirmed to metabolize aspen foliage secretion (phenolic glycosides) [140]. In particular, Klebsiella sp. and Corynebacterium have been isolated from the polyphagous pest larvae of Brithys crini, which participate in the degradation of alkaloids [141]. Gut bacteria protect Trichoplusia ni and Spodoptera eridania from the host plant toxin hydrogen cyanide (HCN) [142]. Some gut bacteria of Trichoplusia ni and S. eridania are capable of detoxifying toxic HCN, producing β-cyanoalanine (nontoxic product) and cysteine [143]. In addition, the E. casseliflavus strain was isolated from the gut and exhibited the ability to tolerate natural latex under laboratory conditions [141]. Xia et al. revealed an important role of Enterobacter cloacae, E. asburiae, and Carnobacterium maltaromaticum in the breakdown of plant cell walls, detoxification of plant phenolics, and synthesis of amino acids of the P. xylostella gut [144]. Members of the genera Pseudomonas, Burkholderia, and Cupriavidus were selected from the moth Retinia resinella and exhibited the ability to degrade specific resin acids such as dehydroabietic or isopimaric acid (diterpenes) [145].
In addition, several polyethylene (PE)-degrading bacteria and fungi have been reported, such as Aspergillus, Acremonium, Fusarium, E. asburiae, and Bacillus [146]. PE is one of the polymer materials that are remarkably resistant to degradation [147]. A fungal strain, Aspergillus flavus, was isolated as a potential microplastic particle-degrading microorganism from the gut contents of wax moth G. mellonella larvae by producing extracellular enzymes [148]. E. asburiae and Bacillus strains isolated from the gut of P. interpunctella can degrade polyethylene by forming biofilms that reduce the hydrophobicity of PE [94]. Subsequently, Ren et al. isolated the Enterobacter sp. strain D1, with the ability to degrade PE films, from the gut of G. mellonella (Ren et al., 2019). Recently, B. mori has also been applied in nanotoxicology studies to assess the potential effects of TiO2 nanoparticles on intestinal microbes [149]. These results suggested that the gut of insects might serve as a potential source for selecting PE-degrading microorganisms. It may also be possible to develop new strategies to reduce the toxic effects of xenobiotics on insects by leveraging their microbial symbionts.
4.4. Potential Application of Gut Symbionts in Controlling Lepidopteran Pests
The frequent application of insecticides has led to the ongoing development of high resistance in the past two decades, enhancing the urgent need for environmentally friendly long-term alternative strategies to control them [6,150]. Recent studies have shown that bacterial symbionts constitute promising microbial control agents (MCAs) with potential applications in controlling major lepidopteran agricultural pests, such as P. xylostella, S. littoralis, and C. fumiferana [151]. B. thuringiensis (Bt) strains have been developed as commercial biopesticides for more than a decade. Xia et al. found that the abundance of some bacteria in the larval midgut was related to the insecticide resistance of P. xylostella. Inoculating larvae with culturable gut microbes (Enterobacter sp. Mn2) reduced larval mortality after infection with B. thuringiensis in other studies, indicating that the gut microbiota can protect taxonomically diverse hosts from pathogen attack [51]. Xenorhabdus nematophila is another entomopathogenic bacterium that is symbiotically associated with parasitic nematodes (Steinernema). It is very effective against lepidopterans, such as the beet armyworm and diamondback moth [152,153].
In addition, Wolbachia species are widespread endosymbionts of lepidopteran insects. Wolbachia species, which are naturally occurring endosymbiotic bacteria found inside the cells of arthropods and filarial nematodes, can manipulate the host reproduction system [82] and are generally known as potential environmentally friendly biopesticides for the control of disease vectors and pests [154]. Recent studies found that Wolbachia species infect approximately 40% of terrestrial arthropod species, such as Lepidoptera, Hymenoptera, and Diptera species [155,156]. Cytoplasmic incompatibility (CI) is one of the most common phenotypes of reproductive manipulation in Wolbachia [157]. For instance, infection of Homona magnanima by multiple Wolbachia strains causes CI in the host, and Wolbachia increases the H. magnanima pupal weight and shortens the host development time [158]. Interestingly, Wolbachia spreads vertically in insects and is inherited maternally due to its presence in the cytoplasm of female gametes. Fukui et al.’s inhibition study of Ostrinia moths found that Wolbachia targets the population masculinization gene of the host to accomplish male killing by a failure of dosage compensation through unproductive mating [159].
These studies suggest that bacterial symbionts are essential in the evolution of insects, and thus, elucidating the role of bacterial symbionts of lepidopterans might help in the development of improved methods of biological control.
5. Conclusions: Implication and Outlook
The gut of lepidopteran insects is the primary site of digestion and absorption. It is the first line of defence against pathogens. With the development of molecular technologies such as high-throughput sequencing of the 16S rRNA gene and metagenome analysis, the research bottleneck has been overcome. Researchers can not only determine the classification and composition of the gut microbiota but also reveal the potential functions of symbionts in the host. The microbial community is closely related to host defence against pathogens. An artificial feeding system with gut wall cells cultured in vitro can be used to simulate the insect gut to evaluate the interactions of the host and gut microbiota.
In addition, we generalized the functions of the gut microbiota in lepidopteran insects (Table 1) to provide an overview of lepidopteran insect gut immune pathways (Figure 2). In this way, we can obtain a better understanding of the mechanisms of the gut microbiota. The established inventory of the microbiota of lepidopteran insects can be supplemented with a large amount of information on its taxonomy and genetics. These findings will help to clarify the microbiological composition of the lepidopteran insect gut and the specific functions of bacteria in such a specialized environment. On the other hand, if we alter the microecological composition of lepidopteran insects, for instance, by manipulating certain intestinal bacteria to improve their disease resistance ability and energy utilization through the digestive tract, enhanced biocontrol strategies against lepidopteran pests can be developed. In addition, the gut microbiota has the potential to be an environmentally friendly alternative control agent for diseases of economically important and beneficial insects and crops.
Author Contributions
Conceptualization, X.Z., F.Z. and X.L.; investigation, X.Z.; writing—original draft preparation, X.Z.; writing—review and editing, F.Z. and X.L. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available in this article only.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the China Agriculture Research System of MOF and MARA, and the National Natural Science Foundation of China (81572027).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Quah S., Hui J.H., Holland P.W. A Burst of miRNA Innovation in the Early Evolution of Butterflies and Moths. Mol. Biol. Evol. 2015;32:1161–1174. doi: 10.1093/molbev/msv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szabó A.K., Várallyay É., Demian E., Hegyi A., Galbács Z.N., Kiss J., Bálint J., Loxdale H.D., Balog A. Local Aphid Species Infestation on Invasive Weeds Affects Virus Infection of Nearest Crops Under Different Management Systems-A Preliminary Study. Front. Plant Sci. 2020;11:684. doi: 10.3389/fpls.2020.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X., Feng H., He J., Liang X., Zhang N., Shao Y., Zhang F., Lu X. The gut commensal bacterium Enterococcus faecalis LX10 contributes to defending against Nosema bombycis infection in Bombyx mori. Pest. Manag. Sci. 2022;78:2215–2227. doi: 10.1002/ps.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massot M., Bagni T., Maria A., Couzi P., Drozdz T., Malbert-Colas A., Maibeche M., Siaussat D. Combined influences of transgenerational effects, temperature and insecticide on the moth Spodoptera littoralis. Environ. Pollut. 2021;289:117889. doi: 10.1016/j.envpol.2021.117889. [DOI] [PubMed] [Google Scholar]
- 5.Chen B., Du K., Sun C., Vimalanathan A., Liang X., Li Y., Wang B., Lu X., Li L., Shao Y. Gut bacterial and fungal communities of the domesticated silkworm (Bombyx mori) and wild mulberry-feeding relatives. ISME J. 2018;12:2252–2262. doi: 10.1038/s41396-018-0174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie S., Lan Y., Sun C., Shao Y. Insect microbial symbionts as a novel source for biotechnology. World J. Microbiol. Biotechnol. 2019;35:25. doi: 10.1007/s11274-019-2599-8. [DOI] [PubMed] [Google Scholar]
- 7.Kaltenpoth M., Florez L.V. Versatile and Dynamic Symbioses Between Insects and Burkholderia Bacteria. Annu. Rev. Entomol. 2020;65:145–170. doi: 10.1146/annurev-ento-011019-025025. [DOI] [PubMed] [Google Scholar]
- 8.Pan X., Wang X., Zhang F. New Insights into Cockroach Control: Using Functional Diversity of Blattella germanica Symbionts. Insects. 2020;11:696. doi: 10.3390/insects11100696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senderovich Y., Halpern M. The protective role of endogenous bacterial communities in chironomid egg masses and larvae. ISME J. 2013;7:2147. doi: 10.1038/ismej.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma J., Wang Z., Li H., Park H.-D., Wu Z. Metagenomes reveal microbial structures, functional potentials, and biofouling-related genes in a membrane bioreactor. Appl. Microbiol. Biotechnol. 2016;100:5109–5121. doi: 10.1007/s00253-016-7312-3. [DOI] [PubMed] [Google Scholar]
- 11.Xiang H., Li M., Zhao Y., Zhao L., Zhang Y., Huang Y. Bacterial community in midguts of the silkworm larvae estimated by PCR/DGGE and 16S rDNA gene library analysis. Jiangxi Sci. 2007;50:222–233. [Google Scholar]
- 12.Brettar I., Christen R., Höfle M.G. Analysis of bacterial core communities in the central Baltic by comparative RNA–DNA-based fingerprinting provides links to structure–function relationships. ISME J. 2012;6:195. doi: 10.1038/ismej.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid N.M., Addison S.L., West M.A., Lloyd-Jones G. The bacterial microbiota of Stolotermes ruficeps (Stolotermitidae), a phylogenetically basal termite endemic to New Zealand. FEMS Microbiol. Ecol. 2014;90:678–688. doi: 10.1111/1574-6941.12424. [DOI] [PubMed] [Google Scholar]
- 14.Alberoni D., Gaggìa F., Baffoni L., Di Gioia D. Beneficial microorganisms for honey bees: Problems and progresses. Appl. Microbiol. Biotechnol. 2016;100:9469–9482. doi: 10.1007/s00253-016-7870-4. [DOI] [PubMed] [Google Scholar]
- 15.Matos R.C., Schwarzer M., Gervais H., Courtin P., Joncour P., Gillet B., Ma D., Bulteau A.L., Martino M.E., Hughes S., et al. D-Alanylation of teichoic acids contributes to Lactobacillus plantarum-mediated Drosophila growth during chronic undernutrition. Nat. Microbiol. 2017;2:1635–1647. doi: 10.1038/s41564-017-0038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang X., Sun C., Chen B., Du K., Yu T., Luang-In V., Lu X., Shao Y. Insect symbionts as valuable grist for the biotechnological mill: An alkaliphilic silkworm gut bacterium for efficient lactic acid production. Appl. Microbiol. Biotechnol. 2018;102:4951–4962. doi: 10.1007/s00253-018-8953-1. [DOI] [PubMed] [Google Scholar]
- 17.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radajewski S., Ineson P., Parekh N.R., Murrell J.C. Stable-isotope probing as a tool in microbial ecology. Nature. 2000;403:646. doi: 10.1038/35001054. [DOI] [PubMed] [Google Scholar]
- 19.Dillon R., Webster G., Weightman A.J., Dillon V., Blanford S., Charnley A.K. Composition of Acridid gut bacterial communities as revealed by 16S rRNA gene analysis. J. Invertebr. Pathol. 2008;97:265–272. doi: 10.1016/j.jip.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Wu K., Yang B., Huang W., Dobens L., Song H., Ling E. Gut immunity in Lepidopteran insects. Dev. Comp. Immunol. 2016;64:65–74. doi: 10.1016/j.dci.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Engel P., Moran N.A. The gut microbiota of insects–diversity in structure and function. FEMS Microbiol. Rev. 2013;37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- 22.Gomes F.M., Carvalho D.B., Machado E.A., Miranda K. Ultrastructural and functional analysis of secretory goblet cells in the midgut of the lepidopteran Anticarsia gemmatalis. Cell Tissue Res. 2013;352:313–326. doi: 10.1007/s00441-013-1563-4. [DOI] [PubMed] [Google Scholar]
- 23.Guarner F., Malagelada J.-R. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 24.Brune A., Friedrich M. Microecology of the termite gut: Structure and function on a microscale. Curr. Opin. Microbiol. 2000;3:263–269. doi: 10.1016/S1369-5274(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 25.Buchon N., Broderick N.A., Chakrabarti S., Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes. Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards M.J., Jacobs-Lorena M. Permeability and disruption of the peritrophic matrix and caecal membrane from Aedes aegypti and Anopheles gambiae mosquito larvae. J. Insect Physiol. 2000;46:1313–1320. doi: 10.1016/S0022-1910(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 27.Egert M., Wagner B., Lemke T., Brune A., Friedrich M.W. Microbial community structure in midgut and hindgut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae) Appl. Environ. Microbiol. 2003;69:6659–6668. doi: 10.1128/AEM.69.11.6659-6668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engel P., Martinson V.G., Moran N.A. Functional diversity within the simple gut microbiota of the honey bee. Proc. Natl. Acad. Sci. USA. 2012;109:11002–11007. doi: 10.1073/pnas.1202970109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genta F.A., Dillon R.J., Terra W.R., Ferreira C. Potential role for gut microbiota in cell wall digestion and glucoside detoxification in Tenebrio molitor larvae. J. Insect Physiol. 2006;52:593–601. doi: 10.1016/j.jinsphys.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Johnson K.S., Barbehenn R.V. Oxygen levels in the gut lumens of herbivorous insects. J. Insect Physiol. 2000;46:897–903. doi: 10.1016/S0022-1910(99)00196-1. [DOI] [PubMed] [Google Scholar]
- 31.Emery H., Johnston R., Rowley A.F., Coates C.J. Indomethacin-induced gut damage in a surrogate insect model, Galleria mellonella. Arch. Toxicol. 2019;93:2347–2360. doi: 10.1007/s00204-019-02508-4. [DOI] [PubMed] [Google Scholar]
- 32.Hongoh Y. Diversity and genomes of uncultured microbial symbionts in the termite gut. Biosci. Biotechnol. Biochem. 2010;74:1145–1151. doi: 10.1271/bbb.100094. [DOI] [PubMed] [Google Scholar]
- 33.Hongoh Y.J.B. Toward the functional analysis of uncultivable, symbiotic microorganisms in the termite gut. Cell. Mol. Life Sci. 2011;68:1311–1325. doi: 10.1007/s00018-011-0648-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X., Cooper A.M., Zhang J., Zhu K.Y. Biosynthesis, modifications and degradation of chitin in the formation and turnover of peritrophic matrix in insects. J. Insect Physiol. 2019;114:109–115. doi: 10.1016/j.jinsphys.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Thong-On A., Suzuki K., Noda S., Inoue J.-i., Kajiwara S., Ohkuma M. Isolation and characterization of anaerobic bacteria for symbiotic recycling of uric acid nitrogen in the gut of various termites. Microbes Environ. 2012;27:286–292. doi: 10.1264/jsme2.ME11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mason C.J., Ray S., Shikano I., Peiffer M., Jones A.G., Luthe D.S., Hoover K., Felton G.W.J. Plant defenses interact with insect enteric bacteria by initiating a leaky gut syndrome. Proc. Natl. Acad. Sci. USA. 2019;116:15991–15996. doi: 10.1073/pnas.1908748116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen B., Xie S., Zhang X., Zhang N., Feng H., Sun C., Lu X., Shao Y. Gut microbiota metabolic potential correlates with body size between mulberry-feeding lepidopteran pest species. Pest. Manag. Sci. 2020;76:1313–1323. doi: 10.1002/ps.5642. [DOI] [PubMed] [Google Scholar]
- 38.Maslowski K.M., Vieira A.T., Ng A., Kranich J., Sierro F., Yu D., Schilter H.C., Rolph M.S., Mackay F., Artis D. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perić-Mataruga V., Ilijin L., Mrdaković M., Todorović D., Prokić M., Matić D., Vlahović M. Parameters of oxidative stress, cholinesterase activity, Cd bioaccumulation in the brain and midgut of Lymantria dispar (Lepidoptera: Lymantriidae) caterpillars from unpolluted and polluted forests. Chemosphere. 2019;218:416–424. doi: 10.1016/j.chemosphere.2018.11.112. [DOI] [PubMed] [Google Scholar]
- 40.Liu H., Chen B., Hu S., Liang X., Lu X., Shao Y. Quantitative proteomic analysis of germination of Nosema bombycis spores under extremely alkaline conditions. Front. Microbiol. 2016;7:1459. doi: 10.3389/fmicb.2016.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darvishzadeh A. Enzymatic activity of alpha-amylase in alimentary tract Spodoptera littoralis (Boisduval)(Lepidoptera: Noctuidae): Characterization and Compartmentalization. Arthropods. 2014;3:138. [Google Scholar]
- 42.Gross E.M., Brune A., Walenciak O.J. Gut pH, redox conditions and oxygen levels in an aquatic caterpillar: Potential effects on the fate of ingested tannins. J. Insect Physiol. 2008;54:462–471. doi: 10.1016/j.jinsphys.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Chen B., Lu X., Guo H., Bartram S., Shao Y. Diversity of the gut microbiota in lepidopteran insects and their interaction with hosts. Acta. Entomol. Sin. 2017;60:710. [Google Scholar]
- 44.Holtof M., Lenaerts C., Cullen D., Broeck J.V. Extracellular nutrient digestion and absorption in the insect gut. Cell Tissue Res. 2019;377:397–414. doi: 10.1007/s00441-019-03031-9. [DOI] [PubMed] [Google Scholar]
- 45.Gao X., Li W., Luo J., Zhang L., Ji J., Zhu X., Wang L., Zhang S. Biodiversity of the microbiota in Spodoptera exigua (Lepidoptera: Noctuidae) J. Appl. Microbiol. 2019;126:1199–1208. doi: 10.1111/jam.14190. [DOI] [PubMed] [Google Scholar]
- 46.Lalles J.P. Recent advances in intestinal alkaline phosphatase, inflammation, and nutrition. Nutr. Rev. 2019;77:710–724. doi: 10.1093/nutrit/nuz015. [DOI] [PubMed] [Google Scholar]
- 47.Douglas A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015;60:17–34. doi: 10.1146/annurev-ento-010814-020822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukatsu T. Next-generation sequencing sheds light on intricate regulation of insect gut. Mol. Ecol. 2012;21:5908–5910. doi: 10.1111/mec.12090. [DOI] [PubMed] [Google Scholar]
- 49.Ni’matuzahroh, Affandi M., Fatimah, Trikurniadewi N., Khiftiyah A.M., Sari S.K., Abidin A.Z., Ibrahim S. Comparative study of gut microbiota from decomposer fauna in household composter using metataxonomic approach. Arch. Microbiol. 2022;204:210. doi: 10.1007/s00203-022-02785-1. [DOI] [PubMed] [Google Scholar]
- 50.Paniagua Voirol L.R., Frago E., Kaltenpoth M., Hilker M., Fatouros N.E. Bacterial Symbionts in Lepidoptera: Their Diversity, Transmission, and Impact on the Host. Front. Microbiol. 2018;9:556. doi: 10.3389/fmicb.2018.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xia X., Zheng D., Zhong H., Qin B., Gurr G.M., Vasseur L., Lin H., Bai J., He W., You M. DNA sequencing reveals the midgut microbiota of diamondback moth, Plutella xylostella (L.) and a possible relationship with insecticide resistance. PLoS ONE. 2013;8:e68852. doi: 10.1371/journal.pone.0068852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang X., Freitak D., Vogel H., Ping L., Shao Y., Cordero E.A., Andersen G., Westermann M., Heckel D.G., Boland W. Complexity and variability of gut commensal microbiota in polyphagous lepidopteran larvae. PLoS ONE. 2012;7:e36978. doi: 10.1371/journal.pone.0036978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Priya N.G., Ojha A., Kajla M.K., Raj A., Rajagopal R.J. Host plant induced variation in gut bacteria of Helicoverpa armigera. PLoS ONE. 2012;7:e30768. doi: 10.1371/journal.pone.0030768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu L., Sun Z., Xu C., Wang J., Mallik A.U., Gu C., Chen D., Lu L., Zeng R., Song Y. High nitrogen in maize enriches gut microbiota conferring insecticide tolerance in lepidopteran pest Spodoptera litura. iScience. 2022;25:103726. doi: 10.1016/j.isci.2021.103726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar D., Sun Z., Cao G., Xue R., Hu X., Gong C. Study of gut bacterial diversity of Bombyx mandarina and Bombyx mori through 16S rRNA gene sequencing. J. Asia Pac. Entomol. 2019;22:522–530. doi: 10.1016/j.aspen.2019.03.005. [DOI] [Google Scholar]
- 56.Prem Anand A.A., Vennison S.J., Sankar S.G., Gilwax Prabhu D.I., Vasan P.T., Raghuraman T., Jerome Geoffrey C., Vendan S.E. Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose, xylan, pectin and starch and their impact on digestion. J. Insect Sci. 2010;10:107. doi: 10.1673/031.010.10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang X., Fu Y., Tong L., Liu H. Microbial shifts of the silkworm larval gut in response to lettuce leaf feeding. Appl. Microbiol. Biotechnol. 2014;98:3769–3776. doi: 10.1007/s00253-014-5532-y. [DOI] [PubMed] [Google Scholar]
- 58.Mogouong J., Constant P., Lavallee R., Guertin C. Gut microbiome of the emerald ash borer, Agrilus planipennis Fairmaire, and its relationship with insect population density. FEMS Microbiol. Ecol. 2020;96:fiaa141. doi: 10.1093/femsec/fiaa141. [DOI] [PubMed] [Google Scholar]
- 59.Gonzalez-Serrano F., Perez-Cobas A.E., Rosas T., Baixeras J., Latorre A., Moya A. The Gut Microbiota Composition of the Moth Brithys crini Reflects Insect Metamorphosis. Microb. Ecol. 2020;79:960–970. doi: 10.1007/s00248-019-01460-1. [DOI] [PubMed] [Google Scholar]
- 60.Zhang N., He J., Shen X., Sun C., Muhammad A., Shao Y. Contribution of sample processing to gut microbiome analysis in the model Lepidoptera, silkworm Bombyx mori. Comput. Struct. Biotechnol. J. 2021;19:4658–4668. doi: 10.1016/j.csbj.2021.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wright G.A., Nicolson S.W., Shafir S. Nutritional physiology and ecology of honey bees. Annu. Rev. Entomol. 2018;63:327–344. doi: 10.1146/annurev-ento-020117-043423. [DOI] [PubMed] [Google Scholar]
- 62.Camus M.F., Huang C.C., Reuter M., Fowler K. Dietary choices are influenced by genotype, mating status, and sex in Drosophila melanogaster. Ecol. Evol. 2018;8:5385–5393. doi: 10.1002/ece3.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrison J.G., Urruty D.M., Forister M.L. An exploration of the fungal assemblage in each life history stage of the butterfly, Lycaeides melissa (Lycaenidae), as well as its host plant Astragalus canadensis (Fabaceae) Fungal. Ecol. 2016;22:10–16. doi: 10.1016/j.funeco.2016.02.001. [DOI] [Google Scholar]
- 64.Dillon R.J., Vennard C.T., Charnley A.K. Diversity of locust gut bacteria protects against pathogen invasion. Ecol. Lett. 2010;8:1291–1298. doi: 10.1111/j.1461-0248.2005.00828.x. [DOI] [Google Scholar]
- 65.Bäckhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 66.Coon K.L., Vogel K.J., Brown M.R., Strand M.R. Mosquitoes rely on their gut microbiota for development. Mol. Ecol. 2014;23:2727–2739. doi: 10.1111/mec.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang H.T., Zou S.S., Zhai L.J., Wang Y., Zhang F.M., An L.G., Yang G.W. Pathogen invasion changes the intestinal microbiota composition and induces innate immune responses in the zebrafish intestine. Fish Shellfish Immunol. 2017;71:35–42. doi: 10.1016/j.fsi.2017.09.075. [DOI] [PubMed] [Google Scholar]
- 68.Ruokolainen L., Ikonen S., Makkonen H., Hanski I. Larval growth rate is associated with the composition of the gut microbiota in the Glanville fritillary butterfly. Oecologia. 2016;181:895–903. doi: 10.1007/s00442-016-3603-8. [DOI] [PubMed] [Google Scholar]
- 69.Visôtto L., Oliveira M., Ribon A., Mares-Guia T., Guedes R.J. Characterization and identification of proteolytic bacteria from the gut of the velvetbean caterpillar (Lepidoptera: Noctuidae) Environ. Entomol. 2009;38:1078–1085. doi: 10.1603/022.038.0415. [DOI] [PubMed] [Google Scholar]
- 70.Pilon F., Visôtto L., Guedes R., Oliveira M. Proteolytic activity of gut bacteria isolated from the velvet bean caterpillar Anticarsia gemmatalis. J. Comp. Physiol. B Biochem. 2013;183:735–747. doi: 10.1007/s00360-013-0744-5. [DOI] [PubMed] [Google Scholar]
- 71.Wang J., Peiffer M., Hoover K., Rosa C., Zeng R., Felton G. Helicoverpa zea gut-associated bacteria indirectly induce defenses in tomato by triggering a salivary elicitor(s) New Phytol. 2017;214:1294–1306. doi: 10.1111/nph.14429. [DOI] [PubMed] [Google Scholar]
- 72.Pinto-Tomás A.A., Sittenfeld A., Uribe-Lorío L., Chavarría F., Mora M., Janzen D.H., Goodman R.M., Simon H.M. Comparison of midgut bacterial diversity in tropical caterpillars (Lepidoptera: Saturniidae) fed on different diets. Environ. Entomol. 2011;40:1111–1122. doi: 10.1603/EN11083. [DOI] [PubMed] [Google Scholar]
- 73.Itoh H., Tago K., Hayatsu M., Kikuchi Y. Detoxifying symbiosis: Microbe-mediated detoxification of phytotoxins and pesticides in insects. Nat. Prod. Rep. 2018;35:434–454. doi: 10.1039/C7NP00051K. [DOI] [PubMed] [Google Scholar]
- 74.Faye T., Brede D.A., Langsrud T., Nes I.F., Holo H. An antimicrobial peptide is produced by extracellular processing of a protein from Propionibacterium jensenii. J. Bacteriol. 2002;184:3649–3656. doi: 10.1128/JB.184.13.3649-3656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ignasiak K., Maxwell A. Antibiotic-resistant bacteria in the guts of insects feeding on plants: Prospects for discovering plant-derived antibiotics. BMC Microbiol. 2017;17:223. doi: 10.1186/s12866-017-1133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oh S.N., Seo M.J., Youn Y.N., Yu Y.M. Antifungfal activity against plant pathogenic fungi on insect enterobacteriaceae. Appl. Microbiol. Biotechnol. 2015;19:71–79. [Google Scholar]
- 77.Jose P.A., Ben-Yosef M., Jurkevitch E., Yuval B.J. Symbiotic bacteria affect oviposition behavior in the olive fruit fly Bactrocera oleae. J. Insect Physiol. 2019;117:103917. doi: 10.1016/j.jinsphys.2019.103917. [DOI] [PubMed] [Google Scholar]
- 78.Oishi S., Moriyama M., Koga R., Fukatsu T. Morphogenesis and development of midgut symbiotic organ of the stinkbug Plautia stali (Hemiptera: Pentatomidae) Zool. Lett. 2019;5:16. doi: 10.1186/s40851-019-0134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Malhotra J., Dua A., Saxena A., Sangwan N., Mukherjee U., Pandey N., Rajagopal R., Khurana P., Khurana J.P. Genome sequence of Acinetobacter sp. strain HA, isolated from the gut of the polyphagous insect pest Helicoverpa armigera. J. Bacteriol. 2012;194:5156. doi: 10.1128/JB.01194-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Habineza P., Muhammad A., Ji T., Xiao R., Yin X., Hou Y., Shi Z. The Promoting Effect of Gut Microbiota on Growth and Development of Red Palm Weevil, Rhynchophorus ferrugineus (Olivier)(Coleoptera: Dryophthoridae) by Modulating Its Nutritional Metabolism. Front. Microbiol. 2019;10:1212. doi: 10.3389/fmicb.2019.01212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Werren J.H., Baldo L., Clark M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 82.Zug R., Hammerstein P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. 2015;90:89–111. doi: 10.1111/brv.12098. [DOI] [PubMed] [Google Scholar]
- 83.Chen B., Zhang N., Xie S., Zhang X., He J., Muhammad A., Sun C., Lu X., Shao Y. Gut bacteria of the silkworm Bombyx mori facilitate host resistance against the toxic effects of organophosphate insecticides. Environ. Int. 2020;143:105886. doi: 10.1016/j.envint.2020.105886. [DOI] [PubMed] [Google Scholar]
- 84.Yun T.-S., Park S.-Y., Yu J., Hwang Y., Hong K.J. Isolation and Identification of Fungal Species from the Insect Pest Tribolium castaneum in Rice Processing Complexes in Korea. Plant Pathol. J. 2018;34:356. doi: 10.5423/PPJ.OA.02.2018.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mondy N., Charrier B., Fermaud M., Pracros P., Corio-Costet M. Mutualism between a phytopathogenic fungus (Botrytis cinerea) and a vineyard pest (Lobesia botrana) J. Insect Physiol. 1998;321:665–671. [Google Scholar]
- 86.Cabezas-Cruz A., Tonk M., Bleackley M.R., Valdés J.J., Barrero R.A., Hernández-Jarguín A., Moutailler S., Vilcinskas A., Richard-Forget F., Anderson M.A.J.T., et al. Antibacterial and antifungal activity of defensins from the Australian paralysis tick, Ixodes holocyclus. Ticks Tick Borne Dis. 2019;10:101269. doi: 10.1016/j.ttbdis.2019.101269. [DOI] [PubMed] [Google Scholar]
- 87.Razinger J., Lutz M., Schroers H.-J., Urek G., Grunder J. Evaluation of insect associated and plant growth promoting fungi in the control of cabbage root flies. J. Econ. Entomol. 2014;107:1348–1354. doi: 10.1603/EC14004. [DOI] [PubMed] [Google Scholar]
- 88.Mueller G.M. Biodiversity of Fungi: Inventory and Monitoring Methods. Elsevier; Amsterdam, The Netherlands: 2011. [Google Scholar]
- 89.Douglas A.E. The microbial dimension in insect nutritional ecology. Funct. Ecol. 2009;23:38–47. doi: 10.1111/j.1365-2435.2008.01442.x. [DOI] [Google Scholar]
- 90.Zhang X.C., Zhang F. The Potential Control Strategies Based on the Interaction between Indoor Cockroaches and Their Symbionts in China. Adv. Insect Physiol. 2018;55:55–122. [Google Scholar]
- 91.Dantur K.I., Enrique R., Welin B., Castagnaro A. Isolation of cellulolytic bacteria from the intestine of Diatraea saccharalis larvae and evaluation of their capacity to degrade sugarcane biomass. AMB Express. 2015;5:15. doi: 10.1186/s13568-015-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lohan O.P. Cell wall constituents and in vitro dry matter digestibility of some fodder trees in Himachal Pradesh. Forage Res. 1980;6:21–28. doi: 10.1042/bj2980249. [DOI] [Google Scholar]
- 93.Chen J., Chen C., Liang G., Xu X., Hao Q., Sun D. In situ preparation of bacterial cellulose with antimicrobial properties from bioconversion of mulberry leaves. Carbohydr. Polym. 2019;220:170–175. doi: 10.1016/j.carbpol.2019.05.062. [DOI] [PubMed] [Google Scholar]
- 94.Yang J., Yang Y., Wu W.-M., Zhao J., Jiang L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 2014;48:13776–13784. doi: 10.1021/es504038a. [DOI] [PubMed] [Google Scholar]
- 95.LeBlanc J.G., Milani C., de Giori G.S., Sesma F., van Sinderen D., Ventura M.J. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013;24:160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 96.Vesga P., Flury P., Vacheron J., Keel C., Croll D., Maurhofer M. Transcriptome plasticity underlying plant root colonization and insect invasion by Pseudomonas protegens. ISME J. 2020;14:2766–2782. doi: 10.1038/s41396-020-0729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Senger K., Harris K., Levine M. GATA factors participate in tissue-specific immune responses in Drosophila larvae. Proc. Natl. Acad. Sci. USA. 2006;103:15957–15962. doi: 10.1073/pnas.0607608103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lemaitre B., Hoffmann J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 99.Konno K., Shimura S., Ueno C., Arakawa T., Nakamura M. Abnormal swelling of the peritrophic membrane in Eri silkworm gut caused by MLX56 family defense proteins with chitin-binding and extensin domains. Phytochemistry. 2018;147:211–219. doi: 10.1016/j.phytochem.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 100.Yang B., Huang W., Zhang J., Xu Q., Zhu S., Zhang Q., Beerntsen B.T., Song H., Ling E. Analysis of gene expression in the midgut of Bombyx mori during the larval molting stage. BMC Genom. 2016;17:866. doi: 10.1186/s12864-016-3162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Konno K., Mitsuhashi W. The peritrophic membrane as a target of proteins that play important roles in plant defense and microbial attack. J. Insect Physiol. 2019;117:103912. doi: 10.1016/j.jinsphys.2019.103912. [DOI] [PubMed] [Google Scholar]
- 102.Lacey L.A., Grzywacz D., Shapiro-Ilan D.I., Frutos R., Brownbridge M., Goettel M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015;132:1–41. doi: 10.1016/j.jip.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 103.Chen K., Lu Z.J.D., Immunology C. Immune responses to bacterial and fungal infections in the silkworm, Bombyx mori. Dev. Comp. Immunol. 2018;83:3–11. doi: 10.1016/j.dci.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 104.Schwechheimer C., Kuehn M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015;13:605. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X., Zhang Y., Zhang R., Zhang J. The diversity of pattern recognition receptors (PRRs) involved with insect defence against pathogens. Curr. Opin. Insect Sci. 2019;33:105–110. doi: 10.1016/j.cois.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 106.Yang P.J., Zhan M.Y., Ye C., Yu X.Q., Rao X.J. Molecular cloning and characterization of a short peptidoglycan recognition protein from silkworm Bombyx mori. Insect Mol. Biol. 2017;26:665–676. doi: 10.1111/imb.12330. [DOI] [PubMed] [Google Scholar]
- 107.Meng X., Zhu F., Chen K. Silkworm: A promising model organism in life science. J. Insect Sci. 2017;17:97. doi: 10.1093/jisesa/iex064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang K., Pan G., Zhao Y., Hao X., Li C., Shen L., Zhang R., Su J., Cui H. A novel immune-related gene HDD1 of silkworm Bombyx mori is involved in bacterial response. Mol. Immunol. 2017;88:106–115. doi: 10.1016/j.molimm.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 109.Motokawa Y., Kokubo M., Kuwabara N., Tatematsu K.I., Sezutsu H., Takahashi H., Sakakura K., Chikamatsu K., Takeda S. Melanoma antigen family A4 protein produced by transgenic silkworms induces antitumor immune responses. Exp. Ther. Med. 2018;15:2512–2518. doi: 10.3892/etm.2018.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaneko T., Goldman W.E., Mellroth P., Steiner H., Fukase K., Kusumoto S., Harley W., Fox A., Golenbock D., Silverman N. Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity. 2004;20:637–649. doi: 10.1016/S1074-7613(04)00104-9. [DOI] [PubMed] [Google Scholar]
- 111.Costa A., Jan E., Sarnow P., Schneider D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS ONE. 2009;4:e7436. doi: 10.1371/journal.pone.0007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu X., Yang R., Zhang X., Chen L., Xiang X., Gong C., Wu X. Molecular cloning and functional characterization of the dual oxidase (BmDuox) gene from the silkworm Bombyx mori. PLoS ONE. 2013;8:e70118. doi: 10.1371/journal.pone.0070118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kausar S., Abbas M., Zhao Y., Cui H. Immune strategies of silkworm, Bombyx mori against microbial infections. Invertebr. Surviv. J. 2019;16:130–140. [Google Scholar]
- 114.Hoffmann D., Hultmark D., Boman H.G. Insect immunity: Galleria mellonella and other lepidoptera have cecropia-P9-like factors active against gram negative bacteria. Insect Biochem. Mol. Biol. 2019;11:537–548. doi: 10.1016/0020-1790(81)90022-6. [DOI] [Google Scholar]
- 115.Landry M., Comeau A.M., Derome N., Cusson M., Levesque R.C. Composition of the spruce budworm (Choristoneura fumiferana) midgut microbiota as affected by rearing conditions. PLoS ONE. 2015;10:e0144077. doi: 10.1371/journal.pone.0144077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9:313. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Senthil-Nathan S. Environmental Sustainability. Springer; Berlin/Heidelberg, Germany: 2015. A review of biopesticides and their mode of action against insect pests; pp. 49–63. [Google Scholar]
- 118.Ding J.L., Hou J., Feng M.G., Ying S.H. Transcriptomic analyses reveal comprehensive responses of insect hemocytes to mycopathogen Beauveria bassiana, and fungal virulence-related cell wall protein assists pathogen to evade host cellular defense. Virulence. 2020;11:1352–1365. doi: 10.1080/21505594.2020.1827886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Malagoli A.Z.a.D. Immune response of Chilo suppressalis Walker (Lepidoptera: Crambidae) larvae to different entomopathogenic fungi. Bull. Entomol. Res. 2014;104:155–163. doi: 10.1017/S0007485313000588. [DOI] [PubMed] [Google Scholar]
- 120.Miltan Chandra Roy Y.K. Toll signal pathway activating eicosanoid biosynthesis shares its conserved upstream recognition components in a lepidopteran Spodoptera exigua upon infection by Metarhizium rileyi, an entomopathogenic fungus. J. Invertebr. Pathol. 2022;188:107707. doi: 10.1016/j.jip.2021.107707. [DOI] [PubMed] [Google Scholar]
- 121.Li G., Zhou Q., Qiu L., Yao Q., Chen K., Tang Q., Hu Z.J. Serine protease Bm-SP142 was differentially expressed in resistant and susceptible Bombyx mori strains, involving in the defence response to viral infection. PLoS ONE. 2017;12:e0175518. doi: 10.1371/journal.pone.0175518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu F., Ma Q., Dang X., Wang Y., Song Y., Meng X., Bao J., Chen J., Pan G., Zhou Z.J. Identification of a new subtilisin-like protease NbSLP2 interacting with cytoskeletal protein septin in Microsporidia Nosema bombycis. J. Invertebr. Pathol. 2017;148:110–117. doi: 10.1016/j.jip.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 123.Suraporn S., Terenius O. Supplementation of Lactobacillus casei reduces the mortality of Bombyx mori larvae challenged by Nosema bombycis. BMC Res. Notes. 2021;14:398. doi: 10.1186/s13104-021-05807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Drezen J.M., Josse T., Bezier A., Gauthier J., Huguet E., Herniou E.A. Impact of Lateral Transfers on the Genomes of Lepidoptera. Genes. 2017;8:315. doi: 10.3390/genes8110315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mondotte J.A., Saleh M.C. Antiviral Immune Response and the Route of Infection in Drosophila melanogaster. Adv. Virus Res. 2018;100:247–278. doi: 10.1016/bs.aivir.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 126.Jakubowska A.K., Vogel H., Herrero S. Increase in gut microbiota after immune suppression in baculovirus-infected larvae. PLoS Pathog. 2013;9:e1003379. doi: 10.1371/journal.ppat.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kolliopoulou A., Van Nieuwerburgh F., Stravopodis D.J., Deforce D., Swevers L., Smagghe G. Transcriptome analysis of Bombyx mori larval midgut during persistent and pathogenic cytoplasmic polyhedrosis virus infection. PLoS ONE. 2015;10:e0121447. doi: 10.1371/journal.pone.0121447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sabin L.R., Hanna S.L., Cherry S. Innate antiviral immunity in Drosophila. Curr. Opin. Immunol. 2010;22:4–9. doi: 10.1016/j.coi.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheng Y., Wang X.Y., Du C., Gao J., Xu J.P. Expression analysis of several antiviral related genes to BmNPV in different resistant strains of silkworm, Bombyx mori. J. Insect Sci. 2014;14:76. doi: 10.1673/031.014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ponnuvel K.M., Nithya K., Sirigineedi S., Awasthi A.K., Yamakawa M. In vitro antiviral activity of an alkaline trypsin from the digestive juice of Bombyx mori larvae against nucleopolyhedrovirus. Arch. Insect Biochem. Physiol. 2012;81:90–104. doi: 10.1002/arch.21046. [DOI] [PubMed] [Google Scholar]
- 131.Van Arnam E.B., Currie C.R., Clardy J.J. Defense contracts: Molecular protection in insect-microbe symbioses. Chem Soc. Rev. 2018;47:1638–1651. doi: 10.1039/C7CS00340D. [DOI] [PubMed] [Google Scholar]
- 132.Hajek A.E., Morris E.E., Hendry T.A. Context dependent interactions of insects and defensive symbionts: Insights from a novel system in siricid woodwasps. Curr. Opin. Insect Sci. 2019;33:77–83. doi: 10.1016/j.cois.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 133.Li X., He J., Li S. Isolation of a chlorpyrifos-degrading bacterium, Sphingomonas sp. strain Dsp-2, and cloning of the mpd gene. Res. Microbiol. 2007;158:143–149. doi: 10.1016/j.resmic.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 134.Singh B.K., Walker A., Morgan J.A., Wright D.J. Effects of soil pH on the biodegradation of chlorpyrifos and isolation of a chlorpyrifos-degrading bacterium. Appl. Environ. Microbiol. 2003;69:5198–5206. doi: 10.1128/AEM.69.9.5198-5206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rayu S., Nielsen U.N., Nazaries L., Singh B.K. Isolation and Molecular Characterization of Novel Chlorpyrifos and 3,5,6-trichloro-2-pyridinol-degrading Bacteria from Sugarcane Farm Soils. Front. Microbiol. 2017;8:518. doi: 10.3389/fmicb.2017.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xia X., Sun B., Gurr G.M., Vasseur L., Xue M., You M. Gut Microbiota Mediate Insecticide Resistance in the Diamondback Moth, Plutella xylostella (L.) Front. Microbiol. 2018;9:25. doi: 10.3389/fmicb.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tago K., Kikuchi Y., Nakaoka S., Katsuyama C., Hayatsu M. Insecticide applications to soil contribute to the development of B urkholderia mediating insecticide resistance in stinkbugs. Mol. Ecol. 2015;24:3766–3778. doi: 10.1111/mec.13265. [DOI] [PubMed] [Google Scholar]
- 138.Ramya S.L., Venkatesan T., Srinivasa Murthy K., Jalali S.K., Verghese A. Detection of carboxylesterase and esterase activity in culturable gut bacterial flora isolated from diamondback moth, Plutella xylostella (Linnaeus), from India and its possible role in indoxacarb degradation. Braz. J. Microbiol. 2016;47:327–336. doi: 10.1016/j.bjm.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Divekar P.A., Narayana S., Divekar B.A., Kumar R., Gadratagi B.G., Ray A., Singh A.K., Rani V., Singh V., Singh A.K., et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022;23:2690. doi: 10.3390/ijms23052690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mason C.J., Lowe-Power T.M., Rubert-Nason K.F., Lindroth R.L., Raffa K.F. Interactions between bacteria and aspen defense chemicals at the phyllosphere–herbivore interface. J. Chem. Ecol. 2016;42:193–201. doi: 10.1007/s10886-016-0677-z. [DOI] [PubMed] [Google Scholar]
- 141.Vilanova C., Baixeras J., Latorre A., Porcar M. The Generalist Inside the Specialist: Gut Bacterial Communities of Two Insect Species Feeding on Toxic Plants Are Dominated by Enterococcus sp. Front. Microbiol. 2016;7:1005. doi: 10.3389/fmicb.2016.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wybouw N., Dermauw W., Tirry L., Stevens C., Grbić M., Feyereisen R., Van Leeuwen T.J.E. A gene horizontally transferred from bacteria protects arthropods from host plant cyanide poisoning. Elife. 2014;3:e02365. doi: 10.7554/eLife.02365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wybouw N., Van Leeuwen T., Dermauw W. A massive incorporation of microbial genes into the genome ofTetranychus urticae, a polyphagous arthropod herbivore. Insect Mol. Biol. 2018;27:333–351. doi: 10.1111/imb.12374. [DOI] [PubMed] [Google Scholar]
- 144.Xia X., Gurr G.M., Vasseur L., Zheng D., Zhong H., Qin B., Lin J., Wang Y., Song F., Li Y., et al. Metagenomic Sequencing of Diamondback Moth Gut Microbiome Unveils Key Holobiont Adaptations for Herbivory. Front. Microbiol. 2017;8:663. doi: 10.3389/fmicb.2017.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vilanova C., Marin M., Baixeras J., Latorre A., Porcar M. Selecting microbial strains from pine tree resin: Biotechnological applications from a terpene world. PLoS ONE. 2014;9:e100740. doi: 10.1371/journal.pone.0100740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Restrepo-Flórez J.-M., Bassi A., Thompson M.R. Microbial degradation and deterioration of polyethylene-A review. Int. Biodeterior. Biodegrad. 2014;88:83–90. doi: 10.1016/j.ibiod.2013.12.014. [DOI] [Google Scholar]
- 147.Muhammad A., Zhou X., He J., Zhang N., Shen X., Sun C., Yan B., Shao Y. Toxic effects of acute exposure to polystyrene microplastics and nanoplastics on the model insect, silkworm Bombyx mori. Environ. Pollut. 2021;285:117255. doi: 10.1016/j.envpol.2021.117255. [DOI] [PubMed] [Google Scholar]
- 148.Zhang J., Gao D., Li Q., Zhao Y., Li L., Lin H., Bi Q., Zhao Y. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020;704:135931. doi: 10.1016/j.scitotenv.2019.135931. [DOI] [PubMed] [Google Scholar]
- 149.Li M., Li F., Lu Z., Fang Y., Qu J., Mao T., Wang H., Chen J., Li B. Effects of TiO2 nanoparticles on intestinal microbial composition of silkworm, Bombyx mori. Sci. Total Environ. 2020;704:135273. doi: 10.1016/j.scitotenv.2019.135273. [DOI] [PubMed] [Google Scholar]
- 150.van den Bosch T.J., Welte C.U. Detoxifying symbionts in agriculturally important pest insects. Microb. Biotechnol. 2017;10:531–540. doi: 10.1111/1751-7915.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ferreira C.M., Soares H.M., Soares E.V. Promising bacterial genera for agricultural practices: An insight on plant growth-promoting properties and microbial safety aspects. Sci. Total Environ. 2019;682:779–799. doi: 10.1016/j.scitotenv.2019.04.225. [DOI] [PubMed] [Google Scholar]
- 152.Mahar A., Al-Siyabi A., Elawad S., Hague N., Gowen S. Application of toxins from the entomopathogenic bacterium, Xenorhabdus nematophila, for the control of insects on foliage. Commun. Agric. Appl. Biol. Sci. 2006;71:233–238. [PubMed] [Google Scholar]
- 153.Shi Y.-M., Bode H.B. Chemical language and warfare of bacterial natural products in bacteria–nematode–insect interactions. Nat. Prod. Rep. 2018;35:309–335. doi: 10.1039/C7NP00054E. [DOI] [PubMed] [Google Scholar]
- 154.Nikolouli K., Colinet H., Renault D., Enriquez T., Mouton L., Gibert P., Sassu F., Cáceres C., Stauffer C., Pereira R. Sterile insect technique and Wolbachia symbiosis as potential tools for the control of the invasive species Drosophila suzukii. J. Pest. Sci. 2018;91:489–503. doi: 10.1007/s10340-017-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kajtoch Ł., Kolasa M., Kubisz D., Gutowski J.M., Ścibior R., Mazur M.A., Holecová M. Using host species traits to understand the Wolbachia infection distribution across terrestrial beetles. Sci. Rep. 2019;9:847. doi: 10.1038/s41598-018-38155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu Q., Zhou J., Zhang C., Dong Q., Ning S., Hui D. Absence of complementary sex determination in Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) Peer J. Prepr. 2019;7:e27871v27871. [Google Scholar]
- 157.Gaunet A., Dincă V., Dapporto L., Montagud S., Vodă R., Schär S., Badiane A., Font E., Vila R. Two consecutive Wolbachia-mediated mitochondrial introgressions obscure taxonomy in Palearctic swallowtail butterflies (Lepidoptera, Papilionidae) Zool. Scr. 2019;48:507–519. doi: 10.1111/zsc.12355. [DOI] [Google Scholar]
- 158.Arai H., Hirano T., Akizuki N., Abe A., Nakai M., Kunimi Y., Inoue M.N. Multiple infection and reproductive manipulations of Wolbachia in Homona magnanima (Lepidoptera: Tortricidae) Microb. Ecol. 2019;77:257–266. doi: 10.1007/s00248-018-1210-4. [DOI] [PubMed] [Google Scholar]
- 159.Fukui T., Kawamoto M., Shoji K., Kiuchi T., Sugano S., Shimada T., Suzuki Y., Katsuma S. The endosymbiotic bacterium Wolbachia selectively kills male hosts by targeting the masculinizing gene. PLoS Pathog. 2015;11:e1005048. doi: 10.1371/journal.ppat.1005048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article only.