Significance
Increased temperatures can disrupt protein conformations, and mammalian cells respond to heat shock (HS) (shift from 37 °C to 43 °C) by enhancing protein degradation presumably by the ubiquitin-proteasome pathway. We found that in 1–2 h at 43 °C, 26S proteasomes become more active in degrading peptides, ubiquitinated proteins, and adenosine 5′-triphosphate, even when purified and assayed at 37 °C. A similar activation occurs upon exposure to arsenite, which also damages cell proteins and increases protein ubiquitination. Unlike the classic HS response, this activation of proteasomes does not require new protein synthesis and seems to occur in response to the increased levels of ubiquitinated proteins in the cells. During proteotoxic stress, increased proteasome activity should help protect cells against the accumulation of damaged proteins.
Keywords: heat shock, 26S proteasome, proteotoxic stress, ubiquitin conjugates, arsenite
Abstract
Heat shock (HS) promotes protein unfolding, and cells respond by stimulating HS gene expression, ubiquitination of cell proteins, and proteolysis by the proteasome. Exposing HeLa and other cells to 43 °C for 2 h caused a twofold increase in the 26S proteasomes’ peptidase activity assayed at 37 °C. This increase in activity occurred without any change in proteasome amount and did not require new protein synthesis. After affinity-purification from HS cells, 26S proteasomes still hydrolyzed peptides, adenosine 5′-triphosphate, and ubiquitinated substrates more rapidly without any evident change in subunit composition, postsynthetic modification, or association with reported proteasome-activating proteins. After returning HS cells to 37 °C, ubiquitin conjugates and proteolysis fell rapidly, but proteasome activity remained high for at least 16 h. Exposure to arsenite, which also causes proteotoxic stress in the cytosol, but not tunicamycin, which causes endoplasmic reticulum stress, also increased ubiquitin conjugate levels and 26S proteasome activity. Although the molecular basis for the enhanced proteasomal activity remains elusive, we studied possible signaling mechanisms. Proteasome activation upon proteotoxic stress required the accumulation of ubiquitinated proteins since blocking ubiquitination by E1 inhibition during HS or arsenite exposure prevented the stimulation of 26S activity. Furthermore, increasing cellular content of ubiquitin conjugates at 37 °C by inhibiting deubiquitinating enzymes with RA190 or b-AP15 also caused proteasome activation. Thus, cells respond to proteotoxic stresses, apparently in response to the accumulation of ubiquitinated proteins, by activating 26S proteasomes, which should help promote the clearance of damaged cell proteins.
Eukaryotic and prokaryotic cells have evolved multiple adaptive mechanisms to cope with an intracellular build-up of misfolded aggregation-prone proteins whose accumulation could impair cell function and viability (1). The primary cellular response to proteotoxic stress in the cytosol and nucleus is the heat shock (HS) response, which enhances the cell’s capacity to block protein aggregation, to promote protein refolding, and to clear damaged proteins by degradation (2). In response to the buildup of thermally damaged proteins, eukaryotic cells enhance transcription of HS genes by activating Heat Shock Factor 1 (HSF1). This transcription factor is normally inhibited by HSP90 or HSP70, but upon HS, these chaperones bind instead to thermally damaged proteins, and the released HSF1 forms homotrimers, is phosphorylated, and begins transcription of HS genes (2).
Many of the induced HS proteins are molecular chaperones (e.g., Hsp70, Hsp90, and Hsp40), which associate with the misfolded proteins, prevent their aggregation, and can function together to catalyze refolding (1). A number of HS proteins are involved in degrading the thermally damaged proteins, including in eukaryotes key components of the ubiquitin (Ub) proteasome system (UPS), Ub, and certain Ub conjugating enzymes (3–5). In bacteria, certain adenosine 5′-triphosphate (ATP)-dependent proteases (e.g., Lon and ClpP) are also HS proteins (6–8). In yeast, proteasome expression is under feedback regulation by the transcription factor, Rpn4, whose activity is influenced by HSF1 (9). However, in mammalian cells, even though proteasomes catalyze 70–80% of the protein degradation (10), the transcription of genes for 26S proteasome genes does not increase during HS (10). One proposed proteasome activating protein, AIRAP, is a HS protein (11). Expression of the Ub ligase, Trim11, appears to enhance the degradation of aggregated proteins and proteasome activity (12).
The transcription of HS genes is induced by shifting cells to slightly higher temperatures; (e.g., typically for mammalian cells shifting from 37 °C to 43 °C). The induction of HS genes serves an adaptive function and leads to increased cell resistance to high, otherwise lethal, temperatures (thermotolerance) and other proteotoxic stresses (13). HS genes are also induced under other conditions that damage cell proteins (e.g., exposure to arsenite or cadmium), or cause an accumulation of misfolded proteins (e.g., incorporation of amino acid analogs or proteasome inhibition) (13–15). These transcriptional adaptations are triggered by the accumulation of misfolded proteins in the nucleus or cytosol (16). A buildup of misfolded proteins in the endoplasmic reticulum (ER) (e.g., upon treatment with tunicamycin) induces the expression of a distinct set of proteins, the unfolded protein response (UPR) or integrated stress response (ISR) (17), which stimulates the expression of several molecular chaperones in the ER (e.g., BIP and GRP90) and proteins that enhance the degradation to misfolded membrane or secretory proteins by ERAD (17).
Although these transcriptional adaptions have been extensively studied, the activation of protein breakdown upon HS has received little attention. Within 30 min of shifting mammalian cells from 37 °C to 43 °C, the overall rate of degradation of normally long-lived proteins increases by approximately twofold (18, 19). This rapid response suggests that it occurs independently of changes in gene expression and activation of HSF1 (18, 19). HS also causes a rapid build-up of K48-linked ubiquitinated proteins, which presumably indicates more rapid ubiquitination and proteasomal degradation of thermally damaged proteins (18, 20). In addition to the E2s, Ubc4 and Ubc5, two Ub ligases, Hul5/Ube3c and Rsp5/Nedd4, appear to contribute to this increased Ub conjugation upon HS (21, 22). Levels of ubiquitinated proteins also rise rapidly with other proteotoxic stresses (19) and presumably indicate more rapid destruction of damaged proteins. Another important role of these Ub conjugates is to facilitate cell recovery after return from HS to the control temperature (23).
The very rapid clearance of aggregation-prone proteins is catalyzed by the 26S proteasome (24, 25). This ATP-dependent proteolytic complex selectively hydrolyzes proteins marked by Ub chains in a multistep process (24, 25). After binding of the ubiquitinated proteins to its 19S regulatory particle, the substrate is deubiquitinated, unfolded, and translocated into the 20S core particle where it is hydrolyzed to small peptides (24). It has been generally assumed that rates of proteolysis by the UPS depend only on the rate of ubiquitination (24). However, under a variety of conditions, 26S proteasomes become more active, leading to more rapid rates of protein degradation (24, 26). Normally in cells, most 26S proteasomes (60–80%) are in a latent, inactive conformation, as shown by cryo-electron microscopy (cryo-EM) (27, 28), and even after purification, most of these particles remain catalytically inactive (24, 26). However, upon binding of a suitable ubiquitinated substrate, they become more active and show a greater capacity to hydrolyze peptides and ATP (29).
A variety of other factors have also been shown to activate the 26S proteasome and enhance overall protein degradation (30–32). Proteins that contain Ub-like (UBL) domains, including shuttling factors (e.g., Rad23) and many enzymes (e.g., Parkin), bind to the 26S complex and enhance its activity (33). Another type of activator is ZFAND5, which is induced during muscle atrophy (34, 35) and stimulates overall protein degradation in cells and the proteasome’s capacity to hydrolyze peptides, ATP, and Ub conjugates (35). Increases in these activities also occurs upon treatment with drugs or hormones that raise adenosine 3′,5′-cyclic monophosphate (cAMP) and activate protein kinase A (36) or raise cGMP and activate protein kinase G (37), which cause phosphorylation of proteasome subunits (37). During the S-M phase of the cell cycle, proteasomes become activated by a distinct protein kinase, DYRK2 (38).
Activation of proteasomes seems important in enabling cells to cope with increased amounts of ubiquitinated substrates. It is noteworthy that in denervated atrophying muscles (39), in liver or muscle on fasting (40), and under conditions where cGMP levels rise, the cellular content of ubiquitinated proteins also increases despite their more rapid degradation. We have investigated here whether 26S proteasomes may also become activated during HS and other proteotoxic conditions, when Ub conjugation and degradation by the UPS rise. We demonstrate here that upon shift of mammalian cells from 37 °C to 43 °C, the 26S proteasome’s degradative capacity increases rapidly, even when assayed at 37 °C. Therefore, we investigated (1) whether this response requires gene transcription and translation and thus the induction of HS proteins and the production of new proteasomes, (2) if the response is rapidly reversed upon return to 37 °C, and (3) if this activation is due to an association with a proteasome activating protein or a change in subunit composition. Our findings indicate that proteasome activation at 43 °C occurs through a postsynthetic modification not only with increased temperatures, but also at 37 °C in other proteotoxic conditions that cause a build-up of ubiquitinated proteins in the cytosol but not in the ER. Finally, we investigated whether proteasome activation is signaled by the increased levels of unfolded proteins, like the transcription of HS genes (2), or perhaps by the accumulation of Ub conjugates, the specific substrates of the 26S proteasome.
Results
HS Stimulates Proteasome Activity Independently of Protein Synthesis.
A variety of studies in eukaryotic and prokaryotic cells have demonstrated a rapid increase in the overall rate of protein degradation upon HS, presumably as cells selectively eliminate thermally damaged proteins (1, 18, 19). To measure degradation of the bulk of cell proteins, HeLa cells were incubated with 3H-phenylalanine for 16 h at 37 °C then washed and resuspended in chase medium containing large amounts of nonradioactive phenylalanine to prevent reincorporation of labeled amino acids. The hydrolysis of labeled proteins was then measured as described previously (41) in cells incubated at 37 °C or 43 °C. The total rate of proteolysis in the HeLa cells increased 70–80% by 1 h at 43 °C (Fig. 1A) and was linear for at least 6 h. After shift to 43 °C, the levels of ubiquitinated proteins also increased by 1 h and remained high for several hours (Fig. 1B). As expected, the increased protein degradation could be blocked almost entirely by proteasome inhibitors (SI Appendix, Fig. S1A).
Fig. 1.
HS stimulates ubiquitination, overall protein degradation, and proteasome activity in cells independently of protein synthesis. (A) The rate of degradation of long-lived proteins in HeLa cells was stimulated upon shift to 43 °C (HS). Cell proteins were labeled with 3H-phenylalanine at 37 °C for 16 h, and proteolysis was measured as described previously (41) after 1 h and 2 h incubation of cells at 37 °C or 43 °C. (B) The levels of Ub conjugates also increased in cells during incubation at 43 °C. Right Panel: relative levels of Ub conjugates. (C) Temperature shift to 43 °C did not increase the levels of proteasome subunits, although it increased the expression of HSP70. The levels of proteasome subunits and HSP70 were determined by Western blots. (D) HS does not alter assembly of 26S complexes, as shown by native PAGE. (B–D) Equal amounts (10 μg) of the lysates were loaded onto SDS/PAGE or native PAGE, and the levels of proteins were determined by Western blots. (E) HS of HeLa cells (shifting from 37 °C to 43 °C) increased the capacity of 26S proteasomes in the cell lysates to hydrolyze short peptides. At each time point, 2 μg of the lysates of each time point were used to measure the chymotrypsin-like activity at 37 °C. (F) Inhibition of protein synthesis did not reduce proteasome activation by HS. To determine whether new protein synthesis is required for proteasome activation, HeLa cells were treated with cycloheximide (10 μg/mL) during HS, and the chymotrypsin-like activity in the cell extracts was assayed at 37 °C. All values are the means of three experiments ± SD. *P < 0.05.
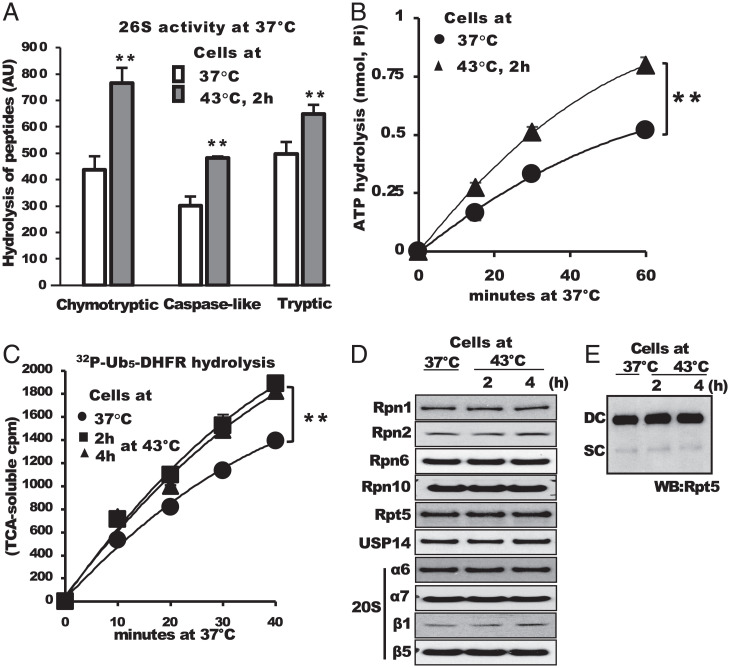
Although overall degradation increased, the content of proteasome subunits assayed by Western blots did not change after switch to 43 °C by SDS-PAGE (Fig. 1C). Furthermore, the levels of neither doubly capped (DC) and singly capped (SC) 26S nor 20S proteasomes were altered by HS for up to 3 h (Fig. 1D). We therefore tested the possibility that the specific activity of the 26S proteasomes increased when Ub-dependent degradation increased. After cells were transferred to 43 °C for 2 h or maintained at 37 °C, they were lysed and proteasomal peptidase activity in the crude extracts was assayed at 37 °C under conditions where 26S proteasomes selectively measured (42). After HS, the proteasomes’ chymotrypsin-like activity increased rapidly and by 2 h reached a maximal activation of approximately twofold (Fig. 1E). This difference in proteasome activity in the extracts was quite stable; it was also evident if cells from 37 °C to 43 °C were frozen before lysis, or if the cell extracts were frozen and thawed repeatedly. Furthermore, exposure of growing HEK293, mouse myoblasts or T-47D cells to 43 °C for 2 h stimulated proteasomal activity similarly (SI Appendix, Fig. S1 B–D). However, shifting to 39 °C did not cause a significant increase either Ub conjugates or proteasome activity (SI Appendix, Fig. S2).
At 43 °C, many HS genes are induced, including chaperones, Ub, and some Ub conjugating enzymes (5, 13). To test whether proteasome activation results from the induction of some HS proteins or requires the synthesis of some other new proteins, we blocked protein synthesis with cycloheximide upon switch to 43 °C. However, inhibition of translation did not diminish the stimulation of peptidase activity upon HS (Fig. 1F). Thus, HS stimulates the activity of preexistent 26S particles and presumably occurs through an adaptive change in proteasome composition or some postsynthetic modification of proteasome subunits.
26S Proteasomes Purified after HS Were Also More Active.
Because HS rapidly stimulated 26S peptidase activity in crude extracts without any change in the expression or assembly of SC or DC proteasomes, we affinity purified these particles to determine if their increased activity was evident after removal of regulatory proteins. HeLa cells were incubated at 37 °C or 43 °C for 2 h or 4 h before lysis, and then 26S proteasomes were purified using the UBL-domain as the affinity ligand (43). The particles from cells at 43 °C showed greater rates of peptide hydrolysis at 37 °C by the chymotrypsin-like, trypsin-like, and caspase-like activities than proteasomes from control cells (SI Appendix, Fig. S2A). The increase in activity of purified 26S was similar to that found in crude extracts (Fig. 1E). Proteasomes isolated from cells at 43 °C also hydrolyzed ATP at 37 °C faster than those from control cells (Fig. 2B). The rate of ATP hydrolysis by proteasomes is proportional to their rate of degradation of ubiquitinated proteins (44). Accordingly, the rate of hydrolysis of radiolabeled ubiquitinated dihydrofolate reductase (DHFR) at 37 °C by proteasomes from HS cells was also greater than those from cells at 37 °C (Fig. 2C). These preparations purified from cells at 43 °C appeared to contain similar levels of 20S and 19S subunits and similar amounts of DC and SC complexes (Fig. 2 D and E). Thus, HS causes an increase in the proteasome’s specific activity, which accounts for the increased activity in crude lysates (Fig. 1E).
Fig. 2.
26S proteasomes purified from cells exposed to 43 °C for 2 h showed greater activity against short peptides, ATP, and a ubiquitinated protein. (A) 26S proteasomes affinity-purified from HeLa cells following exposure to 43 °C for 2 h showed higher peptidase activities assayed at 37 °C. To assay all three proteasomal peptidase activities, the hydrolysis of the fluorogenic peptides (Suc-LLVY-amc, Z-LLE-amc, or Z-VLR-amc) by 26S proteasomes (2 nM) was measured. (B and C) 26S complexes isolated from cells exposed to HS hydrolyzed ATP and ubiquitinated DHFR faster than the controls. (D) The contents of 26S proteasomes purified from cells incubated at 43 °C for 2 or 4 h in SDS-PAGE or (E) the assembly or migration of doubly (DC) and singly (SC) capped particles in native PAGE were not altered. All values are the means of at least three independent experiments ± SD. **P < 0.01.
Mass Spectrometry and Further Purification Showed No Change in 26S Composition.
Although these particles were highly purified (by >95%) and were free of Ub conjugates, a variety of contaminating proteins are still present bound to the proteasomes that may cause the activation upon HS. To test further whether HS may cause a change in the 26S’s subunit composition or in the levels of some associated protein(s) that might cause activation, 26S proteasomes were affinity-purified from cells at 37 °C or at 43 °C for 2 h. Then, the peptides were labeled with tandem mass tags (TMT) and analyzed by mass spectrometry (MS/MS). All fourteen 20S subunits and thirteen 19S subunits were present in similar amounts in the preparations from control and HS-cells (SI Appendix, Table S1). These four preparations also contained similar low amounts of the proteasome regulatory proteins, PA28α, PA28β, PA28γ, PI31, UBE3C, and ECM29, as well as the shuttling factors Rad23b, Ubiquilin 2, and Ubiquilin 4. Although Hsp70 levels increased after HS, the amount of Hsp70 associated with the 26S did not change upon HS. Mass spectrometry did not detect AIRAP or Trim11, which were reported to be induced upon HS, and to stimulate peptidase activity (12, 45).
Because the MS analysis did not suggest any likely mechanism for the increased activity upon HS, we added a second affinity-purification step to better compare subunit composition in control and HS-activated particles. Myoblast cells expressing a Flag-tagged 20S β4 subunit (β4-Flag) were incubated at 37 °C or 43 °C for 2 h. Extracts from these cells showed a similar twofold increase in 26S proteasome activity after HS (SI Appendix, Fig. S1C). Then, 26S proteasomes were purified from each group using two-affinity steps: first, our standard UBL-method (43) and then an anti-Flag antibody. Silver staining of SDS-PAGE gels of these highly purified proteasomes from the control and HS cells were indistinguishable (Fig. 3A). Only the standard 26S subunits were evident, and no other associated proteins, and no minor components were evident in contrast to the 26S isolated only by the UBL-method (Fig. 3A and SI Appendix, Fig. S3). These highly purified preparations from the HS cells still showed a twofold higher peptidase activity at 37 °C than did those from control cells at 37 °C (Fig. 3B). Thus, the increase in activity is quite stable and does not result from some easily dissociable interacting protein or from any obvious loss of a 26S subunit.
Fig. 3.
After two affinity-purification steps, 26S proteasomes purified from beta4-Flag myoblast cells exposed to 43 °C showed higher peptidase than from ones growing at 37 °C. (A) To learn whether the stimulatory effect of HS resulted from proteasome-interacting proteins, we prepared highly pure 26S proteasomes by two-affinity steps (UBL-Flag) using β4-Flag myoblast cells. These highly purified 26S proteasomes showed the characteristic subunits of 19S and 20S complexes, and no additional proteins were observed in silver staining and Western blots. (B) 26S proteasomes purified by two-step from β4-Flag exposed to HS also show higher peptidase activity than ones from cells growing at 37 °C. All values are the means of at least three independent experiments ± SD. **P < 0.01.
Exposing Cells at 37 °C to Arsenite, but Not Tunicamycin, Induced Proteasome Activation.
These findings prompted us to test whether the activation is due to the accumulation of damaged proteins and not some other consequence of increased temperatures. Another treatment that causes proteotoxic stress, increases the expression of HS genes, and leads to an accumulation of ubiquitinated proteins is exposure to arsenite (45). Therefore, HeLa cells at 37 °C were incubated with 25 μM arsenite, and proteasome activity was assayed in cell lysates. In 2 h, this agent caused a large increase in the content of ubiquitinated proteins, composed of K48-linkages, that was even greater at 4 h (Fig. 4A). Simultaneously, proteasome activity in the extracts also increased steadily and by 4 h at 37 °C, was twice as great as in extracts of untreated cells (Fig. 4B). These findings thus resemble those upon shift to 43 °C (Fig. 1). Treatment of cells with arsenite or HS has been reported to induce the expression of a protein activator of proteasomal peptidases, AIRAP (45). However, this stimulation of 26S proteasomes by arsenite was still observed when transcription of AIRAP and other HS proteins was blocked with Actinomycin D (Fig. 4 C and D). In addition, we had shown that purified AIRAP causes only a small increase in 26S peptidase activity and does not enhance the degradation of ubiquitinated proteins (35), unlike the increase upon HS (Fig. 2).
Fig. 4.
Arsenite treatment at 37 °C stimulates proteasomal peptidase activity independently of gene transcription. (A and B) Arsenite treatment (25 μM) for 2 or 4 h at 37 °C caused an accumulation of Ub conjugates and stimulated peptide hydrolysis by proteasomes. (C and D) Inhibition of transcription does not reduce proteasome activation by arsenite, although it prevented the induction of HSP70. HeLa cells were treated with arsenite alone or with actinomycin D (50 μg/mL) for 2 h, and the chymotrypsin-like activity was measured. All values are the means of three experiments ± SD. *P < 0.05.
These findings with arsenite and 43 °C suggest that 26S activation occurs in response to multiple conditions where there is protein unfolding and a buildup of ubiquitinated proteins in the cytosol. We therefore tested if a buildup of misfolded proteins specifically in the secretory pathway, which causes ER stress and triggers the UPR, also stimulates proteasome activity. HeLa cells, C2CI2 myoblasts, or HEK293 cells (SI Appendix, Fig. S4) were treated at 37 °C with tunicamycin for 3 h to prevent protein glycosylation in the ER. However, there was no change in proteasome activity, although there was a clear activation of the UPR as shown by phosphorylation of eIF2a (17). Thus, proteasome activation results from proteotoxic stress in the cytosolic or nuclear compartments, but not in the ER.
HS Increased the Fraction of Cellular 26S Proteasomes That Are Activated.
In hippocampal neurons and algae, most 26S proteasomes are normally in inactive conformations, as shown by cryo-EM (27, 28). After affinity purification, most 26S proteasomes are in a latent form, but can be activated by addition of ATPγS or Ub conjugates, protein kinases (30), and regulatory proteins (e.g., ZFAND5) (24, 33, 46). Such an increase in activity could result from an increase in the number of activated proteasomes or from a further stimulation of the already active particles. To test these possibilities, we used two activity-based probes that react covalently with active sites of the 20S core particles and label them with a fluorescent moiety (47): MVB003, which reacts with the β1, β2, and β5 subunits, or MVB127, which modifies β5. Equal amounts of 26S proteasomes purified by the UBL-method from HeLa cells at 37 °C or 43 °C for 2 h were incubated with these probes for different times at 37 °C, the subunits resolved by SDS-PAGE, and the fluorescent beta subunits quantified. In the presence of ATP, 26S proteasomes from cells at 43 °C for 2 h showed twice as fast labeling with MVB127 or MVB003 in the initial 30 min than those from control cells (Fig. 5 A and B). Thus, HS increased the numbers of activated 26S complexes. We also tested if HS could also stimulate 26S proteasomes in the presence of ATPγS, which stimulates gate opening and substrate entry into the 20S core particle (48). Addition of ATPγS increased the fluorescence of the proteasomes from control cells ∼2.8-fold above the levels with ATP (Fig. 5A). By contrast, ATPγS increased the activity of proteasomes from the HS cells by only ∼50% (Fig. 5A). Thus, HS increases the fraction of proteasomes that are active in a similar fashion to ATPγS. It is noteworthy that the twofold increase in the number of activated particles after HS measured with these probes resembled the twofold increases in the proteasomes’ peptidase activity (Fig. 5C). We also tested whether a similar proteasome activation occurs in vivo during HS by examining the levels of active proteasomes in the HeLa cells using the cell permeable activity probe, LW124 (47). Cells at 37 °C or 43 °C were incubated with the probe and labeled proteasomes were resolved. Upon HS, although proteasome content did not change, the activity of proteasomes in the cells increased (Fig. 5D) in a similar fashion as was observed with 26S complexes purified from the HS cells (Fig. 5A).
Fig. 5.
HS increases the fraction of active cellular 26S proteasomes. To evaluate the fraction of cellular proteasomes that were in an active conformation, we incubated 26S proteasomes isolated from HS and control cells with the activity-based probes (MVB127 or MVB003) or proteasome in cells were labeled with the cell-permeable probe, LW124. (A) In the presence of ATP, 26S proteasomes purified from cells exposed to 43 °C for 2 h (open box) were labeled at 37 °C more than control proteasomes (closed box). Thus, HS increased the amounts of active proteasomes. However, in the presence of ATPγS, the amounts of active proteasomes isolated from cells incubated at both 37 °C and 43 °C for 2 h (open and closed triangles) were similar. (B) The levels of proteasome subunits used in (A) were determined by Western blots, and the same amounts were used for labeling with activity-based probes. (C) In the presence of ATPγS, the chymotrypsin-like activity by 26S proteasomes was not further increased by HS. (D) Upon HS, the fraction of active proteasomes in the HeLa cells increased. Cell extracts were analyzed by SDS PAGE and fluorescent modification of the active site of subunit β1 was quantified. Cells growing at 37 °C were transferred to 43 °C or maintained at 37 °C and with the cell permeable proteasome activity probe, LW124. All values are the means of at least three independent experiments ± SD. *P < 0.05, **P < 0.01, * or ** vs. ATP at 37 °C.
The Enhancement of Proteasome Activity Is Maintained after Cells Are Returned to 37 °C.
We next examined whether the HS-induced increase in 26S activity was rapidly reversed upon return to 37 °C and correlated tightly with the rate of cellular protein degradation. After HS for 2 h, cells were transferred rapidly from 43 °C to fresh medium at 37 °C (by changing media prewarmed at 37 °C), and overall protein degradation rates of proteolysis were measured as in Fig. 1A. HS elevated the overall rate of protein breakdown by about 80%, but after return of these cells to 37 °C, proteolysis decreased to control levels within 1 h (Fig. 6A). To test if proteasome activity also fell to control levels after HS, the cells were returned to 37 °C, lysates prepared at different times, and peptidase activity assayed at 37 °C. To our surprise, incubation at 37 °C for up to 4 h did not decrease the stimulation of peptidase activity (Fig. 6B). In fact, the proteasome’s peptidase activity remained high even after the cells were maintained at 37 °C for 16 h (Fig. 6B). During this period, the cell’s content of 26S proteasome subunits did not change (Fig. 6C). This prolonged proteasome activation was especially surprising because the levels of ubiquitinated proteins, which increased sharply at 43 °C, decreased gradually after return to 37 °C, and reached control levels by 4 h (Fig. 6C). The levels of Hsp70, a measure of the HS response, decreased to control levels by 16 h after return to 37 °C (Fig. 6C). Thus, a high level of ubiquitinated proteins or expression of HS proteins is not necessary for the sustained high proteasome activity. Although unexpected, this prolonged, perhaps irreversible, increase in activity correlates with the finding that this activation is very stable in crude extracts, and withstands freezing, thawing and multiple purification steps. These findings also indicate that 26S activation per se is insufficient to increase overall protein degradation, which probably also requires increased ubiquitination.
Fig. 6.
Unlike protein ubiquitination, degradation, and Hsp70, proteasome activation by HS (43 °C for 2 h) remains high after return of cells to 37 °C even for 16 h. (A) The rate of degradation of long-lived proteins was stimulated by HS for 2 h and then returned to the control levels after incubation at 37 °C for 1 h. (B) Proteasome activity elevated by exposure to 43 °C for 2 h (HS) remained high even after return to 37 °C. The activity of proteasomes in the lysates were all assayed at 37 °C. (C) Upon HS levels of ubiquitinated proteins increased, but returned to control levels by 4 h at 37 °C. Although proteasome content did not change upon HS or return to 37 °C, HSP70 was induced at 43 °C and decreased with time at 37 °C. HeLa cell lysates (10 μg) used in the assay (A) were resolved by SDS-PAGE, and the levels of proteins were determined by Western blots. All values are the means of three independent experiments ± SD *P < 0.05.
The Increase in Proteasome Activity Does Not Require Protein Phosphorylation.
Because the specific activity of the proteasomes increased independently of protein synthesis and without any obvious change in subunit composition, it most likely occurs through some very stable postsynthetic modification or conformational change. Multiple protein kinases can increase proteasome activity and protein degradation by phosphorylation of 26S subunits (36–38). To test whether the 26S activation at 43 °C involves phosphorylation, we initially inhibited various kinases upon exposure of HeLa cells to 43 °C. Addition of H89, which inhibits several serine/threonine kinases including PKA, PKG, AKT, CaMKII, and MSK1 (49), did not decrease the activation of peptide hydrolysis (SI Appendix, Fig. S5A). When proteasomes were purified after HS for 2 h from these cells by the UBL-method, there seemed to be a small increase in phospho-proteins modified by a serine-threonine kinase. However, the sizes of the phosphorylated proteins did not correspond to the size of any proteasome subunit (SI Appendix, Fig. S5B). We also tested if activation may be through phosphorylation by incubating purified proteasomes with protein phosphatase 1 (PP1). This treatment, which can reverse the proteasome activation by protein kinases A and G (36), markedly decreased the phosphorylation of proteins in the 26S preparations (SI Appendix, Fig. S5B), but it did not reduce the elevated peptidase activity (SI Appendix, Fig. S5C). Finally, because mitogen-activated ERK kinase (MEK) was reported to stimulate HSF1 upon HS (50), we tested if MEK or extracellular-regulated kinase (ERK) might be involved in proteasome activation at 43 °C. However, specific inhibitors of these kinases also did not block the stimulation by HS (SI Appendix, Fig. S5D).
The Activation of Proteasomes by HS or Arsenite Requires Protein Ubiquitination.
Further studies explored how the transition to 43 °C may trigger proteasome activation. Because there is a rapid, large accumulation of ubiquitinated proteins upon HS, and because the increase in 26S activity probably serves to promote their degradation (24), we tested whether this buildup of ubiquitinated proteins is required for the activation at 43 °C. To block ubiquitination, HeLa cells were incubated at 37 °C or 43 °C for 2 h in the presence or absence of TAK243, an inhibitor of the Ub activating enzymes, UBE1 and UBA6 (51), and the 26S peptidase activity in the lysates measured. As expected, exposure to TAK243 almost completely eliminated ubiquitination at 37 °C and the large increases at 43 °C. Importantly, TAK243 also completely inhibited the stimulation of proteasomes by HS (Fig. 7 A and B) but did not affect proteasome activity in control cells cultured (Fig. 7 A and B).
Fig. 7.
Inhibition of E1 blocked proteasome activation by HS, arsenite, or Ub accumulation of Ub conjugates. (A and B) Inhibition of the accumulation of Ub conjugates with the Ub activating enzyme inhibitor, TAK243 (1 μM), blocked proteasome activation by HS (43 °C, 2 h). HeLa cells were incubated at 37 °C or 43 °C for 2 h in the presence or absence of TAK243. Proteasomal peptidase activity at 37 °C and the levels of ubiquitination in the lysates were determined. (C) The proteasome stimulation by treatment with arsenite (25 μM) was completely blocked by inhibiting Ub accumulation with TAK243 (1 μM). (D) Inhibitors of deubiquitinases (b-AP15) or conjugate binding to Rpn13 (RA190) not only caused a rapid increase in the levels of Ub conjugates at 37 °C, but (E) also enhanced proteasome activity. (F–H) Treatment with TAK243 at 37 °C also blocked the stimulation of proteasomes and the rise in Ub conjugates levels by RA190 or b-AP15 at 37 °C, suggesting that accumulation of Ub conjugates at 37 °C is required for the stimulation of proteasomes. All values are the means of three independent experiments ± SD. *P < 0.05, 43 °C vs. 37 °C, b-AP15 or RA190 vs. DMSO.
The stimulation of proteasome activity by arsenite was also completely suppressed by simultaneous treatment with TAK243 (Fig. 7C). The finding that the activation of proteasomes upon exposure to 43 °C or arsenite requires ubiquitination suggests that this response is signaled by the buildup of Ub conjugates or alternatively by ubiquitination of a single proteasome regulatory protein, and not simply by the accumulation of damaged proteins, which triggers the activation of HSF1 and thus the transcription of HS genes (2, 52).
A recent study reported that upon HS, Trim11 binds to Rpn1 and stimulates proteasomal peptidase activity by preventing proteasomal binding of the inhibitory DUB, USP14 (12, 45). We examined whether Trim11 may contribute to the activation upon HS. However, levels of Trim11 did not differ in HS and control cells, and knockdown of Trim11 in HeLa cells did not reduce the stimulation of peptidase activity (SI Appendix, Fig. S6).
A Buildup of Ubiquitinated Proteins at 37 °C Can Also Enhance Proteasome Activity.
To determine if the accumulation of Ub conjugates per se can somehow signal the increase in proteasome activity, we tested whether increasing the levels of ubiquitinated proteins pharmacologically at 37 °C may also cause 26S activation. The amounts of ubiquitinated proteins in cells was increased markedly after addition of the inhibitor of deubiquitinases, b-AP15, which was initially reported to inhibit selectively USP14 and UCH37, but also affects multiple DUBs (53). A similar, large accumulation of ubiquitinated proteins in cells was obtained with RA190, which was initially reported to block Rpn13 (Fig. 7D) (54), but its specificity for this site remains unclear (55). Treating HeLa cells at 37 °C with b-AP15 or RA190 not only increased Ub conjugates (Fig. 7D), but also led to a 2- to 2.5-fold stimulation of proteasomal peptidase activity (Fig. 7E). These treatments caused a similar activation in mouse myoblasts (SI Appendix, Fig. S7 A and B) as in HeLa cells (SI Appendix, Fig. S7 C and D). By contrast, the highly selective DUB inhibitor, IU1, which blocks only USP14 (46), did not cause a buildup of Ub conjugates and did not activate the proteasome (Fig. 7 D and E). Furthermore, when the formation of ubiquitinated proteins was blocked with TAK243, the proteasome activation by RA190 or b-AP15 was also completely suppressed (Fig. 7 F–H). Although treating cells with RA190 or b-AP15 causes a similar stimulation of proteasome activity and a similar increase in ubiquitinated proteins (Fig. 7 D and E), RA190 or b-AP15 should not cause protein damage generally. Accordingly, they did not cause Hsp70 induction (SI Appendix, Fig. S7E).
Interestingly, certain treatments that raised Ub conjugate levels did not promote proteasome activity. Inhibition of p97/VCP with NMS872 (56) caused a rapid accumulation of ubiquitinated proteins in HeLa cells and myoblasts, but it did not stimulate proteasome activity (SI Appendix, Fig. S8 A–D). Blocking p97 should cause accumulation of Ub conjugates in the ER or in large protein complexes, but should actually reduce the load of substrates on the proteasome (57). Inhibition of HSP70 with JG-98 (58) and the nonspecific DUB inhibitor, PR-619, caused a buildup of Ub conjugates but failed to stimulate proteasome activity in HeLa cells (SI Appendix, Fig. S8 E–H). These observations together strongly suggest that the initial activation of proteasomes occurs in response to a build-up of a specific type of Ub conjugates or their accumulation in specific parts of the cell, such as ubiquitinated substrates on the proteasome, although clearly the maintenance of their increased activity in cells (Fig. 6) or after purification (Figs. 2 and 3) does not require high levels of ubiquitinated proteins.
Inability to Reproduce HS Activation with Purified Proteasomes or Cell Lysates.
It would clearly be quite informative if we could induce this activation using purified proteasomes or cell-free cell extracts. Unfortunately, our multiple attempts to do so have not been successful. When purified proteasomes were incubated at 43 °C for 2 h in assay buffer, peptidase activity decreased markedly, despite the presence of glycerol and ATP. When we incubated at 43 °C soluble lysates of cells grown at 37 °C, no increase in proteasomal activity was observed. In addition, we examined whether a soluble factor in the HS cells may activate the proteasomes. Cell lysates were prepared after growth at 43 °C for 2 h, and proteasomes were immunodepleted from these lysates with a resin-bound anti-beta5 antibody (SI Appendix, Fig. S9A). Proteasomes from cells at 37 °C were then added to the proteasome-depleted lysates and peptide hydrolysis assayed. The lysates from HS cells did not enhance activity (SI Appendix, Fig. S9B). One other possible approach to test these findings would be addition of ubiquitinated proteins from HS or normal cells to control proteasomes, but such experiments would not be interpretable since addition of a Ub chain or ubiquitinated substrate activates the proteasome (29, 59). These attempts suggest that proteasome activation during HS requires intact cells, although there are many possible reasons why such reconstitution experiments may not be successful.
Discussion
The main physiological consequence of the HS response is that it enables cells to withstand higher, otherwise lethal temperatures as well as other toxic conditions that cause widespread damage to cell proteins (e.g., oxidative stress) (1). This increased thermotolerance is generally attributed to the induction of molecular chaperones that prevent the aggregation and promote the refolding of the thermally damaged proteins (1). However, the ability of certain HS proteins to enhance the degradation of such proteins by the UPS probably also contributes to their increased stress resistance. The present study describes a previously unknown adaptive response to HS, arsenite exposure, and presumably other types of proteotoxic stress, the enhancement of the proteasome’s ability to degrade its physiological substrates, ubiquitinated proteins and ATP, as well as small fluorogenic peptides.
Because the rate of degradation of Ub conjugates by the 26S proteasome is proportional to its rate of ATP hydrolysis (44), this increase in ATPase activity is probably the critical adaptation driving the enhanced degradation of ubiquitinated substrates. The more rapid binding and hydrolysis of ATP can also account for the increased ability to hydrolyze small fluorogenic peptides with HS, since ATP binding triggers gate opening and allows substrate entry into the core 20S complex (44, 48). Although it seems very likely that the HS-induced activation of proteasomes contributes to the acquisition of thermotolerance, it will not be feasible to test rigorously the physiological significance of this increased activity until we clarify its molecular basis and can prevent this increase by mutation or selective inhibition.
The accumulation of Ub conjugates upon HS or treatment with arsenite implies that under these conditions, proteasomal degradation is the rate-limiting step in the UPS, even though it is probably not rate-limiting in nonstressed cells. Following shift to 43 °C, proteasome’s specific activity increased after the rapid rise in Ub conjugates and protein breakdown, and prior to the peak induction of Hsp70 (Fig. 6). By enhancing the cell’s degradative capacity, proteasome activation can help it withstand the buildup of aggregation-prone proteins during HS. However, this adaptation to proteotoxic conditions does not increase the levels of 26S subunits or of DC (30S) or SC (26S) particles (Fig. 2) as occurs upon proteasome inhibition and in certain proteotoxic diseases (60).
None of the 26S subunits or cofactors are products of HS genes. On the contrary, their transcription actually decreases after shift to 43 °C (10). Thus, proteasome activation upon HS differs in several important ways from the classic HS response mediated by HSF-1, especially in that this adaptation does not require gene transcription or translation (Figs. 1F and 4C). It had been asserted that 26S proteasome activity increases only through greater formation of 26S or 30S complexes (61). However, the adaptive increases in 26S activity upon HS, and conditions that raise cGMP (37) or cAMP (e.g., fasting) (37) all occur through activation of preexistent particles without any increase in their formation.
Another surprising feature of the HS-induced doubling of 26S activity is that it is maintained long after the return of cells to 37 °C (i.e., for at least 16 h), in contrast to overall cellular protein degradation, which returned to control levels within 30 min and to the content of ubiquitinated proteins, which fell to control levels in 2–3 h (Fig. 6). Thus, proteasomal specific activity can increase significantly without by itself causing an enhancement of cellular proteolysis. This prolonged increase in 26S activity after return to 37 °C does not appear to be deleterious to the cells. In fact, this response may even leave the cell better equipped to handle prolonged or more severe increases in temperatures, which are probably commonly encountered in natural environments, unlike the abrupt temperature reversal examined in these experiments.
Recent cryo-EM studies (27, 28) have clearly indicated that under normal conditions, the majority of 26S proteasomes in cells are in an inactive conformation, which is maintained in cell lysates. However, the binding of a ubiquitinated protein directly or in a UBL-domain containing the shuttling factor (e.g., Rad23B) to latent proteasomes leads to an activation of peptide hydrolysis, which is rapidly reversed when the substrate is degraded (33). Our experiments using active site-probes which allow only a single round activity on purified proteasomes and on intact cells indicate that HS leads to a greater fraction of the particles in an activated state. Thus, HS must have caused activation of latent 26S particles (Fig. 5) rather than to a further increase in the activity of the previously functioning particles. However, in contrast to the activation by Ub conjugates or shuttling factors, the increased activity upon HS is exceptionally stable and is evident even after two affinity-purification steps, which remove ubiquitinated substrates and visible contaminating proteins. This activation also withstands freezing and thawing of crude cell lysates. These unusual properties and its maintenance of the increased activity following return of cells to 37 °C for at least 16 h suggest that this activation may is irreversible adaptation in vivo.
Although the HS-induced activation does not require new protein synthesis, it may possibly occur through binding of a preexistent stimulatory protein or perhaps through the loss or inactivation of an inhibitory 26S component. However, no consistent alteration in proteasome bands was seen on SDS-PAGE, even in our preparations obtained after two affinity purification steps (Fig. 3). Thus, the activation most likely involves a stable postsynthetic modification of a subunit. Several protein kinases can directly activate the proteasome through phosphorylation (8, 31), but treating cells with general kinase inhibitors and treating the proteasomes from HS cells with phosphatases did not reduce their increased peptidase activity (SI Appendix, Fig. S5). Numerous postsynthetic modifications of proteasomes have been reported (8), but thus far our attempts to use MS have failed to identify a change in subunit composition or a covalent modification of a subunit during HS (SI Appendix, Table S1). Neither AIRAP nor Trim11, previously reported to be proteasome-activating proteins induced upon HS (12, 45), were present in our preparation.
Another possibility is that the proteasome becomes activated during HS through a structural change without any change in its chemical composition. The 26S proteasome exists in a number of distinct conformations, including a sequence of conformations which it assumes during Ub conjugate degradation (25, 62, 63). Perhaps HS, arsenite, or the enhanced degradation of substrates with time induces a novel, stable conformational state, which increases the proteasome’s basal activity without any chemical modification of its components. Our recent studies of proteasome activation by ZFAND5 have revealed unique conformations of the 19S regulatory particle, which are associated with more efficient degradation of ubiquitinated proteins. Hopefully, further MS and cryo-EM analysis of the HS proteasomes will clarify the structural basis of their enhanced activity.
An important clue to the cellular mechanisms signaling proteasome activation upon HS was the finding that a similar stimulation of 26S activity occurred at 37 °C upon treatment with arsenite or with two inhibitors of proteasomal deubiquitinating enzymes, RA190 and b-AP15, all of which increased the cellular levels of Ub conjugates (Fig. 7). We therefore tested with the UBE1 inhibitor whether the increase in protein ubiquitination not only generates more proteasome substrates but may also trigger its activation. These experiments demonstrated that proteasome activation upon HS and with arsenite, RA190, and b-AP15 at 37 °C all required protein ubiquitination (Fig. 7), even though the maintenance of this activated state at 43 °C, after return to 37 °C does not require increased levels of Ub conjugates (Figs. 3 and 6). However, the increased ubiquitination also is important in the recovery of cellular functions after return to 37 °C (e.g., in the disassembly of aggregates formed during HS) (23).
Since the E1 inhibitor cannot prevent the thermal damage to cell proteins, the accumulation of misfolded proteins in these cells per se cannot be the cause of proteasome activation. Instead, this initial response coincides with and requires the build-up of Ub conjugates in the cells. A role for the rise in Ub conjugates in signaling 26S activation is an attractive hypothesis, because the increase in proteolytic activity should assist the cells in clearing these ubiquitinated substrates. An alternative possible explanation for this requirement for Ub conjugation for the initiation, but not the maintenance of proteasome activation, is that the increased activity occurs through irreversible ubiquitination of a proteasome subunit. Ubiquitination of many 26S subunits in normal cells has been observed (8, 30), especially of Rpn10 and Rpn13. However, these modifications are readily reversed in vivo (64–66), and they cause an inhibition of the degradation of ubiquitinated proteins, unlike the activation upon HS. Despite appreciable effort, we have not yet detected any additional subunit ubiquitination upon HS by Western blot.
A regulatory role for the build-up of Ub conjugates in signaling proteasome activation can nicely account for the findings upon HS and with arsenite and certain DUB inhibitors at 37 °C, as does the prevention of 26S activation by blocking Ub conjugation under these conditions. It is also noteworthy that inhibition of the p97/VCP ATPase complex caused an accumulation of Ub conjugates, although it did not cause proteasome activation. Under these conditions, the ubiquitinated proteins do not build-up on the proteasome (57) suggesting that 26S activation may be signaled by the excessive substrate load on the proteasomes, which would necessitate enhanced degradative activity.
Together, these experiments demonstrate a previously unknown very stable proteasome activation that seems to arise by a postsynthetic modification and to be signaled by the accumulation of ubiquitinated proteins, which occurs in proteotoxic conditions. The resulting increase in proteasome activity should help protect cells from the toxic accumulation of damaged proteins, but it remains unclear how this adaptative response develops when the levels of ubiquitinated substrates exceed the cell’s degradative capacity.
Materials and Methods
Conditions for Cell Growth and Induction of HS.
All cells were cultured in DMEM media with 10% FBS and 1% penicillin-streptomycin in a humidified incubator at 37 °C and 5% CO2. To induce HS, cells grown at 37 °C were resuspended in media prewarmed to 43 °C. To examine the effect of returning cells to 37 °C after HS (2 h at 43 °C), cells were transferred to prewarmed media to 37 °C.
Preparation of Cell Extracts and Assays.
Cells were harvested in cold PBS and lysed by sonication in the assay buffer (25 mM Hepes, 5 mM MgCl2, 1 mM DTT, 1 mM ATP, 150 mM NaCl, and 10% glycerol). The crude lysates were centrifuged at 13,000 × g, 4 °C, for 15 min, and the supernatants were used in subsequent assays or for 26S proteasome purification. All assays of hydrolysis of peptides, ATP, and ubiquitinated proteins were performed at 37 °C, as described previously.
Chemicals.
The following stock solutions were prepared: TAK243 (10 mM, kindly provided by Takeda Pharmaceuticals), RA190 (10 mM, Millipore Sigma), b-AP15 (20 mM), NMS873 (10 mM, Millipore Sigma), PR619 (100 mM, Selleck Chemicals), sodium arsenite (50 mM, Millipore Sigma), actinomycin D (500 μM, CalBiochem), and cycloheximide (10 mg/mL, Millipore Sigma).
Knockdown of Trim11.
HeLa cells were transiently transfected with siTrim11 (esiRNA targeting human TRIM11, Millipore Sigma) using Viafect (Promega). After 48 h, cells were exposed to 37 °C or 43 °C for 2 h and harvested. Knockdown of Trim11 was examined with anti-Trim11 antibody (Proteintech).
Purification of 26S Proteasomes.
Cells at 37 °C or 43 °C were harvested, and lysates were prepared. 26S proteasomes were purified by the GST-UBL method as previously described (43). For the two-step (UBL and Flag) purification (Fig. 3), β4-Flag cells were used. The lysates of β4-Flag cells (35) were incubated with GST-UBL bound to glutathione Sepharose 4b (GE Healthcare Life Sciences) for 2 h at 4 °C with gentle rotation. After washing the resin with lysis buffer extensively, proteasomes bound to GST-UBL were eluted with His-UIM. The eluates were incubated with Flag agarose (Millipore Sigma) for 2 h at 4 °C with gentle rotation. After extensive washing, the resin-bound 26S proteasomes were eluted with Flag peptides, which were then removed by dialysis.
Measurement of Protein Degradation.
Briefly, cells were incubated with 3H-Phe (4 μCi/mL) in standard culture medium for 16 h to label long-lived cell proteins and then switched to a chase medium containing 2 mM nonradioactive Phe to prevent reincorporation of released radioactive amino acids for 2 h. After fresh chase medium was added, 10% (vol/vol) of medium were collected at different times. Proteins were precipitated with trichloroacetic acid (TCA, final 10% [vol/vol]), and the amounts of acid-soluble radioactivity were measured and expressed as percentage of the total radioactivity initially incorporated into cell proteins (41).
Labeling 26S Proteasomes with Activity-Based Probes.
26S proteasomes were labeled with the activity-based probes, MVB003, MVB127, or LW124, as described (33, 47). Briefly, 26S proteasomes (2 nM) isolated from cells at 37 °C or 43 °C for 2 h were incubated with either probe (500 nM) in the presence of ATP (1 mM) or ATPγS (0.1 mM) at 37 °C. After proteasomes were resolved by SDS-PAGE, the gels were scanned with an AI600 RGB camera with λex 520 nm and λem 593 nm. Quantification of the labeled bands was performed with the IQTL software (GE Healthcare Life Sciences).
Statistical Analysis.
All analyses were performed using the unpaired Student’s t tests, and significant differences are marked by asterisks (*P < 0.05, **P < 0.01).
Supplementary Material
Acknowledgments
This project was supported by grants to A.L.G. from NIH-National Institute of General Medical Sciences (R01 GM51923) and Cure Alzheimer’s Fund. We thank Dan Finley and Galen Collins for their helpful comments and Amelia Gould for valuable assistance in preparing this manuscript.
Footnotes
Reviewers: G.D., The University of Texas; and M.G., Technion - Israel Institute of Technology.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2122482119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Klaips C. L., Jayaraj G. G., Hartl F. U., Pathways of cellular proteostasis in aging and disease. J. Cell Biol. 217, 51–63 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sala A. J., Bott L. C., Morimoto R. I., Shaping proteostasis at the cellular, tissue, and organismal level. J. Cell Biol. 216, 1231–1241 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bond U., Schlesinger M. J., Ubiquitin is a heat shock protein in chicken embryo fibroblasts. Mol. Cell. Biol. 5, 949–956 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fornace A. J. Jr., Alamo I. Jr., Hollander M. C., Lamoreaux E., Ubiquitin mRNA is a major stress-induced transcript in mammalian cells. Nucleic Acids Res. 17, 1215–1230 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seufert W., Jentsch S., Ubiquitin-conjugating enzymes UBC4 and UBC5 mediate selective degradation of short-lived and abnormal proteins. EMBO J. 9, 543–550 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goff S. A., Casson L. P., Goldberg A. L., Heat shock regulatory gene htpR influences rates of protein degradation and expression of the lon gene in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 81, 6647–6651 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitagawa M., Wada C., Yoshioka S., Yura T., Expression of ClpB, an analog of the ATP-dependent protease regulatory subunit in Escherichia coli, is controlled by a heat shock sigma factor (sigma 32). J. Bacteriol. 173, 4247–4253 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kors S., Geijtenbeek K., Reits E., Schipper-Krom S., Regulation of proteasome activity by (post-)transcriptional mechanisms. Front. Mol. Biosci. 6, 48 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Work J. J., Brandman O., Adaptability of the ubiquitin-proteasome system to proteolytic and folding stressors. J. Cell Biol. 220, e201912041 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sha Z., Goldberg A. L., Proteasome-mediated processing of Nrf1 is essential for coordinate induction of all proteasome subunits and p97. Curr. Biol. 24, 1573–1583 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossi A., et al. , AIRAP, a new human heat shock gene regulated by heat shock factor 1. J. Biol. Chem. 285, 13607–13615 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L., Zhu G., Johns E. M., Yang X., TRIM11 activates the proteasome and promotes overall protein degradation by regulating USP14. Nat. Commun. 9, 1223 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Labbadia J., Morimoto R. I., Rethinking HSF1 in stress, development, and organismal health. Trends Cell Biol. 27, 895–905 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ananthan J., Goldberg A. L., Voellmy R., Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science 232, 522–524 (1986). [DOI] [PubMed] [Google Scholar]
- 15.Goff S. A., Goldberg A. L., Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell 41, 587–595 (1985). [DOI] [PubMed] [Google Scholar]
- 16.Zou J., Guo Y., Guettouche T., Smith D. F., Voellmy R., Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94, 471–480 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Costa-Mattioli M., Walter P., The integrated stress response: From mechanism to disease. Science 368, eaat5314 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parag H. A., Raboy B., Kulka R. G., Effect of heat shock on protein degradation in mammalian cells: Involvement of the ubiquitin system. EMBO J. 6, 55–61 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sha Z., Goldberg A. L., Multiple myeloma cells are exceptionally sensitive to heat shock, which overwhelms their proteostasis network and induces apoptosis. Proc. Natl. Acad. Sci. U.S.A. 117, 21588–21597 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medicherla B., Goldberg A. L., Heat shock and oxygen radicals stimulate ubiquitin-dependent degradation mainly of newly synthesized proteins. J. Cell Biol. 182, 663–673 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang N. N., Ng A. H., Measday V., Mayor T., Hul5 HECT ubiquitin ligase plays a major role in the ubiquitylation and turnover of cytosolic misfolded proteins. Nat. Cell Biol. 13, 1344–1352 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang N. N., et al. , Rsp5/Nedd4 is the main ubiquitin ligase that targets cytosolic misfolded proteins following heat stress. Nat. Cell Biol. 16, 1227–1237 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maxwell B. A., et al. , Ubiquitination is essential for recovery of cellular activities after heat shock. Science 372, eabc3593 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins G. A., Goldberg A. L., The logic of the 26S proteasome. Cell 169, 792–806 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finley D., Prado M. A., The proteasome and its network: Engineering for adaptability. Cold Spring Harb. Perspect. Biol. 12, a033985 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H. T., Goldberg A. L., UBL domain of Usp14 and other proteins stimulates proteasome activities and protein degradation in cells. Proc. Natl. Acad. Sci. U.S.A. 115, E11642–E11650 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asano S., et al. , Proteasomes. A molecular census of 26S proteasomes in intact neurons. Science 347, 439–442 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Albert S., et al. , Direct visualization of degradation microcompartments at the ER membrane. Proc. Natl. Acad. Sci. U.S.A. 117, 1069–1080 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peth A., Kukushkin N., Bossé M., Goldberg A. L., Ubiquitinated proteins activate the proteasomal ATPases by binding to Usp14 or Uch37 homologs. J. Biol. Chem. 288, 7781–7790 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo X., Huang X., Chen M. J., Reversible phosphorylation of the 26S proteasome. Protein Cell 8, 255–272 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VerPlank J. J. S., Goldberg A. L., Regulating protein breakdown through proteasome phosphorylation. Biochem. J. 474, 3355–3371 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg A. L., Kim H. T., Lee D., Collins G. A., Mechanisms that activate 26S proteasomes and enhance protein degradation. Biomolecules 11, 779 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins G. A., Goldberg A. L., Proteins containing ubiquitin-like (Ubl) domains not only bind to 26S proteasomes but also induce their activation. Proc. Natl. Acad. Sci. U.S.A. 117, 4664–4674 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hishiya A., et al. , A novel ubiquitin-binding protein ZNF216 functioning in muscle atrophy. EMBO J. 25, 554–564 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee D., Takayama S., Goldberg A. L., ZFAND5/ZNF216 is an activator of the 26S proteasome that stimulates overall protein degradation. Proc. Natl. Acad. Sci. U.S.A. 115, E9550–E9559 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lokireddy S., Kukushkin N. V., Goldberg A. L., cAMP-induced phosphorylation of 26S proteasomes on Rpn6/PSMD11 enhances their activity and the degradation of misfolded proteins. Proc. Natl. Acad. Sci. U.S.A. 112, E7176–E7185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VerPlank J. J. S., Tyrkalska S. D., Fleming A., Rubinsztein D. C., Goldberg A. L., cGMP via PKG activates 26S proteasomes and enhances degradation of proteins, including ones that cause neurodegenerative diseases. Proc. Natl. Acad. Sci. U.S.A. 117, 14220–14230 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo X., et al. , Site-specific proteasome phosphorylation controls cell proliferation and tumorigenesis. Nat. Cell Biol. 18, 202–212 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen S., Nathan J. A., Goldberg A. L., Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 14, 58–74 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Wing S. S., Haas A. L., Goldberg A. L., Increase in ubiquitin-protein conjugates concomitant with the increase in proteolysis in rat skeletal muscle during starvation and atrophy denervation. Biochem. J. 307, 639–645 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sha Z., Zhao J., Goldberg A. L., Measuring the overall rate of protein breakdown in cells and the contributions of the ubiquitin-proteasome and autophagy-lysosomal pathways. Methods Mol. Biol. 1844, 261–276 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kisselev A. F., Goldberg A. L., Monitoring activity and inhibition of 26S proteasomes with fluorogenic peptide substrates. Methods Enzymol. 398, 364–378 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Besche H. C., Goldberg A. L., Affinity purification of mammalian 26S proteasomes using an ubiquitin-like domain. Methods Mol. Biol. 832, 423–432 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Peth A., Nathan J. A., Goldberg A. L., The ATP costs and time required to degrade ubiquitinated proteins by the 26 S proteasome. J. Biol. Chem. 288, 29215–29222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanhill A., et al. , An arsenite-inducible 19S regulatory particle-associated protein adapts proteasomes to proteotoxicity. Mol. Cell 23, 875–885 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Lee B. H., et al. , Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 467, 179–184 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li N., et al. , Relative quantification of proteasome activity by activity-based protein profiling and LC-MS/MS. Nat. Protoc. 8, 1155–1168 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Smith D. M., et al. , ATP binding to PAN or the 26S ATPases causes association with the 20S proteasome, gate opening, and translocation of unfolded proteins. Mol. Cell 20, 687–698 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Davies S. P., Reddy H., Caivano M., Cohen P., Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang Z., et al. , MEK guards proteome stability and inhibits tumor-suppressive amyloidogenesis via HSF1. Cell 160, 729–744 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hyer M. L., et al. , A small-molecule inhibitor of the ubiquitin activating enzyme for cancer treatment. Nat. Med. 24, 186–193 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Rossi A., et al. , The proteasome inhibitor bortezomib is a potent inducer of zinc finger AN1-type domain 2a gene expression: Role of heat shock factor 1 (HSF1)-heat shock factor 2 (HSF2) heterocomplexes. J. Biol. Chem. 289, 12705–12715 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D’Arcy P., et al. , Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nat. Med. 17, 1636–1640 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Anchoori R. K., et al. , A bis-benzylidine piperidone targeting proteasome ubiquitin receptor RPN13/ADRM1 as a therapy for cancer. Cancer Cell 24, 791–805 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dickson P., et al. , Physical and functional analysis of the putative Rpn13 inhibitor RA190. Cell Chem. Biol. 27, 1371–1382.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magnaghi P., et al. , Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nat. Chem. Biol. 9, 548–556 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Xue L., et al. , Valosin-containing protein (VCP)-adaptor interactions are exceptionally dynamic and subject to differential modulation by a VCP inhibitor. Mol. Cell. Proteomics 15, 2970–2986 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X., et al. , Analogs of the allosteric heat shock protein 70 (Hsp70) inhibitor, MKT-077, as anti-cancer agents. ACS Med. Chem. Lett. 4, 1042–1047 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peth A., Besche H. C., Goldberg A. L., Ubiquitinated proteins activate the proteasome by binding to Usp14/Ubp6, which causes 20S gate opening. Mol. Cell 36, 794–804 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.VerPlank J. J. S., Lokireddy S., Feltri M. L., Goldberg A. L., Wrabetz L., Impairment of protein degradation and proteasome function in hereditary neuropathies. Glia 66, 379–395 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rousseau A., Bertolotti A., An evolutionarily conserved pathway controls proteasome homeostasis. Nature 536, 184–189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de la Peña A. H., Goodall E. A., Gates S. N., Lander G. C., Martin A., Substrate-engaged 26S proteasome structures reveal mechanisms for ATP-hydrolysis-driven translocation. Science 362, eaav0725 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong Y., et al. , Cryo-EM structures and dynamics of substrate-engaged human 26S proteasome. Nature 565, 49–55 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Besche H. C., et al. , Autoubiquitination of the 26S proteasome on Rpn13 regulates breakdown of ubiquitin conjugates. EMBO J. 33, 1159–1176 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Isasa M., et al. , Monoubiquitination of RPN10 regulates substrate recruitment to the proteasome. Mol. Cell 38, 733–745 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacobson A. D., MacFadden A., Wu Z., Peng J., Liu C. W., Autoregulation of the 26S proteasome by in situ ubiquitination. Mol. Biol. Cell 25, 1824–1835 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.