Abstract
Background:
Aerobic exercise has emerged as a useful treatment to improve outcomes among individuals who experience a concussion. However, compliance with exercise recommendations and the effect of exercise volume on symptom recovery require further investigation.
Purposes:
To examine (1) if an 8-week aerobic exercise prescription, provided within 2 weeks of concussion, affects symptom severity or exercise volume; (2) whether prescription adherence, rather than randomized group assignment, reflects the actual effect of aerobic exercise in postconcussion recovery; and (3) the optimal volume of exercise associated with symptom resolution after 1 month of study.
Study Design:
Cohort study; Level of evidence, 2.
Methods:
Individuals randomized to an exercise intervention (n = 17; mean age, 17.2 ± 2.0 years; 41% female; initially tested a mean of 11.3 ± 2.8 days after injury) or standard of care (n = 20; mean age, 16.8 ± 2.2 years; 50% female; initially tested a mean of 10.7 ± 3.2 days after injury) completed an aerobic exercise test within 14 days of injury. They returned for assessments 1 month and 2 months after the initial visit. The aerobic exercise group was instructed to exercise 5 d/wk, 20 min/d (100 min/wk), at a target heart rate based on an exercise test at the initial visit. Participants reported their exercise volume each week over the 8- week study period and reported symptoms at each study visit (initial, 1 month, 2 months). Because of low compliance in both groups, there was no difference in the volume of exercise between the 2 groups.
Results:
There were no significant symptom severity differences between the intervention and standard-of-care groups at the initial (median Post-Concussion Symptom Inventory, 15 [interquartile range = 10, 42] vs 20 [11, 35.5]; P = .26), 1-month (4 [0, 28] vs 5.5 [0.5, 21.5]; P = .96), or 2-month (6.5 [0, 27.5] vs 0 [0, 4]; P = .11) study visits. Exercise volume was similar between groups (median, 115 [54, 225] vs 88 [28, 230] min/wk for exercise intervention vs standard of care; P = .52). Regardless of group, those who exercised < 100 min/wk reported significantly higher symptom severity at the 1-month evaluation compared with those who exercised > 100 min/wk (median, 1.5 [0, 7.5] vs 12 [4, 28]; P = .03). Exercising > 160 min/wk successfully discriminated between those with and those without symptoms 1 month after study commencement (classification accuracy, 81%; sensitivity, 90%; specificity, 78%).
Conclusion and Clinical Relevance:
Greater exercise volume was associated with lower symptom burden after 1 month of study, and an exercise volume > 160 min/wk in the first month of the study was the threshold associated with symptom resolution after the first month of the study. Because our observation on the association between exercise volume and symptom level is a retrospective and secondary outcome, it is possible that participants who were feeling better were more likely to exercise more, rather than the exercise itself driving the reduction in symptom severity.
Keywords: mild traumatic brain injury, adolescent, treatment, rehabilitation, aerobic exercise, symptoms
Introduction
Current sport-related concussion recommendations uniformly emphasize the importance of physical activity and sub-symptom aerobic exercise as a key component of active recovery.5,19,23 Specifics of this recommendation remain vague and do not account for exercise dosage (intensity, frequency, duration),6 leading to variability in clinical practice and potential recovery.17 In fact, a recent randomized clinical trial among adolescents suggested that 21-day sub-symptom at-home aerobic exercise prescription initiated within one week of a concussion led to a faster recovery (by four days) compared to a stretching program,12 although a follow-up analysis failed to show any appreciable difference in mean daily symptom burden compared to stretching or rest within 14 days.27 While this may be interpreted as a lack of harmful impact of rest, it does suggest that sub-symptom aerobic exercise is not more effective than rest. This is somewhat surprising given known benefits of regular exercise and aerobic fitness for overall health,4,22 and among individuals with concussion.6,11
While short-term concussion symptoms may resolve even without exercise, aerobic exercise may have a sizeable impact on persistent post-concussion symptoms (PPCS)29. The focus on short-term (14–21 days) follow-up may be the culprit underlying the apparent lack of effect demonstrated in existing clinical trial reports. Alternatively, compliance with a rehabilitation prescription can be variable.9 The impact of exercise training on physiologic function is highly dependent on compliance,20 and concussion recovery is not an exception. In particular, adolescents tend not to adhere to post-concussion recommendations,24 increasing compliance variability and, consequently, diluting intervention effects. In fact, while prior work suggests 100 minutes/week of aerobic exercise (20 minutes per day, 5 days per week) is a sufficient prescription to reduce symptoms among those who have already developed PPCS,2,3,12,13 the exercise dose necessary to achieve beneficial effects in the acute and sub-acute phases of concussion recovery remains unknown.21
Thus, our objectives were threefold. First, we examined if an 8 week individualized sub-symptom threshold aerobic exercise prescription, provided within the first two weeks of concussion, alleviates symptom severity or affects the amount of exercise performed during the study. Second, we examined whether prescription adherence, rather than randomized group assignment, reflects the actual impact of aerobic exercise in post-concussion recovery. Third, we sought to identify the optimal volume of exercise associated with symptom resolution after one month of study participation. We hypothesized that those randomized to the aerobic exercise intervention would report lower symptom severity at one-month and two-months after study enrollment and would perform a greater amount of exercise compared to the standard-of-care group, and that total aerobic exercise volume, regardless of group randomization assignment, would result in a lower symptom severity.
Materials and Methods
Trial Design
We conducted a block stratified randomized clinical trial of an aerobic exercise prescription compared to standard-of-care (allocation ratio, 1:1). Participants were enrolled, initially tested, and provided with an aerobic exercise prescription (intervention group only: intensity, duration, frequency) within 14 days of concussion. At baseline, participants’ aerobic fitness was determined (described below), and initial exercise intensity prescription was based on this test result (intervention group only). At the mid-point of the study (4-weeks post-enrollment), participants returned to the laboratory for an aerobic exercise test, to adjust the prescribed exercise intensity level (for the intervention group only) and symptom assessment. In order to examine our primary objectives, participants kept a weekly log of aerobic exercise activity, and reported symptom severity at the primary study endpoints: one-month and two-months post-enrollment. (The study flow diagram and original protocol are available as Supplementary Files).
Participants
We identified, recruited, and enrolled study participants (14–21 years old) from a primary care sports medicine clinic or emergency department within a tertiary care regional children’s hospital between September 25, 2018 and March 5, 2020. Inclusion criteria consisted of a clinical concussion diagnosis by a physician (based on the criteria outlined by the 5th International Consensus Statement on Concussion in Sport19), and a post-concussion symptom inventory (PCSI) score >9 at the initial assessment. Exclusion criteria included aerobic exercise contraindications, electrical implants, or a concomitant lower extremity injury at the time of initial assessment. All testing was performed in a dedicated cerebrovascular research laboratory. The study protocol was reviewed and approved by the institutional review board. All participants, and parents/guardians, if under the age of 18, provided written informed consent/assent.
Interventions
All participants completed an in-person assessment within 14 days of injury, including an aerobic exercise test (described below), and follow-up assessments one month and two months following the initial assessment. Based on performance on this test, participants assigned to the intervention group were provided a specific exercise prescription that included an individualized target intensity (heart rate), frequency (5×/week), and duration (20 minutes at the target heart rate) prescription to perform over the subsequent four weeks. When they returned for a follow-up test four weeks of study participation, their target intensity was adjusted based on exercise test performance for the final four weeks of study participation. Specifically, they underwent the same exercise test as the initial visit and participants assigned to the intervention group were given a new aerobic exercise heart rate target corresponding to the test performed at this second visit. They were told to continue aerobic exercise at the same volume (20 min/day, 5×/week). At the second visit, the prescribed target heart rate for the intervention group participants was higher than during the initial visit (mean recommended heart rate: initial visit = 132 [standard deviation = 13], four week visit = 142 [standard deviation = 3.2]). The mode of exercise was left to participant preference. Participants assigned to the standard-of-care group were told to comply with their physician recommended physical activity level. All physicians providing care to participants were board-certified sports medicine physicians, and those caring for participants assigned to the standard-of-care group provided no systematic exercise recommendation.
All participants completed an exercise test using a modified YMCA branching exercise protocol. We selected this approach in order to allow participants of unknown baseline fitness to get to maximum heart rate quickly and avoid endurance-related effects. Performance was used to identify the target heart rate (HR) for the intervention group exercise prescription. After a familiarization period on the stationary bike (Monark 928E G3; HealthCare International, Inc. Langley, WA, USA), participants began a 3 minute warmup, with 50 watts of resistance, pedaling at approximately 60–70 revolutions per minute (RPM). Participants progressed to Stage 1 for 3 minutes (100 watts of resistance) and increased RPM cadence to approximately 70–80. After Stage 1, participants progressed to Stage 2, determined based on their HR in the final 30 seconds of Stage 1. If Warmup-to-Stage 1 change was: <15 beats per minute (bpm), resistance increased to 175 watts; 15–25 bpm, the resistance increased to 150 watts; >25 bpm, the resistance increased to 125 watts. Participants continued for 2 minutes in Stages 3 and 4 (as tolerated) where resistance increased by 50 watts/stage. The protocol stopped if patients experienced significant symptom worsening (a change from rest on the Visual Analog Scale >30 mm; 30% increase in symptom burden), or achieved 85% age-predicted maximum heart rate (220–age). We calculated the exercise prescription intensity for the intervention group as 80% of the HR at the end of the test.
Outcome Measures
At each visit, participants completed a symptom severity assessment (Post-Concussion Symptom Inventory; PCSI). The PCSI is a validated symptom reporting instrument within the age range of participants.16,25 Participants completed 20 questions addressing the severity of different concussion-related symptoms, ranging from 0 (no symptoms) to 6 (maximum severity). Total PCSI scores ranged from 0 (no symptoms) to 120 (maximum severity).
We examined exercise volume (average minutes/week) during the first four weeks and the second four weeks of study separately. Participants completed exercise logs each week via an online reporting platform (REDCap). The diary asked participants to report: if they exercised (yes/no) and the time they spent exercising (in minutes if they reported any exercise) for each day of the week. We excluded participants from analysis if they did not complete a sufficient amount (>50%) of the exercise diaries. Any missing data were treated as such, and no imputations were performed. Among included participants, n=25 (68%) completed 8/8 weekly logs, n=6 (16%) completed 7/8 weekly logs, and n=6 (16%) completed 4–6/8 weekly logs. Participants were given a heart rate monitor to ensure they exercised at the prescribed intensity and to monitor their daily activity. However, only four participants wore the device and instructed, so we did not include these data in the analysis. Therefore, we could not ensure that intervention group participants did or did not exercise at the prescribed target heart rate.
Sample size and randomization
We determined our sample size before the study commencement, based on existing treadmill-based exercise intervention work to induce a 20% symptom burden reduction among patients who had persistent symptoms.15 We estimated we would require n=42 patients in the investigation (n=21 in each arm) would provide 0.80 power. Because of the cessation of all clinical research beginning in March 2020 due to COVID-19, we performed our primary and secondary analyses after n=41 participants had enrolled in the study.
Participants were randomized using a block stratification procedure. We used a block size of 4, and stratification factors included age (14–17 years vs. 18–21 years) and sex (girls/women vs. boys/men).7 Given the nature of the in-person aerobic fitness assessment and exercise prescription, no blinding was possible or meaningful. The lead author generated the random allocation sequence, and investigative team members enrolled participants and assigned them to each group.
Statistical Analysis
Data are presented as means (standard deviation) for continuous variables, and number included and corresponding percentage within group for categorical variables. For our primary purpose, we compared intervention and standard-of-care groups on demographic characteristics using independent samples t-tests and Fisher’s exact tests to ensure randomization was successful. We then compared symptom severity scores between groups at the initial, one-month, and two-month timepoints using Mann-Whitney U tests, given the non-normal distribution of the data. We also compared the exercise volume (average minutes of exercise/week) between groups during the first month and second month of the study using independent samples t-tests.
In order to accomplish our secondary objective, we grouped participants based upon the amount of exercise they reported during the first month of study enrollment, regardless of randomization assignment. We grouped them according to whether they reported ≥100 minutes or <100 minutes of average exercise per week. This classification was done based upon our prescription to the intervention group to exercise 20 minutes/day for 5 days/week, consistent with prior studies.2,3,12,13 Similar to the primary analysis, we compared the symptom severity at the initial, one-month, and two-month timepoints between groups using Mann-Whitney U tests, given the non-normal distribution of the data.
To address our third objective, we grouped participants based on whether or not they reported complete symptom resolution by the one-month study timepoint (PCSI score=0). We then used a receiver operating characteristic (ROC) analysis to calculate how exercise volume during the first four weeks of the study could discriminate by symptom recovery status. We calculated the area under the curve (AUC) value, and classification accuracy between groups at each exercise volume level. The exercise volume with the highest classification accuracy was identified, along with the sensitivity and specificity at that level. Statistical significance was set at α < 0.05 and all tests were two-sided. Statistical analyses were performed using Stata version 15 (StataCorp, College Station, TX).
Results
We assessed 146 patients for eligibility and interest in the study, and n=41 enrolled in the study. Among those who were enrolled in the study, n=20 were allocated to the exercise prescription intervention, and n=21 were allocated to standard-of-care (Figure 1). We included n=17 exercise intervention participants, and n=20 standard-of-care participants in our final analyses. Three exercise intervention participants, and one standard-of-care participant, were not included in the final analysis because they did not complete >50% of the exercise diaries during the study period. No adverse or unintended effects were noted.
Figure 1.
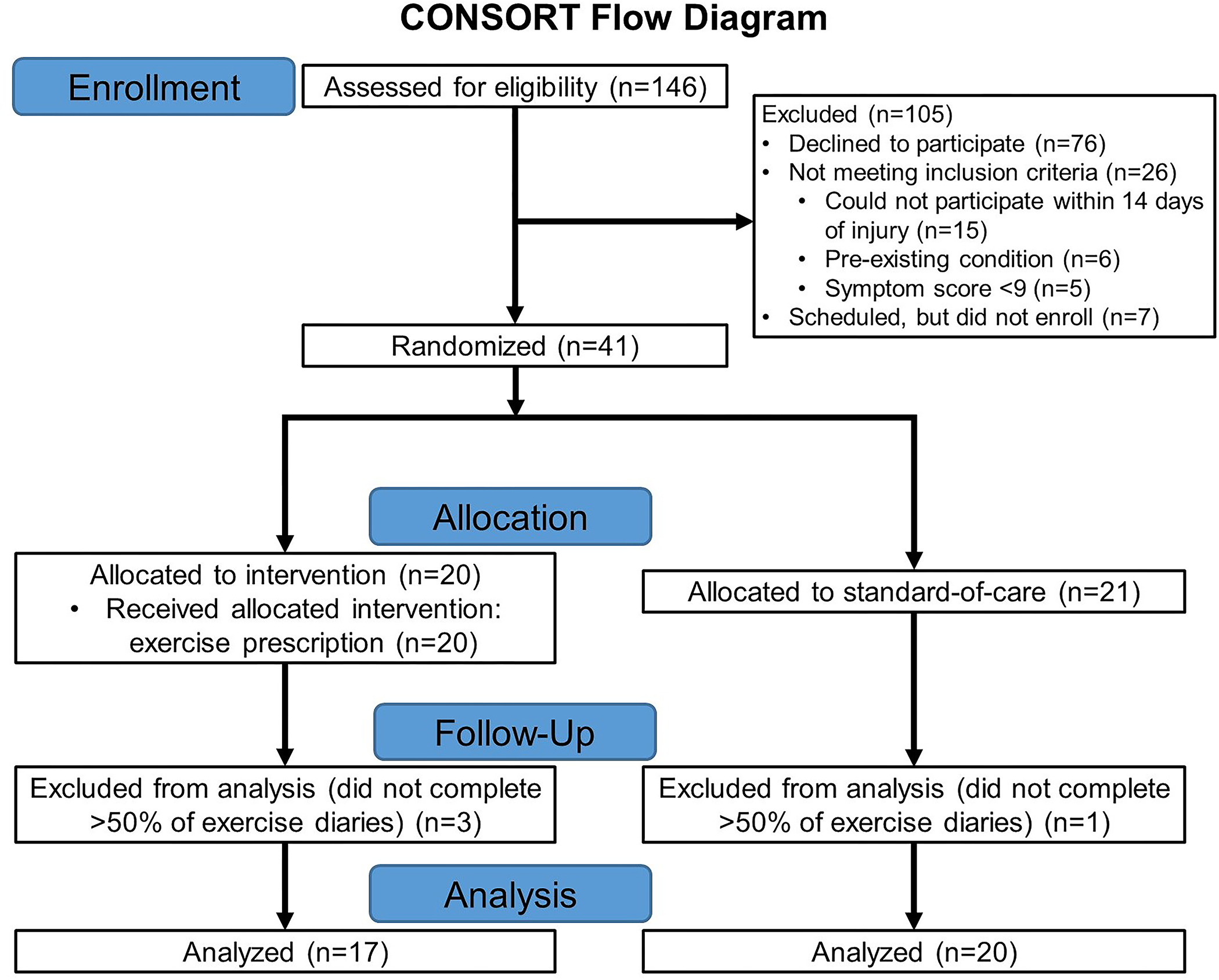
CONSORT (Consolidated Standards of Reporting Trials) diagram.
No demographic differences were observed between those randomized to exercise intervention or standard-of-care (Table 1), however, those in the exercise intervention were seen for a third assessment approximately eight days later post-injury than those randomized to standard-of-care (Table 1).
Table 1.
Participant demographic and exercise volume characteristics, grouped by randomization assignment.
| Variable | Exercise Intervention (n=17) | Standard-of-care (n=20) | P value |
|---|---|---|---|
| Age (years) | 17.2 (2.0) | 16.8 (2.2) | 0.49 |
| Sex (female) | 7 (41%) | 10 (50%) | 0.74 |
| Concussion history | 9 (53%) | 13 (65%) | 0.52 |
| Time of Post-Injury Test 1 (days post-injury) | 11.3 (2.8) | 10.7 (3.2) | 0.53 |
| Time of Post-Injury Test 2 (days post-injury) | 42.3 (7.1) | 39.7 7.5) | 0.30 |
| Time of Post-Injury Test 3 (days post-injury) | 75.6 (2.5) | 67.8 (8.4) | 0.01 |
| Sport/activity of participation during injury | Basketball: 5 (29%) Football: 2 (12%) Ice hockey: 2 (12%) Soccer: 2 (12%) Activity of daily living: 2 (12%) Cheerleading: 1 (6%) Equestrian: 1 (6%) Ice-skating: 1 (6%) Ultimate Frisbee: 1 (6%) |
Soccer: 5 (25%) Skiing/snowboarding: 4 (20%) Football: 2 (10%) Ice hockey: 2 (10%) Volleyball: 2 (10%) Basketball: 1 (5%) Rugby: 1 (5%) Softball: 1 (5%) Wrestling: 1 (5%) Activity of daily living: 1 (5%) |
- |
Objective 1: Effect of Exercise Prescription on Concussion Symptoms and Exercise Volume
Initial symptom severity was not different between the groups (Figure 2A), and no significant differences in symptom severity were found between those randomized to exercise and standard-of-care at the 4-week (Figure 2B) or 8-week (Figure 2C) assessment.
Figure 2.
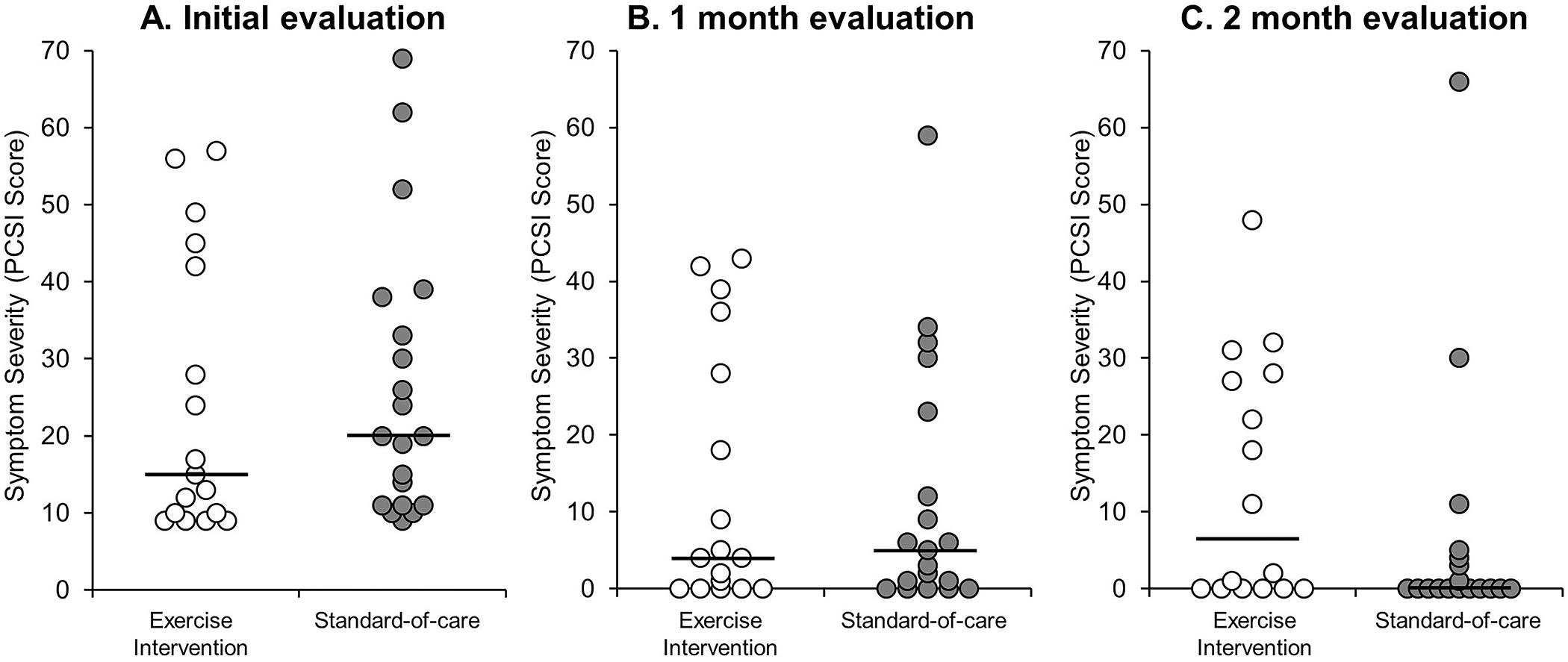
Individual data points describing the distribution of symptom severity for those randomized to the exercise intervention and standard-of-care groups at (A) the initial evaluation (≤14 days post-injury), (B) the 1-month evaluation, and (C) the 2-month evaluation. The solid black line represents the median value for each group. Note: No significant differences were identified between groups at the initial (p=0.26), one-month (p=0.96), or two-month (p=0.11) evaluations.
In addition, there was no significant differences between groups who were randomized to the exercise intervention and standard-of-care for average weekly exercise volume during the first four weeks (Figure 3A) or second four weeks (Figure 3B) of the study. During the first four weeks of the study 65% (n=11/17) of the participants who were provided the exercise prescription were compliant with this recommendation (≥100 min/week), compared to 45% (n=9/20) of the standard-of-care group who exercised ≥100 min/week (p=0.33). During the second four weeks of the study, 71% (n=12/17) of the exercise prescription group exercised ≥100 min/week, compared to 55% (n=11/20) of the standard-of-care group (p=0.50).
Figure 3.
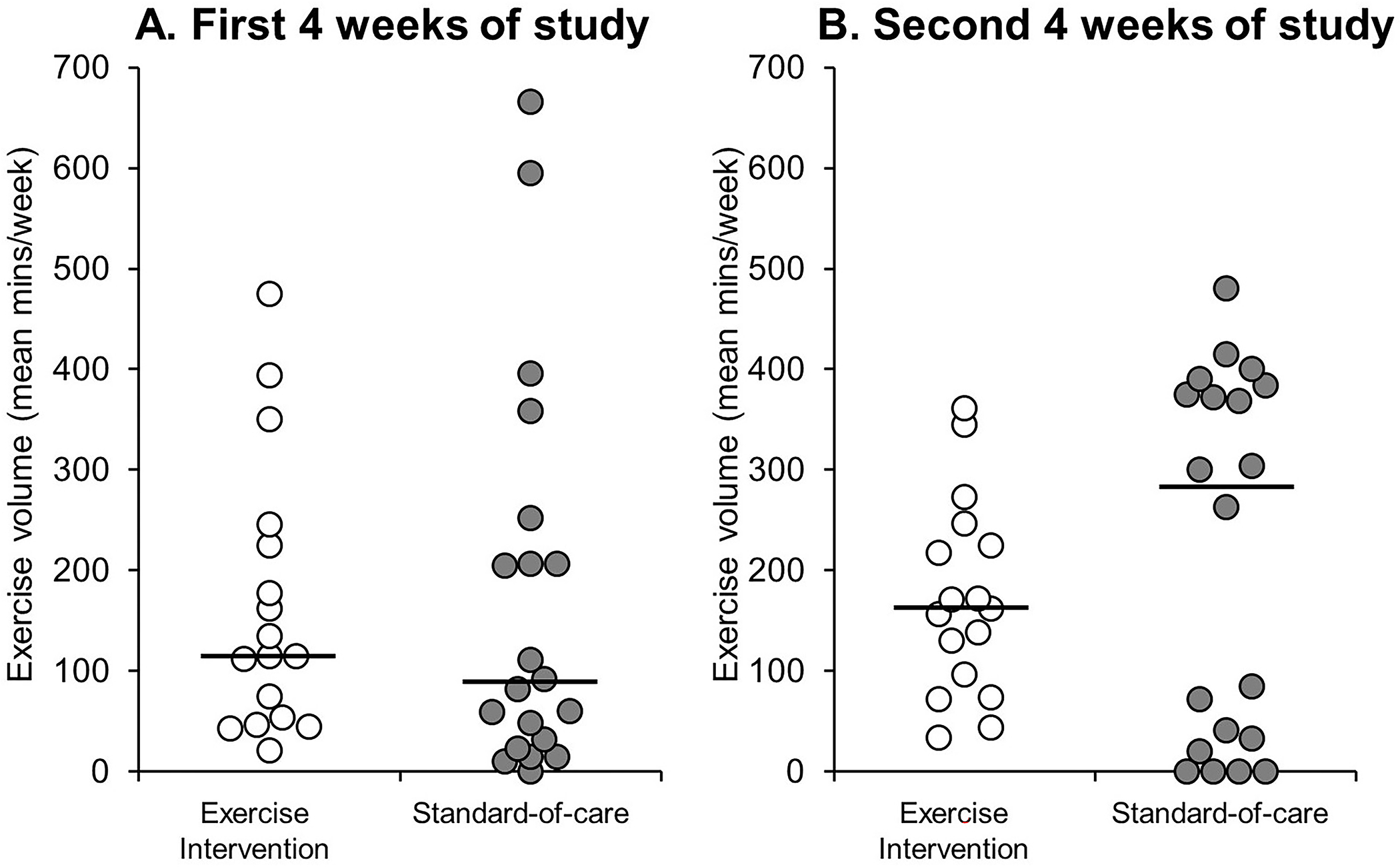
Individual data points describing the distribution of average weekly exercise volume (mins/week) for those randomized to the exercise intervention and standard-of-care groups (A) during the first 4 weeks of the study, and (B) the second 4 weeks of the study. The solid black line represents the group median value. Note: There were no significant differences between groups during the first 4 weeks (p=0.52) or second 4 weeks (p=0.59) of the study.
Objective 2: Effect of Exercise Volume on Concussion Symptoms
When grouped by exercise volume, those who exercised ≥100 min/week were older than those who exercised <100 minutes week (17.8±2.1 vs. 16.0±1.7 years; p=0.008), but the two groups did not differ in the proportion of those who were girls, had a prior history of concussion, or in the time of post-injury testing. The group who exercised ≥100 minutes/week during the first month of the study reported significantly lower symptom severity scores than those who exercised <100 minutes/week (Figure 4B), despite similar initial symptom severity scores (Figure 4A). Those who exercised ≥100 minutes/week during the second month of the study did not report significant symptom severity differences at the two-month study timepoint (Figure 4C).
Figure 4.
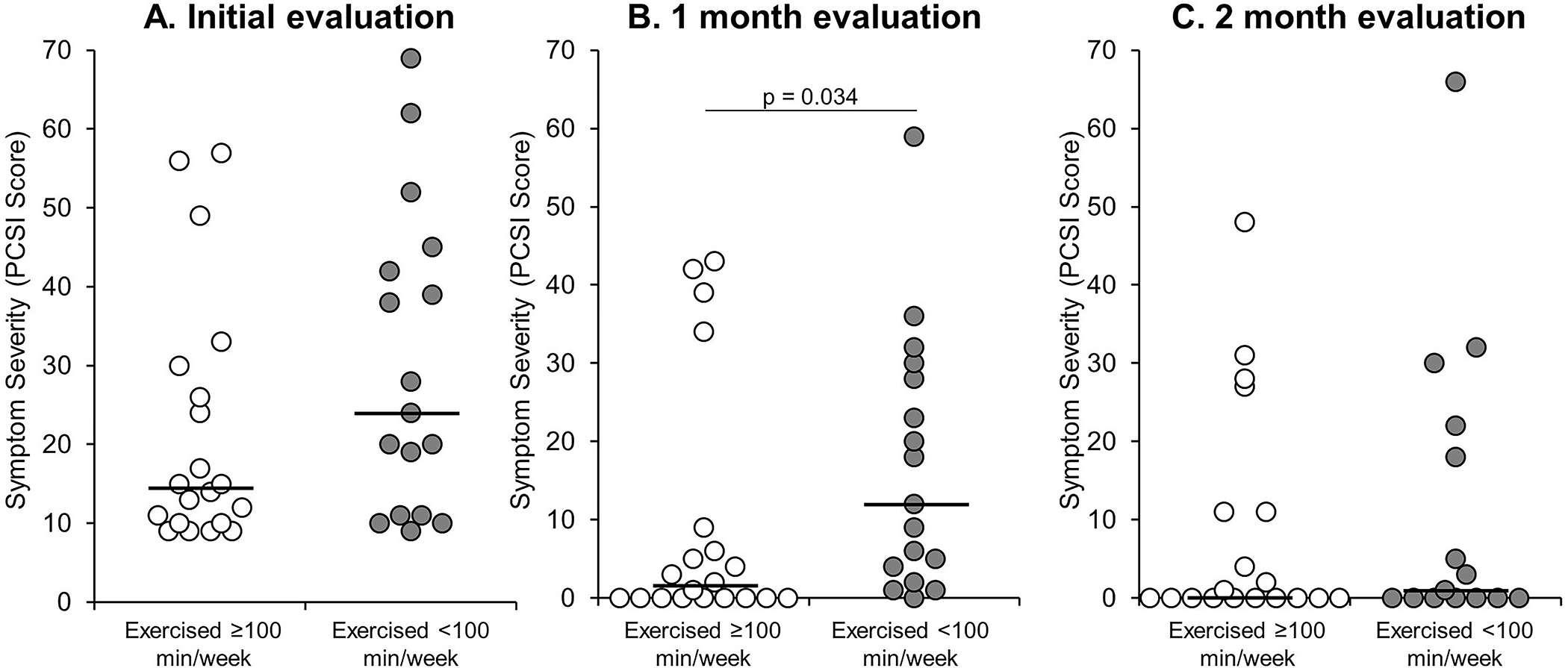
Individual data points describing the distribution of symptom severity, compared between those who did and did not report an average exercise volume ≥100 minutes per week during the first month of the study. The solid black line represents the median value for each group. Note: Those who exercised <100 min/week reported significantly higher symptom severity (p=0.034) at the 1-month evaluation compared to those who exercised ≥100 min/week. No significant differences were identified at the initial (p=0.14) or 2-month (p=0.66) evaluations.
Objective 3: Optimal Exercise Volume for Concussion Symptom Resolution
Participants who reported complete symptom resolution (PCSI score=0) by the one-month timepoint reported significantly more exercise volume during the first month of the study compared to the group who were still symptomatic at the one-month timepoint (mean=326±203 minutes/week vs. 110±107 minutes/week; p<0.001; mean difference=168 minutes/week; 95% CI=113–321 minutes/week). The amount of exercise/week in the first month of the study was able to successfully discriminate between those who were and were not symptomatic at the one-month assessment (AUC = 0.83; 95% confidence interval = 0.66, 0.99; Supplementary Figure 2). From the ROC analysis, we identified an optimal cutoff threshold, where ≥160 minutes/week of exercise successfully discriminated between participants with and without symptoms at one month of the study, with a classification accuracy of 81% (90% sensitivity, 78% specificity). By comparison, the prescription cutoff of ≥100 minutes/week of aerobic exercise provided a classification accuracy of 65% (90% sensitivity, 56% specificity).
Discussion
Randomization to an exercise program within the first two weeks post-concussion did not lead to a greater exercise volume relative to participants who did not receive specific exercise instructions. Furthermore, those randomized to an exercise program demonstrated similar symptom severity as those randomized to standard-of-care. Regardless of group randomization assignment, after one month in the study those who reported exercising at a volume of ≥100 minutes/week had lower symptom severity than those who exercised <100 minutes/week. Over the course of the entire two month study period, those randomized to the exercise prescription did not significantly alter exercise-related behavior, nor did the exercise prescription reduce symptom severity relative to those randomized to standard-of-care, despite an 8-day difference (later in exercise group) in final assessment times. Collectively, these findings suggest that compliance with an exercise prescription, rather than the prescription itself, may be critical to improve post-concussion outcomes. Failure to comply has been observed among adolescents,24 and future studies should seek to better understand how exercise prescription dosage and compliance affects concussion recovery. Furthermore, an aerobic exercise volume higher than 5×/week for 20 minutes/day may be necessary to elicit consistent positive change during concussion recovery; our data suggest a exercise volume of 160 minutes/week (e.g. over 30 minutes/day, 5×/week) was associated with symptom resolution by the one-month follow-up assessment.
Marginal exercise training effects have been reported in previous trials within the first post-concussion month.12,27 The discrepancy between our work and that of previous studies may be due to several factors. Primarily, compliance variability affected outcomes, and typical exercise volume prescription provided may not be sufficient to induce positive effects. The non-intervention group standard-of-care received recommendations from their physician, rather than a non-aerobic exercise such as stretching. Given the clinical practice evolution to now encourage active rehabilitation,5,18,19,23 many standard-of-care participants exceeded the exercise volume level we prescribed to the intervention group. This, in turn, may have affected our symptom outcomes, which were not different between groups. Although the exercise intervention group reported slightly higher PCSI scores than the standard-of-care group at the two month time point (6.5 vs. 0), the clinical relevance of this finding is also likely minimal, given the high prevalence of uninjured individuals who report concussion symptoms at this level without a recent history of concussion.8 At the same time, some of those assigned to the intervention group (perhaps due to high symptom burden) complied poorly with the prescription. Thus, because there were no significant exercise volume differences between groups, group level comparisons based on exercise volume were retrospective and observational, and we cannot conclude any causal effects. Participants may have exercised more because they had fewer symptoms, or had fewer symptoms because they exercised more. Thus, the exercise prescription itself was not sufficient to induce meaningful beneficial effects for concussion recovery. Furthermore, exercise intensity (i.e. heart rate) and perceived exertion (i.e. RPE) during exercise likely resulted in a differential effect on concussion recovery, potentially independent of the exercise volume they reported over the 8 week study period. Currently, clinicians are advised to recommend early physical activity following concussion,19 yet this recommendation remains vague. Alongside this recommendation, consideration of some form of exercise volume and intensity monitoring, via self-recall, daily exercise logs, or actigraphy approaches may help guide optimal post-concussion recovery.
Randomized clinical trials must account for participant intervention compliance.21 Patients do not adhere well to physician recommendations after concussion,24 and therefore may not comply. If patient compliance is not closely monitored,3,13 the observed intervention effect may be misleading. Our data suggest that the prescription itself did not elicit meaningful change. Prescription compliance, especially in the context of rehabilitation, can be highly variable, leading to unknown effects if not documented.9 Compliance variability may dilute the physiological or psychological intervention benefit. Thus, the reason current interventions aimed at alleviating symptoms after TBI do not improve long-term outcomes1 may relate to complex post-concussion pathophysiological restoration processes, and poor compliance with prescribed interventions.
The volume of exercise reported during the first month of the study appears to be associated with lower symptom severity. This may reflect the potential physiological and psychological benefits of exercise following a concussion.10,26 However, it is possible that participants who were feeling better were more likely to exercise more, rather than the exercise itself driving the reduction in symptom severity. Given the groups who exercised more or less than 100 minutes/week during the first month of the study had similar initial symptom burdens, exercise does appear to provide some level meaningful effect. However, this must be confirmed through a dose-escalation study where compliance is ensured, in order to understand the dose-response effect of exercise volume for concussion recovery.
Our sensitivity analysis indicated 160 minutes/week of exercise provided the best discriminatory ability for symptom resolution by one month. This is a higher exercise volume than previous reports,2,3,12,13 and is closer to exercise recommendations for children (>60 minutes/day of moderate-to-vigorous physical activity).28 While 100 minutes/week of exercise was sufficiently sensitive, it also contained low specificity. The increase to 160 minutes/week threshold held the sensitivity at the same level as 100 minutes/week (90%), but also increased specificity (78% vs. 56%). We did not confirm workout intensity, however. Patients recovering from concussion should exercise at an intensity that does not exacerbate symptoms.5,19 While this approach is safe,14 a higher dosage (volume rather than intensity) may provide greater symptom reduction effects, however our study was not designed to address this question specifically.
Limitations
Our study was limited in different ways. First, we do not have data related to exercise intensity or mode. These elements may affect concussion symptom severity as well,6 and should be accounted for in future investigations. Second, we enrolled participants from a single institution in a single geographic area, and injuries primarily occurred during sport participation. Thus, our results are not generalizable to a non-athletic population or to other geographic areas. Third, given the low compliance with the recommended exercise prescription, our study did not succeed in being able to compare the results of two different treatment paths because there was no difference between groups in the proportion that exercised. Future studies should seek to ensure compliance with in-person or virtual monitoring approaches. Fourth, our observation on the association between exercise volume and symptom level is a retrospective and secondary outcome. It is possible that participants who were feeling better were more likely to exercise more, rather than the exercise itself driving the reduction in symptom severity. Fifth, a sample size of n=37 was included in our final analysis. We were unable to recruit beyond this number due to the ramifications of the COVID-19 pandemic in the Spring and Summer of 2020. As such, this sample was smaller than our originally intended size and may have reduced our ability to identify significant between-group differences.
Conclusion
We observed exercise volume contributed to symptom reduction to a greater degree than the intervention recommendation, and that exercising ≥160 min/week during the first month of the study differentiated those who were still symptomatic from those who no longer had symptoms to a greater degree than 100 min/week of aerobic exercise. Clinicians should consider the amount of exercise they prescribe to concussion patients within the first month of injury, as well as monitor compliance with this prescription through the use of daily exercise logs or actigraphy, to understand the effects of this treatment approach on recovery.
Supplementary Material
Supplementary Figure 1. Study design, including timeline of assessments, and randomization procedure.
Supplementary Figure 2. Receiver operating characteristic (ROC) curve, describing how exercise volume during the first four weeks of the study could discriminate those who did and did not report symptom resolution by the one month time point.
Funding Sources
Research reported in this work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (R03HD094560) and the National Institute of Neurological Disorders And Stroke (R03NS106444).
Footnotes
Conflicts of interest
Unrelated to this study, Dr. Howell has received research support from the National Institute of Neurological Disorders And Stroke (R01NS100952 and R43NS108823) and MINDSOURCE Brain Injury Network. Dr. Meehan receives royalties from 1) ABC-Clio publishing for the sale of his books, Kids, Sports, and Concussion: A guide for coaches and parents, and Concussions; 2) Springer International for the book Head and Neck Injuries in the Young Athlete and 3) Wolters Kluwer for working as an author for UpToDate. His research is funded, in part, by philanthropic support from the National Hockey League Alumni Association through the Corey C. Griffin Pro-Am Tournament and a grant from the National Football League. Dr. Tan serves as a data science consultant to Lokavant Inc. and received consultancy fees.
Data sharing statement
De-identified individual-patient data (IPD), and other study-related documents can be shared upon request from the corresponding author.
References
- 1.Anderson V, Godfrey C, Rosenfeld JV, Catroppa C. 10 years outcome from childhood traumatic brain injury. Int J Dev Neurosci Off J Int Soc Dev Neurosci. 2012;30(3):217–224. doi: 10.1016/j.ijdevneu.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 2.Clausen M, Pendergast DR, Willer B, Leddy J. Cerebral Blood Flow During Treadmill Exercise Is a Marker of Physiological Postconcussion Syndrome in Female Athletes. J Head Trauma Rehabil. 2016;31(3):215–224. doi: 10.1097/HTR.0000000000000145 [DOI] [PubMed] [Google Scholar]
- 3.Cordingley D, Girardin R, Reimer K, et al. Graded aerobic treadmill testing in pediatric sports-related concussion: safety, clinical use, and patient outcomes. J Neurosurg Pediatr. 2016;25(6):693–702. doi: 10.3171/2016.5.PEDS16139 [DOI] [PubMed] [Google Scholar]
- 4.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- 5.Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport. Br J Sports Med. 2019;53(4):213–225. doi: 10.1136/bjsports-2018-100338 [DOI] [PubMed] [Google Scholar]
- 6.Howell DR, Andrew Taylor J, Tan CO, Orr R, Meehan WP. The Role of Aerobic Exercise in Reducing Persistent Sport-related Concussion Symptoms. Med Sci Sports Exerc. 2019;51(4):613–619. doi: 10.1249/MSS.0000000000001829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iverson GL, Gardner AJ, Terry DP, et al. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med. 2017;51(12):941–948. doi: 10.1136/bjsports-2017-097729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iverson GL, Silverberg ND, Mannix R, et al. Factors Associated With Concussion-like Symptom Reporting in High School Athletes. JAMA Pediatr. 2015;169(12):1132–1140. doi: 10.1001/jamapediatrics.2015.2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther Clin Risk Manag. 2008;4(1):269–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langevin P, Frémont P, Fait P, Dubé M-O, Bertrand-Charette M, Roy J-S. Aerobic Exercise for Sport-related Concussion: A Systematic Review and Meta-analysis. Med Sci Sports Exerc. Published online June 8, 2020. doi: 10.1249/MSS.0000000000002402 [DOI] [PubMed] [Google Scholar]
- 11.Lawrence DW, Richards D, Comper P, Hutchison MG. Earlier time to aerobic exercise is associated with faster recovery following acute sport concussion. PloS One. 2018;13(4):e0196062. doi: 10.1371/journal.pone.0196062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leddy JJ, Haider MN, Ellis MJ, et al. Early Subthreshold Aerobic Exercise for Sport-Related Concussion: A Randomized Clinical Trial. JAMA Pediatr. 2019;173(4):319–325. doi: 10.1001/jamapediatrics.2018.4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leddy JJ, Haider MN, Hinds AL, Darling S, Willer BS. A Preliminary Study of the Effect of Early Aerobic Exercise Treatment for Sport-Related Concussion in Males. Clin J Sport Med Off J Can Acad Sport Med. Published online September 19, 2018. doi: 10.1097/JSM.0000000000000663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leddy JJ, Hinds AL, Miecznikowski J, et al. Safety and Prognostic Utility of Provocative Exercise Testing in Acutely Concussed Adolescents: A Randomized Trial. Clin J Sport Med. 2018;28(1):13–20. doi: 10.1097/JSM.0000000000000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clin J Sport Med. 2010;20(1):21–27. doi: 10.1097/JSM.0b013e3181c6c22c [DOI] [PubMed] [Google Scholar]
- 16.Ledoux A-A, Tang K, Yeates KO, et al. Natural Progression of Symptom Change and Recovery From Concussion in a Pediatric Population. JAMA Pediatr. 2019;173(1):e183820. doi: 10.1001/jamapediatrics.2018.3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lempke LB, Schmidt JD, Lynall RC. Athletic Trainers’ Concussion-Assessment and Concussion-Management Practices: An Update. J Athl Train. 2020;55(1):17–26. doi: 10.4085/1062-6050-322-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lumba-Brown A, Yeates KO, Sarmiento K, et al. Centers for Disease Control and Prevention Guideline on the Diagnosis and Management of Mild Traumatic Brain Injury Among Children. JAMA Pediatr. Published online September 4, 2018:e182853–e182853. doi: 10.1001/jamapediatrics.2018.2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838–847. doi: 10.1136/bjsports-2017-097699 [DOI] [PubMed] [Google Scholar]
- 20.Miller FL, O’Connor DP, Herring MP, et al. Exercise Dose, Exercise Adherence, and Associated Health Outcomes in the TIGER Study. Med Sci Sports Exerc. 2014;46(1). doi: 10.1249/MSS.0b013e3182a038b9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen RO, Bertelsen ML, Ramskov D, et al. Randomised controlled trials (RCTs) in sports injury research: authors-please report the compliance with the intervention. Br J Sports Med. 2020;54(1):51–57. doi: 10.1136/bjsports-2019-100858 [DOI] [PubMed] [Google Scholar]
- 22.Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. 2005;18(2):189–193. [DOI] [PubMed] [Google Scholar]
- 23.Quatman-Yates CC, Hunter-Giordano A, Shimamura KK, et al. Physical Therapy Evaluation and Treatment After Concussion/Mild Traumatic Brain Injury. J Orthop Sports Phys Ther. 2020;50(4):CPG1–CPG73. doi: 10.2519/jospt.2020.0301 [DOI] [PubMed] [Google Scholar]
- 24.Root JM, McNamara B, Ledda M, Madati PJ. Pediatric Patient Compliance With Recommendations for Acute Concussion Management. Clin Pediatr (Phila). 2019;58(7):731–737. doi: 10.1177/0009922819839230 [DOI] [PubMed] [Google Scholar]
- 25.Sady MD, Vaughan CG, Gioia GA. Psychometric characteristics of the postconcussion symptom inventory in children and adolescents. Arch Clin Neuropsychol Off J Natl Acad Neuropsychol. 2014;29(4):348–363. doi: 10.1093/arclin/acu014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan CO, Meehan WP, Iverson GL, Taylor JA. Cerebrovascular regulation, exercise, and mild traumatic brain injury. Neurology. 2014;83(18):1665–1672. doi: 10.1212/WNL.0000000000000944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willer BS, Haider MN, Bezherano I, et al. Comparison of Rest to Aerobic Exercise and Placebo-like Treatment of Acute Sport-Related Concussion in Male and Female Adolescents. Arch Phys Med Rehabil. 2019;100(12):2267–2275. doi: 10.1016/j.apmr.2019.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Recommended Population Levels of Physical Activity for Health. Glob Recomm Phys Act Health. Published online 2010. Accessed June 11, 2020. https://www.ncbi.nlm.nih.gov/books/NBK305058/
- 29.Zemek R, Barrowman N, Freedman SB, et al. Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA. 2016;315(10):1014–1025. doi: 10.1001/jama.2016.1203 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Study design, including timeline of assessments, and randomization procedure.
Supplementary Figure 2. Receiver operating characteristic (ROC) curve, describing how exercise volume during the first four weeks of the study could discriminate those who did and did not report symptom resolution by the one month time point.
Data Availability Statement
De-identified individual-patient data (IPD), and other study-related documents can be shared upon request from the corresponding author.


