Abstract
Breast cancer is a heterogeneous disease both in its clinical and radiological manifestations and response to treatment. This is largely due to the polymorphism of the histological types as well as diversified molecular profiles of individual breast cancer types. Progress in the understanding of the biology of breast cancer was made with the introduction of immunohistochemical research into the common practice. On this basis, four main breast cancer subtypes were distinguished: luminal A, luminal B, HER2 positive (human epidermal growth factor receptor-2 positive), and triple negative cancer. The classification of a tumour to an appropriate subtype allows for the optimisation of treatment (surgery or pre-operative chemotherapy). In this study, the authors present different patterns of breast cancer subtypes in ultrasound examination and differences in their treatment, with particular emphasis on aggressive breast cancer subtypes, such as triple negative or HER2 positive. They can, unlike the luminal subtypes, create diagnostic problems. Based on multifactorial analysis of the ultrasound image, with the assessment of lesion margins, orientation, shape, echogenicity, vascularity, the presence of calcifications or assessment by sonoelastography, it is possible to initially differentiate individual subtypes.
Keywords: ultrasound, breast cancer, immunohistochemical subtypes, BIRADS lexicon
Introduction
Breast cancer (BC) is a heterogeneous disease of significant social importance. For many years, it has been the most common malignant tumour in women in Poland and in many countries throughout the world. The incidence of BC in women over 30 is systematically increasing. In Poland in 2018, according to the National Cancer Registry, there were 18,869 cases of BC in women and 154 in men. At the same time, a total of 6,895 and 75 deaths were recorded for women and men, respectively(1). The neoplasm is characterised by a varied clinical course and a wide spectrum of morphological images in radiological studies. In recent years, there have been many developments in terms of the knowledge base, diagnostic methods, and new therapeutic options for breast cancer. Currently, BC treatment consists of a comprehensive approach to the diagnostic and therapeutic processes. Three main imaging methods are used in the diagnosis of BC: ultrasonography (US), mammography (MMG), and magnetic resonance imaging (MRI)(2). Each of these methods plays a special role in diagnostics. Ultrasonography is used mainly in young women and those with glandular or mixed breast structure. Contemporary breast US means not only mapping the morphology of focal lesions and their surroundings but also involves a combination of additional techniques such as sonoelastography (SE) and colour Doppler (CD). These techniques allow an increase in the accuracy of imaging and qualification of patients for biopsy or observation(3). Early diagnosis of BC and knowledge of its oncological characteristics on the basis of biopsy findings facilitate the choice of optimal therapy, including surgical treatment which, in selected cases, is preceded by neoadjuvant chemotherapy (NAC). Treatment of early BC is complex and includes a combination of surgical methods (breast conserving therapy, BCT), radiotherapy, systemic therapies (chemotherapy, hormone therapy, molecularly targeted therapies), and adjunctive therapy in various sequences(2). The use of predictive biomarkers such as the histological type of BC (invasive or preinvasive forms), the expression of ER/PgR (estrogen receptor (ER) and progesterone receptor), Ki67 (proliferation index) and HER2 (human epidermal growth factor receptor 2), genomic signatures, if available, stage of the primary tumour, condition of the axillary lymph nodes, and patient’s preferences, affect the choice and sequence of therapies. These methods, especially systemic treatment, have undergone significant changes over the years. NAC, first introduced in 1970, has been used in locally advanced breast cancer (LABC) and inflammatory BC to reduce tumour size and improve the radical nature of surgical treatment, including BCT.
Currently, decisions regarding neoadjuvant treatment should be based on the anticipated sensitivity to particular types of treatment, the benefits of their use, and the individual risk of relapse. Additionally, short-term and longterm toxicity, the biological age of the patients, and their general health and comorbidities should be considered. In the current recommendations of all scientific oncological societies, neoadjuvant chemotherapy is recommended not only in locoregional advanced breast cancer but also in the early stages of the following subtypes: triple-negative breast cancer (TNBC) and in combination with molecularly targeted treatment in the subtypes with the presence of HER2 receptors (luminal B HER2 positive and HER2 positive non-luminal subtypes)(3). NAC can also be used in cases of HER2 negative luminal B cancer with low expression of hormone receptors, high grade (G3), and in young individuals (≤35 years of age), stage II or III(2).
The aim of this study is to present the differential patterns of various subtypes of breast cancer in ultrasound examination based on a review of the literature.
Individual BC subtypes
Triple negative breast cancer (TNBC)
Introduction
A unique challenge for both radiologists and oncologists is presented by TNBC and HER2+ subtypes. TNBC represents 10–15% of the four immunohistochemical subtypes of BC and frequently occurs in young women who are not yet included in the screening programme. In comparison with the other forms, they are characterised by an aggressive clinical course and heterogeneous response to treatment, with a significantly more frequent occurrence of high malignancy grade (G3) and more frequent mutation of the BRCA1/2 genes(2). It is believed that approximately 5% of all breast cancer deaths each year are caused by TNBC. The median overall survival (OS) is currently 10.2 months, with a 5-year survival rate of 65% for locoregional tumours and 11% for disseminated patients(4,5). TNBC is a very heterogeneous group, among which, based on the analysis of gene expression profiles of nearly 600 TNBC cancers, six subtypes of TNBC have been identified(6,7). Systemic treatment can be waived only in very early pT1a tumours or in cases involving special histological subtypes such as secretory carcinomas, apocrine adenocarcinomas or cystadenocarcinoma.
For the treatment of TNBC, NAC has been the only standard preoperative systemic treatment to date(2,8). The reason for heterogeneous response to treatment may be the differential gene expression profile (including BRCA1) in individual TNBC subtypes and in the surrounding stroma. Thanks to the applied neoadjuvant treatment regimens, an important element of further management is obtaining a high pathological complete response (pCR), amounting to approximately 50% of patients, according to the CALGB 40603 and GeparSixto studies(8, 9, 10, 11). In the group of patients with the BRCA1/2 mutations, no improvement in the efficacy of chemotherapy regimens with platinum derivatives was shown(9,10). At the same time, in the remaining patients who did not carry the mutation of the above-mentioned genes, the addition of platinum derivatives to NAC increased the percentage of pCR. The results of research indicate that pCR is a predictor of the risk of recurrence and death in this group of patients. In patients who do not achieve pCR, the administration of 6–8 cycles of capecitabine after surgery prolongs OS. It should be added, though, that the study was conducted in Asian patients(11).
Despite the therapies used, there is no targeted treatment for the subgroup of TNBC patients, as opposed to the HER2+ group. The reason for varied responses to treatment may be the altered gene expression profiles in different TNBC subtypes and in the surrounding stroma. These differences may be reflected by microRNAs (miRNAs) released outside the tumour microenvironment(12).
TNBC – features on ultrasound examination
In imaging studies (US and MMG), this subtype often mimics benign lesions (no acoustic shadow effect behind the lesion and sparse vascularisation). In studies on US imaging features of TNBC subtypes, a characteristic pattern of features has been described. Yang et al. analysed 66 cases of TNBC and showed that microlobular margins occured significantly more often in ER+ and HER2+ subtypes(13). This characterisation of the margins, according to the authors of the study, helps to avoid false negative cases. Another feature that characterised this subtype of BC was the enhancement effect behind the lesion, indicating a high cellularity of the lesions and high degree of histological malignancy. This was confirmed in another study, where the authors analysed 50 TNBC tumours, confirming the presence of microlobular margins and enhancement behind the lesion, and additionally noted their low echogenicity and lack of spicules(14). The authors compared the deformability in SE of TNBC in relation to the other group of non-TNBC, and found no significant differences between the groups based on the 5-grade Tsukuba scale.
On the other hand, Li et al., analysing the material of 543 breast cancers (104 TNBC vs. 439 non-TNBC), showed that, statistically speaking, TNBC with higher histological malignancy (G3-grade 3) often present with benign lesions features. Therefore, they may be disqualified from biopsy, especially in young patients(15). Authors of this study demonstrated the presence of the following features in TNBC: regular shape (OR = 1.8), no spiculated/angular margins (OR = 2.09), acoustic enhancement behind the lesion (2.49), and lack of calcifications (OR = 1.95), which are usually associated with benign lesions. Similarly, in a study published by Dogan et al. calcifications in TNBC were present in only 4.5% of cases(16), whereas well-demarcated margins were identified in 32% of lesions. On the other hand, Ko et al. demonstrated the latter feature in 57% of the TNBC cases studied(17). In such cases, SE seems to be a helpful tool in breast ultrasound diagnostics. Yeo et al. compared fibroadenomas (FA) with TNBC by ultrasound, including only lesions less than 2 cm in length(18). SE proved to be the technique most accurately differentiating these two subtypes with the value of the area under the ROC curve (AUC = 0.869) compared to B-mode (AUC = 0.65) and CD (AUC = 0.576). The authors emphasised that with SE and CD, only two small (6 mm, 8 mm) TNBC cases were evaluated as false negative cases. In contrast, TNBC compared to other forms of BC, are characterised by similarly decreased deformability in the tumour as well as in the surrounding tissue. An example of a deformable TNBC with discrete non-circumscribed margins is shown in Fig. 1.
Fig. 1.
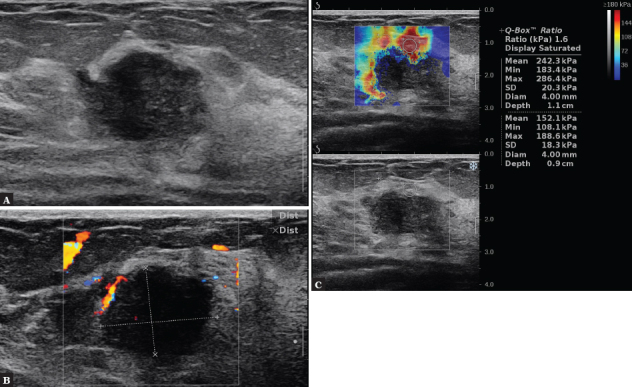
A 35-year-old female patient. In the B-mode test, an oval, solid, hypoechoic lesion with non-circumscribed margins is visible, without an acoustic shadow behind the lesion (A), with a visible broad marginal vessel in CD (B). On SWE, the lesion is hard, Emax = 286 kPa, BIRADS 4c (C). Result of histopathology: TNBC subtype
Zhang L et al., in a study of 1000 BC, described this subtype (n = 125) using two independent patterns on US. One of them was characterised by an oval shape, lobular margins, and a lack of visible vessels. This pattern may mimic lesions that are classified as most likely benign BIRADS 3, and appear as fibroadenoma. In the second model, they were characterised by irregular shape, lobular margins, and lack of calcifications and vessels(19). The presence of irregular shape allows this subgroup to be classified as BIRADS 4, and they will therefore undergo biopsy.
Human epidermal growth factor receptor breast cancer (HER2)
Introduction
Overexpression of HER2 occurs in 15–25% of invasive BC and is associated with a poor prognosis. On the other hand, HER2+ cancers are characterised by a favourable response to targeted therapies(20). HER2 overexpression is associated with increased cell proliferation, cell survival, mobility and invasiveness, and neoangiogenesis due to increased production of vascular endothelial growth factor(21). For this reason, HER2+ carcinomas have a different clinical course and show different features on imaging studies. Molecularly targeted therapy in combination with chemotherapy in patients with HER2 overexpression/ amplification in tumours greater than 1 cm reduces the risk of recurrence and mortality by approximately half, compared to chemotherapy alone, and translates into a 9% gain in 10-year OS(22, 23, 24).
In view of cardiotoxicity, anti-HER2 treatment is not used in combination with anthracyclines. In patients receiving sequential treatment, it begins with chemotherapy according to the AC (Adriamycin, Cyclophosphamide) regimen – 4 courses, then continues with trastuzumab with taxanes(2). In special cases of low risk early BC with tumours <1 cm, encouraging results were obtained when taxanes were used with trastuzumab alone(25). In cases of preoperative treatment, in patients with poor prognostic factors, dual anti-HER2 blockade in combination with chemotherapy results in a higher percentage of pCR than that obtained by chemotherapy with trastuzumab only. The NeoSphere study demonstrated the superiority of the pertuzumab-trastuzumab combination with docetaxel compared to other anti-HER2 combinations with docetaxel. However, the study did not show any effect on disease-free survival (DFS)(26). Based on the pooled analysis (CTNeoBC), it seems that the achievement of pCR after preoperative treatment translates into a better prognosis for these patients, but the analysis covered the full spectrum of different types of BC(27).
A recently reported pooled analysis confirmed the association of pCR with long-term treatment outcomes, although traditional factors of poor prognosis are still relevant even after pCR has been obtained. Hence treatment with adjuvant anti-HER2 with trastuzumab(28) should be continued.
If pCR is not achieved after preoperative treatment, the use of trastuzumab emtansine (T-DM1) in adjuvant treatment reduces the relative risk of recurrence or death by half. However, this therapeutic option is not yet reimbursed in Poland(29).
HER2 breast cancer features on ultrasonography
Several studies have shown that HER2+ cancers are characterised by a higher prevalence of calcifications. In Algazzar’s study(30), calcifications in HER2+ tumours were present in 70% compared to the HER2- group, in which calcifications were observed in 25.5% of lesions (p = 0.007). Similar results were obtained by Sun et al.(31), who reported that calcifications were more common in HER2+ tumours (p = 0.001). On the other hand, Boisserie-Lacroix et al.(32) showed that the presence of calcifications in MMG may indicate HER2 overexpression in cases of inconclusive immunohistochemical findings. Other features assessed by ultrasound, such as shape, margins, orientation, echogenicity, and effect behind the lesion, do not distinguish HER2+ from HER2- tumours(33, 34, 35). In Fig. 2, an example of this type of BC in multiparametric US is presented.
Fig. 2.
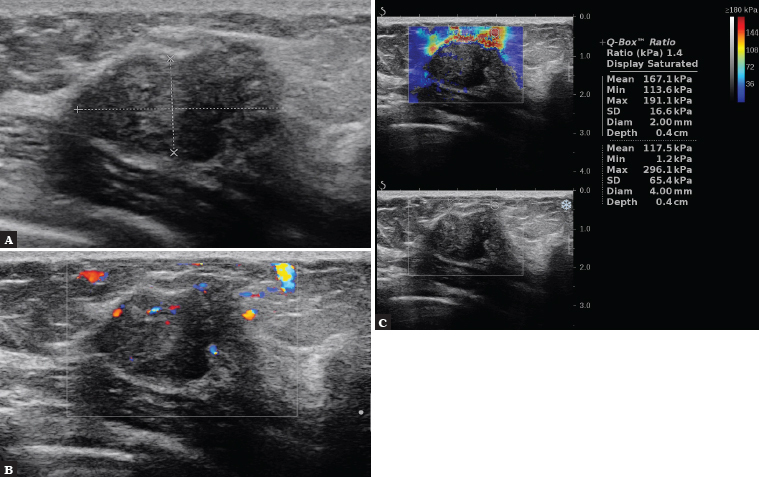
A 52-year-old patient. B-mode shows an oval, solid lesion with mixed echogenicity and partially non-circumscribed margins, with no acoustic shadow behind the lesion, bright echoes present, confirmed by MMG as calcification (A), with visible fine, irregularly shaped vessels located in the margins and within the lesion in CD (B). On SWE, the lesion is hard, Emax = 296 kPa, BIRADS 4c (C). Histopathology: HER2+ subtype
In contrast, another feature associated with HER2+ lesions is the multifocality of tumours; it was shown that unifocal tumours were more common in HER2- than in HER2+ subtypes (92.5% vs. 36.4%, respectively, p <0.001)(35).
Luminal A and B subtypes of BC
Introduction
Luminal breast cancer is an ER-positive type of tumour that accounts for almost 70% of all BC cases in Western populations(36). Luminal A tumours are characterised by high expression of the ER and PR (PR- greater than or equal to 20%), lack of HER2 overexpression or amplification, and Ki-67 proliferation index less than 20%. In this subtype, ER transcription factors activate genes whose expression is characteristic of the luminal epithelium that lines the mammary ducts(37). Additionally, these tumours show low expression of genes related to cell proliferation. Clinically, these cancers are mostly low grade, slow growing, and associated with the best prognosis. They are less sensitive to chemotherapy, and patients benefit more from primary hormone therapy. In general, hormone therapies are used as complementary treatments. In some cases, postmenopausal patients may receive NAC for 4–8 months prior to surgery or until a maximum response is achieved, and continued postoperatively. Aromatase inhibitors are more effective than tamoxifen in reducing tumour size and enabling breast-conserving surgery(38, 39, 40). A good response to preoperative hormone therapy, as expressed by a decrease in Ki67 or the preoperative prognostic index (PEPI), may, in combination with other clinical factors, guide the selection of patients with a favourable prognosis, not requiring adjuvant chemotherapy(41, 42, 43).
In contrast to the A subtype, the prognosis in cases of luminal B tumours is worse, and these tumours have a higher malignancy grade. They are characterised by the expression of ER, low level (<20%) or no expression of PR, the presence or absence of HER2 overexpression, and a higher fraction of Ki67 (>20%). Additionally, they have a high expression of genes related to proliferation, and lower expression of genes and proteins typical of luminal epithelium, such as PR and FOXA (but not of ER, which serves to distinguish luminal from non-luminal cancer types)(44,45). Indications for chemotherapy should include both the risk of recurrence and patient preferences. It could be considered in the absence of response to hormonotherapy and in selected cases of luminal B BC, especially with a high proliferation index, as well as in patients with additional risk factors. In luminal B cancers overexpressing HER2, the principles for the treatment of HER2+ BC are applied, and after surgery, treatment with trastuzumab and hormonotherapy is continued(2).
Luminal subtype – ultrasound features
On imaging examinations, luminal A subtype show some features, the knowledge of which can improve the diagnostic process. On MMG, luminal A and B cancers are far more likely to present as spiculated lesions in comparison with other subtypes(46). Publications describing this subtype in US studies(19) showed that luminal A cancers statistically more frequently show echogenic halo and acoustic shadow or no effect behind the lesion. An example of this type is presented in Fig. 3.
Fig. 3.
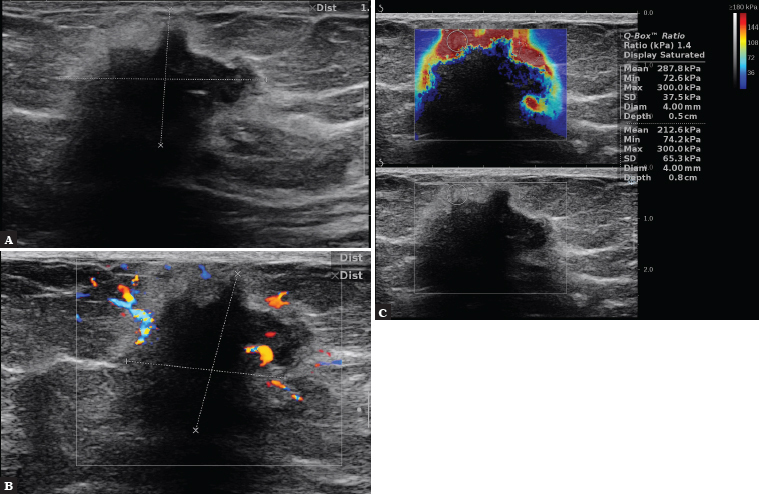
A 76-year-old patient. In B-mode, hypoechoic lesion with spicular and angular margins with irregular shape is visible, with the presence of halo around the lesion and acoustic shadow (A). CD assessment shows a disordered pattern of wide vessels on the periphery (B). On SWE, the lesion is hard in the peripheral parts, Emax = 300 k Pa. BIRADS 5 (C). Histopathology: Luminal A subtype
Histologically, the echogenic halo corresponds to tumour cells infiltrating adipose tissue and interlacing connective tissue fibres. According to the literature, echogenic halomay correspond to very fine, densely packed spicules that are too small to be visualised accurately. A thick, fuzzy, hyperechoic halo may also be a manifestation of peritumoral oedema resulting from inflammation or lymphatic outflow obstruction; in such cases the halo is visible superficially between the tumour and the skin, following the physiological direction of lymph drainage(47).
Tumours showing acoustic shadow are characterised by increased desmoplasia. This is because the slow growth of the tumour allows the surrounding stroma to induce the mobilisation of fibroblasts and inflammatory cells, and the proliferation of vascular structures, which leads to fibrosis(14). The acoustic shadow effect results from the reflection of the ultrasound beam or its attenuation by the neoplasm containing a significant connective/fibrous component(47). The shadow may not be visible behind the entire lesion but only part of it. In a 2019 paper by Liu H. et al.(48), it was shown that luminal A cancers are less likely to contain calcifications, rarely show blood flow, and display an intermediate wave propagation velocity value (SWV mean = 5.13 m/s) in dynamic SE, compared to other subtypes.
Luminal B subtype of BC on ultrasound is characterised by increased vascularity and lack of halo(19). An example of this type is presented in Fig. 4. The lack of halo suggests a higher incidence of recurrence and a high histological grade(32). Subtypes with the presence of HER2 overexpression are also characterised by increased neovasularisation.
Fig. 4.
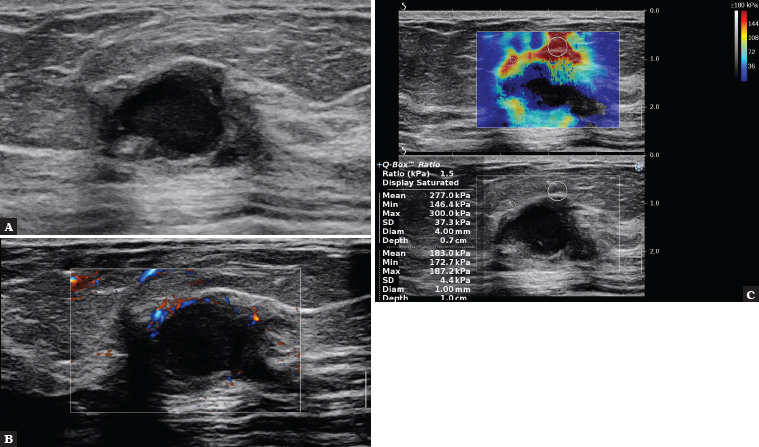
A 56-year-old patient. The B-mode examination shows an irregularly shaped solid-cystic tumour with indistinct margins, with enhancement behind the lesion (A). In the AngioPlus assessment, small vessels are visible on the periphery of the lesion (B); on SWE, the lesion is hard in the peripheral parts, Emax = 300 kPa. BIRADS 5 (C). Histopathology: Luminal B subtype
Conclusions
The authors of this paper wanted to draw attention to differential patterns of various subtypes of breast cancer, especially aggressive ones, such as TNBC and HER2+. Additionally, multiparametric US analysis of BC features is helpful in detecting aggressive subtypes, assigning the appropriate BIRADS classification category, and referring patients for biopsies.
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Author contributions
Original concept of study: KD-S, MG, JM. Writing of manuscript: KD-S, MG, JM, AK-Ć, PG. Analysis and interpretation of data: KD-S, AK-Ć. Final acceptation of manuscript: MG, JM. Collection, recording and/or compilation of data: KD-S. Critical review of manuscript: KD-S, MG, JM, AK-Ć, PG.
References
- 1.Wojciechowska U, Didkowska J. Illnesses and deaths from malignant neoplasms in Poland. National Registry Cancer, National Oncology Institute im. Maria Skłodowska-Curie – National Research Institute. http://onkologia.org.pl/raporty/ Available at.
- 2.Jassem J, Krzakowski M, Bobek-Billewicz B. Breast cancer. Oncol Clin Pract. 2020;16(5):207–260. et al. [Google Scholar]
- 3.Dobruch-Sobczak K. Współczesna ultrasonografia piersi. Medycyna po Dyplomie. 2021;30(9):36–42. [Google Scholar]
- 4.Bonotto M, Gerratana L, Poletto E, Driol P, Giangreco M, Russo S. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncologist. 2014;19:608–615. doi: 10.1634/theoncologist.2014-0002. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehmann BD, Jovanović B, Chen X, Estrada MV, Johnson KN, Shyr Y. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS One. 2016;11:e0157368. doi: 10.1371/journal.pone.0157368. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Yang D, Chen P, Yin X, Sun J, Li H. Efficacy and safety of neoadjuvant chemotherapy regimens for triple-negative breast cancer: a network meta-analysis. Aging (Albany NY) 2019;11:6286–6311. doi: 10.18632/aging.102188. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24:628–637. doi: 10.1038/s41591-018-0009-7. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahnen E, Lederer B, Hauke J, Loibl S, Kröber S, Schneeweiss A. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the GeparSixto Randomized Clinical Trial. JAMA Oncol. 2017;3:1378–1385. doi: 10.1001/jamaoncol.2017.1007. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuda N, Lee SJ, Ohtani S, Im Y-H, Lee E-S, Yokota I. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–2159. doi: 10.1056/NEJMoa1612645. et al. [DOI] [PubMed] [Google Scholar]
- 12.Cui M, Wang H, Yao X, Zhang D, Xie Y, Cui R. Circulating MicroRNAs in cancer: potential and challenge. Front Genet. 2019;10:626. doi: 10.3389/fgene.2019.00626. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Q, Liu HY, Liu D, Song YQ. Ultrasonographic features of triple-negative breast cancer: a comparison with other breast cancer subtypes. Asian Pac J Cancer Prev. 2015;16:3229–3232. doi: 10.7314/apjcp.2015.16.8.3229. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Tian J, Wang X, Wang Y, Zhang L, Jing H. Differences in multi-modal ultrasound imaging between triple negative and non-triple negative breast cancer. Ultrasound Med Biol. 2016;42:882–890. doi: 10.1016/j.ultrasmedbio.2015.12.003. et al. [DOI] [PubMed] [Google Scholar]
- 15.Li J-W, Zhang K, Shi Z-T, Zhang X, Xie J, Liu J-Y. Author correction: Triple-negative invasive breast carcinoma: the association between the sonographic appearances with clinicopathological feature. Sci Rep. 2020;10:4468. doi: 10.1038/s41598-020-61260-3. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dogan BE, Gonzalez-Angulo AM, Gilcrease M, Dryden MJ, Yang WT. Multimodality imaging of triple receptor-negative tumors with mammography, ultrasound, and MRI. AJR Am J Roentgenol. 2010;194:1160–1166. doi: 10.2214/AJR.09.2355. [DOI] [PubMed] [Google Scholar]
- 17.Ko ES, Lee BH, Kim H-A, Noh W-C, Kim MS, Lee S-A. Triple-negative breast cancer: correlation between imaging and pathological findings. Eur Radiol. 2010;20:1111–1117. doi: 10.1007/s00330-009-1656-3. [DOI] [PubMed] [Google Scholar]
- 18.Yeo SH, Kim GR, Lee SH, Moon WK. Comparison of ultrasound elastography and color doppler ultrasonography for distinguishing small triple-negative breast cancer from fibroadenoma. J Ultrasound Med. 2018;37:2135–2146. doi: 10.1002/jum.14564. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Li J, Xiao Y, Cui H, Du G, Wang Y. Identifying ultrasound and clinical features of breast cancer molecular subtypes by ensemble decision. Sci Rep. 2015;5:11085. doi: 10.1038/srep11085. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtis C, Shah SP, Chin S-F, Turashvili G, Rueda OM, Dunning MJ. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou BP, Hung MC. Dysregulation of cellular signaling by HER2/neu in breast cancer. Semin Oncol. 2003;30:38–48. doi: 10.1053/j.seminoncol.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi: 10.1016/S0140-6736(16)32616-2. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez EA, Romond EH, Suman VJ, Jeong J-H, Sledge G, Geter Jr CE. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–141. doi: 10.1056/NEJMoa1406281. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gianni L, Pienkowski T, Im Y-H, Roman L, Tseng L-M, Liu M-C. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. et al. [DOI] [PubMed] [Google Scholar]
- 27.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. et al. [DOI] [PubMed] [Google Scholar]
- 28.Swain SM, Macharia H, Cortes J, Dang C, Gianni L, Hurvitz S. Risk of recurrence and death in patients with early HER2-positive breast cancer who achieve a pathological complete response (pCR) after different types of HER2-targeted therapy: a retrospective exploratory analysis. Cancer Res. 2020;80:P1–18. et al. -01. [Google Scholar]
- 29.von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. et al. [DOI] [PubMed] [Google Scholar]
- 30.Algazzar MA, Elsayed E, Alhanafy AM, Mousa WA. Breast cancer imaging features as a predictor of the hormonal receptor status, HER2neu expression and molecular subtype. Egypt J Radiol Nuclear Med. 2020;51:93. [Google Scholar]
- 31.Sun SS, Zhang B, Zhao HM, Cao XC. Association between mammographic features and clinicopathological characteristics in invasive ductal carcinoma of breast cancer. Mol Clin Oncol. 2014;2:623–629. doi: 10.3892/mco.2014.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boisserie-Lacroix M, Hurtevent-Labrot G, Ferron S, Lippa N, Bonnefoi H, Mac Grogan G. Correlation between imaging and molecular classification of breast cancers. Diagn Interv Imaging. 2013;94:1069–1080. doi: 10.1016/j.diii.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, Seo BK, Lee J, Kim SJ, Cho KR, Lee KY. Correlation of ultrasound findings with histology, tumor grade, and biological markers in breast cancer. Acta Oncol. 2008;47:1531–1538. doi: 10.1080/02841860801971413. et al. [DOI] [PubMed] [Google Scholar]
- 34.Cho N. Molecular subtypes and imaging phenotypes of breast cancer. Ultrasonography. 2016;35:281–288. doi: 10.14366/usg.16030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elias SG, Adams A, Wisner DJ, Esserman L, van’t Veer LJ, Mali WPTM. Imaging features of HER2 overexpression in breast cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1464–1483. doi: 10.1158/1055-9965.EPI-13-1170. et al. [DOI] [PubMed] [Google Scholar]
- 36.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LAG. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014. p. 106. et al. [DOI] [PMC free article] [PubMed]
- 37.Weigelt B, Baehner FL, Reis-Filho JS. The contribution of gene expression profiling to breast cancer classification, prognostication, and prediction: a retrospective of the last decade. J Pathol. 2010;220:263–280. doi: 10.1002/path.2648. [DOI] [PubMed] [Google Scholar]
- 38.Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer J-U. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23:5108–5116. doi: 10.1200/JCO.2005.04.005. et al. [DOI] [PubMed] [Google Scholar]
- 39.Eiermann W, Paepke S, Appfelstaedt J, Llombart- Cussac A, Eremin J, Vinholes J. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol. 2001;12:1527–1532. doi: 10.1023/a:1013128213451. et al. [DOI] [PubMed] [Google Scholar]
- 40.Eggemann H, Ignatov A, Smith BJ, Altmann U, von Minckwitz G, Röhl FW. Adjuvant therapy with tamoxifen compared to aromatase inhibitors for 257 male breast cancer patients. Breast Cancer Res Treat. 2013;137:465–470. doi: 10.1007/s10549-012-2355-3. et al. [DOI] [PubMed] [Google Scholar]
- 41.Sousa B, Moser E, Cardoso F. An update on male breast cancer and future directions for research and treatment. Eur J Pharmacol. 2013;717:71–83. doi: 10.1016/j.ejphar.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 42.Cardoso F, Bartlett JMS, Slaets L, van Deurzen CHM, van Leeuwen-Stok E, Porter P. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol. 2018;29:405–417. doi: 10.1093/annonc/mdx651. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azim HA Jr, Michiels S, Zagouri F, Delaloge S, Filipits M, Namer M. Utility of prognostic genomic tests in breast cancer practice: The IMPAKT 2012 Working Group Consensus Statement. Ann Oncol. 2013;24:647–654. doi: 10.1093/annonc/mds645. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eroles P, Bosch A, Pérez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: Intrinsic subtypes and signaling pathways. Cancer Treat Rev. 2012;38:698–707. doi: 10.1016/j.ctrv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Ades F, Zardavas D. Pugliano L, Fumagalli D, de Azambuja E. Luminal B breast cancer: molecular characterization, clinical management, and future perspectives. J. Clin. Oncol. 2014;32:2794–2803. doi: 10.1200/JCO.2013.54.1870. Bozovic-Spasojevic. et al. [DOI] [PubMed] [Google Scholar]
- 46.Taneja S, Evans AJ, Rakha EA, Green AR, Ball G, Ellis IO. The mammographic correlations of a new immunohistochemical classification of invasive breast cancer. Clin Radiol. 2008;63:1228–1235. doi: 10.1016/j.crad.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Stavros A. Ultrasound of solid breast nodules: distinguishing benign from malignant. In: Breast ultrasound. Lippincott Williams & Wilkins, Philadelphia: 2004. pp. 459–460. –. 488. [Google Scholar]
- 48.Liu H, Wan J, Xu G, Xiang L-H, Fang Y, Ding S-S. Conventional US and 2-D shear wave elastography of virtual touch tissue imaging quantification: correlation with immunohistochemical subtypes of breast cancer. Ultrasound Med Biol. 2019;45:2612–2622. doi: 10.1016/j.ultrasmedbio.2019.06.421. et al. [DOI] [PubMed] [Google Scholar]


