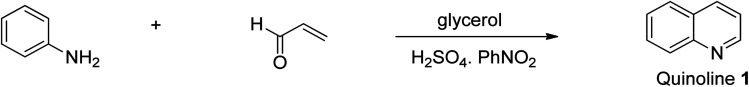Abstract
Quinoline, which consists of benzene fused with N-heterocyclic pyridine, has received considerable attention as a core template in drug design because of its broad spectrum of bioactivity. This review aims to present the recent advances in chemistry, medicinal potential and pharmacological applications of quinoline motifs to unveil their substantial efficacies for future drug development. Essential information in all the current and available literature used was accessed and retrieved using different search engines and databases, including Scopus, ISI Web of Knowledge, Google and PUBMED. Numerous derivatives of the bioactive quinolines have been harnessed via expeditious synthetic approaches, as highlighted herein. This review reveals that quinoline is an indisputable pharmacophore due to its tremendous benefits in medicinal chemistry research and other valuable areas of human endeavour. The recent in vivo and in vitro screening reported by scientists is highlighted herein, which may pave the way for novel drug development. Owing to the array of information available and highlighted herein on the medicinal potential of quinoline and its functionalized derivatives, a new window of opportunity may be opened to medicinal chemists to access more biomolecular quinolines for future drug development.
Quinoline, which consists of benzene fused with N-heterocyclic pyridine, has received considerable attention as a core template in drug design because of its broad spectrum of bioactivity.
Introduction
Heterocyclic compounds are deemed to be found in the total structure of at least four out of the five US top-selling drugs, having pharmaceutical activities such as anticancer, antibacterial, anti-tumor and anti-inflammatory. In biological and pharmaceutical processes, heterocyclic compounds play a vital role in the core structures of many drugs.1 A typical example of bicyclic heterocyclic compounds is quinoline 1, which is the heterocycle of interest in this review. The quinoline core framework exists in many naturally occurring biologically active entities including quinine, chloroquine, bulaquine, primaquine and tafenoquine from Cinchona alkaloids.2 Typical drug design formulations attempt to imitate naturally available heterocycles and exercise potency by disrupting and intercepting regular pathways essential for the growth of pathogenic organisms.3 They have contributed significantly to society through their use as therapeutic agents,4–6 application in human and veterinary medicine,5 use in agriculture,7,8 dyes,9 polymers,10 bioinformatics,11 and molecular engineering12 and they are of increasing importance in many other areas.
Furthermore, functionalized quinoline moieties are highly essential pharmacophoric motifs with undeniable therapeutic propensity owing to their numerous reported biological and pharmacological activities, which include anticancer,13 antimicrobial,14 anti-inflammatory,15 antioxidant,16 antitubercular,17 antimalarial,18 anti-leishmanial,19 antiprotozoal,20 anti-HIV,21 and DNA binding22 among others. However, the previous review by Sharma and coworkers only highlighted the anti-cancer activities of quinoline derivatives,2 while the review by Martins and coworkers focused on the quinoline heterocycle as a tool box in nanomedicine.3 Shiro and coworkers addressed the chemistry and biological activity of quinoline up to 2015,1 while Dib and coworkers reviewed the recent works on the synthesis and biological potential of quinoline-based compound up to 2021.23 Hence, there is a need to bridge the gap regarding these potent pharmacological compounds since then to date. Our present review also presents diverse methods for the synthesis of quinoline, which can pave the way for synthetic chemists to achieve various synthetic modifications to access a new series of quinoline motifs for novel drug design.
Historical background of quinoline
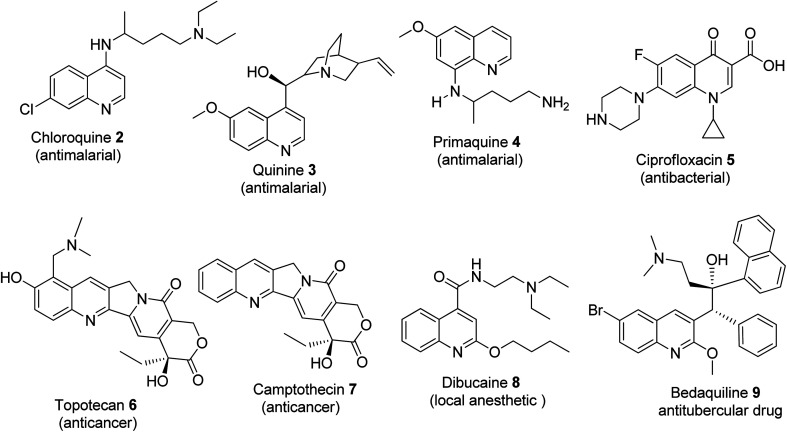
Historically, cinchocaine was the first local anesthetic to be synthesized from the quinoline-based group.24 In 1834, quinoline was first discovered and isolated by Friedlieb Ferdinard Runge from coal tar.25,26 It belongs to the alkaloid family and is a secondary metabolite under the nitrogen-containing natural products. Quinoline and its derivatives are available as drugs, with the outstanding ones being anti-malarial (chloroquine 2, quinine 3, primaquine 4, etc.), antibacterial (fluoroquinolones such as ciprofloxacin 5), anticancer (topotecan 6 and camptothecin 7), local anesthetic (dibucaine 8) and anti-tubercular (bedaquiline 9) drugs. Quinoline derivatives have been reported to be vital agents in the development of anticancer drugs, which induce apoptosis. This is successfully achieved through the elimination of cells that threaten the survival of animals and cell migration disruption, and they also act as angiogenesis inhibitors.27
Compounds that contain a quinoline core template show a wide range of therapeutic properties including anti-malarial, antitumor, and antimicrobial.28 As a lead structure, quinoline is used in the synthesis of many anti-malarial drugs such as chloroquine, pyrimethamine, and mefloquine. Quinine is utilized for the treatment of severe Plasmodium falciparum infection (malaria parasite).29 The quinoline motif can also be used to differentiate various species as quality control markers. Quinine was used in the treatment of the blood stages of Plasmodium, as the core structure for the production of many antimalarial drugs and to treat severe cases of Plasmodium falciparum infections.29
Since the 1980s, fluoroquinolones have been employed in pharmaceutical processes and the treatment of diverse bacterial infections.30 The synthesis of quinoline and its derivatives can be performed using different methods due to the increasing interest in them. This is because of their array of documented applications in pharmaceutical processes. Some of these methods will be discussed further in this review (Fig. 1).
Fig. 1. Selected quinoline-based marketed drugs.
Natural occurrence of quinoline
Quinoline can be derived from different natural sources such as flowering plants, animals and microorganisms.31 The primary source of quinoline is coal tar.32 The bark of the Cinchona plant contains quinine, quinidine, cinchonine and cinchonidine, which are combined and administered as “Quinimax” in malarial therapy.33 Nitidine, an anticancer agent, is a dimethoxylated-quinoline compound obtained from Zanthoxylum nitidum, which belongs to the citrus family. Reticuline, which is isoquinoline, occurs naturally in some notable medicinal plants such as Papaver somniferum, Asimina triloba and Ocotea fasciculata and in opium species. Skimmianine (furoquinoline alkaloid) found in Skimmia japonica is used in sedative and anticonvulsant drugs. Sandramycin, which serves as an antitumor and antibiotic drug, can be found in Nocardioides sp. It is a biomolecule possessing two molecules of 2-amidoquinoline in its core structure.34 Camptothecin (pentacyclic quinoline) can be derived and extracted from the tree bark and stem of Camptotheca acuminata.27 Quinoline alkaloids can be isolated from the Dictamnus species. For instance, robustine is a quinoline alkaloid obtained from the Dictamnus angustifolius plant source, evolitrin, a tricyclic quinoline, which is isolable from Dictamnus albus, ribalinidine is from Dictamnus hispanicus and dictamnine from Dictamnus dasycarpus and Dictamnus angustifolius.35
Physical properties of quinoline
Quinoline is a liquid with a strong odour, which is sparingly miscible with cold water, but completely miscible with hot water. It is readily soluble in many organic solvents at ambient temperature. It possesses the ability to absorb water molecules from the environment.31 It is a colorless hygroscopic liquid, which when aged and exposed to light, changes color to yellow, and then brown.32 Quinoline exhibits a density of 1.093 g mol−1, melting point of −15 °C and boiling point of 238 °C. Similar to monocyclic N-heterocycle (pyridine derivatives) derivative, quinoline congeners have been documented over the years to have ecological effects with buildings/structures related to production of the oil coal, and also water contamination given that aqueous quinoline can be transported in the environment easily.26
Chemical structures of quinoline and its analogs
Quinoline 1 can be identified by its double ring structure, consisting of a pyridine and benzene structure fused together by a change in the state of the benzene ring with pyridine. Pyridine has the chemical formula of C5H5N, similar to the benzene structure, wherein a methine group is replaced with a nitrogen atom.28 Isoquinoline 10 is a unit of quinoline but differs in the position of the nitrogen, which is situated at position 2 in the structure of isoquinoline, whereas the heteroatomic nitrogen in quinoline is at position 1 of the heterocyclic ring portion, as shown in Fig. 2.31
Fig. 2. Chemical structures of quinoline and isoquinoline heterocycles.
The issue of drug resistance and the outbreak of new diverse life-threatening infectious diseases have increased recently and there are many more infectious diseases that are claiming lives in the 21st century than in the past centuries.36 The pandemic nature of Covid 19 is quite sufficient evidence to prove that the scientific world should devote more effort to drug design. Considerable attention has been given to quinoline derivatives such as 8-aminoquinoline because of the potential role of metabolic transformation in its in vivo activation.21 To protect life and make the world a better place to live, there is a need for continuous updates on various scientific research and attempts to use quinoline motifs in drug discovery and medicinal chemistry.
Synthesis of quinoline derivatives
The Skraup synthetic approach
This reaction is very valuable for synthesizing non-substituted quinoline 1. It involves the heating and thermal cyclization of aniline on acrolein in the presence of concentrated sulfuric acid, a mild oxidizing agent, and glycerol at refluxing temperature to give unsubstituted quinoline 1 (Scheme 1). The acid used therein serves a double role because it acts as both a catalyst and dehydrating agent simultaneously.26 The Skraup synthesis enabled the laboratory-scale synthesis of quinoline for the first time, and subsequently other methods were developed for the preparation of diverse substituted quinolines with great medicinal efficacy.
Scheme 1. Synthetic pathway to access unsubstituted quinoline.
Friedlander synthetic approach
The Friedlander synthetic approach was described in 1882 by Friedlander, which is considered one of the most convenient procedures designed and executed for the preparation and synthesis of quinoline scaffolds. It proceeds via the condensation of 2-aminobenzaldehydes with ketones to form quinoline derivatives. This reaction has been catalyzed using agents such as trifluoroacetic acid (TFA), Lewis acids, iodine and p-toluenesulfonic acid. It has been greatly explored for the condensation and cyclodehydration in acidic or basic medium.2,37 Wang and co-workers showed an eco-friendly and cost-effective method for the preparation of 2,3,7-trisubstituted quinoline derivatives 11via the Friedlander reaction of 1-amino-4-bromo benzaldehyde with ethyl acetoacetate using HCl as the catalyst and H2O as the solvent38a (Scheme 2). The Friedlander hetero-annulation reaction was utilized for the synthesis of poly-substituted quinoline in 77–95% yield.38b Generally, the Friedlander technique involves a 2-in-1 step, i.e., the base-catalyzed preparation of a Schiff base followed by intramolecular Claisen condensation. Although, this method has high versatility, its drawback is instability of its aminobenzaldehyde precursor, which can undergo unsolicited intramolecular cyclization of the its amino and aldehydic functional groups; thus, hindering the formation of quinoline.
Scheme 2. Friedlander synthetic route for the formation of substituted quinolines.
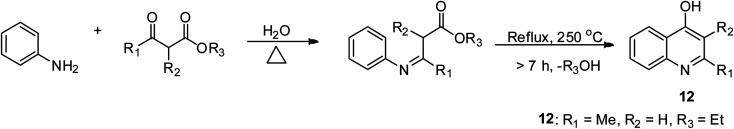
Conrad–Limpach synthetic approach
The Conrad–Limpach method employs aniline derivatives as a precursor, which are condensed with β-ketoesters under suitable reaction conditions to form 4-hydroxy quinolines 12 having a Schiff base as an intermediate.39 4-Oxyquinolines are synthesized by the change in the state of matter of β-keto acids esters, and then reacted with aromatic amines.39,40 The overall reaction includes an addition reaction and condensation reaction. The rate-determining step is that involved in the thermal cyclization via molecular ring closure. The Schiff base is strongly heated 250 °C for this to occur and the nature of the solvent used is also crucial to achieve improved yields of the expected products.39,41 This reaction is difficult to drive to completion at low temperature. However, the extremely high temperature required for product formation can lead to decomposition unless it is carried out in mineral oil (BP > 275 °C) (Scheme 3).
Scheme 3. Conrad–Limpach synthetic route for the formation of 4-hydroxyquinoline.
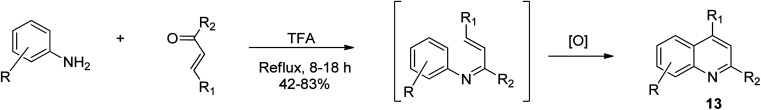
Doebner–Miller synthetic approach
The Doebner–Miller synthesis involves the use of an aldehyde or α,β-unsaturated ketone with aniline.40 This method comprises the condensation of aromatic amines with chalcones to afford quinolines.42 Wu and co-workers reported that when aniline and its substituted derivatives were thermally treated with chalcones in the presence of trifluoroacetic acid solvent,43 an intermediate was formed, which upon oxidative cyclization, afforded trisubstituted quinoline derivatives 13 effortlessly, as shown in Scheme 4. This approach is much simpler than the Skraup method because it is avoids harsh reaction conditions and use of an oxidizing agent.
Scheme 4. Doebner–Miller synthetic route for the formation of substituted quinolines.
Synthesis via intramolecular cyclization of acetanilides
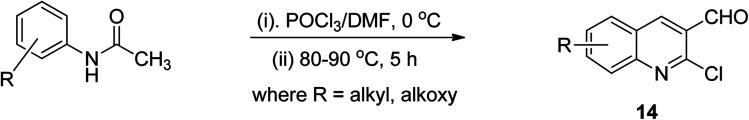
It has been reported that the intramolecular cyclization of acetanilide by phosphorus oxychloride in dimethylformamide at 80–90 °C for 5 h provided easy access to 2-chloroquinoline-3-carboaldehydes 14 through a the Vilsmeier–Haack reaction (Scheme 5). This synthesis could be heat-driven via the conventional technique or new microwave-assisted methods.44
Scheme 5. Synthetic pathway to quinoline derivative 14via Vilsmeier–Haack reaction.
Synthesis via radical induced rearrangement
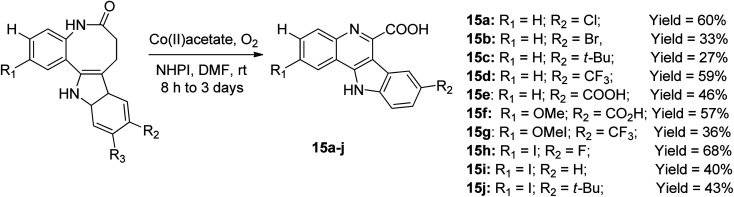
The cobalt-catalyzed radical-induced rearrangement of benzazepinone motifs in the presence of molecular oxygen and N-hydroxyphthalimide (NHPI) at a temperature ranging from ambient temperature to 70 °C afforded a series of ten tetracyclic quinoline derivatives 15a–j, as shown in the Scheme 6.45 The solvent for the optimum reaction yield and effective rearrangement was reported to be dimethylformamide (DMF), while the yields of the products varied from 27–68%. In a recent study, this was further developed by designing the new derivative of 15 with other substitution patterns to afford other products with yields ranging from 13% to 88%. These new compounds were synthesized and investigated for their protein kinase DYRK1A inhibitory properties.46
Scheme 6. Radical-induced rearrangement to afford tetracyclic quinolines.
Pfitzinger synthetic approach
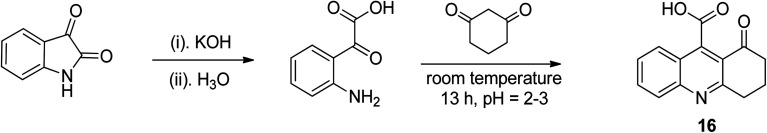
Hydrolysis was carried out on isatin in potassium hydroxide (aqueous), and subsequently the pH was carefully adjusted to 2–3, resulting in ring opening to access anilino-based acid as an intermediate. This intermediate was further reacted immediately with cyclic diketone to furnish the desired tricyclic quinoline 16 in encouraging yield.42 In a condensation reaction under acidic conditions, the obtained keto-acid was reacted with 1,3-diketone to give tricyclic quinoline 16, as shown in the Scheme 7.
Scheme 7. Synthetic pathway to access tricyclic quinolines via Pfitzinger method.
One-pot three-component synthetic approach
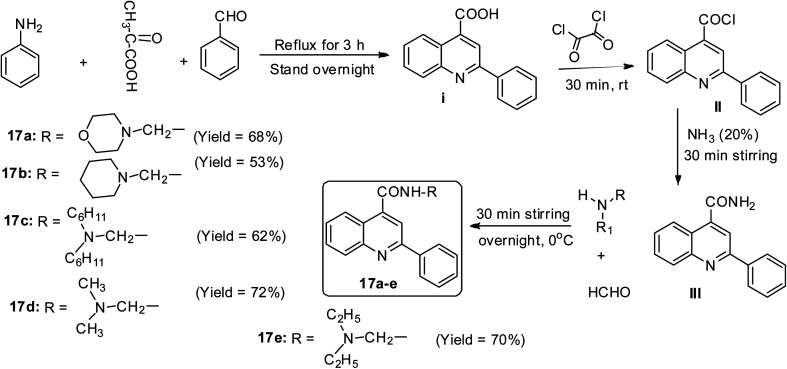
In the report by Jumade and co-workers, a newer Mannich base synthetic approach was utilized to access five different disubstituted quinolines to overcome the water insolubility problem associated with cinchophen-like molecules.47 When a mixture of pyruvic acid, benzaldehyde and aniline was utilized in the presence of ethanol solvent at a refluxing temperature for 3 h, it afforded crystalline 2-phenyl quinoline-4-carboxylic acid I.47 This was synthetically modified at the COOH moiety and allowed to undergo Mannich base reaction to afford five compounds 17a–e in varying yields (53–72%)47 (Scheme 8).
Scheme 8. One-pot synthetic route to access 2-phenylquinoline-4-carboxylic acid.
Povarov synthetic approach
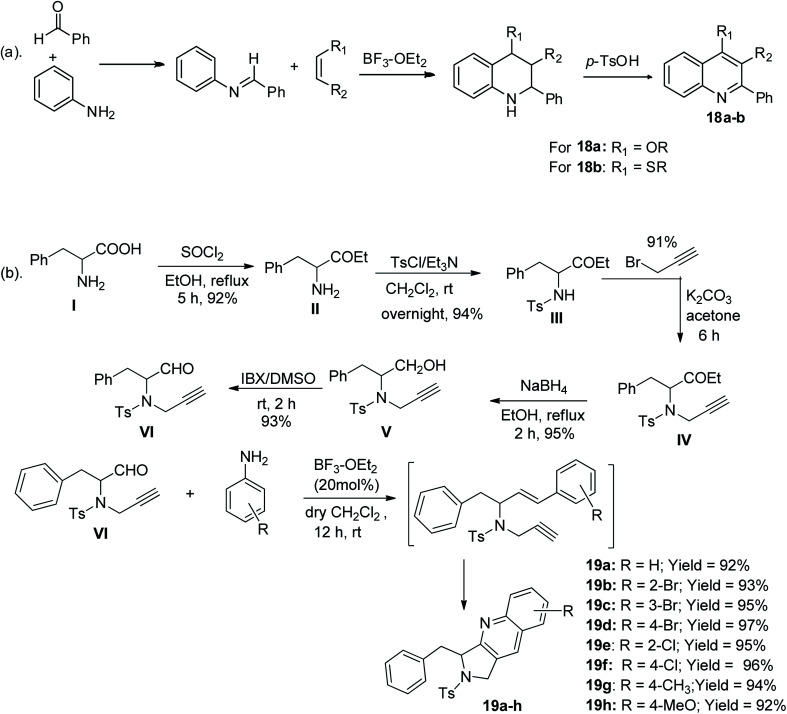
The Povarov route is an aza-Diels–Alder reaction, which was reported originally as a one-pot reaction of aryl aldimines derived from the reaction of benzaldehyde with aminobenzene bearing an electron-rich attachment, particularly ethyl vinyl ether a (where R = H and R′ = OC2H5) or ethyl vinyl sulfide b (where R = H and R′ = SC2H5) in BF3/OEt2 catalyst, resulting in the production of quinoline derivatives with a tetrahydro-hetero-ring, which were further oxidized to the corresponding quinolines 18a and 18b, respectively (Scheme 9a). To address the drawback in the previously reported work, another study showed the five-step transformation of phenylalanine to valuable intermediate VI, which was subsequently reacted with an aniline derivative via Povarov intramolecular reaction to give eight tetrahydropyrrolo-containing tricyclic quinolines 19a–h in 92–97% yield,48 as shown in Scheme 9b.
Scheme 9. (a) Povarov synthetic route for the formation of 2-aryl-substituted quinolines. (b) Povarov synthetic route for the formation of tricyclic quinolines.
Aza-vinylogous Povarov stereoselective synthetic approach
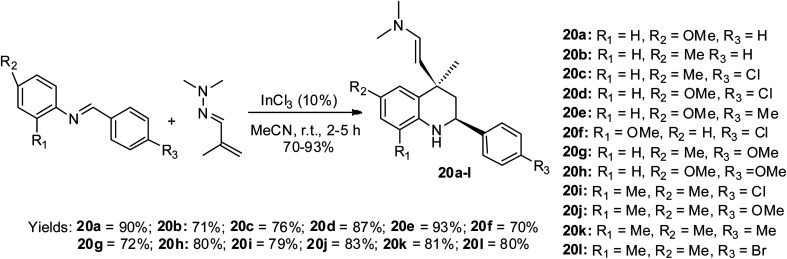
Sridharan and co-workers illustrated the aza-Diels Alder reactive condition for the first time, which involved the use of hydrazonized alkene via the cycloaddition strategy to access twelve final quinoline motifs 20a–lvia InCl3-mediated catalysis.49 This reaction protocol is termed the aza-vinylogous Povarov reaction and the route to this effective preparation is shown in Scheme 10. The reaction for the formation of all the products was executed within 2–3 h except that for 20k, which lasted for 5 h according to the TLC analysis. The reaction was carried out at room temperature to achieve the products in good to excellent yields of 70–93%.
Scheme 10. Aza-vinylogous Povarov stereoselective synthesis of substituted quinolines.
Chemical properties of quinoline
Quinoline is a heterocycle with its two six-membered rings fused. It is also called benzo[b]pyridine or 1-azanaphthalene. It contains a nitrogenous aromatic heterocyclic ring50 with its common chemical reactions being nucleophilic and electrophilic substitution in nature.51 In the presence of acids, it can form a salt and shows similar reactions to that of benzene and pyridine. As a tertiary base, it is weak.50 Quinoline hydrazones (functional group containing R1R2C NNH2) have both polar and non-polar properties, which are beneficial for the penetration of bacterial cells.52
Hydrogenation reaction of quinoline
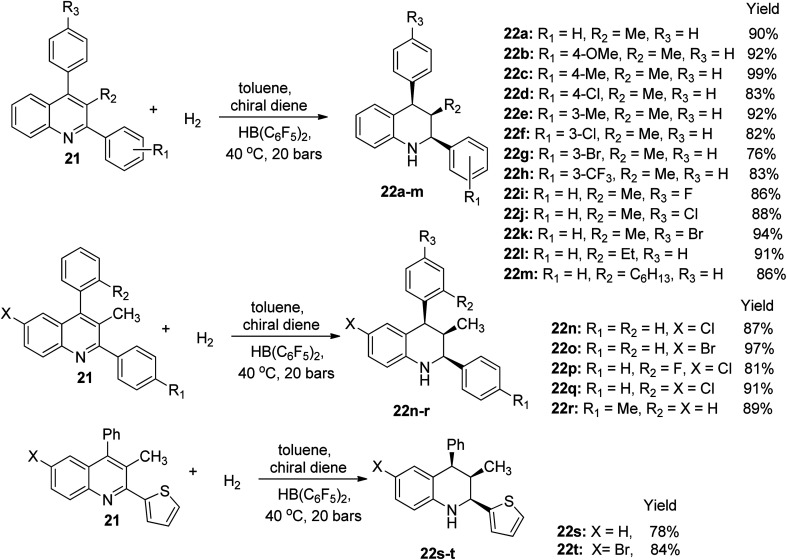
The hydrogenation of 3-alkyl-2,4-s-diphenylquinoline 21 was achieved using HB(C6F5)2 and bubbling about 20 bars of H2 in the presence of toluene solvent and a chiral diene to afford twenty examples of 1,2,3,4-tetrahydroquinoline derivatives 22a–t. The first eighteen derivatives 22a–r (without substitution on the benzene portion of quinoline) were obtained under metal-free enantioselective conditions in yields ranging from 76% to 99%. However, when the 2-substituent was changed to heterocyclic thiophene, hydrogenation in molecular H2 afforded the last two quinoline derivative 22s, 22t in 78% and 84% yield, respectively, as shown in Scheme 11.53 This result was highly interesting because it was an improvement in previous report, and it also authenticated the possibility of realizing asymmetric reactions with the aid of a chiral borane catalyst.
Scheme 11. Hydrogenation reaction of trisubstituted quinoline derivatives.
Electrophilic substitution reaction
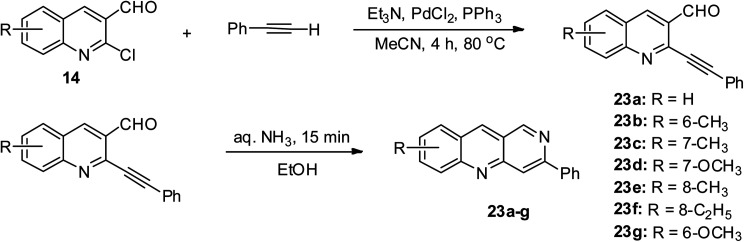
2-Chloroquinoline-3-carbaldehyde 14 conveniently undergo substitution reaction at its 2-chloro position by treating it with phenylacetylene via PdCl2 mediation in CH3CN, triethylamine and triphenylphosphine at 80 °C under an inert atmosphere, giving an 87% yield of 2-(phenylethynyl) quinoline-3-carbaldehydes, which were reacted with aqueous ammonia to give 88% yield of 3-phenylbenzo[b][1,6]naphthyridine 23, as shown in Scheme 12.44 The seven derivatives 23a–g were obtained in varying yields with the highest yield being 88%.
Scheme 12. Electrophilic substitution reaction of disubstituted quinoline derivatives.
Oxidation reaction of quinoline derivatives
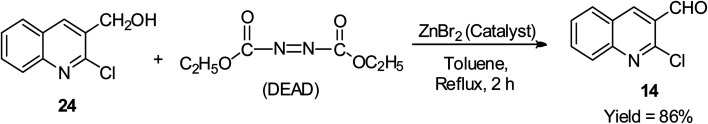
The mild oxidation of the alcoholic side chain of 2-chloroquinolin-3-yl methanol 24 was reported to proceed smoothly using both diethyl diazene-1,2-dicarboxylate (DEAD) and a catalytic amount of zinc bromide in Ph-CH3 at refluxing temperature for 2 h to afford 2-chloroquinoline-3-carbaldehyde 14 in 86% yield, as shown in Scheme 13.44
Scheme 13. Oxidation reaction of disubstituted quinoline derivatives.
Condensation reaction of quinoline derivatives
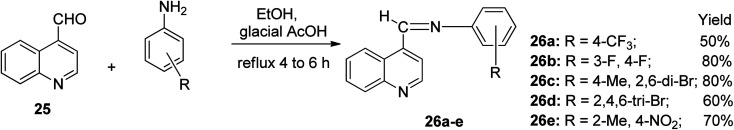
Quinoline-4-carboxaldehyde 25 conveniently underwent a condensation reaction when treated with 4-(trifluoromethyl) aniline and four other substituted anilines in the presence of ethanol solvent using heterogeneous catalysis in glacial CH3COOH to access five Schiff bases 26a–e. The reaction was completed after refluxing for 4 to 6 h with an obvious color change. The reaction mixture was left to stand overnight to afford the imine products 26a–e as colored crystals with yields ranging from 50% to 80%, as shown in Scheme 14.32
Scheme 14. Condensation reaction of quinoline derivatives to afford imines.
Pharmacological activities of quinoline derivatives
The quinoline motif was reported by Lv and co-workers to have various biological activities such as being an anticancer agent and involved in anti-inflammatory, anti-microbial, and anti-HIV activities and lots more.35 There are new developments in quinoline-derived drugs due to the occurrence of drug resistance in bacteria, which is the cause of several diseases, and side effects over the years.54
Antibacterial activities of quinoline derivatives
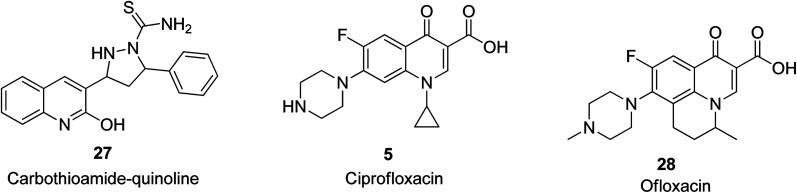
It was reported by Kharb and Kaur that nine quinoline-based compounds were designed with that bearing a carbothioamide-based quinoline motif, 27, exhibiting the most significant antibacterial activity with a zone of inhibition of 20 mm against P. aeruginosa.55 The commercially available quinoline-based antimicrobial drugs include ciprofloxacin 5, which acts synergistically with ZnO nanoparticles against biofilm cells56 and ofloxacin 28 in s fixed-dose combination,57 and their structures are shown in Fig. 3.
Fig. 3. Selected quinoline derivatives with antibacterial activity.
Anti-malarial activities of quinoline derivatives
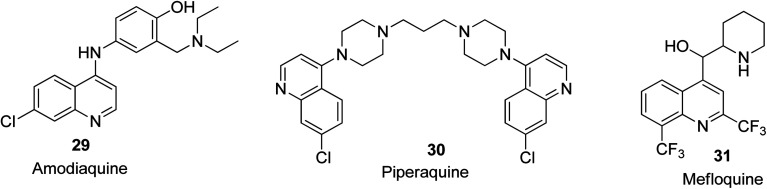
Malaria is known to be among the deadliest pathogenic infections globally. It is a parasitic (hematoprotozoan) disease caused by a specific species of anopheline mosquitoes. The species that affects humans the most is Plasmodium falciparum among the four species causative agents of malaria.58 To date, quinoline derivatives are considered to be the dominant class of heterocyclic compounds used as anti-malarial agents. In the pharmaceutical area, two subclasses of quinoline are used i.e., 4-amino quinolines (chloroquine 2, amodiaquine 29, and piperaquine 30) and aminoalcohols (quinine 3 and mefloquine 31). These two subclasses having different mechanisms of action, depending on haemoglobin digestion inference with the endocytic process.59 Their structures are shown in Fig. 4.
Fig. 4. Selected quinoline derivatives with anti-malarial activity.
c-Met kinase inhibitory activities of quinoline derivatives
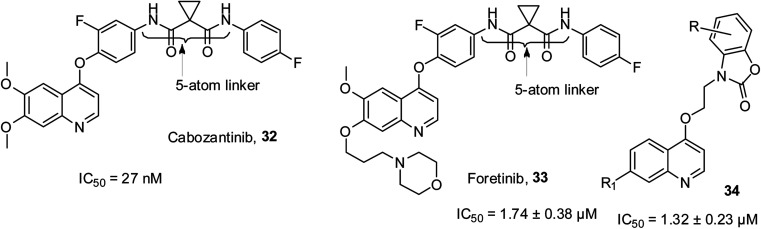
c-Met is a receptor tyrosine kinase, which drives the effective transformation of a pro-enzyme to an active enzyme. When fused with the scatter factor/hematopoietin A, it causes various composite signaling pathways, which show outcomes such as cell migration, proliferation and invasion. Poor clinical results for cancer patients have been associated with an increase in c-Met and SF. Cabozantinib 32 was the first small molecular inhibitor of c-Met, which received approval in 2012. Later, foretinib 33 with a similar 5-atom linker as 32 was reported to have notable inhibitory efficacy on c-Met.60 In recent years, benzo[d]oxazol-2(3H)-one-quinoline 34 has been reported to be a powerful c-Met inhibitor.61 The structures of these kinase inhibitors are shown in Fig. 5.
Fig. 5. Selected quinoline derivatives with c-Met kinase inhibitory activity.
Antileishmanial activities of quinoline derivatives
The occurrence of cutaneous leishmaniasis could be through different strains of Leishmania, some of which are the most prominent in Columbia and other regions of the world. It is a pathogenic disease. Accordingly, quinolinic core frameworks have been reported to exhibit leishmanicidal (killing the leishmania parasite) activities, and when coupled with hydrazone in a single molecule, they are said to become more potent. The leishmanicidal activity of a series of quinoline–hydrazone hybrids was investigated, among which compounds 35 and 36 were found to be potent with high efficacy.62 Their structures are shown in Fig. 6.
Fig. 6. Selected quinoline derivatives with anti-leishmanial activity.
Anti-cancer activities of quinoline derivatives
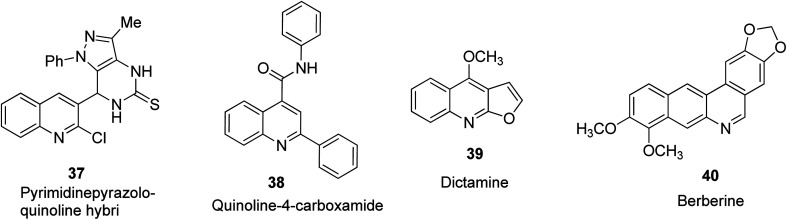
Quinoline-based compound 37 was designed and reported by Abdel-Wahab and co-workers to be a potent anti-cancer agent against breast, lung and CNS tumors.44 Synthetic quinoline structures that possess 2,4-disubstitution such as N-2-diphenylquinol-4-carboxamide 38, as well as naturally occurring quinoline-based alkaloids such as dictamine 39 and berberine 40 have been reported to play a vital function as new anti-cancer agents.31 Their structures are shown in Fig. 7.
Fig. 7. Selected quinoline derivatives with anti-cancer activity.
Antioxidant activities of quinoline derivatives
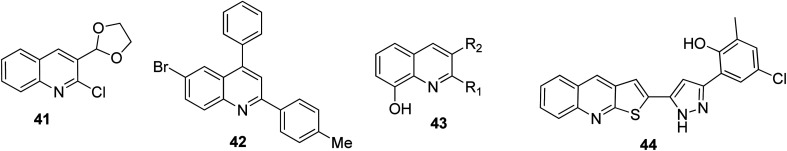
The antioxidant system and free radicals need to be in a state of equilibrium to maintain the health status of an organism. Many health issues over the years are related to oxidative stress. Quinoline motifs are known to be free radical scavengers.63 It was reported by Subashini and co-workers that 3-(1,3-dioxolan-2-yl)quinoline 41 and 3-quinolinecarboxaldehyde 14, which both contain a chlorine substituent on their 2-positions, were potential antioxidants.64 Liberto and co-workers synthesized a series of quinoline derivatives, among which compound 42 was found to be the most potent captor of reactive oxygen species.65 Al-Busafi and co-workers stated that 8-hydroxyquinoline derivatives 43 showed antioxidant activities,66 while according to the research by Mahajan and co-workers,67 among the synthesized and screened thiophene-fused quinolines, 44 emerged as the best antioxidant with an EC50 of 12.03 ± 1.45 μg mL−1. Their structures are shown in Fig. 8.
Fig. 8. Selected quinoline derivatives with anti-oxidant activity.
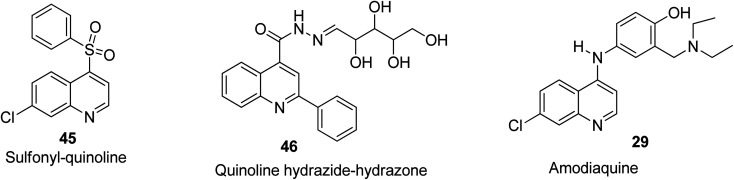
Anti-inflammatory activities of quinoline derivatives
The 7-chloro-4-phenylsulfonyl quinoline 45 bearing a chlorine substituent on the 7-position was screened against inflammation in mice with use of croton oil and was found to exhibit good anti-inflammatory potential.68 Among the 2-phenylquinoline-based designed motifs by Khalifa and co-workers, compound 46 being a nucleoside-linked analogue possessed remarkable anti-inflammatory properties comparable to the standard drug (diclofenac sodium).69 Also, amodiaquine 29 was reported by Mandewale and co-workers to be an anti-inflammatory agent with high efficacy.52 Their structures are shown in Fig. 9.
Fig. 9. Selected quinoline derivatives with anti-inflammatory activity.
Anti-tubercular activities of quinoline derivatives
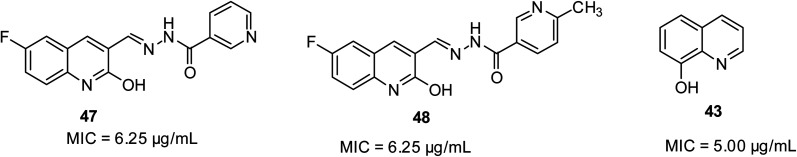
Mycobacterium tuberculosis is the cause of tuberculosis in humans. It is a type of respiratory disease affecting the lungs.54 In the report by Mandewale and co-workers, some quinolone motifs were prepared to evaluate their anti-tubercular potential.52 Their findings showed that quinoline-based compounds 47 and 48 displayed superior efficacy among the motifs, exhibiting 100% inhibition activity at 6.25 μg mL−1. 8-Hydroxyquinoline 43 is well-known as a growth inhibitor for both replicating and non-replicating M. tuberculosis. Their structures are shown in Fig. 10.
Fig. 10. Selected quinoline derivatives with anti-tubercular activity.
Antiviral activities of quinoline derivatives
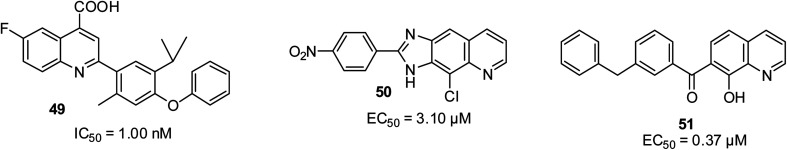
Resistance to drugs can occur due to the rapid mutation of viruses, which can become a problem especially for essential small RNA viruses such as HCV and HIV. In the report by Das and co-workers, compound 49 was found to be a potential drug candidate after showing a wide spectrum of antiviral efficacy.70 It inhibited the growth of human dihydroorotate dehydrogenase (DHODH) at 1 nM. In addition, quinoline compound 50 emerged as the most promising anti-HCV motif with an EC50 of 3.1 μM against the entire tested HCV virus,71 while Zhuang and co-workers reported that 51 possessed antiviral potential at IC50 of 0.37 μM due to the inhibition of HIV-1 integrase.72 Their structures are shown in Fig. 11.
Fig. 11. Selected quinoline derivatives with antiviral activity.
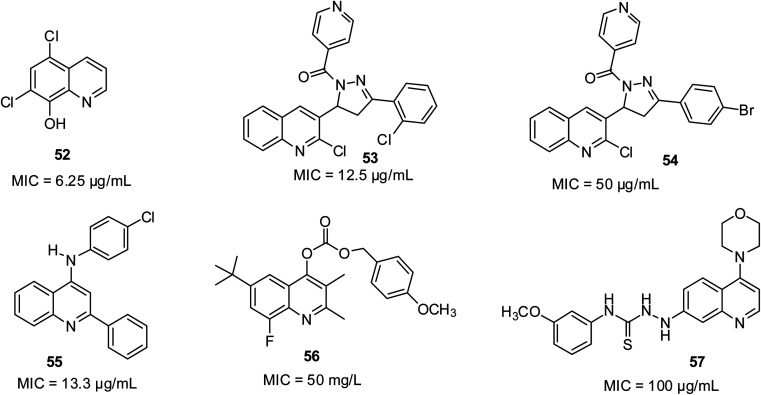
Antifungal activities of quinoline derivatives
According to Al-Busafi and co-workers, 5,7-dichloro- and 5,7-dibromo derivative 52 showed great fungicidal activities.66 According to the report by Desai and co-workers, 2-chlorophenyl-substituted quinoline derivative 53 with an MIC of 12.5 μg mL−1 and 4-bromophenyl-substituted 54 with an MIC of 50 μg mL−1 were shown to possess outstanding growth inhibitory potential against A. clavatus.73 They were more efficient than the standard antifungal drug Griseofulvin (MIC of 100 μg mL−1). The previous review by Dorababu unveiled 55 as eminent antifungal pharmacophore against Cochliobolus lunata at MIC of 13.3 μg mL; 56 against Pyricularia oryzae at 50 mg L; and 57 against C. albicans MTCC 227 at MIC of 100 μg mL−1.74 Their structures are shown in Fig. 12.
Fig. 12. Selected quinoline derivatives with antifungal activity.
Anti-HIV activities of quinoline derivatives
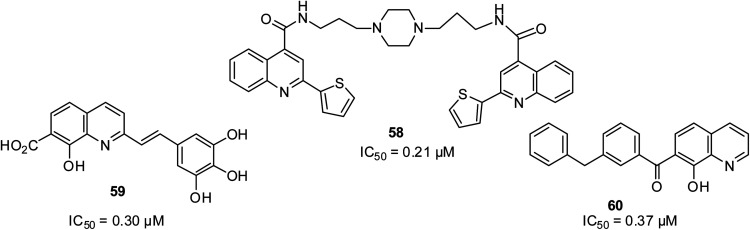
HIV-1 integrase is a crucial target for stopping the viral cycle replication. It is important for integrase inhibitors to show low toxicity but high selectivity given that integrase has no counterpart in the cells of mammals. Luo and co-workers designed quinoline-based structures as HIV-1 inhibitors, and then screened eight quinoline derivatives.75 Among the eight quinoline derivatives, compound 58 was found to be the most active. Among the quinoline motifs designed and screened by Mouscadet and Desmaële, the 3′,4′,5′-trihydroxyphenyl styrylquinolines 59 and 60 were found to be highly active with a strand transfer IC50 of 0.3 μM.76 Their structures are shown in Fig. 13.
Fig. 13. Selected quinoline derivatives with anti-HIV activity.
Antidepressant activities of quinoline derivatives
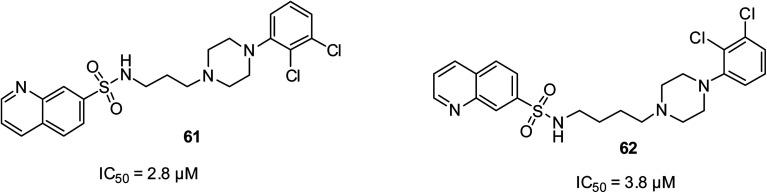
Depression is known globally to be the fourth cause of diseases. Affective disorders, schizophrenia and bipolar disorder are still very disturbing diseases in society, regardless of the development of novel therapy analysis.77 However, the nature of treatment for these affective disorders is still found to be undesirable due to the side effects from anti-depressant drugs such as sexual dysfunction, weight gain and sometimes cardiovascular activities and failure of drugs to achieve remission in patients. Compounds 61 at the IC50 of 2.8 μM and 62 at the IC50 of 3.8 μM were documented to have appreciable antidepressant properties, which were established by their behavioral status as 5-HT7 agonists.77 It is worthy to note that the more active 61 possessed a short alkyl side chain on its amine linker than that of 62. However, although they were the most potent among the ten motifs designed, they were less efficient compared to the standard drug methiothepin, which exhibited an IC50 as low as 2.2 nM. Their structures are shown in Fig. 14.
Fig. 14. Selected quinoline derivatives with anti-depressant activity.
Anticonvulsant activities of quinoline derivatives
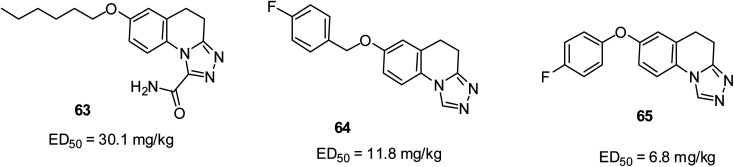
The result from the maxima electroshock (MES) test using intraperitoneally injected mice showed that quinoline-1-carboxamide 63 with an ED50 of 30.1 mg kg (ref. 78) and triazolo[4,3-a]quinoline 64 with an ED50 of 11.8 mg kg−1 were the most active anticonvulsants.79 The careful incorporation of a halogen atom, particularly fluorine as a substituent on the benzene ring tremendously enhanced the anti-MES propensity, which led to the occurrence of 65 with an ED50 of 6.8 mg kg−1 as the most promising in another study.80 Their structures are shown in Fig. 15.
Fig. 15. Selected quinoline derivatives with anti-convulsant activity.
Antidiabetic activities of quinoline derivatives
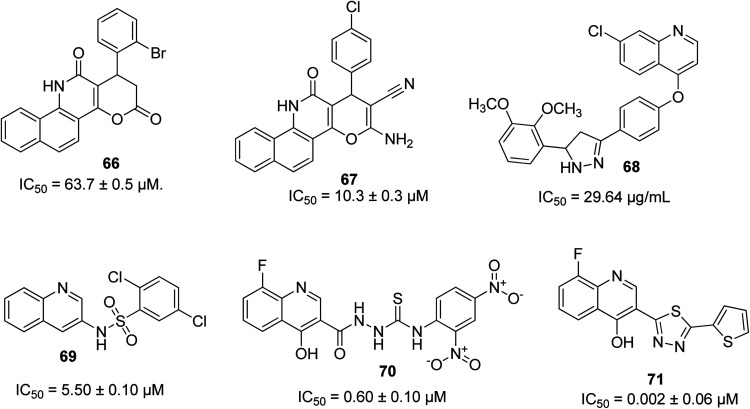
A series of pyrano-quinoline derivatives was synthesized via a two-step approach. The investigation of their α-glucosidase inhibitory potential was carried out using α-glucosidase from Saccharomyces cerevisiae, where 66 was the most active with an IC50 of 63.7 ± 0.5 μM.81 Among the series of pyrano-quinolines designed and synthesized by Nikookar and coworkers, 67 possessed the best activity as an antidiabetic motif (IC50 of 10.3 ± 0.3 μM), which was shown to be 75-times more active than the standard drug acarbose.82 Other outstanding quinoline-based antidiabetic molecules are 68 with an IC50 of 26.94 μg mL−1,8369 with an IC50 of 5.50 ± 0.10 μM,8470 with an IC50 of 0.60 ± 0.01 μM (ref. 85) and 71 with an IC50 of 0.002 ± 0.06 μM.86 Their structures are shown in Fig. 16
Fig. 16. Selected quinoline derivatives with anti-diabetic activity.
Structure activity relationship (SAR) study
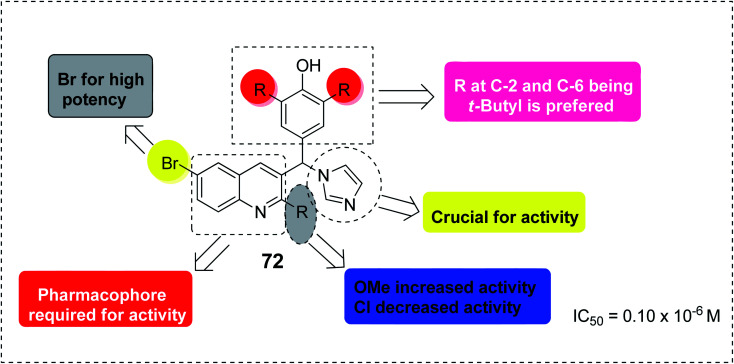
The quinoline template is a pharmacophore of tremendous biological potential for drug design. In addition, various substituents on different positions in the quinoline can enhance the pharmacological efficacy of the quinoline scaffold.87 For instance, SAR studies of the antimalarial capability of quinoline-imidazole hybrid 72 against chloroquine-sensitive strain 3D7 (IC50 = 0.10 μM) showed that the presence of the electron-donating OCH3 at position-2 enhanced its activity, while the presence of the electron-withdrawing Cl at C-2 led to the loss of activity.87 The presence of Br at C-6 is an essential moiety for activity improvement. However, although the presence of imidazole is crucial for activity, substitution on its nucleus was not preferred,87 as shown in Fig. 17.
Fig. 17. SAR-identified moieties responsible for activity in quinoline-imidazole hybrid 72.
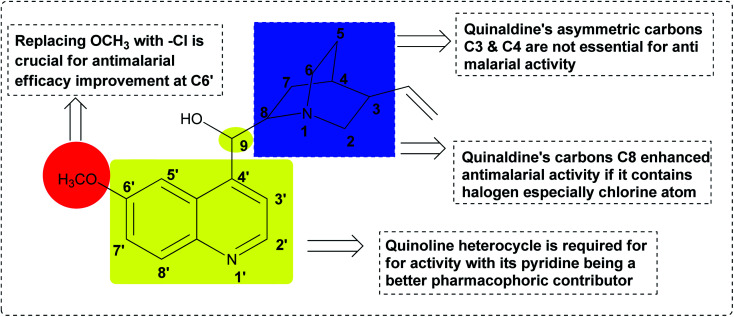
Quinine 3 is another strong antimalarial drug, which is present in nature in the bark of the Cinchona tree.88 It has been an effective treatment of malaria for more than four centuries.89 A summary of the pharmacophoric properties and SAR diversity of the quinaldine and quinoline portions of quinine antimalarial drugs is presented in Fig. 18. The detailed SAR study of quinine showed that at the C3 and C4 positions, asymmetry is not essential for antimalarial potential in quinine. Secondly, halogen substitution at the C8 position increases its antimalarial efficacy. In quinine, the presence of methoxy at the C6′ position is not essential for antimalarial activity. Replacing OCH3 with a halogen such as chloro (Cl) enhances its activity. Placing a phenyl group at the C2 position increases the activity. Modification in the secondary alcohol at the C9 position through oxidation and esterification reduces the antimalarial activity of quinine.90
Fig. 18. SAR-identified moieties responsible for activity in quinine 3.
Combined signaling pathways in oncology
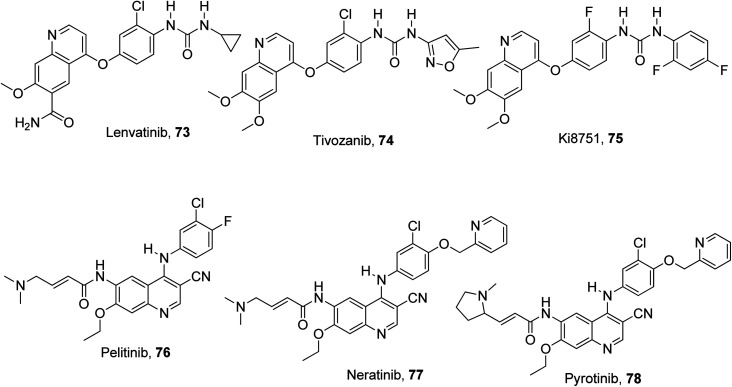
The aberrant signaling pathways of combined interest play a highly effective functional role in the survival, differentiation and proliferation of cancer cells.91 These aberrant signals, which are competent in providing diagnostic tools and oncological therapeutic efficacy, are EGFR, VEGFR and c-Met.92 This is because they are the three most crucial growth factor receptors with direct implication in cancerous cell lines.93 Some designed quinoline-based inhibitors used in the treatment of different types of cancers are lenvatinib 73,94 tivozanib, 74,95 Ki8751 75,96 pelitinib 76,97 neratinib 77,98 pyrotinib 78,99,100 cabozantinib 32,101,102 and foretinib 33.103 They are ligands with good binding affinity to these growth factor receptors, selectively or collectively. The structures of these quinoline-based inhibitors are presented in Fig. 19.
Fig. 19. Structures of quinoline-based inhibitors of the growth factor receptor.
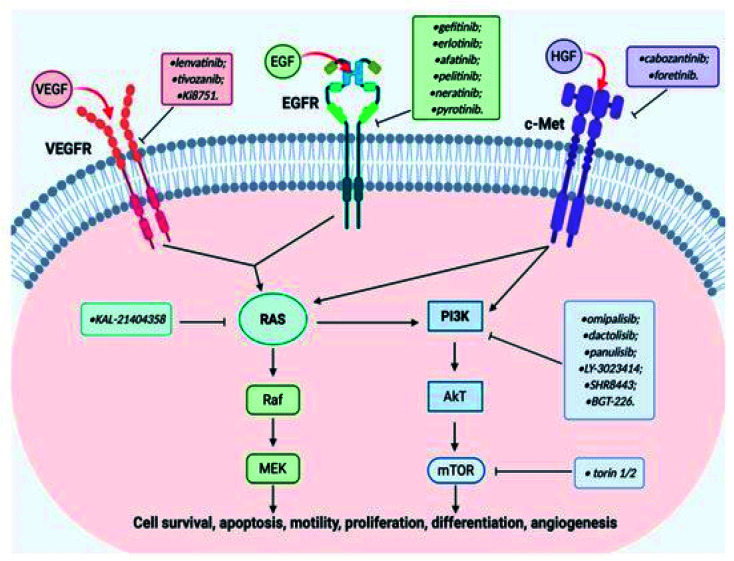
For instance, cabozantinib, 32 which was branded in the USA as Cometriq, is an anticancer drug approved as a selective inhibitor of non-specific tyrosine kinase.104 Tivozanib 74 is a pan-inhibitor of VEGF receptors.95 In addition, foretinib 33 exhibited dual inhibition of the c-Met/VEGFR2 signaling pathway for an improvement in antitumor effect in a gastric cancer model.103 The reference to EGFR as a biomarker of new opportunity also qualified it to be effectively combined with c-Met in cancer therapy.105 Recent work focused on compounds effective on the c-Met, VEGF and EGF receptors, pivotal targets for the activation of important carcinogenic pathways (Ras/Raf/MEK and PI3K/AkT/mTOR).106 A pictorial representation of the cross talk among the EGFR, VEGFR, and c-Met signaling pathways is shown in Fig. 20.106
Fig. 20. Cross-talk among EGFR, VEGFR, and c-Met signaling pathways and quinoline-based ligands as their targeted inhibitors.106.
Conclusion
Quinoline derivatives are privileged heterocyclic nuclei with high bioactivities essential for medicinal chemistry research. The chemistry of quinoline elucidated herein from the effort of various synthetic chemists corroborated the diverse derivatives being used in medicinal chemistry. Based on the reviewed information highlighted herein on the medicinal potential of quinoline and its functionalized derivatives, a new window of opportunity may be opened to medicinal chemists to access more biomolecular quinolines for future drug development.
Conflicts of interest
The authors have declared no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Author contributions
Conceptualization and supervision of the write-up was done by OOA. Writing and Chemistry of Original draft was done by KTI. Writing and pharmacological portion of original draft was done OTA. The review was written through contributions of all authors. All authors have given approval to the final version of the review.
Supplementary Material
Acknowledgments
All authors gratefully acknowledged Covenant University for the support for this work. OOA sincerely thank Royal Society of Chemistry for RSC Research Fund Grant with the Grant No: R21-2456856027.
Biographies
Biography
Olayinka O. Ajani.

Olayinka Oyewale AJANI was born in Ile-ife and attained his elementary and secondary school education in Ibadan. He obtained his Bachelor's and Master's Degree in Chemistry from Obafemi Awolowo University, Ile-ife, Osun State, Nigeria. He joined Covenant University Ota Ogun State, Nigeria in 2005, where he obtained his Doctoral Degree in Chemistry in 2012. Olayinka Ajani is a Professor of Chemistry who has established himself both as an academic and a researcher in the areas of organic synthesis and medicinal chemistry. He is a recipient of many prestigious awards and grants including the German DAAD Research Visit Award and The Royal Society of Chemistry Grant. He had his Postdoctoral Fellowship at Philipps University, Marburg, Germany under the research group of Professor Martin Schlitzer. Currently, Prof. Ajani is the Director of Covenant University Central Instrumentation Research Facility (CUCIRF). He is a member of notable professional bodies such as The Royal Society of Chemistry (RSC) and Chemical Society of Nigeria (CSN). He is happily married and blessed with children.
Biography
King T. Iyaye.

King Tamunodikibugerere IYAYE was born in Okrika and attained his elementary and secondary school education in Rivers State. He obtained his Bachelor's and Master's Degree in Industrial Chemistry from Covenant University, Ota, Ogun State. His BSc dissertation was entitled Synthesis, Characterization and Antibacterial Activity of Novel Hydrazide–Hydrazones of Quinoline. King is a first-class student who finished his BSc with a CGPA of 4.87 out of 5.00, which earned him a scholarship with Covenant University, Ota Ogun State with his research focused on functional and biologically active oxadiazole linked to quinoxaline. His carried out his project under the supervision of Prof. O. O. Ajani. King's Master's thesis was titled Synthesis and Antimicrobial Activity of 1,3,4-Oxadiazole-Linked 1,4-Dihydroquinoxaline Derivatives. He completed his Master's Degree with a CGPA of 5.0/5.0. Furthermore, King has been actively involved in research leading to publications in accredited/peer-reviewed scientific journals. He has, to his credit, publications in reputable journals, which are indexed in the Scopus database.
Biography
Olabisi T. Ademosun.

Mrs Olabisi Ademosun was born in Ota, Ogun State and attained her elementary and secondary school education in the same town, Ota. She obtained her Bachelor's Degree in Pure Chemistry from Olabisi Onabanjo University, Ago Iwoye, Ogun State, Nigeria and Master's Degree in Industrial Chemistry from Covenant University, Ota, Ogun-State, Nigeria, where she is currently a PhD student working on cancer nutrition. Mrs Ademosun has been actively involved in collaboration with other researchers, home and abroad, leading to publications in accredited/peer-reviewed scientific journals. Olabisi T. Ademosun is an Assistant Lecturer who is involved with teaching and projects and research supervision at the undergraduate level at Covenant University. She has served and still serving in various capacities in various committees at the Departmental, College and University levels. She is a member of The Royal Society of Chemistry (RSC). Mrs Ademosun is happily married and blessed with children to the glory of God.
References
- Shiro T. Fukaya T. Tobe M. The chemistry and biological activity of heterocycle-fused quinoline derivatives: a review. Eur. J. Med. Chem. 2015;97:397–408. doi: 10.1016/j.ejmech.2014.12.004. [DOI] [PubMed] [Google Scholar]
- (a) Olateju O. A. Babalola C. P. Olubiyi O. O. Kotila O. A. Kwasi D. A. Oaikhena A. O. Okeke I. N. Quinoline antimalarials increase the antibacterial activity of ampicillin. Front. Microbiol. 2021;12:556550. doi: 10.3389/fmicb.2021.556550. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Duparc S. Chalon S. Miller S. Richardson N. Toovey S. Neurological and psychiatric safety of tafenoquine in Plasmodium vivax relapse prevention: a review. Malar. J. 2020;19:111. doi: 10.1186/s12936-020-03184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Das R. R. Jaiswal N. Dev N. Jaiswal N. Naik S. S. Shankar J. Efficacy and safety of anti-malarial drugs (chloroquine and hydroxy-chloroquine) in treatment of covid-19 infection: a systematic review and meta-analysis. Front. Med. 2020;7:482. doi: 10.3389/fmed.2020.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Sharma V. Mehta D. K. Das R. Synthetic methods of quinoline derivatives as potent anticancer agents. Mini-Rev. Med. Chem. 2017;17(16):1557–1572. doi: 10.2174/1389557517666170510104954. [DOI] [PubMed] [Google Scholar]
- Martins P. Jesus J. Santos S. Raposo L. R. Roma-Rodrigues C. Baptista P. V. Fernandes A. R. Heterocyclic anticancer compounds: recent advances and the paradigm shift towards the use of nanomedicine's tool box. Molecules. 2015;20:16852–16891. doi: 10.3390/molecules200916852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S. Q. Li L. C. Song J. R. Li H. Y. Li D. P. Structurally simple phenanthridine analogues based on nitidine and their antitumor activities. Molecules. 2019;24:437. doi: 10.3390/molecules24030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil V. Barragan E. Patil S. A. Patil S. A. Bugarin A. Direct synthesis and antimicrobial evaluation of structurally complex chalcones. ChemistrySelect. 2016;1(13):3647–3650. [Google Scholar]
- Vijayta G. Vinay K. A review of biological activity of imidazole and thiazole moieties and their derivatives. Sci. Int. 2013;1(7):253–260. [Google Scholar]
- Mohammed A. A mini review: Biological significances of nitrogen heteroatom containing heterocyclic compounds. Int. J. Bioorg. Chem. 2017;2(3):146–152. [Google Scholar]
- Youness B. Younes Z. Jamal T. Ansar M. Pyridazin-3(2H)-ones: synthesis, reactivity, applications in pharmacology and agriculture. J. Chem. Pharm. Res. 2014;6(12):297–310. [Google Scholar]
- Otutu J. O. Synthesis and application of azo dyes derived from 2-amino-1,3,4-thiadiazole-2-thiol on polyester fibre. Int. J. Res. Rev. Appl. Sci. 2013;15(2):292–296. [Google Scholar]
- Emily A. B. Timothy L. E. Imidazole- and imidazolium-containingpolymers for biology and material science applications. Polymer. 2010;51(12):2447–2454. [Google Scholar]
- Anna P. N. Kirtee B. Computer based drug design of various heterocyclic compounds having anticancer activity: a brief review. J. Bioinformat. Genom. Proteom. 2017;2(1):1014. [Google Scholar]
- Chi-Shiang K. Chia-Chia F. Jia-Ying Y. Po-Jung T. Joseph P. R. Chuan-Pin C. Yang-Hsiang C. Molecular engineering and design of semiconducting polymer dots with narrow-band, near-infrared emission for in vivo biological imaging. ACS Nano. 2017;11(3):3166–3177. doi: 10.1021/acsnano.7b00215. [DOI] [PubMed] [Google Scholar]
- Govindarao K. Srinivasan N. Suresh R. Raheja R. K. Annadurai S. Bhandare R. R. Shaik A. B. Quinoline conjugated 2-azetidinone derivatives as prospective anti-breast cancer agents: in vitro antiproliferative and anti-EGFR activities, molecular docking and in-silico drug likeliness studies. J. Saudi Chem. Soc. 2022;26(3):101471. doi: 10.1016/j.jscs.2022.101471. [DOI] [Google Scholar]
- Kania A. Tejchman W. Pawlak A. M. Mokrzyński K. Rózanowski B. Musielak B. M. Greczek-Stachura M. Preliminary studies of antimicrobial activity of new synthesized hybrids of 2-thiohydantoin and 2-quinolone derivatives activated with blue light. Molecules. 2022;27:1069. doi: 10.3390/molecules27031069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K. L. Liu W. Yin W. H. Li J. Y. Yue J. M. Quinoline alkaloids with anti-inflammatory activity from Zanthoxylum avicennae. Org. Biomol. Chem. 2022;20:4176–4182. doi: 10.1039/d2ob00711h. [DOI] [PubMed] [Google Scholar]
- Prasad M. V. V. V. Rao R. H. R. Veeranna V. Chennupalli V. S. Sathish B. Novel quinolone derivatives: synthesis and antioxidant activity. Russ. J. Gen. Chem. 2021;91:2522–2526. doi: 10.1134/S1070363221120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley R. Mashaba C. Rakodi G. H. Ncube N. B. Maphoru M. V. Balogun M. O. Jordan A. Warner D. F. Khan R. Tukulula M. New quinoline–urea–benzothiazole hybrids as promising antitubercular agents: synthesis, in vitro antitubercular activity, cytotoxicity studies, and in silico ADME profiling. Pharmaceuticals. 2022;15:576. doi: 10.3390/ph15050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan K. Panneerselvam C. Subramaniam J. Paulpandi M. Rajaganesh R. Vasanthakumaran M. Madhavan J. Shafi S. S. Roni M. Portilla-Pulido J. S. Mendez S. C. Duque J. E. Wang L. Aziz A. T. Chandramohan B. Dinesh D. Piramanayagam S. Hwang J. S. Synthesis of new series of quinoline derivatives with insecticidal effects on larval vectors of malaria and dengue diseases. Sci. Rep. 2022;12:4765. doi: 10.1038/s41598-022-08397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiseau P. M. Balaraman K. Barratt G. Pomel S. Durand R. Frézard F. Figadère B. The Potential of 2-substituted quinolines as antileishmanial drug candidates. Molecules. 2022;27(7):2313. doi: 10.3390/molecules27072313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorababu A. Quinoline: a promising scaffold in recent antiprotozoal drug discovery. ChemistrySelect. 2021;6(9):2164–2177. [Google Scholar]
- Singh V. K. Mishra R. Kumari P. Som A. Yadav A. K. Ram N. K. Kumar P. Schols D. Singh R. K. In silico design, synthesis and anti-HIV activity of quinoline derivatives as non-nucleoside reverse transcriptase inhibitors (NNRTIs) Comput. Biol. Chem. 2022;98:107675. doi: 10.1016/j.compbiolchem.2022.107675. [DOI] [PubMed] [Google Scholar]
- Krstulović L. Stolić I. Jukić M. Opačak-Bernardi T. Starčević K. Bajić M. Glavaš-Obrovac I. New quinoline-arylamidine hybrids: synthesis, DNA/RNA binding and tumor activity. Eur. J. Med. Chem. 2017;137:196–210. doi: 10.1016/j.ejmech.2017.05.054. [DOI] [PubMed] [Google Scholar]
- Dib M. Ouchetto H. Ouchetto K. Hafid A. Khouili M. Recent developments of quinoline derivatives and their potential biological activities. Curr. Org. Synth. 2021;18(3):248–269. doi: 10.2174/1570179417666201216162055. [DOI] [PubMed] [Google Scholar]
- Ukrainets I., Creation of new local anesthetics based on quinoline derivatives and related heterocycles, in Pain Management-Current Issues and Opinions, ed. R. Gabor and N.E. Carl, InTech, 2012, pp. 63–80 [Google Scholar]
- Alajarin R. and Burgos C., Six-membered heterocyles: Quinoline and Isoquinolin, in Heterocyclic Chemistry, ed. J. Alvarez-Builla, J. J. Vaquero and J. Barluenga, John Wiley & Sons, 2011, p. 1527 [Google Scholar]
- Pandeya S. N. Tyagi A. Synthetic approaches for quinoline and isoquinoline. Int. J. Pharm. Pharm. Sci. 2011;3(3):53–61. [Google Scholar]
- Afzal O. Kumar S. Haider M. R. Ali M. R. Kumar R. Jaggi M. Bawa S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015;97:871–910. doi: 10.1016/j.ejmech.2014.07.044. [DOI] [PubMed] [Google Scholar]
- Amit C. Payal C. Kuldeep K. Mansimran S. Poonam S. Kuldeep S. A review: chemistry of antimicrobial and anticancer quinolines. Can. Open Pharm. J. 2014;1(1):1–12. [Google Scholar]
- Wink M. Medicinal plants: a source of anti-parasitic secondary metabolites. Molecules. 2012;17:12771–12791. doi: 10.3390/molecules171112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. B. Patel S. D. Patel J. N. Patel J. C. Gorgamwala Y. S. Synthesis and antibacterial activity of thioureido amide of fluoroquinolone. Int. J. Biol. Chem. 2011;5(1):37–45. [Google Scholar]
- Jain S. Chandra V. Jain P. K. Pathak K. Pathak D. Vaidya A. Comprehensive review on current developments of quinoline-based anticancer agents. Arab. J. Chem. 2019;12(8):4920–4946. [Google Scholar]
- Kannappan N. Reddy B. S. Sen S. Nagarajan R. Dashpute S. Synthesis and chemical characterization of quinoline imine derivatives. J. Appl. Chem. Res. 2009;9:59–68. [Google Scholar]
- Mistry B. Jauhari S. Synthesis and characterization of some quinoline based azetidinones and thiazolidinones as antimicrobial agents. Sch.Res. Lib. 2010;2(6):332–343. [Google Scholar]
- Diaz G., Miranda I. L. and Diaz M. A., Quinolines, isoquinolines, angustereine, and congeneric alkaloids: Occurrence, chemistry, and biological activity in Phytochemicals Isolation, Characterization and Role in Human Health, ed. A. V. Rao and L. G. Rao, Brazil, Intech, 2015, pp. 142–162 [Google Scholar]
- Lv M. Xu P. Tian Y. Liang J. Gao Y. Xu F. Zhang Z. Sun J. Medicinal uses, phytochemistry and pharmacology of the genus Dictamnus (Rutaceae) J. Ethnopharmacol. 2015;171:247–263. doi: 10.1016/j.jep.2015.05.053. [DOI] [PubMed] [Google Scholar]
- Ajani O. O. Isaac J. T. Owoeye T. F. Akinsiku A. A. Exploration of the chemistry and biological properties of pyrimidine as a privileged pharmacophore in therapeutics. Int. J. Biol. Chem. 2015;9:148–177. [Google Scholar]
- Cheng C. and Yan S. J., The Friedlander synthesis of quinoline, in Organic Reactions, London, John Wiley and Sons, 1982, vol. 28, pp. 37–39 [Google Scholar]
- (a) Wang G. W. Jia C. S. Dong Y. W. Benign and highly efficient synthesis of quinoline from 2-aminoarylketone or 2-aminoarylaldehyde and carbonyl compounds mediated by hydrochloric acid in water. Tetrahedron Lett. 2006;47:1059–1063. [Google Scholar]; (b) Hasaninejad A. R. Zare A. Zolfigol M. A. Abdeshah M. Ghaderi A. Nami-Ana F. Synthesis of poly-substituted quinolines via Friedländer hetero-annulation reaction using silica-supported P2O5 under solvent-free conditions. Iran. J. Chem. Chem. Eng. 2011;30(1):73–81. [Google Scholar]
- Conrad M. Limpach L. Synthesen von Chinolin derivatenmittelst Acetessigester. Ber. Dtsch. Chem. Ges. 1887;20(1):944–948. [Google Scholar]
- Nevalainen T., Synthesis of heterocyclic compounds, 2010, cited February7, 2022, available from: http://www.scripps.edu/chem/baran/heterocycles/
- Brouet J. C. Gu S. Peet N. P. Williams J. D. A survey of solvents for the Conrad Limpach synthesis of 4-hydroxyquinolones. Synth. Commun. 2009;39(9):5193–5196. doi: 10.1080/00397910802542044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madapa S. Tusi Z. Batra S. Advances in the syntheses of quinoline and quinoline-annulated ring systems. Curr. Org. Chem. 2008;12:1116–1183. [Google Scholar]
- Wu Y. C. Liu L. Li H. J. Wang D. Chen Y. J. Skraup-Doebner-Von Miller quinoline synthesis revisited: reversal of the regiochemistry for γ-aryl-β,γ-unsaturated α-keto esters. J. Org. Chem. 2006;71:6592–6595. doi: 10.1021/jo060290n. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab B. F. Khidre R. E. Farahat A. A. El-Ahl A. S. 2-Chloroquinoline-3-carbaldehydes: synthesis, reactions and applications. Arkivoc. 2012;1:211–276. [Google Scholar]
- Becker A. Kohfeld S. Pies T. Wieking K. Preu L. Kunick C. Synthesis of 11H-indolo[3,2-c]quinoline-6-carboxylic acids by cascade autoxidation-ring contractions. Synthesis. 2009;7:1185–1189. [Google Scholar]
- Falke H. Chaikuad A. Becker A. Loaëc N. Lozach O. Jhaisha S. A. Becker W. Jones P. G. Preu L. Baumann K. Knapp S. Meijer L. Kunick C. 10-Iodo-11H indolo[3,2c]quinoline-6-carboxylic acids are selective inhibitors of DYRK1A. J. Med. Chem. 2015;58:3131–3143. doi: 10.1021/jm501994d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumade P. P. Wadher S. J. Chourasia A. J. Kharabe U. V. Mude D. Yeole P. G. Synthesis of newer Mannich bases of quinoline derivative for antimicrobial activity. Int. J. Chem. Sci. 2009;7(3):1518–1530. [Google Scholar]
- Almansour A. I. Arumugam N. Kumar R. S. Menendez J. C. Ghabbour H. A. Fun H. K. Kumar R. R. Straight forward synthesis of pyrrolo[3,4-b]quinolines through intramolecular Povarov reactions. Tetrahedron Lett. 2015;56(49):6900–6903. doi: 10.1016/j.tetlet.2015.10.107. [DOI] [Google Scholar]
- Sridharan V. Perumal P. Avendano C. Menendez C. The first aza Diels-Alder reaction involving an a,b-unsaturated hydrazone as the dienophile: Stereoselective synthesis of C-4 functionalized 1,2,3,4-tetrahydroquinolines containing a quaternary stereocenter. J. Org. Biomol. Chem. 2007;5:1351–1353. doi: 10.1039/b703083e. [DOI] [PubMed] [Google Scholar]
- Ajani O. O. Iyaye K. T. Aderohunmu D. V. Olanrewaju I. O. Germann M. W. Olorunshola S. J. Bello B. L. Microwave-assisted synthesis and antibacterial propensity of N’-(s-benzylidene and s-1H-pyrrol-2-yl)methylene)-2 propylquinol ine-4-carbohydrazide motifs. Arab. J. Chem. 2020;13(1):1809–1820. [Google Scholar]
- Poonam S. Kamaldeep K. Amit C. Ranjodh S. Dhawan R. K. A review on biological activities of quinoline derivatives. BEST: J. Manag. Inf. Technol. Eng. 2016;2(1):1–14. [Google Scholar]
- Mandewale C. M. Patil C. P. Shedge S. V. Dappadwad U. R. Yamgar R. S. A review on quinoline hydrazone derivatives as a new class of potent antitubercular and anticancer agents. Beni-Suef Univ. J. Basic Appl. Sci. 2017;6(4):354–361. doi: 10.1016/j.bjbas.2017.07.005. [DOI] [Google Scholar]
- Zhang Z. Du H. Cis-selective and highly enantioselective hydrogenation of 2,3,4-trisubstituted quinolines. Org. Lett. 2015;17(11):2816–2819. doi: 10.1021/acs.orglett.5b01240. [DOI] [PubMed] [Google Scholar]
- Gomez M. C. Kouznetsov V. V. Recent developments on antimicrobial quinoline chemistry. Microbial pathogens and strategies for combating them. Sci. Tech. Edu. 2013;1:666–677. [Google Scholar]
- Kharb R. Kaur H. Therapeutic significance of quinoline derivatives as antimicrobial agents. Int. Res. J. Pharm. 2013;4(3):63–69. [Google Scholar]
- Yayehrad A. T. Wondie G. B. Marew T. Different nanotechnology approaches for ciprofloxacin delivery against multidrug-resistant microbes. Infect. Drug Resist. 2022;15:413–426. doi: 10.2147/IDR.S348643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulis G. Pradhan R. Kotwani A. Gandra S. India's ban on antimicrobial fixed-dose combinations: winning the battle, losing the war? J. Pharm. Policy Pract. 2022;15:33. doi: 10.1186/s40545-022-00428-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. K. Singh S. A brief history of quinoline as antimalarial agents. Int. J. Pharm. Sci. Rev. Res. 2014;25(1):295–302. [Google Scholar]
- Starkl R. K. Iskra J. Krizaj I. Understanding malarial toxins. Toxicon. 2016;119:319–329. doi: 10.1016/j.toxicon.2016.06.017. [DOI] [PubMed] [Google Scholar]
- Wang L. X. Liu X. Xu S. Tang Q. Duan Y. Xiao Z. Zhi J. Jiang L. Zheng P. Zhu W. Discovery of novel pyrrolo-pyridine/pyrimidine derivatives bearing pyridazinone moiety as c-Met kinase inhibitors. Eur. J. Med. Chem. 2017;141:538–551. doi: 10.1016/j.ejmech.2017.10.027. [DOI] [PubMed] [Google Scholar]
- Lu D. Shen A. Liu Y. Peng X. Xing W. Ai J. Geng M. Hu Y. Design and synthesis of novel benzo[d]oxazol-2(3H)-one derivative bearing 7-substituted-4-ethoxy quinoline moieties as c-Met kinase inhibitors. Eur. J. Med. Chem. 2016;115:191–200. doi: 10.1016/j.ejmech.2016.03.027. [DOI] [PubMed] [Google Scholar]
- Coa J. C. Castrillon W. Cardona W. Carda M. Ospina V. Munoz J. A. Velez I. D. Robledo S. M. Synthesis, leishmanicidal, trypanocidal and cytotoxic activity of quinoline-hydrazone hybrids. Eur. J. Med. Chem. 2015;101:746–753. doi: 10.1016/j.ejmech.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Puskullu M. O. Shirinzadeh H. Nenni M. Gurer-Orhan H. Suzen S. Synthesis and evaluation of antioxidant activity of new quinoline-2-carbaldehydehydrazone derivatives: bioisosteric melatonin analogues. J. Enzym. Inhib. Med. Chem. 2015;31(1):121–125. doi: 10.3109/14756366.2015.1005012. [DOI] [PubMed] [Google Scholar]
- Subashini R. Roopan S. M. Khan F. N. Synthesis and free radical scavenging property of some quinoline derivatives. J. Chil. Chem. Soc. 2010;55(3):317–319. [Google Scholar]
- Liberto N. A. Simoes J. B. Silva S. Modolo L. V. Fatima A. Silvia L. M. Derita M. Zacchino S. Zuniga O. M. P. Romanelli G. P. Fernandes S. A. Quinolines: Microwave-assisted synthesis and their antifungal, anticancer and radical scavenger properties. Bioorg. Med. Chem. 2017;25:1153–1162. doi: 10.1016/j.bmc.2016.12.023. [DOI] [PubMed] [Google Scholar]
- Al-Busafi S. N. Sullman F. E. Al-Alawi Z. 8-Hydroxyquinoline and its derivatives: synthesis and applications. Res. Rev.: J. Chem. 2013;3(1):1–10. [Google Scholar]
- Mahajan P. Nikam M. Asrondkar A. Bobade A. Gill C. Synthesis, antioxidant, and anti-inflammatory evaluation of novel thiophene-fused quinoline based β-diketones and derivatives. J. Heterocycl. Chem. 2017;54(2):1415–1422. [Google Scholar]
- Pinz M. P. Reis A. S. Oliveira R. L. Voss G. T. Vogt A. G. Sacramento M. D. Roehrs J. A. Alves D. Luchese C. Wilhelm E. A. 7-Chloro-4-phenylsulfonyl quinoline, a new antinociceptive and anti-inflammatory molecule: structural improvement of a quinoline derivative with pharmacological activity. Regul. Toxicol. Pharmacol. 2017;90:72–77. doi: 10.1016/j.yrtph.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Khalifa N. M. Al-Omar M. A. AbdEl-Galil A. A. Abd El-Reheem M. Anti-inflammatory and analgesic activities of some novel carboxamides derived from 2-phenylquinoline candidates. Biomed. Res. 2017;28(2):869–874. [Google Scholar]
- Das P. Deng X. Zhang L. Roth G. M. Fontoura B. M. Phillips M. A. De Brabander J. K. SAR-based optimization of a 4-quinoline carboxylic acid analogue with potent antiviral activity. ACS Med. Chem. Lett. 2013;4:517–521. doi: 10.1021/ml300464h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta A. Briguglio I. Piras S. Corona P. Boatto G. Nieddu M. Giunchedi P. Marongiu M. E. Giliberti G. Luliano F. Blois S. Ibba C. Busonera B. La Colla P. Quinoline tricyclic derivatives. Design, synthesis and evaluation of the antiviral activity of three new classes of RNA-dependent RNA polymerase inhibitors. Bioorg. Med. Chem. 2011;19:7070–7084. doi: 10.1016/j.bmc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Zhuang L. Wai J. S. Embrey M. W. Fisher T. E. Egbertson M. S. Payne L. S. Guare Jr J. P. Vacca J. P. Hazuda D. J. Felock A. L. Stillmock K. A. Witmer M. V. Moyer G. Schleif W. A. Gabryelski L. J. Leonard Y. M. Lynch Jr J. J. Michelson S. R. Young S. D. Design and synthesis of 8-hydroxy-[1,6]naphthyridines as novel inhibitors of HIV-1 integrase in vitro and infected cells. J. Med. Chem. 2003;46:453–456. doi: 10.1021/jm025553u. [DOI] [PubMed] [Google Scholar]
- Desai N. C. Patel B. Y. Dave B. P. Synthesis and antimicrobial activity of novel quinoline derivatives bearing derivatives and pyrazoline and pyridine analogues. Med. Chem. Res. 2016;26:109–119. [Google Scholar]
- Dorababu A. Recent update on antibacterial and antifungal activity of quinoline scaffolds. Arch. Pharm. 2021;354(3):e2000232. doi: 10.1002/ardp.202000232. [DOI] [PubMed] [Google Scholar]
- Luo Z. G. Zeng C. C. Wang F. He H. Q. Wang C. X. Du H. G. Hu L. M. Synthesis and biological activities of quinoline derivatives as HIV-1 integrase inhibitors. Chem. Res. Chin. Univ. 2009;25(6):841–845. [Google Scholar]
- Mouscadet J. Desmaële D. Chemistry and structure-activity relationship of the styrylquinoline-type HIV integrase inhibitors. Molecules. 2010;15:3048–3078. doi: 10.3390/molecules15053048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajdel P. Marciniec K. Maślankiewicz A. Grychowska K. Satala G. Duszynska B. Lenda T. Siwek A. Nowak G. Partyka A. Wróbel D. Jastrzębska-Więsek M. Bojarski A. J. Wesołowska A. Pawłowski M. Antidepressant and antipsychotic activity of new quinoline- and isoquinoline-sulfonamide analogs of aripiprazole targeting serotonin 5-HT1A/5-HT7 and dopamine D2/D3 receptors. Eur. J. Med. Chem. 2013;60:42–50. doi: 10.1016/j.ejmech.2012.11.042. [DOI] [PubMed] [Google Scholar]
- Wei C. X. Deng X. Q. Chai K. Y. Sun Z. G. Quan Z. S. Synthesis and anticonvulsant activity of 1-formamide-triazolo[4,3-a]quinoline derivatives. Arch Pharm. Res. 2010;33(5):655–662. doi: 10.1007/s12272-010-0502-0. [DOI] [PubMed] [Google Scholar]
- Jin H. G. Sun X. Y. Chai K. Y. Piao H. R. Quan Z. S. Anticonvulsant and toxicity evaluation of some 7-alkoxy-4,5-dihydro-[1,2,4] triazolo[4,3-a]quinoline-1(2H)- ones. Bioorg. Med. Chem. 2006;14:6868–6873. doi: 10.1016/j.bmc.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Song M. Deng X. Q. Recent developments on triazole nucleus in anticonvulsant compounds: a review. J. Enzym. Inhib. Med. Chem. 2018;33(1):453–478. doi: 10.1080/14756366.2017.1423068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydari Z. Mohammadi-Khanaposhtani M. Imanparast S. Faramarzi M. A. Mahdavi M. Ranjbar P. R. Larijani B. Pyrano[3,2-c]quinoline derivatives as new class of α-glucosidase inhibitors to treat type 2 diabetes: synthesis, in vitro biological evaluation and kinetic study. Med. Chem. 2019;15(1):8–16. doi: 10.2174/1573406414666180528110104. [DOI] [PubMed] [Google Scholar]
- Nikookar H. Mohammadi-Khanaposhtani M. Imanparast S. Faramarzi M. A. Ranjbar P. R. Mahdavi M. Larijani B. Design, synthesis and in vitro α-glucosidase inhibition of novel dihydropyrano[3,2-c]quinoline derivatives as potential anti-diabetic agents. Bioorg. Chem. 2018;77:280–286. doi: 10.1016/j.bioorg.2018.01.025. [DOI] [PubMed] [Google Scholar]
- Kumar A. Kumar P. Shetty C. R. James J. P. Shetty H. C. Synthesis, antidiabetic evaluation and bioisosteric modification of quinoline incorporated 2-pyrazoline derivatives. Indian J. Pharm. Educ. Res. 2021;55(2):574–580. [Google Scholar]
- Bano B. Arshia Khan K. M. Kanwal Fatima B. Taha M. Ismail N. H. Wadood A. Ghufran M. Perveen S. Synthesis, in vitro β-glucuronidase inhibitory potential and molecular docking studies of quinolines. Eur. J. Med. Chem. 2017;139:849–864. doi: 10.1016/j.ejmech.2017.08.052. [DOI] [PubMed] [Google Scholar]
- Zaman K. Rahim F. Taha M. Sajid M. Hayat S. Nawaz M. Salahuddin M. Iqbal N. Khan N. U. Shah S. A. A. Farooq R. K. Bahadar A. Wadood A. Khan K. M. Synthesis, in vitro antiurease, in vivo anti-nematodal activity of quinoline analogs and their in-silico study. Bioorg. Chem. 2021;115:105199. doi: 10.1016/j.bioorg.2021.105199. [DOI] [PubMed] [Google Scholar]
- Taha M. Javid M. T. Imran S. Selvaraj M. Chigurupati S. Ullah H. Rahim F. Khan F. Mohammad J. I. Khan K. M. Synthesis and study of the α-amylase inhibitory potential of thiadiazole quinoline derivatives. Bioorg. Chem. 2017;74:179–186. doi: 10.1016/j.bioorg.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Roy D. Anas M. Manhas A. Saha S. Kumar N. Panda G. Synthesis, biological evaluation, structure-activity relationship studies of quinoline-imidazole derivatives as potent antimalarial agents. Bioorg. Chem. 2022;121:105671. doi: 10.1016/j.bioorg.2022.105671. [DOI] [PubMed] [Google Scholar]
- Uzor P. F. Alkaloids from plants with antimalarial activity: a review of recent studies. Evid Based Complement. Altern. 2020:8749083. doi: 10.1155/2020/8749083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado C. Barnes C. J. Cornett C. Holmfred E. Hansen S. H. Persson C. Antonelli A. Rønsted N. Phylogeny predicts the quantity of antimalarial alkaloids within the iconic yellow cinchona bark (Rubiaceae: Cinchona calisaya) Front. Plant Sci. 2017;8:16. doi: 10.3389/fpls.2017.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renslo A. R. Antimalarial drug discovery: from quinine to the dream of eradication. ACS Med. Chem. Lett. 2013;4(12):1126–1128. doi: 10.1021/ml4004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. Shi P. Zhao G. Xu J. Peng W. Zhang J. Zhang G. Wang X. Dong Z. Chen F. Cui H. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target Ther. 2020;5(8):35. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. Y. Xu X. M. Hong B. Wu Z. G. Qian Y. Weng T. H. Liu Y. Z. Tang T. M. Wang M. H. Yao H. P. Aberrant RON and MET co-overexpression as novel prognostic biomarkers of shortened patient survival and therapeutic targets of tyrosine kinase inhibitors in pancreatic cancer. Front. Oncol. 2019;9:1377. doi: 10.3389/fonc.2019.01377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka T. Kusumoto S. Ando K. Ohba M. Ohmori T. Receptor tyrosine kinase-targeted cancer therapy. Int. J. Mol. Sci. 2018;19:3491. doi: 10.3390/ijms19113491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J. Qi L. Liang J. Zong K. Liu W. Li R. Feng R. Zhai W. Lenvatinib induces anticancer activity in gallbladder cancer by targeting. AKT. J. Cancer. 2021;12(12):3548–3557. doi: 10.7150/jca.50292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momeny M. Sabourinejad Z. Zarrinrad G. Moghaddaskho F. Eyvani H. Yousefi H. et al., Anti-tumour activity of tivozanib, a pan-inhibitor of VEGF receptors, in therapy-resistant ovarian carcinoma cells. Sci. Rep. 2017;7(1):45954. doi: 10.1038/srep45954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H. Guo M. Zhang X. Jiang L. Tan S. Yuan J. Cui H. et al., VEGFR2 inhibition hampers breast cancer cell proliferation via enhanced mitochondrial biogenesis. Cancer Biol. Med. 2021;18(1):139–154. doi: 10.20892/j.issn.2095-3941.2020.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. R. Lee S. Shin J. Y. Kim J. Y. Moon K. S. Jung J. Biomarker LEPRE1 induces pelitinib-specific drug responsiveness by regulating ABCG2 expression and tumor transition states in human leukemia and lung cancer. Sci. Rep. 2022;12:2928. doi: 10.1038/s41598-022-06621-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. Sang X. Wang M. Liu Y. Liu J. Wang X. Liu P. Cheng H. Melatonin potentiates the cytotoxic effect of Neratinib in HER2+ breast cancer through promoting endocytosis and lysosomal degradation of HER2. Oncogene. 2021;40:6273–6283. doi: 10.1038/s41388-021-02015-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W. Hao S. Wang L. Chen Y. Li Z. Luo D. Pyrotinib treatment enhances the radiosensitivity in HER2-positive brain metastatic breast cancer patients. Anticancer Drugs. 2022;33(1):e622–e627. doi: 10.1097/CAD.0000000000001199. [DOI] [PubMed] [Google Scholar]
- Wang C. Deng S. Chen J. Xu X. Hu X. Kong D. Liang G. Yuan X. Li Y. Wang X. The Synergistic effects of pyrotinib combined with adriamycin on HER2-positive breast cancer. Front. Oncol. 2021;11:616443. doi: 10.3389/fonc.2021.616443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallier D. Lesfevre P. Everaert H. Antitumor activity of cabozantinib in metastatic adult ewing sarcoma: A case report. Anticancer Res. 2020;40(4):2265–2269. doi: 10.21873/anticanres.14190. [DOI] [PubMed] [Google Scholar]
- Iuliani M. Simonetti S. Pantano F. Ribelli G. Di Martino A. Denaro V. et al., Antitumor effect of cabozantinib in bone metastatic models of renal cell carcinoma. Biology. 2021;10:781. doi: 10.3390/biology10080781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grojean M. Schwarz M. A. Schwarz J. R. Hassan S. von Holzen U. Zhang C. et al., Targeted dual inhibition of c-Met/VEGFR2 signalling by foretinib improves antitumour effects of nanoparticle paclitaxel in gastric cancer models. J. Cell Mol. Med. 2021;25(11):4950–4961. doi: 10.1111/jcmm.16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metada B. S. Pattanashettar R. Yernale N. G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021;32:115973. doi: 10.1016/j.bmc.2020.115973. [DOI] [PubMed] [Google Scholar]
- Guardiola S. Varese M. Sanchez-Navarro M. Giralt E. A third shot at EGFR: new opportunities in cancer therapy. Trends Pharmacol. Sci. 2019;40:941–955. doi: 10.1016/j.tips.2019.10.004. [DOI] [PubMed] [Google Scholar]
- Martorana A. La Monika G. Lauria A. Quinoline-based molecules targeting c-Met, EGF, and VEGF receptors and the proteins involved in related carcinogenic pathways. Molecules. 2020;25:4279. doi: 10.3390/molecules25184279. [DOI] [PMC free article] [PubMed] [Google Scholar]