Abstract
In early 2022, the Japanese encephalitis virus (JEV) was identified as the cause of stillborn and mummified piglets in pig farms in southeastern Australia. Human cases and additional pig farms with infected piglets were subsequently identified across a widespread area encompassing four states. To inform surveillance and control programs, we synthesized existing information on Australian vectors of JEV, much of which was generated in response to incursions of JEV into the northern state of Queensland between 1995 and 2005. Members of the Culex sitiens subgroup, particularly Culex annulirostris, should be considered the primary vectors of JEV in Australia, as they yielded >87% of field detections of JEV, were highly efficient laboratory vectors of the virus, readily fed on pigs and birds (the key amplifying hosts of the virus) when they were available, and are widespread and often occur in large populations. Three introduced species, Culex quinquefasciatus, Culex gelidus and Culex tritaeniorhynchus may also serve as vectors, but more information on their geographical distribution, abundance and bionomics in the Australian context is required. Mosquitoes from other genera, such as Aedes and Verrallina, whilst considered relatively poor vectors, could play a regional or supplemental role in transmission, especially facilitating vertical transmission as a virus overwintering mechanism. Additional factors that could impact JEV transmission, including mosquito survival, dispersal and genetics, are also discussed. Possible directions for investigation are provided, especially in the context of the virus emerging in a region with different mosquito fauna and environmental drivers than northern Australia.
Keywords: Japanese encephalitis virus, Australia, mosquitoes, virus detection, vector competence, host feeding patterns
1. Introduction
The Japanese encephalitis virus (JEV) is the leading cause of encephalitis in Southeast Asia and is responsible for an estimated 68,000–100,000 cases per year, although this is likely an underestimate [1,2]. It is a positive-sense RNA virus that was classified into five genotypes whose distributions vary due to climate and other environmental factors [3,4,5]. The virus exists in an enzootic transmission cycle involving ardeid wading birds and mosquitoes [6,7]. Pigs are also amplifying hosts and are often involved in the epizootic amplification of the virus, which results in spillover to humans [8,9]. Whilst humans and horses can develop fatal encephalitis, they are considered dead-end hosts of the virus because they do not produce sufficient viremia levels to infect mosquitoes [10]. Pigs can also develop a disease that is characterized by reduced sperm production in boars and fetal abortion in infected sows (reviewed in [11]).
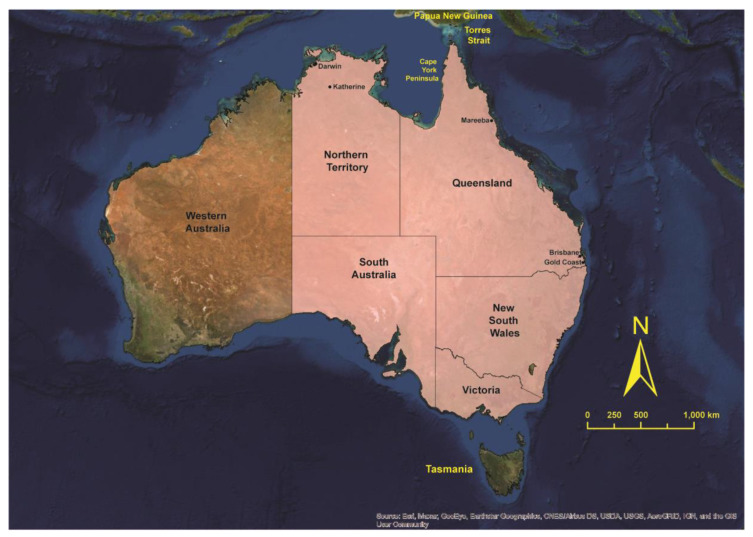
JEV, like other important flaviviruses, such as West Nile virus (WNV) and dengue viruses, shows a strong propensity to spread into new geographic areas [12]. In 1995, JEV emerged in the Australasian zoogeographical region when an outbreak of genotype 2 occurred on the islands of the Torres Strait of northern Australia (Figure 1; [13]). The 1995 outbreak and another, more geographically widespread outbreak in 1998 [14], resulted in five human cases, two of which were fatal. The virus was subsequently identified in the Western Province of Papua New Guinea [15,16], and it was thought that this was the source of incursions into Australia [13,17]. A sentinel pig program detected JEV activity every year (except 1999) in the Torres Strait between 1995 and 2005 [18,19], although identification of only genotype 1 viruses from the year 2000 onward suggested that a separate incursion of the virus into the region had occurred [20]. Activity in 1998 and 2004 spread onto Cape York Peninsula, but there was no evidence that the virus had become established in natural transmission cycles on the mainland [14,21], despite ecological conditions potentially being suitable for its establishment [22]. The status of JEV in northern Australia had become less clear, as most forms of targeted surveillance were gradually phased out after 2011 [19].
Figure 1.
Map of Australia and Papua New Guinea showing locations discussed in the text. The states and territories that are shaded pink had reported human cases of Japanese encephalitis, piggeries with infected pigs and/or positive feral pigs in 2022.
In early 2021, JEV was diagnosed in a resident of the Northern Territory [23], signifying another incursion of the virus into Australia and the first confirmed evidence of virus activity since 2005 [24]. In February 2022, JEV was identified as the virus responsible for an increased number of stillbirths, mummified fetuses and piglets with neurological disease at commercial pig farms in three eastern Australian states [25]. As of 5 May 2022, a total of 70 piggeries across four states had been affected and the virus was detected in feral pigs in the Northern Territory [26]. The first human cases of encephalitis caused by JEV infection were reported in early March 2022 and, as of 25 May 2022, there were 30 confirmed cases, with five deaths, reported across four states [27]. The unprecedented emergence of JEV across such a wide geographical area of southeastern Australia has necessitated a multi-sectoral response aimed at determining the distribution of the virus, the potential for its permanent establishment, ongoing human and animal disease risk, and options for sustainable control of virus transmission.
To inform a “One Health” response to the emergence of JEV, we provide a synthesis of the existing knowledge of Australian mosquito vectors of the virus. To achieve this, we initially employed criteria originally proposed by Reeves [28] and applied by Turell et al. [29] to incriminate different mosquito species in the transmission of WNV in North America. These criteria include (a) detection of the virus in field-collected mosquitoes, (b) a demonstrated ability of the mosquito species to become infected with and transmit the virus, and (c) a demonstrated association between the mosquito species and vertebrate hosts of the virus. Other factors not intrinsically linked to the direct interaction with the virus and a vertebrate host, but which can impact the role a mosquito species plays in transmission cycles, such as mosquito genetics, survival, dispersal, abundance, and geographical and temporal distributions, are also discussed.
2. Incrimination of Australian Mosquitoes as Vectors of JEV
2.1. Detection of JEV in Field Populations of Mosquitoes
In response to recognized JEV incursions or to further characterize the distribution of the virus in the region, mosquitoes were collected between 1995 and 2005 from several locations in northern Australia and Papua New Guinea. Mosquitoes were predominately collected using Centers for Disease Control miniature light traps baited with CO2, which were often supplemented with 1-octen-3-ol. Gravid traps and various propane or solar-powered traps were also deployed on occasion in the Torres Strait [24,30]. Mosquitoes were pooled by species or another taxonomic group, which included damaged specimens and mosquitoes that were morphologically indistinguishable (i.e., the Anopheles farauti species complex; [31]). Of note, difficulty in reliably separating Culex annulirostris, Cx. palpalis and Cx. sitiens [32] meant that for some investigations, they were not identified at the species level and were just referred to as Cx. sitiens subgroup mosquitoes. In some studies, a subsample of mosquitoes or the species composition of positive pools was identified using isoenzyme analysis or molecular assays to indicate the species present [33,34]. JEV was detected using a cell culture immunoassay [35]), or viral RNA was detected using real-time reverse transcriptase (RT) PCR [36].
A total of 540,882 mosquitoes were processed from northern Australia and Papua New Guinea between 1995 and 2000 during periods where JEV was detected from at least one pool of mosquitoes (Supplementary Table S1). This allowed for an assessment of which species or taxonomic groups were infected whilst the virus was actively circulating in a given location. From at least 38 species across 12 genera processed, over 60% of mosquitoes belonged to the genus Culex, from which, 92% were members of the Cx. sitiens subgroup, predominately Cx. annulirostris. Of the remaining genera, Aedes and Verrallina represented 18% and 11% of the mosquitoes processed, respectively.
Between 1995 and 2000, JEV was isolated from 56 pools of mosquitoes, with members of the Culex genus accounting for all but one of the isolates (Table 1). Fifty-four of the isolates were obtained from members of the Cx. sitiens subgroup, whilst single isolates were obtained from Ae. vigilax and Cx. gelidus. Most isolates (93%) were obtained from mosquitoes collected from Badu Island, with all but one from collection trips undertaken in 1995 and 1998. Despite collections from Papua New Guinea comprising 73% of the Cx. sitiens subgroup mosquitoes processed, only three isolates were obtained, although, unlike Badu, there was no other evidence of epizootic activity occurring when the collections were undertaken.
Table 1.
Detection of the Japanese encephalitis virus or viral RNA in mosquitoes collected from northern Australia and Papua New Guinea, 1995–2005.
| Location | Year | Number of Culex Processed | Number of Mosquitoes of Other Genera Processed | Trapping Method a | Number of JEV Positive Pools | Composition of Positive Pools | Reference |
|---|---|---|---|---|---|---|---|
| Badu (Torres Strait) | 1995 | 2987 | 10,313 | CDC-LT | 8 | Culex sitiens subgroup b | [37] |
| 1998 | 26,158 | 5730 | CDC-LT | 42 | Cx. sitiens subgroup | [38] | |
| CDC-LT | 1 | Aedes vigilax | |||||
| 2000 | 7779 | 83,461 | CDC-LT | 1 | Culex gelidus | [39] | |
| 2002 | Not separated by taxonomic group c | MM | 3 | Unknown | [24] | ||
| 2003 | 22,157 | Not processed d | CDC-LT, MM | 18 | Cx. sitiens subgroup | [24,40] | |
| CDC-LT, MM | 2 | Cx. gelidus | [24], this paper | ||||
| CDC-GT | 1 | Culex bitaeniorhynchus | This paper | ||||
| 2004 | 1795 | Not processed | NMT | 1 | Cx. gelidus | [24] | |
| 2005 | 4563 | Not processed | NMT | 1 | Cx. sitiens subgroup | [24] | |
| Moa (Torres Strait) | 2003 | 1400 | Not processed | MM | 2 | Cx. sitiens subgroup | [24] |
| MM | 1 | Cx. gelidus | [24] | ||||
| Cape York Peninsula | 2004 | 23,144 | Not processed | CDC-LT | 1 | Cx. sitiens subgroup | [21] |
| Saibai (Torres Strait) | 2000 | 45,581 | 38,473 | CDC-LT | 1 | Cx. sitiens subgroup | [41] |
| Papua New Guinea | 1997 | 35,038 | 25,445 | CDC-LT | 1 | Cx. sitiens subgroup | [15] |
| 1998 | 210,200 | 49,717 | CDC-LT | 2 | Cx. sitiens subgroup | ||
a Trap used to collect mosquitoes for virus detection: CDC-LT is Centers for Disease Control light trap baited with CO2 with or without 1-octen-3-ol; MM is “Mosquito Magnet®”, a propane-powered updraft trap; CDC-GT is Centers for Disease Control gravid trap; and NMT is the Northern Australia Quarantine Strategy Mozzie Trap, which is a CO2-baited updraft trap. b Includes the morphologically similar species Culex annulirostris, Cx. palpalis and Cx. sitiens. c These mosquitoes were collected as part of a trial of a remote trapping system and were processed without being sorted and processed according to the taxonomic group. d After 2000, only members of the Culex genus were processed.
Based on their role as the primary vectors of JEV, surveillance of the virus in northern Australia conducted between 2003 and 2005 focused predominately on Culex spp. During this period, 33,931 Culex mosquitoes were processed from Badu Island, from which, JEV RNA was detected in 23 pools (Table 1). Viral RNA was also detected in three pools of Culex mosquitoes collected from the nearby Moa Island. All but five of these detections were in pools of Cx. sitiens subgroup mosquitoes, with viral RNA detected in four pools of Cx. gelidus and one pool of Cx. bitaeniorhynchus, with the latter consisting of two mosquitoes collected in a gravid trap. One of the detections in Cx. gelidus and the Cx. bitaeniorhynchus detection are reported here for the first time and are the result of further analysis of data presented in van den Hurk et al. [30]. Between 2002 and 2005, a new trapping strategy was deployed to remotely collect mosquitoes over several weeks, which were then processed in large pool sizes using real-time RT-PCR [24]. In 2002, no attempt was made to identify the mosquitoes from the traps that contained approximately 178,000 mosquitoes; therefore, the taxonomic group composition of the collections from which JEV RNA was detected in three pools is unknown. In 2004, 23,144 Culex spp. were processed from mainland Australia, from which, JEV was detected in a single pool of Cx. sitiens subgroup mosquitoes [21].
Any or a combination of the three Australian members of the Cx. sitiens subgroup could have comprised the species within a JEV-positive pool. Cx. annulirostris were reliably identified during the original field studies on Badu [37] and molecular analysis suggested it was the dominant species during other collection trips [21,40]. Although no isolates have been obtained from mosquitoes identified exclusively as Cx. sitiens in Australia, possibly reflecting the low numbers of mosquitoes of this species processed in the region, this species has yielded isolates in Southeast Asia [42,43,44]. The isolation of JEV from Lake Murray in PNG in 1997 was from pools of Cx. sitiens subgroup mosquitoes, of which, a significant number were subsequently shown to be Cx. palpalis [15,33,34].
2.2. Intrinsic Ability of Australian Mosquitoes to Become Infected with and Transmit JEV
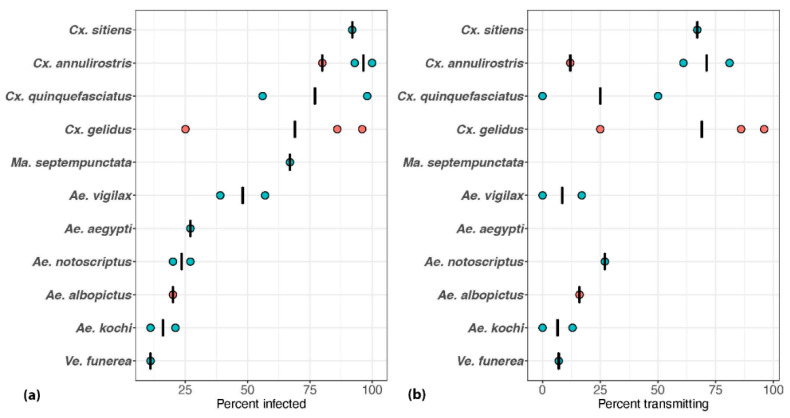
Whilst detection of the virus or viral RNA in field populations can implicate species in arbovirus transmission cycles, laboratory-based vector competence experiments provide evidence that the virus can indeed replicate and be transmitted by implicated species. A total of 17 Australian mosquito species were assessed for their ability to become infected with Australian strains of JEV, with 10 of these species also tested for their ability to transmit the virus [45,46,47,48]. The highest infection and transmission rates were observed in the Culex spp., particularly Cx. annulirostris, Cx. gelidus and Cx. sitiens, with the proportion infected with and transmitting the virus for these three species being >90% and >65%, respectively (Figure 2). The proportion of other genera infected was mostly <50% and <30% of individual mosquitoes tested transmitted the virus. A preliminary investigation of a Northern Territory population of Cx. palpalis revealed that this species was able to transmit the genotype 3 Nakayama strain of JEV to suckling mice (L. Melville, unpublished data).
Figure 2.
Results of the vector competence experiments conducted to assess the ability of Australian mosquito species to (a) become infected with and (b) transmit the Japanese encephalitis virus. Mosquitoes were allowed to feed on an infectious blood meal containing 106−7 infectious units of virus per milliliter and tested at days 10–14 post-exposure. The orange and blue circles represent experiments conducted with genotype 1 and genotype 2 viruses, respectively, and the bars represent the means of data derived from separate virus exposures. Previously published studies [45,46,47,48] provided the source data. A minimum of five mosquitoes was required for inclusion in the analysis. Mansonia septempunctata and Ae. aegypti were not tested for their ability to transmit the virus.
Vector competence for JEV appears to vary within mosquito species, as well as between virus genotypes tested in mosquito species of the same origin. For instance, Cx. annulirostris was a highly efficient laboratory vector of the genotype 2 JEV, which contrasted with its relatively low vector competence for the genotype 1 JEV that appeared to have become the dominant genotype in northern Australia [45,48]. There were also differences in vector competence of two populations of Cx. quinquefasciatus for genotype 2 JEV, with a colony of Gold Coast (southern Queensland) origin having a 50% transmission rate, whilst none of the 16 field-derived mosquitoes from Mareeba (north Queensland) transmitted the virus [48]. Similar differences in vector competence between populations were observed for JEV in Cx. tritaeniorhynchus in Southeast Asia [49,50], and for Murray Valley encephalitis (MVEV) and West Nile virus (Kunjin subtype; WNVKUN) in Cx. annulirostris in Australia [51]. It is also important to note that variation in the vector competence could be due to differences in the experimental methodology, such as the use of colony versus field-collected mosquitoes, the method used to assess transmission or the assay used to detect the virus [52].
The extrinsic incubation period (EIP) of the virus is the time taken from when the mosquito imbibes the virus in an infectious blood meal to when it can transmit it via the saliva during a subsequent blood feed. The EIP plays a critical role in transmission dynamics because if it is short, then a much higher proportion of mosquitoes will survive to transmit the virus. At 28 °C, both Cx. annulirostris and Cx. sitiens were able to transmit the virus to a small proportion of mice on day 7 post-exposure (the earliest day tested), but the highest rates of transmission did not occur until day 14 post-exposure [48]. These results are similar to those reported for Cx. tritaeniorhynchus incubated at 28 °C and tested with an in vitro method of measuring transmission [53]. This latter study found that the EIP increased considerably when mosquitoes were incubated at 20 °C and transmission did not occur until day 20 post-exposure.
2.3. Host-Feeding Patterns of Mosquitoes Implicated as JEV Vectors
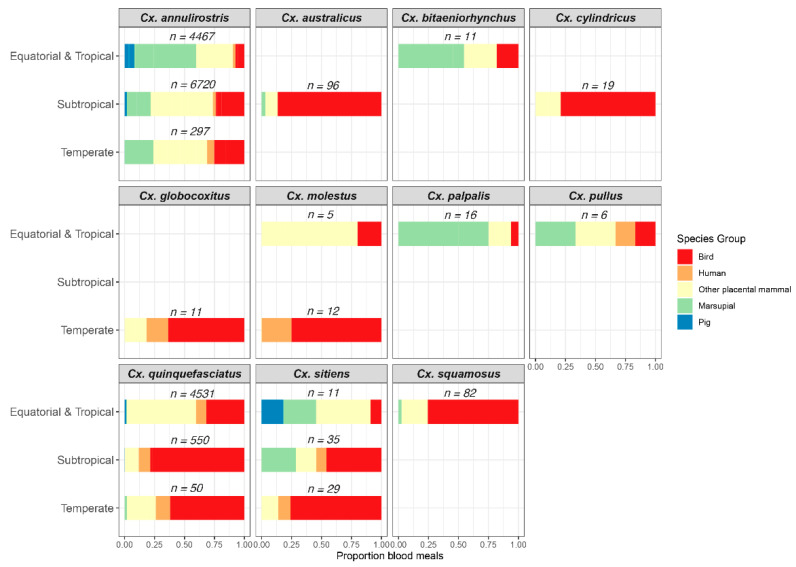
Analysis of host-feeding patterns provides important data on the proportion of blood meals originating from vertebrate hosts of the virus, which for JEV are birds and pigs. In these studies, blood-engorged mosquitoes were collected from the field and the vertebrate source of the blood in the abdomen was identified using either serological or molecular assays [54,55]. We synthesized data from nine studies on the host-feeding patterns of Australian mosquitoes [56,57,58,59,60,61,62,63,64], with a particular focus on Culex spp. (for which ≥5 blood meals had been reported). A total of 16,948 blood meals from 11 mosquito species were identified and patterns were summarized across three climatic regions (Figure 3).
Figure 3.
Identification of the vertebrate source of blood meals of Australian species of Culex, with a focus on animal groups of importance to Japanese encephalitis virus transmission cycles. Source data were from previously published studies [56,57,58,59,60,61,62,63,64].
Across all regions, Cx. annulirostris was an opportunistic feeder, taking blood meals from a diversity of vertebrate groups. Overall, pigs accounted for only a small proportion of blood meals identified. The paucity of blood meals originating from pigs in some sub-tropical and temperate areas could represent the fact that pigs were not present in the collection areas or pig antisera were not included in the panel of antisera used in the serological assays. In equatorial and tropical areas, pig-feeding proportions >30% were identified only where domestic pigs were housed or in locations where feral pigs congregated, such as rubbish dumps [58,64]. Instead, other mammals, particularly macropods, comprised the greater proportion of blood meals identified [64,65]. As wallabies and kangaroos are poor amplifying hosts of the virus [66], it was hypothesized that high feeding rates on macropods may impede the transmission cycle [67]. Whilst Cx. annulirostris did feed on birds, it was only in temperate regions where birds accounted for >20% of the blood meals identified. Interestingly, by employing DNA sequence analysis, Jansen et al. [59] identified Cx. annulirostris blood meals that had originated from the Nankeen night heron Nycticorax caledonicus, one of the ardeid bird species suspected to play a role in JEV transmission in Australia. Across all regions, <10% of Cx. annulirostris blood meals originated from humans.
Of the other Culex spp. implicated in JEV transmission in Australia, birds accounted for >70% of blood meals identified in some regions. Pigs accounted for almost 20% of blood meals identified from Cx. sitiens in the tropical region, but this may have reflected the small number of blood meals tested rather than any specific host-feeding pattern. Except for the temperate populations, Cx. quinquefasciatus obtained >50% of its blood meals from mammals contrasting with its perception as an ornithophilic species. Only a small proportion of blood meals from this species, as well as Cx. sitiens, originated from humans.
3. Australian Vectors of JEV: Reviewing the Evidence
3.1. Mosquitoes Implicated from Field and/or Laboratory Studies
Based on the number of field detections, vector competence and host-feeding patterns [28], members of the Cx. sitiens subgroup should be considered the major vectors of JEV in Australia. Given that Cx. annulirostris is a widely distributed species in Australia, is a highly efficient laboratory vector of JEV and readily feeds on pigs and birds, when available, this species should be considered the primary Australian vector of JEV. This status is not surprising given that it is considered the biological equivalent of Cx. tritaeniorhynchus and Cx. tarsalis, which are two species that are major vectors of encephalitogenic flaviviruses in their respective regions [68]. The high infection and transmission rates observed in Cx. sitiens in the vector competence experiments, coupled with it yielding isolates of the virus in Asia, suggest that this species could act as a vector of JEV, particularly in coastal regions of Australia. Although Cx. palpalis potentially yielded isolates in PNG and was able to transmit the virus in the laboratory, more information on its geographical distribution, biology and interaction with amplifying hosts is required to clarify its role in JEV transmission.
There is no doubt that other Australian species of Culex could potentially play a role in JEV transmission cycles. Of these, Australian populations of Cx. gelidus were highly efficient laboratory vectors of JEV and the virus was detected in this species collected from northern Queensland, despite relatively small numbers being processed. The results of the vector competence experiments suggest that Cx. quinquefasciatus could serve as a vector, especially considering that large populations of this species can be associated with effluent ponds and other larval habitats with high organic content. However, only low numbers of Cx. quinquefasciatus have been processed to detect the virus, as they are not readily collected in CO2-baited light traps and gravid traps were only occasionally deployed to sample them during the JEV investigations of the 1990s and 2000s. Despite only low numbers being processed, the detection in Cx. bitaeniorhynchus indicates that Australian populations of this species can become infected with JEV, but their ability to transmit the virus has not been assessed. All three species have been implicated as JEV vectors in Southeast Asia and the Indian subcontinent [42,69,70,71,72], and have host-feeding patterns that bring them into contact with amplifying hosts (Figure 3; [64]); therefore, they should be considered potential vectors of the virus in Australia.
The status of other mosquito genera as vectors of JEV in Australia is less defined and it could be that they may play only a regional or supplemental role in transmission. The > 50% infection and dissemination rates observed in the Mansonia spp., coupled with the virus being isolated from members of this genus in India [73], suggest that they could also play a role in transmission. Within the Aedes genus, both Ae. vigilax and Ae. notoscriptus were relatively inefficient laboratory vectors of JEV when compared with Culex spp. Although the virus was isolated from Ae. vigilax, the minimum infection rate was considerably lower than that observed in the Cx. annulirostris that were collected at the same time [38]. The lower vector competence of Ae. vigilax and Ae. notoscriptus could be offset by their widespread distribution in Australia and high relative abundance when conditions are suitable [74,75]. Other Aedes spp. (i.e., Ae. kochi and Ae. culiciformis) and Verrallina spp. (i.e., Ve. carmenti and Ve. funerea) were incompetent laboratory vectors and/or yielded no field isolates despite 10,000 s of mosquitoes being collected at the same time that multiple isolates were obtained from Cx. sitiens subgroup mosquitoes. Perhaps the key role that Aedes spp. will play in transmission cycles is virus maintenance through the vertical transmission to progeny via desiccation-resistant eggs [76]. However, in the only experiment assessing this in Australian mosquitoes, there was no evidence that 14 infected Ae. vigilax transmitted the virus to their progeny [48].
3.2. Potential Australian Vectors
Whilst incriminated vectors, such as Cx. annulirostris, Cx. palpalis, Cx. quinquefasciatus and Cx. sitiens, occur in sub-tropical and temperate Australia, there are other species in southern latitudes that could also play an important role in JEV transmission. Potential vectors of JEV would include Culex spp., those that have yielded isolates in Southeast Asia or those that have been associated with closely related flaviviruses. For instance, MVEV and WNVKUN were isolated from Culex australicus, which is susceptible to both viruses in the laboratory [77,78,79,80]. Likely introduced into Australia in the 1940s [81], Culex molestus is a competent laboratory vector of JEV [82]. Furthermore, both species readily feed on birds (Figure 3) and can be locally abundant [83]. There are also some Aedes spp., such as Aedes vittiger and Aedes sagax, that are susceptible to MVEV infection in the laboratory [79,84] and whose populations in southern regions can reach very high levels following flooding rains [83]. If susceptible to JEV infection, then these mosquitoes could provide a mechanism of virus overwintering, similar to other species in the genus.
4. Other Factors That Impact the Role of Mosquitoes in JEV Transmission Cycles
The intrinsic ability for a mosquito to become infected with and transmit an arbovirus, coupled with evidence of infection in the field and interaction with the vertebrate host of the virus, are obviously important criteria for conferring vector status. However, there are numerous other factors that can impact the role that different mosquito species play in transmission cycles. Using studies focused on Cx. annulirostris but including other species where appropriate, we briefly discuss some of these factors and how they could influence the establishment of JEV in Australia.
4.1. Survival
The ability of a mosquito species to survive the EIP of the virus is critical in assessing what role a species can play in transmission. Although Cx. annulirostris could transmit JEV as early as day 7 post-exposure, in modeling the transmission dynamics of JEV in Japan, Wada [85] considered that the time from when Cx. tritaeniorhynchus imbibed an infectious blood meal to being able to transmit the virus was 10 days. Similarly, in analyzing the transmission dynamics of MVEV, Kay et al. [86] considered that mosquitoes did not become epidemiologically important until 8–10 d after feeding on an infectious blood meal in summer and longer at winter temperatures. Based on conservative daily survival rates of 70–75% [87,88], it was estimated that the highest proportion of Cx. annulirostris surviving to 10 days was 0.08 in the summer months.
4.2. Population Dynamics
The impact of survival on arbovirus transmission can be offset by high mosquito populations, which are intimately linked to weather and other environmental variables. In northern Australia, populations of Cx. annulirostris are generally present throughout the year, although peaks in abundance vary within and between years, as well as between locations. In northern and southwestern Queensland, populations tended to peak during the mid-to-late wet season when there was an abundance of larval habitats [89]. This contrasts with the Northern Territory, where populations were at their highest at the end of the wet season (May–June) when constant inundation of larval habitats ceased and they became conducive to colonization by Cx. annulirostris [90].
In southern Australia, Cx. annulirostris populations peak in summer (December–February) before they decline and are not detected during winter months (June–August; [91,92]). Abundant Cx. annulirostris populations, which can exceed 9000 mosquitoes per trap in some locations, were one of the main drivers of the 1974 MVEV and 2011 WNVKUN outbreaks in southeastern Australia [93,94]. In both these instances, above-average rainfall caused by La Niña weather systems provided widespread flooded habitats for the proliferation of Cx. annulirostris and waterbird breeding. Similarly, above-average rainfall associated with the 2021–2022 La Niña weather event led to a convergence of wading birds and mosquitoes, which, coupled with intensive pig farming, produced ideal conditions for JEV to emerge in southeastern Australia.
Whilst rainfall undoubtedly influences the population dynamics of these southern populations, they are also temperature dependent. It was suggested that average daily temperatures need to exceed 17.5 °C to sustain Cx. annulirostris populations [95] and when temperatures are below this, females overwinter as parous adults via quiescence [96]. Russell [91] suggested that infected parous Cx. annulirostris could overwinter viruses in southern Australia.
4.3. Dispersal
Mosquito dispersal can drive the movement of the virus within and between transmission foci and is a factor that requires consideration when formulating control strategies. In the Murray Valley of southeastern Australia, Russell [68] revealed that some Cx. annulirostris females can disperse at least 7 km from larval habitats whilst searching for a blood meal host. Subsequent mark–release–recapture experiments showed that the mean distance traveled by Cx. annulirostris ranged from 3.8 to 4.4 km from a release point, although a small proportion of marked females were able to travel over 12 km [97,98]. Thus, any disease mitigation strategies, such as larval control or relocation of domestic pigs away from productive larval habitats, may need to encompass these flight ranges, especially when mosquito populations are abundant [40,68].
Female Cx. annulirostris are also able to traverse hundreds of kilometers assisted by atmospheric wind transport, providing a mechanism for the long-range dispersal of viruses [99]. Kay and Farrow [100] used kites deployed in the planetary boundary layer (<100 m) to show that, whilst most flights were <150 km, it was estimated that on some nights, Cx. annulirostris had traveled up to 594 km. Similar long-range dispersal of Cx. tritaeniorhynchus was observed in Southeast Asia [101,102]. Using data from backtrack simulations, Ritchie and Rochester [17] suggested that infected Cx. annulirostris carried on low-level winds generated by low-pressure systems may have introduced JEV into the Torres Strait and onto Cape York Peninsula. Long-range mosquito-mediated virus dispersal is certainly plausible, as it is approximately 500 km from potential JEV foci in the New Guinea landmass to the Mitchell River area of Cape York Peninsula, the previous southern limit of the virus in Australia. However, it is over 2500 km from previously recognized foci in northern Australia and the southern limits of JEV reported during the 2022 outbreak. This would suggest that other mechanisms of virus dispersal were responsible, including infected migratory birds or overlapping mosquito–vertebrate cycles that utilize La Niña driven rainfall patterns.
4.4. Genetic Variation between Populations
Hemmerter et al. [103] revealed that five lineages of Cx. annulirostris occur in the Australasian region and suggested that these lineages may vary in their ability to transmit JEV. In particular, they noted that the ann-PNG1 and ann-PNG2 lineages shared a similar southern limit to JEV in northern Australia, hypothesizing that they may be efficient vectors of the genotype 1 JEV that replaced the genotype 2 viruses. Conversely, the ann AUS lineage present in all sampling sites on the Australian mainland and used for most vector competence experiments to date may not be as efficient a vector of the genotype 1 virus when compared with its high competence for the genotype 2 virus. The intensity and spread of the 2022 JEV epizootic in southeastern Australia suggest that the ann AUS and/or the ann S-AUS lineages, with the latter found only in southern locations, are highly competent vectors of the virus responsible.
The population genetics of the Cx pipiens complex, of which, Cx. quinquefasciatus, Cx. australicus, Cx. molestus and Cx. globocoxitus occur in Australia, is poorly understood. Recent analysis suggests that Cx. australicus and Cx. globocoxitus diverged from Cx. quinquefasciatus sometime after the latter species was introduced [104]. Preliminary evidence suggests that there is some genetic diversity in Cx. quinquefasciatus populations in Australia (N. Beebe, unpublished data), which may help to explain the difference in vector competence between the two Queensland populations examined.
4.5. Establishment of Exotic Vectors of JEV
Whilst Cx. annulirostris should be considered the primary vector of JEV, it is concerning that two major vectors of JEV from southeast Asia have become established in Australia in the last 20 years. Cx gelidus was first recognized in Brisbane in 1999 [105] and subsequently found in the Northern Territory [106], far north Queensland [107] and northern Western Australia (M Lindsay and A Broom, pers. comm. cited by Williams et al. [108]). Using climate suitability modeling, Williams et al. [108] concluded that much of Australia is suitable for the establishment of Cx. gelidus. The collection of a single specimen of Cx. gelidus in central New South Wales represents the southernmost record of this species in Australia (Webb, C.E., personal communication). On occasion, high numbers of Cx. gelidus larvae can be found in polluted water sites, such as sewage settlement ponds or run-off from animal processing plants [106]. However, only low numbers of Cx. gelidus are collected in CO2-baited light traps and Whelan et al. [106] suggested that these may not be the most efficient traps for sampling this species.
In 2020, specimens of Cx. tritaeniorhynchus were collected from the Darwin and Katherine regions of the Northern Territory [109]. This species was subsequently identified in northern Western Australia, suggesting that it is now widespread in northern Australia. So far, only low numbers of Cx. tritaeniorhynchus (<40 per trap) are collected relative to Cx. annulirostris (N. Kurucz, personal communication). Like Cx. gelidus, the collection of low numbers of Cx. tritaeniorhynchus may reflect the inefficiency of CO2-baited light traps for sampling this species. Regardless, as it expands its distribution, it may become more abundant as it finds more suitable conditions.
5. Directions for Further Investigation
The original incursions of genotype 1 and 2 viruses into northern Australia led to a considerable amount of research into the role that Australian mosquito species play in transmission cycles. However, much of the work centered on locations in northern Australia where the virus was active or that possessed ecological conditions conducive to virus transmission. In this section, we identify directions for the investigation that will inform our knowledge of the role that Australian mosquitoes could play in the transmission of the emergent virus genotype and how intrinsic and ecological factors could influence transmission dynamics.
5.1. Detection of the Virus in Mosquito Field Populations during Periods of Recognized Virus Activity
These investigations should focus on processing pools according to taxonomic groups so that infection rates in different species can be compared to help incriminate vectors. Collections should be conducted within and between expected transmission seasons to provide information on the temporal and spatial distribution of the virus, as well as its ability to overwinter. Alternatives to the standard CO2-baited light traps used by most jurisdictions should also be utilized, including gravid traps, which collect Cx. quinquefasciatus and, potentially, Cx. gelidus.
5.2. Vector Competence of Australian Mosquito Species for the Newly Emergent JEV Genotype
Representative populations from different latitudes of Australia should be included to determine whether there are within and between species differences in their ability to become infected with and transmit the virus. Once this has been established, the population genetics of key JEV vectors that can influence transmission dynamics should be determined. For instance, it is possible that the southern lineage of Cx. annulirostris could be more efficient vectors of the virus genotype that has emerged in southern Australia. Similar mosquito genotype X virus genotype interactions were proposed for dengue viruses in Aedes aegypti [110].
5.3. The Genetic Diversity of Implicated Vectors
Clearly, there is a considerable amount of genetic diversity within the Cx. sitiens subgroup, particularly within Cx. annulirostris and Cx. palpalis. The population genetics of Cx. quinquefasciatus and other members of the Cx. pipiens complex is even less well known. Thus, widespread sampling of implicated vectors needs to be undertaken so that the genetic structure of these mosquitoes can be determined and the link to bionomics and vector competence established.
5.4. The Potential for JEV to Overwinter in Australian Mosquitoes
Laboratory-based experiments should be undertaken to establish potential filial transmission rates in common floodwater Aedes of southern Australia, such as Ae. vittiger, Ae. sagax and Ae. theobaldi. Detection of the virus in field-collected Aedes spp. males or adults reared from larvae will provide evidence of vertical transmission in nature. Although rarely documented in temperate JEV endemic areas of Southeast Asia [111,112], infected overwintering parous Culex females provide another possible mechanism of virus maintenance that should be investigated.
5.5. Bionomics of Invasive Australian JEV Vectors
The geographical distribution of Cx. gelidus and Cx. tritaeniorhynchus in Australia needs to be defined, along with their population dynamics, preferred larval habitats and biting behavior.
5.6. Host-Feeding Patterns of Incriminated Vectors
These studies need to ascertain the feeding rates on pigs and birds and should focus on mosquitoes found in proximity to intensive pig farms, locations where feral pigs congregate, and ardeid and other waterbird colonies. Feeding on macropods in southeastern Australia also needs to be investigated to determine whether there is a virus dilution effect like that suggested in northern areas. Where possible, vertebrate surveys should be undertaken so that the influence of host abundance on mosquito feeding preference can be quantified [113]. Of particular interest would be the proportion of implicated vectors feeding on domesticated versus feral pigs.
5.7. Australian Vertebrate Fauna as Amplifying Hosts of JEV
There is only limited information on the JEV viremia levels in Australian vertebrates. Experimental inoculation of two ardeid birds, namely, N. caledonicus and intermediate egrets (Egretta intermedia), and brushtail possums (Trichosurus vulpecula) produced moderate viremia levels [66,114]. The latter study also demonstrated that macropods produced only low-level viremia when inoculated with JEV [66]. However, it is difficult to interpret these results, as these viremias may still have been sufficient to infect efficient mosquito vectors. Thus, vertebrate infection studies should be conducted using infected mosquitoes as the source of infection and uninfected recipient mosquitoes used to measure whether candidate vertebrate species are infectious post-exposure. This would show the correlation between vertebrate viremia levels and mosquito infection, and provide a more accurate representation of what would be encountered in the field than if needle inoculation is used for infection and only the viremia levels are measured.
6. Conclusions
Soon after JEV emerged in the mid-1990s, it was postulated that environmental conditions were suitable for the establishment of the virus on the Australian mainland [22]. However, despite at least two introductions of the virus into the region [13,20], there was no evidence to suggest this scenario eventuated [19]. That is what makes the emergence of JEV in southeastern Australia, at least 2500 km below its previous southern geographical limit, such an unanticipated event.
Given the geographical extent of virus activity and evidence the virus was present prior to the widespread 2021–2022 outbreak, it is unlikely that the virus will be eliminated from Australia. Other human and animal health management strategies will have to be implemented, including vaccination, changes in animal husbandry and mosquito control. The synthesis of current knowledge of JEV vectors presented herein reinforces that it is Culex spp., particularly Cx. annulirostris, that will need to be the target of surveillance and control activities. Any programs will need to be sustainable and flexible enough to incorporate data that will arise from investigations conducted to characterize the role that endemic and newly established mosquitoes play in the transmission of JEV in Australia.
Acknowledgments
The authors thank Nina Kurucz, Lorna Melville, Cameron Webb and David Williams for sharing unpublished data, and Alyssa Pyke and Nigel Beebe for useful discussions about various aspects of this review.
Supplementary Materials
The following supporting information can be downloaded from https://www.mdpi.com/article/10.3390/v14061208/s1. Table S1: Mosquitoes collected from the Torres Strait islands of Badu and Saibai, northern Australia, and the Western Province of Papua New Guinea between 1995 and 2000, and processed for the detection of Japanese encephalitis virus.
Author Contributions
Conceptualization, A.F.v.d.H., E.S., S.A.R. and J.S.M.; formal analysis, A.F.v.d.H. and E.S.; writing—original draft preparation, A.F.v.d.H.; writing—review and editing, A.F.v.d.H., E.S., S.A.R. and J.S.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Campbell G.L., Hills S.L., Fischer M., Jacobson J.A., Hoke C.H., Hombach J.M., Marfin A.A., Solomon T., Tsai T.F., Tsu V.D., et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011;89:766–774. doi: 10.2471/BLT.10.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quan T.M., Thao T.T.N., Duy N.M., Nhat T.M., Clapham H. Estimates of the global burden of Japanese encephalitis and the impact of vaccination from 2000–2015. eLife. 2020;9:e51027. doi: 10.7554/eLife.51027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao X., Liu H., Li X., Fu S., Cao L., Shao N., Zhang W., Wang Q., Lu Z., Lei W., et al. Changing Geographic Distribution of Japanese Encephalitis Virus Genotypes, 1935–2017. Vector-Borne Zoonotic Dis. 2019;19:35–44. doi: 10.1089/vbz.2018.2291. [DOI] [PubMed] [Google Scholar]
- 4.Schuh A.J., Ward M.J., Brown A.L., Barrett A.D.T. Phylogeography of Japanese Encephalitis Virus: Genotype is Associated with Climate. PLOS Negl. Trop. Dis. 2013;7:e2411. doi: 10.1371/journal.pntd.0002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon T., Ni H., Beasley D.W.C., Ekkelenkamp M., Cardosa M.J., Barrett A.D.T. Origin and Evolution of Japanese Encephalitis Virus in Southeast Asia. J. Virol. 2003;77:3091–3098. doi: 10.1128/JVI.77.5.3091-3098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buescher E.L., Scherer W.F., McClure H.E., Moyer J.T., Rosenberg M.Z., Yoshii M., Okada Y. Ecologic Studies of Japanese Encephalitis Virus in Japan. Am. J. Trop. Med. Hyg. 1959;8:678–688. doi: 10.4269/ajtmh.1959.8.678. [DOI] [PubMed] [Google Scholar]
- 7.Scherer W.F., Buescher E.L., McClure H.E. Ecologic Studies of Japanese Encephalitis Virus in Japan. Am. J. Trop. Med. Hyg. 1959;8:689–697. doi: 10.4269/ajtmh.1959.8.689. [DOI] [PubMed] [Google Scholar]
- 8.Buescher E.L., Scherer W.F. Ecologic Studies of Japanese Encephalitis Virus in Japan. IX. Epidemiologic correlations and conclusions. Am. J. Trop. Med. Hyg. 1959;8:719–722. doi: 10.4269/ajtmh.1959.8.719. [DOI] [PubMed] [Google Scholar]
- 9.Grossman R.A., Edelman R., Gould D.J. Study of Japanese encephalitis virus in Chiangmai Valley, Thailand. VI. Summary and conclusions. Am. J. Epidemiol. 1974;100:69–76. doi: 10.1093/oxfordjournals.aje.a112010. [DOI] [PubMed] [Google Scholar]
- 10.Endy T.P., Nisalak A. Japanese Encephalitis Virus: Ecology and Epidemiology. Curr. Top. Microbiol. Immunol. 2002;267:11–48. doi: 10.1007/978-3-642-59403-8_2. [DOI] [PubMed] [Google Scholar]
- 11.Ladreyt H., Durand B., Dussart P., Chevalier V. How Central Is the Domestic Pig in the Epidemiological Cycle of Japanese Encephalitis Virus? A Review of Scientific Evidence and Implications for Disease Control. Viruses. 2019;11:949. doi: 10.3390/v11100949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackenzie J.S., Gubler D.J., Petersen L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004;10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 13.Hanna J.N., Ritchie S.A., Phillips D.A., Shield J., Bailey M.C., Mackenzie J.S., Poidinger M., McCall B.J., Mills P.J. An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995. Med. J. Aust. 1996;165:256–260. doi: 10.5694/j.1326-5377.1996.tb124960.x. [DOI] [PubMed] [Google Scholar]
- 14.Hanna J.N., Ritchie S.A., Phillips D.A., Lee J.M., Hills S., van den Hurk A.F., Pyke A., Johansen C.A., Mackenzie J.S. Japanese encephalitis in north Queensland, Australia, 1998. Med. J. Aust. 1999;170:533–536. doi: 10.5694/j.1326-5377.1999.tb127878.x. [DOI] [PubMed] [Google Scholar]
- 15.Johansen C.A., van den Hurk A.F., Ritchie S.A., Zborowski P., Paru R., Bockarie M.J., Drew A.C., Khromykh T.I., Mackenzie J.S. Isolation of Japanese encephalitis virus from mosquitoes (Diptera: Culicidae) collected in the Western Province of Papua New Guinea, 1997–1998. Am. J. Trop. Med. Hyg. 2000;62:631–638. doi: 10.4269/ajtmh.2000.62.631. [DOI] [PubMed] [Google Scholar]
- 16.Mackenzie J.S., Johansen C.A., Ritchie S.A., van den Hurk A.F., Hall R.A. The Emergence and Spread of Japanese Encephalitis Virus in Australasia. Curr. Top. Microbiol. Immunol. 2002;267:49–73. doi: 10.1007/978-3-642-59403-8_3. [DOI] [PubMed] [Google Scholar]
- 17.Ritchie S.A., Rochester W. Wind-Blown Mosquitoes and Introduction of Japanese Encephalitis into Australia. Emerg. Infect. Dis. 2001;7:900–908. doi: 10.3201/eid0705.017524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shield J., Hanna J., Phillips D. Reappearance of the Japanese encephalitis virus in the Torres Strait, 1996. Commun. Dis. Intell. 1996;20:191. [Google Scholar]
- 19.van den Hurk A.F., Pyke A.T., Mackenzie J.S., Hall-Mendelin S., Ritchie S.A. Japanese Encephalitis Virus in Australia: From Known Known to Known Unknown. Trop. Med. Infect. Dis. 2019;4:38. doi: 10.3390/tropicalmed4010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pyke A.T., Williams D.T., Nisbet D.J., van den Hurk A.F., Taylor C.T., Johansen C.A., Macdonald J., Hall R.A., Simmons R.J., Mason R.J.V., et al. The appearance of a second genotype of Japanese encephalitis virus in the Australasian region. Am. J. Trop. Med. Hyg. 2001;65:747–753. doi: 10.4269/ajtmh.2001.65.747. [DOI] [PubMed] [Google Scholar]
- 21.van den Hurk A.F., Montgomery B.L., Northill J.A., Smith I.L., Zborowski P., Ritchie S.A., Mackenzie J.S., Smith G.A. The First Isolation of Japanese Encephalitis Virus from Mosquitoes Collected from Mainland Australia. Am. J. Trop. Med. Hyg. 2006;75:21–25. doi: 10.4269/ajtmh.2006.75.21. [DOI] [PubMed] [Google Scholar]
- 22.Mackenzie J.S. Japanese encephalitis: An emerging disease in the Australasian region, and its potential risk to Australia. Arbovirus Res. Aust. 1997;7:166–170. [Google Scholar]
- 23.Northern Territory Government Japanese Encephalitis. [(accessed on 4 May 2022)]; Available online: https://nt.gov.au/wellbeing/health-conditions-treatments/viral/japanese-encephalitis.
- 24.Ritchie S.A., van den Hurk A.F., Zborowski P., Kerlin T.J., Banks D., Walker J.A., Lee J.M., Montgomery B.L., Smith G.A., Pyke A.T., et al. Operational Trials of Remote Mosquito Trap Systems for Japanese Encephalitis Virus Surveillance in the Torres Strait, Australia. Vector-Borne Zoonotic Dis. 2007;7:497–506. doi: 10.1089/vbz.2006.0643. [DOI] [PubMed] [Google Scholar]
- 25.World Organisation for Animal Health . OIE Immediate Notification—Japanese Encephalitis, Australia. World Organization for Animal Health; Paris, France: 2022. [Google Scholar]
- 26.Australian Government Department of Agriculture, Water and the Environment National Pest and Disease Outbreaks—Japanese Encephalitis. [(accessed on 4 May 2022)]; Available online: https://www.outbreak.gov.au/current-responses-to-outbreaks/japanese-encephalitis.
- 27.Australian Government Department of Health Japanese Encephalitis Virus (JEV) [(accessed on 4 May 2022)]; Available online: https://www.health.gov.au/health-alerts/japanese-encephalitis-virus-jev/about.
- 28.Reeves W.C. Arthropods as vectors and reservoirs of animal pathogenic viruses. In: Hallauer C., Meyer K.F., editors. Handbuch der Virus Forschung. Volume 4. Springer; Vienna, Austria: 1957. pp. 177–202. [Google Scholar]
- 29.Turell M.J., Dohm D.J., Sardelis M.R., O’Guinn M.L., Andreadis T.G., Blow J.A. An Update on the Potential of North American Mosquitoes (Diptera: Culicidae) to Transmit West Nile Virus. J. Med. Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- 30.van den Hurk A.F., Ritchie S.A., Smith G.A., Montgomery B.L., Mackenzie J.S. A Japanese encephalitis odyssey: Entomological studies in northern Australia, 2002–2004. Arbovirus Res. Aust. 2005;9:370–377. [Google Scholar]
- 31.Beebe N.W., Russell T., Burkot T.R., Cooper R.D. Anopheles punctulatus Group: Evolution, Distribution, and Control. Annu. Rev. Entomol. 2015;60:335–350. doi: 10.1146/annurev-ento-010814-021206. [DOI] [PubMed] [Google Scholar]
- 32.Jansen C.C., Hemmerter S., van den Hurk A.F., Whelan P.I., Beebe N.W. Morphological versus molecular identification of Culex annulirostris and Culex palpalis—Key members of the Culex sitiens (Diptera: Culicidae) subgroup in Australasia. Aust. J. Entomol. 2013;52:356–362. doi: 10.1111/aen.12045. [DOI] [Google Scholar]
- 33.Beebe N.W., van den Hurk A.F., Chapman H.F., Frances S.P., Williams C.R., Cooper R.D. Development and evaluation of a species diagnostic polymerase chain reaction-restriction fragment-length polymorphism procedure for cryptic members of the Culex sitiens (Diptera: Culicidae) subgroup in Australia and the southwest Pacific. J. Med. Entomol. 2002;39:362–369. doi: 10.1603/0022-2585-39.2.362. [DOI] [PubMed] [Google Scholar]
- 34.Chapman H.F., Kay B.H., Ritchie S.A., van den Hurk A.F., Hughes J.M. Definition of species in the Culex sitiens subgroup (Diptera: Culicidae) from Papua New Guinea and Australia. J. Med. Entomol. 2000;37:736–742. doi: 10.1603/0022-2585-37.5.736. [DOI] [PubMed] [Google Scholar]
- 35.Broom A.K., Hall R.A., Johansen C.A., Oliveira N., Howard M.A., Lindsay M.D., Kay B.H., Mackenzie J.S. Identification of Australian arboviruses in inoculated cell cultures using monoclonal antibodies in ELISA. Pathology. 1998;30:286–288. doi: 10.1080/00313029800169456. [DOI] [PubMed] [Google Scholar]
- 36.Pyke A.T., Smith I.L., van den Hurk A.F., Northill J.A., Chuan T.F., Westacott A.J., Smith G.A. Detection of Australasian Flavivirus encephalitic viruses using rapid fluorogenic TaqMan RT-PCR assays. J. Virol. Methods. 2004;117:161–167. doi: 10.1016/j.jviromet.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Ritchie S.A., Phillips D., Broom A., Mackenzie J., Poidinger M., Hurk A.V.D. Isolation of Japanese Encephalitis Virus from Culex annulirostris in Australia. Am. J. Trop. Med. Hyg. 1997;56:80–84. doi: 10.4269/ajtmh.1997.56.80. [DOI] [PubMed] [Google Scholar]
- 38.Johansen C.A., van den Hurk A.F., Pyke A.T., Zborowski P., Phillips D.A., Mackenzie J.S., Ritchie S.A. Entomological Investigations of an Outbreak of Japanese Encephalitis Virus in the Torres Strait, Australia, in 1998. J. Med. Entomol. 2001;38:581–588. doi: 10.1603/0022-2585-38.4.581. [DOI] [PubMed] [Google Scholar]
- 39.Van den Hurk A.F., Nisbet D.J., Johansen C.A., Foley P.N., Ritchie S.A., Mackenzie J.S. Japanese encephalitis on Badu Island, Australia: The first isolation of Japanese encephalitis virus from Culex gelidus in the Australasian region and the role of mosquito host-feeding patterns in virus transmission cycles. Trans. R. Soc. Trop. Med. Hyg. 2001;95:595–600. doi: 10.1016/S0035-9203(01)90090-2. [DOI] [PubMed] [Google Scholar]
- 40.Van den Hurk A.F., Ritchie S.A., Johansen C.A., Mackenzie J.S., Smith G.A. Domestic Pigs and Japanese Encephalitis Virus Infection, Australia. Emerg. Infect. Dis. 2008;14:1736–1738. doi: 10.3201/eid1411.071368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansen C.A., Nisbet D.J., Foley P.N., van den Hurk A.F., Hall R.A., Mackenzie J.S., Ritchie S.A. Flavivirus isolations from mosquitoes collected from Saibai Island in the Torres Strait, Australia, during an incursion of Japanese encephalitis virus. Med. Veter. Entomol. 2004;18:281–287. doi: 10.1111/j.0269-283X.2004.00510.x. [DOI] [PubMed] [Google Scholar]
- 42.Vythilingam I., Oda K., Mahadevan S., Abdullah G., Thim C.S., Hong C.C., Vijayamalar B., Sinniah M., Igarashi A. Abundance, Parity, and Japanese Encephalitis Virus Infection of Mosquitoes (Diptera: Culicidae) in Sepang District, Malaysia. J. Med. Entomol. 1997;34:257–262. doi: 10.1093/jmedent/34.3.257. [DOI] [PubMed] [Google Scholar]
- 43.Vythilingam I., Oda K., Tsuchie H., Mahadevan S., Vijayamalar B. Isolation of Japanese encephalitis virus from Culex sitiens mosquitoes in Selangor, Malaysia. J. Am. Mosq. Control Assoc. 1994;10:228–229. [PubMed] [Google Scholar]
- 44.Weng M.H., Lien J.C., Wang Y.M., Lin C.C., Lin H.C., Chin C. Isolation of Japanese encephalitis virus from mosquitoes collected in Northern Taiwan between 1995 and 1996. J. Microbiol. Immunol. Infect. 1999;32:9–13. [PubMed] [Google Scholar]
- 45.Johnson P.H., Hall-Mendelin S., Whelan P.I., Frances S.P., Jansen C.C., Mackenzie D.O., Northill J.A., van den Hurk A.F. Vector competence of Australian Culex gelidus Theobald (Diptera: Culicidae) for endemic and exotic arboviruses. Aust. J. Entomol. 2009;48:234–240. doi: 10.1111/j.1440-6055.2009.00711.x. [DOI] [Google Scholar]
- 46.Nicholson J., Ritchie S.A., van den Hurk A.F. Aedes albopictus (Diptera: Culicidae) as a Potential Vector of Endemic and Exotic Arboviruses in Australia. J. Med. Entomol. 2014;51:661–669. doi: 10.1603/ME13204. [DOI] [PubMed] [Google Scholar]
- 47.van den Hurk A.F., Johnson P.H., Hall-Mendelin S., Northill J.A., Simmons R.J., Jansen C.C., Frances S.P., Smith G.A., Ritchie S.A. Expectoration of Flaviviruses during sugar feeding by mosquitoes (Diptera: Culicidae) J. Med. Entomol. 2007;44:845–850. doi: 10.1603/0022-2585(2007)44[845:EOFDSF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 48.van den Hurk A.F., Nisbet D.J., Hall R.A., Kay B.H., Mackenzie J.S., Ritchie S.A. Vector Competence of Australian Mosquitoes (Diptera: Culicidae) for Japanese Encephalitis Virus. J. Med. Entomol. 2003;40:82–90. doi: 10.1603/0022-2585-40.1.82. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi M. Varaiation in susceptibility among colony strains of Culex tritaeniorhynchus to Japanese encephalitis virus infection. Jap. J. Med. Sci. Biol. 1980;33:321–329. doi: 10.7883/yoken1952.33.321. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi M. Differential transmission efficiency for Japanese encephalitis virus among colonised strains of Culex tritaeniorhynchus. Jap. J. San. Zool. 1982;33:325–333. doi: 10.7601/mez.33.325. [DOI] [Google Scholar]
- 51.Kay B., Fanning I., Carley J. The vector competence of Australian Culex annulirostris with Murray Valley encephalitis and Kunjin viruses. Aust. J. Exp. Biol. Med. Sci. 1984;62:641–650. doi: 10.1038/icb.1984.61. [DOI] [PubMed] [Google Scholar]
- 52.Azar S.R., Weaver S.C. Vector Competence: What Has Zika Virus Taught Us? Viruses. 2019;11:867. doi: 10.3390/v11090867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi M. The Effects of Environmental and Physiological Conditions of Culex tritaeniorhynchus on the Pattern of Transmission of Japanese Encephalitis Virus. J. Med. Entomol. 1976;13:275–284. doi: 10.1093/jmedent/13.3.275. [DOI] [PubMed] [Google Scholar]
- 54.Blackwell A., Mordue A.J.M., Mordue W. Identification of bloodmeals of the Scottish biting midge, Culicoides impunctatus, by indirect enzyme-linked immunosorbent assay (ELISA) Med. Vet. Entomol. 1994;8:20–24. doi: 10.1111/j.1365-2915.1994.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 55.Ngo K.A., Kramer L.D. Identification of Mosquito Bloodmeals Using Polymerase Chain Reaction (PCR) with Order-Specific Primers. J. Med. Entomol. 2003;40:215–222. doi: 10.1603/0022-2585-40.2.215. [DOI] [PubMed] [Google Scholar]
- 56.Flies E.J., Flies A.S., Fricker S.R., Weinstein P., Williams C.R. Regional Comparison of Mosquito Bloodmeals in South Australia: Implications for Ross River Virus Ecology. J. Med. Entomol. 2016;53:902–910. doi: 10.1093/jme/tjw035. [DOI] [PubMed] [Google Scholar]
- 57.Frances S.P., Cooper R.D., Rowcliffe K.L., Chen N., Cheng Q. Occurrence of Ross River Virus and Barmah Forest Virus in Mosquitoes at Shoalwater Bay Military Training Area, Queensland, Australia. J. Med. Entomol. 2004;41:115–120. doi: 10.1603/0022-2585-41.1.115. [DOI] [PubMed] [Google Scholar]
- 58.Hall-Mendelin S., Jansen C.C., Cheah W.Y., Montgomery B.L., Hall R.A., Ritchie S.A., van den Hurk A.F. Culex annulirostris (Diptera: Culicidae) host feeding patterns and Japanese encephalitis virus ecology in northern Australia. J. Med. Entomol. 2012;49:371–377. doi: 10.1603/ME11148. [DOI] [PubMed] [Google Scholar]
- 59.Jansen C.C., Webb C.E., Graham G.C., Craig S.B., Zborowski P., Ritchie S.A., Russell R.C., van den Hurk A.F. Blood Sources of Mosquitoes Collected from Urban and Peri-Urban Environments in Eastern Australia with Species-Specific Molecular Analysis of Avian Blood Meals. Am. J. Trop. Med. Hyg. 2009;81:849–857. doi: 10.4269/ajtmh.2009.09-0008. [DOI] [PubMed] [Google Scholar]
- 60.Johansen C.A., Power S.L., Broom A.K. Determination of Mosquito (Diptera: Culicidae) Bloodmeal Sources in Western Australia: Implications for Arbovirus Transmission. J. Med. Entomol. 2009;46:1167–1175. doi: 10.1603/033.046.0527. [DOI] [PubMed] [Google Scholar]
- 61.Kay B.H., Boreham P.F.L., Fanning I.D. Host-Feeding Patterns of Culex annulirostris and Other Mosquitoes (Diptera: Culicidae) at Charleville, Southwestern Queensland, Australia. J. Med. Entomol. 1985;22:529–535. doi: 10.1093/jmedent/22.5.529. [DOI] [PubMed] [Google Scholar]
- 62.Kay B.H., Boreham P.F.L., Williams G.M. Host preferences and feeding patterns of mosquitoes (Diptera: Culicidae) at Kowanyama, Cape York Peninsula, northern Queensland. Bull. Entomol. Res. 1979;69:441–457. doi: 10.1017/S0007485300018952. [DOI] [Google Scholar]
- 63.Kay B.H., Ryan P.A., Hall R.A., Boyd A.M. Mosquito feeding patterns and natural infection of vertebrates with Ross River and Barmah Forest viruses in Brisbane, Australia. Am. J. Trop. Med. Hyg. 2007;76:417–423. doi: 10.4269/ajtmh.2007.76.417. [DOI] [PubMed] [Google Scholar]
- 64.Van den Hurk A.F., Johansen C.A., Zborowski P., Paru R., Foley P.N., Beebe N.W., Mackenzie J.S., Ritchie S.A. Mosquito host-feeding patterns and implications for Japanese encephalitis virus transmission in northern Australia and Papua New Guinea. Med. Vet. Entomol. 2003;17:403–411. doi: 10.1111/j.1365-2915.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- 65.Van den Hurk A.F., Smith I.L., Smith G.A. Development and evaluation of real-time polymerase chain reaction assays to identify mosquito (Diptera: Culicidae) blood meals originating from native Australian mammals. J. Med. Entomol. 2007;44:85–92. doi: 10.1093/jmedent/41.5.85. [DOI] [PubMed] [Google Scholar]
- 66.Daniels P., Middleton D., Lunt R.A. Assessment of the Potential of Australian Fauna as Maintenance or Amplifying Hosts of Japanese Encephalitis (JE) Virus. CSIRO Australian Animal Health Laboratory; Geelong, Australia: 2000. pp. 1–3. [Google Scholar]
- 67.van den Hurk A.F., Ritchie S.A., Mackenzie J.S. Ecology and Geographical Expansion of Japanese Encephalitis Virus. Annu. Rev. Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- 68.Russell R.C. Dispersal of the arbovirus vector Culex annulirostris Skuse (Diptera: Culicidae) in the Murray VaIIey of Victoria, Australia. Gen. Appl. Entomol. 1986;18:5–9. [Google Scholar]
- 69.Banerjee K., Deshmukh P.K. Transmission of Japanese encephalitis virus to chicks by individual Culex bitaeniorhynchus mosquitoes. Indian J. Med. Res. 1987;86:726–727. [PubMed] [Google Scholar]
- 70.Banerjee K., Deshmukh P.K., Ilkal M.A., Dhanda V. Experimental transmission of Japanese encephalitis virus through Anopheles tessellatus and Culex fatigans mosquitoes. Indian J. Med. Res. 1977;65:746–752. [PubMed] [Google Scholar]
- 71.Gould D.J., Barnett H.C., Suyemoto W. Transmission of Japanese encephalitis virus by Culex gelidus Theobald. Trans. R. Soc. Trop. Med. Hyg. 1962;56:429–435. doi: 10.1016/0035-9203(62)90018-4. [DOI] [PubMed] [Google Scholar]
- 72.Vythilingam I., Oda K., Chew T.K., Mahadevan S., Vijayamalar B., Morita K., Tsuchie H., Igarashi A. Isolation of Japanese encephalitis virus from mosquitoes collected in Sabak Bernam, Selangor, Malaysia in 1992. J. Am. Mosq. Control Assoc. 1995;11:94–98. [PubMed] [Google Scholar]
- 73.Dhanda V., Thenmozhi V., Kumar N.P., Hiriyan J., Arunachalam N., Balasubramanian A., Ilango A., Gajanana A. Virus isolation from wild-caught mosquitoes during a Japanese encephalitis outbreak in Kerala in 1996. Indian J. Med. Res. 1997;106:4–6. [PubMed] [Google Scholar]
- 74.Frances S.P., Mackenzie D.O., Rowcliffe K.L., Corcoran S.K. Comparative Field Evaluation of Repellent Formulations Containing Deet and IR3535 Against Mosquitoes in Queensland, Australia. J. Am. Mosq. Control Assoc. 2009;25:511–513. doi: 10.2987/Moco-09-5938.1. [DOI] [PubMed] [Google Scholar]
- 75.Kay B.H., Watson T.M., Ryan P.A. Definition of productive Aedes notoscriptus (Diptera: Culicidae) habitats in western Brisbane, and a strategy for their control. Aust. J. Entomol. 2008;47:142–148. doi: 10.1111/j.1440-6055.2008.00641.x. [DOI] [Google Scholar]
- 76.Rosen L., Tesh R.B., Lien J.C., Cross J.H. Transovarial Transmission of Japanese Encephalitis Virus by Mosquitoes. Science. 1978;199:909–911. doi: 10.1126/science.203035. [DOI] [PubMed] [Google Scholar]
- 77.Marshall I.D. Epidemiology of Murray Valley encephalitis in eastern Australia—Patterns of arbovirus activity and strategies of arbovirus survival. Arbovirus Res. Aust. 1979;2:47–53. [Google Scholar]
- 78.Marshall I.D., Woodroofe G.M., Hirsch S. Viruses recovered from mosquitoes and wildlife serum collected in the Murray Valley of south-eastern Australia, February 1974, during an epidemic of encephalitis. Aust. J. Exp. Biol. Med. Sci. 1982;60:457–470. doi: 10.1038/icb.1982.51. [DOI] [PubMed] [Google Scholar]
- 79.McLean D.M. Transmission of Murray Valley encephalitis virus by mosquitoes. Aust. J. Exp. Biol. Med. Sci. 1953;31:481–490. doi: 10.1038/icb.1953.52. [DOI] [PubMed] [Google Scholar]
- 80.Van den Hurk A.F., Hall-Mendelin S., Webb C.E., Tan C.S., Frentiu F.D., Prow N.A., Hall R.A. Role of enhanced vector transmission of a new West Nile virus strain in an outbreak of equine disease in Australia in 2011. Parasites Vectors. 2014;7:586. doi: 10.1186/s13071-014-0586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Russell R.C. A Review of the Status and Significance of the Species Within the Culex pipiens Group in Australia. J. Am. Mosq. Control Assoc. 2012;28:24–27. doi: 10.2987/8756-971X-28.4s.24. [DOI] [PubMed] [Google Scholar]
- 82.Weng M.-H., Lien J.-C., Lin C.-C., Yao C.-W. Vector Competence of Culex pipiens molestus (Diptera: Culicidae) from Taiwan for a Sympatric Strain of Japanese Encephalitis Virus. J. Med. Entomol. 2000;37:780–783. doi: 10.1603/0022-2585-37.5.780. [DOI] [PubMed] [Google Scholar]
- 83.Dhileepan K. Mosquito seasonality and arboviral disease incidence in Murray Valley, southeast Australia. Med. Vet. Entomol. 1996;10:375–384. doi: 10.1111/j.1365-2915.1996.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 84.Kay B.H., Fanning I.D., Mottram P. The vector competence of Culex annulirostris, Aedes sagax and Aedes alboannulatus for Murray Valley encephalitis virus at different temperatures. Med. Veter. Entomol. 1989;3:107–112. doi: 10.1111/j.1365-2915.1989.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 85.Wada Y. Theoretical considerations on the epidemic of Japanese encephalitis. Trop. Med. 1975;16:171–199. [Google Scholar]
- 86.Kay B.H., Saul A.J., McCullagh A. A mathematical model for the rural amplification of Murray Valley encephalitis virus in southern Australia. Am. J. Epidemiol. 1987;125:690–705. doi: 10.1093/oxfordjournals.aje.a114582. [DOI] [PubMed] [Google Scholar]
- 87.Kay B.H. Age Structure of Populations of Culex annulirostris (Diptera: Culicidae) at Kowanyama and Charleville, Queensland. J. Med. Entomol. 1979;16:309–316. doi: 10.1093/jmedent/16.4.309. [DOI] [PubMed] [Google Scholar]
- 88.Russell R.C. Population age composition and female longevity of the arbovirus vector Culex annulirostris Skuse near Echuca, Victoria, in the Murray valley of southeastern Australia 1979–1985. Aust. J. Exp. Biol. Med. Sci. 1986;64:595–608. doi: 10.1038/icb.1986.63. [DOI] [PubMed] [Google Scholar]
- 89.Kay B. Seasonal abundance of Culex annulirostris and other mosquitoes at Kowanyama, north Queensland, and Charleville, south west Queensland. Aust. J. Exp. Biol. Med. Sci. 1979;57:497–508. doi: 10.1038/icb.1979.51. [DOI] [PubMed] [Google Scholar]
- 90.Russell R.C. Seasonal Abundance and Age Composition of Two Populations of Culex annulirostris (Diptera: Culicidae) at Darwin, Northern Territory, Australia. J. Med. Entomol. 1986;23:279–285. doi: 10.1093/jmedent/23.3.279. [DOI] [PubMed] [Google Scholar]
- 91.Russell R.C. Seasonal activity and abundance of the arbovirus vector Culex annulirostris Skuse near Echuca, Victoria, in the Murray valley of southeastern Australia 1979-1985. Aust. J. Exp. Biol. Med. Sci. 1986;64:97–103. doi: 10.1038/icb.1986.11. [DOI] [PubMed] [Google Scholar]
- 92.Russell R.C. Culex annuliroslris Skuse (Diptera: Culicidae) at Appin, N.S.W.- bionomics and behaviour. J. Aust. Enlomol. Soc. 1986;25:103–109. doi: 10.1111/j.1440-6055.1986.tb01087.x. [DOI] [Google Scholar]
- 93.Doggett S., Clancy J., Haniotis J., Webb C., Russell R.C., Hueston L., Dwyer D.E. The New South Wales Arbovirus Surveillance and Mosquito Monitoring Program 2010–2011 Annual Report. Department of Medical Entomology, ICPMR, Westmead Hospital; Westmead, Australia: 2011. p. 37. [Google Scholar]
- 94.Marshall I.D., Thibos E., Clarke K. Species composition of mosquitoes collected in the Murray Valley of south-eastern Australia during an epidemic of arboviral encephalitis. Aust. J. Exp. Biol. Med. Sci. 1982;60:447–456. doi: 10.1038/icb.1982.50. [DOI] [PubMed] [Google Scholar]
- 95.McDonald G., McLaren I.W., Shelden G.P., Smith I.R. The effect of temperature on the population growth potential of Culex annulirostris Skuse (Diptera: Culicidae) Austral. Ecol. 1980;5:379–384. doi: 10.1111/j.1442-9993.1980.tb01260.x. [DOI] [Google Scholar]
- 96.Russell R.C. Age composition and overwintering of Culex annulirostris Skuse (Diptera: Culicidae) near Deniliquin, in the Murray Valley of New South Wales. Aust. J. Entomol. 1987;26:93–96. doi: 10.1111/j.1440-6055.1987.tb00269.x. [DOI] [Google Scholar]
- 97.Bryan J.H., O’Donnell M.S., Berry G., Carvan T. Dispersal of adult female Culex annulirostris in Griffith, New South Wales, Australia: A further study. J. Am. Mosq. Control Assoc. 1992;8:398–403. [PubMed] [Google Scholar]
- 98.O’Donnell M.S., Berry G., Carvan T., Bryan J.H. Dispersal of adult females of Culex annulirostris in Griffith, New South Wales, Australia. J. Am. Mosq. Control Assoc. 1992;8:159–165. [PubMed] [Google Scholar]
- 99.Mackenzie J.S., Lindsay M.D., Daniels P.W. The effect of climate on the incidence of vector-borne viral diseases: The potential value of seasonal forecasting. In: Hammer G., Nicholls N., Mitchell C., editors. Applications of Seasonal Climate Forecasting in Agriculture and Natural Ecosystems—The Australian Experience. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2000. pp. 429–452. [Google Scholar]
- 100.Kay B.H., Farrow R.A. Mosquito (Diptera: Culicidae) Dispersal: Implications for the Epidemiology of Japanese and Murray Valley Encephalitis Viruses in Australia. J. Med. Entomol. 2000;37:797–801. doi: 10.1603/0022-2585-37.6.797. [DOI] [PubMed] [Google Scholar]
- 101.Ming J.-G., Jin H., Riley J.R., Reynolds D.R., Smith A.D., Wang R.-L., Cheng J.-Y., Cheng X.-N. Autumn southward “return” migration of the mosquito Culex tritaeniorhynchus in China. Med. Vet. Entomol. 1993;7:323–327. doi: 10.1111/j.1365-2915.1993.tb00699.x. [DOI] [PubMed] [Google Scholar]
- 102.Reynolds D., Smith A.D., Mukhopadhyay S., Chowdhury A.K., De B.K., Nath P.S., Mondal S.K., Das B.K., Mukhopadhyay S. Atmospheric transport of mosquitoes in northeast India. Med. Vet. Entomol. 1996;10:185–186. doi: 10.1111/j.1365-2915.1996.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 103.Hemmerter S., Šlapeta J., van den Hurk A.F., Cooper R.D., Whelan P.I., Russell R.C., Johansen C.A., Beebe N.W. A curious coincidence: Mosquito biodiversity and the limits of the Japanese encephalitis virus in Australasia. BMC Evol. Biol. 2007;7:100. doi: 10.1186/1471-2148-7-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aardema M.L., Vonholdt B.M., Fritz M.L., Davis S.R. Global evaluation of taxonomic relationships and admixture within the Culex pipiens complex of mosquitoes. Parasites Vectors. 2020;13:8. doi: 10.1186/s13071-020-3879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muller M.J., Montgomery B.L., Ingram A., Ritchie S.A. First records of Culex gelidus from Australia. J. Am. Mosq. Control Assoc. 2001;17:79–80. [PubMed] [Google Scholar]
- 106.Whelan P., Hayes G., Carter J., Wilson A., Haigh B. Detection of the exotic mosquito Culex gelidus in the Northern Territory. Commun. Dis. Intell. 2000;24:74–75. doi: 10.33321/cdi.2000.24.11. [DOI] [PubMed] [Google Scholar]
- 107.Ritchie S., Haseler B., Foley P., Montgomery B. Exotic mosquitoes in north Queensland: The true millenium bug. Arbovirus Res. Aust. 2001;8:288–293. [Google Scholar]
- 108.Williams C.R., Ritchie S.A., Whelan P.I. Potential distribution of the Asian disease vector Culex gelidus Theobald (Diptera: Culicidae) in Australia and New Zealand: A prediction based on climate suitability. Aust. J. Entomol. 2005;44:425–430. doi: 10.1111/j.1440-6055.2005.00502.x. [DOI] [Google Scholar]
- 109.Lessard B.D., Kurucz N., Rodriguez J., Carter J., Hardy C.M. Detection of the Japanese encephalitis vector mosquito Culex tritaeniorhynchus in Australia using molecular diagnostics and morphology. Parasites Vectors. 2021;14:411. doi: 10.1186/s13071-021-04911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fansiri T., Fontaine A., Diancourt L., Caro V., Thaisomboonsuk B., Richardson J.H., Jarman R.G., Ponlawat A., Lambrechts L. Genetic Mapping of Specific Interactions between Aedes aegypti Mosquitoes and Dengue Viruses. PLoS Genet. 2013;9:e1003621. doi: 10.1371/journal.pgen.1003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hayashi K., Mifune K., Matsuo S., Shichijo A., Suzuki H., Ura M., Makino Y., Wada Y., Oda T., Mogi M., et al. Ecology of Japanese encephalitis virus in Japan, particularly the results of surveys in every interepidemic season from 1964 to 1976. Trop. Med. 1978;20:81–96. [Google Scholar]
- 112.Lee H.W. Study on overwintering mechanisms of Japanese encephalitis virus in Korea. J. Korean Med. Assoc. 1971;14:871–878. [Google Scholar]
- 113.Stephenson E.B., Murphy A.K., Jansen C.C., Peel A.J., McCallum H. Interpreting mosquito feeding patterns in Australia through an ecological lens: An analysis of blood meal studies. Parasites Vectors. 2019;12:156. doi: 10.1186/s13071-019-3405-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boyle D.B., Dickerman R.W., Marshall I.D. Primary viraemia responses of herons to experimental infection with Murray Valley encephalitis, Kunjin and Japanese encephalitis viruses. Aust. J. Exp. Biol. Med. Sci. 1983;61:655–664. doi: 10.1038/icb.1983.62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.